Abstract
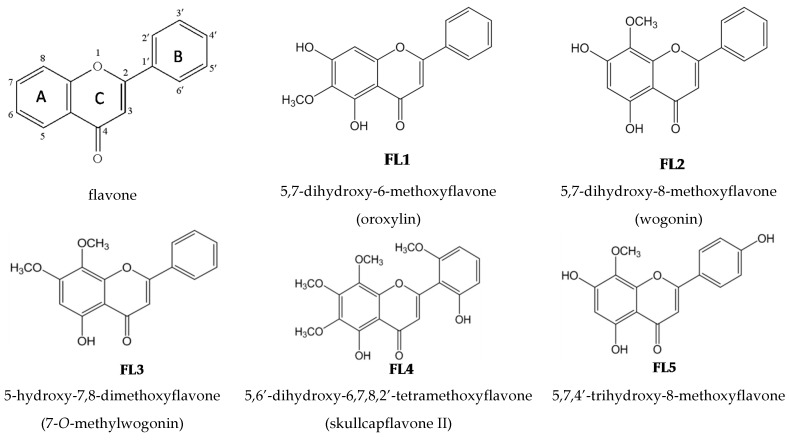
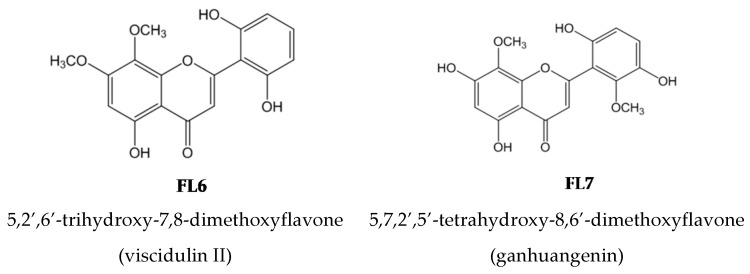
Scutellariae radix, the root of Scutellaria baicalensis, has long been applied in traditional formulations and modern herbal medications. Propionibacterium acnes (P. acnes) in follicles can trigger inflammation and lead to the symptom of inflammatory acnes vulgaris. This study was aimed at evaluating the effect of Scutellariae radix extract and purified components isolated from it on inflammation induced by P. acnes in vitro and in vivo. The results showed the ethyl acetate (EA) soluble fraction from the partition of crude ethanolic extract from Scutellariae radix inhibited P. acnes-induced interleukin IL-8 and IL-1β production in human monocytic THP-1 cells. Seven flavones were isolated from the EA fraction by repeated chromatographies, and identified as 5,7-dihydroxy-6-methoxyflavone (FL1, oroxylin), 5,7-dihydroxy-8-methoxyflavone (FL2, wogonin), 5-hydroxy-7,8-dimethoxyflavone (FL3, 7-O-methylwogonin), 5,6′-dihydroxy-6,7,8,2′-tetramethoxy flavone (FL4, skullcapflavone II), 5,7,4′-trihydroxy-8-methoxyflavone (FL5), 5,2′,6′-trihydroxy-7,8-dimethoxyflavone (FL6, viscidulin II), and 5,7,2′,5′-tetrahydroxy-8,6′-dimethoxyflavone (FL7, ganhuangenin). They all significantly suppressed P. acnes-induced IL-8 and IL-1β production in THP-1 cells, and FL2 exerted the strongest effect with half maximal inhibition (IC50) values of 8.7 and 4.9 μM, respectively. Concomitant intradermal injection of each of the seven flavones (20 μg) with P. acnes effectively attenuated P. acnes-induced ear swelling, and decreased the production of IL-6 and tumor necrosis factor-α in ear homogenates. Our results suggested that all the seven flavones can be potential therapeutic agents against P. acnes-induced skin inflammation.
Keywords: Chinese herb, Scutellariae radix, flavone, anti-inflammation, Propionibacterium acnes
1. Introduction
Acne vulgaris is one of the most common skin diseases. The pathogenesis is complex and incompletely understood, but inflammation is believed to be a key component [1]. Propionibacterium acnes (P. acnes), a Gram-positive anaerobic bacterium species, may play a major role in the initiation of the inflammatory reaction by stimulating the secretion of interleukin (IL)-18, tumor necrosis factor TNFα, IL-8, and IL-12 by monocytic cells, and eventually the development of inflammatory lesions [2,3]. IL-8 is the major inflammatory mediator and a strong chemotactic factor for neutrophils, basophils, and T cells. IL-8 has been implicated in mounting an inflammatory response in acne lesions [4]. In addition, the high levels of IL-1β were observed in human acne lesion, in mouse skin lesion induced by P. acnes, and in P. acnes-exposed human monocytes [5].
Scutellariae radix, the root of Scutellaria baicalensis, has been used as traditional Chinese medicine to treat allergic and inflammatory diseases in Japan and China. It is also often used to treat cardiovascular diseases, respiratory, and gastrointestinal infections [6]. Flavonoids, including baicalin, baicalein, wogonin, and oroxylin-A, have been identified in Scutellariae radix [7]. The extracts and the isolated compounds of Scutellariae radix are pharmacologically-active and show great potential in the treatment of inflammation, cancers, and virus-related diseases [8].
However, the bioactive components of Scutellariae radix have not yet been completely investigated. This study is aimed at exploring the suppressive effects of seven flavones isolated from Scutellaria radix on P. acnes-induced inflammation in vitro and in vivo, and their anti-acne potential.
