Abstract
This study deals with a new and efficient metal-free regioselective synthesis of pyrimido-fused indazoles with nitrogen ring junction motifs. We have developed a metal-free domino type reaction between 3-aminoindazole, aryl aldehydes and aceotophenones in the presence of KOH/DMF that leads to pyrimido[1,2-b]indazole analogues. Response Surface Methodology (RSM) coupled with a Box-Behnken design (BBD) were utilized for exploring the effect of base used (A), temperature of reaction (B) and (C), reaction time. This approach can allow access to a variety of pyrimidoindazole fluorophores and related compounds. The compound N,N-dimethyl-4-(2-phenylpyrimido[1,2-b]indazol-4-yl)aniline (4e) displays the maximum fluorescence intensity at 518 nm and shows a fluorescence quantum yield of 0.068. The synthesized pyramido-fused indazoles have been evaluated for their free radical scavenging activity and compound 4f showed good antioxidant activity.
Keywords: N-fused pyrimidine, Strecker synthesis, A3 coupling, Box-Behnken design (BBD), fluorescence
1. Introduction
Multi-component reactions (MCRs) play an important role in combinatorial chemistry [1]. This method has the capability for producing desired small molecule drugs with various degrees of structural diversity [2]. When three or more than three substances or reactants are reacted simultaneously to produce defined target molecules these reactions are said to be MCRs. The products display features of all the inputs and thus afford greater possibilities for molecular diversity [3]. The cascade/domino/tandem process [4,5,6], involves subsequent transformations of functionalities produced in the previous step [7]. Normally, these reactions are easier to carry out by one pot synthesis than multi-step synthesis [8]. In addition, the isolation of an intermediates are not required in MCR conditions and they are perfect candidates for drug discovery and combinatorial synthesis [9]. The most desirable potential drug candidates are libraries of small organic molecules which have limitations as bioavailable therapeutics. We can built up very large libraries with in short time by using a small set of reactants [10]. Conversely, the significance of MCRs for discovery of novel drugs has been verified certified by substantial industries with academic aspects [11]. The studies have aimed to develop efficient MCR protocols for the generation of a series of heterocyclic compounds. MCRs such as the Strecker synthesis [12], Hantzsch synthesis [13], Biginelli synthesis [14], Mannich reaction [15], Kabachnik-Fields reaction [16], Bucherer-Bergs reaction [17], Gewald reaction [18], Willgerodt-Kindler reaction [19], Ugi reaction [20] and A3 coupling [21] are named reactions which include the carbonyl compounds as one of the reactants. The synthetic arylation via transition metal-free conditions [22] and synthesis of a series of unreported N-fused heterocycles [23,24] was reported by our group. Very recently, our research group proposed a metal catalysed method for the facile preparation of pyrimido-fused indazoles [25]. This is a metal catalysed A3 coupling reaction between aminoindazoles, alkynes and aldehydes, that further proceeds via 6-endo-dig cyclization focusing on the functionalization of pyrimido[1,2-b]indazole derivatives. As a continuation in our synthetic strategy, we report now a method to synthesize highly functionalized pyrimido-fused indazole derivatives in good to excellent yields via cascade/tandem/domino reactions under metal-free conditions. It offers simple reaction conditions, easy protocols and cheap starting materials.
2. Results and Discussion
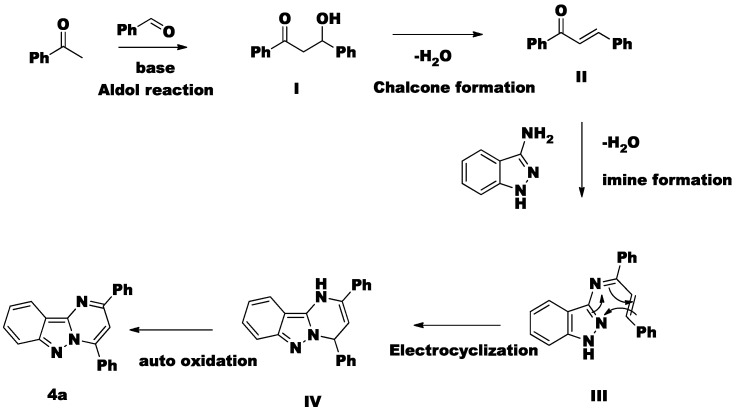
The proposed protocol for the preparation of 2,4-diphenylpyrimido[1,2-b] indazole (4a) via the proposed metal-free cascade process is illustrated in Scheme 1. Initially, acetophenone 2a and benzaldehyde 3a were undergo an aldol reaction in the presence of base providing α,β-unsaturated carbonyl intermediate II. When intermediate II is coupled with an amine with the removal of one water molecule it gives an imine (intermediate III). Then intermediate III undergoes electrocyclization followed by auto-oxidation leading to the formation of 4a.
Scheme 1.
Plausible mechanism of the synthesis of compound 4a.
