Abstract
Bacterial wilt is a destructive disease caused by the phytopathogen Ralstonia solanacearum (R. solanacearum), which is widely found in various tobacco-growing areas all over the world. Botanical bactericidal substances have gradually emerged as a hot topic in modern pesticide research. In this study, the antibacterial activities of two phytochemicals (resveratrol and coumarin) against R. solanacearum and their in vivo and in vitro efficacy for controlling tobacco bacterial wilt were evaluated. We rule out significant biological effects of both phytochemicals using transmission electron microscope (TEM) and fluorescence microscope, which suppressed the growth of R. solanacearum. Furthermore, we demonstrated that the toxicity mechanisms mainly involved damaging bacterial cell membrane and preventing swarming motility and biofilm formation. A further pot experiment demonstrated that coumarin and resveratrol significantly inhibited early adhesion and colonization of R. solanacearum in tobacco plants and the corresponding control efficacies were 68% and 85% after incubation for 13 days, respectively. The findings of this study suggest that both resveratrol and coumarin have potential as non-toxic antimicrobial strategies for controlling tobacco bacterial wilt disease.
Keywords: resveratrol, Coumarin, R. solanacearum, antibacterial activity, bacterial wilt
1. Introduction
Ralstonia solanacearum, belonging to the β-proteobacteria, is a soil-borne phytopathogenic bacterium that can cause bacterial wilt disease with destructive damage to a large number of economic crops, as well as some ornamentals. With the characteristic of genetic polymorphism in R. solanacearum, including worldwide geographic distribution and extraordinarily broad host range, bacterial wilt is one of the most devastating diseases in agriculture [1,2]. Usually, R. solanacearum is mainly distributed in the tropics, subtropics, and partly in the temperate belt, and was first described in tobacco in 1908. As a result of long-term co-evolution with the host and environment, R. solanacearum exhibits a high degree of complexity in its geographic distribution, host range, and pathogenetic mechanisms, making it difficult to prevent from infecting the host plants.
Control measures against R. solanacearum have long relied on traditional chemical pesticides (e.g., zinc thiazole, bismerthiazol, and saisentong) and antibiotics in many countries. However, limited efficacy and microbial resistance strains induced by uncontrolled application of chemical pesticides are a growing problem. Moreover, the use of these methods for field trial is largely limited due to their environmental pollution and potential health risks to human health or non-target organisms [3]. In recent years, increasing research has focused on agricultural and biological control for bacterial wilt disease management, such as applying soil additives [4], the adoption of resistant varieties, sterile grafts, and crop rotation [5,6,7]. However, plant bacterial wilt disease is still a challenging subject in agricultural crop protection. Therefore, there is an immediate need to search for new alternative antibacterial agents that can be used for managing R. solanacearum.
Plants are rich in natural antimicrobial substances, named botanical antimicrobial substances. Botanical pesticides and their derivatives have recently attracted extensive attention from chemists and biologists because of their low phytotoxicity, environment friendly effects, rapid environmental degradation, abundant resources, low cost and because they are renewable [8,9]. Wisely, combined with biotechnology advancement, plant-derived natural products have become a major trend in the development of modern pesticides. Up to now, it has been demonstrated that botanical antimicrobial substances from various plants (Gramineae, Labiatae, Apocynaceae, Rutaceae, Liliaceae and Magnoliaceae) [10,11,12,13], including secondary metabolites, have highly efficient sources of antimicrobial activity against agricultural phytopathogens [14,15,16,17] (Table 1). A great number of plant essential oils have been tested for their antimicrobial activities. A number of natural pesticides such as methyl gallate [18], Lansiumamide B [19], and essential oils [10,20] have been used to inhibit the growth of R. solanacearum and control the phytopathogenic diseases caused by them. In addition, the in vitro and in vivo activities of protocatechualdehyde, a natural polyphenol compound isolated from the roots of traditional Chinese medicine radix Salviae Miltiorrhizae, were evaluated against phytopathogen R. solanacearum [21].
Table 1.
Several phytochemicals with inhibitory activity to R. solanacearum.
| Family | Plant Species | Active Ingredients |
|---|---|---|
| Poaceae | Phyllostachys pubescens Mazel | Bamboo tar |
| Cymbopogon martinii | Palmarosa Oil | |
| Lamiaceae | Thymus serpyllum L. | Essential oil |
| Origanum vulgare “Rogeukuppel” | ||
| Saluia plebeia R. Br. | Ethanol extract | |
| Leucas aspera (Willd.) Link | ||
| Apocynaceae | Herba Catharanthi rosei | Ethanol extract |
| Hunteria zeylanica | ||
| Rutaceae | Atalantia buxifolia | Ethanol extract |
| Micromelum falcatum | ||
| Clausena lansium (Lour.) Skeels | Seed extracts | |
| Liliaceae | Allium sativum L. | Root exudates |
| Asteraceae | Amaranthus tricolor L. | Ethanol extract |
| Eupatorium adenophorum Spreng | Leaf exudates | |
| Elephantopus tomentosus L | Ethanol extract | |
| Magnoliaceae | Magnolia officinalis Rehd. et Wils. | Phenolic compounds |
| Others | Stemona sessilifolia (Miq.) Miq | Petrol ether extract |
| Casuarinaequisetifolia L | Ethanol extract | |
| Sophoraflavescens | Alkaloid | |
| Lithospermum erythrorhizon | Chloroform extract | |
| Toxicodendron sylvestre | Trimethylgallic acid | |
| Litsea cubeba | Ethanol extract |
Coumarin is a benzopyrone that occurs naturally in a wide variety of plants (including tonka bean, sweet clover, cinnamon oil and lavender) and essential oils, and they has been reported to exhibit high antifungal activity [22]. Resveratrol is a natural phytoalexin that is the biologically active ingredient of spermatophyte including red wine, grapes, peanuts, and strawberries, in response to environmental stress, and is commonly used to preserve food and beverages. Both resveratrol and coumarin have multiple biological activities and pharmacological effects that can inhibit the growth of human pathogenic bacteria and fungi [23,24,25], which, more significantly, have been extensively used medicinally for anticancer, anticoagulant, and antimicrobial purposes [23,25,26,27,28]. However, to our knowledge, there have been few reports regarding the effects of resveratrol and coumarin on plant pathogen R. solanacearum.
From another point of view, according to existing research, phytochemicals prevent or control plant pathogens mainly via two mechanisms. It was demonstrated that phytochemicals can directly induce damage to the cell wall and cytomembrane of pathogen, thereby altering cell morphology and permeability [29]. On the other hand, phytochemicals can efficiently manage diseases by increasing host resistance. Their application induces the expression of some plant proteins or polysaccharides such as clerodendrum aculeatum-systemic resistance inducing protein (CA-SRIP), glycoproteins and SL-polysaccharides, which can induce and activate host immune mechanisms to achieve the control effect [30]. In our previous work, we demonstrated antibacterial activities of a series of coumarins against R. solanacearum with different substitution patterns, and the results found that hydroxylation at the C-6, C-7 or C-8 position significantly enhanced the antibacterial activity of coumarins [31]. However, the research was limited to laboratory test.
Therefore, based on the concept of healthy cultivation and safe production in modern agriculture, the current study was performed to examine the antibacterial activity of two phytochemicals (resveratrol and coumarin) against R. solanacearum in vivo and in vitro, and to evaluate the possible antibacterial mechanism of action of these compounds by inhibitory effect assay, fluorescence microscope and transmission electron microscopy observations. Further, their disease control efficacy is also tested. This study provides new ideas for investigating the inhibitory mechanism of phytochemicals against R. solanacearum and studying the prevention and control of bacterial wilt of tobacco. The results lay a solid theoretical and practical foundation for the research and development of new biological pesticides.
