Abstract
Background: Tropaeolum pentaphyllum Lam. tubers (Tropaeolaceae) are known and used as a condiment and for the treatment of skin infections in Southern Brazil. However, its activity and composition has not yet been investigated. Thus, different extracts and the essential oil from the tubers were tested against a range of microorganisms. The most active extracts were submitted to chromatographic analysis. Methods: Hydroalcoholic extract (70%), fractions of it, and the essential oil from the tubers were tested against several bacteria, yeasts and molds, furnishing the corresponding inhibitory, bactericidal and fungicidal minimal concentration values. The most active extracts were submitted to GC-MS investigation. Results: The strongest effects against different strains of microorganisms, such as Gram-positive and negative bacteria, Candida spp. and dermatophytes were observed for the essential oil and the chloroform fraction, with minimal inhibitory concentrations (MICs) well below 200 µg/mL. GC-MS analysis revealed that the major essential oil constituent is benzyl isothiocyanate (BITC), while the chloroform fraction is constituted of BITC, amides, sulfur, fatty acids and its esters, all compounds that may be related to the demonstrated activity. Conclusions: Overall, the results support the popular use of the plant for the treatment of skin infections, and revealed the main active compounds.
Keywords: Tropaeolum pentaphyllum, essential oil, benzyl isothiocyanate, antifungal, antibacterial
1. Introduction
Tropaeolum pentaphyllum Lam. (Tropaeolaceae) is a summer-growing species, native to South America, specifically Uruguay, Northern Argentina and Southern Brazil. It is used as an ornamental, food and medicinal plant. Its flowers make it known and appreciated throughout the world as an ornamental plant, but they are also locally consumed as part of salads [1] and used as an antidiabetic drug [2].
While the flowers of T. pentaphyllum have their applications, the most known and consumed part of the plant is its tubers. These can reach up to 1.5 kg in weight in due time, and are commonly known in south of Brazil as “crem” or “batata-crem” (crem-potato) and also “raiz-amarga” (bitter-root) [3]. “Crem” is a word derived from the slavic “chren”, a common denomination of the roots of Armoracia rusticana (horseradish) in Eastern Europe. T. pentaphyllum’s tubers and horseradish are commonly mistaken, as both are used as spices, prepared in the same way, as homemade pickles with red wine vinegar [4]. T. pentaphyllum’s tubers have similar organoleptic characteristics to those of the horseradish, and its consumption became popular during the 19th century with the settling of immigrants from Europe in the southern region of Brazil.
In traditional medicine, the consumption of the tubers is indicated to prevent and aid in the treatment of flu and scurvy. The decoction of the tubers is recommended as an option for the treatment of dermatosis and dermatological affections [5]. To the best of our knowledge, there is no scientific evidence corroborating this use, nor there are any studies demonstrating the presence of compounds related to these properties.
Bacterial and fungal infections represent part of the known and reported dermatological afflictions. Their treatments can be lengthy and expensive, requiring topical and parenteral medication, and as with other microbial infections, the microorganisms responsible can develop resistance mechanisms [6]. Therefore, the aim of the present study was to evaluate the popular use and the potential of T. pentaphyllum tubers, through an assessment of its in vitro antimicrobial activity and an investigation of the major constituents. For that, phytochemical analysis and antimicrobial evaluation against a series of microorganisms were conducted with the hydroalcoholic extract, its derived fractions and the essential oil obtained from the tubers.
2. Results
2.1. Extraction Methods
The dried tubers, extracted with hydroalcoholic solution (70%), yielded 20.2% of dried crude extract (CE). Yields for the fractions of the CE were 20.2% for the butanol fraction (BuF), 13.4% for the chloroform fraction (CfF) and 3.1% for the ethyl acetate fraction (EaF). The hydrodistillation extraction of the tubers yielded 0.082% of a limpid and pungent oil.
2.2. Antimicrobial Activity
Antimicrobial activity results are summarized in the Table 1, Table 2 and Table 3, divided among bacteria, yeasts and filamentous fungi. The CE and BuF did not show activity at the highest tested concentration (1280 µg/mL) against any of the tested microorganisms (data not shown). Potent bacteriostatic and bactericidal activity were observed for the essential oil (EO) and CfF (Table 1), against both Gram-positive and negative bacteria. Stronger results were those obtained against the Gram-negative E. coli and S. pullorum, with MICs in the same range of the tested reference antimicrobial agent (azithromycin). In addition, bactericidal activity was also verified, albeit at higher concentrations.
Table 1.
Antibacterial activity of the essential oil and fractions from the crude extract (CE) of T. pentaphyllum tubers.
| Bacteria | MIC/MBC (µg/mL) | ||||
|---|---|---|---|---|---|
| EO | CfF | EaF | BITC | Azithromycin | |
| Enterococcus faecalis ATCC 91299 | 40/640 | 80/640 | 80/1280 | 40/640 | 8/- |
| Escherichia coli ATCC 5922 | 10/320 | 10/80 | 20/640 | 10/320 | 8/- |
| Klebsiella pneumoniae ATCC 700603 | 40/1280 | 40/640 | 320/- | 40/640 | 2/- |
| Pseudomonas aeruginosa ATCC 27853 | 20/320 | 40/640 | 40/640 | 20/320 | 4/- |
| Salmonella pullorum ATCC 9140 | 40/640 | 10/- | 80/1280 | 40/640 | 8/- |
| Staphylococcus aureus ATCC 29213 | 40/1280 | 40/640 | 640/- | 40/320 | 2/- |
EO: Essential oil; CfF: Chloroform fraction; EaF: Ethyl acetate fraction; BITC: Benzyl isothiocyanate; -: Not active.