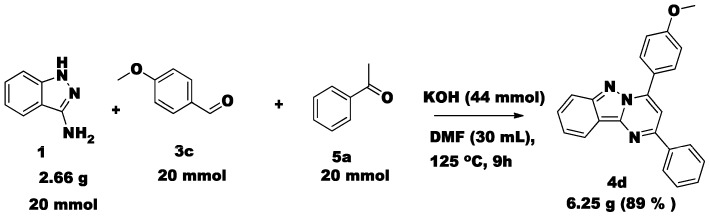
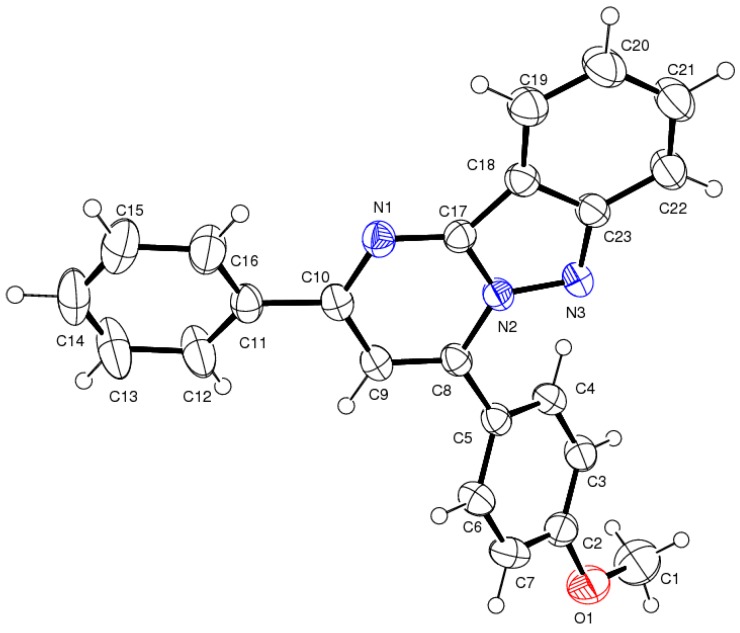
Substituted aldehydes and acetophenones were transformed into the desired products without any difficulty, which clearly indicates that steric hindrance will not affect product formation. Unfortunately, nitro- and hydroxyl-substituted aromatic aldehydes failed to provide the target coupling products. We have investigated the gram scale preparation of compound 4d (Scheme 2) from 20 mmol of 3-aminoindazole (1), 20 mmol of acetophenone (5a) and 20 mmol of 4-methoxy-benzaldehyde (3d) with good yield (89%). The synthesized compound 4d was characterized by melting point, proton and carbon NMR and HRMS. Along with this data, we have confirmed the structure of 4d by single crystal XRD data (CCDC 1456539). The ORTEP diagram is illustrated in Figure 1.
Scheme 2.
Compounds 4d gram scale preparation.
Figure 1.
The ORTEP representation of compound 4d.
RSM is a statistical and mathematical method for empirical modelling and we have optimized a response (output variables) influenced by numerous independent variables (input variables) with a careful design of experiments. It can analyse the effect of multiple or combined variables in a minimal number of experiments and to evaluate the influences of experimental factors on the response by an accurate second order polynomial equation [26,27,28,29,30,31,32]. Aldehydes react with phenylacetylene and aminoindazole to give pyrimido[1,2-b]indazoles by C-2 subsitution in the presence of a metal (Scheme 1). Contrarily, aldehyde substitution occurs at the C-4 position in the absence of a metal catalyst. As part of our continuing effort, our research group has disclosed a simple and highly effective method for the preparation of N-fused pyrimido[1,2-b]indazoles 4(a–s) via a domino process. RSM coupled with BBD was used for various conditions to fine-tune the working parameters. The selection level is based on teach variables (Table 1).
Table 1.
A selection of levels and variables used for BBD.
| Reaction | Variables | Code | Units | Levels | ||
|---|---|---|---|---|---|---|
| −1 | 0 | +1 | ||||
| Metal free cascade Reaction | Base used (equivalent) | A | mg | 1 | 2 | 3 |
| Reaction temperature | B | °C | 110 | 120 | 130 | |
| Reaction time | C | h | 4 | 8 | 12 | |
From the optimized A3 coupling method [25], we have observed that the aldehyde easily substituted at the C-2 position in the pyrimido-fused indazoles. In order to increase the scope of the products, we have developed another novel synthetic route to synthesize pyrimido-fused indazoles via metal-free one pot multi-MCRs. In this case, we found that the aldehydes are certainly substituted at C-4 position in the core structure. This cascade reaction proceeds under metal-free conditions with the reaction between aldehydes, ketones and amines. Our research group started performing reactions between 3-aminoindazole (1), acetophenone (2a) and benzaldehyde (3a) under neat conditions for 8 h, but unfortunately the expected compound was not identified (Table 2, entries 1 and 2). The conditions of reactions are optimized by varying organic solvent, organic bases and inorganic bases (Table 1, entries 3–15) but the expected target molecule was obtained between 16%–93%. After the various combinations of bases and solvents, we have found that the expected product 2,4-diphenylpyrimido[1,2-b]indazole 4a in 56% yield was noted in KOH and EtOH combination at 80 °C for 8 h (Table 1, entry 9). In order to increase the yield further, we have tried with KOH base in various organic solvents like MeOH, DMSO, DMF, 1,4-dioxane, THF and acetonitrile. Finally, we have attained upto 93% yield in KOH/DMF combination at 120 °C for 8 h (Table 1, entry 12).
Table 2.
Optimization of cascade reaction to synthesis compound 2,4-diphenylpyrimido[1,2-b]indazole (4a) via metal free conditions a.
| Entry | Base | Solvent | Temp (°C) | Yield b (%) |
|---|---|---|---|---|
| 1 | - | - | 100 | NR c |
| 2 | - | EtOH | 80 | NR c |
| 3 | Triethylamine | EtOH | 80 | NR c |
| 4 | Na2CO3 | EtOH | 80 | Traces |
| 5 | Piperidine | EtOH | 80 | NR c |
| 6 | NaOtBu | t-BuOH | 100 | 40 |
| 7 | KOtBu | t-BuOH | 100 | 30 |
| 8 | NaOH | EtOH | 80 | 50 |
| 9 | KOH | EtOH | 80 | 56 |
| 10 | KOH | MeOH | 70 | 40 |
| 11 | KOH | DMSO | 120 | 45 |
| 12 | KOH | DMF | 120 | 93 |
| 13 | KOH | 1,4-Dioxane | 80 | 25 |
| 14 | KOH | THF | 80 | 16 |
| 15 | KOH | Acetonitrile | 80 | 16 |
a Reactions proceed with 1 mmol of 1, 2a and 3a in 5 mL of solvent. Optimized condition are denoted in bold letters there are none. The reactions were carried out at various temperatures for 8 h. b Isolated yield and c NR-No reaction. Letters refer to wrong things.
The BBD statistical design is presented in Table 3. We have utilized three variables such as base (A), temperature (B) and time (C) for the reactions without metal. By using multi-regression and backward eradication, the best-fitting models were determined.
Table 3.
