Abstract
A series of benzimidazole phenylhydrazone derivatives (6a–6ai) were synthesized and characterized by 1H-NMR, ESI-MS, and elemental analysis. The structure of 6b was further confirmed by single crystal X-ray diffraction as (E)-configuration. All the compounds were screened for antifungal activity against Rhizoctonia solani and Magnaporthe oryzae employing a mycelium growth rate method. Compound 6f exhibited significant inhibitory activity against R. solani and M. oryzae with the EC50 values of 1.20 and 1.85 μg/mL, respectively. In vivo testing demonstrated that 6f could effectively control the development of rice sheath blight (RSB) and rice blast (RB) caused by the above two phytopathogens. This work indicated that the compound 6f with a benzimidazole phenylhydrazone scaffold could be considered as a leading structure for the development of novel fungicides.
Keywords: benzimidazole, phenylhydrazone, antifungal activity
1. Introduction
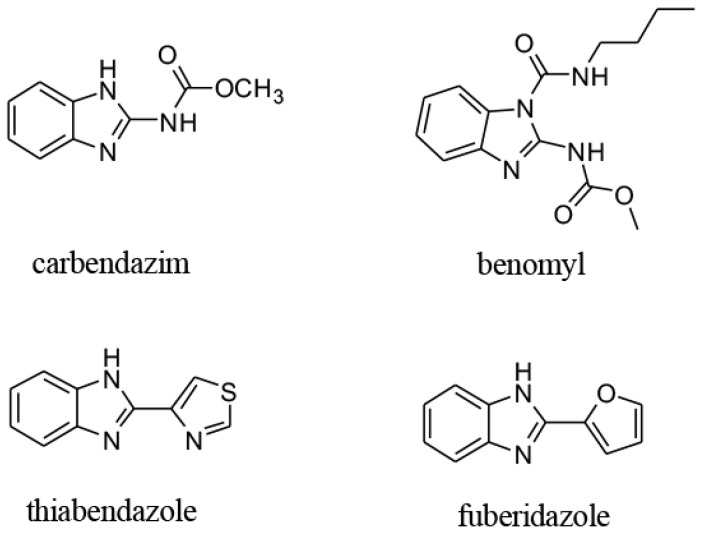
As an important chemical scaffold in medicinal chemistry and agrochemicals [1], benzimidazole derivatives have been attracting the interest of organic chemists for their varieties of biological activities, such as antifungal [2], anticancer [3], antibacterial [4], antioxidant [5], anti-inflammatory [6], and anti-parasitic [7]. In particular, benzimidazole fungicides, with benzimidazole as the core substructure (Figure 1), have been widely used throughout the world to fight against destructive plant pathogens, such as Rhizoctonia solani, Botrytis cinerea, Fusarium graminearum, and Magnaporthe oryzae [8,9]. However, excessive use of benzimidazole fungicides has led to a series of negative problems, such as pesticide residues, drug resistance and serious environmental pollution [10,11]. Therefore, the development of new benzimidazole fungicides with high activity, good selectivity and eco-friendly properties is extremely urgent.
Figure 1.
The commercialization of pesticide.
In our previous work, molecules with phenylhydrazone showed significant antifungal and antioxidant activities [12,13]. Typically, 1,2,3-triazole phenylhydrazone derivatives displayed potent antifungal activity in vitro and in vivo. Inspired by these studies, we initiated a study to synthesize benzimidazole phenylhydrazone derivatives to screen high activity antifungal compounds. Their antifungal activities against two phytopathogenic fungi (R. solani and M. oryzae) were evaluated in vitro and in vivo, and their structure-antifungal activity relationships were also discussed.
2. Results and Discussion
2.1. Synthesis of Compounds
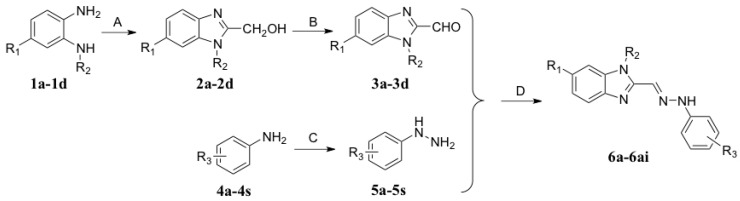
A total of 35 target compounds were synthesized following the routes outlined in Scheme 1. The compounds 2a–2d were synthesized by the reaction of o-phenylenediamine with glycolic acid in 75%–85% yield. Then the compounds 2a–2d were further oxidized to aldehydes (3a–3d) using MnO2 in ethyl acetate. The aldehydes were purified by filtration to remove the excess MnO2, then compounds 3a–3d were obtained as white crystals in 60%–76% yield. The anilines 4a–4s were diazotized by NaNO2, then restored by SnCl2 to obtain substituted phenylhydrazines 5a–5s in 55%–80% yield. Finally, 3a–3d and 5a–5s were condensed to form the -C=N-NH- bond, giving benzimidazole derivatives 6a–6ai in 45%–80% yield. 1H-NMR, MS (ESI), and elemental analysis (CHN) data of the target compounds were in fully accordance with their assigned structures. Among them, compounds 6a, 6q, 6t, 6x, and 6z had been reported previously [14,15], while the remaining 30 compounds were first reported in this study.
Scheme 1.
The synthetic routes to 6a–6ai. Reagents and conditions: (A) (i) glycolic acid, HCl (20% aqueous), 100 °C, 5 h; (ii) NaOH (15% aqueous), pH 9; (B) MnO2, EtOAc, 65 °C, 1 h; (C) (i) NaNO2, HCl, 0 °C, 1 h; (ii) SnCl2, 0 °C; r.t., 2 h; (iii) NaOH (40% aqueous), pH 7; (D) MeOH, r.t., 2 h.
2.2. Crystal Structure of Compound 6b
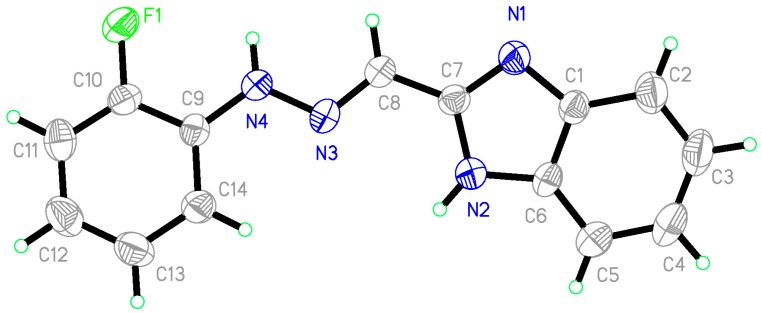
Among these compounds, the crystal structure of compound 6b was determined by X-ray diffraction analysis. Figure 2 gave a perspective view of 6b with the atomic labelling system. The X-ray data had been deposited at the Cambridge Crystallographic Data Centre with the CCDC number 1038194. The result demonstrated that the -C=N-NH- bond bears an (E)-configuration rather than (Z)-configuration.
Figure 2.
X-ray diffraction structure of 6b, thermal ellipsoids was drawn on the 35% probability.
2.3. Antifungal Activities In Vitro
Different concentrations of compounds 6a–6ai were evaluated for their antifungal activities in vitro against R. solani and M. oryzae. The EC50 values were calculated using linear-regression analysis, with validamycin A, carbendazim and isoprothiolane as positive controls (Table 1).
Table 1.