2. Results
2.1. Determination of MIC and MBC
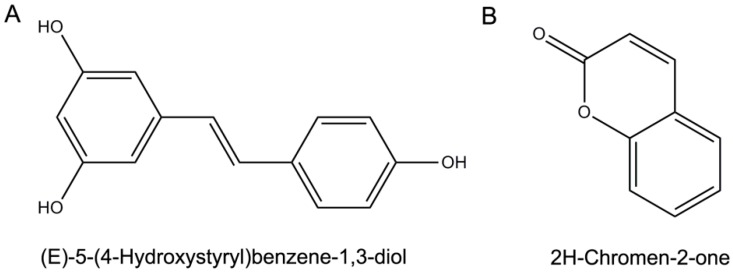
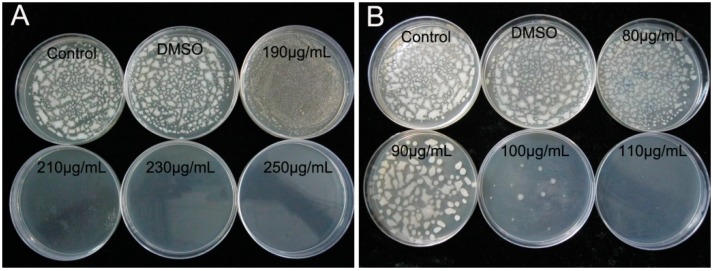
Resveratrol and coumarin are naturally obtained from plant and their molecular structure formulas are shown in Figure 1. As shown in Table 2, the minimum inhibitory concentrations (MICs) of resveratrol and coumarin in liquid medium were determined as 2 and 8 μg/mL, respectively, using a typical microdilution method (ISO 10932|IDF 223:2010). Phytochemicals of less than the MICs had no inhibitory effect on R. solanacearum in the liquid medium (Table 2). The MBCs of the two phytochemicals were measured by plate counting method, and the number of colony grown on phytochemical-containing plates was recorded after 96 h of incubation. The MBCs of resveratrol and coumarin against R. solanacearum were substantially different, 230 and 110 μg/mL, respectively (Figure 2).
Figure 1.
Molecular structural formula and chemical names of: resveratrol (A); and coumarin (B).
Table 2.
Effects of different compounds on the growth of R. solanacearum after 24 h incubation.
| Compounds | Concentration (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| 128 | 64 | 32 | 16 | 8 | 4 | 2 | 1 | |
| resveratrol | + | + | + | + | + | + | + | - |
| coumarin | + | + | + | + | + | - | - | - |
+ represented that compound has inhibitory effect on the growth of R. solanacearum, - represented that compound has no effect on the growth of R. solanacearum.
Figure 2.
The colony growth of R. solanacearum treated with: (A) resveratrol at concentrations ranging from 190 to 250 μg/mL; and (B) coumarin at concentrations ranging from 80 to 110 μg/mL.
2.2. Inhibition of R. solanacearum Growth
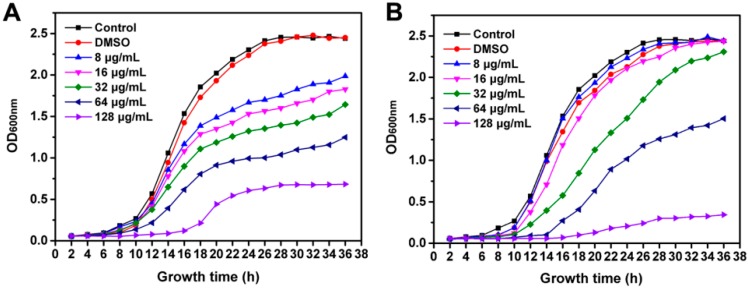
Usually, the antimicrobial activity of compound can be observed by measuring the bacterial growth curve. When R. solanacearum is grown under normal culture conditions, the growth and reproduction period is divided into the lag phase (0–8 h) with less growth, the logarithmic growth phase (8–22 h) with massive growth, and the stationary phase (after 24 h) with stable growth and a gradually decreasing reproduction rate. We plotted the growth curves of R. solanacearum in culture medium in the presence of different concentration of phytochemicals. The same range of concentration was selected between the MIC and MBC of resveratrol and coumarin. As observed in Figure 3, the results showed that both resveratrol and coumarin caused a growth delay of R. solanacearum, depending on the incubation concentration.
Figure 3.
The bacterial growth curve of R. solanacearum in the presence of resveratrol (A) and coumarin (B) at concentrations ranging from 8 to 128 μg/mL. DMSO was indicated as a negative control and Control was used as bacterial cells treatmented with sterile water.
It can be seen from the results that, in the presence of resveratrol, R. solanacearum failed to enter the normal growth cycle. With increasing phytochemical concentration, the logarithmic and stationary phases of R. solanacearum were delayed. It is important to note that the bacteria growth rate significantly decreased with increasing concentration. When the bacteria were treated with 8 μg/mL of resveratrol, there was no major difference versus the control in the beginning; however, bacterial growth became slower after 18 h of incubation. With 128 μg/mL resveratrol, R. solanacearum entered the growth phase after 18 h of incubation and reached the stationary phase after 24 h of incubation; thereafter, no major changes were observed in the bacterial count, displaying significantly inhibitory effects on the bacterial growth (Figure 3A). Similarly, Figure 2B shows that the growth of R. solanacearum cells treated with various concentration of coumarin. Compared with the control, low concentrations (8 and 16 μg/mL) of coumarin had no obvious effects on bacterial growth; however, 32 and 64 μg/mL treatments significantly inhibited the logarithmic and stationary phases of R. solanacearum. Most importantly, bacterial growth was almost completely inhibited by coumarin under 128 μg/mL treatment (Figure 3B).
2.3. Bacterial Cell Morphology Observations
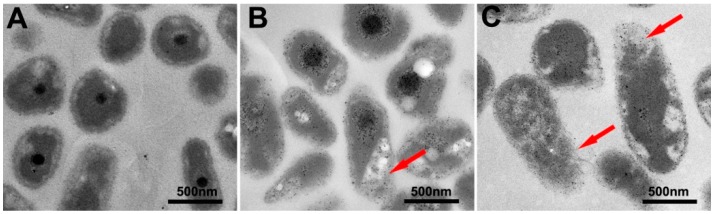
We used TEM to observe cell morphology changes in R. solanacearum when they interacted with the phytochemicals. R. solanacearum cells were treated with resveratrol and coumarin at 128 μg/mL. In the control groups without phytochemical treatment, the cells were intact and uniform in size, with a clear boundary and a smooth surface (Figure 4A). When bacteria were treated with resveratrol, the cell morphology appeared incomplete and turned flat in a dissolved shape, with an unclear boundary (Figure 4B). After treatment with high concentration (128 μg/mL) of coumarin, more severe damage on cell membrane was found, some distorted projections emerged on the cell membrane, and the cell morphology was imperfect, flattened, and partially ruptured (Figure 4C).
Figure 4.
TEM images of R. solanacearum cells after incubation with: sterile water (A); resveratrol (B); and coumarin (C) for 2 h with final concentration 128 μg/mL.
2.4. Suppress the Swarming Motility and Biofilm Formation of R. solanacearum
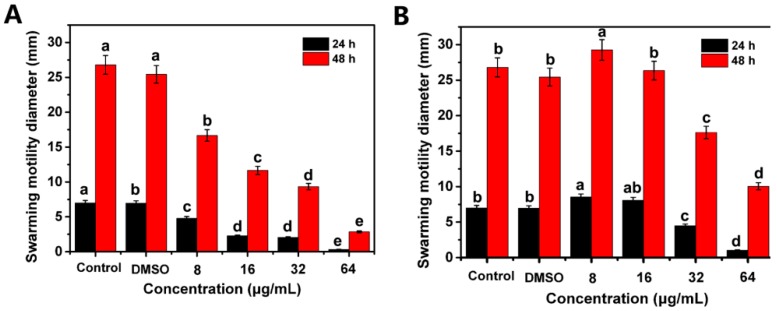
Previous studies, to the best of our knowledge, have reported that flagella-driven swarming motility of R. solanacearum was extremely important to its virulence and contributed significantly to its invasion of host plants. We used the SMM semi-solid medium to evaluate the effects of resveratrol and coumarin on the swarming motility of R. solanacearum. The results displayed that both resveratrol and coumarin restrained the motility diameter of R. solanacearum, revealing significant differences compared with the control group. After incubation for 24 and 48 h, the motility diameter of R. solanacearum were strongly suppressed by resveratrol at different concentrations ranging from 8 to 64 μg/mL in comparison to DMSO and control treatment. The inhibitory effect increased with the rise in resveratrol concentrations. We analyzed the statistical significance using Duncan’s multiple comparison test (p ≤ 0.05) and found significant differences between various concentrations of resveratrol treatment and the control (Figure 5A). Coumarin had slight or no effect on the swarming motility of R. solanacearum at low concentrations (8 and 16 μg/mL). However, at 32 and 64 μg/mL coumarin, bacterial motility was significantly inhibited after both 24 and 48 h of incubation (Figure 5B). Taken together, these results suggest that resveratrol and coumarin inhibited the swarming motility of R. solanacearum in a concentration-dependent manner.
