Abstract
A convenient synthesis of some novel bis-pyrrole derivatives via hydrazonoyl halides is described. Antimicrobial evaluation of some selected examples of the synthesized products was carried out. The bis-pyrrole derivative having chloro substituents showed good activity against all of the used microbes. The molecular docking of the bis-pyrrole derivatives was performed by the Molecular Operating Environment (MOE) program.
Keywords: bis-pyrrole, antibacterial activity, antifungal activity, hydrazonoyl halides, molecular docking
1. Introduction
Bis-heterocycles have attracted much attention because of their diverse biological activities [1,2,3]. These include antibacterial, fungicidal, tuberculostatic, and plant growth regulative properties. Diverse pharmacological activities have been associated with pyrrole derivatives, such as anti-tubulin activity [4], non-Competitive mGluR1 antagonists [5], DNA-Binding applications [6], antitumor activity [7,8], antidepressant activity [9], treatment of hyperlipidemias [10], antiviral [11], and antimicrobial activity [12]. Hydrazonoyl chlorides are highly versatile and useful building blocks for the synthesis of a wide variety of heterocyclic compounds [13,14]. In view of the above-mentioned findings, and as a continuation of my interest in developing new routes for the synthesis of mono- and bis-heterocyclic systems for biological evaluation [15,16,17,18,19,20,21,22,23,24,25], I report herein a convenient route to some novel bis-pyrrole derivatives using hydrazonoyl chlorides and diethyl (Z,Z)-3,3′-(ethane-1,2-diyldiimino)dibut-2-enoate (1) [26,27].
2. Results and Discussion
2.1. Chemistry
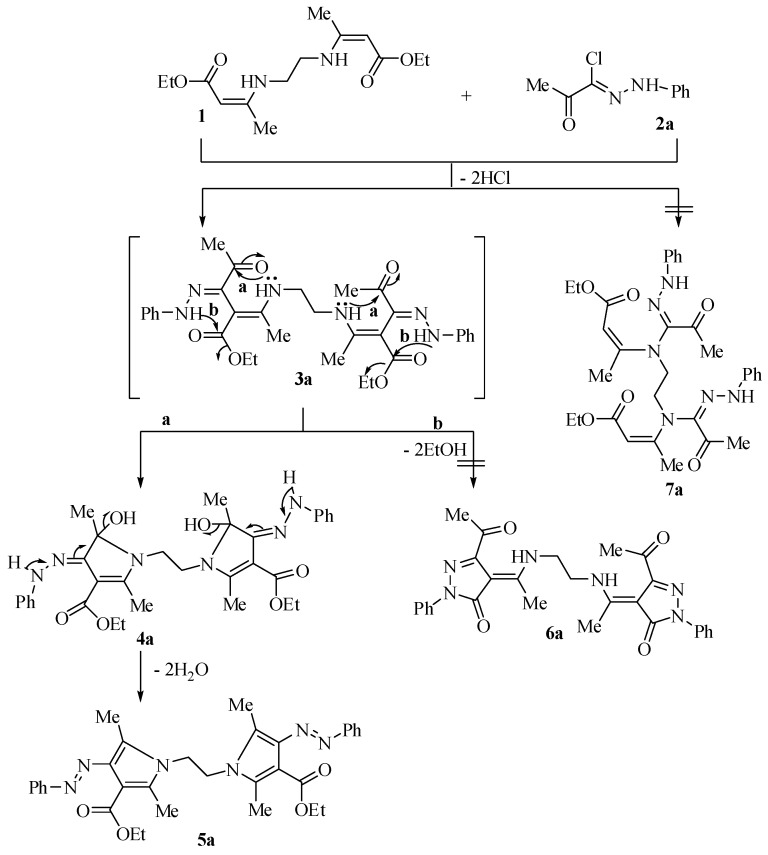
Treatment of compound 1 with a α-ketohydrazonoyl halide (2a) [28,29] in (1:2 molar ratios), in dry benzene at refluxing temperature in the presence of triethylamine (2 molar ratio) afforded the bis-pyrrole derivative 5a (Scheme 1). The structure of compound 5a was elucidated from its spectroscopic, as well as elemental analytical data. Its 1H-NMR spectrum revealed a triplet signal at δ 1.13 (J = 7.1 Hz) due to CH3 protons, two singlet signals at δ 2.17 and 2.34 due to two CH3 protons and a quartet signal at δ 4.14 (J = 7.1 Hz) due to CH2 protons, a singlet signal δ 4.31 due to NCH2 protons, in addition to an aromatic multiplet in the region 7.40–7.68 ppm. This spectroscopic analysis allows ruling out structures 6a and 7a. To account for the formation of the product 5a, it is assumed that the reaction initially proceeds via an initial nucleophilic substitution to give the intermediate 3a, which underwent intramolecular cyclization followed by the elimination of two water molecules to afford the final product 5a.
