Abstract
Palladium nanoparticles, simply and briefly generated in commercial and cheap onium salts using supercritical carbon dioxide, have been found to be an effective catalytic system for additive free N-alkylation reaction using alcohols via cascade oxidation/condensation/reduction steps.
Keywords: N-alkylation, nanoparticle, onium salt
1. Introduction
The amine function plays a major role in many industrial fields such as materials, pharmaceuticals and agrochemicals [1,2,3]. This function is present not only in bioactive structures, such as amino acids and alkaloids, but also in numerous intermediates for polymers and dyes synthesis. N-alkylated amines are mainly obtained either by reaction between an amine and an alkyl halide [4] in the presence of stoichiometric amount of a base or by reductive amination [5] of ketone or aldehyde starting materials. Alternatively, products can be obtained from alcohols, avoiding the presence of potentially mutagenic alkylating reagents or carbonyl compounds as unstable starting materials. Several methods using direct nucleophilic substitution on the hydroxyl group can be considered. However, the main developed methodology relies on a “self-hydrogen transfer” reaction, the so called “hydrogen-borrowing” method (Scheme 1).
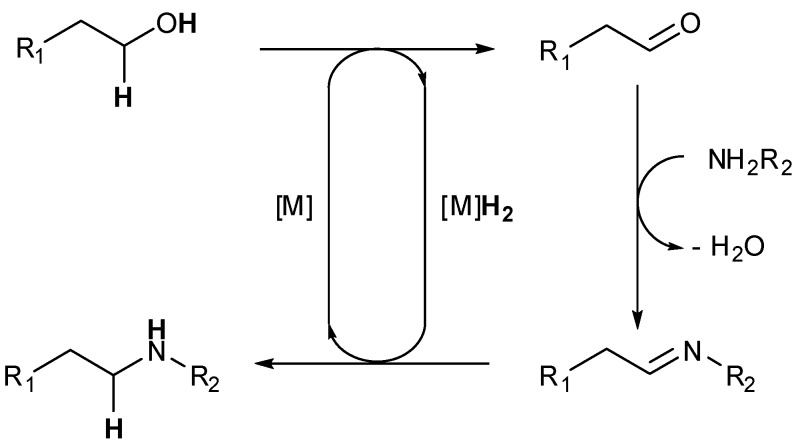
Scheme 1.
Amination of alcohols by borrowing-hydrogen method, cascade oxidation/imine formation/reduction.
In a one pot synthesis, three distinct steps are carried out: first the oxidation step of the starting alcohol takes place, followed by formation of an imine which will finally be reduced to the N-alkyl amine, the hydrogen transfer being performed by the catalyst during oxidation and reduction steps. Water is the only by-product generated and the reaction could theoretically occur in the absence of hydrogen, thereby enhancing the system safety.
By using noble (Pd [6], Ru [7,8,9,10,11,12], Ir [13,14,15,16], Rh [17,18]) and non-noble metals (Cu [19,20,21], Fe [22,23]) as homogenous catalysts for the N-alkylation reaction, several efficient methods have been developed. The improvement of the reaction system allowing easy recycling of the catalyst has led to the emergence of numerous studies advocating the use of heterogeneous catalysts (Pd [24,25,26,27,28], Ru [29,30,31,32], Pt [33,34], Au [35,36,37,38,39,40], Ag [41,42], Ni [43,44,45], Mn [46], Cu [47,48,49], Fe [50,51]) with more or less efficiency.
The synthesis of nanoscaled materials has allowed for the evolution of a new field of catalysis. Indeed, at nanosize, the physicochemical properties of materials can change dramatically, due to a high specific surface area but also to a higher number of metal low valence favouring radical formations. It is known that noble metal nanoparticles are effective in many catalytic processes for both reduction and oxidation reactions and the trend is the preparation of hybrid noble metal nanocatalysts, as simple as can be. Ionic liquids (ILs), as solvents, stabilizers and reducing agents [52,53] are very attractive for an easy and clean synthesis of noble metal-based nanocatalysts [54]. Bringing together the properties of ILs (low surface tension, the presence of charges able to create an electrostatic shell around the metal core, a myriad of structures and so on) [55] with the advantages of supercritical fluids technology (homogeneous single phase reaction media, properties of both liquids—density and solvation capabilities—and gases—high diffusivity, low viscosity and zero surface tension, increased overall reaction rate) highly crystalline metal NPs embedded in a solid matrix and additionally free from any undesired organic parts can be prepared [56]. Among supercritical fluids, supercritical carbon dioxide (scCO2) is especially attractive due to its low critical coordinates of 31 °C and 7.38 MPa and its accessibility, being abundant, cheap, inoffensive, and environmentally friendly, with also no liquid waste generation [57,58].
The present study is focused on the use of palladium NPs as hybrid nanocatalysts for the N-alkylation reaction. The Pd NPs are generated in onium salts (OSs) with the assistance of scCO2 in the absence of molecular solvent. The resulting easy-to-handle powdered OS embedded Pd NPs are stable towards air and moisture and display good catalytic activities in the green synthesis of N-alkylated anilines.
2. Results
2.1. Synthesis and Characterization of the Catalyst
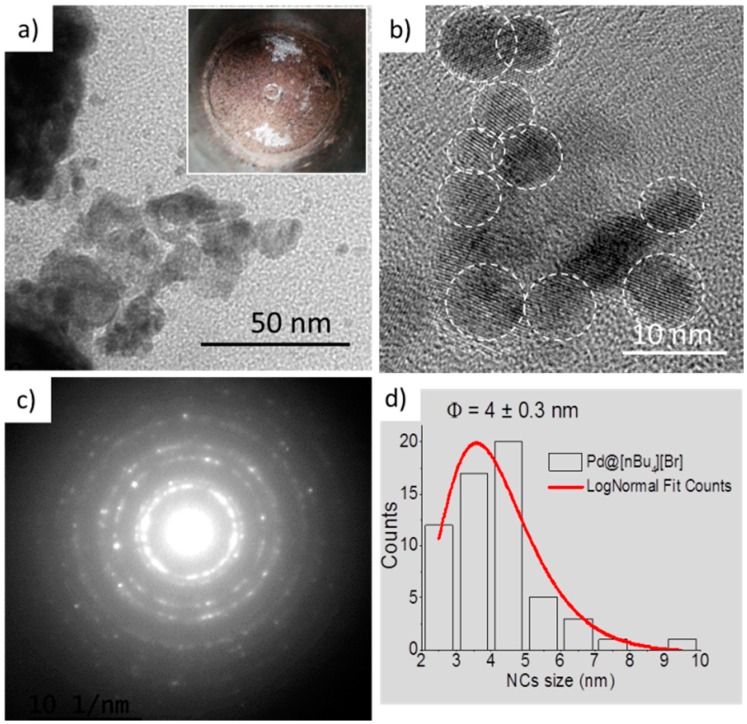
A high pressure/high temperature stirred stainless steel batch reactor (Vreactor = 60 mL) was used to synthesize for the first time the Pd NPs embedded in the solid matrix of quaternary ammonium salts: (1) tetrabutylammonium bromide, [nBu4N][Br]; (2) tetraethylammonium bromide, [Et4N][Br]; (3) benzyltrimethylammonium bromide, [BnNMe3][Br]; (4) cetyltrimethylammonium bromide, [C16NMe3][Br]; (5) cetyltrimethylammonium bis(trifluoromethylsulfonyl)imide [C16NMe3][NTf2] and (6) cetyltrimethylammonium hexafluorophosphate, [C16NMe3][PF6]. Three bar of H2 as reducing agent were first loaded into the reactor containing the metal precursor (Pd(OAc)2) in powder form mixed with the solid OS to give 1 wt % metal loading. The synthesis reaction took place in a scCO2 media, pressurized at 25 MPa, during 60 min at 100 °C, followed by fast depressurization and CO2 venting through the reactor (a few minutes more) at the end of the reaction in order to dry and clean the final material. A more detailed description of the synthesis approach is reported elsewhere [58]. The prepared final nanoparticles embedded in the solid matrix of ammonium salt, Pd@OS were recovered as dark powder (Figure 1a—inset) and used directly in the reaction of N-alkylation.
