Abstract
Two picrotoxane sesquiterpene lactone glycosides, nepalactones A (1) and B (2), and one new coumarin, nepalarin (3), were isolated from the root barks of the poisonous plant Coriaria nepalensis. Their structures were elucidated via HRESIMS and 1D and 2D NMR spectroscopic analyses, and further verified via transformation methods. In addition, compounds 1–3 and five semisynthetic congeners (1a–e) were assayed for the activity to induce neurite outgrowth in rat pheochromocytoma (PC12) cells. As a result, nepalactone A derivative 1c and nepalarin (3) significantly enhanced nerve growth factor (NGF)-mediated neurite outgrowth in PC12 cells.
Keywords: Coriaria nepalensis, Coriariaceae, natural products, picrotoxane, sesquiterpene, neurite outgrowth-potentiating activity, neurotrophic activity
1. Introduction
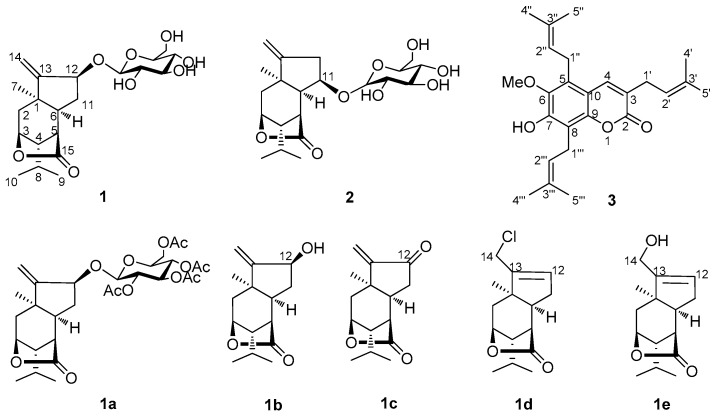
Coriaria nepalensis Wall (Coriaria sinica Maxim) (Coriariaceae) is a Chinese medicine herb mainly distributed in the southwest of China. Traditionally, this herb is used to treat numbness, toothache, traumatic injury, and acute conjunctivitis [1]. Sesquiterpene lactones are the characteristic bioactive constituents from family Coriariaceae [2,3,4,5,6,7,8,9]. A series of sesquiterpenes [6,7,8,9] and prenyled coumarins [10,11] have been previously identified from C. nepalensis. Among these sesquiterpene lactones, coriamytin and tutin have been suggested as main bioactive substances for the treatment of schizophrenia [8,12], and as insecticidal agents [9]. In our recent effort to search for neurotrophic natural products [13,14,15,16], we employed rat dopaminergic PC12 cells as an in vitro cell model to screen various natural compounds for the effect on nerve growth factor (NGF)-induced neurite outgrowth. The active compounds are expected to be potentially useful for the treatment of Alzheimer’s disease and other neurological disorders [17,18]. Here, two new picrotoxane-type sesquiterpene glycosides, nepalactones A (1) and B (2), and one new polyprenyled coumarin, nepalarin (3) (Figure 1), were isolated from the root barks of this plant. We describe herein the isolation and structure elucidation of the three new compounds as well as the assay for the ability to stimulate NGF-mediated neurite outgrowth from PC12 cells.
Figure 1.
Chemical structures of compounds 1–3 and 1a–e.
2. Results and Discussion
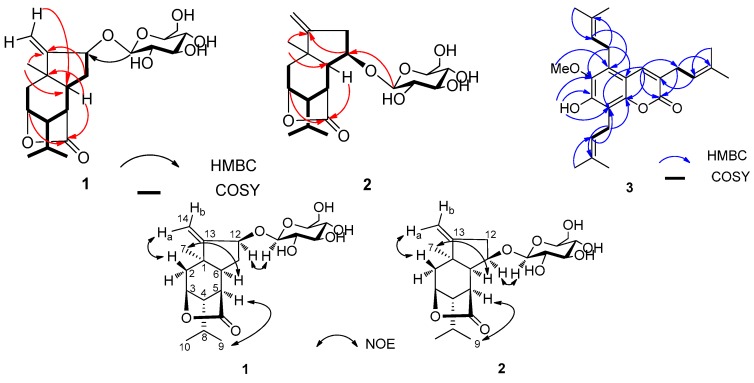
Compound 1 was isolated as a white, amorphous powder. The molecular formula was established as C21H32O8 via HRESIMS (m/z [M + Na]+ 435.1991; calcd. 435.1995) in combination with the 13C-NMR spectrum, requiring six degrees of unsaturation. Its IR spectrum indicated the presence of hydroxy (3563 cm−1) and γ-lactone carbonyl (1765 cm−1) groups. The 13C and DEPT NMR spectra of 1 (Table 1) exhibited 21 carbon signals corresponding to three methyl groups, four methylene groups (including one olefinic carbon at δ 111.5 and one oxygenated carbon at δ 61.6), 11 methine groups (seven oxygenated carbons including five carbons of sugar), and three quaternary carbons (one lactone carbonyl at δ 180.6 and one olefinic carbon at δ 158.2). The 1H and 13C-NMR spectra of 1 displayed signals for tertiary methyl (δ 1.25, s, 3H), isopropyl (δ 0.99, 1.01, d, each 3H, and δ 1.83, m, 1H), and exocyclic olefinic bond (δH 5.21, s, 1H, 5.42, s, 1H) groups. A series of sugar signals were also detected in the 1H-NMR spectrum (Table 1) between δH 3.22–3.87, with an anomeric proton at δH 4.35 (d, 1H, J= 7.5 Hz, H-1′). The 13C-NMR spectrum indicated the presence of a glucose unit due to a set of carbon signals at δC 101.5, 73.5, 76.2, 70.2, 76.6, and 61.6. These data suggested the presence of a glucopyranosyl moiety in 1. The glucose in nepalactone A derivative 1a appeared to be tetraacetylated, based on NMR spectra (Table 1) and HRESIMS (m/z 603.2416 [M + Na]+). Furthermore, the 1H and 13C-NMR data of 1 were similar to those of the known picrotoxane sesquiterpenoid, corialactone D (1b), which has been previously identified from the same plant [8], except for the presence of the glucose unit at C-12 in 1. The attachment of the glucose moiety to C-12 of the aglycone 1b was deduced from the HMBC correlations from H-1′ (δH 4.35) to C-12 (δC 79.4). These spectroscopic data suggested that 1 is a glucoside of corialactone D. The 1H-1H COSY and HSQC correlations indicated the presence of two substructures–CH2(2)–CH(3)–CH(4)–CH(5)–CH(6)–CH(11)–CH(12)–, and –CH(4)–CH(8)[CH3(9)]–CH3(10)–units, whose connectivity was defined by the key HMBC correlations (Figure 2).
