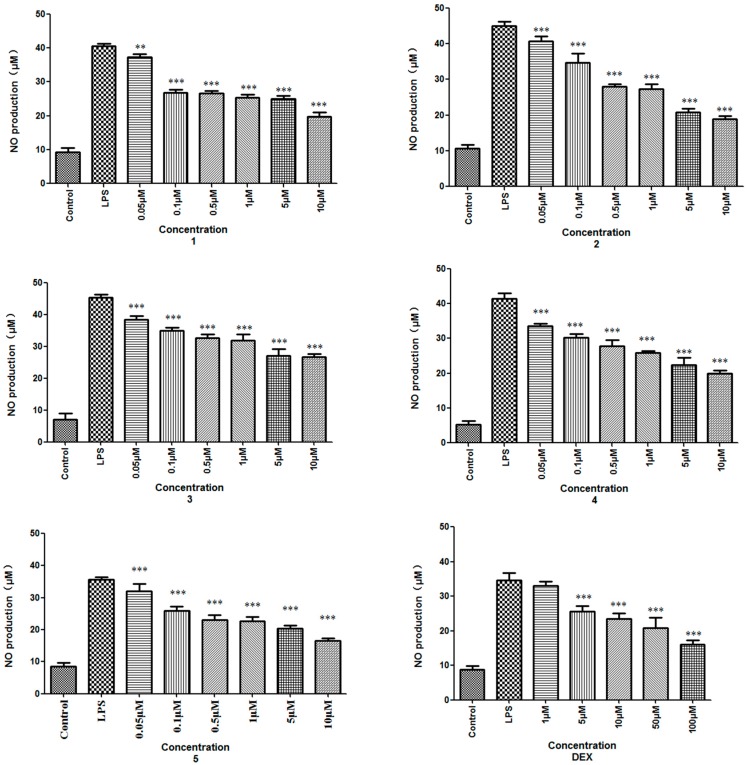
Abstract
Three new C19-norditerpenoid alkaloids (1–3), along with two known C19-norditerpenoid alkaloids (4–5) have been isolated from Aconitum szechenyianum. Their structures were established by extensive spectroscopic techniques and chemical methods as szechenyianine A (1), szechenyianine B (2), szechenyianine C (3), N-deethyl-3-acetylaconitine (4), and N-deethyldeoxyaconitine (5). Additionally, compounds 1–5 were tested for the inhibition of NO production on LPS-activated RAW264.7 cells with IC50 values of 36.62 ± 6.86, 3.30 ± 0.11, 7.46 ± 0.89, 8.09 ± 1.31, and 11.73 ± 1.94 μM, respectively, while the positive control drug dexamethasone showed inhibitory activity with IC50 value of 8.32 ± 1.45 μM. The structure-activity relationship of aconitine alkaloids were discussed.
Keywords: Aconitum szechenyianum, C19-norditerpenoid alkaloids, anti-inflammatory activity, NO production, structure-activity relationship
1. Introduction
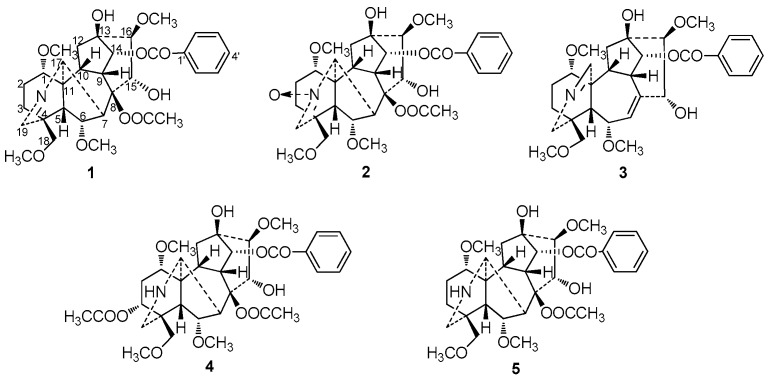
The plant Aconitum szechenyianum Gay., a species in the Aconitum genus of Ranunculaceae, is widely distributed in the west of China and used as a folk medicine in Shaanxi province, known as “Tie-Bang-Chui” [1]. Phytochemical studies revealed that A. szechenyianum contained mainly C19 and C20 diterpenoid alkaloids [2,3,4,5], possessing aconitine-type, 7,17-secoaconitine-type, and napeline-type skeletons. Aconitine-type have no oxygen-containing functionality at C-7, and secoaconitine-type skeleton contains N, C-17, and C-7, C-8 double bonds. Pharmacological studies revealed that these C19 and C20 diterpenoid alkaloids had demonstrated various activities as anti-inflammatory, analgesic, anticancer, anti-epileptiform, antiparasite, and cardiovascular action [6,7]. As part of our research project to explore more bioactive lead compounds from the medicinal herbs in the Qinba mountains of China [8,9,10,11,12,13,14,15,16], the chemical constituents and pharmacological studies of A. szechenyianum were studied, and three new C19-norditerpenoid alkaloids, szechenyianine A (1), szechenyianine B (2), and szechenyianine C (3), along with two known ones, N-deethyl-3-acetylaconitine (4) and N-deethyldeoxyaconitine (5) were isolated (Figure 1). Since the roots of A. szechenyianum were commonly used to treat rheumatism and fracture [17], the isolated compounds were evaluated for their effects on the inhibition of NO production on LPS-activated RAW264.7 cells (Table 2 and Figure 5), and the structure-activity relationship of these compounds were discussed.