2. Results
2.1. Effects of Scutellariae Radix Extracts on P. acnes-Induced IL-1β and IL-8 Production in THP-Cells
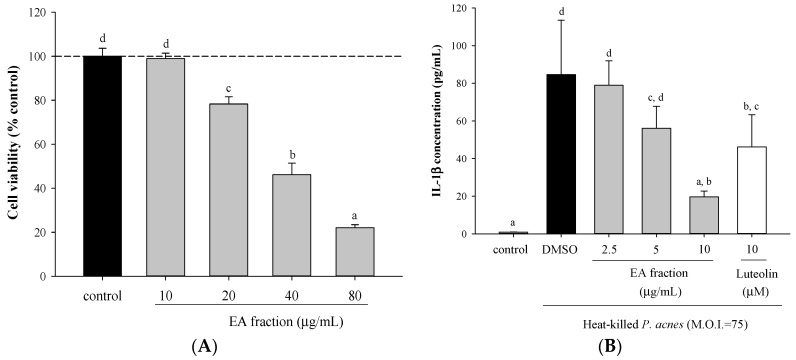
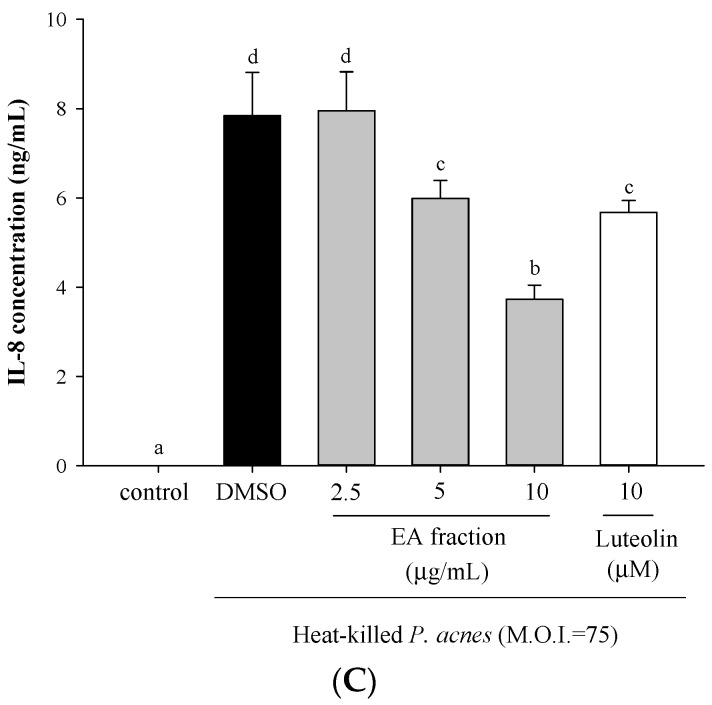
Ethylacetate (EA) fraction was cytotoxic to THP-1 cells when the concentrations applied were higher than 20 μg/mL (Figure 1A). So, the doses of 2.5, 5, and 10 μg/mL were used for the subsequent in vitro experiments. Treatment of THP-1 cells with heat-killed P. acnes evoked the production of IL-1β and IL-8. EA fraction of Scutellariae radix significantly decreased P. acnes-induced IL-1β and IL-8 production in a dose-dependent manner (Figure 1B,C).
Figure 1.
Effect of ethyl acetate (EA) fraction of ethanolic extract from Scutellariae radix on viability of monocytic THP-1 cells, and pro-inflammatory cytokine productions by P. acnes-stimulated THP-1 cells. Cell viability (A) was determined by MTT assay in cells incubated with vehicle control alone, or the indicated concentrations of EA fraction for 24 h. IL-1β (B) and IL-8 (C) were determined in cells co-incubated with P. acnes (M.O.I. = 75) and the indicated concentrations of samples for 24 h. DMSO (0.1%) was a vehicle control, luteolin was a reference control. A control experiment without P. acnes treatment was conducted in parallel. Each column shows the mean ± SD. Values with the same letter are not significantly different as determined by Duncan’s multiple range tests.
The butanol fraction also inhibited IL-1β and IL-8 production by P. acnes-stimulated THP-1 cells, but less effectively than the EA fraction did. The butanol fraction had half maximal inhibition (IC50) values of 80.5 and 135.5 μg/mL for the inhibition of IL-1β and IL-8 secretion, respectively, while the EA fraction had the respective IC50 values of 6.6 and 5.6 μg/mL. Therefore, EA fraction was subjected to chromatography on silica gel and further purification by semi-preparative HPLC. Seven known flavones (FL1-7) were found.
2.2. Effects of the Seven Flavones Isolated from EA Fraction of Scutellariae Radix on P. acnes-Induced IL-8 and IL-1β Production in Human Monocytic THP-1 Cells
Chemical names, common names, and chemical structures of the seven known flavones isolated from EA fraction of Scutellariae radix are shown in Figure 2.
Figure 2.
Structures of flavones isolated from Scutellariae radix.
The concentrations without apparent cytotoxicity toward THP-1 cells, assayed by MTT, were used for the subsequent experiments (Table 1). Seven flavones, at various concentrations, significantly reduced IL-8 and IL-1β levels (Table 1). The potency of the flavones was expressed as IC50 value. IC50 values of FL4 for IL-8, and FL1 and FL6 for IL1β could not be precisely determined because the degree of inhibition provided by the highest test concentration was less than 50%. The rank order of potency of these flavones for IL-8 inhibition was FL2 > FL5 > FL1 > (FL4) FL6 > FL3 > FL7; whereas for IL-1β inhibition was FL2 > FL4 > FL5 > (FL1) > FL3 > (FL6) FL7. Therefore, FL2 had the most potent inhibitory effect on P. acnes-induced IL-1β and IL-8 production in vitro.
