Abstract
Background: Chronic obstructive pulmonary disease (COPD) is characterized by irreversible airflow obstruction and inflammation. Natural products, such as monoterpenes, displayed anti-inflammatory and anti-oxidant activities and can be used as a source of new compounds to COPD treatment. Our aim was to evaluate, in an elastase-induced pulmonary emphysema in mice, the effects of and underlying mechanisms of three related natural monoterpenes (p-cymene, carvacrol and thymol) isolated from essential oil from leaves Lippia sidoides Cham. (Verbenaceae). Methods: Mices received porcine pancreatic elastase (PPE) and were treated with p-cymene, carvacrol, thymol or vehicle 30 min later and again on 7th, 14th and 28th days. Lung inflammatory profile and histological sections were evaluated. Results: In the elastase-instilled animals, the tested monoterpenes reduced alveolar enlargement, macrophages and the levels of IL-1β, IL-6, IL-8 and IL-17 in bronchoalveolar lavage fluid (BALF), and collagen fibers, MMP-9 and p-65-NF-κB-positive cells in lung parenchyma (p < 0.05). All treatments attenuated levels of 8-iso-PGF2α but only thymol was able to reduced exhaled nitric oxide (p < 0.05). Conclusion: Monoterpenes p-cymene, carvacrol and thymol reduced lung emphysema and inflammation in mice. No significant differences among the three monoterpenes treatments were found, suggesting that the presence of hydroxyl group in the molecular structure of thymol and carvacrol do not play a central role in the anti-inflammatory effects.
Keywords: COPD, elastase-induced emphysema, monoterpenes, p-cymene, carvacrol, thymol, lung inflammation
1. Introduction
Pulmonary emphysema is a main constituent of chronic obstructive pulmonary disease (COPD), which is associated to high morbidity and mortality in worldwide but commonly preventable and treatable. It is characterized by alveolar destruction and abnormal permanent enlargement of the alveoli which leads to severe airflow limitation [1]. Inflammatory cells such as neutrophils, macrophages and lymphocytes are activated and release several pro-inflammatory mediators such as interleukin IL1-β, IL-6 and IL-8 [2,3,4,5,6]. Recently, new interleukins had their role recognized which are involved in a wide variety of inflammatory response such as IL-15, IL-18, IL-21, IL-22, IL-23, IL-27, and IL-33 [7]. Commonly, IL-17, which has been become attention since it is produced in a particularly subset of T Helper cells called Th17, is involved in sustaining the inflammation amplifying the inflammatory responses [7]. Moreover, the cyclo-oxygenases that control the production of prostaglandins and thromboxanes [8] are also involved in the modulation of lung inflammation and a flavone chrysin suppressed inflammatory response by the interaction with COX-2 binding site [9]. In addition, the translocation of nuclear transcription factor NF-κB to the nucleus up-regulates the transcription of several cytokines and pro-inflammatory mediators [10,11,12]. The main cause of COPD is the inhalation of cigarette smoke [13] which causes an increase in oxidative stress that plays a central role in this disease because it is both generated due to increased reactive oxygen and nitrogen species induced by smoke as well as produced by activated inflammatory cells [13,14]. Isoprostanes are generated at the end of the membrane lipid peroxidation cascade and has been used as an indirect marker of oxidative stress [15,16,17,18]. Moreover, exhaled nitric oxide, which is mainly produced by inducible nitric oxide enzyme (iNOS) in inflammatory situations, could also have been used as an inflammatory and oxidative stress marker, particularly if it is associated to other parameters [19,20]. Other mechanism involved in emphysema is the imbalance between protease and antiprotease that is responsible for the lung destruction. Neutrophil elastase, metalloproteases and cathepsins are responsible for the degradation of the proteins in alveolar wall extracellular matrix, such as collagen and elastin fibers, thereby causing the lung destruction. Oxidative stress is also involved in the inhibition of the antiprotease activities. The destruction and repair process in response to the presence of chronic inflammation and oxidative stress culminate in a remodeling process which is characterized by an increase in collagen deposition and broken of elastic fibers compromising the mechanical properties of the lung [21,22].
Medicinal plants and their constituents have been widely used to treat several diseases since ancient times and many natural products have been related to effectively counteract lung inflammation in acute and chronic models of lung disease [23,24]. In this regard, drugs that present anti-inflammatory properties have been extensively studied since unwanted side effects are associated to the traditional treatments for inflammation [23,24]. p-Cymene, carvacrol and thymol are structurally related aromatic monoterpenes obtained from essential oils from different plant species [25]. Thymol and carvacrol are phenolic compounds belonging to a class of natural antioxidants due to the presence of one hydroxyl group linked to aromatic ring and the possibility of stabilizing free radicals formation [26], which is associated to preventing lipid peroxidation [27]. A related monoterpene derivative, p-cymene, displayed a similar structure of thymol and carvacrol, but the hydroxyl group is absent in the aromatic ring. Considering that these compounds displayed different biological activities, including antitumoral, antibacterial, antifungal, anti-inflammatory, and antioxidant activities [28,29,30], the aim of the present study was to investigate the effects and underlying mechanisms of natural monoterpenes p-cymene, carvacrol and thymol, isolated from essential oil from leaves Lippia sidoides (Verbenaceae) in an elastase-induced pulmonary emphysema model in mice.
2. Results
2.1. Chemical Analysis of Isolated Monoterpenes from Essential Oil from Leaves of L. sidoides
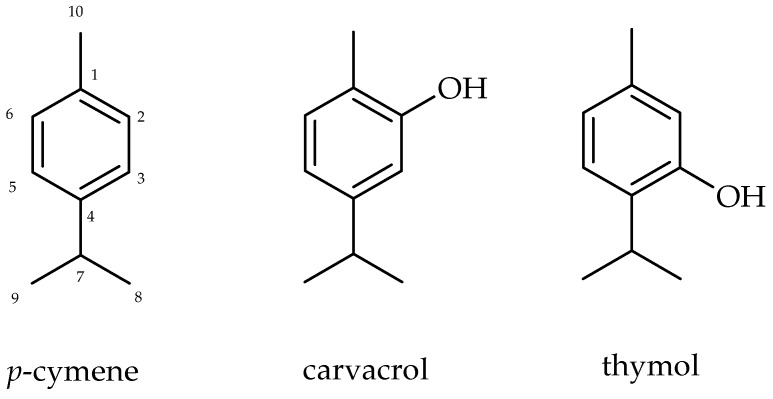
The 13C-NMR spectra of three isolated monoterpenes from essential oil from leaves of L. sidoides showed peaks of sp2 carbons at range δ 112–154 assigned to aromatic ring (C-1 to C-6) and methyl group linked to aromatic ring at δ 15–21 (C-10). Additionally, the presence of two equivalent methyl groups at δ 22–24 and one methyne carbon at δ 26–37 indicated the presence of one isopropyl group. The comparison of obtained spectral data with those reported in the literature [31], associated to LREIMS analysis [32], allowed the identification of the monoterpenes p-cymene, carvacrol and thymol, as shown in Figure 1.
Figure 1.
Structure of monoterpenes p-cymene, carvacrol and thymol.
2.2. Monoterpenes Reduced BALF Lung Inflammation in PPE-Induced Emphysema in Mice
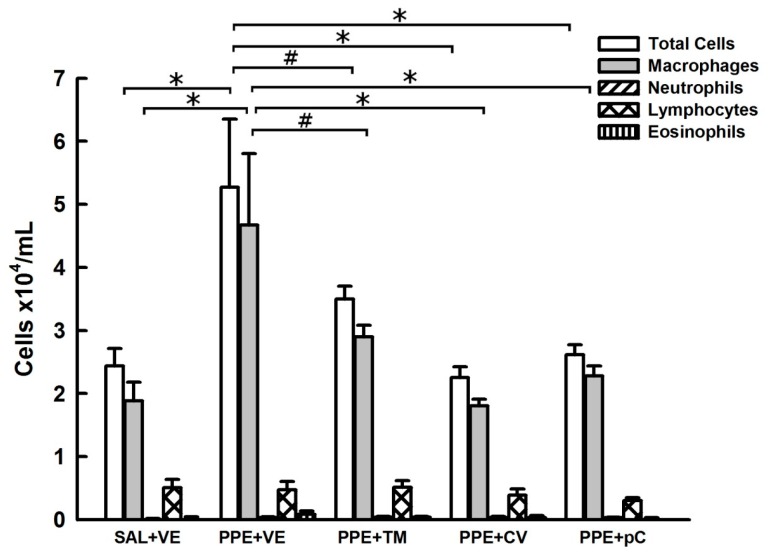
Figure 2 shows the number of total cells as well as the number of macrophages, neutrophils, lymphocytes, and eosinophils recovered in BALF. The PPE instillation increased inflammatory cells in BALF, particularly macrophages, compared to SAL + VE group (p < 0.01 for both comparisons). The treatment with monoterpenes p-cymene, carvacrol and thymol reduced the number of total cells and macrophages (PPE + CA and PPE + pC, p < 0.01 and PPE + TM, p < 0.05) in BALF compared to values obtained in PPE + VE group. No differences were detected in the number of neutrophils, lymphocytes and eosinophils among the experimental groups.