Figure 1.
Structures of compounds 1–5.
Table 2.
IC50 values of the compounds from A. szechenyianum on NO production in LPS-activated RAW264.7 cells.
| Compound | 1 | 2 | 3 | 4 | 5 | Dexamethasone |
|---|---|---|---|---|---|---|
| IC50 (μM) | 36.62 ± 6.86 | 3.30 ± 0.11 | 7.46 ± 0.89 | 8.09 ± 1.31 | 11.73 ± 1.94 | 8.32 ± 1.45 |
Results are expressed as IC50 values in μM and the values are means ± SD; n = 3; dexamethasone was used as a positive control.
Figure 5.
NO inhibitory effects of compounds from A. szechenyianum on LPS-activated RAW264.7 cells. Results represent the mean ± SD of three independent experiments; results differ significantly from the LPS-treated, ** p < 0.01, *** p < 0.001; dexamethasone (DEX) was used as a positive control.
2. Results and Discussion
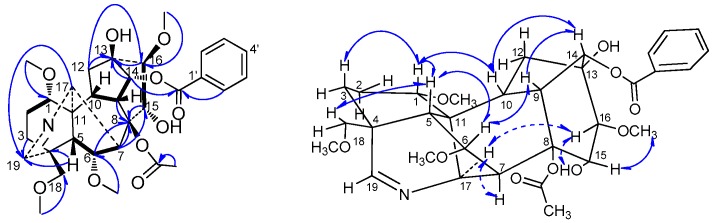
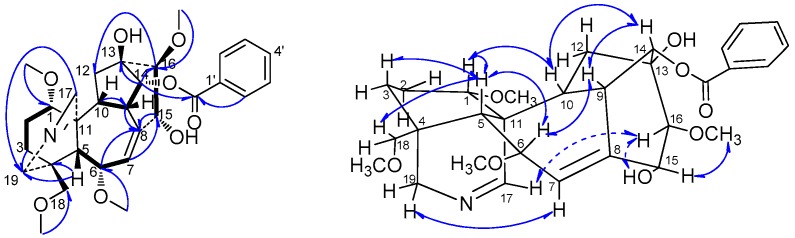
Szechenyianine A (1) was isolated as a white amorphous powder and showed a positive reaction with Dragendorff′s reagent. Its molecular formula C32H41NO10 was derived from a protonated molecular ion peak at m/z 600.2842 [M + H]+ (calcd. 600.2809) of the HR-ESI-MS spectrum. Comparison of the NMR data of 1 and 5, indicated almost similar NMR spectroscopic features, except for the number of C-4, C-17, C-19, this deduction was also confirmed by the chemical shift (Table 1) of C-4 (δC 39.0), C-19 (δC 49.0), and C-17 (δC 56.7) to upfield in 13C-NMR spectra of 5 compared with C-4 (δC 46.8), C-19 (δC 165.9) and C-17 (δC 60.6) of 1, we predicted the existence of N=CH group in compound 1. The 1H-NMR spectrum (Table 1) of 1 showed the presence of five aromatic proton signals due to a monosubstituted benzene at δH 8.02 (2H, d, J = 7.6 Hz), 7.55 (1H, t, J = 7.6 Hz), and 7.43 (2H, t, J = 7.6 Hz); a methine proton of an N = CH group at δH 7.31 (1H, s), four OMe protons at δH 3.75 (3H, s), 3.29 (3H, s), 3.18 (3H, s), and 3.03 (3H, s); and a strongly shielded proton of an acetoxyl group at δH 1.32 (3H, s). The 13C-NMR spectrum (Table 1) displayed 32 carbon resonances. Among them, resonances at δC 166.2, 133.6, 130.0, 129.8 (C × 2), and 128.9 (C × 2) were attributed to a benzoyloxy group; δC 61.3, 59.3, 57.4 and 56.3 were attributed to four OMe groups, δC 172.6 and 21.5 were attributed to an acetoxyl group, and the NMR features of the remained 19 resonances were characteristic to an aconitine-type alkaloid, in which δC 165.9 was attributed to a N=CH group and δC 74.3 and 78.9 were attributed to two oxygenated carbons associated with hydroxyl groups. The assignments of the NMR signals associated with 1 were derived from HSQC, HMBC, and ROESY experiments. In the HMBC spectrum (Figure 2), correlations of H-5 (δH 2.23) and H-17 (δH 3.97) to C-19 (δC 165.9) suggested that C-19 was involved in the N=CH