Abstract
Anthocyanins (ACNs) are plant secondary metabolites from the flavonoid family. Red to blue fruits are major dietary sources of ACNs (up to 1 g/100 g FW), being cyanidin-3-O-glucoside (Cy3G) one of the most widely distributed. Cy3G confers a red hue to fruits, but its content in raspberries and strawberries is low. It has a good radical scavenging capacity (RSC) against superoxide but not hydroxyl radicals, and its oxidative potential is pH-dependent (58 mV/pH unit). After intake, Cy3G can be metabolized (phases I, II) by oral epithelial cells, absorbed by the gastric epithelium (1%–10%) and it is gut-transformed (phase II & microbial metabolism), reaching the bloodstream (<1%) and urine (about 0.02%) in low amounts. In humans and Caco-2 cells, Cy3G’s major metabolites are protocatechuic acid and phloroglucinaldehyde which are also subjected to entero-hepatic recycling, although caffeic acid and peonidin-3-glucoside seem to be strictly produced in the large bowel and renal tissues. Solid evidence supports Cy3G’s bioactivity as DNA-RSC, gastro protective, anti-inflammatory, anti-thrombotic chemo-preventive and as an epigenetic factor, exerting protection against Helicobacter pylori infection, age-related diseases, type 2 diabetes, cardiovascular disease, metabolic syndrome and oral cancer. Most relevant mechanisms include RSC, epigenetic action, competitive protein-binding and enzyme inhibition. These and other novel aspects on Cy3G’s physical-chemistry, foodomics, and health effects are discussed.
Keywords: anthocyanin, cyanidin 3-O-glucoside, cyanidin, antioxidant, bioaccessibility, berries, phenolic compounds, foodomics, splanchnic metabolism
1. Introduction
“An apple a day keeps the doctor away” is a premise that alludes to the fact that many non-communicable chronic diseases (NCCD) could be prevented with a sufficient daily intake of bioactive molecules from fruits and vegetables. Soluble and insoluble dietary fiber, antioxidants, functional carbohydrates and polyunsaturated fatty acids, among others, individually or in a concerted action are responsible for many beneficial health effects. Particularly, dietary antioxidants (AOX) such as pro-vitamins and phenolic compounds (PC), including anthocyanins (ACNs; anthos = flower, kianos = blue), consumed daily will alleviate the oxidative stress associated with many molecular events within our bodies [1,2]. A strong body of evidence supports other ACNs’ bioactivities including anti-inflammatory, neuro-protective, anti-microbial, anti-viral, anti-thrombotic and epigenetic actions [3,4,5,6].
However, not all ACNs are equal [7], neither is their specific metabolic fate and bioactivity. Many physical and chemical factors present in natural or prepared plant foods [8,9,10,11] along with several physiological barriers within our body [12,13,14], could restrain their metabolic action. In fact, the nutraceutical potential of ACNs is structure-specific, an aspect associated with their specific physicochemical behavior within foods and biological systems [15]. In this sense, important pieces of the Cyanidin-3-O-glucoside (Cy3G; A.K.A. chrysanthemin, kuromanin) puzzle, the most widely distributed anthocyanin in edible fruits [16,17], have been published in the last 10 years and some of which are discussed in the following paragraphs.
2. Physical Chemistry of Cy3G
A correct understanding of Cy3G’s bioactivity requires knowing its structural features and physicochemical behavior. For example, its binding potential and radical scavenging capacity (RSC) depend on its REDOX behavior while its absorption and metabolic fate within the gastrointestinal (GI) tract relies on the presence of glucose and/or other glycoside moieties. Pure Cy3G is susceptible to degradation by many physicochemical factors including pH, light, oxygen, solvents, temperature and metal ions [11,18,19]. From a technological perspective, this justifies why ACNs-based products are not widely used as pigments [20] since they are unstable during storage [21]. Also, Cy3G is exposed to many factors along the GI tract (pH, ionic strength), which affect its bioaccessibility, bioavailability and further bioactivity [22,23]. In the following section, some of these features are reviewed.
2.1. Chemical Structure
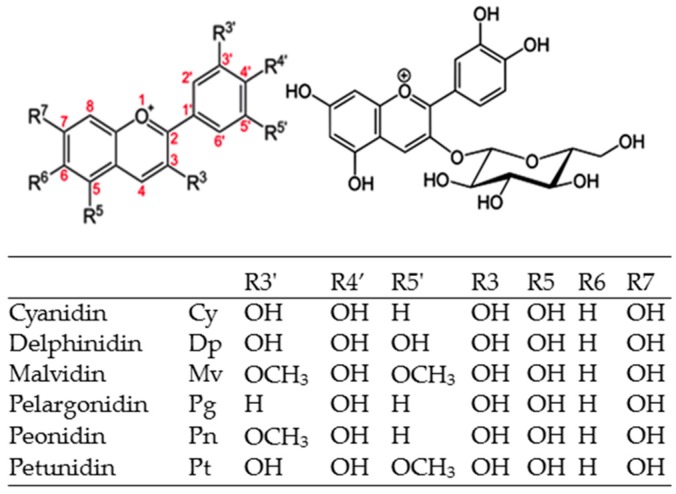
ACNs are anthocyanidin glycosides. Their backbone consists of a benzopyran core [benzoyl ring (A), pyran ring (C)], a phenolic ring (ring B) attached to its (2-position and a sugar moiety mainly at its 3-position in the C-ring (Figure 1). 31 anthocyanidin (aglycones) and more than 600 ACNs have been identified to date [16]. However, 90% of all naturally occurring ACNs are based on six aglycones differing in their B-ring substitution pattern: cyanidin (Cy) about 50%, delphinidin (Dp), pelargodin (Pg) and peonidin (Pn) about 12% each and petunidin (Pt), malvidin (Mv) about 7% each. These aglycons are further classified by the nature and number of bonded sugars and the presence of aliphatic or aromatic carboxylates (attached to their sugar moieties) [10,24], with 3-monosides (mainly glucosides), 3-biosides, 3,5- and 3,7-diglucosides from the 3 non-methylated aglycones (Cy, Dp and Pg) the most common.
Figure 1.
Structure of common anthocyanidins (left) and Cyanidin-3-O-glucoside (Cy3G; right).
From an analytical standpoint, the structural diversity of ACNs represents a challenge for their isolation and identification [10,21]. However, the mass spectral fingerprint of Cy and many of its naturally occurring glycosides have been established so far [14,16,17,25], some included in Table 1.
Table 1.
Mass spectral data and physicochemical characteristics of Cyanidin (Cy) and glycoside-derivates 1.
| Code | Name | [M]+ (m/z) | MS/MS (m/z) | Solubility (mg/mL) | LogP | Å2 |
|---|---|---|---|---|---|---|
| Cy | Cyanidin (anthocyanidin) | 287 | 0.049 | 3.05 | 114.3 | |
| Cy3A | Cy-3-arabinoside (pentose) | 419 | 287 | 0.41 | 1.06 | 173.21 |
| Cy3X | Cy-3-xyloside | 419 | 287 | 0.41 | 1.06 | 173.2 |
| Cy3G | Cy-3-glucoside | 449 | 287 | 0.6 | 0.39 | 193.4 |
| Cy3Ga | Cy-3-galactoside | 449 | 287 | -- | 0.24 | 193.4 |
| Cy3Aga | Cy-3-(6′′-acetyl)-galactoside | 491 | 287 | 0.39 | 0.82 | 199.5 |
| Cy3Sa | Cy-3-sambubioside | 518 | 287 | -- | -- | -- |
| Cy3,3′′MG | Cy-3-(3′′-malonyl)-glucoside | 535 | 287 | 0.47 | 0.68 | 236.8 |
| Cy3,6′′MG | Cy-3-(6′′-malonyl)-glucoside | 535 | 449/287 | 0.45 | 0.68 | 236.8 |
| Cy3Sa | Cy-3-sambubioside | 581 | 287 | 1.17 | -1.1 | 252.4 |
| Cy3dOXG | Cy-3-(dioxaloyl)-glucoside | 593 | 287 | 0.17 | 2.55 | 280.2 |
| Cy3R | Cy-3-rutinoside | 595 | 449/287 | 0.9 | −1.64 | 252.4 |
| Cy3XR | Cy-3-xylosylrutinoside | 727 | 581/287 | 2.52 | −2.1 | 311.3 |
| Cy3GR | Cy-3-glucosylrutinoside | 757 | 287/611 | 3.85 | −2.8 | 331.5 |
| Cy3,5GG | Cy-3,5-diglucoside | 611 | 449/287 | -- | −2.3 | 272.6 |
| Cy3So | Cy-3-sophoroside | 611 | 287 | -- | -- | 260 |
| Cy3Sa5R | Cy-3-sambubioside-5-rhamnoside | 727 | 581/433/287 | -- | -- | -- |
| Cy3So5R | Cy-3-sophoroside-5-rhamnoside | 757 | 611/433/287 | -- | -- | -- |
1 See abbreviations section for non-defined terms.
The pattern of glycosylation and methylation influences Cy’s hydrophobic (octanol)/hydrophilic (water) partition coefficient (LogP), its polar surface area (Å2) and its molecular weight (MW), all having important implications in the metabolic fate of Cy-derivates (ADME: Absorption, Distribution, Metabolism and Excretion) [26]: Cy has a lower MW (287.24 g/mol) and Å2 (114.3) and is less hydrophilic (LogP = 3.05) than Cy3G (449.4 g/mol, Å2 = 191, LogP = 0.39; Table 1). A second glycosylation (Cy-3,5-O-diglucoside, Cy3,5GG) increases its hydrophilic nature but that compromises its absorption capacity [27] while an extra malonyl group (Cy3MG) does the opposite [28]. Other structural features in Cy3G also have important implications on its chemical reactivity in vitro. Fernandes et al. [9] used STD-NMR spectroscopy and molecular dynamics simulations to show that the absence of an extra hydroxyl at R5’ in Cy3G (Figure 1) affects its binding capacity toward citrus pectins when compared to Dp3G. Also, Phan et al. [29] demonstrated that Cy3G (as flavylium cation, pH 3.4) binds spontaneously within 1 min to bacterial (Gluconacetobacter xylinus ATCC 53524)-derived cellulose, steadily increasing up to 2 h. They reported that this binding behavior is not limited by the available interacting sites in cellulose but to the amount of free Cy3G molecules, proposing a Langmuir binding isotherm model (Equation (1)):
| Q = Qmax × [(KL·C) × (1 + KL × C)−1] | (1) |
where Q is the amount of absorbed Cy3G per unit mass of cellulose (µg·mg−1), Qmax is the apparent maximum adsorption capacity (1109 µg·mg−1 of cellulose), KL is the apparent binding affinity constant and C is the free Cy3G concentration at equilibrium (mM).
By applying this equation, a “Cy3G saturation effect” can be observed at about 200 mM. Also, Oliveira and Pintado [30] using an in vitro model to simulate GI conditions demonstrated a “bind-release” behavior between Cy3G and pectin/chitosan at each digestion step (oral, gastric, and intestinal), suggesting a protective mechanism of this polymeric mixture over Gy3G degradation since Cy3G is progressively released from protein and polysaccharide bonds, which are available for its potential absorption by GI epithelial cells.
It is noteworthy that Cy3G also binds to proteins in vitro. In the same experiment reported by Oliveira and Pintado [30], an even stronger binding capacity of Cy3G toward P/C+β-lactoglobulin was observed. Tang et al. [31] reported the differential binding capacity of three ACNs to human serum albumin (HSA; Dp3G> Cy3G> Pg3G) but their capability to induce structural changes in this protein was different (Pg3G> Cy3G> Dp3G). Tang et al. [32], by using multi-spectral techniques and molecular modeling, suggested that Cy3G–protein interactions are established by hydrogen bonding and van der Waals forces and, as a consequence, the secondary structure of bovine serum albumin (BSA), hemoglobin (Hb), and myoglobin (Mb) is partially destroyed (less% α-helixes). These molecular interactions have important implications in Cy3G transport in the bloodstream (HSA) but could also affect the correct functionality of heme-containing proteins (Hb, Mb). Cy3G is commonly represented as a cation (Figure 1), which is only possible under acidic conditions such as in gastric juice, and in silico assays have revealed that cationic Cy3G cannot be absorbed through passive diffusion [33]. However, a simple substitution at R3′ [–H (Pg3G) by –OH (Cy3G)] modifies Cy3G bioaccessibility, absorptivity, and metabolism within enterocytes [34].
Lastly, rare anthocyanidins such as 3-deoxy-anthocyanidins, hydroxylated at the 6th position, 5, 7, 3′, 5′-O-glycosilated, C-glycosylated, or aliphatic (mainly malonic and pyruvic acids)- or PC-acylated ACNs, which are also currently studied [9,10,28,35] because they seem to be more bioactive than conventional counterparts. For instance, Cy-malonyl-glucoside (Cy-Mal-3G) possesses a stronger anti-cancer (colon, liver, prostate, and breast) activity than Cy3G [36]. Also, Cy3G acylated with lauric acid improves its stability because an ester group is more stable than a hydroxyl group [37]. However, the formation of Cy3G adducts with pyruvic acid during wine ageing or fruit juice processing reduces (about 10 times) its radical RSC toward the superoxide anion [38].
2.2. Color
The color of ACNs-rich fruits is a matter of quantity (biosynthesis), molecular inter-play and physicochemical stability. In particular, production and stability of red Cy3G in plants has been extensively studied in horticultural sciences, an aspect that will be further discussed in this article. Many structural features, such as the number of hydroxyl groups, their degree of methylation and the nature and number of sugar moieties bound to the molecule, are related to the color of ACNs. In nature, ACNs show great color diversity from yellow (480 nm) to red (730 nm), and, particularly, Cy3G confers a red hue to fruits [27]. However, the maximum absorption [λmax (εmol) nm] of its flavylium (2-phenyl-1-benzopyrilium) nucleus [10] is more restricted to the six most common aglycones and is related to their B-ring hydroxylation pattern: Cy and Pn (516 nm), Pg (520 nm), and Dp, Pt, Mv (546 nm).
The color stability of ACNs depends on their structure, pH, temperature, light and the presence of complexing agents such as PC and metals [21]. The simple attack by water or sulfites converts the flavylium ion into a colorless pseudobase (nucleophilic addition). However, the color of ACNs also depends on their interactions with other molecules via hydrogen bonds or via “hydrophobic vertical stacking” which is a combination of van der Waals and hydrophobic forces between the planar flavylium and another planar molecule to form a π-π complex. Color enhancement [hyperchromic effect (ΔA) + bathochromic shift (Δλ)] and a stabilization phenomenon called co-pigmentation, results from several inter-molecular associations: (i) between two identical ACNs (self-association); (ii) between one of its aromatic substituents (intra-molecular co-pigmentation) with another non-colored molecule (intermolecular co-pigmentation) or; (iii) with a metal ion, forming π-π and other complexes in solution [10]. For example, Bakowska et al. [39] reported a Δλ = 21.4 nm and ΔA = 0.48 with complexes of Cy3G and flavones isolated from Scutellaria baicalensis Georgi, a Chinese herb used to treat bacterial infections of the respiratory system and GI tract. Pacheco-Palencia [21], when evaluating the influence of the external addition (ratio 1:10 w/w) of rooibos tea (rich in flavone-C-glycosides) to commercial rosemary extracts on the stability of two açai-derived ACNs [40% Cy3G (40%) + 60% Cy-3-O-rutinoside (Cy3R)] model solutions (500 mg/L), found an ΔA = +18% with no changes in Δλ. However, co-pigmentation depends on the ortho-dihydroxyl arrangement in the B-ring in such a way that Cy, Dp, and Pt have the ability to form such complexes, but Mv, Pg or Pn do not [40].
2.3. Temperature
Several industrial processes, such as dyeing of fabrics and food product manufacturing, apply high temperatures to raw/purified sources of ACNs [41,42]. For example, Manosur et al. [20] evaluated the effect of dye bath pH and temperature on the color of wool fabrics by an aqueous extract of Vitis vinifera L. leaves rich in ACNs [acetylated ACNs (69.2%) > Dp3G (10.1%) > Pn3G > Mv3G > Cy3G (3.27%)] showing that processing at 45 °C and 95 °C results in red and brown shades of dyed fabrics, respectively. Also, conventional or microwave (300W) heating at 100 (atmospheric), 38.5, and 7.3 kPa used to concentrate pomegranate juice reduces its Cy3G content but its di-glucoside (CyGG) is not much affected. Also, Mildner-Szkudlarz et al. [43] evaluated the effect of replacing raspberry pomace (0%, 10% and 20%) into wheat flour muffins prepared under various baking conditions (140 °C/30 min, 180 °C/20 min, 240 °C/15 min) showing that low temperatures but longer baking time decrease the amount of Cy3G, independently of the initial percentage incorporated. Slavin et al. [44] found same results when baking black and yellow soybean crackers.