Scheme 1.
Synthesis of diethyl 1,1′-(ethane-1,2-diyl)bis(2,5-dimethyl-4-(phenylazo)-1H-pyrrole-3-carboxylate) (5a).
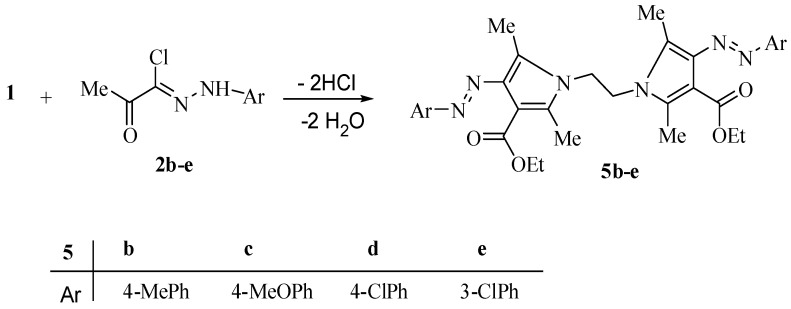
Prompted by the aforementioned results, and to generalize this reaction, the behavior of the hydrazonoyl halides 2b–e [30] towards compound 1 was studied, under the same experimental conditions, which led to the respective bis-pyrrole derivatives 5b–e (Scheme 2).
Scheme 2.
Synthesis of bis-pyrrole derivatives 5b–e.
2.2. Antimicrobial Evaluation
Gram-positive and Gram-negative standard bacterial strains were used in this study to screen synthesized compounds for their potential antibacterial activities. The Gram-positive bacteria were Staphylococcus aureus and Bacillus subtilis. The Gram-negative bacteria were Pseudomonas aeruginosa and Escherichia coli. Four species of fungi known to cause different types of mycoses were also used to test the antifungal activities of synthesized compounds in this study. These fungal species were Aspergillus fumigatus, Geotrichum candidum, Syncephalastrum racemosum, and Candida albicans. Inhibition zone diameter (IZD) in mm was used a criterion for the antimicrobial activity using the agar diffusion well method. The fungicide Itraconazole and the bactericides Penicillin G and Streptomycin were used as references to evaluate the potency of the tested compounds under the same conditions. The results are depicted in Table 1.
Table 1.
Antibacterial and Antifungal Activities of the Synthesized Compounds.
| Microorganisms | Compound Tested | Standard (30 µg/mL) | |||
|---|---|---|---|---|---|
| 5a | 5b | 5d | 5e | ||
| Fungi | Itraconazole | ||||
| Aspergillus fumigatus (RCMB 002003) | 18.2 ± 0.84 | 11.3 ± 0.68 | 21.1 ± 0.2 | 16.3 ± 0.09 | 22 ± 0.1 |
| Geotrichumcandidum (RCMB 052006) | 18.9 ±0.35 | 12.6 ±0.54 | 19.3 ± 0.05 | 15.4 ± 0.1 | 26 ± 0.3 |
| Syncephalastrum racemosum (RCMB 005003) | 11.2 ± 0.44 | NA | 16.4 ± 0.08 | 14.2 ± 0.05 | 19 ± 0.1 |
| Candida albicans (RCMB 005002) | NA | NA | NA | NA | 24 ± 0.1 |
| Gram-positive Bacteria | Penicillin G | ||||
| Staphylococcus aureus (RCMB 000106) (MSSA) | 17.8 ± 0.58 | 10.7 ± 0.36 | 21.4 ± .03 | 17.9 ± 0.03 | 27.4 ± 0.08 |
| Bacillis subtilis (RCMB 000107) | 17.9 ± 0.46 | 11.1 ± 0.72 | 26.1 ± 0.04 | 15.2 ± 0.04 | 28.6 ± 0.03 |
| Gram-negative Bacteria | Streptomycin | ||||
| Pseudomonas aeruginosa (RCMB 000102) | NA | NA | 19.9 ± 0.09 | NA | 26.3 ± 0.03 |
| Escherichia coli (RCMB 000103) | 12.6 ± 0.57 | 12.4 ±0.04 | 17.2 ± 0.2 | 13.4 ± 0.04 | 30.1 ± 0.07 |
NA: No activity, data are expressed in the form of mean ± standard deviation (S.D.)
The results revealed that most of the tested compounds revealed better activity against the Gram-positive bacteria rather than the Gram-negative bacteria. Also, all compounds exhibited almost no activity against Candida albicans. Compound 5d was found to be the most potent relative to the standard drug, Itraconazole, against Aspergillus fumigates.
Additionally, compound 5d has a high degree of antibacterial activity against Gram-positive bacteria Staphylococcus aureus (MSSA) and Bacillus subtilis. All the tested compounds except 5d exhibited no activity against Pseudomonas aeruginosa. The structure antimicrobial activity relationship of the synthesized compounds revealed that the maximum activity was attained with compound 5d, having chloro substituent in the para position of the phenyl ring.
