Abstract
Background
Cyclooxygenase (COX)-2 inhibitors such as celecoxib were designed to preserve anti-inflammatory activity without inhibiting COX-1. Downregulation of COX-2 inhibits colorectal carcinogenesis.
Methods
The Selenium and Celecoxib Trial was a randomized, placebo-controlled trial of once-daily selenium 200 µg and celecoxib 400 mg, alone or together, for colorectal adenoma prevention. Men and women between age 40 and 80 years were eligible following colonoscopic removal of adenomas. The primary outcome was development of new adenomas. Celecoxib was suspended early because of cardiovascular toxicity in other trials. Accrual to selenium or placebo continued. Before suspension, 824 participants were randomly assigned to celecoxib or placebo, of whom 712 (86.4%) were available for analysis. All statistical tests were two-sided.
Results
In the placebo and celecoxib arms of 356 participants each, adenoma detection was 47.5% and 49.7% (relative risk [RR] = 1.04, 95% confidence interval [CI] = 0.90 to 1.21, P = .58), respectively, after median periods of 13.6 and 14.2 months on intervention. Among participants colonoscoped within 12 months of discontinuing intervention (n = 244), overall adenoma recurrence (RR = 0.69, 95% CI = 0.48 to 0.98, P = .04) and recurrence with advanced adenomas (RR = 0.23, 95% CI = 0.07 to 0.80, P = .02) were reduced with celecoxib. Reduction of adenoma recurrence was greatest in participants with previous advanced adenomas. Celecoxib increased risk of hypertension in participants with pre-existing cardiovascular risk factors compared with placebo (hazard ratio = 2.19, 95% CI = 1.07 to 4.50, P = .03).
Conclusions
Limited-duration celecoxib prevents adenoma recurrence in patients with prior high-risk adenomas, in whom strategies to minimize cardiovascular toxicity might be feasible.
Cyclooxygenase (COX)-2 catalyzes production of pro-inflammatory prostaglandin E2 (PGE2) from arachidonic acid (1). Aspirin and other nonsteroidal anti-inflammatory drug inhibitors of COX-2 and PGE2 synthesis prevent experimental colorectal tumorigenesis (2). Prevention of colorectal cancer by aspirin and other NSAIDs is supported by evidence from observational studies and clinical trials (3–6).
Selective COX-2 inhibitors (coxibs), such as celecoxib (Celebrex) and rofecoxib (Vioxx), were introduced to optimize anti-inflammatory inhibition of COX-2 and avoid gastrointestinal and other toxicities attributed to inhibition of constitutive COX-1 expression by aspirin and other nonselective NSAIDS (7). Regression of colorectal polyps in patients with familial adenomatous polyposis while taking coxibs (8,9) led to colorectal neoplasia chemoprevention trials. Three randomized placebo-controlled coxib trials for colorectal adenoma prevention in patients who had undergone colonoscopic clearance of all existing adenomas were initiated between 1999 and 2000: The Adenomatous Polyp Prevention on Vioxx (APPROVe) Trial, comparing rofecoxib 25 mg daily to placebo (10); The Adenoma Prevention with Celecoxib (APC) Trial, comparing celecoxib 200 or 400 mg, both twice daily, to placebo (11); and The Prevention of Colorectal Sporadic Adenomatous Polyps (PreSAP) Trial, comparing celecoxib as a once-daily dose of 400 mg to placebo (12). We launched a randomized, placebo-controlled, two-by-two factorial trial of selenium and celecoxib (Sel/Cel) for the prevention of colorectal adenoma (13). In 2004, increased risk of coxib-associated serious cardiovascular events in APPROVe (14) and APC (11,15) prompted the suspension of all coxib trials, including Sel/Cel. With approval of the Federal Drug Administration and the National Cancer Institute, we continued Sel/Cel as a selenium-only trial.
Adverse event rates varied among the coxib studies according to drug and dosing regimen. The PreSAP once-daily 400 mg dose of celecoxib (also the celecoxib dosing in Sel/Cel) was not associated with an increase in serious cardiovascular events (12), but renal and hypertensive adverse events were more common (relative risk [RR] = 1.35, 95% confidence interval [CI] = 1.08 to 1.69), despite an overall decrease in mean systolic and diastolic blood pressure in both the celecoxib and placebo arms. In a pooled analysis of six colorectal adenoma and other celecoxib trials (16), which included interim data from Sel/Cel, celecoxib-related cardiovascular risk varied with the total dose of the drug and the frequency of its administration (ie, once or twice daily). Pre-existing cardiovascular risk factors compounded this risk. Based on the absence of increased cardiovascular adverse events in PreSAP and Sel/Cel and no apparent adverse effect on blood pressure in PreSAP with a celecoxib dose of 400 mg given once daily, this dosing regimen was deemed less toxic than a total daily dose of 800 mg and a daily dose of 400 mg administered as 200 mg given twice daily (16).
The APPROVe, PreSAP, and APC trials all reported statistically significant reductions in adenoma development with a coxib (RR = 0.55–0.76) (10–12) and evidence that this effect was short lived after coxib withdrawal (10,11,17). While not designed for this purpose, the recently completed Sel/Cel Trial allowed us to evaluate the effect of celecoxib at the 400 mg once-daily dose for a shorter duration than in the earlier trials. We describe these results together with additional data on celecoxib toxicity and adenoma development following withdrawal of the agent in this report. Results of the selenium intervention in Sel/Cel are reported in a companion article (18).
Methods
Trial Design
Sel/Cel was designed as a phase III randomized, placebo-controlled, two-by-two factorial trial of celecoxib crossed with selenium for preventing recurrent colorectal adenomas (Clinical Trials.gov No. NCT00078897). As reported (13), on the recommendation of our External Data and Safety Monitoring Committee (EDSMC), the celecoxib arm of Sel/Cel was suspended in December 2004 because of reports of celecoxib-associated toxicity from other trials (11,12,14,19). Accordingly, the trial was modified to a randomized, two-arm design comparing selenium with placebo. Participants randomly assigned during the factorial phase were retained in the appropriate selenium or placebo arm and were no longer allocated celecoxib or its placebo.
Participants and Eligibility
Participants were recruited through clinical centers in Arizona, Colorado, Texas, and New York following ambulatory colonoscopies. Eligible participants were between age 40 and 80 years and had undergone colonoscopic removal of one or more colorectal adenomas 3 mm or larger within six months prior to random assignment. Patients with a family history of familial adenomatous polyposis or Lynch syndrome or a diagnosis of invasive cancer within the previous five years were excluded. Individuals with unstable cardiac disease, uncontrolled hypertension, poorly controlled diabetes mellitus, or renal insufficiency were excluded. If already taking regular low-dose (≤81 mg daily), continuation was allowed. Adenomatous histology was confirmed by review of pathology reports from local study sites.
All data and biospecimens were collected, managed, and archived at the University of Arizona Cancer Center (Tucson, AZ). The University of Arizona Institutional Review Board (IRB) oversaw the trial in accordance with requirements of the local IRB at each study site. Written informed consent was obtained from all participants.
Intervention
The active intervention was celecoxib 400 mg as a single daily dose of two 200 mg capsules. Celecoxib (Celebrex) 200 mg capsules and matching placebo were provided by Pfizer (New York, NY). The celecoxib content of the drug was assayed annually by high performance liquid chromatography in the University of Arizona Cancer Center Analytical Chemistry Shared Resource and ranged from 189 to 204 mg per capsule (20).