A recent review on the mechanisms of degradation during high thermal processing of ACNs-rich foods [11] indicates that several heating operations (e.g., blanching, pasteurization) and their duration can markedly affect the ACNs content in fruits and vegetables, resulting in a variety of chemical species depending upon the severity and nature of heating. Particularly, Cy3G degradation involves de-glycosylation and further cleavage of covalent bonds leading to phloroglucynaldehyde (PGA) and 4-hydroxybenzoic acid under isothermal conditions. However, ACNs’ degradation follows a non-isothermal kinetic behavior in liquid (concentrated juices) solid (fruits) and semisolid (pomances) food systems. Fortunately, ohmic-, dielectric-, radio frequency- or, microwave-heating, are promising non-thermal technologies that may reduce ACNs loses from prepared foods [45]. The temperature of storage also affects Cy3G stability. Pacheco-Palencia and Talcott [21] evaluated this effect at 5, 20 and 35 °C in Cy3G (40%) /Cy3R (60%) based solutions (500 mg/L), showing that temperature significantly affects Cy3G stability (Equation (2)) and despite there is an important hyperchromic effect (ΔA) due to the addition of phenolic acids, flavone-C-glycosides or procyanidins, they do not protect Cy3G degradation from 20 to 35 °C.
| Half-Life (days) = 12.6e−0.1034(T°C) | (2) |
2.4. pH
At room temperature and minimally acidic conditions, Cy3G exist in four species in equilibrium [22,46]. At more acidic conditions (pH ≤ 4.0), Cy3G exist primarily in the form of flavylium cation (red); as pH increase increases it is transformed to either its carbinol (hemiketal) form (colorless, pH 5.2) as a result of hydration of the flavylium cation or to its quinoidal form (blue color, pH 5.5–6.0) by means of a proton loss. Finally, these species reach an equilibrium through tautomerization forming an open cis-chalcone (light yellow, pH >6.0). In vitro, each form has a different reactivity and antioxidant capacity, since more hydroxyl groups (e.g., hemiketal > quinoidal) means more antioxidant activity [47].
Sui et al. [8] evaluated the stability of Cy3G and Cy3R from black rice in an aqueous system within a pH range from 2.2 to 6.0 and a temperature range from 100 °C to 165 °C. Cy3G was more susceptible to pH or temperature than Cy3R but pH played an important role in stabilizing both molecules under thermal treatment. However, Pacheco-Palencia and Talcott [21] did not find such stabilizing effect at storing temperatures (5, 20 and 35 °C) and nearby its flavylium cation form (pH = 3.0, 3.5, 4.0). Nevertheless, processing wool fabrics with extracts from Vitis Vinifera L. leaves at a high temperature but under acidic conditions improves their color strength [20]. The most profound effect of pH is surely the loss of its RSC, which is discussed below.
2.5. Antioxidant Capacity
An important biological effect of ACNs is their antioxidant capacity, which could be affected by many of the aforementioned factors. This property has been related to the prevention of inflammatory conditions, cardiovascular disease (CVD) and cancer [10]. The antioxidant protection mechanism includes: A) quenching singlet oxygen (1O2), B) scavenging reactive oxygen species (ROS), C) chelation of trace metals involved in free radical production or, D) inhibition of ROS-promoting enzymes [48]. When compared to other flavonoids, ACNs are very efficient quenchers of singlet oxygen: one molecule of ACNs is degraded (by its flavylium cation) by every 125 molecules of 1O2 quenched; the biological relevance relies on the fact that ACNs can be available for many consecutive processes [49]. However, the structural modifications (e.g., type of glycosylation) of ACNs play an important role on the observed antioxidant activity. For instance, the oxidation potential for Cy3G (500 mV vs. Ag/AgCl) is shifted towards a more positive value when compared to its aglycone (Cy) alone (403 mV vs. Ag/AgCl). The more positive oxidation value the less the antioxidant power [50]. The effect on the shift of the oxidation potential has been ascribed to the “steric hindrance” of the glucose moiety in C-ring, which reduces B-ring coplanarity with the rest of the molecule, decreasing the conjugation [51,52].
In general, ACNs have a strong RSC and their electrochemical oxidation shows more than one oxidation process. Three oxidation processes have been reported for Cy3G (Table 2): Electron oxidations in the catechol group (B-ring), the resorcinol group (A-ring) and during the opening of C-ring of Cy3G hemiketal form [47,53]. When a deglucosylation occurs in Cy3GG an oxidation peak potential corresponding to A-ring oxidation occurs at higher values [47].
Table 2.
Voltammetric oxidation of and Cy3G and its aglycone 1.
| Molecule | pH | Epa/mV | Technique | Electrodes | Ref. |
|---|---|---|---|---|---|
| Cy3G | 3.5 | 490, 980 | DPV | WE: Glassy carbon RE: Ag|AgCl |
[47] |
| 4.5 | 420, 815 | ||||
| 7.0 | 310, 500 | ||||
| Cy3G | 2.2 | 548 | CV | WE: Glassy carbon RE: Ag|AgCl |
[52] |
| 4.8 | 400 | ||||
| 5.9 | 310 | ||||
| 6.9 | 230 | ||||
| Cy3G | 2.0 | 500 | ASSWV | WE: Paraffin rod impregnated RE: Ag|AgCl |
[52] |
| Cy | 403 | ||||
| Cy3G | 1.0 | 617 | CV | WE: Platinum RE: SCE |
[53] |
1 See abbreviations section for the meaning of each term.
It is noteworthy that Cy3G oxidative potential is pH-dependent in such way that as pH increases potentials shift to lower values (approximately 58 mV per pH unit [53]. However, Cy3G is easily oxidized at higher pH values due de-protonation of the hydroxyl groups [47,52]. Also, Garcia-Alonso et al. [38] reported that the RSC toward O2− of several ACNs was Mv3G (about 13.3 μM) >Dp3G >Pt3G >Pg3G >Cy3G (33.3 μM) but for •OH was Pg3G>Dp3G >Pt3G >Cy3G >Mv3G at a higher concentration (400–1000 μM); this indicates that the amount of OH− and nature of substitution in B-ring, are important determinants of the RSC of ACNs. As an example, the absence of catechol group seems to affect its activity [54] while the presence of hydroxyl groups at the 3 and 5 position seems to increase its activity [55]. From a physiological stand point, it is generally accepted that flavonoids (and particularly ACNs) have low plasmatic antioxidant activity, this because of that as per aforementioned and its fast metabolism. The maximum plasmatic antioxidant value seems to be reached quickly (between 15 and 30 min) [56] before consumption with important losses (almost all) of its activity in the first plasmatic hour. Pro-oxidant activities have been reported for C3G [54] between 0 and 400 µM (nonexclusive event for ACNs), however, it still remains unclear. However, this event seems to be related to the bioaccessibility, bioavailability and absorption capacity of ACNs within/from the GI milieu, their stability in food products and their bioactivity [57].
3. Cy3G in Plant Biochemistry
Once the many structural and physicochemical limitations of Cy3G in vitro are known, the factors associated to its sufficient intake (convenient plant sources) and in vivo metabolism (consumer’s GI tract) should be studied. Together, these factors are responsible for the ultimate fate and bioactivity of Cy3G in target tissues. First, it is essential to examine its biosynthesis in edible plants which in turn justify their richness in Cy3G. Second, the conflicting results from a plethora of epidemiological and case-control studies involving Cy3G-rich foods or supplements and its health effects could be explained in the context of Cy3G splanchnic metabolism.
3.1. Biosynthesis
ACNs are produced and accumulated in different plant organs in response to several environmental (e.g., stress), genetic (e.g., senescence) and developmental (e.g., ripening) factors [10,37,58]. For example, biosynthesis of ACNs protects certain rice cultivars from drought stress by exerting many antioxidant mechanisms [59]. ACNs are also related to other activities within plants such as visual signals and as antimicrobial agents [57,60]. A comprehensive review on the multiple functional roles of ACNs in plant–environment interactions has been recently published by Landi et al. [61].
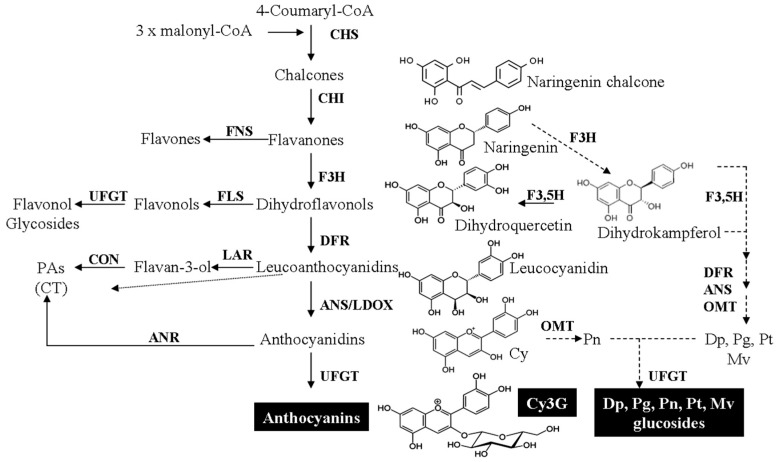
Cy3G is produced through the flavan-3-ol pathway (Figure 2).
Figure 2.
Anthocyanin biosynthesis. Cy3G main steps and crossroads. See abbreviations section for the meaning of each term.
Initially, one molecule of 4 (p) -coumaryl-CoA (coming from phenylalanine) is condensed with three molecules of malonyl-CoA (coming from Acetyl-CoA) to get one naringenin chalcone (tetrahydroxychalcone), a reaction catalyzed by chalcone synthase (CHS). The accumulation of this intermediary in plant tissues is quite rare and so it is further transformed into 2S-naringenin (flavanone) by chalcone isomerase (CHI). In the 1st crossroad in ACNs synthesis, 2S-naringenin can be either directed to flavone synthesis [by flavone synthase (FNS)] or hydroxylated by flavanone-3-hidroxylase (F3H) to produce dihydrokampferol (dihydroflavonol) and this molecule can be either directed to flavonol synthesis (2nd crossroad) by flavonol synthase (FLS) or ACNs’ synthesis by transforming it to a leucocyanidin Dihydroflavonol-4-reductase (DFR) and further by leucoanthocyanidin reductase (LAR) for falvan-3-ol/proanthocyanidin synthesis (3rd cross road).
Dihydrokampferol can be converted into three different intermediary molecules leading to all six common anthocyanidins [62]: (A) to dihydroquercetin catalyzed by flavanone 3′-hydroxylase (F3′H) which further gives Cy and Pn; (B) to dihydromyricetin catalyzed by flavanone 3′,5′-hydroxylase (F3′,5′H) which further gives Dp, Pt and Mv and; (C) to leucopelargodin no additional hydroxylations on the B ring will eventually lead to Pg. These events involve the following enzymes upon request: Anthocyanidin synthase (ANS) also known as leucoanthocyanidin dioxygenase (LDOX), O-methyltransferase (OMT). Finally, all proanthocyanidins (aglycones) are glycosylated by UDP-glucose-flavonoid-3-O-glucosyltransferase (UFGT). It is noteworthy that, ANS catalyzed reaction confers a formal positive charge to the pyran ring, while UFGT is specific to the substitution position [10]. Cy3G can further undergo other structural transformations (e.g., acylation/glycosylation) increasing the diversity of Cy3G derivatives [7,63].
ACNs’ profiles and consequently Cy3G’s natural occurrence depend on the expression of genes involved in their biosynthetic pathway and are characteristic of a particular plant family/species and a specific plant part [10]. According to Zhao et al. [37], two set of genes are needed for ACNs biosynthesis: Structural genes (pathway enzymes) and those encoding their transcription factors (regulatory proteins: MYB, basic Helix-Loop-Helix and WD40). Variations in color intensity can be attributed to different expression patterns. For example, the tight control of MYB over UFGT gene is responsible for the absence of ACNs in white grapes [64], Malay apples [65] and yellow pears [66], and pelargodin-ANS (PgANS) seems to be the limiting enzyme in white pomegranate [37] while mutations in a gene encoding anthocyanidin-3-glycoside rhamnosyl-transferase (3RT) results in black berry cultivars with a lower than normal ACNs content [63]. The concerted action of regulatory and pathway genes also responds to stressors and the ripening process. Kovinich et al. [7] reported that Arabidopsis thaliana preferentially synthesize different Cy3G-derivates characterized by multiple glycosylations/PC acylations in response to distinct stresses during growing (High MgSO4, no phosphate, pH 3.3 or high sucrose), suggesting that each Cy3G derivate imparts a function favorable in a particular stress condition. On the other hand, Song et al. [66] reported that ANS, CHI, F3H, DFR, UFGT and other proteins such as cytochrome c and cytochrome c oxidase subunit 2 all significantly increases during ripening of strawberry fruits (“Honeoye” and “Mira”).
Lastly, novel breeding and biotechnological strategies applied to ACNs-rich fruit cultivars may enhance their nutritional value and nutraceutical potential [67]. Two research lines are currently carried on: (A) new cultivars with improved levels of functional phytochemicals and (e.g., high-throughput technologies for plant genotyping to select improved berry cultivars); and (B) nutrigenomic actions in humans and animal models (e.g., the effect of eating ACNs-overexpressing berries on the expression of cytoprotective genes such as Nrf2. The health effects of these richest ACNs sources will constitute the next generation of scientific studies.
3.2. Dietary Sources
Due to the limited occurrence of anthocyanidins (aglycons) and glycosylation/acylation patterns in edible plants, it is not surprising a more restricted ACNs distribution within fruits and vegetables for human consumption. In this sense, Cy3G is one of the most common [68] but rarely the major CAN; exceptions to this rule are black elderberry, blue hybrid maize and Korean black raspberry. The daily intake and further bioactivity of Cy3G depends largely on the proper selection of their plant sources, as concluded from the previous section. For instance, a higher consumption of conventional (e.g., pomegranate, blackberry) or exotic (e.g., bilberry, elderberry, mulberry) red-to-blue fruits should be recommended. However, ACNs’ (particularly Cy3G) food composition tables are still rather limited [69]. Table 3 summarizes the richness in Cy3G of some edible sources reported in the Phenol Explorer 3.6 database and from other sources of information [36,43,70] discussed in this article. Despite the fact that Cy3G is widely distributed in fruits (mainly in berries and other blue and red fruits and vegetables), it is not necessarily the main ACNs. For instance, strawberry has 15 times more Pg3G and raspberry (fresh/pomace) 1.4–1.5 times more Cy-3-O-sophoriside (Cy3So) than Cy3G.
Table 3.
Cyanidin-3-O-glucoside (Cy3G) content in selected edible sources 1.
| Group | Fruit/Vegetable | Cy3G 2 | Major ACNs 3 |
|---|---|---|---|
| Fruits/Berries | Black elderberry | 794.13 | Cy3G |
| Blackberry raw | 138.72 | Cy3G | |
| Black Aestivalis grape | 18.72 | Cy3G | |
| Gooseberry | 2.95 | Cy3G | |
| Nectarine peeled | 0.56 | Cy3G | |
| Peach peeled | 0.28 | Cy3G | |
| Blackcurrant raw | 25.07 | Dp3R (304.91) | |
| Black chokeberry | 19.64 | Cy3A (252.76) | |
| Blueberry | 14.2 | Dp3G (22.6) | |
| Sweet cherry raw | 18.73 | Cy3R (143.27) | |
| Red raspberry | 14.89 | Cy3So (37.61) | |
| Raspberry pomace (dry) | 41.52 | Cy3So (100.1) | |
| Plum fresh | 8.63 | Cy3R (33.85) | |
| Lowbush blueberry | 7.5 | Mv3G (26.06) | |
| Redcurrant | 3.37 | Cy3XR (11.22) | |
| Strawberry | 2.88 | Pg3G (47.1) | |
| Lingonberry | 1.42 | Cy3Ga (48.69) | |
| Highbush blueberry | 1.37 | Dp3Ga (20.50) | |
| Sour cherry | 1.12 | Cy3GR (43.63) | |
| Black grape | 1.08 | Mv3G (39.23) | |
| American Cranberry | 0.74 | Pn3Ga (22.02) | |
| Cloudberry | 0.62 | Cy3R (1.86) | |
| Juices/wine | Pomegranate pure juice | 3.43 | Cy3G |
| Blood orange pure juice | 1.41 | Cy3MG (1.76) | |
| Red wine | 0.21 | Mv3G (9.97) | |
| Cereals/legumes | Black bean raw | 3.99 | Dp3G (20.50) |
| Blue maize hybrid | 2.25 | Cy3G | |
| Vegetables | Black Olive raw | 10.62 | Cy3R (72.35) |
| Red lettuce raw | 0.62 | Cy3MG (2.91) |
1 See Table 1 and abbreviations section for the meaning of term 2 Average content (mg per 100 mL or 100 g FW), 3 (average content).
