Abstract
This paper presents a comprehensive analysis of the phytochemical profile of a proprietary rosemary (Rosmarinus officinalis L.) extract rich in carnosic acid. A characterization of the (poly)phenolic and volatile fractions of the extract was carried out using mass spectrometric techniques. The (poly)phenolic composition was assessed by ultra-high performance liquid chromatography-electrospray ionization-mass spectrometry (UHPLC-ESI-MSn) and a total of 57 compounds were tentatively identified and quantified, 14 of these being detected in rosemary extract for the first time. The rosemary extract contained 24 flavonoids (mainly flavones, although flavonols and flavanones were also detected), 5 phenolic acids, 24 diterpenoids (carnosic acid, carnosol, and rosmanol derivatives), 1 triterpenoid (betulinic acid), and 3 lignans (medioresinol derivatives). Carnosic acid was the predominant phenolic compound. The volatile profile of the rosemary extract was evaluated by head space solid-phase microextraction (HS-SPME) linked to gas chromatography-mass spectrometry (GC-MS). Sixty-three volatile molecules (mainly terpenes, alcohols, esters, aldehydes, and ketones) were identified. This characterization extends the current knowledge on the phytochemistry of Rosmarinus officinalis and is, to our knowledge, the broadest profiling of its secondary metabolites to date. It can assist in the authentication of rosemary extracts or rosemary-containing products or in testing its bioactivity. Moreover, this methodological approach could be applied to the study of other plant-based food ingredients.
Keywords: rosemary, polyphenol, volatile compound, phytochemical characterization, UHPLC-ESI-MSn, HS-SPME/GC-MS
1. Introduction
Rosemary (Rosmarinus officinalis L.), which belongs to the family Lamiaceae, is an aromatic, evergreen, 1-m high shrub with upright stems, whitish-blue flowers and dark green leaves. The foliage of the plant is usually used as a common household culinary spice for flavoring [1,2,3]. Rosemary extracts, mainly derived from the leaves, are common herbal products used as flavoring and antioxidant agents in food processing and cosmetics. As naturally occurring antioxidants, they are preferred to synthetic antioxidants such as butylated hydroxyanisole (BHA) or butylated hydroxytoluene (BHT) [4]. Moreover, rosemary has been used in traditional and complementary alternative medicine for its digestive, tonic, astringent, diuretic, and diaphoretic properties [1,2,3]. It has also been linked to a broad range of beneficial health effects, having for example antidepressant [5], antihypertensive [6], antiproliferative [7], antibacterial [8], antiatherogenic [9], hypocholesterolemic [10], hepatoprotective [11], and anti-obesity properties [11,12].
The biological properties of rosemary have been attributed to its phytochemical composition rich in (poly)phenolic compounds, mainly diterpenoids such as carnosic acid and carnosol [5,7,10,11,12]. However, the positive contribution of flavonoids to rosemary bioactivity is also reported in the literature [5]. After considering the co-presence of flavonoids and diterpenes in the plant [3,13], the way these compounds are metabolized [14,15,16], and their consequent co-occurrence in circulation, the benefits ascribed to rosemary cannot be unambiguously attributed to a single class of compounds, but rather to the multiple contribution of its different bioactive compounds. Furthermore, the phenolic composition of rosemary extracts has been reported to vary depending on agronomical and processing conditions [17,18,19,20]. For this reason, the phenolic fraction of every rosemary product should be accurately characterized to better understand its technological and bioactivity prospects. In addition, due to the contribution of the volatile profile of any food extract to its potential uses, the characterization of the volatile fraction of rosemary extracts should also be evaluated.
This study aimed to comprehensively profile the phytochemical composition of an extract rich in carnosic acid from a proprietary rosemary line. The (poly)phenolic composition was assessed by means of ultra-high performance liquid chromatography-electrospray ionization-mass spectrometry (UHPLC-ESI-MSn), whereas the volatile profile was studied using head space solid-phase microextraction/gas chromatography-mass spectrometry (HS-SPME/GC-MS).
2. Results and Discussion
2.1. Profiling of the Phenolic Composition
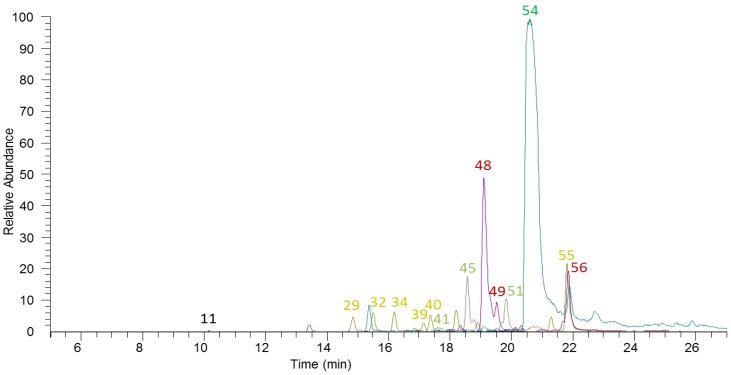
The (poly)phenolic profile of the proprietary rosemary extract rich in carnosic acid was evaluated using an UHPLC-ESI-MSn untargeted method consisting of two complementary mass spectrometry (MS) conditions [21]. About 190 mass spectra were assessed for each analytical replicate and MS operating condition in this comprehensive approach for a complete screening of (poly)phenolic compounds. This procedure allowed a detailed evaluation of the rosemary extract phenolic fraction and the tentative identification of up to 57 phytochemicals (Table 1). The most represented classes of (poly)phenolic compounds in the extract were diterpenoids and flavonoids (flavones, flavanones, and flavonols), with a total of 24 molecules identified for each class. Some phenolic acids and lignans, as well as one triterpenoid, were also identified.
Table 1.
(Poly)phenolic compounds in rosemary extract identified by ultra-high performance liquid chromatography-mass spectrometry (UHPLC-MSn) in negative ionization mode under different mass spectrometry (MS) conditions.
| ID. | Compounds | RT (min) | [M − H]− (m/z) | MS2 ion Fragments (m/z) a | MS3 ion Fragments (m/z) a | Exp. 1 c | Exp. 2 c | Ident. d |
|---|---|---|---|---|---|---|---|---|
| 1 | Caffeic acid | 6.82 | 179 | 135 | x | x | Std | |
| 2 | Medioresinol | 7.18 | 387 | 207 b, 163, 369 | 163 | x | x | [4,12] |
| 3 | p-Coumaric acid | 7.93 | 163 | 119 | 119 | x | x | [4] |
| 4 | Luteolin-rutinoside | 8.78 | 593 | 285 | 285, 241, 175, 199, 217 | x | - | [4,22] |
| 5 | Luteolin-hexoside | 8.98 | 447 | 285, 378 | 285, 241, 267, 199, 175 | x | x | [4] |
| 6 | Isorhamnetin-3-O-hexoside | 9.28 | 477 | 315, 300, 357, 462 | 300 | x | x | [4] |
| 7 | 4-hydroxybenzoic acid | 9.47 | 137 | 93, 137 | x | x | [4] | |
| 8 | Apigenin-7-O-glucoside | 9.82 | 431 | 269 | 225, 149, 201, 183, 281 | x | x | [23] |
| 9 | Hesperidin (Hesperetin-7-O-rutinoside) | 9.87 | 609 | 301 | 286, 242, 257, 283, 125 | x | x | Std |
| 10 | Homoplantaginin (Hispidulin 7-glucoside) | 10.04 | 461 | 299, 446, 284, 341 | 284, 255, 179 | x | x | [3] |
| 11 | Rosmarinic acid | 10.11 | 359 | 161, 179, 197, 223 | 161, 133 | x | x | Std |
| 12 | Luteolin-7-O-glucuronide | 10.28 | 461 | 285 | 241, 217, 175, 199 | x | x | [4] |
| 13 | Dihydroxy-dimethoxyflavone derivative | 10.33 | 387 | 313, 343 | 298 | x | - | [4,24] |
| 14 | Dihydroxy-dimethoxyflavone | 10.71 | 313 | 298 | 269, 283, 297, 280 | x | - | [4,24] |
| 15 | Medioresinol derivative | 11.22 | 593 | 387, 561, 519 | 207, 163, 369 | x | x | [12] |
| 16 | Dihydroxy-dimethoxyflavone | 11.24 | 313 | 298 | 283, 297, 269, 150 | x | - | [4,24] |
| 17 | Luteolin-3′-acetyl-O-glucuronide | 11.25 | 503 | 285, 399, 443 | 241, 243, 217, 199, 175 | x | x | [13,23] |
| 18 | Medioresinol-glucuronide | 11.37 | 563 | 387, 531, 489 | 207, 163, 369 | x | x | [12] |
| 19 | Eriodictyol | 11.46 | 287 | 151 | 107 | x | x | [25] |
| 20 | Isorhamnetin-rutinoside | 11.51 | 623 | 315, 300 | 300 | x | x | [4,22] |
