Abstract
Nerolidol (3,7,11-trimethyl-1,6,10-dodecatrien-3-ol) is a naturally occurring sesquiterpene alcohol that is present in various plants with a floral odor. It is synthesized as an intermediate in the production of (3E)-4,8-dimethy-1,3,7-nonatriene (DMNT), a herbivore-induced volatile that protects plants from herbivore damage. Chemically, nerolidol exists in two geometric isomers, a trans and a cis form. The usage of nerolidol is widespread across different industries. It has been widely used in cosmetics (e.g., shampoos and perfumes) and in non-cosmetic products (e.g., detergents and cleansers). In fact, U.S. Food and Drug Administration (FDA) has also permitted the use of nerolidol as a food flavoring agent. The fact that nerolidol is a common ingredient in many products has attracted researchers to explore more medicinal properties of nerolidol that may exert beneficial effect on human health. Therefore, the aim of this review is to compile and consolidate the data on the various pharmacological and biological activities displayed by nerolidol. Furthermore, this review also includes pharmacokinetic and toxicological studies of nerolidol. In summary, the various pharmacological and biological activities demonstrated in this review highlight the prospects of nerolidol as a promising chemical or drug candidate in the field of agriculture and medicine.
Keywords: cis-nerolidol, trans-nerolidol, sesquiterpene, essential oil, pharmacological activities
1. Introduction
Ever since ancient times, medicinal plants have been explored and used as herbal medicines to treat many diseases [1]. With the advancement of technology, research on herbal medicine has intensified on the efforts to identify the bioactive compounds in medicinal plants that are responsible for their pharmacological and biological activities. Essential oils (EOs) are volatile, natural and complex bioactive compounds which are characterized by a strong odour, and their biological effects are known to be associated to a series of complex interactions with cells, tissues and whole organisms [2]. Besides its well-known application in aromatherapy [3], the uses of EO have been extended into the food, agriculture and pharmaceutical industries [4,5,6]. Among the plants that are rich in EOs are Baccharis dracunculifolia DC, Elettaria cardamomum (L.) Maton, Momordica charantia L., Piper aleyreanum C. DC and Piper claussenianum (Miq.) C. DC [7,8,9,10,11].
Nerolidol (3,7,11-trimethyl-1,6,10-dodecatrien-3-ol), also known as peruviol, is a naturally occurring sesquiterpene alcohol present in the EO of various plants with a floral odour [12,13]. Nerolidol was found to exist as one of the bioactive compounds responsible for the biological activities demonstrated by the EOs of the aforementioned plants.
Statistics showed that the global usage of nerolidol per annum ranges from 10 to 100 metric tonnes [14]. For instance, nerolidol is frequently incorporated in cosmetics (e.g., shampoos and perfumes) and non-cosmetic products (e.g., detergents and cleansers) [13]. Besides, nerolidol is also widely used in the food industry as a flavor enhancer in many food products since its approval by U.S. Food and Drug Administration as a safe food flavoring agent.
Principally, this article aims to review the diverse range of pharmacological and biological activities of nerolidol which include antioxidant, anti-microbial, anti-biofilm, anti-parasitic, insecticidal, anti-ulcer, skin penetration enhancer, anti-tumor, anti-nociceptive and anti-inflammatory properties. The review also covers the chemical structure, physical properties, and the biosynthesis pathway of nerolidol as the intermediate involved in the mechanisms responsible for protection of plants against herbivores and plant pathogens. This article also highlights the pharmacokinetic and toxicological properties of nerolidol in both in vitro and in vivo experimental models.
2. Chemical Structure and Physical Properties
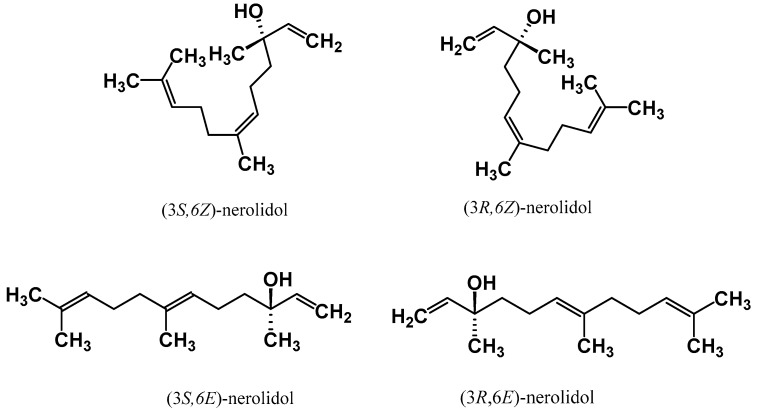
Nerolidol has four different isomeric forms which consist of two enantiomers and two geometric isomers [15]. The existence of these isomeric forms is due to the presence of a double bond at the C-6 position and the asymmetric center at the C-3 position. These isomeric forms of cis- and trans-nerolidol are illustrated in Figure 1. Besides, the synonyms for cis- and trans-nerolidol are listed in Table 1.
Figure 1.
Chemical structures of the two enantiomers both for cis- and trans-isomers of nerolidol.
Table 1.
Synonyms of cis- and trans-nerolidol.
| Cis-Nerolidol | Trans-Nerolidol |
|---|---|
| (i) (±)-cis-nerolidol | (i) (±)-trans-nerolidol |
| (ii) (6Z)-3,7,11-trimethyl-1,6,10-dodecatrien-3-ol | (ii) (6E)-3,7,11-trimethyl-1,6,10-dodecatrien-3-ol |
| (iii) (6Z)-3,7,11-trimethyldodeca-1,6,10-trien-3-ol | (iii) (6E)-3,7,11-trimethyldodeca-1,6,10-trien-3-ol |
| (iv) (6Z)-nerolidol | (iv) (6E)-nerolidol |
| (v) 1,6,10-dodecatrien-3-ol, 3,7,11-trimethyl-, (6Z)- | (v) 1,6,10-dodecatrien-3-ol, 3,7,11-trimethyl-, (6E)- |
| (vi) (Z)-nerolidol | (vi) (E)-nerolidol |
Like other sesquiterpene compounds, nerolidol has high hydrophobicity, thereby allowing easier penetration across the plasma membrane and interaction with intracellular proteins and/or intra-organelle sites [16]. The physical properties of nerolidol (isomer not specified) have been described by Lapczynski et al. [13] as follows:
-
(i)
Physical description: A clear pale yellow to yellow liquid having a faint floral odor reminiscent of rose and apple.
-
(ii)
Chemical formula: C15H26O
-
(iii)
Flash point: >212° F; CC.
-
(iv)
Boiling point: 276 °C.
-
(v)
LogKow (calculated): 5.68.
-
(vi)
Vapor pressure (calculated): 0.1 mm Hg 20 °C.
-
(vii)
Specific gravity: 0.8744.
-
(viii)
Water solubility (calculated): 1.532 mg/L at 25 °C.
3. Sources, Extraction and Analytical Methods of Nerolidol
Numerous extraction methods have been employed for extracting EOs from various plant samples [2]. The hydrodistillation method using the Clevenger-type apparatus appears as the most common method used for extracting nerolidol. Table 2 summarizes the different extraction methods and the yield of nerolidol from various parts of plants such as leaves, flowers, seeds, fruits, resins, twigs and woods. Based on the literature references, leaves are the most common source for extraction of nerolidol. In terms of the percentage of nerolidol in the leaf EO among different plant species, Piper claussenianum (Miq.) C. DC. has the highest percentage of trans-nerolidol (81.4%), followed by Zanthoxylum hyemale A.St.-Hil. (51.0%), Zornia brasiliensis Vogel (48.0%) and Swinglea glutinosa (Blanco) Merr. (28.4%) (Table 2).
Table 2.
Plant sources of nerolidol along with its percentage of nerolidol and extraction method.
| Plant Part | Type of Nerolidol Found in the Essential Oil | Nerolidol Purified from the Essential Oil of the Respective Plants (%) | Extraction Method | Ref. |
|---|---|---|---|---|
| Aerial parts | trans-nerolidol | (i) Warionia saharae ex Benth. & Coss. (23.0%) | Hydrodistillation technique using the Clevenger-type apparatus | [10,26,27,28] |
| (ii) Scutellaria abida L. ssp. albida (9.03%) | ||||
| (iii) Piper aleyreanum C. DC (1.2%) | ||||
| (iv) Leonotis ocymifolia (Burm.f.) Iwarsson (0.41%) | ||||
| Leaf | Nerolidol (n.s.) | (i) Capparis tomentosa Lam. (5.14%) | Hydrodistillation technique using the Clevenger-type apparatus | [29,30] |
| (ii) Virola surinamensis (Rol. ex Rottb.) Warb. (3.0%) | ||||
| Ginkgo biloba L. (0.12%) | Molecular distillation at a feed temperature of 60 °C, distillation temperature of 280 °C, feed flow rate of 180 mL per hour, scraper rate of 300 rpm, and operating pressure of 0.1–0.5 Pa | [31] | ||
| trans-Nerolidol | (i) Baccharis dracunculifolia DC. (33.51%) | Hydrodistillation technique using the Clevenger-type apparatus | [8,9,32,33,34,35,36,37,38,39,40,41,42,43] | |
| (ii) Cassia fistula L. (2.2%) | ||||
| (iii) Comptonia peregrina (L.) Coult. (2.11% and 3.43% after 0–30 min fraction and 30–60 min fraction respectively) | ||||
| (iv) Melaleuca quinquenervia (Cav.) S.T.Blake (24.19%) | ||||
| (v) Myrica rubra (Lour.) Siebold & Zucc. (2%) | ||||
| (vi) Lantana radula Sw. (19.0%) | ||||
| (vii) Peperomia serpens (Sw.) Loudon (38.0%) | ||||
| (viii) Piper aduncum L. (0.2%) | ||||
| (ix) Piper chaba Hunter (5.1%) | ||||
| (x) Piper claussenianum (Miq.) C. DC. (81.4%) | ||||
| (xi) Strychnos spinosa Lam. (0.7%) | ||||
| (xii) Swinglea glutinosa (Blanco) Merr. (28.4%) | ||||
| (xiii) Zanthoxylum hyemale A.St.-Hil. (51.0%) | ||||
| (xiv) Zornia brasiliensis Vogel (48.0%) | ||||
| Stem | trans-Nerolidol | Oplopanax horridus (Sm.) Miq. (54.5%) | Steam distillation using a low pressure system with an external steam source | [44] |
| Flower | trans-Nerolidol | (i) Achillea millefolium L. (11.6%–31.9%) | Hydrodistillation technique using the Clevenger-type apparatus | [42,45,46] |
| (ii) Cananga odorata (Lam.) Hook.f. & Thomson (0.32%) | ||||
| (iii) Cassia fistula L. (38.0%) | ||||
| Root | trans-Nerolidol | Oplopanax horridus (Sm.) Miq. (54.6%) | Steam distillation using a low pressure system with an external steam source | [44] |
| Seed/grain | Nerolidol (n.s.) | Magnolia denudata Desr. (2.18%) | Hydrodistillation technique using the Clevenger-type apparatus | [47] |
| trans-Nerolidol | (i) Elettaria cardamomum (L.) Maton (3.6%) | Hydrodistillation technique using the Clevenger-type apparatus | [7,48] | |
| (ii) Momordica charantia L. (61.6%) | ||||
| Fruit | trans-Nerolidol | Swinglea glutinosa (Blanco) Merr. (19.1%) | Hydrodistillation technique using the Clevenger-type apparatus | [43] |
| Resin | trans-Nerolidol | Canarium schweinfurthii Engl. (14%) | Hydrodistillation technique using the Clevenger-type apparatus | [49] |
| Twig/wood | Trans-Nerolidol | Cinnamomum osmophloeum Kaneh. (1.05%) | Hydrodistillation technique using the Clevenger-type apparatus | [50] |
| Fokienia hodginsii (Dunn) A.Henry & H H.Thomas (34.8%) | Solid-phase microextraction | [51] | ||
| cis-Nerolidol | Myrocarpus fastigiatus Allemao (80.0%) | Hydrodistillation technique using the Clevenger-type apparatus | [52] |
Key: n.s. = not specified.
Microclimatic and environmental factors such as species, season, location, climate, soil type, age of the leaves and the extraction method may influence the concentration of each constituents in EOs [17]. Seasonal variation is one of the main factors that influences the composition of EOs in plants [18,19,20] including the concentration of nerolidol. It was reported by Marques and Kaplan [19] that the harvested leaves from Piper claussenianum (Miq.) C. DC. yielded variable amounts of nerolidol during the year of 2009. The content of trans-nerolidol was higher during the Brazilian spring collection period (September, October and November, 87.0%, 94.0%, 92.0%, respectively) as compared to that during autumn collection period (March, April and May, 78.0%; 77.0%; 80.0%, respectively). Another study conducted by de Sousa et al. [20] has shown that the mean concentration of trans-nerolidol in the leaves of Baccharis dracunculifolia DC. was five fold higher in March 2005 (136.53 mg/100 g of plant) than that in July 2004 (25.03 mg/100 g of plant). All these findings provide important information to identify and determine the most appropriate harvest period to obtain the highest yield of nerolidol from different plants.
Gas chromatography-mass spectrometry (GC-MS) is the analytical method that is most commonly used to detect nerolidol [21]. This is because the boiling points of sesquiterpenes range from ~250 to 280 °C in which suitable for the gas-phase separation technique employed by GC-MS analysis [22,23]. Apart from GC-MS, liquid chromatography-mass spectrometry (LC-MS) method is also widely used due to its high sensitivity and high accuracy [24]. Recently, He et al. [25] suggested that LC-MS could be used for in vivo pharmacokinetic analysis of nerolidol due to its convenience and stability features. The study demonstrated that the lower limit lower quantification (LLOQ) of nerolidol using LC-MS was reported as 10 ng/mL [25]. On the other hand, another study reported the LLOQ of nerolidol as 3.5 ng/mL by using GC-MS [22]. These results suggest that GC-MS may be a more preferable detection method as it was shown to have higher sensitivity than LC-MS in detecting nerolidol.
In order to differentiatie the cis- and trans-isomers of nerolidol, the retention time of different LC-MS and GC-MS chromatography columns as well as the major peaks of the mass spectra (m/z) are the parameters used (Table 3). According to Table 3, cis-nerolidol displayed shorter retention times than trans-nerolidol regardless of the type of GC or LC column used. Besides retention time, one can also discriminate cis- from trans-nerolidol by referring to the retention indices (RIs) and RIs can be used for comparison across different chromatographic systems [53]. RIs, also known as Kováts retention indices, are frequently used along with mass spectrometry because the combination provides a more accurate identification of isomers, which is often difficult to be achieved by mass spectrometry alone [54]. The retention indices of different chromatographic columns of GC are shown in Table 3.
Table 3.
Retention indices of different chromatographic columns of GC and major peaks of mass spectrometry to differentiate cis- and trans-nerolidol.
| Types of Column/Equipment Used | Cis-Nerolidol | Trans-Nerolidol | Ref. |
|---|---|---|---|
| (A) Retention time of different chromatographic columns of GC (minutes) | |||
| (i) A-100 or 154-C column | 14 | 16 | [22] |
| (ii) DB-5 capillary column | n.a. | 10.5 | [21] |
| (iii) TR-5MS capillary column | 5.87 | 5.98 | [22] |
| (B) Retention time of different chromatographic columns of LC (minutes) | |||
| (i) Hypersil BDS C18 column | 11.9 | 13.1 | [25] |
| (C) Major peaks of mass spectrometry (MS) (m/z) | |||
| (i) M-80B gas chromatograph double focusing mass spectrometer | 41, 69, 134, 91, 93, 79 | 69, 41, 93, 43, 71, 55 | [55] |
| (ii) Y2K ion trap (MS) PolarisQ System mass spectrometer | 93, 91, 67, 107, 79, 161, 121, 133, 55, 147, 189, 175 | 93, 121, 67, 107, 79, 161, 136, 55, 189, 148, 175 | [22] |
| (D) Retention indices of different chromatographic columns of GC | |||
| (i) HP-101 | n.a. | 1564 | [56] |
| (ii) HP-20M | n.a. | 2009 | [56] |
| (iii) HP-FFAP | n.a. | 2055 | [56] |
| (iv) Fused silica capillary column coated with DB-5 | n.a. | 1564 | [29] |
| (v) OV-101 | 1533 | 1549 | [55] |
| (vi) PEG 20M | 2028 | 2035 | [55] |
| (vii) DB-5 | 1565 | 1539 | [57] |
| (viii) DB-Wax | 2010 | 2054 | [57] |
| (ix) SPB-1 | 1543 | n.a. | [58] |
| (x) Dimethylsilicone (DIMS) | 1524.4 (a) | 1550.1 (a) | [54] |
| (xi) Dimethylsilicone with 5% phenyl groups (DIMS5P) | 1543.6 (a) | 1560.9 (a) | [54] |
| (xii) Polyethylene glycol (PEG) | 2007.3 (a) | 2036.3 (a) | [54] |
Key: (a) = average value; n.a. = not available.
4. Industrial Synthesis of Nerolidol
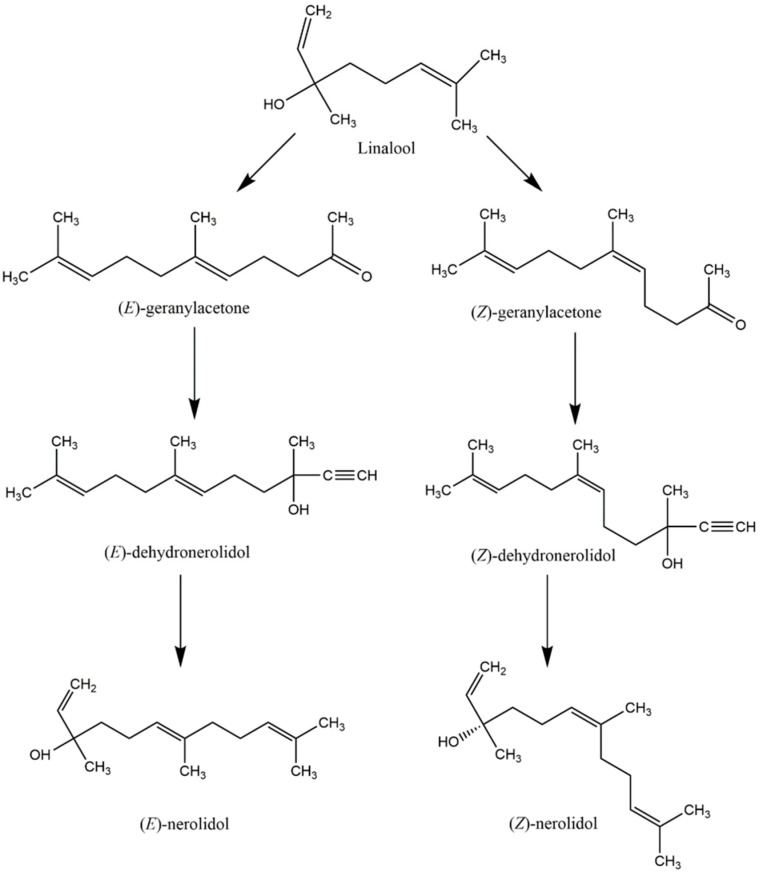
Chemical synthesis of nerolidol is required to increase its production in order to meet the growing industrial demand for nerolidol. Initially, nerolidol was synthesized as an intermediate in the chemical synthesis of geranyl esters from linalool [59]. The process began with the treatment of linalool with diketene or ethyl acetoacetate by the Carroll reaction to yield a mixture of (E)- and (Z)-geranylacetone [59]. Addition of acetylene to both (E)- and (Z)-geranylacetone led to the production of (E)- and (Z)-dehydronerolidol, respectively, which were selectively hydrogenated to trans- and cis-nerolidol, respectively, using a Lindlar catalyst [60]. The overall chemical synthesis of (E)- and (Z)-nerolidol is illustrated as shown in Scheme 1 as described by Nigmatov et al. [59].
