Abstract
Highly active antiretroviral therapy (HAART) has greatly improved health parameters of HIV infected individuals. However, there are several challenges associated with the chronic nature of HAART administration. For populations in health transition, dual use of medicinal plant extracts and conventional medicine poses a significant challenge. There is need to evaluate interactions between commonly used medicinal plant extracts and antiretroviral drugs used against HIV/AIDS. Efavirenz (EFV) and nevirapine (NVP) are the major components of HAART both metabolized by CYP2B6, an enzyme that can potentially be inhibited or induced by compounds found in medicinal plant extracts. The purpose of this study was to evaluate the effects of extracts of selected commonly used medicinal plants on CYP2B6 enzyme activity. Recombinant human CYP2B6 was used to evaluate inhibition, allowing the assessment of herb-drug interactions (HDI) of medicinal plants Hyptis suaveolens, Myrothamnus flabellifolius, Launaea taraxacifolia, Boerhavia diffusa and Newbouldia laevis. The potential of these medicinal extracts to cause HDI was ranked accordingly for reversible inhibition and also classified as potential time-dependent inhibitor (TDI) candidates. The most potent inhibitor for CYP2B6 was Hyptis suaveolens extract (IC50 = 19.09 ± 1.16 µg/mL), followed by Myrothamnus flabellifolius extract (IC50 = 23.66 ± 4.86 µg/mL), Launaea taraxacifolia extract (IC50 = 33.87 ± 1.54 µg/mL), and Boerhavia diffusa extract (IC50 = 34.93 ± 1.06 µg/mL). Newbouldia laevis extract, however, exhibited weak inhibitory effects (IC50 = 100 ± 8.71 µg/mL) on CYP2B6. Launaea taraxacifolia exhibited a TDI (3.17) effect on CYP2B6 and showed a high concentration of known CYP450 inhibitory phenolic compounds, chlorogenic acid and caffeic acid. The implication for these observations is that drugs that are metabolized by CYP2B6 when co-administered with these herbal medicines and when adequate amounts of the extracts reach the liver, there is a high likelihood of standard doses affecting drug plasma concentrations which could lead to toxicity.
Keywords: herb-drug interactions, CYP450, time-dependent inhibition, reversible inhibition, recombinant human CYPs
1. Introduction
It has been shown unequivocally that antiretroviral therapy (ART) reduces HIV/AIDS mortality and prolongs life. However, attention has now shifted from reduction in mortality to improvement of the quality of life for people taking ART [1,2]. Across the world, there are huge disparities in health systems, thus, HIV/AIDS patients in different geographical regions receive different ART regimens mostly due to the different cost of the more than 30 antiretroviral (ARV) drugs approved by the US FDA. In resource constrained settings, the most commonly used ARV drugs include nevirapine (NVP) and efavirenz (EFV) which form the backbone of highly active antiretroviral therapy (HAART) regimens; thus, quite a number of patients use these two drugs during treatment [3,4]. Africa is a continent with a high prevalence of HIV/AIDS making up approximately 67% of the world’s population of HIV infected people [5]. Most ARV drugs used in Africa include EFV and NVP. EFV and NVP are non-nucleoside reverse transcriptase I (NNRTIs) inhibitors which act by inhibiting the reverse transcriptase enzyme. Both EFV and NVP are relatively affordable when compared to other ARV drugs [6]; hence, they are widely used in many African countries. EFV and NVP are substrates of the CYP2B6 enzyme [7,8] and plasma concentrations of these two ARVs are affected by changes in levels and activity of CYP2B6. CYP3A4 also metabolizes these two drugs but its contribution appears to be marginal [9]. EFV and NVP have been associated with a number of side effects including liver damage, nausea, vomiting, fever, diarrhoea, dyslipidemia and headache [10].
CYP2B6 enzyme metabolizes many commonly used drugs including bupropion, propofol and cyclophosphamide which should not be given together with EFV or NVP, to avoid drug-drug interactions. Among populations in health transition, especially those in resource-constrained countries, patients often make use of herbal medicinal plants in the management and treatment of several diseases in addition to use of conventional drugs [11]. HIV infection is an epidemic in resource constrained countries, particularly in sub-Sahara Africa; thus, it is inevitable that there is dual use of HAART and herbal medicinal plants. Herbal medicine is much cheaper and readily available in most settings.
There is substantial evidence supporting the potential of medicinal plants in combating diseases, especially in developing countries [12,13,14,15]. Although effective in the treatment of some diseases, the mechanisms of action for most herbal medicines remain largely unknown. A wide range of compounds (including phytochemicals) have been extracted from some of these medicinal plants so it is not inconceivable to be able to predict possible effects of herbal medicines on enzymes, such as CYP2B6, that metabolize commonly used drugs including EFV and NVP [16,17,18]. Some patients take herbal medicines and conventional medicines at the same time in a bid to get better more quickly, potentiating risk of herb–drug interaction (HDI) [19]. In spite of the wide use of herbal medications among HIV/AIDs patients taking EFV or NVP-based HAART, data on their pharmacokinetic and pharmacodynamics properties in humans remain lacking or scant [11,20]. Clinically significant interaction of herbs with prescribed medications via drug metabolism may worsen the health condition of patients if not detected earlier during treatment.
Cytochrome P450 enzymes (CYP450) are responsible for the metabolism of nearly 75% of phase I-dependent metabolism of clinically used drugs [21]. Relative expression of CYP2B6 ranges from 2% to 10% of the total hepatic content [22,23,24] and contributes to a significant proportion of drug metabolism [25]. Genetic polymorphism in CYP2B6 have been implicated in variations in the activity of the enzyme in metabolizing xenobiotics [26]. Inhibition of CYP2B6 by any drug, conventional and/or herbal, is likely to present in a similar manner as portrayed by polymorphisms that result in deficient enzyme activity. Thus, when herbal medicines are co-administered with ARV drugs, the possibility of herb-ARV drug interaction might be clinically significant and may lead to significant morbidity and mortality.
The purpose of this study was to evaluate the inhibition of CYP2B6 enzyme activity by selected medicinal plant extracts used by HIV patients to treat HIV-associated opportunistic infections. The medicinal plant extracts evaluated were extracted from Hyptis suaveolens, Boerhavia diffusa, Newbouldia laevis, Launaea taraxacifolia and Myrothamnus flabellifolius.
2. Results
This study provides us with an opportunity to understand the effects of five medicinal plants—Hyptis suaveolens, Boerhavia diffusa, Newbouldia laevis, Launaea taraxacifolia and Myrothamnus flabellifolius—on CYP2B6 activity. Data presented here can help to increase awareness among current and future patients of the potential effects of dual use of conventional drugs that are metabolized xenobiotic metabolising enzymes such as CYP2B6 and the above herbal medicinal plants. Our data shows that, indeed, for most of these herbal compounds, there is need for caution when co-administering them with other medications.