Figure 1.
(a,b) TEM and HRTEM images of Pd@[nBu4N][Br] NPs are presented; (c) High NPs crystallinity revealed by selected area diffraction pattern (SADP); (d) LogNormal size distribution of the NPs.
Microscopy images of the prepared nanocatalyst, Pd@[nBu4N][Br], are presented in Figure 1. Using the scCO2 approach the prepared Pd NPs are small but deceivingly more agglomerated than expected. An explanation could be due to the high reactivity of primary Pd nuclei obtained in supercritical media, already observed by our group [59,60]. Although [nBu4N][Br], due to its structure, can act as electrostatic stabilizer preventing NPs growth and agglomeration, in the case of Pd, the electrostatic protection is not strong enough to overcome the agglomeration of the highly reactive NPs.
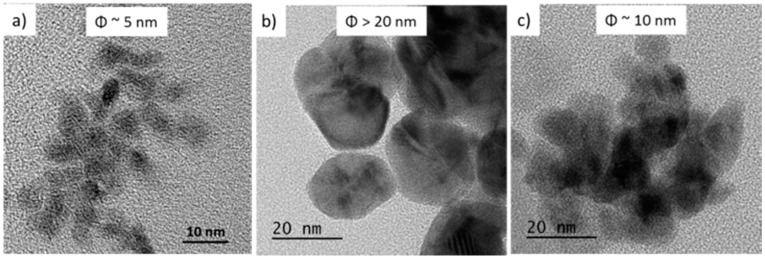
Very small and monodispersed NPs can also be obtained, as we have observed experimentally for other metals such as Ru, Ir, Pt [58]. The Pd NPs are however monocrystalline (Figure 1b,c) with a mean size of about 4 nm (Figure 1d). If this bulkier cation containing four symmetric alkyl chains is replaced by BnNMe3, less symmetrical and containing an aromatic ring, the Pd NPs size, morphology and chemical properties are affected. The obtained NPs are less aggregated, larger in size and less spherical (Figure 2a) leading eventually to different catalytic activity. It was assumed that the use of an OS with a long alkyl chain could stabilize the NPs more efficiently thus avoiding aggregation. With cetyltrimethylammonium bromide, slight improvement in monodispersity and an increase in size for Pd NPs were obtained (Figure 2b,c) with potential repercussions on NPs catalytic activity.
Figure 2.
TEM images of Pd@OS NPs: (a) Pd@[BnNMe3][Br]; (b) Pd@[C16NMe3][Br] and (c) Pd@[C16NMe3][NTf2].
These Pd@OSs systems were then tested in alkylation of anilines using alcohols as alkylating agents. Noticeably, all obtained systems displayed interesting catalytic activities owing to the absence of NP surface modification during the material synthesis process. Indeed, unlike most liquid phase preparation of M(0) NP, surface partial oxidation or residual molecular solvent binding is avoided by the use of scCO2. In all cases, OSs form a protective shell around the NPs allowing for shelf storage at room temperature in standard vials.
2.2. Optimisation of the N-Alkylation Reaction Conditions
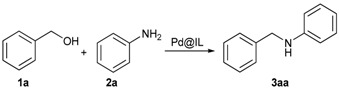
A first screening of the catalytic system and reaction conditions was done on a model reaction between benzyl alcohol (1a) and aniline (2a). Two different simple palladium precursors were tested and only the nanoparticles generated from Pd(OAc)2 precursor in tetrabutylammonium bromide, labelled as Pd@[nBu4N][Br], with 0.2 mol %, gave product 3aa in 48% yield (Table 1, Entry 1). NPs generated from PdCl2 led to less active species, probably due to the passivation of the Pd surface by remaining chlorides.
Table 1.
Optimization of N-alkylation of aniline with benzyl alcohol 1.
| Entry | Ratio | OS | Pd@IL | Solvent | Yield (%) 2 |
|---|---|---|---|---|---|
| 1a:2a | (mol %) | ||||
| 1 | 1:1 | [nBu4N][Br] | 0.2 | - | 48 |
| 2 | 1:1 | [nBu4N][Br] | 0.5 | - | 71 |
| 3 | 1:1 | [nBu4N][Br] | 1 | - | 78 |
| 4 | 1:1 | [nBu4N][Br] | 1 | - | 71 |
| 5 | 2:1 | [nBu4N][Br] | 1 | - | 86 |
| 6 | 5:1 | [nBu4N][Br] | 1 | - | 85 |
| 7 | 1:2 | [nBu4N][Br] | 1 | - | 60 |
| 8 | 2:1 | [nBu4N][Br] | 1 | EG 3 | 44 |
| 9 | 2:1 | [nBu4N][Br] | 1 | diglyme | 46 |
| 10 | 2:1 | [nBu4N][Br] | 1 | toluene | 97 |
| 11 | 2:1 | [nBu4N][Br] | 1 | mesitylene | 78 |
| 12 | 2:1 | [nBu4N][Br] | 1 | anisole | >99 |
| 13 | 2:1 | [nBu4N][Br] | 1 | water | 33 |
| 14 | 2:1 | [Et4N][Br] | 1 | toluene | 28 |
| 15 | 2:1 | [BnNMe3][Br] | 1 | toluene | 43 |
| 16 | 2:1 | [C16NMe3][Br] | 1 | toluene | 63 |
| 17 | 2:1 | [C16NMe3][NTf2] | 1 | toluene | 51 |
| 18 | 2:1 | [C16NMe3][PF6] | 1 | toluene | 62 |
1 General conditions: sealed tube, Ar, limiting reagent (1 mmol), solvent (1 mL), 140 °C, 24 h. 2 GC yields determined by reference to an internal standard. 3 EG: ethylene glycol.
By increasing the catalyst amount to 0.5 and 1 mol %, better yields of 71% and 78% (Table 1, Entries 2–3) were achieved. In situ nanoparticle generation provided the desired product in 71% yield, slightly lower than with the ex situ formed catalytic system (Table 1, Entry 4). Studying the ratio between benzyl alcohol and aniline, the results showed that the use of excess alcohol can be beneficial, leading to 86% yield. A 5 to 1 ratio gave only 85% yield (Table 1, Entries 5–6). In contrast, amine excess reduced the yield to 60%.
Many solvents were evaluated. Among those, ethylene glycol and diglyme had a negative effect, with 44% and 46% yields, respectively (Table 1, Entries 8–9). In the case of ethylene glycol, no competitive N-alkylation with solvent was observed. In the presence of toluene, an excellent yield of 97% was observed (Table 1, Entry 10). Mesitylene was less effective with 78% yield (Table 1, Entry 11). Using a more polar aromatic solvent, such as anisole, compound 3aa was isolated in quantitative yield. The reaction could also take place in an aqueous medium, although leading to a mere 33% yield (Table 1, Entry 12).
Various Pd@OS, OS being chosen from the alkyl ammonium compound class, have been tested. With tetraethylammonium bromide [Et4N][Br] and benzyltrimethylammonium bromide [BnNMe3][Br], the generated nanoparticles seemed less active (28% and 43% yield, respectively, Table 1, Entries 13–14). In addition, the influence of the long alkyl chains of the cationic part and the counter-anion has been tested. Pd@[C16NMe3][Br], Pd@[C16NMe3][NTf2] and Pd@[C16NMe3][PF6] gave less promising results than those obtained with Pd@[nBu4N][Br] (Table 1, Entries 16–18). Bromide and hexafluorophosphate are more effective than triflimidate counter-ions, mostly due to the different Pd NPs size observed. Noticeably, the catalytic properties could arise from the cumulative contribution of the structure and electronic properties of OS with the physicochemical properties (size, morphology, surface chemistry, organization) of Pd NPs.
