Abstract
In this study, novel aminothiazole-paeonol derivatives were synthesized and characterized using 1H-NMR, 13C-NMR, IR, mass spectroscopy, and high performance liquid chromatography. All the new synthesized compounds were evaluated according to their anticancer effect on seven cancer cell lines. The experimental results indicated that these compounds possess high anticancer potential regarding human gastric adenocarcinoma (AGS cells) and human colorectal adenocarcinoma (HT-29 cells). Among these compounds, N-[4-(2-hydroxy-4-methoxyphenyl)thiazol-2-yl]-4-methoxybenzenesulfonamide (13c) had the most potent inhibitory activity, with IC50 values of 4.0 µM to AGS, 4.4 µM to HT-29 cells and 5.8 µM to HeLa cells. The 4-fluoro-N-[4-(2-hydroxy-4-methoxyphenyl)thiazol-2-yl]benzenesulfonamide (13d) was the second potent compound, showing IC50 values of 7.2, 11.2 and 13.8 µM to AGS , HT-29 and HeLa cells, respectively. These compounds are superior to 5-fluorouracil (5-FU) for relatively higher potency against AGS and HT-29 human cancer cell lines along with lower cytotoxicity to fibroblasts. Novel aminothiazole-paeonol derivatives in this work might be a series of promising lead compounds to develop anticancer agents for treating gastrointestinal adenocarcinoma.
Keywords: paeonol, 2-aminothiazole, anti-cancer, sulfonate, adenocarcenoma
1. Introduction
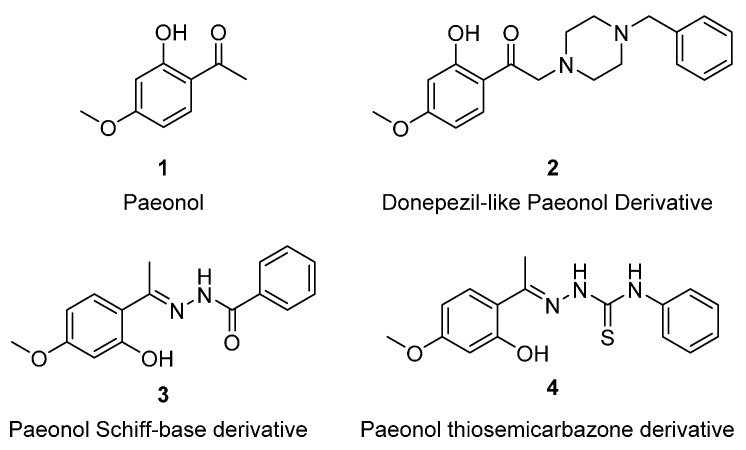
Paeonol, 2-hydroxy-4-methoxy acetophenone (1, Figure 1), is a major component of traditional Chinese medicine. Moutan Cortex, the outer layer of the root of Moutan, is classified in the genus Paeonia and has been used for more than 1000 years. Paeonol is categorized as a flavonoid derivative and exhibits many remarkable biological effects, and it has been applied for anti-inflammatory [1,2], analgesic [2], antioxidant [3], antidiabetic [4], anticancer [5], and antiatherogenic purposes [6]. Paeonol was also found to protect against memory loss after ischemic stroke by reducing amyloid precursor protein (APP), beta-site APP cleaving enzyme (BACE), and apoptosis [7]. Moreover, paeonol derivatives have been reported to show many attractive biological activities. For example, Pan and Hui demonstrated that the donepezil-like paeonol derivative (2, Figure 1) exhibited strong metal-chelating ability for Alzheimer’s disease (AD) treatment [8]. Yang presented a copper ion chelating paeonol Schiff-base derivative (3, Figure 1) complexes that possessed high antioxidant activity and moderate DNA-binding activity as well as high tumor cell cytotoxicity [9]. Moreover, Yu reported a paeonol thiosemicarbazone derivative (4, Figure 1), which exhibited potential mushroom tyrosinase inhibitors [10]. Recently, our group found that phenylsulfonyl moieties-conjugated paeonol derivatives were potential anti-Hepatitis B virus leads [11] and could prevent lipid accumulation at lower doses, and they might be prominent antiatherogenic agents [12].
Figure 1.
Structures of paeonol, donepezil-like paeonol derivative, paeonol Schiff-base derivative, and paeonol thiosemicarbazone derivative.
