Abstract
Multidrug resistance (MDR) to anticancer drugs is a serious health problem that in many cases leads to cancer treatment failure. The ATP binding cassette (ABC) transporter P-glycoprotein (P-gp), which leads to premature efflux of drugs from cancer cells, is often responsible for MDR. On the other hand, a strategy to search for modulators from natural products to overcome MDR had been in place during the last decades. However, Nature limits the amount of some natural products, which has led to the development of synthetic strategies to increase their availability. This review summarizes the research findings on marine natural products and derivatives, mainly alkaloids, polyoxygenated sterols, polyketides, terpenoids, diketopiperazines, and peptides, with P-gp inhibitory activity highlighting the established structure-activity relationships. The synthetic pathways for the total synthesis of the most promising members and analogs are also presented. It is expected that the data gathered during the last decades concerning their synthesis and MDR-inhibiting activities will help medicinal chemists develop potential drug candidates using marine natural products as models which can deliver new ABC transporter inhibitor scaffolds.
Keywords: ABC transporters, P-glycoprotein modulators, marine natural products, multidrug resistance (MDR), synthesis, structure activity relationship (SAR)
1. Introduction
It is well known that cancer figures among the leading causes of morbidity and mortality [1]. Despite many advances in therapy, diagnosis, and prevention, cancer remains a deadly disease. Although the general term cancer covers many different diseases, most disseminated cancers share a common feature of not responding to available chemotherapies. The remorseless onset to resistance to a wide variety of structurally and mechanistically unrelated anticancer drugs, known as multidrug resistance (MDR), is the major obstacle for a successful human cancer therapy [2]. This eventually leads to cancer relapse and death. Some cancers such as gastrointestinal and renal cancers are largely unresponsive to chemotherapy, i.e., they have a high degree of intrinsic MDR, whereas leukemias, lymphomas, ovarian, and breast cancers often respond to initial treatment, but then acquire MDR during the course of the disease [3]. MDR to anticancer drugs is therefore a serious health problem that dramatically affects the efficacy of cancer treatments [4]. MDR is a phenomenon by which cancer cells develop broad resistance to a wide variety of structurally and functionally unrelated compounds which may arise from several mechanisms [5,6] including: (i) decreased cellular drug uptake; (ii) activation of detoxifying enzymes; (iii) alterations in the molecular targets of the drugs; (iv) defective apoptotic pathways; and (v) increased drug efflux [6,7,8,9]. One of the most prominent mechanisms of MDR to cytotoxic drugs is usually associated with the overexpression of ATP-binding cassette (ABC)-transporter proteins in the tumor cells. Due to the increased efflux out of cells, these pumps can lead to a reduced cellular accumulation of anticancer drugs. These proteins mediate MDR not only in drug entry, but also in drug sequestration by lysosomes, phase III metabolism, mechanisms activated after nuclear entry, apoptosis, microenvironment, and signal transduction pathways that lead to the overexpression of genes that codify these ABC transporters [10]. Such proteins belong to a large family of 49 genes, classified in seven subfamilies (A–G), with several transporters involved in MDR [11]. These include P-glycoprotein (P-gp or ABCB1), multidrug resistance associated proteins 1-6 (MRP1-6 or ABCC1-6), and breast cancer resistance protein (BCRP or ABCG2) [12]. P-gp is the best characterized efflux pump mediating MDR due to its broadest substrate specificity and widest tissue and organ distribution. This 170 kDa glycoprotein acts as a drug efflux pump for a wide variety of anticancer agents such as anthracyclines and epidodophyllotoxins, thus limiting their bioavailability and activity. P-gp is encoded by the human MDR-1 gene, which is located at chromosome 7. It contains 1280 amino acids, arranged in two halves, each encompassing a transmembrane domain (TMD) which spans the membrane and an intracellular nucleotide-binding domain (NBD) [13,14].
Several studies have already correlated P-gp expression with resistance to chemotherapeutic drugs, particularly in leukemia cells [15]. Furthermore, down-regulation of P-gp expression was shown to sensitize several tumor-resistant cell lines to chemotherapeutic drugs. Indeed, the use of antisense or rybozyme targeting MDR-1 gene has led to the sensitization of acute myeloid leukemia (AML), ovarian, colon, and breast cancer cells to doxorubicin as well as to increase the sensitivity of chronic and AML cells to daunorubicin [16,17].
It was found that P-gp could be expressed in Chinese hamster ovary cells, selected for colchicine resistance, almost 40 years ago, and since then there has been an ongoing effort to develop therapies that could either block or inactivate this transporter to increase the concentration of anticancer drugs within cells [18]. First generation of P-gp inhibitors referred to drugs already in clinical use or under investigation for therapeutic ability e.g., verapamil, quinidine, and cyclosporine A [19]. However, most of the first generation P-gp inhibitors were found to lack selectivity for P-gp and being substrates for other transporters and enzyme systems; this promiscuity resulted in unpredictable pharmacokinetic interactions in the presence of anticancer drugs [20]. Moreover, low affinity for P-gp, associated with the original therapeutic activity, required the use of high doses which resulted in unacceptable toxicity [6,21]. Second generation of P-gp inhibitors were developed, based on the selective optimization of side activity (SOSA) approach, to increase the potency and reduce toxicity, many of which were single enantiomers of the first generation drugs. An example of these is dexverapamil which is an R-enantiomer of verapamil. Interestingly, many of the second generation P-gp inhibitors such as valspodar (PSC-833), elacridar (GF 120918), and biricodar, also entered clinical trials [6]. Although some of the second generation inhibitors showed better pharmacological profiles in clinical trials, they still had limited use as P-gp modulators since most of them were found to be substrates of cytochrome P450 3A, thus interfering with the metabolism and excretion of co-administered chemotherapeutic agents [22]. The third generation of P-gp inhibitors, which exhibited P-gp inhibitory activity at nanomolar concentrations, were developed to overcome the referred problems through quantitative structure-activity relationships (QSAR) studies and combinatorial chemistry approaches [23]. Since the third generation of inhibitors that entered clinical trials did not interfere with cytochrome P450 3A4, they did not reveal any interference with pharmacokinetics of anticancer drugs [24]. One of the most popular third generation P-gp inhibitors is tariquidar, however, its phase III studies on non-small cell lung cancer patients were terminated due to its high toxicity [25]. The same unwanted results were observed with other third generation inhibitors like zosuquidar, elacridar, laniquidar, and ontogeny [20,26]. Although phase I and II trials were performed with some third generation P-gp inhibitors, an emerging consensus, in 2010, was that P-gp should be taken off the list of druggable targets due to the failure of zosuquidar to show clinical benefits in phase III clinical trials [27]. Until now, the existing P-gp inhibitors have demonstrated limited clinical success due to their limitations in potency and specificity and also to their interactions with anticancer drugs. Moreover, QSAR and docking studies also could not produce promising hits or leads for safe and effective compounds. As there is a permanent need to identify and validate new antitumor agents, with novel mechanisms of action, more efficient P-gp inhibitors are needed.
Recently, there has been a proposal of a new generation of ABC inhibitors which focuses on natural products and natural product mimics [28,29,30], peptidomimetics [31], surfactants and lipids [32], and dual ligands [20]. While the traditional sources of terrestrial plants and microbes will undoubtedly continue to yield valuable new bioactive agents, it is even more important to explore all available pools of molecular diversity. This strategy to seek out new ecosystems for bioprospecting brings with it the opportunity to discover unprecedented molecular structures, with bioactivities unencumbered by known (and evolving) mechanisms of drug resistance [33]. Marine natural products are one of the most interesting targets for global drug discovery since they not only possess structural and chemical uniqueness but also represent novel scaffolds for drug development [34]. Marine natural products have also been studied and tested alongside with synthetic compounds as ABC transporter inhibitors [29,35,36,37,38]. The development of anticancer drugs from marine compounds is one of the most promising approaches in drug discovery with therapeutic agents in clinical practice, such as the anticancer trabectedin [37]. Recently, marine natural products and their analogs with antitumor activity have been developed, and many of them have shown also ability for ABC modulation. Thus, marine natural products are revealing an interesting potential in this field not only for the possibility of being used in combination with other anticancer drugs but also as dual inhibitors of tumor cell growth and P-gp. Thus, they could be of value in the rational design of analogs with high potential and reduced pharmacokinetic interactions.
There are already several reviews which highlighted the importance of marine natural products in cancer and, in particular, as P-gp modulators [29,35,39,40,41,42,43]. However, this review aims to describe not only the effects of the most valuable scaffolds from marine natural products and analogs in overcoming MDR but also their synthetic pathways. These data along with structure-activity relationship herein described could serve to guide medicinal chemists in pursuing innovative inhibitors of ABC transporters using marine natural products as models.
2. Marine Natural Products and Derivatives as Inhibitors of ABC Transporters
2.1. Terpenoids
2.1.1. Sipholane Triterpenoids and Derivatives
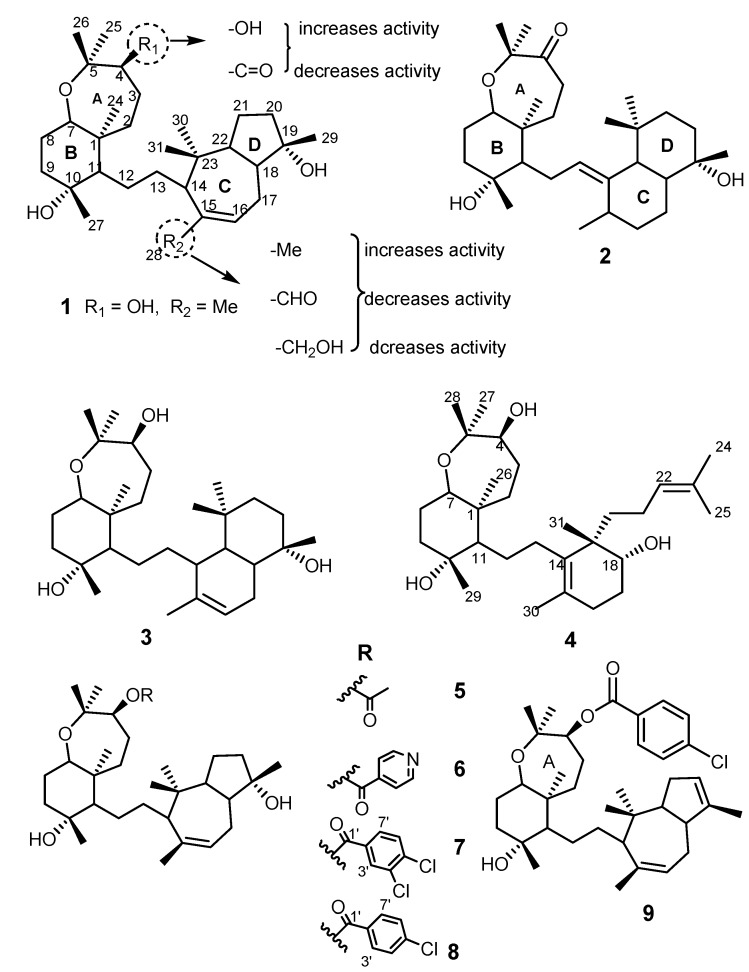
The sipholane triterpenes are compounds that contain a perhydrobenzoxepin (rings A and B) and a [5,3,0] bicyclodecane ring systems (rings C and D) linked together by an ethylene group (Figure 1). This class of triterpenes consists of sipholenol A (1), sipholenone E (2), sipholenol L (3), and siphonellinol D (4), isolated from the Red Sea sponge Callyspongia siphonella [44,45], and semisynthetic derivatives of sipholenol A such as sipholenol A-4-O-acetate (5), sipholenol A-4-O-isonicotinate (6) [39], sipholenol A-4-O-3′,4′-dichlorobenzoate (7) [46], sipholenol A 4-O-4′-chlorobenzoate (8), and 19,20-anhydrosipholenol A 4-O-4′-chlorobenzoate (9) [47] that were synthesized using a ligand-based design approach. Natural and semisynthetic siphonane triterpenoids were shown to reverse MDR activities (1–6), and display antimigratory and antiproliferative activities in breast cancer cell lines (7–9). Sipholenol A (1) was shown to be potent in reversing MDR KB-C2 [48] and KB-V1 tumor cells with overexpressed P-gp, in a concentration-dependent manner. Compound 1 also inhibited P-gp-mediated drug efflux and did not alter the expression of P-gp after treatment in KB-C2 and KB-V1 cells. This compound stimulated the activity of ATPase, and also inhibited the photo-labeling transporter with [125I]-iodoarylazidoprazosin [44]. From SAR studies (Figure 1), it was demonstrated that substitution of the methyl group on C-15 by carbonyl or alcohol reduced the MDR-inhibitory activity, while changing from a hydroxyl to a ketone function at C-4 also reduced the activity [48]. Compounds 2, 3, and 4 also enhanced the cytotoxicity of several P-gp substrates including colchicine, vinblastine, and paclitaxel, and reversed the MDR-phenotype in P-gp-overexpressing MDR KB-C2 tumor cells, in a dose-dependent manner [45]. Compound 2 was reported as a better P-gp inhibitor than 1 in KB-3-1 and KB-C2 cell lines [49], while 3 and 4 also showed a reversal of MDR in the same tumor cells, but did not reveal the effect on cells lacking P-gp expression or expressing MRP1, MRP7, or BCRP transporters [36,45,49].
Figure 1.
Structures of sipholane triterpenes 1–4 and their analogs 5–9. Dashed circles indicate SAR studies performed for MDR activities.
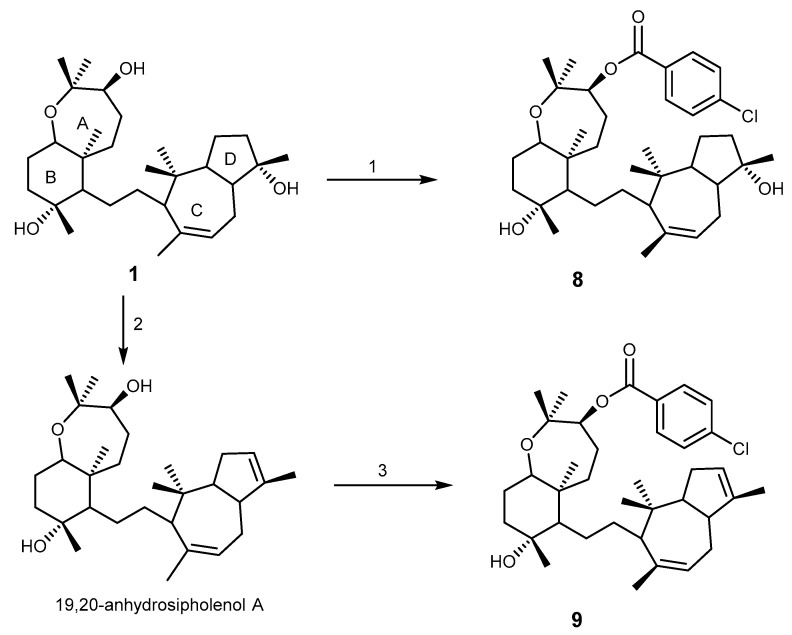
The semisynthetic esters 5 and 6 were found to strongly reverse P-gp (ABCB1)-mediated MDR, however they showed no effect on MRP1/ABCC1 and BCRP/ABCG2-mediated MDR. These analogs increased the intracellular accumulation of paclitaxel through inhibition of its active efflux, and re-sensitized cells that have developed drug-resistance to doxorubicin and paclitaxel. These two compounds stimulated more intensively the ATPase activity of P-gp membranes than 2, 3, and 4. In silico molecular docking study, by the inspection of the virtual binding modes of compounds 5 and 6 into human homology model of P-gp, revealed that both compounds were overlapped but with different molecular orientation of ester substituents. Their strong affinity and specificity to P-gp expressing efflux protein indicated that compounds 5 and 6 may represent a potential reversal agents for treatment of MDR cancers [39]. The synthesis of the ester analogs 8 and 9 is presented in Scheme 1, to highlight the strategy involved (using a ligand-based design approach).
Scheme 1.
The semi-synthesis transformation of sipholenol A (1) into analogs 8 and 9. Reagents and conditions: (1) acid anhydride, MDAP, anhydrous CH2Cl2; (2) p-toluenesulfonic acid, CHCl3; (3) acid anhydride, MDAP, anhydrous CH2Cl2.
2.1.2. Parguerenes and Derivatives
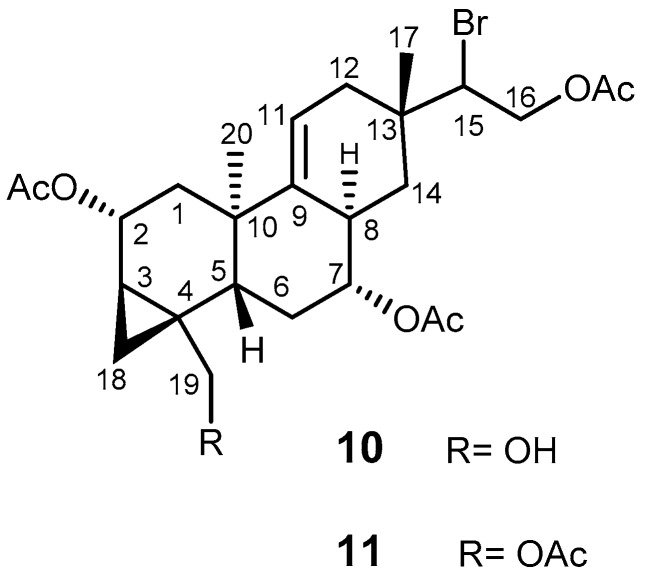
Parguerenes I (10) and II (11) (Figure 2) are bromoditerpenes, isolated from the Australian red alga, Laurencia filiformis [50]. Several brominated diterpenes of the parguerenes and isoparguerenes isolated from red alga Jania rubens were reported to have antitumor, anti-helmintic, and antimicrobial activities [51]. Parguerene derivatives with cytotoxic activity on P388 and HeLa tumor cells possessed an acetoxy group at C-2 and a bromine at C-15 [52]. Compounds 10 and 11 were found to be non-cytotoxic and dose-dependent inhibitors of P-gp mediated drug efflux of verapamil and cyclosporine A. It was also reported that 10 and 11 are capable of reversing P-gp mediated vinblastine, doxorubicin, and paclitaxel in cells overexpressing both P-gp (SW620/ADV300, CEM/VLB100, and HEK93/ABCB1) and MRP1 (2008/MRP1), in a dose-dependent manner. However, their inhibitory effect did not extend to BCRP. Compounds 10 and 11 interact with P-gp by disturbing the extracellular antibody binding epitope of P-gp differently from existing P-gp inhibitors [53]. Therefore, the use of this scaffold as a model for the synthesis of new MDR reversal agents could be of value. To the best of our knowledge, the synthesis of parguerenes has not yet been reported.
Figure 2.
The structures of parguerene I (10) and II (11).
2.2. Sterols
2.2.1. Agosterol and Derivatives
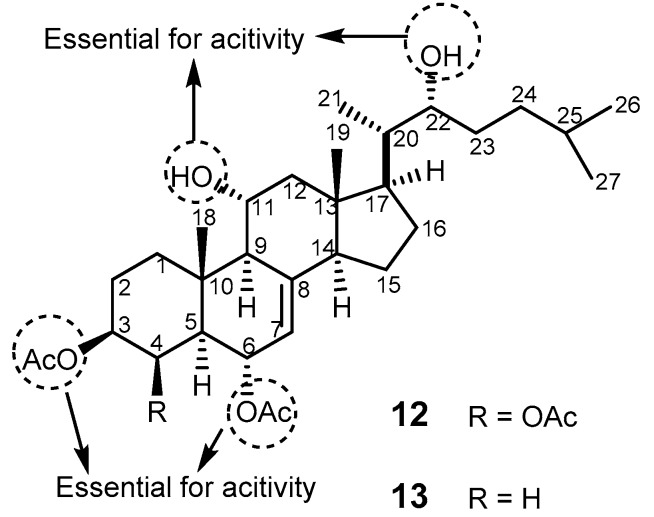
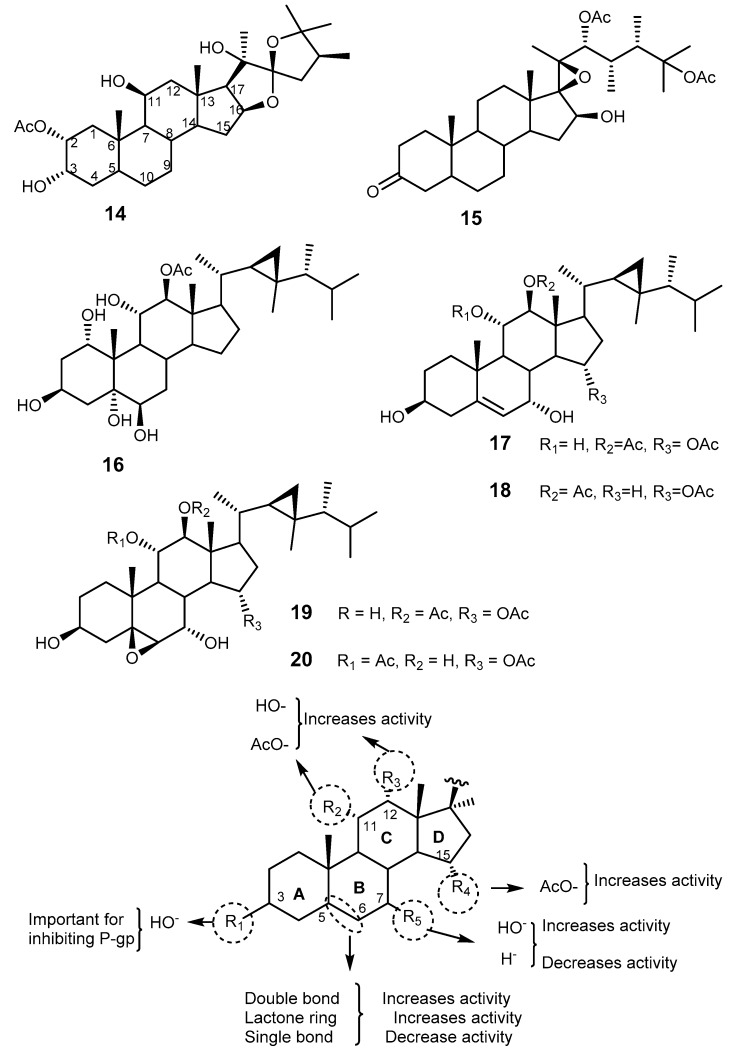
Agosterol A (12, Figure 3), a polyhydroxylated sterol acetate isolated from the marine sponge Spongia sp. [54], was found to completely reverse MDR to colchicine in human carcinoma cells KB-C2 and to vincristine in KB-CV60 (overexpressing MRP1) [55]. Compound 12 was reported to have a dual effect on MRP1 function by reducing MRP1-mediated [3H]-LTC4 and enhancing the accumulation of [3H]-vincristine in KB/MRP cells to the control levels. It also enhances the ATP-dependent efflux and reduces glutathione intracellular concentration [56]. Therefore, 12 has inhibitory effects on both P-gp and MRP1. The effect of analogs of 12, including agosterol B, C, A4, D2, A5 and C6, on MDR in tumor cells was also investigated. Agosterol C was found to be a proteasome inhibitor [57]. From the SAR studies, it was possible to infer that the acetoxy groups on C-3, C-4, and C-6, and the hydroxyl groups on C-11 and C-12 were crucial for MDR reversal activity for binding to the C-terminal of MRP1 (Figure 3) [58,59]. 4-Deacetoxyagostrol A (13) showed a similar MDR-modulating activity against KB CV-60 cell overexpressing MRP [60].
Figure 3.
Structure of agosterol A (12) and 4-deacetoxyagosterol A (13). Dashed circles indicate the groups essential for reversing MDR activity.
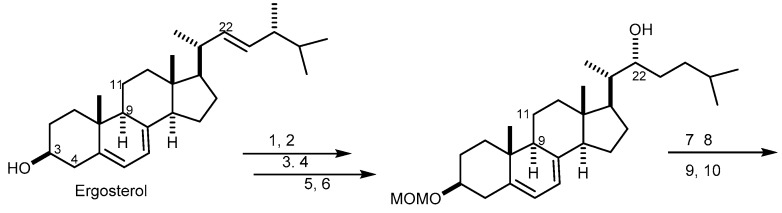
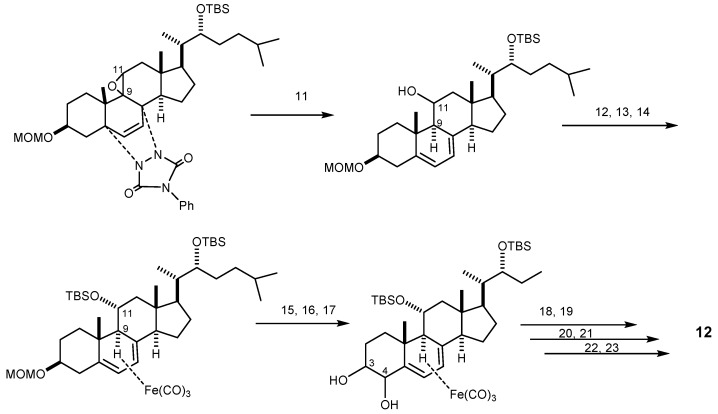
The first synthesis of 12 from ergosterol utilizing regioselective epoxy function cleavage and regioselective dehydroxylation as key reactions, and affording a 3.5% yield after 23 steps, was reported in 2001 [61]. The synthetic pathway started with the oxidative cleavage of the C-22/C-23 double bond of ergosterol to introduce a hydroxyl group to C-22, followed by a regioselective reductive epoxy cleavage of the 9α,11α-epoxide to form an 11α-hydroxyl group. The next step was the introduction of a C-3/C-4 double bond which, after a selective dehydroxylation, afforded 3β,4β-dihydroxyl groups. Finally, a differential removal of the protecting group and acetylation afforded 12 (Scheme 2). Compound 13 was also synthesized from ergosterol by reductive regioselective epoxy-cleavage reaction similar to that of 12 [60].