Figure 2.
Effects of monoterpenes p-cymene, carvacrol and thymol on lung inflammation. Data are presented as mean ± standard error (SE) (n = 7 to 8 mice/group) of number of cells recovered in bronchoalveolar lavage fluid collected at the 28th day of the experimental protocol. SAL + VE: control group with vehicle treatment; PPE + VE: porcine pancreatic elastase instillation and vehicle treatment; PPE + TM: porcine pancreatic elastase instillation and thymol treatment; PPE + CV: porcine pancreatic elastase instillation and carvacrol treatment; PPE + pC: porcine pancreatic elastase instillation and p-cymene treatment. * p < 0.01 and # p < 0.05.
2.3. Monoterpenes Reduced Cytokines in BALF in PPE-Induced Emphysema in Mice
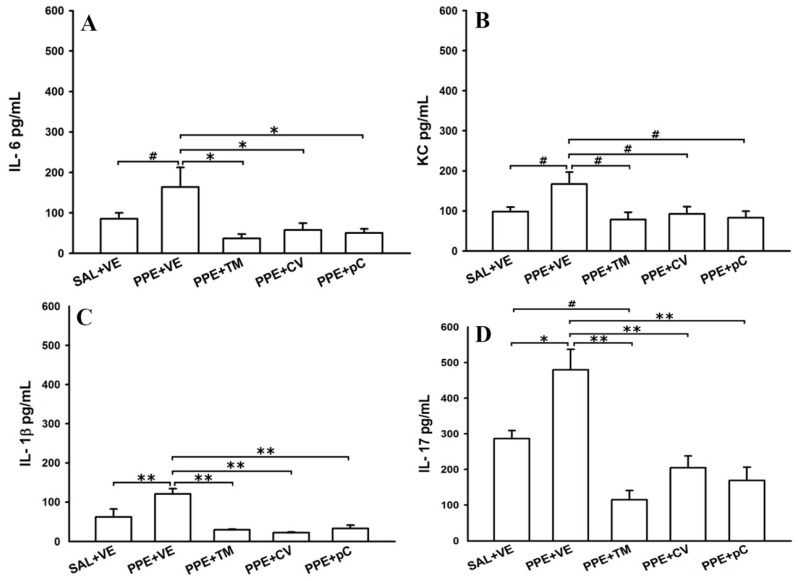
To investigate the role of tested monoterpenes in modulating the secretion of cytokines in BALF, the levels of IL-6 (Figure 3A); KC, which is homologous to IL-8 in humans (Figure 3B); IL-1β (Figure 3C); and IL-17 (Figure 3D) were measured using ELISA. The PPE + VE group showed higher levels of IL-6 (p < 0.05), KC (p < 0.05), IL-1β (p < 0.001) and IL-17 (p < 0.01) compared to SAL + VE group. Moreover, the treatment with monoterpenes p-cymene, carvacrol and thymol reduced the levels of these cytokines in a similar mode to those observed in SAL + VE group (IL-6: p < 0.01; KC: p < 0.05; IL-1β and IL-17: p < 0.001 for the three treatments). Notwithstanding, the levels of IL-17 in thymol treated animals were also reduced in comparison to control animals (p < 0.05).
Figure 3.
Effects of monoterpenes thymol, carvacrol and p-cymene in BALF cytokine levels. Data are presented as mean ± standard error (SE) (n = 6 to 8 mice/group) of levels of: IL-6 (A); KC (B); IL-1β (C); and IL-17 (D) detected in BALF of mice on the 28th day of the experimental protocol. SAL + VE: control group with vehicle treatment; PPE + VE: porcine pancreatic elastase instillation and vehicle treatment; PPE + TM: porcine pancreatic elastase instillation and thymol treatment; PPE + CV: porcine pancreatic elastase instillation and carvacrol treatment; PPE + pC: porcine pancreatic elastase instillation and p-cymene treatment. * p < 0.01, ** p < 0.001 and # p < 0.05.
2.4. Monoterpenes Prevented Alveolar Septa Destruction Reduced the Collagen Fibers Deposition Probably by Controlling MMP-9/TIMP-1 in PPE-Induced Emphysema Model
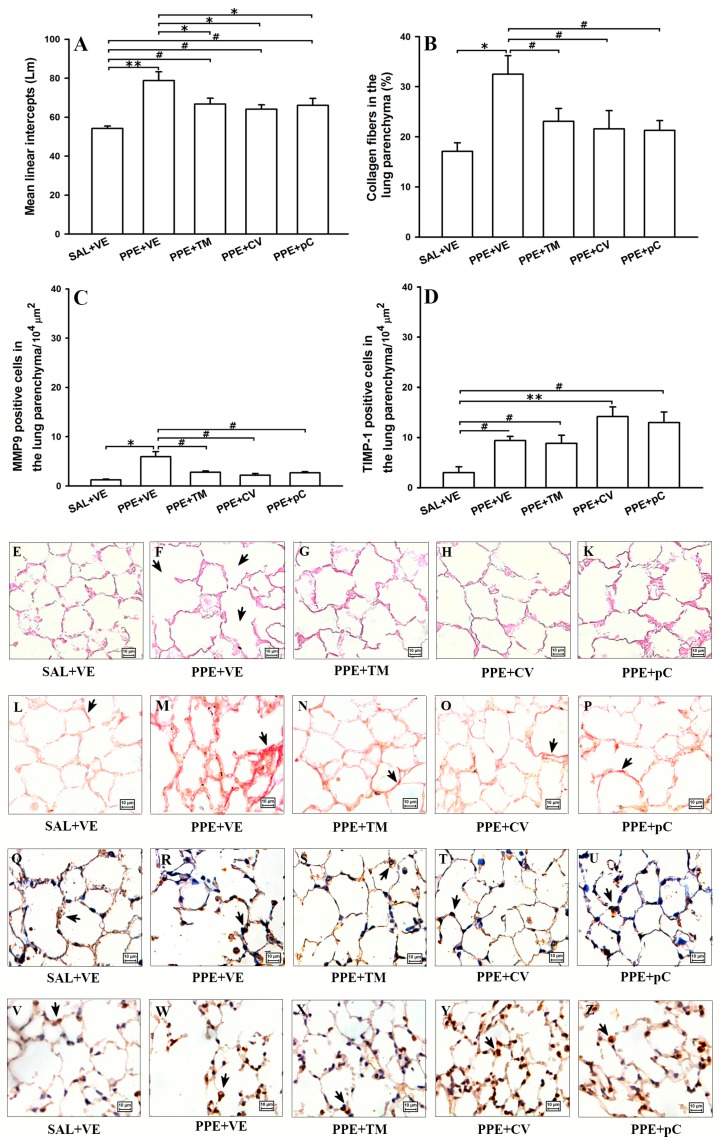
The evaluation of lung emphysema and collagen deposition as well as the MMP-9/TIMP-1 balance is shown in Figure 4. The mean linear intercept (Lm) was used as indicator of alveolar destruction in mice. Mean Linear Intercepts (Lm) increased in PPE + VE group compared to the animals that received saline and vehicle treatment (SAL + VE) (p < 0.001) (Figure 4A). The tested monoterpenes attenuated the emphysema, as shown by the lower values of LM observed in PPE + TM, PPE + CV and PPE + pC groups (p < 0.01) compared with ELA + VE group. However, the treated animals showed high values of LM when compared to SAL + VE group (p < 0.05).
Figure 4.
Effects of monoterpenes thymol, carvacrol and p-cymene on alveolar septa. Data are presented as mean ± standard error (SE) (n = 5 mice/group) of: mean linear intercepts (Lm) (A); volume proportion of collagen fibers (B); and the number of positive cells to MMP-9 (C); and TIMP-1 (D) in lung of mice in the 28th day of the protocol. The photomicrographs represent the lung tissue of one animal from control (E,L,Q,V), PPE treated with vehicle (F,M,R,W) and PPE treated with thymol (G,N,S,X), carvacrol (H,O,T,Y) or p-cymene group (K,P,U,Z) stained with HE (first line), picro-sirius to detect collagen fibers (second line), by immunohistochemistry to MMP-9 and TIMP-1 (third and fourth line respectively). Arrows indicate lung emphysema, collagen deposition and positive cells. SAL + VE: control group with vehicle treatment; PPE + VE: porcine pancreatic elastase instillation and vehicle treatment; PPE + TM: porcine pancreatic elastase instillation and thymol treatment; PPE + CV: porcine pancreatic elastase instillation and carvacrol treatment; PPE + pC: porcine pancreatic elastase instillation and p-cymene treatment. * p < 0.01, ** p < 0.001 and # p < 0.05.