Scheme 1.
The overall chemical synthesis pathway of nerolidol to fulfill the demand of nerolidol in the industrial sector.
Although nerolidol can be obtained via the aforementioned chemical reaction or isolation from natural sources, both methods suffer from the disadvantages that they are expensive and produce low yields of end products. In order to overcome these limitations, researchers have utilized eukaryotes such as yeast to produce higher yields of nerolidol. Therefore, a new method of nerolidol production has been developed and patented [61]. The method involved the cultivation of a yeast strain (particularly Saccharomyces cerevisiae, as it is a natural producer of farnesyl diphosphate (FDP)) lacking functional squalene synthase by modifying one ERG9 squalene synthase gene. This was because the absence of functional squalene synthase prevented the conversion of FDP to squalene, therefore causing FDP to accumulate. The next step involved modifying the yeast to overexpress 3-hydroxy-3-methylglutaryl-coenzyme A (HMG CoA) reductase using an inducible promoter such as GAL1 HMG CoA reductase, leading to a higher throughput of FDP. The last step involved growing the yeast in a synthetic medium which is lacking of uracil so that FDP can be fully hydrolyzed into nerolidol. Further shifting towards nerolidol production can be also enhanced by adjusting the pH of the medium to be more acidic either at the start, during or at the end of the growth cycle.
5. The Ecological Role and Biosynthesis of Nerolidol
Plant secondary metabolites (PSMs) are organic compounds that do not interfere with the primary metabolism of plants. Given that they mediate many ecological functions, PSMs are mainly secreted as plant defenses against herbivore and pathogen damages [62]. PSMs are stored either constitutively in inactive forms or induced in response to insect or microbe attack. To thwart off pathogens and herbivores, PSMs employ different chemical defensive strategies involving secondary metabolite pathways [62]. The first strategy consists of an indirect defense mechanism in which the plants confront herbivores indirectly by secreting herbivore-induced plant volatiles (HIPVs) to attract parasitoids and natural enemies of herbivores [63]. On the other hand, the direct defense mechanism employs another strategy, that is, toxic, volatile and non-volatile metabolites which are stored in specialized cells to be released or activated when plants are attacked by pathogens [64].
Among PSMs, terpenoids are the most structurally diverse group. For instance, monoterpenes and sesquiterpenes are the major volatile terpenoids released from plants [65]. Their function are diverse ranging from basic plant functions such as photosynthesis, respiration, growth and development, to playing role in plant defense mechanism to protect plants against herbivore and pathogen attacks [66].
In general, terpenoids are formed from the universal C5 precursor isopentenyl diphosphate (IPP) and its allylic isomer dimethylallyl diphosphate (DMAPP) [67]. Subsequently, condensation of IPP and DMAPP by prenyltransferases leads to the production of linear isoprenyl diphosphate precursors of many chain lengths such as geranyl diphosphate (GDP), FDP and geranylgeranyl diphosphate (GGDP). The allylic prenyldiphosphates of GDP, FDP and GGDP are then converted by terpene synthases (TPSs) to form monoterpenes (C10), sesquiterpenes (C15) and diterpenes (C20), respectively [68].
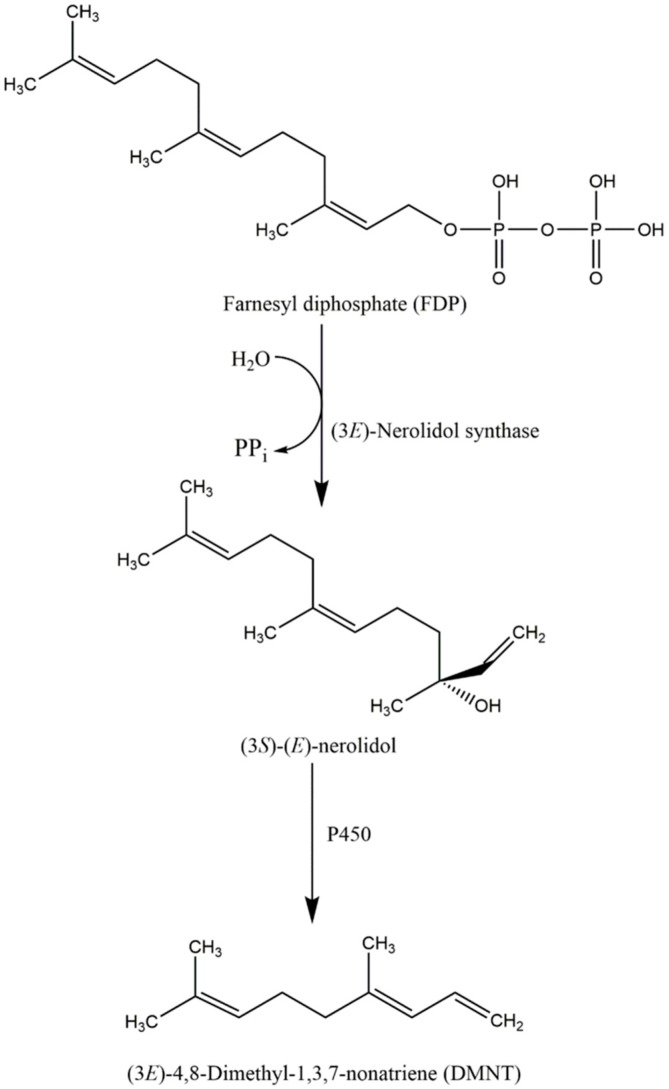
With regard to the production of nerolidol, (E)-nerolidol synthase was recently found to be responsible for the conversion of FDP, the universal precursor of sesquiterpenes to (3S)-(E)-nerolidol. In snapdragon (Antirrhinum majus L.), Nagegowda et al. have recently purified two nerolidol/linalool synthases (AmNES/LIS-1/-2) that are responsible for the production of nerolidol and linalool. AmNES/LIS-1 is found in the cytosol and is responsible for nerolidol biosynthesis, whereas AmNES/LIS-2 is located in the plastids and is responsible for the formation of linalool [69]. Similar to snapdragon, (3S)-(E)-nerolidol synthase activities were demonstrated in maize [70]. Schnee et al. isolated the terpene synthase 1 (TPS1) enzyme, which is encoded by the maize TPS1 gene to produce both 3R- and 3S-enantiomer of (E)-nerolidol [71]. Due to the stimulation of herbivore damage, the expression of tps1 was increased by almost 8-fold, followed by the conversion of (E)-nerolidol to (3E)-4,8-dimethyl-1,3,7-nonatriene (DMNT). Taken all together, the conversion of (E)-nerolidol to DMNT is crucial due to the fact that DMNT acts as an herbivore-induced volatile to protect the plant against herbivore damage. The overall biosynthesis mechanism of nerolidol is illustrated in Scheme 2 as described by Bouwmeester et al. [72].
Scheme 2.
The biosynthesis pathway of (3S)-(E)-nerolidol as an intermediate product for the production of DMNT as an herbivore-induced volatile to protect the plant against herbivore damage.
6. Pharmacological and Biological Activities of Nerolidol
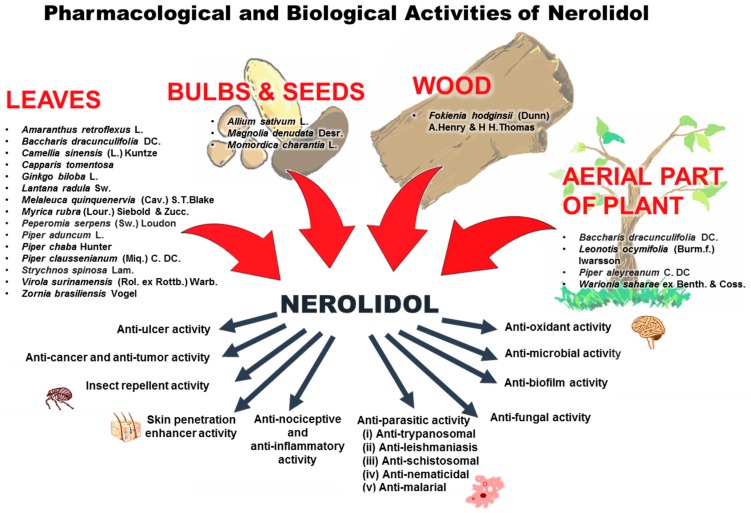
With the knowledge that nerolidol plays a very active role in the defense system of some plants, researchers have been interested to further explore various aspects of its pharmacological and biological activities. To date, various pharmacological and biological activities of nerolidol have been reported such as anti-microbial, anti-biofilm, anti-oxidant, anti-parasitic, skin-penetration enhancer, skin-repellent, anti-nociceptive, anti-inflammatory and anti-cancer. Table 4 summarizes the important information on the pharmacological and biological activities of nerolidol in different in vitro and in vivo models. Besides the pharmacological and biological activities of nerolidol, the sources of nerolidol extraction from various parts of plants are illustrated as well (Figure 2).
Table 4.
A summary of pharmacological and biological activities of nerolidol.
| Bioactivity | Type of Nerolidol | Plant and Part of Plant Used (If Any) | Target Organism(s) | Screening Assay and Methods Used | Results | Possible Mechanisms of Action | Ref. |
|---|---|---|---|---|---|---|---|
| Antioxidant activity | cis-Nerolidol (Aldrich Chemical Co., Milwaukee, WI, USA) | - | - | DPPH and hydroxyl radical scavenging activity | (i) Exhibited DPPH radical scavenging activity | Mediates antioxidant activities via free radical scavenging activity | [73] |
| (ii) Exhibited scavenging activity against hydroxyl radical with IC50 = 1.48 mM | |||||||
| cis-Nerolidol (Sigma-Aldrich, St. Louis, MO, USA) | - | - | Thiobarbituric acid reactive substances (TBARS) assay | (i) Demonstrated 25.60% ± 0.98% malonaldehyde (MDA) reduction in hepatocytes at 1 mM under physiological conditions | Mediates antioxidant activity via lipid peroxidation inhibitory effect | [74] | |
| (ii) Demonstrated higher MDA reduction with value of 36.50% ± 4.47% at 1 mM in hepatocytes under oxidative stress induced by tert-BuOOH | |||||||
| Mixture of cis- and trans-nerolidol (Sigma Chemical Company, St. Louis, MO, USA) | - | - | TBARS assay, nitrite assay, superoxide dismutase (SOD) activity and catalase activity | (i) At doses of 25, 50 and 75 mg/kg of nerolidol caused a significant decrease in lipid peroxidation by 59.97%, 74.79% and 91.31% respectively when compared to negative control | (i) Suggested to prevent oxidation of polyunsaturated fatty acids | ||
| (ii) At doses of 25, 50 and 75 mg/kg of nerolidol caused a significant decrease in nitrite level by 71.1%, 66.6% and 63.35 % respectively when compared to negative control | |||||||
| (iii) At doses of 25, 50 and 75 mg/kg of nerolidol increased superoxide dismutase activity by 31.1%, 34.8% and 66.1%, respectively when compared to negative control | (ii) Suggested to inactivate the enzyme nitric oxide synthase | [75] | |||||
| (iv) At doses of 25, 50 and 75 mg/kg of nerolidol increased catalase enzymatic activity by 109%, 148% and 177.7%, respectively when compared to negative control | |||||||
| Antibacterial activity | Mixture of cis- and trans-nerolidol (Sigma Chemical Company, St. Louis, MO, USA) | - | Staphylococcus aureus FDA 209P, 14 strains of methicillin-susceptible S. aureus (MSSA) and 20 strains of methicillin-resistant S. aureus (MRSA) | Broth-dilution with shaking method (BDS) | Exhibited dose-related inhibition against 34 clinical isolates of S. aureus. Inhibitory dose 50% (ID50) ranged from 5.0 to 22.0 μg/mL and from 2.6 to 10.6 μg/mL against MSSA and MRSA respectively. | Suggested the aliphatic chain of nerolidol mediates the antibacterial activity by damaging the bacterial cell membrane | [76] |
| Mixture of cis- and trans-nerolidol (Sigma Chemical Company, (St. Louis, MO, USA) | - | Staphylococcus aureus FDA209P | Broth dilution with shaking (BDS) method and quantitation of the leakage of K+ ions using K+-selective electrode | Treatment of nerolidol caused a dose-dependent increase in amount of K+ ions leakage from bacterial cells. | Mediates the antibacterial activity via cell membrane-distrupting mechanism and hence resulting in the leakage of K+ ions from bacterial cells | [77] | |
| Mixture of cis- and trans-nerolidol (Sigma Chemical Company, St. Louis, MO, USA) | - | Staphylococcus aureus FDA209P | Broth dilution with shaking (BDS) method and quantitation of the leakage of K+ ions using K+-selective electrode | (i) Caused a dose-dependent increase in K+ ions leakage from bacterial cells | [78] | ||
| (ii) Exhibited minimum inhibitory concentration at 40 μg/mL | |||||||
| trans-Nerolidol | Momordica charantia L., seed | Staphylococcus aureus ATCC 6538 | Broth microdilution method (MIC) | (i) Exhibited anti-microbial activity with MIC ranged from 125–500 μg/mL. | - | [7] | |
| Nerolidol (n.s.) | Camellia sinensis (L.) Kuntze, leaves | Staphylococcus aureus and Streptococcus mutans | Broth dilution method | Exhibited antibacterial activity against S. aureus and S. mutans with MIC measured at 200 and 25 μg/mL respectively | - | [79] | |
| Nerolidol (n.s.) | Ginkgo biloba L., leaves | Salmonella enterica, Staphylococcus aureus and Aspergillus niger | Disc-diffusion and broth dilution methods | (i) Exhibited antibacterial activity against S. enterica, S. aureus and A. niger with MIC, MBC and MFC values measured ranging from 3.9–15.6 μg/mL, 31.3–62.5 μg/mL and 62.5 μg/mL respectively. | - | [31] | |
| cis-Nerolidol and the racemic mixture of cis- and trans-nerolidol (Aldrich Chemical Co., Milwaukee, WI, USA) | - | Escherichia coli and Staphylococcus aureus | Agar-disc diffusion assay | Nerolidol (cis-nerolidol and the racemic mixture of cis- and trans-isomers) potentiated the action of antibiotics: | - | [80] | |
| (i) amoxicillin/clavulanic acid against S. aureus and | |||||||
| (ii) amoxicilline/clavulanic acid, ceftadizine and imipenem against E. coli | |||||||
| Nerolidol (n.s.) (Sigma, St. Louis, MO, USA) | Escherichia coli ATCC 25922 and Staphylococcus aureus | Disc-diffusion assay | (i) Nerolidol concentrations ranged from 0.5 to 2 mM enhanced the susceptibility of S. aureus to ciprofloxacin, clindamycin, erythromycin, gentamicin, tetracycline, and vancomycin | - | [81] | ||
| (ii) Nerolidol (1 mM) enhanced the susceptibility of E. coli to polymyxin B | |||||||
| Racemic mixture of cis- and trans-nerolidol (1:1) (Aldrich, Madrid, Spain) | - | Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 | Antibiotic disc assay | Nerolidol (20 mM) potentiated the susceptibility of E. coli and S. aureus towards ciprofloxacin, erythromycin, gentamicin and vancomycin | - | [82] | |
| Anti-biofilm activity | Mixture of cis- and trans-nerolidol | Black pepper, cananga, and myrrh EOs (Berjé (Bloomfield, NJ, USA), Jin Aromatics (Anyang, Gyeonggi Province, Korea) and Sigma-Aldrich (St. Louis, USA)) | Staphylococcus aureus | Crystal violet biofilm assay | Cis-nerolidol at 0.01% (v/v) inhibited S. aureus biofilm formation by > 80 %; trans-nerolidol at similar concentration exerted 45% inhibition | - | [45] |
| trans-Nerolidol | Piper claussenianum (Miq.) C. DC., leaves | Candida albicans | MTT assay | Concentrations of 0.06%–1.0% inhibited biofilm formation by 30% and 50% after 24 and 48 h incubation respectively | - | [32] | |
| cis,trans-Nerolidol and cis-nerolidol (Sigma Aldrich) | - | Candida albicans | MTT assay | 1.0% of cis,trans-nerolidol exerted 76.1% reduction in the viability of pre-formed biofilm while only 67.0% reduction observed from 1.0% cis-nerolidol | - | [32] | |
| Anti-fungal activity | Nerolidol (n.s.) | Chamaecyparis obtusa (Siebold & Zucc.) Endl. (Japanese cypress) | Microsporum gypseum | Broth microdilution method Skin lesion scoring in guinea pig model | (i) Exhibited MIC concentrations of 0.5%–2% against M. gypseum | - | [83] |
| (ii) Nerolidol-treated group exhibited a significant improvement (p < 0.05) in lesion as compared to eugenol and econazole (positive control) treated groups | |||||||
| trans-Nerolidol | Piper claussenianum (Miq.) C. DC., Piperaceae, leaves | Candida albicans | Broth microdilution and trypan blue exclusion method | (i) Exhibited anti-fungal activity with MIC values ranging from 0.24% to 1.26%. | - | [32] | |
| (ii) Exhibited inhibitory effect on yeast-to-hyphae transition by 81% | |||||||
| Nerolidol (n.s.) (Sigma-Aldrich, Yongin, Korea) | - | Trichophyton mentagrophytes | Agar dilution method | Inhibited the hyphal growth of T. mentagrophytes at the concentration of 0.4 mg/mL. | - | [16] | |
| Nerolidol (n.s.) | Camellia sinensis (L.) Kuntze, leaves | Broth dilution method | Inhibited the growth of T. mentagrophytes at 12.5 μg/mL | - | [79] | ||
| trans-Nerolidol | Lantana radula Sw., leaves | Corynespora cassiicola | Poison food (PF) technique | (i) L. radula EO at the concentration of 1000 mg/L and 3000 mg/L inhibited the growth of C. cassiicola by 17.2% and 40.6% respectively | - | [33] | |
| (ii) L. radula EO at the concentration of 5000 mg/L and 10,000 mg/L completely inhibited the growth of C. cassiicola | |||||||
| trans-Nerolidol | Piper chaba Hunter, leaves | Fusarium oxysporum, Phytophthora capsici, Colletotrichum capsici, Fusarium solani and Rhizoctonia solani | Spore germination assay and agar dilution method | Caused 55.1%–70.3% growth inhibition at concentration ranging from 125 to 500 µg/mL. | - | [34] | |
| trans-Nerolidol | Warionia saharae ex Benth. & Coss., aerial part | Alternaria sp., Penicillium expansum and Rhizopus stolonifer | Poisoned food (PF) technique and volatile activity (VA) assay | Inhibited the fungal spore production of Alternaria sp., P. expansum and R. stolonifera at 1, 2 and 2 µL/mL air respectively | - | [26] | |
| Nerolidol | Allium sativum L., bulb | Sclerotium cepivorum | Disc diffusion method; scanning electron microscopy | (i) Nerolidol ranged from 2.0 to 5.0 µg/disc displayed fungistatic property by inhibiting mycelial growth by ~85% | - | [84] | |
| (ii) Nerolidol ranged from 2.0 to 5.0 µg/disc inhibited the production of sclerotial by ~84% | |||||||
| (ii) Nerolidol at 4.0 µg/disc caused morphological alterations such as shorter branching, hyphal shrinkage and partial distortion | |||||||
| Anti-trypanosomal activity | trans-Nerolidol | Strychnos spinosa Lam., leaves | Trypanosoma brucei | Alamar Blue™ assay. | Exhibited anti-trypanosomal activity with IC50 measured at 1.7 µg/mL (7.6 µM) | - | [35] |
| cis-Nerolidol | Leonotis ocymifolia (Burm.f.) Iwarsson, aerial part | Trypanocidal and cytotoxic assays | Exhibited anti-trypanosomal activity with IC50 measured at 15.78 µg/mL | - | [27] | ||
| Mixture of ±40% cis-nerolidol and ±55% of trans-nerolidol (Merck, Darmstadt, Germany) | - | Trypanosoma evansi | Collection of blood samples from T. evansi-infected mice for observation using light and electron microscopes | (i) Adverse morphological changes observed in nerolidol-treated group. The parasites lost their undulating membrane after 23 day post-treatment. | - | [85] | |
| (ii) Total disfigurement observed after 27 day post-treatment | |||||||
| Anti-leishmanial activity | A mixture of cis- and trans-nerolidol | - | Leishmania (L.) amazonensis, L. braziliensis, and L. chagasi | MTT assay and metabolic labeling with [2-14C] mevalonic acid, [1-14C] acetic acid, [1(n)-3H] farnesyl pyrophosphate and l-[35S]methionine | (i) Inhibited the growth of L. amazonensis, L. braziliensis and L. chagasi promastigotes, and L. amazonensis amastigotes with IC50 of 85, 74, 75, and 67 µM respectively | Inhibition of the isoprenoid biosynthesis pathway | [86] |
| (ii) Nerolidol at 100 µM reduced the percentage of intracellular parasitism of L. amazonensis by 95% from the pre-infected macrophages culture | |||||||