Table 2.
Antifungal activity of the essential oil and fractions from the CE of Tropaeolum pentaphyllum tubers, against yeast fungi.
| Fungus | MIC/MFC (µg/mL) | ||||
|---|---|---|---|---|---|
| EO | CfF | EaF | BITC | Fluconazole | |
| Candida albicans ATCC 14053 | 40/320 | 20/20 | 40/320 | 40/160 | 4/- |
| Candida dubliniensis CBS7987 | 40/320 | 10/10 | 40/640 | 40/160 | 8/- |
| Candida dubliniensis CI FS | 40/160 | 20/20 | 40/640 | 40/160 | 2/- |
| Candida dubliniensis CI FR | 40/160 | 20/20 | 40/640 | 40/160 | 64/- |
| Candida glabrata ATCC 2001 | 20/80 | 40/40 | 40/320 | 20/80 | 32/- |
| Candida glabrata CI FS | 20/160 | 40/40 | 40/640 | 20/160 | 16/- |
| Candida glabrata CI FR | 20/160 | 40/40 | 20/640 | 20/160 | 128/- |
| Candida glabrata CI CR | NT | 160/1280 | NT | 160/640 | 4/- |
| Candida guilliermondii CI | 80/320 | 10/10 | 80/320 | 80/320 | 8/- |
| Candida parapsilosis ATCC 22018 | 80/320 | 10/20 | 80/640 | 80/320 | 16/- |
| Candida parapsilosis CI CR | NT | 320/1280 | NT | 320/1280 | 8/- |
| Candida tropicalis CI | 320/- | 10/10 | 1280/- | 320/- | 8/- |
| Cryptococcus neoformans ATCC 90012 | 10/80 | 2.5/2.5 | 10/320 | 5/80 | 16/- |
| Sacharomyces cerevisiae ATCC 2601 | 40/320 | 2.5/2.5 | 80/320 | 40/160 | 4/- |
EO: Essential oil; CfF: Chloroform fraction; EaF: Ethyl acetate fraction; BITC: Benzyl isothiocyanate; -: Not active; NT: Not tested; CI: Clinical isolate; FS: Fluconazole sensitive; FR: Fluconazole resistant; CR: Caspofungin resistant.
Table 3.
Antifungal activity of the essential oil and fractions from the CE of T. pentaphyllum tubers, against dermatophytes and filamentous fungi.
| Fungus | MIC/MFC (µg/mL) | ||||
|---|---|---|---|---|---|
| EO | CfF | EaF | BITC | Itraconazole | |
| Aspergillus fumigatus CI | 80/1280 | 160/1280 | 320/- | 80/1280 | 8/- |
| Aspergillus fumigatus EI | 20/1280 | 80/640 | 40/- | 20/1280 | 16/- |
| Aspergillus flavus CI | NT | 1280/- | NT | 160/1280 | 1/- |
| Aspergillus niger CI | NT | 1280/- | NT | 160/1280 | 2/- |
| Trichophyton rubrum CI | NT | 2.5/320 | NT | 2.5/40 | 2/- |
| Microsporum canis CI | NT | 20/160 | NF | 20/80 | 8- |
| Fonsecaea pedrosoi CI | NT | 80/640 | NT | 40/320 | 1/- |
| Pseudallescheria boydii CI | NT | 80/1280 | NT | 40/640 | 4/- |
| Fusarium solani CI | NT | 320/- | NT | 160/1280 | >16/- |
| Sporothrix schenckii CI | NT | 40/320 | NT | 80/320 | 4/- |
EO: Essential oil; CfF: Chloroform fraction; EaF: Ethyl acetate fraction; BITC: Benzyl isothiocyanate;-: Not active; NT: Not tested; CI: Clinical isolate; EI: Environmental isolate; FS: Fluconazole sensitive; FR: Fluconazole resistant; CR: Caspofungin resistant.
Antifungal activity is presented on Table 2 and Table 3, divided between yeasts and filamentous fungi. Strong activity was observed for the EO, benzyl isothiocyanate (BITC) standard and CfF, for both fungal forms. Fungicidal effect of these samples was observed for the majority of the tested fungi, the exceptions being some of the filamentous fungi. Fluconazole-resistant yeasts, such as C. dubliniensis and C. glabrata, were sensitive to both BITC and CfF, showing a lower MIC value, as well a fungicidal effect.
2.3. Chromatographic Analysis
The most active tested samples in the antimicrobial activity evaluation were the EO and the CfF, as presented above, and therefore these were submitted to gas chromatography-mass spectrometry (GC-MS) analysis. The chromatograms are shown in Figure 1 and Figure 2. Table 4 complements Figure 2, presenting the composition of the CfF according to the peaks shown in the chromatogram.
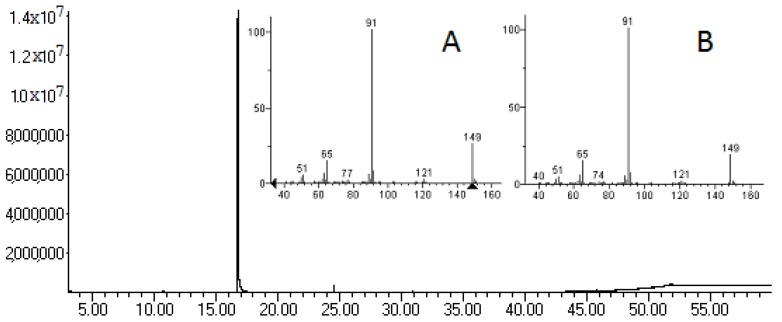
Figure 1.