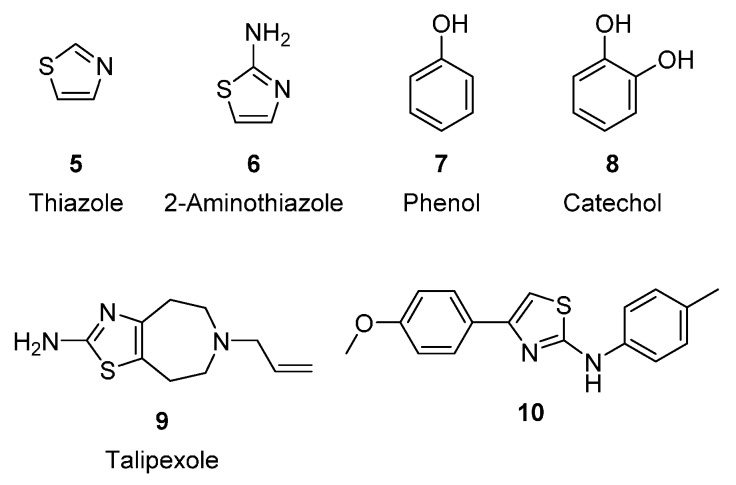
The thiazole ring (5, Figure 2), a five-membered heterocyclic core structure, displays a variety of biological effects, such as antibacterial, antifungal, anti-Human immunodeficiency virus, anti-inflammatory, antidiabetic, antioxidant, and anticancer effects [13]. These heterocyclic rings, notably 2-aminothiazole (6, Figure 2), are considered stable and lipophilic bioisosteres of phenol (7, Figure 2) or catechol (8, Figure 2) moieties, which might retain pharmacological action while having improved oral bioavailability [14]. Talipexole (9, Figure 2), a dopamine agonist for Parkinson’s disease treatment, was designed on the basis of the bioisosteric effect of phenol and 2-aminothiazole [15]. In addition, the 2-aminothiazole core was found to act as the pharmacophore for antitubercular agents, the activity and the cytotoxicity of which could be improved and reduced with appropriate modification [16]. Introducing a phenylsulfonyl moiety in some molecules may increase the solubility of the molecules and trigger antitumor activity [17,18,19].
Figure 2.
Structures of thiazole, 2-aminothiazole, phenol, catechol, talipexole and 2-aminothiazole derivative.
Herein, we present a new series of paeonol derivatives combined with the aminothiazole ring as the core structure and further conjugated with the phenylsulfonyl side-chains. With arylsulfonamidothiazole scaffold decoration, the anticancer activity of paeonol may be enhanced through additional hydrogen bonding interactions while retaining or even improving the solubility of paeonol itself [20,21,22]. This new series of aminothiazole-paeonol derivatives was determined to have potential anticancer effects in human gastric adenocarcinoma (AGS), human cervix adenocarcinoma (HeLa), human pancreas adenocarcinoma (PaTu8988t), human colorectal adenocarcinoma (HT-29), human glioblastoma (U87-MG), human lung adenocarcinoma (A549) and mouse colon carcinoma (CT26.WT) cells. Simultaneously, the toxicity of aminothiazole-paeonol derivatives against normal cells was evaluated by embryonic fibroblast (BALB/3T3) cells. The newly synthesized compounds could be structural templates for designing and developing novel anticancer agents.
2. Results and Discussion
2.1. Chemistry
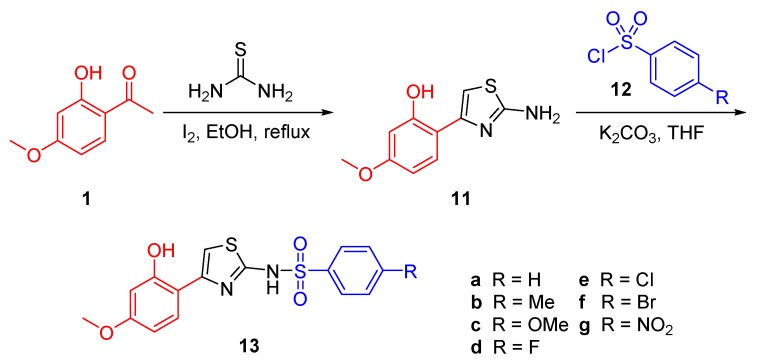
The synthetic methods of preparing the paeonol-2-aminothiazole-phenylsulfonyl derivatives are outlined in Scheme 1. The 2-aminothiazole scaffold was obtained by treating paeonol with thiourea and iodine; the condensation-cyclization of thiourea initiated by iodine afforded compound 11. To produce various paeonol-phenylsulfonyl derivatives, we treated 2-aminothiazole-paeonol 11 with substituted phenylsulfonyl chloride 12 to yield the final desired compounds 13. All these products were obtained in sufficient yield and purified by using recrystallization for anticancer assays.
Scheme 1.
Synthesis of the aminothiazole-paeonol derivatives.