| trans-Nerolidol | Baccharis dracunculifolia DC., leaves | Leishmania donovani | Parasite lactate dehydrogenase (pLDH) assay, antileishmanial assay, schistosomicidal assay and cytotoxicity assay using the mammalian cells Vero. | Exhibited anti-leishmanial activity against promastigotes of L. donovani with IC50 and IC90 values of 42 and 85 µg/mL respectively. | - | [8] | |
| Nerolidol | Piper claussenianum (Miq.) C. DC., Piperaceae, leaves | Leishmania amazonensis | Protozoal arginase activity, nitrite determination and cytotoxicity assay using L929 fibroblast cells (mouse) and Raw cells (mouse macrophages) | (i) Nerolidol inhibited the arginase activity by 62.17% in the promastigotes of Leishmania amazonensis | Interferes with parasite-host cell interaction | [9] | |
| (ii) Nerolidol caused an increase in NO production (20.5%) | |||||||
| Nerolidol (n.s.) (Acros Organics, Geel, Belgium) | - | Promastigotes of Leishmania amazonensis | Anti-proliferative activity assay and electron paramagnetic resonance (EPR) spectroscopy of the spin-labeled 5-doxyl stearic acid | Nerolidol modulated the molecular dynamics of the lipid component in the Leishmania plasma membrane | Insertion of nerolidol into the lipid bilayer increased the fluidity of membranes, thus causing leakage of cytoplasmic content and eventually the death of Leishmania cells | [87] | |
| Anti-schistosomal activity | Nerolidol (n.s.) | Baccharis dracunculifolia DC. (Asteraceae), leaves | Schistosoma mansoni | Schistosomicidal assay | 100% mortality of S. mansoni adult worms after 24 h incubation with 10 to 100 mg/mL of EO containing nerolidol as the main constituent | - | [8] |
| Racemic mixture of cis- and trans-nerolidol (1:1) (Sigma-Aldrich, St. Louis, MO, USA) | - | In vitro anti-schistosomal assay and microscopy studies | Exhibited anti-schistosomal activity by reducing worm motor activity and caused 100% mortality of male and female schistosomes at concentration of 31.2 and 62.5 µM respectively | (i) Induced severe tegumental damage in adult schistosomes. | [88] | ||
| (ii) Caused alterations on the tubercles of male parasites | |||||||
| Anti-malarial activity | Nerolidol (n.s.) | Virola surinamensis (Rol. ex Rottb.) Warb., leaves | Plasmodium falciparum | In vitro anti-plasmodial assay | Treatment with 100 µg/mL of nerolidol caused 100% inhibition in the development of young trophozoite to the schizont stage after 48 h | - | [29] |
| trans-Nerolidol | Piper claussenianum (Miq.) C. DC., leaves | Exerted anti-malarial activity with IC50 of 11.1 μg/mL | - | [89] | |||
| Nerolidol (n.s.) (Sigma, St. Louis, MO, USA) | - | Immunoprecipitation assays and metabolic labeling | Exhibited inhibitory activity on the biosynthesis of the isoprenic side chain of the benzoquinone ring in ubiquinones during the schizont stage | Interferes with the elongation of isoprenic chains via inhibition of isoprenyl diphosphate synthases | [90] | ||
| Nerolidol (n.s.) (Sigma, St. Louis, MO, USA) | - | Nerolidol at 50 nM inhibited the synthesis of the isoprenic chain attached to coenzyme Q at all intraerythrocytic stages | - | [91] | |||
| Nerolidol (n.s.) | - | Isobolographic analysis | Nerolidol mediated supra-additive (the sum of the fractions of IC50 of < 1) interaction with fosmidomycin and squalestatin with average IC50 values of 0.57 and 0.62 µM, respectively in the inhibition of plasmodial isoprenoid pathway | - | [92] | ||
| Other anti-parasite activities | Mixture of cis- and trans-nerolidol (Sigma-Aldrich, St. Louis, MO, USA) | - | Four Babesia species (B. bovis, B. bigemina, B. ovata, and B. caballi) | In vitro growth inhibition assay | Inhibited in vitro growth of B. bovis, B. bigemina, B. ovata, and B. caballi with IC50 values of 21 ± 1, 29.6 ± 3, 26.9 ± 2, and 23.1 ± 1 µM respectively | Inhibits the isoprenoid biosynthesis pathway in a similar mechanism with that of P. falciparum | [93] |
| Mixture of cis- and trans-nerolidol (Sigma Chemical Company, St. Louis, MO, USA) | - | Caenorhabditis elegans | Mortality assay against Caenorhabditis elegans | Caused 74.0% mortality of C. elegans at 50 µg/mL | - | [94] | |
| Nerolidol (n.s.) | - | L3 larvae of Anisakis | In vitro and in vivo larvicidal activity | (i) Nerolidol at both 31.5 and 62.5 µg/mL resulted in 100% mortality of L3 larvae of Anisakis type I after 4 h. | - | [95] | |
| (ii) Only 20% of nerolidol-treated rats were affected by gastric wall lesions caused by Anisakis larvae in comparison to 86% of the control rats | |||||||
| Insecticidal activity | trans-Nerolidol | Siam-wood (Fokienia hodginsii (Dunn) A.Henry & H H.Thomas), wood | Mosquito and house flies | House fly toxicity test | Exhibited insecticidal activity with LD50 measured at 0.17 µmol/fly | - | [51] |
| Combination of nerolidol (n.s.) and linalool | Capparis tomentosa, leaves | Maize weevil (Sitophilus zeamais) | Repellency assay using a glass Y-tube Olfactometer | Exhibited mean repellency value of 58.23% ± 2.95% against S. zeamais at 2 µL | - | [30] | |
| Nerolidol (n.s.) (Moellhausen SpA,Vimercate, Milano, Italy) | Melaleuca alternifolia (Maiden & Betche) Cheel (tea tree oil) | Pediculus capitis (head lice) and its eggs | Pediculicidal and ovicidal activities | Nerolidol in combination with tea tree oil with ratio of 1:2 (tea tree oil 0.5% plus nerolidol 1%), exerted a total killing effect of lice within 30 min and abortive effect of louse eggs after 5 days. | - | [96] | |
| Nerolidol (n.s.) | Magnolia denudata Desr., seeds | Culex pipiens pallens, Aedes aegypti, Aedes albopictus and Anopheles sinensis | Direct-contact mortality bioassay | Exerted larvacidal activity against Culex pipiens pallens, Aedes aegypti, Aedes albopictus and Anopheles sinensis with LD50 value of 9.84, 13.85, 16.34 and 20.84 mg/L respectively | - | [47] | |
| trans-Nerolidol | Melaleuca quinquenervia (Cav.) S.T.Blake, leaves | Aedes aegypti | Larvicidal activity test | Exerted larvicidal activity with ≥ 95% and > 80% mortality of A. aegypti at 0.1 mg/mL and 0.05mg/mL respectively | - | [36] | |
| Piper aduncum L., leaves | Tetranychus urticae Koch | Fumigant, contact, repellency and two-choice assay | Exerted acaricidal activity with repellency value of 83.2% ± 0.59 % at 9.8 µg/mL | - | [37] | ||
| Nerolidol (n.s.) | Baccharis dracunculifolia DC., leaves | Rhipicephalus microplus | Larval packet test (LPT) and engorged female immersion test | (i) Exerted acaricidal activity when concentration more than 5mg/mL and 100% mortality of larvae at 15 mg/mL | - | [97] | |
| (ii) Reduced the quality of the egg and larval hatching rate with increasing concentration from 20 to 50 mg/mL | |||||||
| Antiulcer activity | Nerolidol (n.s.) | Baccharis dracunculifolia DC., leaves | - | In vivo antiulcer activity in male Wistar rat ulcer models induced with ethanol, indomethacin and stress | Nerolidol displayed gastroprotective activity by inhibiting the formation of ulcers induced by all physical and chemical agents in dose-dependent manner (50, 250, 500 mg/kg) | - | [98] |
| Skin penetration enhancer activity | Nerolidol (n.s.) (Aldrich, Gillingham, UK) | - | - | In vitro diffusion studies and stratum corneum-water partitioning studies | Increased diffusion rate by over 20-fold for transdermal delivery of drugs such as 5-fluorouracil | Nerolidol exhibits a chemical structure that allows it to align within the lipid lamellae of the stratum corneum in order to disrupt the organization of stratum corneum | [99] |
| Nerolidol (n.s.) (Alfa Aesar Ltd., Haverhill, MA, USA) | - | - | Solubility studies, ex vivo permeation studies and histopathological studies | The enhancement effect is increased with the increasing lipophilicity; the rank of order (nerolidol > farnesol > limonene > linalool > geraniol > carvone > fenchone > menthol) in facilitating transdermal delivery of alfuzosin hydrochloride | [100] | ||
| Nerolidol (n.s.) (Merck-Schuchardt, Hohenbrunn, Germany) | - | - | In vitro permeation studies | Exhibited the highest permeation enhancing ability with a 3.2-fold increase in permeation of selegiline hydrochloride across the rat skin, followed by the effect of carvone (2.8-fold increase) and anethole (2.6-fold increase) | - | [101] | |
| Nerolidol (n.s.) (Aldrich Chemical Co. Milwaukee, WI, USA) | - | - | In vitro skin permeability studies | Most effective terpene enhancer for percutaneous permeation of four different drug models (nicardipine hydrochloride, hydrocortisone, carbamazepine, and tamoxifen) when compared to fenchone, thymol and limonene | - | [102] | |
| Anti-nociceptive and anti-inflammatory activities | trans-Nerolidol | Peperomia serpens (Sw.) Loudon, leaves | - | (i) Chemical (acetic acid and formalin) and thermal (hot plate) models of nociception | trans-Nerolidol could be responsible for the anti-inflammatory and anti-nociceptive effects displayed by essential oils of both Peperomia serpens (Sw.) Loudon and Piper aleyreanum C. DC | - | [38] |
| (ii) Carrageenan- and dextran-induced paw edema tests in rats croton oil-induced ear edema | |||||||
| (iii) Cell migration, rolling and adhesion activities | |||||||
| trans-Nerolidol | Piper aleyreanum C. DC, aerial parts | - | (i) Nociception induced by formalin | - | [10] | ||
| (ii) Evaluation of locomotor activity | |||||||
| (iii) Induction of acute gastric lesions | |||||||
| Nerolidol (n.s.) (Sigma, St. Louis, MO, USA) | - | - | (i) Rotarod, acetic acid-induced writhing, formalin and hot-plate tests (ii) Involvement of ATP-sensitive opioid and GABAergic K+ channels (iii)Carrageenan-induced paw edema (iv) Analysis of leukocytes, tumor necrosis factor (TNF-α), interleukin 1 beta (IL-1β) and interleukin 6 in peritoneal lavage |
(i) For acetic acid-induced writhing test, at the doses of 200, 300 and 400 mg/kg, nerolidol reduced the frequency of acetic acid-induced writhing at all three doses tested compared to the mice in the control group (55% ± 1.1%, 53% ± 4.5%, and 41% ± 2.4%, respectively) (ii) For formalin test, at the doses of 200, 300 and 400 mg/kg, nerolidol significantly inhibited licking time by 20% ± 3.3%, 33% ± 5.9% and 37% ± 4.8%, respectively when compared to the control mice. (iii) For hot-plate test, no increase in the reaction time to painful stimulation in the mice treated with nerolidol when compared to the control mice. (iv) Reduced leukocytes level by 51% ± 0.7%, 37% ± 0.5% and 57% ± 0.4% at doses of 200, 300 and 400 mg/kg respectively (v) Reduced the level of tumor necrosis factor (TNF-α) at doses of 300 (59.3% ± 30.2%) and 400 (62.2% ± 13.7%) in peritoneal lavage. (vi) IL-1β production was inhibited after treatment with nerolidol (1, 10, 50 and 100 µM) whereas IL-6 level was unchanged |
(i) Anti-nociceptive activtity of nerolidol was indicated to be mediated by GABAA receptors, as the use of bicuculline, a GABAA antagonist inhibited the effect of nerolidol in reducing the paw licking times (ii) Anti-inflammatory activity of nerolidol was suggested to be mediated by inhibiting the production or the activity of pro-inflammatory cytokines such as TNF-α analgesic and IL-1β |
[103] | |
| Anti-cancer or anti-tumor activity | Nerolidol (a combination of cis-nerolidol 40.7%, trans-nerolidol 58.3%, cis-dihydronerolidol 0.4% and trans-dihydro-nerolidol) (Kurt Kitzing Co. Wallerstein, Germany) | - | - | Cytotoxicity assay on HeLa cell lines using CytoTox-96®-assay | Exhibited anticancer effect against HeLa cells with CC50 value at 1.5 ± 0.7 µM | - | [104] |
| cis-Nerolidol (Charabot S.A. Grasse, France) | - | - | Cytotoxicity and cytoproliferative activity on HeLa cell lines using Cytotoxicity Detection Kit (LDH) and the Cell Proliferation Reagent WST-1, respectively | Exhibited cytotoxic effect (16.5 ± 6.7 μM) against HeLa cells | - | [105] | |
| Nerolidol (n.s.) | Camellia sinensis (L.) Kuntze, leaves | - | MTT assay | Exhibited cytotoxic effect with IC50 value of 2.96 and 3.02 µg/mL against BT-20 breast carcinoma and HeLa cells respectively | - | [106] | |
| trans-Nerolidol | Zornia brasiliensis Vogel, leaves | - | In vitro cytotoxic activity assay using Alamar blue assay, and in vivo antitumor activity assay | (i) trans-Nerolidol induced cytotoxic effect on B16-F10, HepG2, HL-60 and K562 cells with IC50 value of >25, >25, 21.99 and 17.58 µg/mL respectively | - | [39] | |
| (ii) The EO at dose of 100 mg/kg containing trans-nerolidol as major constituent reduced the weight of tumor in mice injected with B16-F10 melanoma by 38.61% | |||||||
| Myrica rubra (Lour.) Siebold & Zucc., leaves | - | Neutral red uptake (NRU) test, MTT assay and 2′,7′-dichlorodihydrofluorescein-diacetate (H2DCF-DA) oxidation | Potentiated the action of doxorubicin, an anticancer drug in the modulation of CaCo-2 cancer cells | - | [40] | ||
| Nerolidol (n.s.) (Sigma Aldrich Chemical Company) | - | - | In vivo anti-cancer study | (i) Reduction of incidence of intestinal neoplasia from 82% to 33% in rats fed with nerolidol | Modulation of nerolidol on protein prenylation which responsible for the formation of cancer | [107] | |
| (ii) Reduction of number of tumors/rat from 1.5 to 0.7 in rats fed with nerolidol | |||||||
| Combination of farnesol and nerolidol (n.s.) | - | - | In vitro anti-cancer study | The combination suppressed the proliferation of human HL-60 acute promyelocytic leukemia (HL-60) cells by 20%. Meanwhile, farnesol isomers (2.5 µmol/L) and nerolidol (5 µmol/L) individually suppressed the proliferation of HL-60 cells by 4 and 9%, respectively | Nerolidol induced cell cycle arrest at the G0-G1/S interphase in HL-60 cells and eventually lead to apoptotic cell death | [108] | |
| trans-Nerolidol | Myrica rubra (Lour.) Siebold & Zucc., leaves | - | Cell adhesion and apoptosis luminescent assays | (i) Reduced adhesion of HT29 to collagen. | Nerolidol induced apoptosis in cancer cells | [109] | |
| (ii) Suppressed cell adhesion of HT29 cells in the presence TNFα cytokines | |||||||
| (iii) Decreasing the phosphorylation of NF-κB and increased the activity of caspases |
Key: n.s. = not specified.
Figure 2.
The source of extraction of nerolidol and an overview of the biological activities of nerolidol.
6.1. Antioxidant Activity
Reactive oxygen species (ROS) are formed by the incomplete reduction of oxygen during aerobic metabolism [110]. Superoxide anion (O2–), hydrogen peroxide (H2O2), and hydroxyl radicals (OH•) are some examples of ROS. Under normal circumstance or low level of oxidative stress, an in-built antioxidant defense system in the body helps the cells to counteract with any potential damages by detoxifying ROS with appropriate enzymes such as glutathione (GSH) reductase, GSH peroxidase, superoxide dismutase (SOD) and catalase. However, an imbalance in the antioxidant defense system or overproduction of free radicals which exceeds the detoxification capacity of cell may contribute to the onset of oxidative stress [111]. During oxidative stress, an elevation of intracellular levels of ROS was found to cause damage on biomolecules (lipids, proteins and DNA) [112]. Thus, high level of ROS is detrimental to cells. It is mainly due to the formation and accumulation of cellular damage resulted from oxidative stress and subsequently leading to the loss of cellular functions. If left untreated, it will result in many complications such as cancer, cardiovascular diseases and neurodegenerative disorders [113].
Given the fact that chemical compounds belonging to the sesquiterpene group are well known for their antioxidant properties [113,114], perhaps antioxidant activity may be expected from nerolidol. Furthermore, EOs containing nerolidol derived from medicinal plants were found to exhibit antioxidant activity, suggesting its plausible utilization as an antioxidant agent. Indeed, it has been demonstrated that nerolidol exhibits potent antioxidant properties in counterbalancing the effect of ROS by protecting the cells against oxidative damage to lipids, proteins and DNA [73,74,75]. According to a study conducted by Vinholes et al., the antioxidant activity of cis-nerolidol was evaluated using 1,1-diphenyl-2-picrylhydrazine (DPPH) radical scavenging assay. The study revealed that cis-nerolidol exhibited DPPH scavenging activity [73]. In another study, cis-nerolidol was found to possess higher scavenging activity towards hydroxyl radicals with IC50 measured at 1.48 mM [73]. Due to the ability in scavenging several types of free radicals, cis-nerolidol was evidenced to protect Caco-2 cells against oxidative stress induced by tert-butyl hydroperoxide (tert-BuOOH), suggesting that nerolidol is a good antioxidant that exerts protection against oxidative damage [73]. Another study conducted by Vinholes et al. reported that cis-nerolidol mediated its strong antioxidant activity in protecting the hepatocytes through the inhibition of lipid peroxidation induced by tert-BuOOH, thereby 1mM of cis-nerolidol resulted in 36.50% ± 4.47% of malonaldehyde (MDA) reduction [74].