GC-MS chromatogram from the essential oil (EO) of T. pentaphyllum tubers, with mass spectra of benzyl isothiocyanate (BITC) standard from National Institute of Standards and Technology (NIST) library (A) and from peak with retention time of 16.76 min (B).
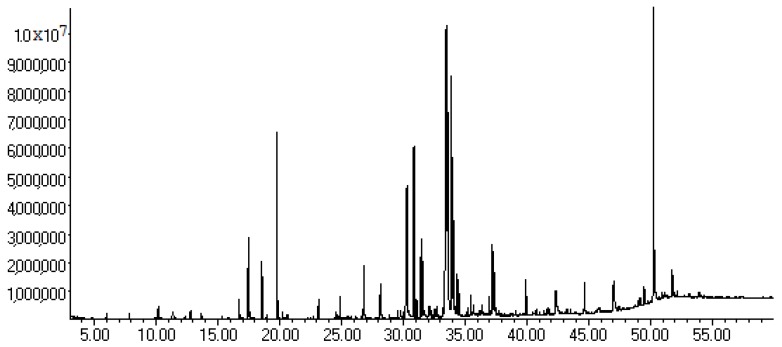
Figure 2.
GC-MS chromatogram from the chloroform fraction of the CE from T. pentaphyllum tubers.
Table 4.
Identified compounds from chloroform fraction (CfF) of the CE from T. pentaphyllum tubers by GC-MS.
| Compound | RT (min) | Relative Area (%) | (M+), m/z (%) | Major Fragment Ions, m/z (%) |
|---|---|---|---|---|
| Benzyl isothiocyanate | 16.72 | 0.65% | 149 (16) | 91 (100) 65 (14) 92 (7) 89 (5) 63 (5) 51 (4) 50 (3) 90 (3) 62 (2) |
| 2-Phenylacetamide | 17.49 | 4.37% | 135 (18) | 91 (100) 92 (91) 65 (20) 44 (12) 63 (8) 89 (7) 51 (5) 93 (5) 90 (4) |
| N-benzylacetamide | 18.56 | 2.21% | 149 (71) | 106 (100) 91 (28) 43 (20) 107 (15) 77 (13) 79 (13) 51 (8) 65 (7) 150 (7) |
| Benzylcarbamide | 19.80 | 6.23% | 150 (50) | 106 (100) 79 (41) 91 (34) 77 (34) 51 (23) 107 (20) 78 (15) 104 (15) 65 (13) |
| Palmitic acid | 30.29 | 6.75% | 256 (30) | 73 (100) 60 (84) 43 (73) 57 (63) 55 (57) 41 (56) 129 (43) 71 (37) 69 (31) 83 (24) |
| Ethyl palmitate | 30.86 | 4.66% | 284 (7) | 88 (100) 101 (58) 43 (26) 55 (22) 41 (20) 89 (17) 57 (16) 70 (16) 157 (15) 73 (15) |
| Elemental sulfur | 31.61 | 1.62% | 256 (70) | 64 (100) 160 (53) 128 (52) 192 (41) 258 (25) 32 (21) 96 (21) 224 (18) 66 (11) |
| Oleic acid | 33.60 | 10.25% | 282 (1) | 55 (100) 69 (72) 41 (67) 83 (58) 97 (49) 67 (45) 43 (45) 81 (37) 84 (34) 57 (32) |
| Ethyl-9,12-octadecadienoate | 33.90 | 6.98% | 308 (8) | 67 (100) 81 (90) 95 (65) 55 (61) 41 (46) 79 (43) 82 (42) 68 (38) 96 (35) 54 (34) |
| Ethyl oleate | 34.02 | 5.88% | 310 (6) | 55 (100) 69 (71) 41 (63) 83 (60) 88 (57) 97 (51) 84 (49) 43 (47) 96 (45) 101 (45) |
| Butyl palmitate | 34.38 | 1.03% | 312 (6) | 56 (100) 57 (47) 257 (41) 43 (35) 41 (32) 55 (28) 73 (24) 60 (24) 239 (20) 129 (20) |
| Unknown phytosterol | 50.26 | 11.35% | 414 (100) | 43(95) 107 (68) 105 (66) 145 (65) 95 (62) 55 (61) 81 (60) 329 (59) 303 (58) |
RT: retention time.
As can be seen in Figure 1, the GC-MS analysis revealed that the major component of the oil is BITC. CfF is a more complex and varied extract, constituted by BITC, amides, sulfur, fatty acids and its esters, with some of the other major components described as a phytosterol and oleic acid (Table 4).
3. Discussion
Overall the antimicrobial activity was strong for the EO and the CfF, with MICs below the cutoff point for promising activity (i.e., lower than 200 µg/mL) [7]. In addition to the bacteriostatic effect demonstrated by the low MIC values of the aforementioned extracts, bactericidal activity was also verified, albeit at higher concentrations. Since mostly of the current antibiotics are growth inhibitors, this bactericidal effect can be a relevant improvement, which also highlights a difference between mechanisms of action, indicating some direct action against the bacteria cell instead of protein synthesis inhibition mechanism (i.e., the mechanism of azithromycin) [8].
