Abstract
Hedyotis diffusa Willd (H. diffusa) is a well-known Chinese medicine with a variety of activities, especially its anti-cancer effect in the clinic. Up to now, 171 compounds have been reported from H. diffusa, including 32 iridoids, 26 flavonoids, 24 anthraquinones, 26 phenolics and their derivatives, 50 volatile oils and 13 miscellaneous compounds. In vitro and in vivo studies show these phytochemicals and plant extracts to exhibit a range of pharmacological activities of anti-cancer, antioxidant, anti-inflammatory, anti-fibroblast, immunomodulatory and neuroprotective effects. Although a series of methods have been established for the quality control of H. diffusa, a feasible and reliable approach is still needed in consideration of its botanical origin, collecting time and bioactive effects. Meanwhile, more pharmacokinetics researches are needed to illustrate the characteristics of H. diffusa in vivo. The present review aims to provide up-to-date and comprehensive information on the phytochemistry, pharmacology, quality control and pharmacokinetic characteristics of H. diffusa for its clinical use and further development.
Keywords: H. diffusa, phytochemistry, pharmacology, quality control, pharmacokinetics
1. Introduction
Hedyotis diffusa Willd (H. diffusa, Family Rubiaceae), known as Oldenlandia diffusa (Willd) Roxb, is a well-known Chinese medicine used for the treatment of inflammation-linked diseases, such as hepatitis, appendicitis and urethritis, for thousands of years in China [1]. In our previous studies, the water extract of H. diffusa has been proved to have an obvious protective effect in lipopolysaccharide-induced renal inflammation in mice. Recently, H. diffusa has gained increasing attention for its properties of anti-proliferative activity in cancer cells and anti-tumor activity in tumor-bearing animals [2,3,4,5]. It has been proved as the most commonly prescribed single Chinese herb used for colon cancer and breast cancer patients [6,7], according to the statistics from the National Health Insurance Research Database of Taiwan.
H. diffusa is an annual herb, widely distributed in the orient and tropical Asia, such as China, Japan and Indonesia [1,8]. Generally, the plant grows in humid fields and ridges of farmlands, ascending to procumbent, to 50 cm tall; the stem is slightly flattened to terete, glabrescent to glabrous and the papilla was observed in the transverse section of the stem; the leaves are opposite, sessile or subsessile and blade drying membranous, linear, narrowly elliptic, 1–4 × 0.1–0.4 cm; the flowers with pedicels are pairs in axillary racemes and the corolla is white [1,9]. Together with these phenotypic characteristics of H. diffusa, methods of thin-layer chromatography (TLC) [10], gas chromatography-mass spectrometer (GC-MS) [11], high performance liquid chromatography (HPLC) [9] and DNA sequencing [8,12] have been developed to differentiate H. diffusa from related species (e.g., Hedyotis corymbosa (L.) Lam) to give the right prescription for illnesses.
Although there are numbers of published scientific literature on the chemical constituents, pharmacological activities and quantitative analysis of H. diffusa, a systematic and updated review is unavailable. Therefore, the aim of this review is to extensively summarize the phytochemistry, pharmacology, quality control and pharmacokinetic characteristics of H. diffusa, as well as being an evidence for clinical uses and further researches of this herb.
2. Phytochemistry
With the advancement of analysis technologies like mass spectrometer (MS), liquid chromatograph–mass spectrometer (LC-MS), nuclear magnetic resonance–mass spectrometer (NMR-MS) etc., many studies on H. diffusa revealed numbers of important phytochemicals, including iridoids, triterpenes, flavonoids, anthraquinones, phenolic acids and their derivatives, sterols, alkaloids, volatile oils, polysaccharides, cyclotides, coumarins and alkaloids. The detailed information for these compounds is summarized in Table 1.
Table 1.
Compounds of the H. diffusa.
| NO. | Compound Name | Molecular Formula | Reference |
|---|---|---|---|
| Iridoids | |||
| 1 | Asperuloside | C18H22O11 | [13,14] |
| 2 | Deacetyl asperuloside | C16H20O10 | [15] |
| 3 | Asperuloside acid | C18H24O12 | [16] |
| 4 | Deacetyl asperulosidic acid | C16H22O11 | [15] |
| 5 | Deacetyl asperulosidic acid methyl ester | C17H24O11 | [15,17] |
| 6 | Geniposidic acid | C16H22O10 | [18] |
| 7 | 10-O-Acetyl geniposidic acid | C18H24O11 | [15] |
| 8 | 10-Dehydro geniposide | C17H22O10 | [17] |
| 9 | 10-Dehydro geniposidic acid | C16H20O10 | [19] |
| 10 | Diffusoside A | C19H28O11 | [20] |
| 11 | Diffusoside B | C19H28O11 | [20] |
| 12 | Lupenylacetate | C32H52O2 | [21] |
| 13 | Alpigenoside | C18H28O12 | [15] |
| 14 | Oldenlandoside III | C34H44O20 | [22] |
| 15 | 5-O-Feruloyl scandoside methyl ester | C27H32O14 | [23] |
| 16 | Hehycoryside C | C23H26O11 | [22] |
| 17 | 6-α-Hydro scandoside | C16H22O11 | [24] |
| 18 | 6-β-Hydro scandoside | C16H22O11 | [24] |
| 19 | 6-Dehydro scandoside | C16H22O10 | [19] |
| 20 | 6-α-Hydro scandoside methyl ester | C17H24O11 | [24] |
| 21 | 6-β-Hydro scandoside methyl ester | C17H24O11 | [24] |
| 22 | 6-α-Hydro-10-acetyl asperuloside acid | C18H24O12 | [24] |
| 23 | 6-β-Hydro-10-acetyl asperuloside acid | C18H24O12 | [24] |
| 24 | 6-O-Methoxyl cinnamoyl scandoside | C27H32O13 | [23] |
| 25 | 6-O-p-Hydro cinnamoyl scandoside | C26H30O13 | [23] |
| 26 | (E)-6-O-p-Coumaroyl-10-O-formoxyl scandoside methyl ester | C27H32O13 | [14] |
| 27 | (E)-6-O-p-Coumaroyl scandoside methyl ester | C26H30O13 | [14,15,25] |
| 28 | (Z)-6-O-p-Coumaroyl scandoside methyl ester | C26H30O13 | [18] |
| 29 | (E)-6-O-p-Methoxy cinnamoyl scandoside methyl ester | C27H32O13 | [15,25,26] |
| 30 | (Z)-6-O-p-Methoxy cinnamoyl scandoside methyl ester | C27H32O13 | [26] |
| 31 | (E)-6-O-Feruloyl scandoside methyl ester | C27H32O14 | [15,25,26] |
| 32 | (Z)-6-O-Feruloyl scandoside methyl ester | C27H32O14 | [27] |
| Triterpenes | |||
| 33 | Arborinone | C30H48O | [28] |
| 34 | Isoarborinol | C30H50O | [28] |
| 35 | Oleanolic acid | C30H48O3 | [19] |
| 36 | Ursolic acid | C30H48O3 | [19] |
| Flavonoids | |||
| 37 | Amentoflavone | C30H18O10 | [26,29] |
| 38 | Chrysin-6-C-glucosyl-8-C-arabinosyl | C26H28O13 | [22] |
| 39 | Chrysin-6-C-arabinosyl-8-C-glucosyl | C26H28O13 | [22] |
| 40 | Oroxylin-A-O-glucuronic acid | C22H20O11 | [22] |
| 41 | Wogonin-O-glucuronic acid | C22H20O11 | [22] |
| 42 | 5,7-Dihydroxy-3-methoxy flavonol | C16H12O5 | [13] |
| 43 | 5,7,4′-Trihydroxy flavonol | C15H10O6 | [13] |
| 44 | 5-Hydroxy-6,7,3′,4′-tetramethoxy flavone | C19H18O7 | [21] |
| 45 | Quercetin | C15H10O7 | [17,19,30] |
| 46 | Rutin | C27H30O16 | [15,25,31] |
| 47 | Quercetin-3-O-β-d-glucopyranside | C21H20O12 | [25,32,33] |
| 48 | Quercetin-3-O-β-d-galactopyranoside | C21H20O12 | [32] |
| 49 | Quercetin-3-O-(2-O-glucopyranosyl)-β-d-glucopyranside | C27H30O17 | [15,25,32,33] |
| 50 | Quercetin-3-O-(2-O-glucopyranosyl)-β-d-galactopyranoside | C27H30O17 | [11,34] |
| 51 | Quercetin-3-O-sambubioside | C26H28O16 | [15,25] |
| 52 | Quercetin-3-O-[2-O-(6-O-E-ferloyl)-β-d-glucopyranosyl]-β-d-galactopyranoside | C37H38O20 | [11,34] |
| 53 | Quercetin-3-O-[2-O-(6-O-E-feruloyl)-β-d-glucopyranosyl]-β-d-glucopyanoside | C37H38O20 | [11,15,25] |
| 54 | Quercetin-3-O-[2-O-(6-O-E-sinapoyl)-β-d-glucopyranosyl]-β-d-glucopyanoside | C38H40O21 | [15] |
| 55 | Quercetin-3-O-[2-O-(6-O-E-sinapoyl)-β-d-glucopyranosyl]-β-d-galactopyranoside | C38H40O21 | [25] |