Table 1.
Effects of flavones isolated from Scutellariae radix on the cell viability and P. acnes-induced cytokine production of THP-1 cells.
| Compound | Flavone (μM) | Cell Viability (% of Control) | IL-8 Level (ng/mL) | IC50 for IL-8 (μM) | IL-1β Level (ng/mL) | IC50 for IL-1β (μM) |
|---|---|---|---|---|---|---|
| Control | 0 | 93.2 ± 4.5 | 0.2 ± 0.2 ** | 0.008 ± 0.007 ** | ||
| DMSO | 0 | 100.0 ± 2.5 | 52.4 ± 5.1 | 2.4 ± 0.2 | ||
| FL1 | 5 | 101.8 ± 7.8 | 39.2 ± 3.8 * | 13.1 | 1.6 ± 0.2 ** | NA (>15) |
| 10 | 93.3 ± 4.0 | 29.3 ± 5.4 ** | 1.5 ± 0.2 ** | |||
| 15 | 97.2 ± 3.0 | 24.1 ± 2.5 ** | 1.2 ± 0.1 ** | |||
| 30 | 64.5 ± 3.0 | ND | ND | |||
| FL2 | 5 | 98.6 ± 12.1 | 55.8 ± 8.0 | 8.7 | 1.2 ± 0.1 ** | 4.9 |
| 10 | 112.0 ± 13.0 | 18.9 ± 3.5 ** | 0.6 ± 0.1 ** | |||
| 15 | 94.7 ± 2.1 | 5.9 ± 1.3 ** | 0.3 ± 0.1 ** | |||
| 30 | 88.4 ± 4.0 | ND | ND | |||
| FL3 | 30 | 99.9 ± 7.1 | 39.1 ± 12.0 * | 55.2 | 1.4 ± 0.1 ** | 72.8 |
| 60 | 101.0 ± 7.8 | 24.7 ± 7.4 ** | 1.4 ± 0.1 ** | |||
| 120 | 103.6 ± 4.5 | 15.8 ± 3.0 ** | 1.0 ± 0.1 ** | |||
| 240 | 65.6 ± 2.4 | ND | ND | |||
| FL4 | 5 | 102.8 ± 2.4 | 56.4 ± 1.9 | NA (>15) | 1.6 ± 0.1 ** | 9.1 |
| 10 | 98.8 ± 3.9 | 39.3 ± 1.6 * | 1.1 ± 0.1 ** | |||
| 15 | 99.6 ± 0.5 | 29.6 ± 2.2 ** | 1.0 ± 0.1 ** | |||
| 30 | 87.1 ± 1.8 | ND | ND | |||
| FL5 | 5 | 103.6 ± 2.0 | 67.7 ± 10.1 ** | 10.2 | 2.0 ± 0.1 * | 11.3 |
| 10 | 100.1 ± 2.0 | 32.4 ± 2.5 ** | 1.3 ± 0.2 ** | |||
| 15 | 100.8 ± 2.5 | 13.5 ± 0.6 ** | 0.8 ± 0.1 ** | |||
| 30 | 78.2 ± 2.8 | ND | ND | |||
| FL6 | 15 | 104.3 ± 5.8 | 41.5 ± 8.2 * | 26.1 | 2.0 ± 0.2 ** | NA (>60) |
| 30 | 101.3 ± 4.6 | 23.8 ± 1.5 ** | 1.6 ± 0.1 ** | |||
| 60 | 100.9 ± 4.2 | 20.3 ± 3.4 ** | 1.4 ± 0.1 ** | |||
| 120 | 87.5 ± 3.1 | ND | ND | |||
| FL7 | 60 | 106.7 ± 6.0 | 33.5 ± 2.3 ** | 124.3 | 1.7 ± 0.1 ** | 84.3 |
| 90 | 110.9 ± 1.1 | 28.6 ± 3.0 ** | 1.0 ± 0.1 ** | |||
| 120 | 120.7 ± 1.2 | 26.3 ± 1.2 ** | 0.8 ± 0.1 ** | |||
| 240 | 89.3 ± 3.2 | ND | ND |
IC50, concentrations that provide 50% inhibition; ND, not-determined; NA, not applicable. Control, cells cultured with DMSO (0.1%) alone for 24 h. DMSO, cells cultured with DMSO (as vehicle) and P. acnes (M.O.I. = 75) for 24 h. FL, cells cultured with the indicated concentrations of flavones and P. acnes (M.O.I. = 75) for 24 h. Cytokine levels were measured using ELISA kits. * p < 0.05, ** p < 0.001, as compared with DMSO.
