Abstract
Eucalyptus oil possesses a wide spectrum of biological activity, including anti-microbial, fungicidal, herbicidal, acaricidal and nematicidal properties. We studied anti-fungal activities of the leaf oil extracted from Eucalyptus. grandis × E. urophylla. Eleven plant pathogenic fungi were tested based on the mycelium growth rates with negative control. The results showed that Eucalyptus oil has broad-spectrum inhibitory effects toward these fungi. Remarkable morphological and structural alterations of hypha have been observed for Magnaporthe grisea after the treatment. The mRNA genome array of M. grisea was used to detect genes that were differentially expressed in the test strains treated by the Eucalyptus oil than the normal strains. The results showed 1919 genes were significantly affected, among which 1109 were down-regulated and 810 were up-regulated (p < 0.05, absolute fold change >2). According to gene ontology annotation analysis, these differentially expressed genes may cause abnormal structures and physiological function disorders, which may reduce the fungus growth. These results show the oil has potential for use in the biological control of plant disease as a green biopesticide.
Keywords: E. grandis × E. urophylla oil, antifungal effects, M. grisea, mRNA Genome Array, biological control
1. Introduction
Agricultural studies have focused on the biocontrol of plant disease for a long time. Discovery of antifungal and germicidal compounds from plants is an efficient way to create new pollution-free pesticides. Many plants have antimicrobial activities that are related to their antimicrobial constituents, including alkaloids, terpenes, polysaccharide, esters, ketones, and quinones [1]. Effective components extracted from plants have promising potential for this purpose because of their high efficacy, low toxicity, and selective characteristics [2]. Various biopesticides have been developed that contain nicotine, rotenone, matrine, toosendanin and other similar compounds as their main component; for example, Green Gold, Econeem, Akign™, Neem Azal™, and Saferin [3].
Essential oils are basic active natural pesticides and disease control compounds found in a wide range of plants. Essential oils have become a major research focus in recent years for their effective disease and insect pest controlling functions. The essential oil of Eucalyptus possesses a wide spectrum of biological activity [4], including anti-microbial, fungicidal, insecticidal/insect repellent, herbicidal, acaricidal and nematicidal properties. The essential oil of Eucalyptus may be obtained from various different plant parts, however, the highest concentration of Eucalyptus essential oil has been found in the leaves. The dominant compounds in Eucalyptus oil include 1,8-cineole, limonene, p-cymene, γ-terpinene, α-pinene, α-terpineol, camphene, linalool, and ocimene [5,6,7,8,9,10,11]. The antimicrobial action of the essential oil of Eucalyptus is well known. Considerable research has indicated that the Eucalyptus extracts such as E. citriodora, E. globulus and E. staigeriana, have clear antimicrobial action against many kinds of pathogenic microbes [7,12,13,14,15]. However, few studies into the inhibitory mechanisms have been conducted.
Eucalyptus agriculture has significant economic and ecological benefits. However, the leaves of Eucalyptus are often burned as fuel or eventually discarded, which have not been well used until now. In this study we analyzed EO, the essential oil of E. grandis × E. urophylla oil, a fast-growing hybrid clone between E. grandis and E. urophylla, which is an economically important pulp tree that is widely grown in many provinces in south China. This pulp tree (Guanglin 9 cultivar) was introduced to Yuping, a small town of Sichuan Province in 2008. The main constituents of local Eucalyptus oil are α-pinene (24.78%), 1,8-cineole (45.57%), terpinyl acetate (8.34%), α-terpineol (2.51%), isoborneol (1.45%), camphene (0.60%) and linalool (0.15%) [16]. Isoborneol, linalool, α-terpineol, 1,8-cineole, α-pinene and camphene have known antimicrobial properties [17,18,19,20,21,22,23]. Known antimicrobial components account for over 75.1% of the total volatile oil. In general, the strong antimicrobial activity is related not only to a high content of one major component such as 1,8-cineole, but also to the presence of moderate and minor compounds [12].
Here we report the findings of systematic research into the main components, the inhibitive activities, the morphological variations, and related molecular mechanisms to improve understanding of the broad-spectrum antifungal effect of the leaf oil of E. grandis × E. urophylla.
2. Results and Discussion
2.1. Inhibitory Activities of the Eucalyptus Oil on Plant Pathogenic Fungi
Table 1 shows the inhibitory activities of the Eucalyptus oil with a final concentration of 2.5 mg/mL on 11 plant pathogenic fungi based on the mycelium growth rates with negative control. It is clear that the Eucalyptus oil had broad-spectrum inhibitory effects to these fungi (Table 1). We observed a higher inhibition rate of serious plant pathogenic fungi like S. turcica, M. grisea, B. cinerea, F. graminearum and B. maydis relative to the control. Based on these results, we conclude that the oil has potential for use in the biological control of plant disease.
Table 1.