Scheme 2.
The synthetic pathway of agosterol A (12). Reagents and conditions: (1) MOMCl, iPr2NEt, CH2Cl2; (2) phthalhydrazide, Pb(OAc)4, CH2Cl2, AcOH, two steps; (3) O3,CH2Cl2, pyridine, then Me2S; (4) 3-methylbutylmagnesium bromide, THF, two steps; (5) TMAD, PMe3, p-OCH3BzOH,THF; (6) LiAlH4, THF; (7) (S)-(−)-MTPA[(R)-(+)-MTPA], EDCl.HCl, DMAP, CH2Cl2; (8)TBSOTf, 2,6-lutidine, DMF-CH2Cl2; (9) Hg(OAc), EtOH/CHCl3/AcOH; (10) 4-phenyl-1,2,4-triazoline-3,5-dione, CH2Cl2; (11) mCPBA, CHCl3; (12) LiAlH4, THF; (13) TBSOTf, 2,6-lutidine, toluene; (14) Fe(CO)3, 1-(4-methyoxyphenyl)-4-pheynyl-1-azabuta-(E,E)-1,3-diene, PhCH3; (15) MgBr2-Et2O, Me2S, CH2Cl2; (16) TsCl, pyridine, quant.; (17) DBN, PhH, quant.; (18) OsO4, pyridine, then aq. NaHSO3; (19) TESOTf, pyridine, quant.; (20) Me3NO, PhH; (21) BH3Me2S, THF, then H2O2, aq. NaOH; (22) TBAF, THF; (23) Ac2O, pyridine; HF, pyridine, THF.
2.2.2. Polyoxygenated Steroids and Derivatives
A number of polyoxygenated steroids has been isolated from gorgonians (Isis hippuris [62] and Leptogorgia sarmentosa [63]), soft corals (Sarcophyton sp. [64] and Sinularia sp. [65]), echinoderms, and sponges [62]. Interestingly, all the polyoxygenated steroids investigated for antitumor [66], antibacterial [64], antifungal [64], and reversing MDR [62] activities contain the characteristic 3β,5α,6β-hydroxyl moiety. Among these derivatives are a spiroketal hippurinstanol (14), hippuristerone (15), which possesses a 3-keto group, and the cyclopropane-containing gorgosterols (16–20) (Figure 4) [62,64]. Polyoxygenated steroids 14–20 also differ in the side chains. Some of them (compounds 16–20) have been tested against KB-C2 overexpressing P-gp cells and showed moderate activity at the concentration of 3 μM by inhibiting the growth of this resistance cell line. Compounds 17 and 18 were the most potent against KB-C2 cells [62].
Figure 4.
The structures of polyoxygenated steroids 14–20, and SAR for polyoxygenated steroids with antitumor activity in overexpressing P-gp cells.
Semi-synthetic derivatives of 3,16,20-polyoxygenated cholestanes, namely (20S)-hydroxy-cholestane-3,6-dione (21), (16S,20S)-dihydroxycholestan-3-one (22), (20S)-hydroxycholest-1-ene-3,16-dione (23), and (20S)-hydroxycholest-4-ene-3,16-dione (24) were tested for cytotoxicity against three tumor cell lines, i.e., human breast adenocarcinoma (MCF7), human small-cell lung carcinoma (NCI-H187), and human epidermoid carcinoma of cavity (KB).
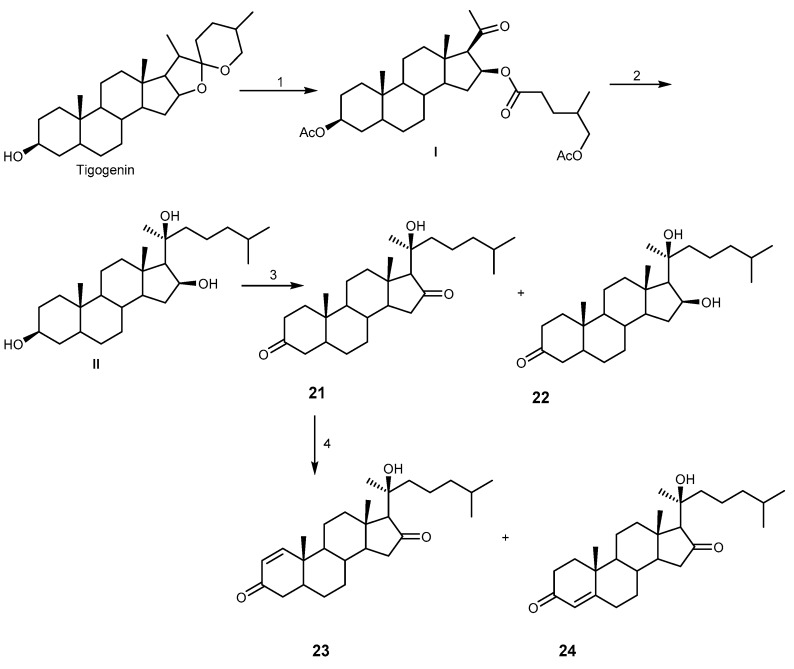
Compounds 23 and 24 showed strong activity against NCI-H187, and moderate activity against MCF-7 and KB, while compound 21 did not show any activity [63]. Although no MDR-reversal activities were described, these compounds provided a promising synthetic approach for natural polyoxygenated steroids. The synthesis of these four analogs, 21–24, was conducted through four reaction steps, using tigogenin as a starting material, as shown in Scheme 3.
Scheme 3.
The synthesis of 3,16,20-polyoxygenated steroids 21–24. Reagents and conditions: (1) I: NH4Cl, pyridine, AcOH; II: CrO3, AcOH, H2O, (CH2Cl2)2; (2) 4-methylpentylmagnesium bromide, THF; (3) PCC, NaOAc, CH2Cl2; (4) Ph2Se2, m-iodomybenzoic acid, toluene, reflux.
The synthetic pathway started with the transformation of a spiroketal functionality of tigogenin to a keto ester using the method previously described by Micovic et al., and modified by Fushs et al. [67]. The keto ester I was then transformed to a trihydroxyl intermediate II by Grignard reagent which, after oxidation, gave compounds 21 and 22. Dehydrogenation of 21 then afforded 23 and 24 [63]. In addition, the introduction of epoxidation and dihydroxylation in suitable positions of steroid nucleus containing oxygenated functions was reported by using transition metal-based oxidants such as methyltrioxorhenium-hydrogen peroxide system, ruthenium tetraoxide, osmium tetraoxide, and potassium permanganate [68].
2.3. Polyketides
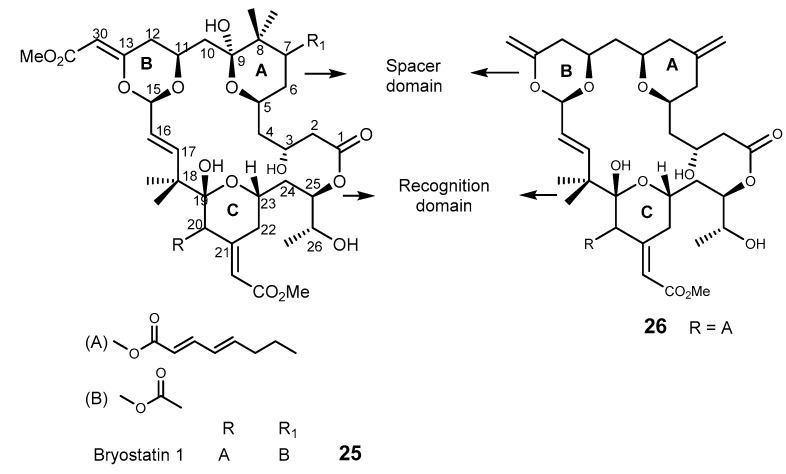
2.3.1. Bryostatin and Derivatives
Bryostatins are highly oxygenated marine macrolides with a polyacetate backbone [69], and more than 20 analogs have been isolated from the marine bryozoans Bugula neritina and Amathia convuluta [70]. Most of bryostatins possess antineoplastic activity in many cancer systems like M5076 sarcoma, L10AB cell, and mouse lymphogenous metastatic model SCID.
Bryostatin 1 (25, Figure 5) was reported to down-regulate MDR-1 and BCL-2, but up-regulates BAX (induction of apoptosis) [71,72]. Spitaler et al. reported that 25 interacted with the mutated MDR-1-V185 and the wild-type MDR-1-G185, but reversed MDR and inhibited drug efflux only in P-gp-V185 mutants [72]. The mechanism of regulating MDR for 25 was also reported as protein kinase C (PKC) independent, and it seems to interact directly with MDR-1 encoded P-gp [73]. However, in a human breast cancer cell line (MCF-7), 25 only decreases P-gp phosphorylation after 24 h treatment in a concentration of 100 nM, but did not affect P-gp function in the intracellular accumulation of [3H]-vinblastine and rhodamine 123 [74]. It was found that C-1 to C-9 segment of bryostatins is suitable for both synthetic and biological investigation, and this segment was prepared from an inexpensive and easily accessible compound 2,2-dimethyl-8-oxabicyclo[3,2,1]oct-6-en-3-one [69]. Merle 23 (26, Figure 5), is a synthetic analog of bryostatin and whose structure differs from that of bryostatin 1 (25) in four positions of the space domain fragment. Interestingly, merle 23 (26) showed phorbol ester-like behavior not that of bryostatin in a human prostate cancer cell line (LNCaP), and this finding provided a powerful tool to dissect bryostatins’ mechanisms and responses [75,76].
Figure 5.
The structures of bryostatin 1 (25) and merle 23 (26).
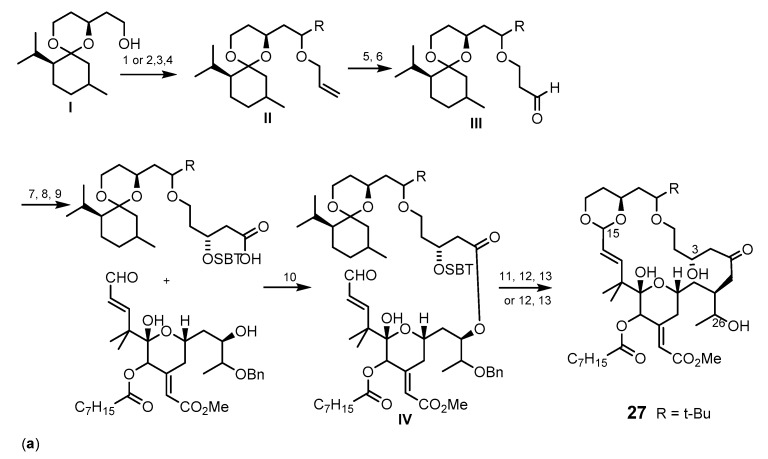
The synthesis of bryostatin analog 27 (Scheme 4a) without ring A and with simplified ring B was carried out by a convergent esterification-macrotransacetalization procedure to couple the recognition (C-17–C-27) and the spacer (C-1–C-16) domain fragments (Figure 5) [70]. The synthetic pathway involves an allylation of an appropriate alcohol I to give the ether intermediate II. Hydroboration of the ether intermediate II, followed by oxidation gave the appropriate aldehyde III, which was then subjected to an oxidative cleavage reaction to furnish the spacer domain. A Yamanguchi esterification was used to produce the next intermediate IV, after which macrotranscetalization and deblocking of C-26 alcohol were accomplished to yield bryostatin analog 27 in 11 steps (Scheme 4a) [77].
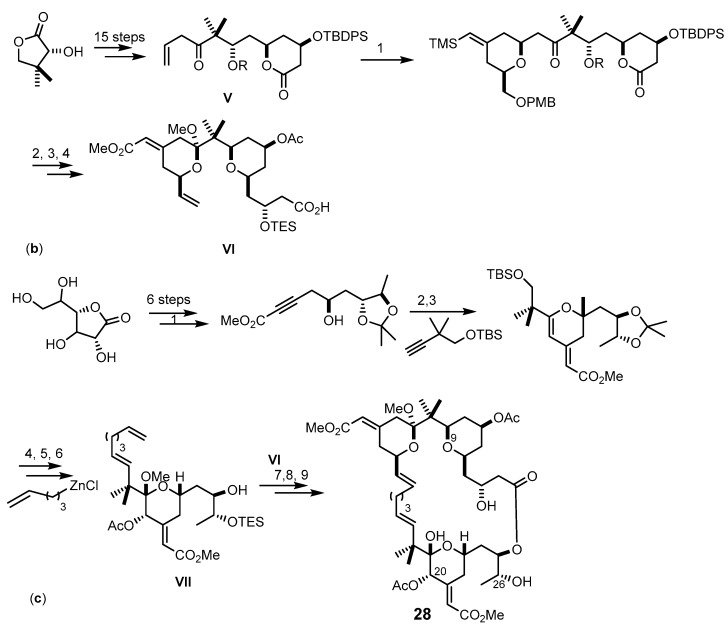
Scheme 4.
Synthesis of the methyl bryostatin 1 (25) analogs 27 (a) and 28 (b,c). Reagents and conditions: (a) (1) NaH, allybromide, THF, rt; (2) Dess-Martin periodinane, CH2Cl2, rt; (3) t-BuLi, Et2O, −78 °C, (recyclable 1:1 diastereomer mixture: Dess-Martin periodinane, CH2Cl2, rt, then NaBH4, CeCl3·7H2O, −40 °C; (4) t-BuOH, allyl bromide, THF, rt; (5) 9-BBN, THF, 66 °C, then NaOH, H2O2; (6) Dess-Martin periodinane, CH2Cl2, rt; (7) (−)-(lpc)BOMe, allylmagnesium bromide, CH2Cl2, −78 °C to rt; (8) TBSCl, imidazole, THF, rt; (9) catalytic KMnO4, NaIO4, rt; (10) 2,4,6-trichloro-benzoylchloride, Et3N, DMAP, CH2Cl2, rt; (11) HF·pyridine, CH3CN, rt; (12) Amberlyst-15 resin, CH2Cl2, rt; (13) Pd(OH)2, H2, EtOAc, 1 atm; (b) (1) 10 mol % of [CpRu(CH3CN)3]PF6, acetone, rt; (2) NBS, DMF then BF3-OEt2, 1,3-propanedithiol, CH2Cl2, 0 °C then PPTS, CH3OH, CH(OCH3)3, refux; (3) TESCl, DMAP, then pyridine, Ac2O; PPTS, MeOH, rt; DMSO, (COCl)2, Et3N, CH2Cl2 −78 °C; Ph3PCH3Br, n-BuLi; (4) TBAF, THF, rt; Me3SnOH, DCE, 140 °C, microwave; Pd(PPh3)4, CO, DMF/CH3OH, 85 °C; TESOTf, 2,6-lutidine, CH2Cl2; (c) (1) n-BuLi, methyl propionate, BF3·OEt2, THF, −78 °C; (2) Pd(OAc)2, tris(2,6-dimethoxyphenyl) phosphine, benzene, then Pd(O2CCF3)2, rt; (3) trifluoroperacetic acid, NaHPO4, CH2Cl2/CH3CN/ CH3OH, 0 °C; Dess-Martin oxidation; NaBH4, CeCl3·7H2O, −30o°C; Ac2O, pyridine, DMAP, CH2Cl2, rt; (4) CrCl2, CHI3, THF, rt; 26% (47% BRSM); Pd(PPh3)4, THF, rt; (5) AcOH/H2O; (6) TESCl, ETA, DMF, −35 to −15 °C; (7) Et3N, DMAP, 2-mehyl-6-nitrobenzoic acid anhydride, CH2Cl2; (8) benzene, 50–80 °C, 17 mol % of Grubbs-Hoveyda catalyst; (9) PPTS, MeOH, rt.
Trost et al. reported the synthesis of the bryostatin analog 28 with cross-metathesis approach at C-16–C-17 bond using ruthenium and palladium coupling methodologies for the synthesis of B- and C-rings [78,79]. The synthetic pathway of the spacer domain started from (R)-pantolactone and a protected alcohol to produce the intermediate V. The coupling of the intermediate V with an alkyne afforded B-ring pyran. The A-ring ketal was formed by deprotection and cyclization reactions, and the spacer domain VI was formed by deprotection, oxidation, and olefination (Scheme 4b). The C-ring fragment was prepared from a lactonized sugar using Roy’s approach [80], and the recognized domain VII was obtained as described in Scheme 4c. Finally, a Shiina esterification was used to join VI and VII to give 28 with 36% yield [79].
2.3.2. Discodermolide
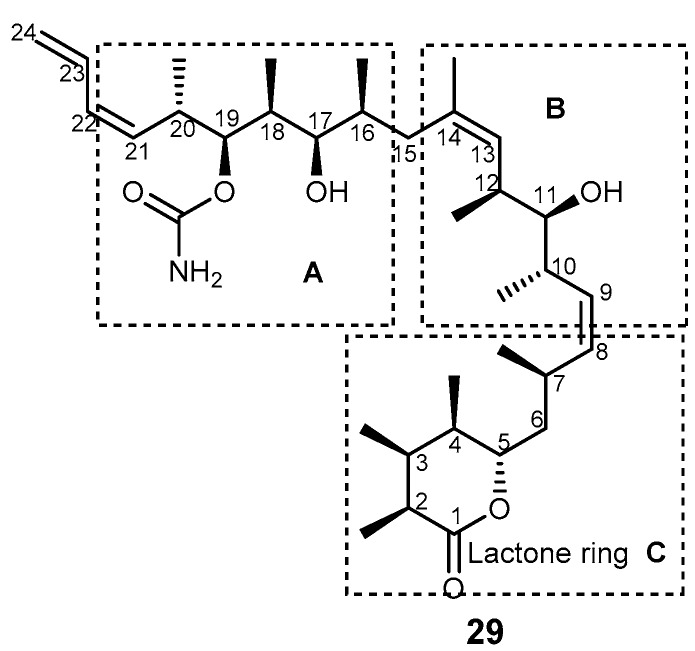
Discodermolide (29, Figure 6) is a polyketide found in the marine sponge Discodermia dissoluta. This marine product was reported to have a mechanism of action similar to that of taxol, i.e., by blocking the cell cycle at G2/M checkpoint, and inducing apoptosis against several cancer cell lines and against taxol-resistant cells.
Figure 6.
Structure of discodermolide (29) with highlighted subunits A, B, and C.
Biological activities of 29 include immunosuppressive properties, antiproliferative and antimitotic properties, and was shown to be a potent inducer of accelerated cell senescence, a neuroprotective agent, and to promote tubulin assembly [81,82,83,84]. In addition, 29 was also found to decrease the MDR to taxol in paclitaxel-resistant colon carcinoma (SW60AD-300) and MDR ovarian carcinoma (A2780Ad) [85].
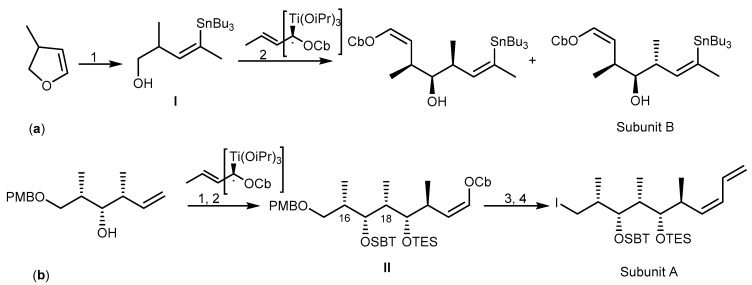
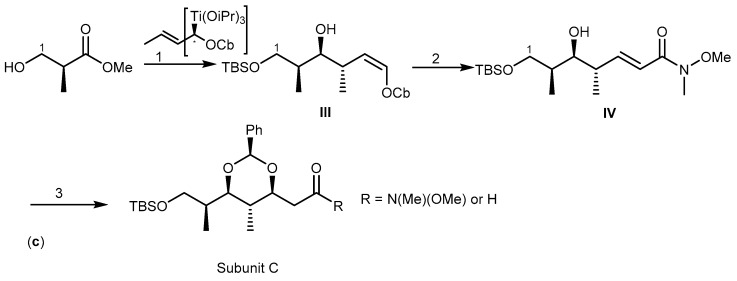
The strategy to synthesize (+)-discodermolide (29) and its analogs was achieved by preparing the three subunits: A (C-15–C-24), B (C-8–C-14), and C (C-1–C-7). The synthetic pathway was designed by two key disconnection points at C-7–C-8 and C-14–C-15 with the two Z double bonds at C-8–C-9 and C-13–C-14 being pivotal in this strategy. Formation of the C-7–C-8 bond was envisaged through an acetylide addition/reduction sequence, whereas formation of the C-14–C-15 linkage was accomplished by a Pd-catalyzed C(sp2)-C(sp3) cross-coupling reaction [86]. Subunit B was prepared in two main stages: a crotyl-titanation reaction for the installation of the stereotriad and a dyotropic rearrangement to build a Z-double bond I (Scheme 5a). The synthesis of subunit A started with the preparation of the C-16–C-18 syn-syn stereotriad II, planned by means of a substrate-based crotylation reaction under Keck’s conditions (Scheme 5b). The third building block, C-1–C-7 subunit C, incorporates four stereocenters. The approach started with standard Brown crotylation of aldehyde to afford syn-anti homoallylic alcohol III. Then, the α,β-unsaturated Weinreb amide was obtained through a classical Horner-Wadsworth-Emmons olefination. Finally, reaction of amide IV with benzaldehyde yielded subunit C (Scheme 5c). The assembly of the three subunits by cross-coupling reaction and deprotection were the last steps to afford 29. Recently, (+)-discodermolide (29) was synthesized by catalytic stereoselective diene hydroboration, and a strategy for alkylation of chiral enolate reaction was established through 36 steps with 13% yield [87].
Scheme 5.
Total synthesis of subunits A, B, and C of (+)-discodermolide (29). Reagents and conditions: (a) (1) t-BuLi, Me2CuLi, LiCN, Et2O/DMS then n-Bu3SnCl; (2) TEMPO, BAIB; (b) (1) TBSOTf, 2,6-lutidine, O3, Sudan III then DMS; (2) TBSOTf, 2,6-lutidine; (3) Ni(acac)2, (CH2=CH4)Sn, MeLi; (4) DDQ, CH2Cl2/H2O, I2, PPh3; (c) (1) TBSCl, imidazole, DIBAL-H, CH2Cl2, (COCl)2, DMSO; (2) O3, Sudan III, (EtO)2P(O)CH2C(O)NMe, NaH; (3) PhCHO, KHMDS.
2.3.3. Halicondrin B and Derivatives
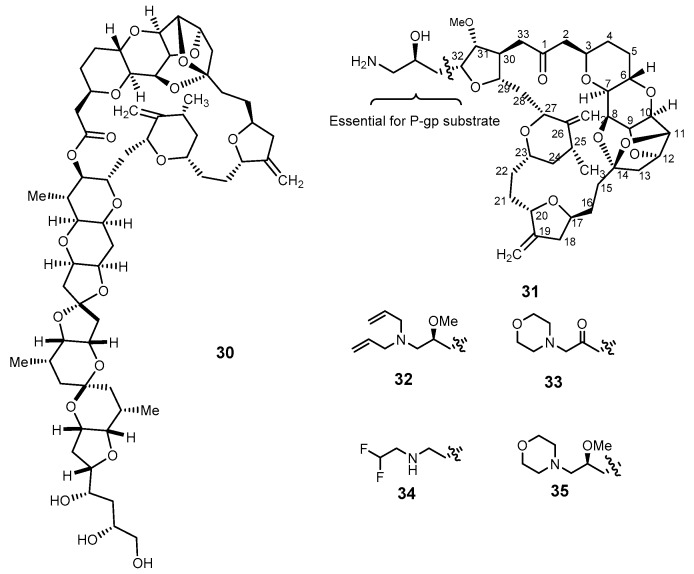
The macrolide halichondrin B (30, Figure 7), a polyether isolated from the marine sponge Halichondria okadai, exhibited both in vitro and in vivo antitumor activity [88]. However, the very limited availability impairs this marine natural product for its therapeutic application [89]. Eribulin mesylate (E7389, or Halaven®, 31), a synthetic analog of 30, was approved in 2010 by FDA for the treatment of locally advanced and metastatic breast cancer by inhibiting the microtubules [89,90], and was reported as a P-gp substrate [91]. The structure of 31 (Figure 7) is complex, containing several oxygen heterocycles, two exocyclic methylene groups, a ketal group, and a pyran ring with trans ring junction, along with 15 stereogenic centers. Several molecular modifications of 31 have been performed, and SAR studies with semi-synthetic analogs revealed that the substituent on C-32 of 31 plays an important role in the P-gp mediated drug efflux. The presence of an amine function in the substituents on C-32 in the analogs 32–35 led to a low susceptibility to P-gp-mediated drug efflux, and compounds 32–35 were active against MDR tumor cell line in vitro and in xenograft modelsin vivo [43,91]. Furthermore, treatment with eribulin in pretreated metastatic breast cancer patients was confirmed as feasible and safe for real-world patients [92].
Figure 7.
The structure of halichondrin B (30), eribulin (31), and the analogs 32–35.