group; correlation of H-14 (δH 4.90) to the carbonyl carbon signal of benzoyl group (δC 166.2) suggested that the benzoyl group was located at C-14; correlation of the proton signal of the acetoxyl group (δH 1.32) to C-8 (δC 90.6) suggested the acetoxyl group was located at C-8; correlations of OCH3 (δH 3.18) to C-1 (δC 82.3), OCH3 (δH 3.03) to C-6 (δC 84.1), OCH3 (δH 3.75) to C-16 (δC 89.9), and OCH3 (δH 3.29) to C-18 (δC 78.2) suggested four methoxyl groups were linked at C-1, C-6, C-16, and C-18, respectively; correlations of H-12 (δH 2.20, 2.21), H-14 (δH 4.90), and H-16 (δH 3.42) to C-13 (δC 74.3), H-9 (δH 2.70) and H-16 (δH 3.42) to C-15 (δC 78.9), suggested two hydroxyl groups were linked at C-13 and C-15, respectively. Thus, the planar structure of 1 was deduced as 14-benzoyloxy-8-acetoxyl-13,15-dihydroxy-1,6,16,18-tetramethoxy-19-en-aconitane. Meanwhile, in the ROSEY spectrum (Figure 2) of 1, the NOE correlations of H-1/H-10, H-10/H-14, H-14/H-9, and H-9/H-6 indicated β-orientation of H-1, H-6, H-9, H-10, and H-14, and α-axial configurations of 1-OCH3, 6-OCH3 and 14-benzoyloxy; NOE correlations of H-6/H-5 and H-5/H-18 revealed β-orientation of H-18 and 18-OCH3, and α-axial of H-19; NOE correlations of H-17/H-7, H-16 and 15-OH, revealed α-axial of H-16, H-17 and 15-OH, and β-orientation of 16-OCH3, 13-OH and 8-acetoxyl. Moreover, the NOE correlations of H-1/H-3 and H-5 while no correlation between H-2 and H-5 indicated 1 had ring A (C-1, C-2, C-3, C-4, C-5, and C-11) in the chair conformation. Thus, according to the literature [18], compound 1 was assigned the name as (A-c)-14α-benzoyloxy-8β-acetoxyl-13β,15α-dihydroxy-1α,6α,16β,18β-tetramethoxy-19-en-aconitane.
Table 1.
1H-NMR and 13C-NMR spectral data of compounds 1–5.
| NO. | 1 | 2 | 3 | 4 | 5 | |||
|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δC | |
| 1 | 82.3 | 3.20 (d, 4.1) | 80.5 | 3.32 (m) | 89.6 | 2.99 (dd, 4.4, 11.3) | 81.0 | 82.3 |
| 2 | 22.9 | 1.66 (m, H-2a) | 22.0 | 1.45 (m, H-2a) | 24.7 | 1.10 (m) | 31.8 | 23.5 |
| 1.57 (m, H-2b) | 1.81 (m, H-2b) | 1.86 (m) | ||||||
| 3 | 28.2 | 1.63 (m, H-3a) | 29.5 | 1.70 (m, H-3a) | 37.4 | 1.55 (m) | 72.3 | 29.0 |
| 1.64 (m, H-3b) | 1.79 (m, H-3b) | 1.69 (m) | ||||||
| 4 | 46.8 | 42.1 | 39.7 | 43.2 | 39.0 | |||
| 5 | 45.8 | 2.23 (d, 7.1) | 44.7 | 2.35 (d, 7.0) | 46.2 | 2.32 (d, 8.9) | 51.2 | 48.7 |
| 6 | 84.1 | 3.92 (d, 7.1) | 82.7 | 4.00 (d, 7.0) | 80.1 | 4.45 (m) | 83.8 | 83.2 |
| 7 | 49.6 | 2.87 (s) | 49.7 | 3.30 (s) | 132.1 | 5.62 (d, 5.5) | 45.2 | 43.6 |
| 8 | 90.6 | 89.3 | 137.5 | 91.7 | 91.5 | |||
| 9 | 42.6 | 2.70 (t, 6.1) | 41.9 | 2.74 (m) | 43.0 | 3.18 (s) | 43.9 | 43.2 |
| 10 | 40.6 | 2.17 (m) | 39.1 | 2.26 (m) | 41.7 | 2.43 (s) | 40.9 | 40.3 |
| 11 | 51.4 | 51.9 | 48.5 | 49.4 | 49.7 | |||
