Abstract
Nowadays, there are some molecules that have shown over the years a high capacity to act against relevant pathologies such as cardiovascular disease, neurodegenerative disorders or cancer. This article provides a brief review about the origin, bioavailability and new research on curcumin and synthetized derivatives. It examines the beneficial effects on health, delving into aspects such as cancer, cardiovascular effects, metabolic syndrome, antioxidant capacity, anti-inflammatory properties, and neurological, liver and respiratory disorders. Thanks to all these activities, curcumin is positioned as an interesting nutraceutical. This is the reason why it has been subjected to several modifications in its structure and administration form that have permitted an increase in bioavailability and effectiveness against different diseases, decreasing the mortality and morbidity associated to these pathologies.
Keywords: curcumin, bioavailability, antioxidant, ROS, anti-inflammatory, cancer, lung diseases, liver disorders, cardiovascular diseases, natural compound
1. Introduction
Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] also called diferuloylmethane, is the main natural polyphenol found in the rhizome of Curcuma longa (turmeric) and in others Curcuma spp. [1]. Curcuma longa has been traditionally used in Asian countries as a medical herb for several pathologies due to its antioxidant, anti-inflammatory [2], antimutagenic, antimicrobial [3,4], and anticancer properties [5,6].
In relation to the solubility properties, curcumin is soluble in alkali or in extremely acidic solvents [7]. It is a crystalline compound with a bright orange-yellow colour so it is used as food colorant [2]. It is a keto-enol tautomeric compound with a predominant keto-form in acid or neutral solutions and the enol-form is predominant in alkalis solutions with good properties as chelator of metal ions [8]. Different activities have been directly associated according to the keto or enol forms of curcumin [9].
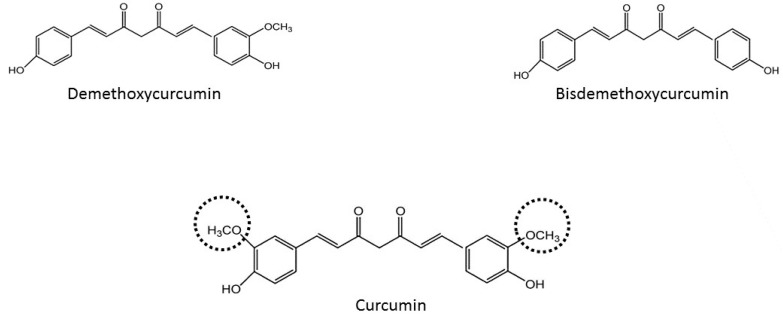
In the last 50 years it has been proven that most of the effects of Curcuma longa are mainly due to curcumin, with a potential effects against diabetes, allergies, arthritis, Alzheimer’s disease [10,11], and other chronic illnesses [9,12]. However, besides curcumin, there are others components in Curcuma longa called the curcuminoids group. They are demethoxycurcumin and bis-demethoxycurcumin. Curcumin is the most abundant of the curcuminoids group (77% of the total weight) [13] and this group constitutes around 5% of the total component of Curcuma longa. According to their structures (Figure 1), some researchers have concluded that the methoxy groups on the phenyl rings in curcumin are important to have health effects [14]. Moreover, it has been described that these curcuminoids have synergistic action for example to have nematocidal activity [15]. The aim of this review is to improve the knowledge of curcumin’s effects on health and try to elucidate its different mechanisms of action against different pathologies.
Figure 1.
Chemical structures of curcuminoids (methoxy groups in curcumin are shown by discontinuous lines).
2. Bioavailability and Metabolism of Curcumin
In the last ten years, many studies with animals and in vitro models have been performed with the objective of stablishing the mechanisms of action of curcumin and its activities against several pathologies [16]. For this reason, there are many studies that aim to understand the bioavailability of curcumin. The first study performed to determine the biological availability was carried out by Wahlstrom and Blennow in 1978, where curcumin was administered to Sprague-Dawley rats at a 1 g/kg dose. In this study a low level of curcumin was observed in blood plasma of rats [17]. However, latter studies demonstrated that when curcumin was administered orally at a dose of 2000 mg/kg to rats, the maximum serum concentration was 1.35 ± 0.23 µg/mL although in humans it was undetectable [18].
Some studies with rats have shown that the oral bioavailability of curcumin was around 1% [19] so very high doses of curcumin are necessary (3600 to 12,000 milligrams) to achieve any beneficial effects. Sharman et al., described no toxic effect of curcuma administered orally in patients with colorectal cancer [20]. This latter study showed that curcumin metabolites were detected in plasma when patients ingested at least 3600 milligrams of curcumin being mainly detected as curcumin glucuronide and curcumin sulphate. In urine samples, curcumin and its metabolites were detected primarily as curcumin, followed by glucuronide and finally as sulphate forms.
If curcumin is administered to rats in a dose of 2000 mg/kg jointly with l-piperoylpiperidine that induces glucuronyl transferase enzymes, the bioavailability of curcumin increases around 154% [18].
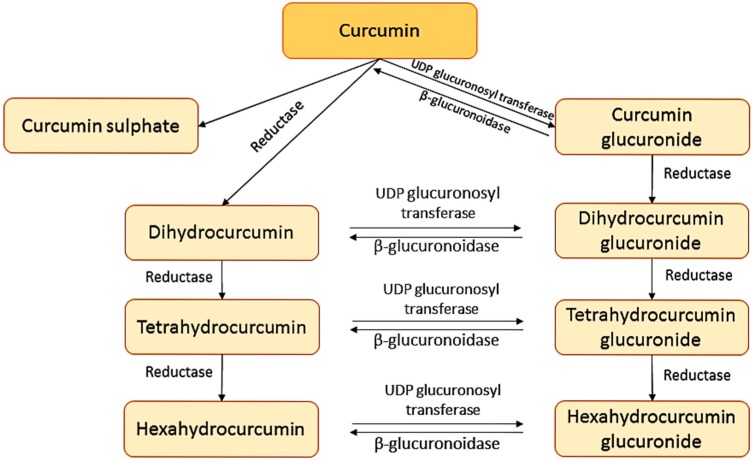
To determine the pharmacokinetic and pharmacodynamic properties of curcumin some researchers have measured the radioactivity after administration of [3H]-curcumin to rats, showing the great difficulty of curcumin in the intestinal absorption process [21,22,23]. In addition, these studies showed that curcumin suffers a biotransformation during the absorption from the bowel to glucuronides of tetrahydrocurcumin and hexahydrocurcumin derivatives. A further study was performed in liver to determine the nature of curcumin metabolites. These studies suggested that curcumin is firstly transformed to dihydrocurcumin and tetrahydrocurcumin by reductases and they are then transformed into monoglucuronide conjugates as dihydrocurcumin-glucuronide and tetrahydrocurcumin-glucuronide by β-glucuronidase [24]. The transformations that curcumin suffers are shown in Figure 2. The transformation in gut and liver can lead to generate curcumin glucuronides and curcumin sulphates or, alternately, reduced molecules like hexahydrocurcumin [25].
Figure 2.
Metabolite derivatives of curcumin.
On the other hand, Ryu et al. [26] demonstrated that curcumin administered intravenously in mice as [18F]-curcumin was accumulated in the liver, spleen, lung and in brain. These authors concluded that curcumin has a specific affinity for some tissues.
Finally, the excretion of curcumin metabolites depends on the vehicle and the route of administration employed [27]. For oral administration, a 75% of curcumin metabolites were found only in faeces but not in urine [17]. However, when the curcumin was administrated intraperitoneally 73% of these metabolites were found in faeces and around 11% in urine [28]. In relation with the administration form, encapsulation in liposomes, polymeric nanoparticles, cyclodextrin encapsulation, lipid complexes or by synthesis of polymer-curcumin complex have been investigated. All of them have helped increase the activity and bioavailability [29] of this compound and to improve the beneficial effect of curcumin against some pathologies such as cancer [30,31] or liver diseases [32].
3. Role of Curcumin in Health
It is known that some diseases are produced by an unhealthy diet (i.e., cardiovascular diseases) and as Wood and Brooks suggest, “We are what we ate” [33]. In this sense, they are evidences that certain diets rich in antioxidants, as the Mediterranean, Indian or Nepalese diets, are excellent against some pathologies related with oxidative stress as cardiovascular diseases [34,35], cancer [36], metabolic disorders [37] or aging, improving the mortality and morbidity of them [38]. Particularly, many of the effects associated to Indian or Nepalese diets have been related with specific compounds such as curcumin and other curcuminoids [39].
3.1. Antioxidant Activity and ROS Scavenger Properties
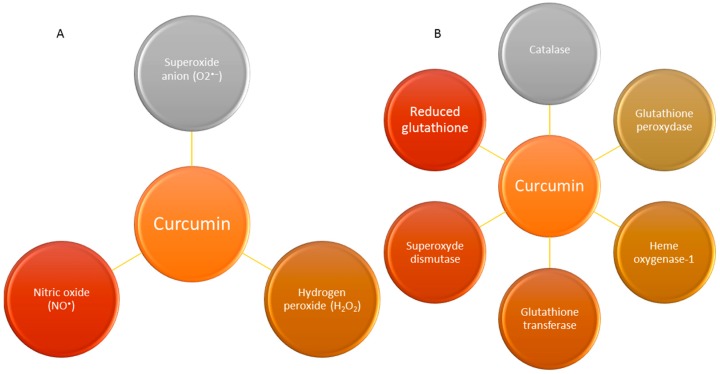
Curcumin is considered as antioxidant due to the β-diketone group in its structure [14,40,41]. Joe and Lokesh determined in 1994 that the most important mechanisms by which curcumin is able to promote the majority of its activities are by inhibition of superoxide radicals, hydrogen peroxide and nitric oxide radical [42]. Other studies proposed that curcumin also enhances the activity of many antioxidant enzymes such as catalase, superoxide dismutase (SOD), glutathione peroxidase (GPx) [43] and heme oxygenase-1 (OH-1) [44]. These activities reduce the lipid peroxidation decreasing the hepatic damage [45,46]. In addition, curcumin is also able to increase the activity of xenobiotic detoxifying enzymes both in liver and kidneys, protecting against carcinogenesis processes [47].
Other studies have described that a dose of 200 mg/kg of curcumin increased the SOD and catalase activities and the hepatic total antioxidant capacity in liver from rats with hepatic damage and high level of oxidative stress provoked by thallium acetate [48].
In human hepatocyte L02 cell line curcumin was able to avoid the ROS formation by increasing SOD activity and reducing glutathione levels after treatment with the antimicrobial feed additive quinocetone as a generator of free radicals [49].
In addition, curcumin can upregulate other enzymes like glutathione transferase and their mRNAs as well as increase both the level of reduced glutathione and the acid-soluble sulphydryl groups [50] and scavenge free radicals [51]. All these effects together have granted it a health- and radioprotective role [42,45,52,53,54]. Moreover, curcumin can increase the levels of reduced gluthathione (GSH) and it can moderate the malondialdehyde levels in a lung carcinogenesis model induced by benzo(a)pyrene (a major carcinogenic pollutant) in mice [55]. The main antioxidant activities and ROS scavenger properties of curcumin in the biological system are described in Figure 3.
Figure 3.
This figure shows a brief summary of the antioxidant properties and ROS scavenger effects of curcumin. (A) Effect of curcumin as ROS and RNS scavengers; (B) Enhanced activities of curcumin on different antioxidant systems.
Some studies have determined that curcumin is able to prevent the brain injury thanks to the suppression of oxidative stress via Akt/Nrf2 (Nuclear factor-E2-related factor 2) pathway, acting then as neuroprotector [56]. Li et al. [57] proved the neuroprotector effect of curcumin by decreasing the reactive oxygen species (ROS)-associated endoplasmic reticulum (ER) stress (related to neuronal damage) through the regulation of AMPK activity. Similar results were obtained by Fu, Yang et al. [58] who tried to determine if curcumin could protect PC12 cells from H2O2-induced neurotoxicity, These authors described that curcumin dysregulated the mitogen-activated protein kinase (MAPK) and AKT pathways, decreasing apoptotic cells through inhibition of ROS accumulation [58].