Inhibitory activities of the Eucalyptus oil on plant pathogenic fungi.
| Strain | Control Group (cm) | EO-Treated Group (cm) | Inhibition Rate (%) |
|---|---|---|---|
| Setosphaeria turcica | 1.7 ± 0.3 | 0.6 ± 0.2 | 91 ± 9 |
| Magnaporthe grisea | 2.4 ± 0.4 | 1.5 ± 0.3 | 81 ± 7 |
| Botrytis cinerea | 3.0 ± 0.8 | 1.1 ± 0.3 | 75 ± 7 |
| Fusarium graminearum | 5.2 ± 1.7 | 1.7 ± 0.4 | 74 ± 6 |
| Bipolaris maydis | 5.9 ± 1.6 | 2.6 ± 0.6 | 62 ± 6 |
| Rhizoctonia solani | 5.4 ± 1.5 | 2.6 ± 0.8 | 56 ± 5 |
| Rhizoctonia solani | 3.5 ± 1.0 | 2.1 ± 0.5 | 46 ± 5 |
| Colletotrichum gloeosporioides | 2.9 ± 0.6 | 2.0 ± 0.4 | 45 ± 5 |
| Alternuria longipes | 4.0 ± 1.1 | 2.9 ± 0.7 | 31 ± 5 |
| Alternaria solani | 3.6 ± 1.0 | 2.8 ± 0.7 | 26 ± 4 |
| Fusarium moniliforme | 4.3 ± 1.1 | 3.3 ± 0.7 | 25 ± 4 |
Diameters of the plaques and inhibition rates (determined by the mycelium growth rate method) are shown.
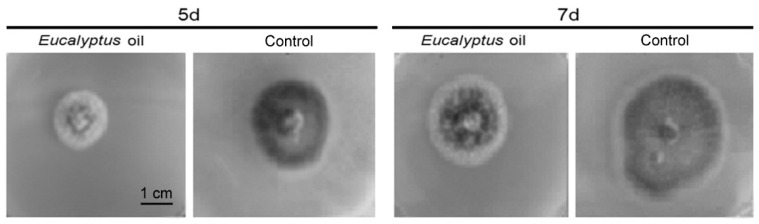
The oil impacts on the growth of M. grisea after 5 days and 7 days of culture are shown in Figure 1. Results indicate that the colony morphology of the strain was larger, the hyphae were relatively dense and more melanin was produced in the control group than in the EO-treated groups. This suggests that EO is able to inhibit hyphal growth and spore sprouting of M. grisea. The EO reached the highest inhibition rate to M. grisea after being cultured for 5 days. After 7 days of culture, the colony morphology of the strain in the EO-treated group became larger, and inhibition rate declined, and spores and melanin were produced, which may be because the oil had evaporated.
Figure 1.
The oil impact on the growth of M. grisea after being cultured for 5 days and 7 days.
2.2. SEM Results
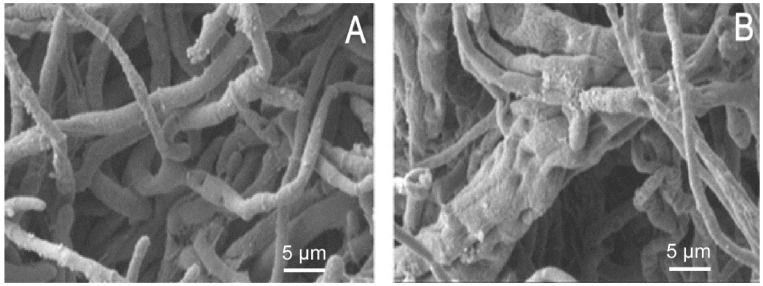
SEM was used to examine the morphological variations of M. grisea (Figure 2). We found remarkable morphological and structural alterations of the hypha. These changes include irregular shape, floccules on the mineral surface, cavities in the outer surface, adhesion of mycelia, and swellings at the top of spores. Both the infective ability and biological activity of M. grisea were largely inhibited by Eucalyptus oil.
Figure 2.
Eucalyptus oil effects on M. grisea hypha growth by the scanning electron microscopy. (A) control group; (B) experimental group.