2.3. Docking and Molecular Modeling
Molecular docking is used to predict the binding mode of ligands within the binding site of target proteins [31]. To validate and specify the target protein for the anti-bacterial activity of newly synthesized bis-pyrrole derivatives E. coli Enoyl reductase protein was selected and downloaded from the Protein Data Bank (PDB ID: 1LXC) [32].
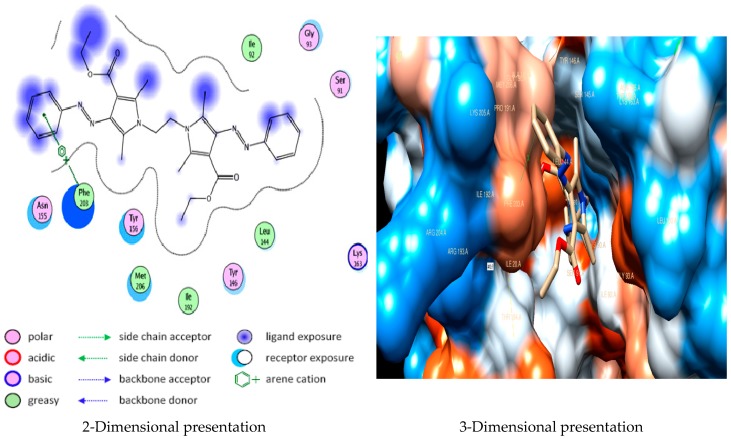
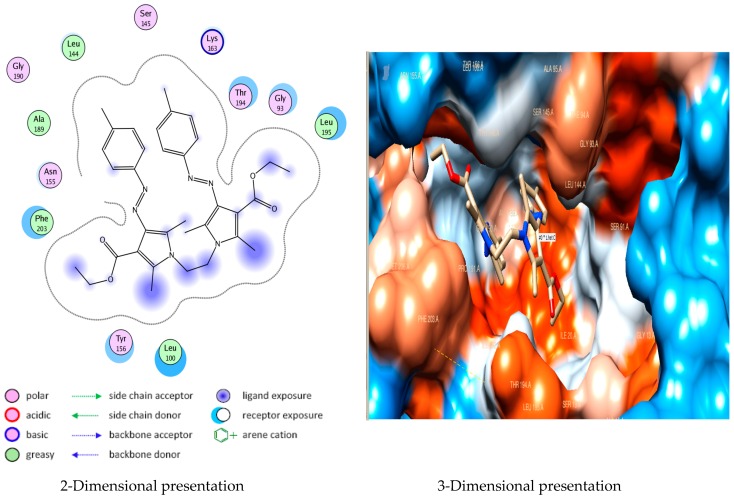
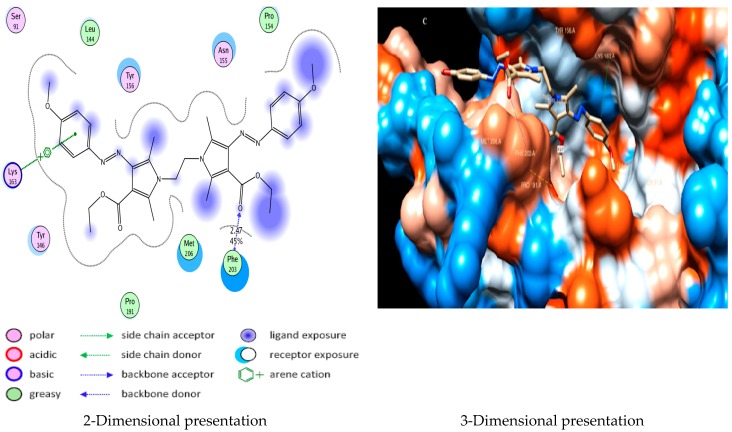
Docking studies of compound 5a into the active site of E. Coli Enoyl reductase Enzyme showed van der Waals bonding between Phe203 and the phenyl ring (Figure 1), while docking studies of compound 5b showed no interactions with E. coli Enoyl reductase Enzyme (Figure 2).
Figure 1.
Docking of compound 5a into E. coli Enoyl reductase Enzyme (PDB ID: 1LXC) showing arene-cation interaction between Phe203 and the phenyl ring.
Figure 2.
Docking of compound 5b into E. coli Enoyl reductase Enzyme (PDB ID: 1LXC).