Curcumin expresses a dichotomy between its antioxidant and pro-oxidant effects, generally depending on the dose used and the presence in the medium of metal ions [50,59,60]. Curcumin can selectively provoke the induction of pro-oxidant effects only in malignant cells for example in cervical cancer cells [61], cell lung cancer NCI-H446 [62] or in murine myelomonocytic leukemia WEHI-3 cells [63]. These studies described that curcumin promotes a powerful increase in the intracellular generation of ROS or ER-stress by provoking a mitochondrial dysfunction.
3.2. Curcumin and Inflammation
The inflammatory cascade plays an important role in the development of chronic illnesses such as autoimmune, cardiovascular, endocrine, neurodegenerative and neoplastic diseases [64,65,66]. Curcumin is able to decrease inflammation by interacting with many inflammatory processes [67,68,69,70].
Oxidative stress can lead to chronic inflammation, causing chronic diseases [71]. ROS production modulates the expression of thebnuclear factor-κβ (NF-κβ) and tumour necrosis factor alpha (TNF-α) pathways which play a central role in the inflammatory response [72]. Curcumin could downregulate oxidative stress and the subsequent inflammation through the Nrf2 pathway. This polyphenol can decrease TNFα production and the cell signalling mediated by TNFα in various types of cells. In vitro and in vivo studies postulate that curcumin is a TNFα blocker due to its direct union to TNFα [73,74]. In this sense, curcumin modulates TNF-α expression by inhibition of p300/CREB-specific acetyltransferase which leads to repression of acetylation of histone/nonhistone proteins and therefore repression of transcription [74]. Regarding NF-κβ the primary transcription factor involved in the start of the inflammatory processes, can be blocked by curcumin [75]. This molecule inhibits the IkBkinase provoking a NF-κβ inhibition.
Curcumin also inhibits inflammatory cytokines, such as interleukins (ILs), chemokines, as well as inflammatory enzymes, such as cycloxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS) and others molecules as cyclinD1 [76]. This natural anti-inflammatory agent is able to inhibit MAPK and NF-κB pathways in TNF-α-treated HaCaT cells and, therefore, IL-1β and IL-6 expression [77]. In others studies in BV2 microglia cell stimulated with lipopolysaccharide (LPS), curcumin also inhibited IL6 and TNF-α [78]. Due to all its anti-inflammatory properties, curcumin is able to improve different chronic diseases as follows.
3.2.1. Inflammation of Respiratory System
Allergy is a pro-inflammatory disease mediated by cytokines [79]. In asthma development, others inflammatory molecules such as eotaxin, monocyte chemoattractant protein-1 (MCP-1) and MCP-3 play also an important role. In this sense, in 2015 Panahi et al., studied the anti-inflammatory properties of curcuminoides in sulphur mustard-intoxicated subjects. These authors found a great effect of curcuminoides vs. placebo in modulating all assessed inflammatory mediators included MCP-1 [80].
There are many studies which confirm the important role of curcumin in respiratory diseases caused by inflammation [81]. In acute allergic asthma in BALB/c mice, the anti-inflammatory effect of curcumin, the down-regulated levels of Notch1/2 receptors and globin transcription factor 3 (GATA3) was reported [82]. Studies of plants extracts with anti-asthmatic components showed that curcumin can act as a scavenger of nitric oxide and could prevent bronchial inflammation in asthmatic patients [83]. Moreover, curcumin can also decrease allergic inflammation in asthma models by regulating Treg/Th17 balance with an important increase of Treg cells [84].
3.2.2. Inflammation of Joints
ROS play a crucial role in joint destruction in rheumatoid arthritis [85,86]. These free radicals can activate many transcription factors such as NF-κβ and activator protein 1 (AP-1), which regulate growth factors, chemokines, inflammatory cytokines and anti-inflammatory molecules [87]. Curcumin as an anti-inflammatory and antioxidant compound possesses anti-rheumatic and anti-arthritic properties [88,89]. In patients with rheumatoid arthritis, curcumin is able to up regulate pro-apoptotic Bax, and down regulate anti-apoptotic B-cell lymphoma 2 (Bcl-2) and X-linked inhibitor of apoptosis protein (XIAP), inducing apoptosis and, therefore, inhibit the growth of synovial fibroblasts [90]. Curcumin also prevents the antiinflammatory response in synovial fibroblasts by inhibition of prostaglandin E2 (PGE2) synthesis due to COX-2 suppression [91].
3.2.3. Digestive System Inflammation
Inflammatory bowel disease is characterized by oxidative stress, nitrosative stress, leukocyte infiltration and production of pro-inflammatory cytokines. NF-κβ also takes part in this disease producing cytokines and chemokines for inflammation [92]. Curcumin is able to suppress STAT3 pathways, reducing the expression of TNFα, and IL-1β. Moreover, this molecule can improve dextran sulphate sodium (DSS)-induced colitis because it decreases myeloperoxidase activity, colon injury, oxidative stress, inflammatory reaction, and apoptotic cell death by blocking c-Jun N-terminal protein kinase (JNK), p38 MAPK pathways [93,94]. According to this, other studies showed that curcumin blocked p38 MAPK and Akt pathways, decreased NF-κβ level and inhibited the expression of vascular cell adhesion molecule 1 (VCAM-1) in human intestinal microvascular endothelial cells (HIMECs) and in 2,4,6-trinitrobenzenesulphonic acid-induced colitis in mice [95,96,97].
In others digestive diseases such as chronic pancreatitis many factors can participate (alcohol tobacco, genetic, environmental, hypertriglyceridemia, hypercalcemia, autoimmune) [98]. Curcumin reduces inflammation by decreasing NF-κβ, AP-1, iNOS, TNF-α, and IL-6 molecules in rats with pancreatitis [99]. In pancreatitis induced by ethanol and cerulein, curcumin improves disease’s severity, acting on serum amylase, pancreatic trypsin, and neutrophil infiltration [100]. Dhillon et al., [101] described that curcumin has biological activity in patients with pancreatic cancer. Curcumin down-regulated expression of NF-κβ and COX-2.
Some studies evaluated the effect of curcumin in gastric mucosa inflammation provoked by Helicobacter pylori. In these studies, curcumin decreased the levels of inflammatory cytokines, chemokines, the expression of toll-like receptors (TLRs) and myeloid differentiation primary Response 88 (MyD88) in mice. These results indicate that curcumin exerts an anti-inflammatory effect in H. pylori-infected mucosa [102].
3.3. Curcumin in Neurological Disease
The World Health Organization (WHO) has defined neurological disorders as different pathologies of the central and peripheral nervous system, including the brain, spinal cord, cranial nerves, peripheral nerves, nerve roots, autonomic nervous system, neuromuscular junction, and muscles. These disorders produce epilepsy, Alzheimer disease (AD), Parkinson’s disease (PD), migraine, brain tumors and traumatic disorders of the nervous system.
Curcumin can interact and modulate many molecular targets such as transcription factors, inflammatory cytokines, kinases, growth factors and antioxidant system. All these actions of curcumin will be responsible, at least in part for its neuroprotective. Many of these beneficial effects mentioned before have been demonstrated by several paths in different types of cells from the nervous system, such as neurons [103], astrocytes [104] and microglia [105]. Some researchers employing primary cell cultures from different regions of the nervous system have also observed the capacity of curcumin to act as neuroprotector due to its antioxidant, anti-inflammatory and anti-protein aggregating properties [106] in cortical [105], mesencephalic [107], hippocampal [108] and spinal cord cells [109].
Furthermore, the capacity of curcumin to decrease neuro-inflammation, which takes place in the progression of neurodegenerative diseases, by reducing the expression of IL-1α, IL-6 and TNF-α in LPS-stimulated BV2 microglia in a dose dependent manner has been well established [77]. AD shows signs of defective phagocytosis that results in an ineffective clearance of Aβ plaques. Curcumin can stimulate microglial phagocytosis and clearance of Aβ plaques in vitro and increase the induction of heat-shock proteins in response to the addition of soluble Aβ aggregates to neuronal cell cultures [110]. It also can attenuate the β-amyloid accumulation and inhibit the formation of Aβ fibrils in these cells [111]. Moreover, in dopaminergic neurons, neurotoxicity triggered by 6-hydroxydopamine (6-OHDA) curcumin attenuated the toxicity in SH-SY5Y and MES23.5 cells through the inhibition of ROS, mitochondrial protection and anti-apoptotic mechanisms [112,113].
Several studies in rodents have proved the neuroprotection of curcumin in neurodegenerative disorders, especially AD and PD. Curcumin can also attenuate oxidative injury, microgliosis, synaptophysin loss, spatial memory deficits, postsynaptic loss, and Aβ deposits produced by intra-cerebral ventricular infusion of Aβ amyloid in rats [114]. Moreover, a study performed in Tg2576 mice (a transgenic mouse model for AD) fed curcumin described a decrease of amyloid plaque formation ROS and reactive nitrogen species (RNS) formation [115]. Finally, in mice overexpressing Aβ (PS1dE9) where curcumin was intravenously administered, a plaque disruption and an attenuation of distorted neuritis were found [116].
In regards to PD, other studies have tested the neuroprotective potential of curcumin against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-OHDA-induced dopaminergic degeneration. Oral and intravenous administration of curcumin were able to modulate dopaminergic damage in 6-OHDA-treated rodents, suppressing apoptosis, inducing microglial activation and improving the locomotion [117]. Other studies performed with CAG140 mice described a powerful capacity of curcumin to decrease Huntington protein aggregation, improving rearing deficits, but impairing climbing behavior [118]. A study in β-amyloid-infused rats showed that curcumin improved spatial memory [119]. Mechanisms through which curcumin can improve memory in AlCl3-challenged mice is decreasing oxidative stress and histopathological damage in brain [120,121].
3.4. Role of Curcumin in Lung Injuries
Respiratory diseases are a complex group of disorders in the respiratory system, from the upper tract into the pleural cavity, including both muscles and the nervous systems that modulates it. The most studied pulmonary pathologies are fibrosis, bronchitis, allergy and asthma in relation with inflammatory process and redox status alterations. The effects of curcumin in these process are decreasing inflammatory cells accumulations [122], cytokines over-production [123] and increasing ROS scavengers [52].
In 1996, the first evidence that curcumin has effects against pulmonary injuries was described by Thresiamma et al. [124] using a radioactive curcumin administered orally in mice. Further studies demonstrated the effect of curcumin in many pulmonary injuries [125,126,127].
Pulmonary fibrosis is characterized by an accumulation of inflammatory cells in the airways [125]. These cells can generate a high increase in the cytokines, ROS and growth factors which promote in turns the basis of the fibrotic scar. In this sense, several studies performed in animal models have demonstrated that curcumin acts against pulmonary fibrosis mainly by decreasing the inflammatory mediators [126,127]. Venkatesan and Chandrakasa [128] showed the beneficial effects of curcumin against cyclophosphamide-induced pulmonary fibrosis in rats. These effects were a reduction in leukocytes and inflammatory mediators and an increment in lung antioxidant defences. Similar results were obtained by Venkatesan [129] who also described that rats injected with paraquat, to provoked pulmonary fibrosis and treated with curcumin had a lower inflammatory response, toxicity and mortality associated to this herbicide.
Asthma is a chronic inflammatory disease identified by a reversible airflow obstruction and bronchospasm with variable intensity. Asthma is also characterized by goblet cell hyperplasia, airway eosinophilia, mucus hypersecretion and hyperresponsiveness to endogenous and exogenous allergens [130]. Several transcription factors are involved in the inflammatory process of asthma, including the NF-κβ, AP-1, cyclic AMP response element binding protein, peroxisome proliferator-activated receptor (PPAR) and the bZIP transcription factor, Nrf2 [131,132]. Eosinophils play a critical role in asthma by producing inflammatory mediators and free radicals [133]. In this sense, there are critical interleukins involved in the activation process of eosinophils such as IL-5, as well as IL-4 but in a lesser degree [134]. Their control can be considered as a novel strategy against asthma [135].
Curcumin has been widely used in ancient Indian medicine for allergy and asthma treatment [81] and currently, several studies have found that curcumin has a powerful activity both in vitro and in vivo against asthma. These effects are produced by an inhibition of IL-5, IL-4, IL-2, immunoglobulin (Ig) E2 production [136,137] and by decreasing the histamine effects in peritoneal mast cells from rats [138,139].