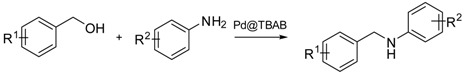
2.3. N-Alkylation of Amines with Benzyl Alcohol Derivatives
Subsequent screening and optimization, Pd@[nBu4N][Br] NPs system was found to be the best nanocatalyst. Therefore, this catalyst was further used to extend the scope of the “hydrogen-borrowing” methodology. Different amines and alcohols derivatives were tested in the previously optimised conditions (Table 2). Model reaction between benzyl alcohol (1a) and aniline (2a) provides N-benzylaniline (3aa) in 96% isolated yield (Table 2, Entry 1). Aniline para substitution with an alkyl group, R2 = Me or tBu provides the desired amines 3ac and 3ab in good yields (95% and 91%, respectively; Table 2, Entries 2–3). In the presence of an electron donating group such as methoxy group, compound 3ad is isolated in 91% yield (Table 2, Entry 4). An electron withdrawing group such as chlorine afforded product 3af in good yield (88%, Table 2, Entry 5). Different aniline substitutions with fluorine in para and meta positions, also led to good yields of 3af and 3ag (Table 2, Entries 6–7). In the case of ortho substitution, no conversion took place, a phenomenon observed for all substituted products at this position. Polysubstituted anilines led to excellent yields. 3ah, 3ai, 3aj are respectively isolated in 94%, 87% and 97% yields (Table 2, Entries 8–10). A strong electron withdrawing group slightly decreased the yield of target 3ak to 76% (Table 2, Entry 11). The reaction is not limited to anilines. With linear primary amines such as benzylamine (2m) and hexylamine (3an), the reaction was effective, leading to 3am and 3an in 93% and 88% yields, respectively (Table 2, Entries 12–13). Secondary amines, such as morpholine (2l), provided compound 3al in 52% yield (Table 2, Entry 14). Further heating at 160 °C improves the yield to 65%, but decomposition of the onium salt and morpholine became non-negligible.
Table 2.
N-alkylation of amines with alcohols 1.
| Entry | Alcohol 1 | Amine 2 | Product | Yield (%) 2 |
|---|---|---|---|---|
 |
 |
 |
||
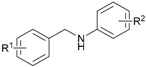
| 1 | 1a (R1 = H) | 2a (R2 = H) | 3aa | 96 |
| 2 | 1a | 2b (R2 = 4-Me) | 3ab | 95 |
| 3 | 1a | 2c (R2 = 4-tBu) | 3ac | 91 |
| 4 | 1a | 2d (R2 = 4-OMe) | 3ad | 91 |
| 5 | 1a | 2e (R2 = 4-Cl) | 3ae | 88 |
| 6 | 1a | 2f (R2 = 4-F) | 3af | 92 |
| 7 | 1a | 2g (R2 = 3-F) | 3ag | 87 |
| 8 | 1a | 2h (R2 = 3,4-Cl) | 3ah | 94 |
| 9 | 1a | 2i (R2 = 3,5-Cl) | 3ai | 87 |
| 10 | 1a | 2j (R2 = 3-Cl,4-F) | 3aj | 97 |
| 11 | 1a | 2k (R2 = 4-CF3) | 3ak | 76 |
| 12 | 1a |
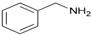 2m |
3am | 93 |
| 13 | 1a |
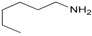 2n |
3an | 88 |
| 14 | 1a |
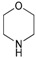 2l |
3al | 52 |
| 15 | 1b (R1 = 4-Me) | 2a | 3ba | 92 |
| 16 | 1c (R1 = 4-OMe) | 2a | 3ca | 97 |
| 17 | 1d (R1 = 4-Cl) | 2a | 3da | 50 |
| 18 |
 1e |
2a | 3ea | 54 |
1 Pd@[nBu4N][Br] (1% in Pd), 1 (2 mmol), 2 (1 mmol), anisole (1 mL), 140 °C, 24 h. 2 isolated yield.
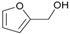
Several benzylic alcohols were tested next (See NMR in Supplementary Materials). In the presence of electron donor groups such as 4-Me 1b and 4-OMe 1c, the reaction was effective with respective yields of 92% and 97% (Table 2, Entries 15–16). In the case of electron withdrawing groups, only the 4-Cl group provided the desired compound 3da (50%, Table 2, Entry 17). In other cases, deactivated benzyl alcohol derivatives and aliphatic alcohols, no conversion was observed showing that the oxidation step is the limiting step. Furfuryl alcohol 1e provides the N-alkylation 3ea with a yield of 54% (Table 2, Entry 18).
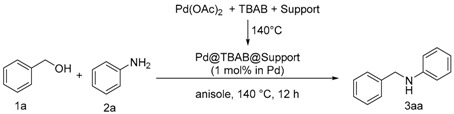
2.4. Heterogeneous Catalysts for Cascade Oxidation/Imine Foration/Hydrogenation Leading to Benzylamine
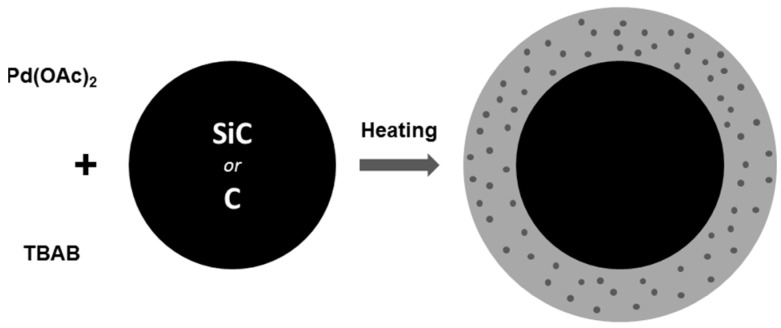
In an attempt to provide an easily accessible heterogeneous catalyst, we prepared coated SiC and C with the previously described Pd@TBAB. The formation of the Pd NPs was directly performed in the presence of the solid material leading to Pd@TBAB@SiC and Pd@TBAB@C respectively (Figure 3). Depending on the TBAB/Support ratio, the material is obtained as beads coated with TBAB or a grey solid of Pd NP and matrix embedded in TBAB.
Figure 3.
Preparation of Pd@TBAB@(Si)C.
In short, silicon carbide turned out to be more efficient than the carbon black particle. This could be attributed to a better inertness of the support not interfering with the low palladium content. This is corroborated by the poor conversion obtained when a large excess of C is used (Table 3, Entry 4). The effect is more limited with silicon carbide, and with a same excess of support, a decent 59% yield is obtained after 24 h in anisole. Nonetheless, the best results were obtained with a Pd:TBAB:SiC ratio of 1:250:500 (Table 3, Entry 8) where both the activity (99% yield) and the form of the catalyst (grey powder) were appealing. When lowering the catalytic charge on the TBAB:SiC mixture, yield remained correct (Table 3, Entries 9–12), only with a 1:250:250 ratio were obtained quantitative yield. However in that case, catalyst is obtained as Pd NPs and SiC particles embedded in TBAB, and practically inconvenient for recycling tests.
Table 3.