Besides the in vitro evidences displaying the antioxidant properties of nerolidol, an in vivo study by Nogueira Neto et al. demonstrated the neuroprotective effects of nerolidol (a mixture of cis- and trans-nerolidol) in adult male Swiss albino mice hippocampus against neuronal damages induced by oxidative stress [75]. The study demonstrated that significant decrease in MDA and nitrite levels were observed for the nerolidol group at doses of 25, 50 and 75 mg/kg when compared to saline, the negative control. Beside that, nerolidol also increased the antioxidant enzymatic activities of superoxide dismutase and catalase at doses of 25, 50 and 75 mg/kg. These observations suggest that nerolidol mediates a potent antioxidant activity by scavenging free radicals, preventing lipid peroxidation and enhancing the production of antioxidant enzymes in cells for protection against oxidative stress [115,116].
6.2. Antibacterial Activity
The decrease in the effectiveness of many antibiotics due to the rise of antimicrobial resistance is a global concern faced by the pharmaceutical, medical and food industries. Consequently, the number of infections caused by multidrug-resistant bacteria is increasing globally, leading to increased risk of mortality and morbidity [117]. Due to this, a large proportion of investments by pharmaceutical industries are being put into drug discovery research of new inhibitory compounds of microbiological, plant, or animal origin to be developed into potentially new anti-microbial drugs.
The studies showed that nerolidol exhibited potent antimicrobial activity against Staphylococcus aureus FDA 209P, 14 strains of methicillin-susceptible S. aureus (MSSA) and 20 strains of methicillin-resistant S. aureus (MRSA) with MIC values ranging from 512 to over 1024 μg/mL [76]. Besides, nerolidol possessed antibacterial activity against various strains of Staphylococcus aureus including MRSA by disrupting the cell membranes as indicated by the increased leakage of K+ ions from the bacterial cells [76,77,78]. The observed effects could be due to the presence of the long aliphatic chain in chemical structure of nerolidol. The hypothesis may comply with the findings of Togashi et al. [118] that the terpene alcohols with carbon chains of C10 to C12 (the numbering is started from the carbon atom connected to a hydroxyl group) was found to exhibit a strong antibacterial activity against S. aureus FDA209P. Since the carbon chain length of nerolidol is C12, it was shown to cause damage to the cell membrane, leading to the leakage of macromolecules and eventually cell lysis [78]. Besides causing membrane disruption, nerolidol was also found to interfere with genes which regulate the pathogenicity of the pathogens. For example, a study conducted by Lee et al. reported that cis-nerolidol present in the black pepper oil was responsible for the down-regulation of the α-hemolysin gene hla expression in S. aureus via quantitative real-time PCR analyses [45].
In a similar way, EOs of Momordica charantia L. seed was found to exhibit strong antibacterial activity against S. aureus ATCC 6538 with MIC value of 125 μg/mL. The high content of trans-nerolidol was suggested to be responsible for the antibacterial activity demonstrated by the EOs of Momordica charantia L. seed [7]. Likewise, the study conducted on the antimicrobial activity of green tea flavor components by Kubo [79] has also shown that nerolidol as one of the ten major green tea flavor compounds, exhibited anti-microbial activity against S. aureus with MIC values of 200 μg/mL. Besides, nerolidol was also found to exert the strongest antibacterial activity against Streptococcus mutans when compared among the ten green tea flavor compounds with a MIC value of 25 μg/mL [79]. Meanwhile, nerolidol derived from the leaf EO of Ginkgo biloba (L.) demonstrated the highest antibacterial activity against Salmonella enterica, and S. aureus when compared to other EOs (isophytol, linalool, β-sitosterol acetate, β-sitosterol, stigmasterol, ergosterol, β-sitosterol-3-O-β-d-gluco-pyranoside and Ginkgo biloba polyprenols (GBP)) [31]. The evidences presented above may suggest nerolidol can be a good antibacterial agent particularly against S. aureus.
Besides the direct antibacterial action of nerolidol, nerolidol (cis-nerolidol and the racemic mixture of cis- and trans-isomers (1:1)) was found to potentiate the action of antibiotics, namely amoxicilline/clavulanic acid against S. aureus and amoxicilline/clavulanic acid, ceftadizine and imipenem against Escherichia coli [80]. The sensitization effect was also observed when nerolidol enhanced the susceptibility of S. aureus to ciprofloxacin, clindamycin, erythromycin, gentamicin, tetracycline, and vancomycin. Other than S. aureus, nerolidol was also found to enhance the susceptibility of E. coli ATCC 25922 to polymyxin B [81]. These findings were further supported by the experiment conducted by Simões et al. as it revealed that the treatment of nerolidol (racemic mixture of the cis- and trans-nerolidol) (1:1) potentiated the susceptibility of E. coli and S. aureus towards the antibiotics (ciprofloxacin, erythromycin, gentamicin and vancomycin), thereby resulted in significantly lower MIC concentrations [82]. Moreover, the study also demonstrated a moderate correlation between cell killing and permeabilization effects of nerolidol against both S. aureus and E. coli, suggesting that nerolidol exerted its action by modifying the bacterial outer layer as evidenced by the increased propidium iodide uptake [82]. All these observations suggest that nerolidol provides an alternative therapeutic option for the development of drug combinations that may be more effective in controlling multi-drug resistant bacteria.
6.3. Anti-Biofilm Activity
Many bacteria are known to possess the ability to produce biofilm, which is defined as a community of microorganisms held together by a self-produced extracellular matrix and attached to living or inert surfaces such as polystyrene, glass, stainless steel and blood components in different environments [119]. Due to the complexity of the biofilm structure formation, microbial biofilms represent a significant challenge to the medical and pharmaceutical industries. The formation of biofilm induces microbial resistance to anti-microbial agents as well as to the body’s immune system. It has also been associated with the increased antibiotic resistance, thereby leading to biofilm-associated infections which complicate the treatment procedure. Therefore, there is a new trend in recent studies focusing on the evaluation of essential oils as potential inhibitors of biofilm formation. In the meantime, nerolidol was found to exhibit anti-biofilm activity against a number of pathogens. For example, a study conducted by Lee et al. has revealed that the EO of Cananga odorata (Lam.) Hook.f. & Thomson exhibited strong biofilm activity in a dose-dependent manner against the biofilm formation of S. aureus ATCC 6538 [45]. The anti-biofilm activity was attributed to the presence of cis- and trans-nerolidol in the essential oil of C. odorata. It was demonstrated that the cis-nerolidol at 0.01% (v/v) inhibited S. aureus biofilm formation by more than 80%, whereas trans-nerolidol at similar concentration exerted 45% inhibition. Another study conducted by Curvelo et al. revealed that the EO of Piper claussenianum (Miq.) C. DC., Piperaceae leaf (trans-nerolidol identified as the main component (81.4%) in this EO) decreases the formation of biofilm by Candida albicans for 30% and 50% after 24 and 48 incubation hours, respectively [32]. The same study also compared the anti-biofilm activities of the cis,trans-nerolidol and cis-nerolidol on the pre-formed biofilm by C. albicans. The study indicated that cis,trans-nerolidol resulted a stronger reduction in the viability of the mature biofilm than that of cis-nerolidol. Thus, it was suggested that trans-nerolidol, which was the main constituent in EO of Piper claussenianum (Miq.) C. DC., Piperaceae leaf, may responsible for the observed anti-biofilm activity in reducing the viability of the pre-formed biofilms.
6.4. Anti-Fungal Activity
Various anti-fungal agents have been developed to control the spread of fungal diseases such as candidiasis [120]. However, there are serious questions concerning the safety of these drugs due to their well-known side-effects as well as the possible development of antifungal drug resistance [121]. Therefore, the attention has been shifted to bio-prospecting the natural products to overcome or control fungal infections. In fact, EOs have been extensively studied and have been proven to be effective against fungal infections [122].
There are many evidences that support the effectiveness of nerolidol in exhibiting anti-fungal activity. Trans-nerolidol, which is a major component of leaf EO of Piper claussenianum (Miq.) C. DC., Piperaceae (81.4%), has been shown to exhibit fungicidal activity against Candida albicans with MIC values measured ranging from 0.24%–1.26% [32]. Similarly, the leaf EO also exerted a strong activity in the inhibition of germ-tube transformation of Candida albicans by 81% [32]. In another study, nerolidol has also been found to possess strong antifungal activity by distorting the hyphal growth of Trichophyton mentagrophytes at the concentration of 0.4 mg/mL [16]. Also, the growth of T. mentagrophytes was inhibited by nerolidol derived from green tea flavor with MIC value measured at 12.5 μg/mL [79].
Lee et al. reported a strong anti-fungal effect of nerolidol against Microsporum gypseum that causes dermatophytosis, a superficial infection in keratinized tissues including hair, nail and stratum corneum of skin [83]. Although nerolidol (0.5%–2%) was found to exhibit lower anti-fungal activity as compared to eugenol (0.01%–0.03%), the study showed that nerolidol was more effective in reducing the skin lesion than eugenol in guinea pig model. Moreover, histopathologic analysis revealed that animals treated with nerolidol had a lower degree of hyperkeratosis and inflammatory cell infiltration than non-treated animals.
Besides its anti-fungal effect against human pathogens, nerolidol also shows promising outcomes in controlling fungal infections in plants caused by phytopathogenic fungi. Trans-nerolidol, extracted from EO of Lantana radula Sw., has demonstrated to exhibit stronger fungistatic activity against the the phytopathogenic fungi Corynespora cassiicola than the EO extracted from Lantana camara [33]. Similarly, the leaf EO of Piper chaba Hunter which contained the trans-nerolidol as one of the major constituents, exhibited antifungal activity against phytopathogenic fungi such as Fusarium oxysporum, Phytophthora capsici, Colletotrichum capsici, Fusarium solani and Rhizoctonia solani with 55.1 to 70.3% growth inhibition and a MIC ranging from 125 and 500 µg/mL [34].
Znini et al. have also reported strong anti-fungal activity of trans-nerolidol extracted from EOs of aerial parts of Warionia saharae ex Benth. & Coss. against the three apple phytopathogenic fungi, Alternaria sp., Penicillium expansum and Rhizopus stolonifer causing the deterioration of apple by significantly inhibiting the mycelial growth of all strains tested. It was also found to inhibit the fungal spore production of Alternaria sp., P. expansum and R. stolonifera at the dosage of 1, 2 and 2 µL/mL air, respectively [26]. Besides this result, another study conducted by Pontin et al. has revealed strong antifungal activity of nerolidol in inhibiting mycelial growth and sclerotial production by ~85% and ~ 84%, respectively [84]. Nerolidol was also found to cause alterations in hyphal morphology and membrane permeability as demonstrated by hyphal shrinkage and partial distortion [84]. In addition, the study revealed an increase in the level of nerolidol in garlic (Allium sativum L.) tissues in response to fungal attack by Sclerotium cepivorum [84]. Based on the number of studies reported on the anti-fungal activity of trans-nerolidol, it could be suggested that trans-nerolidol is a good candidate for the development of anti-fungal drugs.
6.5. Anti-Parasitic Activity
Parasitic diseases such as malaria, leishmaniasis, sleeping sickness and Chagas’ disease continue to affect hundreds of millions of people around the world with a majority of them living in tropical regions [123]. However, most of them live in countries where the prospects of any financial return on investment are too low to support market-driven drug discovery and development of new drugs on parasitic diseases. Moreover, the emergence of parasites resistant to current anti-parasitic drugs thwarts the effort in treating the parasitic diseases. All these challenges underline the importance of plant EO as potential novel anti-parasitic agents [89,124].
6.5.1. Anti-Leishmaniasis
Leishmaniasis is a vector-borne infection caused by protozoan parasites from the genus of Leishmania. Leishmaniasis affects approximately 350 million people in 88 tropical and subtropical countries. The clinical syndromes and manifestations of leishmaniasis vary widely but are often divided into the three clinically distinct syndromes, the visceral leishmaniasis, cutaneous leishmaniasis (CL), and mucosal leishmaniasis (ML), depending on the parasite species and the host’s immune response [125]. CL has affected mankind for centuries, mainly affecting the skin or mucous membranes and is distinguished by the presence of ulcerative skin lesions. On the other hand, VL is fatal if left untreated and the cutaneous forms are disfiguring and mutilating. Although pentavalent antimonials are still widely used to treat leishmaniasis, they are toxic, poorly tolerated and become increasingly ineffective to cure drug-resistant parasites [126]. Therefore, the search of alternative drugs continues.
Recently, trans-nerolidol purified from the leaf EO of Baccharis dracunculifolia DC has been found to mediate strong anti-leishmanial activity against promastigotes of Leishmania (L.) donovani with an IC50 and IC90 values of 42 and 85 µg/mL, respectively [8]. Besides this study, nerolidol also exhibited anti-leishmaniasis activity by inhibiting the growth of L. amazonensis, L. braziliensis, and L. chagasi promastigotes and L. amazonensis amastigotes with in vitro IC50 of 85, 74, 75, and 67 µM, respectively. Moreover, L. amazonensis-infected macrophages treated with 100 µM nerolidol resulted in 95% reduction in the rate of infection. Arruda et al. suggested that nerolidol at 30 μM mediated anti-leishmaniasis activity through the inhibition of isoprenoid biosynthesis in L. amazonensis, as demonstrated by the reduced incorporation of [2-14C] mevalonic acid or [1-14C] acetic acid precursors into dolichol, ergosterol and ubiquinone in the mevalonate pathway [86]. However, nerolidol did not reduce the incorporation of [1(n)-3H] farnesyl pyrophosphate into dolichol and ergosterol, suggesting that nerolidol could be an inhibitor at the early step in the mevalonate pathway [86]. Previously, the inhibition of isoprenoid biosynthesis pathway was shown to result in the arrest of development of Plasmodium falciparum during the intraerythrocytic stages [127]. Marques et al. have also observed similar growth inhibition of promastigotes of L. amazonensis after being treated with trans-nerolidol purified from the leaves of Piper claussenianum (Miq.) C. DC., Piperaceae [9]. Trans-nerolidol was also found to induce (1) a significant inhibition (62.17%) on the arginase activity of L. amazonensis and (2) an increase in the production of nitric oxide (NO) in L. amazonensis-infected macrophages. These results indicated that trans-nerolidol was able to interfere with parasite-host cell interaction, thus reducing the percentage of infected cells. Another study conducted by Camargos et al. have shown that through electron paramagnetic resonance (EPR) spectroscopy, nerolidol was able to increase the molecular dynamics of the lipid component in the Leishmania plasma membrane at IC50 of 0.008 µM [87]. This could be possibly due to the insertion of nerolidol into the lipid bilayer that act as spacers to increase the fluidity of membranes since nerolidol has high hydrophobicity, thus causing major reorganization in cell membranes [128]. Subsequently, this will lead to an increase in the overall molecular dynamics of the membrane, causing leakage of cytoplasmic content and eventually the death of Leishmania cells.
6.5.2. Anti-Trypanosomal Activity
Trypanosomiasis, also known as sleeping sickness, is caused by protozoan parasites of African trypanosomes (e.g., Trypanosoma brucei subspecies) and is fatal if left untreated. Its symptoms include swollen lymph nodes, fever, extreme fatigue and rash. Trans-nerolidol purified from the aerial part of Leonotis ocymifolia (Burm.f.) Iwarsson and leaves of Strychnos spinosa Lam. showed anti-trypanosomal activity with IC50 of 15.78 µg/mL and 1.7 µg/mL, respectively on bloodstream forms of T. brucei brucei [27,35]. Mohd-Shukri et al. conducted an in-depth study about the effects of nerolidol (containing the mixture of ±40% cis-nerolidol and ±55% of trans-nerolidol) compared to a positive control, berenil (a standard anti-trypanosomal drug) on the morphological changes of a protozoan parasite Trypanosoma evansi in mice by using light and electron microscopy [85]. Berenil elicited immediate adverse morphological changes after 2–3 h post-treatment as demonstrated by stiffening and tapering at both ends of the parasite as well as distorted flagella and loss of undulating membranes. On the other hand, nerolidol only induced adverse morphological changes beginning from 23rd to 25th day post-treatment when the parasites became stiff, lost their undulating membrane. At the 27th day post-treatment, total disfigurement was observed, indicating that nerolidol exhibited promising trypanosomatidal activity against the morphology of T. evansi in mice.
6.5.3. Anti-Schistosomal Activity
Schistosomiasis is caused by a trematode blood fluke of the genus Schistosoma and is one of the most significantly neglected tropical diseases in the world [129]. Schistosome transmission involves the contamination of water by faeces or urine containing eggs with a specific freshwater snail as intermediate host, followed by human contact with water inhabited by the freshwater snail [130]. Its acute symptoms include fever, urticaria, diarrhea and eosinophilia. However, schistosomiasis, if left untreated, can progress to its chronic stage, leading to inflammatory and obstructive disease in the urinary system (S. haematobium) or intestinal disease, hepatosplenic inflammation, and liver fibrosis [130]. According to a study by Parreira et al., the EO of Baccharis dracunculifolia DC. (Asteraceae) possessed high schistosomicidal activity since all pairs of Schistosoma mansoni adult worms were dead after 24 h incubation with the EO at concentrations of 10, 50, and 100 µg/mL [8]. However, trans-nerolidol did not display any significant schistosomicidal activity in the tested assays with the concentration ranging from 10 to 100 µM. In contrast, another experiment conducted by Silva et al. have revealed that nerolidol in the form of cis- and trans-nerolidol racemic mixture (1:1) exerted anti-schistosomal activity by reducing worm motor activity and causing the death of all male and female schistosomes of Schistosoma mansoni at concentrations of 31.2 and 62.5 µM, respectively [88]. The differences between these two results could be due to the fact that trans-nerolidol isomer is less active than the racemic mixture of cis- and trans-nerolidol [88]. The study also found that nerolidol induced (1) severe tegumental damage in adult schistosomes and (2) alterations on the tubercles of male parasites in a concentration-dependent manner. With the available findings, a mixture of cis- and trans-nerolidol was shown to be a promising candidate to treat schistosomiasis.
6.5.4. Anti-Malarial Activity
Malaria is an infection caused by the protozoan parasites belonging to the genus of Plasmodium and is transmitted via the bite of Anopheles mosquito [131]. Its symptoms are fever, headache, vomiting, sweating and fatigue. If left untreated, it can cause organ failure, abnormal blood coagulation and ultimately death. According to a study conducted by Lopes et al., nerolidol was found to exhibit strong anti-malarial activity since treatment of Plasmodium falciparum with 100 mg/mL of nerolidol extracted from the leaf EO of Virola surinamensis (Rol. ex Rottb.) Warb. for 48 h resulted in 100% of the inhibition on the development of young trophozoite to the schizont stage without pigment formation [29]. Similarly, nerolidol (23.7%), which is one of the major volatile components extracted from inflorescences oil of Piper claussenianum (Miq.) C. DC., has been demonstrated to exert anti-malarial activity with IC50 of 11.1 μg/mL whereas the crude oil of P. claussenianum showed IC50 of 7.9 μg/mL [89]. The study suggested that nerolidol may exert the inhibition of glycoprotein biosynthesis by repressing the biosynthesis of N-glycoproteins that are otherwise observable in P. falciparum mainly at the ring and young trophozoite stages of the intra-erythrocytic cycles [29]. Besides this mechanism of action, another study conducted by Rodrigues Goulart et al. has shown that the nerolidol inhibited the biosynthesis of the isoprenoid chain attached to the benzoquinone ring in the intraethryocytic stages of Plasmodium falciparum [90]. It was evidenced that nerolidol interfered with isoprenoid biosynthesis of apicoplast by disrupting the elongation of isoprenic chains via inhibition of isoprenyl diphosphate synthases, an enzyme that is responsible for the formation of isoprenoid compounds such as dolichols. Beside isoprenyl diphosphate synthase, nerolidol also inhibited the enzyme octaprenyl phosphate/phytoene synthase which is localized in the cytoplasm and also in mitochondria at the intra-erythrocytic stages of P. falciparum [132]. Moreover, treatment with nerolidol at doses 2.2 times below the IC50 of 0.12 μM was shown to inhibit the production of isoprenic chain attached to coenzyme Q at all intraerythrocytic stages of P. falciparum [91]. These findings indicated that nerolidol possesses strong anti-malarial activity by inhibiting the development of the intraerythrocytic stages of the parasites.