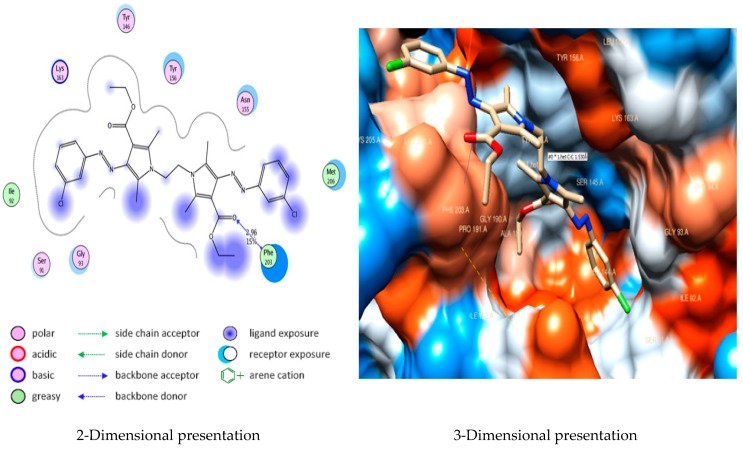
Similarly, docking conformation of compound 5c in the active site of E. coli Enoyl reductase Enzyme showed good interactions with the active site residues of this protein. Compound 5c formed hydrogen bond interaction between carbonyl group moiety, as it acts as a hydrogen bond acceptor with the side chain of Phe203 residue (2.47 Å) with a strength of 45%. Furthermore, it showed van der Waals interaction with Lys163 (Figure 3).
Figure 3.
Docking of compound 5c into E. coli Enoyl reductase Enzyme (PDB ID: 1LXC) showing arene-cation interaction with Lys163 and hydrogen bond with Phe 203.
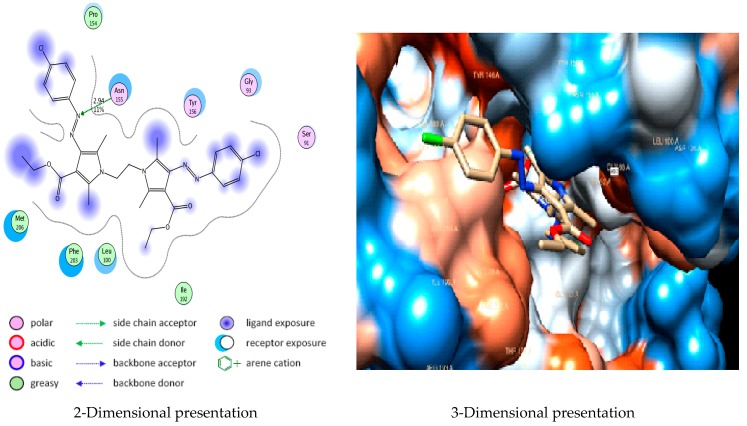
Docking studies of compound 5d showing hydrogen bond interaction between nitrogen atom of azo group moiety, as it acts as a hydrogen bond acceptor with the side chain of Asn155 (2.49 Å) with a strength of 11% (Figure 4). while docking studies of compound 5e showing hydrogen bond interaction between carbonyl group moiety, as it acts as a hydrogen bond acceptor with the side chain of Phe203 (2.96 Å) with a strength of 15% (Figure 5).
Figure 4.
Docking of compound 5d into E. coli Enoyl reductase Enzyme (PDB ID: 1LXC) showing hydrogen bond with Asn155.
Figure 5.
Docking of compound 5e into E. coli Enoyl reductase Enzyme (PDB ID: 1LXC) showing hydrogen bond with Phe203.
3. Materials and Methods
3.1. Chemistry
3.1.1. General
Melting points were measured on a Gallenkamp melting point apparatus and are uncorrected. IR spectra were recorded in potassium bromide discs on Shimadzu FTIR 8101 PC infrared spectrophotometer (Shimadzu, Tokyo, Japan). The NMR spectra were recorded on a BRUKER VX-500 NMR spectrometer (Varian, Palo Alto, CA, USA). 1H spectra were run at 500 MHz and 13C spectra were run at 125 MHz in deuterated dimethylsulphoxide (DMSO-d6) using TMS as an internal standard. Chemical shifts were related to that of the solvent. Mass spectra were recorded on a Shimadzu GCMS-QP 1000 EX mass spectrometer (Shimadzu, Tokyo, Japan) at 70 e.V. Elemental analyzes were measured by using a German made Elementar vario LIII CHNS analyzer (GmbH & Co. KG, Hanau, Germany). The biological evaluation of the products was carried out in the Medical Mycology Laboratory of the Regional Center for Mycology and Biotechnology of Al-Azhar University, Cairo, Egypt. Diethyl (Z,Z)-3,3′-(ethane-1,2-diyldiimino)-dibut-2-enoate (1) [26,27], hydrazonoyl chlorides (2a) [28,29], and 2b–e [30] were prepared according to the procedures reported literature.