Outcomes
The primary outcome was any colorectal adenoma or cancer detected at a follow-up colonoscopy performed six or more months after random assignment. Colorectal cancers diagnosed during follow-up were handled as adenoma recurrences and recorded separately. Colonoscopy follow-up interval was determined by participants’ physicians according to published guidelines (21). Adenoma number, location, size, and histology were abstracted from endoscopic and pathology reports. Cumulative adenoma recurrence was ascertained over all follow-up colonoscopies.
Secondary outcomes included occurrence of multiple (≥3) or advanced adenomas (defined by one or more of the following features: 10 mm or larger, with tubulovillous or villous tissue architecture, and/or with high-grade dysplasia). Toxicity endpoints included serious adverse cardiovascular and gastrointestinal events and hypertension.
Sample Size
Details of the sample size and study power have been reported (13). Sample size for the original factorial design was 400 participants per arm: celecoxib, selenium, celecoxib + selenium, and double placebo. Thus, random assignment of 1600 participants was required and the selenium and celecoxib interventions were to be tested independently. After suspension of celecoxib, the statistical plan was modified with EDSMC approval. The modified plan included a planned test for interaction between selenium and celecoxib. In the absence of a statistically significant interaction, all celecoxib-specific analyses were to be conducted separately from the selenium-specific analyses. The likelihood ratio test (LRT) for interaction between selenium and celecoxib was not statistically significant (P = .78). Thus, the celecoxib-specific analysis includes those 824 participants randomly assigned prior to the suspension of celecoxib.
Random Assignment
Random assignment was conducted using a Structured Query Language function that first checked for previous random assignment and a valid clinic identification number. Random assignment was stratified by clinic site and use of low-dose aspirin. A block size of four was used for the factorial design.
Statistical Methods
Log-binomial regression was used to estimate the relative risk and 95% confidence interval for primary and secondary adenoma outcomes. Poisson regression with robust variance was planned as an alternative method to generate the relative risk and 95% confidence interval in the event of convergence failure of the log-binomial model (22,23). Although the trial was not powered to test for interactions, planned subgroup analyses were conducted to explore potential effect modification by baseline low-dose aspirin use, sex, age, and baseline adenoma category (nonadvanced or advanced). For each potential modifier, an LRT was used to compare a model containing an interaction term between celecoxib and the modifier with a reduced model without an interaction term.
For toxicity analysis, time at risk while taking study intervention was recorded as the period from the second day after starting the intervention (celecoxib or placebo) until the 14th day after stopping. For analysis of postintervention toxicity, the time from the 15th day after stopping celecoxib or placebo until finishing participation in the selenium/placebo arm of the trial was recorded. Event rates per 1000 person-years were calculated, and Cox regression was used to estimate hazard ratios. All Cox regression models were adjusted for the design variables of random assignment to selenium, baseline use of low-dose aspirin, and clinic site. Low-dose aspirin use and sex were assessed in stratified analyses; for each outcome of interest and potential modifier, an LRT was used as described. The proportional hazards assumption was tested in all models using an interaction term between the intervention and log-transformed time.
All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
Results
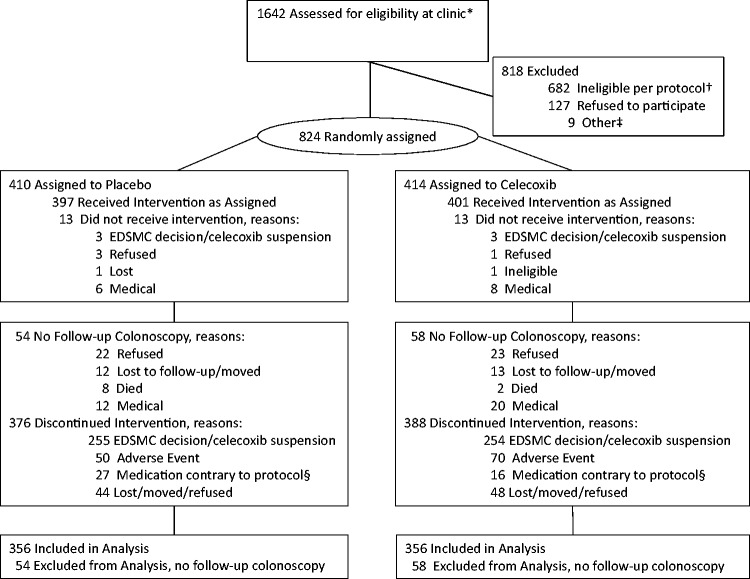
A total of 824 participants were randomly assigned (414 to celecoxib and 410 to placebo) from initiation of the trial on November 27, 2001, through suspension of the celecoxib arm on December 16, 2004 (Figure 1). The celecoxib and placebo groups were well balanced (Table 1). At the time celecoxib was suspended, 5.1% of the placebo arm and 3.1% of the celecoxib arm participants had completed taking the intervention. Of 824 randomly assigned, the median time for taking placebo and celecoxib, respectively, was 13.6 and 14.2 months. Of those randomly assigned, 712 underwent at least one follow-up colonoscopy, with 356 participants each in the placebo (86.8%) and celecoxib (86.0%) groups. The median period from random assignment to last colonoscopy was 57.9 (range = 7.7–130.0) and 63.1 months (range = 7.1–129.1), respectively, in the placebo and celecoxib groups (Table 2). A total of 447 participants underwent at least one colonoscopy within 3.5 years, and 665 within 5.5 years of random assignment. Participant baseline and adenoma characteristics according to colonoscopy surveillance interval are shown in Supplementary Table 1 (available online).
Figure 1.
Consort diagram. *Participants were recruited through clinical centers in Arizona, Colorado, Texas, and New York following ambulatory colonoscopies conducted at high-volume endoscopy facilities. †Not eligible per protocol, including medical conditions (264), medication use (145), regular high-dose aspirin/NSAID use (171), clinical lab results (8), supplemental selenium use (23), other (71). ‡Other, including five lost to follow-up or moved, one deceased, three toxicity during placebo run-in. §Medications contrary to protocol include: Celebrex, VIOXX, other NSAIDs, supplemental selenium, coumadin.
Table 1.