Table 1.
1H-NMR (500 MHz) and 13C-NMR (125 MHz) data for 1, 1a, 1b, and 2 (δ ppm).
| No. | 1 a | 1a b | 2 a | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 42.5 | 46.1 | 42.5 | |||
| 2α | 1.74 (d, 15.5) | 34.0 | 1.63 (d, 15.0) | 33.6 | 1.64 (d, 15.5) | 33.3 |
| 2β | 2.45 (dd, 2.5, 15.5) | 2.40 (dd, 3.5, 15.0) | 2.46 (dd, 3.5, 15.5) | |||
| 3 | 4.72 (m) | 80.2 | 4.63 (m) | 79.6 | 4.68 (m) | 79.7 |
| 4 | 2.02 (m) | 51.5 | 1.96 (m) | 51.5 | 2.02 (m) | 51.4 |
| 5 | 2.35 (m) | 45.5 | 2.27 (m) | 45.5 | 2.50 (m) | 45.0 |
| 6 | 2.36 (m) | 41.7 | 2.26 (m) | 41.3 | 2.39 (d, 3.0) | 49.1 |
| 7 | 1.25 (s) | 31.9 | 1.15 (s) | 31.7 | 1.28 (s) | 32.7 |
| 8 | 1.83 (m) | 25.0 | 1.76 (m) | 24.8 | 1.81 (m) | 25.3 |
| 9 | 0.99 (d, 6.0) | 19.3 | 0.96 (d, 6.0) | 19.6 | 0.99 (d, 6.5) | 19.8 |
| 10 | 1.01 (d, 6.0) | 20.0 | 0.98 (d, 6.0) | 20.7 | 1.03(d, 6.5) | 20.9 |
| 11α | 2.31 (m) | 34.2 | 2.31 (m) | 34.7 | 4.45 (m) | 81.9 |
| 11β | 2.23 (m) | 2.02 (m) | ||||
| 12α | 4.90 (t, 7.5) | 79.4 | 4.77 (t, 7.4) | 81.7 | 2.75 (m) | 39.4 |
| 12β | 2.92 (dd, 8.0, 18.5) | |||||
| 13 | 158.2 | 156.4 | 156.3 | |||
| 14a | 5.21 (d, 1.5) | 111.5 | 5.19 (d, 1.5) | 112.9 | 4.91 (d, 8.0) | 106.1 |
| 14b | 5.42 (d, 1.5) | 5.44 (d, 1.5) | 4.88 (d, 8.0) | |||
| 15 | 180.6 | 179.0 | 178.6 | |||
| 1′ | 4.35 (d, 7.5) | 101.5 | 4.56 (d, 8.0) | 100.4 | 4.33 (d, 7.5) | 102.4 |
| 2′ | 3.22 (m) | 73.5 | 4.96 (m) | 75.1 | 3.27 (s) | 73.5 |
| 3′ | 3.40 (m) | 76.6 | 5.18 (t, 10.0) | 73.2 | 3.44 (m) | 76.4 |
| 4′ | 3.39 (m) | 70.2 | 5.05 (t, 10.0) | 68.5 | 3.44 (m) | 69.7 |
| 5′ | 3.29 (m) | 76.2 | 3.68 (m) | 71.9 | 3.29 (m) | 75.7 |
| 6′a | 3.87 (dd, 2.5, 12.0) | 61.6 | 4.26 (dd, 5.0, 12.0) | 62.2 | 3.87 (dd, 3.0,12.0) | 61.6 |
| 6′b | 3.75 (dd, 5.5, 12.0) | 4.12 (dd, 2.0, 12.0) | 3.78 (dd, 3.0,12.0) | |||
| 2′-Ac | 2.03 (s) | 169.0/20.7 | ||||
| 3′-Ac | 1.99 (s) | 170.8/20.8 | ||||
| 4′-Ac | 2.01 (s) | 169.3/20.7 | ||||
| 6′-Ac | 2.08 (s) | 170.6/20.6 | ||||
a,b: Data were recorded in MeOH-d4, and CDCl3, respectively.