| 21 | Luteolin | 11.75 | 285 | 285, 241, 199, 217, 257, 151, 179, 213 | x | x | [4,22,26] | |
| 22 | Isorhamnetin | 11.91 | 315 | 300, 301, 287 | 300, 216, 228, 256, 272 | x | x | [4,22] |
| 23 | Trihydroxy-methoxyflavone | 11.98 | 299 | 284 | 283, 227, 256, 212, 200 | x | x | [3,27] |
| 24 | Methyl rosmarinate | 12.36 | 373 | 179, 135, 305 | 135 | x | x | [26] |
| 25 | Apigenin-7-O-rutinoside | 12.58 | 577 | 269, 307 | 269, 225, 201, 181, 149 | x | x | [4,22] |
| 26 | Apigenin | 13.02 | 269 | 269, 225, 149, 201, 183 | 181, 197, 169, 224 | x | x | [4,22] |
| 27 | Hispidulin-rutinoside | 13.21 | 607 | 299, 284, 269, 323 | 284 | x | - | [3,27] |
| 28 | Hesperetin | 13.41 | 301 | 286, 242, 257, 283, 125 | 258, 242, 199, 174, 215 | x | x | [28] |
| 29 | 5,6,7,10-tetrahydro-7-hydroxy rosmariquinone derivative | 14.88 | 345 | 301 | 301, 258, 283, 273, 217 | x | x | [14] |
| 30 | Cirsimaritin | 14.98 | 313 | 298 | 283, 297, 269 | x | x | [4,12] |
| 31 | Carnosol methyl ether isomer | 15.35 | 343 | 328, 299 | 313, 299, 285 | x | x | [14] |
| 32 | Rosmanol | 15.46 | 345 | 283, 301, 327 | 268, 240, 227, 265, 239 | x | x | Std |
| 33 | Rosmadial isomer or rosmanol quinone | 15.97 | 343 | 299, 315 | 284, 243, 213, 256, 281 | x | x | [3,4,13] |
| 34 | Rosmanol isomer (epirosmanol) | 16.22 | 345 | 283, 301, 327 | 268, 227, 240, 239, 265 | x | x | [3] |
| 35 | Carnosol quinone | 16.27 | 327 | 299, 258 | 284, 271 | x | x | [29] |
| 36 | Isosakuranetin | 16.44 | 285 | 270, 229, 214, 201, 242 | x | x | [25] | |
| 37 | Genkwanin | 16.45 | 283 | 268 | 268 | x | x | [3,4] |
| 38 | Carnosic acid hexoside | 16.76 | 493 | 331, 373, 313, 179 | 287, 244 | x | x | Std |
| 39 | Rosmanol isomer (epiisorosmanol) | 17.18 | 345 | 301 | 301, 286 | x | x | [12] |
| 40 | 5,6,7,10-tetrahydro-7-hydroxy rosmariquinone derivative | 17.41 | 345 | 301 | 301, 258, 283, 273, 217 | x | x | [14] |
| 41 | Carnosol methyl ether isomer | 17.78 | 343 | 299, 328, 285, 343, 315 | 284, 243, 281, 299, 256 | x | x | [14] |
| 42 | Carnosol methyl ether isomer | 17.99 | 343 | 328, 313, 343, 299, 285 | 313, 300, 285, 257 | x | x | [14] |
| 43 | Carnosic acid derivative | 18.15 | 455 | 331, 287 | 287, 244 | x | x | Std |
| 44 | Rosmanol methyl ether | 18.59 | 359 | 283, 329, 300 | 268, 240, 227, 265, 239 | x | - | [14] |
| 45 | Rosmadial or rosmanol quinone | 18.62 | 343 | 299 | 243, 216, 284 | x | x | [14] |
| 46 | Epiisorosmanol methyl ether | 18.79 | 359 | 315 | 300 | x | - | [14] |
| 47 | Rosmanol methyl ether isomer | 18.96 | 359 | 283, 329, 300 | 268, 240, 227, 265, 239 | x | x | [14] |
| 48 | Carnosol | 19.07 | 329 | 285 | 270, 285, 269, 201, 214 | x | x | Std |
| 49 | Carnosic acid quinone | 19.51 | 329 | 285 | 270, 285, 201, 227 | x | x | [30] |
| 50 | 4′-Methoxytectochrysin | 19.76 | 297 | 282, 269, 297, 254 | 267, 281, 238 | x | x | [20] |
| 51 | Rosmadial | 19.87 | 343 | 315, 299 | 287, 269, 297 | x | x | [3,4,13] |
| 52 | Rosmaridiphenol | 20.09 | 315 | 285, 179, 135 | 285, 214, 201, 270 | x | x | [3,31] |
| 53 | 5,6,7,10-tetrahydro-7-hydroxy rosmariquinone | 20.37 | 301 | 258, 283, 273, 217, 233 | 243, 257, 188, 215, 162 | x | x | [14] |
| 54 | Carnosic acid | 20.85 | 331 | 287 | 287, 244, 272, 217 | x | x | Std |
| 55 | 12-O-Methylcarnosic acid | 21.87 | 345 | 301, 286 | 286 | x | x | Std |
| 56 | Carnosol isomer | 21.88 | 329 | 329, 314, 299, 285 | x | x | [31] | |
| 57 | Betulinic acid | 23.71 | 455.5 | 327, 317, 353, 409, 437 | x | x | Std |
a Fragment ions are listed in order of relative abundance; b MS2 ions in bold were those subjected to MS3 fragmentation; c Exp. 1, detected under experimental condition 1 (carnosol), Exp. 2, experimental condition 2 (rosmarinic acid); d Ident., identification mode: [Reference] or Std (standard, compound identified by comparison of its retention time and MS data with that of a reference compound). Some compounds were defined as “derivatives” since parts of their spectra match those of their corresponding parent compounds but they cannot be fully elucidated.
Table 1 describes the retention time and mass spectrum data for each identified compound. Ten compounds were identified and quantified by comparison with commercial reference standards. The identification of the remaining 47 (poly)phenolic compounds was tentatively carried out by interpreting and comparing their mass spectra, obtained from MS2 and MS3 experiments with data from the literature. Fourteen phytochemicals (compounds 13, 14, 15, 16, 18, 20, 27, 29, 36, 38, 40, 43, 50, and 53) were tentatively identified for the first time, to our knowledge, in rosemary extracts. The description of the MS fragmentation patterns already described in literature is not further discussed unless of special interest.
A total of 24 diterpenoids were identified in the rosemary extract (Figure 1). Most of the detected diterpenoids had already been reported in rosemary (compounds 31, 35, 39, 41, 42, 44–49, 51, 52, and 54–56) [3,4,12,13,17,18,23,29,32]. Compounds 31, 33, 41, 42, 45, and 51 exhibited molecular ions at m/z 343. Carnosol methyl ether isomers (compounds 31, 41, and 42) were distinguished from rosmadial or rosmanol quinone isomers (33, 45, and 51) thanks to the fragment ion at m/z 328 and the neutral loss of 15 amu, characteristic of the methyl group [14]. Unfortunately, distinction between rosmadial or rosmanol quinone isomers was not possible as they share a common fragmentation pattern. The presence in rosemary extracts of several isomers for these molecules has been previously reported [14]. Glycosylated carnosic acid (compound 38) was tentatively identified through its MS2 fragment ions, characterized by the loss of a hexoside (162 amu), and MS3 fragments identical to those registered for the standard of carnosic acid (54). This approach was also used to identify another carnosic acid derivative (compound 43). Compounds 29 and 40 (m/z 345) were tentatively identified as derivatives of 5,6,7,10-tetrahydro-7-hydroxyrosmariquinone. They fragmented to m/z 301 (with neutral loss of 44 amu, likely corresponding to a carboxylic group from the parent ion) and their MS3 fragmentation spectra matched the characteristic fragmentation pattern of 5,6,7,10-tetrahydro-7-hydroxyrosmariquinone (compound 53) [12]. These two derivatives had only been previously described in biological fluids of rats following the intake of a rosemary extract [14].
Figure 1.
Main rosemary (poly)phenolic compounds. Peak numbers refer to components listed in Table 1.
Twenty-four flavonoids, belonging to three subclasses of flavonoids (flavones, flavonols, and flavanones), were tentatively identified. Flavones were the main group of flavonoids in the rosemary extract, with 17 compounds identified. Nine of these were conjugated forms (mainly glycosylated) of luteolin (compounds 4, 5, 12, and 17), apigenin (8 and 25), hispidulin (10 and 27), and a dihydroxy-dimethoxyflavone (13) [3,4,13,24]. A large number of flavone aglycones with different hydroxylation and/or methylation patterns was also detected (14, 16, 21, 23, 26, 30, 37, and 50). The retention time and fragmentation pattern of compound 23 (m/z 299) did not match well with those already reported for other trihydroxy-methoxyflavones previously identified in rosemary extracts such as diosmetin or hispidulin [23]. With respect to flavanones, three aglycones (compounds 19, 28, and 36) and one rutinoside (9) were detected. Isorhamnetin was the only flavonol detected, in both the free (22) and glycosylated forms (6 and 20) [4].