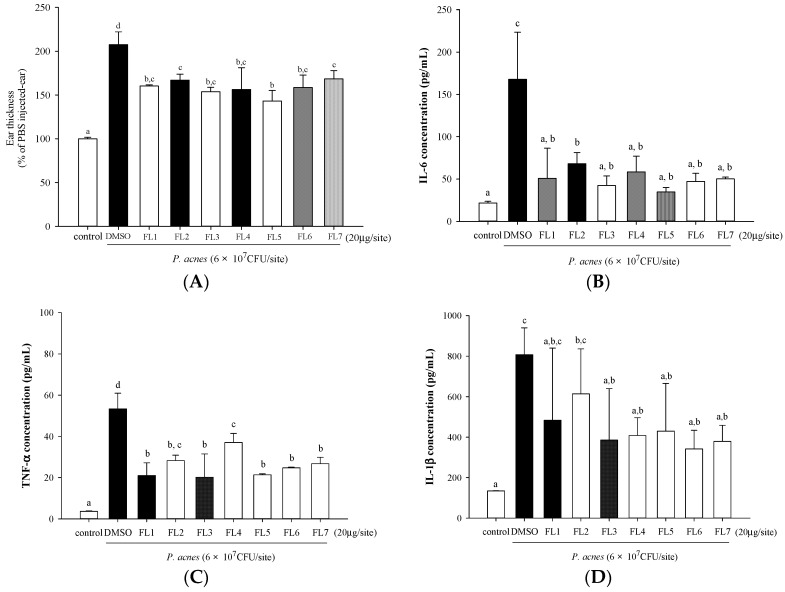
2.3. Effects of the Seven Flavones Isolated from Scutellariae Radix on P. acnes-Induced Ear Edema and Cytokine Production in Mouse Ear Homogenates
Mouse ear edema was induced by intradermal injection of P. acnes to mouse ears. Concomitant injection of each of the seven flavones with P. acnes afforded suppression of P. acnes-induced edema as measured by ear thickness, and all the seven flavones had almost equal effects (Figure 3A). All flavones also significantly inhibited IL-6 (Figure 3B) and TNF-α (Figure 3C) production in P. acnes-treated mouse ears. For the suppression of IL-1β, except FL1 and FL2, all the other five flavones had significant effect (Figure 3D). Our data indicated that these flavones had protective effects against P. acnes-induced skin inflammation.
Figure 3.
In vivo inhibitory effects of seven flavones isolated from Scutellariae radix on P. acnes-induced skin swelling and pro-inflammatory cytokine levels of mice ears. ICR mice were intradermally injected with PBS (as control), DMSO + P. acnes (as vehicle control), or flavones + P. acnes. Mouse ear thickness (A); and IL-6 (B); TNF-α (C); and IL-1β (D) production in mouse ear homogenates were determined. Values with the same letter are not significantly different as determined by Duncan’s multiple range tests.
3. Discussion
The pharmacological effects of the water and organic solvent extracts of Scutellariae radix have been extensively studied [8]. There have been over 40 flavonoids isolated from Scutellariae radix, with flavones and their glycosides being the most abundant [9]. Seven flavones isolated in this study existed in the forms of aglycones. FL1 (oroxylin A) and FL2 (wogonin) are the major flavonoids identified in Scutellariae radix [10]. FL3 to 7 are minor components [8], considerably less attention has been paid to these flavones.
The anti-inflammatory effects of FL1, 2, and 3 have been reported. Oroxylin A (FL1) inhibited LPS-induced iNOS and COX-2 gene expression by blocking NF-κB activation in vitro [11]. It also suppressed LPS-induced angiogenesis by down-regulation of toll-like receptor TLR-4 and the activity of mitogen-activated protein kinases (MAPK) [12]. Wogonin (FL2) had a very potent anti-inflammatory action in vivo on several animal models of inflammation, including carrageenan-induced paw edema, adjuvant-induced arthritis, 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin inflammation and arachidonic acid-induced ear inflammation by oral or topical administration [13,14,15]. 7-O-methylwogonin (FL3) significantly inhibited NO and PGE2 release in LPS-stimulated J774A.1 macrophages [16] and effectively suppressed TNF-α, NO and macrophage inflammatory protein (MIP)-2 levels in LPS/IFN-γ-Stimulated RAW 264.7 macrophages [17]. Fewer studies investigated the biological activities of FL4, 5, 6 and 7. Skullcapflavone II (FL4) inhibited ovalbumin (OVA)-induced airway inflammation by reduction of Th2 cytokines, and OVA-specific IgE levels [18]. 5,7,4′-Trihydroxy-8-methoxyflavone (FL5) exhibited antiviral activity [19]. Viscidulin II (FL6) inhibited activity of adenosine 3′,5′-cyclic monophosphate phosphodiesterase [20]. Ganhuangenin (FL7) alleviated the type I allergic reaction by inhibiting the release of histamine and LBT4 [21]. The in vitro and in vivo P. acnes-induced inflammation models utilized here have never been applied to investigate the effect of Scutellariae radix. Therefore, our results revealed a new function of the seven flavones isolated from Scutellariae radix.
To compare the in vitro inhibitory effects of seven flavones on P. acnes-induced IL-8 and IL-1β production, IC50 values were used. We found wogonin (FL2) was the most potent (Table 1). FL1, 2, 4, and 5 with IC50 values smaller than 20 μM for both IL-1β and IL-8 were more potent than FL3, 6, and 7 which had IC50 values larger than 50 μM. The two hydroxyl groups at C5 and C7 in FL1, 2, 5 might contribute to their high potency. FL7 also has hydroxylated C5 and C7, but showed the lowest potency probably because it has too many substituents in B ring. FL3, 4, 6 with a methoxylated C7, possessing only one hydroxyl group at C5 were assumed to have lower potency. However, FL4 was an exception, probably due to the extra methoxyl group at C6. The number of methoxyl group may also influence the potency. FL1, 2, 5 have only one methoxyl group, while the less potent flavones, FL3, 6, 7 have two methoxyl groups. So, fewer than two methoxyl groups might be better for potency. However, FL4 has four methoxyl groups, but still had high potency. Nikaido, et al. [20] investigated the structure-activity relationship of flavones from Scutellariae radix for cAMP phosphodiesterase inhibition, and found when the flavones had more than five substituents, those with more methoxyl groups were more effective [20]. FL4 and FL7 have six, and FL6 has five substituents. FL4 has four methoxyl groups, while FL6 and 7 has two. The higher potency of FL4 than FL6 and 7 appeared to be consistent with their hypothesis [20].