In addition, Oh et al. [132] demonstrated the ability of curcumin to inhibit NF-κβ production in a dose-dependent form by the inactivation of Iκ-Bα and AKT in pathogen-free BALB/c mice and in murine macrophage-like Raw264.7 cell line [67,140].
Other studies have described that curcumin can improve the elimination of nitric oxide and decrease the nitric oxide synthase activity. This might be a mechanism of curcumin which could prevent the bronchial inflammation in asthmatic patients [83,141]. In addition, curcumin can protect against asthmatic mucus secretion and airway hyperresponsiveness through an increment of Nrf2 and HO-1 levels in lung [94]. In summary, all these beneficial effects of curcumin against asthmatic pathologies and others lung injures immune-, ROS- and inflammation-associated [142,143,144].
3.5. Relationship between Metabolic Syndrome and Curcumin
According to the American Heart, Lung and Blood Institute (NHLBI) metabolic syndrome is defined as a “group of risk factors that raises your risk for heart disease and other health problems, such as diabetes and stroke”. All risk factors are closely linked to obesity, to insulin resistance, lifestyle and diet. For this reason the metabolic syndrome is also called “The Deadly Quartet” of hyperglycaemia, hypertriglyceridemia, hypertension, and obesity [145] and the concurrence of all these together for a long time, finally leads to cardiovascular diseases and diabetes type II [146].
Currently, inflammation is recognized as an overwhelming burden on healthcare status [147]. It is known that an increase of pro-inflammatory molecules (TNF-α, IL-6, MCP-1, and IL-1) directly secreted from adipocytes is related to insulin resistance and chronic inflammation [148,149].
In the study performed by Rains et al. [150], the authors observed that curcumin has immunomodulatory effects in obesity and insulin resistance because it decreases cytokines, TNF-α, MCP-1, glucose and glycosylated haemoglobin in diabetic rats [151,152].
Another study employing C57BL/Ks-db/db diabetic mice demonstrated that curcumin decreased blood glucose and glycosylated haemoglobin levels and increased the plasma insulin levels and hepatic glucokinase activity. In addition, curcumin decreased the glucose-6-phosphatase and phosphoenolpyruvate carboxykinase activities reducing glucose levels in blood [153] and improving the glucose tolerance.
On the other hand, curcumin also decreased plasma free fatty acid, cholesterol, and triglyceride levels and increased hepatic glycogen and skeletal muscle lipoprotein lipase (LPL) [153,154]. Kaur et al. [155] in a study with an extract of curcumin, piperine and quercetin (both of them to enhance the bioavailability of oral curcumin) showed that diabetic rats fed a high fat diet showed decreased plasmatic levels of glucose, triglycerides, total cholesterol and low density lipoprotein (LDL) with a concomitant increase high density lipoprotein (HDL).
Moreover, the decrease in plasma free fatty acid by curcumin in diabetes also promotes lower lipotoxicity, considered as a trigger of insulin resistance through activation of NF-κβ [156]. In relation to obesity, curcumin reduces body weight gain and angiogenesis in adipose tissue, decreases pre-adipocytes differentiation and lipid accumulation in mature adipocytes in mice [157] favouring the non-appearance of one of the risk factors of metabolic syndrome triggers [158,159].
All these results corroborate the powerful activities of curcumin appeasing hyperlipidaemia, insulin resistance and glucose tolerance associated with excess dietary fat intake, obesity, and type 2 diabetes [155,160].
Finally, several clinical trials have been performed in humans with metabolic syndrome employing both curcumin and curcuminoids. In these studies curcumin reduced lipid profile and modified cholesterol-related parameters [161,162]. Furthermore an study suggests the relevance of curcumin improving the anthropometric measurements and body composition when it is associated to diet and lifestyle intervention [163].
3.6. Curcumin as Hepatoprotective
The liver is intensely involved in the metabolism and synthesis of all macronutrients and it presents highly relevant endocrine and exocrine functions. The liver is also responsible for the detoxification of the blood. The liver has a very complex architecture and present different types of cells such as hepatocyte, cholangiocyte/bile duct cell, endothelial cell, liver sinusoidal endothelial cell, pit cell, Kupffer cell and hepatic stellate cell [5,164]. All activities performed by the liver are highly relevant for life and for this reason, when liver diseases appear, these functions are substantially compromised, with a resulting increase in morbidity and mortality [164].
The beneficial effects of curcumin in liver diseases can be due to its anti-inflammatory, antioxidant effects and antifibrogenic properties [165]. In addition, curcumin can decrease levels of thiobarbituric acid reactive substances (TBARS) and increase GSH and SOD levels in the liver homogenates from LPS-challenged rats supplemented with curcumin [166]. Curcumin also reduces the iron-induced hepatic damage by lowering lipid peroxidation [45,46], increase the activity of xenobiotic detoxifying enzymes [47] and the hepatic total antioxidant capacity [48]. Finally, this molecule can up regulate the cytoprotective enzyme HO-1 [167] inhibiting the ROS formation in liver [49].
In this sense, some studies carried out by our research group [168] have demonstrated that curcumin decreased the non-alcoholic steatohepatitis (NASH) and aminotransferase activity, and increased the mitochondrial antioxidants in rabbits with high-fat-induced NASH. Moreover, curcumin also reduced mitochondrial ROS, improved mitochondrial function, and lowered levels of TNF-α. All these results were corroborated by Wang et al. [169] in a rat model.
According to the hepatoprotective effects of curcumin, there are studies which have described a down-regulation of NF-κβ transcription factor [170], an improvement of hepatic fibrosis in alcoholic liver injuries [171], an increase of the survival rates in animals [172] and a reduction of damage in experimental steatohepatitis [173].
3.7. Has Curcumin Got an Antitumor Effect?
Regarding the antitumor properties of curcumin, they are a lot of studies carried out in animals, human leukaemia cell types [12], and several clinical trials in cancer patients. Only few of them have described the anticancer potential of curcumin [20,174] and many of them have investigated the capacity of curcumin as an adjuvant in anti-tumour therapy or reducing adverse effects associated with the treatment [174,175,176,177,178]. In this sense, Sharma et al. [179] showed that curcumin prevent colon cancer in rodent model by inhibition of lipid peroxidation and cyclooxygenase-2 (COX-2) expression and by increase of glutathione S-transferase (GST) enzymes [180].
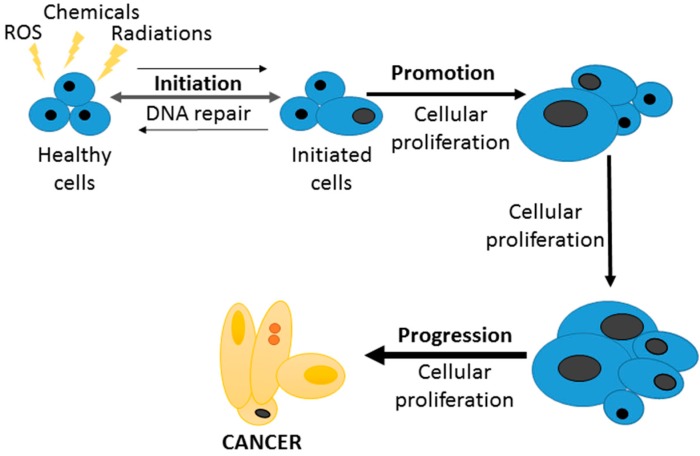
On the other hand, ROS and inflammation are linked to carcinogenesis processes [71,181], mainly acting as initiators in the well-known triphasic theory of cancer: initiation, promotion and progression (Figure 4). For this reason, some anticancer properties of curcumin are due to the antioxidant and ROS scavenger activities already described before. Moreover, the effects of curcumin in different genes and proteins such as Bcl-2, VCAM-1, Cyclin D1, Bax, NF-κβ, VGEF or COX-2, curcumin prevents the promotion and progression stages [182]. Hosseini and Ghorbani [183] have described the anticancer effects of curcumin by multiple actions on mutagenesis, cell cycle regulation, apoptosis and oncogene expression. However, curcumin also can modulate different pathways related to angiogenesis, invasion, tumour growth or metastasis [184] and it can promote apoptosis through interaction with p53 [185], caspase expression [186], and inducing cell cycle arrest [187].
Figure 4.
The three steps in the carcinogenesis process: initiation, promotion and progression.
Several epidemiological studies have determined that inflammation is one of the most important risk factors associated to carcinogenesis. During this process, the activation and interaction between NF-κβ and STAT3 has been identified as a key mechanism in the communication between cancer cells and inflammatory cells [188]. As has already been described previously, curcumin is able to down-regulate different pathways related to both molecules, then decreasing the generation of different inflammatory mediators such as COX-2, lipoxygenase 2 (LOX-2), iNOS, and cytokines associated to them and hence preventing the carcinogenesis process in several types of cancer [189,190,191].
In addition, curcumin inhibits JAK-STAT pathway [192] and inhibits telomerase activity [193], finally driving to the arrest of cell cycle and apoptosis in tumour cells.
Regarding to breast cancer, curcumin has demonstrated similar results by inhibition of COX-1, COX-2, Bax expression, vascular endothelial growth factor (VEGF) and the telomerase activity [71,194,195]. Curcumin also downregulates the inflammatory cytokines CXCL1 and CXCL2 by the inhibition of NF-κβ [196].
In human lung cancer cells, curcumin has similar mechanisms decreasing the migration and invasion of cells through inhibition of MMP-2 and MMP-9 and suppression of VEGF expression [197]. In this sense, Das and Vinayak [198] described how curcumin regulates genes as hypoxia-inducible factor 1-alpha (HIF-1a) and MYC, LDH activity and decreases the angiogenesis process by a MMP-2, MMP-9, PKC-a and VEGF reduction. All these results are in accord with studies performed in a lung cancer mice model which also showed that curcumin can inhibit the neutrophil chemoattractant keratinocyte-derived chemokine expression (CXC-KC) stopping the tumour progression [199].
Finally, several cytokines have been focused as target for prevention and treatment of several types of tumours [200,201,202]. In this sense, curcumin can inhibit some of these cytokines such as IL-1β, IL-6, TNFα or IL-23 so this molecule has an important role in cancer that overexpress them such as oral cavity, forestomach, duodenum, and colon [203].
3.8. How Does Curcumin Prevent Cardiovascular Pathologies?
According to the definition proposed by the World Health Organization, cardiovascular diseases (CVDs) are a complex group of disorders that affect the heart and blood vessels. These disorders are associated with risk factors such as an unhealthy diet, physical inactivity, tobacco use and use of alcohol. There are many signs and symptoms of these disorders as raised blood pressure, raised blood glucose, raised blood lipids, and excess weight and obesity. One of the most important causes of stroke and heart attacks is atherosclerosis. This is a complex and multistep process with many factors involved in the formation and evolution of the atherosclerotic plaque. Lipoprotein oxidation and oxidative processes play a critical role in the pathogenesis atherosclerosis. Some studies have identified the oxidized LDL (ox-LDL) as a powerful atherogenic agent [204,205].
Several mechanisms have been proposed by which ox-LDL can develop the atherogenic process such as the enhanced uptake by macrophages which leads to foam-cell formation, cytotoxic actions and alteration of platelet aggregation mechanism [206,207]. In this sense, some studies performed by our research group, have demonstrated the effectiveness of a curcumin extract to downregulate the LDL and ox-LDL concentration and also increase the HDL level by decreasing the oxidative stress and attenuating aortic fatty streak development in a rabbits fed a high cholesterol diet [208,209]. In addition, curcumin also exhibits an inhibitory effect on the platelet aggregation and eicosanoid metabolism, showing its cardio protective character [210]. In a coronary artery disease study performed in humans, curcumin was able to decrease, very low density lipoprotein (VLDL), LDL cholesterol and serum triglyceride with no effect on inflammatory biomarkers [211].
The strong association between cardiovascular diseases risk and inflammation has been well established [207,212,213]. As has been described before, curcumin decreases the levels of inflammatory molecules such as TNF-α, p38 MAPK, JAK2/STAT3 so it improves cardiovascular risk inflammation-associated [214,215]. Moreover, curcumin suppress the activation of TLR4, inhibits phosphorylation of ERK1/2 and nuclear translocation of NF-κβ and reduces nicotinamide adenine dinucleotide phosphate (NADPH)-mediated intracellular ROS production [216].