Baseline participant and colorectal adenoma characteristics
| Characteristic | Randomly assigned (n = 824) |
Participants with any follow-up (n = 712) |
||
|---|---|---|---|---|
| Placebo No. (%) (n = 410) | Celecoxib No. (%) (n = 414) | Placebo No. (%) (n = 356) | Celecoxib No. (%) (n = 356) | |
| Clinic | ||||
| Baylor | 1 (0.2) | 1 (0.2) | 0 (0.0) | 1 (0.3) |
| Phoenix | 230 (56.1) | 231 (55.8) | 201 (56.5) | 196 (55.1) |
| Colorado | 92 (22.4) | 91 (22.0) | 83 (23.3) | 83 (23.3) |
| Tucson | 84 (20.5) | 88 (21.3) | 69 (19.4) | 74 (20.8) |
| Mayo | 3 (0.7) | 3 (0.7) | 3 (0.8) | 2 (0.6) |
| Mean age ± SD, y | 62.5 ± 9.1 | 63.4 ± 9.5 | 62.6 ± 9.0 | 63.6 ± 9.4 |
| Age group | ||||
| <50 y | 24 (5.9) | 31 (7.5) | 22 (6.2) | 27 (7.6) |
| 0–59 y | 128 (31.2) | 106 (25.6) | 106 (29.8) | 84 (23.6) |
| 60–69 y | 161 (39.3) | 162 (39.1) | 144 (40.4) | 143 (40.2) |
| 70+ y | 97 (23.7) | 115 (27.8) | 84 (23.6) | 102 (28.7) |
| Men | 282 (68.8) | 277 (66.9) | 245 (68.8) | 237 (66.6) |
| Race* | ||||
| White | 385 (94.4) | 393 (94.9) | 338 (95.5) | 340 (95.5) |
| Black/African | 9 (2.2) | 7 (1.7) | 5 (1.4) | 6 (1.7) |
| Asian | 6 (1.5) | 6 (1.4) | 6 (1.7) | 6 (1.7) |
| AmIndian/Alaskan | 2 (0.5) | 2 (0.5) | 0 (0.0) | 0 (0.0) |
| Other/mixed | 6 (1.5) | 6 (1.4) | 5 (1.4) | 4 (1.1) |
| Missing | 2 | 0 | 2 | 0 |
| Hispanic/Latino | 25 (6.1) | 17 (4.1) | 17 (4.8) | 15 (4.2) |
| Education | ||||
| <High school | 6 (1.5) | 12 (2.9) | 3 (0.8) | 8 (2.3) |
| High school or GED | 75 (18.3) | 81 (19.6) | 62 (17.4) | 67 (18.8) |
| Some college | 126 (30.7) | 127 (30.7) | 112 (31.5) | 105 (29.5) |
| Bachelor's | 88 (21.5) | 80 (19.3) | 79 (22.2) | 75 (21.1) |
| Graduate/professional | 115 (28.0) | 114 (27.5) | 100 (28.1) | 101 (28.4) |
| Mean BMI† ± SD | 29.3 ± 5.0 | 29.0 ± 4.8 | 29.3 ± 5.2 | 28.9 ± 4.7 |
| Cigarette smoking status‡ | ||||
| Current | 42 (10.5) | 37 (9.2) | 32 (9.1) | 27 (7.7) |
| Previous | 192 (47.9) | 213 (52.7) | 168 (48.0) | 180 (51.6) |
| Never | 167 (41.6) | 154 (38.1) | 150 (42.9) | 142 (40.7) |
| Missing | 9 | 10 | 6 | 7 |
| History of colorectal polyps | 111 (27.7) | 122 (30.0) | 99 (28.4) | 106 (30.3) |
| Missing | 9 | 7 | 7 | 6 |
| Family history of CRC§ | 71 (18.4) | 89 (22.7) | 63 (18.9) | 78 (23.0) |
| Missing | 25 | 22 | 22 | 17 |
| Diabetes | 32 (7.8) | 36 (8.7) | 28 (7.9) | 27 (7.6) |
| Personal history of cancer∥ | 25 (6.1) | 24 (5.8) | 22 (6.2) | 23 (6.5) |
| Personal history of skin cancer¶ | 62 (15.1) | 55 (13.3) | 56 (15.7) | 48 (13.5) |
| Aspirin use in the past 20 y# | ||||
| <1 y | 232 (56.6) | 249 (60.1) | 200 (56.2) | 217 (61.0) |
| 1–<5 y | 92 (22.4) | 82 (19.8) | 83 (23.3) | 65 (18.3) |
| 5–<10 y | 38 (9.3) | 35 (8.5) | 32 (9.0) | 34 (9.6) |
| ≥10 y | 48 (11.7) | 48 (11.6) | 41 (11.5) | 40 (11.2) |
| Aspirin use at random assignment | 199 (48.5) | 202 (48.8) | 180 (50.6) | 178 (50.0) |
| NSAID use in the past 20 y** | ||||
| <1 y | 367 (89.5) | 372 (89.9) | 321 (90.2) | 324 (91.0) |
| 1–<5 y | 28 (6.8) | 25 (6.0) | 24 (6.7) | 20 (5.6) |
| 5–<10 y | 5 (1.2) | 11 (2.7) | 3 (0.8) | 9 (2.5) |
| ≥10 y | 10 (2.4) | 6 (1.4) | 8 (2.2) | 3 (0.8) |
| Supplement user | 272 (69.2) | 265 (67.6) | 240 (69.6) | 236 (68.4) |
| Missing | 17 | 22 | 11 | 11 |
| No. of adenomas | ||||
| 1 | 250 (61.0) | 249 (60.1) | 220 (61.8) | 203 (57.0) |
| 2 | 92 (22.4) | 90 (21.7) | 75 (21.1) | 84 (23.6) |
| 3+ | 68 (16.6) | 75 (18.1) | 61 (17.1) | 69 (19.4) |
| Proximal adenoma | 242 (62.7) | 228 (60.3) | 209 (62.4) | 203 (61.9) |
| Missing | 24 | 36 | 21 | 28 |
| Distal/rectal adenoma | 223 (57.8) | 218 (57.7) | 194 (57.9) | 188 (57.3) |
| Missing | 24 | 36 | 21 | 28 |
| Large adenoma (≥10 mm) | 113 (28.3) | 111 (28.2) | 102 (29.5) | 98 (29.2) |
| Missing | 11 | 20 | 10 | 20 |
| Tubulovillous or villous tissue architecture | 54 (13.2) | 53 (12.8) | 49 (13.8) | 50 (14.0) |
| High-grade dysplasia | 8 (2.0) | 8 (1.9) | 7 (2.0) | 8 (2.3) |
| Advanced adenoma†† | 130 (32.4) | 128 (32.2) | 114 (32.8) | 115 (33.9) |
| Missing | 9 | 17 | 8 | 17 |
| High-risk‡‡ | 158 (39.3) | 165 (41.5) | 140 (40.1) | 150 (44.1) |
| Missing | 8 | 16 | 7 | 16 |
*Eighteen participants marked more than one race, including 17 who selected a race they identified with primarily (and classified accordingly) and one who was unable to identify with one group (classified as mixed).
†Body mass index calculated as 703*(weight in pounds)/(height in inches)2.
‡Ever smoker defined as 100 or more cigarettes.
§Family history of colorectal cancer in a first-degree relative.
∥Excluding nonmelanoma skin cancer.
¶Including basal cell, squamous cell, and skin cancer of unknown type.
#Number of years in total within the past twenty years taking aspirin or aspirin-containing products at least twice a week for at least six consecutive months.
**Number of years in total within the past twenty years taking a nonsteroidal anti-inflammatory drug, excluding aspirin, either over-the-counter or prescription, at least twice a week for at least six consecutive months.
††Advanced adenoma defined as having one or more of the following features: adenoma ≥ 10 mm, with tubulovillous or villous architecture and/or with high-grade dysplasia.
‡‡High-risk defined as having an advanced adenoma or three or more adenomas.
Table 2.
Duration of intervention and follow-up*
| Randomized participants | Placebo | Celecoxib |
|---|---|---|
| No. (%) | No. (%) | |
| (n = 410) | (n = 414) | |
| Duration of intervention, median (Q1, Q3), mo | 13.6 (4.5, 22.6) | 14.2 (4.9, 22.7) |
| Participants with follow-up within 3.5 y of qualifying colonoscopy (n = 447) | 226 | 221 |
| No. of colonoscopies | ||
| 1 | 217 (96.0) | 215 (97.3) |
| 2 | 9 (4.0) | 6 (2.7) |
| Median mo from random assignment to last follow-up within 3.5 y (Q1, Q3) | 31.2 (28.7, 33.4) | 31.3 (26.5, 33.4) |
| Participants with follow-up within 5.5 y of qualifying colonoscopy (n = 665) | 336 | 329 |
| No. of colonoscopies | ||
| 1 | 298 (88.7) | 290 (88.2) |
| 2 | 35 (10.4) | 34 (10.3) |
| 3 | 3 (0.9) | 5 (1.5) |
| Median mo from random assignment to last follow-up within 5.5 y (Q1, Q3) | 34.8 (31.2, 46.5) | 34.8 (31.3, 45.7) |
| Participants with any follow-up (n = 712) | 356 | 356 |
| No. of colonoscopies | ||
| 1 | 192 (53.9) | 178 (50.0) |
| 2 | 126 (35.4) | 138 (38.8) |
| 3 | 34 (9.6) | 34 (9.6) |
| 4 | 4 (1.1) | 5 (1.4) |
| 5 | 0 (0.0) | 1 (0.3) |
| Median mo from random assignment to last follow-up (Q1, Q3) | 57.9 (35.1, 84.2) | 63.1 (37.2, 90.7) |
*Q1 = 25th percentile; Q3 = 75th percentile.