Figure 5.
Effects of resveratrol (A) and coumarin (B) at different concentration on swarming motility of R. solanacearum. Error bars represent the standard deviation (n = 3). Values presented are means and standard errors from four independent experiments. Different letters indicate that the means are significantly different at p = 0.05.
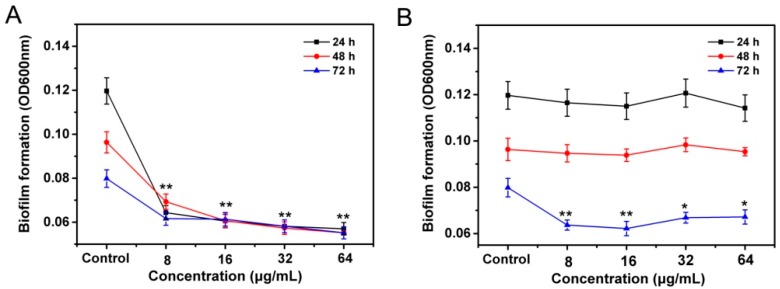
Further, we used a microplate reader to measure the optical density value at 490 nm (OD490) of bacterial biofilms to assess the effects of resveratrol and coumarin on the biofilm-forming ability of R. solanacearum. Bacterial biofilms are a form of bacterial growth on solid surfaces that corresponds to the migratory form of the cells [32]. For most plant pathogens, biofilms constitute a major part of several plant–bacterium interactions, and are involved in locating and infecting host plant roots [33,34]. The current results showed that under the experimental conditions, R. solanacearum exhibited the highest biofilm-forming ability at 24 h of incubation, with an OD490 of 0.120. Biofilm-forming ability gradually decreased with increasing time of incubation, with OD490 = 0.096 and 0.080 after 48 and 72 h, respectively. It is worth noting that treatment concentration plays an important effector role in this interaction process. Differently, the biofilm formation of R. solanacearum was sharply repressed in the presence of resveratrol, even at the low concentration of 8 μg/mL, with the minimum OD490 value of 0.057 after incubation for 24 h (Figure 6A).However, Coumarin had nearly no inhibitory effects on biofilm formation at 24 or 48 h of incubation (Figure 6B), while showed a slight inhibitory effect on biofilm formation of R. solanacearum at 72 h.
Figure 6.
Effects of resveratrol (A) and coumarin (B) at different concentration on biofilm formation of R. solanacearum. Error bars represent the standard deviation (n = 3). * and ** indicate p < 0.05 and p < 0.01, respectively.
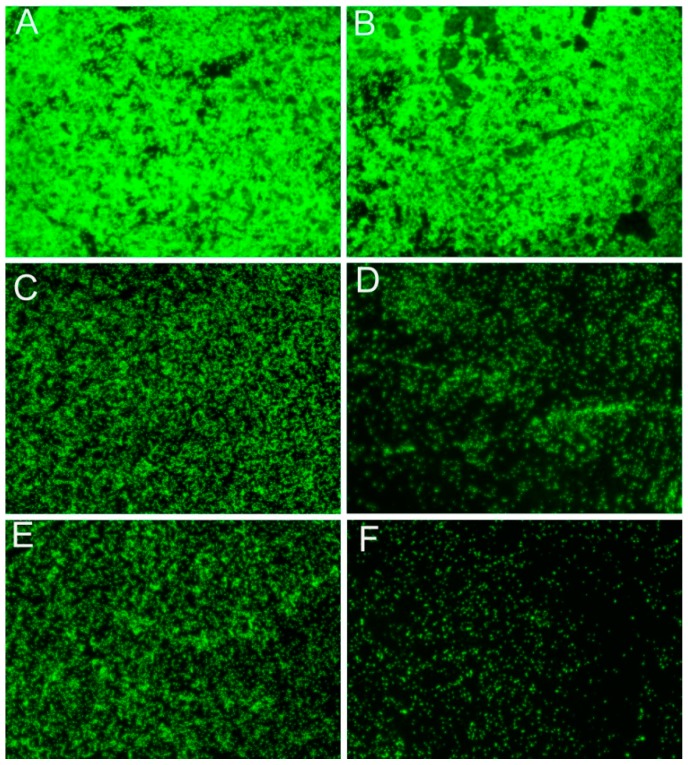
The biofilm inhibitory effects activity of phytochemical against phytopathogens was further substantiated using fluorescent imaging. Fluorescein isothio-cyanate-conjugated concanavalin A (FITC-ConA) dye was used to examine the effects of the two phytochemicals on biofilm formation by R. solanacearum. FITC-ConA can stain extracellular polysaccharides, which is the major component of bacterial biofilms, and emits green fluorescence [35]. The results demonstrated that in the absence of both phytochemical (control group), R. solanacearum biofilm adhered to the carrier in large pieces and formed aggregates of flocculent green fluorescent material (Figure 7A,B). With coumarin (Figure 7C,D) and resveratrol (Figure 7E,F) treatment at 64 μg/mL, only a few bacterial cells adhered to the carrier in a dispersed pattern; the green fluorescent material was markedly reduced and thinned compared with the control. At a higher phytochemical concentration (128 μg/mL), it seems that the number of adherent bacterial cells was reduced even further, while green fluorescent material clustered and overlapped, with enhanced fluorescence. These results further demonstrated that both resveratrol and coumarin inhibited the formation of bacterial biofilms, in agreement with the OD490 measurements.
Figure 7.
Fluorescence microscope images of biofilm formation of R. solanacearum cells after treatment with: (A) sterile water; (B) DMSO; (C,D) coumarin at final concentration of 64 μg/mL and 128 μg/mL respectively; and (E,F) carvacrol at final concentration of 64 μg/mL and 128 μg/mL, respectively. R. solanacearum were incubated with different treatment for 12 h at 30 °C without shaking, and exopolysaccharide matrixes were stained with FITC-ConA. Each treatment was repeated in triplicate.
2.5. Effects of Phytochemicals on Bacterial Adhesion and Colonization
Typical infection of a host plant by R. solanacearum involves three stages: root surface colonization, root cortex infection, and xylem parenchyma infection and invasion [36]. Here, we evaluated the effects of resveratrol and coumarin on bacterial colonization on the tobacco plant. Tobacco plants were soil-soak inoculated with concentration of 10 mL, 1 × 108 cfu/mL of R. solanacearum and incubated at 28 ± 1 °C after irrigating roots with coumarin and resveratrol. Following non-injured root inoculation, resveratrol and coumarin displayed inhibitory effects on the ability of R. solanacearum to adhere to and colonize tobacco roots (Table 3).
Table 3.
Effects of coumarin and resveratrol on rhizosphere colonization of R. solanacearum in tobacco.
| Treatments | On the Root Surface | Within the Root | ||||
|---|---|---|---|---|---|---|
| One Day after Inoculation (cfu/g) | Three Days after Inoculation (cfu/g) | Five Days after Inoculation (cfu/g) | One Day after Inoculation (cfu/g) | Three Days after Inoculation (cfu/g) | Five Days after Inoculation (cfu/g) | |
| Control | 1.03 × 106 b | 6.26 × 106 a | 1.79 × 108 a | 0.00 | 1.50 × 105 b | 5.2 × 106 a |
| Coumarin | 9.30 × 105 b | 4.31 × 106 a,b | 1.83 × 107 c | 0.00 | 4.12 × 104 c | 2.44 × 106 b |
| Resveratrol | 3.44 × 105 c | 3.58 × 106 b | 2.64 × 107 c | 0.00 | 1.41 × 105 b | 2.41 × 106 b |
Values represent means of three independent replicates ± SD. Different letters (a, b, c) within a column indicate statistically significant differences between the means (p < 0.05).