| 56 | Kaempferol | C15H10O6 | [17,35] |
| 57 | Kaempferol-3-O-β-d-glucopyranside | C21H20O11 | [32] |
| 58 | Kaempferol-3-O-β-d-galactopyranoside | C21H20O11 | [32] |
| 59 | Kaempferol-3-O-(2-O-β-d-glucopyranosyl)-β-d-galactopyranoside | C27H30O16 | [11,25,34] |
| 60 | Kaempferol-3-O-(6-O-α-l-rhamnosyl)-β-d-glucopyranside | C27H30O16 | [32] |
| 61 | Kaempferol-3-O-[2-O-(E-6-O-feruloyl)-β-d-glucopyranosyl]-β-d-glucopyranosyl | C37H38O19 | [11,25,33] |
| 62 | Kaempferol-3-O-[2-O-(6-O-E-feruloyl)-β-d-glucopyranosyl]-β-d-galactopyranoside | C37H38O19 | [21,34] |
| Athraquinones | |||
| 63 | 2-Methyl-3-methoxy anthraquinone | C16H12O3 | [19] |
| 64 | 2-Hydroxy-1,3-dimethoxy anthraquinone | C16H12O5 | [29] |
| 65 | 2-Hydroxy-3-methyl-1-methoxy anthraquinone | C16H12O4 | [36] |
| 66 | 2-Hydroxy-3-methyl-4-methoxy anthraquinone | C16H12O4 | [37] |
| 67 | 2-Hydroxy-7-methyl-3-methoxy anthraquinone | C16H12O4 | [36] |
| 68 | 2-Hydroxy-1-methoxy-3-methyl anthraquinone | C16H14O4 | [31] |
| 69 | 2-Hydroxy-3-methyl anthraquinone | C15H10O3 | [17,27] |
| 70 | 2-Hydroxy-1-methoxy anthraquinone | C15H10O4 | [27,29] |
| 71 | 2-Hydroxy-4-methoxy anthraquinone | C15H10O4 | [38] |
| 72 | 2-Hydroxy-3-methoxy-7-methyl anthraquinone | C16H12O4 | [36] |
| 73 | 2-Hydroxy-6-methyl anthraquinone | C15H10O3 | [18] |
| 74 | 2-Hydroxy-3-methoxy-6-methyl anthraquinone | C16H12O4 | [18] |
| 75 | 2,7-Dihydroxy-3-methyl anthraquinone | C15H10O4 | [39] |
| 76 | 3-Hydroxy-2-methyl anthraquinone | C15H10O3 | [19] |
| 77 | 3-Hydroxy-2-methyl-4-methoxy anthraquinone | C16H12O4 | [40] |
| 78 | 2,3-Dimethoxy-6-methyl anthraquinone | C17H14O4 | [18] |
| 79 | 1,3-Dihydroxy-2-methyl anthraquinone | C15H10O4 | [41] |
| 80 | 1,7- Dihydroxy-6-methoxy-2-methyl anthraquinone | C16H12O5 | [41] |
| 81 | 3-Hydroxy-2-methyl-4-methoxy anthraquinone | C16H10O4 | [18] |
| 82 | 2,6-Dihydroxy-3-methyl-4-methoxy anthraquinone | C16H12O5 | [42] |
| 83 | 2,6-Dihydroxy-1-methoxy-3-methyl anthraquinone | C16H12O5 | [31] |
| 84 | 1-Hydroxy-4-methoxy anthraquinone | C15H10O4 | [43] |
| 85 | 2-Hydroxymethy-1-hydroxy anthraquinone | C15H10O4 | [5] |
| 86 | 2-Hydroxymethyl anthraquinone | C15H10O3 | [5] |
| Phenolic acids and their derivatives | |||
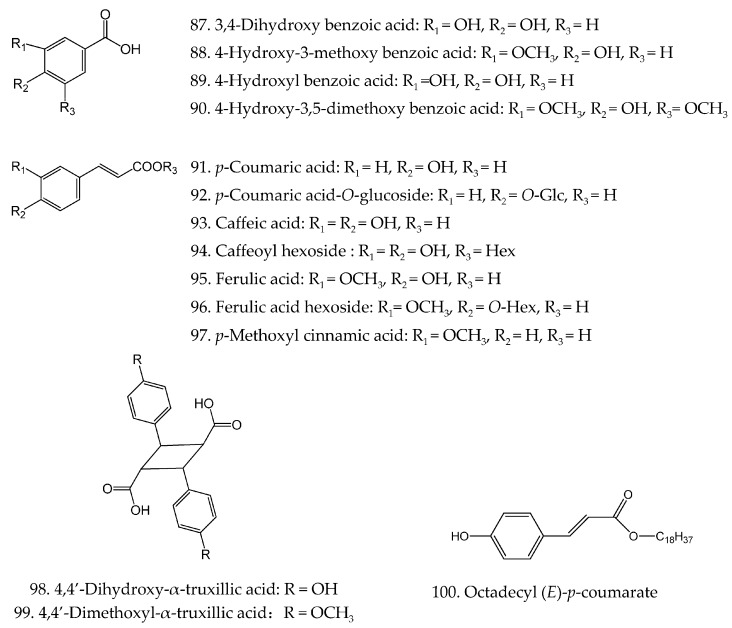
| 87 | 3,4-Dihydroxy benzoic acid | C7H6O4 | [21] |
| 88 | 4-Hydroxy-3-methoxy benzoic acid | C8H8O4 | [30] |
| 89 | trans-Hydroxybenzoic acid | C7H6O3 | [30] |
| 90 | 4-Hydroxy-3,5-dimethoxy benzoic acid | C9H10O5 | [30] |
| 91 | p-Coumaric acid | C9H8O3 | [19,29] |
| 92 | p-Coumaric acid-O-glucopyranside | C15H18O8 | [22] |
| 93 | Caffeic acid | C9H8O4 | [21] |
| 94 | Caffeoyl hexoside | C15H18O9 | [22] |
| 95 | Ferulic acid | C10H10O4 | [41] |
| 96 | Ferulic acid hexoside | C16H20O9 | [22] |
| 97 | p-Methoxy cinnamic acid | C10H10O3 | [44] |
| 98 | 4,4′-Dihydroxy-α-truxillic acid | C18H16O6 | [44] |
| 99 | 4,4′-Dimethoxyl-α-truxillic acid | C19H18O6 | [45] |
| 100 | Octadecyl (E)-p-coumarate | C27H44O3 | [46] |
| 101 | 3-Caffeoyl quinic acid | C16H18O9 | [22] |
| 102 | 4-Caffeoyl quinic acid | C16H18O9 | [22] |
| 103 | 5-Caffeoyl quinic acid | C16H18O9 | [22] |
| 104 | 3-р-Coumaroyl quinic acid | C16H18O8 | [22] |
| 105 | 4-р-Coumaroyl quinic acid | C16H18O8 | [22] |
| 106 | 5-р-Coumaroyl quinic acid | C16H18O8 | [22] |
| 107 | 3-Feruloyl quinic acid | C17H20O9 | [22] |
| 108 | 4-Feruloyl quinic acid | C17H20O9 | [22] |
| 109 | 5-Feruloyl quinic acid | C17H20O9 | [22] |
| Sterols | |||
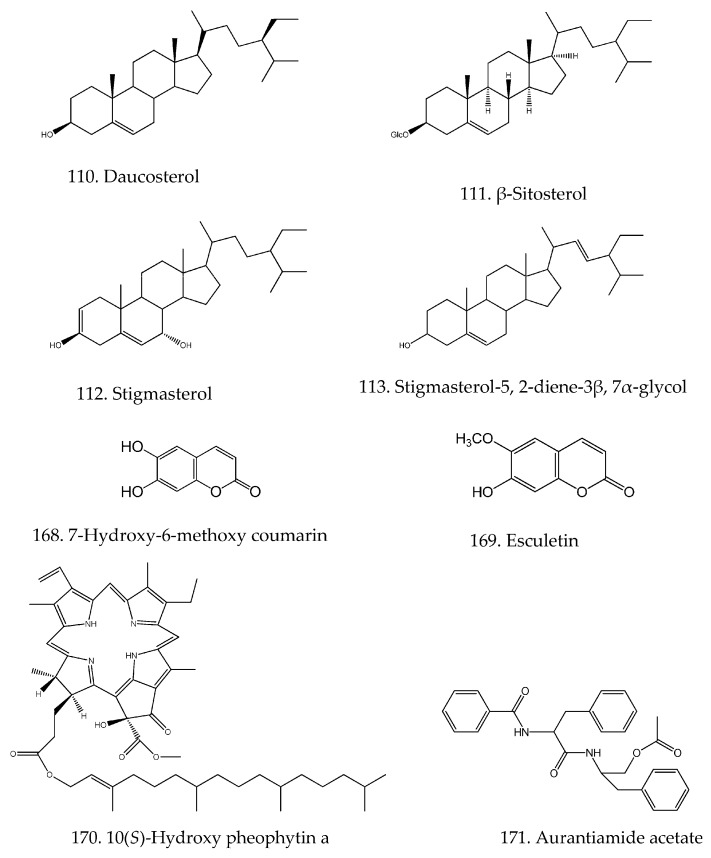
| 110 | Daucosterol | C35H60O6 | [19] |
| 111 | β-Sitosterol | C29H50O | [19] |
| 112 | Stigmasterol | C29H48O | [17,19] |
| 113 | Stigmasterol-5,2-diene-3β, 7α-glycol | C29H48O2 | [47] |
| Volatile oils | |||
| 114 | 6,10,14-Trimethyl-2-pentadecanone | C18H36O | [48] |
| 115 | Phytol | C20H40O | [48] |
| 116 | α-Cedrol | C15H26O | [48] |
| 117 | Tetradecanoic acid | C14H28O2 | [48] |
| 118 | Hexadecanoic acid, methyl ester | C17H34O2 | [48] |
| 119 | Hexadecanoic acid, | C16H32O2 | [48] |
| 121 | 1,2-Benzenediearboxylic acid isobutyl ester | C16H22O4 | [48] |
| 122 | 1,2-Benzenediearboxylic acid, bis(2-methylpropyl)ester | C16H22O4 | [48] |
| 123 | 9,12,15-Octadecatrienoic acid, methyl ester | C19H32O2 | [48] |
| 124 | 9-Octadecenoic acid | C18H34O2 | [48] |
| 125 | 9,12-Octadecenoic acid | C18H32O2 | [48] |
| 126 | Ethyl linoleate | C20H36O2 | [48] |
| 127 | Triethyl phosphate | C6H15O4P | [48] |
| 128 | 4-Vinyl phenol | C8H8O | [48] |
| 129 | 2-Methoxy-4-vinylphenol | C9H10O2 | [48] |
| 130 | n-Pentadecanoic acid | C15H30O2 | [48] |
| 131 | 4,8,12,16-Tetramethyl heptadecan-4-olide | C21H40O2 | [48] |
| 132 | 2,6,10,14,18,22-Tetracosahexaene | C30H50 | [48] |
| 133 | α-Terpineol | C10H18O | [11] |
| 134 | Geranyl acetate | C12H20O2 | [11] |
| 135 | β-Ionone | C13H20O | [11] |
| 136 | Lauric acid | C12H24O2 | [11] |
| 137 | Myristic acid | C14H28O2 | [11] |
| 138 | Palmitic acid | C16H32O2 | [11] |
| 139 | Linoleic acid | C18H32O2 | [11] |
| 140 | β-Linalool | C10H18O | [11] |
| 141 | Isoborneol | C10H18O | [49] |
| 142 | 3-(2-Propenyl)-cyclohexene | C9H14 | [49] |
| 143 | 2-Pentyl-furam | C9H14O | [49] |
| 144 | Cis-2-(2-pentenyl)-furan | C9H12O | [49] |
| 145 | Limonene | C10H18 | [49] |
| 146 | 3,7-Dimethyl-1,6-octadiem-3-ol | C10H18O | [49] |
| 147 | trans-5-Methyl-2-(1-methylethyl)-cyclohexanope | C10H18O | [49] |
| 148 | (1S-endo)-1,7,7-Trimethyl-bicyclo[2,2,1]heptan-2-ol | C10H18O | [49] |
| 149 | p-Menth-1-en-8-ol | C10H18O | [49] |
| 150 | Pulegone | C10H16O | [49] |
| 151 | 4-(2,6,6-Trimethyl-1-cyclohexen-1-yl)-3-buten-2-one | C13H20O | [49] |
| 152 | Hexadecanal | C16H32O | [49] |
| 153 | 2,6,10,14-Tetramethyl-hexadecane | C20H42 | [49] |
| 154 | (Z,Z)-9,12-octadecadienoic acid | C18H32O2 | [49] |
| 155 | (Z)-9,17-octadecadienal | C18H32O | [49] |
| 156 | Cis,cis,cis-7,10,13-hexadecatrienal | C16H26O | [49] |
| 157 | Oleic acid | C18H34O2 | [49] |
| 158 | Hexaldehyde | C6H12O | [49] |
| 159 | Borneol | C10H18O | [49] |
| 160 | Docosane | C22H46 | [49] |
| 161 | Tetracosane | C24H50 | [49] |
| 162 | Hexacosane | C26H54 | [49] |
| 163 | Heptacosane | C27H56 | [49] |
| Polysaccharides | |||
| 164 | ODP-1 | [50] | |
| Cyclotides | |||
| 165 | CD1 | [51] | |
| 166 | CD2 | [51] | |
| 167 | CD3 | [51] | |
| Coumarins | |||
| 168 | 7-Hydroxy-6-methoxy-Coumarin | C10H8O4 | [17] |
| 169 | Esculetin | C9H6O4 | [46] |
| Alkaloids | |||
| 170 | 10(S)-hydroxy pheophytin a | C55H74N4O6 | [52] |
| 171 | Aurantiamide acetate | C27H28N2O4 | [46] |
2.1. Iridoids and Triterpenes
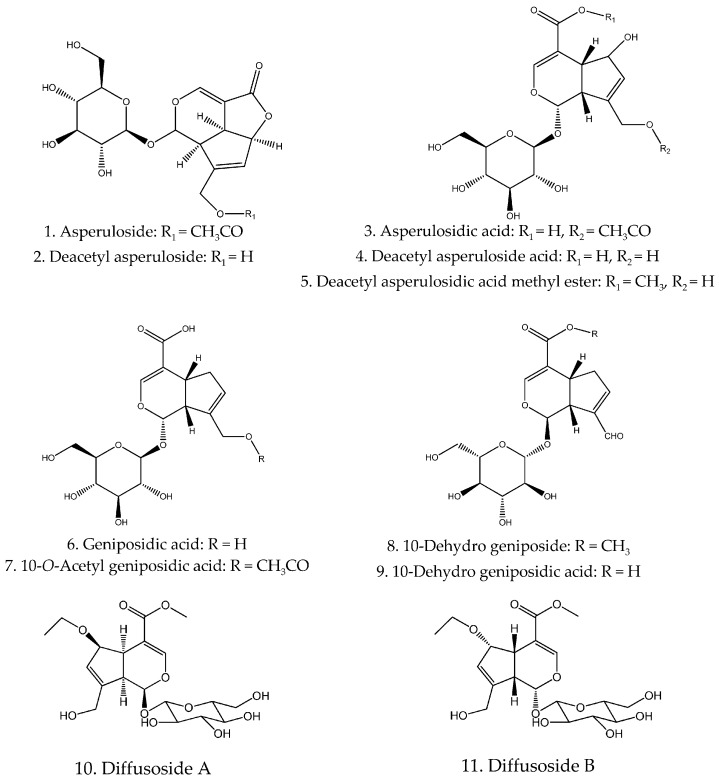
Iridoids are one of the most important components in H. diffusa with various bioactivities, such as anti-oxidant, neuroprotective and anti-inflammatory effects [33,53]. Accompanied with the analysis of the NMR spectra of the pure compounds, the methods of tandem mass spectrometry (MSn) and time-of-flight mass spectrometry (TOF/MS) have become more popular for the identification of these compounds [11,14,15,25,52]. To date, thirty-two iridoids and their iridoid glucosides (1–32) have been isolated and identified from H. diffusa (Figure 1).
Figure 1.
Chemical structures of iridoids and triterpenes in H. diffusa.
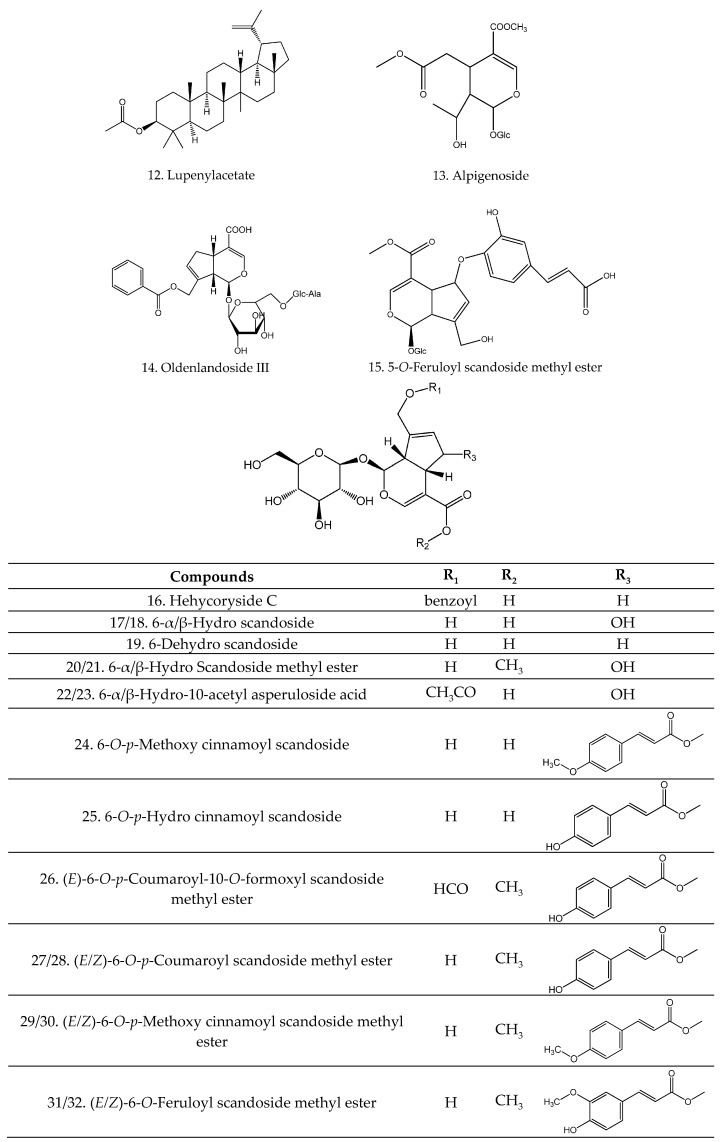
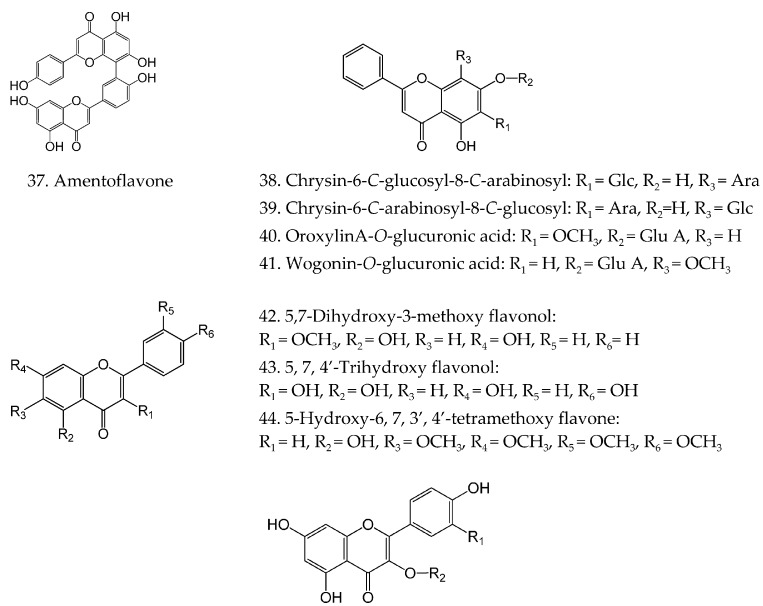
Four triterpenes, named arborinone (33), isoarborinol (34), oleanolic acid (35) and ursolic acid (36), were isolated from H. diffusa and their structures were established by 1D-, 2D-NMR spectroscopic analysis and high-resolution electrospray ionization mass spectroscopy (HRESIMS) [53].