Figure 2.
Selected 2D NMR correlations for compounds 1–3.
The relative configuration of the aglycone (1b) of 1 was defined by a NOESY experiment (Figure 2). The NOE correlations of H3-7/H-6, H3-9/H-5, and H-12/H-1′ indicated that CH3-7, H-5, H-6, H-12, and the isopropyl group were at the same orientation (α-configuration). Additionally, enzymatic hydrolysis of 1 afforded the known intact aglycone 1b, which was identified from 1H NMR, and its absolute configuration (1R,3S,4S,5R,6S,12S) was previously determined from CD spectrum [8]. In addition, the coupling constant (J = 7.5 Hz) of the anomeric proton (δH 4.35, H-1′) suggested a β-configuration for the glucose unit. Indeed, compound 1 was hydrolyzable via β-glucocidase. GC analysis suggested that acid hydrolyzate of 1 contained the glucose moiety with the d-configuration. Hence, the structure of 1 was determined unambiguously as shown, and named nepalactone A.
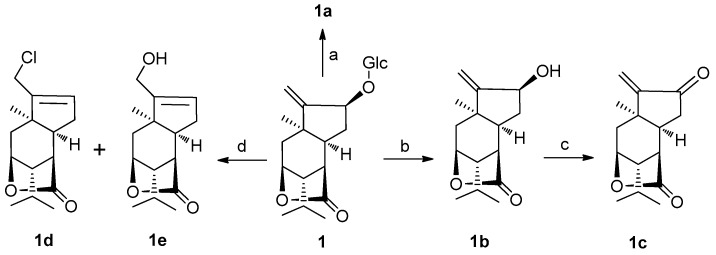
In addition, in order to enhance molecular diversity of sesquiterpene compounds, additional three derivatives of 1. The conversion of the aglycone 1b using Dess-Martin periodinane (DMP) in CH2Cl2 at room temperature afforded 1c; and treatment of 1 with 4 N HCl in dioxane at 100 °C yielded two compounds 1d and 1e (Scheme 1). These three new congeners were characterized by an application of spectroscopic data and HRESIMS.
Scheme 1.
Conversion of 1 into picrotoxane sesquiterpenes 1a–e. Reagents and conditions: (a) acetic anhydride, pyridine, r.t.; (b) citrate buffer, β-glucocidase, rt; (c) Dess–Martin periodinane (DMP), DCM, r.t., 40 min; (d) 4 N HCl-dioxane (1:1), 100 °C, 2 h.
Compound 2 was obtained as white powder with a molecular formula C21H32O8, as established via HRESIMS (m/z [M + Na]+ 435.1987; calcd. 435.2417). Its molecular formula was identical to that of 1, indicating that 2 was a positional isomer of 1. The similar IR spectrum of 2 compared to 1 indicated the presence of similar functional groups. The 1H and 13C-NMR spectral data (Table 1) of 2 were highly similar to those of nepalactone A (1). Compound 1 significantly differed from 2 by the signals for the glucopyranosyl group, attached at C-12 for 1 while at C-11 (δC 81.9) for 2. The HMBC correlations from the anomeric proton H-1′ at δH 4.33 (d, J = 7.5 Hz) to C-11 (δC 81.9) implied that the glucose group is attached to C-11 (Figure 2). The stereochemistry of 2 was established from a NOESY experiment (Figure 2). The most significant NOE correlations observed were between H-6/H3-7, H-5/H3-9, and H-1′/H-11, indicating that H-5, H-6, H-6, the methyl group at C-1, and the isopropyl group at C-4 were all on the same α-face of the molecule. The NMR data of 2 were fully assigned by analysis of the 2D-NMR data including its HSQC, HMBC, and 1H-1H COSY spectra. Thus, the structure of 2 was established as shown for nepalactone B.
Compound 3, yellowish crystalline needles, was assigned the molecular formula C25H32O4 from [M − H]− at m/z 395.2226 in HRESIMS, requiring 10 degrees of unsaturation. The IR spectrum displayed the presence of hydroxy (3319 cm−1), C=O (1691 cm−1) groups and a benzene ring (1627, 1583, and 1458 cm−1). The 13C-NMR and DEPT data showed 25 carbon signals for seven methyls, three methylenes, four methines, and 11 quaternary carbons. The 13C-NMR data suggested the presence of three isopentenyl groups (δC 119.8, δH 5.34, δC 135.2; and δC 121.1, δH 5.33, δC 133.1; and δC 122.6, δH 5.11, δC 132.8), one methoxy group (δC 62.2). Besides three isopentenyl and one methoxy substituent, the NMR characteristic signals and UV spectra were indicative of a coumarin skeleton [11]. One downfield singlet (δH 7.51, s) in the 1H-NMR spectrum of 3 suggested that 3 was a pentasubstituted coumarin. Furthermore, the 1H and 13C-NMR data of 3 were similar to those of the known coumarin, 7-hydroxy-6-methoxy-3,8-bis(3-methyl-2-butenyl)coumarin, which was previously identified from the same plant [11], except for the presence of an additional isopentenyl unit [δH 5.11 (1H, m, H-2′′), 3.56 (2H, d, J = 6.7 Hz, H-1′′), 1.86 (s, H-5′′), 1.75 (s, H-4′′); δC 132.8 (C-3′′), 122.6 (C-2′′), 25.6 (C-4′′), 24.7 (C-1′′), 18.1 (C-5′′)] at C-5 in 3. The location of the isopentenyl unit at C-5 was supported by the HMBC correlations of H-2′′ to C-5, and H-1′′ to C-6 and C-10. Full analyses of the 1H and 13C-NMR spectral data of 3, supported by the 1H-1H COSY, DEPT, HSQC, and HMBC experiments (Figure 2), permitted the assignment of all proton and carbon resonances. Consequently, the structure of 3 was established as 7-hydroxy-6-methoxy-3,5,8-tri(3-methyl-2-butenyl) coumarin and named nepalin C. To our knowledge, this is a rare coumarin with three isopentenyl units in natural source.