2.3. Hybridization Process Results
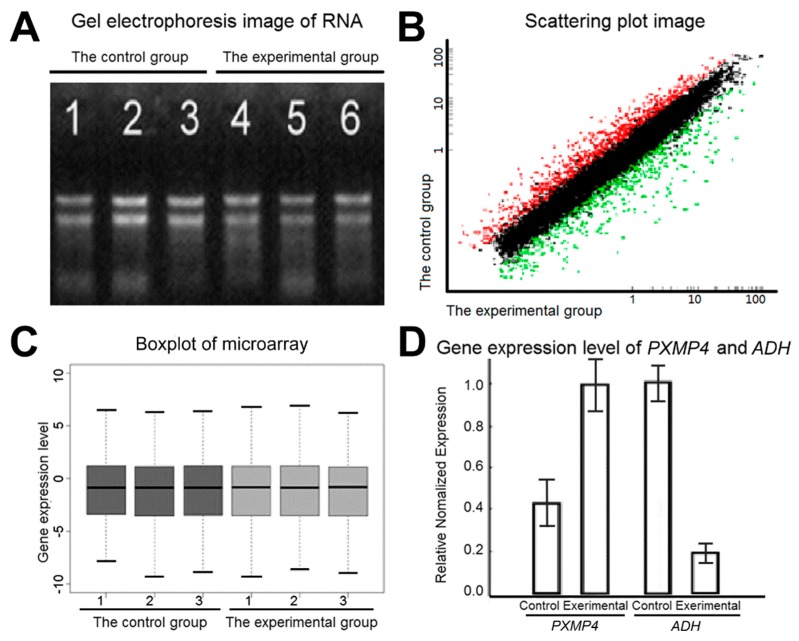
RNA integrity was assessed by standard denaturing agarose gel electrophoresis (Figure 3A). The purity of RNA was A260/280 ≥ 1.80, RNA acquisition ≥1 µg. The electrophoresis of RNA on gel containing formaldehyde showed that the electrophoresis result was satisfactory with clear straps in the correct place (28S:18S rRNA) and the brightness of bands is close to or larger than 2:1. This suggests that the quality control of RNA meet the requirements. Scatter plots (Figure 3B) suggest that there was a significant relationship between the control group and the experimental group. The box diagram (Figure 3C) shows the data processing and normalization results. The mean values of all genes in the control group and the experimental group were at the same level, which reflects the differentially expressed genes between the two groups, and implies that the data were reliable. For each gene, the RT-PCR showed a similar expression pattern to the corresponding results of the gene chip (Figure 3D). The expression levels of PXMP4 (encoding peroxisomal membrane protein 4) increased more than 2.34 times while the chips increased more than 2.24 times. The gene expression level of alcohol dehydrogenase gene ADH decreased 5.21 times, while that of the chips decreased 5.86 times. This shows that the results of RT-PCR were almost the same as those of the chips. In conclusion, the test results of the hybridization process show that hybridization meets the experimental requirements.
Figure 3.
The tests for the hybridization process. RNA integrity (A) was assessed by standard denaturing agarose gel electrophoresis. Scatter plots (B) shows the correlation of signal values between control group and experimental group. The box diagram (C) shows the results of data processing and normalization (to the actin 1 gene). RT-PCR (D) shows a similar expression pattern to the corresponding results of gene chip. The up-regulated gene is peroxisomal membrane protein 4 (PXMP4); the down-regulated gene is alcohol dehydrogenase (ADH). Bars indicate SD.
2.4. Microarray Data Analysis
2.4.1. Identification of Differentially Expressed Genes
The M. grisea mRNA Genome Array was used for detecting the differentially expressed genes between the test strains treated by the Eucalyptus oil and the untreated strains. The results showed 1919 genes were significantly affected, of which 1109 were down-regulated and 810 were up-regulated (p < 0.05, FC > 2).
2.4.2. GO Analysis
Table 2 shows the result of functional annotation and cluster analyses. Based on biological processes, this EO treatment affects metabolism of cells, mycelium development, transmembrane transport and interaction with host via proteins. Based on molecular function, this treatment affects enzyme activities, catalytic activity and transcription factor activity. Based on the cellular component, this treatment affects integrity of membrane, cytoplasm and nucleus.
Table 2.
GO analysis for differentially expressed genes.