2.2. Anticancer Activity and Structure Activity Relationship Analysis
The antitumor effects of the new synthesized compounds against AGS, HeLa, PaTu8988t, HT-29, U87-MG, A549, CT26.WT and BALB/3T3 are described in Table 1. Our results indicated that the aminothiazole-paeonol derivatives exhibited cytotoxic effects toward the tested human cancer cell lines. We observed that compound 13c was the most potent compound, with IC50 values of 4.0 μM to AGS, 4.4 μM to HT-29, 5.8 μM to HeLa, 10.0 μM to CT26.WT, 15.8 μM to PaTu8988t and 22.5 μM to U87-MG. Compound 13c was the only one providing efficient IC50 (less than 50 μM) against U87-MG glioblastoma. Additionally, compound 13c was relatively less toxic to BALB/3T3 (IC50: 32.7 μM) in comparison to 5-FU against BALB/3T3 (IC50: 1.0 μM). Compound 13d was the second most potent compound, showing IC50 values of 7.2, 11.2, 13.8 and 31.4 µM to AGS, HT-29, HeLa and PaTu8988t, respectively. However, compound 13d possessed lower water solubility than compound 13c did (1.55 vs. 3.04 mmol/L, shown in Table 2), which arose from the F and OCH3 groups at the para-positions of the phenylsulfonyl terminals. On the other hand, compound 13g was the most inactive compound. In the HT-29 treatment, the activity of the parent compound 13a was between those of the most potent compound 13c and the most inactive compound 13g. Comparing the structures of 13c and 13g, compound 13c was substituted with the OCH3 group and compound 13g was replaced with the NO2 group at the para-positions of the phenylsulfonyl terminals. In general, the NO2 group manifest electron-withdrawing and lipophilic properties in the structure, which could conceivably alter the pharmacokinetic behaviors of compounds. Moreover, the electron-donating OCH3 contributed an additional oxygen atom to the structure, making it more hydrophilic and thus increasing the water solubility. Some pharmacokinetic properties are listed in Table 2 [23,24]. All the compounds meet Lipinski’s Rule of Five, with the molecular weights under 500 and the log p-values lower than five. In summary, compound 13c presented the most potent activity against AGS and HT-29 cells with IC50 values of 4.0 and 4.4 µM, respectively, which was superior to 5-FU (IC50 values: 43.8 and 7.2 µM against AGS and HT-29). The compound 13c was less toxic against fibroblast cells than 5-FU; therefore, compound 13c might be a promising lead compound for developing an anticancer agent against gastrointestinal tract–related adenocarcinoma.
Table 1.
Cytotoxicity of compounds toward various cell lines.
| Compounds | IC50 (μM) | |||||||
|---|---|---|---|---|---|---|---|---|
| BALB/3T3 | AGS | HeLa | PaTu8988t | HT-29 | U87-MG | A549 | CT26.WT | |
| 13a | >50 | >50 | >50 | 31.1 | 20.7 | >50 | >50 | >50 |
| 13b | >50 | 22.0 | 27.4 | 38.7 | 14.1 | >50 | >50 | >50 |
| 13c | 32.7 | 4.0 | 5.8 | 15.8 | 4.4 | 22.5 | >50 | 10.0 |
| 13d | >50 | 7.2 | 13.8 | 31.4 | 11.2 | >50 | >50 | >50 |
| 13e | >50 | > 50 | >50 | >50 | 13.4 | >50 | >50 | >50 |
| 13f | >50 | > 50 | 16.4 | 22.8 | 11.0 | >50 | >50 | >50 |
| 13g | 32.2 | > 50 | >50 | >50 | 47.8 | >50 | >50 | 30.0 |
| 5-Fu * | 1.0 | 43.8 | 2.2 | 12.5 | 7.2 | >50 | >50 | 9.2 |
| 5-FU ** [25,26,27,28,29,30,31,32] | 13.0 | 79.5 | 0.232 | 11.3 | 19.3 | 4.9 | 10.3 | 61.0 |
| 72 h | 24 h | 48 h | 48 h | 48 h | 48 h | 48 h | 72 h | |
*: the IC50 values were measured at 48 h after cells being treated with 5-FU in our study; **: the IC50 values of 5-FU against different cancer cells published in other references were measured at 24, 48 or 72 h after treatment.
Table 2.
| Compounds | Molecular Weight, Lipophilicity and Water Solubility | |||
|---|---|---|---|---|
| M.W. | clogP | clogS | S (mmol/L) | |
| 13a | 362.418 | 3.26 | −4.14 | 2.625486 |
| 13b | 376.445 | 3.57 | −4.31 | 1.843748 |
| 13c | 392.444 | 3.27 | −4.11 | 3.046335 |
| 13d | 380.4804 | 3.76 | −4.39 | 1.550002 |
| 13e | 396.86 | 3.83 | −4.43 | 1.474475 |
| 13f | 441.314 | 4.07 | −4.57 | 1.187812 |
| 13g | 407.026 | 3.21 | −4.33 | 1.903804 |
3. Experimental Section
3.1. General Experimental Procedures
All reactions were carried out in oven-dried glassware (120 °C) under an atmosphere of nitrogen unless indicated otherwise. Dichloromethane, ethanol, ethyl acetate, hexanes, methanol, and THF were purchased from Mallinckrodt Chemical Co. Ethyl acetate was dried and distilled from CaH2. Tetrahydrofuran was dried by distillation from sodium and benzophenone under an atmosphere of nitrogen. Benzenesulfonyl chloride, 4-bromobenzenesulfonyl chloride, 4-chlorobenzenesulfonyl chloride, 4-fluorobenzenesulfonyl chloride, 4-methoxybenzenesulfonyl chloride, 4-nitrobenzenesulfonyl chloride, peaonol (2′-hydroxy-4-methoxyacetophenone), potassium carbonate, and p-toluenesulfonyl chloride were purchased from Sigma-Aldrich China Inc., Shanghi, China.