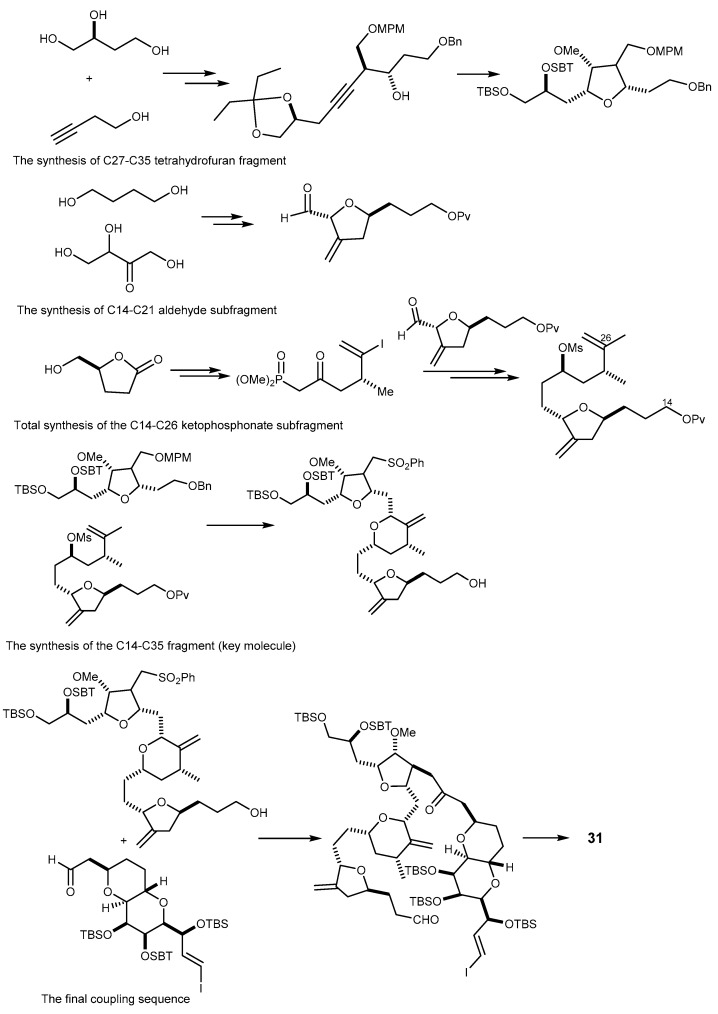
Different methods for the synthesis, to obtain milligram to gram amounts of 31 have been reported [75,93]. Generally, the synthetic pathway of 31 consists of preparation of two fragments, i.e., C-1–C-13 and C-14–C-26, as described by Kishi’s group in 1992, and one key fragment (C-27 to C-35). The final assembly strategy is accomplished by the coupling reaction depicted in Scheme 6 [90,93].
Scheme 6.
Schematic approach for the total synthesis of eribulin (31).
2.4. Alkaloids
2.4.1. Lamellarins and Derivatives
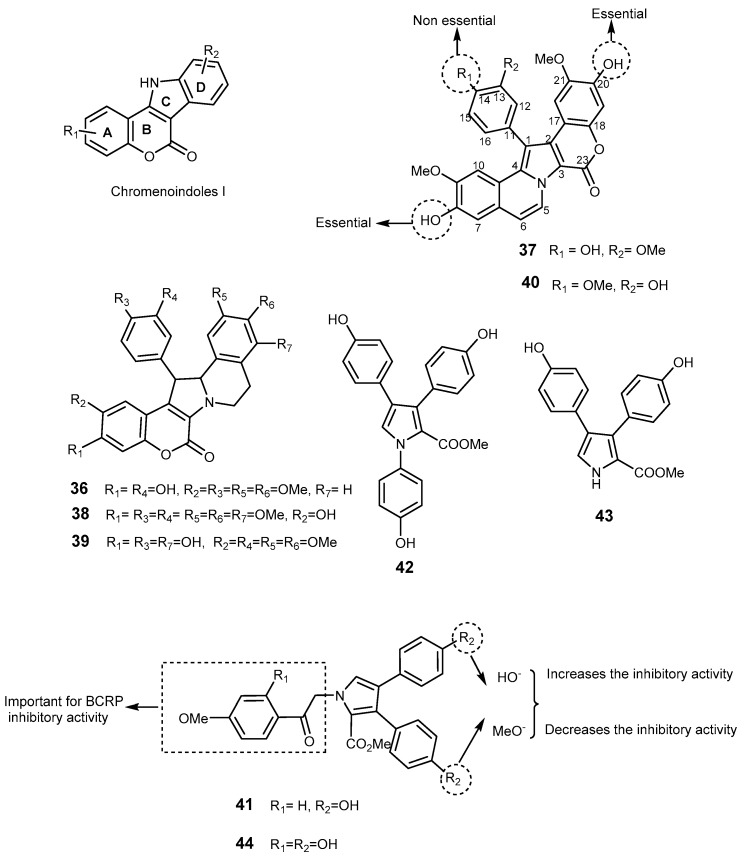
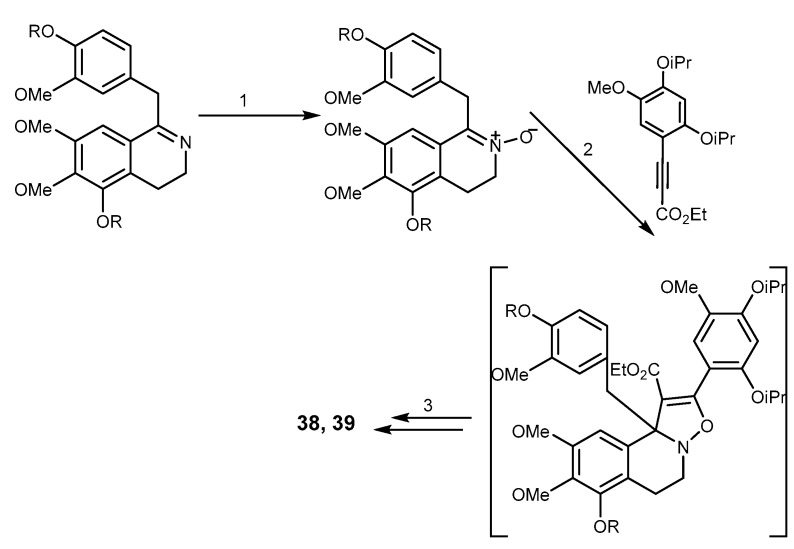
Lamellarins are a group of polycyclic pyrrole-containing alkaloids possessing a common 14-phenyl-6H-[1]-benzopyrano[4′,3′,3,5]pyrrolo-[2,1-α]isoquinoline ring system [94]. Lamellarins were isolated from many marine organisms such as a prosobranch mollusk (Lamellaria sp.) [35,95], an ascidian (Daphniphyllum chartaceum), a sponge (Dendrilla cactos), and unidentified ascidians [35]. More than 50 lamellarins have been described and mainly differ in the number and position of hydroxyl and methoxy groups on the common chromenoindoles I scaffold (Figure 8) [96]. Some lamellarins possess interesting biological activities e.g., lamellarin L (36) which exhibits cytotoxicity against P388 and A549 cancer cell lines [97], lamellarin D (37) which is also an inhibitor of topoisomerase I-targeted antitumor agents and an antiproliferative agent [96,98] and the immunomodulating lamellarin α-20 sulfate which inhibits also HIV [99]. Lamellarin I (38) was the most potent polycyclic pyrrole-containing alkaloid described as a MDR modulator. Methoxy-derivatives of lamellarin D (37), lamellarin K (39), and lamellarin N (40) (Figure 8), exhibit not only potent cytotoxicity against MDR cancer cell lines, but also reverse their MDR at non-cytotoxic concentration [100,101]. Compound 38 was 16 folds more sensitive than verapamil in doxorubicin-resistant Lo Vo/Dx cell line, and it increased the cytotoxicity of doxorubin, vinblastine, and daunorubicine in MDR cells because it directly inhibited P-gp pump function and increased drug accumulation in the cells [102]. Lamellarin O (41) is relevant as a multiple P-gp, BCRP, and MRP1 inhibitor [96], but synthetic analogs of 41, designed by QSAR studies and incorporating the methoxyacetophenone moiety unit, did not show BCRP inhibitory activity [103]. This study also allowed to establish some SAR features: bromo or mono-/dimethyl substituents on the indole moiety (Aryl-R2) in lamellarin O (41) lead to a decrease in the BCRP inhibitory activity [103] (Figure 8).
Figure 8.
The scaffold of lamellarins and their representatives 36–44. SAR studies for lamellarin D (37) and lamellarin O (41) regarding antitumor activity on BCRP overexpressing cells.
The structures of lamellarins are categorized into three types (Figure 8)—type I-a (lamellarin D, 37), type I-b (lamellarin L, 36), and type II [lamellarins O (41), R (42), Q (43)]. The syntheses of these derivatives are illustrated by type as presented in the next subsections.
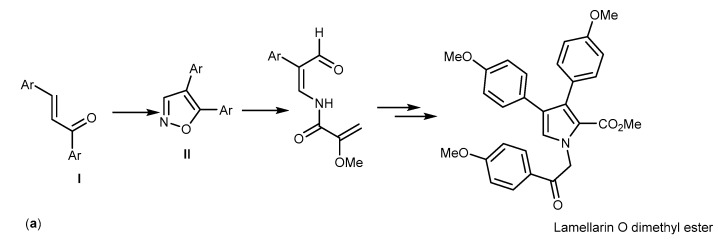
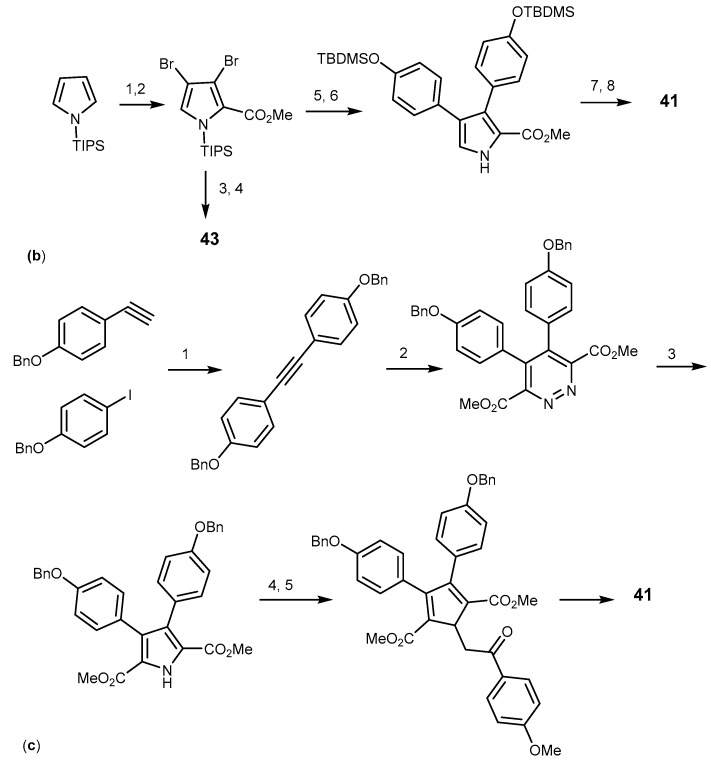
A total synthesis of a type-II lamellarins was described by Furstner in 1995. It was the first characterized pathway by a non-fused pyrrole moiety. The synthetic pathways started with the Sheffer-Weitz-epoxidation of an enone I, as depicted in Scheme 7a, followed by a Lewis acid-mediated rearrangement and condensation with hydroxylamine to afford the corresponding isoxazole II. Cleavage of the N-O bond, condensation, and McMurry cyclization were the subsequent steps to yield lamellarin O dimethyl ester (Scheme 7a) [104]. Another alternative for type-II lamellarin synthesis was performed by cross-coupling using the Stille, Suzuki or Negishi methods, as described by Banwell et al. in 1997. In this process, the pyrrole was protected to form an intermediate which was suitable to afford dibromopyrrole 2-carboxylate. N-desilylation and cross-coupling with aryl stannane produced the coupling product which was subjected to N-alkylation, followed by desilylation to provide 41 with high yield of 45% in six steps (Scheme 7b) [105]. The Banwell strategy was employed by Alvarez et al. with variations on solid phase chemistry and gave lamellarin 43 (in 13% yield), and lamellarin 41 which could not be isolated [106]. In 2008, Iwao et al. used Banwell’s Suzuki-Miyaura cross-coupling approach to synthesize type II lamellarins, yielding compounds 43, 41, and lamellarin P (44) in 7, 11, and 12% respectively, over eight steps [107]. Therefore, increasing the steps of reaction did not increase the reaction yield. Boger et al. described the synthesis of lamellarin 41 using an azadiene Diels-Alder reaction named as 1,2,4,5-tetrazine-1,2-diazine-pyrrole transformation with the overall yield of 34% within seven steps [108]. The pyrrole moiety was assembled by a [4+2] cycloaddition/cycloreversion reaction, followed by a reductive ring transformation. They started by forming the acetylenic precursor using Sonogashira-coupling from aryl acetylene and aryl iodide, followed by the cycloaddition of this precursor to give 1,2-diazine which was then subjected to a reductive ring contraction and N-alkylation. Selective hydrolysis of the symmetrical diester provided the monoacid. The subsequent hydrogenolysis afforded lamellarin 41 (Scheme 7c) [108]. In 2012, Vazquez et al. used the Paal-Knorr pyrrole synthesis to synthesize 41 in 25% yield in seven steps, and 43 in 28% yield in six steps [94].
Scheme 7.
Synthesis of lamellarin O (41) and lamellarins type II. Reagents and conditions: (a) (1) H2O2, NaOH, EtOH/H2O; (2) BF3·Et2O, Et2O, reflux, then NH2OH·HCl, reflux, pyridine, EtOH; (3) H2, Pd/C, THF; (4) ClCOCO2Me, pyridine, THF; (5) Ti-graphide (TiCl:C8K = 1:2), DME, reflux; p-MeO-C6H4COCH2Br, K2CO3, acetone, reflux; (b) (1) NBS (3 eq), THF; (2) PhLi (1 eq), then ClCO2Me (1.05 eq); (3) Pd(PPh3)2Cl2 (10 mol%), 1,4-dioxane, p-TBOMSO-C4H6-SnMe3 (2 eq); (4) Bu4NF (1.1 eq), THF, then 0.5 M HCl; (5) Bu4NF (1.1 eq), THF then 0.5 M HCl; (6) PPd(PPh3)2Cl2 (10 mol %), 1,4-dioxane, p-TBOMSO-C4H6-SnMe3 (2 eq); (7) p-MeO-C4H6-COBr (3 eq), K2CO3 (5 eq), Bu4NCl (20 mol %), THF; (8) Bu4NF (1.1 eq), THF, then 0.5 M HCl; (c) (1) Pd(PP3)2Cl2 (3 mol %), CuI (6 mol %), Et3N; (2) dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate, toluene, reflux; (3) Zn, HOAc; (4) p-MeO-C4H6-COBr, K2CO3, DMF; (5) LiOH, THF/CH3OH/H2O (3:2:1); (6) TFA, CH2Cl2; (7) H2,Pd/C (0.1 wt %), EtOH.
The syntheses of lamellarins type I-a were performed via different approaches such as the halogenation and cross-coupling reaction, N-ylide-mediated pyrrole ring formation, and other miscellaneous approaches. Lamellarin D (37) was synthesized by Alverz et al. [109], starting from the pyrrole ester. The A and B subunits of 37 were established by N-alkylation of pyrrole ester by Baeyer-Villiger oxidation, palladium-catalyzed Heck-type cyclization, followed by a regioselective bromination, providing the tricyclic building block which was subjected to Suzuki-Miyaura cross-coupling sequence O-isopropylation of the phenolic group and to a second bromination-Suzuki cross-coupling sequence. Introduction of a 5,6-double bond was accomplished by oxidation.
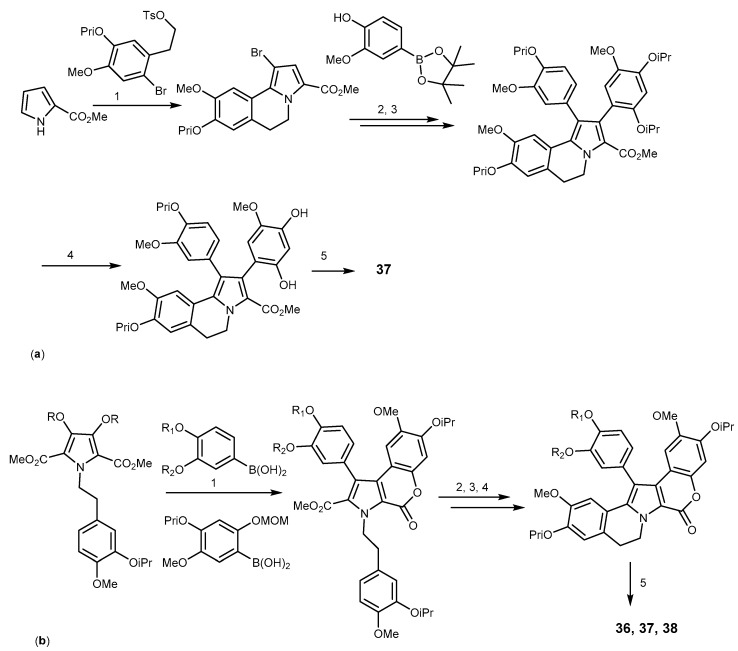
Finally, cleavage of isopropyl esters, followed by acidic lactonization, afforded 37 in 3% yield, over 16 steps (simplified in Scheme 8a) [109]. In the same year, Pla et al. reported a sequential and regioselective bromination/Suzuki cross-coupling reaction to introduce the aryl group at position 1 of methyl 5,6-dihydropyrrolo[2,1-a]isoquinoline-2-carboylate, with the combination of microwave-assisted 2,3-dichloro-5,6-dicynobenzoquinone (DDQ) oxidation, followed by phenol deprotection and lactonization to give 37 in the 18% yield in 8 steps reaction [109]. Iwao et al. also reported the synthesis of 36, 37, and 40 in 19%, 18%, and 16% yields over 11, 12, and 12 steps, respectively. They started from isopropyl-protected isovanillin and applied a nitroaldol condensation, a N-dialkylation and Hinsberg pyrrole cyclization reactions (Scheme 8b) [110].
Scheme 8.
Synthesis of lamellarin D (37). Reagents and conditions: (a) (1) PdCl2(PPh3)2, PPh3, K2CO3, NaH, DMF; (2) PdCl2(PPh3)2, K2CO3, DMF; (3) NBS, THF; (4) DDQ, CHCl3, MW, AlCl3, CH2Cl2; (5) NaH, THF; (b) (1) Pd(PPh3)4, THF, reflux; (2) KOH-EtOH, reflux, then p-TsOH, CH2Cl2, reflux; (3) Cu2O, quinolone; (4) Pd(OAc)2, CH3CN, reflux; (5) BCl3, CH2Cl2.
In 2001, Diaz et al. [101] constructed the pyrrole core in [3 + 2]-cycloaddition of nitrone to synthesize 38 and 39 (Scheme 9).
Scheme 9.
Synthesis of lamellarin I (38) and K (39) via 1,3-dipolar cyclization of nitrones. Reagents and conditions: (1) NaBH4, MeOH, H2O2, Na2WO3, MeOH; (2) toluene, 120 °C, 18 h; (3) AlCl3.
The N-oxides were prepared in moderate yields by reduction of 3,4-dihydro-1-benzylisoquinolines, followed by disodium tungstate-catalyzed oxidation with hydrogen peroxide. Reaction of N-oxide with alkyne produced an intermediate through the 1,3-dipolar cycloaddition-thermal rearrangement. Selective isopropyl group removal from the intermediate, with concomitant lactonization, gave lamellarins 38 and 39 in 4 and 6% yields, respectively [101]. In 2014, Yamaguchi et al. reported the synthesis of lamellarin I (38) using β-selective arylation of pyrroles with aryl iodides and a new double C-H/C-H coupling as a key step to afford 38 in 3% over 8 steps, starting from 2,3,4-trimethoxybenzaldehyde [111].
2.4.2. Ningalins and Derivatives
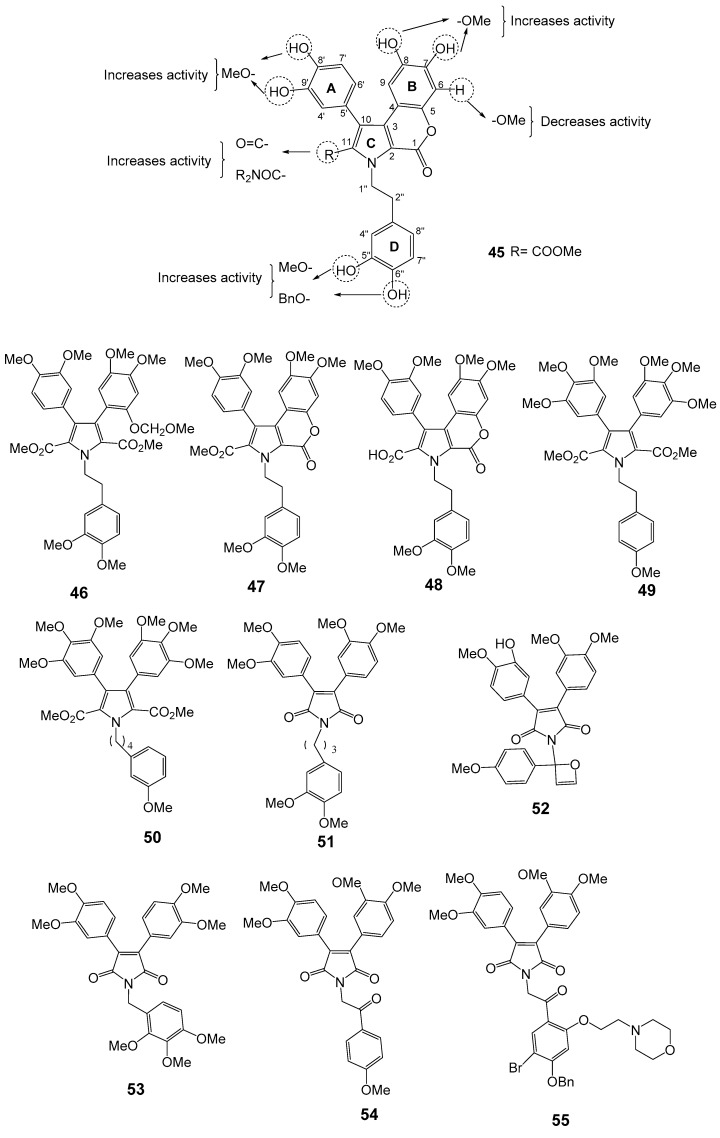
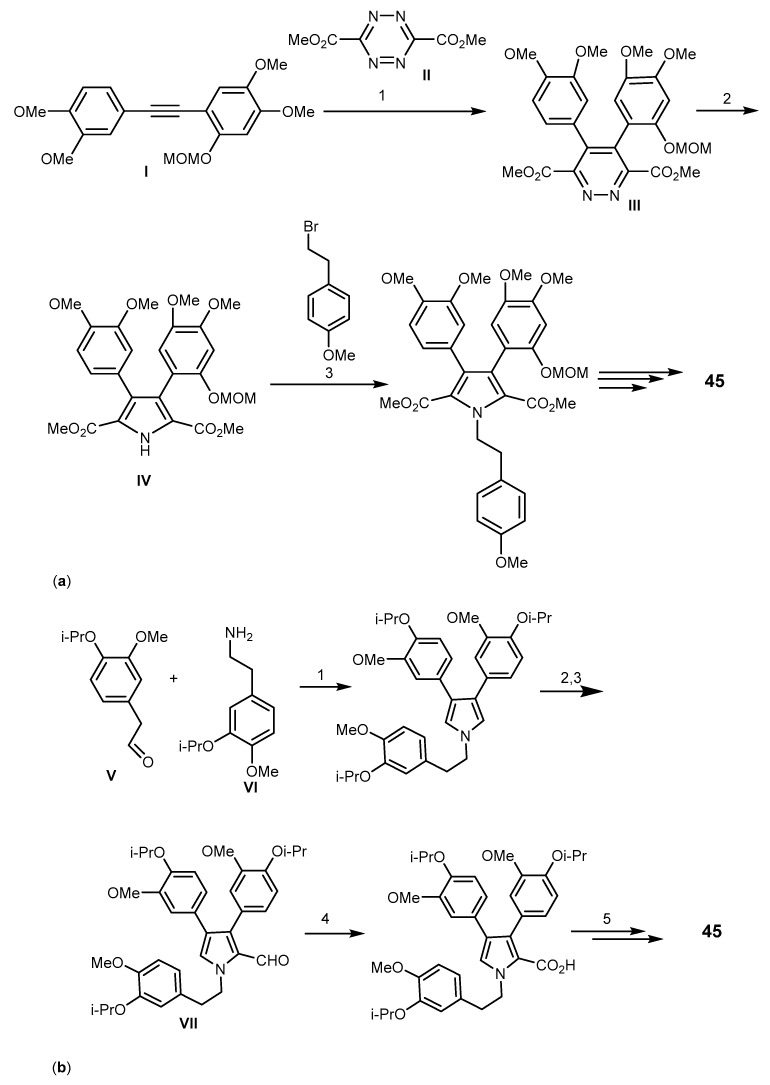
Ningalins are members of 3,4-dihydroxyphenyalanine (DOPA)-derived o-catechol metabolites including the tunichromes [112]. Biological activities of ningalins include HIV-1 integrase inhibition [113], Ningalin B (45, Figure 9), was isolated from an ascidian of the genus Didemnun by Boger et al. in 1999. Compound 45 lacks of an intrinsic cytotoxicity which makes this alkaloid an interesting model as MDR reversal agent. Interestingly, the synthetic analogs 46–50 were shown to significantly sensitize the HCT116/VM46 human colorectal carcinoma cell overexpressing P-gp to vinblastine and doxorubicin better than the marine natural product 45 and verapamil [114]. Studies of the N-aryl homologs of 45 (50–51) revealed that increasing the carbon linker leads to increase in activity. The authors concluded that the N-alkylaryl substituent is necessary for activity, but the analogs which bear free phenolic groups and methyl esters are more cytotoxic and lack potent MDR reversal properties. Analogs of 45 containing tetra- or pentasubstituted pyrroles showed the highest activity as MDR reversal agents [114,115,116] whereas those having 2-carboxylate or 2,5-carboxylates bearing 3,4-diaryl-substituted pyrroles showed reversal ability toward HCT116/VM46 cells. Compound 51 exhibited the greatest potential in reversing the MDR at 1 µM, and at 10 µM with about a 4000-fold increase in sensitivity against the vinblastine resistant MDR-leukemia cell line. This compound was also shown to compete with [3H]-azidopine for P-gp binding site and increased the intracellular accumulation and retention of MDR substrates. In nude mice-xenograft models, the combination of sub-optimal dose of paclitaxel with 51 not only leads to shrinkage the HCT116 tumor size but also to a complete therapeutic remission without increasing toxicity toward the host [116]. The analog 48 showed little or no effect of MDR on HCT116/VM46. The amide analogs of 48 enhanced the MDR activity, while amine derivatives (dimers) were inactive as MDR reversal compounds [115]. The synthetic analog of 46, 1-[2-(4-methoxyphenyl)-2-oxoetyl]-3,4-bis(3,4-dimethoxyphenyl)-1H-pyrrole-2,5-dione (52) which was designed from permethyl ningalin B showed a remarkable enhancement of MDR reversal abilities in concentrations up to 1 μM [117,118]. Compound 53, having a methoxy group on the D-ring, showed a 18.2-fold increase in cell sensitization towards paclitaxel at 1 μM concentration, and a 66 fold one when 0.5 μM of 53 was combined with 0.5 μM of 54 [117]. It was found that increasing the number of methoxy groups on ring B of the ningalin scaffold showed only low to moderate P-gp-modulating activity, while the addition of a benzyloxy group on the D ring enhanced the P-gp modulating activity [119]. SAR studies of a series of novel N-substituted derivatives of 45 on breast P-gp-overexpressing tumor cell line (LCC6MDR) showed that analogs containing one methoxy group and one benzyloxy group on ring C are the most potent P-gp modulators (1 μM desensitized cell line by 42.7 folds) without showing cytotoxicity IC50 for L929 fibroblast >100 μM) [117,119,120]. The analog 55 containing dimethoxy groups on rings A and B, and trisubstitution with o-methoxyethylmorpholine, m-bromo and p-benzyloxyl on ring D showed an effect compatible with P-gp modulation with an effective concentration (EC50) of 423 μM in reversing paclitaxel resistance [118]. Figure 9 summarizes the SAR established for this class of compounds against P-gp modulation highlighting the most promising ningalin derivatives.