Due to degradation of the fibers to be usually followed by a turnover of collagen, collagen content (Figure 4B) in alveolar septa of mice was measured. The obtained results indicated an increase of proportion of collagen fibers in alveolar septa of ELA + VE animals compared with the SAL + VE group (p < 0.01) and the treatments reduced this response (p < 0.05). Since balance between MMPs and TIMPs is involved in the control of the degradation and turnover of the fibers, was quantified the expression of MMP-9 (Figure 4C) and TIMP-1 (Figure 4D) in cells presented in alveolar septa. The obtained results showed an increase of MMP-9 (p < 0.01) and TIMP-1 (p < 0.05) positive cells in alveolar septa from ELA + VE group compared with the control group (SAL + VE). Interestingly, the positive cells to MMP-9 were reduced in lung of the animals that received elastase and were treated with p-cymene, carvacrol and thymol (p < 0.05) compared with ELA + VE group. However, the number of positive cells to TIMP-1 in alveolar septa of animals that received elastase and treated with tested monoterpenes was similar to those observed in ELA + VE group.
The photomicrographs represent the lung tissue of one animal from control, PPE treated with vehicle and PPE treated with thymol, carvacrol or p-cymene groups stained with HE (E to K), with picro-sirius to collagen detection (L to P), as well as immunostained to detect MMP-9 (Q to U) and TIMP-1 (V to Z) positive cells. The PPE group showed large alveolar spaces with intense collagen deposition and increased positive cells to MMP-9 and TIMP-1 (F, M, R and W) compared to control and to treated groups.
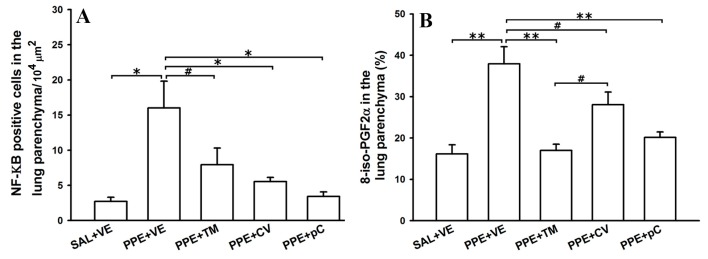
2.5. The Monoterpenes Probably Acts in Reduction of Emphysema and Lung Inflammation in Mice by Reducing Oxidative Stress and in NF-κB Expression in Lung
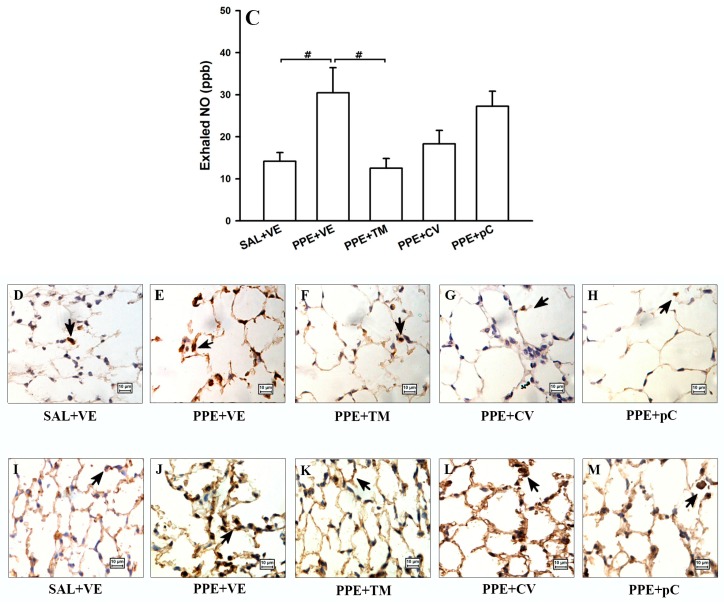
In order to understand some possible mechanisms involved in the effects of monoterpenes p-cymene, carvacrol and thymol in lung emphysema, the expression of the subunit p-65-NF-κB in lung parenchyma by immunohistochemistry was evaluated (Figure 5). This subunit is necessary to activate the NF-κB gene [10], which indirect reflects the activation of this pathway. The obtained results indicated that PPE induced an increase in the number of NF-κB positive cells compared with SAL + VE group (p < 0.01) which was attenuated by monoterpene treatment (p < 0.01 for carvacrol and p-cymene and p < 0.05 for thymol) (Figure 5A). Other possible mechanism involved in the action of tested monoterpenes is associated to reduction of oxidative stress, since these compounds are anti-oxidants. In this context, was evaluated the volume proportion of isoprostane expression (8-iso-PGF2α) in lung tissue. In this aspect, was observed an increase in the expression of isoprostane in PPE + VE animals compared with SAL + VE group (p < 0.001) (Figure 5B) which is also attenuated by monoterpenes treatments (thymol/p-cymene p < 0.01 and carvacrol p < 0.05). Carvacrol-treated animals also presented high levels of isoprostane compared to thymol (p < 0.05). The detected nitric oxide in exhaled air (ENO) is a useful marker of both pulmonary inflammation and oxidative stress. Animals that received PPE instillation and treated with vehicle (PPE + VE) showed high levels of ENO compared with SAL + VE group (P < 0.05) (Figure 5C). In addition, only thymol treated animals showed lower valued of ENO compared to ELA + VE (p < 0.05).
Figure 5.
Effects of monoterpenes thymol, carvacrol and p-cymene in the NF-κB expression in alveolar septa and oxidative stress pathway. Data are presented as mean ± standard error (SE) (n = 5 to 7 mice/group) of the number of positive cells to NF-κB (A) detected in alveolar septa, the volume proportion of 8-iso-PGF-2α expression (B) detected in alveolar septa and exhaled nitric oxide detected in the exhaled air (C) from mice in the day 28 of the protocol. Panels D to H represent tissue stained to detect NF-κB and panels I to M to detect isoprostane by immunohistochemistry. Arrows represent positive cells in lung tissue. SAL + VE: control group with vehicle treatment; PPE + VE: porcine pancreatic elastase instillation and vehicle treatment; PPE + TM: porcine pancreatic elastase instillation and thymol treatment; PPE + CV: porcine pancreatic elastase instillation and carvacrol treatment; PPE + pC: porcine pancreatic elastase instillation and p-cymene treatment. * p < 0.01, ** p < 0.001 and # p < 0.05.
The photomicrographs represent the lung tissue of one control animal, PPE treated with vehicle and PPE treated with thymol, carvacrol or p-cymene group stained with immunohistochemistry to detect positive cells to NF-κB (D to H) and 8-isoprostane (I to M).
3. Discussion
PPE instillation in mice is a useful model to reproduce quickly several features of human emphysema [33,34] and PPE instillation increased macrophages as well as IL-6, KC, IL-1β and IL-17 levels in BALF. PPE also induced lung destruction, collagen deposition and an imbalance between MMP-9 and TIMP-1 expression. These changes were associated to high levels of nitric oxide detected in exhaled air and increase the isoprostane expression in lung tissue. Finally, the number of positive cells to NF-κB in lung was increased in PPE-instilled animals. Most of these features observed in this model have been previously reported in the literature [35,36] and some of these alterations have been already present 60 min after the PPE instillation [37].
In the world, 65 million people have moderate to severe COPD [38] and although there is a range of available drugs, the efficacy of many existing anti-inflammatory drugs have been disappointing in COPD patients. In this context, compounds derived from plants must be investigated as an additional potential therapeutic approach, particularly those which present anti-oxidant effects, since oxidative stress is one of the primary mechanisms involved in the development of COPD [39].
The present study shows that the monoterpenes p-cymene, carvacrol and thymol, extracted from essential oil from leaves of L. sidoides, reduced the inflammatory changes and the alveolar enlargement observed in a murine emphysema model. These changes could be associated, at least in part, with reduced NF-κB expression and cytokine release as well as the control of oxidative stress. Moreover, these treatments also attenuated the MMP-9 expression in cells present in alveolar septa.