| GO Terms | Terms Type | Genes_In_Term | DEG | Up | Down | p-Value |
|---|---|---|---|---|---|---|
| GO:0055114 (oxidation-reduction process) | P | 777 | 109 | 51 | 58 | 1.53 × 10−10 |
| GO:0044271 (cellular nitrogen compound biosynthetic process) | P | 38 | 14 | 0 | 14 | 1.01 × 10−5 |
| GO:0043581 (mycelium development) | P | 468 | 59 | 22 | 37 | 5.60 × 10−4 |
| GO:0009103 (lipopolysaccharide biosynthetic process) | P | 10 | 6 | 4 | 2 | 6.24 × 10−4 |
| GO:0006066 (alcohol metabolic process) | P | 13 | 6 | 4 | 2 | 3.37 × 10−3 |
| GO:0055085 (transmembrane transport) | P | 292 | 37 | 10 | 27 | 1.06 × 10−2 |
| GO:0052051 (interaction with host via protein secreted by type II secretion system) | P | 50 | 11 | 9 | 2 | 1.15 × 10−2 |
| GO:0008152 (metabolic process) | P | 328 | 39 | 27 | 12 | 1.69 × 10−2 |
| GO:0005375 (copper ion transmembrane transporter activity) | P | 8 | 4 | 1 | 3 | 1.69 × 10−2 |
| GO:0035434 (copper ion transmembrane transport) | P | 8 | 4 | 1 | 3 | 1.69 × 10−2 |
| Total P | 1992 | 289 | 129 | 160 | ||
| GO: 0016491 (oxidoreductase activity) | F | 268 | 50 | 23 | 27 | 6.79 × 10−9 |
| GO:0050660 (flavin adenine dinucleotide binding) | F | 90 | 18 | 11 | 7 | 2.27 × 10−3 |
| GO:0008812 (choline dehydrogenase activity) | F | 9 | 5 | 3 | 2 | 6.47 × 10−3 |
| GO:0004181 (metallocarboxypeptidase activity) | F | 6 | 4 | 1 | 3 | 9.44 × 10−3 |
| GO:0003824 (catalytic activity) | F | 175 | 24 | 11 | 13 | 2.44 × 10−2 |
| GO:0000981 (sequence-specific DNA binding RNA polymerase II transcription) factor activity) | F | 113 | 17 | 11 | 6 | 3.99 × 10−2 |
| Total F | 661 | 118 | 60 | 58 | ||
| GO:0016021 (integral to membrane) | C | 742 | 52 | 21 | 31 | 3.37 × 10−6 |
| GO:0005737 (cytoplasm) | C | 259 | 20 | 1 | 19 | 8.13 × 10−3 |
| GO:0005634 (nucleus) | C | 610 | 35 | 18 | 17 | 1.41 × 10−2 |
| Total C | 1611 | 107 | 40 | 67 |
DEG = differentially expressed genes; P: biological_process; C: cellular_component; F: molecular_function.
2.5. Analysis of Treatment Results of the Eucalyptus Oil Combined with Antifungal Drug Targets
2.5.1. Inhibition to Cell-Wall Synthesis, Chitin and Cellulose Synthesis
The fungal cell wall is a special structure of fungi. Cell wall-acting antifungals are inherently selective and fungicidal, features that make them particularly attractive for clinical development. The coordinated synthesis and modification of chitin, a key component of the fungal cell wall, is essential for plant fungal pathogens to maintain cell structure integrity and full pathogenicity. Key enzymes that are related to cell-wall synthesis of fungi are ideal targets for antifungals [24]. For example, polyoxin can interfere with the synthesis of chitin, which inhibits cell-wall synthesis and results in cell death. After polyoxin contact, germ tubes and hyphae become swollen and ruptured, cell contents leak and the organism dies. Polyoxin also inhibits expansion of the disease spot and spore bearing. After the oil treatment, the expression level of chitin deacetylase gene (A_98_P124148, CDAs) decreased significantly (FC = 19.21), which assists in control of the decomposition rate and products [25,26]. Therefore, the down-regulation after treatment may influence the regulation of chitin decomposition, causing chitin to be digested, cell wall structures to be destroyed, and the formation of irregularly shaped hyphae, thus reducing the infection capacity of the strain.
Cellulose is the main component of fungal cell walls. Antifungal agents such as propionamide can affect hyphal development and spore-bearing by influencing cellulose synthesis. Cellobiose dehydrogenase is associated with cellulose production. After the oil treatment, the expression level of cellobiose dehydrogenase gene (A_98_P175762) was significantly up-regulated (FC = 2.92). According to the SEM observations, the Eucalyptus oil may affect cellulose synthesis of M. grisea, leading to abnormal hypha growth.
2.5.2. Interference with Respiration
Mitochondria are important subcellular organs that generate energy for cells. Recent studies suggested that mitochondria are related to cellular aging and cell death, and played a notable role in intracellular communication [27,28]. The antibacterial ability of α-pinene is related to its suppression of the aerobic metabolism of mitochondria [21]. Azoxystrobin can affect energy metabolism by inhibiting cellular respiration, which inhibits the hyphal growth and spore sprouting, causing eventual cell death [29]. Choline dehydrogenase is an important component of electron transport chain in the mitochondrial inner membrane. After the oil treatment, the expression level of choline dehydrogenase gene (A_98_P134755) was decreased significantly (FC = 3.94). Therefore, the down regulation after treatment may affect the respiration of M. grisea, leading to an unfavorable influence on energy metabolism.