2.1. Inhibition Screening and IC50 Determination
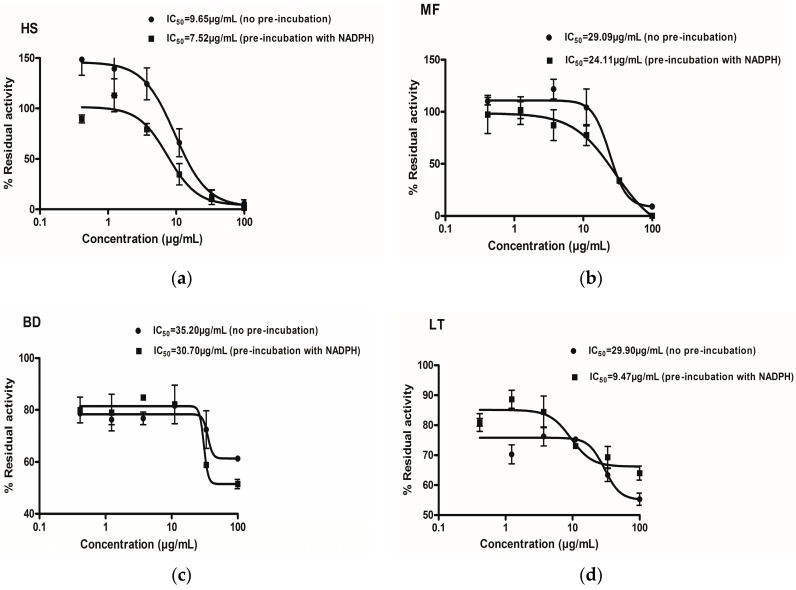
The potential of each medicinal plant extract to inhibit activity of recombinant human CYP2B6 is presented in Figure 1 and Table 1. Four of the medicinal plant extracts inhibited CYP2B6 activity in a concentration dependent manner. The potency of inhibition exhibited by the extracts was in the order of Hyptis suaveolens (HS) > Myrothamnus flabellifolius (MF) > Boerhavia diffusa (BD) > Launaea taraxacifolia (LT). Newbouldia laevis did not show any concentration dependent inhibitory effects.
Figure 1.
IC50 curve shift for Time Dependent Inhibition (TDI) determination. Hyptis suaveolens (HS) (a), Boerhavia diffusa (BD) (c), Myrothamnus flabellifolius (MF) (b) and Launaea taraxacifolia (LT) (d), at various concentrations were incubated with and without NADPH for 30 min. Percentage residual activity for no pre-incubation (closed circles) and 30 min pre-incubation (closed squares) is shown. Residual activity was calculated compared to control activity.
Table 1.
IC50 and IC50 fold shift due to incubation with or without Nicotinamide Adenine Dinucleotide Phosphate (NADPH).
| Inhibitor | Conventional IC50 (µg/mL) (Mean ± SEM) | IC50 (no Pre-Incubation with NADPH) (µg/mL) (Mean ± SEM) | IC50 (Pre-Incubation with NADPH) (µg/mL) (Mean ± SEM) | Fold Shift (−IC50/+IC50) |
|---|---|---|---|---|
| Newbouldia laevi | 100 ± 8.71 | ND | ND | ND |
| Hyptis suaveolens | 19.09 ± 1.16 | 10.60 ± 1.32 | 7.52 ± 1.20 | 1.40 |
| Launeae taraxacifolia | 33.87 ± 1.54 | 29.90 ± 1.32 | 9.47 ± 1.41 | 3.17 |
| Boerhavia diffusa | 34.93± 1.06 | 35.20 ± 2.86 | 30.70 ± 2.02 | 1.20 |
| Myrothamnus flabellifolious | 23.66 ± 4.86 | 29.09 ± 1.74 | 24.11 ± 1.24 | 1.21 |
| Miconazole | 0.53 ± 0.14 | 0.63 ± 0.15 | 0.72 ± 0.20 | 0.88 |
The IC50 values for the medicinal plant extracts were determined as explained in the materials and methods section. The medicinal plant extracts inhibited CYP2B6 in the order Hyptis suaveolens (19.09 ± 1.16 µg/mL), Myrothamnus flabellifolius (23.66 ± 4.86 µg/mL), Launaea taraxacifolia (33.87 ± 1.54 µg/mL) and Boerhavia diffusa (34.93 ± 1.06 µg/mL) (Table 1). Newbouldia laevis indicated an IC50 of 100 ± 8.71 µg/mL, exhibiting very weak inhibitory effects on CYP2B6. Miconazole was used as a standard diagnostic inhibitor of CYP2B6 whose observed IC50 value of 0.55µM is in agreement with what has been reported elsewhere in literature [27].
2.2. Prediction of in Vivo Herb-Drug Interaction for IC50
The % yield and concentration per dose was calculated as shown in Table 2. Since the intestinal absorption and plasma concentrations of each test compound are not known, and with the knowledge that herbal extracts have different bioavailability [28], the estimated bioavailable concentration was calculated using the % yield to give the soluble extract available in the GI tract.
Table 2.
Calculation of herbal medicine concentration in the gut.
| Herbal Extracts | % Yield | Recommended Herbal Dose (Single; mg) | Putative GIT Concentration (µg/mL) | Estimated Bioavailable Concentration (µg/mL) |
|---|---|---|---|---|
| Newbouldia laevis | 14.66 | 200 | 800 | 117.28 |
| Hyptis suaveolens | 6.51 | 400 | 1600 | 104.16 |
| Launaea taraxacifolia | 10.40 | 200 | 800 | 83.3 |
| Boerhavia diffusa | 11.24 | 200 | 800 | 89.92 |
| Myrothamnus flabellifolius | 10.80 | 200 | 800 | 86.4 |
Note: GIT, Gastrointestinal tract, estimated bioavailable concentration = (% yield × putative GIT concentration)/100.
Using this assumption, the putative GIT concentration was calculated as shown in Table 2 and Table 3. The IC50 values obtained from the in vitro assay were then compared with the calculated estimated bioavailable concentration and predictions made. Based on the IC50 values, it was predicted that Hyptis suaveolens, Boerhavia diffusa, Myrothamnus flabellifolius and Launaea taraxacifolia were likely to cause in vivo inhibition of CYP2B6 and with potential HDI (Table 3). It was observed that IC50 values for Hyptis suaveolens, Boerhavia diffusa, Myrothamnus flabellifolius and Launaea taraxacifolia were approximately four times lower than the estimated bioavailable concentration and, therefore, adequate amounts may enter the hepatic portal vein to interact with CYP2B6. However, it was observed that NL had an IC50 value relatively similar to the estimated bioavailable concentration and, therefore, is not expected to enter the hepatic portal vein to interact with CYP2B6.
Table 3.
In vivo prediction of HDI from in vitro for CYP2B6.
| Herbal Extracts | Inhibitor Concentration (µg/mL) | IC50 (µg/mL) | Risk of HDI in the Gut * |
|---|---|---|---|
| Newbouldia laevis | 117.28 | 100 | Unlikely |
| Hyptis suaveolens | 104.16 | 20.33 | Likely |
| Launaea taraxacifolia | 83.20 | 33.87 | Likely |
| Boerhavia diffusa | 89.92 | 34.93 | Likely |
| Myrothamnus flabellifolius | 86.40 | 23.66 | Likely |
Note: HDI, herb-drug interaction, inhibitor concentration = estimated bioavailable concentration (µg/mL), * the likelihood of a clinically relevant interaction when four of these herbal extracts are taken is based on the assumption that the % yield serves as the bioavailable fraction which was used in estimating the bioavailable concentration in the gut and also if there is complete absorption.