2.2. Flavonoids
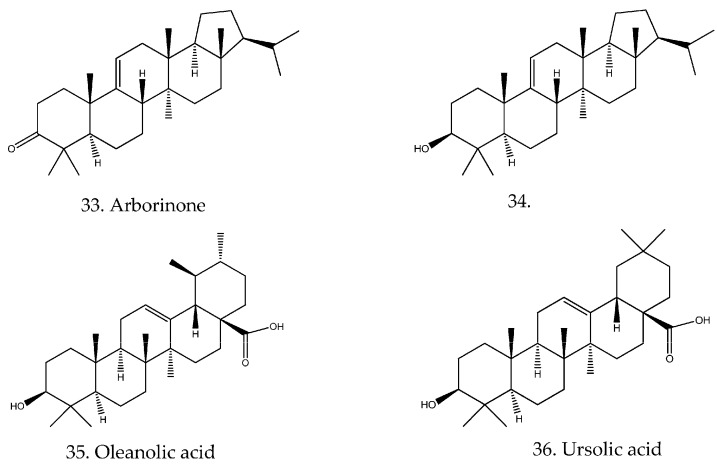
Flavonoids are a major group presented in H. Diffusa, and most of them are derivatives of the flavonol aglycones of kaempferol and quercentin. Recently, other aglycones, such as chrysin, oroxylin and wogonin, have been characterized by ultra-performance liquid chromatography–diode array detector/quadrupole time-of-flight mass spectrometry (UPLC-DAD/Q-TOF-MS). To date, twenty-six flavonoids (37–62) with various substitutions have been identified and their chemical structures are prescribed in Figure 2.
Figure 2.
Chemical structures of flavonoids in H. diffusa.
| Compounds | R1 | R2 |
|---|---|---|
| 45. Quercetin | OH | H |
| 46. Rutin | OH | rutinose |
| 47. Quercetin-3-O-β-d-glucopyranside | OH | β-d-Glc |
| 48. Quercetin-3-O-β-d-galactopyranoside | OH | β-d-Gal |
| 49. Quercetin-3-O-(2-O-glucopyranosyl)-β-d-glucopyranside | OH | β-d-Glc-(1→2)-d-Glc |
| 50. Quercetin-3-O-(2-O-glucopyranosyl)-β-d-galactopyranoside | OH | β-d-Glc-(1→2)-d-Gal |
| 51. Quercetin-3-O-sambubioside | OH | β-d-Xyl-(1→2)-d-Glc |
| 52. Quercetin-3-O-[2-O-(6-O-E-ferloyl)-β-d-glucopyranosyl]-β-d-galactopyranoside | OH | 6′-O-E-feruloyl-β-d-Glc-(1→2)-d-Gal |
| 53. Quercetin-3-O-[2-O-(6-O-E-feruloyl)-β-d-glucopyranosyl]-β-d-glucopyanoside | OH | 6′-O-E-feruloyl-β-d-Glc-(1→2)-d-Glc |
| 54. Quercetin-3-O-[2-O-(6-O-E-sinapoyl)-β-d-glucopyranosyl]-β-d-glucopyanoside | OH | 6′-O-E-sinapoyl-β-d-Glc-(1→2)-d-Glc |
| 55. Quercetin-3-O-[2-O-(6-O-E-sinapoyl)-β-d-glucopyranosyl]-β-d-galactopyranoside | OH | 6′-O-E-sinapoyl-β-d-Glc-(1→2)-d-Gal |
| 56. Kaempferol | H | H |
| 57. Kaempferol-3-O-β-d-glucopyranside | H | β-d-Glc |
| 58. Kaempferol-3-O-β-d-galactopyranoside | H | β-d-Gal |
| 59. Kaempferol-3-O-(2-O-β-d-glucopyranosyl)-β-d-galactopyranoside | H | β-d-Glc-(1→2)-d-Gal |
| 60. Kaempferol-3-O-(6-O-α-l-rhamnosyl)-β-d-glucopyranside | H | α-l-Rha-(1→6)-β-d-Glc |
| 61. Kaempferol-3-O-[2-O-(6-O-E-ferloyl)-β-d-glucopyranosyl]-β-d-glucopyranside | H | 6′-O-E-feruloyl-β-d-Glc-(1→2)-d-Glc |
| 62. Kaempferol-3-O-[2-O-(6-O-E-feruloyl)-β-d-glucopyranosyl]-β-d-galactopyranoside | H | 6′-O-E-feruloyl-β-d-Glc-(1→2)-d-Gal |
2.3. Anthraquinones
Anthraquinones are also a major group of bioactive components in H. diffusa. Up to now, twenty-four anthraquinones with various substitutions (63–86) have been obtained and identified from this herb. These compounds have a typical characteristic of the 9, 10-anthraquinone skeleton with the presence of hydroxy, methyl and/or methoxy groups, for example, 2-hydroxy-3-methoxy-6-methyl anthraquinone (77). Their chemical structures are shown in Figure 3.
Figure 3.
Chemical structures of anthraquinones in H. diffusa.
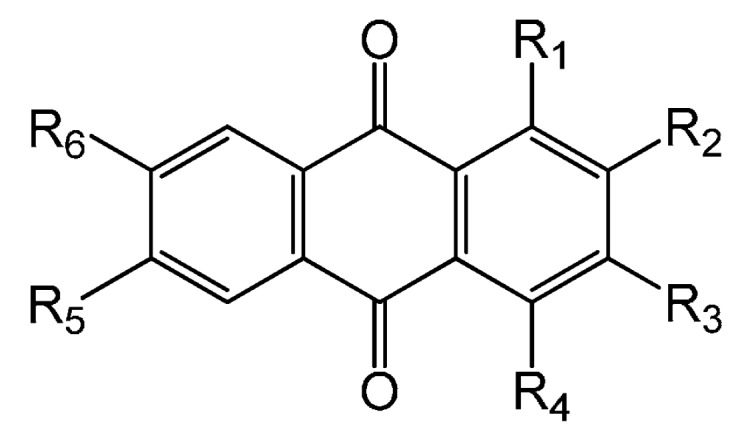
| Compounds | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|
| 63. 2-Methyl-3-methoxy anthraquinone | H | CH3 | OCH3 | H | H | H |
| 64. 2-Hydroxy-1,3-dimethoxy anthraquinone | OCH3 | OH | OCH3 | H | H | H |
| 65. 2-Hydroxy-3-methyl-1-methoxy anthraquinone | OCH3 | OH | CH3 | H | H | H |
| 66. 2-Hydroxy-3-methyl-4-methoxy anthraquinone | H | OH | CH3 | OCH3 | H | H |
| 67. 2-Hydroxy-7-methyl-3-methoxy anthraquinone | H | OH | OCH3 | H | H | CH3 |
| 68. 2-Hydroxy-1-methoxy-3-methyl anthraquinone | OCH3 | OH | CH | H | H | H |
| 69. 2-Hydroxy-3-methyl anthraquinone | H | OH | CH3 | H | H | H |
| 70. 2-Hydroxy-1-methoxy anthraquinone | OCH3 | OH | H | H | H | H |
| 71. 2-Hydroxy-4-methoxy anthraquinone | H | OH | H | OCH3 | H | H |
| 72. 2-Hydroxy-3-methoxy-7-methyl anthraquinone | H | OH | OCH3 | H | H | CH3 |
| 73. 2-Hydroxy-6-methyl anthraquinone | H | OH | H | H | CH3 | H |
| 74. 2-Hydroxy-3-methoxy-6-methyl anthraquinone | H | OH | OCH3 | H | CH3 | H |
| 75. 2,7-Dihydroxy-3-methyl anthraquinone | H | OH | CH3 | H | H | OH |
| 76. 3-Hydroxy-2-methyl anthraquinone | H | CH3 | OH | H | H | H |
| 77. 3-Hydroxy-2-methyl-4-methoxy anthraquinone | H | CH3 | OH | OCH3 | H | H |
| 78. 2,3-Dimethoxy-6-methyl anthraquinone | H | OCH3 | OCH3 | H | CH3 | H |
| 79. 1,3-Dihydroxy-2-methyl anthraquinone | OH | CH3 | OH | H | H | H |
| 80. 1,7- Dihydroxy-6-methoxy-2-methyl anthraquinone | OH | CH3 | H | H | OCH3 | OH |
| 81. 3-Hydroxy-2-methyl-4-methoxy anthraquinone | H | CH3 | OH | OCH3 | H | H |
| 82. 2,6-Dihydroxy-3-methyl-4-methoxy anthraquinone | H | OH | CH3 | OCH3 | OH | H |
| 83. 2,6-Dihydroxy-1-methoxy-3-methyl anthraquinone | OCH3 | OH | CH3 | H | OH | H |
| 84. 1-Hydroxy-4-methoxy anthraquinone | OH | H | H | OCH3 | H | H |
| 85. 2-hydroxymethyl-1-hydroxy anthraquinone | OH | CH2OH | H | H | H | H |
| 86. 2-hydroxymethyl anthraquinone | H | CH2OH | H | H | H | H |
2.4. Phenolic Acids and Their Derivatives
Phenolic acids are very common and important secondary metabolites in nature. To date, twenty-three phenolic acids (87–109) have been identified from the herb of H. Diffusa, including four benzoic acid derivatives (87–90), coumaric acid (91) and its derivative (92), caffeic acid (93) and its derivative (94), ferulic acid (95) and its derivative (96), p-methoxyl cinnamic acid (97), two truxillic acid derivatives (98–99), octadecyl (E)-p-coumarate (100) and nine quinic acid derivatives (101–109). Their chemical structures are prescribed in Figure 4.
Figure 4.
Chemical structures of phenolic acids and their derivatives in H. diffusa.
2.5. Polysaccharides
The polysaccharides in H. diffusa have been researched for their immuno-enhancing activity. They are mainly composed of glucose, galactose and mannose, with the content of 15.10% determined by the spectrophotometry method at 490 nm [54]. Up to now, only one homogeneous polysaccharide, ODP-1, has been separated from H. diffusa, with the relative molecular weight of 20.88 kDa. It consists of mannose, rhamnose, galacturonic acid, glucose, galactose and arabinose, with the molar ratio of 0.005:0.033:0.575:1.000:0.144:0.143 [50].
2.6. Essential Oils
The reports of essential oils in this plant were mainly on isolating many fatty acids, fatty acid esters, etc. [11]. Yang et al. [49] identified 29 compounds representing 81.45% of the total oil content by GC/MS combined with the Kovats Retention index. n-Hexadecanoic acid (119) (31.22%), oleic acid (157) (6.74%), tetracosane (161) (4.94%) and 9,12-octadacadienoic acid (125) (4.87%) were found to be the main constituents. Liu et al. [48] compared the constituents and their content in H. diffusa collected from the provinces of Guangdong, Jiangxi and Guangxi in China and also revealed that the oil of H. diffusa was mainly composed of fatty acids with an oil extraction rate from 0.25% to 0.30%.
2.7. Cyclotides
Three novel cyclotides from H. diffusa, named CD1 (165), CD2 (166) and CD3 (167), with an anti-cancer effect on prostate cancer cells, were reported by Hu et al. [51]. The primary sequences were GAFLKCGESCVYLPCLTTVVGCSCQNSVCYRD, GAVPCGETCVYLPCITPDIGCSCQNKVCYRD and G-TSCGETCVLLPCLSSVLGCTCQNKRCYKD for DC1, DC2 and DC3, respectively.
2.8. Miscellaneous
Only four sterols of daucosterol (110), β-sitosterol (111), stigmasterol (112) and stigmasterol-5,2-diene-3β, 7α-glycol (113), two coumarins of 7-hydroxy-6-methoxy-coumarin (168) and esculetin (169) and two alkaloids of 10-hydroxypheophytin a (170) and aurantiamide acetate (171) have been purified and characterized from H. diffusa and their structures are shown in Figure 5.
Figure 5.
Chemical structures of miscellaneous components in H. diffusa.
3. Pharmacology
H. diffusa has long been used therapeutically in China, due to its broad spectrum of biological and pharmacological activities. Now we have enlisted an overview of the modern pharmacological studies in the following sections (Table 2).
Table 2.