Five phenolic acids were identified in the rosemary extract, a hydroxybenzoic acid (compound 7), two hydroxycinnamic acids (1 and 3), and two rosmarinic acid derivatives (11 and 24). These findings are in agreement with previous works [4,13]. The profiling of the (poly)phenolic fraction of the rosemary extract also allowed the identification of three lignans, namely medioresinol (2) [12] and two medioresinol derivatives (15 and 18), the latter tentatively identified for the first time in this plant material.
Only one triterpenic acid, betulinic acid (57), was detected. Oleanolic acid and ursolic acid, typically present in the triterpenoid fraction of rosemary [3], were not detected in this extract.
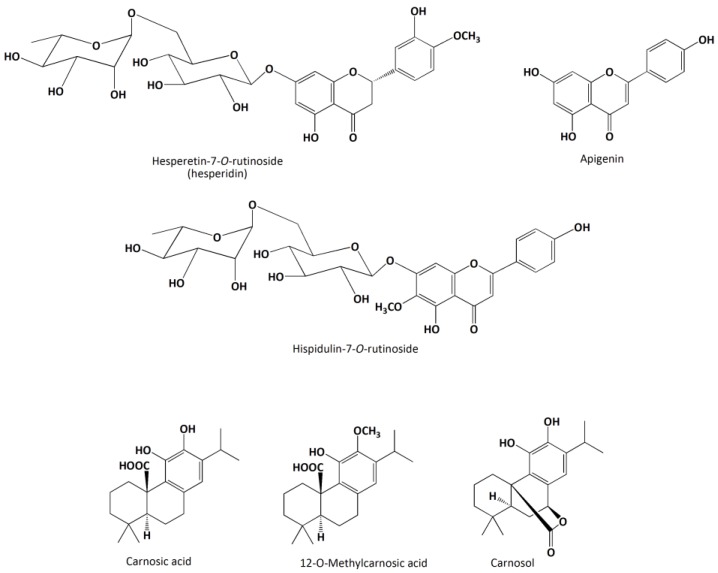
This comprehensive analysis of the phenolic composition of a rosemary extract represents the broadest characterization of its (poly)phenolic fingerprint to date. From the 57 (poly)phenolic compounds tentatively identified, a quarter corresponded to molecules not previously reported as present in this plant. Despite accurate characterizations of rosemary extracts reported in the literature [4,12,17,18,23,32], this work extends the range of molecules contributing to the definition of this food matrix, and may assist in the study of its bioactive properties (Figure 2).
Figure 2.
Some of the (poly)phenolic compounds present in the rosemary extract.
The specific experimental condition in which each compound was detected is reported in Table 1. Interestingly, while some chemical scaffolds could not be identified under experimental condition 2 (optimized for rosmarinic acid analysis), all the structures responded well to the MS settings of experimental condition 1 (optimized for carnosol analysis). In comparison with some other works using the same methodology [21,33], this is the first time that a specific MS configuration was able to detect all the identified compounds of a phenolic-rich plant matrix. This information may account for the versatility of MS experimental condition 1 in identifying varying phenolic structures, such as simple phenolic acids, different kinds of flavonoids, diterpenoids, and triterpenoids.
The quantification of phenolics was carried out by comparison with commercial standards, when available. For those compounds that could not be quantified with their corresponding standards, a reference compound was selected based on structural similarity and considering the functional groups that may affect the ionisation properties (i.e., carnosol derivatives were quantified as carnosol, rosmanol derivatives as rosmanol, flavonols as rutin (quercetin-rutinoside), flavones as luteolin-4-glucoside, etc.). Finally, the molecules responding to the electro-spray ionisation (ESI) source in a unique way with respect to the reference compound of choice, or not reaching the limit of quantification of the corresponding reference compound, were not quantified.
The amount of (poly)phenolic compounds in this rosemary extract was 166.32 ± 11.05 mg/mL. Although the (poly)phenolic profile of the extract was composed of a high number of different phenolic structures (Table 1), diterpenoids accounted for the 97.2% of this phenolic content (161.66 ± 10.64 mg/mL). Furthermore, this was attributed mainly to the amount of carnosic acid derivatives in the extract (77.1% of total phenolics, Table 2). Flavonoids represented about 1.4% (2.38 ± 0.22 mg/mL) of the total amount of detected (poly)phenolic compounds, followed by triterpenoids (1.3% of total phenolics, 2.10 ± 0.25 mg/mL). Phenolic acids made up only 0.1% of the total phenolic fraction. The amount of phenolic compounds previously reported for other rosemary extracts was quite variable and ranged from ~39.3 mg/g [18] to 523 mg/g [12], with some extracts showing a similar content to that reported here [3]. In accordance with our data, other rosemary extracts were composed mainly of carnosic acid, followed by carnosol and other diterpenoids, with flavonoids as minor components [3,12,18]. It should be noted that the amount and relative contribution of each class of (poly)phenolic compounds to rosemary extracts have been reported to be dependent on the extraction procedure and solvent used [17,18,19]. In addition, irrigation conditions, harvest time, storage conditions, and drying treatments are also factors that may affect the final phenolic composition of rosemary extracts [18,20].
Table 2.
Quantitative results for rosemary extract (poly)phenolic compounds.
| ID. a | Compounds | Quantified as… | Concentration (mg/mL) | ||
|---|---|---|---|---|---|
| 1 | Caffeic acid | Caffeic acid b | 0.03 | ± | 0.00 |
| 3 | p-Coumaric acid | Caffeic acid | 0.01 | ± | 0.00 |
| 4 | Luteolin-rutinoside | Luteolin-4-glucoside | 0.00 | ± | 0.00 |
| 5 | Luteolin-hexoside | Luteolin-4-glucoside | 0.01 | ± | 0.00 |
| 6 | Isorhamnetin-3-O-hexoside | Rutin | 0.04 | ± | 0.00 |
| 7 | 4-hydroxybenzoic acid | Caffeic acid | 0.01 | ± | 0.00 |
| 8 | Apigenin-7-O-glucoside | Vitexin (Apigenin-8-C-glucoside) | 0.02 | ± | 0.00 |
| 9 | Hesperidin (Hesperetin-7-O-rutinoside) | Hesperidin (Hesperitin-7-rutinoside) b | 0.26 | ± | 0.02 |
| 10 | Homoplantaginin (Hispidulin 7-glucoside) | Luteolin-4-glucoside | 0.12 | ± | 0.02 |
| 11 | Rosmarinic acid | Rosmarinic acid b | 0.12 | ± | 0.01 |
| 12 | Luteolin-7-O-glucuronide | Luteolin-4-glucoside | 0.01 | ± | 0.00 |
| 13 | Dihydroxy-dimethoxyflavone derivative | Luteolin-4-glucoside | 0.01 | ± | 0.00 |
| 14 | Dihydroxy-dimethoxyflavone | Luteolin-4-glucoside | 0.00 | ± | 0.00 |
| 16 | Dihydroxy-dimethoxyflavone | Luteolin-4-glucoside | 0.02 | ± | 0.00 |
| 17 | Luteolin 3′-O-acetyl-O-glucuronide | Luteolin-4-glucoside | 0.01 | ± | 0.00 |
| 20 | Isorhamnetin rutinoside | Rutin | 0.00 | ± | 0.00 |
| 21 | Luteolin | Luteolin-4-glucoside | 0.14 | ± | 0.03 |
| 22 | Isorhamnetin | Rutin | 0.12 | ± | 0.01 |
| 23 | Trihydroxy-methoxyflavone | Vitexin (Apigenin-8-C-glucoside) | 0.18 | ± | 0.01 |
| 24 | Methyl rosmarinate | Rosmarinic acid | 0.02 | ± | 0.00 |
| 25 | Apigenin-7-O-rutinoside | Vitexin (Apigenin-8-C-glucoside) | 0.00 | ± | 0.00 |
| 26 | Apigenin | Vitexin (Apigenin-8-C-glucoside) | 0.55 | ± | 0.04 |
| 27 | Hispidulin-rutinoside | Luteolin-4-glucoside | 0.89 | ± | 0.15 |
| 29 | 5,6,7,10-tetrahydro-7-hydroxyrosmariquinone derivative | Carnosol | 0.27 | ± | 0.02 |
| 31 | Carnosol methyl ether isomer | Carnosol | 0.00 | ± | 0.00 |
| 32 | Rosmanol | Rosmanol b | 0.15 | ± | 0.01 |
| 33 | Rosmadial isomer or rosmanolquinone | Rosmanol | 0.00 | ± | 0.00 |
| 34 | Rosmanol isomer (epirosmanol) | Rosmanol | 0.14 | ± | 0.01 |
| 35 | Carnosol quinone | Carnosol | 0.02 | ± | 0.00 |
| 38 | Carnosic acid hexoside | Carnosic acid | 0.00 | ± | 0.00 |
| 39 | Rosmanol isomer (epiisorosmanol) | Rosmanol | 0.06 | ± | 0.01 |