Antifungal activity of compounds against two phytopathogens a.
| Compound | R1 | R2 | R3 | EC50 (±SD) μg/mL | |
|---|---|---|---|---|---|
| R. solani | M. oryzae | ||||
| 6a | H | H | H | 2.86 ± 0.15 | 11.09 ± 0.92 |
| 6b b | H | H | 2-F | 2.14 ± 0.12 | 6.73 ± 0.56 |
| 6c b | H | H | 3-F | 2.14 ± 0.18 | 9.08 ± 0.63 |
| 6d b | H | H | 4-F | 1.88 ± 0.13 | 8.29 ± 0.28 |
| 6e b | H | H | 4-OCF3 | 1.50 ± 0.08 | 8.22 ± 0.67 |
| 6f b | H | H | 2,4-2F | 1.20 ± 0.10 | 1.85 ± 0.21 |
| 6g b | H | H | 2,5-2F | 3.98 ± 0.19 | 12.39 ± 0.89 |
| 6h b | H | H | 2-Cl | 3.29 ± 0.17 | 5.47 ± 0.49 |
| 6i b | H | H | 3-Cl | 3.77 ± 0.23 | 13.66 ± 0.19 |
| 6j b | H | H | 4-Cl | 1.00 ± 0.06 | 8.71 ± 0.55 |
| 6k b | H | H | 2,4-2Cl | 2.83 ± 0.15 | 9.27 ± 0.81 |
| 6l b | H | H | 2,5-2Cl | 7.47 ± 0.31 | 14.66 ± 1.17 |
| 6m b | H | H | 2,6-2Cl | 5.85 ± 0.22 | 12.87 ± 0.76 |
| 6n b | H | H | 3,5-2Cl | >25 | >25 |
| 6o b | H | H | 2-Br | 4.18 ± 0.21 | 11.33 ± 1.21 |
| 6p b | H | H | 4-Br | 1.14 ± 0.07 | 8.90 ± 1.05 |
| 6q | H | H | 4-OMe | 7.02 ± 0.26 | 16.53 ± 1.24 |
| 6r b | H | H | 4-CN | 7.60 ± 0.23 | 21.18 ± 1.91 |
| 6s b | H | H | 3,4-2CH3 | 6.95 ± 0.29 | 17.62 ± 1.62 |
| 6t | H | CH3 | H | 10.04 ± 0.25 | >25 |
| 6u b | H | CH3 | 3-F | >25 | >25 |
| 6v b | H | CH3 | 2,4-2F | 2.89 ± 0.04 | 7.89 ± 0.31 |
| 6w b | H | CH3 | 2,5-2Cl | >25 | >25 |
| 6x | H | CH3 | 4-Cl | >25 | >25 |
| 6y b | H | CH3 | 2,4-2Cl | >25 | >25 |
| 6z | H | CH3 | 2-Br | >25 | >25 |
| 6aa b | H | CH3 | 4-OCF3 | >25 | >25 |
| 6ab b | H | CH3 | 4-F | 3.01 ± 0.02 | >25 |
| 6ac b | H | CH3 | 4-CN | >25 | >25 |
| 6ad b | CH3 | H | H | 3.84 ± 0.13 | 19.77 ± 0.65 |
| 6ae b | CH3 | H | 4-Cl | 1.28 ± 0.09 | 11.85 ± 0.34 |
| 6af b | CH3 | H | 2,4-2F | 2.30 ± 0.11 | 6.54 ± 0.19 |
| 6ag b | Cl | H | H | 2.65 ± 0.06 | 14.24 ± 0.58 |
| 6ah b | Cl | H | 4-Cl | 0.93 ± 0.04 | 10.40 ± 0.24 |
| 6ai b | Cl | H | 2,4-2F | 1.15 ± 0.09 | 5.26 ± 0.14 |
| carbendazim | 1.84 ± 0.04 | 1.87 ± 0.10 | |||
| validamycin A | 5.07 ± 0.28 | ||||
| isoprothiolane | 0.02 ± 0.01 | ||||
a The data of the fungicidal activities were statistically analyzed using Excel to give the results of EC50 values. The results were expressed as the mean ± SD of triplicate experiments; b Compounds that were first reported.
Among 35 compounds, 27 compounds showed significant inhibitory activities against R. solani. 6l–6m, 6q–6t were slightly weaker than validamycin A (5.07 μg/mL), while the other 20 compounds were more potent than validamycin A. Interestingly, seven compounds were superior to carbendazim (1.84 μg/mL). Taking single halogen substituents (6k–6m) into account, it was obvious that 6m (2,4-2Cl, 2.83 μg/mL) > 6l (2,6-2Cl, 5.85 μg/mL) > 6k (2,5-2Cl, 7.47 μg/mL) (“>” means more potent). The compound 6ah (0.93 μg/mL) showed the strongest activity, which was much more superior to that of carbendazim (1.84 μg/mL). When R2 was methyl, the activity of compounds (6a and 6t, 6c and 6u) was significantly decreased; H was compared to R2.
Some target compounds showed great inhibitory activities against M. oryzae. Among these 35 compounds, 6f showed potent inhibiting effects with EC50 of 1.85 μg/mL, compared to carbendazim (EC50 of 1.87 μg/mL). Taking single halogen substituents (6b–6d) and (6h–6j) into account, it was obvious that 6b (2F, 6.73 μg/mL) > 6d (4F, 8.29 μg/mL) > 6c (3F, 9.08 μg/mL), 6h (2Cl, 5.47 μg/mL) > 6j (4Cl, 8.71, μg/mL) > 6i (3Cl, 13.66 μg/mL).
Based on the results of the antifungal activities shown in Table 1, 6f was selected for further antifungal activity tests in vivo.
2.4. Inhibition of 6f on the Sclerotia Germination of R. solani
As shown in Table 2, when grain dry sclerotia was inoculated, germination could be observed in the water of the blank control after a certain incubation period (about four days) at 25 °C in the dark. 6f had better controlling effects than sclerotia.
Table 2.
The inhibition rate of 6f on the germination of sclerotia of R. solani a.
| Compound | Treatment (μg/mL) | ||
|---|---|---|---|
| 1 | 10 | 50 | |
| 6f | 0 | 24.4% | 53.1% |
| validamycin A | 26.3% | 52.7% | 100% |
a Values are the average of 3 replicates.
2.5. Protective Activity of 6f against Rice Sheath Blight (RSB) In Vivo
Compound 6f was selected for evaluating the protective activity against RSB caused by R. solani. As shown in Figure 3 and Table 3, four days after inoculating with R. solani, significant differences could be observed between the treated and untreated groups. Tan spots appeared on the leaf sheath of the blank control, and the scab length reached 16.4 mm. When at a concentration of 200 μg/mL, the protective effect of 6f could reach 68.9% in vivo, close to validamycin A (73.8%), which was commonly used as a fungicide against RSB. When at a concentration of 100 μg/mL, the protective effect of 6f and validamycin A reached 66.5% and 61.0% respectively. Thus, 6f showed similar activity with validamycin A against RSB in vivo.
Figure 3.
Protective activity of 6f against rice sheath blight (RSB).
Table 3.
Protective activity of 6f against RSB a.
| Compound | Treatment (μg/mL) | Lesion Lengtha (mm) | Protection Efficacy (%) |
|---|---|---|---|
| 6f | 200 | 5.1 | 68.9 |
| 100 | 5.5 | 66.5 | |
| validamycin A | 200 | 4.3 | 73.8 |
| 100 | 6.4 | 61.0 | |
| blank control | 16.4 |
a Values are the average of 20 replicates.