NeuriteOutgrowth-Promoting Activity
Compounds (1–3, and 1a–1e) were evaluated for the effect on NGF-induced neurite outgrowth in rat pheochromocytoma PC12 cells according to our previously reported procedures [17,18].
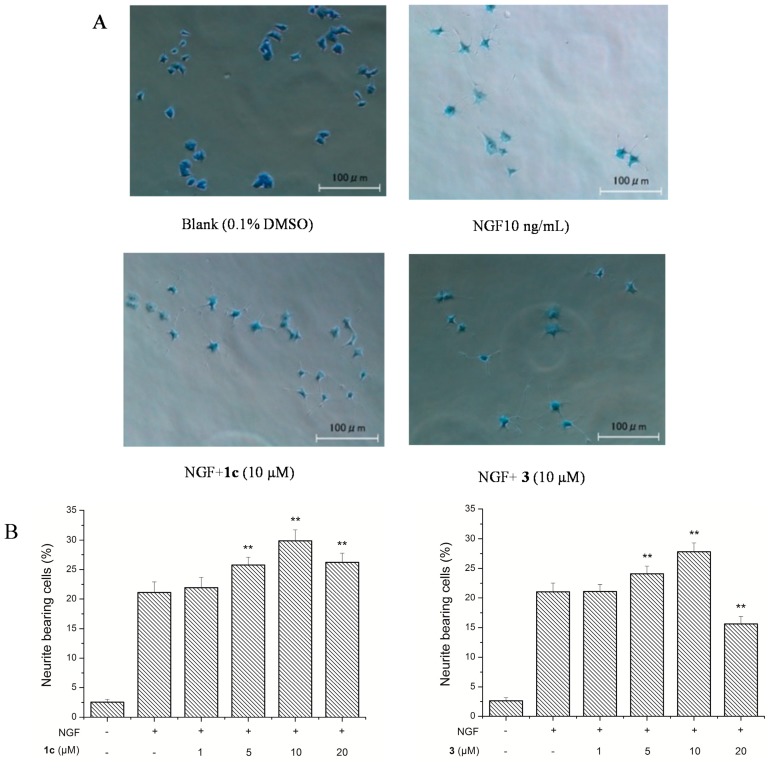
All these compounds at the concentrations of 1, 5, 10, and 20 μM failed to induce neurite outgrowth from PC12 cells in the absence of NGF. However, in the presence of 2 ng/mL NGF, compounds 2, 3, and 1c–1e at a concentration of 10 μM markedly increased the NGF-induced neurite outgrowth based on the numbers of neurite-bearing cells by 21.67% ± 2.64% to 24.40% ± 2.04%, greater than those (17.60% ± 0.24%) of control NGF (2 ng/mL). In particular, compounds 1c and 3 were selected for the assay of neurotrophic activity. As shown in Figure 3, compounds 1c and 3 at concentrations ranging from 1 to 10 μM increased the numbers of neurite-bearing cells in a concentration dependent manner. Compared with 21.11% ± 1.62% for 10 ng/mL NGF alone, compounds 1c and 3 at a concentration of 10 μM increased the numbers of neurite-bearing cells by 29.89% ± 1.86% and 27.81% ± 1.48%, respectively. Nevertheless, compounds 1c and 3 at 20 μM showed somewhat toxicity to PC12 cells. These results suggested that the attachment of the sugar unit to the picrotoxane skeleton might reduce the neurotrophic activity.
Figure 3.
Neurite outgrowth-promoting activity of 1c and 3 in PC12 cells. (A) Morphology of PC12 cells; (B) Quantitative analysis of neurite outgrowth promoted by 1c and 4. Compounds 1c and 3 (1, 5, 10, and 20 μM) + NGF (10 ng/mL). Data are expressed as the means ± SD (n = 3) (** p < 0.01 vs. NGF; Dunnett’s t test). In control experiments, the percentages of neurite-bearing cells were 21.11% ± 1.62% following incubation with 10 ng/mL of NGF after 48 h.