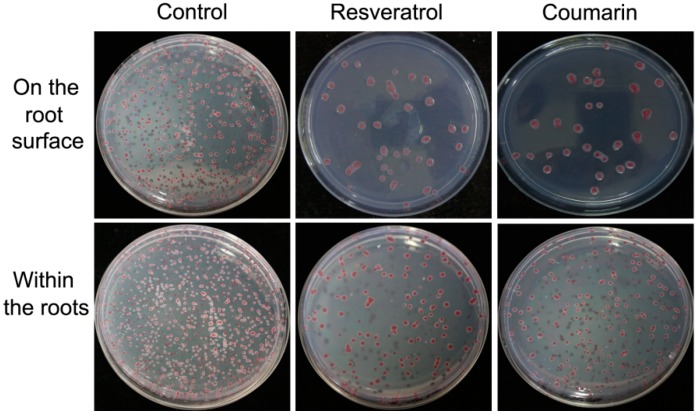
In the control, the bacterial count on the tobacco root surface increased with increasing inoculation time. However, no bacteria were detected in the root interior on Day 1 after inoculation, and the bacterial count increased on Day 3 after inoculation. In the coumarin and resveratrol treatments, the bacterial counts on the root surface were deceased on Day 1 and 3 after inoculation and in the root interior on Day 3 after inoculation compared with the control. The bacterial counts in the phytochemical treatments, either on the root surface or in the root interior, were significantly lower than that in the control group on Day 5 after inculcation. The inhibitory effects were visually shown in SMSA-agar plate (Figure 8).These results showed that resveratrol and coumarin had similar effects on the adhesion and colonization ability of R. solanacearum in the rhizosphere of tobacco plants. Both phytochemicals inhibited R. solanacearum colonization on the root surface and in the root interior.
Figure 8.
Representative SMSA-agar plate images of R. solanacearum colony in root exterior and interior after treatment with 128 μg/mL of coumarin and resveratrol on Day 5 after inoculation. After treatment with both phytochemical for five days, 10−3 to 10−5 bacterial suspensions were used for the test.
2.6. Control Efficacy of Phytochemicals to Bacterial Wilt of Tobacco
We performed a laboratory pot experiment to assess the antibacterial activity of phytochemicals against R. solanacearum in vivo on seven-week-old tobacco seedlings. The high concentration was selected for evaluating the control efficacy test of phytochemicals on bacterial wilt of tobacco. The results were recorded from the emergence of disease symptoms and expressed as disease incidence, disease index, and control efficacy. As shown in Table 4, the coumarin and resveratrol treatments suppressed the development of bacterial wilt, showing significant differences in disease incidence and disease index. The control efficiency of coumarin and resveratrol on tobacco bacterial wilts are 99%, 68%, and 37% and 100%, 85%, and 20% at 9, 13, and 19 days after root irrigation, respectively. After nine days post inoculation, the representative pictures of efficiently controlling the bacterial wilt disease are shown in Figure 9. On Day 13 after inoculation, the disease incidence and disease index in the control plants were 67% and 47%, respectively, both of which were markedly greater than those in the coumarin-treated (28% and 15%) and resveratrol-treated (22% and 7%) plants. Furthermore, the corresponding control efficacy of coumarin and resveratrol were 68% and 85%, respectively. When the disease incidence reached 100% in the control, the control efficacy was most significant in the phytochemical treatments (37% and 20%). Additionally, the emergence of wilting symptoms was markedly delayed, and the course of disease development was slower in phytochemical-treated plants compared with the control plants. Though the control efficacy was decreased with the deterioration of the disease after 19 days incubation, the values were still maintained at 37% and 20%, respectively. It is noteworthy that, when implementing pot experiment, higher inoculation concentration was needed in order to shorten experimental period, while stillensuring the occurrence of tomato bacterial wilt. The control efficacy in field application may be sufficiently higher than laboratory test. Therefore, based on the non-injured root inoculation, both phytochemicals significantly inhibit the incidence of tobacco bacterial wilt disease, demonstrating positive control efficacy by delaying or slowing the occurrence of disease.
Table 4.
Inhibition effects of coumarin and resveratrol against tobacco bacterial wilt under greenhouse.
| Days after Inoculation | Control | Coumarin Treatment | Resveratrol Treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Disease Incidence (%) | Disease Index | Control Efficacy (%) | Disease Incidence (%) | Disease Index | Control Efficacy (%) | Disease Incidence (%) | Disease Index | Control Efficacy (%) | |
| 7 | 0 | 0 | - | 0 | 0 | 100 | 0 | 0 | 100 |
| 9 | 13 | 3 | - | 0.3 | 0 | 99 | 0 | 0 | 100 |
| 13 | 67 | 47 | - | 28 | 15 | 68 | 22 | 7 | 85 |
| 19 | 100 | 92 | - | 80 | 58 | 37 | 86 | 74 | 20 |
Figure 9.
Control efficiency of sterile water (control) (A); resveratrol (B); and coumarin (C) in reducing wilting disease severity caused by R. solanacearum on tobacco seedlings at nine days post inoculation. The treatment concentration was 128 μg/mL. The experiments were performed a minimum of five times.
3. Discussion
Coumarin and resveratrol, a wide group of naturally occurring compounds isolated from a variety of plants, due to their wide range of biological properties, including anticancer, anti-leukemic, antifungal and antibacterial activity, have attracted considerable researcher attention [25,37,38]. However, there have been few studies focused on the antibacterial activity of phytopathgen and its innovative application in the agriculture field. In this study, we aimed to investigate the antibacterial activity of two phytochemicals against R. solanacearum and their control efficacy towards tobacco bacterial wilt, which is one of the catastrophic soil-borne diseases around the world. In vitro evidence from the platecount method, followed by the measurements of MIC and MBC, manifested that resveratrol and coumarin exhibited high bactericidal activity against R. solanacearum in both liquid and solid culture. To some extent, these results are consistent with previous studies on antibacterial activity of the essential oils. Pare et al. reported that thymol, palmarosa oil, and lemongrass oil have markedly antibacterial effects and there was no bacteria detected in ginger plants in pot experiments, significantly reducing the incidence of bacterial wilt. However, no single treatment sufficiently controlled the disease after 180 days of growth [16]. Meanwhile, there were significant differences in the effects of the different phytochemicals and the growth inhibitory effects of phytochemical on R. solanacearum in NB medium are concentration dependent. A comparison of R. solanacearum growth in different treatments revealed that both resveratrol and coumarin can affect the normal growth cycle and inhibit the growth of R. solanacearum. A high concentration (128 μg/mL) of coumarin resulted strongest inhibitory effect with nearly no bacterial growth while different concentrations of resveratrol all showed inhibitory effects on bacterial growth.
Previous studies have shown that the cell membrane is the primary target for the antimicrobial activity of most phytochemicals [18,29,39]. These compounds interact with the lipid bilayer of the cytoplasmic membrane and increase the space between fatty acid chains, destabilizing the membrane structure and enhancing its fluidity and permeability [29,40]. Coumarin has been considered as a naturally active component of essential oil. Another study illustrated that the active components of the essential oil might interact with the cytoplasmic membrane, penetrating to the membrane-bound enzymes on the surface and the phospholipid bilayer, then disturb the subsequent macromolecules synthesis process, such as DNA, RNA, protein, or polysaccharides, eventually causing the death of the cells [41]. Presently, resveratrol and coumarin have shown to be active mainly against medical pathogens, especially methicillin-resistant Staphylococcus aureus (MRSA) and food-derived pathogens [39,40]. Thus far, relevant research on plant pathogens are still is rare, and no study has reported their antibacterial activity against R. solanacearum or the associated mechanism. In the current study, we observed the cell morphology of R. solanacearum by TEM images after treatment with two phytochemicals. The biocontrol mechanism is most likely due to the action of cell morphology deformation and cell membrane damage induced by the phytochemical. We found that in the presence of resveratrol, bacterial cells showed anon-uniform size and irregular shape, the cell surface was wrinkled, and some cells were ruptured. We speculate that the antibacterial action of two phytochemicals is also reflected in physical damage and impacts on cell metabolism. Paulo et al. reported the antibacterial effects of resveratrol against several dozen human pathogenic bacteria [40].They found that resveratrol can alter the morphological structure and DNA content and also affect the metabolic cycle of bacteria, which was in agreement with the antibacterial action of resveratrol and coumarin in our study. Similarly, Ultee et al. [42] indicated that carvacrol can interfere with H+ and K+ transport across the cell membrane of the foodborne pathogen Bacillus cereus, leading to ion gradient disorders and affecting the basic cellular functions, ultimately leading to cell death. La Storia et al. performed anatomic force microscopy analysis and proved that the cell membrane is the primary target site of carvacrol [43].The reduction in cell size, length and diameter adds to the evidence that carvacrol alters the cell surface structure of pathogenic bacteria. In short, TEM images illustrate that resveratrol and coumarin caused significant damage to R. solanacearum cells, showing incomplete cell morphology and a rough outer membrane. That may be because the polarity of the phenolic compounds affects the interaction between resveratrol and membranes [44]. These observations indicate that phytochemicals may inhibit the bacterial cell wall or destroy the outer membrane to change its permeability, leading to the leakage of intracellular substances and cell rupture or retraction (Figure 4). Like other polyphenols, they can also bind to membrane, disturbing the hydrophobic part of the lipid bilayer [44].Previous study also found sorbic acids can most likely interact with the headgroups and shallowly embed near the top of the phospholipid head groups [45]. Thus, the cells show an incomplete morphology and therefore decreased bacterial growth. These findings are of great significance to the study of new agricultural phytochemicals and the development of new pesticides.