| 12 | 36.4 | 2.20 (m, H-12a) | 36.5 | 2.27 (m, H-12a) | 38.9 | 2.45 (m) | 35.2 | 35.4 |
| 2.21 (m, H-12b) | 1.98 (m, H-12b) | |||||||
| 13 | 74.3 | 74.1 | 75.6 | 74.4 | 74.0 | |||
| 14 | 79.3 | 4.90 (d, 4.9) | 78.8 | 4.89 (d, 4.8) | 79.4 | 5.08 (d, 4.2) | 79.1 | 78.9 |
| 15 | 78.9 | 4.48 (dd, 2.9, 5.3) | 78.7 | 4.48 (dd, 3.0, 4.9) | 74.1 | 4.80 (dd, 3.0, 5.8) | 79.2 | 79.0 |
| 16 | 89.9 | 3.42 (d, 5.3) | 89.6 | 3.45 (d, 5.0) | 92.2 | 3.30 (d, 6.0) | 90.0 | 89.5 |
| 17 | 60.6 | 3.97 (s) | 72.9 | 4.02 (s) | 166.4 | 7.86 (br s) | 55.8 | 56.7 |
| 18 | 78.2 | 3.78 (d, 8.5, H-18a) | 77.9 | 3.79 (d, 8.5, H-18a) | 80.6 | 3.16 (d, 8.4) | 73.8 | 79.8 |
| 3.42 (d, 8.5, H-18b) | 3.33 (d, 8.5, H-18b) | 3.86 (d, 8.4) | ||||||
| 19 | 165.9 | 7.31 (s) | 138.9 | 6.70 (d, 1.2) | 58.3 | 3.53 (m) | 41.6 | 49.0 |
| 3.45 (m) | ||||||||
| 8-OAc | 172.6 | 172.1 | 172.3 | 172.0 | ||||
| 21.5 | 1.32 (s) | 21.4 | 1.32 (s) | 21.5 | 21.3 | |||
| 1-OCH3 | 56.3 | 3.18 (s) | 56.6 | 3.21 (s) | 58.2 | 3.20 (s) | 56.0 | 55.4 |
| 6-OCH3 | 57.4 | 3.03 (s) | 57.3 | 3.05 (s) | 56.9 | 3.19 (s) | 58.3 | 57.9 |
| 16-OCH3 | 61.3 | 3.75 (s) | 61.4 | 3.77 (s) | 61.8 | 3.75 (s) | 61.4 | 61.1 |
| 18-OCH3 | 59.3 | 3.29 (s) | 59.3 | 3.27 (s) | 59.1 | 3.27 (s) | 59.1 | 59.1 |
| ArC=O | 166.2 | 166.2 | 166.4 | 166.2 | 165.9 | |||
| ArC-1′ | 130.0 | 129.9 | 130.0 | 130.0 | 130.7 | |||
| 3′, 5′ | 128.9 | 7.43 (t, 7.6) | 129.0 | 7.44 (t, 7.3) | 128.7 | 7.42 (t, 7.5) | 128.9 | 128.6 |
| 2′, 6′ | 129.8 | 8.02 (d, 7.6) | 129.9 | 8.01 (d, 7.3) | 130.1 | 8.03 (d, 7.5) | 129.8 | 129.6 |
| 4′ | 133.6 | 7.55 (t, 7.6) | 133.8 | 7.57 (t, 7.3) | 133.5 | 7.53 (t, 7.5) | 133.5 | 133.3 |
δ in CDCl3, in ppm from TMS; coupling constants (J) in Hz; 1H-NMR at 400 MHz and 13C-NMR at 100 MHz.
Figure 2.
Key 1H-1H COSY (H H), HMBC (H→C) and ROESY (H↔H) correlations of compound 1.
H), HMBC (H→C) and ROESY (H↔H) correlations of compound 1.
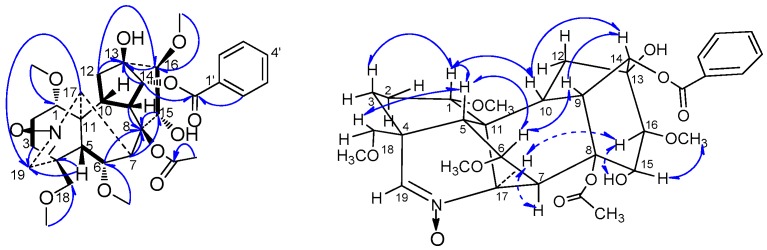
Szechenyianine B (2) was isolated as a white amorphous powder. The NMR spectroscopic data indicated that 2 was an analogu of 1 with similar skeleton and substituent groups. However, the molecular formula of 2 was deduced as C32H41NO11 from the protonated molecular ion peak at m/z 616.2783 [M + H]+ (calcd. 616.2758), suggesting that an N-oxidation group was included in compound 2. This deduction was also confirmed by the chemical shift (Table 1) of C-4 (δC 42.1) and C-19 (δC 138.9) to upfield, and C-17 (δC 72.9) to downfield in 13C-NMR spectra of 2 compared with C-4 (δC 46.8), C-19 (δC 165.9) and C-17 (δC 60.6) of 1. Thus, compound 2 was identified by HSQC, 1H-1H COSY, HMBC, and ROESY experiments (Table 1 and Figure 3) as (A-c)-14α-benzoyloxy-8β-acetoxyl-13β,15α-dihydroxy-1α,6α,16β,18β-tetra-methoxy-19-en-aconitane-N-oxide.