N-Alkylation of amines with alcohols using a heterogeneous catalyst 1.
| Entry | Pd:TBAB:Support Ratio | Support | Yield (%) 2 |
|---|---|---|---|
| 1 | 1:250:250 | C | 60 |
| 2 | 1:250:500 | C | 35 |
| 3 | 1:250:1000 | C | 36 |
| 4 | 1:250:2000 | C | 9 |
| 5 | 1:500:1000 | C | 39 |
| 6 | 1:1000:1000 | C | 46 |
| 7 | 1:250:250 | SiC | 99 |
| 8 | 1:250:500 | SiC | 99 |
| 9 | 1:250:1000 | SiC | 73 |
| 10 | 1:250:2000 | SiC | 59 |
| 11 | 1:500:1000 | SiC | 77 |
| 12 | 1:1000:1000 | SiC | 80 |
1 Pd@[nBu4N][Br]@M (1% in Pd), 1 (2 mmol), 2 (1 mmol), anisole (1 mL), 140 °C, 24 h. 2 isolated yield.
Recycling experiments were performed using the carbon support, and unsurprisingly, the catalytic activity in the first cycle was deceiving. In that case, a complete loss of activity was observed after recovering the catalyst by filtration (Table 4, Entry 2).
Table 4.
Recycling of heterogeneous catalyst 1.
| Entry | Run | Support | Time | Yield (%) 2 | |
|---|---|---|---|---|---|
| 1 | 1 | C (1:250:250) | anisole 3 | 4 h | 60 |
| 2 | 2 | C (1:250:250) | anisole 3 | 4 h | 0 |
| 3 | 1 | SiC (1:250:500) | anisole 3 | 4 h | 99 |
| 4 | 2 | SiC (1:250:500) | anisole 3 | 4 h | 98 |
| 5 | 3 | SiC (1:250:500) | anisole 3 | 4 h | 85 |
| 6 | 4 | SiC (1:250:500) | anisole 3 | 4 h | 60 |
| 7 | 5 | SiC (1:250:500) | anisole 3 | 4 h | 0 |
| 8 | 1 | SiC (1:250:500) | scCO2 4 | 4 h | <5 |
| 9 | 1 | SiC (1:250:500) | scCO2 5 | 6 h | 51 |
| 10 | 2 | SiC (1:250:500) | scCO2 5 | 6 h | 50 |
| 11 | 3 | SiC (1:250:500) | scCO2 5 | 6 h | 50 |
1 Pd@[nBu4N][Br]@M (1% in Pd), 1 (2mmol), 2 (1 mmol); 2 isolated yield; 3 anisole (1 mL), 140 °C; 4 CO2, 100 °C, 80 bar; 5 CO2, 140 °C, 100 bar.
This phenomenon, due to PdNP release in the reaction mixture was also observed using the 1:250/500 SiC catalysts but only after five cycles (Table 4, Entries 3–7). This is due to the relatively high solubility of TBAB in anisole, leading to leaching of Pd NPs into the reaction mixtures. In an attempt to limit this phenomenon, the solvent was switched to CO2 under supercritical conditions. TBAB is insoluble in scCO2 but scCO2 is soluble in the TBAB phase, favouring reagent/product exchange between scCO2 phase and TBAB phase. While the reaction is inconclusive at 100 °C 80 bar, (Table 4, Entry 8), harsher reaction conditions led to a decent yield, which was maintained for three cycles owing to the insolubility of the catalytic active phase (Pd@TBAB) in the bulk solvent (scCO2) (Table 4, Entries 9–11).
3. Experimental Section
3.1. General Information
GC-MS analysis were performed with a Agilent 7890A GC system (Agilent, Santa Clara, CA, USA) equipped with a J & W Scientific (Agilent) DB-1701 capillary column, a 5975C VL MSD with triple axis detector (EI) using the following program: 70 °C for 1 min then 20 °C·min−1 until 230 °C then 6 min at 230 °C. 1H-, 13C-NMR were recorded on 300 MHz Avance I spectrometers (Bruker, Billerica, MA, USA). The chemical shifts (δ) and coupling constants (J) are expressed in ppm and Hertz respectively. The following abbreviations were used to explain the multiplicities: s = singlet, d =doublet, m = multiplet.
3.2. Chemicals
Amines and alcohols were used without further purification, except for furfuryl alcohol and morpholine which were distillated over CaH2. All catalytic reactions were carried out under argon atmosphere. All chemicals were stored under argon. Silica gel (230–400 mesh) purchased from Merck (Merck KGaA, Darmstadt, Germany) was used for flash chromatography. Analytical TLC silica gel 60 F254 was used.
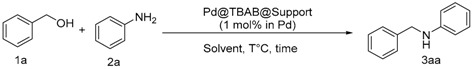
3.3. General Procedure
Pd@TBAB (1 mol % Pd in 250 mg of TBAB synthetized in scCO2), alcohol (2 mmol, 2 eq), amine (1 mmol, 1 eq) and anisole (1 mL) were introduced into a sealed tube under argon. The reaction was then heated at 140 °C for 12 h. After cooling the reaction at room temperature, the mixture was filtrated on Celite and washed with Et2O and the filtrate was concentrated under vacuum. The crude oil was purified by column chromatography.
3.4. Analyses
N-Phenylbenzenemethanamine (3aa). CAS: 103-32-2; white solid; Yield: 96%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.42–7.27 (m, 5H), 7.24–7.14 (m, 2H), 6.78–6.69 (m, 1H), 6.66 (dd, J = 8.6, 1.0 Hz, 2H), 4.35 (s, 2H), 4.04 (bs, 1H) 13C-NMR (75 MHz, CDCl3, 25 °C) δ 148.0, 139.4, 129.4 (2C), 128.8 (2C), 127.8 (2C), 127.4, 118.0, 113.2 (2C), 48.6 MS (m/z) 183 (78), 106 (18), 91 (100), 77 (20), 65 (14), 51 (8).
N-(4-Methylphenyl)-benzenemethanamine (3ab). CAS: 5405-15-2; Pale yellow oil; Yield: 95%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.42–7.27 (m, 5H), 6.99 (d, J = 7.0 Hz, 2H), 6.57 (d, J = 7.0 Hz, 2H), 4.31 (s, 2H), 3.90 (bs, 1H), 2.24 (s, 3H) 13C-NMR (75 MHz, CDCl3, 25 °C) δ 145.1, 139.1, 130.2, 129.9 (2C), 128.7 (2C), 127.9 (2C), 127.4, 113.9 (2C), 49.3, 20.6 MS (m/z) 197 (74), 120 (21), 91 (100), 77 (10), 65 (14), 51 (4).
N-(4-tert-Butylphenyl)-benzenemethanamine (3ac). CAS : 255835-93-9; White solid; Yield: 91%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.49–7.37 (m, 4H), 7.38–7.31 (m, 2H), 7.19–7.10 (m, 1H), 6.80–6.68 (m, 2H), 4.45 (s, 2H), 4.36 (s, 1H), 1.48 (s, 9H) 13C-NMR (75 MHz, CDCl3, 25 °C) δ 165.8, 162.6, 149.9, 138.8, 130.5, 130.4, 128.9 (2C), 127.6 (2C), 127.5, 108.9, 104.4, 104.1, 99.9, 99.6, 48.4 MS (m/z) 239 (46), 224 (28), 196 (5), 132 (9), 120 (8), 106 (8), 91 (100), 77 (6), 56 (8), 51 (2).
N-(4-Methoxyphenyl)-benzenemethanamine (3ad). CAS: 17377-95-6; Pale yellow oil; Yield: 91%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.40 (d, J = 7.3 Hz, 2H), 7.30 (t, J = 6.3 Hz, 2H), 7.00 (dd, J = 5.3, 2.5 Hz, 2H), 6.83 (d, J = 5.7 Hz, 1H), 6.74 (d, J = 7.6 Hz, 2H), 4.35 (s, 2H), 4.02 (bs, 1H), 3.90 (s, 3H) 13C-NMR (75 MHz, CDCl3, 25 °C) δ 158.9, 148.3, 131.5, 129.3 (2C), 129.3 (2C), 117.6, 114.1 (2C), 112.9 (2C), 55.4, 47.9 MS (m/z) 213 (100), 198 (9), 168 (5), 136 (9), 122 (97), 91 (89), 77 (6), 65 (14), 51 (4).