3. Experimental Section
3.1. General Procedures
Optical rotations were recorded on an Autopol III automatic polarimeter. UV spectra were obtained using a Thermo Scientific Evolution 300 UV-VIS Spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). 1H and 13C-NMR spectra were recorded on 400 MHz and 500 MHz NMR instruments. Chemical shifts were reported using the solvent residual peak as the internal standard. ESI-MS spectra were performed on a Thermo Fisher LTQ Fleet instrument spectrometer (Thermo Fisher Scientific Inc.). HRESIMS data were obtained on an API QSTAR time-offlight spectrometer. Column chromatography (CC) was performed on silica gel (90–150 μm) (Qingdao Marine Chemical Inc., Qingdao, China), Sephadex LH-20 (40–70 μm), and Lichroprep RP-18 gel (40–63 μm; Merck, Darmstadt, Germany). GF254 plates were used for thin-layer chromatography (TLC). Preparative TLC (PTLC) was carried out on silica gel 60 GF254; semi-preparative HPLC was performed via a Thermo BDS Hypersil column (250 mm× 4.6 mm and 250 mm× 10 mm, 5 μm).
3.2. Plant Materials
The dried root barks of Coriaria nepalensis were collected from the Qinling mountains, in the Shaanxi province of China in November 2013. The plant was authenticated by Professor Wu. A voucher specimen (0023466) was deposited at the Herbarium of College of Life Sciences, Northwest A&F University, P. R. China.
3.3. Extraction, Isolation, and Purification
The dried and powdered root-bark of C. nepalensis (4.2 kg) were extracted by 95% EtOH at room temperature for three times. After removal of the solvent, the residue (1.9 kg) obtained was suspended in water and then extracted successively with petroleum ether (PE), EtOAc, and n-BuOH. The EtOAc portion (911 g) was chromatographed on silica gel over CC eluting with PE/EtOAc mixtures of increasing polarity (9:1, 8:2, 7:3, 6:4, 4:6) to yield five fractions (Frs. 1–5). Fr. 2 was separated by repeated CC over silica gel (EtOAc/MeOH 9:1 to 6:4) to yield five fractions (2-1–2-5). Fraction 2-2 (2 g) was submitted to a RP-18 column (MeOH/H2O, 10:90 to 100:0) to yield two further fractions (2-2-1 and 2-2-2), and Fraction 2-2-1 was separated by Sephadex LH-20 (CHCl3/MeOH 1:1) and silica gel CC (CHCl3/MeOH 8:2) to yield 1 (216 mg). Fraction 2-3 was separated by silica gel CC (PE/acetone 20:1) to afford four fractions (2-3-1–2-3-4). Fraction 2-3-3 was separated by CC on LH-20 (CHCl3/MeOH 1:1), RP-18 CC (70% MeOH), and then silica gel with (PE/acetone, 50:1 to 40:1) to afford 3 (1.23 g). The n-butanol fraction (242 g) was separated by silica gel CC using EtOAc-MeOH (30:1–15:1) as eluent to afford seven fractions (Frs. A–G). Fr. C was separated by CC over silica gel (CHCl3/MeOH, 12:1 to 5:1), Sephadex LH-20 (MeOH), and then RP-18 to yield 1 (500 mg). Purification of Fr. C-3 by LH-20 CC (MeOH) followed by HPLC (40% EtOH, flow rate = 2 mL/min) provided 2 (2 mg, tR = 17.8 min).
Nepalactone A (1). White, amorphous powder; −26.3 (c 0.24, MeOH); IR (KBr)νmax 3402, 1765, 1076, 1025 cm−1; 1H and 13C-NMR data, see Table 1, Figures S1–S9; ESIMS m/z 435.17 [M + Na]+, 846.62 [2M + Na]+; HRESIMS m/z 435.1991 [M + Na]+ (calcd. for C21H32O8Na, 435.1995).
Nepalactone B (2). White powder; + 6.9 (c 0.24, MeOH); IR (KBr) νmax 3390, 1769, 1076, 1028 cm−1; 1H and 13C-NMR data, see Table 1, Figures S19–27; ESIMS m/z 823.15 [2M − H]−; HRESIMS m/z 435.1987 [M + Na]+ (calcd. for C21H32O8Na, 435. 1995).
Nepalarin (3). Pale yellow needles; UV(MeOH): λmax (logε) nm: 221 (4.28), 336 (4.22); IR (KBr) νmax 3355, 1731, cm−1; IR (KBr) νmax 3319, 1692, 1583, 1458, 1394, 1306, 1067, 1041, 1015 cm−1; 1H-NMR (500 MHz, CDCl3) δ 7.51 (1H, s, H-4), 5.34 (1H, m, H-2′), 5.33 (1H, m, H-2′′′), 5.11 (1H, m, H-2′′), 3.84 (3H, s, 6-OCH3), 3.58 (2H , d, J = 7.5 Hz, H-1′′′), 3.56 (2H, d, J = 6.7 Hz, H-1′′), 3.26 (2H, d, J = 7.0 Hz, H-1′), 1.89 (3H, s, H-4′′′), 1.86 (3H, s, H-5′′), 1.83 (3H, s, H-4′), 1.75(3H, s, H-4′′), 1.72 (3H, s, H-5′′′), 1.71 (3H, s, H-5′); 13C-NMR (125 MHz, CDCl3) δ 162.3 (C-2), 149.5 (C-7), 149.3 (C-9), 142.0 (C-6), 135.9 (C-4), 135.2 (C-3′), 133.1 (C-3′′′), 132.8 (C-3′′), 128.2 (C-10), 124.6 (C-3), 122.6 (C-2′′), 121.1 (C-2′′′), 119.8 (C-2′), 114.3 (C-8), 111.8 (C-5), 62.2 (6-OCH3), 28.5 (C-1′), 25.9 (C-4′), 25.8 (C-5′′′), 25.6 (C-4′′), 24.7 (C-1′′), 22.4 (C-1′′′), 18.1 (C-5′′), 18.0 (C-4′′′), 17.8 (C-5′); ESI-MS m/z 419.49 [M + Na]+, 815.18 [2M + Na]+, 341.19 [M − C4H7]+; ESI-MS m/z 395.41 [M − H]−; HRESIMS m/z 395.2226 [M − H]− (calcd. for C25H31O4, 395.2222).