Glycolytic reactions provide energy for organisms and are crucial to their function. The glycerol, which enters glycolysis, is closely related to the infection of M. grisea. The antimicrobial mechanism of early copper-based and mercury-based pesticides was to block glycolysis and energy metabolism by interfering with the important glycolytic enzymes, such as pyruvate kinase, hexokinase and pyruvate decarboxylase [30]. After the EO treatment, the differential expressions of genes that play a vital role in glycolysis and energy metabolism indicate that this EO treatment affects the glycolysis of M. grisea. Spore germination and appressorium formation of M. grisea require a constant source of energy, most of which is derived from oxidation of glucose. Glucose oxidase may affect the glucose metabolism and cause a deficiency of energy supply, which influences spore germination and appressorium formation of M. grisea, and its physiological activity and pathogenicity [31]. After the oil treatment, the expression level of glucose oxidase gene (A_98_P114990) decreased significantly (FC = 3.50). Phosphoglycerate kinase (PGK) as a key glycolytic enzyme, is necessary for life and the lack of PGK results in severe dysfunction, such as a metabolic disorder [32]. After the oil treatment, the expression level of PGK (A_98_P132671) decreased significantly (FC = 4.47). Therefore, the down regulation after treatment may affect the glycometabolism and energy metabolism of M. grisea, leading to abnormal hyphae growth and weakening of the pathogenicity of hyphae and spores.
2.5.3. Inhibition of Nucleic Acid Synthesis
After the oil treatment, the expression level of ribose 5-phosphate isomerase gene (A_98_P136903) was decreased significantly (FC = 2.01). Nucleotide coenzymes and nucleic acid were synthesized with 5-phosphate isomerase as the main materials, which are well known for healing effects after injury (tissue regeneration) [33]. Therefore, the down regulation after treatment may also affect self-healing and tissue regeneration.
2.6. Potential Application
Eucalyptus oil can be regarded as a new original plant biopesticide that is highly efficient, broad spectrum and safe. The practical application of Eucalyptus oil as a biological pesticide should involve consideration of the application modes and time according to the different bacteriostatic rates (i.e., weather, temperature, and other properties). Besides, some additives to the Eucalyptus oil also should be developed to reduce its volatilization.
According to GO annotation analysis, this EO treatment affect mycelium development, nitrogen metabolism, carbohydrate metabolism, lipid metabolism, protein synthesis, protein synthesis, signal transduction and material transport in M. grisea. This may cause abnormal structures and physiological function disorder in M. grisea and correspondingly reduce its survival and infective ability. The SEM result showed that the Eucalyptus oil plays an important role in damaging mycelium morphology and structure. The influence of this EO treatment on the growth of M. grisea conforms to some current antifungal drug targets, and thus has the potential to be developed into a future biological pesticide.
The Eucalyptus oil resulted in at least 80% control of rice blast when applied at concentrations over 2.5 mg/mL. Minimum inhibitory concentrations(MIC) values between 0.55 and 4.45 mg/mL (geometric mean 1.26 mg/mL) were found against 22 different Malassezia furfur strains while very low MIC values for miconazole were found for M. furfur (geometric mean 2.34 mg/mL) [34].
3. Experimental Section
3.1. Extraction of Eucalyptus Oil (EO)
The leaves (3 years of growth) of E. grandis × E. urophylla were collected from Yuping, Hongya, a small town in Sichuan Province, China, in August 2012. The fresh leaves were harvested at random from 15–20 trees in a forest. After natural drying, grinding and sieving (20 mesh) of leaf samples, 100 g of samples were collected. Oils were extracted from the leaves of Eucalyptus using a laboratory scale supercritical fluid extraction (SFE) system using CO2. The oil extracted by hydro-distillation contained only volatile compounds while the oil from the SFE and Soxhlet contained both volatile and higher molecular weight compounds [35].
The oil was extracted as follows: extraction temperature of 80 °C, extraction time of 8 h, and an extraction pressure of 400 MPa. The resultant yield of oil was 7.87%. The EO was preserved at 4 °C in a dark brown vial under shaded conditions until further analysis [16].
3.2. Inhibitory Test
Fungal strains including Fusarium graminearum, Rhizoctonia solani, Magnaporthe grisea (A47), Colletotrichum gloeosporioides, Alternuria longipes, Alternaria solani, Botrytis cinerea, Bipolaris maydis, Fusarium moniliforme, Rhizoctonia solani and Setosphaeria turcica were obtained from the Institute of Plant Protection, Sichuan Academy of Agricultural Sciences (Chengdu, China). Fungal strains were maintained on potato dextrose agar (PDA) medium. Fungal cultures were sub-cultured (1% inoculum) in PDA broth at 28 °C for at least 2–4 days before their use in the screening assays.
Oil dilutions were prepared by adding 500 μL (0.396 g) of oil to Tween 20 with final concentration of 1% (w/v), then we added distilled water to dilute samples to the treatment concentrations. An antifungal activity test was carried out using the mycelium growth rate method at 28 °C for 5 days [36]. The oil was mixed with the melting PDA medium, until a final test concentration of 2.5 mg/mL was reached. Tween 20 was used as cosolvent. Blank controls were the fungi cultured in normal PDA medium and negative controls were the fungi cultured in PDA medium with Tween 20.
3.3. Morphological Study of the Inhibitory Activities of EO on M. grisea
The samples were prepared as described in the Section 3.2. Scanning electron microscopy (SEM, Gemini, Carl-Zeiss Comp., Schwabhausen, Germany) was used to examine the morphological variations of M. grisea after the 5-days treatment described previously.