N-(4-Chlorophenyl)-benzenemethanamine (3ae). CAS: 2948-37-0; Pale yellow oil; Yield: 88%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.35 (d, J = 4.5 Hz, 2H), 7.34–7.25 (m, 3H), 7.11 (d, J = 8.9 Hz, 2H), 6.55 (d, J = 8.9 Hz, 2H), 4.31 (s, 2H), 4.07 (bs, 1H). 13C-NMR (75 MHz, CDCl3, 25 °C) 145.3, 138.0, 129.28 (2C), 128.9 (2C), 127.9 (2C), 127.6, 123.7, 115.2 (2C), 49.3. MS (m/z) 217 (40), 140 (7), 111 (6), 91 (100), 65 (9), 51 (3).
N-(4-Fluorophenyl)-benzenemethanamine (3af). CAS: 370-77-4; Pale yellow oil; Yield: 92 %. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.43–7.27 (m, 5H), 6.97–6.84 (m, 2H), 6.63–6.53 (m, 2H), 4.31 (s, 2H), 3.94 (s, 1H). 13C-NMR (75 MHz, CDCl3, 25 °C) δ 157.8 (s), 154.7 (s), 143.8 (s), 138.7 (s), 128.7 (s), 127.6 (JCF = 18.1 Hz), 115.9 (s), 115.6 (s), 114.3 (JCF = 7.5 Hz), 77.5 (s), 77.1 (s), 76.7 (s), 49.3 (s). MS (m/z) 201 (53), 124 (9), 91 (100), 65 (10), 51 (3).
N-(3-Fluorophenyl)-benzenemethanamine (3ag). CAS: 123330-53-0; Pale yellow oil; Yield: 87%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.37–7.36 (m, 4H), 7.32–7.28 (m, 1H), 7.12–7.08 (m, 1H), 6.41–6.39 (m, 2H), 6.34–6.31(m, 1H), 4.32 (s, 2H), 4.18 (s, 1H). 13C-NMR (75 MHz, CDCl3, 25 °C) δ 164.4 (JCF = 241 Hz), 150.1 (JCF = 11Hz), 139.0, 130.5 (JCF = 10 Hz), 129.0, 127.7, 127.6, 109.0, 104.2 (JCF = 21 Hz), 99.7 (JCF = 25 Hz), 48.4. MS (m/z) 201 (52), 124 (8), 91 (100), 65 (16), 51 (5).
N-(3,4-Dichlorophenyl)-benzenemethanamine (3ah). CAS: 51597-75-2; Pale yellow oil; Yield: 94%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.44–7.26 (m, 1H), 7.18 (d, J = 8.7 Hz, 1H), 6.71 (d, J = 2.7 Hz, 1H), 6.46 (dd, J = 8.7, 2.8 Hz, 1H), 4.29 (s, 1H), 4.23 (s, 1H). 13C-NMR (75 MHz, CDCl3, 25 °C) 147.6, 138.4, 132.9, 130.7, 128.9 (2C), 127.7, 127.5 (2C), 120.2, 114.1, 112.7, 48.3. MS (m/z) 251 (27), 174 (4), 145 (4), 91 (100), 65 (8), 51 (2).
N-(3,5-Dichlorophenyl)-benzenemethanamine (3ai). CAS: 65089-00-1; Pale yellow oil; Yield: 87%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.47 – 7.32 (m, 5H), 6.72 (t, J = 1.7 Hz, 1H), 6.53 (d, J = 1.7 Hz, 2H), 4.35 (s, 1H), 4.33 (s, 2H). 13C-NMR (75 MHz, CDCl3, 25 °C) δ 140.8, 136.1, 129.6, 128.6 (2C), 127.6, 126.9 (2C), 123.2, 120.2, 113.4, 48.2. MS (m/z) 251 (27), 174 (4), 145 (4), 91 (100), 65 (8), 51 (2).
N-(3-Chloro-4-fluorophenyl)-benzenemethanamine (3aj). CAS: 131776-32-4; Pale yellow oil; Yield: 97%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.36–7.26 (m, 4H), 7.24–7.20 (m, 1H), 7.06 (t, J = 9.2 Hz,1H), 6.63 (dd, J = 2.8, 6.4 Hz, 1H), 6.51 (dt, J = 3.2, 9.2 Hz, 1H), 6.43 (t, J = 5.8 Hz, 1H), 4.22 (s, 2H, J = 6.0 Hz). 13C-NMR (75 MHz, CDCl3, 25 °C) 153.2, 146.2, 146.1, 138.7, 128.9, 128.7, 127.8, 127.6, 127.6, 117.0, 113.9, 112.1, 48.0. MS (m/z) 235 (32), 158 (5), 129 (6), 91 (100), 65 (8), 51 (2).
N-(4-(Trifluoromethyl)phenyl)-benzenemethanamine (3ak). CAS: 405-81-2; Pale yellow oil; Yield: 76%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.49–7.16 (m, 6H), 7.02–6.90 (m, 1H), 6.86 (m, 1H), 6.76 (dd, J = 8.2, 2.3 Hz, 1H), 4.35 (s, 2H), 1.54 (s, 1H). 13C-NMR (75 MHz, CDCl3, 25 °C) δ 150.6, 138.6, 128.9, 127.6, 127.5, 126.8, 123.7, 119.2 (q, JCF = 32.5 Hz), 48.0. MS (m/z) 251 (42), 174 (7), 145 (11), 91 (100), 65 (8), 51 (2).
N-(Phenylmethyl)-benzenemethanamine (3am). CAS: 103-49-1; Pale yellow oil; Yield: 93%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.61–7.29 (m, 10H), 3.93 (s, 4H), 1.76 (s, 1H). 13C-NMR (75 MHz, CDCl3, 25 °C) δ 140.4 (2C), 128.4 (4C), 128.2 (4C), 127.0 (2C), 53.2 (2C). MS (m/z) 196 (14), 120 (8), 106 (57), 91 (100), 77 (6), 65 (14), 51 (5).
N-Hexylbenzenemethanamine (3an). CAS: 25468-44-4; Pale yellow oil; Yield: 88%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.47–7.22 (m, 5H), 3.84 (s, 2H), 2.67 (t, J = 9.3 Hz, 2H), 1.64–1.29 (m, 6H), 0.94 (t, J = 9.3 Hz, 2H). 13C-NMR (75 MHz, CDCl3, 25 °C) δ 140.7, 128.5 (2C), 128.2 (2C), 126.9, 54.2, 49.7, 31.9, 30.2, 27.2, 22.7, 14.2. MS (m/z) 191(2), 120 (65), 106 (8), 91 (100), 65 (6).
4-(Phenylmethyl)-morpholine (3al). CAS: 10316-00-4; yellow oil; Yield: 52%. 1H-NMR (300 MHz, CDCl3, 25 °C), 7.45–7.28 (m, 5H), 4.68 (s, 2H), 2.1–2.0 (m, 8H). 13C-NMR (75 MHz, CDCl3, 25 °C), 140.5, 129.4, 128.1, 126.9, 66.3, 64.9, 60.4. MS (m/z) 177 (30), 146 (31), 91 (100), 86 (17), 65 (11), 56 (6).