In relation to cardioprotective effects associated to oxidative stress, curcumin attenuates oxidative stress by up-regulating eNOS expression, decreasing p47phox NADPH oxidase and superoxide generation the vascular tissues. All these effects promote a descent of blood pressure and hind limb vascular resistance and increased hind limb blood flow [217]. In addition, curcumin increases Nrf2 expression and inhibits NF-κβ activation in an obesity-induced heart injury both in in vivo and in vitro models [218]. In 5/6 nephrectomised rats curcumin also promoted the Nrf2 activation, and prevented of hemodynamic changes and glomerular hypertension [219,220]. In the study carried out by Abo-Salem et al. [221], curcumin increased cardiac antioxidant enzymes such as catalase, SOD, glutathione-S-transferase and glutathione reduced in streptozotocin-induced heart injury in rats.
Finally, curcumin can also act as cardioprotector in an ischemia reperfusion injury model. This effect is produced because curcumin can activate SIRT1 signalling which reduces mitochondrial damage. SIRT1 reduces some molecules as SOD, succinate dehydrogenase, cytochrome c oxidase, methane dicarboxylic aldehyde and H2O2 levels in mitochondria [222]. In summary, the natural cardioprotective activity of curcumin is in part due to a reduction of the oxidative stress.
4. Conclusions
Through this review, the beneficial effects of curcumin against different diseases highly relevant in modern societies has been proved. In summary it can be concluded that curcumin has protective health effects mainly through anti-inflammatory and antioxidant mechanisms. Thus, curcumin has an important role against neurological, cancer, cardiovascular and lung diseases, and also against metabolic syndrome and liver disorders. Likewise, several specific mechanisms of action have been postulated for curcumin, to understand its beneficial effects and its role as a nutraceutical compound.
Author Contributions
All authors have participated actively in the conception and design of the review. They also have stablished different points of view about the molecule studied and its role in health, being inescapable part in this study. M.C. Ramirez-Tortosa and Cesar Ramirez-Tortosa have also participated in the paper revision and interpretation. M. Pulido-Moran and Jorge Moreno-Fernandez have written this paper and have actively participated in the revision and conceptualization of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- 1.Aggarwal B.B., Kumar A., Bharti A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 2.Lestari M.L., Indrayanto G. Curcumin. Profiles Drug Subst. Excip. Relat. Methodol. 2014;39:113–204. doi: 10.1016/B978-0-12-800173-8.00003-9. [DOI] [PubMed] [Google Scholar]
- 3.Mahady G.B., Pendland S.L., Yun G., Lu Z.Z. Turmeric (Curcuma longa) and curcumin inhibit the growth of Helicobacter pylori, a group 1 carcinogen. Anticancer Res. 2002;22:4179–4181. [PubMed] [Google Scholar]
- 4.Reddy R.C., Vatsala P.G., Keshamouni V.G., Padmanaban G., Rangarajan P.N. Curcumin for malaria therapy. Biochem. Biophys. Res. Commun. 2005;326:472–474. doi: 10.1016/j.bbrc.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 5.Vera-Ramirez L., Perez-Lopez P., Varela-Lopez A., Ramirez-Tortosa M., Battino M., Quiles J.L. Curcumin and liver disease. Biofactors. 2013;39:88–100. doi: 10.1002/biof.1057. [DOI] [PubMed] [Google Scholar]
- 6.Wright L.E., Frye J.B., Gorti B., Timmermann B.N., Funk J.L. Bioactivity of turmeric-derived curcuminoids and related metabolites in breast cancer. Curr. Pharm. Des. 2013;19:6218–6125. doi: 10.2174/1381612811319340013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao E.V., Sudheer P. Revisiting curcumin chemistry part I: A new strategy for the synthesis of curcuminoids. Indian J. Pharm. Sci. 2011;73:262–270. doi: 10.4103/0250-474X.93508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 9.Manolova Y., Deneva V., Antonov L., Drakalska E., Momekova D., Lambov N. The effect of the water on the curcumin tautomerism: A quantitative approach. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014;132:815–820. doi: 10.1016/j.saa.2014.05.096. [DOI] [PubMed] [Google Scholar]
- 10.Brondino N., Re S., Boldrini A., Cuccomarino A., Lanati N., Barale F., Politi P. Curcumin as a therapeutic agent in dementia: A mini systematic review of human studies. Sci. World J. 2014 doi: 10.1155/2014/174282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chin D., Huebbe P., Pallauf K., Rimbach G. Neuroprotective properties of curcumin in Alzheimer’s disease—Merits and limitations. Curr. Med. Chem. 2013;20:3955–3985. doi: 10.2174/09298673113209990210. [DOI] [PubMed] [Google Scholar]
- 12.Aggarwal B.B., Surh Y.-J., Shishodia S. The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease. Springer; New York, NY, USA: 2007. [Google Scholar]
- 13.Naksuriya O., Okonogi S., Schiffelers R.M., Hennink W.E. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35:3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 14.Sandur S.K., Pandey M.K., Sung B., Ahn K.S., Murakami A., Sethi G., Limtrakul P., Badmaev V., Aggarwal B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–1773. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 15.Kiuchi F., Goto Y., Sugimoto N., Akao N., Kondo K., Tsuda Y. Nematocidal activity of turmeric: Synergistic action of curuminoids. Chem. Pharm. Bull. 1993;41:1640–1643. doi: 10.1248/cpb.41.1640. [DOI] [PubMed] [Google Scholar]
- 16.Schneider C., Gordon O.N., Edwards R.L., Luis P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015;63:7606–7614. doi: 10.1021/acs.jafc.5b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahlstrom B., Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. 1978;43:86–92. doi: 10.1111/j.1600-0773.1978.tb02240.x. [DOI] [PubMed] [Google Scholar]
- 18.Shoba G., Joy D., Joseph T., Majeed M., Rajendran R., Srinivas P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64:353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 19.Yang K.-Y., Lin L.-C., Tseng T.-Y., Wang S.-C., Tsai T.-H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007;853:183–189. doi: 10.1016/j.jchromb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Sharma R.A., Euden S.A., Platton S.L., Cooke D.N., Shafayat A., Hewitt H.R., Marczylo T.H., Morgan B., Hemingway D., Plummer S.M., et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 21.Ravindranath V., Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16:259–265. doi: 10.1016/0300-483X(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 22.Ravindranath V., Chandrasekhara N. In vitro studies on the intestinal absorption of curcumin in rats. Toxicology. 1981;20:251–257. doi: 10.1016/0300-483X(81)90056-1. [DOI] [PubMed] [Google Scholar]
- 23.Ravindranath V., Chandrasekhara N. Metabolism of curcumin—Studies with [3H]curcumin. Toxicology. 1981;22:337–344. doi: 10.1016/0300-483X(81)90027-5. [DOI] [PubMed] [Google Scholar]
- 24.Pan M.H., Huang T.M., Lin J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. Biol. Fate Chem. 1999;27:486–494. [PubMed] [Google Scholar]
- 25.Ireson C.R., Jones D.J.L., Orr S., Coughtrie M.W.H., Boocock D.J., Williams M.L., Farmer P.B., Steward W.P., Gescher A.J. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol. Biomark. Prev. 2002;11:105–111. [PubMed] [Google Scholar]
- 26.Ryu E.K., Choe Y.S., Lee K.-H., Choi Y., Kim B.-T. Curcumin and dehydrozingerone derivatives: Synthesis, radiolabeling, and evaluation for beta-amyloid plaque imaging. J. Med. Chem. 2006;49:6111–6119. doi: 10.1021/jm0607193. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez-Tortose M.C., Pulido-Moran M., Granados S., Gaforio J.J., Quiles J.L. Hydroxytyrosol as a Component of the Mediterranean Diet and Its Role in Disease Prevention. In: Preedy V.R., Watson R.R., editors. The Mediterranean Diet: An Evidence-Based Approach. Elsevier Science; London, United Kingdom: 2014. pp. 205–215. [Google Scholar]
- 28.Holder G.M., Plummer J.L., Ryan A.J. The metabolism and excretion of curcumin (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) in the rat. Xenobiotica. 1978;8:761–768. doi: 10.3109/00498257809069589. [DOI] [PubMed] [Google Scholar]
- 29.Prasad S., Tyagi A.K., Aggarwal B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014;46:2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bisht S., Feldmann G., Soni S., Ravi R., Karikar C., Maitra A., Maitra A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): A novel strategy for human cancer therapy. J. Nanobiotechnol. 2007;5 doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie M., Fan D., Zhao Z., Li Z., Li G., Chen Y., He X., Chen A., Li J., Lin X., et al. Nano-curcumin prepared via supercritical: Improved anti-bacterial, anti-oxidant and anti-cancer efficacy. Int. J. Pharm. 2015;496:732–740. doi: 10.1016/j.ijpharm.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Maiti K., Mukherjee K., Gantait A., Saha B.P., Mukherjee P.K. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007;330:155–163. doi: 10.1016/j.ijpharm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 33.Wood B., Brooks A. Human evolution. We are what we ate. Nature. 1999;400:219–220. doi: 10.1038/22227. [DOI] [PubMed] [Google Scholar]
- 34.Estruch R., Martinez-Gonzalez M.A., Corella D., Salas-Salvado J., Ruiz-Gutierrez V., Covas M.I., Fiol M., Gomez-Gracia E., Lopez-Sabater M.C., Vinyoles E., et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006;145 doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 35.Goncalves I., Andersson Georgiadou E., Mattsson S., Skog G., Pedro L., Fernandes E.F.J., Dias N., Engstrom G., Nilsson J., Stenstrom K. Direct association between diet and the stability of human atherosclerotic plaque. Sci. Rep. 2015;5 doi: 10.1038/srep15524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Couto E., Boffetta P., Lagiou P., Ferrari P., Buckland G., Overvad K., Dahm C.C., Tjonneland A., Olsen A., Clavel-Chapelon F., et al. Mediterranean dietary pattern and cancer risk in the EPIC cohort. Br. J. Cancer. 2011;104:1493–1499. doi: 10.1038/bjc.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howes M.J., Simmonds M.S. The role of phytochemicals as micronutrients in health and disease. Curr. Opin. Clin. Nutr. Metab. Care. 2014;17:558–566. doi: 10.1097/MCO.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 38.Sadowska-Bartosz I., Bartosz G. Effect of antioxidants supplementation on aging and longevity. Biomed. Res. Int. 2014 doi: 10.1155/2014/404680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eigner D., Scholz D. Ferula asa-foetida and Curcuma longa in traditional medical treatment and diet in Nepal. J. Ethnopharmacol. 1999;67 doi: 10.1016/S0378-8741(98)00234-7. [DOI] [PubMed] [Google Scholar]
- 40.Wright J.S. Predicting the antioxidant activity of curcumin and curcuminoids. J. Mol. Struct. THEOCHEM. 2002;591:207–217. doi: 10.1016/S0166-1280(02)00242-7. [DOI] [Google Scholar]
- 41.Priyadarsini K.I., Maity D.K., Naik G.H., Kumar M.S., Unnikrishnan M.K., Satav J.G., Mohan H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003;35:475–484. doi: 10.1016/S0891-5849(03)00325-3. [DOI] [PubMed] [Google Scholar]