Figure 9.
Structures of ningalin B (45), analogs 46–55, and SAR studies for P-gp modulation.
The synthesis of ningalin B (45) was described by Boger et al. using the Diels-Alder reaction of the acetylene I with 1,2,4,5-tetrazine II to give the 1,2-diazine III. The synthetic pathway includes the transformation of 1,2-diazine III to the pyrrole IV, followed by N-alkylation, lactonization and decarboxylation, and demethylation to yield 45 (Scheme 10a) [121,122]. Another synthesis of ningalin B (45) started from the aldehyde V and the amine VI, as depicted in Scheme 10b, and was accomplished by oxidative coupling reaction to yield the pyrrole moiety, followed by Vilsmeier-Haack formylation under traditional heating to give the formylpyrrole VII which was subsequently converted to the lactone and then to ningalin B (45) [113].
Scheme 10.
Synthesis of ningalin B (45). Reagents and conditions: (a) (1) N2; (2) Zn, AcOH; (3) K2CO3; (b) (1) AgOAc, NaOAc, THF; (2) POCl3, DMF; (3) DMSO/H2O or THF: t-BuOH:H2O; (4) Pb(OAc)4, EtOAc; (5) BBr3, CH2Cl2.
2.4.3. Welwitindolinones and Derivatives
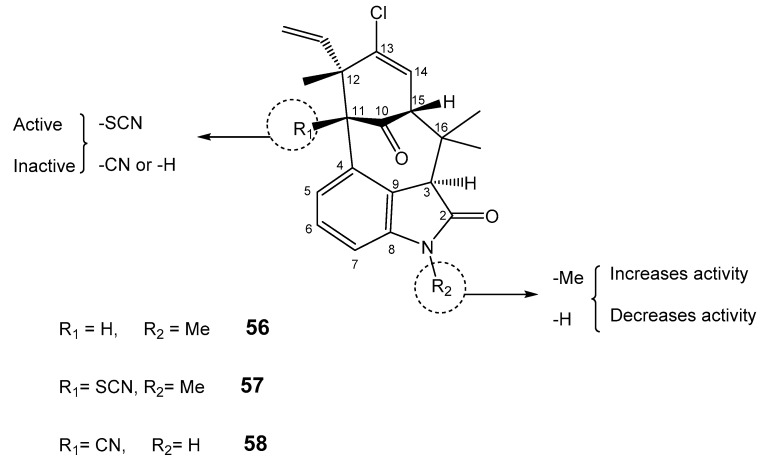
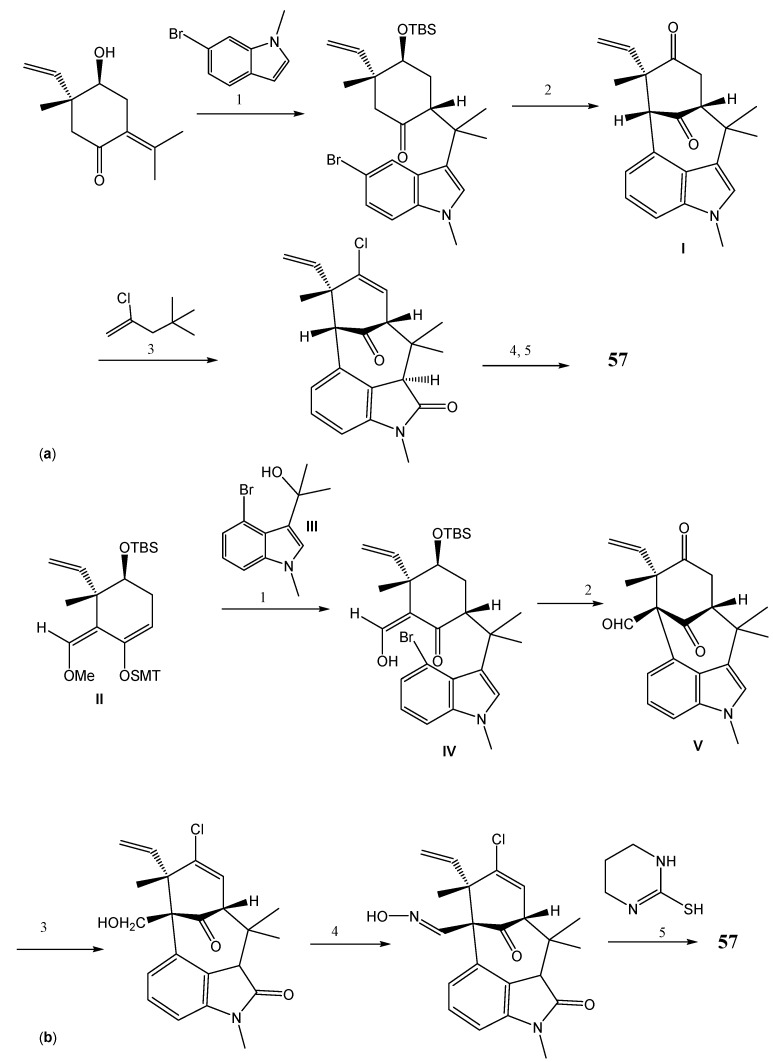
Welwitindolinones are alkaloids found in the cyanobacteria Hapalosiphon weltischii, Westiella intracta, Fischerella muscicola, and F. major [123,124]. The extracts which significantly contain N-methylwelwitindolinone (56) were found to have potent biological activities including antifungal, larvicidal, and insecticidal properties [124]. Most welwitindolinones possess a densely functionalized oxindole-fused bicyclo[4,3,1]decane ring system (Figure 10). The core structure of these alkaloids contains two contiguous stereogenic centers. These alkaloids exhibit a significant activity in reversing P-gp-mediated drug resistance in human tumor cells [125]. Among them, N-methylwelwitindolinone C (56) enhanced cytotoxicity of anticancer drugs like vinblastine, taxol, actinomycin D, colchicine, and daunomycin in MDR breast carcinoma cell line. The absence of the N-methyl group reduced the activity, while the absence of the isothiocyanate group completely abolished the activity, as observed for welwitindolinone C isothiocyanate (57) and welwitindolinone isonitrile (58), respectively. Welwitindolinone 57 was found to be the most potent derivative in increasing the accumulation of [3H]-vinblastine and [3H]-paclitaxel by blocking P-gp in SK-VLB-1 cells. This compound also inhibits the P-gp photoaffinity labeling by [3H]-azidopine in MDR cells [36].
Figure 10.
Structures of welwitindolinones 56–58 and SAR studies. The dashed circle indicates the groups important for P-gp.
Compound 57 was synthesized by two different approaches—Gang’s and Rawal’s. In Gang’s method, the synthetic pathway begins with coupling of a functional cyclohexanone using iodine promoted bromoindole addition, followed by ring closure to form the key intermediate compound I. Thereafter, 57 was formed by chlorination through vinyltrimethylstannane oxidation to oxindole, isotopically enhanced tethered nitrene insertion, and isothiocyanate introduction (Scheme 11a) [126,127,128]. In Rawal’s approach (Scheme 11b), the coupling of silylenolether II with nucleophile bromoindole III was achieved by Lewis acid to form the corresponding cyclohexanone IV which then produces the key intermediate V by palladium-catalyzed enolate arylation [124,129]. The key intermediate compound V was subjected to aldehyde reduction via a hydrazine (oxidation to the oxindole), alcohol oxidation and oxime formation, and Curtius rearrangement of oxime to isothiocynate to furnish 57.
Scheme 11.
Synthesis of N-methylwelwitindolinone C isothiocyanate (57) by Gang’s approach (a) and Rawal’s approach (b). Reagents and conditions: (a) (1) iodine promoted bromination; (2) NaNH2, t-BuOH, THF; (3) trimethyethylstannane; (4) LiEt3B-D, THF, Cl3CCONCO, CH2Cl2, K2CO3, MeOH; AgOTf, PhI(OAc)2, CH3CN, bathophenantroline; (5) NaH, air, THF; (b) (1) TiCl4, toluene; (2) Pd(OAc)2, P-tBu3, KO-tBu, toluene; (3) NaBH(OMe)3, THF/EtOH then N2H2, AcOH, EtOH; NCS, pyridine; MMPP, TFA, AcOH; (4) Dess-Martin periodinane, NaHCO3, CH2Cl2; NH2OH.HCl, pyridine, MeOH; (5) NCS, DMF, THF, then Et3N.
2.4.4. Harmine and Derivatives
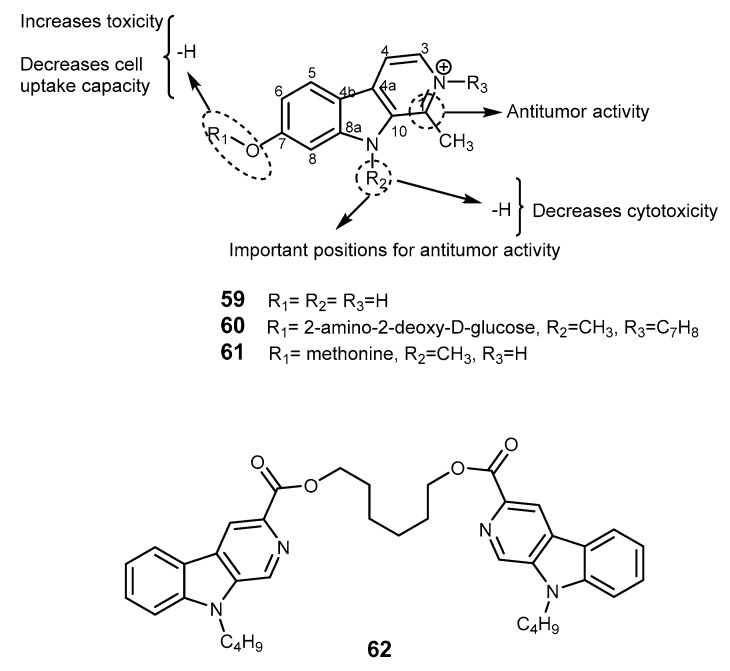
Harmine (59) is a β-carboline alkaloid found in plants, mammalians, insects, and marine organisms [130], e.g., the marine brown alga Melanothamnus afaqhusainii [131,132]. The plant extracts containing 59 were traditionally used to treat alimentary tract cancer and malaria in Northwest China [133]. These extracts have a broad spectrum of biological activities, including antimicrobial, antifungal, antitumor, antiplasmodial, antioxidant, antimutagenic, antigenotoxic, as well as hallucinogenic properties [130]. Compound 59 also inhibits topoisomerase I, cyclin-dependent kinases, monoamine oxidase A, and intercalates into DNA [134]. Studies on the biological function of 59 reported this alkaloid as a potent antiproliferative and cytotoxic agent, with MDR reversal activity by inhibiting BCRP in a BCRP overexpressing breast cancer cell line (MDA-MB-123) and by reducing the resistance of mitoxantrone and camptothecin mediated by BCRP, but did not inhibit MDR-1 overexpressing cells growth [135]. More detailed studies of 59 revealed that this compound failed clinical in applications due to its low pharmacological effects and neurotoxicity [132,134]. To improve the therapeutic efficacy of 59, several derivatives were synthesized by modification of substituents in positions 2, 7, and 9 of harmine (59) ring and with other two different groups—2-amino-2-deoxy-d-glucose and methionine—two derivatives were obtained, 2DG-Har-01 (60) and MET-Har-02 (61), respectively. This study found that substitution at position 7 reduced natural toxicity and increased tumor cell uptake ability, at position 9 enhanced cytotoxicity, and at position 2 enhanced the antiproliferative effect [132], and also showed that substituents at position 1 could be crucial to antitumor potency [136]. SAR studies confirmed that substituents in position 2 and 9 displayed an important role in modulation of antitumor activities [134]. Derivatives of 59 that possess in R1, R2, and, R3 (Figure 11) a benzyl and 3’-fluorobenzyl groups are more likely to act as protein synthesis inhibitors [137].
Figure 11.
Harmine (59) and analogs 60–62. The dashed circles indicate groups important for biological functions.
The scaffold of harmine (59, β-carboline) was a model for the synthesis of novel compounds which are bivalent β-carbolines. The different number of methylene units in the linker between two β-carbolines produced agents which displayed good and selective cytotoxicity against 769-P and KB cell lines, being the six methylene unit derivative 62 a potent antitumor compound against Lewis lung cancer in mice [136].
The synthesis of β-carboline-based alkaloids has been reported using tryptophan as starting material [138,139]. To the best of our knowledge, there is no report on the total synthesis of 59. Most of the harmine analogs were synthesized from the harmine scaffold using the Friedel-Crafts reaction, molecular modifications, and N-oxidation [132,140,141,142].
2.4.5. Indolcarbazoles and Derivatives
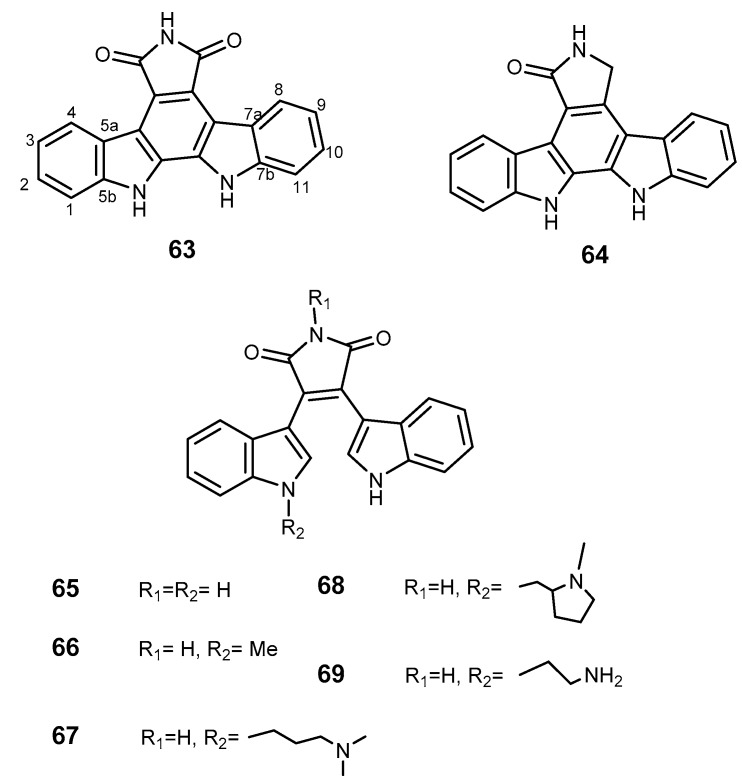
Indolcarbazoles are alkaloids isolated from marine-derived actinomycetes strain Z039-2 which possess a broad spectrum of biological activities including anticancer activity [143]. The mechanisms of their antitumor effects include topoisomerase I poisoning, inhibition of PKC, PKA, pyruvate dehydrogenase kinase (PDK)/cyclin B, and cyclin-dependent kinase 5 (CDK5)/p5 [144,145]. The naturally occurring arcyriaflavin (63) and indolcarbazole K252c or staurosporin aglycone (64, Figure 12) showed the most potent effects in BCRP inhibition in the BCRP-transferred HEK-293 cell line, with low toxicity in BCRP-transfected cells, and reduced the relative resistance of ABCG2-transfected cells SN-38 [29,146]. Bisindolylmaleimide analogs 65–69 (Figure 12) were found to be able to inhibit [125I]iodoarylazidoprazosin labeling of BCRP by 65% to 80% at 20 μM [146]. These findings revealed that indolocarbazole and bisindolylmaleimide analogs directly interact with BCRP protein and may increase oral bioavailability of BCRP substrates.
Figure 12.
Structures of indolcarbazole derivatives 63–69.
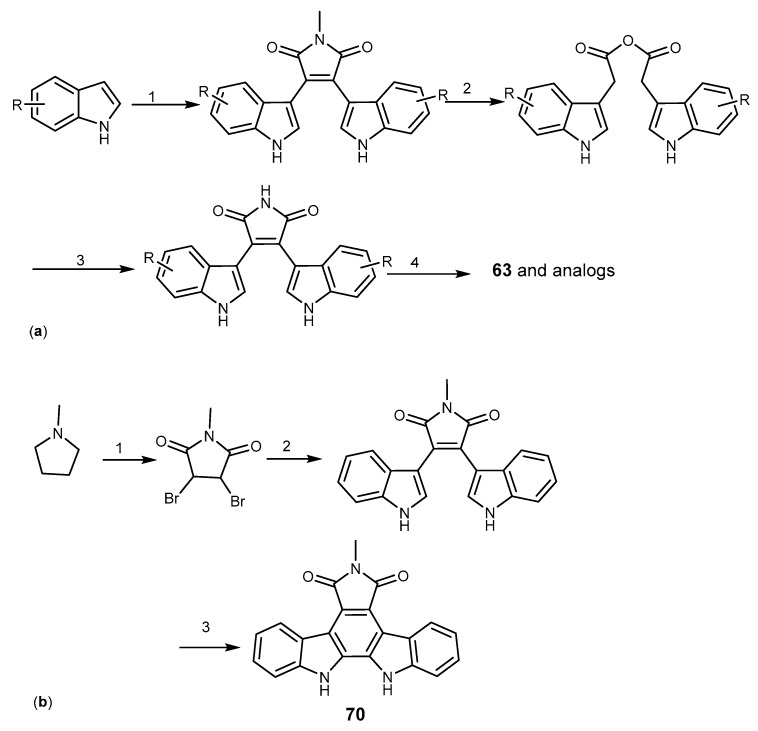
The indolocabazole nucleus was prepared from bis-indolylmaleimides, followed an oxidative cyclisation to give the indolocarbazole (Scheme 12a). Another way to obtain the indolocarbazole nucleus was applied in the preparation of N-methyl indolocarbazole (70) which was obtained from N-methylpyrrole by bromination and oxidation to give N-methyldibromomaleimide which was coupled with indolylmagnesium bromide to afford bis-indolylmaleimide. Oxidative cyclization of the later compound led to indolocarbazole 70, as depicted in Scheme 12b [145,147,148].
Scheme 12.
Synthesis of indolcarbazole derivatives 63 and 70. Reagents and conditions: (a) (1) i. EtMgBr, THF, benzene, ii. N-methylmaleimide; (2) KOH, MeOH or dioxane; (3) NH4OAc; (4) DDQ, PTSA, toluene; (b) (1) Br2, HNO3; (2) CH3CH2Br, Mg, THF, indole, toluene; (3) DDQ, toluene.
2.4.6. Ecteinascidin 743 (Trabectedin) and Derivatives
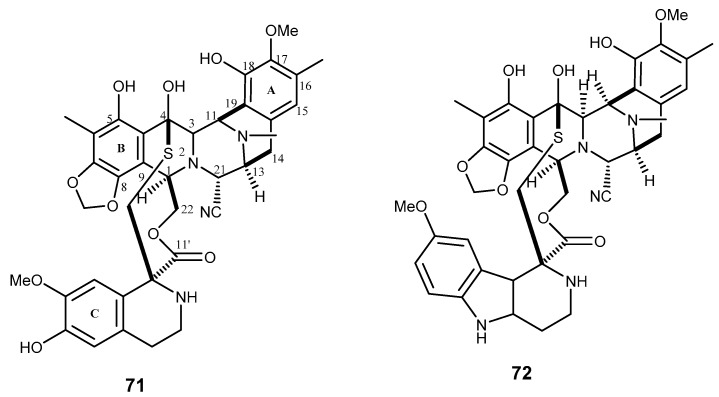
Esteinascidin 743 or trabectedin (71, Figure 13) is an anticancer tetrahydroisoquinolone alkaloid already approved by the FDA. This compound was first isolated from the Caribbean tunicate Ecteinascidia turbinata in a minute quantity. However, this compound could be synthesized from cyanosafracin B, a starting material obtained from fermentation of Pseudomonas fluorescens [149]. It is a highly potent anticancer agent and has activity against a number of human solid tumor cells. The compound was also showed to reverse the resistance to doxorubicin and vincristine in KB-C2 and KB-8-5 cells overexpressing P-gp in concentrations of 0.1 nM [150,151]. Trabectedin (71) was approved as the medicine Yondelis® for the treatment of advanced soft-tissue sarcomas and ovarian cancer. Although 71 is a substrate for P-gp, this potential mechanism of resistance has only been shown to be significant at concentrations exceeding clinically relevant values [151]. Moreover, this marine natural product also inhibits the activation of the MDR-1 gene that encodes for P-gp [152]. Therefore, 71 could be considered a promising model to overcome MDR. For instance, the synthetic agent lurbinectedin or PM01183 (72), which was obtained by modification of the subunit C of 71, showed a potent cytotoxic activity against tumor cell line of different origin, and it was introduced in phase I clinical trials for solid tumor, and phase II for metastatic pancreatic cancer. PM01183 (72) showed enhanced activity to cisplatin- and oxaliplatin-resistant cell line, and combination of 72 and cisplatin was the most synergistic toward parental and cisplatin-resistant ovarian carcinoma cells [153].
Figure 13.
Structures of trabectedin (71) and lurbinectedin (72).
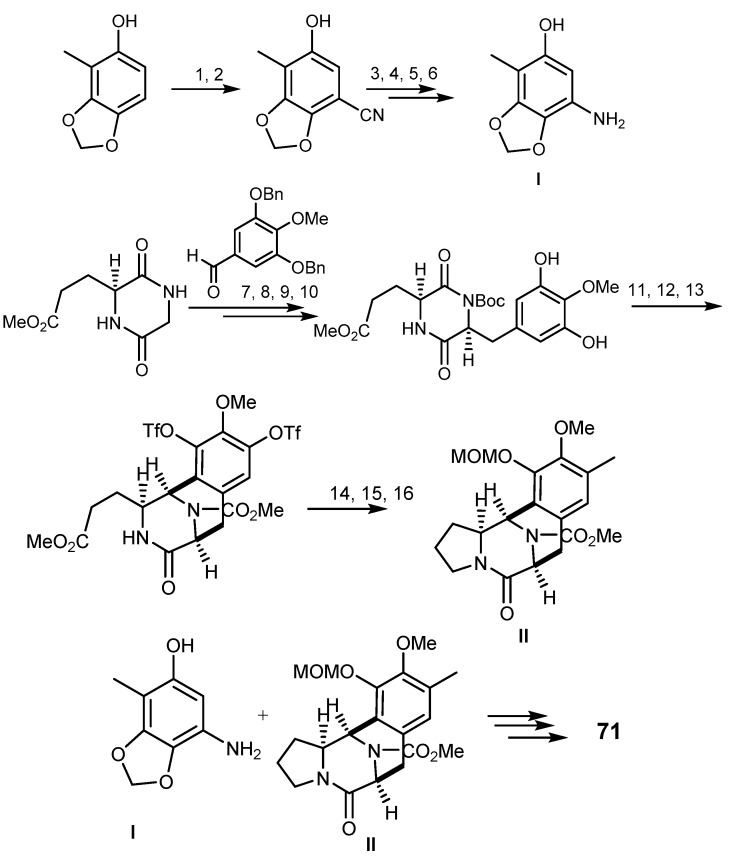
Syntheses of trabectedin (71) have been described by many researchers. In summary, there are three important pathways (Scheme 13): (A) combination of two fragments (I and II) via an oxazolidine intermediate, (B) acid-promoted macrocyclization via creation of carbon-sulfur bond with concomitant formation of a 10-membered ring, and (C) the intramolecular Pictet-Spengler reaction to obtain 71 [154,155,156,157]. The straightforward synthesis of 71, using 28 steps with 1.1% yield, started from l-glutamic acid as the single chiral source [158] while a 31 steps synthesis, with 1.7% yield, began from 3-methylcatechol [159]. The first conditions lead to the B-ring formation by stereoselective Heck reaction between diazonium salt and enamide, oxidative cleavage of the resulting alkane and intramolecular ortho substitution of phenol by aldehyde. This pathway can form a diketopiperazine by Perkin condensation and the bicyclo[3.3.3]system by an N-acyliminium ion-mediated cyclization, and a regioselective Suzuki-Miyaura coupling (Scheme 13) [158]. In 2000, Cuevas et al. [149] established the synthesis of 71 and 72 from cyanosafracin B, which is an antibiotic obtained from fermentation of the bacterium Pseudomonas fluorescens. The methoxy-p-quinone of cyanosafracin B was treated with bromochloromethane, followed by Edman degradation to form thiourea (Scheme 13). The critical substitution of the amino group by an alcohol group gave the key intermediate to yield 71 through the Corey method [149].