Melting points were obtained with a Fargo MP-2D melting point apparatus (melting point range up to ~400 °C). Analytical thin layer chromatography (TLC) was performed on precoated plates (silica gel 60 F-254), purchased from Merck Inc. Infrared (IR) spectra were measured on a Perkin-Elmer Model Spectrum 100 spectrophotometer. Absorption intensities are recorded by the following abbreviations: s = strong; m = medium; and w = weak. Proton NMR spectra were obtained on a Varian Mercury-400 (400 MHz) spectrometer or Bruker AC-400 (400 MHz) spectrometer by use of chloroform-d (CDCl3) and dimethylsulfoxide-d6 (DMSO-d6) as the solvents. Proton NMR chemical shifts were referenced to residual protonated solvents (δ 7.24 for chloroform and δ 2.49 for dimethylsulfoxide). Carbon-13 NMR spectra were obtained on a Varian Mercury-400 (100 MHz) spectrometer by use of chloroform-d (CDCl3) and dimethylsulfoxide-d6 (DMSO-d6) as the solvents. Carbon-13 chemical shifts are referenced to the center of the CDCl3 triplet (δ 77.0 ppm) and DMSO septet (δ 39.5 ppm). Multiplicities are recorded by the following abbreviations: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; J, coupling constant (hertz). High-resolution mass spectra were obtained by means of a JEOL JMS-700 mass spectrometer.
3.2. Procedure for the Preparation of Aminothiazole-Paeonol (11)
To obtain aminothiazole-paeonol (11), paeonol (1) (1.0 equiv) was reacted with iodine (1.1 equiv) and thiourea (3.0 equiv) in ethanol under reflux condition for 12.0–16.0 h. Then, the reaction mixture was quenched with NaOH(aq) (2.0 equiv) and the ethanol was removed under reduced pressure. The residue was extracted with ethyl acetate and the combined organic layer were washed the brine and dried over MgSO4(s). After being filtered and condensed under reduced pressure, the crude product was purified by column chromatography on silica gel (ethyl acetate and hexane as eluent) to give compound 11.
2-(2-Aminothiazol-5-yl)-5-methoxyphenol (11): 1H-NMR (CDCl3, 400 MHz): δ 7.40 (d, J = 8.4 Hz, 1 H, H-3), 6.54 (s, 1 H, CH), 6.47 (s, 1 H, H-6), 6.42 (dd, J = 8.4, 2.0 Hz, 1 H, H-4), 5.05 (s, 2 H, NH2), 3.78 (s, 3 H, OMe) ppm. 13C-NMR (CDCl3, 100 MHz): δ 166.8, 161.0, 157.3, 148.9, 126.6, 111.0, 106.8, 101.6, 98.9, 55.2 (OMe) ppm; HRMS (ESI+) m/z [M + H]+ calculated for C16H14N2O4S2, 223.0463, found 223.0462.
3.3. Standard Procedure for the Preparation of Aminothiazole-Paeonol Derivatives (13)
To a solution containing aminothiazole-paeonol (11, 1.0 equiv) in anhydrous THF (2.0–3.0 mL) was added potassium carbonate (1.3 equiv) and a sulfonyl chloride 12 (1.1 equiv). After the reaction mixture was stirred at 25 °C for 2.0–3.0 h, it was diluted with dichloromethane (5.0 mL). Inorganic solids were filtered off and the filtrate was concentrated under reduced pressure to afford the residue. It was then purified by use of column chromatography on silica gel (various ratio of methanol to dichloromethane) to give the desired conjugates 13.
N-[4-(2-Hydroxy-4-methoxyphenyl)thiazol-2-yl]benzenesulfonamide (13a): Yield 80%, green solid product. IR (film): ν 3569.1, 2811.2, 1560.1, 1481.2, 1374.2, 1131.6, 853.4 cm−1. 1H-NMR (CDCl3, 400 MHz): δ 7.58–7.55 (m, 2 H, 2 × ArH), 7.53–7.51 (m, 1 H, H-6), 7.43–7.33 (m, 3 H, 3 × ArH), 6.84 (d, J = 2.0 Hz, 1 H, H-3), 6.79–6.77 (m, 1 H, H-5), 6.55 (s, 1 H, SCH), 3.75 (s, 3 H, OMe) ppm. 13C NMR (CDCl3, 100 MHz): δ 166.8, 163.9, 158.3, 147.2, 143.7, 131.5, 125.9, 121.1, 113.6, 113.2, 108.8, 106.2, 55.6 (OMe) ppm; HRMS (ESI+) m/z [M + H]+ calculated for C16H14N2O4S2, 363.0395, found 363.0396.
N-[4-(2-Hydroxy-4-methoxyphenyl)thiazol-2-yl]-4-methylbenzenesulfonamide (13b): Yield 84%, off-white solid product. IR (film): ν 3579.1, 2921.2, 1580.1, 1491.2, 1384.1, 1141.6, 843.4 cm−1. 1H-NMR (CDCl3, 400 MHz): δ 7.44 (d, J = 8.8 Hz, 1 H, H-6), 7.39 (d, J = 8.2 Hz, 2 H, 2 × ArH), 7.06 (d, J = 8.2 Hz, 2 H, 2 × ArH), 6.81 (d, J = 2.4 Hz, 1 H, H-3), 6.74 (dd, J = 8.8, 2.4 Hz, 1 H, H-5) 6.52 (s, 1 H, SCH), 3.72 (s, 3 H, OMe), 2.29 (s, 3 H, CH3) ppm. 13C-NMR (CDCl3, 100 MHz): δ 167.8, 159.3, 147.0, 145.3, 144.4, 132.1, 130.5, 129.1, 128.2, 120.9, 113.1, 108.7, 105.9, 55.4 (OMe), 21.4 (CH3) ppm; HRMS (ESI+) m/z [M + H]+ calculated for C17H16N2O4S2, 377.0551, found 377.0551.