3.4. Nepalactones A Tetraacetate 1a
To a solution of 1 (3.2 mg, 0.03 mmol) in pyridine (1 mL), acetic anhydride (1 mL) was added, and the mixture stood at RT for 24 h. The reaction mixture was concentrated to dryness, and the residue were purified on silica gel CC using EtOAc, affording tetraacetate 1a (ca. 3 mg) as an off-white gum. −20.9 (c 0.2, MeOH); 1H and 13C-NMR data, see Table 1, Figures S10–18; HRESIMS m/z [M + Na]+ 603.2416 (calcd. for C29H40O12Na, 603.2417).
3.5. Acid Hydrolysis of 1 and theDetermination of Sugar
Compound 1 (2 mg) was hydrolyzed with 2N HCl-dioxane (1:1, 1 mL) at 100 °C for 2 h. The reaction mixture was partitioned between chloroform and H2O three times. The aqueous layer was neutralized with 2 M Ag2CO3 and evaporated in vacuo. The residue was dissolved in pyridine (0.5 mL), to which l-cysteine methyl ester hydrochloride in pyridine (0.1 M, 0.5 mL) was added. After reacting at 60 °C for 1 h, trimethylsilylimidazole (0.05 mL) was added to the reaction mixture and kept at 4 °C for another 8 h. The mixture was analyzed via GC–MS. By comparison of the retention time of the authentic sample, the monosaccharide of 1 was determined to be d-glucose (tR 11.85 min) (tR 12.15 min of l-glucose).
3.6 Enzymatic Hydrolysis of 1
To a solution of 1 (20 mg) in a mixture of a citrate buffer (pH = 5, 1 mL) and water (1 mL), β-gluosidase (1.3 mg, Sigma-Aldrich, St. Louis, MO, USA) was added. After the reaction mixture was incubated at 30 °C for 5 days, it was extracted with EtOAc, dried over anhydrous sodium sulfate, concentrated, and purified via column chromatography using MeOH/CHCl3 (1:15) to yield aglycon 1b as a white solid (8.2 mg, 68%), which was identified as a sesquiterpenoid, corialactone D, via comparison with the 1H-NMR spectrum. 1H-NMR (500 MHz, CDCl3) δ 1.73 (d, J = 15.0 Hz, H-2α), 2.46 (dd, J = 3.5, 15.0 Hz, H-2β), 4.68 (m, H-3), 2.02 (m, H-4), 2.36 (m, H-5), 2.36 (m, H-6), 1.30 (s, 3H, H-7), 1.83 (m, 1H, H-8), 1.02 (d, J = 6.9 Hz, 3H, H-9), 1.00 (d, J = 6.9 Hz, 3H, H-10), 2.37 (m, 1H, H-11α), 2.10 (m, 1H, H-11β), 4.86 (m, 1H, H-12), 5.33 (s, 1H, H-14a), 5.18 (s, 1H, H-14b).
3.7 Preparation of 1c
To a solution of 1b (8.2 mg, 0.03 mmol) in CH2Cl2 (1 mL) at room temperature, NaHCO3 (13.8 mg, 0.16 mmol, 5 equiv.) and Dess-Martin periodinane (DMP) (20.9 mg, 0.05 mmol, 1.5 equiv.) were added. After 40 min of stirring, the reaction mixture was poured into a mixture of a saturated aqueous solution of Na2S2O3 (5 mL), a saturated aqueous solution of NaHCO3 (5 mL), and water (5 mL). The layers were separated, and the aqueous phase was extracted with CH2Cl2. The combined organic extracts were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure. The crude product was purified via flash chromatography on silica gel (PE/EtOAc 5/1 to 2/1), affording 1c (7.8 mg, yield 98%) as a slightly yellow oil; 1H-NMR (500 MHz, CDCl3) δ 6.06 (s, 1H, H-14a), 5.25 (s, 1H, H-14b), 4.66 (t, J = 4.0 Hz, 1H, H-3), 2.65 (dd, J = 19.3, 8.8 Hz, 1H, H-11α), 2.46 (d, J = 19.3 Hz, 1H, H-11β), 2.38 (m, 1H, H-2β), 2.36 (m, 1H, H-5), 2.34 (m, 1H, H-6), 1.99 (m, 1H, H-4), 1.78 (m, 1H, H-8), 1.77 (m, 1H, H-2α), 1.15 (s, 3H, H-7), 0.96 (d, J = 7.0 Hz, 3H, H-10), 0.94 (d, J = 7.0 Hz, 3H, H-9); 13C-NMR (125 MHz, CDCl3) δ 203.7 (C-12), 176.7 (C-15), 151.9 (C-13), 117.5 (C-14), 79.0 (C-3), 51.2 (C-4), 45.0 (C-5), 40.1 (C-1), 39.8 (C-11), 35.5 (C-6), 33.5 (C-2), 32.2 (C-7), 25.3 (C-8), 20.6 (C-10), 19.73 (C-9); ESIMS m/z 249.08 [M + H]+, 271.15 [M + Na]+; HRESIMS m/z 271.1307 [M + Na]+ (calcd. for C15H20O3Na, 271.1305).