Pharmacological effects of H. diffusa.
| Activities | Model | Formulation/Dosage/Extract | Reference | |
|---|---|---|---|---|
| Anti-tumor activity | ||||
| Colorectal cancer | HT-29 cells | Ethanol extract | The extract suppressed HT-29 cell growth and induced apoptosis via inactivation of the IL-6/STAT3-signaling pathway. | [2] |
| HT-29 cells | Ethanol extract | The extract reduced HT-29 cell viability and survival. It could suppress cancer cell proliferation by blocking the cell cycle, preventing G1 to S progression, and reducing mRNA expression of pro-proliferative PCNA, Cyclin D1 and CDK4, but increasing that of anti-proliferative p21. | [55] | |
| HT-29 cells | Ethanol extract | The extract induced the HT-29 cell morphological changes and reduced cell viability. In addition, the extract treatment resulted in DNA fragmentation, loss of plasma membrane asymmetry, collapse of mitochondrial membrane potential, activation of caspase-9 and caspase-3 and increase of the ratio of pro-apoptotic Bax to anti-apoptotic Bcl-2. | [56] | |
| HT-29 cells | Ethanol extract | The extract treatment downregulated the mRNA and protein expression levels of VEGF-A in HT-29 human colon carcinoma cells. | [57] | |
| HT-29 cells | Ethanol extract | The extract inhibits colorectal cancer growth in vivo via inhibition of SHH-mediated tumor angiogenesis. | [58] | |
| CRC mouse xenograft model | Ethanol extract | The extract inhibited the expression of the gene VEGF-A and VEGFR2, thus, suppressed the activation of Sonic hedgehog (SHH)-signaling in CRC xenograft tumors; it inhibits colorectal cancer growth. | [58] | |
| CRC mouse xenograft model | Ethanol extract | The extract suppressed the STAT3 pathway by suppressing STAT3 phosphorylation in tumor tissues, altering the expression pattern of target genes of Cyclin D1, CDK4 and Bcl-2, as well as upregulating p21 and Bax. | [59] | |
| CT-26 cells | Ethanol extract | The extract can inhibit the proliferation of CT-26 colon cancer cells from BALB/c mice in a time- and dose- dependent manner. | [60] | |
| HCT-8/5-FU cells | Ethanol extracts | The extract treatment significantly reduced the cell viability of HCT-8/5-FU cells by downregulating the expression of P-gp and ABCG2. | [61] | |
| Caco-2 cells | Aqueous extracts | The decoction of H. diffusa and its fraction 9 contained sufficient ursolic acid and oleanolic acid to possibly induce apoptosis of Caco-2 cells. | [62] | |
| Caco-2 cells | Nine pure compounds isolated from H. diffusa | 2-Hydroxymethy-1-hydroxy anthraquinone (IC50 45 mM) and ursolic acid (IC50 71 mM) exhibited the highest inhibition of Caco-2 cell proliferation. | [5] | |
| Leukemia | CEM cells | Aqueous extract | The extract inhibited Leukemia CEM cells growth in time- and concentration-dependent manners. And the inhibition mechanism has greater correlation with the upregulation of P53 expression. | [63] |
| BALB/c mice | Aqueous extract | The extract had anti-leukemia effects on WEHI-3 cell-induced leukemia in vivo. | [64] | |
| HL-60 cells | H. diffusa injection | The extract could induce HL-60 cells differentiation, and suppress the expression of the anti-apoptosis-related gene to inhibit the growth of HL-60 cells. | [65] | |
| HL-60 cells, WEHI-3 cells |
Ethanol extract | The extract inhibited the cell proliferation of HL-60 cells. It triggered an arrest of HL-60 cells at the G0/G1 phase and sub-G1 population, provoked DNA condensation and DNA damage, but the activities of caspase-3, caspase-8, and caspase-9 were elevated in H. diffusa-treated HL-60 cells. | [66] | |
| U937 cells | 2-Hydroxy-3-methyl anthraquinone | 2-Hydroxy-3-methyl anthraquinone enhanced apoptosis of U937 cells through the activation of p-p38MAPK and downregulation of p-ERK1/2. | [67] | |
| THP-1 Cells | 2-Hydroxy-3-methyl anthraquinone | 2-Hydroxy-3-methyl anthraquinone induced THP-1 cell apoptosis, which was associated with a more prominent induction expression of Fas/FasL, DR4 and TRAIL. Moreover, 2-Hydroxy-3-methylanthraquinone treatment resulted in activation of caspase-8. | [68] | |
| Liver cancer | H22 mice | Aqueous extract | The extract had an inhibitory effect on the metastasis of hepatocarcinoma in blood. | [69] |
| HepG2 cells | Aqueous extract | The extract remarkably inhibited HepG2 cell proliferation via arrest of HepG2 cells at the G0/G1 phase and induction of S phase delay. In addition, the extract potentiated the anticancer effect of low-dose 5-FU in the absence of overt toxicity by downregulating the mRNA and protein levels of CDK2, cyclin E and E2F1. | [70] | |
| MHCC97-H cells | Total flavones extract | The extract treatment reduced the level of E-cadherin protein and increased the expression of vimentin protein in TGF-β1-induced MHCC97-H. | [71] | |
| HepG2 cells | 1,3-Dihydroxy-2-Methylanthraquinone Ethyl acetate extract | Both 1,3-Dihydroxy-2-Methylanthraquinone and ethyl acetate extract exhibited an inhibitory effect on HepG2 cells, resulting in in upregulation of Bax, p53, Fas, FasL, p21 and cytoplasmic cytochrome C levels and caspase-3, -8, -9 proteases activities, while downregulating Bcl-2, mitochondrial cytochrome C, cyclin E and CDK 2 in a dose-dependent manner. | [72] | |
| HepG2 cells | Nine pure compounds isolated from H. diffusa | Ursolic acid exhibited a strong inhibition of cell survival with C50 37 mM. | [5] | |
| HepG2 cells | 2-Hydroxy-3-methyl anthraquinone 1-Methoxy-2-hydroxy anthraquinone | Both compounds showed inhibitory activity against protein tyrosine kinases v-src and pp60src and arrested the growth of HepG2 cancer cells. | [38] | |
| Lung cancer | A549 cells, H1355 cells, LLC cells |
Ethanol extract | The extract suppressed the cell proliferation of A549 and H1355 cells as well as reduced cell viability in a concentration-dependent manner. | [66] |
| SPC-1-A cells | 2-Hydroxy-3-methyl anthraquinone 1-Methoxy-2-hydroxy anthraquinone | Both compounds showed inhibitory activity against protein tyrosine kinases v-src and pp60src and arrested the growth of SPC-1-A. | [38] | |
| Breast cancer | MCF-7 cells | Compounds of anthraquinones, iridoid glucosides, stigmasterols and alkaloids/flavonoids | Alkaloids/flavonoids possessed antitumor activity against the human breast cancer cell line MCF7 | [73] |
| MCF-7 cells | Methyl anthraquinone | Methyl anthraquinone-induced MCF-7 cells apoptosis via Ca2+/calpain/caspase-4 pathway. | [74] | |
| Bcap37 cells | 2-Hydroxy-3-methyl anthraquinone, 1-Methoxy-2-hydroxy anthraquinone | Both compounds showed inhibitory activity against protein tyrosine kinases v-src and pp60src and arrested the growth of Bcap37 cells. | [38] | |
| Cervical tumor | Nude mouse model | Aqueous extract | The extract had an inhibitory effect on cervical cancer cells with the expression of Ki-67 protein significantly decreased, and the mean survival time of the mice was significantly extended. | [3] |
| HeLa cells | Nine pure compounds isolated from H. diffusa | 2-Hydroxymethy-1-hydroxy anthraquinone exhibited the strongest inhibitory effect on cell viability. | [5] | |
| Prostate Cancer | DU145 cells, PC-3 cells LNCaP cells |
Nine pure compounds isolated from H. diffusa | 2-Methyl-3-methoxy anthraquinone, 2-hydroxy-3-methyl anthraquinone and ursolic acid exhibited inhibitory effects on prostate cancer cell survival. | [5] |
| PC3 cells LNCaP cells |
6-O-(E)-p-Coumaroyl scandoside methyl ester 10(S)-Hydroxy pheophytin |
Two compounds showed a moderate anti-proliferation effect on PC3 human androgen-independent prostate cancer cells, while 10(S)-hydroxy pheophytin also showed a strong anti-proliferation effect on LNCaP human androgen-sensitive prostate cancer cells. | [52] | |
| Multiple myeloma | RPMI 8226 cells | Nine pure compounds isolated from H. diffusa | 2-Hydroxymethy-1-hydroxy anthraquinone exhibited the strongest inhibition of RPMI 8226cells growth. | [5] |
| RPMI 8226 cells | Polysaccharides extracts | Polysaccharides extracts suppressed the growth of RPMI 8226 cells in a dose- and time-dependent manner. | [75] | |
| RPMI 8226 cells | H. diffusa injection | H. diffusa injection could inhibit the proliferation of RPMI 8226 cells. | [76] | |
| Others | B16F10 cells | Ethanol extract | The extract suppressed the cell proliferation of B16F10 cells as well as reducing cell viability in a concentration-dependent manner. | [66] |
| S180 cells | Decoction, lipophilic extract, crude polysaccharide | Lipophilic extract and crude polysaccharide showed anti-tumor activities and a protective effect on chemotherapeutic damage. However, the aqueous extract had no marked anti-tumor effect on S-180 cells. | [77] | |
| MG-63cells | H. diffusa injection | H. diffusa injection could inhibit the proliferation of MG-63 cells, and Bax gene expression was significantly increased. | [78] | |
| MG-63 cells | H. diffusa injection | H. diffusa injection could induce the apoptosis of MG-63 cells by increasing Bax gene expression in a concentration-dependent manner. | [79] | |
| MG-63 cells | Aqueous extract | H. diffusa, combined with cisplatin, had a stronger inhibitory effect than the single agents in MG-63 cells with IC50164.6 and 5.0 μL/mL, respectively. As a result, H. diffusa could alter anti-apoptotic (Bax and Bad) and pro-apoptotic protein (Bcl-xl and Bcl-2) expression, and it elevated the levels of caspase-3 and caspase-8. | [80] | |
| U87 cells | Aqueous extract | The extract suppressed U87 cells growth in a dose- and time-dependent manner. | [4] | |
| Angiogenesis | 1.Breast tumor-bearing BALB/c mice 2. Zebrafish embryo model 3. Human endothelial cells 4. C57BL/6 mice |
4-Vinyl phenol | 4-Vinyl phenol was demonstrated with anti-angiogenic activity in vitro and in vivo. | [81] |
| Immunomodulatory effect | ||||
| Normal BALB/c mice | Ethanol Extract | The extract has promoted immune responses in normal BALB/c mice. | [82] | |
| Immunosuppression mice induced by cyclophosphamide | Polysaccharides extracts | The extract could improve the clearance index, phagocytic index, and the index of the thymus and spleen of immunosuppression mice. | [50] | |
| Inmmunosuppressed mice induced by cyclophosphamide | Total flavonoids extract | The extract enhanced specific and non-specific immunity. | [83] | |
| Antioxidant effects | ||||
| The extract from methanol, acetone and 80% alcohol | The extraction with 80% alcohol has the strongest antioxidant activity on DPPH assay. | [84] | ||
| The extract from water, ethanol, acetone, chloroform, ether, petroleum benzine | Acetone extract had the strongest antioxidant effect. | [85] | ||
| LO2 cells | Aqueous extract | The aqueous extract exerted a good antioxidant effect in DPPH assay with a 50% scavenging concentration at 0.153 mg/mL. Aqueous extract treatment reversed H2O2-induced activation of the MEK/ERK pathway and H2O2-induced inhibition of the P13-K/AKT/GSK3b pathway in LO2 cells. This may be due to the improvement activity of the aqueous extract of H. diffusa on the antioxidant defense system. | [86] | |
| Twelve pure compounds isolated from H. diffusa | All compounds showed antioxidant effects on xanthine oxidase inhibition, xanthine-xanthine oxidase cytochrome c and TBA-MDA systems. | [33] | ||
| Anti-inflammatory effect | ||||
| Lipopolysaccharide-induced renal inflammation mice | Aqueous extract | The extract protected renal tissues, significantly suppressed the production of TNF-α, IL-1, IL-6 and MCP-1, as well as significantly promoted the production of IL-10 in serum and renal tissues. | [87] | |
| RAW 264.7 cells | Total flavonoids extract | The extract treatment on LPS-stimulated RAW 264.7 cells, reduced expression of iNOS, TNF-α, IL-6 and IL-1β, as well as suppressing phosphorylation of IκB p38, JNK and ERK1/2 in a concentration-dependent manner, indicating that the anti-inflammatory activity of total flavonoids had a close relationship with the NF-κB and MAPK signaling pathways. | [88] | |
| Neuroprotective effect | ||||
| Rat cortical cells damaged by l-glutamate | Methanolic extract, five flavonoids and four O-acylated iridoid glycosides | All compounds exhibited significant neuroprotective activity in primary cultures of rat cortical cells damaged by l-glutamate. | [34] | |
| Anti-fibrosis effect | ||||
| Ras oncogene-transformed R6 cells | Oleanolic acid | Oleanolic acid inhibits the growth of ras oncogene-transformed R6 cells. Oleanolic acid-mediated growth inhibition of transformed cells does not require direct cell–cell contact between normal and ras-transformed cells. | [89] | |
3.1. Anti-Cancer Activity
3.1.1. Anti-Colorectal Cancer Activity
H. diffusa has been used as a major formula for the clinical treatment of colorectal cancer (CRC). In vitro, ethanol extract of H. diffusa treatment significantly suppresses proliferation and induced apoptosis of HT-29 cells, resulting in DNA fragmentation, loss of plasma membrane asymmetry, collapse of mitochondrial membrane potential, activation of caspase-9 and caspase-3, increase of the ratio of pro-apoptotic Bax to anti-apoptotic Bcl-2, reduction of the mRNA expression levels of cyclin D1, cyclin-dependent kinase 4 and B-cell lymphoma-2 (Bcl-2), upregulation of the expression levels of Bcl-2-associated X protein, prevention of G1–S progression, and reduction of mRNA expression of pro-proliferative PCNA, Cyclin D1 and CDK4. These results indicated that the anti-colorectal cancer cells effect of H. diffusa might be carried out via multiple approaches, such as the mitochondria-dependent pathway, IL-6/STAT3 pathway and cell cycle arrest [2,55,56,57,58]. The mechanism was also confirmed by animal experiments [58,59]. Meanwhile, the ethanolic extract of H. diffusa displayed an inhibition effect on CT-26 cells with inhibitory rates from 35.46% ± 3.59% to 71.84% ± 3.12% at different concentrations (0.06 mg/mL, 0.08 mg/mL, 0.10 mg/mL, 0.12 mg/mL, 0.14 mg/mL, 0.16 mg/mL, 0.18 mg/mL and 0.20 mg/mL) and showed a stronger inhibition effect with an increase of concentration [60]. Li et al. [61] revealed that the ethanolic extract treatment could overcome 5-fluorouracil resistance in HCT-8/5-FU cells by downregulating the expression of P-gp and ABCG2. In addition, 2-hydroxymethy-1-hydroxy anthraquinone (IC50 45 μM) and ursolic acid (IC50 71 μM)) isolated from H. diffusa exhibited inhibition effects on Caco-2 cell proliferation [5], and the mechanism of the inhibition effect for ursolic acid might include the cleavage of the Poly (ADP-ribose) Polymerase (PARP) [62].