2.6. Inhibition of 6f on the Conidium Germination of M. oryzae
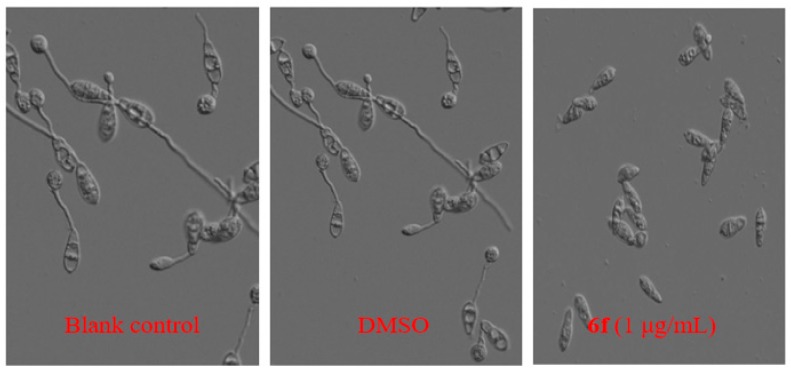
As shown in Figure 4, conidia of M. oryzae were inoculated after 24 h, germination could be observed in the water of the blank control under the microscope with a germination rate of 100%, and 1% DMSO had no effect on the conidium germination. The conidia could not form the germ tube or appressorium when incubated with 6f at the concentration of 1.0 μg/mL (1% DMSO).
Figure 4.
The effect of 6f on the conidium germination rate of M. oryzae.
2.7. Protective Activity of 6f against Rice Blast (RB) In Vivo
The efficacy of the protective activity experiment is shown in Figure 5 and Table 4, fungus spores were inoculated on leaves, blast lesions could be observed after 7 days. At a concentration of 50 μg/mL, the protective effect of 6f could reach 21.6%, whereas carbendazim treatment resulted in 88.0%.
Figure 5.
Protective activity of 6f against rice blast (RB).
Table 4.
Protective activity of 6f against RB a.
| Compound | Treatment (μg/mL) | Blast Lesions | Protection Efficacy (%) |
|---|---|---|---|
| 6f | 50 | 32.7 | 21.6 |
| 100 | 26 | 37.6 | |
| carbendazim | 25 | 11.3 | 72.9 |
| 50 | 5.0 | 88.0 | |
| blank control | 41.7 |
a Values are the average of 10 replicates.
3. Materials and Methods
3.1. Reagents and Analysis
All reagents bought from Alfa Aesar (Ward Hill, MA, USA), Aladdin (Shanghai, China) or Sinopharm Chemical Reagent Co., Ltd. (Beijing, China) were pure analytical grades and used without further treatments. Reactions were monitored by TLC using silica gel coated glass slides (silica-gel 60 GF254, Qingdao Haiyang Chemical, Qingdao, China). Melting points were measured on WRS-1B digital melting-point apparatus (SPSIC, Shanghai, China), uncorrected. 1H-NMR spectra were recorded on a Bruker Avance III 400 NMR spectrometer (Bruker, Stuttgart, Germany). The chemical shifts (δ) are reported in ppm with reference to internal TMS, and coupling constants (J) are given in Hz. ESI-MS spectra were recorded on a Bruker UHR-TOF maxis (Bruker, Billerica, MA, USA). X-ray single crystal diffraction analysis was conducted on a Bruker D8 Venture diffractometer (Bruker, Karlsruhe, German). Elemental analyses were performed on a Elementar Vario MICRO instrument (Elementar, Langenselbold, German) and were within ±0.4% of the theoretical values.
3.2. Synthesis
3.2.1. Synthesis and Purification of Compound 2a–2d
The procedures were conducted according to the literature [16]. Glycolic acid (15.24 g, 0.2 mol) was dissolved in 40 mL HCl (20% aqueous) in the flask. When the temperature reached 65 °C, compound 1a–1d (16.27 g, 0.15 mol) was added at three times, then refluxed at 100 °C for 5 h. After cooling to room temperature, the solution was basified with NaOH (15%, aqueous) until it reached pH 9.0 to precipitate the desired compounds 2a–2d (in 75%–85% yield).
3.2.2. Synthesis and Purification of Compound 3a–3d
A solution of 2a–2d (2 mmol), MnO2 (3.48 g, 40 mmol) was added to EtOAc (120 mL), then refluxed at 65 °C for 1 h (monitored by TLC). Afterwards the solution was filtered, and concentrated in vacuo to give pure compounds 3a–3d (in 60%–76% yield) [17].
3.2.3. Synthesis and Purification of Compound 5a–5s
Substituted aniline 4a–4s (50 mmol) was dissolved in the 50 mL HCl (18%, aqueous) in the ice bath. NaNO2 (50 mmol) dissolved in 50 mL water was added dropwise. The reaction mixture was stirred for 1 h to obtain a clear solution. Then the solution of SnCl2 (0.1 mol) in 30 mL of concentrated HCl was added dropwise at 0 °C. The mixture was stirred at room temperature for 2 h. Afterwards, the mixture was extracted with 50 mL EtOAc and the organic impurities were discarded. Then the solution was basified with NaOH (40%, aqueous) until it reached pH 7.0. The reaction mass was extracted with EtOAc three times. Finally, substituted phenylhydrzine 5a–5s was afforded after being vapored under reduced pressure (in 55%–80% yield) [12].
3.2.4. Synthesis and Purification of Compound 6a–6ai
The equimolar aldehyde 3a–3d (1 mmol) and substituted phenylhydrazine 5a–5s (1 mmol) were mixed in CH3OH (10 mL) and stirred at room temperature [18]. After about 2 h, the reaction was completed (monitored by TLC). The residual crude was purified via silica gel column chromatogram using a gradient mixture of petroleum ether and ethyl acetate to obtain the pure target compounds 6a–6ai (in 45–80% yield).
(E)-2-((2-Phenylhydrazono)methyl)-1H-benzo[d]imidazole (6a): Yellow power, yield 64.2%, m.p. 185.3–186.1 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.52 (s, 1H), 10.90 (s, 1H), 7.88 (s, 1H), 7.53 (s, 2H), 7.31–7.26 (m, 2H), 7.25 (t, J = 4.2 Hz, 2H), 7.18 (d, J = 2.8 Hz, 2H), 6.84 (dd, J = 9.7, 4.2 Hz, 1H). MS (ESI): 237.10 (C14H13N4, [M + H]+). Anal. Calcd. For C14H12N4: C, 71.17; H, 5.12; N, 23.71. Found: C, 70.95; H, 5.19; N, 23.49.
(E)-2-((2-(2-Fluorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6b): Sandybrown power, yield 72.8%, 201.8–202.4 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.64 (s, 1H), 10.89 (s, 1H), 8.18 (s, 1H), 7.83 (t, J = 8.2 Hz, 1H), 7.62–7.49 (m, 2H), 7.25–7.14 (m, 4H), 6.86 (dd, J = 13.0, 7.0 Hz, 1H). MS (ESI): 255.10 (C14H12FN4, [M + H]+). Anal. Calcd. For C14H11FN4: C, 66.13; H, 4.36; N, 22.04. Found: C, 66.34; H, 4.11; N, 21.89.
(E)-2-((2-(3-Fluorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6c): Yellow power, yield 69.3%, m.p. 203.5–205.9 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.61 (s, 1H), 11.09 (s, 1H), 7.90 (d, J = 0.6 Hz, 1H), 7.55 (s, 2H), 7.33–7.26 (m, 1H), 7.26–7.17 (m, 3H), 6.91 (dd, J = 8.1, 1.2 Hz, 1H), 6.63 (td, J = 8.3, 2.0 Hz, 1H). MS (ESI): 255.10 (C14H12FN4, [M + H]+). Anal. Calcd. For C14H11FN4: C, 66.13; H, 4.36; N, 22.04. Found: C, 66.34; H, 4.11; N, 21.89.
(E)-2-((2-(4-Fluorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6d): Yellow power, yield 76.8%, m.p. 199.2–199.4 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.54 (s, 1H), 10.90 (s, 1H), 7.86 (s, 1H), 7.52 (s, 2H), 7.24 (dt, J = 10.2, 4.1 Hz, 2H), 7.21–7.11 (m, 4H). MS (ESI): 255.10 (C14H12FN4, [M + H]+). Anal. Calcd. For C14H11FN4: C, 66.13; H, 4.36; N, 22.04. Found: C, 66.34; H, 4.11; N, 21.89.