4-Methyl-N-phenylbenzenemethanamine (3ba). CAS: 15818-64-1; Pale yellow oil; Yield: 92%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.31 (d, J = 7.9 Hz, 1H), 7.27–7.15 (m, 2H), 6.82–6.73 (m, 1H), 6.73–6.65 (m, 1H), 4.33 (s, 1H), 4.11 (s, 1H), 2.40 (s, 1H). 13C-NMR (75 MHz, CDCl3, 25 °C) δ 148.2, 136.9, 136.4, 129.3 (2C), 129.2 (2C), 127.6 (2C), 117.5, 113.9, 112.9, 48.1, 21.2. MS (m/z) 197 (48), 105 (100), 77 (18), 65 (4), 51 (4).
4-Methoxy-N-phenylbenzenemethanamine (3ca). CAS: 3526-43-0; Pale yellow oil; Yield: 97%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.40 (d, J = 7.3 Hz, 2H), 7.30 (t, J = 6.3 Hz, 2H), 7.00 (dd, J = 5.3, 2.5 Hz, 2H), 6.83 (d, J = 5.7 Hz, 1H), 6.74 (d, J = 7.6 Hz, 2H), 4.35 (s, 2H), 4.02 (s, 1H), 3.90 (s, 3H). 13C-NMR (75 MHz, CDCl3, 25 °C) δ 159.0, 148.3, 131.5, 129.3 (2C), 128.9 (2C), 117.6, 114.1 (2C), 112.9 (2C), 55.4, 47.9. MS (m/z) 213 (20), 121 (100), 77 (11), 51 (3).
4-Chloro-N-phenylbenzenemethanamine (3da). CAS: 4750-61-2; Pale yellow oil; Yield : 50% 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.54–7.31 (m, 4H), 7.31–7.17 (m, 2H), 6.80 (td, J = 7.3, 1.0 Hz, 1H), 6.67 (dd, J = 7.8, 0.7 Hz, 2H), 4.37 (s, 2H), 4.12 (s, 1H). 13C-NMR (75 MHz, CDCl3, 25 °C) δ 147.9, 138.1, 133.0, 129.4 (2C), 128.9 (2C), 128.8 (2C), 117.9, 113.0 (2C), 47.7. MS (m/z) 214 (100), 104 (14), 89 (10), 77 (63), 51 (18).
N-Phenyl-2-furanmethanamine (3ea). CAS: 4439-56-9; Pale yellow oil; Yield: 54%. 1H-NMR (300 MHz, CDCl3, 25 °C) δ 7.48–7.44 (m, 1H), 7.34–7.22 (m, 2H), 6.83 (td, J = 7.3, 1.0 Hz, 1H), 6.76 (dd, J = 7.7, 0.9 Hz, 2H), 6.40 (dd, J = 3.0, 1.7 Hz, 1H), 6.37–6.28 (m, 1H), 4.39 (s, 2H), 4.08 (s, 1H). 13C-NMR (75 MHz, CDCl3, 25 °C) δ 152.8, 148.7, 142.0, 129.3 (2C), 118.1, 113.3 (2C), 110.4, 107.0, 41.5. MS (m/z) 173 (48), 81 (100), 77 (12), 65 (6), 53 (7).
4. Conclusions
Owing to the key combination of onium salts and scCO2, a flexible, robust, solvent-free preparation of Pd NPs has been done. In contrast with many other methods, it provided a ready-to-use, stable, storable powder containing Pd NPs with unmodified surface leading to high catalytic activity. The N-alkylation of amines with benzylic alcohols was optimized in the absence of base or other additives classically used in this case, and generated only water as by-product in the presence of nanocatalysts. Recycling of Pd NP embedded in OS coated on SiC has yet to be improved to achieve a high conversion while ensuring a low leaching level. Supercitical millifluidics is currently under study to obtain a relevant continuous production system for benzylation of amines.
Acknowledgments
All authors acknowledge the financial support of the Aquitaine Region and the ANR-12-CDII-010-NANOCAUSYS.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/8/1042/s1.
Author Contributions
M.P. and C.A. conceived and designed the experiments; B.C. and O.P. performed the experiments; B.C. and O.P. analyzed the data; M.P. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Lawrence S.A. Amines: Synthesis, Properties and Applications. Cambridge University Press; Cambridge, UK: 2006. p. 384. [Google Scholar]
- 2.Seayad A., Ahmed M., Klein H., Jackstell R., Gross T., Beller M. Internal olefins to linear amines. Science. 2002;297:1676–1678. doi: 10.1126/science.1074801. [DOI] [PubMed] [Google Scholar]
- 3.Brown B.R. The Organic Chemistry of Aliphatic Nitrogen Compounds. Oxford University Press; Oxford, UK: 1994. p. 800. [Google Scholar]
- 4.Buchwald S.L., Mauger C., Mignani G., Scholz U. Industrial-scale palladium-catalyzed coupling of aryl halides and amines—A personal account. Adv. Synth. Catal. 2006;348:23–39. doi: 10.1002/adsc.200505158. [DOI] [Google Scholar]
- 5.Alonso F., Riente P., Yus M. Nickel nanoparticles in hydrogen transfer reactions. Acc. Chem. Res. 2011;44:379–391. doi: 10.1021/ar1001582. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Asencio A., Yus M., Ramon D.J. Palladium (II) acetate as catalyst for the N-alkylation of aromatic amines, sulfonamides, and related nitrogenated compounds with alcohols by a hydrogen autotransfer process. Synthesis. 2011;22:3730–3740. doi: 10.1002/chin.201210035. [DOI] [Google Scholar]
- 7.Bui T.K., Concilio C., Porzi G. A facile synthesis of symmetrical secondary amines from primary amines promoted by the homogeneous catalyst RuCl2 (Ph3P) 3. J. Organomet. Chem. 1981;208:249–251. [Google Scholar]
- 8.Pridmore S.J., Slatford P.A., Daniel A., Whittlesey M.K., Williams J.M.J. Ruthenium-catalyzed conversion of 1,4-alkynediols into pyrroles. Tetrahedron Lett. 2007;48:5115–5120. doi: 10.1016/j.tetlet.2007.05.070. [DOI] [Google Scholar]
- 9.Hamid M.H.S.A., Allen C.L., Lamb G.W., Maxwell A.C., Maytum H.C., Watson A.J.A., Williams J.M.J. Ruthenium-Catalyzed N-alkylation of Amines and Sulfonamides Using Borrowing Hydrogen Methodology. J. Am. Chem. Soc. 2009;131:1766–1774. doi: 10.1021/ja807323a. [DOI] [PubMed] [Google Scholar]
- 10.Baehn S., Imm S., Mevius K., Neubert L., Tillack A., Williams J.M.J., Beller M. Selective ruthenium-catalyzed N-alkylation of indoles by using alcohols. Chem. Eur. J. 2010;16:3590–3593. doi: 10.1002/chem.200903144. [DOI] [PubMed] [Google Scholar]
- 11.Imm S., Baehn S., Neubert L., Neumann H., Beller M. An Efficient and General Synthesis of Primary Amines by Ruthenium-Catalyzed Amination of Secondary Alcohols with Ammonia. Angew. Chem. Int. Ed. 2010;49:8126–8129. doi: 10.1002/anie.201002576. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y., Chen W., Feng C., Deng G. Ruthenium-Catalyzed One-Pot Aromatic Secondary Amine Formation from Nitroarenes and Alcohols. Chem. Asian J. 2011;6:1142–1146. doi: 10.1002/asia.201000945. [DOI] [PubMed] [Google Scholar]