2.3. Time Dependent Inhibition (TDI) by IC50 Curve-Shift and Single Point NADPH Inhibition Screening Evaluation
The IC50 curve shift assay was used to evaluate the TDI potencies of the various extracts. A comparison was made between the IC50 values obtained with or without NADPH. The IC50-curve shift showing the potency of the plant extracts to cause TDI is shown in Figure 1.
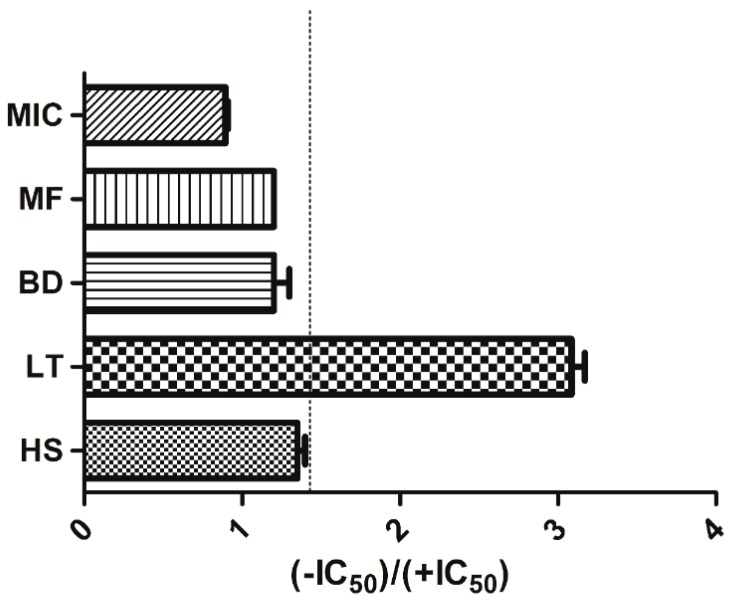
A fold decrease in IC50 was used to categorize a plant extract as a potential TDI. Extracts with an IC50 ratio of ≥1.5 were classified as positive TDIs as shown in Figure 2. Table 1 shows the shifted IC50 obtained for the various extracts. Launaea taraxacifolia showed TDI potency with an IC50 fold decrease of 3.17. However, Hyptis suaveolens, Boerhavia diffusa and Myrothamnus flabellifolius did not exhibit a TDI effect on CYP2B6.
Figure 2.
Time dependent inhibition (TDI) classification of medicinal plant extract incubation based on IC50 curve shift.
Extracts of Hyptis suaveolens (HS), Boerhavia diffusa (BD), Myrothamnus flabellifolius (MF) and Launaea taraxacifolia (LT) on recombinant CYP2B6. The bars represent ratio of IC50 values from the no pre-incubation assays for the herbal extracts listed. Extracts that were >1.5 (dashed line) were classified as TDI candidates. Data shown are the mean ± SEM (n = 2).
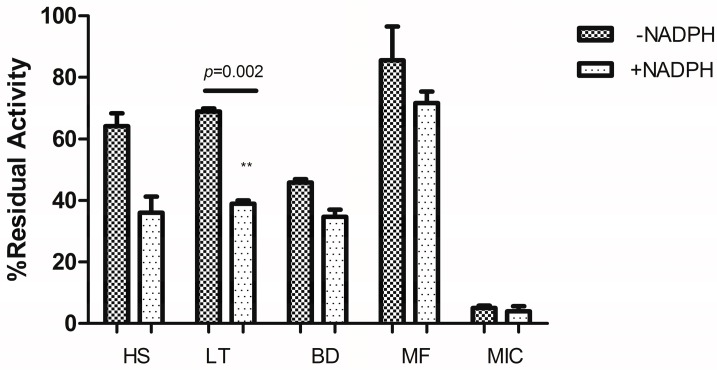
Specific concentrations of the plant extracts were pre-incubated with or without NADPH and the percentage residual activity was compared (Figure 3). Medicinal plant extracts that were pre-incubated with NADPH showed a reduction in the percentage activity of CYP2B6 compared to those without NADPH with a significant effect observed for the Launaea taraxacifolia extract indicating its time dependent inhibitory potency without the possibility of enzyme activity recovery.
Figure 3.
Inhibitory effects of 50 µg/mL of each extract after pre-incubation in the presence and absence of NADPH. ** Significant p value.
Hyptis suaveolens (HS), Boerhavia diffusa (BD), Myrothamnus flabellifolius (MF) and Launaea taraxacifolia (LT) on recombinant CYP2B6 after pre-incubation with or without NADPH for 30 min. Percentage residual activities were plotted against respective fractions. Two-tailed unpaired t-test was used to compare the percentage residual activity of each fraction with p < 0.05 considered significant.
2.4. Relative Quantification of Selected Phenolic Compounds Using UPLC-MS
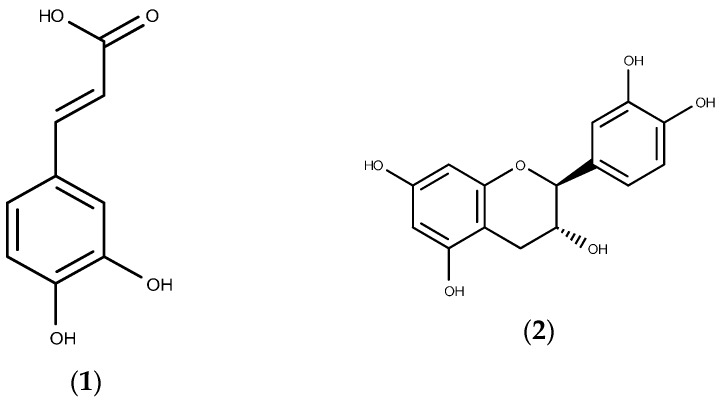
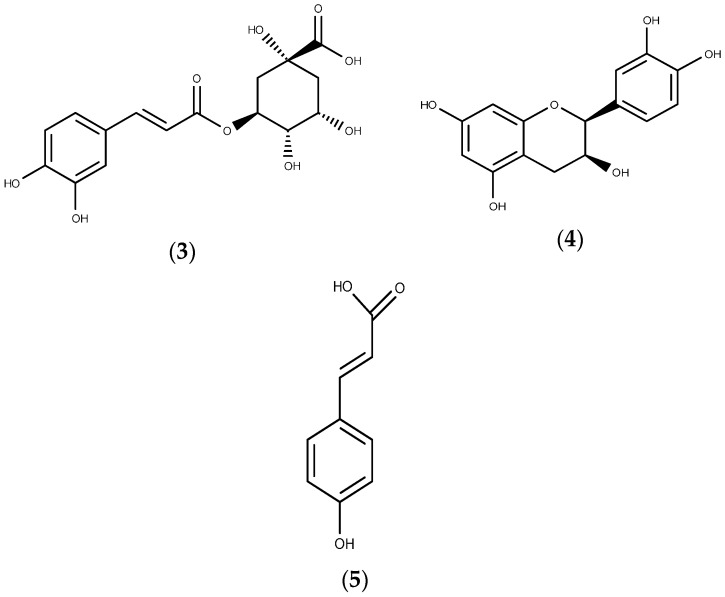
The relative quantification of phenolic compounds after UPLC-MS was performed using XCMS data analysis software. Table 4 shows the relative quantification of the phenolic compounds analyzed using MS detection expressed in mg·kg−1 of original starting material used in the extraction, whereas Figure 4 shows structures for some of the identified compounds. Hyptis suaveolens had the highest content of chlorogenic acid (1665.33 ± 0.87 mg·kg−1) followed by Launaea taraxacifolia (1662.98 ± 2.10 mg·kg−1). Launaea taraxacifolia however contained the highest content of caffeic acid (998.59 ± 1.48 mg·kg−1) followed by Hyptis suaveolens (100.00 ± 0.97 mg·kg−1). Non-significant traces of epicatechin and catechin were found in all the medicinal plant extracts except Myrothamnus flabellifolius.