(E)-2-((2-(4-(Trifluoromethoxy)phenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6e): White power, yield 54.1%, m.p. 207.3–207.9 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.61 (s, 1H), 11.07 (s, 1H), 7.91 (d, J = 0.6 Hz, 1H), 7.54 (s, 2H), 7.33–7.28 (m, 4H), 7.19 (d, J = 2.8 Hz, 2H). MS (ESI): 321.09 (C15H12F3N4O, [M + H]+). Anal. Calcd. For C15H11F3N4O: C, 56.25; H, 3.46; N, 17.49. Found: C, 56.27; H, 3.65; N, 17.07.
(E)-2-((2-(2,4-Difluorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6f): Brown power, yield 65.5%, m.p. 200.2–200.3 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.68 (s, 1H), 11.05 (s, 1H), 8.18 (s, 1H), 7.70–7.64 (m, 1H), 7.61 (d, J = 7.8 Hz, 1H), 7.50 (d, J = 7.7 Hz, 1H), 7.29–7.21 (m, 2H), 7.18 (dd, J = 13.4, 5.6 Hz, 1H), 6.64 (ddd, J = 11.9, 8.5, 3.3 Hz, 1H). MS (ESI): 273.09 (C14H11F2N4, [M + H]+). Anal. Calcd. For C14H10F2N4: C, 61.76; H, 3.70; N, 20.58. Found: C, 61.89; H, 3.83; N, 20.49.
(E)-2-((2-(2,5-Difluorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6g): Pale yellow power, yield 59.6%, m.p. 247.1–248.2 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.67 (s, 1H), 11.04 (s, 1H), 8.18 (s, 1H), 7.70–7.63 (m, 1H), 7.61 (d, J = 7.7 Hz, 1H), 7.50 (d, J = 7.7 Hz, 1H), 7.29–7.21 (m, 2H), 7.21–7.14 (m, 1H), 6.67–6.59 (m, 1H). MS (ESI): 273.09 (C14H11F2N4, [M + H]+). Anal. Calcd. For C14H10F2N4: C, 61.76; H, 3.70; N, 20.58. Found: C, 61.89; H, 3.83; N, 20.49.
(E)-2-((2-(2-Chlorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6h): Brown power, yield 72.6%, m.p. 198.8–199.3 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.65 (s, 1H), 10.54 (s, 1H), 8.35 (s, 1H), 7.88 (d, J = 8.2 Hz, 1H), 7.56 (s, 2H), 7.38 (d, J = 7.9 Hz, 1H), 7.33 (t, J = 7.7 Hz, 1H), 7.21 (s, 2H), 6.88 (dd, J = 10.9, 4.3 Hz, 1H). MS (ESI): 271.05 (C14H12ClN4, [M + H]+). Anal. Calcd. For C14H11ClN4: C, 62.11; H, 4.10; N, 20.70. Found: C, 61.94; H, 4.19; N, 20.29.
(E)-2-((2-(3-Chlorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6i): Pale yellow power, yield 66.7%, m.p. 212.0–212.2 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.62 (s, 1H), 11.06 (s, 1H), 7.89 (s, 1H), 7.59 (d, J = 7.9 Hz, 1H), 7.49 (d, J = 7.7 Hz, 1H), 7.43 (s, 1H), 7.28 (t, J = 8.0 Hz, 1H), 7.22 (t, J = 7.5 Hz, 1H), 7.16 (t, J = 7.5 Hz, 1H), 7.03 (d, J = 8.2 Hz, 1H), 6.86 (dd, J = 7.8, 1.2 Hz, 1H). MS (ESI): 271.05 (C14H12ClN4, [M + H]+). Anal. Calcd. For C14H11ClN4: C, 62.11; H, 4.10; N, 20.70. Found: C, 61.94; H, 4.19; N, 20.29.
(E)-2-((2-(4-Chlorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6j): Yellow power, yield 72.3%, m.p. 194.4–194.5 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.59 (s, 1H), 11.02 (s, 1H), 7.88 (s, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.48 (d, J = 7.8 Hz, 1H), 7.34 (d, J = 2.0 Hz, 1H), 7.33–7.31 (m, 1H), 7.28–7.25 (m, 1H), 7.24 (d, J = 2.0 Hz, 1H), 7.24–7.19 (m, 1H), 7.16 (t, J = 7.6 Hz, 1H). MS (ESI): 271.05 (C14H12ClN4, [M + H]+). Anal. Calcd. For C14H11ClN4: C, 62.11; H, 4.10; N, 20.70. Found: C, 61.94; H, 4.19; N, 20.29.
(E)-2-((2-(2,4-Dichlorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6k): Yellow power, yield 69.4%, m.p. 212.7–212.8 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.71 (s, 1H), 10.65 (s, 1H), 8.36 (d, J = 0.8 Hz, 1H), 7.89 (d, J = 8.9 Hz, 1H), 7.53 (d, J = 2.3 Hz, 3H), 7.41 (dd, J = 8.9, 2.3 Hz, 1H), 7.21 (s, 2H). MS (ESI): 305.02 (C14H11Cl2N4, [M + H]+). Anal. Calcd. For C14H10Cl2N4: C, 55.10; H, 3.30; N, 18.36. Found: C, 55.58; H, 3.56; N, 17.88.
(E)-2-((2-(2,5-Dichlorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6l): Yellow power, yield 60.1%, m.p. 215.2–215.3 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.87 (s, 1H), 10.70 (s, 1H), 8.38 (s, 1H), 7.94 (d, J = 2.5 Hz, 1H), 7.58 (dd, J = 5.6, 3.5 Hz, 2H), 7.40 (t, J = 6.1 Hz, 1H), 7.26–7.20 (m, 2H), 6.91 (dd, J = 8.5, 2.5 Hz, 1H). MS (ESI): 305.02 (C14H11Cl2N4, [M + H]+). Anal. Calcd. For C14H10Cl2N4: C, 55.10; H, 3.30; N, 18.36. Found: C, 55.58; H, 3.56; N, 17.88.
(E)-2-((2-(2,6-Dichlorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6m): Pale yellow power, yield 72.3%, m.p. 212.5–212.9 °C.1H-NMR (400 MHz, DMSO-d6) δ 12.38 (s, 1H), 10.13 (s, 1H), 7.87 (s, 1H), 7.56 (d, J = 8.1 Hz, 3H), 7.43 (s, 1H), 7.25 (t, J = 8.1 Hz, 1H), 7.16 (s, 2H). MS (ESI): 305.02 (C14H11Cl2N4, [M + H]+). Anal. Calcd. For C14H10Cl2N4: C, 55.10; H, 3.30; N, 18.36. Found: C, 55.58; H, 3.56; N, 17.88.
(E)-2-((2-(3,5-Dichlorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6n): Yellow power, yield 55.8%, m.p. 260.8–261.0 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.71 (s, 1H), 11.20 (s, 1H), 7.91 (s, 1H), 7.56 (s, 2H), 7.24 (d, J = 1.2 Hz, 2H), 7.21 (s, 2H), 6.97 (t, J = 1.8 Hz, 1H). MS (ESI): 305.02 (C14H11Cl2N4, [M + H]+). Anal. Calcd. For C14H10Cl2N4: C, 55.10; H, 3.30; N, 18.36. Found: C, 55.58; H, 3.56; N, 17.88.