3.1.2. Anti-Leukemia Activity
The anti-leukemia effects of both aqueous and ethanolic extracts of H. diffusa have been investigated in several cancer cell lines. H. diffusa aqueous extract treatment with 0.01–4150 μg/mL restrained the growth of the CEM cells by enhancing the expression of P53 in vitro [63] and influenced murine leukemia WEHI-3 cells, as well as promoting T- and B-cell proliferation in leukemic mice administrated with 16 and 32 mg/kg in vivo [64]. The ethanolic extract of H. diffusa could trigger an arrest of HL-60 cells at the G0/G1 phase and sub-G1 population, provoke DNA condensation and DNA damage, but elevate the activities of caspase-3, caspase-8 and caspase-9, thus, inhibiting the cell proliferation of HL-60 cells with the half maximal inhibitory concentration (IC50) value of 4.62 mg/mL [65,66]. Wang et al. [67] found that 2-hydroxy-3-methyl anthraquinone treatment (0–80 μM) could enhance apoptosis of U937 cells in a dose-dependent manner through the activation of p-p38MAPK and downregulation of p-ERK1/2. Further study verified it could alter the expression of Fas/FasL and activation of caspase-8, thus inducing THP-1 cell apoptosis [68].
3.1.3. Anti-Liver Cancer Activity
Li et al. [69] reported the inhibition of aqueous extract of H. diffusa on blood metastasis in H22 mice. The body and immune organs weights increased after administration with H. diffusa extract at three doses of 0.25, 0.5 and 1.0 mg/kg. In vitro, the aqueous extract of H. diffusa treatment (1.25–10 mg/mL) remarkably inhibited HepG2 cell proliferation in a dose-dependent manner, probably via the arrest of HepG2 cells at the G0/G1 phase and the induction of S phase delay [70]. Treatment with total flavones extract from H. diffusa could reverse the invasion of MHCC97-H cells in epithelial-mesenchymal transition induced by TGF-β1 at the dose of 200μg/mL, and the effect might be carried out by decreasing the level of E-cadherin protein and increasing the expression of vimentin protein [71]. Li et al. found that both 1,3-Dihydroxy-2-Methylanthraquinone (79 and 157 μmol/L) and ethyl acetate extract (100 and 200 μg/mL) induced apoptosis on HepG2 cells, resulting in upregulation of Bax, p53, Fas, FasL, p21 and cytoplasmic cytochrome C levels and caspase-3, -8, -9 proteases activities, while downregulation of Bcl-2, mitochondrial cytochrome C, cyclin E and CDK 2 in a dose-dependent manner [72]. Nine compounds from H. diffusa, namely, ethyl 13 (S)-hydroxy-chlorophyllide a, 2-methyl-3-methoxy anthraquinone, 2-hydroxymethyl anthraquinone, 2-hydroxy-3-methyl anthraquinone, 2-hydroxymethy-1-hydroxy anthraquinone, 2-hydroxy-1-methoxy anthraquinone, 2-hydroxy-3-methyl-1-methoxy anthraquinone, oleanolic acid and ursolic acid, have been researched for their anti-liver cancer effect within the concentration range from 1 to 200 μM. As a result, ursolic acid exhibited a strong inhibition of HepG2 cell survival (IC50 36.63 μM) [5]. Another study revealed that the inhibitory activity of 2-hydroxy-3-methyl anthraquinone (IC50 51 μM) and 2-hydroxy-1-methoxy anthraquinone (IC50 62 μM) might be achieved by activity against protein tyrosine kinases v-src and pp60src [38].
3.1.4. Anti-Lung Cancer Activity
Aqueous extract of H. diffusa treatment (0–200 μg/mL) showed a suppression effect on A549 and H1355 cells in a concentration-dependent manner. But this effect was not found in LLC cells [66]. Further, Shi et al. [38] confirmed that two compounds of 2-hydroxy-3-methyl anthraquinone (IC50 66 μM) and 2-hydroxy-1-methoxy anthraquinone (IC50 79 μM) from H. diffusa could induce apoptosis on SPC-1-A cells with a close relationship to the mitochondrial apoptotic pathway.
3.1.5. Anti-Breast Cancer Activity
Anthraquinones, iridoid glucosides, stigmasterols and alkaloid/flavonoid extracts were evaluated for anti-breast cancer using human breast cancer cell line MCF7. Dong et al. [73] found that the crude alkaloid/flavonoid extract, but not its three major components, possessed antitumor activity against the human breast cancer cell line MCF7. However, Liu et al. [74] observed that methyl anthraquinone from H. diffusa exhibited an inhibition effect on MCF7 cells with half maximal effective concentration (EC50) of 18.62 ± 2.71 and 42.19 ± 3.84 μM for 24 and 48 h, respectively, and induced MCF-7 cells apoptosis via the Ca2+/calpain/caspase-4 pathway. Moreover, compounds of 2-hydroxy-3-methyl anthraquinon (IC50 57 μM) and 2-hydroxy-1-methoxy anthraquinone) (IC50 65 μM) inhibited protein tyrosine kinases v-src and pp60src and the growth of Bcap37 cells [38].
3.1.6. Anti-Cervical Tumor Activity
Zhang et al. [3] discovered that the aqueous extract of H. diffusa treated (0.5 g/kg bw) by intragastric administration on human cervical carcinoma xenograft in nude mice showed an inhibitory effect on cervical cancer cells and induced apoptosis of Hela cells. Meanwhile, anthraquinones, especially 2-hydroxymethy-1-hydroxy anthraquinone, showed a strong inhibitory effect on Hela cells with IC50 45 μM in vitro [5].
3.1.7. Anti-Prostate Cancer Activity
The potential anti-prostate cancer effect of H. diffusa, mainly the active compounds, has previously been provided on a variety of cell lines. 2-Methyl-3-methoxy anthraquinone (IC50 64.72–105.90 μM), 2-hydroxy-3-methyl anthraquinone (IC50 28.82–159.20 μM) and ursolic acid (IC50 22.33–36.08 μM) exhibited inhibitory effects on DU145, PC-3 and LNCaP cells [5]. 6-O-(E)-p-coumaroyl scandoside methyl esterand 10(S)-hydroxy pheophytin a showed an anti-proliferation effect on PC-3 cells in a dose-dependent manner from 0 to 60 μM, while 10(S)-hydroxy pheophytin a also showed a strong anti-proliferation effect on LNCaP cells with a significant effect, with an IC50 value of 20 μM [52]. Hu et al. [51] isolated three cyclotides (DC 1-3) and studied their anti-prostate cancer effect. Thus, three cyclotides, especially DC 3 (1 mg/kg) showed inhibition against PC3, DU145 and LNCap cells. In addition, DC3 significantly inhibited development of the tumor in weight and size in the model of a prostate xenograft, and showed significant anti-cancer effect (p < 0.01) at a dose of 1 mg/kg, with 40.23% inhibition of the rate of tumor growth (weight).
3.1.8. Anti-Multiple Myeloma Activity
Up to now, the anti-multiple myeloma effect of H. diffusa has been proved in RPMI 8226 cells. The polysaccharides extracts (1, 2 and 3 mg/mL) [75], the compound of 2-hydroxymethyl-1-hydroxy anthraquinone (1–200 μM) [5], as well as H. diffusa injection (20, 40 and 60 μL/mL) [76], exhibited an inhibitory effect on RPMI 8226 cells growth in a dose-dependent manner.
3.1.9. Other Anti-Cancer Effects
Other anti-cancer effects have also been reported during these years. The ethanolic extract of H. diffusa (0–200 μg/mL) suppressed the proliferation of B16F10 cells in a dose-dependent manner [66]. The lipophilic extract (50 and 100 mg/kg) and crude polysaccharide (31.25 and 62.5 mg/kg) from H. diffusa showed anti-tumor activities on S-180 cells and a protective effect on chemotherapeutic damage [77]. H. diffusa injection could induce the apoptosis of MG-63 cells by increasing the Bax gene expression in a concentration-dependent manner from 50 to 400 μg/mL [78,79]. When it is used with cisplatin, the combined use exhibited a stronger inhibitory effect than the single agents. This might be due to its property of elevating the levels of Bax, Bad, caspase-3 and caspase-8 expression and decreasing the levels of Bcl-xl and Bcl-2 [80]. Meanwhile, Zhang et al. [4] found that the aqueous extract of H. diffusa (2–8 mg/mL) inhibited the growth of U87 cells in a dose-dependent manner by inducing mitochondrial apoptosis via the AKT/ERK pathways. Moreover, the compound, 4-vinyl phenol, was demonstrated to have anti-angiogenic activity in human endothelial cells of HUVEC (IC50 15.31 μg/mL) and HMEC-1 (IC50 21.43 μg/mL), breast tumor-bearing BALB/c mice (0.2–2 mg/kg), C57BL/6 mice (20–100 μg/mL matrigel) and zebrafish embryo models (6.25–12.5 μg/mL matrigel), and this effect had a close relationship with the PI3K/AKT pathway [81].
3.2. Immunomodulatory Effect
Lin et al. [64] found that aqueous extract of H. diffusa (16 and 32 mg/kg) affected immune responses by promoting T- and B-cell proliferation in leukemic mice in WEHI-3-generated leukemia mice. Meanwhile, Kuo et al. [82] discovered that the ethanolic extract (16, 32 and 64 mg/kg) could also promote immune responses in normal BALB/c mice by promoting CD11b, CD19 and Mac-3 levels, increasing phagocytosis activity of macrophages obtained from the peritoneal cavity and increasing NK cell activity and B- and T-cell proliferation. The polysaccharides extracts (2.25, 4.5 and 9.0 mg/mL) could improve the clearance index, phagocytic index and the index of the thymus and spleen of immunosuppression mice [50]. When inmmunosuppressed mice were orally administrated total flavonoids of H. diffusa (15, 30 and 60 mg/kg), the levels of interleukin-2 (IL-2) and interferon-γ (INF-γ) were enhanced and the proliferation of T and B lymphocytes was increased, indicating the immunomodulatory effect of total flavonoids [83].
3.3. Antioxidant Effect
The aqueous, methanolic and 80% acetonic extracts were evaluated for antioxidant activity and the extraction from 80% alcohol (0.1–4.5 mg/mL) showed the strongest antioxidant activity, by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay [84]. Yu et al. [85] compared the antioxidant effects of aqueous, alcoholic, acetonic, chloroform, ether and petroleum benzene extracts from H. diffusa, and found that the acetonic extracts (0.03%–0.18%), especially the 0.12% acetone extract, had the strongest antioxidant effect, by determination of peroxide value. In addition, the aqueous extract (0.3–10 mg/mL) treatment could protect human hepatocyte cells from H2O2-induced cytotoxicity in a dose-dependent manner as the probable result of the improvement activity of the aqueous extract of H. diffusa on the antioxidant defense system by reversing H2O2-induced activation of the MEK/ERK pathway and H2O2-induced inhibition of the P13-K/AKT/GSK3β pathway in LO2 cells [86]. The antioxidant effect of H. diffusa may be due to its compounds, like flavonoids and iridoids. Three flavonol glycosides (quercetin 3-O-sambubioside, kaempferol-3-O-[2-O-(E-6-O-feruloyl)-β-d- glucopyranosyl]-β-d-galactopyranoside and quercetin 3-O-sophoroside) and six known iridoid glycosides (asperuloside, asperulosidic acid methyl ester, (E)-6-O-p-methoxy cinnamoyl scandoside methyl ester, (E)-6-O-feruloyl scandoside methyl ester and (E)-6-O-coumaroyl scandoside methyl ester) were determined for their antioxidant effects on xanthine oxidase inhibition, xanthine-xanthine oxidase cytochrome c and TBA-MDA systems. In consequence, asperuloside (IC50 118.5 ± 0.70 μM) and kaempferol-3-O-(2-O-β-d-glucopyranosyl)-β-d-galactopyranoside (IC50 98.7 ± 0.16 μM) showed a minor anti-lipid peroxidation effect and quercetin di-glycosides exerted a remarkable antioxidant effect as superoxide anion scavengers [33].
3.4. Anti-Inflammatory Effect
The aqueous extract (5.0 g/kg bw) treatment exhibited an anti-inflammatory effect in lipopolysaccharide-induced renal inflammation of mice by significantly suppressing the production of tumor necrosis factor-α (TNF-α), IL-1, IL-6 and monocyte chemotactic protein 1 (MCP-1) in renal tissues, as well as significantly promoting the production of IL-10 in serum and renal tissues. Moreover, two main chemotypes, including eight flavonoids and four iridoid glycosides were found in renal tissues after H. diffusa treatment, indicating that the anti-inflammatory effect may be due to these constituents [87]. In vitro, Chen et al. found that the flavonoids extract treatment (50−100 μg/mL) on LPS-stimulated RAW 264.7 cells reduced expression of iNOS, TNF-α, IL-6 and IL-1β, as well as suppressing phosphorylation of IκB p38, JNK and ERK1/2 in a concentration-dependent manner, indicating that the anti-inflammatory activity of total flavonoids had a close relationship with the NF-κB- and MAPK-signaling pathways [88].
3.5. Others
Five flavonol glycosides (kaempferol-3-O-[2-O-(6-O-E-feruloyl)-β-d-glucopyranosyl]-β-d-galactopyranoside, quercetin-3-O-[2-O-(6-O-E-feruloyl)-β-d-glucopyranosyl]-β-d-galactopyranoside, quercetin-3-O-[2-O-(6-O-E-feruloyl)-β-d-glucopyranosyl]-β-d-glucopyranoside, kaempferol-3-O-(2-O-β-d-glucopyranosyl)-β-d-galactopyranoside and quercetin-3-O-(2-O-β-d-glucopyranosyl)-β-d-galactopyranoside) and four O-acylated iridoid glycosides (6-O-Z-p-methoxy cinnamoyl scandoside methyl ester, 6-O-E-p-methoxy cinnamoyl scandoside methyl ester, 6-O-Z-p-coumaroyl scandoside methyl ester and 6-O-E-p-coumaroyl scandoside methyl ester) isolated from H. diffusa exhibited a significant neuroprotective effect on l-glutamate-damaged rat cortical cells in the concentration from 0.1 to 10 μM; further, the structure–activity study proved di-OH in the B ring and an acyl substituent in flavonoids, a p-methoxy group in the aromatic ring and a trans double bond in the acyl moiety of acylated iridoid glycosides might be crucial for the biological response [34]. Wu et al. [89] found the inhibitory effect of oleanolic acid (2 and 8 μg/mL) isolated from H. diffusa against ras-transformed fibroblasts on R6 cells, and this inhibition might cause normal cells to secrete an inhibitory factor against the transformed cells, but did not require direct cell–cell contact.