Table 4.
Quantification of compounds identified in herbal extracts using MS detection.
| Phenolic Compound | Newbouldia laevis (Mean ± SEM) | Hyptis suaveolens (Mean ± SEM) | Launaea taraxacifolia (Mean ± SEM) | Boerhavia diffusa (Mean ± SEM) | Myrothamnus flabellifolius (Mean ± SEM) |
|---|---|---|---|---|---|
| Catechin | Trace | Trace | Trace | 1.40 ± 0.03 | 27.58 ± 0.10 |
| p-Coumaric acid | 7.54 ± 0.10 | 75.25 ± 0.10 | 751.41 ± 1.30 | 13.89 ± 0.10 | 39.13 ± 0.21 |
| Caffeic acid | 10.01± 0.01 | 100.00 ± 0.97 | 998.59 ± 1.48 | 10.59 ± 0.06 | 3.98 ± 0.01 |
| Epicatechin | Trace | Trace | Trace | Trace | 170.06 ± 0.39 |
| Chlorogenic acid | 166.77 ± 0.20 | 1665.33 ± 0.87 | 1662.98 ± 2.10 | Trace | 5.84 ± 0.10 |
Note: values are expressed as mg·kg−1 for four biological replicates ± SD.
Figure 4.
Structures of the phenolic compounds identified and quantified from crude herbal extracts (1) Caffeic acid; (2) Catechin; (3) Chlorogenic acid; (4) Epicatechin; (5) p-Coumaric acid.
3. Discussion
The use of medicinal plants for therapeutic purpose is a globally known phenomenon. When adverse effects are experienced or cure fails, like in the case of HIV infection and cancer, limited resources could be used to promote the use of herbal medicines. Thus, studies such as this one, which seek to scientifically validate the safety of herbal medicines, are imperative. Herbal medicine is being used for the treatment of a wide spectrum of disease conditions including cardiovascular and viral disease, cancer and diabetes [29]. There is increasing evidence to also show that there is concomitant use of herbal medicines with conventional medicines which leads to clinical complications resulting from herb–drug interactions [11,19,30]. Studies on the drug interaction potential of herbal medicines, especially those that have been in use for centuries, will help to create an awareness of the potential for complications [31,32]. It is also imperative that identification of the phytochemical constituents in these herbal medicines be performed to identify those likely to cause drug interactions. The advantage of phytochemical profiling is that similar drug interacting constituents could be identified in other related herbs that are used for therapeutic purposes and that necessary mechanistic studies are performed on them. Guidelines from the FDA relating to drug interaction and identification of compounds which interact with drug metabolizing enzymes and transporters is of high value since these compounds play a major role in affecting the pharmacokinetic and pharmacodynamics profiles of allopathic medications [33].
In this study, five herbal medicines commonly taken to treat and manage the effects of HIV/AIDS or its comorbidities by patients were evaluated for their ability to modulate activity of CYP2B6 in vitro using recombinant human CYPs. Crude extracts of the herbal medicines were used because since patients take these extracts in their crude from, it became important that the effect of the crude form is evaluated. The method of extraction used for this study was also performed to mimic the indigenous mode of extraction using water. The study showed that Launaea taraxacifolia Hyptis suaveolens, Boerhavia diffusa and Myrothamnus flabellifolius extracts caused a concentration-dependent inhibition in CYP2B6. The US FDA, EMA and pharmaceutical industries have published opinion documents and guidelines for the conduct of drug enzyme inhibition studies [34,35,36] which include reversible and time dependent inhibition profiles of new chemical entities (NCE). A reversible inhibition profile was thus conducted for the herbal extracts. It was observed that Hyptis suaveolens, Myrothamnus flabellifolius, Launaea taraxacifolia and Boerhavia diffusa had a reversible inhibitory effect on the activity of CYP2B6. Newbouldia laevis, however, did not show any significant inhibitory effects on the activity of CYP2B6 based on the concentration range assessed.
Based on the recommended dosage taken by patients, the GIT concentration was calculated and % yield used to estimate the bioavailable concentration (Table 2) and the possibility of causing HDI in vivo determined. Here, a comparison was made between the amount of extract in the gut and the IC50 concentrations obtained. If the amount in the gut is at least two-fold (2×) or more higher than the IC50, then it is expected that adequate amounts of the extract will reach hepatic CYP2B6 to cause clinically significant HDI [37]. It was observed that Hyptis suaveolens, Myrothamnus flabellifolius, Launaea taraxacifolia and Boerhavia diffusa were likely to cause HDI in the gut (Table 3). Newbouldia laevis, however, seems unlikely to cause an HDI in the gut (Table 3). Thus, patients taking Hyptis suaveolens, Myrothamnus flabellifolius, Launaea taraxacifolia and Boerhavia diffusa would need to exercise caution as these herbs are likely to inhibit CYP2B6 and could render drugs that are CYP2B6 substrates—which include efavirenz and nevirapine—toxic.
According to US FDA, EMA and pharmaceutical industries guidelines on the conduct of drug enzyme inhibition studies [34,36,38], NCE that show significant reversible inhibition should also be assessed for time dependent inhibitory (TDI) effects. The IC50 shift approach is one of the recommended methods for TDI assessment [39,40]. A significant shift towards the left in the inhibition curves after pre-incubation shows potency for TDI. The method was validated with miconazole which has not been known to show any TDI effects. According to Berry and Zhao [39], a fold shift or IC50-shift decrease of ≥1.5 is significant for a NCE to be called a TDI compound. In this study, therefore, this criterion was used to classify the herbal extracts as potential TDI candidates. Launaea taraxacifolia showed an IC50-shift decrease of 3.17 and was thus classified to be a potential TDI candidate. Hyptis suaveolens, Myrothamnus flabellifolius and Boerhavia diffusa showed fold decreases of 1.40, 1.21 and 1.20, respectively. The effect of NCE that causes TDI is that even if the usage of the compound is discontinued, there is permanent destruction of enzyme which requires de novo synthesis of the enzyme of interest. Potential TDI compounds asserts inhibitory effects through formation of more inhibitory metabolites or the irreversible inactivation of enzymes by metabolic products that form haem or protein adducts [41]. Launaea taraxacifolia thus having been classified as a potential TDI candidate in this study demonstrates potential in inhibiting CYP2B6. Hyptis suaveolens, Myrothamnus flabellifolius and Boerhavia diffusa exhibited a non-TDI effect suggesting the potential of these three herbs interacting reversibly with CYP2B6.