Figure 3.
Key 1H-1H COSY (H H), HMBC (H→C) and ROESY (H↔H) correlations of compound 2.
H), HMBC (H→C) and ROESY (H↔H) correlations of compound 2.
Szechenyianine C (3) was isolated as a white amorphous powder. Its molecular formula C30H39NO8 was derived from a protonated molecular ion peak at m/z 542.2783 [M + H]+ (calcd. 542.2754) of the HR-ESI-MS spectrum. The 1H-NMR spectrum (Table 1) of 3 showed the presence of five aromatic protons signals due to a monosubstituted benzene at δH 8.03 (2H, d, J = 7.5 Hz), 7.53 (1H, t, J = 7.5 Hz), and 7.42 (2H, t, J = 7.5 Hz); two olefinic protons signals at δH 7.86 (1H, brs) due to N=CH and δH 5.62 (1H, d, J = 5.5 Hz) due to C=CH, respectively; and four OMe protons at δH 3.75 (3H, s), 3.27 (3H, s), 3.20 (3H, s) and 3.19 (3H, s). The 13C-NMR spectrum (Table 1) displayed 30 carbon resonances. Among them, resonances at δC 166.4, 133.5, 130.0, 130.1 (C × 2) and 128.7 (C × 2) were attributed to a benzoyl group; δC 61.8, 59.1, 58.2 and 56.9 were attributed to four OMe groups; and the NMR features of the remained 19 resonances were characteristic to a 7, 17-secoaconitine alkaloid, in which δC 166.4 was attributed to a N=CH group, and δC 132.1 and 137.5 were attributed to an olefinic bond. In the HMBC spectrum (Figure 4), correlations of H-1 (δH 2.99), H-5 (δH 2.32), H-10 (δH 2.43), and H-19 (δH 3.53) to C-17 (δC 166.4) suggested that C-17 was involved in the N=CH group, and correlations of H-5 (δH 2.32), H-6 (δH 4.45) to C-7 (δC 132.1), H-6 (δH 4.45), H-14 (δH 5.08), and H-15 (δH 4.80) to C-8 (δC 137.5) suggested the olefinic bond was located at C-7 and C-8, which supported the presence of skeleton of the 7,17-secoaconitine alkaloid. Moreover, HMBC correlation of H-14 (δH 5.08) to the carbonyl carbon signal of benzoyl group (δC 166.4) suggested that the benzoyl group was located at C-14; correlations of OCH3 (δH 3.20) to C-1 (δC 89.6), OCH3 (δH 3.19) to C-6 (δC 80.1), OCH3 (δH 3.75) to C-16 (δC 92.2), and OCH3 (δH 3.27) to C-18 (δC 80.6) suggested four methoxyl groups were linked at C-1, C-6, C-16 and C-18, respectively; correlations of H-10 (δH 2.43) and H-14 (δH 5.08) to C-13 (δC 75.6), H-7 (δH 5.62) and H-16 (δH 3.30) to C-15 (δC 74.1) suggested two hydroxyl group were linked at C-13 and C-15, respectively. Thus, the planar structure of 3 was deduced as 14-benzoyloxy-13,15-dihydroxy-1,6,16,18-tetramethoxy-7(8),17-dien-7,17-secoaconitane. Meanwhile, in the ROSEY spectrum (Figure 4) of 3, the NOE correlations of H-1/H-10, H-10/H-14 and H-14/H-9 indicated β-orientation of H-1, H-9, H-10 and H-14, and α-axial configurations of 1-OCH3 and 14-benzoyloxy; the NOE correlations of H-6/H-5 and H-5/H-18 revealed β-orientation of H-18 and 18-OCH3, and α-axial of 6-OCH3; NOE correlations of H-17/H-16 and 15-OH, H-15/16-OCH3 revealed α-axial of H-16 and 15-OH, and β-orientation of 16-OCH3 and 13-OH. Moreover, the NOE correlations of H-1/H-3 and H-5 while no correlation between H-2 and H-5 indicated 3 had ring A (C-1, C-2, C-3, C-4, C-5, and C-11) in the chair conformation. Thus, according to the literature [18], compound 3 was assigned the name as (A-c)-14α-benzoyloxy-13β, 15α-dihydroxy-1α,6α,16β,18β-tetramethoxy-7(8),17-dien-7,17-secoaconitane.
Figure 4.
Key 1H-1H COSY (H H), HMBC (H→C) and ROESY (H↔H) correlations of compound 3.
H), HMBC (H→C) and ROESY (H↔H) correlations of compound 3.