Increased number of inflammatory cells, such as neutrophil and macrophage, are presented in the lung and in BALF from COPD patients [2]. In animals models, the activation and increase in the number of neutrophils are observed particularly in the early phase. Other authors have found increased neutrophils only four days after elastase instillation, whereas 21 or 28, the mainly was characterized by the presence of macrophages in BALF [40,41]. The treatment with monoterpenes was able to reduce the number of macrophage recovered in the BALF in the present study.
Macrophage is an important cell involved in emphysema physiopathology and is able to release several inflammatory mediators involved in the destruction of the alveolar parenchyma [42,43,44]. Tumor necrose factor (TNF) –α, interferon-γ and various classes of interleukins as the IL-1β, IL-6, IL-8, IL-10, IL-17, IL-18, IL-32 and others are involved in the progression of emphysema [4,45]. The monoterpenes reduced both levels of IL-6, and KC which is homologous with IL-8 in humans [46]. Increase levels of IL-6 and IL-8 were detected during COPD exacerbation in serum and in induces sputum and in BALF [47,48].
The IL-17, and its receptor IL17R, was first identified in CD4 + T cells. However, the IL-17, a pro-inflammatory cytokine, has become of a great interest because it is produced by a specifically cell subtype called Th17. The IL-17 pathway is involved in the host defense and plays a critical role in both acute and chronic inflammatory diseases. IL-17 is involved in either benefic or malefic effects for health protective because it can exacerbate immune responses and is produced highly by patients with chronic inflammatory diseases [49,50]. Moreover, some authors demonstrated that IL-17 is involved mainly in sustaining inflammatory responses rather than to initiate an inflammatory reaction [51]. In lung, the blockade of IL-17R reduced airway inflammation and hyperreactivity in an asthma model [52].
The expression of IL-17 has previously been shown in COPD patients and it seems that IL-17A induces the secretion of neutrophils-recruiting chemokines such as the expression of IL-6 in bronchial epithelial cells and fibroblasts [6,40,53] as well as increase IL-8 production [54]. More importantly, the levels of IL-17 were inversely correlated with lung function [55] in patients with COPD. Monoterpenes p-cymene, carvacrol and thymol reduced IL-17 levels in BALF. Kurimoto et al. [40] have shown that IL-17 is necessary to elastase-induce emphysema in experimental models. Interestingly, we also found a reduction in the levels of IL-1β induced by monoterpenes treatment. The IL1-β can be released by monocytes, macrophages and fibroblast and is increased in the peripheral blood, in sputum and in BALF from COPD patients [4]. In an experimental model, Lappalainen et al. [3] demonstrated that genetically modified mice, which expressed human IL-1β, developed an intense inflammatory process characterized by increased neutrophils and macrophages as well as emphysema.
Other authors showed the effects of other monoterpenes in the reduction of inflammation in experimental lung diseases [56,57,58,59,60]. In this regard, Boskabady and Gholami [57] showed that Zataria multiflora and it main constituent carvacrol reduced the inflammatory cells recovered from BALF and the levels of IL-8 in an emphysema model. Zhou et al. [60] using an asthma model showed that thymol isolated from L. sidoides reduced inflammatory cells in airways and the levels of Th2 cytokines in BALF. Sun et al. [58] observed that the monoterpene paeoniflorin reduced inflammation and cytokines after OVA exposure. Recently, Zhao et al. [59] showed that 1,8-cineol reduced TNF-α and IL-1β levels in BALF in an acute lung injury model. Finally, Xie et al. [56] showed that p-cymene reduced inflammatory cells and cytokines in ALI model. However, no data were found comparing the biological effects of structural related monoterpenes in emphysema.
The imbalance between proteases and anti-proteases can contribute to the lung destruction in emphysema. The obtained results showed that monoterpenes thymol, carvacrol and p-cymene attenuate alveolar destruction in this model. Moreover, these treatments reduced collagen deposition in alveolar septa, suggesting that the reduction in lung destruction and inflammation could avoid the fibers remodeling. It is well known that MMP-9 is increased in blood, sputum and BALF from COPD patients [61,62]. Additionally, was found a reduction in positive cells to MMP-9 induced by monoterpenes treatment in PPE animals. The activity of MMP-9 is controlled by TIMP-1 [63] and although in this study the monoterpenes reduced MMP-9, this treatment did not affect TIMP-1 positive cells. These findings suggest that monoterpenes contribute to the balance between MMP-9 and TIMP-1 since it reduced the MMP-9 but maintained the levels of TIMP-1 in lung, avoiding the tissue destruction and consequently remodeling. Although other authors have shown the remodeling process in PPE models, few studies have evaluated the effects of monoterpenes in this process.
The NF-κB is a signaling pathway that positive regulates pro-inflammatory cytokines in lung diseases [64,65]. Monoterpenes thymol, carvacrol and p-cymene reduced the positive cells to NF-κB in lung and it justified the effects of these compounds in reducing inflammation. In COPD, the NF-κB activation could occur in response to increase levels of IL-1β [66] and the reduction of IL-1β observed by monoterpenes treatment could justified the effects in NF-κB.
The NF-κB pathway is regulated by redox system and could be influenced by an unbalance between oxidant and anti-oxidants enzymes [67]. The isoprostane 8-PGF-2α is a product formed in the lipid peroxidation and could be used as an indirect marker of oxidative stress, being elevated in COPD patients [68,69] and in emphysema models [18,70,71]. Interestingly, only thymol reduced the exhaled nitric oxide, which has been used as a marker to both detect the levels of lung inflammation and oxidative stress [72]. A possible explanation could be related to the contribution from constitutive isoforms in ENO, which could be modified for one compound or the other. However these finding should be detailed investigated in the future.
The monoterpenes p-cymene, carvacrol and thymol are bioactive molecules with anti-inflammatory potential [73], which could be associated to anti-oxidant activity. In this case, thymol and carvacrol displayed higher potential in comparison to p-cymene since these are phenolic compounds and showed one hydroxyl group bearing to benzene ring, which could be associated to the capacity of established free radicals preventing the lipid peroxidation [26,27]. Interestingly in this study was observed an isoprostane reduction in lung tissue after treatment with these three related compounds suggesting that the structural differences (presence of absence of hydroxyl group in the molecular structure) do not interfere significantly in biological effects in this model. This could be partially explained by metabolization of p-cymene in vivo affording active carvacrol and other oxygenated derivatives [74]. Previous studies have reports the effects of carvacrol in a model of COPD and showed that the increase in thiol group in COPD animals is the main mechanism involved in the benefic effects of carvacrol [57].
It is also important to consider that the effects of thymol and carvacrol could occur in the elastase activity, an enzyme that displayed an important role in the development of emphysema [75,76,77]. Even considering that the effects of these compounds are in the elastase activity, the clinical interest is relevant since the direct action of a drug in the activity of elastase could be benefic to COPD patients since this enzyme is involved in the progression and development of the disease.
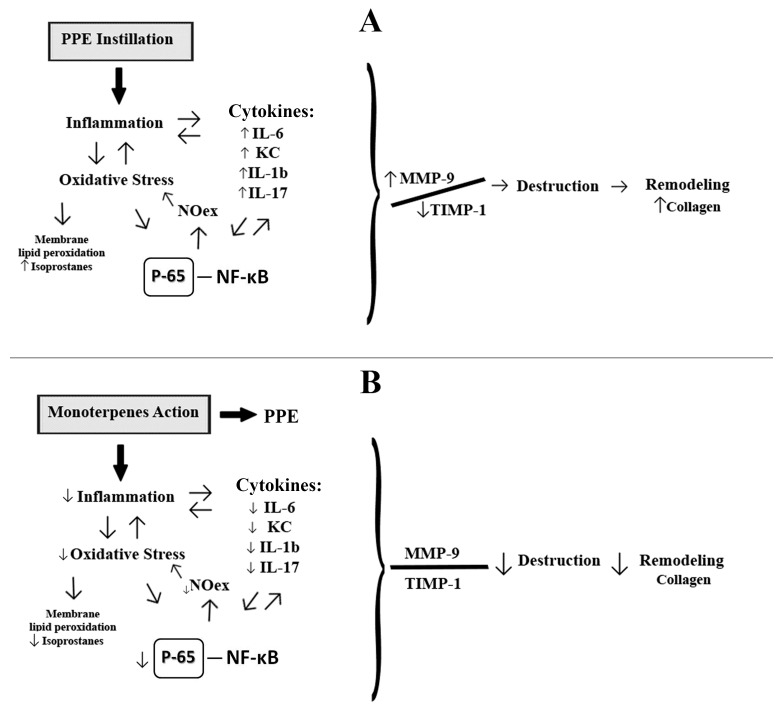
In conclusion, the results of the present study indicate a preventive effect of monoterpenes on lung emphysema by controlling the inflammation, avoiding the lung remodeling through NF-κB and isoprostane reduction (Figure 6). These findings could be of great clinical interest suggesting a potential role of monoterpenes on lung inflammation and oxidative stress in COPD.