Launaea taraxacifolia has been used for centuries in West African countries such as Ghana and Nigeria as a vegetable and also for managing dyslipidemia and liver diseases [42] which are complications of HIV/AIDS, its comorbidities [43,44,45,46] and HAART [10,47,48,49]. Patients on HAART regimens containing EFV and NVP are likely to experience adverse drug effects from Launaea taraxacifolia when used together. In resource-limited settings, Launaea taraxacifolia is a commonly used vegetable; thus, herb–drug interactions are highly likely to be reported.
Although the study analyzed effects of the crude herbal extracts as taken by patients and did not evaluate the effects of the individual phytochemical constituents or fractions, UPLC-MS was used to quantify some phenolic compounds known for their therapeutic effects and some that have been implicated in CYP inhibition. Quantification of catechin, p-coumaric acid, caffeic acid, epicatechin and chlorogenic acid was performed with known standards and analyzed using XCMS data analysis software. Extract from Launaea taraxacifolia showed high content of chlorogenic acid (1662.98 mg·kg−1) and caffeic acid (998.59 mg·kg−1) (Table 4) with though these were also present in Hyptis suaveolens, Myrothamnus flabellifolius and Boerhavia diffusa also contained varying amounts. Caffeic acid [50] and chlorogenic acid [51,52] have been implicated in the inhibition of CYP450 activity in vitro. It is therefore postulated that the inhibitory effects observed on CYP2B6 in the study could be via the effects of these phenolic compounds. Other phenolic compounds—for example catechin, epicatechin [53] and p-coumaric acid [54], known for their antioxidant and anti-inflammatory effects—were also found in the extracts which also explains some of their therapeutic uses.
4. Materials and Methods
4.1. Chemicals and Reagents
Miconazole nitrate was purchased from Sigma-Aldrich (St. Louis, MO, USA). Black costar 96 well plates were obtained from Thermo Fischer Scientific (Pittsburgh, PA, USA). Vivid® CYP450 Blue Screening Kit and Vivid® Substrate, 7-benzyl-oxymethyloxy-3-cyanocoumarin, (BOMCC) were purchased from Life Technologies, (Grand Island, NY, USA). Standard for epicatechin, catechin, chlorogenic acid, caffeic acid and p-coumaric acid for identification of constituents of herbal extracts were purchased from Chromadex (Wesel, Germany). Purified water (double-distilled and deionized) was obtained from Millipore (Bedford, MA, USA).
4.2. Plant Material
The following plants Hyptis suaveolens (UCC/BS/687), Boerhavia diffusa (UCC/BS/688), Newbouldia laevis (UCC/BS/689) and Launaea taraxacifolia (UCC/BS/690), Table 5, used in the study were obtained and authenticated by botanists from the University of Cape Coast and samples were kept in the herbarium in the department of Biological sciences with voucher numbers as indicated. Myrothamnus flabellifolius, Table 5, was obtained from Zimbabwe and authenticated by a botanist. Leaves of the plants were air dried and made into a powder using mortar and pestle. Ethical approval was obtained from the University of Cape Town Human Research Ethics committee with number HREC REF: 826/2014.
Table 5.
Herbal plants and their medicinal value.
| Plant Species | Commonly Found African Countries | Purported Medicinal Value |
|---|---|---|
| Newbouldia laevis | Ghana, Togo, Nigeria, Congo | Anti-malaria, immune booster, anti-bacterial, anti-fungal |
| Hyptis suaveolens | Ghana, Togo, Nigeria, Congo, Benin, Guinea | Anti-bacterial, anti-fungal, anti-malaria, anti-cholesterol |
| Launaea taraxacifolia | Ghana, Togo, Nigeria, Congo, Benin, Guinea, Cote d’Ivoire | Anti-bacterial, anti-fungal, anti-malaria, anti-cholesterol, urinary infections, anti-diabetic |
| Boerhavia diffusa | Ghana, Togo, Nigeria, Congo, Benin, Guinea, Cote d’Ivoire, South Africa | Anti-bacterial, hepatoprotective, anti-nociceptive |
| Myrothamnus flabellifolious | Zimbabwe, Botswana, South Africa, Uganda, Egypt | Anti-viral, immune booster |
4.3. Extraction of Plant Material
A sample of 10 g of leaves from each plant was prepared in 100 mL of distilled water and heated for one hour at 60 °C to mimic the indigenous mode of extraction. The material was allowed to extract for 72 h at room temperature during which supernatant was decanted every 24 h and the solid residue reconstituted in the same volume of purified water for the extraction process to be repeated. The supernatants were pooled and centrifuged (14,000× g, 10 min) and filtered using filter paper (8 µm, Whatman International LTD, Maidstone, UK). The filtrates were freeze-dried using a Virtis sentry freeze dryer (the Virtis Company, NC, Gardiner, NY, USA) and the resulting powders were weighed. The dried extracts were stored in an airtight container and stored at −20 °C until needed for use.
4.4. Screening for Inhibition
The medicinal plant extracts were screened for inhibitory effects on CYP2B6 using a two point screening assay that used two concentrations of the extracts, 10 µg/mL and 100 µg/mL, respectively. The assays were performed using the Vivid® CYP450 Screening Kits (Life Technologies, Grand Island, NY, USA) with the protocol provided by the supplier. The Vivid® CYP450 screening kits are designed to assess the metabolic activity and inhibition of the predominant human CYP450 isozymes involved in hepatic drug metabolism by the use of 7-benzyl-oxymethyloxy-3-cyanocoumarin (BOMCC) as a probe substrate. Briefly, the two concentrations of medicinal plants extracts mentioned above were pre-incubated with a mixture of CYP2B6 BACULOSOME plus reagent and regeneration system (consisting of glucose-6-phosphate and glucose-6-phosphate dehydrogenase) in Vivid® reaction buffer I (Master pre-mix) in a black Costar 96-well plate for 20 min at 37 °C. The reaction was initiated by adding a mixture of reconstituted BOMCC and NADP+ in Vivid® reaction buffer I and incubated for 30 min at 37 °C. The reaction was stopped by adding ice-cold 20% Tris base/80% acetonitrile (ACN). The activity of the enzymes was monitored by measuring the formation of fluorescent metabolite at an excitation and emission wavelength of 405/460 nm. Flourescence (SSN instruments, Set Point Technology, South Africa) was measured using a Varian Cary eclipse advanced reads software. The two point screening assay was followed by IC50 determination which included preparation of serial dilutions of medicinal plant extracts from a concentration of 100 µg/mL. A mixture of CYP2B6 BACULOSOME plus reagent and regeneration system in Vivid® reaction buffer I (Master Pre-mix) in a black costar 96-well plate were pre-incubated for 20 min at 37 °C. The reaction was carried out in a similar manner to the two-point screening assay.