(E)-2-((2-(2-Bromophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6o): Brown power, yield 65.9%, m.p. 202.2–202.3 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.65 (s, 1H), 10.32 (s, 1H), 8.39 (s, 1H), 7.86 (dd, J = 8.3, 1.3 Hz, 1H), 7.64–7.50 (m, 3H), 7.39–7.34 (m, 1H), 7.19 (t, J = 12.5 Hz, 2H), 6.83 (td, J = 7.9, 1.5 Hz, 1H). MS (ESI): 315.02 (C14H12BrN4, [M + H]+). Anal. Calcd. For C14H11BrN4: C, 53.35; H, 3.52; N, 17.78. Found: C, 53.38; H, 3.67; N, 17.28.
(E)-2-((2-(4-Bromophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6p): Brown power, yield: 75.2%, m.p. 202.2–202.5 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.59 (s, 1H), 11.02 (s, 1H), 7.88 (s, 1H), 7.53 (s, 2H), 7.45 (d, J = 8.8 Hz, 2H), 7.23–7.16 (m, 4H). MS (ESI): 315.02 (C14H12BrN4, [M + H]+). Anal. Calcd. For C14H11BrN4: C, 53.35; H, 3.52; N, 17.78. Found: C, 53.38; H, 3.67; N, 17.28.
(E)-2-((2-(4-Methoxyphenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6q): Brown power, yield: 69.5%, m.p. 196.8–196.9 °C. 1H-NMR (400 MHz, DMSO-d6) δ:12.46 (s, 1H), 10.74 (s, 1H), 7.81 (s, 1H), 7.51 (dd, J = 5.2, 3.6 Hz, 2H), 7.19 (d, J = 2.1 Hz, 1H), 7.17 (d, J = 3.2 Hz, 2H), 7.15 (d, J = 3.2 Hz, 1H), 6.91 (d, J = 2.1 Hz, 1H), 6.90–6.88 (m, 1H), 3.72 (s, 3H). MS (ESI): 267.10 (C15H15N4O, [M + H]+). Anal. Calcd. For C15H14FN4O: C, 67.65; H, 5.30; N, 21.04. Found: C, 67.38; H, 5.21; N, 21.29.
(E)-4-(2-((1H-Benzo[d]imidazol-2-yl)methylene)hydrazinyl)benzonitrile (6r): Brown power, yield 67.9%, m.p. 260.1–260.2 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.71 (s, 1H), 11.41 (s, 1H), 7.72 (d, J = 8.9 Hz, 2H), 7.53 (d, J = 35.5 Hz, 2H), 7.35 (d, J = 8.5 Hz, 2H), 7.28–7.15 (m, 2H). MS (ESI): 262.09 (C15H12N5, [M + H]+). Anal. Calcd. For C15H11N5: C, 68.95; H, 4.24; N, 26.80. Found: C, 68.74; H, 4.13; N, 26.99.
(E)-2-((2-(3,4-Dimethylphenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6s): Brown power, yield 56.7%, m.p. 203.9–204.5 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.48 (s, 1H), 10.76 (s, 1H), 7.82 (s, 1H), 7.56–7.47 (m, 2H), 7.17 (dd, J = 5.8, 2.9 Hz, 2H), 7.08–7.00 (m, 2H), 6.99–6.94 (m, 1H), 2.22 (s, 3H), 2.16 (s, 3H). MS (ESI): 265.12 (C16H17N4, [M + H]+). Anal. Calcd. For C16H16N4: C, 72.70; H, 6.10; N, 21.20. Found: C, 72.24; H, 6.19; N, 21.09.
(E)-1-Methyl-2-((2-phenylhydrazono)methyl)-1H-benzo[d]imidazole (6t): Yellow power, yield: 65.2%, m.p. 201.5–201.7 °C. 1H-NMR (400 MHz, DMSO-d6) δ 10.91 (s, 1H), 8.04 (s, 1H), 7.65–7.55 (m, 2H), 7.34–7.25 (m, 3H), 7.21 (t, J = 7.1 Hz, 1H), 7.11 (d, J = 7.7 Hz, 2H), 6.85 (t, J = 7.3 Hz, 1H), 4.14 (s, 3H). MS (ESI): 251.10 (C15H15N4, [M + H]+). Anal. Calcd. For C15H14N4: C, 71.98; H, 5.64; N, 22.38. Found: C, 71.73; H, 5.31; N, 22.69.
(E)-2-((2-(3-Fluorophenyl)hydrazono)methyl)-1-methyl-1H-benzo[d]imidazole (6u): Yellow power, yield: 54.6%, m.p. 219.7–319.8 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.09 (s, 1H), 8.06 (s, 1H), 7.64–7.58 (m, 2H), 7.30 (dddd, J = 9.4, 7.2, 5.2, 2.7 Hz, 2H), 7.22 (ddd, J = 8.2, 7.2, 1.2 Hz, 1H), 6.91–6.84 (m, 2H), 6.68–6.61 (m, 1H), 4.13 (s, 3H). MS (ESI): 269.14 (C15H14FN4, [M + H]+). Anal. Calcd. For C15H13FN4: C, 67.15; H, 4.88; N, 20.88. Found: C, 67.23; H, 4.71; N, 20.69.
(E)-2-((2-(2,4-Difluorophenyl)hydrazono)methyl)-1-methyl-1H-benzo[d]imidazole (6v): Yellow power, yield: 64.1%, m.p. 191.6–191.8 °C. 1H-NMR (400 MHz, DMSO-d6) δ 10.81 (s, 1H), 8.31 (s, 1H), 7.61 (t, J = 8.4 Hz, 2H), 7.47 (td, J = 9.3, 5.9 Hz, 1H), 7.30 (ddd, J = 14.6, 8.2, 2.8 Hz, 2H), 7.23 (dd, J = 11.1, 4.0 Hz, 1H), 7.08 (t, J = 8.6 Hz, 1H), 4.12 (s, 3H). MS (ESI): 287.11 (C15H13F2N4, [M + H]+). Anal. Calcd. For C15H12F2N4: C, 62.93; H, 4.22; N, 19.57. Found: C, 62.83; H, 4.11; N, 19.66.
(E)-2-((2-(2,5-Dichlorophenyl)hydrazono)methyl)-1-methyl-1H-benzo[d]imidazole (6w): Yellow power, yield: 72.3%, m.p. 206.5–207.2 °C. 1H-NMR (400 MHz, DMSO-d6) δ 10.68 (s, 1H), 8.56 (s, 1H), 7.64 (dd, J = 16.3, 8.0 Hz, 2H), 7.44 (d, J = 8.2 Hz, 2H), 7.32 (t, J = 7.6 Hz, 1H), 7.24 (t, J = 7.5 Hz, 1H), 6.93 (dd, J = 8.5, 2.2 Hz, 1H), 4.13 (s, 3H). MS (ESI): 319.06 (C15H13Cl2N4, [M + H]+). Anal. Calcd. For C15H12Cl2N4: C, 56.44; H, 3.79; N, 17.55. Found: C, 56.31; H, 4.01; N, 17.61.
(E)-2-((2-(4-Chlorophenyl)hydrazono)methyl)-1-methyl-1H-benzo[d]imidazole (6x): Yellow power, yield: 67.5%, m.p. 213.2–213.5 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.02 (s, 1H), 8.05 (d, J = 1.1 Hz, 1H), 7.63–7.57 (m, 2H), 7.34 (d, J = 2.0 Hz, 1H), 7.33–7.30 (m, 1H), 7.29–7.26 (m, 1H), 7.22 (ddd, J = 8.2, 7.1, 1.3 Hz, 1H), 7.11 (d, J = 2.1 Hz, 1H), 7.09 (d, J = 2.1 Hz, 1H), 4.12 (s, 3H). MS (ESI): 285.09 (C15H14ClN4, [M + H]+). Anal. Calcd. For C15H13ClN4: C, 63.27; H, 4.60; N, 19.68. Found: C, 63.11; H, 4.69; N, 19.76.