The antibacterial activity presented for the EO is due to its chemical composition. As presented in Figure 1, the GC-MS analysis revealed that the major component of the oil is BITC. While it is not a new compound to the genus, as it was already identified in Tropaeolum majus [9], this is the first report of its presence in T. pentaphyllum. The occurrence of BITC in T. pentaphyllum tubers explains their pronounced organoleptic properties, the reason behind its use as a condiment.
The antibacterial activity of standard BITC was tested (Table 1), with results highly corresponding those of the EO. This is in accordance with previous publications, which demonstrated BITC bactericidal activity against a range of Gram-negative bacteria, while the tested Gram-positive bacteria were less susceptible [10]. Kim and Lee [11] demonstrated BITC activity against some harmful intestinal bacteria such as Clostridium difficile and E. coli, while not inhibiting other intestinal bacteria, such as Bifidobacterium spp. and Lactobacillus acidophilus, indicating that T. pentaphyllum tubers consumption could have a similar effect against intestinal bacteria. Dias et al. [12] showed that BITC is a stronger growth inhibitor against methicillin-resistant Staphylococcus aureus (MRSA) than allyl and 2-phenylethyl isothiocyanate, with MICs ranging from 2.9 to 110 µg/mL against several MRSA strains, the closest comparison that can be made with our results is that both BITC and EO had a 40 µg/mL MIC against a standard non-MRSA strain in our work.
Antimicrobial mechanisms of action of isothiocyanates (and BITC as well) are not well established, nonetheless several modes of action are proposed such as effects on membranes, inhibition of regulatory systems (quorum sensing), inhibition of respiratory enzymes, induction of heat-shock response, oxidative stress and stringent response [13]. Studies specifically with BITC demonstrated that it can cause the loss of membrane integrity, conversely to what was observed for ampicillin (a reference antibiotic) [10], and it was also the most potent inhibitor of the quorum sensing system CviIR on Chromobacterium violaceum when compared to allyl isothiocyanate and 2-phenylethyl isothiocyanate [14]. Other mechanisms of action verified with BITC were the induction of a heat-shock-like response, reduction of O2 consumption and protein aggregation on Campylobacter jejuni [15,16].
While the EO verified antibacterial activity is solely due to BITC, it is not the case for the CfF, which is a mixture of low polarity compounds as seen in Table 4. BITC is in fact part of this mixture, and it is followed in the chromatographic run by three structurally related compounds: 2-phenylacetamide, N-benzylacetamide and benzylcarbamide. To this moment it is not clear if these compounds are of natural occurrence, or derived from BITC, during the processing and extraction of the tubers. Similar amides were already found in plant extracts [17]. On the contrary, there is some evidence on isothiocyanate reaction products in aqueous medium, including the formation of elemental sulfur [18,19] a minor bacterial growth inhibitor [20], which may be related to the observed effect, since it was encountered in the CfF at 31.61 min.
Additionally contributing to the demonstrated antibacterial activity of CfF are fatty acids and their esters. They comprise the bulk of the fraction and possess antimicrobial activity [21]. Recently Tamokou et al. [22] purified a fraction from the ethyl acetate extract of Albizia adianthifolias stem bark containing only oleic and palmitic acid, two of the major constituents of the CfF, and it was active against E. faecalis and S. aureus, presenting MICs of 400 µg/mL and 200 µg/mL, respectively, which is less pronounced inhibitory activity than that exerted by CfF against the same bacteria (MICs of 80 and 40 µg/mL, respectively). In our study, we highlight the potent effect of the combined compounds of the fraction, rather than the action of individual constituents.
Not only was the antibacterial activity strong, but antifungal activity (Table 2 and Table 3) was also remarkable, observed for the EO, standard BITC and CfF, for both fungal forms. T. rubrum (Table 3), one of the major causes of dermatophytosis, was highly sensitive to both BITC and CfF, with a MIC value of 2.5 µg/mL, almost the same value obtained for the tested antifungal agent, itraconazole (MIC of 2.5 µg/mL). However, itraconazole is a fungistatic agent, whereas BITC and CfF also exerted a fungicidal effect, presenting MFCs of 40 µg/mL and 320 µg/mL, respectively. Another common causative agent of dermatophytosis, M. canis, was also very sensitive to BITC and CfF (MICs of 20 µg/mL). These data corroborates the widespread use of the tubers’ decoction to treat dermatophytosis [5]. Other filamentous fungi that causes cutaneous and subcutaneous infections, such as F. pedrosoi (chromoblastomycosis causative agent) and S. schenckii (sporotrichosis causative agent) were also sensitive to both BITC and CfF, while Aspergillus spp. presented mixed results, A. flavus and A. niger clinical isolates were resistant to CfF (MIC of 1280 µg/mL and with no observed fungicidal activity at the same concentration).
Antifungal activity of isothiocyanates against few fungi species was reported by Drobnica et al. [23] who showed that BITC is in general 3.6 time more potent than allyl isothiocyanate. Equivalent results can be seen, as the authors obtained a MIC of 26.86 µg/mL against filamentous fungus A. niger after four days of incubation, while we observed a MIC of 160 µg/mL for our clinical isolate of A. niger, with two days of incubation. More recently Manici et al. [24] showed isothiocyanates have fungitoxic activity against plant pathogenic fungi, and the proposed mechanisms of action were inactivation of intracellular enzymes by breakdown of disulfide bonds, inhibition of metabolic enzymes by thiocyanate radical (indicated as degradation product of isothiocyanates) and uncoupler action on oxidative phosphorylation.