| 40 | 5,6,7,10-tetrahydro-7-hydroxyrosmariquinone derivative | Carnosol | 0.08 | ± | 0.01 |
| 41 | Carnosol methyl ether isomer | Carnosol | 0.00 | ± | 0.00 |
| 42 | Carnosol methyl ether isomer | Carnosol | 0.00 | ± | 0.00 |
| 43 | Carnosic acid derivative | Carnosic acid | 0.00 | ± | 0.00 |
| 44 | Rosmanol methyl ether | Rosmanol | 0.00 | ± | 0.00 |
| 45 | Rosmadial or rosmanol quinone | Rosmanol | 0.89 | ± | 0.08 |
| 46 | Epiisorosmanol methyl ether | Rosmanol | 0.01 | ± | 0.00 |
| 47 | Rosmanol methyl ether isomer | Rosmanol | 0.00 | ± | 0.00 |
| 48 | Carnosol | Carnosol b | 28.89 | ± | 2.24 |
| 49 | Carnosic acid quinone | Carnosic acid | 0.17 | ± | 0.14 |
| 51 | Rosmadial | Rosmanol | 1.25 | ± | 0.07 |
| 52 | Rosmaridiphenol | Carnosol | 0.57 | ± | 0.04 |
| 53 | 5,6,7,10-tetrahydro-7-hydroxyrosmariquinone | Carnosol | 0.01 | ± | 0.00 |
| 54 | Carnosic acid | Carnosic acid b | 121.08 | ± | 7.67 |
| 55 | 12-O-Methylcarnosic acid | 12-O-Methylcarnosic acid | 6.90 | ± | 0.58 |
| 56 | Carnosol isomer | Carnosol b | 1.16 | ± | 0.07 |
| 57 | Betulinic acid | Betulinic acid b | 2.10 | ± | 0.25 |
| Hydroxybenzoic acids c | 0.01 | ± | 0.00 | ||
| Hydroxycinnamic acids d | 0.04 | ± | 0.00 | ||
| Rosmarinic acid derivatives e | 0.14 | ± | 0.01 | ||
| Flavones f | 1.82 | ± | 0.18 | ||
| Flavonols g | 0.31 | ± | 0.02 | ||
| Flavanones h | 0.26 | ± | 0.02 | ||
| Carnosic acid derivatives i | 128.15 | ± | 8.11 | ||
| Carnosol derivatives j | 30.08 | ± | 2.31 | ||
| Rosmanol derivatives k | 1.25 | ± | 0.11 | ||
| Other diterpene derivatives l | 2.18 | ± | 0.12 | ||
| Triterpenic acids m | 2.10 | ± | 0.25 | ||
| Total phenolics | 166.32 | ± | 11.05 | ||
a See Table 1 for peak assignment; b Quantified by comparison with its corresponding standard; c Hydroxybenzoic acids include compound 7; d Hydroxycinnamic acids, compounds 1 and 3; e Rosmarinic acid derivatives, compounds 11 and 24; f Flavones, compounds 4, 5, 8, 10, 12–14, 16, 17, 23, and 25–27; g Flavonols, compounds 6 and 20–22; h Flavanones, compound 9; i Carnosic acid derivatives, compounds 38, 43, 49, 54, and 55; j Carnosol derivatives, compounds 31, 35, 41, 42, 48, and 56; k Rosmanol derivatives, compounds 32–34, 39, and 44–47; l Other diterpene derivatives, compounds 29, 40, and 51–53; and m Triterpenic acids, compound 57. Mean (n = 3) ± standard deviation(SD).
2.2. Volatile Profile of Rosemary Extract
The composition of the volatile fraction of rosemary extract was investigated by means of HS-SPME/GC-MS technique. The obtained profile was composed of 63 different gas-chromatographic signals. Two approaches were combined for peak identification: the comparison of registered mass spectra with those present in the instrument library (NIST 14), and the calculation of LRIs (linear retention index) obtained on two different stationary phase columns (SUPELCOWAX 10 and BP5MS). The relative amounts of all identified compounds were calculated based on comparison to an internal standard (toluene). Results are listed in Table 3.
Table 3.
Identification of rosemary extract volatile compounds, with relative aromatic notes, calculated linear retention indices (LRIs) on two different stationary phases (“wax” polar and “BP5” a-polar), identification methods, references, and relative amounts (mean ± SD).
| ID. | Identification | Flavor Note (Flavornet.org) | LRI-wax | LRI-BP5 | Identif. Method | Reference | Concentration (µg/g) |
|---|---|---|---|---|---|---|---|
| 1 | 1R-α-Pinene | Intense woody, pine | 1022 | 928 | MS + LRI | [34] | 4.34 ± 0.65 |
| 2 | Hexanal | Green | 1087 | 776 | MS + LRI | [35] | 2.81 ± 0.28 |
| 3 | α-Thujene | Woody | 1128 | 948 | MS + LRI | [34] | 76.26 ± 13.13 |
| 4 | β-Myrcene | Peppery, terpenic | 1170 | 983 | MS + LRI | [34] | 6.36 ± 0.91 |
| 5 | (+)-4-Carene | 1185 | 1080 | MS | 15.96 ± 2.11 | ||
| 6 | Heptanal | Fresh, aldehydic | 1194 | 890 | MS + LRI | [36] | 4.90 ± 0.44 |
| 7 | D-Limonene | Sweet, citrus, peely | 1205 | 1024 | MS + LRI | [35] | 11.78 ± 2.80 |
| 8 | Eucalyptol | Eucalyptus, herbal | 1213 | 1025 | MS + LRI | [34] | 20.22 ± 2.58 |
| 9 | Cosmene | Dahlia, Laurus nobilis | 1223 | 998 | MS + LRI | [33] | 3.39 ± 0.28 |
| 10 | Not Identified | 1231 | 984 | 5.88 ± 1.36 | |||
| 11 | 2-Pentylfuran | Fruity | 1239 | MS + LRI | [37] | 3.01 ± 0.79 | |
| 12 | γ-Terpinene | Terpy, citrus | 1251 | 1052 | MS + LRI | [35] | 6.26 ± 1.17 |
| 13 | 3-Octanone | Mushroom, ketonic, cheesy and moldy | 1261 | MS + LRI | [37] | 0.61 ± 0.19 | |
| 14 | o-Cymene | Lavender and cypress oil | 1276 | 1017 | MS + LRI | [33] | 15.14 ± 1.87 |
| 15 | α-Terpinene | Terpy, woody, | 1287 | 1011 | MS + LRI | [36] | 5.93 ± 0.67 |
| 16 | 1-Octen-3-one | Intense creamy, earthy | 1308 | MS + LRI | [34] | 0.44 ± 0.28 | |
| 17 | 2,4-Hexadienal | Green, creamy | 1323 | MS + LRI | [38] | 0.48 ± 0.09 | |
| 18 | 2-Heptenal | Green, fatty | 1331 | MS + LRI | [37] | 2.58 ± 0.44 | |
| 19 | 6-Methyl-5-hepten-2-one | Citrus | 1344 | MS+LRI | [37] | 1.06 ± 0.32 | |
| 20 | 3-Octanol | Musty, mushroom | 1396 | MS | 0.74 ± 0.14 | ||
| 21 | Nonanal | Waxy, aldehydic | 1400 | 1094 | MS + LRI | [35] | 3.47 ± 0.91 |
| 22 | (E)-2-Octenal | Fatty, green, herbal | 1437 | 1048 | MS + LRI | [37] | 2.83 ± 0.59 |
| 23 | Ethyl caprylate | Fruity, waxy | 1441 | MS + LRI | [39] | 7.66 ± 2.43 | |
| 24 | p-Cymenene | Phenolic | 1445 | MS | 34.70 ± 5.71 | ||
| 25 | Ylangene | 1487 | 1369 | MS + LRI | [40] | 8.06 ± 1.50 | |
| 26 | α-Copaene | Woody, spicy, honey | 1495 | 1374 | MS + LRI | [37] | 1.02 ± 0.30 |
| 27 | trans-2,4-Heptadienal | Sweet creamy, fatty | 1503 | MS + LRI | [37] | 0.77 ± 0.10 | |
| 28 | Camphor | Camphoreous | 1524 | MS + LRI | [39] | 41.52 ± 6.00 | |
| 29 | 2-Nonenal | Fatty, green, melon | 1543 | MS + LRI | [35] | 0.31 ± 0.14 | |
| 30 | β-Linalool | Floral | 1553 | 1092 | MS + LRI | [33] | 18.79 ± 3.38 |
| 31 | Isopulegol | Minty, herbaceous | 1570 | MS | 0.37 ± 0.09 | ||
| 32 | Pinocarvone | Minty | 1576 | 1154 | MS + LRI | [41] | 3.56 ± 0.56 |
| 33 | Bornyl acetate | Camphoreous, woody | 1590 | 1278 | MS + LRI | [42] | 54.02 ± 8.77 |
| 34 | β-Caryophyllene | Spicy, peppery | 1604 | 1420 | MS + LRI | [37] | 26.44 ± 4.84 |
| 35 | Terpinen-4-ol | Peppery, woody | 1608 | 1174 | MS + LRI | [34] | 16.48 ± 3.65 |
| 36 | Hotrienol | Sweet, tropical | 1616 | 1105 | MS + LRI | [33] | 1.42 ± 0.76 |
| 37 | α-Thujenal | 1638 | MS | 1.39 ± 0.27 | |||
| 38 | Ethyl caprate | Sweet, waxy | 1646 | 1385 | MS + LRI | [39] | 12.41 ± 1.93 |
| 39 | Humulene | Woody | 1654 | 1456 | MS + LRI | [43] | 2.16 ± 0.38 |
| 40 | α-Caryophyllene | Woody, spicy, earthy | 1677 | 1404 | MS+LRI | [44] | 38.53 ± 7.24 |
| 41 | α-Muurolene | 1697 | 1478 | MS + LRI | [45] | 9.57 ± 1.98 | |
| 42 | α-Terpineol | Pine, lilac, citrus | 1704 | MS + LRI | [46] | 24.70 ± 4.46 | |
| 43 | Borneol | Pine, woody, camphoreous | 1708 | 1165 | MS + LRI | [47] | 11.92 ± 2.01 |