- 13.Prades A., Corberan R., Poyatos M., Peris E. [IrCl2Cp*(NHC)] complexes as highly versatile efficient catalysts for the cross-coupling of alcohols and amines. Chem. Eur. J. 2008;14:11474–11479. doi: 10.1002/chem.200801580. [DOI] [PubMed] [Google Scholar]
- 14.Kawahara R., Fujita K.-I., Yamaguchi R. Multialkylation of Aqueous Ammonia with Alcohols Catalyzed by Water-Soluble Cp*Ir-Ammine Complexes. J. Am. Chem. Soc. 2010;132:15108–15111. doi: 10.1021/ja107274w. [DOI] [PubMed] [Google Scholar]
- 15.Saidi O., Blacker A.J., Farah M.M., Marsden S.P., Williams J.M.J. Iridium-catalysed amine alkylation with alcohols in water. Chem. Commun. 2010;46:1541–1543. doi: 10.1039/b923083a. [DOI] [PubMed] [Google Scholar]
- 16.Saidi O., Williams J.M.J. Iridium-catalyzed hydrogen transfer reactions. Top. Organomet. Chem. 2011;34:77–106. [Google Scholar]
- 17.Grigg R., Mitchell T.R.B., Sutthivaiyakit S., Tongpenyai N. Transition metal-catalyzed N-alkylation of amines by alcohols. J. Chem. Soc. Chem. Commun. 1981:611–612. doi: 10.1039/c39810000611. [DOI] [Google Scholar]
- 18.Feng S.L., Liu C.Z., Li Q., Yu X.C., Xu Q. Rhodium-catalyzed aerobic N-alkylation of sulfonamides with alcohols. Chin. Chem. Lett. 2011;22:1021–1024. doi: 10.1016/j.cclet.2011.03.014. [DOI] [Google Scholar]
- 19.Cui X., Shi F., Tse M.K., Goerdes D., Thurow K., Beller M., Deng Y. Copper-Catalyzed N-alkylation of Sulfonamides with Benzylic Alcohols: Catalysis and Mechanistic Studies. Adv. Synth. Catal. 2009;351:2949–2958. doi: 10.1002/adsc.200900490. [DOI] [Google Scholar]
- 20.Martinez-Asencio A., Ramon D.J., Yus M. N-alkylation of poor nucleophilic amine and sulfonamide derivatives with alcohols by a hydrogen autotransfer process catalyzed by copper (II) acetate. Tetrahedron Lett. 2010;51:325–327. doi: 10.1016/j.tetlet.2009.11.009. [DOI] [Google Scholar]
- 21.Martinez-Asencio A., Ramon D.J., Yus M. N-alkylation of poor nucleophilic amines and derivatives with alcohols by a hydrogen autotransfer process catalyzed by copper (II) acetate: Scope and mechanistic considerations. Tetrahedron. 2011;67:3140–3149. doi: 10.1016/j.tet.2011.02.075. [DOI] [Google Scholar]
- 22.Cui X., Shi F., Zhang Y., Deng Y. Fe (II)-catalyzed N-alkylation of sulfonamides with benzylic alcohols. Tetrahedron Lett. 2010;51:2048–2051. doi: 10.1016/j.tetlet.2010.02.056. [DOI] [Google Scholar]
- 23.Zhao Y., Foo S.W., Saito S. Iron/Amino Acid Catalyzed Direct N-alkylation of Amines with Alcohols. Angew. Chem. Int. Ed. 2011;50:3006–3009. doi: 10.1002/anie.201006660. [DOI] [PubMed] [Google Scholar]
- 24.De Luca L., Porcheddu A. Microwave-Assisted Synthesis of Polysubstituted Benzimidazoles by Heterogeneous Pd-Catalyzed Oxidative C-H Activation of Tertiary Amines. Eur. J. Org. Chem. 2011;2011:5791–5795. doi: 10.1002/ejoc.201101001. [DOI] [Google Scholar]
- 25.Zhang Y., Qi X., Cui X., Shi F., Deng Y. Palladium catalyzed N-alkylation of amines with alcohols. Tetrahedron Lett. 2011;52:1334–1338. doi: 10.1016/j.tetlet.2011.01.059. [DOI] [Google Scholar]
- 26.Zhang Y.-Q., Wei X.-W., Yu R. Fe3O4 Nanoparticles-Supported Palladium-Bipyridine Complex: Effective Catalyst for Suzuki Coupling Reaction. Catal. Lett. 2010;135:256–262. doi: 10.1007/s10562-010-0293-4. [DOI] [Google Scholar]
- 27.Anderson M., Afewerki S., Berglund P., Córdova A. Total Synthesis of Capsaicin Analogues from Lignin-Derived Compounds by Combined Heterogeneous Metal, Organocatalytic and Enzymatic Cascades in One Pot. Adv. Synth. Catal. 2014;356:2113–2118. doi: 10.1002/adsc.201301148. [DOI] [Google Scholar]
- 28.Deiana L., Jiang Y., Palo-Nieto C., Afewerki S., Incerti-Pradillos C.A., Verho O., Tai C.-W., Johnston E.V., Córdova A. Combined Heterogeneous Metal/Chiral Amine: Multiple Relay Catalysis for Versatile Eco-Friendly Synthesis. Angew. Chem. Int. Ed. 2014;53:3447–3451. doi: 10.1002/anie.201310216. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi K., Koike T., Kim J.W., Ogasawara Y., Mizuno N. Highly dispersed ruthenium hydroxide supported on titanium oxide effective for liquid-phase hydrogen-transfer reactions. Chem. Eur. J. 2008;14:11480–11487. doi: 10.1002/chem.200801655. [DOI] [PubMed] [Google Scholar]
- 30.Kim J. W., Yamaguchi K., Mizuno N. Heterogeneously catalyzed selective N-alkylation of aromatic and heteroaromatic amines with alcohols by a supported ruthenium hydroxide. J. Catal. 2009;263:205–208. doi: 10.1016/j.jcat.2009.01.020. [DOI] [Google Scholar]
- 31.Shi F., Tse M. K., Zhou S., Pohl M.-M., Radnik J., Huebner S., Jaehnisch K., Brueckner A., Beller M. Green and Efficient Synthesis of Sulfonamides Catalyzed by Nano-Ru/Fe3O4. J. Am. Chem. Soc. 2009;131:1775–1779. doi: 10.1021/ja807681v. [DOI] [PubMed] [Google Scholar]
- 32.Cano R., Ramon D.J., Yus M. Impregnated Ruthenium on Magnetite as a Recyclable Catalyst for the N-alkylation of Amines, Sulfonamides, Sulfinamides, and Nitroarenes Using Alcohols as Electrophiles by a Hydrogen Autotransfer Process. J. Org. Chem. 2011;76:5547–5557. doi: 10.1021/jo200559h. [DOI] [PubMed] [Google Scholar]
- 33.He W., Wang L., Sun C., Wu K., He S., Chen J., Wu P., Yu Z. Pt-Sn/γ-Al2O3-Catalyzed Highly Efficient Direct Synthesis of Secondary and Tertiary Amines and Imines. Chem. Eur. J. 2011;17:13308–13317. doi: 10.1002/chem.201101725. [DOI] [PubMed] [Google Scholar]
- 34.Wang L., He W., Wu K., He S., Sun C., Yu Z. Heterogeneous bimetallic Pt-Sn/γ-Al2O3 catalyzed direct synthesis of diamines from N-alkylation of amines with diols through a borrowing hydrogen strategy. Tetrahedron Lett. 2011;52:7103–7107. doi: 10.1016/j.tetlet.2011.10.100. [DOI] [Google Scholar]
- 35.He L., Lou X.-B., Ni J., Liu Y.-M., Cao Y., He H.-Y., Fan K.-N. Efficient and clean Gold-catalyzed one-pot selective N-alkylation of amines with alcohols. Chem. Eur. J. 2010;16:13965–13969. doi: 10.1002/chem.201001848. [DOI] [PubMed] [Google Scholar]