Scheme 13.
Total synthesis of trabectedin (71). Reagents and conditions: (1) PhI(OAc)2, MeOH; (2) NaCN, DMF/H2O; (3) BnBr, K2CO3, DMF; (4) aq H2O2, K2CO3, DMSO; (5) PhI(OAc)2, KOH, MeOH; (6) LiOH, EtOH/H2O, reflux; (7) t-BuOK, THF, DBU, (8) Boc2O, DMAP, THF, (2 steps); (9) H2 (750 psi), Pd/C, EtOAc; (10) H2NNH2·H2O, THF, evaporation; NaBH4, MeOH, (2 steps); (11) TFA, CF3CH2OH; evaporation; PhNTf2, DMAP, Cs2CO3, MeCN; (12) trimethylboroxine, Pd(PPh3)4, K3PO4, 1,4-dioxane; (13) HCl, EtOAc; ClCO2Me, NaHCO3, H2O; (14) l-Selectride, THF; (15) CSA, toluene, reflux (2 steps); (16) aq KOH, 1,4-dioxane, rt, MOMCl.
2.5. Diketopiperazines
2.5.1. Nocardioazines
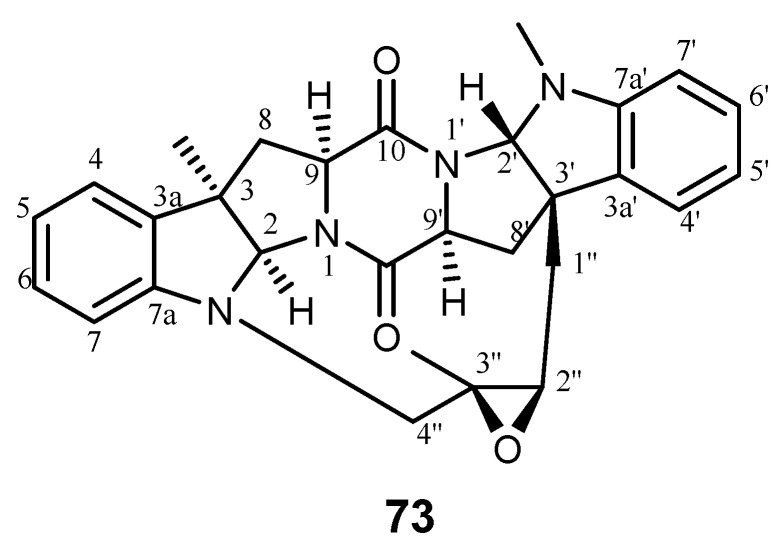
Nocardioazines are bridged diketopiperazine alkaloids which were isolated from a non-saline liquid culture of Nocardiopsis sp. (CMB-M0232). Nocardioazine A (73) is composed of cyclo-(l-Trp-l-Trp) and cyclo-(l-Trp-d-Trp), as shown in Figure 14. This compound has revealed an effect compatible with P-gp inhibition in a P-gp overexpressing colon cancer cell line (SW60Ad300) [160].
Figure 14.
Structure of nocardioazine A (73).
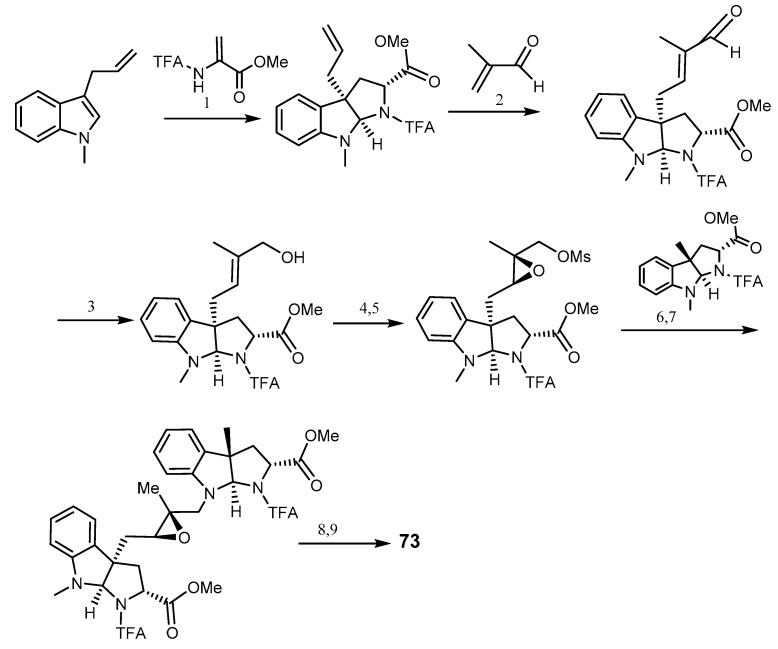
The first synthesis of (+)-nocardioazine A (73) was reported in 2014 by Wang and Reisman. The synthetic pathway comprises the building of the pyrroloindoline unit from an enantioselective formal [3 + 2] cycloaddition, and an unusual intramolecular diketopiperazine formation. The synthesis was performed from 3-allylindole in nine steps which gave an overall yield of 11% of 73 (Scheme 14) [161].
Scheme 14.
Total synthesis of nocardioazine A (73). Reagents and conditions: (1) (S)-BINOL, SnCl4, DCM; (2) OiPr-HG II, DCM, reflux; (3) NaBH4, CeCl3·7H2O, MeOH; (4) (+)-diethyl tartrate, Ti(OiPr)4, tBuOOH, 4Å MS, DMC; (5) MsCl, Et3N, THF; (6) TBAI, DIPEA, CH3CN; (7) Pd2(dba)3, dppb, DMBA, DEC; (8) LiOH, THF/H2O; (9) PyBrop, DIPEA, DMF.
2.5.2. Fumitremorgins and Derivatives
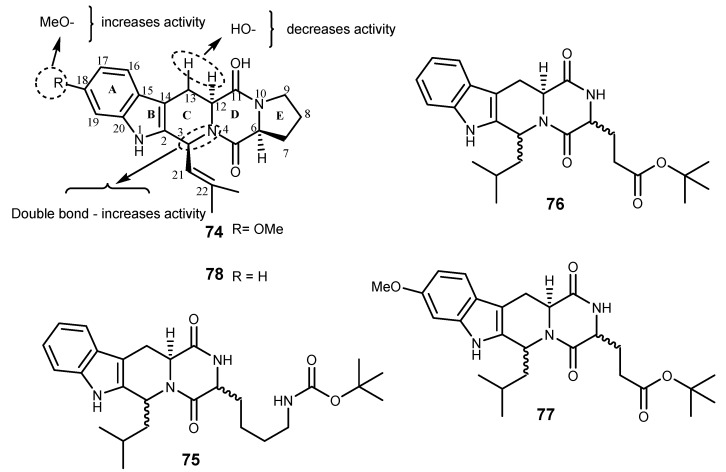
Fumitremorgin C (FTC, 74) is an indolyl diketopiperazine alkaloid found in several marine fungi such as the fungal strain BM939, fungal A-f-11, Aspergillus sydowii, and Aspergillus fumigatus (YK-7) [162]. Fumitremorgins were found to be tremorgenic mycotoxins by interfering the releasing neurotransmitters and also showed inhibitory activity on cell cycle [163]. Compound 74 was reported as a chemosensitizing agent that could reverse a drug-resistant cell lines that do not overexpress P-gp and MRP [163,164]. This effect was shown to be due to BCRP expression in the S1-M1-3.2 cell line resistant to mitoxantrone, topotecan, and doxorubicin [164]. Fumitremorgin C (74) almost completely reversed resistance mediated by BCRP in MCF-7 cells transfected with this protein [165]. Moreover, 75 was found to inhibit nitrofurantoin and mitoxantrone (BCRP’s substrates) transcellular transport in MDCKII and MEF3.8 cell lines [166]. The SAR study using the ftm gene cluster revealed that the moieties that are essential for inhibitory activity of 74 against BCRP are the double bond between C-3 and N-4, as well as the methoxy group at C-18, however, the hydroxyl groups at C-12 and C-13 lead to a decrease in activity (Figure 15) [167]. Unfortunately, 74 was found to induce tremors or convulsion in mice and other animals [168]. Two derivatives, Ko132 (75) and Ko134 (76), were found to be potent inhibitors of the BCRP-mediated drug efflux in T6400 mouse and T8 human cell lines with low cytotoxicity at an effective concentration of 1 μM [163,169]. In addition, 77 was the most effective inhibitor of BCRP, with very low activity against P-gp or other known drug transporters [168,170]. In addition, the stereospecificity was shown to be very critical in the Ko family, for example, compounds having the 3S,6S,12αS configuration were 18 times more potent than those with 3S,6R,12αS configuration in inhibiting BCRP; however, this stereoselective effect was not observed in P-gp and MRP-1 [171].
Figure 15.
Structures of fumitremorgin C (74) and derivatives 75–78 and SAR for BCRP inhibition.
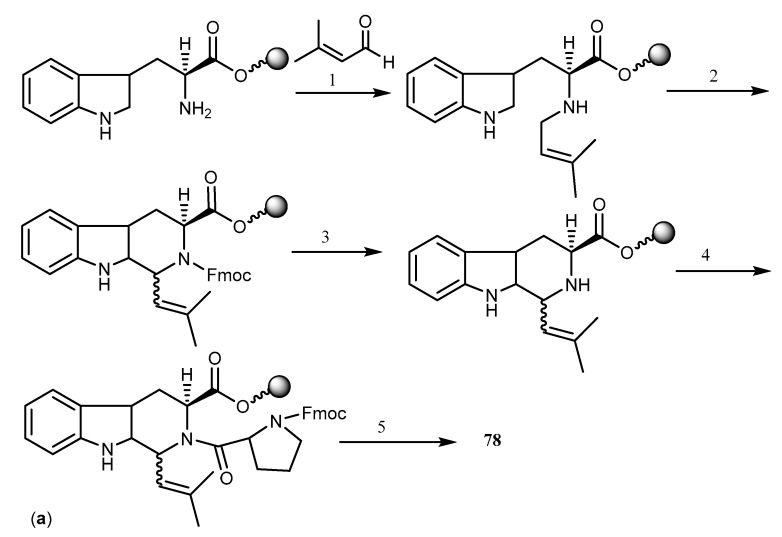
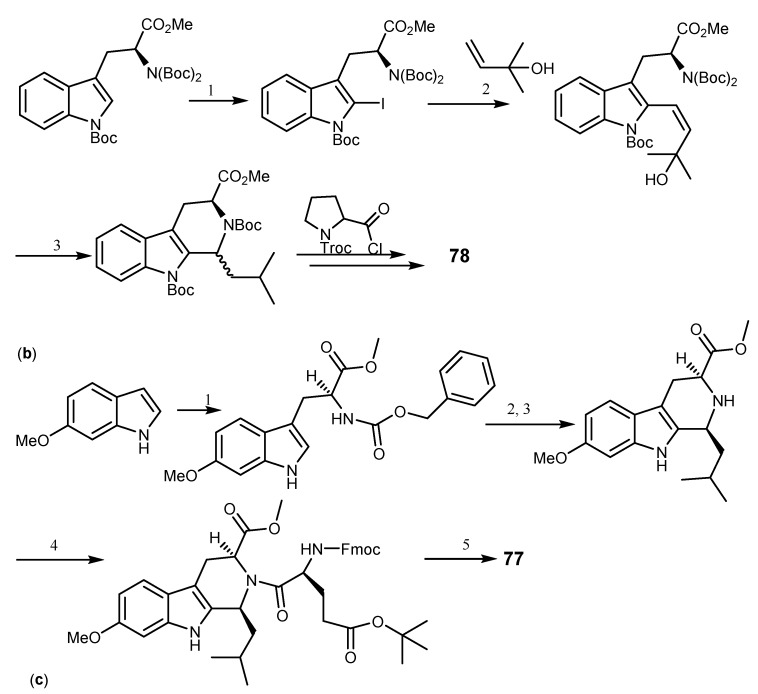
The first synthesis of 74 was reported by Hino et al. who prepared N-propyl-7-methoxy-β-carboline as the key intermediate [172]. Loevezijn et al. reported a solid phase synthesis of demethoxy-FTC (78), also an inhibitor of BCRP-mediated MDR, by a cyclization/cleavage multiple parallel syntheses method (Scheme 15a). This synthetic pathway was based on the formation of the diketopiperazine rings system, followed by simultaneous cleavage of solid support, which acted as a leaving group at the cyclization step. The reaction was performed by the Pictet-Spengler condensation of the hydroxyethyl functionalized polystyrene resin linked l-tryptophan with excess of aldehyde Scheme 15a [163,169].
Scheme 15.
Total synthesis of FTC derivatives, dimethoxy-FTC (78) and Ko143 (77). Reagents and conditions: (a) (1) CH(OMe)3; (2) Fmoc-HCl, pyridine, CH2Cl2; (3) piperidine, DMF; (4) Fmoc-l-pro-OH, CIP, DiPEA, MNP; (5) piperidine, THF; (b) (1) Hg(OOCCF3)2, KI, I2, CH2Cl2, then Boc2O, DMAP, MeCN; (2) Pd(OAc)2, Ag2CO3, toluene; (3) Mg(ClO4)2, MeCN; (c) (1) 1-benzyl-2-methyl-(S)-1,2-aziridinedicarboxylate, ytterbium triflate, CH2Cl2; (2) H2 balloon, MeOH, 10% Pd/C; (3) isovaleraldehyde, TFA, CH2Cl2; (4) N-Fmoc-5-t-butyl l-glutamic acid ester, diisopropylethylamine 2-chloro-1,3-dimethylimmidazolinium hexaflorophosphate, N-methylpyrrolidinone; (5) piperidine, THF.
Another alternative to obtain 78 was achieved by Mg(ClO4)2-catalyzed intramolecular allylic amination with carbamate or sulfonamide as nucleophiles to form substituted piperidine and pyrrolidine (Scheme 15b) [173]. The synthesis of Ko143 (77), a potent BCRP inhibitor of the FTC series was accomplished and optimized by Yuexian et al. [174] which involved ytterbium triflate-promoted coupling between 6-methoxyindole and optically active 1-benzyl-2-methyl-(S)-1,2-aziridinedicarboxylate (Scheme 15c) [174].
2.5.3. Halimide and Derivatives
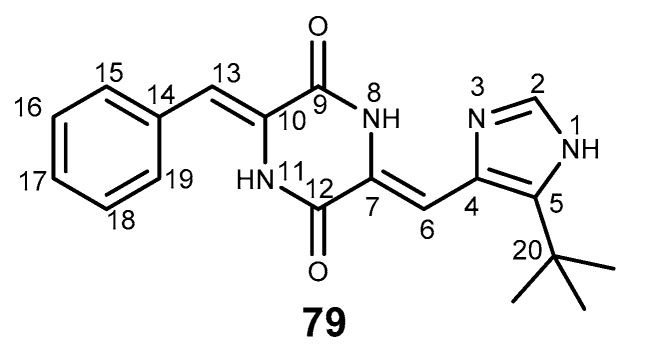
Halimide, is a diketopiperazine secondary metabolite isolated from the marine fungus Aspergillus ustus. This compound exhibited potential in vitro cytotoxic activity against human colon and ovarian carcinoma cells [175]. Structurally, halimide is composed of a phenylalanine, a histidine, and a tertiary butyl group. An analog of halimide, plinabulin (KPU-2, 79, Figure 16), that was obtained by molecular modification at the phenyl ring, has revealed a potent in vitro antitumor activity not only against various human tumor cell lines, but also against those with various MDR profiles [176]. Moreover, 79 was reported to have a similar mechanism of action to that of eribulin (31) as microtubule-disrupting agent with colchicine-like tubulin-depolymerizing activity, but the main problem concerning 79 is its poor solubility (<0.1 μM), which can trigger further studies on this scaffold. The phase I/II clinical trial of 79 in combination with docetaxel was completed in patients with advanced non-small cell lung cancer, and compound 79 was shown to act synergistically with docetaxel in murine models of NSCLC [177].
Figure 16.
Structure of plinabulin (79).
2.6. Peptides
2.6.1. Hapalosin and Derivatives
Hapalosin (80, Figure 17) is a cyclic depsipeptide, isolated from the lipophilic fraction of the extract of a cyanobacterium Hapalosiphon welwitschii W. & G.S West, and was found to reverse MDR in a P-gp overexpressing, vinblastine-resistant human ovarian adenocarcinoma cell line with higher effect than the known P-gp inhibitor verapamil [178,179].
Figure 17.
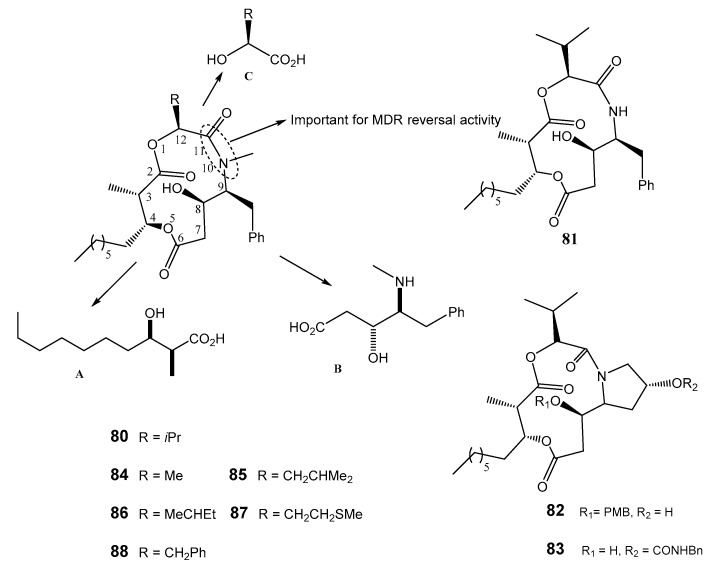
Structure of hapalosin (80) and analogs 81–88 highlighting the important domains for synthesis (A–C).
At 20 μM concentration, compound 80 significantly enhanced the accumulation of [3H]-paclitaxel in SKVLB1 cells, and exhibited a similar activity to verapamil in breast cancer cell MCF-7/ADR in the range of 1.5–10 μM [180,181]. Several syntheses for 80 and its respective analogs have been reported, with the purpose of obtaining compounds with similar or better MDR reversal activity. Some of the analogs did not fulfill this objective; for example, the glucose mimic of hapalosin (80) and 8-deoxyhapalosin, the triamide analogs, and N-demethylhapalosin 81 possessing a trans-amide, exhibited weak MDR reversal activity [180,181,182]. Through SAR studies, proline-containing congeners (82 and 83) were found to be more potent against MCF-7/ADR cells than 81 [182]. Substitution at C-12 in 80 furnished analogs 84–88, which exhibited higher vincristine accumulation than verapamil and 80 in MDR 2780AD cells at 10 μM [183]. It was postulated that the cis-peptide might be essential to express the MDR-reversing activity, and the bioactive function of 80 and analogs depends on the S-cis or S-trans configuration [182,184]. Also, a free hydroxyl and aromatic groups may be important for the anti-MDR activity of hapalosin (80) and its analogs.
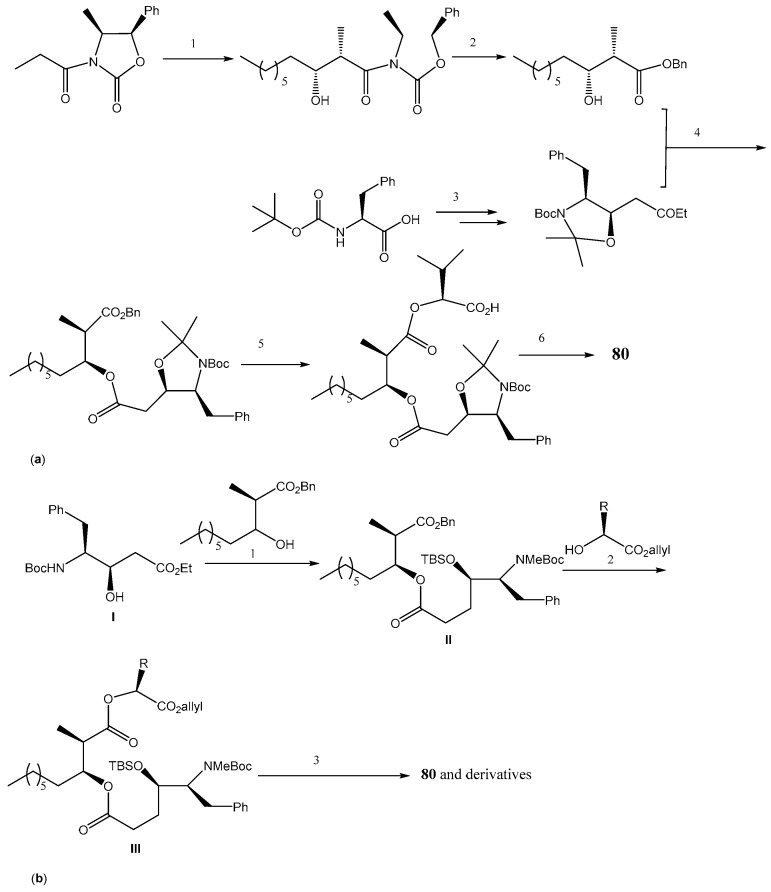
The structure of hapalosin (80) was investigated for synthesis, and it was divided into 3 main domains: a β-hydroxy acid (A), a γ-amino-β-hydroxy acid (B), and an α-hydroxy acid (C). Hapalosin (80) was initially synthesized by macrolactonization and by cycloamidation [185]. Nobuki et al. reported the synthesis of 80 and analogs by cyclization, producing the peptide bond at the end of the process which allowed obtaining 80 in 44% yield. They used the coupling of N-propionyl-(4S,5R)-4-methyl-5-phenyl-2-oxazolidinone with octanal (Scheme 16a) [181,186]. Another synthesis of hapalosin (80) and its analogs was accomplished by a macrolactonization strategy [187,188,189]. The method was based on a chiral acetate reagent, which offered advantages to the aldol reaction and avoided intramolecular cyclization during amino group deprotection and segment coupling [189]. Shigeru et al. presented the synthesis of hapalosin (80) and derivatives with modification at C-12 by cyclization producing the peptide bond at the final stage [183]. According to their strategy, hapalosin (80) and derivatives were commenced by using γ-amino-β-hydroxy acid I which was obtained by either Evans aldol/Curtius combination route [190] or asymmetric dihydroxylation route [191].
Scheme 16.
Synthesis of hapalosin (80) and derivatives. Reagents and conditions: (a) (1) Bu2BOTf, Et3N/CH2Cl2, then Me(CH2)6CHO; (2) n-BuLi, BnOH/THF; (3) Ref. [193]; (4) NaOH, DCC, DMAP/CH2Cl2; (5) H2, Pd(OH)2/EtOH, vinyl-2-hydroxy-3-methylbutanoate, DCC, DMAP/CH2Cl2; (6) TFA/CH2Cl2, DPPA, i-Pr2NEt/DMF; (b) (1) i. DCC, DMAP/CH2Cl2, ii. H2, Pd(OH)2/EtOH, (2) DCC, DMAP/CH2Cl2; (3) i. HF-pyridine, ii. (Ph3P)4Pd, morpholine, then TFA, iii. DPPA, EtNt-Pr.
Then, the catalytic hydrogenation of the benzyl ester II was performed, and the generated carboxylic acid was coupled with an appropriate allyl α-hydroxylate, leading to allyl ester III. After sequential deprotection, cyclization produced 80 and derivatives (Scheme 16b). Kumar et al. also described a flexible and highly diastereoselective synthesis of hapalosin (80) with the addition of an organometallic reagent to N-tert-butanesulfinylimine, the non-aldol aldol reaction, and the Yamaguchi esterification as key steps in this strategy [192].