4.5. Time-Dependent Inhibition (TDI) Assessment Using the IC50 Curve-Shift
Determination of time-dependent inhibition (TDI) followed the same procedure as that of the IC50 determination with slight modifications. Medicinal plant extracts with a starting concentration of 100 µg/mL were serially diluted in duplicate six consecutive times in the black costar 96-well plate. The black Costar 96-well plate was divided into two halves; reaction mixture A (CYP2B6 BACULOSOME, regeneration system, NADP+ and Vivid® reaction buffer I) was applied to the first half and reaction mixture B (CYP2B6 BACULOSOME, regeneration system and Vivid® reaction buffer I) was added into the second half of the plate. The mixture was pre-incubated at 37 °C for 30 min. After pre-incubation, reconstituted BOMCC and NADP+ in Vivid® reaction buffer I was added to each well to start the reaction. The mixture was incubated for an additional 30 min and the reaction was terminated by adding ice-cold 20% Tris base/80% ACN. The activity of the enzymes was monitored by measuring the formation of fluorescent metabolite at the same excitation and emission wavelength as in the IC50 determination. For single point NADPH screening, based on the shift in the IC50 curve, 50 µg/mL concentrations of the extracts were pre-incubated with or without NADPH for 30 min. The procedure for TDI by IC50 curve-shift was followed as explained above. This single point NADPH screening is carried out in order to assess the significance of the effect of pre-incubating with NADPH at a single concentration.
4.6. Relative Quantification of Selected Phenolic Compounds Using UPLC-MS
In order to identify and quantify constituents in the medicinal plant extracts, chromatographic separation was performed on a Waters Acquity UPLC system (Waters Corporation, Milford, MA, USA) coupled to a PDA detector and Synapt G2 (ESI negative) following the procedure described by Farag and Wessjohann [55]. Quantification of glycosylated derivatives of epicatechin, catechin, chlorogenic acid, caffeic acid and p-coumaric acid was calculated from calibration curves of epicatechin, catechin, chlorogenic acid, and caffeic acid and p-coumaric acid standards, respectively. Calibration curves for each reference compound ranged from 0.1 to 100 µg/mL.
4.7. Data Analysis
4.7.1. IC50 Determination
The data generated was exported into an excel spreadsheet and the amount of metabolite formed at the various concentrations relative to the control (residual activity) was calculated using Equation (1)
| % Residual Activity = (Test − Test blank)/(Control − Control blank) × 100 | (1) |
The percentage residual activity was plotted against the log transformed concentrations of the medicinal plant extracts and the miconazole (positive CYP2B6 inhibitor). A sigmoid curve was then fitted using non-linear regression analysis and the IC50 value was calculated using GraphPad Prism version 5.0 (GraphPad Software Inc., San Diego, CA, USA). IC50 values were calculated with the remaining enzyme activity and inhibitor concentration using Equation (2)
| Y = 100 − [(100 × [I]H)/(IC50H + [I]H)] | (2) |
Y: remaining CYP2B6 activity (percentage of control), [I]: concentration of medicinal plant extract, H: Hill coefficient.
4.7.2. IC50 Curve-Shift for TDI Prediction, Point Screening and Relative Quantification of Phenolic Compound
The difference between the calculated IC50 values for pre-incubation with or without NADPH for each medicinal plant extract and diagnostic inhibitor was compared [56]. A ratio of IC50 value for pre-incubation without NADPH (−IC50) and pre-incubation with IC50 with NADPH (+IC50) was then calculated to establish the fold-shift (−IC50/+IC50). Medicinal plant extracts that showed an IC50 fold-shift of ≥1.5 were thus classified as TDI and those below classified as non-TDI based on the recommendation of Berry and Zhang [39]. For single point NADPH inhibition screening, a bar graph was plotted showing the percentage residual activity with the concentration of extracts with or without NADPH. The percent remaining activity was compared using the unpaired t-test with p < 0.05 considered significant. Relative quantification of metabolite profiles after UPLC-MS was performed using XCMS data analysis software.
4.7.3. Prediction of in Vivo Herb-Drug Interaction for IC50
The assumptions to risk rank the HDI potential of all extracts used in this study was done according to the method of Awortwe et al. [57]. Percentage yield of each extract was calculated from amounts extracted from each herbal plant material. To predict the likelihood of medicinal plant extracts interacting with hepatic CYP2B6, two assumptions were made: firstly, that extracts were completely soluble in the human Gastro intestinal tract (GIT) fluid volume of 250 mL. The commonly used dose of the various extracts was estimated to be 200–400 mg. Based on the recommended dose used in humans, an estimated concentration per dose in GIT depending on the % yield was calculated; Secondly, that adequate amount of each extract enters the hepatic portal vein to interact with CYP2B6, if the IC50 values were lower than the estimated amount in the GIT.
5. Conclusions
We have shown that some of the medicinal plant extracts that have been used traditionally to cure different diseases may need to be carefully considered in the era of HAART treatment, especially when considering regimens containing EFV and NVP because of the effects of these herbal treatments on CYP2B6, an enzyme that is necessary in the deposition of many therapeutically used drugs.
Acknowledgments
Research reported in this publication was supported by the National Research Foundation (NRF) under an Indigenous Knowledge Systems (IKS) Research Grant to Collet Dandara. The views and opinions expressed are not those of the NRF but of the authors of the material published. Nicholas Ekow Thomford is funded by an NRF grant-holder linked bursary from the IKS research grant awarded to Collet Dandara.
Author Contributions
Collet Dandara, Ambroise Wonkam, Nicholas Ekow Thomford, Michelle Skelton, Dee Blackhurst and Faustina Adu contributed to the design of the study. All authors have materially participated in the manuscript preparation. Nicholas Ekow Thomford and Charles Awortwe performed the laboratory and data analysis. Collet Dandara and Nicholas Ekow Thomford wrote the draft manuscript and interpretation of data. Denis Chopera, Kevin Dzobo, Ambroise Wonkam, Dee Blackhurst and Michelle Skelton commented. All authors reviewed the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Basavaraj K.H., Navya M.A., Rashmi R. Quality of life in HIV/AIDS. Indian J. Sex. Transm. Dis. 2010;31:75–80. doi: 10.4103/0253-7184.74971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oguntibeju O.O. Quality of life of people living with HIV and AIDS and antiretroviral therapy. HIV/AIDS. 2012;4:117–124. doi: 10.2147/HIV.S32321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Antiretroviral Therapy for HIV Infection in Adults and Adolescents. World Health Organization; Geneva, Switzerland: 2010. [PubMed] [Google Scholar]