The specific Cy3G profile in any plant food could be affected by several factors including the varietal and ripening stage. For example, Niño-Medina et al. [71] evaluated the ACNs profile of different eggplant (Solanum melongena L.) cultivars grown in a same location, showing that a Philippine varietal had the highest ACNs content (161.10 mg Cy3G equivalents/100 g FW) and the higher RSC (92.5% inhibition of DPPH radical) as compared to other Chinese, American, Hindu and Thai varieties. Song et al. [66] reported important changes in Cy3G (0.008/0.015–0.69/0.48 mg/100 g FW) and Pg3G (0.23/0.15–47.07/31.39 mg/100 g FW) content in strawberry fruit (‘Honeoye’ and ‘Mira’) at different ripening stages (white-to-red). Also, major ACNs in red pomegranate (skin and aryls) are Cy3G, Pg3G and Cy3GG, all of them affected by the ripening process but white pomegranate do not have detectable levels of any ACN [37].
Dietary surveys with detailed information on total and specific intake of ACNs are also scarce. From the very few national surveys, a conclusion can be drawn: the daily intake of ACNs and particularly Cy3G do not depend on the richness of their sources. Per capita daily intake (mean) of ACNs was estimated to be 12.5 mg/day in US adults in 2000–2002 [16], 80% coming from blueberry, grape, onion, grape 100% juices, raspberry, red cabbage, wine and cherry sweet [17,72]; in 2007–2008, this intake was 11.2 mg/day adding banana, red/purple vegetables and yogurt to the list [73]. In Europeans, mean intake of total anthocyanidins is about 20 mg/d, being Cy the most common [68] and in Polish adults participants of the HAPPIEE study, 56% of the daily ACNs intake came from black currant, beans and strawberries [74]. Eastern countries seem to have a higher intake of flavonoids than Americans or Europeans but their ACNs sources appear to be lesser than their isoflavone/proantocyanidin sources. According to KNHANES 2007–2012 [75], the mean daily intake of total flavonoids in Korean adults was 318 mg/d/person, from proanthocyanidins (22.3%), flavonols (20.3%), isoflavones (18.1%), flavan-3-ols (16.2%), anthocyanidins (11.6%), flavanones (11.3%) and flavones (0.3%); major contributing food groups to flavonoid intake were vegetables (20.5%) such as onions (9.6%) and fruits (54.4%) such as apples (21.9%), mandarins (12.5%), grapes (9.0%) and other fruits (1.4%). It should be remembered that the habitual intake of ACNs and Cy3G may vary widely among populations, regions, and seasons and among individuals with different education, financial status and the lack of adequate dietary assessment instruments (e.g., 24FR vs. FFQ) or incompleteness of ACNs food composition tables [76].
In conclusion, dietary choices can have a substantial impact on both the amount of ACNs (and Cy3G) consumed and the associated health effects. In this sense, recent advances in agricultural and food technology have driven the international market of berry fruits at a lower cost. For example, according to the Agri-food and Fisheries Service the production of berries in Mexico has increased almost three-fold in the last years. As a consequence, the intake of ACNs and particularly of Cy3G will steadily increase in the near future.
4. Foodomics
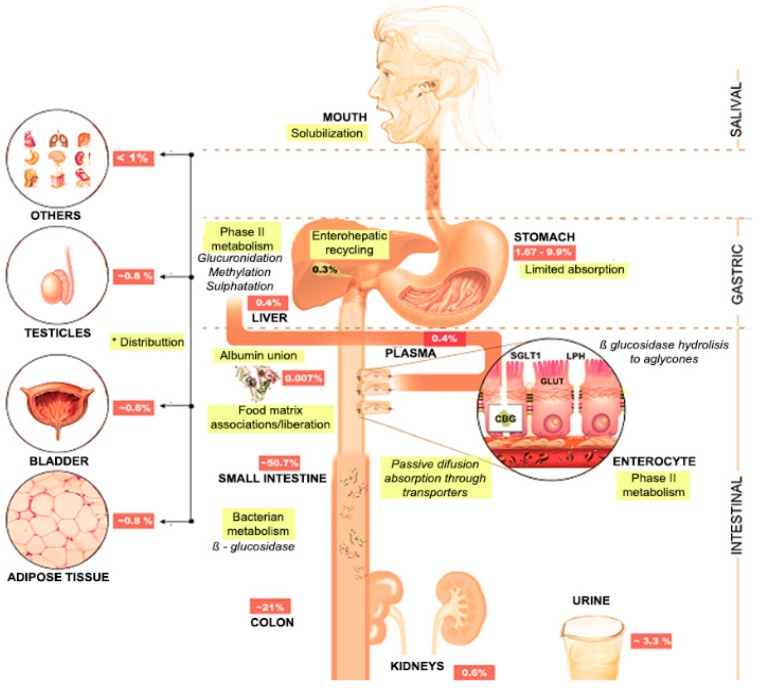
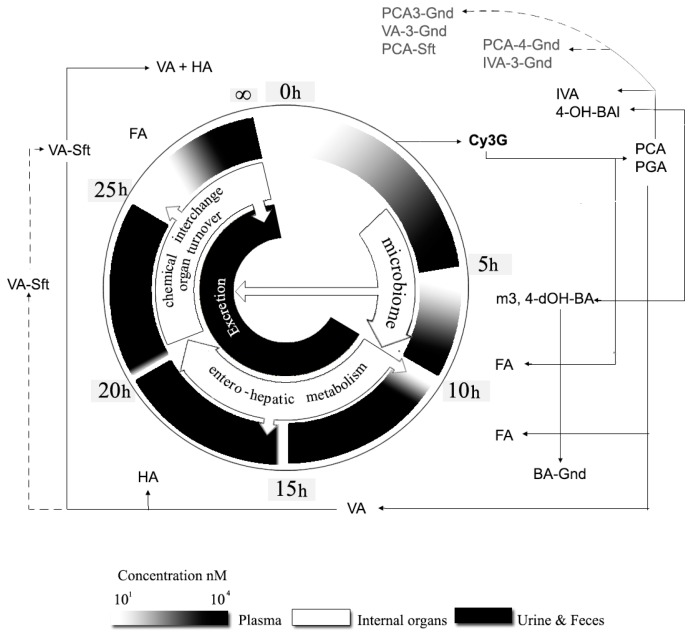
According to Capozzi and Bordoni [77], the study of the food domain as a whole to reach an optimized human health and well-being is referred as to foodomics. Accordingly, after intake, Cy3G within a food matrix must be subject to different bioprocesses [26] in order to exert its functional action within target organs. It must be releasable (bioaccessible) from its food matrix, presented to and absorbed by gut epithelial cells, transported in the bloodstream, bio-transformed in target tissues and finally excreted in urine and feces [78]. During ADME, Cy3G undergoes many transformations that reduce or enhance its bioactivity such as acid and enzymatic modifications, transport across gut epithelium, phase I and II metabolism and delivering mechanisms to name a few [26]. Particularly, the specific enzymatic action on Cy3G along with its enhanced capacity to be absorbed (as compared to its aglycone) have a great influence on its metabolic fate. A step-by-step review on Cy3G’s metabolic physiological fate (Figure 3) and foodomics (Figure 4) is discussed in detail in the following paragraphs.
Figure 3.
Cy3G metabolic fate in humans. Expressed as percentage of original Cy3G intake. See abbreviations section for the meaning of term.
Figure 4.
Time-course bioavailability of Cy3G and its main metabolites. Note: The absorption and biotransformation of cyanidin-3-O-glucoside (Cy3G) into derivates and metabolites are depicted clockwise in this figure and explained in detail within the text. See abbreviations section for the meaning of terms.
4.1. Oral Cavity
ACNs-rich edible sources are firstly subject to oral metabolism. The oral pH (6.8 ± 0.2) favors the quinoidal form of Cy while the glucose moiety improves its solubility (Table 1). Many mechanical and chemical factors in the oral cavity lead to the first release of PC (including Cy3G) by changing the texture of foods and dissolving their components [79]. Oral processing of foods involves mastication, lubrication (saliva), enzymatic hydrolysis and epithelial transportation before the bolus is propelled to the esophagus (swallowing). Food composition, structure, rheology and flavor are important determinants of bolus formation [80] and the proper release of ACNs. For example, the oral perception of dryness (astringency) and roughness (puckering) for positively charged emulsions such as ACNs-based beverages is related to their strong interaction with saliva components such as His/Pro-rich proteins [81] causing their precipitation.
As previously mentioned, Cy3G can bind to human proteins by non-covalent forces mainly in acidic conditions [31,32]. Since a high intake of Cy3G (e.g., from berry juices) results in α-amylase inhibition at neutral conditions [82], it is probably the case that Cy3G undergoes a slow sequence of water addition (hemiketal formation) and subsequent C-ring opening (chalcone formation). Here, the concentration and time of exposure are important regulators of Cy3G’ fate. Kamonpatana et al. [83] reported that almost 50% of Cy3G, Cy3Ga, Cy3A and, Cy3X (see Table 1 for nomenclature) were degraded by oral enzymes in a time-dependent manner during 60 min ex vivo incubation at 37 °C with human saliva suggesting a limited impact of type of sugar moiety.
Nevertheless, Cy3G can be bio-transformed in the oral cavity. Mallery et al. [84], in an experiment involving three sequential mouthwashes with a black raspberry solution (BRB; 10% w/v) rich in Cy3R, Cy3G, Cy3XR and, Cy3Sa (see Table 1 for nomenclature), demonstrated that these molecules are efficiently de-glycosylated by salivary β-glycosidase (microbial derived) and bio transformed to protocatechuic acid (PCA) and Cy-glucoronides, implying an ADME process within the oral cavity; These events are surely related to Cy3G’s preventive action toward smoking-related periodontal diseases [4] or prevention from oral cancer [85]. However, whether these important events are related to the oral transit time or epithelial accumulation of Cy3G at this level needs further investigations.
4.2. Stomach
Gastric conditions represent the second barrier for the bioaccessibility and bioavailability of Cy3G (Figure 3). In simulated gastric conditions, Cy3G (as flavylium cation) seems to be stable to acid pH (1.3 ± 0.2) and pepsin action and can be easily released from complex and pH-denatured food matrices [79,86,87]. Since certain studies involving rats and humans indicate that Cy3G reaches plasma very rapidly (0.25–2 h) after intake (Figure 4) [26,27,88], this has led to the conclusion that Cy3G is efficiently absorbed by the gastric epithelia (1%–10% intact, 10%–20% as first-pass metabolites) by active but not by passive diffusion [33,56]. Many authors have suggested active transport mechanisms in gastric epithelial cells, mainly the bilitranslocase transporter [88,89], NA+-glucose transporter 1 (SGLT1), glucose transporter 1 (GLUT1) and 3 (GLUT3) and mono-carboxylated transporter 1 (MCT1) [86,87]. The molecular mechanism by which Cy3G crosses the gastric epithelia seems to be related to a specific conformation in its B-ring and glucose moiety with the transporter [30,90]. If that is the case, then it seems likely a pH-dependent competition between d-(+)-glucose and Cy3G [56].
In situ gastric perfusion studies have confirmed this absorption phenomenon but also an extensive first-pass metabolism that reduces Cy3G bioavailability [12,13,56]. In particular, Felgines et al. [28] demonstrated that the percentage of absorption of ACNs in red oranges [Cy3G and Cy-3-malonylglucoside (Cy3MG)] after an intra-gastric injection of about 54 nmol (each) was 21% and 18%, respectively. This finding coincides with the hydrophilic nature of each molecule (Table 1). Ex vivo experiments have confirmed that Cy3G is absorbed and metabolized by human gastric cells (AGS and KATO III) but its anti-proliferative activity (AAP) is lower than that of its aglycone [91,92]. Lastly, as stated earlier, in acidic conditions, many intermolecular binding events can occur between Cy3G and dietary and/or gastric components negatively charged [9,29]. However, whether any other phytochemicals exerts a synergistic or an antagonistic effect on Cy3G’s bioavailability at this level remains obscure.
4.3. Small Bowel
The third moment on Cy3G metabolism is within the small bowel. Unlike gastric conditions, the physical and chemical microenvironment in the small intestine reduces Cy3G’s bioavailability by 40–50% (Figure 3). Studies performed in mice [28], humans [14,26] and rats [12,93] confirm this finding. Factors such as pH, Cy3G’s releasability from the food matrix, pancreatic and brush border enzyme action, transportation mechanisms and enterocyte’s phase I/II metabolism, are responsible for the bioavailability of Cy3G, Cy and their metabolites (degradation products or phase II metabolites). At intestinal pH (8.2 ± 0.2) Cy3G returns to its quinoidal form (negatively charged and highly unstable) while its glucose moiety remains neutral. Also, uncertain factors/enzymes perform the further de-glucosylation of Cy3G since lactase-phlorizin hydrolase (LPH; EC 3.2.1.62) or cytosolic β-glucosidases do not seem to participate [12]; however, other authors still consider the action of LPH on Cy3G molecule [94]. Nevertheless, it is noteworthy that the cleavage of its glucose moiety is not a prerequisite for Cy3G chemical breakdown and splanchnic metabolism [93,95] although it is an important mediator of its trans-epithelial transport [27].
Cy3G metabolism at this level largely depends on its ability to release from the food matrix. For example, Kuntz et al. [96] demonstrated in a self-crossover intervention with humans that a grape/blueberry juice (low viscosity) was as good as a smoothie (high viscosity) in terms of ACNs’ bioavailability. Also, Ribnicky et al. [97] used an automated upper GI-simulation system to study the bioaccessibility and ileal recovery of blueberry-ACNs rich extract (500 mg) in the absence (fasting state) and presence of fat (fed state), showing a clear effect of fat over the BA and IE of Cy-glycosides. Also, the inhibitory capacity of Cy3G toward pancreatic enzymes is dose-dependent. For example, Sui et al. [98] performed in vitro and in silico studies to evaluate the inhibitory capacity of several ACNs against porcine pancreatic α-amylase, showing the following trend: Cy3G (Ki = 0.014 mM) > CyR > Cy3, 5GG > Pn3G (Ki = 0.045 mM). Lastly, Akkarachiyasit et al. [99] showed that Cy3Ga and Cy3G are potent inhibitors of sucrase and α-amylase with IC50 values of 0.50 ± 0.05 and 0.30 ± 0.01 mM.
Many aspects of Cy3G’s transport mechanisms have been learned from the use of Caco-2 cells as an absorption model. Most of these studies suggest that, unlike other flavonoids, ACNs could be transported in intact aglycone forms from berries (and their products) except for black currant and some grape varieties [22]. Kuntz et al. [96] evaluated the uptake of ACNs from a grape/blueberry extract (with 63 mg/L of Cy3G) in Caco-2 (ATCCqHTB37e) monolayers showing that Cy3G, although not being the major ACN, has a higher absorption efficiency when compared to Pt3G (103 mg/L) or Dp3G (96.4 mg/L) but this efficiency was better at acidic but not neutral pH. Zou et al. [90] showed that the transport of Cy3G from apical to basolateral is mediated mainly by glucose-transporters (SGLT1 and GLUT2) but a “saturation” effect (up to 40 μM) during Cy3G active transport was observed. Lastly, the presence of other food components has been shown to have a major impact on ACNs transport [22]. In particular, the presence of phospholipids and terpenes enhances Cy3G and Cy3R absorption in Caco-2 cell monolayers [100], contrary to what was found by Ribnicky et al. [97] using TIM-1.