Intention-to-treat analyses were conducted over follow-up periods of varying duration: 1) 712 of the 824 participants (86.4%) with any follow-up procedure at least six months after random assignment; 2) 447 participants (54.2%) with a follow-up colonoscopy within 3.5 years of qualifying colonoscopy; and 3) 665 participants (80.7%) with a follow-up colonoscopy within 5.5 years of qualification. The 3.5- and 5.5-year time intervals, respectively, correspond to guidelines for colonoscopy surveillance intervals following removal of advanced and nonadvanced adenomas (21). The median time on intervention was similar among the three groups of participants as defined by the time between qualifying and follow-up colonoscopies, which was, respectively, 14.8 (range = 0–35.8), 15.3 (range = 0–34.2), and 14.9 (range = 0–35.7) months. For participants undergoing any follow-up colonoscopy at least six months after qualifying colonoscopy, or within 3.5 or 5.5 years of qualifying colonoscopy, respectively, the median time between discontinuing intervention and follow-up colonoscopy was 47.0 (range = 0–112.8), 11.3 (range = 0–35.9), and 22.3 (range = 0–59.9) months. The distribution of time on- and off-intervention at follow-up is shown in Supplementary Figure 1 (available online).
Among 712 participants, recurrent adenomas occurred in 47.5% and 49.7%, respectively, of those randomly assigned to placebo and celecoxib (Table 3). Recurrences included eight cancers that were diagnosed before celecoxib was suspended (2 in the double placebo, 2 in the celecoxib, 3 in the selenium, and 1 in the celecoxib + selenium group). The relative risk of adenoma recurrence with celecoxib was 1.04 (95% CI = 0.90 to 1.21, P = .58). For those undergoing follow-up colonoscopy within 3.5 and 5.5 years, respectively, there were statistically nonsignificant 21% (RR = 0.79, 95% CI = 0.61 to 1.01, P = .06) and 6% (RR = 0.94, 95% CI = 0.77 to 1.13, P = .50) reductions of adenoma recurrence with celecoxib.
Table 3.
Recurrent colorectal adenoma among participants randomly assigned to celecoxib and placebo*
| Outcome | Any colorectal adenoma at follow-up colonoscopy within 3.5 y of qualification (n = 447) |
Any metachronous neoplasia at follow-up colonoscopy within 5.5 y of qualification (n = 665) |
Any colorectal neoplasia at follow-up colonoscopy (n = 712) |
|||
|---|---|---|---|---|---|---|
| Placebo | Celecoxib | Placebo | Celecoxib | Placebo | Celecoxib | |
| Primary outcome | ||||||
| Neoplasia/total (%) | 90/226 (39.8) | 69/221 (31.2) | 135/336 (40.2) | 124/329 (37.7) | 169/356 (47.5) | 177/356 (49.7) |
| RR (95% CI), P | 0.79 (0.61 to 1.01), .06 | 0.94 (0.77 to 1.13), .50 | 1.04 (0.90 to 1.21), .58 | |||
| Secondary outcome | ||||||
| Advanced neoplasia† (%) | 18/223 (8.1) | 8/214 (3.7) | 32/332 (9.6) | 28/319 (8.8) | 43/349 (12.3) | 39/346 (11.3) |
| RR (95% CI), P | 0.46 (0.20 to 1.03), .06 | 0.91 (0.56 to 1.47), .70 | 0.92 (0.62 to 1.39), .70 | |||
| Multiple neoplasms‡ (%) | 11/218 (5.0) | 5/218 (2.3) | 28/328 (8.5) | 27/323 (8.4) | 53/350 (15.1) | 50/347 (14.4) |
| RR (95% CI), P | 0.47 (0.16 to 1.32), .15 | 0.97 (0.59 to 1.60), .91 | 0.96 (0.67 to 1.37), .83 | |||
| Proximal neoplasia§ (%) | 63/223 (28.3) | 42/219 (19.2) | 98/333 (29.4) | 86/324 (26.5) | 132/354 (37.3) | 138/351 (39.3) |
| RR (95% CI), P | 0.68 (0.48 to 0.96), .03 | 0.90 (0.71 to 1.15), .41 | 1.05 (0.87 to 1.27), .58 | |||
| Distal neoplasia∥ (%) | 40/223 (17.9) | 34/219 (15.5) | 67/333 (20.1) | 60/324 (18.5) | 84/354 (23.7) | 83/351 (23.6) |
| RR (95% CI), P | 0.86 (0.57 to 1.31), .49 | 0.91 (0.67 to 1.24), .55 | 0.98 (0.76 to 1.28), .90 | |||
| Potential modifier | ||||||
| Aspirin users (%) | 42/112 (37.5) | 33/107 (30.8) | 63/168 (37.5) | 60/162 (37.0) | 82/180 (45.6) | 88/178 (49.4) |
| RR (95% CI), P | 0.83 (0.58 to 1.20), .33 | 0.99 (0.74 to 1.31), .92 | 1.08 (0.87 to 1.35), .47 | |||
| Non-aspirin users (%) | 48/114 (42.1) | 36/114 (31.6) | 72/168 (42.9) | 64/167 (38.3) | 86/176 (48.9) | 89/178 (50.0) |
| RR (95% CI), P | 0.74 (0.53 to 1.05), .09 | 0.89 (0.68 to 1.15), .36 | 1.00 (0.81 to 1.23), .99 | |||
| LRT Pinteraction | .68 | .59 | .59 | |||
| Men (%) | 68/155 (43.9) | 42/138 (30.4) | 101/230 (43.9) | 83/217 (38.2) | 124/245 (50.6) | 122/237 (51.5) |
| RR (95% CI), P | 0.69 (0.51 to 0.94), .02 | 0.87 (0.70 to 1.09), .22 | 1.01 (0.85 to 1.20), .92 | |||
| Women (%) | 22/71 (31.0) | 27/83 (32.5) | 34/106 (32.1) | 41/112 (36.6) | 45/111 (40.5) | 55/119 (46.2) |
| RR (95% CI), P | 1.03 (0.65 to 1.62), .91 | 1.16 (0.81 to 1.66), .43 | 1.14 (0.86 to 1.53), .36 | |||
| LRT Pinteraction | .15 | .22 | .49 | |||
| Age ≤ 63 y (%) | 41/119 (34.5) | 29/101 (28.7) | 61/172 (35.5) | 60/156 (38.5) | 74/179 (41.3) | 86/172 (50.0) |
| RR (95% CI) | 0.83 (0.56 to 1.23), .36 | 1.07 (0.80 to 1.42), .65 | 1.18 (0.94 to 1.49), .16 | |||
| Age > 63 y (%) | 49/107 (45.8) | 40/120 (33.3) | 74/164 (45.1) | 64/173 (37.0) | 95/177 (53.7) | 91/184 (49.5) |
| RR (95% CI), P | 0.72 (0.52 to 1.00), .05 | 0.81 (0.62 to 1.04), .10 | 0.91 (0.75 to 1.11), .36 | |||
| LRT Pinteraction | .57 | .15 | .07 | |||
| Baseline nonadvanced adenoma (%) | 44/127 (34.7) | 39/128 (30.5) | 77/219 (35.2) | 69/202 (34.2) | 94/234 (40.2) | 102/224 (45.5) |
| RR (95% CI), P | 0.89 (0.63 to 1.27), .53 | 0.96 (0.74 to 1.25), .78 | 1.13 (0.91 to 1.39), .27 | |||
| Baseline advanced adenoma (%) | 44/93 (47.3) | 24/80 (30.0) | 55/110 (50.0) | 45/110 (40.9) | 70/114 (61.4) | 64/115 (55.7) |
| RR (95% CI), P | 0.61 (0.41 to 0.89), .01 | 0.81 (0.61 to 1.09), .17 | 0.91 (0.73 to 1.13), .37 | |||
| LRT Pinteraction | .14 | .38 | .14 | |||
*Two-sided log-binomial regression analysis adjusted for random assignment to selenium, regular aspirin use, and clinic. CI = confidence interval; LRT = two-sided likelihood ratio test; RR = relative risk.