Besides displaying anti-malarial activity alone, nerolidol has also been found to exhibit a synergistic effect with either fosmidomycin or squalestatin against malarial parasites. The combination of nerolidol with either fosmidomycin or squalestatin resulted in strong supra-additive (the sum of the fractions of IC50 of <1) interaction in mediating inhibition of plasmodial isoprenoid pathway against P. falciparum with strong combinatorial IC50 of 0.57 and 0.54 μM respectively [92].
6.5.5. Other Anti-Parasitic Activities
Nerolidol demonstrated strong nematicidal activity against a nematode, Caenorhabditis elegans with its LC50 value of 12 µg/mL as well as 74.0% mortality at 50 µg/mL [94]. Besides nematicidal activity, nerolidol (a mixture of cis- and trans-nerolidol) inhibited the in vitro growth of four Babesia species with IC50 values of 21 ± 1, 29.6 ± 3, 26.9 ± 2, and 23.1 ± 1 µM for B. bovis, B. bigemina, B. ovata, and B. caballi, respectively. This anti-parasitic activity could be due to inhibition of the isoprenoid pathway by nerolidol by a similar mechanism similar to that found with Plasmodium falciparum [93]. Nerolidol was also found to be the most active compound among the tested sesquiterpenes (nerolidol, farnesol and elemol) that caused the death of nematodes, L3 larvae of Anisakis simplex type I with the mortality at 4 hours of 100% at the concentrations of 31.5 and 62.5 µg/mL [95]. Moreover, only 20% of nerolidol-treated rats were affected by gastric wall lesions caused by Anisakis larvae in comparison to 86% of the control rats [95].
6.6. Insect Repellent Activity
There is a growing concern about the usage of current commercial synthetic insecticides due to the increasing difficulty in the management of pesticide resistance [133]. For this reason, the researchers have focused on research of EOs that have been traditionally used as repellants. Studies in several countries have shown that certain plant EOs are effective not only in repelling insects, but have contact and fumigant insecticidal activity against specific pests without harmful side-effects to humans and animals [134,135].
The combination of nerolidol and linalool (that are purified from EO of Capparis tomentosa fresh leaves) showed significant repellence activity against maize weevil Sitophilus zeamais at all tested doses (0.002, 0.02, 0.2 and 2 µL) [30]. In another study, trans-nerolidol derived from the EO of Siam-wood (Fokienia hodginsii (Dunn) A.Henry & H H.Thomas) was shown to possess insecticidal activity with LD50 value at 0.17 µmol/fly [51].
A mixture of nerolidol and tea tree oil with a ratio of 2:1 (tea tree oil 0.5% plus nerolidol 1%) was shown to exert insecticidal and ovicidal activity against Pediculus capitis (head lice) and its eggs [96]. Besides, nerolidol purified from the seeds of Magnolia denudata Desr. also showed larvacidal activity against third-instar larvae of insecticide-susceptible Culex pipiens pallens and Aedes aegypti as well as the wild Aedes albopictus and Anopheles sinensis with lethal dose (LD)50 values of 9.84, 13.85, 16.34 and 20.84 mg/L respectively [47]. Similarly, trans-nerolidol, which is one of the components of EO from the leaves of Melaleuca quinquenervia (Cav.) S.T.Blake, at its concentration of 0.1 mg/mL exhibited strong larvicidal activity with ≥ 95% mortality against Aedes aegypti [36].
Meanwhile, the trans-nerolidol that was purified from the leaves of Piper aduncum L. possessed strong acaricidal activity due to its highest repellency of 83.2% ± 0.59% compared to α-humulene (73.3% ± 0.83%) and β-caryophyllene (70.7% ± 0.88%) against the two-spotted spider mite, Tetranychus urticae Koch that causes damage to many agricultural crops [37]. The EO of aerial parts of Baccharis dracunculifolia DC. containing nerolidol as one of the major components was discovered to demonstrate strong acaricidal activity by causing 100% mortality of Rhipicephalus microplus larvae (cattle tick that infests cattle) at 20.0 mg/mL [97]. Meanwhile, a 100% mortality of Rhipicephalus microplus larvae was achieved at a lower concentration of nerolidol (15.0mg/mL). The study also demonstrated that nerolidol reduced the quality of the egg and larval hatching rate with increasing concentration from 20 to 50mg/mL [97].
6.7. Anti-Ulcer Activity
Gastric ulcer affects thousands of people around the world and is known to be caused by an imbalance between aggressive (acid, pepsin) and protective factor (secretion and action of mucus and bicarbonate) in the stomach [98]. It is induced by several factors, such as stress, smoking, nutritional deficiencies and ingestion of non-steroidal anti-inflammatory drugs (NSAIDs). The current therapy for ulcers usually involves the use of histamine H2-antagonists, proton pump inhibitors and anti-muscarinics for the inhibition of gastric acid secretion. However, these drugs pose severe side-effects, particularly hypersensitivity, arrhythmia and impotence [136]. With this in mind, plant EOs have recently been exploited as they have been shown to produce promising results for alternative therapies to treat gastric ulcers with lesser side-effects.
A study has been conducted by Klopell et al. on the anti-ulcer property of nerolidol using different experimental models such as ethanol-, indomethacin- and stress-induced ulceration in rat [98]. In the stress-induced ulceration model of experiment, nerolidol treatment at 50, 250 and 500 mg/k caused a significant reduction in the ulcerative lesion index (ULI) by 41.22, 51.31 and 56.57, respectively when compared to the control group animals. With regard to the ethanol-induced ulceration model of experiment, treatment with nerolidol at 250 and 500 mg/kg significantly inhibited the formation of ulcer at 52.63% and 87.63%, respectively as compared to the control group. On the other hand, indomethacin-induced ulceration model of experiment, the treatment at 250 and 500 mg/kg of nerolidol had significantly inhibited the gastric ulcer for 51.02% and 46.93%. These findings indicate that nerolidol could be used as an active component in gastroprotective and anti-ulcer treatments.
6.8. Skin Penetration Enhancer Activity
Transdermal delivery has gained a lot of attention as an attractive alternative route to intravenous and oral drug delivery systems [137]. However, the application of transdermal delivery is limited by poor drug permeability as the stratum corneum plays as a rate-limiting lipophilic barrier against the uptake of chemical and biological agents [138]. Therefore, terpenes are often used as topical skin penetration enhancers due to their wide range of physicochemical properties such as low cutaneous irritancy and good toxicological profile as well as adsorption enhancement ability [139].
Nerolidol has been found to be a potent skin penetration enhancer. It was found to increase the diffusion rate by over 20-fold for transdermal delivery of several drugs especially on 5-fluorouracil [99]. This high permeation-enhancing ability was attributed to the structure of nerolidol which is suitable for the alignment within lipid lamellae of the stratum corneum in order to disrupt the organization of stratum corneum. This view has been further supported by Prasanthi and Lakshmi [100] who reported that nerolidol with highest lipophilicity (log P = 5.36 ± 0.38) have the highest enhancement effect with its rank of order of nerolidol > farnesol > limonene > linalool > geraniol > carvone > fenchone > menthol in facilitating transdermal delivery of alfuzosin hydrochloride. Similarly, nerolidol has the highest permeation enhancing ability with a 3.2-fold increase in permeation of selegiline hydrochloride across the rat skin, followed by carvone (2.8-fold increase) and anethole (2.6-fold increase) [101]. Another study conducted by El-Kattan et al. has shown that nerolidol was the most effective percutaneous permeation enhancer for four model drugs (nicardipine hydrochloride, hydrocortisone, carbamazepine, and tamoxifen) when compared to other terpenes (fenchone, thymol, nerolidol and d-limonene) [102].
6.9. Anti-Nociceptive and Anti-Inflammatory Activity
Pain is an unpleasant sensation and emotional experience that is associated with actual or potential tissue damage [140]. On the other hand, the stimulation of nociception is associated with the detection of real tissue injury or a potentially damaging event by nociceptors as a stimuli (transduction) followed by its transmission of encoded information to the brain [141]. Under normal circumstances, the presence of an injury activates the inflammatory response as follows: firstly the inflammatory mediators are released from damaged cells such as ions (K+, H+), bradykinin, histamine, 5-hydroxytryptamine (5-HT), ATP and nitric oxide. Subsequent activation of arachidonic acid pathway leads to the production of prostanoids and leukotrienes that would then lead to the release of more inflammatory mediators such as cytokines and growth factors. These mediators will ultimately activate peripheral nociceptors directly, resulting in spontaneous pain. Beside this action, they also act to convert responses of primary afferent neurons to subsequent stimuli (peripheral sensitization) to be transmitted to the brain. Due to exacerbated physiological response, chronic exposure to pain is very harmful to an individual as it can cause organ damage and ultimately death, if left untreated [142].
Non-steroid anti-inflammatory drugs (NSAIDs) are well-known analgesic drugs that act to reduce inflammation and pain by acting as an inhibitor of cyclooxygenases (COXs) in the arachidonic acid pathway. However, one major disadvantage of administration of NSAIDs is their serious side-effects such as significant gastrointestinal upset, gastritis, ulceration, hemorrhage, and even death [143]. In order to address this issue, EOs extracted from various medicinal plants have been increasingly explored as alternative traditional medicines to treat inflammation and pain without posing harmful side-effects.
Pinheiro et al. investigated the anti-nociceptive and anti-inflammatory effects of EO of Peperomia serpens (Sw.) Loudon in rodents [38]. The EO has been found to possess anti-inflammatory and anti-nociceptive activities which could possibly be mediated by one of its major compounds, trans-nerolidol (38.0%). In a similar experiment, Lima et al. reported anti-nociceptive and anti-inflammatory activities of the EO of the aerial parts of Piper aleyreanum C. DC which is attributable to the presence of trans-nerolidol (1.2%) [10]. In order to strengthen the findings, Fonsêca et al. investigated the anti-nociceptive and anti-inflammatory activities of nerolidol using mouse models of pain [103]. The study found that nerolidol has no effect on the locomotor activity. Meanwhile, anti-nociceptive activity was evaluated via acetic acid-induced writhing and the formalin tests. The results demonstrated that oral administration of nerolidol was able to cause lesser acetic acid-induced abdominal contractions and also inhibition in paw licking behavior in the respective tests when compared to the control group (Table 4). These results implied that nerolidol modulates its effect on neuropathic pain and inflammatory processes as demonstrated by the formalin test [103]. However, the anti-nociceptive effect of nerolidol did not involve the thermal stimulation of centrally mediated nociception as shown by the negative hot-plate test result. In addition, the researchers also further elucidated the possible anti-nociceptive mechanisms of nerolidol by examining its effects on the parameters GABAergic system, opioidergic and ATP-sensitive K+ channels. The results have shown a positive association of nerolidol with the GABAergic system but not with opioidergic or ATP-sensitive K+ channels, implying that the anti-nociceptive activtity of nerolidol is mediated through GABAA receptors [103]. In order to evaluate the anti-inflammatory activity of nerolidol, carrageenan-induced paw edema was used as a model of inflammation. It was found that nerolidol exhibited inhibitory effect on inflammation. Further investigation of carrageenan-induced peritonitis model revealed that nerolidol decreased the levels of polymorphonuclear cells and tumor necrosis factor (TNF-α) in peritoneal lavage as well as interleukin 1 beta (IL-1b) in LPS-stimulated, peritoneal macrophages (Table 4) [103]. Taken these results together, nerolidol has been shown to demonstrate promising analgesic and anti-inflammatory activities.
6.10. Anti-Cancer and Anti-Tumor Activity
Cancer is one of the most alarming causes of death, with an estimated over six million deaths have been reported around the world annually. It is a multifactorial disease that leads to uncontrolled growth and invasion of abnormal cells, ultimately leading to the formation of tumor. Chemotherapy, radiosurgery and surgery are some of the effective treatments against various type of tumors. Despite that, these treatments still pose many side-effects that lead to acute and chronic organ damage such as bone marrow suppression, hepatic, pulmonary, cardiac, renal and gastrointestinal toxicities [144,145]. Moreover, the development of drug resistance in tumors have also been reported during the courses of chemotherapy. This may be due to the occurrence of mutations in the tumor cells that negates the apoptotic pathway during cancer treatment [146]. These drawbacks of chemotherapy treatment have urged researchers to find alternative treatments without harming the growth of normal cells and triggering anti-tumor drug resistance. Among the alternative approaches, plant phytochemicals have been recently explored for their possible beneficial (anti-proliferative and cytotoxic) effects on cancer cells in vitro or in vivo models [147,148].
6.10.1. In Vitro Studies
Several studies have shown anti-tumor properties of nerolidol on cancer cell lines. A study conducted by Ryabchenko et al. has demonstrated strong anti-tumor effects of nerolidol (a combination of cis-nerolidol 40.7%, trans-nerolidol 58.3%, cis-dihydronerolidol 0.4% and trans-dihydronerolidol). It was found to reduce the viability of HeLa cells at its concentration (CC50) of less than 5 µM (1.5 ± 0.7 µM) [104]. Beside this study, nerolidol (isomer not specified), which is one of the ten major compounds found in the green tea flavor, exhibited strong cytotoxicity effect with an IC50 value of 2.96 and 3.02 µg/mL against BT-20 breast carcinoma cells and HeLa cells, indicating that nerolidol potentially exhibit strong anti-cancer activity against the two tumor cell lines [106]. This has been further supported by the fact that trans-nerolidol, which was purified from the leaf EO of Zornia brasiliensis Vogel, had a strong cytotoxicity activity against cancer cell lines such as B16-F10 (mouse melanoma), HepG2 (human hepatocellular carcinoma), HL-60 (human promyelocytic leukemia) and K562 (human chronic myelocytic leukemia) with IC50 values of >25, >25, 21.99 and 17.58 µg/mL, respectively using Alamar blue assay [39]. The study also reported no cytotoxicity effect of trans-nerolidol on non-tumor cells, particularly the peripheral blood mononuclear cells (PBMCs) [39]. Another study conducted by Boris et al. have shown that cis-nerolidol exhibited the strongest cytotoxic activity (16.5 ± 6.7 μM) against HeLa cells among other sesquiterpene alcohols such as α-bisabolol, cedrol, patchoulol, and santalol [105]. Beside green tea, trans-nerolidol extracted from leaf EO of Comptonia peregrina (L.) Coult. has been found to induce strong cytotoxic effect against human lung carcinoma A-549 and colon adenocarcinoma DLD-1 cell lines with IC50 values of 6.4 ± 0.4 and 5.8 ± 0.4 µg/mL, respectively [149]. Another anti-proliferative study conducted by Ambrož et al. has shown that trans-nerolidol potentiated the action of doxorubicin, an anticancer drug, by increasing killing of CaCo-2 cancer cells [40]. The study also reported no cytotoxicity effect of trans-nerolidol on rat hepatocytes that serve as non-tumor cells [145]. Based on the literature, it can be suggested nerolidol is a good candidate for the development of anticancer agent that selectively targets specific cancerous cells with no cytotoxicity towards PBMCs and rat hepatocytes.
When examining the cytotoxic effect of nerolidol with regard to the apoptotic pathway, the combination of two acylic isoprenoids, farnesol and nerolidol was found to suppress the proliferation of human HL-60 acute promyelocytic leukemia (HL-60) cells by 20%, which was slightly synergistic (slightly exceeding the 13% suppression obtained from the sum of both compounds); farnesol isomers (2.5 µmol/L) and nerolidol (5 µmol/L) individually suppressed the proliferation of HL-60 cells by 4% and 9%, respectively [108]. The mechanism of action involved prolonging the cell cycle arrest of HL-60 cells at the G0-G1/S interphase and lead to apoptotic cell death. Another study conducted by Hanušová et al. focused on the anti-proliferative effect of EO from leaves of Myrica rubra (Lour.) Siebold & Zucc. (MEO) and its major compound, trans-nerolidol on the adhesion, expression of adhesion molecules (ICAM-1; E-cadherin; β-catenin and apoptotic molecules (NF-κB, caspases) in colorectal cancer cell line HT29 [147]. The study showed that only the MEO reduced the cell adhesion to collagen. Meanwhile, both MEO and trans-nerolidol (30 µg/mL) significantly suppressed cell adhesion of HT29 cells in the presence of TNFα and it was suggested due to the down-regulation of ICAM-1 [109]. Besides, trans-nerolidol (30 µg/mL) also significantly increased the expression of E-cadherin [109], a cell adhesion molecule that mediates the suppression of epithelial cell tumor invasiveness [150]. MEO and trans-nerolidol were also found to decrease the phosphorylation of NF-κB and activate caspases activity in TNFα-induced HT29, thereby leading to apoptosis of cancer cells [109].
6.10.2. In Vivo Studies
In animal studies, nerolidol has been found to possess strong anti-tumor activity by inhibiting the intestinal carcinogenesis induced by azoxymethane (15 mg/kg body weight) administered twice per week for a duration of three weeks in male F344 rats [107]. The result showed the reduction of incidence of intestinal neoplasia from 82% to 33% in rats fed with nerolidol. Moreover, the number of tumors/rat was reduced from 1.5 to 0.7 in rats fed with nerolidol. The improvement of intestinal carcinogenesis was possibly due to the modulatory effect nerolidol on protein prenylation, a post-translational process that is required to cause cancer [151]. Another study conducted by Costa et al. has demonstrated that the EO of Zornia brasiliensis Vogel leaf at dose of 100 mg/kg containing trans-nerolidol as major constituent reduced the weight of tumor in mice injected with B16-F10 melanoma by 38.61% when compared to the untreated group [39].
7. Pharmacokinetic Studies
Although there is an increasing popularity of herbal medicines and essential oils, they are not properly screened for purity and potency which may raise serious questions regarding their possible herb-drug interaction with conventional medicine that may cause serious adverse effects on human health [152]. In order to address this issue, more pharmacokinetic and toxicological research have been conducted to examine the efficacy and safety of essential oils.
7.1. In Vitro Studies
In order to determine the recovery of nerolidol, Saccharomyces cerevisiae prototrophic haploid strain IWD72 was incubated in YEPD medium comprised of yeast extract, bacteriological peptone, glucose, adenine, and uracil. Nerolidol (100 μg/mL) was subsequently added to the 50 mL bacteria culture in YEPD medium. The aerobic culture cells were harvested by centrifugation after 24 h, and the cells were collected for the recovery of nerolidol. Residual nerolidol recovered at 24 h was 79.0 µg/mL [14,153].
7.2. In Vivo Studies
Rats were fed 20 and 40 mg of nerolidol that were mixed with 1 mL of cottonseed oil and 30–35 mL of evaporated milk per day, for eight days. The average daily excretion on the 1st to 4th and 4th to 8th day was monitored. Rats fed with 20 mg/day of nerolidol excreted 0.3 and 0.7 mg of nerolidol per day with a maximum average of 0.9 mg. On the other hand, the rats fed with 40 mg/day of nerolidol excreted 1.0 and 1.6 mg per day with a maximum average of 2.1 mg [14,154].