On the other hand, Hassimotto et al. [56] using jejunal everted sacs from rats demonstrated that Cy3G is efficiently transported by SGLT1 in a dose dependent way but Cy is not. However, in the mouse small intestine, Cy3G absorption not only depends on SGLT1 transporter but there might be an exclusive transporting mechanism for flavonoid-like molecules since quercetin-3-glucose seems to inhibit Cy3G absorption [101]. At this point, it should be mentioned that there is not much evidence on the para-cellular transport of Cy since it is more hydrophobic than Cy3G (Table 1). Lastly, luminal Cy3G’s metabolites are efficiently absorbed at intestinal level due the contribution of those derived from entero-hepatic (EHM; Figure 4) or from oral metabolisms [12,13,84].
Once inside the enterocyte, Cy and Cy3G could be either transformed to other PC (particular phenolic acids) and derivatives in phase I metabolism or to several conjugates (methylated, glucuronidated or sulphated) in phase II metabolism. Microbial but not host metabolic machinery is responsible for producing phase I metabolites, although there is a controversy if this event takes place in the small bowel or it is also a result of EHM. In phase II metabolism, many enzymes such as phenyl sulfotransferases (PST), uridine 5′diphosphate glucuronosyltransferases (UGT) and catechol-O-methyltransferase (COMT) activities can modify the Cy (and other anthocyanidins) structure making it more water-soluble and facilitating their further elimination by the kidneys [12,13,28,93].
PCA and PGA have been consistently reported as the main phase I Cy-derived metabolites/degradants [23,27]. Cy and PGA are more hydrophobic molecules than PCA and so, they can passively diffuse through biological membranes, reaching the plasma in the first 2 h (Figure 4). Another reported reaction, although it is not clear where exactly it happens, involves Cy (or Cy3G) methylation to produce Pn (or Pn3G) both having almost the same in vivo bioactivity [9,14]. Recently, Fang [13] proposed the major pathways for Cy3G metabolism in liver microsomes: after deglycosylation, Cy produces PGA from its A-ring, ferulic acid (FA), 3,4-dihydroxyphenyl acetic and 4-hydroxyphenylacetic acids from its B-ring and 3,4-dihydroxybenzaldehyde (PCA immediate precursor) also from its B-ring.
Lastly, PCA undergoes several structural transformations by certain phase I enzymes to produce hippuric (HA), vanillic (VA) or isovanillic (IVA) acids. Cy3G, Cy, PCA, VA and IVA are further metabolized to their specific glucuronide or sulfate conjugates by phase II enzymes. According to Ferrars et al. [14], the tmax for both Cy3G and Cy3-glucoronide is 16 times lower (1.8 h) than that observed for VA-sulphate (30.1 h) while Cmax for PCA-3-O-glucuronide is 177 times lower (11 nM) than for HA (1,962 nM). Lastly, most of these metabolites have been found in rats but they also produce β-resorcylic acid (βRA: 2,4-dihydroxybenzoic acid) [102] and other methylated derivates [93].
4.4. Large Bowel
Cy3G and derived metabolites that surpassed absorption from the small bowel can be finally released from fibrous food matrices (also known as macromolecular antioxidants), transformed by the microbiome [102] and then absorbed by colonocytes. The large bowel contributes to the remaining deglucosylation, phenolic acid production and phase II conjugation events and so less than 0.005% of Cy3G is excreted intact [14]. As occurred in the small bowel, the C-ring rupture and the Cy chalcone formation [103] leads to the apparition of molecules derived from hydroxybenzoic (OH-BA) and phenylacetic acids, VA, IVA, FA and HA (Figure 4) which can be further eliminated in feces and urine (by means of EHM). These metabolic transformations are favored by the slightly basic pH present at this level [79] where Cy3G and Cy are highly unstable. However, certain metabolites such as 2-OH-4-methoxybenzoic acid, 4-methoxybenzaldehyde, methyl-VA and caffeic acid are specifically produced within the large bowel [14].
Hanske et al. [102] evaluated the impact of human intestinal bacteria on the fate of Cy3G in a rat model as compared to germ-free (GF) rats. HMA rats excreted 3× and 2× more phase I (unconjugated: OH-BA derivates) and phase II (conjugated) Cy3G metabolites than GF rats. Also, Pn and 3-OH-cinnamic acid were excreted in urine from HMA but not GF rats. Also, during microbial fermentation of purple sweet potato (a rich source of Cy-mono and di-glycosides, and Cy3G), produces a substantial amount of short-chain- and lactic acids and partially fragmented to phenolic acids. Whether microbial populations are benefited by the dietary fiber, phenolic compounds or both in a synergistic or specific interaction is currently under investigation [103]. Ozdal et al. [104] recently reviewed the state of the art in this area.
4.5. Splanchnic Metabolism
Isotope tracer studies have been very useful to establish the extent to which Cy3G is metabolized by splanchnic organs [94,105]. Czank et al. [26] evaluated the post-prandial kinetics of 13C5-Cy3G (500 mg) after intake. The relative 13C5 bioavailability was 12.4% (5.4% urine, 7.0% breath) and the percentage of recovery in feces, breath and urine was 44%. Cy3G metabolites peaked (Cmax = 6 μM) at 10.3 h but their half-life ranged from about 12.4 to 51.6 h. Despite that 13C elimination was faster by urine (0–1 h, 90.3 μg/h) than for breath (6 h, 132.9 μg/h) or feces (6–24 h, 557.3 μg/h), the highest concentration of 13C was recovered in feces (43.2 μM) and urine (10.8 μM), respectively. The authors concluded that Cy3G (and its metabolites) was as bioavailable as flavan-3-ols and flavones (2.5%–18.5%) but their HPLC-MS/MS method failed to detect other diverse breakdown products and metabolites (56%).
De Ferrars et al. [14], using HPLC-ESI-MS/MS and non-compartmental pharmacokinetic modelling, reported the metabolic fate of Cy3G using the same oral dose and tracer as Czank et al. [26]. Plasma pharmacokinetic parameters for 13C5-Cy3G were: maximal concentration Cmax = 141 ± 70 nM, tmax = 1.8 ± 0.2 h, t1/2 = 0.4 h and, area under the curve (AUC)0–48h = 279 ± 170 mM. For metabolic products, Cmax, tmax and t1/2 ranged from 10 to 2000 nM, 2 to 30 h and 0.5 to 96 h, respectively. Moreover, the abundance of 13C-metabolites was 42 times higher than 13C5-Cy3G at their respective maximum serum concentrations. The authors confirmed the relative bioavailability of Cy3G reported by Czank et al. [26] but also identified 35 important metabolites of which 17, 31 and 28 of them were identified in plasma, urine and feces, respectively [13] which indirectly sustain the aforementioned EHM of Cy3G’s metabolites. Also, typical urinary recoveries of ACNs are <2% of the intake [88] but for Cy3G seems to be much lower (0.024%) [14], although that of its metabolites is not accounting for about 3.0%. In fact, metabolites such as Cy-glucoronides and Pn3G are only found in urine.
Figure 4 attempts to integrate the most recent findings on Cy3G’s foodomics but also postulates new questions for further research. For example, ADME studies (acute consumption) provide enough evidence to ensure that Cy3G has a very short half-life in plasma since it is rapidly metabolized during absorption and EHM [14]. However, regular consumption of ACNs-rich foods could results in a different molecular turnover than that observed after acute consumption. In support of this idea, Khymenets et al. [106] recently reported that a sustained consumption (human volunteers) of a functional beverage based on grape skin extract increased the number of molecular species derived from microbial metabolism of flavan-3-oles, as compared to acute consumption. Also, the metabolic integration of many organs with specific antioxidants needs could result in a different fate for Cy. Lastly, Cy3G’s metabolites can be deposited in hydrophobic (e.g., adipose tissue, liver, testicles) or hydrophilic (e.g., bladder, kidneys) tissues and so long-lasting metabolites such as VA, HA and FA may suffer chemical interchange reactions upon their entry into these tissues. For instance, de Ferrars et al. [14] indicated that urine contains trace amounts of Pn3G which strictly come from the methylation of Cy3G only in renal tissues. Also, more than 100 and 700 organic acids and derivates and aromatic heteropolyciclic compounds have been identified in the human urine metabolome including HA (HMDB00714), OH-HA (HMDB13678), trans-FA (HMDB00954), benzoic acid (BA; HMD01870), 4-OH-BA (HMDB13678), VA-lactic acid (HMDB00913) and VA-mandelic acid (HMBD00291), which are commonly found as breakdown products from a wide range of foods, drinks, drugs, environmental contaminants, endogenous metabolites and bacterial by-products [107]. Nevertheless, more in vitro studies on the specific effects of the physical-chemical (e.g., pH, ionic strength) and biochemical (e.g., phase I and II enzymatic modifications) changes in Cy3G combined with in vivo or ex vivo studies are needed in order to elucidate many important physiological implications for the bioactivity of this molecule.
5. Cy3G Health Effects
Generally speaking, anthocyanins seem to be non-toxic molecules within the margin of a normal consumption. Their safe intake (LD50) has been set up to 25,000 mg in mice and 20,000 in rats per kg body weight with no apparent adverse effects. Rabbits orally fed with ACNs (6 g/kg bw) had no pressure changes while, in guinea pigs and dogs, no sub-chronic toxic effects were observed (3 g/kg bw) [108]. Moreover, Charoensin et al. [109] provide information concerning the safety and anti-mutagenic potency of Cy3G. On the other hand, and despite their apparent chemical instability and low bioavailability, ACNs-rich foods have demonstrated their biological efficacy in a wide range of human diseases (acute and chronic) and the molecular mechanisms by which they exert their benefits have recently been reviewed by Li et al. [15]. However, a critical and thorough analysis of this evidence provides little insight into the specific role of Cy3G. Nevertheless, epidemiological studies, experimental trials using laboratory animals, in vitro assays using cell lines and ex vivo tissues and clinical studies with humans all together provide enough metabolomic evidence on Cy3G’s bioactivity and nutraceutical potential. In this last part, some of the most recognized health benefits of Cy3G (and metabolites) are systematically evaluated with a special emphasis on metabolomic responses.
5.1. Human Studies
The first evidence comes from very few epidemiological studies. The wide range of ACN intakes from dietary sources observed in many population-based surveys has been related to certain benefits against NCCD. Cassidy et al. [110] observed that a higher ACN intake (mostly coming from apples, pears, red wine, and strawberries) was inversely associated with many inflammatory biomarkers. Mehta et al. [111] demonstrated that consuming ≥2 cups/week of blueberries (or strawberry to a lesser extend) was associated with a slower rate of annual decline in lung function but no significant associations were observed for red wine intake. Also, Ponzo et al. [112] reported that a higher anthocyanidin intake is associated to a lower incidence of CVD events and all-cause mortality. Although we cannot ensure a priori other health benefits, these studies demonstrate that in vivo RSC of ACNs could be the main mechanism of action. At this point, we must remember that regular consumption of berry fruits makes important contributions to total ACNs and Cy3G daily consumption since the latter is the most common ACN (Table 3).
Clinical studies in humans have provided additional pieces of information on Cy3G’s bioactivity. For example, Cy3G has benefits for cardiovascular health. Hassellund and others [113] performed a randomized and double-blind crossover study [placebo vs. 640 mg/d/4 weeks (purified ACNs supplement)] showing that several ACNs peaked within 1–3 h but so did HDL-cholesterol, while Davinelli and others [114] using the same protocol but different ACN sources [placebo vs. 486 mg/d/4 weeks (Delphinol® from maqui berry, Huechuruba, Santiago, Chile] observed a decrement in LDLox and F2-isoprostanes levels but both studies did not show any other apparent benefit for CVD markers. According to this, Zhu et al. [115] showed significant changes in serum HDL- (up) and LDL- (down) cholesterol as well as high sensitivity C-reactive protein (hsCRP) and soluble vascular cell adhesion molecule-1 (sVCAM-1) levels after the 24-week ACNs supplementation. The same results have been reported with ACNs from strawberry, modulating these inflammatory biomarkers and indirectly improving insulin action [116]. It is noteworthy that endothelial health is related to the regulation of nitric oxide production, and the latter could be modified by several types of flavonoids including ACNs [117,118]. As to Cy3G’s anticancer activity, Mallery and others [84] confirmed that Cy derivatives (including Cy3G) from black raspberries (BRB) are extensively metabolized and retained within the oral cavity of healthy humans and Knobloch and others [85] showed that providing oral troches of freeze-dried BRB (FD-BRB) to patients with oral squamous cell carcinomas (OSCCs) for 14 day enhances the expression of pro-survival genes (AURKA, BIRC5, EGFR) and reduces other pro-inflammatory genes (NFKB1, PTGS2). Moreover, the acute intake of a blueberry dry extract regulates DNA methylation in patients with colorectal adenocarcinomas, despite the inter-individual variability [119]. Most if not all of these metabolic effects are related to Cy’s or Cy3G’s binding capacity toward different macromolecules including key proteins and enzymes, some of which will be discussed in Section 5.3.
5.2. Cell Lines and Rodent Models
Most findings regarding Cy3G’s bioactivity come from in vitro cell-based assays (Table 4) and in vivo experimental studies with rodents (Table 5). Cellular and rodent models provide concurrent and complementary evidence in many cases. Cy3G’s bioactivities include most commonly DNA-RSC, gastro-protective, anti-inflammatory, anti-thrombotic, insulinotropic, anti-microbial, chemopreventive and epigenetic effects. In turn, these actions may help to prevent H. pylory infection, periodontal and age-related diseases, type 2 diabetes mellitus, CVD, metabolic syndrome and oral cancer, to name just a few [3,4,5,6,120,121,122,123,124], involving specific molecular mechanisms (for both Cy and Cy3G, either pure or within plant food matrices) including RSC, epigenetic action, protein binding capacity and enzyme inhibition and many other mechanisms [125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149] some of which are discussed in the following paragraphs.
Table 4.
Cy3G associated effects-cell lines.
| Cell Line | Cy3G dose | Mechanism | Ref. |
|---|---|---|---|
| Erythrocytes | 10–100 μM | ↓ cholesterol and TBAR in cell membranes | [1] |
| Human adherent macrophages (from U937 cells), oral epithelial cells (GMSM-K) and gingival fibroblasts (HGF-1) | 5–25 μg/mL | ↓ IL-6 level (macrophages), cytoprotection (GMSM-K, HGF-1) against nicotine toxicity | [4] |
| Colon (Caco2), liver (HepG2), prostate (PC3) | Blue maize ACNs (189–500 μg/g)-extract | ↓ cell proliferation | [36] |
| Gastric cancer (KATO III) | 12.5 μM | ↓ Helicobacter pylori VacA-induced cell death | [91] |
| Adipocytes (3T3-L1) | 50 μM | ↓ FoxO1-mediated transcription of lipase | [123] |
| Hepatome (HepG2) | 1–100 μM | ↑ fatty acid oxidation and AMPK activity | [124] |
| Adipocyte | 0.5–50 μM + docosahexanoic acid | ↓ basal lipolysis , inflammatory markers | [127] |
| Breast cancer (BT474m MD-MB231, MCF7) | 10 μM | ↓ invasion / increased expression of ErB2 | [133] |
| Murine thymoma (EL-4T) | 2.5–5.0 μg/mL | ↓ Il-3 & IL-4 by GATA-3 inhibition | [134] |
| Pheochromocytoma (PC-12) | IC50, 15.3 μg/mL | ↓ ATP-induced [Ca2+] increase | [135] |
| Colon cancer (HT-29) | 25 μM | ↓ IL-8, nitrite, PGE2 | [136] |
| human aortic epithelial cells | 0.5–50 μM | ↑ oxiesterol efflux, ↑ABCG1/ABCA1 expression | [137] |
| Heart (isolated mitochondria) | 20 Μm | ↑ phosphorylation, ATP production, ↑e− carrier | [138] |
| Adipocytes (steam cells) | 100 μg/mL | ↓ IL-6 level | [139] |
| Ovarian cancer (HO-8910PM) | IC50, 13.8 μg/mL | ↑ apoptosis, ↓mucin 4 expression | [140] |
Table 5.