(E)-2-((2-(2,4-Dichlorophenyl)hydrazono)methyl)-1-methyl-1H-benzo[d]imidazole (6y): Yellow power, yield: 75.4%, m.p. 211.8–212.9 °C. 1H-NMR (400 MHz, DMSO-d6) δ 10.62 (s, 1H), 8.53 (d, J = 1.1 Hz, 1H), 7.67–7.61 (m, 2H), 7.55 (d, J = 2.4 Hz, 1H), 7.52 (d, J = 8.9 Hz, 1H), 7.39 (dd, J = 8.9, 2.4 Hz, 1H), 7.31 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H), 7.24 (ddd, J = 8.2, 7.2, 1.2 Hz, 1H), 4.13 (s, 3H). MS (ESI): 319.06 (C15H13Cl2N4, [M + H]+). Anal. Calcd. For C15H12Cl2N4: C, 56.44; H, 3.79; N, 17.55. Found: C, 56.31; H, 4.01; N, 17.61.
(E)-4-(2-((1-Methyl-1H-benzo[d]imidazol-2-yl)methylene)hydrazinyl)benzonitrile (6z): Yellow power, yield: 55.8%, m.p. 258.8–259.0 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.42 (s, 1H), 8.14 (s, 1H), 7.71 (d, J = 8.6 Hz, 2H), 7.63 (t, J = 8.3 Hz, 2H), 7.31 (t, J = 7.5 Hz, 1H), 7.25 (d, J = 7.8 Hz, 1H), 7.19 (d, J = 8.5 Hz, 2H), 4.13 (s, 3H). MS (ESI): 329.05 (C15H14BrN4, [M + H]+). Anal. Calcd. For C15H13BrN4: C, 54.73; H, 3.98; N, 17.02. Found: C, 54.51; H, 4.01; N, 17.11.
(E)-1-Methyl-2-((2-(4-(trifluoromethoxy)phenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6aa): Pale yellow power, yield: 68.2%, m.p. 196.8–196.9 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.07 (s, 1H), 8.07 (s, 1H), 7.61 (t, J = 7.9 Hz, 2H), 7.29 (dd, J = 11.2, 4.7 Hz, 3H), 7.25–7.20 (m, 1H), 7.16 (d, J = 9.0 Hz, 2H), 4.13 (s, 3H). MS (ESI): 335.07 (C16H14F3N4O, [M + H]+). Anal. Calcd. For C16H13F3N4O: C, 57.49; H, 3.92; N, 16.76. Found: C, 57.17; H, 3.89; N, 16.76.
(E)-2-((2-(4-Fluorophenyl)hydrazono)methyl)-1-methyl-1H-benzo[d]imidazole (6ab): Yellow power, yield: 60.8%, m.p. 183.2–184.1 °C. 1H-NMR (400 MHz, DMSO-d6) δ 10.91 (s, 1H), 8.03 (s, 1H), 7.60 (t, J = 8.5 Hz, 2H), 7.27 (dd, J = 11.1, 4.1 Hz, 1H), 7.22 (dd, J = 11.0, 4.0 Hz, 1H), 7.15(s,1H), 7.14–7.07 (m, 3H), 4.12 (s, 3H). MS (ESI): 269.14 (C15H14FN4, [M + H]+). Anal. Calcd. For C15H13FN4: C, 67.15; H, 4.88; N, 20.88. Found: C, 67.23; H, 4.71; N, 20.69.
(E)-2-((2-(2-Bromophenyl)hydrazono)methyl)-1-methyl-1H-benzo[d]imidazole (6ac): Brown power, yield: 56.6%, m.p. 190.1–190.9 °C. 1H-NMR (400 MHz, DMSO-d6) δ 10.29 (s, 1H), 8.56 (s, 1H), 7.63 (dd, J = 14.4, 8.0 Hz, 2H), 7.53 (ddd, J = 17.2, 8.1, 1.2 Hz, 2H), 7.37 (t, J = 7.3 Hz, 1H), 7.30 (dd, J = 11.2, 4.0 Hz, 1H), 7.26–7.20 (m, 1H), 6.87–6.81 (m, 1H), 4.13 (d, J = 7.6 Hz, 3H). MS (ESI): 276.13 (C16H14N5, [M + H]+). Anal. Calcd. For C16H13N5: C, 69.80; H, 4.76; N, 25.44. Found: C, 69.75; H, 4.74; N, 25.23.
(E)-6-Methyl-2-((2-phenylhydrazono)methyl)-1H-benzo[d]imidazole (6ad): Yellow power, yield: 64.3%, m.p. 204.6–205.3 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.40 (s, 1H), 10.87 (s, 1H), 7.84 (s, 1H), 7.48 (t, J = 7.1 Hz, 1H), 7.39 (t, J = 8.1 Hz, 1H), 7.30 (s, 1H), 7.29–7.25 (m, 2H), 7.25–7.20 (m, 2H), 6.84 (t, J = 7.1 Hz, 1H), 2.41 (s, 3H). MS (ESI): 251.10 (C15H15N4, [M + H]+). Anal. Calcd. For C15H14N4: C, 71.98; H, 5.64; N, 22.38. Found: C, 71.73; H, 5.31; N, 22.69.
(E)-2-((2-(4-Chlorophenyl)hydrazono)methyl)-6-methyl-1H-benzo[d]imidazole (6ae): Yellow power, yield: 72.5%, m.p. 216.3–217.5 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.48 (s, 1H), 11.00 (s, 1H), 7.86 (s, 1H), 7.42 (d, J = 8.2 Hz, 1H), 7.34 (s, 1H), 7.31 (s, 2H), 7.24 (d, J = 8.7 Hz, 2H), 7.00 (d, J = 8.1 Hz, 1H), 2.41 (s, 3H). MS (ESI): 285.09 (C15H13ClN4, [M + H]+). Anal. Calcd. For C15H13ClN4: C, 63.27; H, 4.60; N, 19.68. Found: C, 63.11; H, 4.69; N, 19.76.
(E)-2-((2-(2,4-Difluorophenyl)hydrazono)methyl)-6-methyl-1H-benzo[d]imidazole (6af): Pale yellow power, yield: 59.4%, m.p. 221.9–222.7 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.51 (s, 1H), 10.81 (s, 1H), 8.13 (s, 1H), 7.80 (td, J = 9.3, 5.9 Hz, 1H), 7.46–7.38 (m, 1H), 7.35–7.23 (m, 2H), 7.10 (tt, J = 8.8, 1.9 Hz, 1H), 7.01 (d, J = 8.2 Hz, 1H), 2.41 (s, 3H). MS (ESI): 287.11 (C15H13F2N4, [M + H]+). Anal. Calcd. For C15H12F2N4: C, 62.93; H, 4.22; N, 19.57. Found: C, 62.83; H, 4.11; N, 19.66.
(E)-6-Chloro-2-((2-phenylhydrazono)methyl)-1H-benzo[d]imidazole (6ag): Pale yellow power, yield: 72.1%, m.p. 215.6–215.9 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.73 (s, 1H), 11.03 (s, 1H), 7.87 (s, 1H), 7.63–7.46 (m, 2H), 7.30 (dd, J = 8.6, 6.9 Hz, 2H), 7.28–7.23 (m, 2H), 7.21 (dd, J = 8.5, 2.1 Hz, 1H), 6.87 (t, J = 7.0, 1.4 Hz, 1H). MS (ESI): 271.05 (C14H12ClN4, [M + H]+). Anal. Calcd. For C14H11ClN4: C, 62.11; H, 4.10; N, 20.70. Found: C, 61.94; H, 4.19; N, 20.29.