Based on a recent in vivo pharmacokinetic study conducted by Saito et al., nerolidol (cis-/trans-nerolidol, 1:3; w/w) was quantitatively determined in mouse plasma using GC-MS method [22]. Three BALB/c mice weighing 20 ± 2 g were firstly fed orally with a single oral dose of 1000 mg/kg of nerolidol. Blood samples were then taken at 30 min, 1, 2, 3, 4, 5, 6, 8, and 12 h after oral administration, followed by separation of plasma from blood via centrifugation and GC-MS analysis. The level of nerolidol was observed in the plasma with its maximum concentration of ∼0.27 ± 0.07 μg/mL within 30 min after oral administration and remained constant for up to 3 h after administration, reaching a maximum concentration of ~0.35 ± 0.05 μg/mL after 6 h of administration. The concentration of nerolidol in plasma after 6 hours was twice the IC50 concentration of in vitro nerolidol administration on P. falciparum (0.169 μg/mL) [155]. Moreover, this maximum concentration was detected to be ~1460 times lower than the concentration required to induce 50% hemolysis (∼511 μg/mL). The concentration of nerolidol in plasma decreased to near zero 12 h after oral administration. These results indicated that the maximum concentration in mouse plasma after oral administration was below the hemolytic concentration, thus the safe oral dose is up to 1000 mg/kg.
In another recent study, He et al. utilized LC-MS instead of GC-MS method to quantitatively determine the in vivo pharmacokinetics of nerolidol (cis-/trans-nerolidol, 2:3) in rat plasma [25]. Sprague-Dawley rats weighing 250–300 g were firstly administered only once with 25 mg/kg of nerolidol via intraperitoneal injection. Blood samples were then collected at 10, 20, 30, 60, 90, 120, 240 and 360 min after injection, followed by separation of plasma from blood via centrifugation. Through the LC-MS analysis, they revealed that the maximum concentration of nerolidol observed in rat plasma was 8.30 ± 1.07 μg/mL at 20 min after single intraperitoneal injection. The concentration of nerolidol in plasma decreased to near zero two hours after intraperitoneal injection.
A comparative pharmacokinetic study between work by Saito et al. [22] and He et al. [25] revealed that the oral administration of nerolidol exhibited lower peak plasma concentration than that of intraperitoneal administration (Table 5). This is because orally administered nerolidol must first undergo first-pass effect by which it must pass through the intestinal wall and then to the portal circulation and liver [157]. As a result, a portion of the oral dose of nerolidol was lost during the first pass metabolism in the liver, thus contributing to low bioavailability as compared to that of intraperitoneal injection [158]. Another key point of the pharmacokinetic comparison between the two studies is that the human equivalent doses (HEDs) of both administration calculated in Table 5 can be used as appropriate starting doses of nerolidol in human clinical trials.
Table 5.
| Parameters | Saito et al. [22] | He et al. [25] |
|---|---|---|
| Type of nerolidol | Mixture of cis- and trans-nerolidol (1:3) | Mixture of cis- and trans-nerolidol (2:3) |
| Analytical method used | GC-MS | LC-MS |
| Animal used | BALB/c mice | Sprague-Dawley rats |
| Route of administration | Oral | Intraperitoneal injection |
| Dosage (mg/kg) | 1000 | 25 |
| Type of sample used | Plasma | |
| Time collection taken (min) | 30, 60, 120, 180, 240, 300, 360, 480 and 720 | 10, 20, 30, 60, 90, 120, 240 and 360 |
| Peak plasma concentration (Cmax) (µg/mL) | ~0.27 ± 0.07 | 8.30 ± 1.07 |
| Peak time (Tmax) (min) | 30 | 20 |
| Elimination half life (T1/2) (min) | n.a. | 20.98 ± 7.71 |
| Mean residence time (MRT) (min) | n.a. | 27.72 ± 2.14 |
| Clearance (L/min/kg) | n.a. | 0.082 ± 0.012 |
| Time for drug to be eliminated to almost near zero | 12 | ~2 |
| Human equivalent dose a (HED) (mg/kg) | 81.08 | 4.05 |
Key: (i) n.a. = data not available; (ii) a = HED values were calculated based on the formula for dose translation based on body surface area (BSA) [156] as follows: Human equivalent dose (HED) (mg/kg) = Animal dose (mg/kg) × with adult human, rat and mouse Km factors of 37, 6 and 3 respectively [156].
7.3. Toxicological Studies
Despite the long history of therapeutic uses of EOs and its general acknowledgment by the public, the assumption that the HEDs can be used for the first clinical trial has however seldom been verified, thus raising the concern of safety issues pertaining the usage of EOs [159]. For that reason, toxicological screenings are required to assess the potential toxicities induced by the EOs.
7.3.1. Acute Toxicity
The dermal LD50 values of nerolidol (isomer not specified) in rabbit was found to be higher than 2000 mg/kg body weight, thus indicating low acute toxicity via transdermal route [13]. In terms of oral administration, the oral LD50 values of nerolidol in rats and mice were higher than 2000 mg/kg body weight (>5000 and 9976 mg/kg body weight, respectively), indicating low acute toxicity via oral route [13].
7.3.2. Skin Irritation and Sensitization Studies
In human studies, 4% nerolidol (isomer not specified) administration in a pre-test for a maximization study with single occlusive application for 48 h did not cause skin irritation [13]. On the other hand, the application of undiluted nerolidol (isomer not specified) on intact and abraded skin caused well-defined erythema or slight edema in rabbits after 48 h, whereas 5% nerolidol in diethylphthalate caused very slight edema in one animal and was cleared after 48 h [13]. With regard to skin sensitization, human studies have shown that nerolidol (isomer not specified) (4%) did not cause any positive reactions in maximization tests or repeated insult patch tests. In animal studies, the administration of nerolidol in guinea pigs resulted in weak reactions in two adjuvant tests with concentrations of 3% and 10% [160,161]. However, no cross-sensitization was observed when guinea pigs induced with farnesyl acetate were cross-sensitized with nerolidol.
7.3.3. Mucous Membrane Irritation
No human data is available for mucous membrane irritation studies [13]. However, in animal studies, undiluted nerolidol initially caused very slight redness, but was cleared by 2 h. On the other hand, 5% nerolidol in DEP poses no mucous membrane irritation [13].
7.3.4. Phototoxicity and Photoallergenicity
UV spectra for nerolidol indicated that it did not absorb UVB light (290–320 nm) [162]. However, nerolidol peaked at the UVC range (220–240 nm) with a very slight absorption at 250–300 nm range, thus do not possibly induce phototoxicity or photoallergy under the current conditions of use as fragrance ingredients [13].
7.3.5. Reproductive and Developmental Toxicity
In order to investigate the development of fetal epidermal permeability barrier in vitro, the activators of the receptors for vitamin D3 and retinoids, and of the peroxisome proliferator activated receptors (PPARs) and the farnesoid X-activated receptor (FXR) were examined. Sprague-Dawley rats were firstly impregnated (plug date = day 0) in order for the skin of 17-day old fetus to be cultured for the measurement of barrier function. The effect of activators of FXR, isoprenoid precursors and metabolites on the development of epidermal barrier was monitored. Explants were incubated in the presence of 100 μM nerolidol for two days. Full-thickness flank skin was excised from fetal rats for skin analysis with light and electron microscopy. The results have shown that nerolidol did not activate the FXR, thus did not alter the epidermal barrier during skin development [14,163].
7.3.6. Cytotoxicity and Genotoxicity
7.3.6.1. In Vitro Studies
Due to nerolidol being used as a potent skin permeation enhancer with low toxicity, a number of studies were conducted on the toxicological properties of nerolidol. Mendanha et al. compared the hemolytic and toxic effects of nerolidol and various monoterpenes on fibroblast cells as well as their effect on erythrocyte membrane fluidity [155]. By using the 3T3 NRU assay to evaluate the cytotoxicity of nerolidol and various monoterpenes (α-terpineol, l-(−)-carvone, (+)-limonene, l-menthone, d,l-menthol, pulegone and 1,8-cineole) on fibroblast cells, nerolidol was found to be the most cytotoxic with its IC50 value of 0.06 ± 0.01 mM. Besides, nerolidol caused 50% hemolysis at 2.3 ± 0.8 mM and induced a significant increase in the fluidity of erythrocyte membrane at 2.5 × 109 molecules/cell, indicating that the nerolidol possessed the highest hemolytic effect on erythrocyte membrane fluidity when compared to terpenes. In addition, electron paramagnetic resonance (EPR) spectroscopy of the spin label 5-doxyl stearic acid (5-DSA) was used to investigate the effect of terpenes on membrane fluidity in erythrocyte and fibroblast cells. Nerolidol was found to be more potent than terpenes that caused an increase in the membrane fluidity. The results implied that nerolidol was able to increase membrane fluidity but also had increased ability to disrupt the membrane and had higher cytotoxic potential.
Ferreira et al. further examined the toxicity of nerolidol (a racemic mixture of cis- and trans-isomers) (1:1) on mitochondrial and cellular energetics in in vivo model using Wister rat liver mitochondria and in vitro model using HepG2 (human hepatocellular liver carcinoma) cells [12]. In the in vitro study, nerolidol exertes hepatic cell cytotoxicity due to a decrease in ATP/ADP levels by negatively interfering with hepatic mitochondrial bioenergetics in concentrations lower than 2.4 μM. Consequently, nerolidol induced cell arrest and cell death which is possibly due to the inhibition of F0F1-ATP synthase in a concentration-dependent manner. In the in vivo study, nerolidol (low doses up to 2.4 μM) induced a decrease in transmembrane electric potential in the mitochondrial membrane isolated from rat liver in a concentration-dependent manner [12]. By decreasing transmembrane electric potential, nerolidol could negatively affect the hepatic mitochondrial bioenergetics as it would cause mitochondrial dysfunction, thus leading to hepatic cell cytotoxicity and eventually cell death [164].
Marques et al. investigated the cytotoxicity effect of EO extracted from leaves of P. claussenianum and reported no toxicity effect in the fibroblasts nor macrophages cell lines in any concentration tested (ranging from 40 to 0.56 mg/mL) [9]. Similarly, the EO extracted from leaves of P. claussenianum did not induce toxicity on the L929 mouse fibroblast cells [78]. Peres et al. investigated the cytotoxicity effect of EO purified from Piper gaudichaudianum Kunth in which its major compounds were trans-nerolidol, α-humulene, (E)-caryophyllene and bicyclogermacrene [165]. Although dose-dependent cytotoxicity effect of the EO as well as single-strand DNA breakage were observed in the Chinese hamster lung fibroblast cells (V79 cells), however, no double-strand breaks occurred. Furthermore, the EO induced a significant increase in lipid peroxidation at higher dose. The results indicated that the EO possessed strong cytotoxicity, genotoxic and mutagenic effects which were attributed to the role of nerolidol. Due to this reason, Sperotto et al. further investigated the cytotoxic and mutagenic properties of the EO of P. gaudichaudianum as well as its major compound trans-nerolidol using Saccharomyces cerevisiae as a model organism [166]. P. gaudichaudianum EO was found to induce cytotoxic effects in the XV185-14c and N123 strains of S. cerevisiae but induced mutagenesis only at the lys1 locus at the highest concentration of 100 µg/mL. On the other hand, nerolidol (a racemic mixture of cis- and trans-isomers) (1:1) was found to be cytotoxic in XV185-14c at concentration of 25, 50 and 100 µg/mL and did not significantly cause any induction of mutagenicity at the three loci evaluated. Moreover, EO and nerolidol were discovered to generate ROS via DCF-DA probing assay in superoxide dismutase (Sod)-deficient strains. Based on these findings, Sperotto et al. [166] claimed that nerolidol shows a weak mutagenic effect but exerts strong cytotoxicity effect which could be attributed to the formation of reactive oxygen species (ROS) and the formation of single-strand breaks. Despite that, more in depth analysis involving molecular techniques could be conducted to elucidate the mechanisms that are responsible for the strong cytotoxic but weak mutagenic effects of nerolidol. Furthermore, toxicogenomics could be an option to be considered in further evaluating the safety of nerolidol. Briefly, toxicogenomic is based on the integration of genomics and toxicology with the aim of studying the toxicity of xenobiotics on the biological systems via global analysis of genome-wide mRNA expression (transcriptomics), protein (proteomics) and metabolite patterns (metabonomics) [167]. Due to its wide usage in the research of plant-based medicinal natural products, particularly in traditional Chinese medicine (TCM) [168], toxicogenomics could perhaps be an effective tool in evaluating the safety of nerolidol at the genomic level to ensure that it is safe for humans. Besides that, more in vitro experiments investigating the induction of micronuclei, chromosome aberration or telomere shortening effect of nerolidol may also be performed in order to confirm the genotoxicity status of nerolidol in human or mammalian cells.
7.3.6.2. In Vivo Studies
Pículo et al. had investigated the genotoxicity and clastogenicity (ability to cause DNA damage by inducing chromosomal aberrations) effects of trans-nerolidol in blood and liver cells of 12-week-old male Swiss albino mice (Mus musculus) using comet and micronucleus assays respectively [169]. The experiment was based on the cytotoxicity effect analysis by scoring 200 consecutive total polychromatic (PCE) and normochromatic (NCE) erythrocytes (PCE:NCE ratio) in bone marrow cells and no significant decrease in PCEs:NCEs ratios at the three doses tested (250, 500 and 2000 mg kg−1) was observed, indicating the absence of cytotoxic effects of trans-nerolidol at these doses. Nevertheless, weak genotoxic effects of trans-nerolidol in the blood and liver cells were observed and a slight increase in the DNA damage at higher doses. Based on the available studies, it can be deduced that the non-toxic dose of trans-nerolidol for animal is up to 2000 mg/kg. Moreover, no DNA damage was observed in the peripheral blood cells and liver of the animals. In order to determine the safety of nerolidol consumption in humans, more clinical trials should be conducted to assess and validate the toxicity and side effects of nerolidol in humans.
8. Conclusions
Nerolidol is one of the common components found in the essential oil of various medicinal plants. A majority of the studies reveal that nerolidol is the major constituent in many plants that accounts for their pharmacological and biological activities such as anti-microbial, anti-parasitic, anti-biofilm, anti-oxidant, anti-nociceptive, anti-inflammatory, anti-ulcer, skin penetration enhancer, insect repellent and anti-cancer properties. Based on pharmacokinetic and toxicological data available, the dosage of nerolidol is considered safe to be translated from animal to clinical studies in order to evaluate its efficacy. Taken all together, nerolidol has a great potential to be used as a new chemical or therapeutic drug in the field of agriculture and medicine, respectively and sufficient baseline information is available for guiding future works and commercial exploitation.
Acknowledgments
This work was supported by the Monash University Malaysia ECR Grant (5140077-000-00), Ministry of Science, Technology and Innovation Malaysia (MOSTI), eScience Funds (02-02-10-SF0215 & 06-02-10-SF0300), University of Malaya for High Impact Research Grant (UM-MOHE HIR Nature Microbiome Grant No. H-50001-A000027 and No.A000001-50001), External Industry Grants from Biotek Abadi Sdn Bhd (vote no. GBA-808138 and GBA-808813).