Another explanation for the mode of action of resveratrol and coumarin may involve the prevention of biofilm and inhibition of bacterial motility (Figure 5 and Figure 6). It is well known that the R. solanacearum–host pathogenic interaction is generally described by a pathogenesis progress of strains of R. solanacearum into hosts generally involving three stages: moves to the host plant, attaches to the plant roots, and infects the cortex and colonizes the xylem parenchyma [46,47]. This invasion process results from the coordination of multiple pathogenic factors, including extracellular polysaccharides, a type III secretion system and its effectors, extracellular proteins, lipopolysaccharides, and a type IV flagellar system [48,49,50]. When pathogenic bacteria R. solanacearum colonized biotic or abiotic surfaces, surface motility and biofilm play essential role in plant root infection and early colonization process [49,51].
From our results, coumarin and resveratrol can inhibit the swarming motility of R. Solanacearum cells, even in the shorter incubation time of 24 h. Swarming motility, aflagella-dependent surface motility, is migratory of bacterial cells from inoculation point as a group on the surface of solid medium [52]. Once the bacteria motion are restrained, a series of behaviors will be affected including replication and recruitment that prompt the formation of aggregates of bacteria, then inhibit mature biofilm development [53], as observed in this study (Figure 6). Bacterial biofilms, as film-like composites, are a structured community of bacterial cells enclosed in a self-produced polymeric matrix and adherent to host surface, which consist of bacterial cells, extracellular polysaccharides, extracellular proteins, and water [33]. For plant pathogens, biofilm formation mainly plays a role in clogging the xylem conduit, resisting antimicrobial agents, and increasing colonization within a particular environment [25,54]. In the current study, we used a fluorescently labeled lectin (FITC-ConA) to enable the specific staining and qualitative analysis of polysaccharides in bacterial biofilm. We found that both coumarin and resveratrol inhibited biofilm formation by R. solanacearum, which was similar with some natural plant extracts such as cinnamon, protocatechualdehyde and eucalyptus essential oils [21,55]. Therefore, not surprisingly, R. solanacearum could not aggressively adhere to and colonizes tobacco roots surfaces, as shown in Table 3. We can speculate that the important antibacterial mechanism of resveratrol and coumarin is very likely through decreased levels of expression of a number of virulence factors related to cell motility and pathogenicity, suggesting decreased pathogenicity of the bacterium after treatment.
Further, laboratory pot experiments in vivo showed that both resveratrol and coumarin can suppress the adhesion ability of R. solanacearum cells and thereby delay or slow the occurrence of bacterial wilt of tobacco, the control efficacy of which were up to 68% and 85% at 13 days after inoculation, respectively. It seems that resveratrol and coumarin exhibited high efficacy for controlling tobacco bacterial wilt before the bacteria blocked the plant vascular. This can be expected as the phytochemicals might thereby perform as a potential natural antimicrobial agent in bacterial wilt management. Though the values were reduced after 19 days of irrigating, continuous irrigation may be resorted to manage the bacterial wilt. These results may be related to the complex pathogenic regulatory system of R. solanacearum. The process of bacterial wilt is controlled by a complex pathogenic regulatory system via the coordinated expression of multiple genes. Extensive trials are needed to further explore the effect of phytochemicals on the pathogenic genes of R. solanacearum.
4. Materials and Methods
4.1. Bacterial Strain and Culture Conditions
The test R. solanacearum strain (Biovar3, Phylotype I) was obtained from Laboratory of Natural Product Pesticide of Southwest university, which was identified to be a highly pathogenic strain. The strain was cultured on NA agar medium (yeast extract 1.0 g, beef extract 3.0 g, peptone 5.0 g, glucose 10.0 g, agar 20.0 g, deionized water 1000 mL, pH 7.0) at 30 °C for 48 h. Single colonies were picked and transferred into NB liquid medium (NA medium without agar) for 12–16 h. The cells were harvested, by centrifugation, at an optical density at 600 nm (OD600) of 1.0 and stored at −80 °C before use.
Resveratrol and coumarin used in this study were purchased from Shanghai Yuanye Bio-technology (Shanghai, China) and had ≥98% purity (by HPLC analysis). Stock solutions (20 mg/mL resveratrol or coumarin) were prepared in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) and filter-sterilized through a bacterial filter before use.
4.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Assays
The bacteriostatic and bactericidal activities of resveratrol and coumarin against R. solanacearum were examined referring to the typical microdilution method (ISO 10932|IDF 223:2010) to determine the minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC). The stock solution of resveratrol or coumarin (20 mg/mL) was added to 10 mL of NB liquid medium to produce phytochemical-containing media with final concentrations of 1, 2, 4, 8, 16, 32, 64, and 128 μg/mL. The phytochemical-containing media were inoculated with 10 μL of bacterial suspension at OD600 = 1.0 (≈109 cfu/mL) and incubated at 30 °C with shaking at 180 r/min for 24 h. The OD values of the culture solution were read using a Thermo electron Nicolet Evolution 300 UV-Vis spectrophotometer (Thermo Sci. Inc., Wilmington, NC, USA). The minimum inhibitory concentration (MIC) was defined as the lowest concentration of phytochemicals that inhibited bacterial growth.
The minimum bactericidal or lethal concentration (MBC) was determined based on the viability of the strain on solid medium. NA medium was melted and cooled at room temperature to 45 °C. Resveratrol or coumarin was then added to obtain phytochemical-containing media at final concentrations of 10–250 μg/mL. The phytochemical-containing media were thoroughly mixed before being poured into 9-cm diameter Petri dishes (15 mL each). A bacterial suspension at OD600 = 1.0 (≈109 cfu/mL) was then diluted to 1 × 105 cfu/mL, and 100 μL of diluted suspension was spread evenly on the phytochemical-containing plates. The inoculated plates were inverted and incubated at 30 °C for 96 h. The experimental results were then photographed and recorded. The MBC was defined as the lowest concentration of phytochemical that yielded no colony growth. DMSO with a final concentration of 0.1% was used as the negative control, and a blank control (Control) was also prepared in each experiment.
4.3. Growth Curve Assay
The stock solution of resveratrol and coumarin were pipetted into 100 mL of NB liquid medium to produce phytochemical-containing media with final concentrations of 128, 64, 32, 16, and 8 μg/mL, respectively. Phytochemical-containing medium was inoculated with 100 µL of bacterial suspension and incubated at 30 °C with shaking at 180 r/min for 36 h. The OD600 values of the samples were measured every 2 h during incubation using a Nicolet Evolution 300 UV-Vis spectrophotometer. A solvent control was prepared with DMSO at a final concentration of 0.1%, and a blank control was also included in each experiment. The OD600 values (vertical axis) were plotted against the sampling time (horizontal axis) to generate growth curves for R. solanacearum under the effect of the phytochemicals. The experiments were repeated multiple times, and the results were expressed as arithmetic means.
4.4. Observation of Bacterial Cell Morphology by TEM
To determine the antibacterial efficacy of the both phytochemicals and the morphological changes of the bacteria, TEM observation was performed on the tested bacteria according to the method as described previously [56]. First, the stock solution of resveratrol and coumarin (64 and 32 μL; 20 mg/mL each) were added into 10 mL of NB medium to obtain phytochemical-containing media with final concentrations of 128 μg/mL, respectively. Then, 10 μL of bacterial suspension (OD600 = 1.0) was inoculated and thoroughly mixed. The culture mixture was incubated at 30 °C with shaking at 180 r/min for 2 h. Twenty-four hours later, the culture mixture was dispensed into 1.5-mL centrifuge tubes and centrifuged at 6000 r/min for 5 min to collect the cells. The cell pellet was resuspended in 0.1 M phosphate-buffered saline (PBS, pH 7.2), washed three times with PBS to remove the impurities, and the cell concentration was adjusted to 107–108 cfu/mL finally. Bacterial cells were resuspended in 1 mL of 2.5% glutaraldehyde, and the cells were fixed overnight at 4 °C. After that, the cells were collected by centrifugation at 6000 r/min for 5 min and dehydrated using an ethanol a serie of gradient (1 mL; 30%, 50%, 70%, 85%, and 100%). One drop of the cell suspension was evenly dispersed on foil, freeze-dried, and coated with gold. Thin sections were placed on the copper grids and Cell morphology was observed and photographed under Tecnai G20 microscopy (FEI, Brno, Czech Republic).