4. Quality Control
Quality control of herbal medicines is necessary to ensure their stability, efficiency and safety. Modern analytical techniques provide simpler, more accurate and reliable methods for the quality control for H. diffusa. Besides the macroscopic and microscopic characters of H. diffusa [9], DNA sequence has become a powerful tool for the distinguishing H. diffusa from counterfeits, such as H. corymbosa and H. tenelliflora [8,12]. Chemical fingerprint is a comprehensive method accepted by the Food and Drug Administration, European Medicines Agency, and China Food and Drug Administration [90]. It can provide information about the types of compounds, as well as their relative ratios. A HPLC-MS fingerprint method was applied to 10 batches of H. diffusa materials from nine regions in China. The results showed that this method could differentiate samples from different geographical origins or processing methods [91]. Liang et al. [92] analyzed the chemical fingerprints of 17 batches of H. diffusa and found that the contents of asperuloside and (E)-6-O-p-coumaroyl scandoside methyl ester were quite different in samples collected from different habitats.
The quantitative analysis for the quality control of H. diffusa has mostly focused on the diversity of components by a series of analytical methods, such as UV, HPLC, TLC and LC/MS. Up to now, triterpenes (ursolic acidand oleanulic acid), iridoids ((E)-6-O-p-coumaroyl scandoside methyl ester), geniposidic acid, deacetyl asperulosidic acid methyl ester, asperuloside acid, asperulosideand (E)-6-O-feruloyl scandoside methyl ester), phenolic acid (p-coumaric acid and ferulic acid), flavonoids (quercetin, rutin, quercetin-3-O-β-d-glucopyranside, quercetin-3-O-sambubioside, kaempferol, kaempferol-3-O-β-d-glucopyranside), anthraquinones (2-hydroxy-3-methoxy-7-methyl anthraquinone and 2-hydroxy-1-methoxy anthraquinone), polysassharides and one miscellaneous compound have been quantified as mark compounds for the quality control of H. diffusa. However, there were wide variations in the contents of these compounds, caused by samples from different sources and different collecting times (Table 3). Therefore, it is very urgent that a comprehensive method for ensuring the quality of H. diffusa be established.
Table 3.
Quantitative analysis for the quality control of H. diffusa.
| Analytes | Method | Results | Reference |
|---|---|---|---|
| Deacetyl asperulosidic acid methyl ester | HPLC | The contents of deacetyl asperulosidic acid methyl ester of 22 batches were from 0.31 to 3.34 mg/g. | [93] |
| Oleanolic acid | TLC | The contents of oleanolic acid of 3 batches were from 1.63% to 1.72% | [94] |
| Isoscutellarein | HPLC | The contents of isoscutellarein have a close relationship with the collecting times and were also different in leaves (1.11–2.72 mg/g) and stem (0.35–0.94 mg/g). | [95] |
| p-Coumaric acid | HPLC | The contents of p-coumaric acid in the injection of H. diffusa from four manufacturers ranged from 0.34 to 0.49 mg/mL. | [96] |
| p-Coumaric acid | HPLC | The contents of p-coumaric acid of 13 batches were from 0.46 to1.88 mg/mL | [97] |
| 3,4-Dihydroxy methyl benzoate | HPLC | The contents of 3,4-dihydroxy methyl benzoate of 8 batches were from 40.8 to 87.0 μg/g. | [98] |
| Polysassharides | UV | Polysassharides have been determined by the phenol-sulfuric acid method by spectrophosured at 490 nm, and the content was 15.10%. | [99] |
| Ursolic acid Oleanolic acid |
HPLC | Six batches have been determined with the contents of 1.75–3.37 mg/g for ursolic acid and 0.50–0.80 mg/g for oleanolic acid, indicating that the ursolic acid and oleanolic acid content in the samples from different sources were significantly different. | [100] |
| Ursolic acid Oleanolic acid |
HPLC | The contents of ursolic acid and oleanolic acid have a close relationship with the collecting time. The range of contents was 1.17–3.75 and 0.19–0.96 mg/g for ursolic acid and oleanolic acid, respectively. | [101] |
| Ursolic acid Oleanolic acid |
HPLC | The contents of ursolic acid and oleanolic acid were 0.51%–0.58% and 0.11%–0.14%, respectively. And the contents of the whole herb were slightly lower than those of the overground part for both of the two compounds. | [102] |
| Ursolic acid Oleanolic acid |
HPLC-MS/MS | The contents of ursolic acid and oleanolic acid for 10 batches were 0.15%–0.65% and 0.06%–0.17%, respectively. | [103] |
| 2-Hydroxy-3-methoxy-7-methyl anthraquinone 2-Hydroxy-1-methoxy anthraquinone |
HPLC | The contents were 0.16–0.51 and 0.22–0.49 mg/g for 2-hydroxy-3-methoxy-7-methyl anthraquinone and 2-hydroxy-1-methoxyanthraquinone, respectively. | [104] |
| Asperuloside E-6-O-p-Coumaroyl scandoside methyl ester E-6-O-p-Coumaroyl scandoside methyl ester-10-methyl ether |
HPLC | The contents of asperuloside, E-6-O-p-coumaroyl scandoside methyl ester and E-6-O-p-coumaroyl scandoside methyl ester-10-methyl ether have been determined in twenty-three batches. The result was that the contents of the compounds were significantly varied among the different samples. The concentration ranges were 0–7.885, 1.104–7.159 and 0–1.795 mg/g for asperuloside, E-6-O-p-coumaroyl scandoside methyl ester and E-6-O-p-coumaroyl scandoside methyl ester-10-methyl ether, respectively. | [105] |
| 3,4-Dihydroxy methyl benzoate p-Coumaric acid Ferulic acid (E)-6-O-p-Coumaroyl scandoside methyl ester |
HPLC | Four compounds have been quantified in the injection of H. diffusa with contents of 2.25–2.63, 7.02–7.15, 0.96–1.17 and 7.16–7.33 g/L for 3,4-dihydroxy methyl benzoate, p-coumaric acid, ferulic acid and (E)-6-O-p-coumaroyl scandoside methyl ester, respectively. | [106] |
| Geniposidic acid Ursolic acid Quercetin p-Coumaric acid |
CE | Four compounds have been quantified in the injection of H. diffusa with contents of 1.004, 1.182, 0.110 and 0.067 mg/g for ursolic acid, geniposidic acid, quercetin and p-coumaric acid, respectively. | [107] |
| Asperuloside acid Asperuloside (E)-6-O-Feruloyl scandoside methyl ester (E)-6-O-p-Coumaroyl scandoside methyl ester Scandoside methyl ester |
HPLC | The contents were 1.57–5.93, 1.45–3.86, 1.82–3.23, 1.54–3.82 and 1.49–4.11 mg/g for asperuloside acid, asperuloside, (E)-6-O-feruloyl scandoside methyl ester, (E)-6-O-p-coumaroyl scandoside methyl ester and scandoside methyl ester, respectively, and they were very different in different batches. | [108] |
| Quercetin-3-O-sambubioside Quercetin-3-O-β-d-glucopyranside Kaempferol-3-O-β-d-glucopyranside Rutin Quercetin Kaempferol |
HPLC | Six compounds from eight batches of H. diffusa have been quantified with contents of 1.36–6.32, 0.98–10.23, 0.79–7.98, 4.92–15.78, 0.52–1.72 and 0.75–2.15 mg/g for quercetin-3-O-sambubioside, quercetin-3-O-β-d-glucopyranside, kaempferol-3-O-β-d-glucopyranside, rutin, quercetin and kaempferol, respectively, indicating that the contents for these compounds were quite different from different regions. | [109] |
| Desacetyl asperulosidic acid Asperuloside Aesculetin Coumaric acid Ferulic acid Quercetin Kaempferol |
HPLC | Seven compounds from six batches of H. diffusa have been quantified with contents of 42.48 ± 1.43, 63.76 ± 1.01, 1765 ± 0.69, 881.9 ± 0.74, 86.99 ± 1.65, 1395 ± 0.731 and 902.2 ± 0.82 μg/g for desacetyl asperulosidic acid, asperuloside, aesculetin, coumaric acid, ferulic acid, quercetin, kaempferol, respectively. | [110] |
5. Pharmacokinetics
The investigations about pharmacokinetics of H. diffusa are very scarce. After oral administration of H. diffusa in lipopolysaccharide-induced renal inflammation in mice, most compounds, including flavonoids, iridoid glycosides and anthraquinone, were found in plasma, and 12 compounds (eight flavonoids and four iridoid glycosides) were found in kidney, determined by UPLC-Q-TOF-MS/MS. The results indicated that flavonoids, iridoids and anthraquinones might be responsible for the protective effect of H. diffusa on renal inflammation [87]. Liu et al. [111] found that p-coumaric acid was a major metabolite of (E)-6-O-p-coumaroyl scandoside methyl ester in rat plasma after oral administration of a dose of 20 mg/kg. Compared with direct administration of p-coumaric acid, the absorption and elimination of p-coumaric acid were slower with administration of (E)-6-O-p-coumaroyl scandoside methyl ester. This was also confirmed by Yan et al. at 2011 [112]. Moreover, Ganbold et al. [62] investigated the bioavailability of H. diffusa by production of post-absorption samples using the Caco-2 cell model and confirmed that the decoction has good permeability (Papp = 3.575 × 10−6 cm/s) in vitro with no cytotoxic effect.
6. Conclusions
Although H. diffusa has been used in China for thousands of years as a heat-clearing and detoxifying medicine, it has become popular for its anti-cancer effect, especially in the Taiwan district. Modern research on H. diffusa has provided much evidence for its anti-cancer effect using in vitro and in vivo experiments and has tried to clarify the mechanism of its action. Meanwhile, its other activities, such as anti-oxidant, anti-inflammatory, anti-fibroblasts, immunomodulatory and neuroprotective effects, have been reported. The achievement of these therapeutic effects is due to the chemical composition of H. diffusa. One hundred and seventy-one compounds have been reported, including iridoids, flavonoids, anthraquinones, phenolic acids and their derivatives, sterols, triterpenes, polysaccharides, cyclotides, coumarins, alkaloids and volatile oils. Among these constituents, iridoids, flavonoids and anthraquinones are three main ingredients and may play an essential role in its activities. However, there is no official quality standard for the quality control of H. diffusa. The contents of bioactive compounds are significantly different in the samples from different sources and different collecting times. So, a feasible and reliable approach is urgently needed in considering the botanical origin and bioactive effects. Moreover, a relatively small number of pharmacokinetics studies have been summarized, and, therefore, it is difficult to evaluate the function of H. diffusa in the human body. Altogether, this review gives comprehensive information about H. diffusa and provides evidence for its clinical application and further development.
Acknowledgments
The project was financially supported by the National Natural Science Foundation of China (81503376) and Guangdong Natural Science Foundation (2014A030310280).