3.4. Total RNA Extraction
M. grisea was cultured as previously described, leading to final test concentrations of EO at 2 mg/mL. The total RNA was extracted using Trizol (Invitrogen, Gaithersburg, MD, USA) and purified with mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA) according to manufacturer’s protocols. RNA integrity was determined by capillary electrophoresis using the RNA 6000 Nano Lab-on-a-Chip kit and the Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). Only RNA extracts with RNA integrity number values >6 underwent in further analysis. Furthermore, the total RNA was isolated and purified using a NucleoTrap®RNA clean-up Kit (Macherey-Nagel, Düren, Deutschland), and analyzed by agarose gel electrophoresis.
3.5. Fluorescent Dye Labelling, Chip Hybridization and Scanning
Subsequent data processing was analyzed for data summarization, normalization and quality control by using the GeneSpring GX software (Capital Bio, San Diego, CA, USA) [37]. Normalization was conducted using the percentile shift method. To screen the genes differentially expressed by threshold values of ≥2 and ≤−2-fold change and a Benjamini-Hochberg corrected p value of 0.05, and Flag was marked as Detected. The data was Log2 transformed and median centered by genes using of CLUSTER 3.0 software (CapitalBio, Beijing, China) [38]. The functional enrichment of up- or down-regulated genes was assessed based on the GO terms [39].
3.6. RT-PCR Analysis
RT-PCR was used to validate the results for two genes from M. grisea. Actin was used as the reference gene. Gene expression level of peroxisomal membrane protein 4 and alcohol dehydrogenase after the treatment was compared with the results of gene chip. Primer design: Actin 1-F: 5′-TCGACGTCCGAAAGGATCTGT-3′; Actin 1-R: 5′-ACTCCTGCTTCGAGATCCACATC -3′; Peroxisomal membrane protein 4-F: 5′-GCCCGTATTCGCTGCCACAA-3′; Peroxisomal membrane protein 4-R: 5′-CTCAGGCCATCCCATTCGTT-3′; Alcohol dehydrogenase-F: 5′-AGATGCCCAGCGTCATTTCC-3′; Alcohol dehydrogenase-R: 5′-GCCAATGTGCTTCTTGTTCCA-3′.
3.7. Statistical Analysis
All results presented here were confirmed in at least three independent experiments. Data were expressed as mean ± SD. Statistical comparisons were made by Student’s t-test. A value of p < 0.05 was considered statistically significant.
4. Conclusions
Expression pattern of genes that play an important part in glycolysis and energy metabolism indicated that Eucalyptus oil can influence the normal physiology activity of fungal pathogens, probably through inhibiting glycolysis which in turn influenced cell energy metabolism. This means that the original balance of M. grisea is broken. Furthermore, the Eucalyptus oil had characteristics of high activity and low toxicity, therefore, it could be developed as an ideal pesticide for the agricultural industry. Eucalyptus oil could have direct potential as a green biopesticide because of its high content of antifungal components.
Acknowledgments
This work was supported by International Science and Technology Cooperation Program of China (2015DFR31060) and open research fund of Key Laboratory of Central South Fast-growing Timber Cultivation of Forestry Ministry of China, Guangxi University.
Author Contributions
Lin-Han Bai and Zhi-Rong Yang conceived and designed the experiments; Li-Jun Zhou and Li-Jie Huang performed the experiments; Fu-Rong Li assisted the experiments; Shu Yuan analyzed the data; Li-Jun Zhou wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds from Eucalyptus leaves oil are available from the authors.