4.5. Bacterial Motility Assay
Motility assays were undertaken in petri dishes (polystyrene, diameter of 90 mm), referred to the previous report [53]. The stock solution of resveratrol or coumarin (20 mg/mL) was added to the semi-solid medium (0.35% agar) (SMM) at 45 °C to obtain final concentrations of 64, 32, 16, and 8 μg/mL. Fifteen milliliters of phytochemical-containing medium was dispensed into each Petri dish. Five microliters of bacterial suspension at OD600 = 0.1 (≈3 × 108 cfu/mL) was dropped onto the prepared culture plates and spread evenly. The plates were kept horizontal during incubation to prevent experimental errors caused by the flow of the semi-solid medium. The diameters of the swarming motility zones around the bacterial colonies were measured after incubation (30 °C) for 24 and 48 h, respectively. Bacterial motility was calculated as follows:
| Swarming motility diameters = Measured diameter (mm) − Initial inoculation diameter (6 mm) | (1) |
A solvent control was prepared with DMSO at a final concentration of 0.1%, and a blank control was also included in each experiment. The experiment was repeated three times.
4.6. Bacterial Biofilm Formation Assay
The bacterial culture under different treatment was dispensed into sterile polystyrene microtiter plate (96-well plates) and mixed. No culture was added to wells at the edge of the plates. All experiments were performed in triplicate at least. All the 96-well plates were incubated at 30 °C under 24-h static culture. After that, the culture was removed carefully and gently with eppendof and, immediately, the 96-well plates were washed with 200 μL of distilled water. Bacterial biofilm was stained with 220 μL of 0.1% crystal violet and incubating at room temperature for 30 min without shaking. Then, crystal violet was removed, and the biofilm was washed twice with 200 μL of distilled water. After the floating color was eliminated, the biofilm was dried for 30 min at room temperature. Then, 200 μL of 95% ethanol was added to dissolve and adsorb the crystal violet. The absorbance of the solution at 490 nm was measured using a microplate reader to reflect biofilm formation by R. solanacearum under the experimental conditions [57]. The same procedure was followed to measure biofilm formation at 48 and 72 h. Tests were performed in triplicate.
4.7. Biofilm Observations by Fluorescence Microscopy
We further observed R. solanacearum biofilm formation by staining the main extracellular component of the biofilm using fluorescein isothiocyanate-conjugated concanavalin A (FITC-ConA), which can emit green fluorescence and be imaged via fluorescence microscopy [58]. Firstly, phytochemical-containing media with final concentrations of 64 and 128 μg/mL were prepared. Phytochemical-containing medium was then inoculated with 10 μL of bacterial suspension (OD600 = 1.0) and thoroughly mixed. Next, a 10 mm × 10 mm sterile coverslip was placed into each well of the 24-well plates as a carrier for biofilm adhesion, followed by the addition of 1 mL of culture mixture per well. After the inoculated plates were covered with plastic wrap and incubated for 12 h at 30 °C, the coverslips were removed and washed with PBS and then fixed with 2.5% glutaraldehyde for 4 h at 4 °C. After removed the residual fixative solution, the coverslips were stained with FITC-ConA in the dark for 30 min at 4 °C, followed by three washes with PBS to remove the redundant dye. Then, the coverslips were dried on absorbent paper and mounted on glass slides using 40% glycerol. Biofilms were observed on an inverted fluorescence microscope (Eclipse Ti, Nikon, Tokyo, Japan).
4.8. Bacterial Adhesion Test
The stock solutions of resveratrol and coumarin (20 mg/mL) were diluted to 128 μg/mL with sterile water. Then, 30 mL of diluted phytochemical solution were respectively evenly irrigated into tobacco roots. Twelve hours later, R. solanacearum cells were inoculated via non-injured root inoculation at OD600 = 0.1 (≈108 cfu/mL), 10 mL per plant. The inoculated tobacco seedlings were cultivated in a plant growth chamber at a temperature of 30 ± 1 °C, relative humidity of 85%–90%, and light period of 14 h. Control plants were irrigated with sterile water in the experiment. Each treatment was designed nine plants and three replicates. One day after inoculation, three plants, which were randomly selected from each treatment, were washed to obtain clean and intact roots. The root weights were recorded. The weighed tobacco roots were placed in a flask containing 50 mL of sterile water and incubated for 30 min at 30 °C, with shaking at 180 r/m. The roots were then rinsed with sterile water and thoroughly mixed with sterile water in the flask. The mixed solution was allowed to stand for 5 min, and 1 mL of tobacco root exterior bacterial suspension was taken from the mid-upper portion with a pipette.
The tobacco roots were surface-dried with absorbent paper and disinfected for 20 min in a Petri dish containing 75% ethanol. After that, the disinfected roots were thoroughly ground in a sterile mortar. Ten milliliters of sterile water was added to the mortar to thoroughly mix the root homogenate. The mixture was allowed to stand for 5 min, and 1 mL of supernatant was then taken as the tobacco root interior bacterial suspension. The bacterial suspensions obtained from the tobacco roots were prepared in serial dilutions at 10−1 to 10−7. The number of R. solanacearum cells in the suspensions was measured by plate counting on selective SMSA medium. Three dilutions of the root exterior (10−2 to 10−4) and interior bacterial suspensions (10−1 to 10−3) were evenly spread on duplicate SMSA plates (0.1 mL each). The inoculated plates were incubated for 48 h at 30 °C and R. solanacearum colonies were recorded. The number of R. solanacearum cells in the bacterial suspensions was calculated at different dilutions to obtain root-exterior and interior bacterial counts. The same procedure was repeated at 3 and 5 days after inoculation. However, the dilutions were changed to 10−3 to 10−5 for root-exterior bacterial suspension and 10−2 to10−4 for root-interior bacterial suspension on Day 3 and 10−3 to 10−5 for both root exterior and interior bacterial suspensions on Day 5.
4.9. Controlling Tobacco Bacterial Wilt In Vivo under Greenhouse Conditions
Firstly, the uniformly grown four-week-old tobacco seedlings were selected to carry out the effects of resveratrol and coumarin on tobacco bacterial wilt. Furthermore, 30 mL of diluted phytochemical solution (128 μg/mL) were respectively evenly irrigated into tobacco root soil. Deionized water without phytochemicals or bacteria was set as blank control in this experiment. Nine tobacco seedlings were carried out for each treatment. Twelve hours later, R. solanacearum was inoculated via non-injured root inoculation at OD600 = 0.1 (≈108 cfu/mL) per plant. The inoculated tobacco seedlings were cultivated in a plant growth chamber at a temperature of 30 ± 1 °C, relative humidity of 85%–90%, and light period of 14 h. Following the inoculation of R. solanacearum, the disease occurrence of the tobacco seedlings and disease index were investigated at 7, 9, 13 and 19 days since the first observation of bacterial wilt diseased plants (7 days in our experiment). Bacterial wilt disease classification of tobacco plants in the laboratory was evaluated and recorded according to the “Protocols of Disease Investigation and Classification” [59]. The disease incidence, disease index, and control efficacy were calculated using Equations (2)–(4) as follows:
| Disease incidence (%) = Number of diseased plants/total number of plants × 100 | (2) |
| Disease index = [Σ (Number of diseased plants × number of diseased plants)/(total number of plants × representative value of the highest grade)] × 100 | (3) |
| Control efficacy (%) = (Disease index of the control − disease index of the treatment)/disease index of the control × 100 | (4) |
4.10. Statistic Analysis
All the experiments were performed in triplicate and the results were expressed as mean values ± SD (standard deviation). Statistical analysis was implemented using Statistical Product and Service Solutions software (SPSS 11.0) (SPSS Inc., Chicago, IL, USA). The differences between the groups were assessed using the analysis of variance test. The results were considered statistically significant when the p value was <0.05 or <0.01.