- 42.Joe B., Lokesh B.R. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim. Biophys. Acta. 1994;1224:255–263. doi: 10.1016/0167-4889(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 43.Reddy A.C., Lokesh B.R. Effect of dietary turmeric (Curcuma longa) on iron-induced lipid peroxidation in the rat liver. Food Chem. Toxicol. 1994;32:279–283. doi: 10.1016/0278-6915(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 44.Jeong G.S., Oh G.S., Pae H.-O., Jeong S.-O., Kim Y.-C., Shin M.-K., Seo B.Y., Han S.Y., Lee H.S., Jeong J.-G., et al. Comparative effects of curcuminoids on endothelial heme oxygenase-1 expression: Ortho-methoxy groups are essential to enhance heme oxygenase activity and protection. Exp. Mol. Med. 2006;38:393–400. doi: 10.1038/emm.2006.46. [DOI] [PubMed] [Google Scholar]
- 45.Reddy A.C., Lokesh B.R. Effect of curcumin and eugenol on iron-induced hepatic toxicity in rats. Toxicology. 1996;107:39–45. doi: 10.1016/0300-483X(95)03199-P. [DOI] [PubMed] [Google Scholar]
- 46.Rukkumani R., Aruna K., Varma P.S., Menon V.P. Curcumin influences hepatic expression patterns of matrix metalloproteinases in liver toxicity. Ital. J. Biochem. 2004;53:61–66. [PubMed] [Google Scholar]
- 47.Iqbal M., Sharma S.D., Okazaki Y., Fujisawa M., Okada S. Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: Possible role in protection against chemical carcinogenesis and toxicity. Pharmacol. Toxicol. 2003;92:33–38. doi: 10.1034/j.1600-0773.2003.920106.x. [DOI] [PubMed] [Google Scholar]
- 48.Abdel-Daim M.M., Abdou R.H. Protective Effects of Diallyl Sulfide and Curcumin Separately against Thallium-Induced Toxicity in Rats. Cell J. 2015;17:379–388. doi: 10.22074/cellj.2016.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai C., Tang S., Li D., Zhao K., Xiao X. Curcumin attenuates quinocetone-induced oxidative stress and genotoxicity in human hepatocyte L02 cells. Toxicol. Mech. Methods. 2015;25:340–346. doi: 10.3109/15376516.2015.1045659. [DOI] [PubMed] [Google Scholar]
- 50.Joe B., Vijaykumar M., Lokesh B.R. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 51.Kocaadam B., Sanlier N. Curcumin, an Active Component of Turmeric (Curcuma longa), and Its Effects on Health. Crit. Rev. Food Sci. Nutr. 2015 doi: 10.1080/10408398.2015.1077195. [DOI] [PubMed] [Google Scholar]
- 52.Biswas S.K., McClure D., Jimenez L.A., Megson I.L., Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: Mechanism of free radical scavenging activity. Antioxid. Redox Signal. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 53.Khopde S.M., Priyadarsini K.I., Guha S.N., Satav J.G., Venkatesan P., Rao M.N. Inhibition of radiation-induced lipid peroxidation by tetrahydrocurcumin: Possible mechanisms by pulse radiolysis. Biosci. Biotechnol. Biochem. 2000;64:503–509. doi: 10.1271/bbb.64.503. [DOI] [PubMed] [Google Scholar]
- 54.Sreejayan N., Rao M.N. Free radical scavenging activity of curcuminoids. Arzneimittel-Forschung. 1996;46:169–171. [PubMed] [Google Scholar]
- 55.Liu Y., Wu Y.M., Zhang P.Y. Protective effects of curcumin and quercetin during benzo(a)pyrene induced lung carcinogenesis in mice. Eur. Rev. Med. Pharmacol. Sci. 2015;19:1736–1743. [PubMed] [Google Scholar]
- 56.Wu J., Li Q., Wang X., Yu S., Li L., Wu X., Chen Y., Zhao J., Zhao Y. Neuroprotection by curcumin in ischemic brain injury involves the Akt/Nrf2 pathway. PLoS ONE. 2013;8:e59843. doi: 10.1371/journal.pone.0059843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y., Li J., Li S., Li Y., Wang X., Liu B., Fu Q., Ma S. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicol. Appl. Pharmacol. 2015;286:53–63. doi: 10.1016/j.taap.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 58.Fu X.Y., Yang M.F., Cao M.Z., Li D.W., Yang X.Y., Sun J.Y., Zhang Z.Y., Mao L.L., Zhang S., Wang F.Z., et al. Strategy to Suppress Oxidative Damage-Induced Neurotoxicity in PC12 Cells by Curcumin: The Role of ROS-Mediated DNA Damage and the MAPK and AKT Pathways. Mol. Neurobiol. 2016;53:369–378. doi: 10.1007/s12035-014-9021-1. [DOI] [PubMed] [Google Scholar]
- 59.Ahsan H., Hadi S.M. Strand scission in DNA induced by curcumin in the presence of Cu(II) Cancer Lett. 1998;124:23–30. doi: 10.1016/S0304-3835(97)00442-4. [DOI] [PubMed] [Google Scholar]
- 60.Ahsan H., Parveen N., Khan N.U., Hadi S.M. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem. Biol. Interact. 1999;121:161–175. doi: 10.1016/S0009-2797(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 61.Kim B., Kim H.S., Jung E.J., Lee J.Y., Tsang B.K., Lim J.M., Song Y.S. Curcumin induces ER stress-mediated apoptosis through selective generation of reactive oxygen species in cervical cancer cells. Mol Carcinog. 2015 doi: 10.1002/mc.22332. [DOI] [PubMed] [Google Scholar]
- 62.Yang C.L., Ma Y.G., Xue Y.X., Liu Y.Y., Xie H., Qiu G.R. Curcumin induces small cell lung cancer NCI-H446 cell apoptosis via the reactive oxygen species-mediated mitochondrial pathway and not the cell death receptor pathway. DNA Cell Biol. 2012;31:139–150. doi: 10.1089/dna.2011.1300. [DOI] [PubMed] [Google Scholar]
- 63.Huang A.-C., Chang C.-L., Yu C.-S., Chen P.-Y., Yang J.-S., Ji B.-C., Lin T.-P., Chiu C.-F., Yeh S.-P., Huang Y.-P., et al. Induction of apoptosis by curcumin in murine myelomonocytic leukemia WEHI-3 cells is mediated via endoplasmic reticulum stress and mitochondria-dependent pathways. Environ. Toxicol. 2013;28:255–266. doi: 10.1002/tox.20716. [DOI] [PubMed] [Google Scholar]
- 64.Aggarwal B.B., Shishodia S., Sandur S.K., Pandey M.K., Sethi G. Inflammation and cancer: How hot is the link? Biochem. Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 65.Amor S., Peferoen L.A.N., Vogel D.Y.S., Breur M., van der Valk P., Baker D., van Noort J.M. Inflammation in neurodegenerative diseases—An update. Immunology. 2014;142:151–166. doi: 10.1111/imm.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amor S., Puentes F., Baker D., van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Basnet P., Skalko-Basnet N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jurenka J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. J. Clin. Ther. 2009;14:141–153. [PubMed] [Google Scholar]
- 70.Recio M.C., Andujar I., Rios J.L. Anti-inflammatory agents from plants: Progress and potential. Curr. Med. Chem. 2012;19:2088–2103. doi: 10.2174/092986712800229069. [DOI] [PubMed] [Google Scholar]
- 71.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sethi G., Sung B., Aggarwal B.B. Nuclear factor-kappaB activation: From bench to bedside. Exp. Biol. Med. 2008;233:21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- 73.Anthwal A., Thakur B.K., Rawat M.S.M., Rawat D.S., Tyagi A.K., Aggarwal B.B. Synthesis, characterization and in vitro anticancer activity of C-5 curcumin analogues with potential to inhibit TNF-alpha-induced NF-kappaB activation. BioMed Res. Int. 2014 doi: 10.1155/2014/524161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gupta S.C., Tyagi A.K., Deshmukh-Taskar P., Hinojosa M., Prasad S., Aggarwal B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch Biochem. Biophys. 2014;559:91–99. doi: 10.1016/j.abb.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Singh S., Aggarwal B.B. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) J. Biol. Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 76.Cretu E., Trifan A., Vasincu A., Miron A. Plant-derived anticancer agents - curcumin in cancer prevention and treatment. Rev. Medico-Chir. Soc. Med. Nat. Iasi. 2012;116:1223–1229. [PubMed] [Google Scholar]
- 77.Cho J.W., Lee K.S., Kim C.W. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int. J. Mol. Med. 2007;19:469–474. [PubMed] [Google Scholar]
- 78.Jin C.Y., Lee J.D., Park C., Choi Y.H., Kim G.Y. Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta Pharmacol. Sin. 2007;28:1645–1651. doi: 10.1111/j.1745-7254.2007.00651.x. [DOI] [PubMed] [Google Scholar]
- 79.Locksley R.M. Asthma and allergic inflammation. Cell. 2010;140:777–783. doi: 10.1016/j.cell.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moqbel R., Odemuyiwa S.O. Allergy, Asthma, and Inflammation: Which Inflammatory Cell Type Is More Important? Allergy Asthma Clin. Immunol. 2008;4:150–156. doi: 10.1186/1710-1492-4-4-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kurup V.P., Barrios C.S. Immunomodulatory effects of curcumin in allergy. Mol. Nutr. Food Res. 2008;52:1031–1039. doi: 10.1002/mnfr.200700293. [DOI] [PubMed] [Google Scholar]
- 82.Chong L., Zhang W., Nie Y., Yu G., Liu L., Lin L., Wen S., Zhu L., Li C. Protective effect of curcumin on acute airway inflammation of allergic asthma in mice through Notch1-GATA3 signaling pathway. Inflammation. 2014;37:1476–1485. doi: 10.1007/s10753-014-9873-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nilani P., Kasthuribai N., Duraisamy B., Dhamodaran P., Ravichandran S., Ilango K., Suresh B. In vitro antioxidant activity of selected antiasthmatic herbal constituents. Anc. Sci. Life. 2009;28:3–6. [PMC free article] [PubMed] [Google Scholar]
- 84.Ma C., Ma Z., Fu Q., Ma S. Curcumin attenuates allergic airway inflammation by regulation of CD4+CD25+ regulatory T cells (Tregs)/Th17 balance in ovalbumin-sensitized mice. Fitoterapia. 2013;87:57–64. doi: 10.1016/j.fitote.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 85.Ramadan G., El-Menshawy O. Protective effects of ginger-turmeric rhizomes mixture on joint inflammation, atherogenesis, kidney dysfunction and other complications in a rat model of human rheumatoid arthritis. Int. J. Rheum. Dis. 2013;16:219–229. doi: 10.1111/1756-185X.12054. [DOI] [PubMed] [Google Scholar]
- 86.Tyagi P., Khan H.A. Amelioration of oxidative stress in the joint tissue may be the basis for the antiarthritic activity of Terminalia arjuna bark extract. Int. J. Rheum. Dis. 2014 doi: 10.1111/1756-185X.12429. [DOI] [PubMed] [Google Scholar]
- 87.Godin A.M., Araujo D.P., Menezes R.R., Brito A.M., Melo I.S., Coura G.M., Soares D.G., Bastos L.F., Amaral F.A., Ribeiro L.S., et al. Activities of 2-phthalimidethanol and 2-phthalimidethyl nitrate, phthalimide analogs devoid of the glutarimide moiety, in experimental models of inflammatory pain and edema. Pharmacol. Biochem. Behav. 2014;122:291–298. doi: 10.1016/j.pbb.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 88.Chandran B., Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother. Res. 2012;26:1719–1725. doi: 10.1002/ptr.4639. [DOI] [PubMed] [Google Scholar]
- 89.Kloesch B., Becker T., Dietersdorfer E., Kiener H., Steiner G. Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes. Int. Immunopharmacol. 2013;15:400–405. doi: 10.1016/j.intimp.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 90.Park C., Moon D.O., Choi I.W., Choi B.T., Nam T.J., Rhu C.H., Kwon T.K., Lee W.H., Kim G.Y., Choi Y.H. Curcumin induces apoptosis and inhibits prostaglandin E(2) production in synovial fibroblasts of patients with rheumatoid arthritis. Int. J. Mol. Med. 2007;20:365–372. doi: 10.3892/ijmm.20.3.365. [DOI] [PubMed] [Google Scholar]