Figure 6.
Monoterpenes probably mechanism is related to reduction in inflammation, oxidative stress and in NF-κB in lung of PPE-instilated animals. (A) The PPE instillation induces inflammation and increase marker of oxidative stress. Cytokines such as IL-6, KC, IL-1β and IL-17 are released and induce the perpetuation of inflammation. Together with oxidative stress, there is activation in NF-κB pathway, which in turn up-regulated transcription of proinflammatory cytokines and iNOS, that can induce an increase in exhaled nitric oxide. Augment in MMP-9 positive cells can amplify inflammation and contribute to the destruction of lung tissue culminating with collagen fibers deposition; (B) The administration of monoterpenes led to a reduction of the inflammatory process and consequent reduction in oxidative stress, which is related to the effects of monoterpenes in cytokines reduction (IL-6, KC, IL-1b and IL-17), NF-κB and isoprostane. These anti-inflammatory and antioxidant effects contributed to reduction in tissue destruction.
4. Materials and Methods
4.1. General Experimental Procedures
13C-NMR spectra were recorded 75 MHz in an Ultrashield 300 Advance III spectrometer (Bruker, Fremont, CA, USA) using CDCl3 (Tedia Brazil, Rio de Janeiro, Brazil) as solvent and TMS (Aldrich, St. Louis, MO, USA) as internal standard. Silica gel for flash chromatography (Merck, Kenilworth, NJ, USA, 230–400 mesh) and silica gel 60 F254 (Merck, Kenilworth, NJ, USA) were used for column chromatography and for analytical (0.25 mm) TLC, respectively. GC-FID chromatograms were obtained on a GC-2010 gas chromatograph (Shimadzu, Kyoto, Japan) equipped with an flame ionization detector (FID) and one automatic injector AOC-20i (Shimadzu, Kyoto, Japan) using RtX-5 capillary column (5% phenyl, 95% polydimethylsiloxane (Restek, Bellefonte, PA, USA, 30 m × 0.32 mm × 0.25 μm film thickness). These analyses were performed by injecting 1.0 μL of a 1.0 mg/mL solution of sample material in pentane in a split mode (1:10) employing helium as the carrier gas (1 mL/min) under the following conditions: injector and detector temperatures of 250 °C and 280 °C, respectively; oven programmed temperature from 100–260 °C at 5 °C/min, holding 15 min at 260 °C. LREIMS analysis was carried out using a MS-QP-5050A mass spectrometer (Shimadzu, Kyoto, Japan), operating under an ionization voltage of 70 eV and an ion source temperature of 230 °C.
4.2. Chemical and Reagents
All solvents used were of analytical grade and purchased from CAAL (São Paulo, Brazil) while elastase was obtained from Sigma (St. Louis, MO, USA, 6.6 units/mg, E1250, type I). ELISA and antibodies were purchased from R&D Systems (Minneapolis, MN, USA).
4.3. Plant Material
L. sidoides was collected in the Atlantic Forest Biome in São Paulo State, Brazil, in March, 2014. The studied species was identified by Prof. Euder G.A. Martins and its identification of was possible comparing the voucher specimens with those deposited in the Herbarium of the Universidade de São Paulo—SP.
4.4. Essential Oil Extraction
Fresh leaves (200 g) were subjected to hydrodistillation in a Clevenger type apparatus for 4 h. After extraction using CH2Cl2 the essential oil was dried using anhydrous Na2SO4, filtered and the solvent was evaporated under reduced pressure to afford 644 mg of crude essential oil (yield 0.32%).
4.5. Isolation of Monoterpenes
Part of the crude essential oil of L. sidoides (400 mg) was subjected to separation over flash silica gel column chromatography eluted with CH2Cl2 and CH2Cl2:MeOH (99:1, 98:2 and 95:5). Using this procedure, were obtained 45 fractions (5 mL each) which were analyzed by GC-FID and then pooled into eight groups (A–H). Group B (12 mg) was composed by pure p-cymene (98.5%) whereas groups D (16 mg) and G (66 mg) were composed by carvacrol (purity 99.0%) and thymol (purity 99.6%), respectively.
4.5.1. p-Cymene
Colorless oil. 13C-NMR (75 MHz, CDCl3) δC: 135.1 (C-1), 126.3 (C-2/C-6), 129.0 (C-3/C-5), 33.8 (C-7), 24.1 (C-8/C-9), 20.9 (C-10). LREIMS m/z (rel. int.): 134 (25), 119 (100), 103, 91 (28), 77, 65 (11).
4.5.2. Carvacrol
Amorphous solid. 13C-NMR (75 MHz, CDCl3) δC: 130.8 (C-1), 153.6 (C-2), 121.0 (C-3), 148.4 (C-4), 118.7 (C-5), 112.9 (C-6), 33.6 (C-7), 23.9 (C-8/C-9), 15.3 (C-10). LREIMS m/z (rel. int.): 150 (28), 135 (100), 115, 107 (16), 91 (20), 77 (14).
4.5.3. Thymol
Amorphous solid. 13C-NMR (75 MHz, CDCl3) δC: 131.3 (C-1), 126.2 (C-2), 152.5 (C-3), 136.5 (C-4), 121.6 (C-5), 116.0 (C-6), 26.6 (C-7), 22.6 (C-8/C-9), 20.8 (C-10). LREIMS m/z (rel. int.): 150 (27), 135 (100), 115 (18), 107 (15), 91 (24), 77 (12).
4.6. Animals
Male C57BL/6 (6–8 weeks) mice received humane care according to the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources-1996, Washington, DC, USA). All surgical procedures were performed while the animals were under general anesthesia. The male mice used in the present study were maintained in a temperature-controlled room at 21–23 °C with a 12-h light/dark cycle and ad libitum access to water and food. This study was approved by the Ethics Committees of Federal University of São Paulo (São Paulo, Brazil, protocol number 715701/15) and of University of São Paulo (protocol number 029/15).
4.7. Experimental Design
Forty male C57BL6J mice were aleatory distributed among five experimental groups: A, SAL + VE group: animals that received nasal instillation of saline + vehicle treatment (50% DMSO + 50% Saline); B, PPE + VE group: animals that received nasal instillation of porcine pancreatic elastase (PPE) + vehicle (50% DMSO + 50% Saline) treatment; C, PPE + TM group: animals that received nasal instillation of elastase + thymol treatment; D, PPE + CV group: animals that received nasal instillation of elastase plus carvacrol treatment; and E, PPE + pC group: animals that received nasal instillation of elastase and p-cymene treatment. DMSO was used to dissolve the tested monoterpenes.
4.8. PPE-Induced Emphysema in Mice
Mice were anesthetized with xylazine (40 mg/kg) and ketamin (5 mg/kg) and intranasal instilled with 50 μL of PPE (6.6 units/mg, E1250, type I, Sigma-Aldrich) [78] with a concentration of de 0.677 UI (day zero). Control group received an intranasal instillation of the same volume of saline. All animals were evaluated 28 days after the intranasal instillation.
4.9. Monoterpenes Treatment
Ten microliters of p-cymene, carvacrol, thymol or vehicle was administered by intranasal instillation 30 min after PPE or saline instillation, and then repeated on 7th, 14th, 21st, and 28th days of the protocol [18]. The dose of p-cymene, carvacrol, thymol used were 20 mg/kg chosen based in previous studies of our lab in acute lung injury models (unpublished results) diluted in DMSO and saline in 1:1 proportion. Previous studies determined that this vehicle was not toxic for animals [18,79].
4.10. Exhaled Nitric Oxide and BALF Collection
Animals were anesthetized with injection of thiopental (70 mg/kg), tracheostomized and connected to a ventilator for small animals (Harvard 683; Harvard Apparatus, South Natick, MA, USA) to collect the exhaled nitric oxide (ENO). The concentrations of ENO were measured by chemiluminescence using a fast-responding analyzer (NOA 280; Sievers Instruments, Boulder, CO, USA), as previously described [19]. We measured ENO levels at the expiratory port of the ventilator using a Mylar bag during 10 min. A NO filter was attached to the breathing circuit in order to avoid environment contamination. After NO collection, animals were exsanguinated, and the BALF was collected by washing the lungs with 0.5 mL of sterile saline and withdrawing the fluid (3 times). For total and differential cell counting, the BALF was centrifuged (Model GS-6R Centrifuge, Beckman Instruments, Fullerton, CA, USA) at 800 g for 8 min at 5 °C, and the cell pellet was resuspended in 0.2 mL of sterile saline. The total viable cell count was determined using a Neubauer haemocytometer chamber (Carl Roth, Karlsruhe, Germany), and differential cells (at least 300 cells) were counted using an optical microscope (Model BX40 Olympus Optical Co, Tokyo, Japan) after the cytocentrifuge preparations of the BALF (450 rpm for 6 min) (model: Cytospin 3, Shandon Instruments, Sewickley, PA, USA) and Diff-Quick staining (Biochemical Sciences Inc., Swedesboro, NJ, USA) [18].