- 36.Peng Q., Zhang Y., Shi F., Deng Y. Fe2O3-supported nano-gold catalyzed one-pot synthesis of N-alkylated anilines from nitroarenes and alcohols. Chem. Commun. 2011;47:6476–6478. doi: 10.1039/c1cc11057h. [DOI] [PubMed] [Google Scholar]
- 37.Tang C.-H., He L., Liu Y.-M., Cao Y., He H.-Y., Fan K.-N. Direct One-Pot Reductive N-alkylation of Nitroarenes by using Alcohols with Supported Gold Catalysts. Chem. Eur. J. 2011;17:7172–7177. doi: 10.1002/chem.201100393. [DOI] [PubMed] [Google Scholar]
- 38.He L., Qian Y., Ding R.-S., Liu Y.-M., He H.-Y., Fan K.-N., Cao Y. Highly Efficient Heterogeneous Gold-catalyzed Direct Synthesis of Tertiary and Secondary Amines from Alcohols and Urea. ChemSusChem. 2012;5:621–624. doi: 10.1002/cssc.201100581. [DOI] [PubMed] [Google Scholar]
- 39.Ishida T., Takamura R., Takei T., Akita T., Haruta M. Support effects of metal oxides on gold-catalyzed one-pot N-alkylation of amine with alcohol. Appl. Catal. A: Gen. 2012;413–414:261–266. doi: 10.1016/j.apcata.2011.11.017. [DOI] [Google Scholar]
- 40.Zotova N., Roberts F.J., Kelsall G.H., Jessiman A.S., Hellgardt K., Hii K.K. Catalysis in flow: Au-catalyzed alkylation of amines by alcohols. Green Chem. 2012;14:226–232. doi: 10.1039/C1GC16118K. [DOI] [Google Scholar]
- 41.Shimizu K., Nishimura M., Satsuma A. γ-Alumina-Supported Silver Cluster for N-Benzylation of Anilines with Alcohols. ChemCatChem. 2009;1:497–503. doi: 10.1002/cctc.200900209. [DOI] [Google Scholar]
- 42.Cui X., Zhang Y., Shi F., Deng Y. Organic Ligand-Free Alkylation of Amines, Carboxamides, Sulfonamides, and Ketones by Using Alcohols Catalyzed by Heterogeneous Ag/Mo Oxides. Chem. Eur. J. 2011;17:1021–1028. doi: 10.1002/chem.201001915. [DOI] [PubMed] [Google Scholar]
- 43.Winans C.F., Adkins H. The alkylation of amines as catalyzed by nickel. J. Am. Chem. Soc. 1932;54:306–312. doi: 10.1021/ja01340a046. [DOI] [Google Scholar]
- 44.Imabeppu M., Kiyoga K., Okamura S., Shoho H., Kimura H. One-step amination of α,ω-alkylenediols over Cu/Ni-based catalysts. Catal. Commun. 2009;10:753–757. doi: 10.1016/j.catcom.2008.10.046. [DOI] [Google Scholar]
- 45.Cui X., Dai X., Deng Y., Shi F. Development of a general non-noble metal catalyst for the benign amination of alcohols with amines and ammonia. Chem. Eur. J. 2013;19:3665–3675. doi: 10.1002/chem.201203417. [DOI] [PubMed] [Google Scholar]
- 46.Yu X., Liu C., Jiang L., Xu Q. Manganese Dioxide Catalyzed N-alkylation of Sulfonamides and Amines with Alcohols under Air. Org. Lett. 2011;13:6184–6187. doi: 10.1021/ol202582c. [DOI] [PubMed] [Google Scholar]
- 47.Likhar P.R., Arundhathi R., Kantam M.L., Prathima P.S. Amination of Alcohols Catalyzed by Copper-Aluminum Hydrotalcite: A Green Synthesis of Amines. Eur. J. Org. Chem. 2009;2009:5383–5389. doi: 10.1002/ejoc.200900628. [DOI] [Google Scholar]
- 48.He J., Yamaguchi K., Mizuno N. Selective synthesis of secondary amines via N-alkylation of primary amines and ammonia with alcohols by supported copper hydroxide catalysts. Chem. Lett. 2010;39:1182–1183. doi: 10.1246/cl.2010.1182. [DOI] [Google Scholar]
- 49.Shimizu K.-I., Shimura K., Nishimura M., Satsuma A. Silver cluster-promoted heterogeneous copper catalyst for N-alkylation of amines with alcohols. RSC Adv. 2011;1:1310–1317. doi: 10.1039/c1ra00560j. [DOI] [Google Scholar]
- 50.Martinez R., Ramon D.J., Yus M. Selective N-monoalkylation of aromatic amines with benzylic alcohols by a hydrogen autotransfer process catalyzed by unmodified magnetite. Org. Biomol. Chem. 2009;7:2176–2181. doi: 10.1039/b901929d. [DOI] [PubMed] [Google Scholar]
- 51.Reddy B.V.S., Krishna A.S., Ganesh A.V., Kumar G.G.K.S.N. Nano Fe3O4 as magnetically recyclable catalyst for the synthesis of α-aminophosphonates in solvent-free conditions. Tetrahedron Lett. 2011;52:1359–1362. doi: 10.1016/j.tetlet.2011.01.074. [DOI] [Google Scholar]
- 52.Parvulescu V.I., Hardacre C. Catalysis in Ionic Liquids. Chem. Rev. 2007;107:2615–2665. doi: 10.1021/cr050948h. [DOI] [PubMed] [Google Scholar]
- 53.Scholten J.D., Leal B.C., Dupont J. Transition Metal Nanoparticle Catalysis in Ionic Liquids. ACS Catal. 2012;2:184–200. doi: 10.1021/cs200525e. [DOI] [Google Scholar]
- 54.Hassine F., Pucheault M., Vaultier M. Exceptional efficiency of palladium nanoparticle catalyzed Heck cross-coupling in binary task specific ionic liquids. Comptes Rendus Chim. 2011;14:671–679. doi: 10.1016/j.crci.2010.07.001. [DOI] [Google Scholar]
- 55.Richter K., Campbell P.S., Baecker T., Schimitzek A., Yaprak D., Mudring A.-V. Ionic liquids for the synthesis of metal nanoparticles. Phys. Status Solidi B. 2013;250:1152–1164. doi: 10.1002/pssb.201248547. [DOI] [Google Scholar]
- 56.Cansell F., Aymonier C. Design of functional nanostructured materials using supercritical fluids. J. Supercrit. Fluids. 2009;47:508–516. doi: 10.1016/j.supflu.2008.10.002. [DOI] [Google Scholar]
- 57.Zhang Y., Erkey C. Preparation of supported metallic nanoparticles using supercritical fluids: A review. J. Supercrit. Fluids. 2006;38:252–267. doi: 10.1016/j.supflu.2006.03.021. [DOI] [Google Scholar]
- 58.Pascu O., Liautard V., Vaultier M., Pucheault M., Aymonier C. Catalysed stereodivergent hydrosilylation with Onium Salts stabilised M(0) Nanocatalysts prepared in scCO2. RSC Adv. 2014;4:59953–59960. doi: 10.1039/C4RA09881A. [DOI] [Google Scholar]
- 59.Pascu O., Marciasini L., Marre S., Vaultier M., Pucheault M., Aymonier C. Continuous coflow synthesis of hybrid palladium nanocrystals as catalysts for borylation reaction. Nanoscale. 2013;5:12425–12431. doi: 10.1039/c3nr03858k. [DOI] [PubMed] [Google Scholar]
- 60.Pascu O., Moisan S., Marty J.-D., Aymonier C. Highly Reactive Pd NCs by Versatile Continuous Supercritical Fluids Synthesis for the Preparation of Metal-Nonmetal Pd-Based NCs. J. Phys. Chem. C. 2014;118:14017–14025. doi: 10.1021/jp503503p. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.