| 44 | Verbenone | Camphor, menthol | 1720 | 1203 | MS + LRI | [34] | 77.59 ± 12.85 |
| 45 | τ-Elemene | 1730 | MS | 4.00 ± 0.96 | |||
| 46 | p-Methen-3-one | 1737 | 1246 | MS | 2.57 ± 0.58 | ||
| 47 | Carvone | Minty, licorice | 1743 | 1213 | MS | 0.89 ± 0.23 | |
| 48 | δ-Cadinene | Thyme, herbal, woody | 1763 | 1517 | MS + LRI | [34] | 4.20 ± 1.04 |
| 49 | Myrtenol | Minty, camphoreous | 1798 | 1315 | MS + LRI | [41] | 0.76 ± 0.15 |
| 50 | 2-Phenylethyl acetate | Floral | 1826 | MS + LRI | [39] | 0.98 ± 0.12 | |
| 51 | Calamenene | Herb spice | 1840 | MS + LRI | [48] | 1.76 ± 0.46 | |
| 52 | p-Cymen-8-ol | Sweet, fruity, coumarinic | 1857 | 1183 | MS + LRI | [33] | 3.06 ± 0.80 |
| 53 | 2-Phenyl ethanol | Floral, rose | 1920 | MS + LRI | [39] | 1.00 ± 0.22 | |
| 54 | α-Calacorene | Woody | 1925 | MS + LRI | [49] | 2.46 ± 0.63 | |
| 55 | Eucarvone | Minty | 1933 | MS | 8.52 ± 2.22 | ||
| 56 | 5,5-Dimethyl-1-ethyl-1,3-cyclopentadiene | 1971 | 984 | MS | 0.78 ± 0.25 | ||
| 57 | 5,5-Dimethyl-1-ethyl-1,3-cyclopentadiene-like | 2008 | MS | 1.93 ± 0.49 | |||
| 58 | Eugenol methyl ether | Sweet, spicy, cinnamon | 2022 | MS | 1.29 ± 0.43 | ||
| 59 | 2-Ethylcyclohexanone | 2095 | MS | 0.58 ± 0.13 | |||
| 60 | Eugenol | Spicy | 2165 | 1345 | MS + LRI | [33] | 4.19 ± 1.11 |
| 61 | Thymol | Herbal | 2180 | 1293 | MS + LRI | [33] | 0.36 ± 0.13 |
| 62 | p-Thymol | 2195 | MS | 0.46 ± 0.11 | |||
| 63 | Carvacrol | Spicy | 2205 | MS + LRI | [33] | 0.73 ± 0.19 |
The aromatic profile of the rosemary extract was composed of about 628 µg/g of volatile compounds. These results differed from those obtained by Szumny et al. [50], who reported a total volatile amount of 135 g/kg (135,000 µg/g) in a rosemary mixture of fresh leaves, branches, and stems. However, they also showed a decrease of 44% in volatiles during the rosemary drying process [50]. Therefore, it is possible that the lower volatile amount found in our sample may be attributed to both the drying and extraction procedure used. Moreover, it should be mentioned that the characteristic rosemary volatiles pertain to the terpene class, which are usually contained in the non-polar fraction of rosemary: the essential oil. It was demonstrated that different extraction methods, such as extraction with solvents (hexane-acetone), distillation, use of supercritical CO2 or microwaves, utilized on rosemary leaves to obtain the essential oil, lead to different yields in term of volatile percentage [51]. The solvent used for extraction of the rosemary sample in this study was focused on recovery of the (poly)phenolic fraction and not on the essential oil. For this reason, it seems reasonable to find a lower concentration of volatile compounds in contrast to other processes targeting the extraction of rosemary essential oil, or its volatile fraction.
As expected, the class of molecules that mainly contribute to the volatile profile of rosemary extract are the terpenes (primarily mono- and sesquiterpenes), with more than 40 peaks representing 90% of the total volatile amount, followed by alcohols and esters (4% of total volatiles), and aldehydes (3% of total volatiles), as shown in Table 3. Small amounts of some ketones, one furan, and other non-fully identified compounds were also detected.
Among terpenes, verbenone and α-thujene were the most abundant compounds, in combination representing 24% of the total volatiles (77.59 ± 12.85 µg/g and 76.26 ± 13.13 µg/g, respectively). They were followed by bornyl acetate (54.02 ± 8.77 µg/g), camphor (41.52 ± 6.00 µg/g), α-caryophyllene (38.53 ± 7.24 µg/g), p-cymenene (34.70 ± 5.71 µg/g), β-caryophyllene (26.44 ± 4.84 µg/g), α-terpineol (24.70 ± 4.46 µg/g), and eucalyptol (20.22 ± 2.58 µg/g). All of these molecules contributed to give woody, camphoreous, mentholic, and phenolic aromatic notes to the rosemary extract. Our results were in agreement with those already reported in literature for rosemary essential oil, in which terpenes represented the prevalent compounds of the volatile profile. Li et al. [52] investigated the volatile composition of rosemary essential oils extracted from 18 different rosemary cultivars collected from the Mediterranean area, and found a prevalence of terpenes in the volatile fractions of all the selected rosemary cultivars. In particular, α- and β-pinene and myrcene emerged among the monoterpene hydrocarbons, while 1,8-cineol (eucalyptol), camphor, verbenone, and bornyl acetate were the prevalent compounds in the oxygenated monoterpenes sub-group [52]. Many compounds detected in the volatile fraction of this rosemary extract were also identified in Brazilian rosemary essential oil by Lemos et al. [53], who further demonstrated that the volatile fraction of rosemary could depend on seasonality. In 2012, Lakušić et al. [54] demonstrated the existence of two major oil chemotypes while studying the chemical composition of rosemary essential oil from the Balkan peninsula. One chemotype was characterized by the predominance of camphor in the aromatic fraction, while a second was defined by the predominance of 1,8-cineol (eucalyptol) [54]. Similarly, rosemary chemotypes characterized by verbenone, 1,8-cineol, and camphor, or by verbenone and α-pinene as major constituents have been identified and associated with geographical origin and climatic conditions of growth [55,56]. In the current study, a prevalence of verbenone (77.59 ± 12.85 µg/g), camphor (41.52 ± 6.00 µg/g), and lower concentrations of eucalyptol (20.22 ± 2.58 µg/g) were recorded. Thus, it is possible that the rosemary line utilized may be related to a chemotype in which verbenone and camphor are preferably bio-synthesized. Besides verbenone and camphor, considerable amounts of borneol and α-pinene were observed (11.92 ± 2.01 µg/g and 4.34 ± 0.65 µg/g, respectively). This is expected since they are major components of the rosemary aromatic profile [57,58]. On the contrary, camphene, a volatile compound typically present in rosemary, could not be identified among all the detected molecules.
Among minor compounds, small amounts of alcohols, esters, 2-phenyl ethanol and 2-phenylethyl acetate, were detected (1.00 ± 0.22 µg/g and 0.98 ± 0.12 µg/g, respectively). These compounds could confer floral aromatic notes to the sample, with the former associated with notes of rose. In addition, considerable amounts of ethyl caprylate and ethyl caprate were found (7.66 ± 2.34 µg/g and 12.41 ± 1.93 µg/g, respectively). These compounds are observed in other matrices, such as wine [39]. Finally, volatiles belonging to the aldehyde class, such as hexanal, heptanal, 2-heptenal, and nonanal, were also identified. Aldehydes have been recently reported as components in the volatile profile of rosemary essential oil extracted from Rosmarinus eriocalyx [59].