To our best knowledge, this is the first study to test the suppression effects of these flavones on P. acnes-induced inflammation in vivo. Due to the complicated cell populations involved in ear inflammation, and a fixed dose used for all the flavones, the effectiveness of each flavone cannot be completely compared as was ranked in vitro. However each of the seven flavones effectively reduced P. acnes-induced ear swelling and strongly suppressed the production of TNF-α and IL-6 in mice (Figure 3). IL-6, a pro-inflammatory and chemotactic factor [22], was measured in mice instead of IL-8 that we measured in human THP-1 cells because mice do not produce IL-8 [23].
Luteolin, a positive control in this study, is also a flavone, but without any methoxyl group. Our previous study found it inhibited P. acnes-induced pro-inflammatory cytokines expression by inactivating NF-κB through the suppression of mitogen-activated protein kinases (MAPK) phosphorylation [24]. Although the molecular mechanisms by which these seven flavones from Scutellariae radix modulate pro-inflammatory cytokine levels are not elucidated, our in vitro and in vivo results suggest each of the seven flavones has promising therapeutic potential against inflammatory acne vulgaris. Our results open a new aspect of the pharmacological role of these flavones.
4. Experimental Section
4.1. Isolation and Structural Elucidation
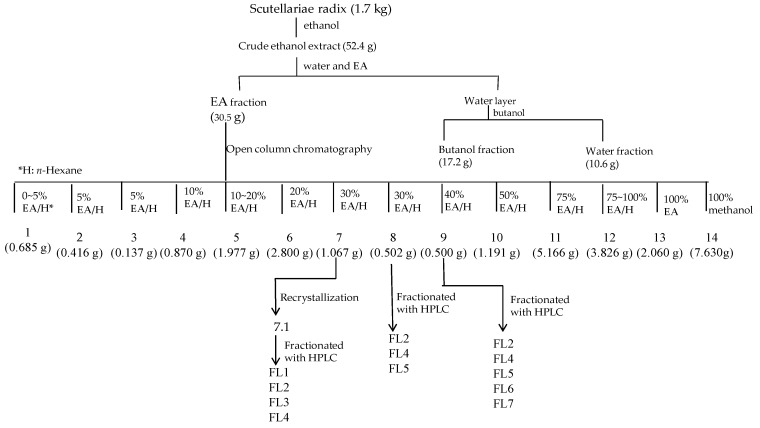
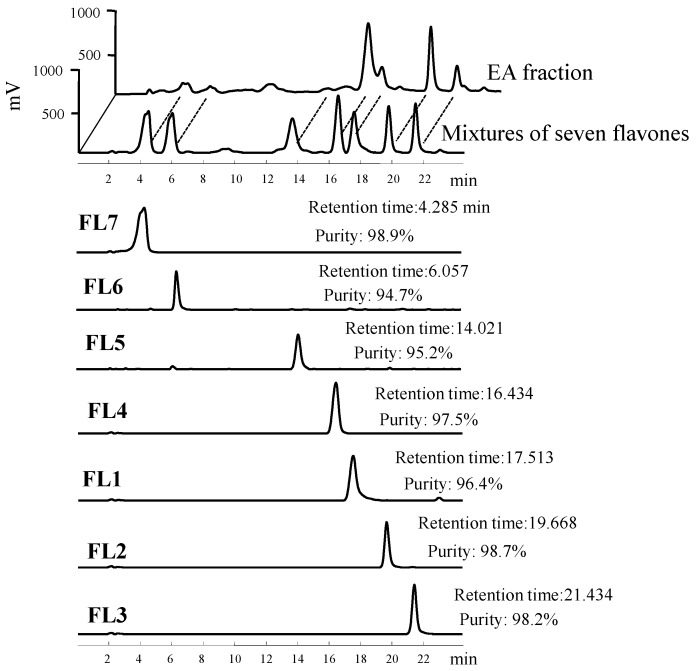
Scutellariae radix is a Chinese herb and commercially available. We bought it from Sun Ten Pharmaceutical Co. (Taichung, Taiwan). The isolation flowchart of FL1 to 7 from Scutellariae radix is shown in Figure 4. Air dried pieces of Scutellariae radix (1.7 kg) were extracted twice with ethanol at room temperature (five days each time). The extract was evaporated under reduced pressure using a rotavapor to give brown residue (52.4 g). The residue was suspended in 1.5 L of H2O and then partitioned with 2 L of ethyl acetate (EA) twice. The water layer was partitioned with n-butanol. The combined EA soluble layer was subjected to chromatography using silica gel and further purification using a high-performance liquid chromatography (HPLC) system (Knauer, Berlin, Germany) with a Phenomenex Luna C18 column (250 × 10 mm, 5 µm) to furnish seven known flavones (Figure 4). Flavones were identified by comparing their physical and spectral data with literature values as 5,7-dihydroxy-6-methoxyflavone (FL1, oroxylin A) [25,26], 5,7-Dihydroxy-8-methoxyflavone (FL2, wogonin) [25,26,27], 5-hydroxy-7,8-dimethoxyflavone (FL3, 7-O-methylwogonin) [17], 5,6′-dihydroxy-6,7,8,2′-tetramethoxyflavone (FL4, skullcapflavone II) [28], 5,7,4′-trihydroxy -8-methoxyflavone (FL5) [29,30], 5,2′,6′-trihydroxy-7,8-dimethoxyflavone (FL6, viscidulin II) [31], and 5,7,2′,5′-tetrahydroxy-8,6′-dimethoxyflavone (FL7, ganhuangenin) [32] (Figure 2). Nuclear magnetic resonance and infrared data of the seven known compounds are available as Supplementary figures. The purity of flavones was measured by HPLC (Ecom, Prague, Czech Republic) equipped with gradient pumps (Ecom LCP 4100), a UV detector (Ecom LCD 2084) and a LiChrospher® 100 RP-18E (5 µm) HPLC column (125 × 4 mm i.d., Merck Millipore, Darmstadt, Germany). The mobile phase consisting of a mixture of solvent A (water/methanol, 98:2), and solvent B (methanol/acetic acid, 98:2) was run in the following gradient mode: 0–7 min, from 50% A to 40% A with a flow rate of 0.5 mL/min; 7–12 min, from 40% A to 30% A with a flow rate of 0.3 mL/min; and 12–28 min, from 30% A to 20% A with a flow rate of 0.3 mL/min. The UV detector was at 280 nm. Chromatographic processing was done using the Peak-ABC Chromatography Data Handling System. The purities of FL1, FL2, FL3, FL4, FL5, FL6, and FL7 were 96.4%, 98.7%, 98.2%, 97.5%, 95.2%, 94.7%, and 98.9%, respectively (Figure 5).