The protein concentrations of IL-6, IL-8, IL-1β and IL-17 in the BALF were assayed by ELISA according to the manufacturer’s protocol for a Duo-Set kit from R&D Research and Development (San Diego, CA, USA) [18] and were expressed in pg of cytokine/mL BALF.
4.11. Lung Histopathology and Immunohistochemistry
Lungs were removed in bloc, the right lung was fixed, and histological sections (3 µm thickness) were stained with hematoxylin and eosin (H&E) to evaluate the mean linear intercepts, an index that is widely used to characterize the presence of emphysema [21] or picro-sirius (Direct Red 80, CI 35780; Sigma-Aldrich) to identify collagen fibers [18]. With a 50-line, 100-point grid connected to the ocular of the microscope (Model BX40 Olympus Co.), was assessed the Mean Linear Intercepts (Lm) and the volume proportion of collagen fibers in the alveolar tissue using a point-counting technique. The mean linear intercept (Lm) was determined at a magnification of 200× in 20 non-overlapping fields of the distal lung parenchyma per animal, and the number of times that the lines of the integrating eyepiece intersected with the alveolar septum was counted. The volume proportion of collagen was determined in 10 lung fields of each animal by dividing the number of points corresponding to collagen by the total number of points corresponding to alveolar septa (400×).
Immunohistochemistry was performed using anti-MMP-9 (goat polyclonal IgG diluted to 1:500), anti-TIMP-1 (rabbit polyclonal IgG diluted to 1:100), anti-NF-κB p65 (rabbit polyclonal igG diluted to 1:300) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and the biotin–streptavidin–peroxidase method. We used the Vectastain ABC Kit (Vector Elite PK-6105, Burlingame, CA, USA) and DAB (Sigma-Aldrich) to reveal the staining. We determined the number of cells that were positive for MMP-9, TIMP-1 and NF-κB using the point-counting technique as described above. The number of points corresponding to alveolar septa and the number of positive cells within the alveolar septa were counted, and the results were expressed as cells per unit area (104 µm2) [18].
4.12. Statistical Analyses
The results were statistically analyzed using the SigmaStat (Jandel Scientific, San Rafael, CA, USA) and analysis of variance ANOVA factor was used. The data were obtained and therefore parametric test was used for post hoc Student-Newman-Keuls test. A p < 0.05 was considered statistically significant.
Acknowledgments
The authors would like to thank the CNPq (300546/2012-2; 304465/2012-7; 476877/2012-1, 306278/2015-4), the FAPESP (2011/51739-0/ 2013/02881-4, 2008/55359-5, 2015/11936-2 and 2014/25689-4) and the Laboratório de Investigação Médica–LIM20, Hospital das Clínicas, Faculdade de Medicina, University of São Paulo for providing financial support and fellowships.
Author Contributions
E.G., F.D.T.Q.S.L., C.R.O., I.F.L.C.T., M.A.M., J.H.G.L. and C.M.P. substantially contributed to the conception and design of the work. J.H.G.L. and E.A.O. isolated and characterized the monoterpenes. E.G., M.G., F.R.S., N.M.P., E.A.O., F.D.T.Q.S.L., C.R.O., J.H.G.L. and C.M.P. contributed to data acquisition and performed experiments. E.G., M.G., F.R.S., N.M.P., E.A.O., F.D.T.Q.S.L., C.R.O., I.F.L.C.T., M.A.M., J.H.G.L. and C.M.P. analysed and interpreted the data. EG, J.H.G.L. and C.M.P. drafted the paper. E.G., M.G., F.R.S., N.M.P., E.A.O., F.D.T.Q.S.L., C.R.O., I.F.L.C.T., M.A.M., J.H.G.L. and C.M.P. revised the manuscript critically for important intellectual content and final approval of the version to be published.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.GOLD Global strategies for diagnosis, management and prevention of chronic obstructive pulmonary, NHLBI/WHO Workshop report. Am. J. Respir. Crit. Care. Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 2.Silva O.R., Montes J.F., García-Valero J., Olloquequi J. Cellular effectors of the inflammatory response in chronic obstructive pulmonary disease (COPD) Rev. Med. Chil. 2015;143:1162–1171. doi: 10.4067/S0034-98872015000900009. [DOI] [PubMed] [Google Scholar]
- 3.Lappalainen U., Whitsett J.A., Wert S.E., Tichelaar J.W., Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am. J. Respir. Cell. Mol. Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 4.Caramori G., Adcock I.M., Di Stefano A., Chung K.F. Cytokine inhibition in the treatment of COPD. Int. J. Chron. Obstruct. Pulm. Dis. 2014;9:397–412. doi: 10.2147/COPD.S42544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Stefano A., Caramori G., Gnemmi I., Contoli M., Vicari C., Capelli A., Magno F., D’Anna S.E., Zanini A., Brun P., et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin. Exp. Immunol. 2009;157:316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozinovski S., Seow H.J., Chan S.P., Anthony D., McQualter J., Hansen M., Jenkins B.J., Anderson G.P., Vlahos R. Innate cellular sources of interleukin-17A regulate macrophage accumulation in cigarette- smoke-induced lung inflammation in mice. Clin. Sci. (Lond.) 2015;129:785–796. doi: 10.1042/CS20140703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Acquisto F., Maione F., Pederzoli-Ribeil M. From IL-15 to IL-33: The never-ending list of new players in inflammation. Is it time to forget the humble aspirin and move ahead? Biochem. Pharmacol. 2010;79:525–534. doi: 10.1016/j.bcp.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Rainsford K.D. Anti-inflammatory drugs in the 21st century. Subcell. Biochem. 2007;42:3–27. doi: 10.1007/1-4020-5688-5_1. [DOI] [PubMed] [Google Scholar]
- 9.Rauf A., Khan R., Raza M., Khan H., Pervez S., de Feo V., Maione F., Mascolo N. Suppression of inflammatory response by chrysin, a flavone isolated from Potentilla evestita Th. Wolf. In silico predictive study on its mechanistic effect. Fitoterapia. 2015;103:129–135. doi: 10.1016/j.fitote.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Panday A., Inda M.E., Bagam P., Sahoo M.K., Osorio D., Batra S. Transcription Factor NF-κB: An Update on Intervention Strategies. Arch. Immunol. Ther. Exp. 2016 doi: 10.1007/s00005-016-0405-y. [DOI] [PubMed] [Google Scholar]
- 11.Hang C.H., Shi J.X., Li J.S., Li W.Q., Wu W. Expressions of intestinal NF-κB, TNF-alpha, and IL-6 following traumatic brain injury in rats. J. Surg. Res. 2005;123:188–193. doi: 10.1016/j.jss.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Stegmaier J.C., Kirchhoff C., Bogner V., Matz M., Kanz K.G., Mutschler W., Biberthaler P. Dynamics of neutrophilic NF-κB translocation in relation to IL-8 mRNA expression after major trauma. Inflamm. Res. 2008;57:547–554. doi: 10.1007/s00011-008-7207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boots A.W., Haenen G.R., Bast A. Oxidant metabolism in chronic obstructive pulmonary disease. Eur. Respir. J. Suppl. 2003;46:14s–27s. doi: 10.1183/09031936.03.00000403a. [DOI] [PubMed] [Google Scholar]
- 14.Barnes P.J., Shapiro S.D., Pauwels R.A. Chronic obstructive pulmonary disease: Molecular and cellular mechanisms. Eur. Respir. J. 2003;22:672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 15.Antczak A., Ciebiada M., Pietras T., Piotrowski W.J., Kurmanowska Z., Górski P. Exhaled eicosanoids and biomarkers of oxidative stress in exacerbation of chronic obstructive pulmonary disease. Arch. Med. Sci. 2012;8:277–285. doi: 10.5114/aoms.2012.28555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inonu H., Doruk S., Sahin S., Erkorkmaz U., Celik D., Celikel S., Seyfikli Z. Oxidative stress levels in exhaled breath condensate associated with COPD and smoking. Respir. Care. 2012;57:413–419. doi: 10.4187/respcare.01302. [DOI] [PubMed] [Google Scholar]