References
- 1.Farzaei M.H., Bahramsoltani R., Abbasabadi Z., Rahimi R. A comprehensive review on phytochemical and pharmacological aspects of Elaeagnus angustifolia L. J. Pharm. Pharmacol. 2015;67:1467–1480. doi: 10.1111/jphp.12442. [DOI] [PubMed] [Google Scholar]
- 2.Xu X.C., Zhang Z.W., Chen Y.E., Yuan M., Yuan S., Bao J.K. Antiviral and antitumor activities of the lectin extracted from Aspidistra elatior. Z. Naturforsch. 2015;70c:7–13. doi: 10.1515/znc-2014-4108. [DOI] [PubMed] [Google Scholar]
- 3.Mondal D., Barat S., Mukhopadhyay M.K. Toxicity of neem pesticides on a fresh water loach, Lepidocephalichthys guntea (Hamilton Buchanan) of Darjeeling district in West Bengal. J. Environ. Biol. 2007;28:119–122. [PubMed] [Google Scholar]
- 4.Batish D.R., Singh H.P., Kohli R.K., Kaur S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008;256:2166–2174. doi: 10.1016/j.foreco.2008.08.008. [DOI] [Google Scholar]
- 5.Hajer N.B.M., Mehrez R., Ahmed L., Florence M., François C., Manef A., Mohamed L.K., Jalloul B. Eucalyptus oleosa essential oils: Chemical composition and antimicrobial and antioxidant activities of the oils from different plant parts (stems, leaves, flowers and fruits) Molecules. 2011;16:1695–1709. doi: 10.3390/molecules16021695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng S.S., Huang C.G., Chen Y.J., Yu J.J., Chen W.J., Chang S.T. Chemical compositions and larvicidal activities of leaf essential oils from two Eucalyptus species. Bioresour. Technol. 2009;100:452–456. doi: 10.1016/j.biortech.2008.02.038. [DOI] [PubMed] [Google Scholar]
- 7.Gilles M., Zhao J., An M., Agboola S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem. 2010;119:731–737. doi: 10.1016/j.foodchem.2009.07.021. [DOI] [Google Scholar]
- 8.Macie M.V., Morais S.M., Bevilaqua C.M.L., Silva R.A., Barros R.S., Sousa R.N., Brito E.S., Souza-Neto M.A. Chemical composition of Eucalyptus spp. essential oils and their insecticidal effects on Lutzomyia longipalpis. Vet. Parasitol. 2010;167:1–7. doi: 10.1016/j.vetpar.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 9.Dušan B., Slavenko G., Dejan O., Dragana M.C., Jelena K.V., Neda M.D. Essential Oil of Eucalyptus Gunnii. Hook. As a Novel Source of Antioxidant, Antimutagenic and Antibacterial Agents. Molecules. 2014;19:19007–19020. doi: 10.3390/molecules191119007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ameur E., Zyed R., Samia M., Karima B.H.S., Mahjoub A., Mohamed L.K., Farhat F., Rachid C., Fethia H.S. Correlation between chemical composition and antibacterial activity of essential oils from fifteen Eucalyptus species growing in the Korbous and Jbel Abderrahman arboreta (North East Tunisia) Molecules. 2012;17:3044–3057. doi: 10.3390/molecules17033044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar P., Mishra S., Malik A., Satya S. Compositional analysis and insecticidal activity of Eucalyptus globulus (family: Myrtaceae) essential oil against housefly (Musca domestica) Acta Trop. 2012;122:212–218. doi: 10.1016/j.actatropica.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Cimanga K., Kambu K., Tona L., Apers S., De Bruyne T., Hermans N., Totté J., Pieters L., Vlietinck A.J. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002;79:213–220. doi: 10.1016/S0378-8741(01)00384-1. [DOI] [PubMed] [Google Scholar]
- 13.Ramezani H., Singh H.P., Batish D.R., Kohli R.K. Antifungal activity of the volatile oil of Eucalyptus citriodora. Fitoterapia. 2002;73:261–262. doi: 10.1016/S0367-326X(02)00065-5. [DOI] [PubMed] [Google Scholar]
- 14.Tyagi A.K., Malik A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem. 2011;126:228–235. doi: 10.1016/j.foodchem.2010.11.002. [DOI] [Google Scholar]
- 15.Rocha Caldas G.F., Oliveira A.R., Araújo A.V., Lafayette S.S., Albuquerque G.S., Silva-Neto Jda C., Costa-Silva J.H., Ferreira F., Costa J.G., Wanderley A.G. Gastroprotective mechanisms of the monoterpene 1,8-cineole (eucalyptol) PLoS ONE. 2015;10:e0134558. doi: 10.1371/journal.pone.0134558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou L.J., Huang L.J., Yang Z.R., Bai L.H. Optimization of supercritical CO2 extraction conditions for essential oil from Eucalyptus grandis × Eucalyptus urophylla using Box-Behnken design-response surface methodology. J. Sichuan Univ. (Nat. Sci. Ed.) 2014;51:1319–1324. [Google Scholar]
- 17.Armaka A., Papanikolaou E., Sivropoulou A., Arsenakis M. Antiviral properties of isoborneol, a potent inhibitor of herpes simplex virus type 1. Antivir. Res. 1999;43:79–92. doi: 10.1016/S0166-3542(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 18.Park S.N., Lim Y.K., Freire M.O., Cho E., Jin D., Kook J.K. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe. 2012;18:369–372. doi: 10.1016/j.anaerobe.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Park M.J., Gwak K.S., Yang I., Kim K.W., Jeung E.B., Chang J.W., Choi I.G. Effect of citral, eugenol, nerolidol and α-terpineol on the ultrastructural changes of Trichophyton mentagrophytes. Fitoterapia. 2009;80:290–296. doi: 10.1016/j.fitote.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Marei G.I.Kh., Rasoul M.A.A., Abdelgaleil S.A.M. Comparative antifungal activities and biochemical effects of monoterpenes on plant pathogenic fungi. Pestic. Biochem. Phys. 2012;103:56–61. doi: 10.1016/j.pestbp.2012.03.004. [DOI] [Google Scholar]