Figure 4.
Isolation flowchart of FL1–7.
Figure 5.
Retention time and purity analysis of FL1–7 in HPLC.
4.2. Culture of P. acnes and Preparation of Heat-Killed Bacteria
The strain of P. acnes (BCRC10723, isolated from facial acne) was obtained from the Bioresource Collection and Research Center (Hsinchu, Taiwan). P. acnes was cultured in brain heart infusion (BHI) broth (Difco, Detroit, MI, USA) with 1% glucose in an anaerobic atmosphere using BBL GasPak systems (Becton Dickinson Microbiology Systems, Cockeysville, MD, USA) at 37 °C. A spectrophotometer OD600 (Chrom Tech, Apple Valley, MN, USA) was used to determine the bacterial log phase of growth. The log-phase bacterial culture was harvested, washed thrice with phosphate-buffered saline (PBS) and re-suspended in PBS for induction of mouse ear edema. For the in vitro experiments, heat-killed P. acnes was prepared. Log-phase bacteria were washed with PBS, incubated at 100 °C for 30 min, re-suspended in RPMI medium (Gibco, Carlsbad, CA, USA) and stored at 4 °C until use.
4.3. Determination of the Viability of THP-1 Cells
The human monocytic THP-1 cell line (BCRC 60430) was obtained from the Bioresource Collection and Research Center (Hsinchu, Taiwan). Cells were maintained in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco), penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C in a humidified atmosphere with 5% CO2. To determine the cytotoxicity of tested samples, a suspension of THP-1 cells (1 × 106 cells/mL) was treated with various concentrations of tested samples in 96-well culture plates for 24 h at 37 °C. Whole cell suspension was taken from each well and centrifuged for 4 min at 600 g. Supernatant was removed, and the pellet was incubated with 100 μL of MTT reagent (Sigma-Aldrich) for 3 h at 37 °C. Samples were centrifuged for 2 min at 4500 g, and supernatant was gently removed. Finally, the converted dye from the MTT reagent in cell pellets was solubilized with 500 μL of isopropanol/HCl, and then 200 μL of each sample was transferred in duplicates to a 96-well plate. The absorbance was measured using a Synergy HT multi-detection micro-plate reader (BioTek, Winooski, VT, USA) at 540 nm with 690 nm as the reference wavelength.
4.4. Measurement of Cytokine Production in Human Monocytic THP-1 Cells
THP-1 cells were seeded at 1 × 106 cells/mL in 24-well plates with serum-free medium, and were treated with heat-killed P. acnes (7.5 × 107 CFU/mL; multiplicity of infection (M.O.I.) = 75) alone or in combination with different concentrations of tested samples for a 24-h incubation. Cell-free supernatants were collected, and concentrations of IL-1β and IL-8 were analyzed with respective enzyme immunoassay kits (BioLegend, San Diego, CA, USA). The half maximal inhibitory concentration (IC50) values were estimated using a nonlinear regression algorithm (SigmaPlot 12; SPSS Inc. Chicago, IL, USA).
4.5. P. acnes-Induced Ear Edema and Measurement of Cytokine Levels In Vivo
Eight-week-old male ICR mice were purchased from the Animal Center of College of Medicine, National Taiwan University, Taipei, Taiwan. Animal experiments were approved by the Animal Care Committee of the National Taiwan Normal University. Mice were fed with chow diet and water ad libitum. To examine the anti-inflammatory effect of flavones in vivo, an intradermal injection model was employed [24]. In the preliminary test, 10 μL of flavones (up to 20 μg/site) was injected into mice ears. No noticeable skin irritation occurred (data not shown). Hence, flavones (20 μg/site) were used for the following experiments. Mice were randomly grouped (n = 5 per group). P. acnes (6 × 107 CFU per 10 μL in PBS) was injected into the left ear of ICR mice. Right ears received an equal amount (10 μL) of PBS. Ten microliters of flavones in 5% DMSO in PBS was injected into the same location of both ears right after P. acnes or PBS injection. Twelve hours after bacterial injection, the increase in ear thickness was measured using a micro-caliper (Mitutoyo, Kanagawa, Japan). The increase in ear thickness of the P. acnes-injected ear was calculated and expressed as percentage of the PBS-injected control.