- 17.Antus B., Kardos Z. Oxidative stress in COPD: Molecular background and clinical monitoring. Curr. Med. Chem. 2015;22:627–650. doi: 10.2174/092986732205150112104411. [DOI] [PubMed] [Google Scholar]
- 18.Taguchi L., Pinheiro N.M., Olivo C.R., Choqueta-Toledo A., Grecco S.S., Lopes F.D., Caperuto L.C., Martins M.A., Tiberio I.F., Câmara N.O., et al. A flavanone from Baccharis retusa (Asteraceae) prevents elastase-induced emphysema in mice by regulating NF-κB, oxidative stress and metalloproteinases. Respir. Res. 2015;16:79. doi: 10.1186/s12931-015-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prado C.M., Leick-Maldonado E.A., Yano L., Leme A.S., Capelozzi V.L., Martins M.A., Tibério I.F. Effects of nitric oxide synthases in chronic allergic airway inflammation and remodeling. Am. J. Respir. Cell. Mol. Biol. 2006;35:457–465. doi: 10.1165/rcmb.2005-0391OC. [DOI] [PubMed] [Google Scholar]
- 20.Gemicioglu B., Musellim B., Dogan I., Guven K. Fractional exhaled nitric oxide (FeNo) in different asthma phenotypes. Allergy Rhinol. 2014;5:157–161. doi: 10.2500/ar.2014.5.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2005;2:258–266. doi: 10.1513/pats.200504-045SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grzela K., Litwiniuk M., Zagorska W., Grzela T. Airway Remodeling in Chronic Obstructive Pulmonary Disease and Asthma: The Role of Matrix Metalloproteinase-9. Arch. Immunol. Ther. Exp. (Warsz.) 2016;64:47–55. doi: 10.1007/s00005-015-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maione F., Russo R., Khan H., Mascolo N. Medicinal plants with anti-inflammatory activities. Nat. Prod. Res. 2016;30:1343–1352. doi: 10.1080/14786419.2015.1062761. [DOI] [PubMed] [Google Scholar]
- 24.Khan H. Medicinal Plants in Light of History: Recognized Therapeutic Modality. J. Evid. Based Complement. Altern. Med. 2014;19:216–219. doi: 10.1177/2156587214533346. [DOI] [PubMed] [Google Scholar]
- 25.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 26.Riachi L.G., De Maria C.A. Peppermint antioxidants revisited. Food Chem. 2015;176:72–81. doi: 10.1016/j.foodchem.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Soares S.E. Ácidos fenólicos como antioxidantes. Rev. Nutr. 2002;15:71–81. doi: 10.1590/S1415-52732002000100008. [DOI] [Google Scholar]
- 28.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Ruberto G., Baratta M.T., Deans S.G., Dorman H.J. Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med. 2000;66:687–693. doi: 10.1055/s-2000-9773. [DOI] [PubMed] [Google Scholar]
- 30.De Araújo D.A., Freitas C., Cruz J.S. Essential oils components as a new path to understand ion channel molecular pharmacology. Life Sci. 2011;89:540–544. doi: 10.1016/j.lfs.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Rahman A.U., Ahmad V.U. 13C-NMR of Natural Products: Monoterpenes and sesquiterpenes. Volume 1 Plenum Press; New York, NY, USA: 1992. [Google Scholar]
- 32.Adams R.P. Identification of Essential oils by Ion Trap Mass Spectroscopy. Academic Press; San Diego, CA, USA: 1995. p. 465. [Google Scholar]
- 33.Wright J.L., Cosio M., Churg A. Animal models of chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295:L1–L15. doi: 10.1152/ajplung.90200.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes F.D., Toledo A.C., Olivo C.R., Prado C.M., Leick E.A., Medeiros M.C., Santos A.B., Garippo A., Martins M.A., Mauad T. A comparative study of extracellular matrix remodeling in two murine models of emphysema. Histol. Histopathol. 2013;28:269–276. doi: 10.14670/HH-28.269. [DOI] [PubMed] [Google Scholar]
- 35.Antunes M.A., Rocco P.R. Elastase-induced pulmonary emphysema: Insights from experimental models. An. Acad. Bras. Cienc. 2011;83:1385–1396. doi: 10.1590/S0001-37652011005000039. [DOI] [PubMed] [Google Scholar]
- 36.Ohnishi S., Nagaya N. Tissue regeneration as next-generation therapy for COPD—Potential applications. Int. J. Chron. Obstruct Pulm. Dis. 2008;3:509–514. doi: 10.2147/copd.s1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muñoz-Barrutia A., Ceresa M., Artaechevarria X., Montuenga L.M., Ortiz-de-Solorzano C. Quantification of lung damage in an elastase-induced mouse model of emphysema. Int. J. Biomed. Imaging. 2012;2012:734734. doi: 10.1155/2012/734734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WORLD HEALTH ORGANIZATION (WHO) [(accessed on 2 July 2016)]. Available online: http://www.who.int/respiratory/copd/burden/en/
- 39.Cavalcante A.G., de Bruin P.F. The role of oxidative stress in COPD: Current concepts and perspectives. J. Bras. Pneumol. 2009;35:1227–1237. doi: 10.1590/s1806-37132009001200011. [DOI] [PubMed] [Google Scholar]
- 40.Kurimoto E., Miyahara N., Kanehiro A., Waseda K., Taniguchi A., Ikeda G., Koga H., Nishimori H., Tanimoto Y., Kataoka M., et al. IL-17A is essential to the development of elastase-induced pulmonary inflammation and emphysema in mice. Respir. Res. 2013;14:5. doi: 10.1186/1465-9921-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lourenço J.D., Neves L.P., Olivo C.R., Duran A., Almeida F.M., Arantes P.M., Prado C.M., Leick E.A., Tanaka A.S., Martins M.A., et al. A treatment with a protease inhibitor recombinant from the cattle tick (Rhipicephalus Boophilus microplus) ameliorates emphysema in mice. PLoS ONE. 2014;9:e98216. doi: 10.1371/journal.pone.0098216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnes P.J. Alveolar macrophages as orchestrators of COPD. COPD. 2004;1:59–70. doi: 10.1081/COPD-120028701. [DOI] [PubMed] [Google Scholar]
- 43.Murugan V., Peck M.J. Signal transduction pathways linking the activation of alveolar macrophages with the recruitment of neutrophils to lungs in chronic obstructive pulmonary disease. Exp. Lung Res. 2009;35:439–485. doi: 10.1080/01902140902759290. [DOI] [PubMed] [Google Scholar]
- 44.Pappas K., Papaioannou A.I., Kostikas K., Tzanakis N. The role of macrophages in obstructive airways disease: Chronic obstructive pulmonary disease and asthma. Cytokine. 2013;64:613–625. doi: 10.1016/j.cyto.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Rufino R., Lapa e Silva J.R. Cellular and biochemical bases of chronic obstructive pulmonary disease. J. Bras. Pneumol. 2006;32:241–248. doi: 10.1590/S1806-37132006000300011. [DOI] [PubMed] [Google Scholar]
- 46.Bozic C.R., Gerard N.P., von Uexkull-Guldenband C., Kolakowski L.F., Conklyn M.J., Breslow R., Showell H.J., Gerard C. The murine interleukin 8 type B receptor homologue and its ligands. Expression and biological characterization. J. Biol. Chem. 1994;269:29355–29358. [PubMed] [Google Scholar]
- 47.Tanino M., Betsuyaku T., Takeyabu K., Tanino Y., Yamaguchi E., Miyamoto K., Nishimura M. Increased levels of interleukin-8 in BAL fluid from smokers susceptible to pulmonary emphysema. Thorax. 2002;57:405–411. doi: 10.1136/thorax.57.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soler N., Ewig S., Torres A., Filella X., Gonzalez J., Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur. Respir. J. 1999;14:1015–1022. doi: 10.1183/09031936.99.14510159. [DOI] [PubMed] [Google Scholar]
- 49.Lorè N.I., Bragonzi A., Cigana C. The IL-17A/IL-17RA axis in pulmonary defence and immunopathology. Cytokine Growth Factor Rev. 2016;30:19–27. doi: 10.1016/j.cytogfr.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Krstic J., Obradovic H., Kukolj T., Mojsilovic S., Okic-Dordevic I., Bugarski D., Santibanez J.F. An Overview of Interleukin-17A and Interleukin-17 Receptor A Structure, Interaction and Signaling. Protein Pept. Lett. 2015;22:570–578. doi: 10.2174/0929866522666150520145554. [DOI] [PubMed] [Google Scholar]