2.6.2. Botryllamides and Derivatives
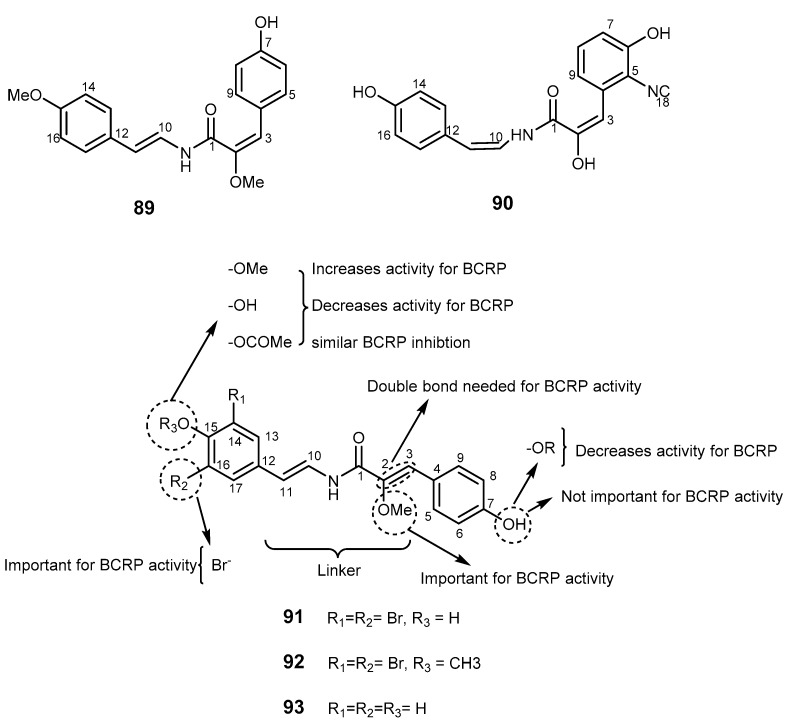
Botryllamides are a group of dehydrotyrosine derivatives, isolated from styelid ascidians Botryllus sp. [194,195]. Some botryllamides exhibited weak cytotoxicity against the HCT-116 tumor cells [195]. Botryllamides exhibited selectivity toward BCRP, but have different specificity to other ABC transporters. Two naturally occurring botryllamides, botryllamide I (89) and J (90) were reported to have activity against BCRP by inhibiting BCRP-mediated BODIPY FL prazosin transport in BCRP-transfected HEK293 cells (Figure 18). Both 89 and 90 competed with [125I]-iodoaryl-azidoprazosin labeling of BCRP, and were shown to stimulate BCRP-associated ATPase activity, reversing BCRP-mediated resistance [196]. In a different study, botryllamide G (91) was reported as the most potent botryllamide BCRP inhibitor, but did not inhibit the efflux of rhodamine out of P-gp overexpressing cells. In contrast, botryllamide A (92), which is less potent against BCRP, showed that activity against P-gp [197]. These differences were explained by the present or absent of an o-methyl group at C-15. The biological investigation of analogs of botryllamides 92 and 93 suggested that the 2-methoxy-p-coumaric acid moiety and the number of conjugated double bonds with this group were important for BCRP inhibition [197]. This study also found that: (i) the phenolic hydroxyl group at C-7 was not important for activity; (ii) the binding region with BCRP was between C-4 and C-9 (right side of Figure 18) of the aryl ring; and (iii) conjugation through C-1 to C-9 was necessary for activity, but extended conjugation from C-10 to C-17 (left side of Figure 18) of the aryl ring was not required, however, it might contribute to specificity of activity.
Figure 18.
Botryllamides 89–93 and SAR studies on BCRP inhibitory activity.
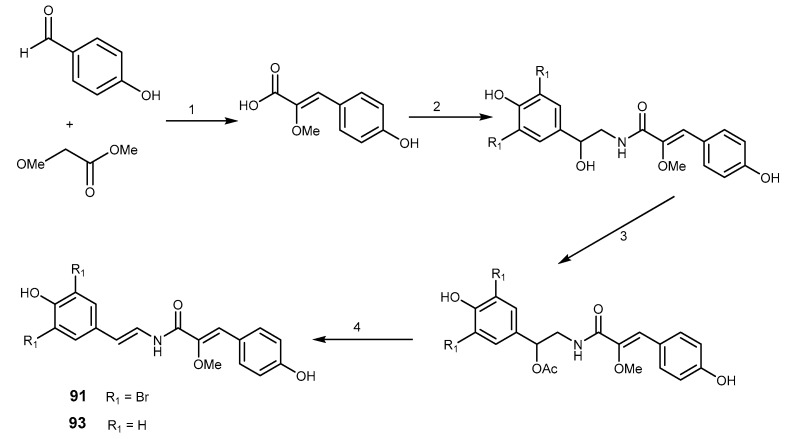
The syntheses of botryllamides G (91) and F (93) were already described, and are depicted in Scheme 17. The strategy started with a condensation of octopamine with 2-methoxy-p-coumaric acid to generate an amide bond, followed by a dehydration of the adjacent hydroxyl group to provide the key enamine amide functionality [197].
Scheme 17.
Synthesis of boryllamides G (91) and F (93). Reagents and conditions: (1) NaOMe, MeOH, reflux, overnight; (2) octopamine·HCl or bis-brominated octopamine, WSCl, HOBt, Et3N; (3) Ac2O, pyridine; (4) K2CO3, DMSO.
2.6.3. Kendarimide
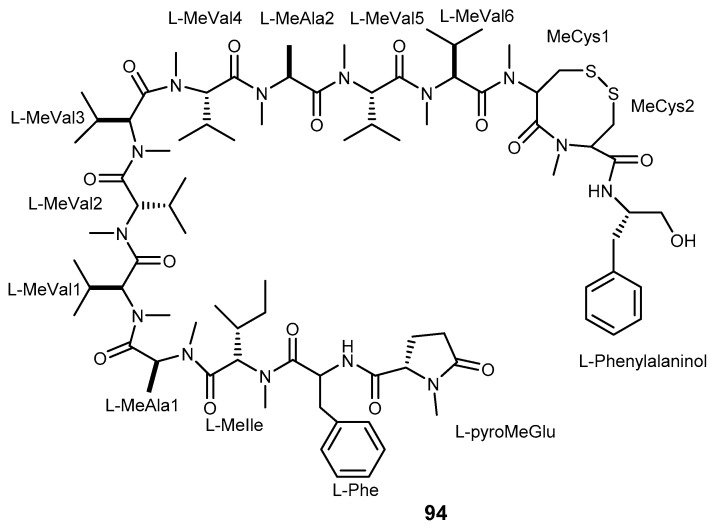
Kendarimide A (94, Figure 19) is an oligopeptide found in the Indonesian sponge Haliclona sp. This compound showed the potential of MDR reversal in human carcinoma (KB-C2) cell line overexpressing P-gp at the concentration of 6 μM, however, it was not active against KB-3-1 at the same concentration. The combination of 94 (6 μM) with colchicine (0.1 µg/mL) was found to inhibit KB-C2 cell growth by 87% [198]. Kendarimide A (94) is composed of several amino acids such as N-methylpyroglutamic acid (pyroMeGlu), N-methylated eight membered cysteinyl-cysteine (ox-[MeCys-MeCys]) together with many N-methyl amino acid residues, similar to cyclosporine A, a well-known P-gp inhibitor [199]. These data highlight that peptides can be considered as a valuable scaffold to reverse MDR.
Figure 19.
Structure of kendarimide A (94).
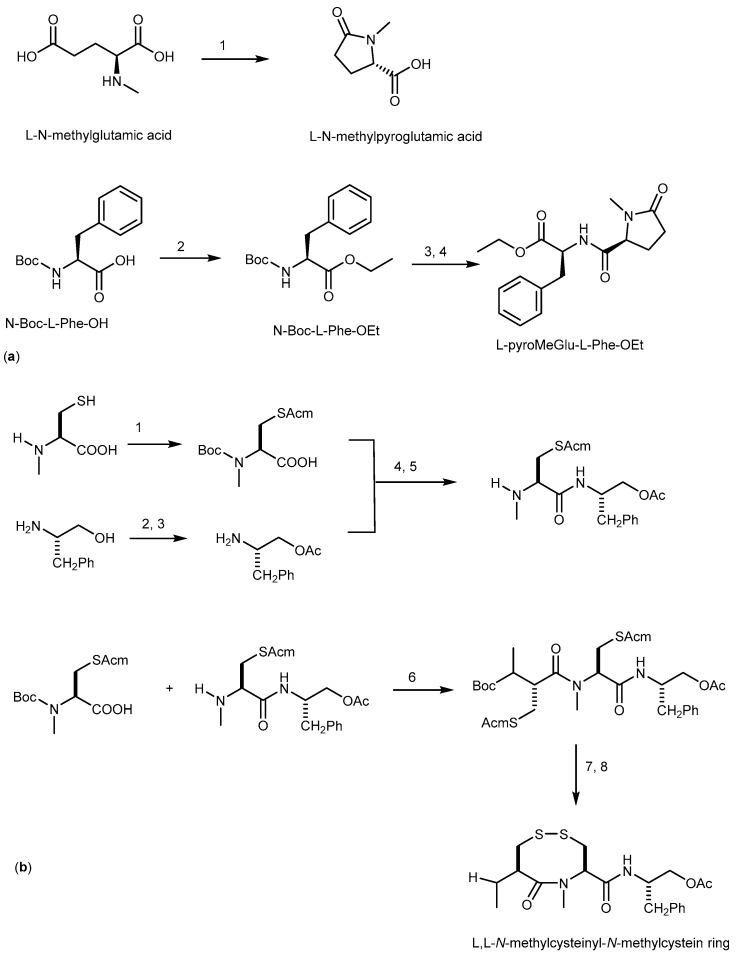
From our understanding the total synthesis of compound 94 has not yet been reported. l-pyroMeGlu-l-Phe-OEt is the moiety that was synthetized as a model compound to represent pyroMeGlu of 94 as described in Scheme 18a [198]. Another synthetic model compound (N-methylcysteinyl-N-methylcysteine ring) to study the absolute stereostructure of the C-terminal tetrapeptides (N-MeCys-N-MeCys) in 94 was synthesized, and the absolute configuration of both of the two adjacent N-methylcysteines in 94, which form an eight-membered disulfide ring, was elucidated to be L. The synthetic pathway was described in Scheme 18b [199].
Scheme 18.
Synthesis of l-pyroMeGlu-l-Phe-OEt (a) and l,l-N-methylcysteinyl-N-methylcystein ring (b). Reagents and Conditions: (a) (1) H2O, 132 °C, 1.9 kg/cm2 (autoclave); (2) Na2HCO3, C2H5I, DMF; (3) TFA, CH2Cl2; (4) l-N-pyroMeGlu, DEPC, TEA, DMF, 0 °C; (b) (1) acetamidomethanol, conc. HCl, (Boc)2O, 1N NaOH; (2) (Boc)2O, 1N NaOH, Ac2O, pyridine; (3) TFA, CH2Cl2; (4) DEPC, Et3N, DMF; (5) TFA, CH2Cl2, quant.; (6) EDCI, HOAt, CH2Cl2/DMF; (7) I2, CH2Cl2/MeOH; (8) TFA, CH2Cl2, quant.
2.6.4. Patellamide and Derivatives
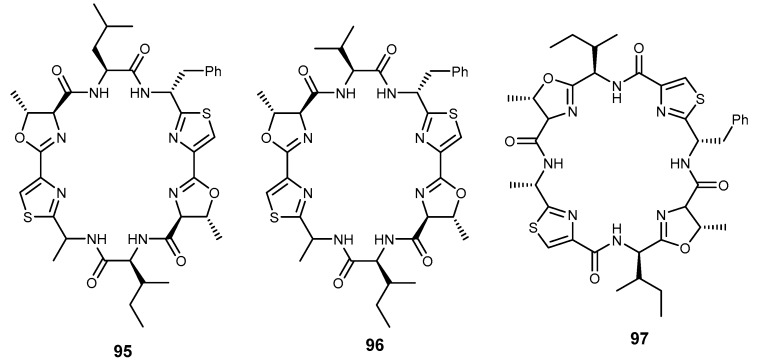
Patellamides are thiazole and oxazoline lipophilic cyclic peptides isolated from the tunicate Lissoclinum patella. These compounds exhibited cytotoxicity and reversed MDR in tumor cells. Patellamides B (95), C (96), and D (97) showed reversal activity and enhanced the activity of P-gp inhibitors such as vinblastine, colchicine, and doxorubicin in CEM/VLB100 cell line (Figure 20) [200,201].
Figure 20.
Structures of patellamides B (95), C (96), and D (97).
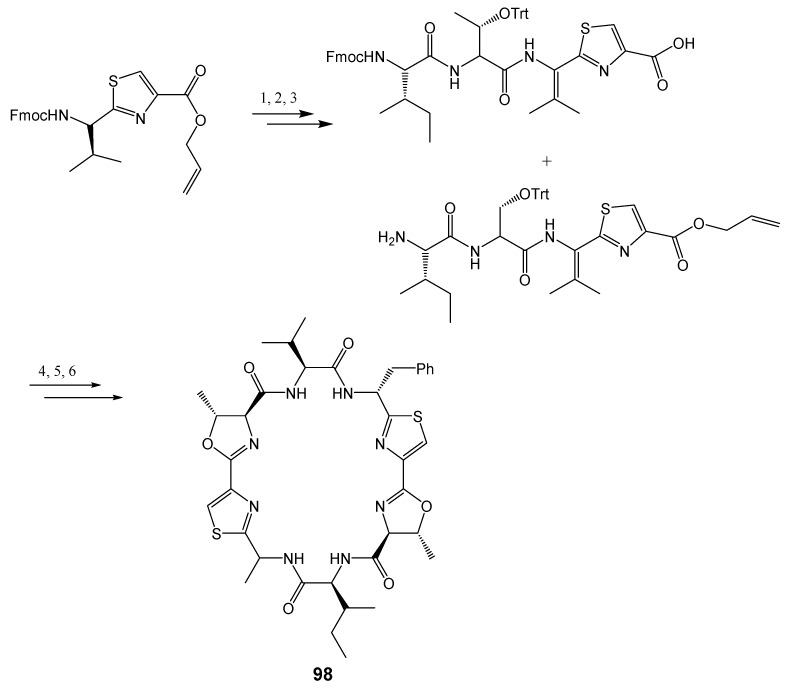
Patellamide D (97) showed a better result than verapamil in modulating drug resistance in vitro, and colchicine cytotoxicity were enhanced by 2.8 fold. Doxorubicin toxicity was reduced from IC50 > 1000 ng/mL to 110 ng/mL, by 97. This result indicated that patellamide D (97) acts as a selective antagonist in MDR, and thus can be considered as a potential modulator for drug resistance [7]. The synthesis of patellamide derivatives has been accomplished, using thiazole as starting material, via two contemporary heterocyclization approaches to form oxazolines and thiazoline via coupling and cyclodehydration reactions. The example illustrating the synthesis of patellamide A (98) is described in Scheme 19 [202].
Scheme 19.
Synthesis of patellamide A (98). Reagents and conditions: (1) 20% piperidine/DMF, HOBt, HBTU, DIEA, Fmoc-aThr(Trt)-OH; (2) 20% piperidine/DMF, HOBt, HBTU, DIEA, Fmoc-Ile-OH; (3) Pd(PPh3)4, PhSiH3, CH2Cl2, 6 h and 20% piperidine/DMF; (4) HOBt, HBTU, DIEA, CH2Cl2, 2% TFA, PhSH, CH2Cl2; (5) Burgess reagent, THF, 55 °C, 1 h then 77 °C, 4 h; (6) Pd(PPh3)4, PhSiH3, CH2Cl2, 2 h.
2.7. Miscellaneous
2.7.1. Irciniasulfonic Acid Derivatives
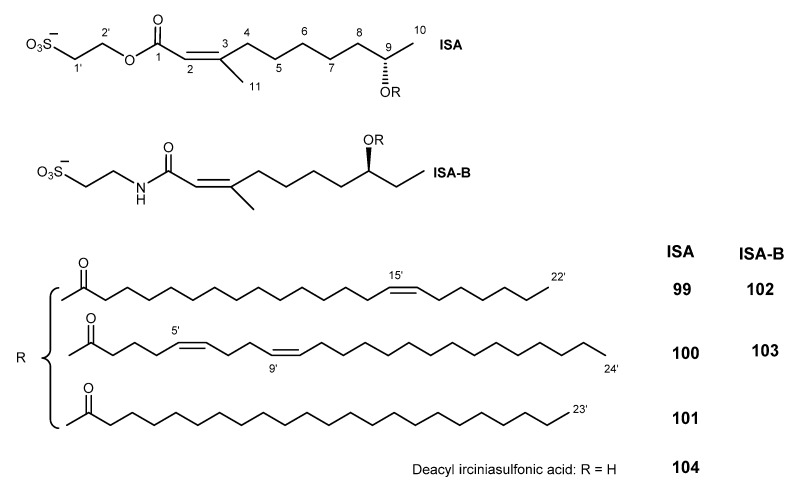
Irciniasulfonic acids consist of ISA (compounds 99–101, [203]) and ISA-B (compounds 102, 103, [204]). These acids are esters that were isolated from the marine sponge Ircinia sp. [203], and deacyl irciniasulfonic acid was isolated from tropical Coscinoderma sp. sponge (Figure 21) [205]. Irciniasulfonic acids 99–103 were found to reverse MDR against KB/VJ300 overexpressing P-gp at the concentration of 100 μM in the present of vincristine [203,204,206]. Compound 99–101 were shown to reverse MDR against KB/VJ300 overexpressing P-pg in the presence of verapamil at the concentration of 25 μM, and the simplified analog (deacyl ISA, 104) showed to be 13-fold more potent than other irciniasulfonic acids in reversal the MDR phenotype [203,204].
Figure 21.
Structure of ISA (99–101), ISA-B (102, 103), and deacyl ISA (104).
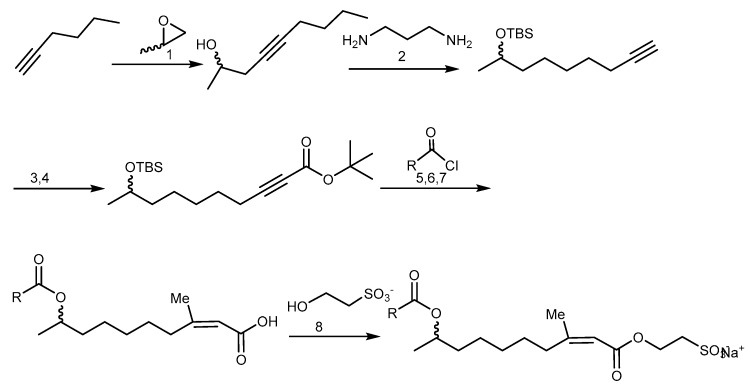
The synthesis of ISA derivatives was accomplished using propylene oxide as starting material via a convergent approach to achieve both enantiomers of the natural product and allowed also the incorporation of a range of side chains for optimization of their biological activity. The procedure of this synthesis is illustrated in Scheme 20 [207].
Scheme 20.
The total synthesis of ISA derivatives. Reagents and conditions: (1) n-BuLi, Et2AlCl, toluene; (2) KH; (3) TBS-Cl, imidazole, DMAP, DMF; (4) n-BuLi, Boc2O; (5) CuBr2.SMe2, MeLi, THF; (6) HF-pyridine; (7) TFA, CH2Cl2; (8) (COCl)2, DMF cat, CH2Cl2, toluene, reflux.
2.7.2. Secalonic Acid D
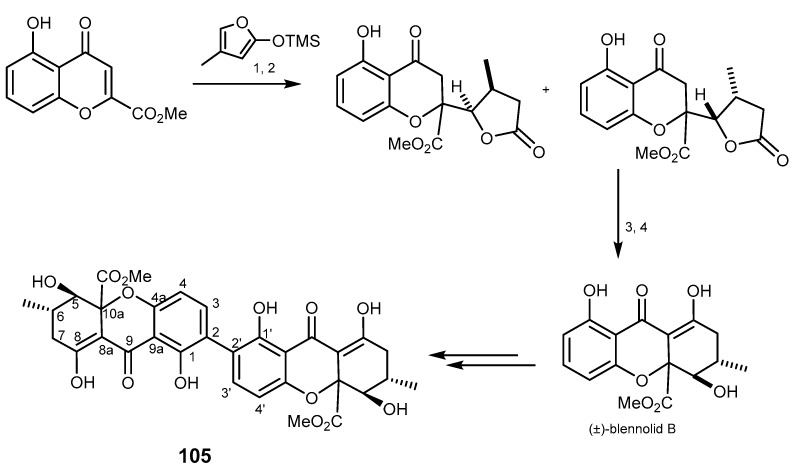
Secalonic acid D (105, Scheme 21) is a mycotoxin isolated from the marine fungi Penicillium oxalicum [208] and Gliocladium sp. T31 [209], which was investigated for antitumor properties as a DNA topoisomerase I inhibitor [210]. This compound showed a potent cytotoxicity on MDR cells (P-gp, MRP1, and BCRP-overexpressing cells) and on their parental cells by down-regulating the expression of BCRP protein [211]. It also decreased the percentage of side population cell in lung cancer cells. These results highlight 105 as an interesting molecule that has a potent cytotoxic activity by enduring BCRP degradation, and was considered as a lead compound for the development of new BCRP inhibitors [212].
Scheme 21.
Total synthesis of secalonic acid D (105). Reagents and conditions: (1) 2,6-lutidine, iPrSi(OTf)2, CH2Cl2, then Et3N·3HF; (2) Rh/Al2O3, H2, MeOH; (3) NaH, THF; (4) CH2Cl2, then NaH, THF.
Chemically, secalonic acid D (105) is the chiral dimeric natural product of ergochrome xanthone [213]. The synthesis of secalonic acid D (105) started from the intermediate compound (±)-blennolide B, which was synthesized from 5-hydroxychromone, with a construction of a hemisecalonic derivative, followed by the conversion with iodide and stannane. This reaction is a copper-catalyzed C–C bond-forming between the two molecules under oxidative conditions as the key dimerization step. This reaction is the first challenge to oxidize the xanthone framework which was previously shown as unsuitable for a direct oxidation. The iodide compound can be preactivated at an o-position to make the dimerization regioselective. The total synthesis of secalonic acid D (105) is illustrated in Scheme 21 [213,214,215,216].
2.7.3. Terrein
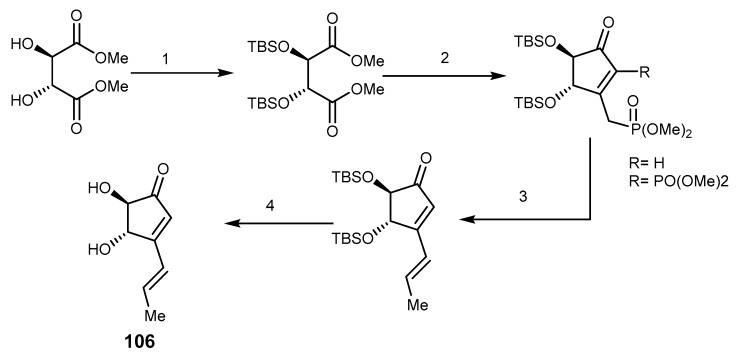
Terrein (106, Scheme 22) is a fungal metabolite isolated from the marine-derived thermophilic fungus Aspergillus terreus. This metabolite has been already reported to inhibit cell proliferation, to induce cell cycle arrest in human ovarian tumor cells (SKOV3,) and to inhibit the proliferation of ovarian cancer stem-like cells [217], to inhibit the epidermal proliferation of skin [218], as well as to inhibit melanogenesis [219]. It also displayed a strong cytotoxicity against breast cancer (MCF-7) cell and suppressed the growth of MCF-7 expressing BCRP cells by inducing apoptosis caspase-7 pathway, and inhibiting the Akt signaling pathway [220].
Scheme 22.
Synthesis of (+)-terrein (106). Reagents and conditions: (1) TBSCl, imidazole, DMF; (2) n-BuLi, MePO(OMe)2, THF, then benzene-H2O, reflux; (3) NaH, MeCHO, THF; (4) (Et4NCl), MeCN or HF·MeCN or TBAF, MeCN.
A simple synthesis of (+)-terrein (106) was reported by using l-tartrate or dimethyl l-tartrate as starting materials. The whole process included cyclization, Horner-Wadsworth-Emmons, and deprotection of bis-t-butyldimethylsilyl (bis-TBS) group as shown in Scheme 22 [221].
2.7.4. Shornephine A
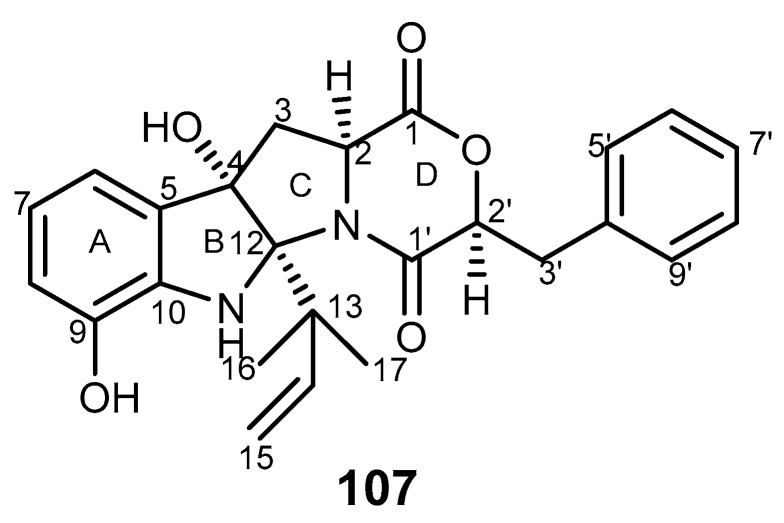
Shornephine A (107, Figure 22) is a diketomorpholine isolated from the marine fungus Aspergillus sp. (CMC-M081F) which was collected from marine sediment. This compound was described as non-cytotoxic against bacteria, fungus, and tumor cell lines and an inhibitor of P-gp in MDR human colon tumor cells (SW60 Ad300) at the concentration of 20 μM [222].
Figure 22.
Structure of shornephine A (107).