3. Materials and Methods
3.1. Materials
Acetonitrile, methanol, formic acid, caffeic acid, hesperidin, rutin, vitexin, C8-C20 alkane solution, and toluene were purchased from Sigma-Aldrich (St. Louis, MO, USA). Carnosic acid, carnosol, 12-O-methylcarnosic acid, rosmanol, rosmarinic acid, and betulinic acid were purchased from PhytoLab (Vestenbergsgreuth, Germany). Luteolin-4-glucoside was obtained from AASC Ltd. (Southampton, UK). Ultrapure water from MilliQ system (Millipore, Bedford, MA, USA) was used throughout the experiment. The proprietary rosemary extract rich in carnosic acid was provided by Kemin Foods, L.C. (Des Moines, IA, USA). It was prepared from dried leaves by a proprietary acetone-based extraction.
3.2. Identification and Quantification of (Poly)phenolic Compounds by UHPLC-ESI-MSn
The (poly)phenolic compounds in the sample were extracted according to previous reports [33,60], with some modifications. A mixture of 150 µL of extract and 1 mL of acetonitrile acidified with formic acid (2%) was ultrasonicated for 10 min and subsequently centrifuged at 10,480× g for 5 min at room temperature. The supernatant was directly injected into the UHPLC-MS system. Aliquots diluted with acidified acetonitrile (1/100 and 1/10,000) were also analyzed to quantify within the linearity range of the reference compounds, avoiding MS signal saturation. The sample was extracted in triplicate.
The extract of rosemary was analyzed using an Accela UHPLC 1250 equipped with a linear ion trap-mass spectrometer (LTQ XL, Thermo Fisher Scientific Inc., San Jose, CA, USA) fitted with a heated-ESI probe (H-ESI-II; Thermo Fisher Scientific Inc.). Separations were performed using a XSELECTED HSS T3 (50 mm × 2.1 mm), 2.5 µm particle size (Waters, Milford, MA, USA). The volume injected was 5 µL and the column oven was set to 30 °C. Two MS experiments were performed in negative mode [21].
An MS experiment optimized in negative mode for carnosol analysis (experimental condition 1) was carried out using conditions as follows. The MS worked with a capillary temperature equal to 275 °C and the source heater temperature set to 250 °C. The sheath gas flow was 50 units, while the auxiliary gas was set to 12 units. The source voltage was 3 kV. The capillary voltage and tube lens were −49 and −148 V, respectively. Elution was performed at a flow rate of 0.3 mL/min. The gradient started with 99% of 0.1% aqueous formic acid, isocratic conditions were maintained for 1 min, and then a 13-min linear gradient from 1% to 40% acetonitrile with 0.1% formic acid was applied. From 14 to 27 min the acidified acetonitrile was increased to 99%, followed by 2 min of 99% acetonitrile, and 6 min at the start conditions to re-equilibrate the column. Analyses were carried out using full scan mode, data-dependent MS3 scanning from m/z 100 to 1500, with collision induced dissociation (CID) equal to 35 (arbitrary units). Pure helium gas was used for CID.
In a second experimental framework, the MS worked with conditions optimized for rosmarinic acid analysis (experimental condition 2). Since the ionization of carnosic acid and carnosol was similar, rosmarinic acid (with diverse ionization/structure characteristics) was selected to optimize the secondary experimental condition in an attempt to cover a wider range of phenolic structures. The capillary temperature was set to 275 °C, while the source heater temperature was 50 °C. The sheath gas flow was 40 units, while auxiliary and sweep gas flow were set to 5 and 0 units, respectively. The source voltage was 4 kV. The capillary and tube lens voltage were −26 and −78 V, respectively. Analyses were carried out using full scan mode, data-dependent MS3 scanning from m/z 100 to 1500, with CID equal to 30 (arbitrary units). The chromatographic conditions were identical to those used for Experimental Conditions 1.
Quantification was performed in selected ion monitoring (SIM) mode by selecting the relative base peak at the corresponding mass to charge ratio (m/z) under Experimental Conditions 1.
3.3. HS-SPME/GC-MS Analysis
The volatile fraction composition of the rosemary extract sample was investigated according to the protocol of Cirlini et al. [33]. Briefly, 100 mg of rosemary extract were exactly weighted and placed in a 30 mL vial adding 20 µL of an aqueous toluene standard solution (348 mg/L). Sampling was performed in a thermostatted water bath at 40 °C for 35 min. During this time the sample was stirred at a constant speed and a fiber was inserted in the sample head space. For each SPME analysis, a silica fiber coated with 50/30 µm of divinylbenzene-carboxen-polydimethylsiloxane (DVB/Carboxen/PDMS, Supelco, Bellefonte, PA, USA) was used. After the sampling time, the fiber was removed from the vial and inserted into the GC-MS injector for the desorption of the volatiles over 2 min at 230 °C. The analysis was replicated twice.
All the analyses were performed on a Thermo Scientific Trace 1300 gas-chromatograph coupled to a Thermo Scientific ISQ MS equipped with an electronic impact (EI) source (Thermo Fisher Scientific Inc.). Separation was performed on a SUPELCOWAX 10 capillary column (Supelco, 30 m × 0.25 mm, f.t. 0.25 µm). All the injections were performed in splitless mode keeping the valve closed for 2 min. Temperature increase in the column was as follows: initiation at 50 °C for 3 min, increase to 200 °C at 5 °C per min, followed by a holding time of 12 min. The injector and transfer line temperatures were set at 230 °C and helium was used as carrier gas. Full scan mode was chosen as acquisition mode in the range of 41–500 m/z.
Peak identification was performed by comparing registered mass spectra with those present in the instrument library (NIST 14). LRIs were calculated for each detected signal on two different stationary phase columns, SUPELCOWAX 10 capillary column (Supelco, 30 m × 0.25 mm, f.t. 0.25 µm) and BP5MS (SGE Analytical Science, 30 m × 0.25 mm, f.t. 0.25 µm), according to retention times of a C8–C20 alkane standard solution analyzed under the same GC conditions applied for sample analyses. The semi-quantification of all detected GC signals was performed based on comparison to an internal standard (toluene).
4. Conclusions
This study described the phytochemical composition of a proprietary rosemary extract rich in carnosic acid with respect to its (poly)phenolic and volatile compounds. The use of an untargeted approach based on two chromatographic techniques coupled to mass spectrometry (UHPLC-ESI-MSn and GC-MS) allowed elucidation of a broad array of compounds characterizing the phenolic and volatile fractions of this herb with multiple applications. This is, to our knowledge, the broadest profiling of rosemary secondary metabolites to date.
The UHPLC-ESI-MSn−-based characterization of the phenolic fraction of the rosemary extract allowed the tentative identification of 57 (poly)phenolic compounds belonging to different phenolic groups (24 flavonoids, 5 phenolic acids, 24 diterpenes, 1 triterpenic acid, and 3 lignans). Fourteen of these phenolic compounds are being described for the first time in this rosemary-based food ingredient. From a quantitative point of view, diterpenoids were the main class of (poly)phenolic structures, representing 97.2% of the phenolic content. With respect to the volatile fraction, 63 gas-chromatographic signals were detected and semi-quantified, describing the volatile profile and characteristics of this extract. The vast phytochemical characterization of this plant extract with food/pharma applications extends the number of molecules previously defined for rosemary and may assist in the study of their biological properties. This complete mass spectrometric analysis could be utilized to evaluate other rosemary-based products as well as other plant foodstuffs/extracts in order to fully unravel their phytochemical properties.
Acknowledgments
This study was partly supported by Kemin Food, L.C.
Abbreviations
The following abbreviations are used in this manuscript:
| CID | collision-induced dissociation |
| EI | electronic impact |
| ESI | electrospray ionisation |
| GC-MS | gas chromatography-mass spectrometry |
| HS-SPME | head space solid-phase microextraction |
| LRI | linear retention indices |
| MS | mass spectrometry |
| SIM | selected ion monitoring |
| UHPLC-ESI-MSn | ultra-high performance liquid chromatography-electrospray ionization-mass spectrometry |
Author Contributions
D.D.R., P.M., and K.A.H. conceived and designed the experiments; P.M. and M.C. performed the experiments; P.M. and M.C. analyzed the data; D.D.R., K.A.H., and C.D.A. contributed reagents/materials/analysis tools; P.M., M.C., M.T., and D.D.R. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the collection, analyses, or interpretation of data, and in the writing of the manuscript.
Footnotes
Sample Availability: Samples are available from the authors.