- 4.Bhatt N.B., Baudin E., Meggi B., da Silva C., Barrail-Tran A., Furlan V., Grinsztejn B., Bonnet M., Taburet A.M., Jani I.V., et al. Nevirapine or efavirenz for tuberculosis and HIV coinfected patients: Exposure and virological failure relationship. J. Antimicrob. Chemother. 2014;70:225–232. doi: 10.1093/jac/dku348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodney P., Ndjakani Y., Ceesay F.K., Wilson N.O. Addressing the Impact of HIV/AIDS on Women and Children in Sub-Saharan Africa: PEPFAR, the U.S. Strategy. Afr. Today. 2011;57:64–76. doi: 10.2979/AFT.2010.57.1.64. [DOI] [Google Scholar]
- 6.Yimer G., Amogne W., Habtewold A., Makonnen E., Ueda N., Suda A., Worku A., Haefeli W.E., Burhenne J., Aderaye G., et al. High plasma efavirenz level and CYP2B6 *6 are associated with efavirenz-based HAART-induced liver injury in the treatment of naïve HIV patients from Ethiopia: A prospective cohort study. Pharmacogenom. J. 2012;12:499–506. doi: 10.1038/tpj.2011.34. [DOI] [PubMed] [Google Scholar]
- 7.Ward B.A., Gorski J.C., Jones D.R., Hall S.D., Flockhart D.A., Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: Implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J. Pharmacol. Exp. Ther. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- 8.Haas D.W., Gebretsadik T., Mayo G., Menon U.N., Acosta E.P., Shintani A., Floyd M., Stein C.M., Wilkinson G.R. Associations between CYP2B6 polymorphisms and pharmacokinetics after a single dose of nevirapine or efavirenz in African americans. J. Infect. Dis. 2009;199:872–880. doi: 10.1086/597125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuchiya K., Gatanaga H., Tachikawa N., Teruya K., Kikuchi Y., Yoshino M., Kuwahara T., Shirasaka T., Kimura S., Oka S. Homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz-containing regimens. Biochem. Biophys. Res. Commun. 2004;319:1322–1326. doi: 10.1016/j.bbrc.2004.05.116. [DOI] [PubMed] [Google Scholar]
- 10.Mugusi S., Ngaimisi E., Janabi M., Minzi O., Bakari M., Riedel K.D., Burhenne J., Lindquist L., Mugusi F., Sandstrom E., et al. Liver enzyme abnormalities and associated risk factors in HIV patients on efavirenz-based HAART with or without tuberculosis co-infection in Tanzania. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomford N.E., Dzobo K., Chopera D., Wonkam A., Skelton M., Blackhurst D., Chirikure S., Dandara C. Pharmacogenomics Implications of Using Herbal Medicinal Plants on African Populations in Health Transition. Pharmaceuticals. 2015;8:637–663. doi: 10.3390/ph8030637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H.G., Oh M.S. Herbal medicines for the prevention and treatment of Alzheimer’s disease. Curr. Pharm. Des. 2012;18:57–75. [PubMed] [Google Scholar]
- 13.Chien C.F., Wu Y.T., Tsai T.H. Biological analysis of herbal medicines used for the treatment of liver diseases. Biomed. Chromatogr. 2011;25:21–38. doi: 10.1002/bmc.1568. [DOI] [PubMed] [Google Scholar]
- 14.Dhiman R.K., Chawla Y.K. Herbal medicines for liver diseases. Dig. Dis. Sci. 2005;50:1807–1812. doi: 10.1007/s10620-005-2942-9. [DOI] [PubMed] [Google Scholar]
- 15.Dahanukar S.A., Kulkarni R.A., Rege N.N. Pharmacology of medicinal plants and natural products. Indian J. Pharmacol. 2000;32:81–118. [Google Scholar]
- 16.Efferth T., Koch E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr. Drug Targets. 2011;12:122–132. doi: 10.2174/138945011793591626. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy D., Wightman E. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. Int. Rev. J. 2011;2:32–50. doi: 10.3945/an.110.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koffuor G.A., Woode E., Amaning-Danquah C. Potential Drug Interactions of a Polyherbal Antihypertensive Mixture in Ghana. Pharmacol. Sin. 2011;2:39–45. [Google Scholar]
- 19.Chen X.W., Serag E.S., Sneed K.B., Liang J., Chew H., Pan S.-Y., Zhou S.-F. Clinical herbal interactions with conventional drugs: From molecules to maladies. Curr. Med. Chem. 2011;18:4836–4850. doi: 10.2174/092986711797535317. [DOI] [PubMed] [Google Scholar]
- 20.He S.M., Chan E., Zhou S.F. ADME properties of herbal medicines in humans: Evidence, challenges and strategies. Curr. Pharm. Des. 2011;17:357–407. doi: 10.2174/138161211795164194. [DOI] [PubMed] [Google Scholar]
- 21.Ingelman-Sundberg M. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: The past, present and future. Trends Pharmacol. Sci. 2004;25:193–200. doi: 10.1016/j.tips.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Turpeinen M., Zanger U.M. Cytochrome P450 2B6: Function, genetics, and clinical relevance. Drug Metab. Drug Interact. 2012;27:185–197. doi: 10.1515/dmdi-2012-0027. [DOI] [PubMed] [Google Scholar]
- 23.Mo S.L., Liu Y.H., Duan W., Wei M.Q., Kanwar J.R., Zhou S.F. Substrate specificity, regulation, and polymorphism of human cytochrome P450 2B6. Curr. Drug Metab. 2009;10:730–753. doi: 10.2174/138920009789895534. [DOI] [PubMed] [Google Scholar]
- 24.Wang H., Tompkins L.M. CYP2B6: New insights into a historically overlooked cytochrome P450 isozyme. Curr. Drug Metab. 2008;9:598–610. doi: 10.2174/138920008785821710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turpeinen M., Raunio H., Pelkonen O. The functional role of CYP2B6 in human drug metabolism: Substrates and inhibitors in vitro, in vivo and in silico. Curr. Drug Metab. 2006;7:705–714. doi: 10.2174/138920006778520633. [DOI] [PubMed] [Google Scholar]
- 26.Zanger U.M., Klein K. Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): Advances on polymorphisms, mechanisms, and clinical relevance. Front. Genet. 2013;4:24. doi: 10.3389/fgene.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGinnity D.F., Tucker J., Trigg S., Riley R.J. Prediction of CYP2C9-mediated drug-drug interactions: A comparison using data from recombinant enzymes and human hepatocytes. Drug Metab. Dispos. 2005;33:1700–1707. doi: 10.1124/dmd.105.005884. [DOI] [PubMed] [Google Scholar]
- 28.Abourashed E. Bioavailability of Plant-Derived Antioxidants. Antioxidants. 2013;2:309–325. doi: 10.3390/antiox2040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stout C.W., Weinstock J., Homoud M.K., Wang P.J.N., Estes A.M., Link M.S. Herbal medicine: Beneficial effects, side effects, and promising new research in the treatment of arrhythmias. Curr. Cardiol. Rep. 2003;5:395–401. doi: 10.1007/s11886-003-0097-x. [DOI] [PubMed] [Google Scholar]
