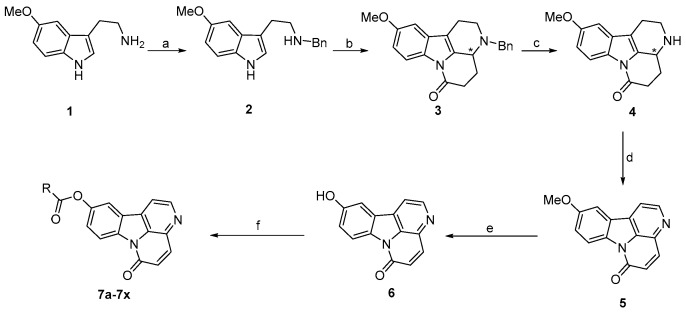
Scheme 1.
Synthetic route and chemical structures of compounds 4–6 and 7a–7x. Reagents and conditions: (a) i: benzaldehyde, methanol, r.t.; ii: NaBH4, r.t., 98%; (b) α-ketoglutaric acid, p-TSA, dry toluene: dioxane = 3:2, DST, reflux, 83%; (c) HCOONH4, 5% Pd/C, methanol: toluene = 1:1, reflux, 75%; (d) 5% Pd/C, xylene, reflux, 90%; (e) BBr3, dry DCM, −78 °C to r.t., 63%; (f) acyl chloride, TEA, dry DCM, 0 °C, 48%–86%.
| 7b: R = 2′-pyridyl | 7h: R = 4′-ClC6H4 | 7n: R = 2′-CF3C6H4 | 7t: R = CH3CH2 |
| 7c: R = 2′-CH3C6H4 | 7i: R = 2′,4′-Cl2C6H3 | 70: R = 3′-CF3C6H4 | 7u: R = CH3(CH2)2 |
| 7d: R = 3′-CH3C6H4 | 7j: R = 4′-CH3OC6H4 | 7p: R = 4′-CF3C6H4 | 7v: R = CH3(CH2)3 |
| 7e: R = 4′-CH3C6H4 | 7k: R = 2′-FC6H4 | 7q: R = 1′-naphthyl | 7w: R = CH3(CH2)4 |