- 21.Abrahim D., Francischini A.C., Pergo E.M., Kelmer-Bracht A.M., Ishii-Iwamoto E.L. Effects of α-pinene on the mitochondrial respiration of maize seedlings. Plant. Physiol. Biochem. 2003;41:985–991. doi: 10.1016/j.plaphy.2003.07.003. [DOI] [Google Scholar]
- 22.Hammer K., Carson C.F., Riley T.V. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol. 2003;95:853–860. doi: 10.1046/j.1365-2672.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 23.Ojeda-Sana A.M., Van Baren C.M., Elechosa M.A., Juárez M.A., Moreno S. New insights into antibacterial and antioxidant activities of rosemary essential oils and their main components. Food Control. 2013;31:189–195. doi: 10.1016/j.foodcont.2012.09.022. [DOI] [Google Scholar]
- 24.Georgopapadakou N.H. Update on antifungals targeted to the cell wall: Focus on beta-1,3-glucan synthase inhibitors. Expert Opin. Investig. Drugs. 2001;10:269–280. doi: 10.1517/13543784.10.2.269. [DOI] [PubMed] [Google Scholar]
- 25.Guo W., Li G.X., Pang Y., Wang P. A novel chitin-binding protein identified from the peritrophic membrane of the cabbage looper, Trichoplusia ni, Insect Biochem. Mol. Biol. 2005;35:1224–1234. doi: 10.1016/j.ibmb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Lenardon M.D., Munro C.A., Gow N.A. Chitin synthesis and fungal pathogenesis. Curr. Opin. Microbiol. 2010;13:416–423. doi: 10.1016/j.mib.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanlerberghe G.C., Robson C.A., Yip J.Y.H. Induction of mitochondrial alternative oxidase in response to a cell signal pathway down-regulation the cytochrome c pathway prevent programmed cell death. Plant Physiol. 2002;129:1829–1842. doi: 10.1104/pp.002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiorani F., Umbach A.L., Siedow J.N. The alternative oxidase of plant mitochondria is involved in the acclimation of shoot growth at low temperature. A study of Arabidopsis AOX1a transgenic plants. Plant Physiol. 2005;139:1795–1805. doi: 10.1104/pp.105.070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartlett D.W., Clough J.M., Godwin J.R., Hall A.A., Hamer M., Parr-Dobrzanski B. The strobilurin fungicides. Pest. Manag. Sci. 2002;58:649–662. doi: 10.1002/ps.520. [DOI] [PubMed] [Google Scholar]
- 30.Graham J., Williams T., Morgan M.A., Ratcliffe R., Sweetlove L. Glycolytic enzymes associate dynamically with mitochondria in response to respiratory demand and support substrate channeling. Plant Cell. 2007;19:3723–3738. doi: 10.1105/tpc.107.053371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong C.M., Wong K.H., Chen X.D. Glucose oxidase: Natural occurrence, function, properties and industrial applications. Appl. Microbiol. Biotechnol. 2008;78:927–938. doi: 10.1007/s00253-008-1407-4. [DOI] [PubMed] [Google Scholar]
- 32.Scopes R.K. 3-Phosphoglycerate kinase. Enzyme. 1973;8:335–351. [Google Scholar]
- 33.Hove-Jensen B. Mutation in the phosphoribosylpyrophosphate synthetase gene (prs) that results in simultaneous requirements for purine and pyrimidine nucleosides, nicotinamide nucleotide, histidine, and tryptophan in Escherichia coli. J. Bacteriol. 1988;170:1148–1152. doi: 10.1128/jb.170.3.1148-1152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nenoff P., Haustein U.F., Brandt W. Antifungal activity of the essential oil of Melaleuca alternifolia (tea tree oil) against pathogenic fungi in vitro. Skin Pharmacol. 1996;9:388–394. doi: 10.1159/000211450. [DOI] [PubMed] [Google Scholar]
- 35.Zhao S., Zhang D. Supercritical CO2 extraction of Eucalyptus leaves oil and comparison with Soxhlet extraction and hydro-distillation methods. Sep. Purif. Technol. 2014;133:443–451. doi: 10.1016/j.seppur.2014.07.018. [DOI] [Google Scholar]
- 36.Scheckhuber C.Q. Penicillium chrysogenum as a model system for studying cellular effects of methylglyoxal. BMC Microbiol. 2015;15:138. doi: 10.1186/s12866-015-0472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J., Yang D., Liu S., Li S., Xu G., Zheng G., Xie L., Zhang R. Microarray: A global analysis of biomineralization-related gene expression profiles during larval development in the pearl oyster, Pinctada fucata. BMC Genom. 2015;16:325. doi: 10.1186/s12864-015-1524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisen M.B., Spellman P.T., Brown P.O., Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conesa A., Gotz S., Garcia-Gomez J.M., Terol J., Talon M., Robles M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]