Cy3G associated effects-rodent models.
| Model | Protocol (Dose) | Effects | Ref. |
|---|---|---|---|
| Mice (nude), SKH-1 | Oral | ↓ lipid per oxidation ↑ Glutathione | [3] |
| Mice, C57BL/6 | Oral, 24 h before, (2 mg/kg) | ↓ Neuronal apoptosis reducing factor, superoxide level, infarct size | [5] |
| Mice (obese), C57BL/6 | Oral, 5 w, 0.02% diet, (ad libitum) | Antidiabetic by modulating c-jun N-terminal kinase | [122] |
| Mice, apoE (-) | Oral, 12 w, 0.06% diet, (ad libitum) | ↓ expression of hepatic cholesterol 7a-hydroxylase | [137] |
| Mice, ovarian cancer | Oral, 2 w, (5 mg/kg) | ↓ Growth of ovarian xenograft tumors | [140] |
| Mice, C57BL/6 | Oral, 12 w, (40–200 mg/kg) | ↓ weigh gain, insulin resistance, adiposity, leptin | [141] |
| Mice, apoE (-) | Oral, 8 w, 0.2% diet (ad libitum) | ↓ atherogenesis, ↑endothelial repair | [142] |
| Mice, with peritonitis/edema | Oral, 30–60 min before (40 mg/kg) | ↓ inflammation, expression COX-2/PGE2 | [143] |
| Mice, diabetic | Oral, 4 w, (300 µg/10g) | ↓ blood glucose | [144] |
| Mice, KK-Ay | Oral | ↓ visceral fat ↑ lipoprotein lipase | [145] |
| Mice, C57BL/6 w/acute alcohol-induced liver injury | Oral, 24–48 h, (10 mg/kg) | ↓ plasma IL-6 ,TNF-α, ALT and AST and ↑ SIRT1 p-c-Jun and Bax expression | [146] |
| Rats (retinal degeneration) | Oral, 5 w, 100 mg/kg | ↓ Loss of photoreceptors | [147] |
| Mice (fetus) | Intra-peritoneal 10–30 mg/kg | ↓Neuronal damage by caspase 3 inhibition | [148] |
| Rats (β-amyloidosis) | Oral, 30 day, 10 mg/kg | ↓ cognitive impairment induced by Aβ via the modulation of GSK-3β/tau | [149] |
The RSC and molecular competition ability of Cy3G (and Cy) may help to prevent certain inflammatory processes, CVD, aging and cancer [10]. For example, senescent and cancer cells are also susceptible to DNA cleavage due to environmental stressors which generate free radicals and activate oxidative enzymes such as xanthine oxidase that can be attenuated by Cy and Cy3G [120]. Also, results from animal studies suggest that Cy3G may slow or inhibit the absorption of lipids and glucose in the intestine [15,100,121,122] confirming the postulated mechanisms in cells [1,118,119]) and the physiological impact in humans [113]. Also, Ziberna et al. [125] indicate that Cy3G provides protection against oxidative stress-related CVD since it is transported in EA.hy926 cells by a specific bilitranslocase and accumulates in the vascular endothelium exerting anti-ischemic activity in the isolated rat heart. A special mention should be made of the metabolic bioactivity of Cy3G in adipose cells, both in vivo and in vitro. Tsuda et al. [126] showed that Cy3G and Cy both up-regulate human adiponectin, uncoupling protein-2, acylCoA oxidase-1 and perilipin but down-regulate plasminogen activator inhibitor-1 and IL-6. Björk et al. [127] observed that exposing white adipose cells to the omega-3-fatty acid docosahexaenoic acid + Cy3G suppresses the secretion of interleukin-6 and monocyte chemoattractant protein-1 (MCP-1/CCL2) and decreases its basal lipolytic activity. However, Tsuda et al. [128] found that Cy3G and Cy up-regulates the hormone-sensitive lipase gene and enhances the lipolytic activity of rat adipocytes. These findings suggest a species-specific effect of Cy and Cy3G.
However, the biological effects demonstrated in laboratory animals and in vitro assays could be overstated. In many cases, the amount required to achieve a specific biological action in general is much larger than that obtained from dietary sources. For example, the average amount of Cy3G employed in rat/mice bioassays far exceeds (about 30–60 times) the amount that can be obtained from a single dietary source (Table 3) [132], although in many cases the amount derived from an habitual diet is sufficient to achieve certain benefits. Moreover, as stated in previous sections, Cy3G from natural sources or manufactured nutraceuticals is largely limited by its splanchnic metabolism and so its fate in target internal tissues is limited. In this case, entrapping agents such as malto/cyclodextrins or liposomes are convenient alternatives to preserve its bioaccessibility and bioavailability within the GI tract. In this sense, concentrated sources of Cy3G such as purees or freeze-dried fruits could result not only in a much higher intake of Cy3G but also constitute a way to protect it from GI harsh conditions [87,129]. Other bioactivities related to Cy3G’s RSC, molecular competition and epigenetic action are shown in Table 4 and Table 5 [133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149].
5.3. Physiologically-Relevant Molecular Mechanisms
The aforementioned bioactivities of Cy3G, its aglycone (Cy) and derived metabolites rely mostly on the following mechanisms: RSC, epigenetic action, competitive protein-binding and enzyme inhibition. The unspecific RSC mechanism of these molecules has been extensively discussed in Section 2 as related to their structural features [130,131], while their epigenetic mechanisms have been postulated in almost all studies involving cancer cell lines and tumor xenografts. On the latter, important epigenetic modifications known to regulate gene expression are exerted by many flavonoids such as naringenin, kaempferol and quercetin [150] and the combination of flavonoids and chemotherapy seems to be an interesting approach for cancer treatment [151]. However, molecular studies involving Cy3G or Cy as epigenetic effectors are still scarce.
As to their macromolecular-binding and enzyme inhibition capacities, the first body of evidence comes from all in vitro and in silico studies using pure DNA (152) or protein (31–32, 153–156) systems. Zhang et al. [152] studied the binding capacity of Cy and Cy3G to calf thymus DNA demonstrating a Δλ to the ultraviolet-visible spectra of this nucleic acid, indicating the formation of the DNA-Cy and DNA-Cy3G complexes with an intercalative binding mode evidenced in their fluorescence spectra and that Cy3G binds to DNA more efficiently than Cy. Both molecules also bind salivary [81,83,84] and blood [31,32] proteins, a fact that may modify their fate within GI and their bloodstream transport. Spectroscopic studies suggest that Cy3G spontaneously binds albumins by means of weak forces such as hydrogen bonds and Van der Waals forces and hydrophobic interaction on a minor scale [31,32]. Cy3G binds to BSA in its IIA sub domain and it is surrounded by key hydrophobic and non-polar (Ala, Leu, Phe, Gly, Trp and Tyr) and polar (Arg, Asp, Glu and Lys) residues within the hydrophobic cavity of site II’ [153]. The same phenomenon has been observed in HAS, but also certain structural features of both anthocyanidins/ACNs, modify their binding capacity toward HAS: At pH 7.0 (high) and pH 4.0 (low), there is a differential electrostatic environment affecting the binding capacity of ACNs in their quinoidal form [154,155]. Also, the binding constant of Cy3G has been reported to be higher for myoglobin than for BSA hemoglobin, structurally associated to its binding capacity toward α-helices [32]. Lastly, evidence on the differential binding and inhibitory capacity of Cy and Cy3G toward GI enzymes has been disentangled. Akkarachiyasit et al. [99] revealed that Cy3G is a much stronger inhibitor of both intestinal α-glucosidase and pancreatic α-amylase as compared to Cy. While Adisakwattana et al. [156] found that glucose substitution at 3-O (increases) and 5-O (reduces) positions in Cy, it modifies its inhibitory activity toward α-glucosidase.
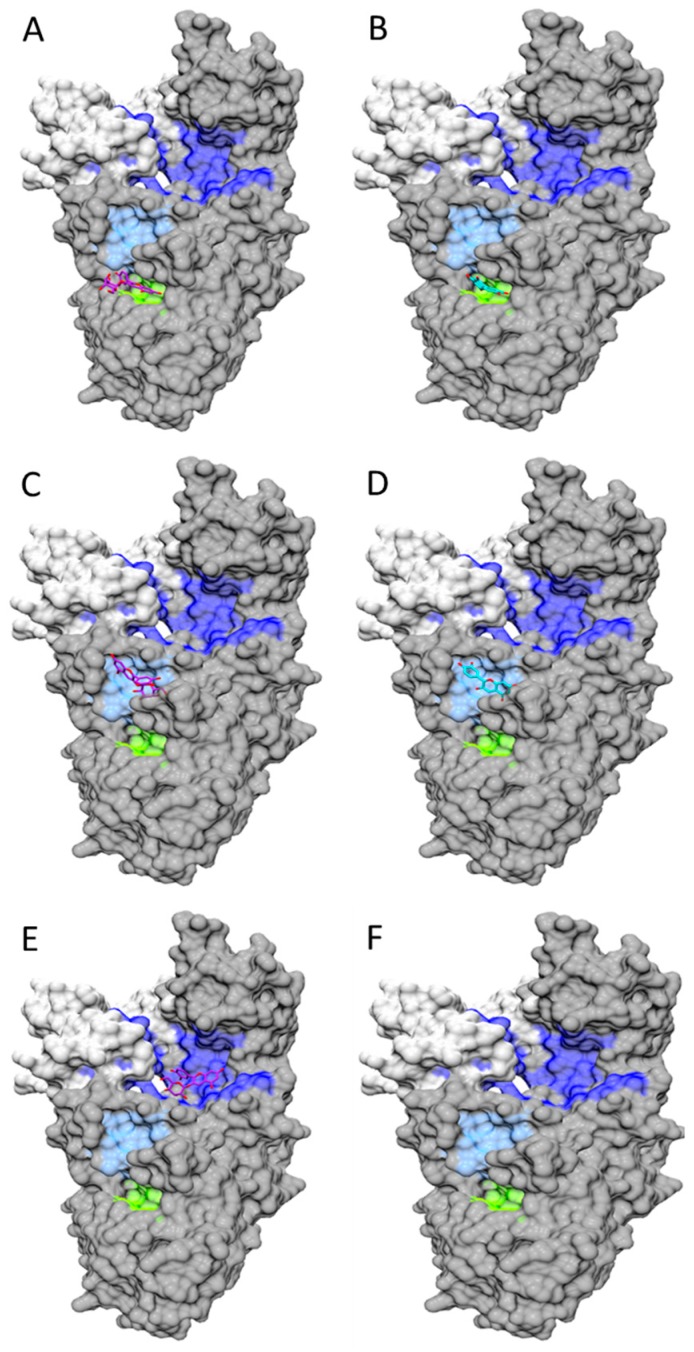
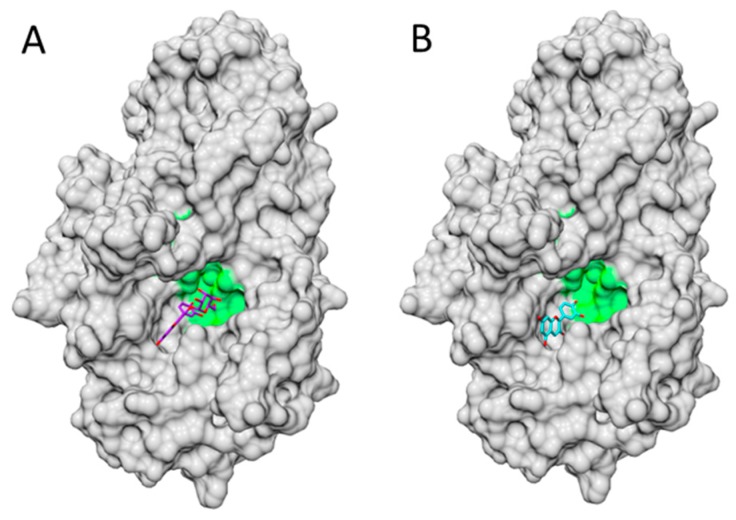
Molecular docking simulations have provided further evidence on the structural features and mechanisms involved in Cy3G’s and Cy’s binding capacity toward different proteins. Oliveira et al. [86] studied the binding capacity of Cy, Dp and Mv glucosides toward hGLUT1 showing that the pattern of hydroxylation determines their hydrogen-bonding with this protein at ΔGbinding = −9.07, −8.13 and −7.36 kcal/mol, respectively [86] while Perez-Diaz et al. [157] showed the ability of four glutathione S-transferases (H-site, N-terminal domain) from Vitis vinifera to bind and even transport Cy3G (ΔGbinding = −2.43 to −2.03). However, similar studies comparing Cy’s and Cy3G’s differential binding capacity toward GI enzymes have not been reported yet. Docking simulations [flexible ligand/rigid enzyme (100 × 100 × 100 grid points) docking] using AutoDock Vina [158] and UCSF Chimera [159] packages, Cy3G (PubChem: 92131208) and Cy (PubChem: 128861) as ligands and porcine lipase/colipase complex (RCSB PDB-1ETH) and porcine amylase (RCSB PDB-1PIF) as templates are depicted in Figure 5 and Figure 6. Cy3G and Cy have three potential binding sites for porcine lipase–colipase complex (Figure 5) within (A, B) and near (C, D) the active site and at the binding region with colipase (E, F). While Cy presumably binds more effectively than Cy3G within (ΔGbinding = −9.8 vs. −7.0 kcal/mol) and near (ΔGbinding = −8.8 vs. −8.0 kcal/mol) the active site, an inverse phenomenon is observed at the lipase-colipase region (ΔGbinding = 0.0 vs. −7.7 kcal/mol). According to Figure 6, Cy also binds more strongly to amylase when compared to Cy3G (ΔGbinding = −8.9 vs. −7.8 kcal/mol). Based on this data, a different lipase-colipase and amylase inhibitory activity could be expected and possibly a different hypolipidemic and hypoglycemic effect in clinical studies. However, these statements should be proven in future studies.
Figure 5.
Docking simulation of Cy3G and Cy toward porcine lipase/colipase complex. Docking simulation details are described in Section 5.3. Cy3G (left) and Cy (right) within (A,B, light green) and near (C,D; light blue) the active site of lipase (dark grey) and at the binding region with colipase (intense blue; E,F).
Figure 6.
Docking simulation of Cy3G and Cy toward porcine amylase. Docking simulation details are described in Section 5.3. Cy3G (A); Cy (B) within enzyme’s (light grey) active site (green).
6. Future Prospects
Cy3G is a fascinating PC in many ways. For this reason, it has been studied in many disciplines including chemical, agricultural and biomedical sciences. Unlike other ACNs, its chemical nature has a profound effect on its in vitro and in vivo behavior, sometimes improving or otherwise limiting its antioxidant capacity and stability, bioaccessibility and bioavailability in the gastrointestinal tract and its nutraceutical effects in living systems. However, an adequate and sustained consumption of Cy3G-rich sources such as certain berry-based juices, purees or concentrates could contribute to maintaining an adequate amount of metabolites in plasma and certain tissues to ensure its nutraceutical effects. However, the Cy3G puzzle is not solved yet since more foodomic studies in plants, animals and man are required to discover new chemical and biological aspects of ACNs widely distributed in nature and their therapeutic effects in people with serious illnesses. Lastly, more studies on the assessment of Cy and Cy3G in equilibrium (bound/unbound) toward physiologically relevant macromolecules are needed in order to understand their physiological relevance
Acknowledgments
Partial financial support from the Mexico’s Faculty Improvement Program (PROMEP) and the National Council of Science and Technology (CONACyT) through the granted Basic Science project (CB-2015-1/ 254063) and doctorate scholarship to FJOA, are gratefully acknowledged. All authors are indebted to all academic authorities (UACJ, UAQ, CIAD) for their institutional support. Lastly, all authors are grateful to Dr. Angel G. Diaz Sanchez for its excellent graphical assistance (molecular docking simulation).