While the antifungal activity of BITC is more established and explains the EO antifungal properties, the CfF presents a different situation, where activity can be ascribed to the whole set of compounds, instead of selected ones. Elemental sulfur (RT of 31.61 min), at 1.62% of the CfF, can play a major role in the observed antifungal activity. In fact, it is regarded as the oldest of all pesticides, with well-established antifungal action, whether in its inorganic or organic forms [25].
There are no reports in the literature regarding the activity of the amides of the CfF composition (eluted between 17.49 min and 19.80 min). However there are some indications that they can present some inhibitory properties. Antimicrobial activity of the ethyl acetate extract from the algae Trichodesmium erythraeum [26], containing, among other compounds, 2-phenylacetamide (benezeneacetamide) at 17.48%, inhibited the growth of some fungi such as T. rubrum and Trichophyton simii, with MICs of 500 µg/mL and 16.2 µg/mL. CfF was stronger against T. rubrum, with a MIC of 2.5 µg/mL, and 4.37% of 2-phenylacetamide in its composition, suggesting that these amides do not play a prominent role in the antifungal activity of the CfF, albeit a synergistic contribution is possible.
In addition to the BITC and elemental sulfur contribution to the observed antifungal activity, the fatty acids and its esters, which comprise the bulk of the CfF, may also have an important role. Extracts and fractions containing fatty acids have been reported to possess at least fungistatic activity. The results of Tamokou et al. [22] for a purified mixture of oleic and palmitic acid obtained from ethyl acetate extract of Albizia adianthifolia were active against different Candida sp. and C. neoformans, with MICs ranging from 100 µg/mL to 400 µg/mL, higher values than those observed for CfF against a wide range of tested yeasts.
Palmitic and oleic acid, major constituents of CfF (6.75% and 10.25%, respectively), are among the fatty acids reviewed for their antifungal properties [27]. A proposed possible mechanism of antifungal action of fatty acids indicates that they can alter fungal membrane fluidity, causing cell membrane disorganization and leakage of vital components, eventually leading to cell disintegration [28].
The importance of fatty acids in the plant, and in the extract, may not be restricted only to the antimicrobial activity, but also for their action as penetration enhancers, a supporting role in the utilization of the plant against skin infections. Fatty acids and their esters can penetrate the skin, but in addition they could also promote the penetration of other active compounds such as the hydrophobic BITC, the amides and sulfur, to deeper layers of the skin, by disruption and alteration of the stratum corneum lipid strucure [29,30]. An extract from the algae Botryococcus braunii rich in palmitic and oleic acid [31], fatty acids we identified in the CfF, enhanced the skin penetration of flurbiprofen, and while the extract were less effective than the purified fatty acids, it was also less irritating to the skin. Another work [32] demonstrated that fatty acids can penetrate and accumulate to different degrees in the skin, and, in addition, the authors have shown that oleic acid significantly enhanced tolnaftate penetration.
To the moment, of the compounds encountered in the EO and CfF of the tubers from T. pentaphyllum, only the fatty acids have an established relation with the skin. We have not found specific studies concerning BITC, amides and elemental sulfur. Their hydrophobic, and low molecular weight structures put them among candidates for skin penetration and enhancement by combined use along with fatty acids, although experimentation with these compounds is necessary to a better understanding of the behavior towards skin.
4. Materials and Methods
4.1. Plant Material and Extraction Methods
Tubers of T. pentaphyllum were acquired from local farmers in the municipality of Gaurama (Rio Grande do Sul, Brazil), in August 2011, along with the material required for identification. A dried voucher specimen, containing leaves and flowers, is preserved in the herbarium of the Biology Department at the Federal University of Santa Maria under the registration code SMDB-13137.
About 1.9 kg of fresh tubers were initially shredded, after that an amount of 200 g was separated for essential oil extraction and the remaining material was submitted to drying, at 40 °C, in an oven with air flow, during seven days. These shredded and dried tubers were then ground in a knife mill. The resulting dried tuber powder (517 g) was submitted to maceration, at room temperature during a week, with 70% ethanol in water, with a proportion of 8 mL of solvent for each g of plant material. The obtained crude extract (CE) was filtered and the alcoholic solution was evaporated under reduced pressure, in order to eliminate the ethanol. The remaining aqueous solution was successively partitioned with chloroform, ethyl acetate and n-butanol until depletion of the color visible components, which was achieved extracting 300 mL of the aqueous crude extract three times with 300 mL of chloroform, followed by three extractions with 300 mL of ethyl acetate and three extractions with butanol, using 300 mL, 200 mL and 150 mL of solvent. Each organic extract was subsequently dried under reduced pressure, resulting in chloroform (CfF), ethyl acetate (EaF) and n-butanol (BuF) dried fractions from the CE. In addition, an aliquot of the referred crude extract was fully dried, and stored for further use.
Essential oil (EO) from the tubers was obtained from the shredded fresh material (200 g), separated as mentioned above. Extraction was performed through hydrodistillation at 60 °C during four hours, using a Clevenger apparatus. Separation of the oil from the water was achieved in a separatory funnel.