- 91.Moon D.O., Kim M.O., Choi Y.H., Park Y.M., Kim G.Y. Curcumin attenuates inflammatory response in IL-1beta-induced human synovial fibroblasts and collagen-induced arthritis in mouse model. Int. Immunopharmacol. 2010;10:605–610. doi: 10.1016/j.intimp.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 92.Atreya I., Atreya R., Neurath M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 93.Topcu-Tarladacalisir Y., Akpolat M., Uz Y.H., Kizilay G., Sapmaz-Metin M., Cerkezkayabekir A., Omurlu I.K. Effects of curcumin on apoptosis and oxidoinflammatory regulation in a rat model of acetic acid-induced colitis: The roles of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. J. Med. Food. 2013;16:296–305. doi: 10.1089/jmf.2012.2550. [DOI] [PubMed] [Google Scholar]
- 94.Liu L., Shang Y., Li M., Han X., Wang J., Wang J. Curcumin ameliorates asthmatic airway inflammation by activating nuclear factor-E2-related factor 2/haem oxygenase (HO)-1 signalling pathway. Clin. Exp. Pharmacol. Physiol. 2015;42:520–529. doi: 10.1111/1440-1681.12384. [DOI] [PubMed] [Google Scholar]
- 95.Epstein J., Sanderson I.R., Macdonald T.T. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Br. J. Nutr. 2010;103:1545–1557. doi: 10.1017/S0007114509993667. [DOI] [PubMed] [Google Scholar]
- 96.Hanai H., Sugimoto K. Curcumin has bright prospects for the treatment of inflammatory bowel disease. Curr. Pharm. Des. 2009;15:2087–2094. doi: 10.2174/138161209788489177. [DOI] [PubMed] [Google Scholar]
- 97.Motawi T.K., Rizk S.M., Shehata A.H. Effects of curcumin and Ginkgo biloba on matrix metalloproteinases gene expression and other biomarkers of inflammatory bowel disease. J. Physiol. Biochem. 2012;68:529–539. doi: 10.1007/s13105-012-0168-9. [DOI] [PubMed] [Google Scholar]
- 98.Bhardwaj P., Yadav R.K. Chronic pancreatitis: Role of oxidative stress and antioxidants. Free Radic. Res. 2013;47:941–949. doi: 10.3109/10715762.2013.804624. [DOI] [PubMed] [Google Scholar]
- 99.Gulcubuk A., Haktanir D., Cakiris A., Ustek D., Guzel O., Erturk M., Karabagli M., Akyazi I., Cicekci H., Altunatmaz K., et al. Effects of curcumin on proinflammatory cytokines and tissue injury in the early and late phases of experimental acute pancreatitis. Pancreatology. 2013;13:347–354. doi: 10.1016/j.pan.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 100.Nagar A.B., Gorelick F.S. Acute pancreatitis. Curr. Opin. Gastroenterol. 2004;20:439–443. doi: 10.1097/00001574-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 101.Dhillon N., Aggarwal B.B., Newman R.A., Wolff R.A., Kunnumakkara A.B., Abbruzzese J.L., Ng C.S., Badmaev V., Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 102.Santos A.M., Lopes T., Oleastro M., Gato I.V., Floch P., Benejat L., Chaves P., Pereira T., Seixas E., Machado J., et al. Curcumin inhibits gastric inflammation induced by Helicobacter pylori infection in a mouse model. Nutrients. 2015;7:306–320. doi: 10.3390/nu7010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lavoie S., Chen Y., Dalton T.P., Gysin R., Cuenod M., Steullet P., Do K.Q. Curcumin, quercetin, and tBHQ modulate glutathione levels in astrocytes and neurons: Importance of the glutamate cysteine ligase modifier subunit. J. Neurochem. 2009;108:1410–1422. doi: 10.1111/j.1471-4159.2009.05908.x. [DOI] [PubMed] [Google Scholar]
- 104.Karlstetter M., Lippe E., Walczak Y., Moehle C., Aslanidis A., Mirza M., Langmann T. Curcumin is a potent modulator of microglial gene expression and migration. J. Neuroinflamm. 2011;8 doi: 10.1186/1742-2094-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang J., Zhang Y.J., Du S. The protective effect of curcumin on Abeta induced aberrant cell cycle reentry on primary cultured rat cortical neurons. Eur. Rev. Med. Pharmacol. Sci. 2012;16:445–454. [PubMed] [Google Scholar]
- 106.Darvesh A.S., Carroll R.T., Bishayee A., Novotny N.A., Geldenhuys W.J., Van der Schyf C.J. Curcumin and neurodegenerative diseases: A perspective. Expert Opin. Investig. Drugs. 2012;21:1123–1140. doi: 10.1517/13543784.2012.693479. [DOI] [PubMed] [Google Scholar]
- 107.Ortiz-Ortiz M.A., Moran J.M., Ruiz-Mesa L.M., Niso-Santano M., Bravo-SanPedro J.M., Gomez-Sanchez R., Gonzalez-Polo R.A., Fuentes J.M. Curcumin exposure induces expression of the Parkinson’s disease-associated leucine-rich repeat kinase 2 (LRRK2) in rat mesencephalic cells. Neurosci. Lett. 2010;468:120–124. doi: 10.1016/j.neulet.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 108.Ye J., Zhang Y. Curcumin protects against intracellular amyloid toxicity in rat primary neurons. Int. J. Clin. Exp. Med. 2012;5:44–49. [PMC free article] [PubMed] [Google Scholar]
- 109.Jiang H., Tian X., Guo Y., Duan W., Bu H., Li C. Activation of nuclear factor erythroid 2-related factor 2 cytoprotective signaling by curcumin protect primary spinal cord astrocytes against oxidative toxicity. Biol. Pharm. Bull. 2011;34:1194–1197. doi: 10.1248/bpb.34.1194. [DOI] [PubMed] [Google Scholar]
- 110.Cole G.M., Teter B., Frautschy S.A. Neuroprotective effects of curcumin. Adv. Exp. Med. Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ono K., Hasegawa K., Naiki H., Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J. Neurosci. Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 112.Jaisin Y., Thampithak A., Meesarapee B., Ratanachamnong P., Suksamrarn A., Phivthong-Ngam L., Phumala-Morales N., Chongthammakun S., Govitrapong P., Sanvarinda Y. Curcumin I protects the dopaminergic cell line SH-SY5Y from 6-hydroxydopamine-induced neurotoxicity through attenuation of p53-mediated apoptosis. Neurosci. Lett. 2011;489:192–196. doi: 10.1016/j.neulet.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 113.Wang J., Du X.X., Jiang H., Xie J.X. Curcumin attenuates 6-hydroxydopamine-induced cytotoxicity by anti-oxidation and nuclear factor-kappa B modulation in MES23.5 cells. Biochem. Pharmacol. 2009;78:178–183. doi: 10.1016/j.bcp.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 114.Frautschy S.A., Hu W., Kim P., Miller S.A., Chu T., Harris-White M.E., Cole G.M. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol. Aging. 2001;22:993–1005. doi: 10.1016/S0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 115.Begum A.N., Jones M.R., Lim G.P., Morihara T., Kim P., Heath D.D., Rock C.L., Pruitt M.A., Yang F., Hudspeth B., et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garcia-Alloza M., Borrelli L.A., Rozkalne A., Hyman B.T., Bacskai B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- 117.Tripanichkul W., Jaroensuppaperch E.O. Curcumin protects nigrostriatal dopaminergic neurons and reduces glial activation in 6-hydroxydopamine hemiparkinsonian mice model. Int. J. Neurosci. 2012;122:263–270. doi: 10.3109/00207454.2011.648760. [DOI] [PubMed] [Google Scholar]
- 118.Hickey M.A., Zhu C., Medvedeva V., Lerner R.P., Patassini S., Franich N.R., Maiti P., Frautschy S.A., Zeitlin S., Levine M.S., et al. Improvement of neuropathology and transcriptional deficits in CAG 140 knock-in mice supports a beneficial effect of dietary curcumin in Huntington’s disease. Mol. Neurodegener. 2012;7 doi: 10.1186/1750-1326-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ahmed T., Enam S.A., Gilani A.H. Curcuminoids enhance memory in an amyloid-infused rat model of Alzheimer’s disease. Neuroscience. 2010;169:1296–1306. doi: 10.1016/j.neuroscience.2010.05.078. [DOI] [PubMed] [Google Scholar]
- 120.Kakkar V., Kaur I.P. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem. Toxicol. 2011;49:2906–2913. doi: 10.1016/j.fct.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 121.Pan R., Qiu S., Lu D.X., Dong J. Curcumin improves learning and memory ability and its neuroprotective mechanism in mice. Chin. Med. J. 2008;121:832–839. [PubMed] [Google Scholar]
- 122.Hu M., Du Q., Vancurova I., Lin X., Miller E.J., Simms H.H., Wang P. Proapoptotic effect of curcumin on human neutrophils: Activation of the p38 mitogen-activated protein kinase pathway. Crit. Care Med. 2005;33:2571–2578. doi: 10.1097/01.CCM.0000186760.20502.C7. [DOI] [PubMed] [Google Scholar]
- 123.Literat A., Su F., Norwicki M., Durand M., Ramanathan R., Jones C.A., Minoo P., Kwong K.Y. Regulation of pro-inflammatory cytokine expression by curcumin in hyaline membrane disease (HMD) Life Sci. 2001;70:253–267. doi: 10.1016/S0024-3205(01)01398-4. [DOI] [PubMed] [Google Scholar]
- 124.Thresiamma K.C., George J., Kuttan R. Protective effect of curcumin, ellagic acid and bixin on radiation induced toxicity. Indian J. Exp. Biol. 1996;34:845–847. [PubMed] [Google Scholar]
- 125.Crouch E. Pathobiology of pulmonary fibrosis. Am. J. Physiol. 1990;259:L159–L184. doi: 10.1152/ajplung.1990.259.4.L159. [DOI] [PubMed] [Google Scholar]
- 126.Punithavathi D., Venkatesan N., Babu M. Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. Br. J. Pharmacol. 2000;131:169–172. doi: 10.1038/sj.bjp.0703578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Punithavathi D., Venkatesan N., Babu M. Protective effects of curcumin against amiodarone-induced pulmonary fibrosis in rats. Br. J. Pharmacol. 2003;139:1342–1350. doi: 10.1038/sj.bjp.0705362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Venkatesan N., Chandrakasan G. Modulation of cyclophosphamide-induced early lung injury by curcumin, an anti-inflammatory antioxidant. Mol. Cell. Biochem. 1995;142:79–87. doi: 10.1007/BF00928916. [DOI] [PubMed] [Google Scholar]
- 129.Venkatesan N. Pulmonary protective effects of curcumin against paraquat toxicity. Life Sci. 2000;66:PL21–PL28. doi: 10.1016/S0024-3205(99)00576-7. [DOI] [PubMed] [Google Scholar]
- 130.Broaddus V.C., Mason R.C., Ernst J.D., King T.E., Lazarus S.C., Murray J.F., Nadel J.A., Slutsky A., Gotway M. Murray & Nadel’s Textbook of Respiratory Medicine. Elsevier Health Sciences; Philadelphia, PA, USA: 2015. [Google Scholar]
- 131.Roth M., Black J.L. Transcription factors in asthma: Are transcription factors a new target for asthma therapy? Curr. Drug Targets. 2006;7:589–595. doi: 10.2174/138945006776818638. [DOI] [PubMed] [Google Scholar]
- 132.Oh S.W., Cha J.Y., Jung J.E., Chang B.C., Kwon H.J., Lee B.R., Kim D.Y. Curcumin attenuates allergic airway inflammation and hyper-responsiveness in mice through NF-kappaB inhibition. J. Ethnopharmacol. 2011;136:414–421. doi: 10.1016/j.jep.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 133.Walsh G.M., Al-Rabia M., Blaylock M.G., Sexton D.W., Duncan C.J.A., Lawrie A. Control of eosinophil toxicity in the lung. Curr. Drug Targets Inflamm. Allergy. 2005;4:481–486. doi: 10.2174/1568010054526296. [DOI] [PubMed] [Google Scholar]
- 134.Leckie M.J. Anti-interleukin-5 monoclonal antibodies: Preclinical and clinical evidence in asthma models. Am. J. Respir. Med. Drugs Devices Other Interv. 2003;2:245–259. doi: 10.1007/BF03256653. [DOI] [PubMed] [Google Scholar]
- 135.Pease J.E. Asthma, allergy and chemokines. Curr. Drug Targets. 2006;7:3–12. doi: 10.2174/138945006775270204. [DOI] [PubMed] [Google Scholar]