3.8 Preparation of 1d and 1e
Compound 1 (30 mg) was hydrolyzed with 4 N HCl–dioxane (1:1, 15 mL) at 100 °C for 2 h. The reaction mixture was partitioned between chloroform and H2O three times. The organic layer was neutralized with 20 M Ag2CO3 and filtered. The resulting solution was washed with brine (50 mL), dried over Na2SO4, and then filtered and evaporated. The residue was subjected to column chromatography on silica gel using petroleum ether and ethyl acetate as eluent to yield 1d (11 mg, 56%) and 1e (7 mg, 38%) as a yellowish oil.
1d: amorphous powder; IR (KBr)νmax 3402, 1765, 1076, 1025 cm−1; 1H-NMR (500 MHz, CDCl3) δ 5.74 (s, 1H, H-12), 4.64 (s, 1H, H-3), 4.23 (d, J = 13.0 Hz, 1H, H-14a), 4.12 (d, J = 13.0 Hz, 1H, H-14b), 1.79 (m, 1H, H-8), 1.25 (s, 3H, H-7), 0.99 (d, J = 6.5 Hz, 3H, H-10), 0.96 (d, J = 6.5 Hz, 3H, H-9); 13C-NMR (125 MHz, CDCl3) δ 178.1 (C-15), 144.9 (C-13), 130.2 (C-12), 79.6 (C-3), 50.9 (C-4), 46.4 (C-1), 44.7 (C-5), 43.8 (C-6), 40.8 (C-14), 34.0 (C-11), 30.8 (C-2), 29.1 (C-7), 25.8 (C-8), 20.6 (C-10), 19.8 (C-9); HRESIMS m/z 291.1128 [M + Na]+ (calcd. for C15H21O2ClNa, 291.1127).
1e: amorphous powder; IR (KBr)νmax 3402, 1765, 1076, 1025 cm−1; 1H-NMR (500 MHz, CDCl3) δ 5.59 (s, 1H, H-12), 4.65 (s, 1H, H-3), 4.18 (s, 2H, H-14), 1.79 (m, 1H, H-8), 1.15 (s, 3H, H-7), 1.00 (d, J = 6.5 Hz, 3H, H-10), 0.97 (d, J = 6.5 Hz, 3H, H-9); 13C-NMR (125 MHz, CDCl3) δ 178.3 (C-15), 149.1 (C-13), 126.4 (C-12), 79.9 (C-3), 59.8 (C-14), 51.0 (C-4), 46.1 (C-1), 44.8 (C-5), 42.7 (C-6), 34.2 (C-11), 31.4 (C-2), 28.4 (C-7), 25.2 (C-8), 20.7 (C-10), 19.8 (C-9); HRESIMS m/z 273.1470 [M + Na]+ (calcd. for C15H22O3Na, 273.1471).
3.9. Neurite Outgrowth-Promoting Assay
Neurite outgrowth-promoting activity of these isolated compounds was assessed as described previously by our group [17,18]. Experiments were repeated at least three times, and data are expressed as mean ± SD (** p < 0.01). Statistical analyses were performed using Dunnett’s t-test.
4. Conclusions
In conclusion, the present study reported the identification of three new compounds, including two new picrotoxane sesquiterpene glycosides nepalactones A (1) and B (2) and one new coumarin nepalarin (3), from the root barks of C. nepalensis. In addition, five congeners 1a–e were synthesized from nepalactone A. We for the first time discovered that these compounds, especially compound 1c and 3, could potentiate NGF-induced neurite outgrowth in PC12 cells.
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (21572182, 31300283), and the Natural Science Foundation of Shaanxi Province (2014JZ2-001, 2015JM3111), as well as the Chinese Universities Scientific Fund (Z109021547, 2014YB025).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/10/1344/s1.
Author Contributions
J.-M.G. designed the research; Y.-Y.W., J.-M.T., C.-C.Z. and B.L. performed the research and analyzed the data; J.-M.G. wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not Available.