Abbreviations
The following abbreviations are used in this manuscript:
| ΔA | Hyperchromic effect |
| Δλ | Bathochromic shift |
| ADME | Absorption, distribution, metabolism and excretion |
| ANR | Anthocyanidin reductase |
| ANS | Anthocyanidin synthase |
| ACNs | Anthocyanins |
| Å2 | Polar surface area |
| AOX | Antioxidants |
| ASSWV | Abrasive stripping square wave voltammetry |
| βRA | β-Resorcylic acid |
| BA | Benzoic acid-4-glucoronide |
| BA-Gnd | Benzoic acid-4-glucoronide |
| BRB | Black raspberry |
| BSA | Bovine serum albumin |
| CHI | Chalcone isomerase |
| CHS | Chalcone synthase |
| COMT | Catechol-O-methyltransferase |
| CON | Unknown condensing enzyme(s) |
| CT | Condensed tannins |
| CV | Cyclic voltammetry |
| CVD | Cardiovascular disease |
| Cy | Cyanidin |
| Cy3G | Cyanidin-3-O-glucoside |
| DFR | Dihydroflavonol-4-reductase |
| Dp | Delphinidin |
| Dp3G | Delphinidin-3-O-glucoside |
| Dp3Ga | Delphinidin-3-O-galactoside |
| Dp3R | Delphinidin-3-O-rutinoside |
| DPV | Differential of pulses voltammetry |
| EHM | Entero-hepatic metabolism |
| Epa | Anodic peak potential |
| F3,5H | Flavanone-3,5-hidroxylase |
| F3H | Flavanone-3-hidroxylase |
| FA | Ferulic acid |
| FLS | Flavonol synthase |
| FNS | Flavone synthase |
| GI | Gastrointestinal |
| GLUT1,2,3 | Glucose transporter 1,2,3 |
| HA | Hippuric acid |
| HAS | Human serum albumin |
| Hb | Hemoglobin |
| IVA | Isovainillinic acid |
| IVA-3-Gnd | IVA-3-glucoronide |
| LAR | Leucoanthocyanidin reductase |
| LDOW | Leucoanthocyanidin dioxigenase |
| LogP | Octanol/water partition coefficient |
| LPH | Lactase-phlorizin hydrolase |
| Mb | Myoglobin |
| MCP-1/CCL2 | Monocyte chemoattractant protein-1 |
| MCT1 | Mono-carboxylated transporter 1 |
| Mv | Malvidin |
| Mv3G | Malvidin-3-O-glucoside |
| MW | Molecular weight |
| NCCD | Non-communicable chronic diseases |
| OMT | Malvidin (Mv), O-methyltransferase |
| Pas | Proanthocyanidins |
| PC | Phenolic compounds |
| PCA | Protocatechuic acid |
| PCA-3-Gnd | PCA-3-glucoronide |
| PCA-3-Gnd | PCA-4-glucoronide |
| PCA-Sft | PCA-sulfate |
| Pg | Pelargoddin |
| PGA | Phloroglucinaldehyde |
| PgANS | Pelargodin specific-anthocyanidin synthase |
| Pn | Peonidin |
| Pn3Ga | Peonidin-3-O-galactoside |
| PST | Phenyl sulfotranferases |
| Pt | Petunidin |
| RE | Reference electrode |
| ROS | Reactive oxygen species |
| RSC | Radical scavenging capacity |
| SGLT1 | Na+-glucose transporter |
| UFGT | UDP-glucose-flavonoid-3-o-glucosyltransferase |
| UGT | Uridine-5′-diphosphate glucoronosyltransferases |
| VA | Vainillinic acid |
| VA-3-Gnd | VA-3-glucoronide |
| VA-Sft | VA-sulfate |
| WE | Working electrode |
| 3,4-dOH-BA | Methyl-3,4-dihydroxybenzoate |
| 3RT | Anthocyanidin-3-glycoside rhamnosyl-transferase |
| 4-OH-BA | 4-Hydroxy-benzaldehyde |
Author Contributions
All authors contributed equally to the design, production and preparation of the manuscript. AICR and SOMD drafted the “physical-chemistry” section; JRG, GAGA and EAP “plant biochemistry” section; LAR and AWM “foodomics” section and FJOA, ARJ and NRMR “health effects” section.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Duchnowicz P., Broncel M., Podsędek A., Koter-Michalak M. Hypolipidemic and antioxidant effects of hydroxycinnamic acids, quercetin, and cyanidin 3-glucoside in hypercholesterolemic erythrocytes (in vitro study) Eur. J. Nutr. 2012;51:435–443. doi: 10.1007/s00394-011-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreyra M.F., Rius S.P., Casati P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant. Sci. 2012;3:1–15. doi: 10.3389/fpls.2012.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pratheeshkumar P., Son Y.O., Wang X., Divya S.P., Joseph B., Hitron J.A., Wang L., Kim D., Yin Y., Roy R.V., et al. Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating MAP kinase and NF-κB signaling pathways in SKH-1 hairless mice skin. Toxicol. Appl. Pharmacol. 2014;280:127–137. doi: 10.1016/j.taap.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desjardins J., Tanabe S., Bergeron C., Gafner S., Grenier D. Anthocyanin-rich black currant extract and cyanidin-3-O-glucoside have cytoprotective and anti-inflammatory properties. J. Med. Food. 2012;15:1045–1050. doi: 10.1089/jmf.2011.0316. [DOI] [PubMed] [Google Scholar]
- 5.Min J., Yu S.W., Baek S.H., Nair K.M., Bae O.N., Bhatt A., Kassab M., Majid A. Neuroprotective effect of cyanidin-3-O-glucoside anthocyanin in mice with focal cerebral ischemia. Neurosci. Lett. 2011;500:157–161. doi: 10.1016/j.neulet.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 6.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovinich N., Kayanja G., Chanoca A., Riedl K., Otegui M.S., Grotewol E. Not all anthocyanins are born equal: Distinct patterns induced by stress in Arabidopsis. Planta. 2014;240:931–940. doi: 10.1007/s00425-014-2079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sui X., Dong X., Zhou W. Combined effect of pH and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chem. 2014;163:163–170. doi: 10.1016/j.foodchem.2014.04.075. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes I., Marques F., de Freitas V., Mateus N. Antioxidant and antiproliferative properties of methylated metabolites of anthocyanins. Food Chem. 2013;141:2923–2933. doi: 10.1016/j.foodchem.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 10.Cheynier V., Gómez C., Ageorges A. Flavonoids: Anthocyanins. In: Nollet L.M.C., Toldrá F., editors. Handbook of Analysis of Active Compounds in Functional Foods. 1st ed. CRC Press/Taylor & Francis Group; Boca Raton, FL, USA: 2012. pp. 379–403. [Google Scholar]
- 11.Patras A., Brunton N.P., O′Donnell C., Tiwari B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends. Food Sci. Technol. 2010;21:3–11. doi: 10.1016/j.tifs.2009.07.004. [DOI] [Google Scholar]
- 12.Fang J. Bioavailability of anthocyanins. Drug. Metab. Rev. 2014;46:508–520. doi: 10.3109/03602532.2014.978080. [DOI] [PubMed] [Google Scholar]
- 13.Fang J. Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa: Extensive presystemic metabolism reduces apparent bioavailability. J. Agric. Food Chem. 2014;62:3904–3911. doi: 10.1021/jf405356b. [DOI] [PubMed] [Google Scholar]
- 14.De Ferrars R.M., Czank C., Zhang Q., Botting N.P., Kroon P.A., Cassidy A., Kay C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014;171:3268–3282. doi: 10.1111/bph.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D., Wang P., Luo Y., Zhao M., Chen F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2015 doi: 10.1080/10408398.2015.1030064. [DOI] [PubMed] [Google Scholar]
- 16.Wu X., Prior R.L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J. Agric. Food Chem. 2005;53:2589–2599. doi: 10.1021/jf048068b. [DOI] [PubMed] [Google Scholar]
- 17.Wu X., Beecher G.R., Holden J.M., Haytowitz D.B., Gebhardt S.E., Prior R.L. Concentration of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006;54:4069–4075. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- 18.Pala Ç.U., Toklucu A.K. Effect of UV-C light on anthocyanin content and other quality parameters of pomegranate juice. J. Food Comp. Anal. 2011;24:790–795. doi: 10.1016/j.jfca.2011.01.003. [DOI] [Google Scholar]
- 19.Hernández-Herrero J.A., Frutos M.J. Influence of rutin and ascorbic acid in colour, plum anthocyanins and antioxidant capacity stability in model juices. Food Chem. 2015;173:495–500. doi: 10.1016/j.foodchem.2014.10.059. [DOI] [PubMed] [Google Scholar]
- 20.Mansour R., Ezzli B., Farouk M. Dyeing properties of wool fabrics dyed with Vitis vinifera L. (black grenache) leaves extract. Fibers Polym. 2013;14:786–792. doi: 10.1007/s12221-013-0786-z. [DOI] [Google Scholar]
- 21.Pacheco-Palencia L.A., Talcott S.T. Chemical stability of açai fruit (Euterpe oleracea Mart.) anthocyanins as influenced by naturally occurring and externally added polyphenolic cofactors in model systems. Food Chem. 2010;118:17–25. [Google Scholar]
- 22.Kamiloglu S., Capanoglu E., Grootaert C., Van Camp J. Anthocyanin absorption and metabolism by human intestinal Caco-2 cells-A review. Int. J. Mol. Sci. 2015;16:21555–21574. doi: 10.3390/ijms160921555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie L., Lee S.G., Vance T.M., Wang Y., Kim B., Lee J.Y., Chun O.K., Bolling B.W. Bioavailability of anthocyanins and colonic polyphenol metabolites following consumption of Aronia berry extract. Food Chem. 2016;211:860–868. doi: 10.1016/j.foodchem.2016.05.122. [DOI] [PubMed] [Google Scholar]
- 24.Castañeda-Ovando A., Pacheco-Hernández M.D.L., Páez-Hernández M.E., Rodríguez J.A., Galán-Vidal C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009;113:859–871. doi: 10.1016/j.foodchem.2008.09.001. [DOI] [Google Scholar]
- 25.Flamini R., de Rosso M., Bavaresco L. Study of grape polyphenols by liquid chromatography-high-resolution mass spectrometry (UHPLC/QTOF) and suspect screening analysis. J. Anal. Methods Chem. 2015 doi: 10.1155/2015/350259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Czank C., Cassidy A., Zhang Q., Morrison D.J., Preston T., Kroon P.A., Botting N.P., Kay C.D. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: A (13)C-tracer study. Am. J. Clin. Nutr. 2013;97:995–1003. doi: 10.3945/ajcn.112.049247. [DOI] [PubMed] [Google Scholar]
- 27.Miyazawa T., Nakagawa K., Kudo M., Muraishi K., Someva K. Direct intestinal absorption of red fruit anthocyanins, cyanidin-3-glucoside and cyanidin-3,5-diglucoside into rats and humans. J. Agric. Food Chem. 1999;47:1083–1091. doi: 10.1021/jf9809582. [DOI] [PubMed] [Google Scholar]
- 28.Felgines C., Krisa S., Mauray A., Besson C., Lamaison J.L., Scalbert A., Mérillon J.M., Texier O. Radiolabelled cyanidin 3-O-glucoside is poorly absorbed in the mouse. Br. J. Nutr. 2010;103:1738–1745. doi: 10.1017/S0007114510000061. [DOI] [PubMed] [Google Scholar]
- 29.Phan A.D.T., Netzel G., Wang D., Flanagan B.M., D’Arcy B.R., Gidley M.J. Binding of dietary polyphenols to cellulose: Structural and nutritional aspects. Food Chem. 2015;171:388–396. doi: 10.1016/j.foodchem.2014.08.118. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira A., Pintado M. In vitro evaluation of the effects of protein-polyphenol-polysaccharide interactions on (+)-catechin and cyanidin-3-glucoside bioaccesibility. Food Funct. 2015;6:3444–3453. doi: 10.1039/C5FO00799B. [DOI] [PubMed] [Google Scholar]
- 31.Tang L., Zuo H., Li S. Comparison of the interaction between three anthocyanins and human serum albumins by spectroscopy. J. Lumin. 2014;153:54–63. doi: 10.1016/j.jlumin.2014.03.004. [DOI] [Google Scholar]
- 32.Tang L., Li S., Bi H., Gao X. Interaction of cyanidin-3-O-glucoside with three proteins. Food Chem. 2016;196:550–559. doi: 10.1016/j.foodchem.2015.09.089. [DOI] [PubMed] [Google Scholar]
- 33.Molinspiration Cheminformatics. [(accessed on 25 July 2016)]. Available online: http://www.molinspiration.com/about.html.