- 136.Kobayashi T., Hashimoto S., Horie T. Curcumin inhibition of Dermatophagoides farinea-induced interleukin-5 (IL-5) and granulocyte macrophage-colony stimulating factor (GM-CSF) production by lymphocytes from bronchial asthmatics. Biochem. Pharmacol. 1997;54:819–824. doi: 10.1016/S0006-2952(97)00220-7. [DOI] [PubMed] [Google Scholar]
- 137.Chung S.-H., Choi S.H., Choi J.A., Chuck R.S., Joo C.-K. Curcumin suppresses ovalbumin-induced allergic conjunctivitis. Mol. Vis. 2012;18:1966–1972. [PMC free article] [PubMed] [Google Scholar]
- 138.Yano S., Terai M., Shimizu K.L., Futagami Y., Horie S., Tsuchiya S., Ikegami F., Sekine T., Takamoto K., Saito K., et al. Antiallergic Activity of Curcuma longa (II): Features of inhibitory actions on histamine release from mast cells. Nat. Med. 2000;54:325–329. [Google Scholar]
- 139.Li X., Lu Y., Jin Y., Son J.-K., Lee S.H., Chang H.W. Curcumin inhibits the activation of immunoglobulin e-mediated mast cells and passive systemic anaphylaxis in mice by reducing serum eicosanoid and histamine levels. Biomol. Ther. 2014;22:27–34. doi: 10.4062/biomolther.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Deeb D., Jiang H., Gao X., Al-Holou S., Danyluk A.L., Dulchavsky S.A., Gautam S.C. Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1-6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-kappaB via inhibition of the prosurvival Akt signaling pathway. J. Pharmacol. Exp. Ther. 2007;321:616–625. doi: 10.1124/jpet.106.117721. [DOI] [PubMed] [Google Scholar]
- 141.Moon D.-O., Kim M.-O., Lee H.-J., Choi Y.H., Park Y.-M., Heo M.-S., Kim G.-Y. Curcumin attenuates ovalbumin-induced airway inflammation by regulating nitric oxide. Biochem. Biophys. Res. Commun. 2008;375:275–279. doi: 10.1016/j.bbrc.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 142.Karaman M., Firinci F., Cilaker S., Uysal P., Tugyan K., Yilmaz O., Uzuner N., Karaman O. Anti-inflammatory effects of curcumin in a murine model of chronic asthma. Allergol. Immunopathol. 2012;40:210–214. doi: 10.1016/j.aller.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 143.Jagetia G.C., Aggarwal B.B. “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 2007;27:19–35. doi: 10.1007/s10875-006-9066-7. [DOI] [PubMed] [Google Scholar]
- 144.Ram A., Das M., Ghosh B. Curcumin attenuates allergen-induced airway hyperresponsiveness in sensitized guinea pigs. Biol. Pharm. Bull. 2003;26:1021–1024. doi: 10.1248/bpb.26.1021. [DOI] [PubMed] [Google Scholar]
- 145.Kaplan N.M. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch. Intern. Med. 1989;149:1514–1520. doi: 10.1001/archinte.1989.00390070054005. [DOI] [PubMed] [Google Scholar]
- 146.Alberti K.G.M.M., Zimmet P., Shaw J., IDF Epidemiology Task Force Consensus Group The metabolic syndrome—A new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 147.Edwards T. Inflammation, pain, and chronic disease: An integrative approach to treatment and prevention. Altern. Ther. Health Med. 2005;11:20–27. [PubMed] [Google Scholar]
- 148.Berg A.H., Scherer P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 149.Lastra G., Manrique C. Perivascular adipose tissue, inflammation and insulin resistance: Link to vascular dysfunction and cardiovascular disease. Horm. Mol. Biol. Clin. Investig. 2015;22:19–26. doi: 10.1515/hmbci-2015-0010. [DOI] [PubMed] [Google Scholar]
- 150.Jain S.K., Rains J., Croad J., Larson B., Jones K. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid. Redox Signal. 2009;11:241–249. doi: 10.1089/ars.2008.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aggarwal B.B. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu. Rev. Nutr. 2010;30:173–199. doi: 10.1146/annurev.nutr.012809.104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Talpur N., Echard B., Ingram C., Bagchi D., Preuss H. Effects of a novel formulation of essential oils on glucose-insulin metabolism in diabetic and hypertensive rats: A pilot study. Diabetes Obes. Metab. 2005;7:193–199. doi: 10.1111/j.1463-1326.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- 153.Seo K.-I., Choi M.-S., Jung U.J., Kim H.-J., Yeo J., Jeon S.-M., Lee M.-K. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol. Nutr. Food Res. 2008;52:995–1004. doi: 10.1002/mnfr.200700184. [DOI] [PubMed] [Google Scholar]
- 154.Shin S.-K., Ha T.-Y., McGregor R.A., Choi M.-S. Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism. Mol. Nutr. Food Res. 2011;55:1829–1840. doi: 10.1002/mnfr.201100440. [DOI] [PubMed] [Google Scholar]
- 155.Kaur G., Meena C. Amelioration of obesity, glucose intolerance, and oxidative stress in high-fat diet and low-dose streptozotocin-induced diabetic rats by combination consisting of “curcumin with piperine and quercetin”. ISRN Pharmacol. 2012 doi: 10.5402/2012/957283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shimabukuro M., Zhou Y.T., Levi M., Unger R.H. Fatty acid-induced beta cell apoptosis: A link between obesity and diabetes. Proc. Natl. Acad. Sci. USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ejaz A., Wu D., Kwan P., Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J. Nutr. 2009;139:919–925. doi: 10.3945/jn.108.100966. [DOI] [PubMed] [Google Scholar]
- 158.Shao W., Yu Z., Chiang Y., Yang Y., Chai T., Foltz W., Lu H., Fantus I.G., Jin T. Curcumin prevents high fat diet induced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PLoS ONE. 2012;7:e28784. doi: 10.1371/journal.pone.0028784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weisberg S.P., Leibel R., Tortoriello D.V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology. 2008;149:3549–3558. doi: 10.1210/en.2008-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jang E.-M., Choi M.-S., Jung U.J., Kim M.-J., Kim H.-J., Jeon S.-M., Shin S.-K., Seong C.-N., Lee M.-K. Beneficial effects of curcumin on hyperlipidemia and insulin resistance in high-fat-fed hamsters. Metab. Clin. Exp. 2008;57:1576–1583. doi: 10.1016/j.metabol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 161.Yang Y.-S., Su Y.-F., Yang H.-W., Lee Y.-H., Chou J.I., Ueng K.-C. Lipid-lowering effects of curcumin in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled trial. Phytother. Res. PTR. 2014;28:1770–1777. doi: 10.1002/ptr.5197. [DOI] [PubMed] [Google Scholar]
- 162.Panahi Y., Khalili N., Hosseini M.S., Abbasinazari M., Sahebkar A. Lipid-modifying effects of adjunctive therapy with curcuminoids-piperine combination in patients with metabolic syndrome: Results of a randomized controlled trial. Complement. Ther. Med. 2014;22:851–857. doi: 10.1016/j.ctim.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 163.Di Pierro F., Bressan A., Ranaldi D., Rapacioli G., Giacomelli L., Bertuccioli A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: Preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2015;19:4195–4202. [PubMed] [Google Scholar]
- 164.Si-Tayeb K., Lemaigre F.P., Duncan S.A. Organogenesis and development of the liver. Dev. Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 165.Ghosh N., Ghosh R., Mandal V., Mandal S.C. Recent advances in herbal medicine for treatment of liver diseases. Pharm. Biol. 2011;49:970–988. doi: 10.3109/13880209.2011.558515. [DOI] [PubMed] [Google Scholar]
- 166.Kaur G., Tirkey N., Bharrhan S., Chanana V., Rishi P., Chopra K. Inhibition of oxidative stress and cytokine activity by curcumin in amelioration of endotoxin-induced experimental hepatoxicity in rodents. Clin. Exp. Immunol. 2006;145:313–321. doi: 10.1111/j.1365-2249.2006.03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cerny D., Lekic N., Vanova K., Muchova L., Horinek A., Kmonickova E., Zidek Z., Kamenikova L., Farghali H. Hepatoprotective effect of curcumin in lipopolysaccharide/-galactosamine model of liver injury in rats: Relationship to HO-1/CO antioxidant system. Fitoterapia. 2011;82:786–791. doi: 10.1016/j.fitote.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 168.Ramirez-Tortosa M.C., Ramirez-Tortosa C.L., Mesa M.D., Granados S., Gil A., Quiles J.L. Curcumin ameliorates rabbits’s steatohepatitis via respiratory chain, oxidative stress, and TNF-alpha. Free Radic. Biol. Med. 2009;47:924–931. doi: 10.1016/j.freeradbiomed.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 169.Wang L., Lv Y., Yao H., Yin L., Shang J. Curcumin prevents the non-alcoholic fatty hepatitis via mitochondria protection and apoptosis reduction. Int. J. Clin. Exp. Pathol. 2015;8:11503–11509. [PMC free article] [PubMed] [Google Scholar]
- 170.Kim K.M., Pae H.O., Zhung M., Ha H.Y., Ha Y.A., Chai K.Y., Cheong Y.K., Kim J.M., Chung H.T. Involvement of anti-inflammatory heme oxygenase-1 in the inhibitory effect of curcumin on the expression of pro-inflammatory inducible nitric oxide synthase in RAW264.7 macrophages. Biomed. Pharmacother. 2008;62:630–636. doi: 10.1016/j.biopha.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 171.Samuhasaneeto S., Thong-Ngam D., Kulaputana O., Suyasunanont D., Klaikeaw N. Curcumin decreased oxidative stress, inhibited NF-kappaB activation, and improved liver pathology in ethanol-induced liver injury in rats. J. Biomed. Biotechnol. 2009 doi: 10.1155/2009/981963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shapiro H., Ashkenazi M., Weizman N., Shahmurov M., Aeed H., Bruck R. Curcumin ameliorates acute thioacetamide-induced hepatotoxicity. J. Gastroenterol. Hepatol. 2006;21:358–366. doi: 10.1111/j.1440-1746.2005.03984.x. [DOI] [PubMed] [Google Scholar]
- 173.Leclercq I.A., Farrell G.C., Sempoux C., dela Pena A., Horsmans Y. Curcumin inhibits NF-kappaB activation and reduces the severity of experimental steatohepatitis in mice. J. Hepatol. 2004;41:926–934. doi: 10.1016/j.jhep.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 174.Garcea G., Berry D.P., Jones D.J.L., Singh R., Dennison A.R., Farmer P.B., Sharma R.A., Steward W.P., Gescher A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomark. Prev. 2005;14:120–125. [PubMed] [Google Scholar]
- 175.Bayet-Robert M., Kwiatkowski F., Leheurteur M., Gachon F., Planchat E., Abrial C., Mouret-Reynier M.-A., Durando X., Barthomeuf C., Chollet P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010;9:8–14. doi: 10.4161/cbt.9.1.10392. [DOI] [PubMed] [Google Scholar]
- 176.Belcaro G., Hosoi M., Pellegrini L., Appendino G., Ippolito E., Ricci A., Ledda A., Dugall M., Cesarone M.R., Maione C., et al. A controlled study of a lecithinized delivery system of curcumin (Meriva) to alleviate the adverse effects of cancer treatment. Phytother. Res. PTR. 2014;28:444–450. doi: 10.1002/ptr.5014. [DOI] [PubMed] [Google Scholar]
- 177.Francis M., Williams S. Effectiveness of Indian Turmeric Powder with Honey as Complementary Therapy on Oral Mucositis : A Nursing Perspective among Cancer Patients in Mysore. Nurs. J. India. 2014;105:258–260. [PubMed] [Google Scholar]
- 178.Panahi Y., Saadat A., Beiraghdar F., Sahebkar A. Adjuvant therapy with bioavailability-boosted curcuminoids suppresses systemic inflammation and improves quality of life in patients with solid tumors: A randomized double-blind placebo-controlled trial. Phytother. Res. PTR. 2014;28:1461–1467. doi: 10.1002/ptr.5149. [DOI] [PubMed] [Google Scholar]