N-[4-(2-Hydroxy-4-methoxyphenyl)thiazol-2-yl]-4-methoxybenzenesulfonamide (13c): Yield 83%, off-white solid product. IR (film): ν 3672.1, 2931.1, 1560.6, 1473.2, 1388.1, 1142.7, 817.4 cm−1. 1H-NMR (CDCl3, 400 MHz): δ 7.46–7.42 (m, 3 H, H-6 + 2 × ArH), 6.86 (d, J = 2.8 Hz, 1 H, H-3), 6.76–6.72 (m, 3 H, H-5 + 2 × ArH), 6.57 (s, 1 H, SCH), 3.75 (s, 3 H, OMe), 3.74 (s, 3 H, OMe) ppm. 13C-NMR (CDCl3, 100 MHz): δ 166.8, 163.9, 159.4, 147.1, 144.7, 130.5, 125.9, 121.0, 113.7, 113.2, 108.8, 106.2, 55.6 (OMe), 55.5 (OMe) ppm; HRMS (ESI+) m/z [M + H]+ calculated for C17H16N2O5S2, 393.0501, found 393.0498.
4-Fluoro-N-[4-(2-hydroxy-4-methoxyphenyl)thiazol-2-yl]benzenesulfonamide (13d): Yield 81%, white solid product. IR (film): ν 3695.5, 2943.3, 1589.7, 1493.9, 1378.3, 1157.7, 837.7 cm−1. 1H-NMR (CDCl3, 400 MHz): δ 7.51–7.48 (m, 2 H, 2 × ArH), 7.41 (d, J = 8.8 Hz, 1 H, H-6), 6.95–6.91 (m, 2 H, 2 × ArH), 6.86 (d, J = 2.6 Hz, 1 H, H-3), 6.75 (dd, J = 8.8, 2.6 Hz, 1 H, H-5), 6.46 (s, 1 H, SCH), 3.74 (s, 3 H, OMe) ppm. 13C NMR (CDCl3, 100 MHz): δ 167.3, 167.1, 164.5, 159.4, 146.8, 144.5, 131.2, 130.6, 120.9, 115.9, 115.6, 113.3, 108.9, 105.8, 55.5 (OMe) ppm; HRMS (ESI+) m/z [M + H]+ calculated for C16H13FN2O4S2, 381.0301, found 381.0302.
4-Chloro-N-[4-(2-hydroxy-4-methoxyphenyl)thiazol-2-yl]benzenesulfonamide (13e): Yield 81%, white solid product. IR (film): ν 3708.8, 2796.7, 1566.74, 1459.55, 1314.15, 1256.54, 984.95 cm−1. 1H-NMR (CDCl3, 400 MHz): δ 7.53 (d, J = 8.8 Hz, 1 H, H-6), 7.48–7.46 (m, 2 H, 2 × ArH), 7.29–7.27 (m, 2 H, 2 × ArH), 6.94 (d, J = 2.4 Hz, 1 H, H-3), 6.82 (dd, J = 8.8, 2.4 Hz, 1 H, H-5), 6.56 (s, 1 H, SCH), 3.81 (s, 3 H, OMe) ppm. 13C-NMR (CDCl3, 100 MHz): δ 167.2, 159.5, 146.8, 144.7, 140.7, 133.2, 130.7, 129.6, 128.7, 120.9, 113.5, 109.1, 106.0, 55.6 (OMe) ppm; HRMS (ESI+) m/z [M + H]+ calculated for C16H13ClN2O4S2, 397.0005, found 397.0006.
4-Bromo-N-[4-(2-hydroxy-4-methoxyphenyl)thiazol-2-yl]benzenesulfonamide (13f): Yield 83%, white solid product. IR (film): ν 3691.2, 2838.7, 1591.3, 1479.1, 1362.1, 1143.7, 821.7 cm−1. 1H-NMR (CDCl3, 400 MHz): δ 7.36–7.33 (m, 3 H, H-5 + 2 × ArH), 7.28–7.26 (m, 2 H, 2 × ArH), 6.80 (d, J = 1.6 Hz, 1 H, H-3), 6.71 (dd, J = 8.8, 1.6 Hz, 1 H, H-5), 6.35 (s, 1 H, SCH), 3.69 (s, 3 H, OMe) ppm. 13C-NMR (CDCl3, 100 MHz): δ 167.2, 164.3, 158.4, 145.7, 143.6, 132.3, 121.2, 115.6, 113.2, 108.7, 105.2, 56.4 (OMe) ppm; HRMS (ESI+) m/z [M + H]+ calculated for C16H13BrN2O4S2, 440.9500, found 440.9502.