Since the roots of A. szechenyianum are commonly used to treat rheumatism and fracture [17], in which inflammation is involved in the pathophysiological process and inhibitors of NO release are considered as potential anti-inflammatory agents for the treatment of these diseases [18,19,20], the isolated compounds from A. szechenyianum were evaluated using the Griess assay [21] for their effects on the inhibition of NO production in LPS-activated RAW264.7 cells. Dexamethasone (DEX) was selected as a positive control. As shown in Table 2 and Figure 5, all compounds with aconitine or 7,17-secoaconitine skeleton exhibited anti-inflammatory activities in a dose-dependent manner. Compared the activity with the substituent groups of 1, 2, 4, and 5, the structure-activity relationship may be due to the chemical environment of N atom. The compound 1 could hinder the inhibition of NO production with IC50 value of 36.62 ± 6.86 μM. The compound 2 exhibited excellent active performance with IC50 value of 3.30 ± 0.11 μM, indicated that the presence of N→O might increase anti-inflammatory activities. Moreover, compound 4 exhibited effective inhibitory activity with IC50 value of 8.09 ± 1.31 μM and compound 5 showed inhibitory activity with IC50 value of 11.73 ± 1.94 μM. In addition, compound 3 as a 7,17-secoaconitine type alkaloid also exhibited potent inhibitory activity on NO production with IC50 value of 7.46 ± 0.89 μM.
3. Experimental Section
3.1. General Information
ESI-MS was performed on a Quattoro Premier instrument (Waters, Milford, MA, USA). The HR-ESI-MS spectra were recorded on an Agilent Technologies 6550 Q-TOF (Santa Clara, CA, USA). 1D and 2D-NMR spectra were recorded on Bruker-AVANCE 400 instrument (Bruker, Rheinstetten, Germany) with TMS as an internal standard. The analytical HPLC was performed on a Waters e2695 Separations Module coupled with a 2998 Photodiode Array Detector and a Accurasil C-18 column (4.6 mm × 250 mm, 5 μm particles, Ameritech, Chicago, IL, USA). Semipreparative HPLC was performed on a system comprising an LC-6AD pump equipped with an SPD-20A UV detector (Shimadzu, Kyoto, Japan) and an Ultimate XB-C18 (10 mm × 250 mm, 5 μm particles) or YMS-Pack-ODS-A (10 mm × 250 mm, 5 μm particles). Silica gel was purchased Qingdao Haiyang Chemical Group Corporation (Qingdao, China).
3.2. Plant Material
The roots of Aconitum szechenyianum Gay. were collected from the Xi Mountains of Gansu Province of China in July 2014, and identified by senior experimentalist Jitao Wang. A voucher specimen (herbarium No. 20140728) has been deposited in the Medicinal Plants Herbarium (MPH), Shaanxi University of Chinese Medicine, Xianyang, China.
3.3. Extraction and Isolation
The air-dried and powdered underground parts of A. szechenyianum (5.0 kg) were extracted with 80% EtOH at 80 °C for three times (each time 40 L for 1.5 h). After removal of EtOH solvent under reduced pressure, the extract (2 L) was dispersed in water (1.5 L), adjusted with 9% HCl solution to pH 0.8, and extracted with petroleum ether (PE). The acidic water solution was alkalized to pH 10.26 with 25% ammonia solution, extracted with CHCl3 three times, and evaporated under pressure to give crude alkaloids (50 g). The crude alkaloids (47 g) were chromatographed on silica gel column, eluting with gradient solvent system (PE/acetone/diethylamine, 50:1:0.1–1:1:0.1) to give 12 fractions (Fr.1–Fr.12). Fr.6 (3.2 g) was purified by HPLC (YMC-Pack-ODS-A, 10 mm × 250 mm, 5 μm particles, flow rate: 1.0 mL·min−1) with CH3OH/H2O (70:30) as mobile phase to obtained Fr.6-1 (30 mg; tR = 5 min), Fr.6-2 (120 mg; tR = 30 min), Fr.6-3 (130 mg; tR = 42 min), Fr.6-4 (120 mg; tR = 63 min), Fr.6-5 (140 mg; tR = 77 min), and Fr.6-6 (200 mg; tR = 63 min). Fr.6-3 (130mg) was purified by HPLC with CH3OH/H2O (60:40) as mobile phase to afford 1 (13 mg; tR = 50 min) and 2 (12 mg; tR = 65 min). Fr.6-4 (120 mg) was purified by HPLC with CH3OH/H2O (65:35) as mobile phase to afford 3 (10 mg; tR = 45 min), 4 (20 mg; tR = 65 min), and 5 (25 mg; tR = 75 min). See more detailed spectrums in the supplementary materials.
(A-c)-14α-Benzoyloxy-8β-acetoxyl-13β,15α-dihydroxy-1α,6α,16β,18β-tetramethoxy-19-en-aconitane (szechenyianine A): A white amorphous powder, +60.2 (c 0.38, MeOH), IR (KBr) νmax: 3508, 2936, 1718, 1637 and 714 cm−1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3) spectral data, see Table 1; m/z 600.2842 [M + H]+ (calcd. for C32H41NO10, 600.2809).