3.1.2. Synthesis of Bis-Pyrrole Derivatives 5a–e
General procedure: To a solution of compound 1 (0.284 g, 1 mmol) and the appropriate hydrazonoyl halide 2a–e (2 mmol) in dry benzene (20 mL), was added triethylamine (0.2 mL, 2 mmol) and the reaction mixture was heated under reflux for 6 h. After cooling, the mixture was filtered off to remove the precipitated triethylamine hydrochloride and the solvent was distilled under reduced pressure. The oil residue was triturated with MeOH and the solid product was collected by filtration, washed with methanol, and recrystallized for purification to afford the corresponding bis-pyrrole derivatives 5a–e.
Diethyl 1,1′-(ethane-1,2-diyl)bis(2,5-dimethyl-4-(phenylazo)-1H-pyrrole-3-carboxylate) (5a). Yield (40%), mp. 230–232 °C (EtOH/DMF); IR (KBr) νmax: 2960 (aliphatic CH), 1695 (C=O) cm−1; 1H-NMR (DMSO-d6): δ 1.13 (t, 6H, 2CH3, J = 7.1 Hz), 2.17 (s, 6H, 2CH3), 2.34 (s, 6H, 2CH3), 4.14 (q, 4H, 2CH2, J = 7.1 Hz), 4.31 (s, 4H, 2CH2), 7.40–7.68 (m, 10H, ArH); 13C-NMR (DMSO-d6): δ 8.97, 9.56, 14.13, 42.94, 59.62, 106.04, 121.48, 129.12, 129.41, 131.99, 133.27, 136.56, 152.45, 165.73; MS m/z (%) 570 (8.77), 569 (M+, 9.89), 463 (4.98), 105 (13.22), 77 (90.33). Anal. Calcd for C32H36N6O4: C, 67.59; H, 6.38; N, 14.78. Found: C, 67.64; H, 6.46; N, 14.66%.
Diethyl 1,1′-(ethane-1,2-diyl)bis(2,5-dimethyl-4-(4-methylphenylazo)-1H-pyrrole-3-carboxylate) (5b). Yield (38%), mp. 230–231 °C (EtOH/DMF); IR (KBr) νmax: 2978 (aliphatic CH), 1693 (C=O) cm−1; 1H-NMR (DMSO-d6): δ 1.13 (t, 6H, 2CH3, J = 6.9 Hz), 2.15 (s, 6H, 2CH3), 2.31 (s, 6H, 2CH3), 2.35 (s, 6H, 2CH3), 4.11 (q, 4H, 2CH2, J = 6.9 Hz), 4.28 (s, 4H, 2CH2), 7.26 (d, 4H, J = 7.8 Hz), 7.55 (d, 4H, J = 7.8 Hz); MS m/z (%) 598 (M+ + 1, 100), 100), 505 (13.52), 477 (6.3), 297 (2.3), 119 (30.76), 91 (56.18). Anal. Calcd for C34H40N6O4: C, 68.43; H, 6.76; N, 14.08. Found: C, 68.37; H, 6.68; N, 14.18%.
Diethyl 1,1′-(ethane-1,2-diyl)bis(2,5-dimethyl-4-(4-methoxyphenylazo)-1H-pyrrole-3-carboxylate) (5c). Yield (35%), mp. 228–230 °C (EtOH/DMF); IR (KBr) νmax: 2921(aliphatic CH), 1696 (C=O), 1600 (C=N) cm−1; 1H-NMR (DMSO-d6): δ 1.13 (t, 6H, 2CH3, J = 6.6 Hz), 2.15 (s, 6H, 2CH3), 2.30 (s, 6H, 2CH3), 3.8 (s, 6H, 2CH3), 4.12 (q, 4H, 2CH2, J = 6.6 Hz), 4.27 (s, 4H, 2CH2), 7.02 (d, 4H, J = 8.1 Hz), 7.56 (d, 4H, J = 8.1 Hz); 13C-NMR (DMSO-d6): 8.92, 9.53, 14.13, 42.93, 55.39, 59.48, 106.03, 114.24, 123.07, 130.30, 132.89, 136.44, 146.64, 160.35, 165.80. Anal. Calcd for C34H40N6O6: , C, 64.95; H, 6.41; N, 13.37. Found: C, 64.82; H, 6.38; N, 13.35%
Diethyl 1,1′-(ethane-1,2-diyl)bis(2,5-dimethyl-4-(4-chlorophenylazo)-1H-pyrrole-3- carboxylate) (5d). Yield (43%), mp. 214–215 °C (EtOH/DMF); IR (KBr) νmax: 2978 (aliphatic CH), 1701 (C=O) cm−1; 1H-NMR (DMSO-d6): δ 1.12 (t, 6H, 2CH3, J = 6.9 Hz), 2.16 (s, 6H, 2CH3), 2.32 (s, 6H, 2CH3), 4.12 (q, 4H, 2CH2, J = 6.9 Hz), 4.31 (s, 4H, 2CH2), 7.52 (d, 4H, J = 8.7 Hz), 7.68 (d, 4H, J = 8.7 Hz); 13C-NMR (DMSO-d6): δ 8.97, 9.55, 14.12, 42.95, 59.63, 106.06, 120.64, 123.04, 129.16, 132.72, 133.49, 136.45, 151.07, 165.55; MS m/z (%) 637 (M+, 5.46), 622 (0.89), 497 (0.52), 139 (2.18), 111 (8.72). Anal. Calcd for C32H34Cl2N6O4: C, 60.28; H, 5.38; N, 13.18. Found: C, 60.25; H, 5.29; N, 13.07%.