N-[4-(2-Hydroxy-4-methoxyphenyl)thiazol-2-yl]-4-nitrobenzenesulfonamide (13g): Yield 84%, orange solid product. IR (film): ν 3607.7, 2685.6, 1554.65, 1360.65, 1325.25, 1267.54, 964.95 cm−1. 1H-NMR (CDCl3, 400 MHz): δ 8.06 (d, J = 8.0 Hz, 2 H, 2 × ArH), 7.59 (d, J = 8.0 Hz, 2 H, 2 × ArH), 7.29 (d, J = 8.6 Hz, 1 H, H-6), 6.90 (s, 1 H, H-3), 6.77 (d, J = 8.6 Hz, 1 H, H-5), 6.32 (s, 1 H, SCH), 3.76 (s, 3 H, OMe) ppm. 13C-NMR (CDCl3, 100 MHz): δ 167.2, 162.0, 156.2, 151.1, 147.8, 145.9, 132.9, 128.2, 124.2, 112.8, 107.4, 105.0, 104.2, 55.8 (OMe) ppm; HRMS (ESI+) m/z [M + H]+ calculated for C16H13N3O6S2, 408.0246, found 408.0247.
3.4. Cell Culture
Cell lines, including human gastric adenocarcinoma (AGS), human cervix adenocarcinoma (HeLa), human pancreas adenocarcinoma (PaTu8988t), human colorectal adenocarcinoma (HT-29), human glioblastoma (U87-MG), human lung adenocarcinoma (A549), mouse colon carcinoma (CT26.WT) cells and embryonic fibroblast (BALB/3T3) were purchased from Bioresource Collection and Research Center (BCRC, Taiwan) to evaluate anticancer activity of aminothiazole-paeonol derivatives. Five cell lines were cultured in different media from Gibco (Life Technologies, Grand Island, NY, USA) and Hyclone (GE Healthcare Life Science). Cells were cultured in 90% medium mixed with 10% serum at 37 °C in humidified atmosphere with 5% CO2 and grown in T-75 flask with a feeding cycle of two to three days. The composition and procedures of preparing culture media for different cell lines were followed by instruction manual of suppliers [5,33].
3.5. Drug Treatment and Cell Viability Assay
The different cell lines were seeded into 96-well tissue culture plates at a concentration of 5 × 103 cells/100 µL/well overnight. Subsequently, the cells were treated with serial concentrations of seven paeonol derivatives. After 48 h of incubation, cell viability of each cell line was determined by MTT colorimetric assay. Briefly, 100 µL of 2 mg/mL MTT reagent (Sigma-Aldrich, St. Louis, MO, USA) was added to each well and incubated for 4 h at 37 °C. Later, the medium was aspirated and 100 µL of dimethyl sulphoxide (DMSO) added to each well; finally, the OD595 of each well was measured by ELISA reader (TECAN, Wien, Austria).
4. Conclusions
Various phenylsulfonyl side-chains were directly conjugated to paeonol, the major component of a traditional Chinese medicine, Moutan cortex, through chemical synthesis to generate a new series of aminothiazole-paeonol derivatives. The substituents on the phenylsulfonyl side-chain included F, Cl, Br, NO2, Me, and OMe. All the synthesized compounds were characterized using 1H-NMR, 13C-NMR, and mass spectra data. The cytotoxic effects of all compounds were evaluated against fibroblast cells (BALB/3T3) and seven cancer cell lines, including AGS, HeLa, PaTu8988t, HT-29, U87-MG, A549 and CT26.WT. We observed that the thiazole-paeonol-phenylsulfonyl derivatives demonstrated cytotoxic effects against the tested cancer cell lines. We also discussed the structural activity relationship on the basis of screening results and pharmacokinetic properties. Results indicated that 13c exhibited the most potent activity against AGS and HT-29 cells with IC50 values of 4.0 and 4.4 µM, respectively, which is superior to 5-FU (IC50 values: 43.8 and 7.2 µM against AGS and HT-29) and may be a promising lead compound to develop an anticancer agent for gastrointestinal tract–related adenocarcinoma.
Acknowledgments
For financial support, we thank the Ministry of Science and Technology of the Republic of China and National Tsing Hua University. Authors are grateful of financial supports from National Health Research Institutes (NHRI-BN-104-PP-27 for JKC) and Ministry of Science and Technology (MOST 103-2113-M-400-001 for JKC) (MOST-103-2113-M-007-020 and MOST-104-2119-M-007-017 for MHH).