(E)-6-Chloro-2-((2-(4-chlorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6ah): Brown power, yield: 62.8%, m.p. 235.5–236.9 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.77 (s, 1H), 11.13 (s, 1H), 7.87 (s, 1H), 7.67–7.46 (m, 3H), 7.35 (d, J = 2.2 Hz, 1H), 7.34 (d, J = 2.3 Hz, 1H), 7.26 (d, J = 2.2 Hz, 1H), 7.25 (d, J = 2.1 Hz, 1H), 7.21 (d, J = 8.8 Hz, 1H). MS (ESI): 305.02 (C14H11Cl2N4, [M + H]+). Anal. Calcd. For C14H10Cl2N4: C, 55.10; H, 3.30; N, 18.36. Found: C, 55.58; H, 3.56; N, 17.88.
(E)-6-Chloro-2-((2-(2,4-difluorophenyl)hydrazono)methyl)-1H-benzo[d]imidazole (6ai): Yellow power, yield: 68.2%, m.p. 230.2–23.0.7 °C. 1H-NMR (400 MHz, DMSO-d6) δ 12.79 (s, 1H), 10.94 (s, 1H), 8.12 (s, 1H), 7.80 (td, J = 9.3, 5.9 Hz, 1H), 7.67–7.58 (m, 1H), 7.49 (d, J = 7.9 Hz, 1H), 7.30 (ddd, J = 11.8, 8.9, 2.8 Hz, 1H), 7.22 (dd, J = 20.5, 8.3 Hz, 1H), 7.12 (tt, J = 8.9, 2.1 Hz, 1H). MS (ESI): 306.99 (C14H10ClF2N4, [M + H]+). Anal. Calcd. For C14H9ClF2N4: C, 54.83; H, 2.96; N, 18.27. Found: C, 54.61; H, 3.01; N, 17.98.
3.3. Crystallographic Study
X-ray single-crystal diffraction data for compound 6b were measured on a Bruker SMART APEX CCD area detector diffractometer using MoKα radiation (λ = 0.71073 Å) by the π and ω scan mode. The program SAINT was used for integration of the diffraction profiles. The structure was solved by direct methods using the SHELXS program of the SHELXTL package and refined by full-matrix least-squares methods with SHELXL. All non-hydrogen atoms of compound 6b were refined with anisotropic thermal parameters. All hydrogen atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms.
3.4. Biological Assay
The test fungi R. solani and M. oryzae were provided by the Laboratory of Plant Disease Control at Nanjing Agricultural University. After removing strains from the storage tube, the strains were incubated in potato dextrose agar (PDA) at 25 °C for a week to develop new mycelia for the antifungal assay.
3.4.1. Antifungal Activity Assays in Vitro
The in vitro antifungal activities of the target compounds against R. solani and M. oryzae were evaluated using the mycelium growth rate method [19,20]. The compounds were dissolved in DMSO and then mixed with 30 mL sterile molten PDA to obtain final concentrations of 0.625, 1.25, 2.5, 5, 10 and 20 μg/mL (containing 4‰ DMSO) at 50 °C, then PDA with different compounds was poured into 90 mm petri dishes (15 mL/dish), on which 0.5 cm mycelial disks of the two fungi were planted in the center. Each measurement consisted of at least three replicates. After a certain incubation period (1.5 days for R. solani, 5 days for M. oryzae, according to their different mycelial growth rates) at 25 °C in the dark, mycelia growth diameters were measured. DMSO was served as negative control. The inhibition rate was calculated according to the formula: Inhibition rate = (A − B)/(A − 0.5 cm) × 100%. Where A is the diameter (cm) of the negative control and B is the mycelial diameter (cm) in petri dishes with compounds. Carbendazim, validamycin A and isoprothiolane were co-assayed as the positive control. Those compounds were treated to calculate their median effective concentration (EC50) values.
3.4.2. Inhibition of 6f on the Sclerotia Germination of R. solani
Sclerotia of R. solani were cultured and dried for germination test [21]. A series of concentrations of tested samples were prepared, then two layers of filter paper were soaked with the solution. These filter paper layers were put in culture dishes and each concentration consisted of at least three replicates. A replicate included 50 sclerotia. Validamycin A and distilled water was served as the positive control and blank control, respectively. These dishes were incubated at 25 °C for 4 days. Then the inhibition rate of the compounds on the germination of sclerotia was calculated as: Inhibition rate = (average germination number of blank control − average germination number of treatment)/average germination number of blank control × 100%.
3.4.3. Protective Activity of 6f against RSB In Vivo
For evaluating the antifungal activity of 6f against RSB in vivo, rice cultivar (Shanyou 63) was sown and grown in plastic pots in the greenhouse [22]. The compound 6f at the concentration of 200 μg/mL and 100 μg/mL was sprayed on the cultivar, respectively, and inoculated with R. solani 24 h later. Validamycin A at the concentration of 200 μg/mL and 100 μg/mL was used as the positive control. All the treatments were replicated for 20 plants and incubated in the greenhouse with an average midday relative humidity of 85%. A visual disease assessment was made 7 days after inoculating with R. solani by measuring the lesion length. The protective efficacy was calculated as: Protection efficacy = ((average lesion length of control − average lesion length of treated group)/average lesion length of control) × 100%.
3.4.4. Inhibition of 6f on the Conidium Germination of M. oryzae
Abundant spores of M. oryzae were collected and suspended to a concentration of 5 × 104 spores per milliliter in sterile water for conidium germination test. A series of concentrations of tested samples (6f and control) were mixed with conidial suspension, respectively. Aliquots of 10 μL of prepared conidial suspension were placed on separate glass slides in triplicate, which were incubated in a moisture chamber at 25 °C for 24 h. Each slide was then observed under the microscope, and the appressorium formation percentage was examined [23].
3.4.5. Protective Activity of 6f against RB In Vivo
Compound 6f was further measured for its antifungal activity in vivo against RB on rice (Shanyou 63). Different concentrations (50 μg/mL and 100 μg/mL) of 6f (4 mL) were sprayed on two-week old seedlings of rice leaves. After 24 h, previously prepared spore suspension of M. oryzae was also sprayed with 0.2% (w/v) gelatin. Plants were kept at 25 °C with 90% humidity and in a 12/12 h light/dark cycle. The positive control is carbendazim of 25 and 50 μg/mL. Lesion formation was observed daily and the protective efficacies were calculated after 7 days [24].
4. Conclusions
In conclusion, a series of benzimidazole derivatives had been synthesized and investigated for the antifungal activities against R. solani and M. oryzae in vitro and in vivo. The result showed that many of the compounds had significant antifungal activities. Compound 6f exhibited most potent antifungal activity with an EC50 value of 1.20 μg/mL against R. solani, which was better than carbendazim (1.84 μg/mL). The protective activity of 6f in vivo reached 66.5% at 100 μg/mL, whereas validamycin A was 61.0%. At the same time, 6f displayed moderate activity against M. oryzae with EC50 value of 1.85 μg/mL, and inhibited the conidium germination at 1.0 μg/mL. The result indicated that the compound 6f with a benzimidazole phenylhydrazone scaffold could be considered as a leading structure for the development of novel fungicides.
Acknowledgments
This work was co-supported by the National Natural Science Foundation of China (31572043), the Jiangsu Agriculture Science and Technology Innovation Fund (CX(15)1054), and the Natural Science Foundation of Jiangsu Province (BK20151428).