3. Future Perspectives
We are now witnessing a renaissance of the interest in efflux transporters, not only due to regulatory requirements but also to the significant role of these carriers in the adsorption, distribution, metabolism, excretion, and toxicity (ADMET) process of drug discovery. Therefore, P-gp and other ABC transporters that mediate drug efflux are recognized as a “team to beat”. Marine organisms have proven to be an important source in the discovery and development of interesting compounds, and, in particular, of anticancer agents. These marine-derived compounds, which can be grouped mainly as alkaloids, polyoxygenated sterols, terpenoids, and peptides, have demonstrated a reversal effect of MDR by themselves. Some compounds were shown to increase rather than decrease cytotoxicity towards MDR cell lines and can be defined as collateral sensitizing agents or this effect was shown in combination with anticancer drugs, being non-cytotoxic MDR reversal agents. The major sources of marine MDR reversal agents and mechanisms have been scarcely studied, and very little is known about their mechanism of action. Several marine compounds which have shown potent and selective MDR activity against cancer cell lines belong to the alkaloids; however, they present some toxicity. Among marine natural products, trabectedin has proved to be the most promising against MDR with in vitro and in vivo efficacy. Chemically, synthetic analogs of marine compounds acting as inhibitors have been investigated to achieve higher activity, less toxicity and less pharmacokinetic interaction properties, and to establish SAR. With this diversity of scaffolds it is not possible to extract common features essential for MDR reversal activity; however, most of the derivatives are highly lipophilic and contain fused rings and multiple chiral centers, which in turn reflect their complex total synthesis procedures. Among the synthetic analogs, eriburin has been shown to be the most promising P-gp modulator, active against MDR in vitro and with proved safety in clinical patients. These features also open new challenges for medicinal chemists to accomplish viable synthetic routes and new avenues in the design of analogs with suitable drug-like properties that could render potential drug candidates. Joining together all the pieces of the chemical-biological-pharmaceutical puzzle, we can anticipate more effective chemotherapies based on molecules from the sea.
Acknowledgments
The authors thank to national funds provided by FCT—Foundation for Science and Technology and European Regional Development Fund (ERDF) and COMPETE under the projects PEst-C/MAR/LA0015/2013, PTDC/MAR-BIO/4694/2014, and INNOVMAR—Innovation and Sustainability in the Management and Exploitation of Marine Resources, reference NORTE-01-0145-FEDER-000035, Research Line NOVELMAR. S.L. thanks Erasmus Mundus Action 2 (LOTUS+, LP15DF0205) for full PhD scholarship.
Author Contributions
The manuscript was conceived by E.S., S.L. collected the primary data and compiled draft manuscripts. E.S., A.K. and M.M.M.P. supervised development of the manuscript, and assisted in data interpretation, manuscript evaluation, and editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cancer. [(accessed on 16 March 2016)]. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/
- 2.Che X.F., Nakajima Y., Sumizawa T., Ikeda R., Ren X.Q., Zheng C.L., Mukai M., Furukawa T., Haraguchi M., Gao H., et al. Reversal of p-glycoprotein mediated multidrug resistance by a newly synthesized 1,4-benzothiazipine derivative, jtv-519. Cancer Lett. 2002;187:111–119. doi: 10.1016/S0304-3835(02)00359-2. [DOI] [PubMed] [Google Scholar]
- 3.Gustav L. P-glycoprotein as a drug target in the treatment of multidrug resistant cancer. Curr. Drug Targets. 2000;1:85–99. doi: 10.2174/1389450003349443. [DOI] [PubMed] [Google Scholar]
- 4.Higgins C.F. Multiple molecular mechanisms for multidrug resistance transporters. Nature. 2007;446:749–757. doi: 10.1038/nature05630. [DOI] [PubMed] [Google Scholar]
- 5.Ambudkar S.V., Kim I.-W., Sauna Z.E. The power of the pump: Mechanisms of action of P-glycoprotein (ABCB1) Eur. J. Pharm. Sci. 2006;27:392–400. doi: 10.1016/j.ejps.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Thomas H., Coley H.M. Overcoming multidrug resistance in cancer: An update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control. 2003;10:159–165. doi: 10.1177/107327480301000207. [DOI] [PubMed] [Google Scholar]
- 7.Williams A.B., Jacobs R.S. A marine natural product, patellamide d, reverses multidrug resistance in a human leukemic cell line. Cancer Lett. 1993;71:97–102. doi: 10.1016/0304-3835(93)90103-G. [DOI] [PubMed] [Google Scholar]
- 8.Li X., Yuan H., Wu J., Li J., Qu X., Xu W., Tang W. Strategies to overcome or circumvent P-glycoprotein mediated multidrug resistance. Curr. Med. Chem. 2008;15:470–476. doi: 10.2174/092986708783503258. [DOI] [PubMed] [Google Scholar]
- 9.Li X., Li J.P., Yuan H.Y., Gao X., Qu X.J., Xu W.F., Tang W. Recent advances in P-glycoprotein-mediated multidrug resistance reversal mechanisms. Methods Find. Exp. Clin. Pharmacol. 2007;29:607–617. doi: 10.1358/mf.2007.29.9.1139054. [DOI] [PubMed] [Google Scholar]
- 10.Baguley B.C. Multiple drug resistance mechanisms in cancer. Mol. Biotechnol. 2010;46:308–316. doi: 10.1007/s12033-010-9321-2. [DOI] [PubMed] [Google Scholar]
- 11.Gillet J.-P., Gottesman M.M. Overcoming multidrug resistance in cancer: 35 years after the discovery of abcb1. Drug Resist. Updates. 2012;15:2–4. doi: 10.1016/j.drup.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Ween M.P., Armstrong M.A., Oehler M.K., Ricciardelli C. The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit. Rev. Oncol. Hematol. 2015;2:220–256. doi: 10.1016/j.critrevonc.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Leslie E.M., Deeley R.G., Cole S.P.C. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Lopes-Rodrigues V., Seca H., Sousa D., Sousa E., Lima R.T., Vasconcelos M.H. The network of P-glycoprotein and micrornas interactions. Int. J. Cancer. 2014;135:253–263. doi: 10.1002/ijc.28500. [DOI] [PubMed] [Google Scholar]
- 15.Rubnitz J.E., Gibson B., Smith F.O. Acute myeloid leukemia. Pediatr. Clin. N. Am. 2008;55:21–51. doi: 10.1016/j.pcl.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Steinbach D., Legrand O. ABC transporters and drug resistance in leukemia: Was P-gp nothing but the first head of the hydra? Leukemia. 2007;21:1172–1176. doi: 10.1038/sj.leu.2404692. [DOI] [PubMed] [Google Scholar]
- 17.Rubnitz J.E. How I treat pediatric acute myeloid leukemia. Blood. 2012;119:5980–5988. doi: 10.1182/blood-2012-02-392506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessel D., Botterill V., Wodinsky I. Uptake and retention of daunomycin by mouse leukemic cells as factors in drug response. Cancer Res. 1968;28:938–941. [PubMed] [Google Scholar]
- 19.Srivastava S., Choudhary B.S., Sharma M., Malik R. Pharmacophore modeling and 3D-QSAR studies of galloyl benzamides as potent P-gp inhibitors. Med. Chem. Res. 2016;25:1140–1147. doi: 10.1007/s00044-016-1556-4. [DOI] [Google Scholar]
- 20.Palmeira A., Sousa E., Vasconcelos M.H., Pinto M.M. Three decades of P-gp inhibitors: Skimming through several generations and scaffolds. Curr. Med. Chem. 2012;19:1946–2025. doi: 10.2174/092986712800167392. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Victorias F.J., Conseil G., Munoz-Martinez F., Perez-Victoria J.M., Dayan G., Marsaud V., Castanys S., Gamarro F., Renoir J.M., di Pietro A. Ru49953: A non-hormonal steroid derivative that potently inhibits P-glycoprotein and reverts cellular multidrug resistance. Cell. Mol. Life Sci. 2003;60:526–535. doi: 10.1007/s000180300044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Modok S., Mellor H.R., Callaghan R. Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr. Opin. Pharmacol. 2006;6:350–354. doi: 10.1016/j.coph.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Krishna R., Mayer L.D. Multidrug resistance (MDR) in cancer—Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000;11:265–283. doi: 10.1016/S0928-0987(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 24.Coley H.M. Overcoming multidrug resistance in cancer: Clinical studies of P-glycoprotein inhibitors. In: Zhou J., editor. Multi-Drug Resistance in Cancer. Humana Press; Totowa, NJ, USA: 2010. pp. 341–358. [DOI] [PubMed] [Google Scholar]
- 25.Discontinuation of two phase III trials of tariquidar in non-small-cell lung cancer. Inpharma Wkly. 2013;1387:10. doi: 10.2165/00128413-200313870-00027. [DOI] [Google Scholar]
- 26.Wandel C., Kim R.B., Kajiji S., Guengerich F.P., Wilkinson G.R., Wood A.J.J. P-glycoprotein and cytochrome p-450 3a inhibition: Dissociation of inhibitory potencies. Cancer Res. 1999;59:3944–3948. [PubMed] [Google Scholar]
- 27.Cripe L.D., Uno H., Paietta E.M., Litzow M.R., Ketterling R.P., Bennett J.M., Rowe J.M., Lazarus H.M., Luger S., Tallman M.S. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: A randomized, placebo-controlled trial of the eastern cooperative oncology group 3999. Blood. 2010;116:4077–4085. doi: 10.1182/blood-2010-04-277269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C., Zhou J., Ji C. Quercetin: A potential drug to reverse multidrug resistance. Life Sci. 2010;87:333–338. doi: 10.1016/j.lfs.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Cherigo L., Lopez D., Martinez-Luis S. Marine natural products as breast cancer resistance protein inhibitors. Mar. Drugs. 2015;13:2010–2029. doi: 10.3390/md13042010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes N., Lefranc F., Kijjoa A., Kiss R. Can some marine-derived fungal metabolites become actual anticancer agents? Mar. Drugs. 2015;13:3950–3991. doi: 10.3390/md13063950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann K., Bekeredjian R., Schmidt J., Büchler M.W., Märten A. Effects of the high-affinity peptide reversin 121 on multidrug resistance proteins in experimental pancreatic cancer. Tumor Biol. 2009;29:351–358. doi: 10.1159/000178142. [DOI] [PubMed] [Google Scholar]
- 32.Bogman K., Erne-Brand F., Alsenz J., Drewe J. The role of surfactants in the reversal of active transport mediated by multidrug resistance proteins. J. Pharm. Sci. 2003;92:1250–1261. doi: 10.1002/jps.10395. [DOI] [PubMed] [Google Scholar]
- 33.Capon R.J. Marine bioprospecting—Trawling for treasure and pleasure. Eur. J. Org. Chem. 2001:633–645. doi: 10.1002/1099-0690(200102)2001:4<633::AID-EJOC633>3.0.CO;2-Q. [DOI] [Google Scholar]
- 34.Zhang G., Li J., Zhu T., Gu Q., Li D. Advanced tools in marine natural drug discovery. Curr. Opin. Biotechnol. 2016;42:13–23. doi: 10.1016/j.copbio.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Lopez D., Martinez-Luis S. Marine natural products with P-glycoprotein inhibitor properties. Mar. Drugs. 2014;12:525–546. doi: 10.3390/md12010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abraham I., El Sayed K., Chen Z.S., Guo H. Current status on marine products with reversal effect on cancer multidrug resistance. Mar. Drugs. 2012;10:2312–2321. doi: 10.3390/md10102312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kathawala R.J., Gupta P., Ashby C.R., Chen Z.S. The modulation of ABC transporter-mediated multidrug resistance in cancer: A review of the past decade. Drug Resist. Updates. 2015;18:1–17. doi: 10.1016/j.drup.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Stonik V.A., Fedorov S.N. Marine low molecular weight natural products as potential cancer preventive compounds. Mar. Drugs. 2014;12:636–671. doi: 10.3390/md12020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., Zhang Y.K., Wang Y.J., Vispute S.G., Jain S., Chen Y., Li J., Youssef D.T.A., El Sayed K.A., Chen Z.S. Esters of the marine-derived triterpene sipholenol a reverse P-gp-mediated drug resistance. Mar. Drugs. 2015;13:2267–2286. doi: 10.3390/md13042267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shanthi J., Senthil A., Gopikrishnan V., Balagurunathan R. Characterization of a potential β-lactamase inhibitory metabolite from a marine streptomyces sp. Pm49 active against multidrug-resistant pathogens. Appl. Biochem. Biotechnol. 2015;175:3696–3708. doi: 10.1007/s12010-015-1538-x. [DOI] [PubMed] [Google Scholar]
- 41.Huang X.C., Kumar P., Anreddy N., Xiao X., Yang D.H., Chen Z.S. Handbook of Anticancer Drugs from Marine Origin. Springer; Berlin, Germany: 2015. P-gp inhibitory activity from marine sponges, tunicates and algae; pp. 593–619. [Google Scholar]
- 42.Dinić J., Podolski-Renić A., Stanković T., Banković J., Pešić M. New approaches with natural product drugs for overcoming multidrug resistance in cancer. Curr. Pharm. Design. 2015;21:5589–5604. doi: 10.2174/1381612821666151002113546. [DOI] [PubMed] [Google Scholar]
- 43.Patel A., Wang D.-S., Sim H.-M., Ambudkar S.V., Chen Z.-S. ABC transporter modulatory drugs from marine sources: A new approach to overcome drug resistance in cancer. In: Efferth T., editor. Resistance to Targeted abc Transporters in Cancer. Springer International Publishing; Cham, Switzerland: 2015. pp. 183–208. [Google Scholar]
- 44.Shi Z., Jain S., Kim I.W., Peng X.X., Abraham I., Youssef D.T.A., Fu L.W., El Sayed K., Ambudkar S.V., Chen Z.S. Sipholenol a, a marine-derived sipholane triterpene, potently reverses P-glycoprotein (ABCB1)-mediated multidrug resistance in cancer cells. Cancer Sci. 2007;98:1373–1380. doi: 10.1111/j.1349-7006.2007.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abraham I., Jain S., Wu C.P., Khanfar M.A., Kuang Y., Dai C.L., Shi Z., Chen X., Fu L., Ambudkar S.V., et al. Marine sponge-derived sipholane triterpenoids reverse P-glycoprotein (ABCB1)-mediated multidrug resistance in cancer cells. Biochem. Pharmacol. 2010;80:1497–1506. doi: 10.1016/j.bcp.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akl M.R., Foudah A.I., Ebrahim H.Y., Meyer S.A., El Sayed K.A. The marine-derived sipholenol A-4-O-3′,4′-dichlorobenzoate inhibits breast cancer growth and motility in vitro andin vivo through the suppression of brk and fak signaling. Mar. Drugs. 2014;12:2282–2304. doi: 10.3390/md12042282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foudah A.I., Sallam A.A., Akl M.R., El Sayed K.A. Optimization, pharmacophore modeling and 3D-QSAR studies of sipholanes as breast cancer migration and proliferation inhibitors. Eur. J. Med. Chem. 2014;73:310–324. doi: 10.1016/j.ejmech.2013.11.039. [DOI] [PubMed] [Google Scholar]
- 48.Jain S., Laphookhieo S., Shi Z., Fu L.W., Akiyama S.I., Chen Z.S., Youssef D.T.A., van Soest R.W.M., El Sayed K.A. Reversal of p-glycoprotein-mediated multidrug resistance by sipholane triterpenoids. J. Nat. Prod. 2007;70:928–931. doi: 10.1021/np0605889. [DOI] [PubMed] [Google Scholar]
- 49.Jain S., Abraham I., Carvalho P., Kuang Y.H., Shaala L.A., Youssef D.T.A., Avery M.A., Chen Z.S., El Sayed K.A. Sipholane triterpenoids: Chemistry, reversal of ABCB1/P-glycoprotein-mediated multidrug resistance, and pharmacophore modeling. J. Nat. Prod. 2009;72:1291–1298. doi: 10.1021/np900091y. [DOI] [PubMed] [Google Scholar]
- 50.Rochfort S.J., Capon R.J. Parguerenes revisited: New brominated diterpenes from the southern australian marine red alga laurencia filiformis. Aust. J. Chem. 1996;49:19–26. [Google Scholar]
- 51.Awad N.E. Bioactive brominated diterpenes from the marine red alga Jania Rubens (L.) lamx. Phytother. Res. 2004;18:275–279. doi: 10.1002/ptr.1273. [DOI] [PubMed] [Google Scholar]
- 52.Takeda S., Kurosawa E., Komiyama K., Suzuki T. The structures of cytotoxic diterpenes containing bromine from the marine red alga Laurencia obtusa (hudson) lamouroux. Bull. Chem. Soc. Jpn. 1990;63:3066–3072. doi: 10.1246/bcsj.63.3066. [DOI] [Google Scholar]
- 53.Huang X.-C., Sun Y.-L., Salim A.A., Chen Z.-S., Capon R.J. Parguerenes: Marine red alga bromoditerpenes as inhibitors of P-glycoprotein (ABCB1) in multidrug resistant human cancer cells. Biochem. Pharmacol. 2013;85:1257–1268. doi: 10.1016/j.bcp.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 54.Aoki S., Chen Z.-S., Higasiyama K., Setiawan I., Akiyama S.-I., Kobayashi M. Reversing effect of agosterol a, a spongean sterol acetate, on multidrug resistance in human carcinoma cells. Jpn. J. Cancer Res. 2001;92:886–895. doi: 10.1111/j.1349-7006.2001.tb01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aoki S., Yoshioka Y., Miyamoto Y., Higuchi K., Setiawan A., Murakami N., Chen Z.S., Sumizawa T., Akiyama S., Kobayashi M. Agosterol a, a novel polyhydroxylated sterol acetate reversing multidrug resistance from a marine sponge of spongia sp. Tetrahedron Lett. 1998;39:6303–6306. doi: 10.1016/S0040-4039(98)01336-7. [DOI] [Google Scholar]
- 56.Chen Z.-S., Aoki S., Komatsu M., Ueda K., Sumizawa T., Furukawa T., Okumura H., Ren X.-Q., Belinsky M.G., Lee K., et al. Reversal of drug resistance mediated by multidrug resistance protein (MRP) 1 by dual effects of agosterol a on MRP1 function. Int. J. Cancer. 2001;93:107–113. doi: 10.1002/ijc.1290. [DOI] [PubMed] [Google Scholar]
- 57.Mayer A.M.S., Rodríguez A.D., Berlinck R.G.S., Hamann M.T. Marine pharmacology in 2003-4: Marine compounds with anthelmintic antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiplatelet, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007;145:553–581. doi: 10.1016/j.cbpc.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aoki S., Setiawan A., Yoshioka Y., Higuchi K., Fudetani R., Chen Z.-S., Sumizawa T., Akiyama S.-I., Kobayashi M. Reversal of multidrug resistance in human carcinoma cell line by agosterols, marine spongean sterols. Tetrahedron. 1999;55:13965–13972. doi: 10.1016/S0040-4020(99)00870-4. [DOI] [Google Scholar]
- 59.Mayer A.M.S., Gustafson K.R. Marine pharmacology in 2001–2: Antitumour and cytotoxic compounds. Eur. J. Cancer. 2004;40:2676–2704. doi: 10.1016/j.ejca.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 60.Murakami N., Sugimoto M., Morita M., Akiyama S.-I., Kobayashi M. Synthesis and evaluation of 4-deacetoxyagosterol a as an mdr-modulator. Bioorg. Med. Chem. Lett. 2000;10:2521–2524. doi: 10.1016/S0960-894X(00)00502-3. [DOI] [PubMed] [Google Scholar]
- 61.Murakami N., Sugimoto M., Morita M., Kobayashi M. Total synthesis of agosterol a: An MDR-modulator from a marine sponge. Chem. Eur. J. 2001;7:2663–2670. doi: 10.1002/1521-3765(20010618)7:12<2663::AID-CHEM26630>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka J., Trianto A., Musman M., Issa H.H., Ohtani I.I., Ichiba T., Higa T., Yoshida W.Y., Scheuer P.J. New polyoxygenated steroids exhibiting reversal of multidrug resistance from the gorgonian isis hippuris. Tetrahedron. 2002;58:6259–6266. doi: 10.1016/S0040-4020(02)00625-7. [DOI] [Google Scholar]
- 63.Boonananwong S., Kongkathip B., Kongkathip N. First synthesis of 3,16,20-polyoxygenated cholestanes, new cytotoxic steroids from the gorgonian leptogorgia sarmentosa. Steroids. 2008;73:1123–1127. doi: 10.1016/j.steroids.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 64.Wang Z., Tang H., Wang P., Gong W., Xue M., Zhang H., Liu T., Liu B., Yi Y., Zhang W. Bioactive polyoxygenated steroids from the south china sea soft coral, sarcophyton sp. Mar. Drugs. 2013;11:775–787. doi: 10.3390/md11030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li R., Shao C.L., Qi X., Li X.B., Li J., Sun L.L., Wang C.Y. Polyoxygenated sterols from the south China sea soft coral sinularia sp. Mar. Drugs. 2012;10:1422–1432. doi: 10.3390/md10071422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang P., Tang H., Liu B.-S., Li T.-J., Sun P., Zhu W., Luo Y.-P., Zhang W. Tumor cell growth inhibitory activity and structure-activity relationship of polyoxygenated steroids from the gorgonian menella kanisa. Steroids. 2013;78:951–958. doi: 10.1016/j.steroids.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 67.Kim S., Sutton S.C., Guo C., LaCour T.G., Fuchs P.L. Synthesis of the north 1 unit of the cephalostatin family from hecogenin acetate1. J. Am. Chem. Soc. 1999;121:2056–2070. doi: 10.1021/ja9817139. [DOI] [Google Scholar]
- 68.Musumeci D., Sica D., Zollo F. Synthesis of polyoxygenated steroids with transition metal-based oxidants: Methyltrioxorhenium-hydrogen peroxide system, ruthenium tetraoxide, osmium tetraoxide and potassium permanganate. Curr. Org. Synth. 2005;2 doi: 10.2174/1570179052996919. [DOI] [Google Scholar]
- 69.Weiss J.M., Hoffmann H.M.R. Synthesis of the c1–c9 segment of bryostatin. Tetrahedron Asymmetry. 1997;8:3913–3920. doi: 10.1016/S0957-4166(97)00564-8. [DOI] [Google Scholar]
- 70.Ohmori K., Ogawa Y., Obitsu T., Ishikawa Y., Nishiyama S., Yamamura S. Total synthesis of bryostatin 3. Angew. Chem. Int. Ed. 2000;39:2290–2294. doi: 10.1002/1521-3773(20000703)39:13<2290::AID-ANIE2290>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 71.Pettit G.R., Herald C.L., Hogan F. Chapter 12—Biosynthetic products for anticancer drug design and treatment: The bryostatins. In: Kerr B.C.B.J., editor. Anticancer Drug Development. Academic Press; San Diego, CA, USA: 2002. pp. 203–235. [Google Scholar]
- 72.Spitaler M., Utz I., Hilbe W., Hofmann J., Grunicke H. PKC-independent modulation of multidrug resistance in cells with mutant (V185) but not wild-type (G185) P-glycoprotein by bryostatin 1. Biochem. Pharmacol. 1998;56:861–869. doi: 10.1016/S0006-2952(98)00107-5. [DOI] [PubMed] [Google Scholar]
- 73.Utz I., Hofmann J., Grunicke H. Bryostatin 1 regulates multi drug resistance by a PKC-independent mechanism. Eur. J. Cancer. 1995;31(Suppl. 3):S13. doi: 10.1016/0959-8049(95)99872-W. [DOI] [Google Scholar]
- 74.Scala S., Dickstein B., Regis J., Szallasi Z., Blumberg P.M., Bates S.E. Bryostatin 1 affects P-glycoprotein phosphorylation but not function in multidrug-resistant human breast cancer cells. Clin. Cancer Res. 1995;1:1581–1587. [PubMed] [Google Scholar]
- 75.Kedei N., Telek A., Czap A., Lubart E.S., Czifra G., Yang D., Chen J., Morrison T., Goldsmith P.K., Lim L., et al. The synthetic bryostatin analog merle 23 dissects distinct mechanisms of bryostatin activity in the lncap human prostate cancer cell line. Biochem. Pharmacol. 2011;81:1296–1308. doi: 10.1016/j.bcp.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kedei N., Telek A., Michalowski A.M., Kraft M.B., Li W., Poudel Y.B., Rudra A., Petersen M.E., Keck G.E., Blumberg P.M. Comparison of transcriptional response to phorbol ester, bryostatin 1, and bryostatin analogs in lncap and u937 cancer cell lines provides insight into their differential mechanism of action. Biochem. Pharmacol. 2013;85:313–324. doi: 10.1016/j.bcp.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wender P.A., De Brabander J., Harran P.G., Hinkle K.W., Lippa B., Pettit G.R. Synthesis and biological evaluation of fully synthetic bryostatin analogues. Tetrahedron Lett. 1998;39:8625–8628. doi: 10.1016/S0040-4039(98)01955-8. [DOI] [Google Scholar]