Diethyl 1,1′-(ethane-1,2-diyl)bis(2,5-dimethyl-4-(3-chlorophenylazo)-1H-pyrrole-3-carboxylate) (5e). Yield (44%), mp. 198–199 °C (EtOH); IR (KBr) νmax; 2974 (aliphatic CH), 1709 (C=O) cm−1; 1H-NMR (DMSO-d6): δ 1.17 (t, 6H, 2CH3, J = 6.9 Hz), 2.19 (s, 6H, 2CH3), 2.36 (s, 6H, 2CH3), 4.17 (q, 4H, 2CH2, J = 6.9 Hz), 4.34 (s, 4H, 2CH2), 7.15-7.66 (m, 8H, ArH); 13C-NMR (DMSO-d6): δ 9.12, 9.52, 14.12, 42.94, 59.71, 106.20, 119.57, 121.70, 128.88, 130.92, 133.06, 133.88, 133.92, 136.41, 153.61, 165.52. Anal. Calcd for C32H34Cl2N6O4: C, 60.28; H, 5.38; N, 13.18. Found: C, 60.17; H, 5.24; N, 13.25%.
3.2. Agar Diffusion Well Method to Determine the Antimicrobial Activity
The microorganism inoculums were uniformly spread using sterile cotton swabs on a sterile Petri dish malt extract agar (for fungi) and nutrient agar (for bacteria). One hundred µL of each sample was added to each well (10 mm diameter holes cut in the agar gel, 20 mm apart from one another). Then, the systems were incubated for 24–48 h at 37 °C (for bacteria) and at 28 °C (for fungi). After incubation, the microorganism’s growth was observed. Inhibition of the bacterial and fungal growth were measured in mm. Tests were performed in triplicate [33].
3.3. Docking Studies
Docking studies for the synthesized products were performed using Molecular Operating Environment (MOE) version 2008.10 (Chemical Computing Group Inc., Montreal, QC, Canada). Compounds 5a–e were built using the builder interface of the MOE program and subjected to energy minimization using the included Forcefield MMFF94x calculations. The X-crystallographic structure of E. coli Enoyl reductase (PDB ID: 1LXC) which is complexed with ((E)-3-(6-aminopyridin-3-yl)-N-methyl-N-((1-methyl-1H-indol-2-yl)methyl) acrylamide (PDB ID: AYM)) (NAD), that was obtained from Protein Data Bank.
4. Conclusions
A new simple approach to bis-pyrrole derivatives from hydrazonoyl halides has been achieved. The maximum antimicrobial activity was attained with compound 5d, having chlorine in the para position. The molecular docking of bis-pyrrole derivatives 5a–e was performed using the MOE program.
Acknowledgments
I am thankful to Ahmed Abdelshafy–faculty of science–Cairo University for the Molecular Docking.
Conflicts of Interest
The author declares that there is no conflict of interests regarding the publication of this paper.
Footnotes
Sample Availability: Samples of the compounds 5a–e are available from the author.