BBD matrix response and its design a.
| Run | Metal Free Condition | ||||
|---|---|---|---|---|---|
| A | B | C | Y (%) | X (%) | |
| 2 | 120 | 8 | 93.01 | 90.80 | |
| 2 | 120 | 8 | 89.00 | 90.80 | |
| 1 | 120 | 4 | 40.33 | 30.12 | |
| 1 | 130 | 8 | 62.03 | 65.62 | |
| 3 | 130 | 8 | 82.09 | 77.12 | |
| 2 | 110 | 4 | 34.12 | 39.00 | |
| 3 | 110 | 8 | 80.31 | 76.37 | |
| 3 | 120 | 4 | 50.41 | 48.62 | |
| 2 | 120 | 8 | 91.01 | 90.80 | |
| 3 | 120 | 12 | 71.00 | 80.88 | |
| 1 | 110 | 8 | 50.21 | 54.87 | |
| 2 | 110 | 12 | 82.07 | 75.75 | |
| 2 | 130 | 4 | 41.09 | 47.25 | |
| 1 | 120 | 12 | 65.21 | 66.37 | |
| 2 | 120 | 8 | 88.44 | 90.80 | |
| 2 | 130 | 12 | 84.51 | 79.00 | |
| 2 | 120 | 8 | 93.01 | 90.80 | |
a Y—Experimental Yield, X—Predicted Yield.
Depending on the reaction phenomenon, we have found the yield range was 41%–93%. Due to the reaction performed by the above method the correlation between the process variables and the isolated yield (Y) with a quadratic polynomial model is represented in Equation (1):
| Y = 90.80 + 8.25A + 2.88 B + 17.13C − 2.50 AB − 1.00 AC − 1.25 BC − 13.03 A − 9.28 B − 21.27C | (1) |
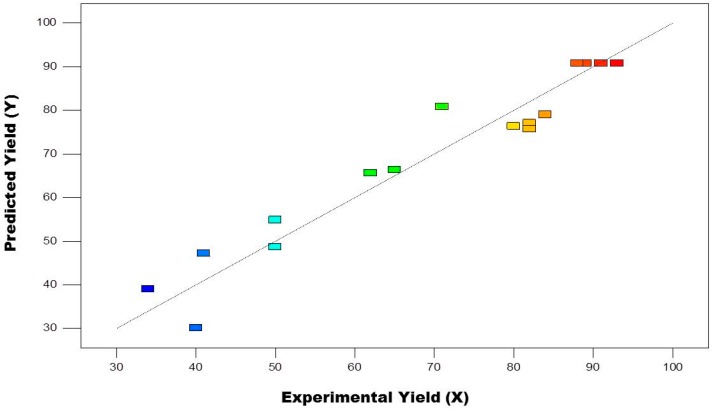
where A, B and C represents the variables coded and Y represents isolated yield. Figure 2 depicts the respectable correlation linear between actual and predicted yields.
Figure 2.
The predicted and actual isolated yield values.
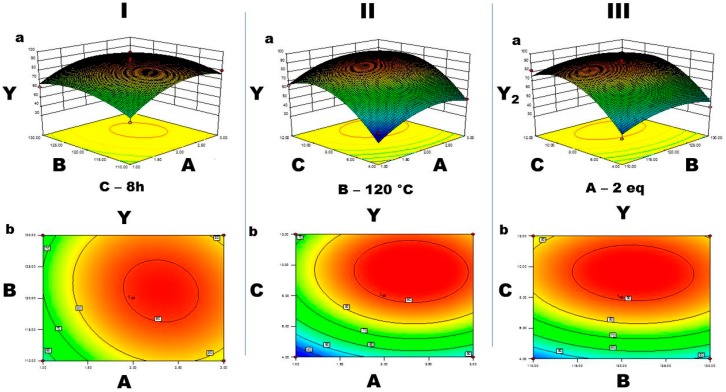
ANOVA results for response surface quadratic models are shown in Table 4. The F-value model is 11.57 and it suggests the model was significant. F value has a chance of 0.002% of being due to noise. For this model A, C, A2, B2, C2 are significant model terms. The values <0.1 represent that the model terms are non-significant. The lack of fit F value of 25.69 suggests that lack of fit is significant. The “Pred R-Squared” of 0.0372 is not as close to the “Adj R-Squared” of 0.8560 as one might usually expect; i.e., the difference is more than 0.002. All empirical models should be tested by doing confirmation runs. “Adeq Precision” noise to signal ratio measures and ratio <4 is desirable. The adequate signal with the ratio of 10.194 implies that we utilized the above model for space design navigation. Effect of interaction between base (A) and temperature of the reaction (B) time constant of reaction of 8 h (C) (Figure 3).
Table 4.
Quadratic model by ANOVA for response surface a.
| Source | Sum of Squares | DF | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 6271.98 | 9 | 696.89 | 11.57 | 0.0020 | S |
| A | 544.50 | 1 | 544.50 | 9.04 | 0.0197 | |
| B | 66.13 | 1 | 66.13 | 1.10 | 0.3295 | |
| C | 2346.12 | 1 | 2346.12 | 38.96 | 0.0004 | |
| AB | 25.00 | 1 | 25.00 | 0.42 | 0.5399 | |
| AC | 4.00 | 1 | 4.00 | 0.066 | 0.8040 | |
| BC | 6.25 | 1 | 6.25 | 0.10 | 0.7567 | |
| A | 714.32 | 1 | 714.32 | 11.86 | 0.0108 | |
| B | 362.21 | 1 | 362.21 | 6.01 | 0.0439 | |
| C | 1905.79 | 1 | 1905.79 | 31.65 | 0.0008 | |
| Residual | 421.55 | 7 | 60.22 | |||
| Lack of Fit | 400.75 | 3 | 133.58 | 25.69 | 0.0045 | S |
| Pure Error | 20.80 | 4 | 5.20 | |||
| Correlation Total | 6693.53 | 16 |
a Where S—Significant, DF—Degree of freedom.
Figure 3.
Contour plots for parameters combined yield for reaction conditions without metal. (I) Effect of A and B; (II) Effect of A and C; (III) Effect of B and C.
Plots of contour base (A) and temperature (B) (Figure 3IB) are circular, which result in lower a interaction between A and B. Plots of contour base (A) and time (C) (Figure 3IIB), temperature (B) and time (C) (Figure 3IIIC) are elliptical, which shows a good relation of interaction between A and C, and between B and C. The optimized condition for predicted and actual isolated yields is revealed in Table 5.