Author Contributions
Rui Chen wrote the Introduction, Phytochemistry and Pharmacology sections; Jingyu He wrote the Quality control and Pharmacokinetics sections; Xueli Tong sorted out the references; Lan Tang performed the English editing; Menghua Liu designed this review and wrote the Conclusions section.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tao C., Taylor C.M. Rubiaceae. In: Wu Z.Y., Raven P.H., Hong D.Y., editors. Flora of China. Volume 19. Science Press; Beijing, China: Missouri Botanical Garden Press; St. Louis, MO, USA: 2011. pp. 147–174. [Google Scholar]
- 2.Lin J.M., Li Q.Y., Chen H.W., Lin H., Lai Z.J., Peng J. Hedyotis diffusa Willd extract suppresses proliferation and induces apoptosis via IL-6-inducible STAT3 pathway inactivation in human colorectal cancer cells. Oncol. Lett. 2015;9:1962–1970. doi: 10.3892/ol.2015.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang P.Y., Zhang B., Gu J., Hao L., Hu F.F., Han C.H. The study of the effect of Hedyotis diffusa on the proliferation and the apoptosis of the cervical tumor in nude mouse model. Cell Biochem. Biophys. 2015;72:783–789. doi: 10.1007/s12013-015-0532-9. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y., Xie R.F., Xiao Q.G., Li R., Shen X.L., Zhu X.G. Hedyotis diffusa Willd extract inhibits the growth of human glioblastoma cells by inducing mitochondrial apoptosis via AKT/ERK pathways. J. Ethnopharmacol. 2014;158:404–411. doi: 10.1016/j.jep.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 5.Meng Q.X., Roubin H.R., Hanranhan R.J. Ethnopharmacological and bioactivity guided investigation of five TCM anticancer herbs. J. Ethnopharmacol. 2013;148:229–238. doi: 10.1016/j.jep.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Chao T.H., Fu P.K., Chang C.H., Chang S.N., Mao F.C., Lin C.H. Prescription patterns of Chinese herbal products for post-surgery colon cancer patients in Taiwan. J. Ethnopharmacol. 2014;156:702–708. doi: 10.1016/j.jep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Yeh Y.C., Chen H.Y., Yang S.H., Lin Y.H., Chiu J.H., Lin Y.H., Chen C.L. Hedyotis diffusa combined with scutellaria barbata Are the core treatment of Chinese herbal medicine used for breast cancer patients: A population-based Study. Evid. Based Complement Alternat. Med. 2014;2014 doi: 10.1155/2014/202378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M., Wong Y.L., Jiang L.L., Wong K.L., Wong Y.T., Lau C.B., Shaw P.C. Application of novel loop-mediated isothermal amplification (LAMP) for rapid authentication of the herbal tea ingredient Hedyotis diffusa Willd. Food Chem. 2013;141:2522–2525. doi: 10.1016/j.foodchem.2013.05.085. [DOI] [PubMed] [Google Scholar]
- 9.Lee H.Z., Bau D.T., Kuo C.L., Tsai R.Y., Chen C.Y., Chang Y.H. Clarification of the phenotypic characteristics and anti-tumor activity of Hedyotis diffusa. Am. J. Chin. Med. 2011;39:201–213. doi: 10.1142/S0192415X11008750. [DOI] [PubMed] [Google Scholar]
- 10.Lau C.B., Cheng L., Cheng B.W., Yue G.G., Wong E.C., Lau C.P., Leung P.C., Fung K.P. Development of a simple chromatographic method for distinguishing between two easily confused species, Hedyotis diffusa and Hedyotis corymbosa. Nat. Prod. Res. 2012;26:1446–1450. doi: 10.1080/14786419.2011.603317. [DOI] [PubMed] [Google Scholar]
- 11.Wang L., Zhou C., Mai H.Z. Analysis of volatile compounds in Hedyotis diffusa and Hedyotis corymbosa. J. Chin. Mater. Med. 2003;26:563–564. [Google Scholar]
- 12.Liu Z.M., Hao M.G., Wang J.L. Application of allele-specific primer in the identification of Hedyotis diffusa. J. Chin. Mater. Med. 2004;27:484–487. [PubMed] [Google Scholar]
- 13.Yang Y.B., Yang X.Q., Ding Z.T. Chemical constituents from Hedyotis diffusa. Chin. J. Yunnan Univ. (Nat. Sci.) 2007;29:187–189. [Google Scholar]
- 14.Liang Z.T., He M.F., Fong W.F., Jiang Z.H., Zhao Z.Z. A comparable, chemical and pharmacological analysis of the traditional Chinese medicinal herbs Oldenlandia diffusa and O. corymbosa and a new valuation of their biological potential. Phytomedicine. 2008;15:259–267. doi: 10.1016/j.phymed.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Liu E.H., Zhou T., Li G.B., Li J., Huang X.N., Pan F., Gao N. Characterization and identification of iridoid glucosides, flavonoids and anthraquinones in Hedyotis diffusa by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2012;35:263–272. doi: 10.1002/jssc.201100780. [DOI] [PubMed] [Google Scholar]
- 16.Nishihama Y., Masuda K., Yamaki M., Takagi S., Sakina K. Three new iridoid glucosides from Hedyotis diffusa. DlantaMedica. 1981;43:28–33. doi: 10.1055/s-2007-971468. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y.Y., Luo J.B. Studies on the chemical constituents in Herb of Hedyotis diffusa. J. Chin. Mater. Med. 2008;31:522–524. [PubMed] [Google Scholar]
- 18.Ji B.Y., Fan C.Q., Fei L.X., Ma Y. Advance on the chemical and pharmacological effects studies of Hedyotis diffusa. Chin. J. Exp. Tradit. Med. Form. 2014;20:235–240. [Google Scholar]
- 19.Si J.Y., Chen D.H., Pan R.L., Zhao X.H. Chemical constituents of Hedyotis Diffusa. Nat. Prod. Res. Dev. 2006;18:942–944. [Google Scholar]
- 20.Zhang Y.Y., Chen Y., Fan C.L., Ye W.C., Luo J.B. Two new iridoids from Hedyotis diffusa. Fitoterapia. 2010;81:515–517. doi: 10.1016/j.fitote.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Liu J.Z., Wang L. Studies on chemical constituents of Hedyotis diffusa Willd. J. Heibei Med. Univ. 2007;28:188–190. [Google Scholar]
- 22.Li C.M., Zhao Y.Y., Guo Z.M., Zhang X.L., Xue X.Y., Liang X.M. Effective 2D-RPLC/RPLC enrichment and separation of micro-components from Hedyotis diffusa Willd and characterization by using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2014;99:35–44. doi: 10.1016/j.jpba.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 23.Chen W.C., Gu D.W., Zhang H., Zhu Z.Y., Zhang G.Q., Chai Y.F. HPLC-TOEMS in rapid separation and identification of chemical components in Oldenlanda diffusa and its injection preparations. Acad.J. Second Mil. Med. Univ. 2010;31:292–296. doi: 10.3724/SP.J.1008.2010.00292. [DOI] [Google Scholar]
- 24.Li C., Xue X., Zhou D., Zhang F., Xu Q., Ren L., Liang X. Analysis of iridoid glucosides in Hedyotis diffusa by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2008;48:205–211. doi: 10.1016/j.jpba.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Li D.X., Schmitz O.J. Comprehensive two-dimensional liquid chromatography tandem diode array detector (DAD) and accurate mass QTOF-MS for the analysis of flavonoids and iridoid glycosides in Hedyotis diffusa. Anal. Bioanal. Chem. 2015;407:231–240. doi: 10.1007/s00216-014-8057-4. [DOI] [PubMed] [Google Scholar]
- 26.Xu G.H., Kim Y.H., Chi S.W., Choo S.J., Ryoo I.J., Ahn J.S., Yoo I.D. Evaluation of human neutrophil elastase inhibitory effect of iridoid glycosides from Hedyotis diffusa. Bioorg. Med. Chem. Lett. 2010;20:513–515. doi: 10.1016/j.bmcl.2009.11.109. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q.M., Sun Z.Y. Study on chemical constituents of Oldenlandia diffusa. Chin. J. Chin. Mater. Med. 2014;37:2216–2218. [PubMed] [Google Scholar]
- 28.Ren R.A. Identification of Chinese Drug. Shanghai Scientific & Technological Publishers; Shanghai, China: 1986. p. 491. [Google Scholar]
- 29.Wu K.S., Zhang K., Tan G.S., Zeng G.Y., Zhou Y.J. Study on constituents of Oldenlandia diffusa. Chin. Pharm. J. 2005;40:817–818. [Google Scholar]
- 30.Liang S.Y., Chen F.L., Tang Q.F., Luo J.B., Zeng Y.C. Study of Chemical Constituents from Herba Hedyotis diffusa. Tradit. Chin. Drug Res. Clin. Pharm. 2012;23:655–657. [Google Scholar]
- 31.Zhou Y.J., Wu K.S., Zeng G.R., Tang J.B., Xu K.P., Li F.S., Tang G.S. Study on chemical constituents of Oldenlandia diffusa. Chin. J. Chin. Mater. Med. 2007;32:590–593. [PubMed] [Google Scholar]
- 32.Zhang H.J., Chen Y.G., Huang R. Study on flavonoids constituents of Oldenlandia diffusa. Chin. J. Chin. Mater. Med. 2005;28:385–387. [PubMed] [Google Scholar]
- 33.Lu C.M., Yang J.J., Wang P.Y., Lin C.C. A new acylated flavonol glycoside and antioxidant effects of Hedyotis diffusa. Planta Med. 2000;66:374–377. doi: 10.1055/s-2000-8544. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y., Park E.J., Kim J., Kim Y., Kim S.R., Kim Y.Y. Neuroprotective constituents from Hedyotis diffusa. J. Nat. Prod. 2001;64:75–78. doi: 10.1021/np000327d. [DOI] [PubMed] [Google Scholar]
- 35.Ren F.Z., Liu G.S., Zhang L., Niu G.Y. Study on Chemical constituents of Hedyotis diffusa. Chin. Pharm. J. 2005;40:502–504. [Google Scholar]
- 36.Kang X.D., Li X., Mao Y., Zhao C.C., Li N., Meng D.L. Chemical constituents of Hedyotis diffusa Willd. J. Shenyang Pharm. Univ. 2007;24:479–481. [Google Scholar]
- 37.Liu Y.Q., Ying W.J., Liu Y., Feng Y.N., Lv Q.T. Summarization on the chemical constituents of Oldenlandia Diffusa Willd. Shandong J. Tradit. Chin. Med. 2014;33:709–712. [Google Scholar]
- 38.Shi Y., Wang C.H., Gong X.G. Apoptosis-inducing effects of two anthraquinones from Hedyotis diffusa Wild. Biol. Pharm. Bull. 2008;31:1075–1078. doi: 10.1248/bpb.31.1075. [DOI] [PubMed] [Google Scholar]
- 39.Yu L., Li J.M., Jiang Z., Guo X.J. A new anthraquinone from Hedyotis diffusa. Chin. J. Med. Chem. 2008;18:298–300. [Google Scholar]
- 40.Tai D.F., Lin Y.M., Chen F.C. Component of Hedyotis diffusa willd. Chemistry. 1979;3:60–61. [Google Scholar]
- 41.Huang W.H., Li Y.B., Jiang J.Q. Chemical constituents from Hedyotis diffusa. Chin. J. Chin. Mater. Med. 2008;33:524–526. [PubMed] [Google Scholar]
- 42.Kang X.D., Li X., Mao Y. A new anthraquinone from Hedyotis diffusa Willd. Chin. J. Chin. Mater. Med. 2006;16:368–370. [Google Scholar]
- 43.Zhou Y., Gao W.Y., Wang Y., Liu X.J. Studies on constituents of Oldenlandia diffusa. Chin. Tradit. Herb. Drug. 2007;38:55–57. [Google Scholar]
- 44.Lv H.C., He J. A study on chemical constituents of Oldenlandia diffusa (Willd) Roxb. Nat. Prod. Res. Dev. 1996;8:34–37. [Google Scholar]
- 45.Ruehle P.H., Browne C.E., Vickery E.H., Beller N.R., Eisenbraun E.J., Loghry R.A., Van der Helm D. Synthesis and antifertility activity of 3,9-dihydroxy-5,6,6a alpha,6b beta,11,12,12a beta,12b alpha-octahydrodibenzo[a,g]biphenylene, a structural relative of diethylstilbestrol. J. Med. Chem. 1980;23:1410–1414. doi: 10.1021/jm00186a023. [DOI] [PubMed] [Google Scholar]
- 46.Huang W., Li Y., Jiang J. Chemical constituents from Hedyotis diffusa. Chin. J. Chin. Mater. Med. 2009;34:712–714. [PubMed] [Google Scholar]
- 47.Tan N.H., Wang S.M., Yang Y.B., Tian F. Anticancer activity and principles of Hedyotis diffusa. Nat. Prod. Res. Dev. 2002;14:33–36. [Google Scholar]
- 48.Liu Z.G., Luo J.B., Chen F.L. The Pilot Study of Volatile Compounds in Hedyotis diffusa from different sources. Tradit. Chin. Drug Res. Clin. Pharm. 2005;16:132–134. [Google Scholar]
- 49.Yang S., Yang W.W., Hu J.F., Lv Q.F., Rong R., Jiang H.Q., Gong L.L. GC-MS combined with Kovats Index analysis for volatile compounds in Hedyoti diffusae. Chin. J. Exp. Tradit. Med. Form. 2012;18:93–95. [Google Scholar]
- 50.Ma H., Cheng Y.L., Zhang J.J., Cao G.S., Yang P.M. Effect of preliminary immune activity and structural identification of a polysaccharide extracted from Oldenlandia diffusa. Chin. J. Exp.Tradit. Med. Form. 2014;20:37–40. [Google Scholar]
- 51.Hu E., Wang D.G., Chen J.Y., Tao X.L. Novel cyclotides from Hedyotis diffusa induce apoptosis and inhibit proliferation and migration of prostate cancer cells. Int. J. Clin. Exp. Med. 2015;8:4059–4065. [PMC free article] [PubMed] [Google Scholar]
- 52.Li M., Jiang R.W., Hon P.M., Cheng L., Li L.L., Zhou J.R., Shaw P.C., But P.P. Authentication of the anti-tumor herb Baihuasheshecao with bioactive marker compounds and molecular sequences. Food Chem. 2010;119:1239–1245. doi: 10.1016/j.foodchem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X., Cheng W.M., Yao X.M., Guo X.J. Qualitative analysis of the chemical constituents in Hedyotis diffusa by HPLC-TOF-MS. Nat. Prod. Res. 2012;26:167–172. doi: 10.1080/14786419.2010.537275. [DOI] [PubMed] [Google Scholar]
- 54.Qi J.Y., Fan W.P., Ju P.P. Extraction and deputation of polysaccharide from Hedyotis diffusa willd. Acta Universitatis Medicinalis Nanjing. 2001;21:558. [Google Scholar]
- 55.Lin M.H., Lin J.M., Wei L.H., Xu W., Hong Z.F., Cai Q.Y., Peng J., Zhu D.Z. Hedyotis diffusa Willd extract inhibits HT-29 cell proliferation via cell cycle arrest. Exp. Ther. Med. 2012;4:307–310. doi: 10.3892/etm.2012.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin J.M., Chen Y.Q., Wei L.H., Chen X.Z., Xu W., Hong Z.F. Hedyotis diffusa Willd extract induces apoptosis via activation of the mitochondrion-dependent pathway in human colon carcinoma cells. Int. J. Oncol. 2010;37:1331–1338. doi: 10.3892/ijo_00000785. [DOI] [PubMed] [Google Scholar]
- 57.Lin J.M., Wei L.H., Xu W., Hong Z.F., Liu X.X., Peng J. Effect of Hedyotis diffusa Willd extract on tumor angiogenesis. Mol. Med. Rep. 2011;4:1283–1288. doi: 10.3892/mmr.2011.577. [DOI] [PubMed] [Google Scholar]
- 58.Lin J.M., Wei L.H., Shen A.L., Cai Q.Y., Xu W., Li H. Hedyotis diffusa Willd extract suppresses Sonic hedgehog signaling leading to the inhibition of colorectal cancer angiogenesis. Int. J. Oncol. 2013;42:651–656. doi: 10.1016/j.ijrobp.2013.06.1723. [DOI] [PubMed] [Google Scholar]