References
- 1.Mabkhot Y.N., Barakat A., Al-Majid A.M., Alshahrani S., Yousuf S., Choudhary M.I. Synthesis, reactions and biological activity of some new bis-heterocyclic ring compounds containing sulphur atom. Chem. Cent. J. 2013;7:112–120. doi: 10.1186/1752-153X-7-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar R., Panwar H. Synthesis and pharmacological evaluation: antimicrobial, anti-inflammatory, analgesic, ulcerogenic properties of several bis-heterocyclic derivatives. Indonesian J. Pharm. 2015;26 doi: 10.14499/indonesianjpharm26iss1pp1. [DOI] [Google Scholar]
- 3.Bhanu Prakash T., Dinneswara Reddy G., Padmaja A., Padmavathi V. Synthesis and antimicrobial activity of amine linked bis- and tris-heterocycles. Eur. J. Med. Chem. 2014;82:347–354. doi: 10.1016/j.ejmech.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Tripathi A., Fornabaio M., Kellogg G.E., Gupton J.T., Gewirtz D.A., Yeudall W.A., Vega N.E., Mooberry S.L. Docking and hydropathic scoring of polysubstituted pyrrole compounds with antitubulin activity. Bioorg. Med. Chem. 2008;16:2235–2242. doi: 10.1016/j.bmc.2007.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Micheli F., di Fabio R., Cavanni P., Rimland J.M., Capelli A.M., Chiamulera C., Corsi M., Corti C., Donati D., Feriani A., et al. Synthesis and pharmacological characterisation of 2,4-dicarboxy-pyrroles as selective non-competitive mGluR1 antagonists. Bioorg. Med. Chem. 2003;11:171–183. doi: 10.1016/S0968-0896(02)00424-8. [DOI] [PubMed] [Google Scholar]
- 6.Krutzik P.O., Chamberlin A.R. Rapid solid-phase synthesis of DNA-binding pyrrole-imidazole polyamides. Bioorg. Med. Chem. Lett. 2002;12:2129–2132. doi: 10.1016/S0960-894X(02)00359-1. [DOI] [PubMed] [Google Scholar]
- 7.Lv K., Wang L.L., Liu M.L., Zhou X.B., Fan S.Y., Liu H.Y., Zheng Z.B., Li S. Synthesis and antitumor activity of 5-[1-(3-(dimethylamino)propyl)-5-halogenated-2-oxoindolin-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxamides. Bioorg. Med. Chem. Lett. 2011;21:3062–3065. doi: 10.1016/j.bmcl.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 8.Baraldi P.G., Romagnoli R., Pavani M.G., Nunez M.D.C., Bingham J.P., Hartley J.A. Benzoyl and cinnamoyl nitrogen mustard derivatives of benzoheterocyclic analogues of the tallimustine: Synthesis and antitumour activity. Bioorg. Med. Chem. 2002;10:1611–1618. doi: 10.1016/S0968-0896(01)00425-4. [DOI] [PubMed] [Google Scholar]
- 9.Kang S.Y., Park E.J., Park W.K., Kim H.J., Jeong D., Jung M.E., Song K.S., Lee S.H., Seo H.J., Kim M.J., et al. Arylpiperazine-containing pyrrole 3-carboxamide derivatives targeting serotonin 5-HT2A, 5-HT2C and the serotonin transporter as a potential antidepressant. Bioorg. Med. Chem. Lett. 2010;20:1705–1711. doi: 10.1016/j.bmcl.2010.01.093. [DOI] [PubMed] [Google Scholar]
- 10.Holub J.M., O’Toole-Colin K., Getzel A., Argenti A., Evans M.A., Smith D.C., Dalglish G.A., Rifat S., Wilson D.L., Taylor B.M., et al. Lipid-lowering effects of ethyl 2-phenacyl-3-aryl-1H-pyrrole-4-carboxylates in rodents. Molecules. 2004;9:134–157. doi: 10.3390/90300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almerico A.M., Diana P., Barraja P., Dattolo G., Mingoia F., Putzolu M., Perra G., Milia C., Musiu V., Marongiu M.E. Glycosidopyrroles. Part 2. Acyclic derivatives: 1-(1,3-dihydroxy-2-propoxy)methyl pyrroles as potential antiviral agents. Farmaco. 1997;52:667–672. [PubMed] [Google Scholar]
- 12.Ramazanzadeh R., Nasiri F. Dimethyl 2-hydroxy-1-methyl-3-[2-Oxo-2-Phenylethylidene]-2-Phenyl-1,2-Dihydro-3H-Pyrrole-4,5-dicarboxylate: A potential lead compound as anti-gram-positive and anti-gram-negative agent. J. Applied Sci. 2009;9:2198–2200. doi: 10.3923/jas.2009.2198.2200. [DOI] [Google Scholar]
- 13.Shawali A.S., Abdallah M.A. The Chemistry of Heterocyclic Hydrazonoyl Halides. Adv Heterocycl. Chem. 2001;80:277–338. [Google Scholar]
- 14.Shawali A.S., Edrees M.M. Reactions of nitrilimines with heterocyclic amines and enamines. Convenient methodology for synthesis and annulation of Heterocycles. Arkivoc. 2006;9:292–365. doi: 10.1002/chin.200725234. [DOI] [Google Scholar]