Author Contributions
Tsai C.Y., Huang Y.P., and Kapoor M. performed the synthesis and structure elucidation. Tsai C.Y., Lin Y.L., Lin H.H., Huang S.C. and Liao W.N. contributed to evaluation of bioactivity. Huang J.S., Liang Y.C., Tsai, F.Y., Chen J.K. and Hsu M.H. prepared the manuscript and supervised whole research project.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Fu P.K., Wu C.L., Tsai T.H., Hsieh C.L. Anti-inflammatory and anticoagulative effects of paeonol on lps-induced acute lung injury in rats. Evid. Based Complement. Altern. Med. 2012;2012:837513. doi: 10.1155/2012/837513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou T.C. Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia. Br. J. Pharmacol. 2003;139:1146–1152. doi: 10.1038/sj.bjp.0705360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen B., Ning M., Yang G. Effect of paeonol on antioxidant and immune regulatory activity in hepatocellular carcinoma rats. Molecules. 2012;17:4672–4683. doi: 10.3390/molecules17044672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau C.H., Chan C.M., Chan Y.W., Lau K.M., Lau T.W., Lam F.C., Law W.T., Che C.T., Leung P.C., Fung K.P., et al. Pharmacological investigations of the anti-diabetic effect of cortex moutan and its active component paeonol. Phytomedicine. 2007;14:778–784. doi: 10.1016/j.phymed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Yin J., Wu N., Zeng F., Cheng C., Kang K., Yang H. Paeonol induces apoptosis in human ovarian cancer cells. Acta Histochem. 2013;115:835–839. doi: 10.1016/j.acthis.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Zhao J.F., Jim Leu S.J., Shyue S.K., Su K.H., Wei J., Lee T.S. Novel effect of paeonol on the formation of foam cells: Promotion of LXRα-ABCA1-dependent cholesterol efflux in macrophages. Am. J. Chin. Med. 2013;41:1079–1096. doi: 10.1142/S0192415X13500730. [DOI] [PubMed] [Google Scholar]
- 7.Su S.Y., Cheng C.Y., Tsai T.H., Hsieh C.L. Paeonol protects memory after ischemic stroke via inhibiting β-secretase and apoptosis. Evid. Based Complement. Altern. Med. 2012;2012:932823. doi: 10.1155/2012/932823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou A., Wu H., Pan J., Wang X., Li J., Wu Z., Hui A. Synthesis and evaluation of paeonol derivatives as potential multifunctional agents for the treatment of alzheimer's disease. Molecules. 2015;20:1304–1318. doi: 10.3390/molecules20011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin D.D., Yang Z.Y., Zhang F.H., Du B., Wang P., Li T.R. Evaluation of the antioxidant, DNA interaction and tumor cell cytotoxicity activities of copper(II) complexes with paeonol schiff-base. Inorg. Chem. Commun. 2010;13:727–729. doi: 10.1016/j.inoche.2010.03.030. [DOI] [Google Scholar]
- 10.Zhu T.H., Cao S.W., Yu Y.Y. Synthesis, characterization and biological evaluation of paeonol thiosemicarbazone analogues as mushroom tyrosinase inhibitors. Int. J. Biol. Macromol. 2013;62:589–595. doi: 10.1016/j.ijbiomac.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 11.Huang T.J., Chuang H., Liang Y.C., Lin H.H., Horng J.C., Kuo Y.C., Chen C.W., Tsai F.Y., Yen S.C., Chou S.C., et al. Design, synthesis, and bioevaluation of paeonol derivatives as potential anti-HBV agents. Eur. J. Med. Chem. 2015;90:428–435. doi: 10.1016/j.ejmech.2014.11.050. [DOI] [PubMed] [Google Scholar]
- 12.Pao K.C., Zhao J.F., Lee T.S., Huang Y.P., Han C.C., Huang L.C.S., Wu K.H., Hsu M.H. Low-dose paeonol derivatives alleviate lipid accumulation. Rsc. Adv. 2015;5:5652–5656. doi: 10.1039/C4RA13986K. [DOI] [Google Scholar]
- 13.Nevagi R.J. Biological and medicinal significance of 2-aminothiazoles. Der Pharm. Lett. 2014;6:134–150. [Google Scholar]
- 14.Jaen J.C., Wise L.D., Caprathe B.W., Tecle H., Bergmeier S., Humblet C.C., Heffner T.G., Meltzer L.T., Pugsley T.A. 4-(1,2,5,6-tetrahydro-1-alkyl-3-pyridinyl)-2-thiazolamines: A novel class of compounds with central dopamine agonist properties. J. Med. Chem. 1990;33:311–317. doi: 10.1021/jm00163a051. [DOI] [PubMed] [Google Scholar]
- 15.Van Vliet L.A., Rodenhuis N., Wikstrom H., Pugsley T.A., Serpa K.A., Meltzer L.T., Heffner T.G., Wise L.D., Lajiness M.E., Huff R.M., et al. Thiazoloindans and thiazolobenzopyrans: A novel class of orally active central dopamine (partial) agonists. J. Med. Chem. 2000;43:3549–3557. doi: 10.1021/jm000087z. [DOI] [PubMed] [Google Scholar]
- 16.Pieroni M., Wan B., Cho S., Franzblau S.G., Costantino G. Design, synthesis and investigation on the structure-activity relationships of n-substituted 2-aminothiazole derivatives as antitubercular agents. Eur. J. Med. Chem. 2014;72:26–34. doi: 10.1016/j.ejmech.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Niwata S., Fukami H., Sumida M., Ito A., Kakutani S., Saitoh M., Suzuki K., Imoto M., Shibata H., Imajo S., et al. Substituted 3-(phenylsulfonyl)-1-phenylimidazolidine-2,4-dione derivatives as novel nonpeptide inhibitors of human heart chymase. J. Med. Chem. 1997;40:2156–2163. doi: 10.1021/jm960793t. [DOI] [PubMed] [Google Scholar]