†Advanced neoplasia defined as colorectal cancer or advanced adenoma. Advanced status uncertain for 17 of the 712 participants with any follow-up.
‡Number of adenomas uncertain for 15 of the 712 participants with any follow-up.
§Proximal neoplasia defined as any adenoma or cancer detected in the cecum, ascending colon, hepatic flexure, or transverse colon; missing for seven of the 712 participants with any follow-up.
∥Distal neoplasia defined as any adenoma or cancer detected in the splenic flexure, descending colon, sigmoid colon, or rectum; missing for seven of the 712 participants with any follow-up.
Among participants undergoing endpoint colonoscopy within 3.5 years, recurrences with advanced (3.7% vs 8.1%) and proximal (19.2% vs 28.3%) adenomas were reduced with intervention (Table 3); the difference was statistically significant only for recurrence with proximal adenomas (RR = 0.68, 95% CI = 0.48 to 0.96, P = .03). In this same subgroup, adenoma recurrence was statistically significantly reduced with celecoxib (RR = 0.61, 95% CI = 0.41 to 0.89, P = .01) among participants with advanced adenomas at baseline.
In the subgroup with a shorter interval of 12 months or less between stopping intervention and follow-up colonoscopy (n = 244), recurrence with any adenoma was statistically significantly reduced in the celecoxib group (RR = 0.69, 95% CI = 0.48 to 0.98, P = .04) (Table 4); this effect was no longer observed when the analysis was extended to include individuals (n = 400) for whom the interval between intervention suspension and follow-up colonoscopy was increased to two years. Additionally, in the subgroup with the shorter 12-month or less interval between stopping intervention and follow-up colonoscopy, recurrence with advanced adenomas was reduced with celecoxib (RR = 0.23, 95% CI = 0.07 to 0.80, P = .02) (Table 4).
Table 4.
Recurrent colorectal adenomas, restricted to participants with follow-up colonoscopy performed within 1 y of discontinuing intervention*
| Outcomes and potential modifiers | Colorectal adenoma at follow-up within 1 y of intervention cessation (n = 244) |
|||
|---|---|---|---|---|
| Placebo total at risk (%) | Celecoxib total at risk (%) | |||
| Any neoplasia | 50/121 (41.3) | 34/123 (27.6) | ||
| RR (95% CI), P | 0.69 (0.48 to 0.98), .04 | |||
| Advanced neoplasia | 12/119 (10.1) | 3/119 (2.5) | ||
| RR (95% CI), P | 0.23 (0.07 to 0.80), .02 | |||
| Multiple neoplasms | 5/115 (4.4) | 4/122 (3.3) | ||
| RR (95% CI), P | 0.86 (0.24 to 3.07), .82 | |||
| Proximal neoplasia | 36/118 (30.5) | 22/122 (18.0) | ||
| RR (95% CI), P | 0.61 (0.38 to 0.98), .04 | |||
| Distal neoplasia | 18/118 (15.3) | 18/122 (14.8) | ||
| RR (95% CI), P | 1.00 (0.55 to 1.81), .99 | |||
| Potential modifiers | ||||
| Aspirin users | 25/56 (44.6) | 14/50 (28.0) | ||
| RR (95% CI), P | 0.61 (0.36 to 1.03), .07 | |||
| Non-aspirin users | 25/65 (38.5) | 20/73 (27.4) | ||
| RR (95% CI), P | 0.77 (0.48 to 1.24), .28 | |||
| LRT Pinteraction | .61 | |||
| Men | 37/85 (43.5) | 22/81 (27.2) | ||
| RR (95% CI), P | 0.62 (0.41 to 0.94), .03 | |||
| Women | 13/36 (36.1) | 12/42 (28.6) | ||
| RR (95% CI), P | 0.68 (0.35 to 1.36), .28 | |||
| LRT Pinteraction | .44 | |||
| Age ≤ 63 y | 23/70 (32.9) | 18/58 (31.0) | ||
| RR (95% CI), P | 1.00 (0.61 to 1.65), .99 | |||
| Age > 63 y | 27/51 (52.9) | 16/65 (24.6) | ||
| RR (95% CI), P† | 0.47 (0.28 to 0.77), .003 | |||
| LRT Pinteraction | .04 | |||
| Nonadvanced baseline adenoma | 22/66 (33.3) | 22/74 (29.7) | ||
| RR (95% CI), P | 0.91 (0.56 to 1.50), .72 | |||
| Advanced baseline adenoma | 26/52 (50.0) | 12/47 (25.5) | ||
| RR (95% CI), P | 0.53 (0.30 to 0.92), .02 | |||
| LRT Pinteraction | .11 | |||
*Two-sided log-binomial regression analysis adjusted for random assignment to selenium, regular aspirin use, and clinic. CI = confidence interval; LRT = two-sided likelihood ratio test; RR = relative risk.
†The convergence of the log-binomial regression model was questionable for participants older than age 63 years; the relative risk estimates presented for the age-stratified analysis were generated by Poisson regression with robust variance, an alternative for generating relative risk estimates.
As a result of the early suspension of celecoxib, the length of time for which celecoxib or placebo was taken correlated with the date of random assignment. To explore the influence of the length of time on celecoxib, we analyzed adenoma recurrence by year of random assignment (Supplementary Table 2, available online). For participants undergoing follow-up colonoscopy within 3.5 years of discontinuing celecoxib, similar statistically nonsignificant reductions in adenoma recurrence with celecoxib were seen for participants randomly assigned between 2001 and 2002 (25% reduction, median celecoxib exposure = 24.9 months), 2003 (22% reduction, median celecoxib exposure = 17.3 months), and 2004 (17% reduction, median celecoxib exposure = 3.7 months).