- 15.Kheder N.A., Mabkhoot Y.N. Synthesis and antimicrobial studies of some novel bis-[1,3,4]thiadiazole and bis-thiazole pendant to thieno[2,3-b]thiophene moiety. Int. J. Mol. Sci. 2012;13:3661–3670. doi: 10.3390/ijms13033661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mabkhot Y.N., Kheder N.A., Al-Majid A.M. Facile and convenient synthesis of new thieno[2,3-b]thiophene derivatives. Molecules. 2010;15:9418–9426. doi: 10.3390/molecules15129418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darwish E.S., Kheder N.A., Farag A.M. Synthesis and antimicrobial evaluation of some new pyridine based Heterocycles. Heterocycles. 2010;81:2247–2256. [Google Scholar]
- 18.Mabkhot Y.N., Kheder N.A., Farag A.M. An efficient synthesis of new thiazole based heterocycles. Heterocycles. 2010;81:2369–2376. [Google Scholar]
- 19.Dawood K.M., Kheder N.A., Ragab E.A., Mohamed S.N. A facile access to some new pyrazole, 1,3,4-thiadiazole, and thiophene derivatives via B-ketosulfones. Phosphorus Sulfur Silicon Relat. Elem. 2010;185:330–339. doi: 10.1080/10426500902796980. [DOI] [Google Scholar]
- 20.Farag A.M., Kheder N.A., Mabkhot Y.N. Synthesis and antimicrobial evaluation of new pyrazole, thiophene, thiazole and 1,3,4-thiadiazole derivatives incorporating pyrimidine ring. Heterocycles. 2009;78:1787–1798. doi: 10.3987/COM-09-11682. [DOI] [Google Scholar]
- 21.Kheder N.A. Convenient synthesis of novel bis(hydrazone) and bis(indole) derivatives. Heterocycles. 2009;78:1281–1288. doi: 10.3987/COM-08-11615. [DOI] [Google Scholar]
- 22.Kheder N.A. Synthesis of some novel bis(pyrazole), bis(pyridine) and bis-pyrazolo[5,1-c]-1,2,4-triazine derivatives. Heterocycles. 2009;78:1815–1822. doi: 10.3987/COM-09-11676. [DOI] [Google Scholar]
- 23.Kheder N.A., Mabkhoot Y.N., Farag A.M. Synthesis and antimicrobial evaluation of some bis(thioxopyridine), bis(pyrazolo[3,4-b]pyridine), bis(thieno[2,3-b]pyridine), bis(1,3,4-thiadiazole) and bis(thiophene) derivatives. Heterocycles. 2008;75:2937–2948. [Google Scholar]
- 24.Kheder N.A., Mabkhot Y.N., Farag A.M. Facile and convenient synthesis of pyrazole, pyridine, pyridazine, pyrazolo[3,4-b]pyridine, and pyrazolo[5,1-c][1,2,4]-triazine derivatives. Synth. Commun. 2008;38:3170–3182. doi: 10.1080/00397910802109257. [DOI] [Google Scholar]
- 25.Kheder N.A., Farghaly T.A. Bis-Hydrazonoyl chloride as precursors for synthesis of novel polysubstituted bis-azoles. Arab. J. Chem. 2013 doi: 10.1016/j.arabjc.2013.11.040. [DOI] [Google Scholar]
- 26.Shyadligeri A.S., Gadaginamath G.S., Subramanian L.R. One-pot synthesis of a novel 1,1′-bridged bis(benzo(g)indole) diester and the preparation of some of its derivatives. J. Chem. Res. 1996;2:114–115. doi: 10.1002/chin.199631119. [DOI] [Google Scholar]
- 27.Zhang Z.L., Jin S., Shang Z., Huang S., Liu B., Guo J. Diethyl (Z,Z)-3,3′-(ethane-1,2-diyldi-imino)dibut-2-enoate. Acta Crystallogr. C. 2004;60:o176–o177. doi: 10.1107/S0108270104000241. [DOI] [PubMed] [Google Scholar]
- 28.Dieckmann W., Platz L. Ueber eine neue Bildungsweise von Osotetrazonen. Ber. Dtsch. Chem. Ges. 1905;38:2986–2990. doi: 10.1002/cber.190503803103. [DOI] [Google Scholar]
- 29.Abushamleh A.S., Al-Aqarbeh M.M., Day V. Transition Metal Complexes of Derivatized Chiral Dihydro-1,2,4-triazin-6-ones. Template Synthesis of Nickel(II) Tetraaza-(4N-M) Complexes Incorporating the Triazinone Moiety. Am. J. Appl. Sci. 2008;5:750–754. doi: 10.3844/ajassp.2008.750.754. [DOI] [Google Scholar]
- 30.Eweiss N.F., Abdelhamid A.O. Synthesis of Heterocycles. Part II. New routes to acetyl thiadiazolines and alkylazothiazoles. J. Heterocycl. Chem. 1980;17:1713–1717. doi: 10.1002/jhet.5570170814. [DOI] [Google Scholar]
- 31.Tanoli S.A.K., Tanoli N.U., Usmani S., Ferreira A.G. The exploration of interaction studies of smaller size, mostly ignored yet intrinsically inestimable molecules towards BSA; An example of STD and DOSY NMR. Cent. Eur. J. Chem. 2014;12:332–340. doi: 10.2478/s11532-013-0380-7. [DOI] [Google Scholar]
- 32.Protein Data Bank. [(accessed on 1 March 2016)]. Available online: http://www.rcsb.org/pdb.
- 33.Smania A., Monache F.D., Smania E.F.A., Cuneo R.S. Triterpenes and sterols from ganoderma australe (Fr) Pat. (Aphyllophoromycetideae) Int. J. Med. Mushrooms. 1999;1:325–334. [Google Scholar]