Table 5.
Model validation for metal free reaction.
| Parameter | Base Equivalent (A) | Reaction Temperature °C (B) | Reaction Time (min) (C) | % Yield |
|---|---|---|---|---|
| Predicted | 2.23 | 125.3 | 9.17 | 93.5 |
| Experimental | 2.0 | 120.0 | 8.0 | 93.0 |
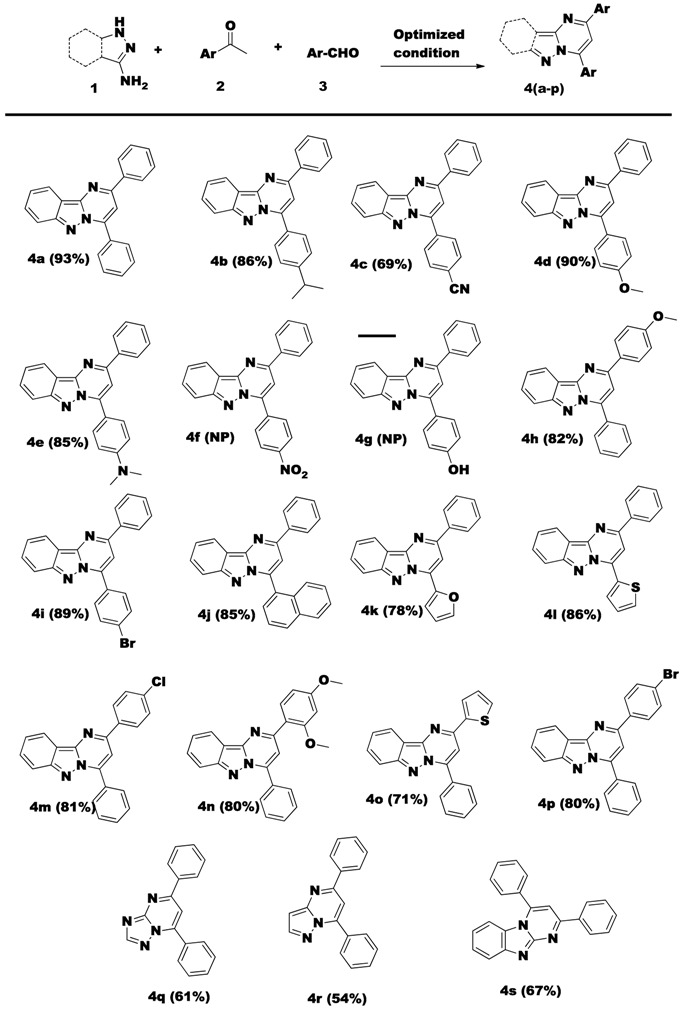
To further confirm the suitability for this model for identifying a higher yield, three confirmation runs were performed using the optimum parameters. The isolated yield results of compound 4a were 93.0%, 92.4% and 93.7%. To validate the efficiency of the metal-free conditions, the choice of the reactants were explored under the optimized condition and the results are indicated in Table 6. A series of amines 1(a–d), aryl aldehydes 3(a–k) and acetophenones 2(a–f) were subsequently converted into the corresponding pyrimido-fused indazoles 4(a–s) in good to moderate yields.
Table 6.
Syntheses of N-fused pyrimidine derivatives 4(a–p).
 |
The synthesized N-fused analogues 4(a–s) were orange to red powders. The solvatochromism spectra of compound 4a was studied with fifteen organic solvents (Figure S1). Ethyl acetate shows higher UV/Vis absorbance and we have recorded UV/Vis absorbances (Figure S2) and fluorescence emission spectra for all synthesized compounds 4(a–p) in EtOAc at 10−5 M concentration.
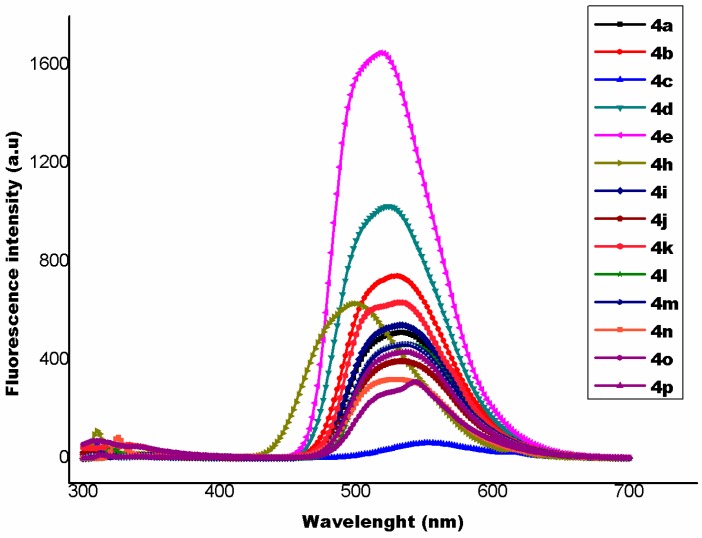
Compound 4e displays a maximum fluorescence intensity at 518 nm (Figure 4). The fluorescence quantum yield [33] (ΦF) of the synthesized compounds 4(a–p) have been calculated by using following Equation (2):
| ΦF = (ΦR ×IS × ODR × ղs)/(IR × ODS × ղR) | (2) |
where ΦR = fluorescence quantum yield of reference, IS and IR = integral area of reference and sample, respectively, ODS and ODR = excited absorbance of sample and reference respectively, ղs and ղR = refractive index of sample solvent and reference solvent respectively. We have used tryptophan as a standard for calculating the emission quantum yield.
Figure 4.
Fluorescence emission spectra the compounds 4(a–p).
The fluorescence quantum yield of the synthesized compounds 4(a–p) ocurrs in the range of 0.010 to 0.068. The compound N,N-dimethyl-4-(2-phenylpyrimido[1,2-b]indazol-4-yl)aniline (4e) shows a fluorescence quantum yield of 0.068 (Table 7).
Table 7.