4.2. Antimicrobial Assays
The essential oil, the hydroalcoholic extract and its fractions, as well as standard benzyl isothiocyanate (BITC) were tested against bacteria, yeasts and molds. Susceptibility tests were performed according to the Clinical and Laboratory Standards Institute (CLSI) microdilution technique, M07-A9 [33] for bacteria, M27-A3 [34] for yeasts and M38-A2 [35] for molds. Compounds were solubilized in dimethyl sulfoxide (DMSO) to obtain stock solutions with final concentration of 128,000 µg/mL. The solutions were used immediately after preparation, and all the analysis were executed in triplicate. Final concentration of DMSO in the testing wells was of 0.01%, experimentally determined to not interfere with the analysis. The tested concentrations ranged from 2.5 µg/mL to 1280 µg/mL for all of the microorganisms. The results of the tests were expressed as the minimal inhibitory concentration (MIC), minimal fungicidal concentration (MFC) and minimal bactericidal concentration (MBC).
4.2.1. Antibacterial Activity
The following six bacteria strains were tested: Enterococcus faecalis ATCC 91299, Escherichia coli ATCC 5922, Klebsiela pneumoniae ATCC 700603, Pseudomonas aeruginosa ATCC 27853, Salmonella pullorum ATCC 9140, Staphylococcus aureus ATCC 29213. The isolates were stored in 10% glycerol solution at −70 °C and revived by subculturing in Mueller-Hinton Agar (MHA). The compounds were tested by microdilution broth assay using Mueller-Hinton Broth (MHB) medium in 96-well microplates. The inoculum was prepared by growing bacteria at 37 °C for 24 h in MHA. Colonial growth was suspended in 2 mL of saline, to an approximate 0.5 McFarland turbidity or 1 × 108 colony-forming unit per mL (CFU/mL). The inoculum was diluted (1:20) in MHB and added to all wells, except negative control. The plates were incubated at 37 °C during 24 h. Antibacterial activity was detected by adding 20 µL of 0.5% TTC (triphenyltetrazolium chloride, Merck, Darmstadt, Germany) solution. MIC was defined as the lowest concentration of compounds that inhibited visible growth, as indicated by the TTC staining. Bacterial suspensions from all tests wells were subcultured in sterile agar medium in order to evaluate bactericidal activity.
4.2.2. Antifungal Activity
Dilutions of the compounds were prepared in RPMI-MOPS 1640 medium, in order to obtain two times the final concentrations, and 100 µL of each concentration of extracts was added to columns 1 to 10 of each 96-well microplates. After, 100 µL of standard inoculum was added to all wells, except negative control. The plates were incubated for 48 h at 35 °C.
Strains of fourteen species of yeasts were used, including clinical isolates, caspofungin/fluconazole sensitive or resistant species and standard ATCC/CBS strains. The clinical isolates were obtained from the private collection of the Mycology Research Laboratory, Federal University of Santa Maria, Santa Maria, Brazil (LAPEMI-UFSM) and identified based on carbohydrate assimilation profiles using by ID32-C test (bioMérieux). The strains were grown in Sabouraud Dextrose Agar (SDA) for 48 h at 35 °C to inoculum preparation. The following yeast were used (Candida albicans ATCC 140053, Candida dubliniensis CBS 7987, Candida dubliniensis fluconazole-sensitive, Candida dubliniensis fluconazole-resistant, Candida glabrata ATCC 2001, Candida glabrata fluconazole-sensitive, Candida glabrata fluconazole-resistant, Candida glabrata caspofungin-resistant, Candida guilliermondii, Candida parapsilosis ATCC 22018, Candida parapsilosis caspofungin-resistant, Candida tropicalis, Cryptococcus neoformans ATCC 90012, Saccharomyces cerevisiae ATCC 260).
Strains of ten species of molds were used, including clinical isolates (CI) and environmental isolates (EI) as follows (Aspergillus fumigatus (CI), Aspergillus fumigatus (EI-isolated from maize), Aspergillus flavus (CI), Aspergillus niger (CI), Trichophyton rubrum (CI), Microsporum canis (CI), Fonsecaea pedrosoi (CI), Pseudallescheria boydii (CI), Fusarium solani (CI), Sporothrix schenckii (CI)). The clinical and environmental isolates were obtained from the private collection of the Mycology Research Laboratory, Federal University of Santa Maria, Santa Maria, Brazil (LAPEMI-UFSM) and identified by macroscopic and microscopic examination. The strains were grown in Potato Dextrose Agar (PDA) from 48 h until seven days at 35 °C for inoculum preparation.
The MIC is defined as the lowest concentration of the compound that inhibits the visible growth of a microorganism after 48 h of incubation, as indicated by the TTC staining. Fungal suspensions from all tests wells were subcultured in sterile Sabouraud Dextrose Agar medium in order to evaluate fungicidal activity.
4.3. Chromatographical Analysis
The essential oil and the chloroform fraction of the crude extract were submitted to separation through gas chromatography, with detection by mass spectrometry (GC-MS). The ethyl acetate and n-butanol fractions were not suitable for this analysis due to their physical and chemical properties, moreover, chloroform fraction exhibited better results in the antimicrobial activity assay making it the logical choice for a more in-depth investigation.
The analysis was performed in a model 6890N chromatograph, coupled with a 5975B model mass detector, both from Agilent Technologies. Chromatographic conditions were as follows: oven initial temperature was 50 °C, during one minute, followed by heating at a rate of 5 °C/min until 300 °C, remaining this temperature during 9 min, of a total of 60 min run; separation was achieved in a DB-5MS column (30 m × 320 µm × 0.25 µm), at a constant rate of 1.5 mL/min of helium; detection was performed in the quadrupole equipment using electron ionization. Peak identification was carried by comparison of the experimental mass spectrum with those of the National Institute of Standards and Technology (NIST) standard library and published papers.