- 18.Schroder J., Henke A., Wenzel H., Brandstetter H., Stammler H.G., Stammler A., Pfeiffer W.D., Tschesche H. Structure-based design and synthesis of potent matrix metalloproteinase inhibitors derived from a 6H-1,3,4-thiadiazine scaffold. J. Med. Chem. 2001;44:3231–3243. doi: 10.1021/jm010887p. [DOI] [PubMed] [Google Scholar]
- 19.Bachovchin D.A., Zuhl A.M., Speers A.E., Wolfe M.R., Weerapana E., Brown S.J., Rosen H., Cravatt B.F. Discovery and optimization of sulfonyl acrylonitriles as selective, covalent inhibitors of protein phosphatase methylesterase-1. J. Med. Chem. 2011;54:5229–5236. doi: 10.1021/jm200502u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbosa M.L.D., Lima L.M., Tesch R., Sant’Anna C.M.R., Totzke F., Kubbutat M.H.G., Schachtele C., Laufer S.A., Barreiro E.J. Novel 2-chloro-4-anilino-quinazoline derivatives as EGFR and VEGFR-2 dual inhibitors. Eur. J. Med. Chem. 2014;71:1–14. doi: 10.1016/j.ejmech.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 21.Abouzid K., Shouman S. Design, synthesis and in vitro antitumor activity of 4-aminoquinoline and 4-aminoquinazoline derivatives targeting EGFR tyrosine kinase. Bioorg. Med. Chem. 2008;16:7543–7551. doi: 10.1016/j.bmc.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z.C., Duan Y.T., Qiu H.Y., Huang W.Y., Wang P.F., Yan X.Q., Zhang S.F., Zhu H.L. Novel metronidazole-sulfonamide derivatives as potent and selective carbonic anhydrase inhibitors: Design, synthesis and biology analysis. Rsc. Adv. 2014;4:33029–33038. doi: 10.1039/C4RA03819C. [DOI] [Google Scholar]
- 23.Tetko I.V., Gasteiger J., Todeschini R., Mauri A., Livingstone D., Ertl P., Palyulin V., Radchenko E., Zefirov N.S., Makarenko A.S., et al. Virtual computational chemistry laboratory—Design and description. J. Comput. Aided Mol. Des. 2005;19:453–463. doi: 10.1007/s10822-005-8694-y. [DOI] [PubMed] [Google Scholar]
- 24.Alagille D., Baldwin R.M., Tamagnan G.D. One-step synthesis of 2-arylbenzothiazole (“BTA”) and -benzoxazole precursors for in vivo imaging of β-amyloid plaques. Tetrahedron Lett. 2005;46:1349–1351. doi: 10.1016/j.tetlet.2004.12.111. [DOI] [Google Scholar]
- 25.Flis S., Splwinski J. Inhibitory effects of 5-fluorouracil and oxaliplatin on human colorectal cancer cell survival are synergistically enhanced by sulindac sulfide. Anticancer Res. 2009;29:435–441. [PubMed] [Google Scholar]
- 26.Bhattacharya B., Akram M., Balasubramanian I., Tam K.K., Koh K.X., Yee M.Q., Soong R. Pharmacologic synergy between dual phosphoinositide-3-kinase and mammalian target of rapamycin inhibition and 5-fluorouracil in PIK3CA mutant gastric cancer cells. Cancer Biol. Therapy. 2012;13:34–42. doi: 10.4161/cbt.13.1.18437. [DOI] [PubMed] [Google Scholar]
- 27.Lu D.Y., Huang M., Xu C.H., Yang W.Y., Hu C.X., Lin L.P., Tong L.J., Li M.H., Lu W., Zhang X.W., et al. Anti-proliferative effects, cell cycle G2/M phase arrest and blocking of chromosome segregation by probimane and MST-16 in human tumor cell lines. BMC Pharmacol. 2005;5:11. doi: 10.1186/1471-2210-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nambaru P.K., Hubner T., Kock K., Mews S., Grube M., Payen L., Guitton J., Sendler M., Jedlitschky G., Rimmbach C., et al. Drug efflux transporter multidrug resistance-associated protein 5 affects sensitivity of pancreatic cancer cell lines to the nucleoside anticancer drug 5-fluorouracil. Drug Metabol. Dispos. Biol. Fate Chem. 2011;39:132–139. doi: 10.1124/dmd.110.033613. [DOI] [PubMed] [Google Scholar]
- 29.Nita M.E., Nagawa H., Tominaga O., Tsuno N., Fujii S., Sasaki S., Fu C.G., Takenoue T., Tsuruo T., Muto T. 5-fluorouracil induces apoptosis in human colon cancer cell lines with modulation of BCL-2 family proteins. Br. J. Cancer. 1998;78:986–992. doi: 10.1038/bjc.1998.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blois J., Smith A., Josephson L. The slow cell death response when screening chemotherapeutic agents. Cancer Chemother. Pharmacol. 2011;68:795–803. doi: 10.1007/s00280-010-1549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan X., Zhang X., Sun H., Zhang J., Yan M., Zhang H. Autophagy inhibition promotes 5-fluorouraci-induced apoptosis by stimulating ros formation in human non-small cell lung cancer a549 cells. PLoS ONE. 2013;8:e56679. doi: 10.1371/journal.pone.0056679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Da S., Gomide M., de O. Lemos F., Lopes M.T.P., de Alves T.M., Viccini L.F., Coelho C.M. The effect of the essential oils from five different lippia species on the viability of tumor cell lines. Braz. J. Pharm. 2013;23:895–902. [Google Scholar]
- 33.Li M., Tan S.Y., Wang X.F. Paeonol exerts an anticancer effect on human colorectal cancer cells through inhibition of PGE2 synthesis and COX-2 expression. Oncol. Rep. 2014;32:2845–2853. doi: 10.3892/or.2014.3543. [DOI] [PubMed] [Google Scholar]