The optical properties of the synthesized pyrimido[1,2-b]indazolederivatives 4(a–p).
| Entry | Λmax (abs, nm) | Λmax (em, nm) | Stokes Shift (nm) | OD | I | ΦF |
|---|---|---|---|---|---|---|
| Tryptophan [32] | 280 | 355 | 75 | 0.384 | 158,517 | 0.130 |
| 4a | 306 | 533 | 227 | 0.774 | 46,182 | 0.019 |
| 4b | 306 | 530 | 224 | 0.957 | 90,759 | 0.031 |
| 4c | 308 | 553 | 245 | 1.064 | 31,279 | 0.010 |
| 270 (Sh) | 553 | 283 | 0.838 | 31,279 | 0.012 | |
| 4d | 310 | 524 | 214 | 1.505 | 115,328 | 0.025 |
| 4e | 300 | 518 | 218 | 0.870 | 165,016 | 0.062 |
| 344 (Sh) | 518 | 174 | 0.788 | 165,016 | 0.068 | |
| 4h | 310 | 524 | 214 | 1.505 | 115,328 | 0.025 |
| 4i | 306 | 534 | 228 | 1.883 | 73,195 | 0.013 |
| 270 | 534 | 264 | 1.251 | 73,195 | 0.019 | |
| 4j | 300 | 534 | 234 | 1.140 | 61,312 | 0.017 |
| 4k | 314 | 532 | 218 | 1.950 | 83,407 | 0.014 |
| 4l | 320 | 540 | 220 | 2.070 | 65,088 | 0.010 |
| 4m | 308 | 535 | 227 | 1.794 | 70,535 | 0.013 |
| 4n | 272 | 530 | 258 | 0.449 | 54,760 | 0.040 |
| 322 (Sh) | 530 | 208 | 0.439 | 54,760 | 0.041 | |
| 4o | 270 | 543 | 273 | 1.761 | 29,458 | 0.005 |
| 4p | 306 | 499 | 193 | 1.176 | 59,599 | 0.016 |
| 268 (Sh) | 499 | 231 | 0.827 | 59,599 | 0.023 |
Sh = shoulder; abs = absorbance; em = emission; OD = excited absorbance; I = integral area; ΦF = Fluorescence quantum yield.
We have evaluated the free radical scavenging property of the synthesized pyrimido-fused indazoles at 0.001 mM and 0.002 mM and its IC50 values were measured. We have calculated the IC50 values and compound 4f showed excellent antioxidant properties as 3.08 and compounds 4g (8.91), 4n (9.24), 4k (8.59) and 4o (5.60) showed significant antioxidant properties compared to ascorbic acid (4.50; Supplementary Materials Table S1).
3. Materials and Methods
3.1. General Information
All commercially available reagents were used without any further purification and the reactions were monitored by TLC. 1H- (400 MHz) and 13C-NMR (100 MHz) were obtained using an Avance 400 Mz spectrometer (Bruker, Oestliche Rheinbrueckenstr, Karlsruhe, Germany, Europe) in CDCl3 with TMS as an internal standard. Chemical shift values (δ) are expressed in parts per million (ppm). Abbreviations are as follows: s, singlet; d, doublet; t, triplet; m, multiplet. Melting points were measured on an Elchem microprocessor- based DT apparatus (Geninune Sientific Instruments, Chennai, Tamilnadu, India) using open capillary tubes and are corrected with benzoic acid. Mass spectra were obtained on a high resolution mass spectrometer (Bruker, Oestliche Rheinbrueckenstr, Karlsruhe, Germany, Europe). UV-vis spectra were obtained on a UV-2550 instrument (Shimadzu Corporation, Kyoto, Japan). The fluorescence spectra were obtained on a F-7000 FL spectrophotometer (HitachiPerkin-Elmer, Bengaluru, Karnataka, India).
3.2. General Procedure for the Synthesis of 2,4-Diphenylpyrimido[1,2-b]indazoles 4(a–s) via Metal Free Conditions
To a mixture of 1H-indazol-3-amine (1 mmol), aldehyde (1 mmol) and acetophenone (1 mmol) in 5 mL of dimethylformamide potassium hydroxide (2.2 mmol) was added at room temperature. The reaction mixture was heated to 125 °C for 9 h. The progress of the reaction was monitored by TLC. After the completion of the reaction, the mixture was poured into crushed ice. The mixture was extracted by ethyl acetate and water. The organic layer was separated and dried over sodium sulphate and the solvent evaporated. The crude product was purified by column chromatography to afford the product as a solid.
3.3. Characterization Data
4-(4-Isopropylphenyl)-2-phenylpyrimido[1,2-b]indazole (4b); Brown solid; Isolated yield 86%; m.p.: 170–172 °C; 1H-NMR (CDCl3) δ 8.42 (d, J = 8.0 Hz, 1H), 8.28–8.26 (m, 2H), 8.18–8.16 (m, 2H), 7.96–7.85 (m, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.74 (s, 1H), 7.63–7.549 (m, 7H), 7.32–7.28 (m, 1H), 3.08–3.01 (m, 1H), 1.35 (d, J = 6.8 Hz, 6H); 13C-NMR (CDCl3) δ 23.8, 34.3, 108.4, 113.9, 116.5, 120.6, 121.2, 126.5, 127.0, 127.2, 128.0, 128.3, 128.5, 129.0, 129.2, 129.5, 130.0, 133.0, 137.4, 145.0, 145.4, 151.6, 152.2, 152.6; HRMS (EI): m/z calcd. for C25H21N4 363.1735 found 363.1735.
4-(4-Methoxyphenyl)-2-phenylpyrimido[1,2-b]indazole (4d); Yellow solid; Isolated yield 90%; m.p.: 188–190 °C; 1H-NMR (CDCl3) δ 8.40 (d, J = 8.0 Hz, 1H), 8.26–8.22 (m, 4H), 7.84 (d, J = 8.8 Hz, 1H), 7.69 (s, 1H), 7.56–7.49 (m, 4H), 7.29–7.18 (m, 1H), 7.14–7.11 (m, 2H), 3.91 (s, 3H); 13C-NMR (CDCl3) δ 55.5, 107.8, 113.9, 114.2, 116.5, 120.5, 121.2, 123.9, 127.2, 129.0, 129.7, 130.0, 131.2, 137.5, 145.0, 145.1, 151.5, 152.5, 161.7; HRMS (EI): m/z calcd. for C23H17N3O 351.1372 found 351.1370.