Toxicity analyses, including duration of exposure to celecoxib or placebo, for all 824 randomly assigned participants and various subgroups are shown in Table 5; participant subgroup baseline characteristics are provided in Supplementary Table 3 (available online). The risk of serious adverse events, including cardiovascular and thromboembolic disorders, did not differ between the placebo and celecoxib groups, and there was no evidence of an increased risk of death in participants while on (n = 1) or off (n = 2) celecoxib when compared with the respective placebo groups (n = 3 and 6) (Supplementary Table 4, available online). The hazard ratio (HR) for gastrointestinal ulceration and hemorrhage with celecoxib compared with placebo was 1.26 (95% CI = 0.65 to 2.43) and statistically nonsignificant (P = .50). For those participants also taking low-dose aspirin, the hazard ratio for gastrointestinal ulceration and hemorrhage with celecoxib was 2.48 (95% CI = 0.78 to 7.95, Pinteraction = .13).
Table 5.
Serious adverse events, cardiovascular and thromboembolic disorders, hypertension, and gastrointestinal ulceration and hemorrhage by low-dose aspirin (ASA) at baseline*
| Adverse events | Median time at risk, mo | Events/participants |
Event rate/1000 person-years (95% CI) |
HR (95% CI), P | LRT Pinteraction | ||
|---|---|---|---|---|---|---|---|
| Placebo | Celecoxib | Placebo | Celecoxib | ||||
| Serious adverse events | |||||||
| Intention-to-treat analysis† | |||||||
| Overall (n = 824) | 29.6 | 93/410 | 104/414 | 100.8 (82.3 to 123.5) | 112.8 (93.1 to 136.7) | 1.13 (0.85 to 1.49), .40 | |
| Yes ASA (n = 401) | 29.6 | 52/199 | 53/202 | 113.4 (86.4 to 148.8) | 121.5 (92.8 to 159.0) | 1.06 (0.72 to 1.56), .76 | |
| No ASA (n = 423) | 29.4 | 41/211 | 51/212 | 88.4 (65.1 to 120.1) | 105.0 (79.8 to 138.1) | 1.20 (0.80 to 1.82), .38 | .66 |
| While on intervention‡ | |||||||
| Overall (n = 798) | 13.2 | 41/397 | 53/401 | 86.9 (64.0 to 118.0) | 112.5 (86.0 to 147.3) | 1.29 (0.86 to 1.94), .22 | |
| Yes ASA (n = 393) | 13.4 | 22/196 | 30/197 | 95.5 (62.9 to 145.0) | 131.3 (91.8 to 187.8) | 1.36 (0.78 to 2.37), .27 | |
| No ASA (n = 405) | 13.1 | 19/201 | 23/204 | 78.7 (50.2 to 123.4) | 94.8 (63.0 to 142.7) | 1.20 (0.65 to 2.21), .55 | .77 |
| After intervention cessation§ | |||||||
| Overall (n = 679) | 12.2 | 57/336 | 60/343 | 121.7 (93.9 to 157.7) | 127.8 (99.2 to 164.6) | 1.06 (0.73 to 1.52), .77 | |
| Yes ASA (n = 328) | 12.3 | 31/165 | 28/163 | 131.0 (92.1 to 186.3) | 126.9 (87.6 to 183.8) | 0.96 (0.57 to 1.60), .87 | |
| No ASA (n = 351) | 11.5 | 26/171 | 32/180 | 112.2 (76.4 to 164.7) | 128.6 (90.9 to 181.8) | 1.15 (0.68 to 1.94), .60 | .59 |
| Serious adverse cardiovascular and thromboembolic disorders | |||||||
| Intention-to-treat analysis† | |||||||
| Overall (n = 824) | 31.4 | 38/410 | 35/414 | 37.5 (27.3 to 51.6) | 34.2 (24.5 to 47.6) | 0.93 (0.59 to 1.47), .75 | |
| Yes ASA (n = 401) | 31.5 | 26/199 | 20/202 | 52.3 (35.6 to 76.8) | 41.8 (27.0 to 64.8) | 0.78 (0.43 to 1.40), .40 | |
| No ASA (n = 423) | 31.4 | 12/211 | 15/212 | 23.3 (13.2 to 41.0) | 27.5 (16.6 to 45.6) | 1.17 (0.54 to 2.51), .69 | .35 |
| While on intervention‡ | |||||||
| Overall (n = 798) | 14.6 | 18/397 | 22/401 | 36.8 (23.2 to 58.3) | 44.2 (29.1 to 67.1) | 1.18 (0.63 to 2.20), .60 | |
| Yes ASA (n = 393) | 14.2 | 13/196 | 14/197 | 55.2 (32.0 to 95.0) | 58.5 (34.7 to 98.8) | 1.03 (0.48 to 2.20), .93 | |
| No ASA (n = 405) | 15.0 | 5/201 | 8/204 | 19.7 (8.2 to 47.3) | 30.9 (15.5 to 61.9) | 1.63 (0.53 to 4.99), .40 | .52 |
| After intervention cessation§ | |||||||
| Overall (n = 679) | 13.6 | 20/336 | 16/343 | 39.1 (25.2 to 60.6) | 31.0 (19.0 to 50.6) | 0.78 (0.40 to 1.51), .46 | |
| Yes ASA (n = 328) | 13.6 | 13/165 | 8/163 | 50.3 (29.2 to 86.7) | 33.2 (16.6 to 66.4) | 0.62 (0.26 to 1.49), .28 | |
| No ASA (n = 351) | 13.5 | 7/171 | 8/180 | 27.6 (13.2 to 57.9) | 29.1 (14.5 to 58.1) | 0.98 (0.34 to 2.79), .97 | .47 |
| Cardiovascular and thromboembolic disorders∥ | |||||||
| Intention-to-treat analysis† | |||||||
| Overall (n = 824) | 30.7 | 71/410 | 65/414 | 73.0 (57.7 to 92.5) | 67.1 (52.7 to 85.6) | 0.91 (0.65 to 1.28), .58 | |
| Yes ASA (n = 401) | 30.9 | 46/199 | 38/202 | 100.5 (75.3 to 134.2) | 85.1 (61.9 to 116.9) | 0.85 (0.55 to 1.31), .46 | |
| No ASA (n = 423) | 30.4 | 25/211 | 27/212 | 47.2 (31.4 to 71.0) | 51.8 (35.5 to 75.5) | 1.03 (0.60 to 1.78), .91 | .57 |
| While on intervention‡ | |||||||
| Overall (n = 798) | 13.8 | 39/397 | 40/401 | 81.4 (59.5 to 111.4) | 83.2 (61.0 to 113.4) | 1.01 (0.65 to 1.57), .96 | |
| Yes ASA (n = 393) | 13.4 | 30/196 | 25/197 | 132.1 (92.3 to 188.9) | 109.4 (73.9 to 161.9) | 0.81 (0.47 to 1.37), .43 | |
| No ASA (n = 405) | 14.5 | 9/201 | 15/204 | 35.7 (18.6 to 68.6) | 59.5 (35.9 to 98.7) | 1.68 (0.73 to 3.83), .22 | .15 |
| After intervention cessation§ | |||||||
| Overall (n = 679) | 12.6 | 35/336 | 31/343 | 71.8 (51.6 to 100.0) | 61.6 (43.3 to 87.6) | 0.86 (0.53 to 1.39), .53 | |
| Yes ASA (n = 328) | 12.5 | 20/165 | 16/163 | 80.5 (51.9 to 124.8) | 68.7 (42.1 to 112.1) | 0.85 (0.43 to 1.65), .62 | |
| No ASA (n = 351) | 12.7 | 15/171 | 15/180 | 62.8 (37.8 to 104.1) | 55.5 (33.5 to 92.1) | 0.84 (0.40 to 1.74), .63 | .96 |
| Hypertension | |||||||
| Intention-to-treat analysis† | |||||||
| Overall (n = 824) | 31.0 | 43/410 | 55/414 | 43.3 (32.1 to 58.3) | 57.8 (44.4 to 75.3) | 1.34 (0.90 to 1.99), .16 | |
| Yes ASA (n = 401) | 31.2 | 21/199 | 29/202 | 42.2 (27.5 to 64.7) | 64.9 (45.1 to 93.3) | 1.49 (0.85 to 2.62), .17 | |
| No ASA (n = 423) | 30.8 | 22/211 | 26/212 | 44.4 (29.2 to 67.4) | 51.6 (35.1 to 75.8) | 1.16 (0.66 to 2.05), .61 | .51 |
| While on intervention‡ | |||||||
| Overall (n = 798) | 13.3 | 20/397 | 34/401 | 41.7 (26.9 to 64.7) | 71.3 (51.0 to 99.8) | 1.72 (0.99 to 2.98), .06 | |
| Yes ASA (n = 393) | 13.5 | 8/196 | 18/197 | 33.7 (16.9 to 67.4) | 78.1 (49.2 to 123.9) | 2.33 (1.01 to 5.37), .05 | |