- 179.Sharma R.A., Ireson C.R., Verschoyle R.D., Hill K.A., Williams M.L., Leuratti C., Manson M.M., Marnett L.J., Steward W.P., Gescher A. Effects of dietary curcumin on glutathione S-transferase and malondialdehyde-DNA adducts in rat liver and colon mucosa: Relationship with drug levels. Clin. Cancer Res. 2001;7:1452–1458. [PubMed] [Google Scholar]
- 180.Townsend D.M., Tew K.D. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene. 2003;22:7369–7375. doi: 10.1038/sj.onc.1206940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lu H., Ouyang W., Huang C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 182.Cheng A.L., Hsu C.H., Lin J.K., Hsu M.M., Ho Y.F., Shen T.S., Ko J.Y., Lin J.T., Lin B.R., Ming-Shiang W., et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 183.Hosseini A., Ghorbani A. Cancer therapy with phytochemicals: Evidence from clinical studies. Avicenna J. Phytomed. 2015;5:84–97. [PMC free article] [PubMed] [Google Scholar]
- 184.Sung B., Prasad S., Yadav V.R., Aggarwal B.B. Cancer cell signaling pathways targeted by spice-derived nutraceuticals. Nutr. Cancer. 2012;64:173–197. doi: 10.1080/01635581.2012.630551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.He Z.Y., Shi C.B., Wen H., Li F.L., Wang B.L., Wang J. Upregulation of p53 expression in patients with colorectal cancer by administration of curcumin. Cancer Investig. 2011;29:208–213. doi: 10.3109/07357907.2010.550592. [DOI] [PubMed] [Google Scholar]
- 186.Guo L.D., Chen X.J., Hu Y.H., Yu Z.J., Wang D., Liu J.Z. Curcumin inhibits proliferation and induces apoptosis of human colorectal cancer cells by activating the mitochondria apoptotic pathway. Phytother. Res. 2013;27:422–430. doi: 10.1002/ptr.4731. [DOI] [PubMed] [Google Scholar]
- 187.Lee S.J., Langhans S.A. Anaphase-promoting complex/cyclosome protein Cdc27 is a target for curcumin-induced cell cycle arrest and apoptosis. BMC Cancer. 2012;12 doi: 10.1186/1471-2407-12-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fan Y., Mao R., Yang J. NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176–185. doi: 10.1007/s13238-013-2084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Aggarwal B.B., Vijayalekshmi R.V., Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: Short-term friend, long-term foe. Clin. Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 190.Kunnumakkara A.B., Diagaradjane P., Anand P., Harikumar K.B., Deorukhkar A., Gelovani J., Guha S., Krishnan S., Aggarwal B.B. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int. J. Cancer. 2009;125:2187–2197. doi: 10.1002/ijc.24593. [DOI] [PubMed] [Google Scholar]
- 191.Yang C.-L., Liu Y.-Y., Ma Y.-G., Xue Y.-X., Liu D.-G., Ren Y., Liu X.-B., Li Y., Li Z. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PLoS ONE. 2012;7:e37960. doi: 10.1371/journal.pone.0037960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rajasingh J., Raikwar H.P., Muthian G., Johnson C., Bright J.J. Curcumin induces growth-arrest and apoptosis in association with the inhibition of constitutively active JAK-STAT pathway in T cell leukemia. Biochem. Biophys. Res. Commun. 2006;340:359–368. doi: 10.1016/j.bbrc.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 193.Chakraborty S., Ghosh U., Bhattacharyya N.P., Bhattacharya R.K., Roy M. Inhibition of telomerase activity and induction of apoptosis by curcumin in K-562 cells. Mutat. Res. 2006;596:81–90. doi: 10.1016/j.mrfmmm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 194.Schindler R., Mentlein R. Flavonoids and vitamin E reduce the release of the angiogenic peptide vascular endothelial growth factor from human tumor cells. J. Nutr. 2006;136:1477–1482. doi: 10.1093/jn/136.6.1477. [DOI] [PubMed] [Google Scholar]
- 195.Shao Z.-M., Shen Z.-Z., Liu C.-H., Sartippour M.R., Go V.L., Heber D., Nguyen M. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int. J. Cancer J. Int. Cancer. 2002;98:234–240. doi: 10.1002/ijc.10183. [DOI] [PubMed] [Google Scholar]
- 196.Bachmeier B.E., Mohrenz I.V., Mirisola V., Schleicher E., Romeo F., Hohneke C., Jochum M., Nerlich A.G., Pfeffer U. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis. 2008;29:779–789. doi: 10.1093/carcin/bgm248. [DOI] [PubMed] [Google Scholar]
- 197.Lin S.S., Lai K.C., Hsu S.C., Yang J.S., Kuo C.L., Lin J.P., Ma Y.S., Wu C.C., Chung J.G. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and -9 and Vascular Endothelial Growth Factor (VEGF) Cancer Lett. 2009;285:127–133. doi: 10.1016/j.canlet.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 198.Das L., Vinayak M. Long term effect of curcumin in regulation of glycolytic pathway and angiogenesis via modulation of stress activated genes in prevention of cancer. PLoS ONE. 2014;9:e99583. doi: 10.1371/journal.pone.0099583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Moghaddam S.J., Barta P., Mirabolfathinejad S.G., Ammar-Aouchiche Z., Garza N.T., Vo T.T., Newman R.A., Aggarwal B.B., Evans C.M., Tuvim M.J., et al. Curcumin inhibits COPD-like airway inflammation and lung cancer progression in mice. Carcinogenesis. 2009;30:1949–1956. doi: 10.1093/carcin/bgp229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Klampfer L. Cytokines, inflammation and colon cancer. Curr. Cancer Drug Targets. 2011;11:451–464. doi: 10.2174/156800911795538066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fantini M.C., Pallone F. Cytokines: From gut inflammation to colorectal cancer. Curr. Drug Targets. 2008;9:375–380. doi: 10.2174/138945008784221206. [DOI] [PubMed] [Google Scholar]
- 202.Grivennikov S.I., Karin M. Inflammatory cytokines in cancer: Tumour necrosis factor and interleukin 6 take the stage. Ann. Rheum. Dis. 2011;70:104–108. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 203.Aggarwal B.B., Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 204.Jialal I., Devaraj S. The role of oxidized low density lipoprotein in atherogenesis. J. Nutr. 1996;126:1053S–1057S. doi: 10.1093/jn/126.suppl_4.1053S. [DOI] [PubMed] [Google Scholar]
- 205.Steinberg D., Parthasarathy S., Carew T.E., Khoo J.C., Witztum J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 206.Reaven P.D., Witztum J.L. Oxidized low density lipoproteins in atherogenesis: Role of dietary modification. Annu. Rev. Nutr. 1996;16:51–71. doi: 10.1146/annurev.nu.16.070196.000411. [DOI] [PubMed] [Google Scholar]
- 207.Tsimikas S., Miller Y.I. Oxidative modification of lipoproteins: Mechanisms, role in inflammation and potential clinical applications in cardiovascular disease. Curr. Pharm. Des. 2011;17:27–37. doi: 10.2174/138161211795049831. [DOI] [PubMed] [Google Scholar]
- 208.Quiles J.L., Mesa M.D., Ramirez-Tortosa C.L., Aguilera C.M., Battino M., Gil A., Ramirez-Tortosa M.C. Curcuma longa extract supplementation reduces oxidative stress and attenuates aortic fatty streak development in rabbits. Arterioscler. Thromb. Vasc. Biol. 2002;22:1225–1231. doi: 10.1161/01.ATV.0000020676.11586.F2. [DOI] [PubMed] [Google Scholar]
- 209.Ramirez-Tortosa M.C., Mesa M.D., Aguilera M.C., Quiles J.L., Baro L., Ramirez-Tortosa C.L., Martinez-Victoria E., Gil A. Oral administration of a turmeric extract inhibits LDL oxidation and has hypocholesterolemic effects in rabbits with experimental atherosclerosis. Atherosclerosis. 1999;147:371–378. doi: 10.1016/S0021-9150(99)00207-5. [DOI] [PubMed] [Google Scholar]
- 210.Srivastava K.C. Extracts from two frequently consumed spices—Cumin (Cuminum cyminum) and turmeric (Curcuma longa)—inhibit platelet aggregation and alter eicosanoid biosynthesis in human blood platelets. Prostaglandins Leukot. Essent Fat. Acids. 1989;37:57–64. doi: 10.1016/0952-3278(89)90187-7. [DOI] [PubMed] [Google Scholar]
- 211.Mirzabeigi P., Mohammadpour A.H., Salarifar M., Gholami K., Mojtahedzadeh M., Javadi M.R. The Effect of Curcumin on some of Traditional and Non-traditional Cardiovascular Risk Factors: A Pilot Randomized, Double-blind, Placebo-controlled Trial. Iran. J. Pharm. Res. IJPR. 2015;14:479–486. [PMC free article] [PubMed] [Google Scholar]
- 212.Libby P. Inflammation and cardiovascular disease mechanisms. Am. J. Clin. Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 213.Mason J.C., Libby P. Cardiovascular disease in patients with chronic inflammation: Mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur. Heart J. 2015;36:482c–489c. doi: 10.1093/eurheartj/ehu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Chen T.H., Yang Y.C., Wang J.C., Wang J.J. Curcumin treatment protects against renal ischemia and reperfusion injury-induced cardiac dysfunction and myocardial injury. Transplant. Proc. 2013;45:3546–3549. doi: 10.1016/j.transproceed.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 215.Duan W., Yang Y., Yan J., Yu S., Liu J., Zhou J., Zhang J., Jin Z., Yi D. The effects of curcumin post-treatment against myocardial ischemia and reperfusion by activation of the JAK2/STAT3 signaling pathway. Basic Res. Cardiol. 2012;107 doi: 10.1007/s00395-012-0263-7. [DOI] [PubMed] [Google Scholar]
- 216.Meng Z., Yan C., Deng Q., Gao D.F., Niu X.L. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-kappaB pathways. Acta Pharmacol. Sin. 2013;34:901–911. doi: 10.1038/aps.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Boonla O., Kukongviriyapan U., Pakdeechote P., Kukongviriyapan V., Pannangpetch P., Prachaney P., Greenwald S.E. Curcumin improves endothelial dysfunction and vascular remodeling in 2K-1C hypertensive rats by raising nitric oxide availability and reducing oxidative stress. Nitric Oxide. 2014;42:44–53. doi: 10.1016/j.niox.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 218.Zeng C., Zhong P., Zhao Y., Kanchana K., Zhang Y., Khan Z.A., Chakrabarti S., Wu L., Wang J., Liang G. Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-kappaB both in vitro and in vivo. J. Mol. Cell. Cardiol. 2015;79 doi: 10.1016/j.yjmcc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 219.Tapia E., Soto V., Ortiz-Vega K.M., Zarco-Marquez G., Molina-Jijon E., Cristobal-Garcia M., Santamaria J., Garcia-Nino W.R., Correa F., Zazueta C., et al. Curcumin induces Nrf2 nuclear translocation and prevents glomerular hypertension, hyperfiltration, oxidant stress, and the decrease in antioxidant enzymes in 5/6 nephrectomized rats. Oxid. Med. Cell. Longev. 2012 doi: 10.1155/2012/269039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tapia E., Zatarain-Barron Z.L., Hernandez-Pando R., Zarco-Marquez G., Molina-Jijon E., Cristobal-Garcia M., Santamaria J., Pedraza-Chaverri J. Curcumin reverses glomerular hemodynamic alterations and oxidant stress in 5/6 nephrectomized rats. Phytomedicine. 2013;20:359–366. doi: 10.1016/j.phymed.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 221.Abo-Salem O.M., Harisa G.I., Ali T.M., El-Sayed el S.M., Abou-Elnour F.M. Curcumin ameliorates streptozotocin-induced heart injury in rats. J. Biochem. Mol. Toxicol. 2014;28:263–270. doi: 10.1002/jbt.21562. [DOI] [PubMed] [Google Scholar]
- 222.Yang Y., Duan W., Lin Y., Yi W., Liang Z., Yan J., Wang N., Deng C., Zhang S., Li Y., et al. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic. Biol. Med. 2013;65:667–679. doi: 10.1016/j.freeradbiomed.2013.07.007. [DOI] [PubMed] [Google Scholar]