References
- 1.Jiangsu New Medical College . Dictionary of Traditional Chinese Medicine. Shanghai Science & Technology; Shanghai, China: 1977. p. 294. [Google Scholar]
- 2.Okuda T., Yoshida T. Structure of corianin. Tetrahedron Lett. 1971;47:4499–4502. doi: 10.1016/S0040-4039(01)97512-4. [DOI] [Google Scholar]
- 3.Aguirre-Galviz L.E., Templeton W. Toxic sesquiterpenoid lactones from the leaves of Coriaria microphylla. Planta Med. 1990;56:244. doi: 10.1055/s-2006-960944. [DOI] [PubMed] [Google Scholar]
- 4.Larsen L., Joyce N.I., Sansom C.E., Cooney J.M., Jensen D.J., Perry N.B. Sweet poisons: Honeys contaminated with glycosides of the neurotoxin tutin. J. Nat. Prod. 2015;78:1363–1369. doi: 10.1021/acs.jnatprod.5b00241. [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita T., Itaki N., Hikita M., Aoyagi Y., Hitotsuyanagi Y., Takeya K. The isolation andstructure elucidation of a new sesquiterpene lactone from the poisonous plant Coriariajaponica (Coriariaceae) Chem. Pharm. Bull. 2005;53:1040–1042. doi: 10.1248/cpb.53.1040. [DOI] [PubMed] [Google Scholar]
- 6.Shen Y.H., Li S.H., Li R.T., Han Q.B., Zhao Q.S., Li L., Sun H.D., Lu Y., Cao P., Zheng Q.T. Coriatone and corianlactone, two novel sesquiterpenes from Coriaria nepalensis. Org. Lett. 2004;6:1593–1595. doi: 10.1021/ol049660p. [DOI] [PubMed] [Google Scholar]
- 7.Wei H., Zeng F.J., Lu M.Y., Tang R.J. Studies on chemical constituents from the root of Coriaria nepalensis wall (Coriaria sinica Maxim) Acta Pharm. Sin. 1998;33:688–692. [PubMed] [Google Scholar]
- 8.Zhao F., Liu Y.B., Ma S.G., Qu J., Yu S.S., Fang Z.F., Li L., Si Y.K., Zhang J.J. New sesquiterpenes from the roots of Coriaria nepalensis. Tetrahedron. 2012;68:6204–6210. doi: 10.1016/j.tet.2012.05.067. [DOI] [Google Scholar]
- 9.Li M.L., Cui J., Qin R.H., Gao J.M., Zhang Y.B., Guo X.R., Zhang W. Semisynthesis and antifeedant activity of new acylated derivatives of tutin, a sesquiterpene lactone from Coriariasinica. Heterocycles. 2007;71:1155–1162. [Google Scholar]
- 10.Yang J.H., Pu J.X., Du X., Zhang H.B., Xiao W.L., Sun H.D. Two new compounds from Coriaria nepalensis. Chin. Chem. Lett. 2011;22:1078–1080. doi: 10.1016/j.cclet.2011.04.002. [DOI] [Google Scholar]
- 11.Shen Y.H., Li S.-H., Han Q.B., Li R.T., Sun H.D. Coumarins from Coriaria nepalensis. J. Asian Nat. Prod. Res. 2006;8:345–350. doi: 10.1080/10286020500044724. [DOI] [PubMed] [Google Scholar]
- 12.Okuda T., Yoshida T., Chen X.M., Xie J.X., Fukushima M. Corianin from Coriaria japonica A. Gray, and sesquiterpene lactones from Loranthus parasiticus Merr. used for treatment of schizophrenia. Chem. Pharm. Bull. 1987;35:182–187. doi: 10.1248/cpb.35.182. [DOI] [PubMed] [Google Scholar]
- 13.Shi X.W., Zhang A.L., Pescitelli G., Gao J.M. Secoscabronine M, a novel diterpenoid from the Chinese bitter mushroom Sarcodon scabrosus. Chirality. 2012;24:386–390. doi: 10.1002/chir.22031. [DOI] [PubMed] [Google Scholar]
- 14.Gao J.M., Qin J.C., Pescitelli G., Pietro S.D., Ma Y.T., Zhang A.L. Structure and absolute configuration of toxic polyketide pigments from the fruiting bodies of the fungus Cortinarius rufo-olivaceus. Org. Biomol. Chem. 2010;8:3543–3551. doi: 10.1039/c002773a. [DOI] [PubMed] [Google Scholar]
- 15.Liu L., Shi X.W., Zong S.C., Tang J.J., Gao J.M. Scabronine M, a novel inhibitor of NGF-induced neurite outgrowth from PC12 cells from the fungus Sarcodon scabrosus. Bioorg. Med. Chem. Lett. 2012;22:2401–2406. doi: 10.1016/j.bmcl.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Liu H.W., Yu X.Z., Padula D., Pescitelli G., Lin Z.W., Wang F., Ding K., Lei M., Gao J.M. Lignans from Schisandra sphenathera Rehd. et Wils. and semisynthetic schisantherin A analogues: Absolute configuration, and their estrogenic and anti-proliferative activity. Eur. J. Med. Chem. 2013;59:265–273. doi: 10.1016/j.ejmech.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Shi X.W., Liu L., Gao J.M., Zhang A.L. Cyathane diterpenes from Chinese mushroom Sarcodon scabrosus and their neurite outgrowth-promoting activity. Eur. J. Med. Chem. 2011;46:3112–3117. doi: 10.1016/j.ejmech.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C.C., Yin X., Cao C.Y., Wie J., Zhang Q., Gao J.M. Chemical constituents from Hericium erinaceus and their ability to stimulate NGF-mediated neurite outgrowth on PC12 cells. Bioorg. Med. Chem. Lett. 2015;25:5078–5082. doi: 10.1016/j.bmcl.2015.10.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.