- 59.Cai Q.Y., Lin J.M., Wei L.H., Zhang L., Wang L.L., Zhan Y.Z. Hedyotis diffusa Willd inhibits colorectal cancer growth In Vivo via inhibition of STAT3 signaling pathway. Int. J. Mol. Sci. 2012;13:6117–6128. doi: 10.3390/ijms13056117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu Z.P., Jin C.G., Li J., Chen X.Q., Yao Q., Zhu Q.S. Inhibition of colon cancer cells by ethanol extract of Oldenlandia diffusa. J. Kunming Med. Univ. 2013;34:31–34. [Google Scholar]
- 61.Li Q.Y., Wang X.F., Shen A.L., Zhang Y.C., Chen Y.Q., Thomas J.S., Lin J.M., Peng J. Hedyotis diffusa Willd overcomes 5-fluorouracil resistance in human colorectal cancer HCT-8/5-FU cells by downregulating the expression of P-glycoprotein and ATP-binding casette subfamily G member 2. Exp. Ther. Med. 2015;10:1845–1850. doi: 10.3892/etm.2015.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ganbolda M., Barkera J., Ma R., Jones L., Carew M. Cytotoxicity and bioavailability studies on a decoction of Oldenlandia diffusa and its fractions separated by HPLC. J. Ethnopharmacol. 2010;131:396–403. doi: 10.1016/j.jep.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 63.Zhu D.C., Pan R.B., Wang Q. Research on the mechanisms of inhibiting effects of the aqueous extract of Hedyotis diffusa Willd on CEM cells. Lishizhen Med. Mater. Med. Res. 2014;25:827–829. [Google Scholar]
- 64.Lin C.C., Kuo C.L., Lee M.H., Hsu S.C., Huang A.C., Tang N.Y., Lin J.P., Yang J.S., Lu C.C., Chiang J.H. Extract of Hedyotis diffusa Willd influences murine leukemia WEHI-3 cells in vivo as well as promoting T- and B-cell proliferation in leukemic mice. In Vivo. 2011;25:633–640. [PubMed] [Google Scholar]
- 65.Chen X.H., Gao R.L., Qian X.D., Wang X., Tan P.L., Yin L.M., Zhou Y.H. Inhibition effect of Hedyotis diffusa wild injection on HL-60 cells and its mechanism. J. Exp. Hematol. 2008;16:1035–1038. [PubMed] [Google Scholar]
- 66.Kuo Y.J., Yang J.S., Lu C.C., Chiang S.Y., Lin J.G., Chung J.G. Ethanol extract of Hedyotis diffusa willd upregulates G0/G1 phase arrest and induces apoptosis in human leukemia cells by modulating caspase cascade signaling and altering associated genes expression was assayed by cDNA microarray. Environ. Toxicol. 2015;30:1162–1177. doi: 10.1002/tox.21989. [DOI] [PubMed] [Google Scholar]
- 67.Wang N., Li D.Y., Niu H.Y., Zhang Y., He P., Wang J.H. 2-Hydroxy-3-methylanthraquinone from Hedyotis diffusa Willd induces apoptosis in human leukemic U937 cells through modulation of MAPK pathways. Arch. Pharm. Res. 2013;36:752–758. doi: 10.1007/s12272-013-0096-4. [DOI] [PubMed] [Google Scholar]
- 68.Wang J.H., Shu L.H., Yang L.L., Zhang M., Zhang M., He P. 2-Hydroxy-3-methylanthraquinone from Hedyotis diffusa WILLD Induces apoptosis via alteration of Fas/FasL and activation of caspase-8 in human Leukemic THP-1 Cells. Arch. Med. Res. 2011;42:577–583. doi: 10.1016/j.arcmed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Li J., Sun J., Song J. Experimental research on effect of Hedyotis diffusa Willd on blood metastasis in H22 mice. Lishizhen Med. Mater. Med. Res. 2012;23:2434–2435. [Google Scholar]
- 70.Chen X.Z., Cao Z.Y., Chen T.S., Zhang Y.Q., Liu Z.Z., Su Y.T. Water extract of Hedyotis Diffusa Willd suppresses proliferation of human HepG2 cells and potentiates the anticancer efficacy of low-dose 5-fluorouracil by inhibiting the CDK2-E2F1 pathway. Oncol. Rep. 2012;28:742–748. doi: 10.3892/or.2012.1834. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y.B., Zhu J., Xiao J.X., Guo Y.H., Liao Z.J., Xu R. Effect and mechanism of total flavones of Oldenlendia diffusa willd on epithelial-mesenchymal transition of cell line MHCC97-H induced by TGF-β1. J. Xi’an Jiaotong Univ. (Med. Sci.) 2016;37:279–282. [Google Scholar]
- 72.Li Y.L., Zhang J., Min D., Hongyan Z., Lin N., Li Q.S. Anticancer effects of 1,3-dihydroxy-2-methyl anthraquinone and the ethyl acetate fraction of Hedyotis diffusa willd against HepG2 carcinoma cells mediated via apoptosis. PLoS ONE. 2016 doi: 10.1371/journal.pone.0151502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dong Q., Ling B., Gao B., Maley J., Sammynaiken R., Yang J. Hedyotis diffusa water extract diminished the cytotoxic effects of chemotherapy drugs against human breast cancer MCF7 cells. Nat. Prod. Commun. 2014;9:699–700. [PubMed] [Google Scholar]
- 74.Liu Z., Liu M., Liu M., Li J.C. Methyl anthraquinone from Hedyotis diffusa WILLD induces Ca2+-mediated apoptosis in human breast cancer cells. Toxicol. In Vitro. 2010;24:142–147. doi: 10.1016/j.tiv.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Lin S.Y., Shen C.Y., Jiang J.P., Wu L.Q., Dai T.Y., Qian W.B. Meng HT Apoptosis of multiple myeloid cells induced by polysaccharides extracts from Hedyotis diffusa and its mechanism. Chin. J. Hematol. 2013;34:337–340. doi: 10.3760/cma.j.issn.0253-2727.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 76.Zhang X., Ye B.D., Lin S.Y. Effects of Hedyotis diffusa Willd injection on the proliferation of RPMI 8226 cells. Chin. J. Integr. Tradit. West. Med. 2012;32:1658–1662. [PubMed] [Google Scholar]
- 77.Zhao H.R., Li R., Lin Y.N., Cheng N.L. Influence of extraction on process of Hedyotis diffusa on anti-tumor activity. J. Chin. Pharm. Univ. 2002;33:510–513. [Google Scholar]
- 78.Huang Y.L., Tang Y.J., Wang J.L., Xie K.G., Huang K. Effect of Hedyotic diffusa willd injection on osteosarcoma MG-63 cells bax gene expression. Chongqing Med. 2014;43:4708–4710. [Google Scholar]
- 79.Xie K.G., Tang Y.J., Huang Y.L., Huang K., Lu L., Lin J.J., Lu X.Z. Effect of different concentration and action time spreading Hedyotis herb injection induced MG-63 cells apoptosis. Med. Innov. China. 2016;13:255–263. [Google Scholar]
- 80.Pu F., Chen F., Lin S., Chen S., Zhang Z., Wang B., Shao Z. The synergistic anticancer effect of cisplatin combined with Oldenlandia diffusa in osteosarcoma MG-63 cell line in vitro. OncoTargets Ther. 2016;9:255–263. doi: 10.2147/OTT.S90707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yue G.G.L., Lee J.K.M., Kwok H.F., Cheng L., Wong E.C.W., Jiang L., Yu H., Leung H.W., Wong Y.L., Leung P.C., et al. Novel PI3K/AKT targeting anti-angiogenic activities of 4-vinylphenol, a new therapeutic potential of a well-known styrene metabolite. Sci. Rep. 2015;5 doi: 10.1038/srep11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuo Y.J., Lin J.P., Hsiao Y.T., Chou G.L., Tsai Y.H., Chiang S.Y., Lin J.G., Chuang J.G. Ethanol extract of Hedyotis diffusa Willd affects immune responses in vivo. In Vivo. 2015;29:453–460. [PubMed] [Google Scholar]
- 83.Wang Y.L., Zhang Y., Fang M., Li Q.J., Jiang Q., Ming L. Immunomdulatory effects of total flavones of Oldenlandia diffusa willd. Chin. Pharmacol. Bull. 2005;21:444–447. [Google Scholar]
- 84.Yang X.Z., Hao Z.Y., Zhu Y.C., Dong Y. Effects of different solvents and extraction methods on antioxidant activity of Hedyotis diffusa Extract. Guizhou Agric. Sci. 2014;42:43–45. [Google Scholar]
- 85.Yu X., Du Z.J., Chen Y.J., Huang T.Q. Study on antioxidant effect from Oldenlandia diffusa Willd. Food Ferment. Ind. 2002;28:10–13. [Google Scholar]
- 86.Gao X., Li C., Tang Y.L., Zhang H., Chan S.W. Effect of Hedyotis diffusa water extract on protecting human hepatocyte cells (LO2) from H2O2-induced cytotoxicity. Pharm. Biol. 2015 doi: 10.3109/13880209.2015.1056310. [DOI] [PubMed] [Google Scholar]
- 87.Ye J.H., Liu M.H., Zhang X.L., He J.Y. Chemical profiles and protective effect of Hedyotis diffusa Willd in lipopolysaccharide-induced renal inflammation mice. Int. J. Mol. Sci. 2015;16:27252–27269. doi: 10.3390/ijms161126021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Y., Lin Y., Li Y., Li C. Total flavonoids of Hedyotis diffusa willd inhibit inflammatory responses in LPS-activated macrophages via suppression of the NF-κB and MAPK signaling pathways. Exp. Ther. Med. 2016;11:1116–1122. doi: 10.3892/etm.2015.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu P.K., Tai W.C.S., Liang Z.T., Zhao Z.Z., Hsiao W.L.W. Oleanolic acid isolated from Oldenlandia diffusa exhibits a unique growth inhibitory effect against ras-transformed fibroblasts. Life Sci. 2009;85:113–121. doi: 10.1016/j.lfs.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 90.Liu L.S., Liu M.H., He J.Y. Hypericum japonicum Thunb. ex murray: Phytochemistry, pharmacology, quality Ccontrol and pharmacokinetics of an important herbal medicine. Molecules. 2014;19:10733–10754. doi: 10.3390/molecules190810733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang T., Yang Y.H., Yang J.Y., Chen B.M., Chen Y.X., Yu S.Y., Duan J.P., Ouyang H.T., Cheng J.P. Finger print of Hedyotis diffusa Willd by LC-MS. Chin. Med. Herb. 2007;4:21–23. [Google Scholar]
- 92.Liang Z.T., Jiang Z.H., Ho H., Zhao Z.Z. Comparative analysis of Oldenlandia diffusa and its substitues by high performance liquid chromatographic fingerprint and mass spectrometric analysis. Planta Med. 2007;73:1502–1508. doi: 10.1055/s-2007-990259. [DOI] [PubMed] [Google Scholar]
- 93.Fan C.Q., Li R.R., Jin Y., Li H.X., Feng X.F. Quality standard of Hedyotis diffusae. Chin. J. Exp. Tradit. Med. Form. 2014;20:98–101. [Google Scholar]
- 94.Lu W.B. Quantitative determination of oleanolic acid in Oldenlandia diffusa (Willd) Roxb. by TLC-scaning. Lishizhen Med. Mater. Med. Res. 2001;12:961–962. [Google Scholar]
- 95.Cao G.S., Yang P.M., Wang X.F., Li H., Gao P. Determination of isoseutellarein in different parts of Hedyotis diffusa in different harvest time by HPLC. Chin. J. Exp. Tradit. Med. Form. 2014;20:49–51. [Google Scholar]
- 96.Zhang C.H., Guo X.J., Xue X.F., Yang L., Ran G.M., Li F.M. Determination of p-comnaric acid in Baihua Sheshecao injection by HPLC. Chin. Pharm. J. 2004;39:854–855. [Google Scholar]
- 97.Zhang C.H., Guo X.J., Bao L.D., Qin F., Li M. Determination of p-coumaric acid in Hedyotis diffusae Willd. From different source by reversed-phase high-performance liquid chromatography. Chin. J. Chromatogr. 2005;23:180–182. [PubMed] [Google Scholar]
- 98.Ma L., Li J.M., Chen Y.Q., Li Y., Guo X.J. Determination of 3,4-dihydroxy Methyl Benzoate in Hedyotis diffusa Willd by HPLC. Lishizhen Med. Mater. Med. Res. 2009;20:528–529. [Google Scholar]
- 99.Ling Y.Z. Separation and content determination of polysassharides in Hedyotis diffusa Willd. Biotechnology. 2005;15:48–50. [Google Scholar]
- 100.Yang Y.C., Wei M.C., Chiu H.F., Huang T.C. Development and validation of a modified ultrasound-assisted extraction method and a HPLC method for the quantitative determination of two triterpenic acids in Hedyotis diffusa. Nat. Prod. Commun. 2013;8:1683–1686. [PubMed] [Google Scholar]
- 101.Zhang Y., Tan X.H., Cui X.B., Jiang G.B., Zhu Y.L. Quantitative determination of ursolic acid and oleanolic acid in Baihuasheshecao (Herba Hedyotis diffusae) produced from different places by HPLC. J. Beijing Univ. Tradit. Chin. Med. 2010;33:274–276. [Google Scholar]
- 102.Zhou S.Q., Leng G.H. Mensunation of content of oleanolic acid and maloic acid of Hedyotis diffuse Wild by HPLC. J. Anhui Agric. Sci. 2006;34:1785–1787. [Google Scholar]
- 103.Yang Y.H., Chen Y.X. Determination of oleanolic acid and ursolic acid in Oldenlandia diffusa (Willd) Roxb. by LC/MS. Herb. Med. 2008;27:589–591. [Google Scholar]
- 104.Liu Y.Q., Ying W.J., Zuo L., Man Q.Q., Lv H.T., Jiang H.Q., Gong L.L. Determination of two anthraquinones in Hedyotis diffusa by HPLC. Chin. J. Exp. Tradit. Med. Form. 2014;20:42–44. [Google Scholar]
- 105.Liang Z.T., Jiang Z.H., Leung K.S.Y., Zhao Z.Z. Determination of iridoid glucosides for quality assessment of herba oldenlandiae by high-performance liquid chromatography. Chem. Phann. Bull. 2006;54:1131–1137. doi: 10.1248/cpb.54.1131. [DOI] [PubMed] [Google Scholar]
- 106.Zhang H.F., Zhang H.Y., Li Y., Guo X.J. Simultaneous determination of four components in Hedyotis diffusa oral solution by reversed-phase HPLC. Chin. J. Chin. Mater. Med. 2008;33:2329–2331. [PubMed] [Google Scholar]
- 107.Cheung H.Y., Cheung S.H., Law M.L., Lai W.P. Simultaneous determination of key bioactive components in Hedyotis diffusa by capillary electrophoresis. J. Chromatogr. B. 2006;834:195–198. doi: 10.1016/j.jchromb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 108.Yang P.M., Cao G.S., Li F., Gao P. Contents of five iridoids in Ooldenlandia diffusa (Wind) Roxb. based on HPLC-DAD. Chin. Hosp. Pharm. J. 2015;35:9–12. [Google Scholar]
- 109.Cao G.S., Yang P.M., Li F., Li J. Determination of six active flavonoids in Oldenlandia diffusa based on HPLC-DAD. Chin. J. Exp. Tradit. Med. Form. 2014;20:52–55. [Google Scholar]
- 110.Zhai X., Lv Y. Simultaneous determination of 7 active components in Hedyotis diffuse by HPLC. Chin. Pharm. 2016;19:70–72. [Google Scholar]
- 111.Liu K., Yan L.Q., Yao G.C., Guo X.J. Estimation of p-coumaric acid as metabolite of E-6-O-p-coumaroyl scandoside methyl ester in rat plasma by HPLC and its application to a pharmacokinetic study. J. Chromatogr. B. 2006;831:303–306. doi: 10.1016/j.jchromb.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 112.An L.Z., Wang Y.F., Yu Q.R. HPLC-TOF-MS analysis of metabolites of Oldenlandia diffusa effective extracts in rats. Chin. J. Chin. Mater. Med. 2011;36:1301–1304. [PubMed] [Google Scholar]