4.6. Measurement of Cytokine Levels In Vivo
After thickness had been measured, the ears were excised (n = 5 per group). The ear samples were homogenized using a BioMasher III® (Nippi Inc., Tokyo, Japan) in radioimmunoprecipitation (RIPA) buffer (G-Biosciences, St. Louis, MO, USA) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF) for 1 min on ice. The homogenates were vortexed and centrifuged at 10,000 g for 10 min at 4 °C. The supernatant was reserved and stored at −80 °C for the determinations of TNF-α, IL-1β, and IL-6 levels according to the manufacturer’s instruction (BioLegend).
4.7. Statistical Analysis
All data are presented as the mean ± standard deviation (SD). Statistical analyses were performed using the SPSS 19.0 statistical package. The data were evaluated for statistical significance with the one-way ANOVA followed by Duncan’s multiple range tests. A p value of < 0.05 was considered statistically significant.
Acknowledgments
This work was supported by research grants from the National Taiwan Normal University (Grant No. 100T0700), Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002) and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/1/15/s1.
Author Contributions
Po-Jung Tsai, Wen-Huey Wu, and Yueh-Hsiung Kuo designed the research and wrote the paper; Ping-Jyun Sung analyzed the spectroscopic data and determined the chemical structures; Po-Jung Tsai and Ming-Chi Hsieh, and Wen-Cheng Huang performed the experimental work.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Farrar M.D., Ingham E. Acne: Inflammation. Clin. Dermatol. 2004;22:380–384. doi: 10.1016/j.clindermatol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Koreck A., Pivarcsi A., Dobozy A., Kemény L. The role of innate immunity in the pathogenesis of acne. Dermatology. 2003;20:96–105. doi: 10.1159/000068476. [DOI] [PubMed] [Google Scholar]
- 3.Kurokawa I., Danby F.W., Ju Q., Wang X., Xiang L.F., Xia L., Chen W., Nagy I., Picardo M., Suh D.H., et al. New developments in our understanding of acne pathogenesis and treatment. Exp. Dermatol. 2009;18:821–832. doi: 10.1111/j.1600-0625.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 4.Trivedi N.R., Gilliland K.L., Zhao W., Liu W., Thiboutot D.M. Gene array expression profiling in acne lesions reveals marked upregulation of genes involved in inflammation and matrix remodeling. J. Investig. Dermatol. 2006;126:1071–1079. doi: 10.1038/sj.jid.5700213. [DOI] [PubMed] [Google Scholar]
- 5.Kistowska M., Gehrke S., Jankovic D., Kerl K., Fettelschoss A., Feldmeyer L., Fenini G., Kolios A., Navarini A., Ganceviciene R., et al. IL-1beta drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J. Investig. Dermatol. 2014;134:677–685. doi: 10.1038/jid.2013.438. [DOI] [PubMed] [Google Scholar]
- 6.Shang X., He X., He X., Li M., Zhang R., Fan P., Zhang Q., Jia Z. The genus Scutellaria an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2010;128:279–313. doi: 10.1016/j.jep.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Li H.B., Jiang Y., Chen F. Separation methods used for Scutellaria baicalensis active components. J. Chromatogr. B. 2004;812:277–290. doi: 10.1016/S1570-0232(04)00545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C., Lin G., Zuo Z. Pharmacological effects and pharmacokinetics properties of Radix Scutellariae and its bioactive flavones. Biopharm. Drug Dispos. 2011;32:427–445. doi: 10.1002/bdd.771. [DOI] [PubMed] [Google Scholar]
- 9.Wagner H., Bauer R., Melchart D., Xiao P.G., Staudinger A. Radix Scutellariae—Huangqin. In: Wagner H., Bauer R., Melchart D., Xiao P.G., Staudinger A., editors. Chromatographic Fingerprint Analysis of Herbal Medicines. Volume 2. Springer; Vienna, Austria: 2011. pp. 755–765. Chapter 63. [Google Scholar]
- 10.Li K.L., Sheu S.J. Determination of flavonoids and alkaloids in the scute-coptis herb couple by capillary electrophoresis. Anal. Chim. Acta. 1995;313:113–120. doi: 10.1016/0003-2670(95)00190-B. [DOI] [Google Scholar]
- 11.Chen Y., Yang L., Lee T.J. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-κB activation. Biochem. Pharmacol. 2000;59:1445–1457. doi: 10.1016/S0006-2952(00)00255-0. [DOI] [PubMed] [Google Scholar]
- 12.Song X., Chen Y., Sun Y., Lin B., Qin Y., Hui H., Li Z., You Q., Lu N., Guo Q. Oroxylin A, a classical natural product, shows a novel inhibitory effect on angiogenesis induced by lipopolysaccharide. Pharmacol. Rep. 2012;64:1189–1199. doi: 10.1016/S1734-1140(12)70915-5. [DOI] [PubMed] [Google Scholar]
- 13.Kubo M., Matsuda H., Tanaka M., Kimura Y., Okuda H., Higashino M., Tani T., Namba K., Arichi S. Studies on Scutellaria radix. VII. Anti-arthritic and anti-inflammatory actions of methanol extract and flavonoid components from Scutellaria radix. Chem. Pharm. Bull. 1984;32:2724–2729. doi: 10.1248/cpb.32.2724. [DOI] [PubMed] [Google Scholar]