References
- 1.Hassani F.V., Shirani K., Hosseinzadeh H. Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: A review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016;389:931–949. doi: 10.1007/s00210-016-1256-0. [DOI] [PubMed] [Google Scholar]
- 2.Cui L., Kim M.O., Seo J.H., Kim I.S., Kim N.Y., Lee S.H., Park J., Kim J., Lee H.S. Abietane diterpenoids of Rosmarinus officinalis and their diacylglycerol acyltransferase-inhibitory activity. Food Chem. 2012;132:1775–1780. doi: 10.1016/j.foodchem.2011.11.138. [DOI] [Google Scholar]
- 3.Kontogianni V.G., Tomic G., Nikolic I., Nerantzaki A.A., Sayyad N., Stosic-Grujicic S., Stojanovic I., Gerothanassis I.P., Tzakos A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013;136:120–129. doi: 10.1016/j.foodchem.2012.07.091. [DOI] [PubMed] [Google Scholar]
- 4.Hossain M.B., Rai D.K., Brunton N.P., Martin-Diana A.B., Barry-Ryan A.C. Characterization of phenolic composition in lamiaceae spices by LC-ESI-MS/MS. J. Agric. Food Chem. 2010;58:10576–10581. doi: 10.1021/jf102042g. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki K., El Omri A., Kondo S., Han J., Isoda H. Rosmarinus officinalis polyphenols produce anti-depressant like effect through monoaminergic and cholinergic functions modulation. Behav. Brain Res. 2013;238:86–94. doi: 10.1016/j.bbr.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Kwon Y.I., Vattem D.A., Shetty K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006;15:107–118. [PubMed] [Google Scholar]
- 7.Tai J., Cheung S., Wu M., Hasman D. Antiproliferation effect of rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine. 2012;19:436–443. doi: 10.1016/j.phymed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Wang W., Li N., Luo M., Zu Y., Efferth T. Antibacterial activity and anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules. 2012;17:2704–2713. doi: 10.3390/molecules17032704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ullevig S.L., Zhao Q., Zamora D., Asmis R. Ursolic acid protects diabetic mice against monocyte dysfunction and accelerated atherosclerosis. Atherosclerosis. 2011;219:409–416. doi: 10.1016/j.atherosclerosis.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afonso M.S., De O Silva A.M., Carvalho E.B., Rivelli D.P., Barros S.B., Rogero M.M., Lottenberg A.M., Torres R.P., Mancini-Filho J. Phenolic compounds from Rosemary (Rosmarinus officinalis L.) attenuate oxidative stress and reduce blood cholesterol concentrations in diet-induced hypercholesterolemic rats. Nutr. Metab. 2013;10:19. doi: 10.1186/1743-7075-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harach T., Aprikian O., Monnard I., Moulin J., Membrez M., Béolor J.C., Raab T., MacÉ K., Darimont C. Rosemary (Rosmarinus officinalis L.) Leaf extract limits weight gain and liver steatosis in mice fed a high-fat diet. Planta Med. 2010;76:566–571. doi: 10.1055/s-0029-1240612. [DOI] [PubMed] [Google Scholar]
- 12.Romo-Vaquero M., Yáñez-Gascón M.J., Villalba R., Larrosa M., Fromentin E., Ibarra A., Roller M., Tomás-Barberán F., de Gea J.C., García-Conesa M.T. Inhibition of gastric lipase as a mechanism for body weight and plasma lipids reduction in Zucker rats fed a rosemary extract rich in carnosic acid. PLoS ONE. 2012;7:e39773. doi: 10.1371/journal.pone.0039773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pérez-Fons L., Garzón M.T., Micol V. Relationship between the antioxidant capacity and effect of rosemary (Rosmarinus officinalis L.) polyphenols on membrane phospholipid order. J. Agric. Food Chem. 2010;58:161–171. doi: 10.1021/jf9026487. [DOI] [PubMed] [Google Scholar]
- 14.Romo-Vaquero M., García-Villalba R., Larrosa M., Yáñez-Gascón M.J., Fromentin E., Flanagan J., Roller M., Tomás-Barberán F.A., Espín J.C., García-Conesa M.T. Bioavailability of the major bioactive diterpenoids in a rosemary extract: Metabolic profile in the intestine, liver, plasma, and brain of Zucker rats. Mol. Nutr. Food Res. 2013;57:1834–1846. doi: 10.1002/mnfr.201300052. [DOI] [PubMed] [Google Scholar]
- 15.Del Rio D., Rodríguez-Mateos A., Spencer J.P.E., Tognolini M., Borges G., Crozier A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Mateos A., Vauzour D., Krueger C.G., Shanmuganayagam D., Reed J., Calani L., Mena P., Del Rio D., Crozier A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014;88:1803–1853. doi: 10.1007/s00204-014-1330-7. [DOI] [PubMed] [Google Scholar]
- 17.Ribeiro A., Caleja C., Barros L., Santos-Buelga C., Barreiro M.F., Ferreira I.C.F.R. Rosemary extracts in functional foods: Extraction, chemical characterization and incorporation of free and microencapsulated forms in cottage cheese. Food Funct. 2016;7:2185–2196. doi: 10.1039/C6FO00270F. [DOI] [PubMed] [Google Scholar]
- 18.Mulinacci N., Innocenti M., Bellumori M., Giaccherini C., Martini V., Michelozzi M. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta. 2011;85:167–176. doi: 10.1016/j.talanta.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 19.Bicchi C., Binello A., Rubiolo P. Determination of phenolic diterpene antioxidants in rosemary (Rosmarinus officinalis L.) with different methods of extraction and analysis. Phytochem. Anal. 2000;11:236–242. doi: 10.1002/1099-1565(200007/08)11:4<236::AID-PCA503>3.0.CO;2-B. [DOI] [Google Scholar]
- 20.Almela L., Sánchez-Muñoz B., Fernández-López J.A., Roca M.J., Rabe V. Liquid chromatograpic-mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J. Chromatogr. A. 2006;1120:221–229. doi: 10.1016/j.chroma.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 21.Mena P., Calani L., Dall’Asta C., Galaverna G., García-Viguera C., Bruni R., Crozier A., Del Rio D. Rapid and comprehensive evaluation of (poly)phenolic compounds in pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules. 2012;17:14821–14840. doi: 10.3390/molecules171214821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNab H., Ferreira E.S.B., Hulme A.N., Quye A. Negative ion ESI-MS analysis of natural yellow dye flavonoids-An isotopic labelling study. Int. J. Mass Spectrom. 2009;284:57–65. doi: 10.1016/j.ijms.2008.05.039. [DOI] [Google Scholar]
- 23.Borrás Linares I., Arráez-Román D., Herrero M., Ibáñez E., Segura-Carretero A., Fernández-Gutiérrez A. Comparison of different extraction procedures for the comprehensive characterization of bioactive phenolic compounds in Rosmarinus officinalis by reversed-phase high-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight mass spectrometry. J. Chromatogr. A. 2011;1218:7682–7690. doi: 10.1016/j.chroma.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Sawada Y., Nakabayashi R., Yamada Y., Suzuki M., Sato M., Sakata A., Akiyama K., Sakurai T., Matsuda F., Aoki T., Hirai M.Y., Saito K. RIKEN tandem mass spectral database (ReSpect) for phytochemicals: A plant-specific MS/MS-based data resource and database. Phytochemistry. 2012;82:38–45. doi: 10.1016/j.phytochem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Fabre N., Rustan I., de Hoffmann E., Quetin-Leclercq J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass. Spectrom. 2001;12:707–715. doi: 10.1016/S1044-0305(01)00226-4. [DOI] [PubMed] [Google Scholar]
- 26.Moharram F.A.E., Marzouk M.S., El-Shenawy S.M., Gaara A.H., El Kady W.M. Polyphenolic profile and biological activity of Salvia splendens leaves. J. Pharm. Pharmacol. 2012;64:1678–1687. doi: 10.1111/j.2042-7158.2012.01544.x. [DOI] [PubMed] [Google Scholar]
- 27.Cuyckens F., Claeys M. Mass spectrometry in the structural analysis of flavonoids. J. Mass Spectrom. 2004;39:1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- 28.Bresciani L., Calani L., Cossu M., Mena P., Sayegh M., Ray S., Del Rio D. (Poly)phenolic characterization of three food supplements containing 36 different fruits, vegetables and berries. PharmaNutrition. 2015;3:11–19. doi: 10.1016/j.phanu.2015.01.001. [DOI] [Google Scholar]
- 29.Masuda T., Kirikihira T., Takeda Y. Recovery of antioxidant activity from carnosol quinone: Antioxidants obtained from a water-promoted conversion of carnosol quinone. J. Agric. Food Chem. 2005;53:6831–6834. doi: 10.1021/jf050685s. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Smuts J.P., Dodbiba E., Rangarajan R., Lang J.C., Armstrong D.W. Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J. Agric. Food Chem. 2012;60:9305–9314. doi: 10.1021/jf302179c. [DOI] [PubMed] [Google Scholar]