5. Conclusions
In summary, two phytochemicals, resveratrol and coumarin, significantly inhibited the propagation of R. solanacearum and demonstrated a bactericidal effect in vitro and in vivo. On the one hand, the cell membrane structure of R. solanacearum was damaged after phytochemical treatment, thereby affecting bacterial growth. On the other hand, both resveratrol and coumarin inhibited the formation of bacterial biofilms and impeded the motility of R. solanacearum, which plays an important role in preventing R. solanacearum infection of tobacco plants. Furthermore, in vitro experiments demonstrated that resveratrol and coumarin inhibited early adhesion and colonization by R. solanacearum during infection, thereby significantly controlling bacterial wilt of tobacco. However, this study was limited to pot experiments, and it is therefore necessary to perform field trials on the prevention and control of bacterial wilt. Meanwhile, in-depth studies are needed to further elucidate the antibacterial mechanism of resveratrol and coumarin.
Acknowledgments
This work was supported by the National Natural Science Foundation China (31272041), and the Key Project of the China National Tobacco Corporation (110201202002 and 110201502019)
Author Contributions
J.C., W.D and Y.Y. conceived and designed the experiments; Y.Y. and S.L performed the experiments; J.C. and Y.Y. analyzed the data; and J.C and Y.Y. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds (Resveratrol and Coumarin) are available from Shanghai Yuanye Bio-technology Co., Ltd. (Shanghai, China).
References
- 1.Hayward A.C. Biology and epidemiology of bacterial wilt caused by Pseudomonas-solanacearum. Annu. Rev. Phytopathol. 1991;29:65–87. doi: 10.1146/annurev.py.29.090191.000433. [DOI] [PubMed] [Google Scholar]
- 2.Buddenhagen I., Kelman A. Biological and physiological aspects of bacterial wilt caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 1964;2:203–230. doi: 10.1146/annurev.py.02.090164.001223. [DOI] [Google Scholar]
- 3.Yi Y.J., Liu R.S., Yin H.Q., Luo K., Liu E.M., Liu X.D. Isolation, identification and field control efficacy of an endophytic strain against tobacco bacterial wilt (Ralstonia solanacarum) Chin. J. Appl. Ecol. 2007;18:554–558. (in Chinese) [PubMed] [Google Scholar]
- 4.Zhang S., Raza W., Yang X., Hu J., Huang Q., Xu Y., Liu X., Ran W., Shen Q. Control of Fusarium wilt disease of cucumber plants with the application of a bioorganic fertilizer. Biol. Fert. Soils. 2008;44:1073–1080. doi: 10.1007/s00374-008-0296-0. [DOI] [Google Scholar]
- 5.King S.R., Davis A.R., Liu W., Levi A. Grafting for disease resistance. Hort Sci. 2008;43:1673–1676. [Google Scholar]
- 6.Zhang N., Wu K., He X., Li S.Q., Zhang Z.H., Shen B., Yang X.M., Zhang R.F., Huang Q.W., Shen Q.R. A new bioorganic fertilizer can effectively control banana wilt by strong colonization with Bacillus subtilis N11. Plant Soil. 2011;344:87–97. doi: 10.1007/s11104-011-0729-7. [DOI] [Google Scholar]
- 7.Ren X., Zhang N., Cao M., Wu K., Shen Q., Huang Q. Biological control of tobacco black shank and colonization of tobacco roots by a Paenibacillus polymyxa strain C5. Biol. Fert. Soils. 2012;48:613–620. doi: 10.1007/s00374-011-0651-4. [DOI] [Google Scholar]
- 8.Zheng S., Zhou X., Xu S., Zhu R., Bai H., Zhang J. Synthesis and antimicrobial characterization of half-calycanthaceous alkaloid derivatives. Molecules. 2016;21:1207. doi: 10.3390/molecules21091207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon M.Y., Cha B., Kim J.C. Recent trends in studies on botanical fungicides in agriculture. Plant Pathol. J. 2013;29:1–9. doi: 10.5423/PPJ.RW.05.2012.0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pradhanang P., Momol M., Olson S., Jones J. Effects of plant essential oils on Ralstonia solanacearum population density and bacterial wilt incidence in tomato. Plant Dis. 2003;87:423–427. doi: 10.1094/PDIS.2003.87.4.423. [DOI] [PubMed] [Google Scholar]
- 11.Deberdt P., Perrin B., Coranson-Beaudu R., Duyck P.F., Wicker E. Effect of Allium fistulosum extract on Ralstonia solanacearum populations and tomato bacterial wilt. Plant Dis. 2012;96:687–692. doi: 10.1094/PDIS-07-11-0601. [DOI] [PubMed] [Google Scholar]
- 12.Feng J., Yuan F.H., Gao Y., Liang C.G., Xu J., Zhang C.L., He L.Y. A novel antimicrobial protein isolated from potato (Solanum tuberosum) shares homology with an acid phosphatase. Biochem. J. 2003;376:481–487. doi: 10.1042/bj20030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai K., Ye Y., Wei Z., Wang G., Yang H. Study on antimicrobial activities of α-(l)-terpineol against plant pathogens. Technol. Dev. Chem. Ind. 2007;12:5–7. [Google Scholar]
- 14.Dorman H., Deans S. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 15.Shafi P.M., Nambiar M.G., Clery R.A., Sarma Y., Veena S. Composition and antifungal activity of the oil of Artemisia nilagirica (Clarke) Pamp. J. Essent. Oil Res. 2004;16:377–379. doi: 10.1080/10412905.2004.9698748. [DOI] [Google Scholar]
- 16.Paret M.L., Cabos R., Kratky B., Alvarez A.M. Effect of plant essential oils on Ralstonia solanacearum race 4 and bacterial wilt of edible ginger. Plant Dis. 2010;94:521–527. doi: 10.1094/PDIS-94-5-0521. [DOI] [PubMed] [Google Scholar]
- 17.Ji P., Momol M., Olson S., Pradhanang P., Jones J. Evaluation of thymol as biofumigant for control of bacterial wilt of tomato under field conditions. Plant Dis. 2005;89:497–500. doi: 10.1094/PD-89-0497. [DOI] [PubMed] [Google Scholar]
- 18.Fan W.W., Yuan G.Q., Li Q.Q., Lin W. Antibacterial mechanisms of methyl gallate against Ralstonia solanacearum. Australas. Plant Path. 2014;43:1–7. doi: 10.1007/s13313-013-0234-y. [DOI] [Google Scholar]
- 19.Li L., Feng X., Tang M., Hao W., Han Y., Zhang G., Wan S. Antibacterial activity of Lansiumamide B to tobacco bacterial wilt (Ralstonia solanacearum) Microbiol. Res. 2014;169:522–526. doi: 10.1016/j.micres.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y.H., Choi C.W., Kim S.H., Yun J.G., Chang S.W., Kim Y.S., Hong J.K. Chemical pesticides and plant essential oils for disease control of tomato bacterial wilt. Plant Pathol. J. 2012;28:32–39. doi: 10.5423/PPJ.OA.10.2011.0200. [DOI] [Google Scholar]
- 21.Li S., Yu Y., Chen J., Guo B., Yang L., Ding W. Evaluation of the Antibacterial Effects and Mechanism of Action of Protocatechualdehyde against Ralstonia solanacearum. Molecules. 2016;21:754. doi: 10.3390/molecules21060754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiew P., Ioset J., Kokpol U., Chavasiri W., Hostettmann K. Antifungal, antioxidant and larvicidal activities of compounds isolated from the heartwood of Mansonia gagei. Phytother. Res. 2003;17:190–193. doi: 10.1002/ptr.1260. [DOI] [PubMed] [Google Scholar]
- 23.Yasunaka K., Abe F., Nagayama A., Okabe H., Lozada-Pérez L., López-Villafranco E., Muñiz E.E., Aguilar A., Reyes-Chilpa R. Antibacterial activity of crude extracts from Mexican medicinal plants and purified coumarins and xanthones. J. Ethnopharmacol. 2005;97:293–299. doi: 10.1016/j.jep.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Wu C., Wang J., Hou Z., Li H., Bi Y. Inhibitory activity of resveratrol and its derivatives against phytopathogenic fungi. Chin. J. Pestic. Sci. 2012;14:283–290. [Google Scholar]
- 25.Paulo L., Oleastro M., Gallardo E., Queiroz J., Domingues F. Science against Microbial Pathogens: Communicating Current Research and Technological Advances. Volume 1. Formatex Research Center; Badajoz, Spain: 2001. Antimicrobial properties of resveratrol: A review; pp. 1225–1235. [Google Scholar]