- 30.Djuv A., Nilsen O.G., Steinsbekk A. The co-use of conventional drugs and herbs among patients in Norwegian general practice: A cross-sectional study. BMC Complement. Altern. Med. 2013;13 doi: 10.1186/1472-6882-13-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fugh-berman A. Herb-drug interactions. Lancet. 2000;355:134–138. doi: 10.1016/S0140-6736(99)06457-0. [DOI] [PubMed] [Google Scholar]
- 32.Chen X.W., Sneed K.B., Pan S.Y., Cao C., Kanwar R.J., Chew H., Zhou S.F. Herb-drug interactions and mechanistic and clinical considerations. Curr. Drug Metab. 2012;13:640–651. doi: 10.2174/1389200211209050640. [DOI] [PubMed] [Google Scholar]
- 33.Huang S.M., Strong J.M., Zhang L., Reynolds K.S., Nallani S., Temple R., Abraham S., Al Habet S., Baweja R.K., Burckart G.J., et al. New era in drug interaction evaluation: US Food and Drug Administration update on CYP enzymes, transporters, and the guidance process. J. Clin. Pharmacol. 2008;48:662–670. doi: 10.1177/0091270007312153. [DOI] [PubMed] [Google Scholar]
- 34.Bjornsson T.D., Callaghan J.T., Einolf H.J., Fischer V., Gan L., Grimm S., Kao J., King S.P., Miwa G., Ni L., et al. The conduct of in vitro and in vivo drug-drug interaction studies: A PhRMA perspective. J. Clin. Pharmacol. 2003;43:443–469. [PubMed] [Google Scholar]
- 35.Zhang L., Zhang Y.D., Zhao P., Huang S.M. Predicting drug-drug interactions: An FDA perspective. AAPS J. 2009;11:300–306. doi: 10.1208/s12248-009-9106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.European Medicines Agency . Guideline on the Investigation of Drug Interactions. Committee for Human Medicinal Products (CHMP); London, UK: 2015. pp. 1–59. CPMP/EWP/560/560/95/Rev. [Google Scholar]
- 37.Manda V.K., Dale O.R., Awortwe C., Ali Z., Khan I., Walker L., Khan S.I. Evaluation of drug interaction potential of Labisia pumila (Kacip Fatimah) and its constituents. Front. Pharmacol. 2014;5 doi: 10.3389/fphar.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obach R.S., Walsky R.L., Venkatakrishnan K. Mechanism-based inactivation of human cytochrome p450 enzymes and the prediction of drug-drug interactions. Drug Metab. Dispos. 2007;35:246–255. doi: 10.1124/dmd.106.012633. [DOI] [PubMed] [Google Scholar]
- 39.Berry L.M., Zhao Z. An examination of IC50 and IC50-shift experiments in assessing time-dependent inhibition of CYP3A4, CYP2D6 and CYP2C9 in human liver microsomes. Drug Metab. Lett. 2008;2:51–59. doi: 10.2174/187231208783478407. [DOI] [PubMed] [Google Scholar]
- 40.Li P., Lu C., Balani S.K., Gan L.-S. A refined cytochrome P540 IC50 shift assay for reliably identifying CYP3A time-dependent inhibitors. Drug Metab. Dispos. 2011;39:1054–1057. doi: 10.1124/dmd.111.038208. [DOI] [PubMed] [Google Scholar]
- 41.Grimm S.W., Einolf H.J., Hall S.D., He K., Lim H.K., Ling K.H.J., Lu C., Nomeir A., Seibert E., Skordos K.W., et al. The conduct of in vitro studies to address time-dependent inhibition of drug-metabolizing enzymes: A perspective of the Pharmaceutical Research and Manufacturers of America. Drug Metab. Dispos. 2009;37:1355–1370. doi: 10.1124/dmd.109.026716. [DOI] [PubMed] [Google Scholar]
- 42.Adinortey M.B., Sarfo J.K., Quayson E.T., Weremfo A., Adinortey C.A. Phytochemical Screening, Proximate and Mineral Composition of Launaea taraxacifolia Leaves. Res. J. Med. Plant. 2012;6:171–179. doi: 10.3923/rjmp.2012.171.179. [DOI] [Google Scholar]
- 43.Ocama P., Katwere M., Piloya T., Feld J., Opio K.C., Kambugu A., Katabira E., Thomas D., Colebunders R., Ronald A. The spectrum of liver diseases in HIV infected individuals at an HIV treatment clinic in Kampala, Uganda. Afr. Health Sci. 2008;8:8–12. [PMC free article] [PubMed] [Google Scholar]
- 44.Price J.C., Thio C.L. Liver disease in the HIV-infected individual. Clin. Gastroenterol. Hepatol. 2010;8:1002–1012. doi: 10.1016/j.cgh.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Husain N.E.O., Ahmed M.H. Managing dyslipidemia in HIV/AIDS patients: Challenges and solutions. HIV/AIDS. 2015;7:1–10. doi: 10.2147/HIV.S46028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo J. Dyslipidemia and lipid management in HIV-infected patients. Curr. Opin. Endocrinol. Diabetes Obes. 2011;18:144–147. doi: 10.1097/MED.0b013e328344556e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feeney E.R., Mallon P.W.G. HIV and HAART-Associated Dyslipidemia. Open Cardiovasc. Med. J. 2011;5:49–63. doi: 10.2174/1874192401105010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Da Neto S.P.L.F., das Neves M.B., Ribeiro-Rodrigues R., Page K., Miranda A.E. Dyslipidemia and fasting glucose impairment among HIV patients three years after the first antiretroviral regimen in a Brazilian AIDS outpatient clinic. Braz. J. Infect. Dis. 2013;17:438–443. doi: 10.1016/j.bjid.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Estrada V., Portilla J. Dyslipidemia related to antiretroviral therapy. AIDS Rev. 2011;13:49–56. [PubMed] [Google Scholar]
- 50.Rastogi H., Jana S. Evaluation of Inhibitory Effects of Caffeic acid and Quercetin on Human Liver Cytochrome P450 Activities. Phytother. Res. 2014;28:1873–1878. doi: 10.1002/ptr.5220. [DOI] [PubMed] [Google Scholar]
- 51.Baer-Dubowska W., Szaefer H., Krajka-Kuzniak V. Inhibition of murine hepatic cytochrome P450 activities by natural and synthetic phenolic compounds. Xenobiotica. 1998;28:735–743. doi: 10.1080/004982598239155. [DOI] [PubMed] [Google Scholar]
- 52.Huynh H.T., Teel R.W. Effects of plant-derived phenols on rat liver cytochrome P450 2B1 activity. Anticancer Res. 2002;22:1699–1703. [PubMed] [Google Scholar]
- 53.Nagao T., Komine Y., Soga S., Meguro S., Hase T., Tanaka Y., Tokimitsu I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005;81:122–129. doi: 10.1093/ajcn/81.1.122. [DOI] [PubMed] [Google Scholar]
- 54.Kannan R.R.R., Arumugam R., Thangaradjou T., Anantharaman P. Phytochemical constituents, antioxidant properties and p-coumaric acid analysis in some seagrasses. Food Res. Int. 2013;54:1229–1236. doi: 10.1016/j.foodres.2013.01.027. [DOI] [Google Scholar]
- 55.Farag M.A., Wessjohann L.A. Metabolome classification of commercial Hypericum perforatum (St. John’s Wort) preparations via UPLC-qTOF-MS and chemometrics. Planta Med. 2012;78:488–496. doi: 10.1055/s-0031-1298170. [DOI] [PubMed] [Google Scholar]
- 56.Berry L.M., Zhao Z., Lin M.J. Dynamic Modeling of Cytochrome P450 Inhibition in vitro: Impact of Inhibitor Depletion on IC50 Shifts. Drug Metab. Dispos. 2013;41:1433–1441. doi: 10.1124/dmd.113.051508. [DOI] [PubMed] [Google Scholar]
- 57.Awortwe C., Bouic P.J., Masimirembwa C.M., Rosenkranz B. Inhibition of Major Drug Metabolizing CYPs by Common Herbal Medicines used by HIV/AIDS Patients in Africa—Implications for Herb-Drug Interactions. Drug Metab. Lett. 2013;7:83–95. doi: 10.2174/1872312808666140129123210. [DOI] [PMC free article] [PubMed] [Google Scholar]