5. Conclusions
The in vitro antimicrobial activity tested and reported corroborates the popular use of the plant’s tubers against cutaneous infections, signaling a possible alternative for the treatment of resistant skin infections, especially of fungal origin. The chemical findings suggest that more than one compound may be related to this effect, with the pro-eminence of benzyl isothiocyanate. Its presence not only explains and is related to the antimicrobial activity, but it is also relevant to its edible aspects. The reported results should pave the way for more investigations, aiming at determining the in vivo toxicity and pharmacological effects, and a more in-depth evaluation of the plant composition.
Acknowledgments
We would like to thank Margareth Linde Athayde (Universidade Federal de Santa Maria), for her advice and support in the development of the several aspects of this work. We are also very grateful to Professor Renato Aquino Záchia (Universidade Federal de Santa Maria), for the identification of the voucher specimen.
Author Contributions
Ritiel Corrêa da Cruz, Sydney Hartz Alves and Marli Matiko Anraku de Campos conceived and designed the experiments; Ritiel Corrêa da Cruz, Laura Bedin Denardi, Natalia Jank Mossmann and Mariana Piana performed the experiments; Ritiel Corrêa da Cruz and Laura Bedin Denardi analyzed the data; Sydney Hartz Alves and Marli Matiko Anraku de Campos contributed with materials, equipment and tools for the execution of the experiments; Ritiel Corrêa da Cruz wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of all the extracts and the essential oil are available from the authors.
References
- 1.Rix M. Tropaeolaceae: 687. Tropaeolum pentaphyllum. Curtis’s Bot. Mag. 2010;27:296–300. doi: 10.1111/j.1467-8748.2010.01706.x. [DOI] [Google Scholar]
- 2.Trojan-Rodrigues M., Alves T.L.S., Soares G.L.G., Ritter M.R. Plants used as antidiabetics in popular medicine in Rio Grande do Sul, southern Brazil. J. Ethnopharmacol. 2012;139:155–163. doi: 10.1016/j.jep.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 3.Zuchiwschi E., Fantini A.C., Alves A.C., Peroni N. Limitações ao uso de espécies florestais nativas pode contribuir com a erosão do conhecimento ecológico tradicional e local de agricultores familiares. Acta Bot. Bras. 2010;24:270–282. doi: 10.1590/S0102-33062010000100029. [DOI] [Google Scholar]
- 4.Agneta R., Möllers C., Rivelli A.R. Horseradish (Armoracia rusticana), a neglected medical and condiment species with a relevant glucosinolate profile: A review. Genet. Resour. Crop. Evol. 2013;60:1923–1943. doi: 10.1007/s10722-013-0010-4. [DOI] [Google Scholar]
- 5.Fenner R., Betti A.H., Mentz L.A., Rates S.M.K. Plantas utilizadas na medicina popular brasileira com potencial atividade antifúngica. Braz. J. Pharm. Sci. 2006;42:369–394. doi: 10.1590/S1516-93322006000300007. [DOI] [Google Scholar]
- 6.Aly R. Microbial infections of skin and nail. In: Baron S., editor. Medical Microbiology. 4th ed. University of Texas Medical Branch; Galveston, TX, USA: 1996. [PubMed] [Google Scholar]
- 7.Cos P., Vlietinck A.J., Berghe V.D., Maes L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006;106:290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Pubchem Azithramycine. [(accessed on 20 February 2016)]; Available online: https://pubchem.ncbi.nlm.nih.gov/compound/55185?from=summary#section=Top.
- 9.Kjaer A., Madsen Ø., Maeda Y. Seed volatiles within the family Tropaeolaceae. Phytochemistry. 1978;17:1285–1287. doi: 10.1016/S0031-9422(00)94575-8. [DOI] [Google Scholar]
- 10.Sofrata A., Santagelo E.M., Azeem M., Borg-Karlson A.-K., Gustafsson A., Pütsep K. Benzyl isothiocyanate, a major component from the roots of Salvadora persica is highly active against Gram-negative bacteria. PLoS ONE. 2011;6:e23045. doi: 10.1371/journal.pone.0023045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim M.G., Lee H.S. Growth-inhibiting activities of phenethyl isothiocyanate and its derivatives and against intestinal bacteria. J. Food. Sci. 2009;74:467–471. doi: 10.1111/j.1750-3841.2009.01333.x. [DOI] [PubMed] [Google Scholar]
- 12.Dias C., Aires A., Saavedra M.J. Antimicrobial activity of isothiocyanates from cruciferous plants against Methicillin-Resistant Staphylococcus aureus (MRSA) Int. J. Mol. Sci. 2014;15:19552–19561. doi: 10.3390/ijms151119552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dufour V., Stahl M., Baysse C. The antibacterial properties of isothiocyanates. Microbiology. 2015;161:229–243. doi: 10.1099/mic.0.082362-0. [DOI] [PubMed] [Google Scholar]
- 14.Borges A., Serra S., Abreu A.C., Saavedra M.J., Salgado A., Simões M. Evaluation of the effects of selected phytochemicals on quorum sensing inhibition and in vitro cytotoxicity. Biofouling. 2014;30:183–195. doi: 10.1080/08927014.2013.852542. [DOI] [PubMed] [Google Scholar]