- 31.Ivanović J., Dilas S., Jadranin M., Vajs V., Babović N., Petrović S., Žižović I. Supercritical carbon dioxide extraction of antioxidants from rosemary (Rosmarinus officinalis L.) and sage (Salvia officinalis L.) J. Serb. Chem. Soc. 2009;74:717–732. doi: 10.2298/JSC0907717I. [DOI] [Google Scholar]
- 32.Vallverdú-Queralt A., Regueiro J., Martínez-Huélamo M., Rinaldi Alvarenga J.F., Leal L.N., Lamuela-Raventos R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014;154:299–307. doi: 10.1016/j.foodchem.2013.12.106. [DOI] [PubMed] [Google Scholar]
- 33.Cirlini M., Mena P., Tassotti M., Herrlinger K.A., Nieman K.M., Dall’Asta C., Rio D.D. Phenolic and volatile composition of a dry spearmint (Mentha spicata L.) extract. Molecules. 2016;21:1007. doi: 10.3390/molecules21081007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Högnadóttir Á., Rouseff R.L. Identification of aroma active compounds in orange essence oil using gas chromatography-olfactometry and gas chromatography-mass spectrometry. J. Chromatogr. A. 2003;998:201–211. doi: 10.1016/S0021-9673(03)00524-7. [DOI] [PubMed] [Google Scholar]
- 35.Cirlini M., Dall’Asta C., Silvanini A., Begh D., Fabbri A., Galaverna G., Ganino T. Volatile fingerprinting of chestnut flours from traditional Emilia Romagna (Italy) cultivars. Food Chem. 2012;134:662–668. doi: 10.1016/j.foodchem.2012.02.151. [DOI] [PubMed] [Google Scholar]
- 36.Goodner K.L. Practical retention index models of OV-101, DB-1, DB-5, and DB-Wax for flavor and fragrance compounds. LWT Food Sci. Technol. 2008;41:951–958. doi: 10.1016/j.lwt.2007.07.007. [DOI] [Google Scholar]
- 37.Bianchi F., Careri M., Mangia A., Musci M. Retention indices in the analysis of food aroma volatile compounds in temperature-programmed gas chromatography: Database creation and evaluation of precision and robustness. J. Sep. Sci. 2007;30:563–572. doi: 10.1002/jssc.200600393. [DOI] [PubMed] [Google Scholar]
- 38.Brunton N.P., Cronin D.A., Monahan F.J. Volatile components associated with freshly cooked and oxidized off-flavours in Turkey breast meat. Flavour Fragr. J. 2002;17:327–334. doi: 10.1002/ffj.1087. [DOI] [Google Scholar]
- 39.Dall’Asta C., Cirlini M., Morini E., Galaverna G. Brand-dependent volatile fingerprinting of Italian wines from Valpolicella. J. Chromatogr. A. 2011;1218:7557–7565. doi: 10.1016/j.chroma.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 40.Olivero J., Gracia T., Payares P., Vivas R., Díaz D., Daza E., Geerlings P. Molecular structure and gas chromatographic retention behavior of the components of Ylang-Ylang oil. J. Pharm. Sci. 1997;86:625–630. doi: 10.1021/js960196u. [DOI] [PubMed] [Google Scholar]
- 41.Bicchi C., Rubiolo P., Saranz Camargo E.E., Vilegas W., de Souza Gracioso J., Monteiro Souza Brito A.R. Components of Turnera diffusa Willd. var. afrodisiaca (Ward) Urb. essential oil. Flavour Fragr. J. 2003;18:59–61. doi: 10.1002/ffj.1155. [DOI] [Google Scholar]
- 42.Priestap H.A., Van Baren C.M., Lira P.D.L., Coussio J.D., Bandoni A.L. Volatile constituents of Aristolochia argentina. Phytochemistry. 2003;63:221–225. doi: 10.1016/S0031-9422(02)00751-3. [DOI] [PubMed] [Google Scholar]
- 43.Frizzo C.D., Serafini L.A., Dellacassa E., Lorenzo D., Moyna P. Essential oil of Baccharis uncinella DC. from Southern Brazil. Flavour Fragr. J. 2001;16:286–288. doi: 10.1002/ffj.998. [DOI] [Google Scholar]
- 44.Choi H.S. Character impact odorants of Citrus hallabong [(C. unshiu Marcov × C. sinensis Osbeck) × C. reticulata Blanco] cold-pressed peel oil. J. Agric. Food Chem. 2003;51:2687–2692. doi: 10.1021/jf021069o. [DOI] [PubMed] [Google Scholar]
- 45.Karioti A., Skaltsa H., Demetzos C., Perdetzoglou D., Economakis C.D., Salem A.B. Effect of nitrogen concentration of the nutrient solution on the volatile constituents of leaves of Salvia fruticosa Mill. in solution culture. J. Agric. Food Chem. 2003;51:6505–6508. doi: 10.1021/jf030308k. [DOI] [PubMed] [Google Scholar]
- 46.Culleré L., Escudero A., Cacho J., Ferreira V. Gas chromatography-olfactometry and chemical quantitative study of the aroma of six premium quality Spanish aged red wines. J. Agric. Food Chem. 2004;52:1653–1660. doi: 10.1021/jf0350820. [DOI] [PubMed] [Google Scholar]
- 47.Chisholm M.G., Wilson M.A., Gaskey G.M. Characterization of aroma volatiles in key lime essential oils (Citrus aurantifolia Swingle) Flavour Fragr. J. 2003;18:106–115. doi: 10.1002/ffj.1172. [DOI] [Google Scholar]
- 48.Cavalli J.F., Tomi F., Bernardini A.F., Casanova J. Composition and chemical variability of the bark oil of Cedrelopsis grevei H. Baillon from Madagascar. Flavour Fragr. J. 2003;18:532–538. doi: 10.1002/ffj.1263. [DOI] [Google Scholar]
- 49.Davies N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A. 1990;503:1–24. doi: 10.1016/S0021-9673(01)81487-4. [DOI] [Google Scholar]
- 50.Szumny A., Figiel A., Gutiérrez-Ortíz A., Carbonell-Barrachina A.A. Composition of rosemary essential oil (Rosmarinus officinalis) as affected by drying method. J. Food Eng. 2010;97:253–260. doi: 10.1016/j.jfoodeng.2009.10.019. [DOI] [Google Scholar]
- 51.Presti M.L., Ragusa S., Trozzi A., Dugo P., Visinoni F., Fazio A., Dugo G., Mondello L. A comparison between different techniques for the isolation of rosemary essential oil. J. Sep. Sci. 2005;28:273–280. doi: 10.1002/jssc.200400037. [DOI] [PubMed] [Google Scholar]
- 52.Li G., Cervelli C., Ruffoni B., Shachter A., Dudai N. Volatile diversity in wild populations of rosemary (Rosmarinus officinalis L.) from the tyrrhenian Sea vicinity cultivated under homogeneous environmental conditions. Ind. Crops Prod. 2016;84:381–390. doi: 10.1016/j.indcrop.2016.02.029. [DOI] [Google Scholar]
- 53.Lemos M.F., Pacheco H.P., Endringer D.C., Scherer R. Seasonality modifies rosemary’s composition and biological activity. Ind. Crops Prod. 2015;70:41–47. doi: 10.1016/j.indcrop.2015.02.062. [DOI] [Google Scholar]
- 54.Lakušić D.V., Ristić M.S., Slavkovska V.N., Åinžar-Sekulić J.B., Lakušić B.S. Environment-related variations of the composition of the essential oils of rosemary (Rosmarinus officinalis L.) in the Balkan penninsula. Chem. Biodivers. 2012;9:1286–1302. doi: 10.1002/cbdv.201100427. [DOI] [PubMed] [Google Scholar]
- 55.Celiktas O.Y., Kocabas E.E.H., Bedir E., Sukan F.V., Ozek T., Baser K.H.C. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 2007;100:553–559. doi: 10.1016/j.foodchem.2005.10.011. [DOI] [Google Scholar]
- 56.Pintore G., Usai M., Bradesi P., Juliano C., Boatto G., Tomi F., Chessa M., Cerri R., Casanova J. Chemical composition and antimicrobial activity of Rosmarinus officinalis L. oils from Sardinia and Corsica. Flavour Fragr. J. 2002;17:15–19. doi: 10.1002/ffj.1022. [DOI] [Google Scholar]
- 57.Díaz-Maroto M.C., Pérez-Coello M.S., Sánchez-Palomo E., González Viñas M.A. Impact of drying and storage time on sensory characteristics of rosemary (Rosmarinus officinalis L.) J. Sens. Stud. 2007;22:34–48. doi: 10.1111/j.1745-459X.2007.00093.x. [DOI] [Google Scholar]
- 58.Pino J.A., Estarrón M., Fuentes V. Essential oil of rosemary (Rosmarinus officinalis L.) from Cuba. J. Essent. Oil Res. 1998;10:111–112. doi: 10.1080/10412905.1998.9700854. [DOI] [Google Scholar]
- 59.Bendif H., Boudjeniba M., Djamel Miara M., Biqiku L., Bramucci M., Caprioli G., Lupidi G., Quassinti L., Sagratini G., Vitali L.A., Vittori S., Maggi F. Rosmarinus eriocalyx: An alternative to Rosmarinus officinalis as a source of antioxidant compounds. Food Chem. 2017;218:78–88. doi: 10.1016/j.foodchem.2016.09.063. [DOI] [PubMed] [Google Scholar]
- 60.Sánchez-Salcedo E.M., Mena P., García-Viguera C., Martínez J.J., Hernández F. Phytochemical evaluation of white (Morus alba L.) and black (Morus nigra L.) mulberry fruits, a starting point for the assessment of their beneficial properties. J. Funct. Foods. 2015;12:399–408. doi: 10.1016/j.jff.2014.12.010. [DOI] [Google Scholar]