- 14.Yasukawa K., Takido M., Takeuchi M., Nakagawa S. Effect of chemical constituents from plants on 12-O-tetradecanoylphorbol-13-acetate induced inflammation in mice. Chem. Pharm. Bull. 1989;37:1071–1073. doi: 10.1248/cpb.37.1071. [DOI] [PubMed] [Google Scholar]
- 15.Chi Y.S., Lim H., Park H., Kim H.P. Effects of wogonin, a plant flavone from Scutellaria radix, on skin inflammation: In vivo regulation of inflammation-associated gene expression. Biochem. Pharmacol. 2003;66:1271–1278. doi: 10.1016/S0006-2952(03)00463-5. [DOI] [PubMed] [Google Scholar]
- 16.Chandrasekaran C.V., Thiyagarajan P., Deepak H.B., Agarwal A. In vitro modulation of LPS/calcimycin induced inflammatory and allergic mediators by pure compounds of Andrographis paniculata (King of bitters) extract. Int. Immunopharmacol. 2011;11:79–84. doi: 10.1016/j.intimp.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Chao W.W., Kuo Y.H., Lin B.F. Anti-inflammatory activity of new compounds from Andrographis paniculata by NF-κB transactivation inhibition. J. Agric. Food Chem. 2010;58:2505–2512. doi: 10.1021/jf903629j. [DOI] [PubMed] [Google Scholar]
- 18.Jang H.Y., Ahn K.S., Park M.J., Kwon O.K., Lee H.K., Oh S.R. Skullcapflavone II inhibits ovalbumin-induced airway inflammation in a mouse model of asthma. Int. Immunopharmacol. 2012;12:666–674. doi: 10.1016/j.intimp.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Nagai T., Suzuki Y., Tomimori T., Yamada H. Antiviral activity of plant flavonoid, 5,7,4′-trihydroxy-8-methoxyflavone, from the roots of Scutellaria baicalensis against influenza A (H3N2) and B viruses. Biol. Pharm. Bull. 1995;18:295–299. doi: 10.1248/bpb.18.295. [DOI] [PubMed] [Google Scholar]
- 20.Nikaido T., Ohmoto T., Sankawa U., Tomimori T., Miyaichi Y., Imoto Y. Inhibition of adenosine 3′,5′-cyclic monophosphate phosphodiesterase by flavonoids. II. Chem. Pharm. Bull. 1988;36:654–661. doi: 10.1248/cpb.36.654. [DOI] [PubMed] [Google Scholar]
- 21.Lim B.O. Effect of ganhuangenin obtained from Scutellaria radix on the chemical mediator production of peritoneal exudate cells and immunoglobulin E level of mesenteric lymph node lymphocytes in Sprague-Dawley rats. Phytother. Res. 2002;16:166–170. doi: 10.1002/ptr.1034. [DOI] [PubMed] [Google Scholar]
- 22.Clahsen T., Schaper F. Interleukin-6 acts in the fashion of a classical chemokine on monocytic cells by inducing integrin activation, cell adhesion, actin polymerization, chemotaxis, and transmigration. J. Leukoc. Biol. 2008;84:1521–1529. doi: 10.1189/jlb.0308178. [DOI] [PubMed] [Google Scholar]
- 23.Singer M., Sansonetti P.J. IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J. Immunol. 2004;173:4197–4206. doi: 10.4049/jimmunol.173.6.4197. [DOI] [PubMed] [Google Scholar]
- 24.Huang W.C., Tsai T.H., Huang C.J., Li Y.Y., Chyuan J.H., Chuang L.T., Tsai P.J. Inhibitory effects of wild bitter melon leaf extract on Propionibacterium acnes-induced skin inflammation in mouse and cytokine production in vitro. Food Funct. 2015;6:2550–2560. doi: 10.1039/C5FO00550G. [DOI] [PubMed] [Google Scholar]
- 25.Huang W.H., Chien P.Y., Yang C.H., Lee A.R. Novel synthesis of flavonoids of Scutellaria baicalensis Georgi. Chem. Pharm. Bull. 2003;51:339–340. doi: 10.1248/cpb.51.339. [DOI] [PubMed] [Google Scholar]
- 26.Lin Y.L., Ou J.C., Chen C.F., Kuo Y.H. Flavonoids from the roots of Scutellaria luzonica Rolfe. J. Chin. Chem. Soc. 1991;38:619–623. doi: 10.1002/jccs.199100101. [DOI] [Google Scholar]
- 27.Hua Y., Wang H.Q. Chemical components of Anaphalis sinica Hance. J. Chin. Chem. Soc. 2004;51:409–415. doi: 10.1002/jccs.200400063. [DOI] [Google Scholar]
- 28.Furukawa M., Suzuki H., Makino M., Ogawa S., Iida T., Fujimoto Y. Studies on the constituents of Lagochilus leiacanthus (Labiatae) Chem. Pharm. Bull. 2011;59:1535–1540. doi: 10.1248/cpb.59.1535. [DOI] [PubMed] [Google Scholar]
- 29.Jang J., Kim H.P., Park H. Structure and antiinflammatory activity relationships of wogonin derivatives. Arch. Pharm. Res. 2005;28:877–884. doi: 10.1007/BF02973870. [DOI] [PubMed] [Google Scholar]
- 30.Stevens J.F., Wollenweber E., Ivancic M., Hsu V.L., Sundberg S., Deinzer M.L. Leaf surface flavonoids of Chrysothamnus. Phytochemistry. 1999;51:771–780. doi: 10.1016/S0031-9422(99)00110-7. [DOI] [Google Scholar]
- 31.Tanka T., Iinuma M., Mizuno M. Synthesis of flavonoids in Scutellaria spp. II. Synthesis of 2′,6′-dioxygenated flavones. Yakugaku Zasshi. 1987;107:827–829. [Google Scholar]
- 32.Iinuma M., Tanaka T., Mizuno M. Flavonoids synthesis II. Synthesis of flavones with a 2′,3′,6′-trioxygenated ring B. Chem. Pharm. Bull. 1985;33:4034–4036. doi: 10.1248/cpb.33.4034. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.