- 15.Dufour V., Alazzam B., Ermel G., Thepaut M., Rossero A., Tresse O., Baysse C. Antimicrobial activities of isothiocyanates against Campylobacter jejuni isolates. Front. Cell Infect. Microbiol. 2012;2:1–13. doi: 10.3389/fcimb.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dufour V., Stahl M., Rosenfeld E., Stintzi A., Baysse C. Insights into the mode of action of benzyl isothiocyanate on Campylobacter jejuni. Appl. Environ. Microbiol. 2013;79:6958–6968. doi: 10.1128/AEM.01967-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalil A.T. Benzylamides from Salvadora persica. Arch. Pharm. Res. 2006;29:952–956. doi: 10.1007/BF02969277. [DOI] [PubMed] [Google Scholar]
- 18.Kawakishi S., Namiki M. Decomposition of allyl isothiocyanate in aqueous solution. Agric. Biol. Chem. 1968;33:452–459. doi: 10.1080/00021369.1969.10859329. [DOI] [Google Scholar]
- 19.Pecháček R., Velišek J., Hrabcová H. Decomposition products of allyl isothiocyanate in aqueous solution. J. Agric. Food Chem. 1997;45:4584–4588. doi: 10.1021/jf970316z. [DOI] [Google Scholar]
- 20.Libenson L., Hadley F.P., McIlroy A.P., Wetzel V.M., Mellon RR. Antibacterial effect of elemental sulfur. J. Infect. Dis. 1953;93:28–35. doi: 10.1093/infdis/93.1.28. [DOI] [PubMed] [Google Scholar]
- 21.Kabara J.J., Swieczkowski D.M., Conley A.J., Truant J.P. Fatty acids and derivatives as antimicrobial agents. Antimicrob. Agents Chemother. 1972;2:23–28. doi: 10.1128/AAC.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamokou J.D., Mpetga D.J.S., Lunga P.K., Tene M., Tane P., Kuiate J.R. Antioxidant and antimicrobial of ethyl acetate extract, fractions and compounds from stem bark of Albizia adianthifolia (Mimosoideae) BMC Complement. Altern. Med. 2012;12:1–10. doi: 10.1186/1472-6882-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drobnica L., Zemanová M., Nemec P., Antos K., Kristián P., Stullerová A., Knoppová V., Nemec P., Jr. Antifungal activity of isothiocyanates and related compounds I. Naturally ocurring isothiocyanates and their analogues. Appl. Environ. Microb. 1967;15:701–709. doi: 10.1128/am.15.4.701-709.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manici L.M., Lazzeri L., Palmieri S. In vitro Fungitoxic activity of some glucosinolates and their enzyme-derived products toward plant pathogenic fungi. J. Agric. Food Chem. 1997;45:2768–2773. doi: 10.1021/jf9608635. [DOI] [Google Scholar]
- 25.Tweedy B.G. Inorganic sulfur as a fungicide. In: Gunther F.A., Gunther J.D., editors. Residue Reviews. Springer; New York, NY, USA: 1981. pp. 43–68. [Google Scholar]
- 26.Thillairajasekar K., Duraipandiyan V., Perumal P., Ignacimuthu S. Antimicrobial activity of Trichodesmium erythraeum (Ehr) (microalga) from South East coast of Tamil Nadu, India. Int. J. Integr. Biol. 2009;5:167–170. [Google Scholar]
- 27.Pohl C.H., Kock J.L.F., Thibane V.S. Antifungal free fatty acids: A review. In: Méndez-Vilaz A., editor. Science against Microbial Pathogens: Communicating Current Research and Technological Advances. Formatex; Badajoz, Spain: 2011. pp. 61–71. [Google Scholar]
- 28.Avis T.J., Bélanger R.B. Specificity and mode of action of the antifungal fatty acid cis-9-Heptadecenoic acid produced by Pseudozyma flocculosa. Appl. Environ. Microbiol. 2001;67:956–960. doi: 10.1128/AEM.67.2.956-960.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moser K., Kriwet K., Naik A., Kalia Y.N., Guy R.H. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2001;52:103–112. doi: 10.1016/S0939-6411(01)00166-7. [DOI] [PubMed] [Google Scholar]
- 30.Fox L.T., Gerber M., Du Pleiss J., Hamman J.H. Transdermal drug delivery enhancement by compounds of natural origin. Molecules. 2011;16:10507–10540. doi: 10.3390/molecules161210507. [DOI] [Google Scholar]
- 31.Fang J.-Y., Chiu H.-C., Wu J.-T., Chiang Y.-R., Hsu S.-H. Fatty acids in Botryococcus braunii accelerate topical delivery of flurbiprofen into and across skin. Int. J. Pharm. 2004;276:163–173. doi: 10.1016/j.ijpharm.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 32.Kezutyte T., Desbenoit N., Brunelle A., Briedis V. Studying the penetration of fatty acids into human skin by ex vivo TOF-SIMS imaging. Biointerphases. 2013;8:1–8. doi: 10.1186/1559-4106-8-3. [DOI] [PubMed] [Google Scholar]
- 33.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically. Approved Standard, M07-A9. 9th ed. CLSI; Wayne, MI, USA: 2012. [Google Scholar]
- 34.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically. Approved Standard, M27-A3. 3th ed. CLSI; Wayne, MI, USA: 2008. [Google Scholar]
- 35.Clinical and Laboratory Standards Institute . Reference Method for Broth Dilution Antifungals Susceptibility Testing of Conidium-Forming Filamentous Fungi: Approved Standard, M38-A2. 2nd ed. CLSI; Wayne, MI, USA: 2008. [Google Scholar]