Author Contributions
All authors contributed equally.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Petrovska B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012;6:1–5. doi: 10.4103/0973-7847.95849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 3.Ali B., Al-Wabel N.A., Shams S., Ahamad A., Khan S.A., Anwar F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015;5:601–611. doi: 10.1016/j.apjtb.2015.05.007. [DOI] [Google Scholar]
- 4.Perricone M., Arace E., Corbo M.R., Sinigaglia M., Bevilacqua A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isman M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- 6.Edris A.E. Pharmaceutical and therapeutic Potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007;21:308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 7.Braca A., Siciliano T., D’Arrigo M., Germanò M.P. Chemical composition and antimicrobial activity of Momordica charantia seed essential oil. Fitoterapia. 2008;79:123–125. doi: 10.1016/j.fitote.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Parreira N.A., Magalhaes L.G., Morais D.R., Caixeta S.C., de Sousa J.P., Bastos J.K., Cunha W.R., Silva M.L., Nanayakkara N.P., Rodrigues V., et al. Antiprotozoal, schistosomicidal, and antimicrobial activities of the essential oil from the leaves of Baccharis dracunculifolia. Chem. Biodivers. 2010;7:993–1001. doi: 10.1002/cbdv.200900292. [DOI] [PubMed] [Google Scholar]
- 9.Marques A.M., Barreto A.L.S., Curvelo J.A.d.R., Romanos M.T.V., Soares R.M.d.A., Kaplan M.A.C. Antileishmanial activity of nerolidol-rich essential oil from Piper claussenianum. Rev. Bras. Farmacogn. 2011;21:908–914. doi: 10.1590/S0102-695X2011005000157. [DOI] [Google Scholar]
- 10.Lima D.K.S., Ballico L.J., Rocha Lapa F., Gonçalves H.P., de Souza L.M., Iacomini M., Werner M.F.d.P., Baggio C.H., Pereira I.T., da Silva L.M., et al. Evaluation of the antinociceptive, anti-inflammatory and gastric antiulcer activities of the essential oil from Piper aleyreanum C.DC in rodents. J. Ethnopharmacol. 2012;142:274–282. doi: 10.1016/j.jep.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Tan L.T., Lee L.H., Yin W.F., Chan C.K., Abdul Kadir H., Chan K.G., Goh B.H. Traditional uses, phytochemistry, and bioactivities of Cananga odorata (ylang-ylang) Evid. Based Complement. Altern. Med. 2015 doi: 10.1155/2015/896314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira F.M., Palmeira C.M., Oliveira M.M., Santos D., Simões A.M., Rocha S.M., Coimbra M.A., Peixoto F. Nerolidol effects on mitochondrial and cellular energetics. Toxicol. In Vitro. 2012;26:189–196. doi: 10.1016/j.tiv.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Lapczynski A., Bhatia S.P., Letizia C.S., Api A.M. Fragrance material review on nerolidol (isomer unspecified) Food Chem. Toxicol. 2008;46:S247–S250. doi: 10.1016/j.fct.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 14.McGinty D., Letizia C.S., Api A.M. Addendum to fragrance material review on nerolidol (isomer unspecified) Food Chem. Toxicol. 2010;48(Suppl. 3):S43–S45. doi: 10.1016/j.fct.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Schubert V., Dietrich A., Ulrich T., Mosandl A. The stereoisomers of nerolidol: Separation, analysis and olfactoric properties. Z. Naturforsch. C. 1992;47:304–307. [Google Scholar]
- 16.Park M.J., Gwak K.S., Yang I., Kim K.W., Jeung E.B., Chang J.W., Choi I.G. Effect of citral, eugenol, nerolidol and α-terpineol on the ultrastructural changes of Trichophyton mentagrophytes. Fitoterapia. 2009;80:290–296. doi: 10.1016/j.fitote.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Batish D.R., Singh H.P., Kohli R.K., Kaur S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008;256:2166–2174. doi: 10.1016/j.foreco.2008.08.008. [DOI] [Google Scholar]
- 18.Grulova D., de Martino L., Mancini E., Salamon I., de Feo V. Seasonal variability of the main components in essential oil of Mentha × piperita L. J. Sci. Food Agric. 2015;95:621–627. doi: 10.1002/jsfa.6802. [DOI] [PubMed] [Google Scholar]
- 19.Marques A.M., Kaplan M.A.C. Seasonal evaluation and chemical composition of volatile fractions from Piper claussenianum by hydrodistillation and SPME. J. Essent. Oil Res. 2011;23:15–19. doi: 10.1080/10412905.2011.9700475. [DOI] [Google Scholar]
- 20.De Sousa J.P.B., Jorge R.F., Leite M.F., Furtado N.A.J.C., Bastos J.K., da Silva Filho A.A., Queiroga C.L., de Magalhães P.M., Soares A.E.E. Seasonal variation of the (E)-nerolidol and other volatile compounds within ten different cultivated populations of Baccharis dracunculifolia D.C. (Asteraceae) J. Essent. Oil Res. 2009;21:308–314. doi: 10.1080/10412905.2009.9700179. [DOI] [Google Scholar]
- 21.Ma C., Qu Y., Zhang Y., Qiu B., Wang Y., Chen X. Determination of nerolidol in teas using headspace solid phase microextraction-gas chromatography. Food Chem. 2014;152:285–290. doi: 10.1016/j.foodchem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Saito A.Y., Sussmann R.A.C., Kimura E.A., Cassera M.B., Katzin A.M. Quantification of nerolidol in mouse plasma using gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2015;111:100–103. doi: 10.1016/j.jpba.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez S., Kirby J., Denby C.M., Keasling J.D. Production and quantification of sesquiterpenes in Saccharomyces cerevisiae, including extraction, detection and quantification of terpene products and key related metabolites. Nat. Protoc. 2014;9:1980–1996. doi: 10.1038/nprot.2014.132. [DOI] [PubMed] [Google Scholar]
- 24.Pitt J.J. Principles and applications of liquid chromatography-mass spectrometry in clinical biochemistry. Clin. Biochem. Rev. 2009;30:19–34. [PMC free article] [PubMed] [Google Scholar]
- 25.He Y.-S., Sun W., Zhang B.-Y., Xu L.-H., Yang J., Gao W., Qi L.-W., Li P., Wen X.-D. Application of a sensitive liquid chromatography-mass spectrometry method to a pharmacokinetic study of nerolidol in rat plasma. Anal. Methods. 2016;8:785–789. doi: 10.1039/C5AY02575C. [DOI] [Google Scholar]
- 26.Znini M., Cristofari G., Majidi L., El Harrak A., Paolini J., Costa J. In vitro antifungal activity and chemical composition of Warionia saharae essential oil against 3 apple phytopathogenic fungi. Food Sci. Biotechnol. 2013;22:113–119. doi: 10.1007/s10068-013-0056-2. [DOI] [Google Scholar]
- 27.Nibret E., Wink M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine. 2010;17:911–920. doi: 10.1016/j.phymed.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Skaltsa H.D., Lazari D.M., Mavromati A.S., Tiligada E.A., Constantinidis T.A. Composition and antimicrobial activity of the essential oil of Scutellaria albida ssp. albida from Greece. Planta Med. 2000;66:672–674. doi: 10.1055/s-2000-8650. [DOI] [PubMed] [Google Scholar]
- 29.Lopes N.P., Kato M.J., Eloısa H.d.A., Maia J.G., Yoshida M., Planchart A.R., Katzin A.M. Antimalarial use of volatile oil from leaves of Virola surinamensis (Rol.) Warb. by Waiapi Amazon Indians. J. Ethnopharmacol. 1999;67:313–319. doi: 10.1016/S0378-8741(99)00072-0. [DOI] [PubMed] [Google Scholar]
- 30.Ndung’u M., Gitu L. Repellent activity of the essential oil from Capparis tomentosa against maize weevil Sitophilus zeamais. J. Resour. Dev. Manag. 2013;1:9–13. [Google Scholar]
- 31.Tao R., Wang C.-Z., Kong Z.-W. Antibacterial/antifungal activity and synergistic interactions between polyprenols and other lipids isolated from Ginkgo biloba L. Leaves. Molecules. 2013;18:2166–2182. doi: 10.3390/molecules18022166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curvelo J.A.R., Marques A.M., Barreto A.L.S., Romanos M.T.V., Portela M.B., Kaplan M.A.C., Soares R.M.A. A novel nerolidol-rich essential oil from Piper claussenianum modulates Candida albicans biofilm. J. Med. Microbiol. 2014;63:697–702. doi: 10.1099/jmm.0.063834-0. [DOI] [PubMed] [Google Scholar]
- 33.Passos J.L., Barbosa L.C.A., Demuner A.J., Alvarenga E.S., Silva C.M.d., Barreto R.W. Chemical characterization of volatile compounds of Lantana camara L. and L. radula Sw. and their antifungal activity. Molecules. 2012;17:11447–11455. doi: 10.3390/molecules171011447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman A., Al-Reza S., Kang S. Antifungal activity of essential oil and extracts of Piper chaba Hunter against phytopathogenic fungi. J. Am. Oil Chem. Soc. 2011;88:573–579. doi: 10.1007/s11746-010-1698-3. [DOI] [Google Scholar]
- 35.Hoet S., Stevigny C., Hérent M.-F., Quetin-Leclercq J. Antitrypanosomal compounds from the leaf essential oil of Strychnos spinosa. Planta Med. 2006;72 doi: 10.1055/s-2005-916255. [DOI] [PubMed] [Google Scholar]
- 36.Park H.-M., Kim J., Chang K.-S., Kim B.-S., Yang Y.-J., Kim G.-H., Shin S.-C., Park I.-K. Larvicidal activity of myrtaceae essential oils and their components against Aedes aegypti, acute toxicity on Daphnia magna, and aqueous residue. J. Med. Entomol. 2011;48:405–410. doi: 10.1603/ME10108. [DOI] [PubMed] [Google Scholar]
- 37.Araújo M.C., Câmara C.G., Born F., Moraes M., Badji C. Acaricidal activity and repellency of essential oil from Piper aduncum and its components against Tetranychus urticae. Exp. Appl. Acarol. 2012;57:139–155. doi: 10.1007/s10493-012-9545-x. [DOI] [PubMed] [Google Scholar]
- 38.Pinheiro B.G., Silva A.S.B., Souza G.E.P., Figueiredo J.G., Cunha F.Q., Lahlou S., da Silva J.K.R., Maia J.G.S., Sousa P.J.C. Chemical composition, antinociceptive and anti-inflammatory effects in rodents of the essential oil of Peperomia serpens (SW.) Loud. J. Ethnopharmacol. 2011;138:479–486. doi: 10.1016/j.jep.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 39.Costa E.V., Menezes L.R.A., Rocha S.L.A., Baliza I.R.S., Dias R.B., Rocha C.A.G., Soares M.B.P., Bezerra D.P. Antitumor properties of the leaf essential oil of Zornia brasiliensis. Planta Med. 2015;81:563–567. doi: 10.1055/s-0035-1545842. [DOI] [PubMed] [Google Scholar]
- 40.Ambrož M., Boušová I., Skarka A., Hanušová V., Králová V., Matoušková P., Szotáková B., Skálová L. The influence of sesquiterpenes from Myrica rubra on the antiproliferative and pro-oxidative effects of doxorubicin and its accumulation in cancer cells. Molecules. 2015;20:15343–15358. doi: 10.3390/molecules200815343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simionatto E., Porto C., Dalcol I.I., da Silva U.F., Morel A.F. Essential oil from Zanthoxylum hyemale. Planta Med. 2005;71:759–763. doi: 10.1055/s-2005-864184. [DOI] [PubMed] [Google Scholar]
- 42.Tzakou O., Loukis A., Said A. Essential oil from the flowers and leaves of Cassia fistula L. J. Essent. Oil Res. 2007;19:360–361. doi: 10.1080/10412905.2007.9699305. [DOI] [Google Scholar]
- 43.Stashenko E., Martínez J.R., Medina J.D., Durán D.C. Analysis of essential oils isolated by steam distillation from Swinglea glutinosa fruits and leaves. J. Essent. Oil Res. 2015;27:276–282. doi: 10.1080/10412905.2015.1045087. [DOI] [Google Scholar]
- 44.Garneau F.-X., Collin G., Gagnon H., Jean F.-I., Strobl H., Pichette A. The essential oil composition of devil’s club, Oplopanax horridus J.E. Smith Miq. Flavour. Fragr. J. 2006;21:792–794. doi: 10.1002/ffj.1716. [DOI] [Google Scholar]
- 45.Lee K., Lee J.-H., Kim S.-I., Cho M., Lee J. Anti-biofilm, anti-hemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014;98:9447–9457. doi: 10.1007/s00253-014-5903-4. [DOI] [PubMed] [Google Scholar]
- 46.Judzentiene A., Mockutë D. Chemical composition of essential oils produced by pink flower inflorescences of wild Achillea millefolium L. Chemija. 2004;15:28–32. [Google Scholar]
- 47.Wang Z.Q., Perumalsamy H., Wang M., Shu S., Ahn Y.J. Larvicidal activity of Magnolia denudata seed hydrodistillate constituents and related compounds and liquid formulations towards two susceptible and two wild mosquito species. Pest Manag. Sci. 2015 doi: 10.1002/ps.4064. [DOI] [PubMed] [Google Scholar]
- 48.Kapoor I., Singh B., Singh G., Isidorov V., Szczepaniak L. Chemistry, antifungal and antioxidant activities of cardamom (Amomum subulatum) essential oil and oleoresins. Int. J. Essent. Oil Ther. 2008;2:29–40. [Google Scholar]
- 49.Koudou J., Abena A.A., Ngaissona P., Bessière J.M. Chemical composition and pharmacological activity of essential oil of Canarium schweinfurthii. Fitoterapia. 2005;76:700–703. doi: 10.1016/j.fitote.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Tung Y.-T., Chua M.-T., Wang S.-Y., Chang S.-T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008;99:3908–3913. doi: 10.1016/j.biortech.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 51.Gretchen E.P., Junwei Z., Lyric B., Joel R.C. Pesticides in Household, Structural and Residential Pest Management. Volume 1015. American Chemical Society; Washington, DC, USA: 2009. Amyris and siam-wood essential oils: Insect activity of sesquiterpenes; pp. 5–18. [Google Scholar]
- 52.Maurer B., Hauser A., Ohloff G. New sesquiterpenoids from cabreuva oil. Helv. Chim. Acta. 1986;69:2026–2037. doi: 10.1002/hlca.19860690826. [DOI] [Google Scholar]
- 53.Lucero M., Estell R., Tellez M., Fredrickson E. A retention index calculator simplifies identification of plant volatile organic compounds. Phytochem. Anal. 2009;20:378–384. doi: 10.1002/pca.1137. [DOI] [PubMed] [Google Scholar]
- 54.Babushok V.I., Zenkevich I.G. Retention indices for most frequently reported essential oil compounds in GC. Chromatographia. 2008;69:257–269. doi: 10.1365/s10337-008-0872-3. [DOI] [Google Scholar]
- 55.Orav A. Current Practice of Gas Chromatography-Mass Spectrometry. Marcel Dekker, Inc.; New York, NY, USA: 2001. Identification of terpenes by gas chromatography-mass spectrometry; pp. 483–494. [Google Scholar]
- 56.Chung T.Y., Eiserich J.P., Shibamoto T. Volatile compounds isolated from edible Korean chamchwi (Aster scaber Thunb) J. Agric. Food Chem. 1993;41:1693–1697. doi: 10.1021/jf00034a033. [DOI] [Google Scholar]
- 57.Choi H.-S. Character impact odorants of citrus hallabong [(C. unshiu Marcov × C. sinensis Osbeck) × C. reticulata Blanco] cold-pressed peel oil. J. Agric. Food Chem. 2003;51:2687–2692. doi: 10.1021/jf021069o. [DOI] [PubMed] [Google Scholar]
- 58.Behera S., Nagarajan S., Jagan Mohan Rao L. Microwave heating and conventional roasting of cumin seeds (Cuminum cyminum L.) and effect on chemical composition of volatiles. Food Chem. 2004;87:25–29. doi: 10.1016/j.foodchem.2003.10.012. [DOI] [Google Scholar]
- 59.Nigmatov A.G., Serebryakov é.P., Yanovskaya L.A. Improved method for the isolation of geranyl esters of (4E/Z,8E)- and (4E/Z,8Z)-farnesylacetic acid. Pharm. Chem. J. 1987;21:529–533. doi: 10.1007/BF00758769. [DOI] [Google Scholar]
- 60.Ofner A., Kimel W., Holmgren A., Forrester F. Synthetisches nerolidol und verwandte c15-alkohole. Helv. Chim. Acta. 1959;42:2577–2584. doi: 10.1002/hlca.19590420729. [DOI] [Google Scholar]
- 61.McNeil C.V., Morlacchi P., Baevich A., Matsuda S.P.T. Nerolidol, Terpene, and Terpene Deriviative Synthesis. 8173405. U.S. Patent and Trademark Office; Washington, DC, USA: U.S. Patent. 2012 May 8;
- 62.Iason G. The role of plant secondary metabolites in mammalian herbivory: Ecological perspectives. Proc. Nutr. Soc. 2005;64:123–131. doi: 10.1079/PNS2004415. [DOI] [PubMed] [Google Scholar]
- 63.Pichersky E., Gershenzon J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant Biol. 2002;5:237–243. doi: 10.1016/S1369-5266(02)00251-0. [DOI] [PubMed] [Google Scholar]
- 64.Turlings T.C., Ton J. Exploiting scents of distress: The prospect of manipulating herbivore-induced plant odours to enhance the control of agricultural pests. Curr. Opin. Plant Biol. 2006;9:421–427. doi: 10.1016/j.pbi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 65.Dudareva N., Pichersky E., Gershenzon J. Biochemistry of plant volatiles. Plant Physiol. 2004;135:1893–1902. doi: 10.1104/pp.104.049981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dudareva N., Negre F., Nagegowda D.A., Orlova I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006;25:417–440. doi: 10.1080/07352680600899973. [DOI] [Google Scholar]
- 67.Cheng A.-X., Lou Y.-G., Mao Y.-B., Lu S., Wang L.-J., Chen X.-Y. Plant terpenoids: Biosynthesis and ecological functions. J. Integr. Plant Biol. 2007;49:179–186. doi: 10.1111/j.1744-7909.2007.00395.x. [DOI] [Google Scholar]
- 68.Degenhardt J., Köllner T.G., Gershenzon J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry. 2009;70:1621–1637. doi: 10.1016/j.phytochem.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 69.Nagegowda D.A., Gutensohn M., Wilkerson C.G., Dudareva N. Two nearly identical terpene synthases catalyze the formation of nerolidol and linalool in snapdragon flowers. Plant J. 2008;55:224–239. doi: 10.1111/j.1365-313X.2008.03496.x. [DOI] [PubMed] [Google Scholar]
- 70.Degenhardt J., Gershenzon J. Demonstration and characterization of (E)-nerolidol synthase from maize: A herbivore-inducible terpene synthase participating in (3E)-4,8-dimethyl-1,3,7-nonatriene biosynthesis. Planta. 2000;210:815–822. doi: 10.1007/s004250050684. [DOI] [PubMed] [Google Scholar]
- 71.Schnee C., Köllner T.G., Gershenzon J., Degenhardt J. The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-β-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage. Plant Physiol. 2002;130:2049–2060. doi: 10.1104/pp.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bouwmeester H.J., Verstappen F.W., Posthumus M.A., Dicke M. Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean. The first dedicated step in acyclic c11-homoterpene biosynthesis. Plant Physiol. 1999;121:173–180. doi: 10.1104/pp.121.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vinholes J., Gonçalves P., Martel F., Coimbra M.A., Rocha S.M. Assessment of the antioxidant and antiproliferative effects of sesquiterpenic compounds in in vitro Caco-2 cell models. Food Chem. 2014;156:204–211. doi: 10.1016/j.foodchem.2014.01.106. [DOI] [PubMed] [Google Scholar]
- 74.Vinholes J., Rudnitskaya A., Gonçalves P., Martel F., Coimbra M.A., Rocha S.M. Hepatoprotection of sesquiterpenoids: A quantitative structure-activity relationship (QSAR) approach. Food Chem. 2014;146:78–84. doi: 10.1016/j.foodchem.2013.09.039. [DOI] [PubMed] [Google Scholar]
- 75.Nogueira Neto J., de Almeida A., da Silva Oliveira J., dos Santos P., de Sousa D., de Freitas R. Antioxidant effects of nerolidol in mice hippocampus after open field test. Neurochem. Res. 2013;38:1861–1870. doi: 10.1007/s11064-013-1092-2. [DOI] [PubMed] [Google Scholar]
- 76.Hada T., Shiraishi A., Furuse S., Inoue Y., Hamashima H., Matsumoto Y., Masuda K., Shiojima K., Shimada J. Inhibitory effects of terpenes on the growth of Staphylococcus aureus. Nat. Med. 2003;57:64–67. [Google Scholar]
- 77.Inoue Y., Shiraishi A., Hada T., Hirose K., Hamashima H., Shimada J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol. Lett. 2004;237:325–331. doi: 10.1111/j.1574-6968.2004.tb09714.x. [DOI] [PubMed] [Google Scholar]
- 78.Togashi N., Hamashima H., Shiraishi A., Inoue Y., Takano A. Antibacterial activities against Staphylococcus aureus of terpene alcohols with aliphatic carbon chains. J. Essent. Oil Res. 2010;22:263–269. doi: 10.1080/10412905.2010.9700321. [DOI] [Google Scholar]
- 79.Kubo I. Bioactive Volatile Compounds from Plants. Volume 525. American Chemical Society; Washington, DC, USA: 1993. Antimicrobial activity of green tea flavor components; pp. 57–70. [Google Scholar]
- 80.Gonçalves O., Pereira R., Gonçalves F., Mendo S., Coimbra M.A., Rocha S.M. Evaluation of the mutagenicity of sesquiterpenic compounds and their influence on the susceptibility towards antibiotics of two clinically relevant bacterial strains. Mutat. Res. Genet. Toxicol. Environ. 2011;723:18–25. doi: 10.1016/j.mrgentox.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 81.Brehm-Stecher B.F., Johnson E.A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother. 2003;47:3357–3360. doi: 10.1128/AAC.47.10.3357-3360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simões M., Rocha S., Coimbra M.A., Vieira M.J. Enhancement of Escherichia coli and Staphylococcus aureus antibiotic susceptibility using sesquiterpenoids. Med. Chem. 2008;4:616–623. doi: 10.2174/157340608786242016. [DOI] [PubMed] [Google Scholar]