N,N-Dimethyl-4-(2-phenylpyrimido[1,2-b]indazol-4-yl)aniline (4e); Brown solid; Isolated yield 85%; m.p.: 180–182 °C; 1H-NMR (CDCl3) δ 8.44 (d, J = 8.0 Hz, 1H), 8.33–8.30 (m, 4H), 7.99 (d, J = 8.4 Hz, 1H), 7.75 (s, 1H), 7.65–7.51 (m, 4H), 7.32–7.28 (m, 1H), 6.92 (d, J = 8.0 Hz, 2H), 3.13 (s, 6H); 13C-NMR (CDCl3) δ 40.1, 106.8, 111.6, 113.8, 116.4, 118.4, 120.1, 121.3, 127.2, 128.9, 129.5, 129.8, 130.8, 137.8, 145.2, 145.7, 151.5, 152.1, 152.5; HRMS (EI): m/z calcd. for C24H20N4 364.1688 found 364.1688.
4-(Naphthalen-1-yl)-2-phenylpyrimido[1,2-b]indazole (4j); Brown solid; Isolated yield 85%; m.p.: 248–250 °C; 1H-NMR (CDCl3) δ 8.47 (d, J = 8.0 Hz, 1H), 8.30 (d, J = 7.6 Hz, 2H), 8.12 (d, J = 8.0 Hz, 1H), 8.00 (d, J = 8.0 Hz, 1H), 7.86 (d, J = 6.8 Hz, 1H), 7.79 (s, 1H), 7.76–7.65 (m, 2H), 7.57–7.31 (m, 8H); 13C-NMR (CDCl3) δ 110.8, 114.0, 116.8, 120.8, 121.1, 125.2, 125.4, 126.6, 127.1, 127.2, 128.2, 128.7, 129.1, 129.7, 129.8, 130.2, 130.7, 131.1, 133.7, 137.2, 144.7, 145.1, 151.7, 152.1; HRMS (EI): m/z calcd. for C26H17N3 371.1422 found 371.1422.
4-(Furan-2-yl)-2-phenylpyrimido[1,2-b]indazole (4k); Brown solid; Isolated yield 78%; m.p.: 172–174 °C; 1H-NMR (CDCl3) δ 8.49–8.48 (m, 1H), 8.36 (d, J = 8.4 Hz, 1H), 8.25 (d, J = 8.0 Hz, 2H), 8.16 (s, 1H), 7.84 (d, J = 8.8 Hz, 1H), 7.70 (s, 1H), 7.83–7.79 (m, 4H), 7.25 (t, J = 8.0 Hz, 1H), 6.73-6.72 (m, 1H); 13C-NMR (CDCl3) δ 103.4, 113.2, 113.8, 116.4, 119.7, 120.7, 121.3, 127.2, 129.0, 130.0, 134.4, 137.6, 144.7, 145.0, 145.4, 151.8; HRMS (EI): m/z calcd. for C20H13N3O 311.1059 found 311.1058.
2-Phenyl-4-(thiophen-2-yl)pyrimido[1,2-b]indazole (4l); Brown solid; Isolated yield 86%; m.p.: 190–192 °C; 1H-NMR (CDCl3) δ 8.59–8.58 (m, 1H), 8.42 (d, J = 7.6 Hz, 1H), 8.28 (d, J = 8.0 Hz, 2H), 8.09 (s, 1H), 7.94 (d, J = 8.8 Hz, 1H), 7.79 (d, J = 5.2 Hz, 1H), 7.68–7.52 (m, 4H), 7.36–7.30 (m, 2H); 13C-NMR (CDCl3) δ 104.8, 113.9, 116.5, 120.8, 121.4, 127.2, 127.7, 129.0, 130.0, 130.1, 131.3, 132.1, 132.2, 137.5, 138.5, 145.2, 151.4, 152.0; HRMS (EI): m/z calcd. for C20H13N3S 327.0830 found 327.0830.
3.4. Experimental Design and Mathematical Model
An experimental design was established for the series of parameters used for the synthesis of 2,4-diphenylpyrimido [1,2-b] indazoles by two reaction methods such as metal mediated and metal-free conditions. The model was built by Response Surface Methodology (RSM) with the Design-Expert Version 9.0.5.1 software (Stat-Ease, Inc., Minneapolis, MN, USA). Levels of selection for each variable were based on the results of the preliminary studies. The three components for each reaction method, such as the catalyst loading (A1), reaction temperature (B1) and response time (C1) were utilized for metal mediated reaction. For metal free reaction, we have used base equivalent (A2), reaction temperature (B2) and reaction time (C2) as three factors. The actual isolated yields Y1 and Y2 were chosen to be the target or response parameter as dependent variables. The values X1 and X2 are the predicted isolated yields. Seventeen sets of experiments were performed for each both reaction methods according to Box-Behnken experimental design (BBD). The variables were tested at the three levels by associating negative sign (−1) for lower level, Zero (0) indicating the core value and plus signs (+1) for higher stages (Table 1). The experimental design matrix and their effects are presented in Table S1. The quadratic polynomial equation recommended by RSM was used to predict the optimal value and examine the interaction between the response of experimental design (actual yield) and the variables (process parameters). The general form of quadratic polynomial was as follows:
| Y= β0 + β1X1 + β2X2 + β3X3 + β11X12 + β22X22 + β33X32 + β12X1X2 + β13X1X2+ β23X2X3 | (3) |
where β0 is constant coefficient of the models. The regression coefficients (β1, β2 and β3), (β11, β22 and β33) and (β12, β13 and β23) respectively represent linear, quadratic and interaction effects of the model estimated by multiple regression analysis.
3.5. Antioxidant Activity
The free radical scavenging activity of the pyrimido-fused indazoles was determined according to the method using our earlier reports [34].
4. Conclusions
A metal-free MCR has been developed to synthesize a series of pyrimido-fused indazoles via cascade/tandem transformation. This KOH/DMF mediated three component cascade type transformation offers good to moderate yields. It offers good functional group tolerance. The RSM coupled with BBD results displayed the significance of the quadratic model and offered fine-tuned conditions for the preparation of pyrimido[1,2-b]indazole derivatives. The synthesized analogues showed better fluorescence properties. The synthesized pyrimido-fused indazoles were evaluated as good free radical scavengers.