| No ASA (n = 405) | 13.0 | 12/201 | 16/204 | 49.6 (28.2 to 87.3) | 65.0 (39.8 to 106.1) | 1.28 (0.61 to 2.71), .51 | .28 |
| After intervention cessation∥ | |||||||
| Overall (n = 679) | 12.8 | 20/336 | 21/343 | 39.4 (25.4 to 61.1) | 42.6 (27.8 to 65.4) | 1.09 (0.59 to 2.00), .79 | |
| Yes ASA (n = 328) | 12.8 | 10/165 | 11/163 | 38.6 (20.8 to 71.8) | 47.7 (26.4 to 86.1) | 1.19 (0.50 to 2.83), .69 | |
| No ASA (n = 351) | 12.8 | 10/171 | 10/180 | 40.3 (21.7 to 74.8) | 38.2 (20.6 to 71.0) | 0.94 (0.39 to 2.26), .89 | .72 |
| Gastrointestinal ulceration and hemorrhage | |||||||
| Intention-to-treat analysis† | |||||||
| Overall (n = 824) | 31.6 | 16/410 | 20/414 | 15.5 (9.5 to 25.4) | 19.6 (12.6 to 30.4) | 1.26 (0.65 to 2.43), .50 | |
| Yes ASA (n = 401) | 31.8 | 7/199 | 14/202 | 13.5 (6.4 to 28.4) | 29.0 (17.2 to 49.0) | 2.08 (0.84 to 5.17), .11 | |
| No ASA (n = 423) | 31.4 | 9/211 | 6/212 | 17.6 (9.2 to 33.8) | 11.1 (5.0 to 24.8) | 0.61 (0.22 to 1.74), .36 | .09 |
| While on intervention‡ | |||||||
| Overall (n = 798) | 14.5 | 8/397 | 13/401 | 16.2 (8.1 to 32.4) | 26.3 (15.3 to 45.3) | 1.59 (0.66 to 3.84), .30 | |
| Yes ASA (n = 393) | 14.5 | 4/196 | 10/197 | 16.7 (6.3 to 44.4) | 41.6 (22.4 to 77.3) | 2.48 (0.78 to 7.95), .13 | |
| No ASA (n = 405) | 14.7 | 4/201 | 3/204 | 15.8 (5.9 to 42.0) | 11.8 (3.8 to 36.6) | 0.73 (0.16 to 3.28), .69 | .21 |
| After intervention cessation§ | |||||||
| Overall (n = 679) | 13.6 | 7/336 | 6/343 | 13.4 (6.4 to 28.0) | 11.6 (5.2 to 25.9) | 0.87 (0.29 to 2.61), .81 | |
| Yes ASA (n = 328) | 14.4 | 3/165 | 3/163 | 11.2 (3.6 to 34.7) | 12.3 (4.0 to 38.1) | 1.17 (0.23 to 5.87), .85 | |
| No ASA (n = 351) | 13.4 | 4/171 | 3/180 | 15.6 (5.9 to 41.7) | 11.0 (3.6 to 34.2) | 0.69 (0.15 to 3.14), .64 | .68 |
*Two-sided Cox regression model adjusted for random assignment to selenium, aspirin use (unless stratified), and clinic. ASA = aspirin; CI = confidence interval; HR = hazard ratio; LRT = two-sided likelihood ratio test.
†Time at risk from random assignment to study withdrawal.
‡Time at risk from the second day after participant received the intervention until 14 days after intervention cessation.
§Time at risk from 15 days after intervention cessation until study withdrawal.
∥Two participants, both randomized to placebo, experienced a cardiovascular/thromboembolic event on the date of random assignment (time = 0). These two participants are included in the event counts but not included in the rate calculations or the Cox models because they had no time-at-risk to contribute.
In participants on celecoxib, the overall hazard ratio for hypertension was 1.72 (95% CI = 0.99 to 2.98, P = .06) compared with placebo; the risk was statistically significant in men (HR = 2.30, 95% CI = 1.19 to 4.43, P = .01) and participants taking low-dose aspirin (HR = 2.33, 95% CI = 1.01 to 5.37, P = .05). When stratified for prerandomization cardiovascular risk according to the Modified Framingham Risk Score (24), the hazard for hypertension while on intervention increased two-fold with celecoxib in participants at high risk for cardiovascular disease on study entry (HR = 2.19, 95% CI = 1.07 to 4.50, P = .03) (Supplementary Table 5, available online). This excess risk was not observed after discontinuation of celecoxib.
Discussion
With early suspension of celecoxib, a study limitation is that we were unable to test the original hypothesis that 400 mg once-daily celecoxib for three to five years would prevent colorectal adenomas. Nonetheless, our results are consistent with the findings from completed trials that coxibs statistically significantly reduce adenoma recurrence (10–12). Premature termination of the celecoxib arm did provide the opportunity for a fuller analysis of the differing effects, beneficial and adverse, of variable lengths of exposure to once-daily 400 mg celecoxib than in the earlier trials. Uninterrupted continuation of the selenium arm allowed us to analyze the durability of the effects of celecoxib after its withdrawal during prolonged surveillance. We provide supportive evidence that celecoxib-related gastrointestinal ulceration and hemorrhage is exacerbated by concurrent use of low-dose aspirin. As in the earlier report that included interim data from our trial (16), we did not find evidence that celecoxib 400 mg taken once daily for a median of 14.8 months is associated with serious cardiovascular events or death. However, our results do indicate that celecoxib at this dose and for this duration may increase the risk of hypertension in patients already at increased risk for cardiovascular disease. And while limited to few events, our results support the findings of others (25) that the risk of gastrointestinal ulceration and hemorrhage may be exacerbated by concurrent use of low-dose aspirin and celecoxib.
We found no evidence that duration of exposure to celecoxib influenced its effect on adenoma recurrence. The reduction in adenoma recurrence of 25% found in early participants with a median exposure to celecoxib of 25.7 months was similar to the 17% reduction in recurrence found in participants randomly assigned later, whose median exposure was 3.7 months. By contrast, as in follow-up reports of PreSAP (17) and APPROVe (10), the adenoma-preventive effects, in this case of celecoxib, waned quickly after withdrawal. Adenoma recurrence was statistically significantly reduced (by 31%) in the celecoxib arm of our trial compared with placebo in participants undergoing follow-up colonoscopy within 12 months of discontinuing the active agent; benefit was lost on longer time between stopping and follow-up. The lack of a sustained clinical effect is consistent with the in vitro reversibility of NSAID effects in human colon cancer cell lines (26) and the largely cell growth–inhibitory effects of PGE2 inhibition in vivo in experimental animals and humans (27,28).