- 83.Lee S.-J., Han J.-I., Lee G.-S., Park M.-J., Choi I.-G., Na K.-J., Jeung E.-B. Antifungal effect of eugenol and nerolidol against Microsporum gypseum in a guinea pig model. Biol. Pharm. Bull. 2007;30:184–188. doi: 10.1248/bpb.30.184. [DOI] [PubMed] [Google Scholar]
- 84.Pontin M., Bottini R., Burba J.L., Piccoli P. Allium sativum produces terpenes with fungistatic properties in response to infection with Sclerotium cepivorum. Phytochemistry. 2015;115:152–160. doi: 10.1016/j.phytochem.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 85.Mohd-Shukri H., Zainal-Abidin B. The effects of nerolidol, allicin and berenil on the morphology of Trypanosoma evansi in mice: A comparative study using light and electron microscopic approaches. Malays. Appl. Biol. 2011;40:25–32. [Google Scholar]
- 86.Arruda D.C., D’Alexandri F.L., Katzin A.M., Uliana S.R.B. Antileishmanial activity of the terpene nerolidol. Antimicrob. Agents Chemother. 2005;49:1679–1687. doi: 10.1128/AAC.49.5.1679-1687.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Camargos H.S., Moreira R.A., Mendanha S.A., Fernandes K.S., Dorta M.L., Alonso A. Terpenes increase the lipid dynamics in the Leishmania plasma membrane at concentrations similar to their IC50 values. PLoS ONE. 2014;9:e104429. doi: 10.1371/journal.pone.0104429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silva M.P., Oliveira G.L., de Carvalho R.B., de Sousa D.P., Freitas R.M., Pinto P.L., de Moraes J. Antischistosomal activity of the terpene nerolidol. Molecules. 2014;19:3793–3803. doi: 10.3390/molecules19033793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marques A.M., Peixoto A.C.C., de Paula R.C., Nascimento M.F.A., Soares L.F., Velozo L.S., Guimarães E.F., Kaplan M.A.C. Phytochemical investigation of anti-plasmodial metabolites from Brazilian Native Piper species. J. Essent. Oil Bear. Plants. 2015;18:74–81. doi: 10.1080/0972060X.2014.974075. [DOI] [Google Scholar]
- 90.Rodrigues Goulart H., Kimura E.A., Peres V.J., Couto A.S., Aquino Duarte F.A., Katzin A.M. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob. Agents Chemother. 2004;48:2502–2509. doi: 10.1128/AAC.48.7.2502-2509.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Macedo C.S., Uhrig M.L., Kimura E.A., Katzin A.M. Characterization of the isoprenoid chain of coenzyme Q in Plasmodium falciparum. FEMS Microbiol. Lett. 2002;207:13–20. doi: 10.1111/j.1574-6968.2002.tb11021.x. [DOI] [PubMed] [Google Scholar]
- 92.Da Silva M.F., Saito A.Y., Peres V.J., Oliveira A.C., Katzin A.M. In vitro antimalarial activity of different inhibitors of the plasmodial isoprenoid synthesis pathway. Antimicrob. Agents Chemother. 2015;59:5084–5087. doi: 10.1128/AAC.04161-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.AbouLaila M., Sivakumar T., Yokoyama N., Igarashi I. Inhibitory effect of terpene nerolidol on the growth of Babesia parasites. Parasitol. Int. 2010;59:278–282. doi: 10.1016/j.parint.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 94.Abdel-Rahman F.H., Alaniz N.M., Saleh M.A. Nematicidal activity of terpenoids. J. Environ. Sci. Health B. 2013;48:16–22. doi: 10.1080/03601234.2012.716686. [DOI] [PubMed] [Google Scholar]
- 95.Navarro-Moll M.C., Romero M.C., Montilla M.P., Valero A. In vitro and in vivo activity of three sesquiterpenes against L3 larvae of Anisakis type I. Exp. Parasitol. 2011;127:405–408. doi: 10.1016/j.exppara.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 96.Di Campli E., di Bartolomeo S., Delli Pizzi P., Di Giulio M., Grande R., Nostro A., Cellini L. Activity of tea tree oil and nerolidol alone or in combination against Pediculus capitis (head lice) and its eggs. Parasitol. Res. 2012;111:1985–1992. doi: 10.1007/s00436-012-3045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Assis Lage T.C., Montanari R.M., Fernandes S.A., de Oliveira Monteiro C.M., de Oliveira Souza Senra T., Zeringota V., da Silva Matos R., Daemon E. Chemical composition and acaricidal activity of the essential oil of Baccharis dracunculifolia De Candole (1836) and its constituents nerolidol and limonene on larvae and engorged females of Rhipicephalus microplus (Acari: Ixodidae) Exp. Parasitol. 2015;148:24–29. doi: 10.1016/j.exppara.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 98.Klopell F.C., Lemos M., Sousa J.P.B., Comunello E., Maistro E.L., Bastos J.K., Andrade S.F.d. Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae) Z. Naturforsch. C. 2007;62:537–542. doi: 10.1515/znc-2007-7-812. [DOI] [PubMed] [Google Scholar]
- 99.Cornwell P.A., Barry B.W. Sesquiterpene components of volatile oils as skin penetration enhancers for the hydrophilic permeant 5-fluorouracil. J. Pharm. Pharmacol. 1994;46:261–269. doi: 10.1111/j.2042-7158.1994.tb03791.x. [DOI] [PubMed] [Google Scholar]
- 100.Prasanthi D., Lakshmi P.K. Terpenes: Effect of lipophilicity in enhancing transdermal delivery of alfuzosin hydrochloride. J. Adv. Pharm. Technol. Res. 2012;3:216–223. doi: 10.4103/2231-4040.104712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krishnaiah Y.S., Al-Saidan S.M., Jayaram B. Effect of nerodilol, carvone and anethole on the in vitro transdermal delivery of selegiline hydrochloride. Pharmazie. 2006;61:46–53. [PubMed] [Google Scholar]
- 102.El-Kattan A.F., Asbill C.S., Kim N., Michniak B.B. The effects of terpene enhancers on the percutaneous permeation of drugs with different lipophilicities. Int. J. Pharm. 2001;215:229–240. doi: 10.1016/S0378-5173(00)00699-2. [DOI] [PubMed] [Google Scholar]
- 103.Fonsêca D.V., Salgado P.R.R., de Carvalho F.L., Salvadori M.G.S.S., Penha A.R.S., Leite F.C., Borges C.J.S., Piuvezam M.R., Pordeus L.C.d.M., Sousa D.P., et al. Nerolidol exhibits antinociceptive and anti-inflammatory activity: Involvement of the GABAergic system and proinflammatory cytokines. Fundam. Clin. Pharmacol. 2015;30:14–22. doi: 10.1111/fcp.12166. [DOI] [PubMed] [Google Scholar]
- 104.Ryabchenko B., Tulupova E., Schmidt E., Wlcek K., Buchbauer G., Jirovetz L. Investigation of anticancer and antiviral properties of selected aroma samples. Nat. Prod. Commun. 2008;3:1085–1088. [Google Scholar]
- 105.Boris R., Elena T., Erich S., Walter J., Gerhard B., Leopold J. Cytotoxic properties of selected sesquiterpene alcohols on human cervix carcinoma cell lines. J. Essent. Oil Bear. Plants. 2011;14:316–319. doi: 10.1080/0972060X.2011.10643940. [DOI] [Google Scholar]
- 106.Kubo I., Morimitsu Y. Cytotoxicity of green tea flavor compounds against two solid tumor cells. J. Agric. Food Chem. 1995;43:1626–1628. doi: 10.1021/jf00054a039. [DOI] [Google Scholar]
- 107.Wattenberg L.W. Inhibition of azoxymethane-induced neoplasia of the large bowel by 3-hydroxy-3,7,11-trimethyl-l,6,10-dodecatriene (nerolidol) Carcinogenesis. 1991;12:151–152. doi: 10.1093/carcin/12.1.151. [DOI] [PubMed] [Google Scholar]
- 108.Tatman D., Mo H. Volatile isoprenoid constituents of fruits, vegetables and herbs cumulatively suppress the proliferation of murine B16 melanoma and human HL-60 leukemia cells. Cancer Lett. 2002;175:129–139. doi: 10.1016/S0304-3835(01)00723-6. [DOI] [PubMed] [Google Scholar]
- 109.Hanušová V., Skálová L., Ambrož M., Králová V., Langhansová L., Matoušková P. The effect of Myrica rubra essential oil and its components α-humulene and trans-nerolidol on adhesion and apoptosis of colorectal cancer cells. Cancer Cell Microenviron. 2015;2 doi: 10.14800/ccm.1058. [DOI] [Google Scholar]
- 110.Lushchak V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014;224:164–175. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 111.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging. 2007;2:219–236. [PMC free article] [PubMed] [Google Scholar]
- 112.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hana B., Veronika H., Lenka S., Martin A., Iva B. Antioxidant, pro-oxidant and other biological activities of sesquiterpenes. Curr. Top. Med. Chem. 2014;14:2478–2494. doi: 10.2174/1568026614666141203120833. [DOI] [PubMed] [Google Scholar]
- 114.Gonzalez-Burgos E., Gomez-Serranillos M.P. Terpene compounds in nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012;19:5319–5341. doi: 10.2174/092986712803833335. [DOI] [PubMed] [Google Scholar]
- 115.Halliwell B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996;16:33–50. doi: 10.1146/annurev.nu.16.070196.000341. [DOI] [PubMed] [Google Scholar]
- 116.Wang C.Y., Wang S.Y., Chen C.T. Increasing antioxidant activity and reducing decay of blueberries by essential oils. J. Agric. Food Chem. 2008;56:3587–3592. doi: 10.1021/jf7037696. [DOI] [PubMed] [Google Scholar]
- 117.Blair J.M.A., Webber M.A., Baylay A.J., Ogbolu D.O., Piddock L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 118.Kubo I., Muroi H., Masaki H., Kubo A. Antibacterial activity of long-chain alcohols: The role of hydrophobic alkyl groups. Bioorg. Med. Chem. Lett. 1993;3:1305–1308. doi: 10.1016/S0960-894X(00)80336-4. [DOI] [Google Scholar]
- 119.Donlan R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002;8:881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ashley E.S.D., Lewis R., Lewis J.S., Martin C., Andes D. Pharmacology of systemic antifungal agents. Clin. Infect. Dis. 2006;43:S28–S39. doi: 10.1086/504492. [DOI] [Google Scholar]
- 121.Kalemba D., Kunicka A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003;10:813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- 122.Pattnaik S., Subramanyam V.R., Kole C. Antibacterial and antifungal activity of ten essential oils in vitro. Microbios. 1996;86:237–246. [PubMed] [Google Scholar]
- 123.Renslo A.R., McKerrow J.H. Drug discovery and development for neglected parasitic diseases. Nat. Chem. Biol. 2006;2:701–710. doi: 10.1038/nchembio837. [DOI] [PubMed] [Google Scholar]
- 124.Anthony J.P., Fyfe L., Smith H. Plant active components—A resource for antiparasitic agents? Trends Parasitol. 2005;21:462–468. doi: 10.1016/j.pt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 125.Kaye P., Scott P. Leishmaniasis: Complexity at the host-pathogen interface. Nat. Rev. Microbiol. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 126.Tiuman T.S., Santos A.O., Ueda-Nakamura T., Filho B.P.D., Nakamura C.V. Recent advances in leishmaniasis treatment. Int. J. Infect. Dis. 2011;15:e525–e532. doi: 10.1016/j.ijid.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 127.Couto A.S., Kimura E.A., Peres V.J., Uhrig M.L., Katzin A.M. Active isoprenoid pathway in the intra-erythrocytic stages of Plasmodium falciparum: Presence of dolichols of 11 and 12 isoprene units. Biochem. J. 1999;341:629–637. doi: 10.1042/0264-6021:3410629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mendanha S.A., Alonso A. Effects of terpenes on fluidity and lipid extraction in phospholipid membranes. Biophys. Chem. 2015;198:45–54. doi: 10.1016/j.bpc.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 129.Elbaz T., Esmat G. Hepatic and intestinal schistosomiasis: Review. J. Adv. Res. 2013;4:445–452. doi: 10.1016/j.jare.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ross A.G.P., Bartley P.B., Sleigh A.C., Olds G.R., Li Y., Williams G.M., McManus D.P. Schistosomiasis. N. Engl. J. Med. 2002;346:1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 131.Tangpukdee N., Duangdee C., Wilairatana P., Krudsood S. Malaria diagnosis: A brief review. Korean. J. Parasitol. 2009;47:93–102. doi: 10.3347/kjp.2009.47.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tonhosolo R., D’Alexandri F.L., Genta F.A., Wunderlich G., Gozzo F.C., Eberlin M.N., Peres V.J., Kimura E.A., Katzin A.M. Identification, molecular cloning and functional characterization of an octaprenyl pyrophosphate synthase in intra-erythrocytic stages of Plasmodium falciparum. Biochem. J. 2005;392:117–126. doi: 10.1042/BJ20050441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ukeh D.A., Birkett M.A., Pickett J.A., Bowman A.S., Jennifer Mordue A. Repellent activity of alligator pepper, Aframomum melegueta, and ginger, Zingiber officinale, against the maize weevil, Sitophilus zeamais. Phytochemistry. 2009;70:751–758. doi: 10.1016/j.phytochem.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 134.Isman M.B. Plant essential oils for pest and disease management. Crop Prot. 2000;19:603–608. doi: 10.1016/S0261-2194(00)00079-X. [DOI] [Google Scholar]
- 135.Prajapati V., Tripathi A.K., Aggarwal K.K., Khanuja S.P.S. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour. Technol. 2005;96:1749–1757. doi: 10.1016/j.biortech.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 136.Chan F.K.L., Leung W.K. Peptic-ulcer disease. Lancet. 2002;360:933–941. doi: 10.1016/S0140-6736(02)11030-0. [DOI] [PubMed] [Google Scholar]
- 137.Prausnitz M.R., Mitragotri S., Langer R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004;3:115–124. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 138.Fang J.-Y., Leu Y.-L., Hwang T.-L., Cheng H.-C., Hung C.-F. Development of sesquiterpenes from Alpinia oxyphylla as novel skin permeation enhancers. Eur. J. Pharm. Sci. 2003;19:253–262. doi: 10.1016/S0928-0987(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 139.Gao S., Singh J. In vitro percutaneous absorption enhancement of a lipophilic drug tamoxifen by terpenes. J. Control. Release. 1998;51:193–199. doi: 10.1016/S0168-3659(97)00168-5. [DOI] [PubMed] [Google Scholar]
- 140.Riedel W., Neeck G. Nociception, pain, and antinociception: Current concepts. Z. Rheumatol. 2001;60:404–415. doi: 10.1007/s003930170003. [DOI] [PubMed] [Google Scholar]
- 141.Kidd B.L., Urban L.A. Mechanisms of inflammatory pain. Br. J. Anaesth. 2001;87:3–11. doi: 10.1093/bja/87.1.3. [DOI] [PubMed] [Google Scholar]
- 142.Liew F.Y. The role of innate cytokines in inflammatory response. Immunol. Lett. 2003;85:131–134. doi: 10.1016/S0165-2478(02)00238-9. [DOI] [PubMed] [Google Scholar]
- 143.Maroon J.C., Bost J.W., Maroon A. Natural anti-inflammatory agents for pain relief. Surg. Neurol. Int. 2010;1 doi: 10.4103/2152-7806.73804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chan C.K., Supriady H., Goh B.H., Kadir H.A. Elephantopus scaber induces apoptosis through ROS-dependent mitochondrial signaling pathway in HCT116 human colorectal carcinoma cells. J. Ethnopharmacol. 2015;168:291–304. doi: 10.1016/j.jep.2015.03.072. [DOI] [PubMed] [Google Scholar]
- 145.Singh P., Singh A. Ocular adverse effects of anti-cancer chemotherapy. J. Cancer Ther. Res. 2012;1 doi: 10.7243/2049-7962-1-5. [DOI] [Google Scholar]
- 146.Johnstone R.W., Ruefli A.A., Lowe S.W. Apoptosis: A link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/S0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 147.Gautam N., Mantha A.K., Mittal S. Essential oils and their constituents as anticancer agents: A mechanistic view. Biomed. Res. Int. 2014 doi: 10.1155/2014/154106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goh B.H., Chan C.K., Kamarudin M.N.A., Abdul Kadir H. Swietenia macrophylla King induces mitochondrial-mediated apoptosis through p53 upregulation in HCT116 colorectal carcinoma cells. J. Ethnopharmacol. 2014;153:375–385. doi: 10.1016/j.jep.2014.02.036. [DOI] [PubMed] [Google Scholar]
- 149.Sylvestre M., Pichette A., Lavoie S., Longtin A., Legault J. Composition and cytotoxic activity of the leaf essential oil of Comptonia peregrina (L.) Coulter. Phytother. Res. 2007;21:536–540. doi: 10.1002/ptr.2095. [DOI] [PubMed] [Google Scholar]
- 150.Golias C., Tsoutsi E., Matziridis A., Makridis P., Batistatou A., Charalabopoulos K. Leukocyte and endothelial cell adhesion molecules in inflammation focusing on inflammatory heart disease. In Vivo. 2007;21:757–769. [PubMed] [Google Scholar]
- 151.Casey P.J., Solski P.A., Der C.J., Buss J.E. p21ras is modified by a farnesyl isoprenoid. Proc. Natl. Acad. Sci. USA. 1989;86:8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.De Smet P.A.G.M. Clinical risk management of herb-drug interactions. Br. J. Clin. Pharmacol. 2007;63:258–267. doi: 10.1111/j.1365-2125.2006.02797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.King A.J., Dickinson J.R. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Res. 2003;3:53–62. doi: 10.1111/j.1567-1364.2003.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 154.Longenecker H.E., Musulin R.R., Tully R.H., King C.G. An acceleration of Vitamin C synthesis and excretion by feeding known organic compounds to rats. J. Biol. Chem. 1939;129:445–453. [Google Scholar]
- 155.Mendanha S.A., Moura S.S., Anjos J.L.V., Valadares M.C., Alonso A. Toxicity of terpenes on fibroblast cells compared to their hemolytic potential and increase in erythrocyte membrane fluidity. Toxicol. In Vitro. 2013;27:323–329. doi: 10.1016/j.tiv.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 156.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 157.Rosenbaum S. Basic Pharmacokinetics and Pharmacodynamics: An Integrated Textbook and Computer Simulations. 1st ed. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2011. Drug administration, absorption, and bioavailability; pp. 36–59. [Google Scholar]
- 158.Jambhekar S.S., Breen P.J. Basic Pharmacokinetics. 2nd ed. Pharmaceutical Press; London, UK: 2012. Bioavailability/bioequivalence; pp. 137–159. [Google Scholar]
- 159.Bunel V., Ouedraogo M., Nguyen A.T., Stévigny C., Duez P. Methods applied to the in vitro primary toxicology testing of natural products: State of the art, strengths, and limits. Planta Med. 2014;80:1210–1226. doi: 10.1055/s-0033-1360273. [DOI] [PubMed] [Google Scholar]
- 160.Hausen B.M., Evers P., Stüwe H.T., König W.A., Wollenweber E. Propolis allergy (IV) studies with further sensitizers from propolis and constituents common to propolis, poplar buds and Balsam of Peru. Contact Dermat. 1992;26:34–44. doi: 10.1111/j.1600-0536.1992.tb00865.x. [DOI] [PubMed] [Google Scholar]
- 161.Hausen B.M., Simatupang T., Bruhn G., Evers P., Koenig W.A. Identification of new allergenic constituents and proof of evidence for coniferyl benzoate in Balsam of Peru. Am. J. Contact Dermat. 1995;6:199–208. doi: 10.1016/1046-199X(95)90043-8. [DOI] [Google Scholar]
- 162.Belsito D., Bickers D., Bruze M., Calow P., Greim H., Hanifin J.M., Rogers A.E., Saurat J.H., Sipes I.G., Tagami H. A safety assessment of non-cyclic alcohols with unsaturated branched chain when used as fragrance ingredients: The RIFM expert panel. Food Chem. Toxicol. 2010;48(Suppl. 3):S1–S42. doi: 10.1016/j.fct.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 163.Hanley K., Jiang Y., Crumrine D., Bass N.M., Appel R., Elias P.M., Williams M.L., Feingold K.R. Activators of the nuclear hormone receptors PPARalpha and FXR accelerate the development of the fetal epidermal permeability barrier. J. Clin. Investig. 1997;100:705–712. doi: 10.1172/JCI119583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brand M.D., Nicholls D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Péres V.F., Moura D.J., Sperotto A.R.M., Damasceno F.C., Caramão E.B., Zini C.A., Saffi J. Chemical composition and cytotoxic, mutagenic and genotoxic activities of the essential oil from Piper gaudichaudianum Kunth leaves. Food Chem. Toxicol. 2009;47:2389–2395. doi: 10.1016/j.fct.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 166.Sperotto A.R.M., Moura D.J., Péres V.F., Damasceno F.C., Caramão E.B., Henriques J.A.P., Saffi J. Cytotoxic mechanism of Piper gaudichaudianum Kunth essential oil and its major compound nerolidol. Food Chem. Toxicol. 2013;57:57–68. doi: 10.1016/j.fct.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 167.Heijne W.H.M., Kienhuis A.S., van Ommen B., Stierum R.H., Groten J.P. Systems toxicology: Applications of toxicogenomics, transcriptomics, proteomics and metabolomics in toxicology. Expert Rev. Proteom. 2005;2:767–780. doi: 10.1586/14789450.2.5.767. [DOI] [PubMed] [Google Scholar]
- 168.Youns M., Hoheisel J.D., Efferth T. Toxicogenomics for the prediction of toxicity related to herbs from traditional Chinese medicine. Planta Med. 2010;76:2019–2025. doi: 10.1055/s-0030-1250432. [DOI] [PubMed] [Google Scholar]
- 169.Pículo F., Guiraldeli Macedo C., de Andrade S.F., Luis Maistro E. In vivo genotoxicity assessment of nerolidol. J. Appl. Toxicol. 2011;31:633–639. doi: 10.1002/jat.1607. [DOI] [PubMed] [Google Scholar]