Acknowledgments
The corresponding author acknowledges the support provided by research grant DST-SERB (No. SB/FT/CS-126/2012) fund. Naif Abdullah Al-Dhabi and Valan Arasu extend their sincere thanks to the Deanship of Scientific Research, King Saud University for its funding to the Prolific Research Group (PRG-1437-28). Also we express our sincere thanks to VIT-SIF for giving permission to record the NMR, UV/Vis and fluorescence spectra.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/11/1571/s1.
Author Contributions
The molecules have been synthesized by Jeyakannu Palaniraja. Also manuscript has been written by him. The work plan has been suggested by Selvaraj Mohana Roopan. G Mokesh Rayalu helped for RSM optimization. Mariadhas Valan Arasu grown the single crystal. Antioxidant activity has been carried out by Naif Abdullah Al-Dhabi. Scientific manuscript has been verified and approved by Selvaraj Mohana Roopan.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Fiorot R.G., Filho J.F.A., Pereria T.M.C., Lacerda V., Jr., Santos R.B.D., Romao W., Greco S.J., Keating T.A. A simple and convenient method for synthesis of new aminonaphthoquinones derived from lawsone by catalytic multicomponent Mannich reaction. Tetrahedron Lett. 2014;55:4373–4377. doi: 10.1016/j.tetlet.2014.06.031. [DOI] [Google Scholar]
- 2.Rahmati A., Ahmadi S., Varzaneh M.A. One-pot synthesis of 1,2,4,5-tetrahydro-2,4-dioxobenzo[b]-[1,4]diazepine and malonamide derivatives using multi-component reactions. Tetrahedron. 2014;70:9512–9521. doi: 10.1016/j.tet.2014.10.060. [DOI] [Google Scholar]
- 3.Gore R.P., Rajput A.P. A review on recent progress in multicomponent reactions of pyrimidines synthesis. Drug Invent. Today. 2013;2:148–152. doi: 10.1016/j.dit.2013.05.010. [DOI] [Google Scholar]
- 4.Terzidis M.A., Drosos N.M., Kasapidou P.M., Stephanatou J.S., Tsiaras V.G., Buth G., Tsoleridis C.A., Kostakis G.E. Chromeno[2,3-c]pyrroles by one-pot multicomponent domino addition–amination reaction. Tetrahedron Lett. 2014;55:5601–5604. doi: 10.1016/j.tetlet.2014.08.048. [DOI] [Google Scholar]
- 5.Feng H., Zhao P., Sun Z. CuI/CuBr2-catalyzed decarboxylative/A3 reaction of propiolic acids for the facile synthesis of 1,4-diheterocycle-2-butynes. Tetrahedron Lett. 2015;56:5676–5680. doi: 10.1016/j.tetlet.2015.08.075. [DOI] [Google Scholar]
- 6.Roopan S.M., Bharathi A., Palaniraja J., Gengan R.M., Anand K. Unexpected regiospecific Michael addition product: Synthesis of 5,6-dihydrobenzo[1,7]phenanthrolines. RSC Adv. 2015;48:38640–38645. doi: 10.1039/C4RA16640J. [DOI] [Google Scholar]
- 7.Subhashini R., Roopan S.M., Khan F.R.N. Synthesis and free radical scavenging property of some quinoline derivatives. J. Chil. Chem. Soc. 2010;55:317–319. doi: 10.4067/S0717-97072010000300008. [DOI] [Google Scholar]
- 8.Wang J., Shen Q., Zhang J., Song G. Metal-free multicomponent coupling reaction of aliphatic amines, formaldehyde, organoboronic acids, and propiolic acids for the synthesis of diverse propargylamines. Tetrahedron Lett. 2015;56:903–906. doi: 10.1016/j.tetlet.2014.12.142. [DOI] [Google Scholar]
- 9.Bondzic B.P. Rh catalyzed multicomponent tandem and one-pot reactions under hydroformylation conditions. J. Mol. Catal. A Chem. 2015;408:310–334. doi: 10.1016/j.molcata.2015.07.026. [DOI] [Google Scholar]
- 10.Devi N., Rawal R.K., Singh V. Diversity-oriented synthesis of fused-imidazole derivatives via Groebke–Blackburn–Bienayme reaction: A review. Tetrahedron. 2015;71:183–232. doi: 10.1016/j.tet.2014.10.032. [DOI] [Google Scholar]
- 11.Ugi I. Recent progress in the chemistry of multicomponent reactions. Pure Appl. Chem. 2001;73:187–191. doi: 10.1351/pac200173010187. [DOI] [Google Scholar]
- 12.Surendra K., Krishnaveni N.S., Mahesh A., Rao K.R. Supramolecular Catalysis of Strecker Reaction in Water under Neutral Conditions in the Presence of β-Cyclodextrin. J. Org. Chem. 2006;71:2532–2534. doi: 10.1021/jo052510n. [DOI] [PubMed] [Google Scholar]
- 13.Janardhan B., Rajitha B., Crooks P.A. Poly(4-vinylpyridinium)hydrogen sulfate: An efficient heterogeneous catalyst for the one-pot synthesis of polyhydroquinolines via unsymmetrical Hantzsch reaction in aqueous medium. J. Saudi Chem. Soc. 2014;18:722–727. doi: 10.1016/j.jscs.2014.01.009. [DOI] [Google Scholar]
- 14.Zamani F., Izadi E. Synthesis and characterization of sulfonated-phenylacetic acid coated Fe3O4 nanoparticles as a novel acid magnetic catalyst for Biginelli reaction. Catal. Commun. 2013;42:104–108. doi: 10.1016/j.catcom.2013.08.006. [DOI] [Google Scholar]
- 15.Dongare S.B., Chavan H.V., Bhale P.S., Mule Y.B., Kotmale A.S., Bandgar B.P. A catalyst- and solvent-free multicomponent synthesis of 7-azagramine analogues via a Mannich type reaction. Chin. Chem. Lett. 2016;27:99–103. doi: 10.1016/j.cclet.2015.07.029. [DOI] [Google Scholar]