Our finding that reduction in new adenoma development with celecoxib was largely confined to participants with advanced baseline adenomas was somewhat unexpected. The other coxib trials cited (10–12) excluded patients with adenomas smaller than 6 mm in size, contrasting with our trial, in which patients with diminutive adenomas 3 mm or larger were eligible; it is possible that propensity for developing recurrent adenomas was reduced in the latter lower-risk group with adenomas 3 to 6 mm in size. The greater benefit among higher-risk advanced adenoma subgroups might suggest that COX-2 inhibition may have additional effects on adenoma biology beyond simply inhibiting growth. While speculative, this would be consistent with evidence favoring a role for stromal microenvironmental factors in the COX-2/PGE2–mediated pathogenesis of clinically more serious advanced adenomas that are absent in diminutive adenomas (29,30).
As noted, a total daily dose of celecoxib 400 mg administered as 200 mg twice daily in the APC trial was associated with an increase in mean blood pressure (11). Here, celecoxib 400 mg once daily was associated with increased risk of new-onset hypertension, but the risk was largely confined to individuals with pre-existing cardiovascular risk factors and diminished after the withdrawal of celecoxib.
Our study demonstrates that even short exposure to celecoxib can be effective for adenoma prevention. We confirm that any coxib-mediated effect is short lived after withdrawal of the agent. New-onset hypertension in participants receiving celecoxib 400 mg once daily, which was previously thought to be a safer regimen than 200 mg twice daily, was most evident in participants with pre-existing cardiovascular risk factors. Because of the cardiovascular risk, coxibs are not considered safe for chemoprevention in the general population. Our finding of little celecoxib benefit in patients with small adenomas compared with sizable benefit for patients with high-risk adenomas suggests that judicious use of celecoxib 400 mg once daily, with blood pressure monitoring, might prove beneficial for carefully selected high-risk patients.
Funding
This trial is supported by grants P01 CA041108 (to PL), R01 CA151708 (to PL and PAT), and P30 CA23074 (to ASK).
Notes
The study funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors thank the research nurses and clinic staff: Mildred Arnold, Patricia Blair, Darlene Bunpian, Amy Carrier, Marita Clifford, Ann Dejong-Ruhnau, Theresa Dunn, Pat Graham, Dianne Parish, Eugenia M. Schleski, and Christina Yang-Hellewell; and the staff of the study laboratory and data management team at the University of Arizona Cancer Center: Carole Kepler, Christina Preece, Jerilyn San Jose, and Manuel Snyder.
Supplementary Material
References
- 1. Honn KV, Bockman RS, Marnett LJ. Prostaglandins and cancer: a review of tumor initiation through tumor metastasis. Prostaglandins. 1981;21(5):833-864. [DOI] [PubMed] [Google Scholar]
- 2. Gupta RA, DuBois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1(1):11-21. [DOI] [PubMed] [Google Scholar]
- 3. Thun MJ, Namboodiri MM, Heath CW., Jr. Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325(23):1593-1596. [DOI] [PubMed] [Google Scholar]
- 4. Rothwell PM, Wilson M, Elwin C-E, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 376(9754):1741-1750. [DOI] [PubMed] [Google Scholar]
- 5. Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13(5):518-527. [DOI] [PubMed] [Google Scholar]
- 6. Wang D, DuBois RN. The Role of Anti-Inflammatory Drugs in Colorectal Cancer. Annu Rev Med. 2013;64(1):131-144. [DOI] [PubMed] [Google Scholar]
- 7. Hochberg MC. COX-2 selective inhibitors in the treatment of arthritis: a rheumatologist perspective. Curr Top Med Chem. 2005;5(5):443-448. [DOI] [PubMed] [Google Scholar]
- 8. Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342(26):1946-1952. [DOI] [PubMed] [Google Scholar]
- 9. Higuchi T, Iwama T, Yoshinaga K, et al. A randomized, double-blind, placebo-controlled trial of the effects of rofecoxib, a selective cyclooxygenase-2 inhibitor, on rectal polyps in familial adenomatous polyposis patients. Clin Cancer Res. 2003;9(13):4756-4760. [PubMed] [Google Scholar]
- 10. Baron JA, Sandler RS, Bresalier RS, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131(6):1674-1682. [DOI] [PubMed] [Google Scholar]
- 11. Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873-884. [DOI] [PubMed] [Google Scholar]
- 12. Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355(9):885-895. [DOI] [PubMed] [Google Scholar]
- 13. Thompson P, Roe DJ, Fales L, et al. Design and Baseline Characteristics of Participants in a Phase III Randomized Trial of Celecoxib and Selenium for Colorectal Adenoma Prevention. Cancer Prev Res (Phila). 2012;5(12):1381-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352(11):1092-1102. [DOI] [PubMed] [Google Scholar]
- 15. Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352(11):1071-1080. [DOI] [PubMed] [Google Scholar]
- 16. Solomon SD, Wittes J, Finn PV, et al. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 2008;117(16):2104-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arber N, Spicak J, Racz I, et al. Five-year analysis of the prevention of colorectal sporadic adenomatous polyps trial. Am J Gastroenterol. 2011;106(6):1135-1146. [DOI] [PubMed] [Google Scholar]
- 18. Thompson PA, Ashbeck EL, Roe DJ. et al. Selenium supplementation for prevention of colorectal adenomas and risk of associated type 2 diabetes. J Natl Cancer Inst. 2016:108(12):djw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solomon SD, Pfeffer MA, McMurray JJ, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114(10):1028-1035. [DOI] [PubMed] [Google Scholar]
- 20. Chow HH, Anavy N, Salazar D, et al. Determination of celecoxib in human plasma using solid-phase extraction and high-performance liquid chromatography. J Pharm Biomed Anal. 2004;34(1):167-174. [DOI] [PubMed] [Google Scholar]
- 21. Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56(3):143-159; quiz 184-185. [DOI] [PubMed] [Google Scholar]
- 22. Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199-200. [DOI] [PubMed] [Google Scholar]
- 23. Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol. 1986;123(1):174-184. [DOI] [PubMed] [Google Scholar]
- 24. Schisterman EF, Whitcomb BW. Coronary age as a risk factor in the modified Framingham risk score. BMC Med Imaging. 2004;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lanas A, Garcia-Rodriguez LA, Arroyo MT, et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. Gut. 2006;55(12):1731-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruschoff J, Wallinger S, Dietmaier W, et al. Aspirin suppresses the mutator phenotype associated with hereditary nonpolyposis colorectal cancer by genetic selection. Proc Natl Acad Sci U S A. 1998;95(19):11301-11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328(18):1313-1316. [DOI] [PubMed] [Google Scholar]
- 28. Takayama T, Nagashima H, Maeda M, et al. Randomized double-blind trial of sulindac and etodolac to eradicate aberrant crypt foci and to prevent sporadic colorectal polyps. Clin Cancer Res. 2011;17(11):3803-3811. [DOI] [PubMed] [Google Scholar]
- 29. Gounaris E, Tung CH, Restaino C, et al. Live Imaging of Cysteine-Cathepsin Activity Reveals Dynamics of Focal Inflammation, Angiogenesis, and Polyp Growth. PLoS ONE. 2008;3(8):e2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu Y, Hua P, Lance P. Cyclooxygenase-2 expression and prostanoid biogenesis reflect clinical phenotype in human colorectal fibroblast strains. Cancer Res. 2003;63(2):522-526. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.