- 51.Maione F., Paschalidis N., Mascolo N., Dufton N., Perretti M., D’Acquisto F. Interleukin 17 sustains rather than induces inflammation. Biochem. Pharmacol. 2009;77:878–887. doi: 10.1016/j.bcp.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 52.Willis C.R., Siegel L., Leith A., Mohn D., Escobar S., Wannberg S., Misura K., Rickel E., Rottman J.B., Comeau M.R., et al. IL-17RA Signaling in Airway Inflammation and Bronchial Hyperreactivity in Allergic Asthma. Am. J. Respir. Cell. Mol. Biol. 2015;53:810–821. doi: 10.1165/rcmb.2015-0038OC. [DOI] [PubMed] [Google Scholar]
- 53.Jones C.E., Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am. J. Respir. Cell. Mol. Biol. 2002;26:748–753. doi: 10.1165/ajrcmb.26.6.4757. [DOI] [PubMed] [Google Scholar]
- 54.Rahman M.S., Yang J., Shan L.Y., Unruh H., Yang X., Halayko A.J., Gounni A.S. IL-17R activation of human airway smooth muscle cells induces CXCL-8 production via a transcriptional-dependent mechanism. Clin. Immunol. 2005;115:268–276. doi: 10.1016/j.clim.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Zhang L., Cheng Z., Liu W., Wu K. Expression of interleukin (IL)-10, IL-17A and IL-22 in serum and sputum of stable chronic obstructive pulmonary disease patients. COPD. 2013;10:459–465. doi: 10.3109/15412555.2013.770456. [DOI] [PubMed] [Google Scholar]
- 56.Xie G., Chen N., Soromou L.W., Liu F., Xiong Y., Wu Q., Li H., Feng H., Liu G. p-Cymene protects mice against lipopolysaccharide-induced acute lung injury by inhibiting inflammatory cell activation. Molecules. 2012;17:8159–8173. doi: 10.3390/molecules17078159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boskabady M.H., Gholami Mahtaj L. Lung inflammation changes and oxidative stress induced by cigarette smoke exposure in guinea pigs affected by Zataria multiflora and its constituent, carvacrol. BMC Complement. Altern. Med. 2015;15:39. doi: 10.1186/s12906-015-0574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun J., Wu J., Xu C., Luo Q., Li B., Dong J. Paeoniflorin attenuates allergic inflammation in asthmatic mice. Int. Immunopharmacol. 2015;24:88–94. doi: 10.1016/j.intimp.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 59.Zhao C., Sun J., Fang C., Tang F. 1,8-cineol attenuates LPS-induced acute pulmonary inflammation in mice. Inflammation. 2014;37:566–572. doi: 10.1007/s10753-013-9770-4. [DOI] [PubMed] [Google Scholar]
- 60.Zhou E., Fu Y., Wei Z., Yu Y., Zhang X., Yang Z. Thymol attenuates allergic airway inflammation in ovalbumin (OVA)-induced mouse asthma. Fitoterapia. 2014;96:131–137. doi: 10.1016/j.fitote.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 61.Cataldo D.D., Bettiol J., Noël A., Bartsch P., Foidart J.M., Louis R. Matrix metalloproteinase-9, but not tissue inhibitor of matrix metalloproteinase-1, increases in the sputum from allergic asthmatic patients after allergen challenge. Chest. 2002;122:1553–1559. doi: 10.1378/chest.122.5.1553. [DOI] [PubMed] [Google Scholar]
- 62.Gagliardo R., La Grutta S., Chanez P., Profita M., Paternò A., Cibella F., Bousquet J., Viegi G., Gjomarkaj M. Non-invasive markers of airway inflammation and remodeling in childhood asthma. Pediatr. Allergy Immunol. 2009;20:780–790. doi: 10.1111/j.1399-3038.2009.00945.x. [DOI] [PubMed] [Google Scholar]
- 63.Atkinson J.J., Senior R.M. Matrix metalloproteinase-9 in lung remodeling. Am. J. Respir. Cell. Mol. Biol. 2003;28:12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- 64.Blackwell T.S., Christman J.W. The role of nuclear factor-kappa B in cytokine gene regulation. Am. J. Respir. Cell. Mol. Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 65.Schuliga M. NF-κB Signaling in Chronic Inflammatory Airway Disease. Biomolecules. 2015;5:1266–1283. doi: 10.3390/biom5031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Edwards M.R., Bartlett N.W., Clarke D., Birrell M., Belvisi M., Johnston S.L. Targeting the NF-κB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol. Ther. 2009;121:1–13. doi: 10.1016/j.pharmthera.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rajendrasozhan S., Yang S.R., Edirisinghe I., Yao H., Adenuga D., Rahman I. Deacetylases and NF-κB in redox regulation of cigarette smoke-induced lung inflammation: Epigenetics in pathogenesis of COPD. Antioxid. Redox Signal. 2008;10:799–811. doi: 10.1089/ars.2007.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ko F.W., Lau C.Y., Leung T.F., Wong G.W., Lam C.W., Hui D.S. Exhaled breath condensate levels of 8-isoprostane, growth related oncogene alpha and monocyte chemoattractant protein-1 in patients with chronic obstructive pulmonary disease. Respir. Med. 2006;100:630–638. doi: 10.1016/j.rmed.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 69.Tufvesson E., Ekberg M., Bjermer L. Inflammatory biomarkers in sputum predict COPD exacerbations. Lung. 2013;191:413–416. doi: 10.1007/s00408-013-9473-5. [DOI] [PubMed] [Google Scholar]
- 70.Anciães A.M., Olivo C.R., Prado C.M., Kagohara K.H., Silva Pinto T., Moriya H.T., Mauad T., Arruda Martins M., Lopes F.D. Respiratory mechanics do not always mirror pulmonary histological changes in emphysema. Clinics (Sao Paulo) 2011;66:1797–1803. doi: 10.1590/S1807-59322011001000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toledo A.C., Magalhaes R.M., Hizume D.C., Vieira R.P., Biselli P.J., Moriya H.T., Mauad T., Lopes F.D., Martins M.A. Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur. Respir. J. 2012;39:254–264. doi: 10.1183/09031936.00003411. [DOI] [PubMed] [Google Scholar]
- 72.Malerba M., Radaeli A., Olivini A., Damiani G., Ragnoli B., Montuschi P., Ricciardolo F.L. Exhaled nitric oxide as a biomarker in COPD and related comorbidities. BioMed Res. Int. 2014;2014:271918. doi: 10.1155/2014/271918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baratta M.T., Dorman H.J., Deans S.G., Figueiredo A.C., Barroso J.G., Ruberto G. Antimicrobial and antioxidant properties of some commercial essential oils. Flavour Fragr. J. 1998;13:235–244. doi: 10.1002/(SICI)1099-1026(1998070)13:4<235::AID-FFJ733>3.0.CO;2-T. [DOI] [Google Scholar]
- 74.Walde A., Ve B., Scheline R.R., Monge P. p-Cymene metabolism in rats and guinea-pigs. Xenobiotica. 1983;13:503–512. doi: 10.3109/00498258309052290. [DOI] [PubMed] [Google Scholar]
- 75.Khan M.S., Ahmad I., Cameotra S.S. Carum copticum and Thymus vulgaris oils inhibit virulence in Trichophyton rubrum and Aspergillus spp. Braz. J. Microbiol. 2014;45:523–531. doi: 10.1590/S1517-83822014000200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sivamani P., Singaravelu G., Thiagarajan V., Jayalakshmi T., Ramesh Kumar G. Comparative molecular docking analysis of essential oil constituents as elastase inhibitors. Bioinformation. 2012;8:457–460. doi: 10.6026/97320630008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kacem R., Meraihi Z. Effects of essential oil extracted from Nigella sativa (L.) seeds and its main components on human neutrophil elastase activity. Yakugaku Zasshi. 2006;126:301–305. doi: 10.1248/yakushi.126.301. [DOI] [PubMed] [Google Scholar]
- 78.Toledo A.C., Sakoda C.P., Perini A., Pinheiro N.M., Magalhães R.M., Grecco S., Tibério I.F., Câmara N.O., Martins M.A., Lago J.H., et al. Flavonone treatment reverses airway inflammation and remodelling in an asthma murine model. Br. J. Pharmacol. 2013;168:1736–1749. doi: 10.1111/bph.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perea S.E., Baladron I., Garcia Y., Perera Y., Lopez A., Soriano J.L., Batista N., Palau A., Hernández I., Farina H., et al. CIGB-300, a synthetic peptide-based drug that targets the CK2 phosphoaceptor domain. Translational and clinical research. Mol. Cell. Biochem. 2011;356:45–50. doi: 10.1007/s11010-011-0950-y. [DOI] [PubMed] [Google Scholar]