(A-c)-14α-Benzoyloxy-8β-acetoxyl-13β,15α-dihydroxy-1α,6α,16β,18β-tetramethoxy-19-en-aconitane-N-oxide (szechenyianine B): A white amorphous powder, [α] +10.5 (c 0.44, MeOH), IR (KBr) νmax: 3510, 2938, 1719, 1603 and 716 cm−1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3) spectral data, see Table 1; m/z 616.2783 [M + H]+ (calcd. for C32 H41NO11, 616.2758).
(A-c)-14α-Benzoyloxy-13β,15α-dihydroxy-1α,6α,16β,18β-tetramethoxy-7(8),17-dien-7,17-secoaconitane (szechenyianine C): A white amorphous powder, [α] +21.6 (c 0.64, MeOH), IR (KBr) νmax: 3513, 2930, 2824, 1716, 1645, 1453, 1367, 1275, 1102 and 715 cm−1; 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3) spectral data, see Table 1; m/z 542.2783 [M + H]+ (calcd. for C30 H39NO8, 542.2754).
3.4. Inhibitory Assay of NO Production
Assays for NO production were carried out according to the Griess reaction, using dexamethasone as positive control. Briefly, RAW264.7 cells were seeded into 96-well microplates at a density of 2 × 105 mL−1 and allowed to adhere for 4 h. RPMI1640 (100 μL) containing test samples (final concentration of 10, 5, 1, 0.5, 0.1, and 0.05 μM) dissolved in DMSO (final concentration less than 0.2%) and LPS (final concentration of 1 μg·mL−1) were added. After incubation at 37 °C for 18 h, 50 μL of cell-free supernatant was mixed with 50 μL of Griess Reagent I and 50 μL of Griess Reagent II to determine NO production. Absorbance was measured at 550 nm against a calibration curve with NaNO2 standard. The NO productions of the isolated compounds were tested (Figure 5), the inhibitory rate on NO production induced by LPS was calculated by the NO2− levels as follows: Inhibitory rate (%) = 100 × ([NO2−]LPS − [NO2−]LPS+sample)/([NO2−]LPS − [NO2−]untreated), the IC50 values were calculated (Table 2). Values are mean ± SD, n = 3, ** p < 0.01, *** p < 0.001 vs. LPS treated.
Acknowledgments
This project was financially supported by the National Natural Science Foundations of China (grant No. 81102805, 81373978), the Open Projects Program of the Key Laboratory of Tibetan Medicine Research, Chinese Academy of Sciences (grant No. 2014-TMR-01), the Program of Shannxi Provincial Department of Education (grant No. 15JK1204), the Innovative Research Team in TCM Material Foundation and Key Preparation Technology (grant No. 2012KCT-20), the Innovation Projects of Science and Technology in Shaanxi Province (grant No. 2011KTCQ03-02, 2013KTCQ03-14), and the Key Program of Shaanxi University of Chinese Medicine (grant No. 201425).
Supplementary Materials
IR, HR-ESI-MS, 1H-NMR, 13C-NMR and 2D NMR spectra for compounds 1–3 can be found, in the online version, at http://www.mdpi.com/1420-3049/21/9/1175/s1.
Author Contributions
Every author has participated in the research and did his or her individual contribution to the article: F.W. and Z.Y. conducted the experiments and collected the data; P.X. And Z.L. carried out the cytotoxicity biology experiments; L.Z. coordinated the experiments; B.S. and Z.T. analyzed the data; X.S. designed the study, and Z.Y. planned and oversaw the research project and drafted the paper. Finally, All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 4–5 are available from the authors.