- 34.Kosińska-Cagnazzo A., Diering S., Prim D., Andlauer W. Identification of bioaccessible and uptaken phenolic compounds from strawberry fruits in in vitro digestion/Caco-2 absorption model. Food Chem. 2015;170:288–294. doi: 10.1016/j.foodchem.2014.08.070. [DOI] [PubMed] [Google Scholar]
- 35.Fernandes A., Brás N.F., Mateus N., de Freitas V. Understanding the molecular mechanisms of anthocyanin binding to pectin. Langmuir. 2014;30:8516–8527. doi: 10.1021/la501879w. [DOI] [PubMed] [Google Scholar]
- 36.Urías-Lugo D.A., Heredia J.B., Muy-Rangel M.D., Valdez-Torres J.B., Serna-Saldívar S.O., Gutiérrez-Uribe J.A. Anthocyanins and phenolic acids of hybrid and native blue maize (Zea mays L.) extracts and their antiproliferative activity in mammary (MCF7), liver (HepG2), colon (Caco2 and HT29) and prostate (PC3) cancer cells. Plant. Foods Hum. Nutr. 2015;70:193–199. doi: 10.1007/s11130-015-0479-4. [DOI] [PubMed] [Google Scholar]
- 37.Zhao X., Yuan Z., Feng L., Fang Y. Cloning and expression of anthocyanin biosynthetic genes in red and white pomegranate. J. Plant Res. 2015;128:687–696. doi: 10.1007/s10265-015-0717-8. [DOI] [PubMed] [Google Scholar]
- 38.García-Alonso M., Rimbach G., Sasai M., Nakahara M., Matsugo S., Uchida Y., Rivas-Gonzalo J.C., de Pascual-Teresa A. Electron spin resonance spectroscopy studies on the free radical scavenging activity of wine anthocyanins and pyranoanthocyanins. Mol. Nutr. Food Res. 2015;49:1112–1119. doi: 10.1002/mnfr.200500100. [DOI] [PubMed] [Google Scholar]
- 39.Bakowska A., Kucharska A.Z., Oszmiański J. The effects of heating, UV irradiation, and storage on stability of the anthocyanin–Polyphenol copigment complex. Food Chem. 2003;81:349–355. doi: 10.1016/S0308-8146(02)00429-6. [DOI] [Google Scholar]
- 40.Boulton R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001;52:67–87. [Google Scholar]
- 41.Fazaeli M., Yousefi S., Emam-Djomeh Z. Investigation on the effects of microwave and conventional heating methods on the phytochemicals of pomegranate (Punica granatum L.) and black mulberry juices. Food Res. Int. 2013;50:568–573. doi: 10.1016/j.foodres.2011.03.043. [DOI] [Google Scholar]
- 42.Cavalcanti R.N., Santos D.R., Meireles M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems—An overview. Food Res. Int. 2011;44:499–509. doi: 10.1016/j.foodres.2010.12.007. [DOI] [Google Scholar]
- 43.Mildner-Szkudlarz S., Bajerska J., Górnaś P., Segliņa D., Pilarska A., Jesionowski T. Physical and bioactive properties of muffins enriched with raspberry and cranberry pomace powder: A promising application of fruit by-products rich in biocompounds. Plant Foods Hum. Nutr. 2016;71:165–173. doi: 10.1007/s11130-016-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slavin M., Lu Y., Kaplan N., Yu L.L. Effects of baking on cyanidin-3-glucoside content and antioxidant properties of black and yellow soybean crackers. Food Chem. 2013;141:1166–1174. doi: 10.1016/j.foodchem.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 45.Rawson A., Patras A., Tiwari B.K., Noci F., Koutchma T., Brunton N. Effect of thermal and non-thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Res. Int. 2011;44:1875–1887. doi: 10.1016/j.foodres.2011.02.053. [DOI] [Google Scholar]
- 46.Wang H. Rapid quantitative analysis of individual anthocyanin content based on high-performance liquid chromatography with diode array detection with the pH differential method. J. Sep. Sci. 2014;37:2535–2544. doi: 10.1002/jssc.201400364. [DOI] [PubMed] [Google Scholar]
- 47.Janeiro P., Brett A.M.O. Redox behavior of anthocyanins present in Vitis vinifera L. Electroanalysis. 2007;19:1779–1786. doi: 10.1002/elan.200703941. [DOI] [Google Scholar]
- 48.Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. 5th ed. Oxford University Press; Oxford, UK: 2015. pp. 77–79. [Google Scholar]
- 49.Moran V.F.E., Boggetti H.J., Zampini I.C., Ordoñez R.M., Isla M.I., Alvarez R.M.S., de Rosso V., Mercadante A.Z., Borsarelli C.D. Singlet oxygen quenching and radical scavenging capacities of structurally-related flavonoids present in Zuccagnia punctata Cav. Free Radic. Res. 2009;43:553–564. doi: 10.1080/10715760902912264. [DOI] [PubMed] [Google Scholar]
- 50.Komorsky-Lovric S., Novak I. Abrasive stripping square-wave voltammetry of blackberry, raspberry, strawberry, pomegranate, and sweet and blue potatoes. J. Food Sci. 2011;76:916–920. doi: 10.1111/j.1750-3841.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- 51.Zhao C.L., Yu Y.Q., Chen Z.J., Wen G.S., Wei F.G., Zheng Q., Wang C.-D., Xiao X.L. Stability-increasing effects of anthocyanin glycosyl acylation. Food Chem. 2017;214:119–128. doi: 10.1016/j.foodchem.2016.07.073. [DOI] [PubMed] [Google Scholar]
- 52.De Lima A.A., Sussuchi E.M., Giovani W.F. Electrochemical and antioxidant properties of anthocyanins and anthocyanidins. Croatia Chem. Acta. 2007;80:29–34. [Google Scholar]
- 53.Moncada M.C., de Mesquita M.F., dos Santos M.M.C. Electrochemical oxidation of the synthetic anthocyanin analogue 4-methyl-7,8-dihydroxyflavylium salt. J. Electroanal. Chem. 2009;636:60–67. doi: 10.1016/j.jelechem.2009.09.004. [DOI] [Google Scholar]
- 54.Fukumoto L.R., Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000;48:3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- 55.Yordi E.G., Pérez E.M., Matos M.J., Villares E.U. Antioxidant and pro-oxidant effects of polyphenolic compounds and structure-activity relationship evidence. In: Bouayed J., Bohn T., editors. Nutrition, Well-Being and Health. InTech; Rijeka, Croatia: 2012. pp. 23–48. [Google Scholar]
- 56.Hassimotto N.M.A., Genovese M.I., Lajolo F.M. Absorption and metabolism of cyanidin-3-glucoside and cyanidin-3-rutinoside extracted from wild mulberry (Morus nigra L.) in rats. Nutr. Res. 2008;28:198–207. doi: 10.1016/j.nutres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 57.Lacombe A., Wu V.C., Tyler S., Edwards K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids against Escherichia coli O157:H7. Int. J. Food Microbiol. 2010;139:102–107. doi: 10.1016/j.ijfoodmicro.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 58.He F., Mu L., Yang G.L., Liang N.N., Pan Q.H., Wang J., Reeves M.J., Duan C.Q. Biosynthesis of anthocyanins and their regulation in colored grapes. Molecules. 2010;15:9057–9091. doi: 10.3390/molecules15129057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ploenlap P., Pattanagul W. Effects of exogenous abscisic acid on foliar anthocyanin accumulation and drought tolerance in purple rice. Biologia. 2015;70:915–921. doi: 10.1515/biolog-2015-0107. [DOI] [Google Scholar]
- 60.Cisowska A., Wojnicz D., Hendrich A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011;6:149–156. [PubMed] [Google Scholar]
- 61.Landi M., Tattini M., Gould K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015;119:4–17. doi: 10.1016/j.envexpbot.2015.05.012. [DOI] [Google Scholar]
- 62.Bulgakov V.P., Avramenko T.V., Tsitsiashvili G.S. Critical analysis of protein signaling networks involved in the regulation of plant secondary metabolism: Focus on anthocyanins. Crit. Rev. Biotechnol. 2016:1–16. doi: 10.3109/07388551.2016.1141391. [DOI] [PubMed] [Google Scholar]
- 63.Dossett M., Lee J., Finn C.E. Characterization of a novel anthocyanin profile in wild black raspberry mutants: An opportunity for studying the genetic control of pigment and color. J. Funct. Foods. 2011;3:207–214. doi: 10.1016/j.jff.2011.04.003. [DOI] [Google Scholar]
- 64.Walker A.R., Lee E., Bogs J., McDavid D.A.J., Thomas M.R., Robinson S.P. White grapes arose through the mutation of two similar and adjacent regulatory genes. Plant J. 2007;49:772–785. doi: 10.1111/j.1365-313X.2006.02997.x. [DOI] [PubMed] [Google Scholar]
- 65.Pierantoni L., Dondini L., De Franceschi P., Musacchi S., Winkel B.S.J., Sansavini S. Mapping of an anthocyanin-regulating MYB transcription factor and its expression in red and green pear, Pyrus communis. Plant Physiol. Biochem. 2010;48:1020–1026. doi: 10.1016/j.plaphy.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Song J., Du L., Li L., Kalt W., Palmer L.C., Fillmore S., Zhang Y., Zang Z.Q., Li X. Quantitative changes in proteins responsible for flavonoid and anthocyanin biosynthesis in strawberry fruit at different ripening stages: A targeted quantitative proteomic investigation employing multiple reaction monitoring. J. Proteom. 2015;122:1–10. doi: 10.1016/j.jprot.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 67.Mazzoni L., Perez-Lopez P., Giampieri F., Alvarez-Suarez J.M., Gasparrini M., Forbes-Hernandez T.Y., Quiles J.L., Mezzetti B., Battino M. The genetic aspects of berries: From field to health. J. Sci. Food Agric. 2016;96:365–371. doi: 10.1002/jsfa.7216. [DOI] [PubMed] [Google Scholar]
- 68.Vogiatzoglou A., Mulligan A.A., Lentjes M.A., Luben R.N., Spencer J.P., Schroeter H., Khaw H., Kuhnle G.G. Flavonoid intake in European adults (18 to 64 years) PLoS ONE. 2015;10:e0128132. doi: 10.1371/journal.pone.0128132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Department of Agriculture, Agricultural Research Service USDA National Nutrient Database for Standard Reference, Release 28. [(accessed on 30 July 2016)];2005–2011 Available online: http://www.ars.usda.gov/Services/docs.htm?docid = 8964.
- 70.Diaconeasa Z., Leopold L., Rugină D., Ayvaz H., Socaciu C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant Juice. Int. J. Mol. Sci. 2015;16:2352–2365. doi: 10.3390/ijms16022352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niño-Medina G., Muy-Rangel D., Gardea-Bejar A., González-Aguilar G., Heredia B., Baez-Sanudo M., Siller-Cepeda J., Velez de la Rocha R. Nutritional and nutraceutical components of commercial eggplant types grown in Sinaloa, México. Not. Bot. Horti Agrobot. Cluj-Napoca. 2014;42:538–544. doi: 10.15835/nbha.42.2.9573. [DOI] [Google Scholar]
- 72.Nimptsch K., Zhang X., Cassidy A., Song M., O’Reilly É.J., Lin J.H., Pischon T., Rimm E.B., Willet W.C., Fuchs C.S., et al. Habitual intake of flavonoid subclasses and risk of colorectal cancer in 2 large prospective cohorts. Am. J. Clin. Nutr. 2016;103:184–191. doi: 10.3945/ajcn.115.117507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sebastian R.S., Enns C.W., Goldman J.D., Martin C.L., Steinfeldt L.C., Murayi T., Moshfegh A.J. A new database facilitates characterization of flavonoid intake, sources, and positive associations with diet quality among US Adults. J. Nutr. 2015;145:1239–1248. doi: 10.3945/jn.115.213025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grosso G., Stephania U., Micek A., Stefler D., Bobak M., Paja A. Dietary polyphenols are inversely associated with metabolic syndrome in Polish adults of the HAPIEE study. Eur. J. Nutr. 2016 doi: 10.1007/s00394-016-1187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jun S., Shin S., Joung H. Estimation of dietary flavonoid intake and major food sources of Korean adults. Br. J. Nutr. 2016;115:480–489. doi: 10.1017/S0007114515004006. [DOI] [PubMed] [Google Scholar]
- 76.Peterson J.J., Dwyer J.T., Jacques P.F., McCullough M.L. Improving the estimation of flavonoid intake for study of health outcomes. Nutr. Rev. 2015;73:553–576. doi: 10.1093/nutrit/nuv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Capozzi F., Bordoni A. Foodomics: A new comprehensive approach to food and nutrition. Genes Nutr. 2013;8:1–4. doi: 10.1007/s12263-012-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Velderrain-Rodríguez G., Palafox H., Wall-Medrano A., Ayala-Zavala J.F., Chen C.Y.O., Robles-Sánchez R.M., Astiazaran-García H., Álvarez-Parrilla H., González-Aguilar G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014;5:189–197. doi: 10.1039/C3FO60361J. [DOI] [PubMed] [Google Scholar]
- 79.Kopf-Bolanz K.A., Schwander F., Gijs M., Vergères G., Portmann R., Egger L. Validation of an in vitro digestive system for studying macronutrient decomposition in humans. J. Nutr. 2012;142:245–250. doi: 10.3945/jn.111.148635. [DOI] [PubMed] [Google Scholar]
- 80.Chen J. Food oral processing: Some important underpinning principles of eating and sensory perception. Food Struct. 2014;1:91–105. doi: 10.1016/j.foostr.2014.03.001. [DOI] [Google Scholar]
- 81.Carpenter G.H. The secretion, components, and properties of saliva. Ann. Rev. Food Sci. Technol. 2013;4:267–276. doi: 10.1146/annurev-food-030212-182700. [DOI] [PubMed] [Google Scholar]
- 82.Wiese S., Gärtner S., Rawel H.M., Winterhalter P., Kulling S.E. Protein interactions with cyanidin-3-glucoside and its influence on α-amylase activity. J. Sci. Food Agric. 2009;89:33–40. doi: 10.1002/jsfa.3407. [DOI] [Google Scholar]
- 83.Kamonpatana K., Giusti M.M., Chitchumroonchokchai C., Moren Cruz M., Riedl K.M., Kumar P., Failla M.L. Susceptibility of anthocyanins to ex vivo degradation in human saliva. Food Chem. 2012;135:738–747. doi: 10.1016/j.foodchem.2012.04.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mallery S.R., Budendorf D.E., Larsen M.P., Pei P., Tong M., Holpuch A.S., Larsen P.E., Stoner G.D., Fields H.W., Chan K.K., et al. Effects of human oral mucosal tissue, saliva, and oral microflora on intraoral metabolism and bioactivation of black raspberry anthocyanins. Cancer Prev. Res. 2011;4:1209–1221. doi: 10.1158/1940-6207.CAPR-11-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Knobloch T.J., Uhrig L.K., Pearl D.K., Casto B.C., Warner B.M., Clinton S.K., Sardo-Molmenti C.L., Ferguson J.M., Daly B.T., Riedl K., et al. Suppression of pro-inflammatory and pro-survival biomarkers in oral cancer patients consuming a black raspberry phytochemical-rich troche. Cancer Prev. Res. 2016;9:159–171. doi: 10.1158/1940-6207.CAPR-15-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oliveira H., Fernandes I., Brás N.F., Faria A., De Freitas V., Calhau C., Mateus N. Experimental and theoretical data on the mechanism by which red wine anthocyanins are transported through a human MKN-28 gastric cell model. J. Agric. Food Chem. 2015;63:7685–7692. doi: 10.1021/acs.jafc.5b00412. [DOI] [PubMed] [Google Scholar]
- 87.Del Bo’ C., Riso P., Brambilla A., Gardana C., Rizzolo A., Simonetti P., Bertolo G., Klimis-Zacas D., Porrini M. Blanching improves anthocyanin absorption from highbush blueberry (Vaccinium corymbosum L.) purée in healthy human volunteers: A pilot study. J. Agric. Food Chem. 2012;60:9298–9304. doi: 10.1021/jf3021333. [DOI] [PubMed] [Google Scholar]
- 88.McGhie T.K., Walton M.C. The bioavailability and absorption of anthocyanins: Towards a better understanding. Mol. Nutr. Food Res. 2007;51:702–713. doi: 10.1002/mnfr.200700092. [DOI] [PubMed] [Google Scholar]
- 89.Passamonti S., Terdoslavich M., Franca R., Vanzo A., Tramer F., Braidot E., Petrussa E., Angelo V. Bioavailability of flavonoids: A review of their membrane transport and the function of bilitranslocase in animal and plant organisms. Curr. Drug Metab. 2009;10:369–394. doi: 10.2174/138920009788498950. [DOI] [PubMed] [Google Scholar]
- 90.Zou T.B., Feng D., Song G., Li H.W., Tang H.W., Ling W.H. The role of sodium-dependent glucose transporter 1 and glucose transporter 2 in the absorption of cyanidin-3-O-β-glucoside in Caco-2 cells. Nutrients. 2014;6:4165–4177. doi: 10.3390/nu6104165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim S.H., Woo H., Park M., Rhee K.J., Moon C., Lee D., Seo W.D., Kim J.B. Cyanidin 3-O-glucoside reduces Helicobacter pylori VacA-induced cell death of gastric KATO III cells through inhibition of the SecA pathway. Int. J. Med. Sci. 2014;11:742–747. doi: 10.7150/ijms.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shih P.H., Yeh C.T., Yen G.C. Effects of anthocyanidin on the inhibition of proliferation and induction of apoptosis in human gastric adenocarcinoma cells. Food Chem. Toxicol. 2005;43:1557–1566. doi: 10.1016/j.fct.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 93.Felgines C., Talavera S., Texier O., Bensson C., Fogliano V., Lamaison J.L., la Fauci L., Galvano G., Remes C., Galvano F. Absorption and metabolism of red orange anthocyanins in rats. Br. J. Nutr. 2006;95:898–904. doi: 10.1079/BJN20061728. [DOI] [PubMed] [Google Scholar]
- 94.Rodriguez-Mateos A., Vauzour D., Krueger C.G., Shanmuganayagam D., Reed J., Calani L., Mena P., del Rio D., Crozier A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014;88:1803–1853. doi: 10.1007/s00204-014-1330-7. [DOI] [PubMed] [Google Scholar]
- 95.Woodward G.M., Needs P.W., Kay C.D. Anthocyanin-derived phenolic acids form glucuronides following simulated gastrointestinal digestion and microsomal glucuronidation. Mol. Nutr. Food Res. 2011;55:378–386. doi: 10.1002/mnfr.201000355. [DOI] [PubMed] [Google Scholar]