- 16.Wang H., Deng T., Cai C. Fluorousbis(oxazolines) ligand: Synthesis and application in Kabachnik-Fields reaction. J. Flour. Chem. 2014;168:144–150. doi: 10.1016/j.jfluchem.2014.09.024. [DOI] [Google Scholar]
- 17.Montagne C., Montagne M., Shipman M., Shipman M. Modified Bucherer-Bergs Reaction for the One-Pot Synthesis of 5,5′-Disubstituted Hydantoins from Nitriles and Organometallic Reagents. Synlett. 2006;14:2203–2206. doi: 10.1002/chin.200650139. [DOI] [Google Scholar]
- 18.Han Y., Tang W.Q., Yan C.G. Gewald-type reaction of double activated 2,3-diarylcyclopropanes with elemental sulfur for synthesis of polysubstituted 2-aminothiophenes. Tetrahedron. 2014;55:1441–1443. doi: 10.1016/j.tetlet.2014.01.043. [DOI] [Google Scholar]
- 19.Salim S.D., Pathare S.P., Akamanchi K.G. Sulfated tungstate: A green catalyst for synthesis of thiomorpholides via Willgerodt–Kindler reaction. Catal. Commun. 2011;13:78–81. doi: 10.1016/j.catcom.2011.06.022. [DOI] [Google Scholar]
- 20.Borah P., Boarh J.M., Chowdhury P. Microwave (MW) irradiated Ugi four-component reaction (Ugi-4CR): Expedited synthesis of steroid–amino acid conjugates—A novel class of hybrid compounds. Steroids. 2015;98:49–57. doi: 10.1016/j.steroids.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Varyani M., Khatri P.K., Jain S.L. Amino acid ionic liquid bound copper Schiff base catalyzed highly efficient three component A3-coupling reaction. Catal. Commun. 2016;77:113–117. doi: 10.1016/j.catcom.2016.01.020. [DOI] [Google Scholar]
- 22.Roopan S.M., Palaniraja J. Synthetic journey towards transition metal-free arylations. Res. Chem. Intermed. 2015;41:8111–8146. doi: 10.1007/s11164-014-1880-6. [DOI] [Google Scholar]
- 23.Palaniraja J., Roopan S.M. UV-light induced domino type reactions: Synthesis and photophysical properties of unreported nitrogen ring junction quinazolines. RSC Adv. 2015;5:37415–37423. doi: 10.1039/C5RA00229J. [DOI] [Google Scholar]
- 24.Palaniraja J., Roopan S.M. Iodine-mediated synthesis of indazolo-quinazolinones via a multi-component reaction. RSC Adv. 2015;12:8640–8646. doi: 10.1039/C4RA13779E. [DOI] [Google Scholar]
- 25.Palaniraja J., Roopan S.M., Rayalu G.M. One-pot synthesis of highly functionalized pyrimido[1,2-b]indazoles via 6-endo-dig cyclization. RSC Adv. 2016;6:24610–24616. doi: 10.1039/C6RA02596J. [DOI] [Google Scholar]
- 26.Noshadia I., Amina N.A.S., Parnasb R.S. Continuous production of biodiesel from waste cooking oil in a reactive distillation column catalyzed by solid heteropolyacid: Optimization using response surface methodology (RSM) Fuel. 2012;94:156–164. doi: 10.1016/j.fuel.2011.10.018. [DOI] [Google Scholar]
- 27.Wie L., Li X., Yanxun L., Tingliang L., Guoji L. Process Variables of the Esterification Reaction of 3-Pentadecylphenol and Acryloyl Chloride Catalyzed by an Ionic Liquid. Chem. Eng. Technol. 2013;36:559–566. doi: 10.1002/ceat.201200575. [DOI] [Google Scholar]
- 28.Yin P., Chen L., Wang Z., Qu R., Liu X., Xu Q., Ren S. Biodiesel production from esterification of oleic acid over aminophosphonic acid resin D418. Fuel. 2012;102:499–505. doi: 10.1016/j.fuel.2012.05.027. [DOI] [Google Scholar]
- 29.Yin P., Chen W., Liu W., Chen H., Qu R., Liu X., Tang Q., Xu Q. Efficient bifunctional catalyst lipase/organophosphonic acid-functionalized silica for biodiesel synthesis by esterification of oleic acid with ethanol. Bioresour. Technol. 2013;140:146–151. doi: 10.1016/j.biortech.2013.04.082. [DOI] [PubMed] [Google Scholar]
- 30.Xin L., Jinyu X., Weimin L. Response surface design of solid waste based geopolymer. RSC Adv. 2015;5:1598–1604. doi: 10.1039/C4RA05458J. [DOI] [Google Scholar]
- 31.Divsar F., Habibzadeh K., Shariati S., Shahriarinour M. Aptamer conjugated silver nanoparticles for the colorimetric detection of arsenic ions using response surface methodology. Anal. Methods. 2015;7:4568–4576. doi: 10.1039/C4AY02914C. [DOI] [Google Scholar]
- 32.Ye M., Edmunds A.J.F., Morris J.A., Sale D., Zhang Y., Yu J.Q. A Robust Protocol for Pd(II)-catalyzed C-3 Arylation of (1H) Indazoles and Pyrazoles: Total Synthesis of NigellidineHydrobromide. Chem. Sci. 2013;4:2374–2379. doi: 10.1039/c3sc50184a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemealtha K., Madhumitha G., Vasavi C.S., Munusami P. 2,3-Dihydroquinazolin-4(1H)-ones: Visible light mediated synthesis, solvatochromism and biological activity. J. Photochem. Photobiol. B. 2013;143:139–147. doi: 10.1016/j.jphotobiol.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 34.Rajesh S., Roopan S.M., Al-Dhabi N.A., Suthindhiran K., Gargi S., Arasu M.A. 1,2,4-Triazolo-quinazoline-thiones: Non-conventional synthetic approach, study of solvatochromism and antioxidant assessment. J. Photochem. Photobiol. B. 2016;162:232–239. doi: 10.1016/j.jphotobiol.2016.06.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.