References
- 1.Song X.M., Liu H.J. Research and Application of "Qi-Medicines" in Taibai Mountains. People's Medical Publishing House; Beijing, China: 2011. [Google Scholar]
- 2.Fan Z.C., Zhang Z.Q. Crystal and molecular structure of songorine from the root of Aconitum szechenyianum Gay. J. Chem. Crystallogr. 2008;38:635–639. doi: 10.1007/s10870-008-9361-7. [DOI] [Google Scholar]
- 3.Jie W.Y., Zeng C.J., Yao Z., Zhang J., Zhang Y., Zhang F. Diterpene alkaloids from the roots and processed products of Aconitum pendulum. Chin. Tradit. Herbal Drugs. 2010;41:347–351. [Google Scholar]
- 4.Liu L.M., Wang H.C., Zhu Y.L. Studies on Chinese drug Aconitum spp. XIX. The alkaloids of Aconitum pendulum and their chemical structure. Yao Xue Xue Bao. 1983;18:39–44. [PubMed] [Google Scholar]
- 5.Wang Y.J., Zhang J., Zeng C.J., Yao Z., Zhang Y. Three new C19-diterpenoid alkaloids from Aconitum pendulum. Phytochem. Lett. 2011;4:166–169. doi: 10.1016/j.phytol.2011.02.008. [DOI] [Google Scholar]
- 6.Wada K., Ohkoshi E., Morris-Natschke S.L., Bastow K.F., Lee K.H. Cytotoxic esterified diterpenoid alkaloid derivatives with increased selectivity against a drug-resistant cancer cell line. Bioorg. Med. Chem. Lett. 2012;22:249–252. doi: 10.1016/j.bmcl.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan J.F., Zhang Z.Q., Kang X., Liu J.L. LC-MS analysis for the components captured by ECV304 cell from extract of Aconitum szechenyianum Gay. Biomed. Chromatogr. 2009;23:406–411. doi: 10.1002/bmc.1131. [DOI] [PubMed] [Google Scholar]
- 8.Fan L., Li Y., Sun Y., Han J., Yue Z., Meng J., Zhang X., Zhang F., Mei Q. Paris Saponin VII Inhibits the Migration and Invasion in Human A549 Lung Cancer Cells. Phytother. Res. 2015;29:1366–1372. doi: 10.1002/ptr.5389. [DOI] [PubMed] [Google Scholar]
- 9.Fan L., Li Y., Sun Y., Yue Z., Meng J., Zhang X., Zhang R., Zhang D., Zhang F., Mei Q. Paris saponin VII inhibits metastasis by modulating matrix metalloproteinases in colorectal cancer cells. Mol. Med. Rep. 2015;11:705–711. doi: 10.3892/mmr.2014.2728. [DOI] [PubMed] [Google Scholar]
- 10.Li Y., Fan L., Sun Y., Miao X., Zhang F., Meng J., Han J., Zhang D., Zhang R., Yue Z., et al. Paris saponin VII from trillium tschonoskii reverses multidrug resistance of adriamycin-resistant MCF-7/ADR cells via P-glycoprotein inhibition and apoptosis augmentation. J. Ethnopharmacol. 2014;154:728–734. doi: 10.1016/j.jep.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Wang X., He H., Zhang D., Jiang Y., Yang X., Wang F., Tang Z., Song X., Yue Z. Steroidal Saponins from the Roots and Rhizomes of Tupistra chinensis. Molecules. 2015;20:13659–13669. doi: 10.3390/molecules200813659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song X., Zhang D., He H., Li Y., Yang X., Deng C., Tang Z., Cui J., Yue Z. Steroidal glycosides from Reineckia carnea. Fitoterapia. 2015;105:240–245. doi: 10.1016/j.fitote.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Yue Z., Qin H., Li Y., Sun Y., Wang Z., Yang T., Liu L., Wang M., Feng F., Mei Q. Chemical constituents of the root of Jasminum giraldii. Molecules. 2013;18:4766–4775. doi: 10.3390/molecules18044766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chai J., Song X., Wang X., Mei Q., Li Z., Cui J., Tang Z., Yue Z. Two new compounds from the roots and rhizomes of Trillium tschonoskii. Phytochem. Lett. 2014;10:113–117. doi: 10.1016/j.phytol.2014.08.010. [DOI] [Google Scholar]
- 15.Song X., Li Y., Zhang D., Jiang Y., Wang W., Song B., Tang Z., Cui J., Yue Z. Two new spirostanol saponins from the the roots and rhizomes of Tupistra chinensis. Phytochem. Lett. 2015;13:6–10. doi: 10.1016/j.phytol.2015.05.004. [DOI] [Google Scholar]
- 16.Zhang D., Wang W., Li Y., Li Z., Jiang Y., Tang Z., Song X., Yue Z. Two new pregnane glycosides from Reineckia carnea. Phytochem. Lett. 2016;15:142–146. doi: 10.1016/j.phytol.2015.12.005. [DOI] [Google Scholar]
- 17.Xiao P.G., Wang F.P., Gao F., Yan L.P., Chen D.L., Liu Y. A pharmacophylogenetic study of Aconitum L. (Ranunculaceae) from China. Acta Phytotaxon. Sin. 2006;44:1–46. doi: 10.1360/aps050046. [DOI] [Google Scholar]
- 18.Li Z., Scott M.J., Fan E.K., Li Y., Liu J., Xiao G., Li S., Billiar T.R., Wilson M.A., Jiang Y., et al. Tissue damage negatively regulates LPS-induced macrophage necroptosis. Cell Death Differ. 2016;23:1428–1447. doi: 10.1038/cdd.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X., Sun J., Xin W., Li Y., Ni L., Ma X., Zhang D., Zhang T., Du G. Anti-inflammation effect of methyl salicylate 2-O-beta-d-lactoside on adjuvant induced-arthritis rats and lipopolysaccharide (LPS)-treated murine macrophages RAW264.7 cells. Int. Immunopharmacol. 2015;25:88–95. doi: 10.1016/j.intimp.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Loi F., Cordova L.A., Pajarinen J., Lin T.H., Yao Z., Goodman S.B. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dirsch V.M., Stuppner H., Vollmar A.M. The Griess assay: Suitable for a bio-guided fractionation of anti-inflammatory plant extracts? Planta Med. 1998;64:423–426. doi: 10.1055/s-2006-957473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.