- 96.Kuntz S., Rudloff S., Asseburg H., Borsch C., Fröhling B., Unger F., Dold S., Spengler B., Romp A., Kunz C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015;113:1044–1055. doi: 10.1017/S0007114515000161. [DOI] [PubMed] [Google Scholar]
- 97.Ribnicky D.M., Roopchand D.E., Oren A., Grace M., Poulev A., Lila M.A., Havenaar R., Raskin I. Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1) Food Chem. 2014;142:349–357. doi: 10.1016/j.foodchem.2013.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sui X., Zhang Y., Zhou W. In vitro and in silico studies of the inhibition of anthocyanins against pancreatic α-amylase. J. Funct. Foods. 2016;21:50–57. doi: 10.1016/j.jff.2015.11.042. [DOI] [Google Scholar]
- 99.Akkarachiyasit S., Charoenlertkul P., Yibchok-anun S., Adisakwattana S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010;11:3387–3396. doi: 10.3390/ijms11093387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cardona J.A., Mertens-Talcott S.U., Talcott S.T. Phospholipids and terpenes modulate Caco-2 transport of açaí anthocyanins. Food Chem. 2015;175:267–272. doi: 10.1016/j.foodchem.2014.11.119. [DOI] [PubMed] [Google Scholar]
- 101.Walton M.C., McGhie T.K., Reynolds G.W., Hendriks W.H. The flavonol quercetin-3-glucoside inhibits cyanidin-3-glucoside absorption in vitro. J. Agric. Food Chem. 2006;54:4913–4920. doi: 10.1021/jf0607922. [DOI] [PubMed] [Google Scholar]
- 102.Hanske L., Engst W., Loh G., Sczesny S., Blaut M., Braune A. Contribution of gut bacteria to the metabolism of cyanidin 3-glucoside in human microbiota-associated rats. Br. J. Nutr. 2013;109:1433–1441. doi: 10.1017/S0007114512003376. [DOI] [PubMed] [Google Scholar]
- 103.Valdés L., Cuervo A., Salazar N., Ruas-Madiedo P., Gueimonde M., González S. The relationship between phenolic compounds from diet and microbiota: Impact on human health. Food Funct. 2015;6:2424–2439. doi: 10.1039/C5FO00322A. [DOI] [PubMed] [Google Scholar]
- 104.Ozdal T., Sela D.A., Xiao J., Boyacioglu D., Chen F., Capanoglu E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients. 2016;8:78. doi: 10.3390/nu8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang O., Botting N.P., Kay C. A gram scale synthesis of a multi-13C-labelled anthocyanin, [6,8,10,3′,5′-13C5] cyanidin-3-glucoside, for use in oral tracer studies in humans. Chem. Commun. 2011;47:10596–10598. doi: 10.1039/c1cc14323a. [DOI] [PubMed] [Google Scholar]
- 106.Khymenets O., Andres-Lacueva C., Urpi-Sarda M., Vázquez-Fresno R., Mart M.M., Reglero G., Torres M., Llorach R. Metabolic fingerprint after acute and under sustained consumption of a functional beverage based on grape skin extract in healthy human subjects. Food Funct. 2015;6:1288–1298. doi: 10.1039/C4FO00684D. [DOI] [PubMed] [Google Scholar]
- 107.Bouatra S., Aziat F., Mandal R., Guo A.C., Wilson M.R., Knox C., Bjorndahl T.C., Krishnamurthy R., Saleem F., Liu P., et al. The human urine metabolome. PLoS ONE. 2013;8:e73076. doi: 10.1371/journal.pone.0073076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific opinion on the re-evaluation of anthocyanins (e 163) as food additives. EFSA J. 2013;11:3145. [Google Scholar]
- 109.Charoensin S., Taya S., Wongpornchai S., Wongpoomchai R. Assessment of genotoxicity and antigenotoxicity of an aqueous extract of Cleistocalyx nervosum var. paniala in in vitro and in vivo models. Interdiscip. Toxicol. 2012;5:201–206. doi: 10.2478/v10102-012-0033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cassidy A., Rogers G., Peterson J.J., Dwyer J.T., Lin H., Jacques P.F. Higher dietary anthocyanin and flavonol intakes are associated with anti-inflammatory effects in a population of US adults. Am. J. Clin. Nutr. 2015;102:172–181. doi: 10.3945/ajcn.115.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mehta A.J., Cassidy A., Litonjua A.A., Sparrow D., Vokonas P., Schwartz J. Dietary anthocyanin intake and age-related decline in lung function: Longitudinal findings from the VA Normative Aging Study. Am. J. Clin. Nutr. 2016;103:542–550. doi: 10.3945/ajcn.115.121467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ponzo V., Goitre I., Fadda M., Gambino R., De Francesco A., Soldati L., Gentile L., Magistroni P., Cassander M., Bo S. Dietary flavonoid intake and cardiovascular risk: A population-based cohort study. J. Transl. Med. 2015;13:218. doi: 10.1186/s12967-015-0573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hassellund S.S., Flaa A., Kjeldsen S.E., Seljeflot I., Karlsen A., Erlund I., Rostrup M. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: A double-blind randomized placebo-controlled crossover study. J. Hum. Hypertens. 2013;27:100–106. doi: 10.1038/jhh.2012.4. [DOI] [PubMed] [Google Scholar]
- 114.Davinelli S., Bertoglio J.C., Zarrelli A., Pina R., Scapagnini G. A randomized clinical trial evaluating the efficacy of an anthocyanin–maqui berry extract (Delphinol®) on oxidative stress biomarkers. J. Am. Coll. Nutr. 2015;34 doi: 10.1080/07315724.2015.1080108. [DOI] [PubMed] [Google Scholar]
- 115.Zhu Y., Ling W., Guo H., Song F., Ye Q., Zou T., Li D., Zhang Y., Li G., Xiao Y., et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013;23:843–849. doi: 10.1016/j.numecd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 116.Edirisinghe I., Banaszewski K., Cappozzo J., Sandhya K., Ellis C.L., Tadapaneni R., Kappagoda C.T., Burton-Freeman B.M. Strawberry anthocyanin and its association with postprandial inflammation and insulin. Br. J. Nutr. 2011;106:913–922. doi: 10.1017/S0007114511001176. [DOI] [PubMed] [Google Scholar]
- 117.Rodriguez-Mateos A., Rendeiro C., Bergillos-Meca T., Tabatabaee S., George T.W., Heiss C., Spencer J.P. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J Clin. Nutr. 2013;98:1179–1191. doi: 10.3945/ajcn.113.066639. [DOI] [PubMed] [Google Scholar]
- 118.Zhu Y., Xia M., Yang Y., Liu F., Li Z., Hao Y., Mi M., Jinm T., Ling W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin. Chem. 2011;57:1524–1533. doi: 10.1373/clinchem.2011.167361. [DOI] [PubMed] [Google Scholar]
- 119.Wang L.S., Arnold M., Huang Y.W., Sardo C., Seguin C., Martin E., Huang T.H., Riedl K., Schwartz S., Frankel W., et al. Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: A phase I pilot study. Clin. Cancer Res. 2011;17:598–610. doi: 10.1158/1078-0432.CCR-10-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Acquaviva R., Russo A., Galvano F., Galvano G., Barcellona M.L., Volti G.L., Vanella A. Cyanidin and cyanidin 3-O-β-d-glucoside as DNA cleavage protectors and antioxidants. Cell Biol. Toxicol. 2003;19:243–252. doi: 10.1023/B:CBTO.0000003974.27349.4e. [DOI] [PubMed] [Google Scholar]
- 121.Yu R.Q., Wu X.Y., Zhou X., Zhu J., Ma L.Y. Cyanidin-3-glucoside attenuates body weight gain, serum lipid concentrations and insulin resistance in high-fat diet-induced obese rats. Chin. J. Contemp. Pediatr. 2014;16:534–538. [PubMed] [Google Scholar]
- 122.Guo H., Xia M., Zou T., Ling W., Zhong R., Zhang W. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J. Nutr. Biochem. 2012;23:349–360. doi: 10.1016/j.jnutbio.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 123.Guo H., Guo J., Jiang X., Li Z., Ling W. Cyanidin-3-O-β-glucoside, a typical anthocyanin, exhibits antilipolytic effects in 3T3-L1 adipocytes during hyperglycemia: Involvement of FoxO1-mediated transcription of adipose triglyceride lipase. Food Chem. Toxicol. 2012;50:3040–3047. doi: 10.1016/j.fct.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 124.Guo H., Liu G., Zhong R., Wang Y., Wang D., Xia M. Cyanidin-3-O-β-glucoside regulates fatty acid metabolism via an AMP-activated protein kinase-dependent signaling pathway in human HepG2 cells. Lipids Health Dis. 2012;11 doi: 10.1186/1476-511X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ziberna L., Tramer F., Moze S., Vrhovsek U., Mattivi F., Pssamonti S. Transport and bioactivity of cyanidin 3-glucoside into the vascular endothelium. Free Radic. Biol. Med. 2012;52:1750–1759. doi: 10.1016/j.freeradbiomed.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 126.Tsuda T., Ueno Y., Yoshikawa T., Kojo H., Osawa T. Microarray profiling of gene expression in human adipocytes in response to anthocyanins. Biochem. Pharmacol. 2006;71:1184–1197. doi: 10.1016/j.bcp.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 127.Björk C., Wilhelm U., Mandrup S., Larsen B.D., Bordoni A., Hedén P., Rydén M., Arner P., Laurencikiene J. Effects of selected bioactive food compounds on human white adipocyte function. Nutr. Metab. (Lond.) 2016;13 doi: 10.1186/s12986-016-0064-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tsuda T., Ueno Y., Kojo H., Yoshikawa T., Osawa T. Gene expression profile of isolated rat adipocytes treated with anthocyanins. Biochim. Biophys. Acta. 2005;1733:137–147. doi: 10.1016/j.bbalip.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 129.Pimpão R.C., Ventura M.R., Ferreira R.B., Williamson G., Santos C.N. Phenolic sulfates as new and highly abundant metabolites in human plasma after ingestion of a mixed berry fruit purée. Br. J. Nutr. 2015;113:454–463. doi: 10.1017/S0007114514003511. [DOI] [PubMed] [Google Scholar]
- 130.Chemaxon. [(accessed on 10 August 2016)]. Available online: https://www.chemaxon.com/
- 131.Wishart D.S., Jewison T., Guo A.C., Wilson M., Knox C., Liu Y., Djoumbou Y., Mandal R., Aziat F., Dong E., et al. HMDB 3.0—The human metabolome database in 2013. Nucleic Acids Res. 2013;41:D801–D807. doi: 10.1093/nar/gks1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Phenol-Explorer Data Base on Polyphenol Content in Foods, Version 3.6 (Internet Database) [(accessed on 10 August 2016)]. Available online: http://phenol-explorer.eu/
- 133.Xu M., Bower K.A., Wang S., Frank J.A., Chen G., Ding M., Luo J. Cyanidin-3-glucoside inhibits ethanol-induced invasion of breast cancer cells overexpressing ErbB2. Mol. Cancer. 2010;9:285. doi: 10.1186/1476-4598-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pyo M.Y., Yoon S.J., Yu Y., Park S., Jin M. Cyanidin-3-glucoside suppresses Th2 cytokines and GATA-3 transcription factor in EL-4 T cells. Biosci. Biotechnol. Biochem. 2014;78:1037–1043. doi: 10.1080/09168451.2014.912115. [DOI] [PubMed] [Google Scholar]
- 135.Perveen S., Yang J.S., Ha T.J., Yoon S.H. Cyanidin-3-glucoside inhibits ATP-induced intracellular free Ca2+ concentration, ROS formation and mitochondrial depolarization in PC12 cells. Korean J. Physiol. Pharmacol. 2014;18:297–305. doi: 10.4196/kjpp.2014.18.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Serra D., Paixão J., Nunes C., Dinis T.C., Almeida L.M. Cyanidin-3-glucoside suppresses cytokine-induced inflammatory response in human intestinal cells: Comparison with 5-aminosalicylic acid. PLoS ONE. 2013;8:e73001. doi: 10.1371/journal.pone.0073001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang D.A., Xia M., Gao S., Li D., Zhang Y., Kin T., Ling W. Cyanidin-3-O-β-glucoside upregulates hepatic cholesterol 7α-hydroxylase expression and reduces hypercholesterolemia in mice. Mol. Nutr. Food Res. 2012;56:610–621. doi: 10.1002/mnfr.201100659. [DOI] [PubMed] [Google Scholar]
- 138.Skemiene K., Liobikas J., Borutait V. Anthocyanins as substrates for mitochondrial complex I—protective effect against heart ischemic injury. FEBS J. 2015;282:963–971. doi: 10.1111/febs.13195. [DOI] [PubMed] [Google Scholar]
- 139.Zhou Z., Nair M.G., Claycombe K.J. Synergistic inhibition of interleukin-6 production in adipose stem cells by tart cherry anthocyanins and atorvastatin. Phytomedicine. 2012;19:878–881. doi: 10.1016/j.phymed.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 140.Zeng L., Gao J., Zhang R. Study on anti-tumor effect of cyanidin-3-glucoside on ovarian cancer. Zhongguo Zhong Yao Za Zhi. 2012;37:1651–1654. [PubMed] [Google Scholar]
- 141.Wu T., Qi X., Liu Y., Guo J., Zhu R., Chen W., Yu T. Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chem. 2013;141:482–487. doi: 10.1016/j.foodchem.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 142.Zhang Y., Wang X., Wang Y., Liu Y., Xia M. Supplementation of cyanidin-3-O-β-glucoside promotes endothelial repair and prevents enhanced atherogenesis in diabetic apolipoprotein E–deficient mice. J. Nutr. 2013;143:1248–1253. doi: 10.3945/jn.113.177451. [DOI] [PubMed] [Google Scholar]
- 143.Hassimotto N.M., Moreira V., do Nascimento N.G., Souto P.C., Teixeira C., Lajolo F.M. Inhibition of carrageenan-induced acute inflammation in mice by oral administration of anthocyanin mixture from wild mulberry and cyanidin-3-glucoside. BioMed Res. Int. 2013;2013:146716. doi: 10.1155/2013/146716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sun C.D., Zhang B., Zhang J.K., Xu C.J., Wu Y.L., Li X., Chen K.S. Cyanidin-3-glucoside-rich extract from Chinese bayberry fruit protects pancreatic β cells and ameliorates hyperglycemia in streptozotocin-induced diabetic mice. J. Med. Food. 2012;15:288–298. doi: 10.1089/jmf.2011.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wei X., Wang D., Yang Y., Xia M., Li D., Li G., Zhu Y., Xiao Y., Ling W. Cyanidin-3-O-β-glucoside improves obesity and triglyceride metabolism in KK-Ay mice by regulating lipoprotein lipase activity. J. Sci. Food Agric. 2011;91:1006–1013. doi: 10.1002/jsfa.4275. [DOI] [PubMed] [Google Scholar]
- 146.Liu J., Zhou J., Wu Z., Wang X., Liu L., Yao C. Cyanidin-3-O-β-Glucoside ameliorates ethanol-induced acute liver injury by attenuating oxidative stress and apoptosis: The role of SIRT1/FOXO1 signaling. Alcohol. Clin. Exp. Res. 2016;40:457–466. doi: 10.1111/acer.12982. [DOI] [PubMed] [Google Scholar]
- 147.Lee S.H., Jeong E., Paik S.S., Jeon J.H., Jung S.W., Kim H.B., Kim M., Chun M.H., Kim I.B. Cyanidin-3-glucoside extracted from mulberry fruit can reduce N-methyl-N-nitrosourea-induced retinal degeneration in rats. Curr. Eye Res. 2013;39:79–87. doi: 10.3109/02713683.2013.825275. [DOI] [PubMed] [Google Scholar]
- 148.Ke Z., Liu Y., Wang X., Fan Z., Chen G., Xu M., Bower K.A., Frank J.A., Ou X., Shi X., et al. Cyanidin-3-glucoside ameliorates ethanol neurotoxicity in the developing brain. J. Neurosci. Res. 2011;89:1676–1684. doi: 10.1002/jnr.22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Qin L., Zhang J., Qin M. Protective effect of cyanidin 3-O-glucoside on beta-amyloid peptide-induced cognitive impairment in rats. Neurosci. Lett. 2013;534:285–288. doi: 10.1016/j.neulet.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 150.Busch C., Burkard M., Leischner C., Lauer U.M., Frank J., Venturelli S. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin. Epigenetics. 2015;7:1–18. doi: 10.1186/s13148-015-0095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kiemlian Kwee J. Yin and Yang of polyphenols in cancer prevention: A short review. Anti-Cancer Agents Med. Chem. 2016;16:832–840. doi: 10.2174/1871520616666151116124549. [DOI] [PubMed] [Google Scholar]
- 152.Zhang C., Guo X., Cai W., Ma Y., Zhao X. Binding characteristics and protective capacity of Cyanidin-3-glucoside and its aglycon to calf thymus DNA. J. Food Sci. 2016;80:H889–H893. doi: 10.1111/1750-3841.12823. [DOI] [PubMed] [Google Scholar]
- 153.Shi J.H., Wang J., Zhu Y.Y., Chen J. Characterization of intermolecular interaction between cyanidin-3-glucoside and bovine serum albumin: Spectroscopic and molecular docking methods. Luminescence. 2014;29:522–530. doi: 10.1002/bio.2579. [DOI] [PubMed] [Google Scholar]
- 154.Cahyana Y., Gordon M.H. Interaction of anthocyanins with human serum albumin: Influence of pH and chemical structure on binding. Food Chem. 2013;141:2278–2285. doi: 10.1016/j.foodchem.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 155.Sheng F., Wang Y., Zhao X., Tian N., Hu H., Li P. Separation and identification of anthocyanin extracted from mulberry fruit and the pigment binding properties toward human serum albumin. J. Agric. Food Chem. 2014;62:6813–6819. doi: 10.1021/jf500705s. [DOI] [PubMed] [Google Scholar]
- 156.Adisakwattana S., Yibchok-Anun S., Charoenlertkul P., Wongsasiripat N. Cyanidin alleviates postprandial hyperglycemia and its synergism with acarbose by inhibition of intestinal α glucosidase. J. Clin. Biochem. Nutr. 2011;49:36–41. doi: 10.3164/jcbn.10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pérez-Díaz R., Madrid-Espinoza J., Salinas-Cornejo J., González-Villanueva E., Ruiz-Lara S. Differential roles for VviGST1, VviGST3, and VviGST4 in proanthocyanidin and anthocyanin transport in Vitis vinífera. Front Plant Sci. 2016;7 doi: 10.3389/fpls.2016.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Trott O., Olson A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]