Author Contributions
Y.-H.Y. designed the research; X.W. and Y.-F.C. performed the synthesis, separation and structure elucidation. W.Y. and L.-L.C. contributed to evaluation of antifungal activities. X.W. and Y.-H.Y. coordinated writing the paper to which all co-authors contributed.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Woolley D.W. Some biological effects producted by benzimidazole and their reversal by purines. J. Biol. Chem. 1944;152:225–232. [Google Scholar]
- 2.Madkour H., Farag A.A., Ramses S.S., Ibrahiem N. Synthesis and fungicidal activity of new imidazoles from 2-(chloromethyl)-1H-benzimidazole. Phosphorus Sulfur. 2006;181:255–265. doi: 10.1080/104265090970241. [DOI] [Google Scholar]
- 3.Singla P., Luxami V., Paul K. Synthesis and in vitro evaluation of novel triazine analogues as anticancer agents and their interaction studies with bovine serum albumin. Eur. J. Med. Chem. 2016;117:59–69. doi: 10.1016/j.ejmech.2016.03.088. [DOI] [PubMed] [Google Scholar]
- 4.Song D., Ma S.T. Recent development of benzimidazole-containing antibacterial agents. ChemMedChem. 2016;11:646–659. doi: 10.1002/cmdc.201600041. [DOI] [PubMed] [Google Scholar]
- 5.Kus C., Ayhan-Kilcigil G., Ozbey S., Kaynak F.B., Kaya M., Coban T., Can-Eke B. Synthesis and antioxidant properties of novel N-methyl-1,3,4-thiadiazol-2-amine and 4-methyl-2H-1,2,4-triazole-3(4H)-thione derivatives of benzimidazole class. Bioorg. Med. Chem. 2008;16:4294–4303. doi: 10.1016/j.bmc.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca T., Gigantea B., Gilchrist L. A short synthesis of phenanthro[2,3-d]imidazoles from dehydroabietic acid. Application of the methodology as a convenient route to benzimidazoles. Tetrahedron. 2001;57:1793–1799. doi: 10.1016/S0040-4020(00)01158-3. [DOI] [Google Scholar]
- 7.Perez-Villanueva J., Santos R., Hernandez-Campos A., Giulianotti M.A., Castillo R., Medina-Franco J.L. Structure-activity relationships of benzimidazole derivatives as antiparasitic agents: Dual activity-difference (DAD) maps. MedChemComm. 2011;2:44–49. doi: 10.1039/C0MD00159G. [DOI] [Google Scholar]
- 8.Bi C.W., Qiu J.B., Zhou M.G., Chen C.J., Wang J.X. Effects of carbendazim on conidial germination and mitosis in germlings of Fusarium graminearum and Botrytis cinerea. Int. J. Pset Manag. 2009;55:157–163. [Google Scholar]
- 9.Tanejk M., Grover R.K. Efficacy of benzimidazole and related fungicides against Rhizoctonia solain and R. bataticola. Ann. Appl. Biol. 1982;100:425–432. doi: 10.1111/j.1744-7348.1982.tb01409.x. [DOI] [Google Scholar]
- 10.Liu L.X., Tom H. Estimating benzimidazole residues in thatch and turfgrass by bioassay. Pestic. Sci. 1996;46:139–143. doi: 10.1002/(SICI)1096-9063(199602)46:2<139::AID-PS326>3.0.CO;2-3. [DOI] [Google Scholar]
- 11.Zhu L.F., Hou Z., Zhou K., Tong Z.B., Kuang Q., Geng H.L., Zhou L. Synthesis, bioactivity and structure-activity relationships of new 2-aryl-8-OR-3,4-dihydroisoquinolin-2-iums salts as potential antifungal agents. Bioorg. Med. Chem. Lett. 2016;26:2413–2417. doi: 10.1016/j.bmcl.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M., Dai Z.C., Qian S., Liu J., Xiao Y., Lu A., Zhu H.L., Wang J.X., Ye Y.H. Design, synthesis, antifungal, and antioxidant activities of (E)-6-((2-phenylhydrazono)methyl)quinoxaline derivatives. J. Agric. Food Chem. 2014;62:9637–9643. doi: 10.1021/jf504359p. [DOI] [PubMed] [Google Scholar]
- 13.Dai Z.C., Chen Y.F., Zhang M., Li S.K., Yang T.T., Shen L., Wang J.X., Qian S., Zhu H.L., Ye Y.H. Synthesis and antifungal activity of 1,2,3-triazole phenylhydrazone derivatives. Org. Biomol. Chem. 2015;13:477–486. doi: 10.1039/C4OB01758G. [DOI] [PubMed] [Google Scholar]
- 14.Ellis G.P., Wathey W.B. Benzimidazoles. Part 2. A new synthesis of [1,2,4] triazino [4,5-a] benzimidazol-1-ones. J. Chem. Res. 1984;12:384–385. [Google Scholar]
- 15.Le B., Marie T., Wahl H. Monoazo dye derivatives of 1,2-dimethylbenzimidazole. C. R. 1957;245:2058–2060. [Google Scholar]
- 16.Eom Y.W., Oh S., Woo H.B., Ham J., Ahn C.M., Lee S. Cytotoxicity of substituted benzimidazolyl curcumin mimics against multi-drug resistance cancer cell. Bull. Korean Chem. Soc. 2013;34:1272–1274. doi: 10.5012/bkcs.2013.34.4.1272. [DOI] [Google Scholar]
- 17.Hagiwara H., Okada S. A polymorphism-dependent T1/2 shift of 100 K in a hysteretic spin-crossover complex related to differences in intermolecular weak CH⋯X hydrogen bonds (X = S vs. S and N) Chem. Commun. 2016;52:815–818. doi: 10.1039/C5CC08215C. [DOI] [PubMed] [Google Scholar]
- 18.Ayati A., Falahati M., Irannejad H., Emami S. Synthesis, in vitro antifungal evaluation and in silico study of 3-azolyl-4-chromanone phenylhydrazones. Daru J. Pharm. Sci. 2012;20:46. doi: 10.1186/2008-2231-20-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye Y.H., Ma L., Dai Z.C., Xiao Y., Zhang Y.Y., Li D.D., Wang J.X., Zhu H.L. Synthesis and antifungal activity of Nicotinamide derivatives as succinate dehydrogenase inhibitors. J. Agric. Food Chem. 2014;62:4063–4071. doi: 10.1021/jf405437k. [DOI] [PubMed] [Google Scholar]
- 20.Xiao Y., Li H.X., Li C., Wang J.X., Li J., Wang M.H., Ye Y.H. Antifungal screening of endophytic fungi from Ginkgo biloba for discovery of potent anti-phytopathogenic fungicides. FEMS Microbiol. Lett. 2013;339:130–136. doi: 10.1111/1574-6968.12065. [DOI] [PubMed] [Google Scholar]
- 21.Tang Z.H., Wang H.C., Wang J.X., Chen C.J., Zhou M.G. Fungicidal activity of propiconazole to Rhizoctonia. solani and its control efficacy against rice sheath blight. Plant Prot. 2012;38:158–161. [Google Scholar]
- 22.Peng D., Li S.D., Wang J.X., Chen C.J., Zhou M.G. Integrated biological and chemical control of rice sheath blight by Bacillus subtilis NJ-18 and jinggangmycin. Pest Manag. Sci. 2014;70:258–263. doi: 10.1002/ps.3551. [DOI] [PubMed] [Google Scholar]
- 23.Cao L.L., Zhang Y.Y., Liu Y.J., Yang T.T., Zhang J.L., Zhang Z.G., Ye Y.H. Anti-phytopathogenic activity of sporothriolide, a metabolite from endophyte Nodulisporium. sp. A21 in Ginkgo biloba. Pestic. Biochem. Phys. 2016;129:7–13. doi: 10.1016/j.pestbp.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H.F., Tang W., Liu K., Huang Q., Zhang X., Yan X. Eight RGS and RGS-like proteins orchestrate growth, differentiation, and pathogenicity of Magnaporthe oryzae. PLoS Pathog. 2011;7:e100245012. doi: 10.1371/journal.ppat.1002450. [DOI] [PMC free article] [PubMed] [Google Scholar]