- 26.Houillé B., Papon N., Boudesocque L., Bourdeaud E., Besseau S., Courdavault V., Enguehard-Gueiffier C., Delanoue G., Guérin L., Bouchara J.P. Antifungal activity of resveratrol derivatives against Candida species. J. Nat. Prod. 2014;77:1658–1662. doi: 10.1021/np5002576. [DOI] [PubMed] [Google Scholar]
- 27.Nawrot-Modranka J., Nawrot E., Graczyk J. In vivo antitumor, in vitro antibacterial activity and alkylating properties of phosphorohydrazine derivatives of coumarin and chromone. Eur. J. Med. Chem. 2006;41:1301–1309. doi: 10.1016/j.ejmech.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Rosselli S., Maggio A., Bellone G., Formisano C., Basile A., Cicala C., Alfieri A., Mascolo N., Bruno M. Antibacterial and anticoagulant activities of coumarins isolated from the flowers of Magydaris tomentosa. Planta Med. 2007;73:116–120. doi: 10.1055/s-2006-951772. [DOI] [PubMed] [Google Scholar]
- 29.Nguefack J., Budde B.B., Jakobsen M. Five essential oils from aromatic plants of Cameroon: Their antibacterial activity and ability to permeabilize the cytoplasmic membrane of Listeria innocua examined by flow cytometry. Lett. Appl. Microbiol. 2004;39:395–400. doi: 10.1111/j.1472-765X.2004.01587.x. [DOI] [PubMed] [Google Scholar]
- 30.Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L., Ding W., Xu Y., Wu D., Li S., Chen J., Guo B. New insights into the antibacterial activity of hydroxycoumarins against Ralstonia solanacearum. Molecules. 2016;21:468. doi: 10.3390/molecules21040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao J., Allen C. The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host. J. Bacteriol. 2007;189:6415–6424. doi: 10.1128/JB.00398-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris C.E., Monier J.M. The ecological significance of biofilm formation by plant-associated bacteria. Annu. Rev. Phytopathol. 2003;41:429–453. doi: 10.1146/annurev.phyto.41.022103.134521. [DOI] [PubMed] [Google Scholar]
- 34.Davey M.E., O’toole G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000;64:847–867. doi: 10.1128/MMBR.64.4.847-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 36.Monteiro F., Genin S., van Dijk I., Valls M. A luminescent reporter evidences active expression of Ralstonia solanacearum type III secretion system genes throughout plant infection. Microbiology. 2012;158:2107–2116. doi: 10.1099/mic.0.058610-0. [DOI] [PubMed] [Google Scholar]
- 37.Sashidhara K.V., Kumar A., Kumar M., Sarkar J., Sinha S. Synthesis and in vitro evaluation of novel coumarin-chalcone hybrids as potential anticancer agents. Bioorg. Med. Chem. Lett. 2010;20:7205–7211. doi: 10.1016/j.bmcl.2010.10.116. [DOI] [PubMed] [Google Scholar]
- 38.Riveiro M., De Kimpe N., Moglioni A., Vazquez R., Monczor F., Shayo C., Davio C. Coumarins: Old compounds with novel promising therapeutic perspectives. Curr. Med. Chem. 2010;17:1325–1338. doi: 10.2174/092986710790936284. [DOI] [PubMed] [Google Scholar]
- 39.Thati B., Noble A., Rowan R., Creaven B.S., Walsh M., McCann M., Egan D., Kavanagh K. Mechanism of action of coumarin and silver(I)-coumarin complexes against the pathogenic yeast Candida albicans. Toxicol. In Vitro. 2007;21:801–808. doi: 10.1016/j.tiv.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 40.Paulo L., Ferreira S., Gallardo E., Queiroz J.A., Domingues F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J. Microbiol. Biotechnol. 2010;26:1533–1538. doi: 10.1007/s11274-010-0325-7. [DOI] [Google Scholar]
- 41.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 42.Ultee A., Kets E., Smid E. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 1999;65:4606–4610. doi: 10.1128/aem.65.10.4606-4610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.La Storia A., Ercolini D., Marinello F., Di Pasqua R., Villani F., Mauriello G. Atomic force microscopy analysis shows surface structure changes in carvacrol-treated bacterial cells. Res. Microbiol. 2011;162:164–172. doi: 10.1016/j.resmic.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Yu X.T., Chu S.D., Hagerman A.E., Lorigan G.A. Probing the interaction of polyphenols with lipid bilayers by solid-State NMR spectroscopy. J. Agric. Food Chem. 2011;12:6783–6789. doi: 10.1021/jf200200h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu S.D., Hawes J.W., Lorigan G.A. Solid-state NMR spectroscopic studies on the interaction of sorbic acid with phospholipid membranes at different pH levels. Magn. Reson. Chem. 2009;8:651–657. doi: 10.1002/mrc.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasse J., Frey P., Trigalet A. Microscopic studies of intercellular infection and protoxylem invasion of tomato roots by Pseudomonas-solanacearum. Mol. Plant Microbe Interact. 1995;8:241–251. doi: 10.1094/MPMI-8-0241. [DOI] [Google Scholar]
- 47.Genin S., Denny T.P. Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 2012;50:67–89. doi: 10.1146/annurev-phyto-081211-173000. [DOI] [PubMed] [Google Scholar]
- 48.Liu H., Zhang S., Schell M.A., Denny T.P. Pyramiding unmarked deletions in Ralstonia solanacearum shows that secreted proteins in addition to plant cell-wall-degrading enzymes contribute to virulence. Mol. Plant Microbe Interact. 2005;18:1296–1305. doi: 10.1094/MPMI-18-1296. [DOI] [PubMed] [Google Scholar]
- 49.Tans-Kersten J., Brown D., Allen C. Swimming motility, a virulence trait of Ralstonia solanacearum, is regulated by FlhDC and the plant host environment. Mol. Plant Microbe Interact. 2004;17:686–695. doi: 10.1094/MPMI.2004.17.6.686. [DOI] [PubMed] [Google Scholar]
- 50.Tran T.M., Macintyre A., Hawes M., Allen C. Escaping underground nets: Extracellular DNases degrade plant extracellular traps and contribute to virulence of the plant pathogenic bacterium Ralstonia solanacearum. PLoS Pathog. 2016;12:e1005686. doi: 10.1371/journal.ppat.1005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tans-Kersten J., Huang H., Allen C. Ralstonia solanacearum needs motility for invasive virulence on tomato. J. Bacteriol. 2001;183:3597–3605. doi: 10.1128/JB.183.12.3597-3605.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rashid M.H., Kornberg A. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA. 2000;97:4885–4890. doi: 10.1073/pnas.060030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’May C., Tufenkji N. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl. Environ. Microbiol. 2011;77:3061–3067. doi: 10.1128/AEM.02677-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bogino P.C., Oliva M.d.L., Sorroche F.G., Giordano W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013;14:15838–15859. doi: 10.3390/ijms140815838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hosseinzadeh S., Shams-Bakhsh M., Hosseinzadeh E. Effects of sub-bactericidal concentration of plant essential oils on pathogenicity factors of Ralstonia solanacearum. Arch. Phytopathol. Plant Prot. 2013;46:643–655. doi: 10.1080/03235408.2012.749698. [DOI] [Google Scholar]
- 56.Chen J., Peng H., Wang X., Shao F., Yuan Z., Han H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale. 2014;6:1879–1889. doi: 10.1039/C3NR04941H. [DOI] [PubMed] [Google Scholar]
- 57.Li X., Yan Z., Xu J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology. 2003;149:353–362. doi: 10.1099/mic.0.25932-0. [DOI] [PubMed] [Google Scholar]
- 58.Holden C., Trounson A. Staining of the inner acrosomal membrane of human spermatozoa with concanavalin A lectin as an indicator of potential egg penetration ability. Fertil. Steril. 1991;56:967–974. doi: 10.1016/S0015-0282(16)54673-1. [DOI] [PubMed] [Google Scholar]
- 59.China State Tobacco Monopoly Administration . YC/T39−1996, Grade and Investigating Method of Tobacco Disease. China Standard Press; Beijing, China: 1996. [Google Scholar]