- 78.Trost B.M., Yang H., Dong G. Total syntheses of bryostatins: Synthesis of two ring-expanded bryostatin analogues and the development of a new-generation strategy to access the c7–c27 fragment. Chem. Eur. J. 2011;17:9789–9805. doi: 10.1002/chem.201002932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trost B.M., Yang H., Thiel O.R., Frontier A.J., Brindle C.S. Synthesis of a ring-expanded bryostatin analogue. J. Am. Chem. Soc. 2007;129:2206–2207. doi: 10.1021/ja067305j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roy R., Rey A.W., Charron M., Molino R. Enantiospecific synthesis of the c-17–c-20 and c-21–c-27 synthons of the antineoplastic macrolide bryostatins. J. Chem. Soc. Chem. Commun. 1989:1308–1310. doi: 10.1039/C39890001308. [DOI] [Google Scholar]
- 81.Honore S., Kamath K., Braguer D., Horwitz S.B., Wilson L., Briand C., Jordan M.A. Synergistic suppression of microtubule dynamics by discodermolide and paclitaxel in non-small cell lung carcinoma cells. Cancer Res. 2004;64:4957–4964. doi: 10.1158/0008-5472.CAN-04-0693. [DOI] [PubMed] [Google Scholar]
- 82.Loggley R.E., Ccddigan D., Harmody D., Gunasekera M., Gunasekera S.P. Discodermolide-a new, marine-derived immunosuppressive compound: I. in vitro studies. Transplantation. 1991;52:650–655. doi: 10.1097/00007890-199110000-00014. [DOI] [PubMed] [Google Scholar]
- 83.Longley R.E., Gunasekera S.P., Faherty D., McLane J., Dumont F. Immunosuppression by discodermolide. Ann. N. Y. Acad. Sci. 1993;696:94–107. doi: 10.1111/j.1749-6632.1993.tb17145.x. [DOI] [PubMed] [Google Scholar]
- 84.Dimri G.P., Lee X., Basile G., Acosta M., Scott G., Roskelley C., Medrano E.E., Linskens M., Rubelj I., Pereira-Smith O. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kowalski R.J., Giannakakou P., Gunasekera S.P., Longley R.E., Day B.W., Hamel E. The microtubule-stabilizing agent discodermolide competitively inhibits the binding of paclitaxel (taxol) to tubulin polymers, enhances tubulin nucleation reactions more potently than paclitaxel, and inhibits the growth of paclitaxel-resistant cells. Mol. Pharmacol. 1997;52:613–622. [PubMed] [Google Scholar]
- 86.Betzer J.F., Ardisson J. Strategies and Tactics in Organic Synthesis. Volume 11. Elsevier; San Diego, CA, USA: 2015. Discodermolide: Total synthesis of natural product and analogues; pp. 51–84. [Google Scholar]
- 87.Yu Z., Ely R.J., Morken J.P. Synthesis of (+)-discodermolide by catalytic stereoselective borylation reactions. Angew. Chem. Int. Ed. 2014;53:9632–9636. doi: 10.1002/anie.201405455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Towle M.J., Salvato K.A., Budrow J., Wels B.F., Kuznetsov G., Aalfs K.K., Welsh S., Zheng W., Seletsky B.M., Palme M.H., et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–1021. [PubMed] [Google Scholar]
- 89.Cortes J., Montero A.J., Glück S. Eribulin mesylate, a novel microtubule inhibitor in the treatment of breast cancer. Cancer Treat. Rev. 2012;38:143–151. doi: 10.1016/j.ctrv.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 90.Huyck T.K., Gradishar W., Manuguid F., Kirkpatrick P. Eribulin mesylate. Nat. Rev. Drug Discov. 2011;10:173–174. doi: 10.1038/nrd3389. [DOI] [PubMed] [Google Scholar]
- 91.Narayan S., Carlson E.M., Cheng H., Du H., Hu Y., Jiang Y., Lewis B.M., Seletsky B.M., Tendyke K., Zhang H., et al. Novel second generation analogs of eribulin. Part I: Compounds containing a lipophilic c32 side chain overcome P-glycoprotein susceptibility. Bioorg. Med. Chem. Lett. 2011;21:1630–1633. doi: 10.1016/j.bmcl.2011.01.111. [DOI] [PubMed] [Google Scholar]
- 92.Garrone O., Montemurro F., Saggia C., la Verde N., Vandone A.M., Airoldi M., de Conciliis E., Donadio M., Lucio F., Polimeni M.A., et al. Eribulin in pretreated metastatic breast cancer patients: Results of the trotter trial—A multicenter retrospective study of eribulin in real life. SpringerPlus. 2016;5:1–8. doi: 10.1186/s40064-016-1700-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu M.J., Zheng W., Seletsky B.M. From micrograms to grams: Scale-up synthesis of eribulin mesylate. Nat. Prod. Rep. 2013;30:1158–1164. doi: 10.1039/c3np70051h. [DOI] [PubMed] [Google Scholar]
- 94.Imbri D., Tauber J., Opatz T. Synthetic approaches to the lamellarins—A comprehensive review. Mar. Drugs. 2014;12:6142–6177. doi: 10.3390/md12126142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ishibashi F., Tanabe S., Oda T., Iwao M. Synthesis and structure-activity relationship study of lamellarin derivatives. J. Nat. Prod. 2002;65:500–504. doi: 10.1021/np0104525. [DOI] [PubMed] [Google Scholar]
- 96.Bailly C. Anticancer properties of lamellarins. Mar. Drugs. 2015;13:1105–1123. doi: 10.3390/md13031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ploypradith P., Jinaglueng W., Pavaro C., Ruchirawat S. Further developments in the synthesis of lamellarin alkaloids via direct metal-halogen exchange. Tetrahedron Lett. 2003;44:1363–1366. doi: 10.1016/S0040-4039(02)02887-3. [DOI] [Google Scholar]
- 98.Facompre M., Tardy C., Bal-Mahieu C., Colson P., Perez C., Manzanares I., Cuevas C., Bailly C. Lamellarin d: A novel potent inhibitor of topoisomerase i. Cancer Res. 2003;63:7392–7399. [PubMed] [Google Scholar]
- 99.Ridley C.P., Reddy M.V., Rocha G., Bushman F.D., Faulkner D.J. Total synthesis and evaluation of lamellarin alpha 20-sulfate analogues. Bioorg. Med. Chem. 2002;10:3285–3290. doi: 10.1016/S0968-0896(02)00237-7. [DOI] [PubMed] [Google Scholar]
- 100.Ohta T., Fukuda T., Ishibashi F., Iwao M. Design and synthesis of lamellarin d analogues targeting topoisomerase i. J. Org. Chem. 2009;74:8143–8153. doi: 10.1021/jo901589e. [DOI] [PubMed] [Google Scholar]
- 101.Díaz M., Guitián E., Castedo L. Syntheses of lamellarins i and k by [3 + 2] cycloaddition of a nitrone to an alkyne. Synlett. 2001:1164–1166. doi: 10.1055/s-2001-15143. [DOI] [Google Scholar]
- 102.Quesada A.R., García Grávalos M.D., Fernández Puentes J.L. Polyaromatic alkaloids from marine invertebrates as cytotoxic compounds and inhibitors of multidrug resistance caused by P-glycoprotein. Br. J. Cancer. 1996;74:677–682. doi: 10.1038/bjc.1996.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang X.C., Xiao X., Zhang Y.K., Talele T.T., Salim A.A., Chen Z.S., Capon R.J. Lamellarin o, a pyrrole alkaloid from an australian marine sponge, ianthella sp., reverses bcrp mediated drug resistance in cancer cells. Mar. Drugs. 2014;12:3818–3837. doi: 10.3390/md12073818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fuerstner A., Weintritt H., Hupperts A. A new, titanium-mediated approach to pyrroles: First synthesis of lukianol a and lamellarin o dimethyl ether. J. Org. Chem. 1995;60:6637–6641. doi: 10.1021/jo00125a068. [DOI] [Google Scholar]
- 105.Banwell M.G., Flynn B.L., Hamel E., Hockless D.C.R. Convergent syntheses of the pyrrolic marine natural products lamellarin-O, lamellarin-Q, lukianol-A and some more highly oxygenated congeners. Chem. Commun. 1997:207–208. doi: 10.1039/a606793j. [DOI] [Google Scholar]
- 106.Marfil M., Albericio F., Álvarez M. Solid-phase synthesis of lamellarins Q and O. Tetrahedron. 2004;60:8659–8668. doi: 10.1016/j.tet.2004.05.110. [DOI] [Google Scholar]
- 107.Fukuda T., Sudo E.-I., Shimokawa K., Iwao M. Palladium-catalyzed cross-coupling of N-benzenesulfonyl-3,4-dibromopyrrole and its application to the total syntheses of lamellarins O, P, Q, and R. Tetrahedron. 2008;64:328–338. doi: 10.1016/j.tet.2007.10.105. [DOI] [Google Scholar]
- 108.Boger D.L., Boyce C.W., Labroli M.A., Sehon C.A., Jin Q. Total syntheses of ningalin A, lamellarin O, lukianol A, and permethyl storniamide a utilizing heterocyclic azadiene diels-alder reactions. J. Am. Chem. Soc. 1999;121:54–62. doi: 10.1021/ja982078+. [DOI] [Google Scholar]
- 109.Pla D., Marchal A., Olsen C.A., Albericio F., Álvarez M. Modular total synthesis of lamellarin d. J. Org. Chem. 2005;70:8231–8234. doi: 10.1021/jo051083a. [DOI] [PubMed] [Google Scholar]
- 110.Fujikawa N., Ohta T., Yamaguchi T., Fukuda T., Ishibashi F., Iwao M. Total synthesis of lamellarins D, L, and N. Tetrahedron. 2006;62:594–604. doi: 10.1016/j.tet.2005.10.014. [DOI] [Google Scholar]
- 111.Ueda K., Amaike K., Maceiczyk R.M., Itami K., Yamaguchi J. B-selective C–H arylation of pyrroles leading to concise syntheses of lamellarins c and i. J. Am. Chem. Soc. 2014;136:13226–13232. doi: 10.1021/ja508449y. [DOI] [PubMed] [Google Scholar]
- 112.Kang H., Fenical W. Ningalins A–D: Novel aromatic alkaloids from a western australian ascidian of the genus didemnum. J. Org. Chem. 1997;62:3254–3262. doi: 10.1021/jo962132+. [DOI] [PubMed] [Google Scholar]
- 113.Li Q., Jiang J., Fan A., Cui Y., Jia Y. Total synthesis of lamellarins D, H, and R and ningalin B. Org. Lett. 2011;13:312–315. doi: 10.1021/ol1027877. [DOI] [PubMed] [Google Scholar]
- 114.Soenen D.R., Hwang I., Hedrick M.P., Boger D.L. Multidrug resistance reversal activity of key ningalin analogues. Bioorg. Med. Chem. Lett. 2003;13:1777–1781. doi: 10.1016/S0960-894X(03)00294-4. [DOI] [PubMed] [Google Scholar]
- 115.Tao H., Hwang I., Boger D.L. Multidrug resistance reversal activity of permethyl ningalin b amide derivatives. Bioorg. Med. Chem. Lett. 2004;14:5979–5981. doi: 10.1016/j.bmcl.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 116.Chou T.C., Guan Y., Soenen D.R., Danishefsky S.J., Boger D.L. Potent reversal of multidrug resistance by ningalins and its use in drug combinations against human colon carcinoma xenograft in nude mice. Cancer Chemother. Pharmacol. 2005;56:379–390. doi: 10.1007/s00280-005-1019-y. [DOI] [PubMed] [Google Scholar]
- 117.Zhang P.Y., Wong I.L.K., Yan C.S.W., Zhang X.Y., Jiang T., Chow L.M.C., Wan S.B. Design and syntheses of permethyl ningalin B analogues: Potent multidrug resistance (MDR) reversal agents of cancer cells. J. Med. Chem. 2010;53:5108–5120. doi: 10.1021/jm100035c. [DOI] [PubMed] [Google Scholar]
- 118.Wang Z., Wong I.L.K., Li F.X., Yang C., Liu Z., Jiang T., Jiang T.F., Chow L.M.C., Wan S.B. Optimization of permethyl ningalin B analogs as P-glycoprotein inhibitors. Bioorg. Med. Chem. 2015;23:5566–5573. doi: 10.1016/j.bmc.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 119.Yang C., Wong I.L.K., Jin W.B., Jiang T., Chow L.M.C., Wan S.B. Modification of marine natural product ningalin B and SAR study lead to potent P-glycoprotein inhibitors. Mar. Drugs. 2014;12:5209–5221. doi: 10.3390/md12105209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bin J.W., Wong I.L.K., Hu X., Yu Z.X., Xing L.F., Jiang T., Chow L.M.C., Biao W.S. Structure-activity relationship study of permethyl ningalin B analogues as P-glycoprotein chemosensitizers. J. Med. Chem. 2013;56:9057–9070. doi: 10.1021/jm400930e. [DOI] [PubMed] [Google Scholar]
- 121.Hamasaki A., Zimpleman J.M., Hwang I., Boger D.L. Total synthesis of ningalin D. J. Am. Chem. Soc. 2005;127:10767–10770. doi: 10.1021/ja0526416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saracoglu N. Recent advances and applications in 1,2,4,5-tetrazine chemistry. Tetrahedron. 2007;63:4199–4236. doi: 10.1016/j.tet.2007.02.051. [DOI] [Google Scholar]
- 123.Fu T.H., McElroy W.T., Shamszad M., Martin S.F. Formal syntheses of naturally occurring welwitindolinones. Org. Lett. 2012;14:3834–3837. doi: 10.1021/ol301424h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.MacKay J.A., Bishop R.L., Rawal V.H. Rapid synthesis of the N-methylwelwitindolinone skeleton. Org. Lett. 2005;7:3421–3424. doi: 10.1021/ol051043t. [DOI] [PubMed] [Google Scholar]
- 125.Smith C.D., Zilfou J.T., Stratmann K., Patterson G.M.L., Moore R.E. Welwitindolinone analogues that reverse P-glycoprotein-mediated multiple drug resistance. Mol. Pharmacol. 1995;47:241–247. [PubMed] [Google Scholar]
- 126.Grundmann A., Kuznetsova T., Afiyatullov S., Li S.M. FTMPT2, an N-prenyltransferase from aspergillus fumigatus, catalyses the last step in the biosynthesis of fumitremorgin B. Chembiochem. 2008;9:2059–2063. doi: 10.1002/cbic.200800240. [DOI] [PubMed] [Google Scholar]
- 127.Fu T.H., McElroy W.T., Shamszad M., Heidebrecht R.W., Jr., Gulledge B., Martin S.F. Studies toward welwitindolinones: Formal syntheses of N-methylwelwitindolinone C isothiocyanate and related natural products. Tetrahedron. 2013;69:5588–5603. doi: 10.1016/j.tet.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wood J.L. Total synthesis: Welwitindolinone is well worth it. Nat. Chem. 2012;4:341–343. doi: 10.1038/nchem.1335. [DOI] [PubMed] [Google Scholar]
- 129.Komine K., Nomura Y., Ishihara J., Hatakeyama S. Total synthesis of (−)-N-methylwelwitindolinone C isothiocyanate based on a Pd-catalyzed tandem enolate coupling strategy. Org. Lett. 2015;17:3918–3921. doi: 10.1021/acs.orglett.5b01952. [DOI] [PubMed] [Google Scholar]
- 130.Patel K., Gadewar M., Tripathi R., Prasad S.K., Patel D.K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “harmine”. Asian Pac. J. Trop. Biomed. 2012;2:660–664. doi: 10.1016/S2221-1691(12)60116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khan A.M., Noreen S., Imran Z.P., Attaur R., Choudhary M.I. A new compound, jolynamine, from marine brown alga jolyna laminarioides. Nat. Prod. Res. 2011;25:898–904. doi: 10.1080/14786419.2010.509722. [DOI] [PubMed] [Google Scholar]
- 132.Li S., Wang A., Gu F., Wang Z., Tian C., Qian Z., Tang L., Gu Y. Novel harmine derivatives for tumor targeted therapy. Oncotarget. 2015;6:8988–9001. doi: 10.18632/oncotarget.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen Q., Chao R., Chen H., Hou X., Yan H., Zhou S., Peng W., Xu A. Antitumor and neurotoxic effects of novel harmine derivatives and structure-activity relationship analysis. Int. J. Cancer. 2005;114:675–682. doi: 10.1002/ijc.20703. [DOI] [PubMed] [Google Scholar]
- 134.Cao R., Fan W., Guo L., Ma Q., Zhang G., Li J., Chen X., Ren Z., Qiu L. Synthesis and structure-activity relationships of harmine derivatives as potential antitumor agents. Eur. J. Med. Chem. 2013;60:135–143. doi: 10.1016/j.ejmech.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 135.Ma Y., Wink M. The β-carboline alkaloid harmine inhibits BCRP and can reverse resistance to the anticancer drugs mitoxantrone and camptothecin in breast cancer cells. Phytother. Res. 2010;24:146–149. doi: 10.1002/ptr.2860. [DOI] [PubMed] [Google Scholar]
- 136.Wu Q., Bai Z., Ma Q., Fan W., Guo L., Zhang G., Qiu L., Yu H., Shao G., Cao R. Synthesis and biological evaluation of novel bivalent β-carbolines as potential antitumor agents. Med. Chem. Commun. 2014;5:953–957. doi: 10.1039/c4md00098f. [DOI] [Google Scholar]
- 137.Frédérick R., Bruyére C., Vancraeynest C., Reniers J., Meinguet C., Pochet L., Backlund A., Masereel B., Kiss R., Wouters J. Novel trisubstituted harmine derivatives with original in vitro anticancer activity. J. Med. Chem. 2012;55:6489–6501. doi: 10.1021/jm300542e. [DOI] [PubMed] [Google Scholar]
- 138.Dighe S.U., Khan S., Soni I., Jain P., Shukla S., Yadav R., Sen P., Meeran S.M., Batra S. Synthesis of β-carboline-based n-heterocyclic carbenes and their antiproliferative and antimetastatic activities against human breast cancer cells. J. Med. Chem. 2015;58:3485–3499. doi: 10.1021/acs.jmedchem.5b00016. [DOI] [PubMed] [Google Scholar]
- 139.Pohl B., Luchterhandt T., Bracher F. Total syntheses of the chlorinated β-carboline alkaloids bauerine a, b, and c. Synth. Commun. 2007;37:1273–1280. doi: 10.1080/00397910701226228. [DOI] [Google Scholar]
- 140.Nurmaganbetov Z.S., Shultz E.E., Chernov S.V., Turmukhambetov A.Z., Seydakhmetova R.B., Shakirov M.M., Tolstikov G.A., Adekenov S.M. Synthesis of substituted indolizino[8,7-b]indoles from harmine and their biological activity. Chem. Heterocycl. Compd. 2011;46:1494–1499. doi: 10.1007/s10593-011-0698-z. [DOI] [Google Scholar]
- 141.Mukusheva G.K., Nurmaganbetov Z.S., Ismagulova N.M., Ponamareva O.A., Burdel’naya E.V., Turmukhambetov A.Z., Kazantsev A.V., Adekenov S.M. Synthesis and phagocytosis-stimulating activity of harmine and glaucine n-oxides. Pharm. Chem. J. 2011;45:458–460. doi: 10.1007/s11094-011-0654-3. [DOI] [Google Scholar]
- 142.Begum S., Ali S., Farhat F., Hassan S., Siddiqui B. A simple, rapid and mild one pot synthesis of benzene ring acylated and demethylated analogues of harmine under solvent-free conditions. Molecules. 2008;13:1584–1598. doi: 10.3390/molecules1301584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sanchez C., Mendez C., Salas J.A. Indolocarbazole natural products: Occurrence, biosynthesis, and biological activity. Nat. Prod. Rep. 2006;23:1007–1045. doi: 10.1039/B601930G. [DOI] [PubMed] [Google Scholar]
- 144.Prudhomme M. Biological targets of antitumor indolocarbazoles bearing a sugar moiety. Curr. Med. Chem. Anticancer Agents. 2004;4:509–521. doi: 10.2174/1568011043352650. [DOI] [PubMed] [Google Scholar]
- 145.Li Z., Zhai F., Zhao L., Guo Q., You Q. Design and synthesis of N-methylmaleimide indolocarbazole bearing modified 2-acetamino acid moieties as topoisomerase i inhibitors. Bioorg. Med. Chem. Lett. 2009;19:406–409. doi: 10.1016/j.bmcl.2008.11.061. [DOI] [PubMed] [Google Scholar]
- 146.Robey R.W., Shukla S., Steadman K., Obrzut T., Finley E.M., Ambudkar S.V., Bates S.E. Inhibition of ABCG2-mediated transport by protein kinase inhibitors with a bisindolylmaleimide or indolocarbazole structure. Mol. Cancer Ther. 2007;6:1877–1885. doi: 10.1158/1535-7163.MCT-06-0811. [DOI] [PubMed] [Google Scholar]
- 147.Slater M.J., Cockerill S., Baxter R., Bonser R.W., Gohil K., Gowrie C., Robinson J.E., Littler E., Parry N., Randall R., et al. Indolocarbazoles: Potent, selective inhibitors of human cytomegalovirus replication. Bioorg. Med. Chem. 1999;7:1067–1074. doi: 10.1016/S0968-0896(99)00032-2. [DOI] [PubMed] [Google Scholar]
- 148.Slater M.J., Baxter R., Bonser R.W., Cockerill S., Gohil K., Parry N., Robinson E., Randall R., Yeates C., Snowden W., et al. Synthesis of n-alkyl substituted indolocarbazoles as potent inhibitors of human cytomegalovirus replication. Bioorg. Med. Chem. Lett. 2001;11:1993–1995. doi: 10.1016/S0960-894X(01)00352-3. [DOI] [PubMed] [Google Scholar]
- 149.Cuevas C., Pérez M., Martín M.J., Chicharro J.L., Fernândez-Rivas C., Flores M., Francesch A., Gallego P., Zarzuelo M., de la Calle F., et al. Synthesis of ecteinascidin ET-743 and phthalascidin Pt-650 from cyanosafracin B. Org. Lett. 2000;2:2545–2548. doi: 10.1021/ol0062502. [DOI] [PubMed] [Google Scholar]
- 150.Kanzaki A., Takebayashi Y., Ren X.Q., Miyashita H., Mori S., Akiyama S., Pommier Y. Overcoming multidrug drug resistance in P-glycoprotein/MDR1-overexpressing cell lines by ecteinascidin 743. Mol. Cancer Ther. 2002;1:1327–1334. [PubMed] [Google Scholar]
- 151.Beumer J.H., Buckle T., Ouwehand M., Franke N.E.F., Lopez-Lazaro L., Schellens J.H.M., Beijnen J.H., van Tellingen O. Trabectedin (ET-743, yondelis™) is a substrate for P-glycoprotein, but only high expression of P-glycoprotein confers the multidrug resistance phenotype. Investig. New Drugs. 2007;25:1–7. doi: 10.1007/s10637-006-7773-9. [DOI] [PubMed] [Google Scholar]
- 152.Jin S., Gorfajn B., Faircloth G., Scotto K.W. Ecteinascidin 743, a transcription-targeted chemotherapeutic that inhibits MDR1 activation. Proc. Natl. Acad. Sci. USA. 2000;97:6775–6779. doi: 10.1073/pnas.97.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Soares D.G., Machado M.S., Rocca C.J., Poindessous V., Ouaret D., Sarasin A., Galmarini C.M., Henriques J.A.P., Escargueil A.E., Larsen A.K. Trabectedin and its c subunit modified analogue pm01183 attenuate nucleotide excision repair and show activity toward platinum-resistant cells. Mol. Cancer Ther. 2011;10:1481–1489. doi: 10.1158/1535-7163.MCT-11-0252. [DOI] [PubMed] [Google Scholar]
- 154.Chen X., Chen J., de Paolis M., Zhu J. Synthetic studies toward ecteinascidin 743. J. Org. Chem. 2005;70:4397–4408. doi: 10.1021/jo050408k. [DOI] [PubMed] [Google Scholar]
- 155.Enomoto T., Yasui Y., Takemoto Y. Synthetic study toward ecteinascidin 743: Concise construction of the diazabicyclo[3.3.1]nonane skeleton and assembly of the pentacyclic core. J. Org. Chem. 2010;75:4876–4879. doi: 10.1021/jo100788j. [DOI] [PubMed] [Google Scholar]
- 156.Zheng S., Chan C., Furuuchi T., Wright B.J., Zhou B., Guo J., Danishefsky S.J. Stereospecific formal total synthesis of ecteinascidin 743. Angew. Chem. Int. Ed. Engl. 2006;45:1754–1759. doi: 10.1002/anie.200503983. [DOI] [PubMed] [Google Scholar]
- 157.Chen J., Chen X., Willot M., Zhu J. Asymmetric total syntheses of ecteinascidin 597 and ecteinascidin 583. Angew. Chem. Int. Ed. Engl. 2006;45:8028–8032. doi: 10.1002/anie.200603179. [DOI] [PubMed] [Google Scholar]
- 158.Kawagishi F., Toma T., Inui T., Yokoshima S., Fukuyama T. Total synthesis of ecteinascidin 743. J. Am. Chem. Soc. 2013;135:13684–13687. doi: 10.1021/ja408034x. [DOI] [PubMed] [Google Scholar]
- 159.Chen J., Chen X., Bois-Choussy M., Zhu J. Total synthesis of ecteinascidin 743. J. Am. Chem. Soc. 2006;128:87–89. doi: 10.1021/ja0571794. [DOI] [PubMed] [Google Scholar]
- 160.Raju R., Piggott A.M., Huang X.C., Capon R.J. Nocardioazines: A novel bridged diketopiperazine scaffold from a marine-derived bacterium inhibits P-glycoprotein. Org. Lett. 2011;13:2770–2773. doi: 10.1021/ol200904v. [DOI] [PubMed] [Google Scholar]
- 161.Wang H., Reisman S.E. Enantioselective total synthesis of (−)-lansai b and (+)-nocardioazines a and b. Angew. Chem. Int. Ed. 2014;53:6206–6210. doi: 10.1002/anie.201402571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kato N., Suzuki H., Okumura H., Takahashi S., Osada H. A point mutation in ftmd blocks the fumitremorgin biosynthetic pathway in aspergillus fumigatus strain af293. Biosci. Biotechnol. Biochem. 2013;77:1061–1067. doi: 10.1271/bbb.130026. [DOI] [PubMed] [Google Scholar]
- 163.Van Loevezijn A., van Maarseveen J.H., Stegman K., Visser G.M., Koomen G.-J. Solid phase synthesis of fumitremorgin, verruculogen and tryprostatin analogs based on a cyclization/cleavage strategy. Tetrahedron Lett. 1998;39:4737–4740. doi: 10.1016/S0040-4039(98)00931-9. [DOI] [Google Scholar]
- 164.Rabindran S.K., He H., Singh M., Brown E., Collins K.I., Annable T., Greenberger L.M. Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin c. Cancer Res. 1998;58:5850–5858. [PubMed] [Google Scholar]
- 165.Rabindran S.K., Ross D.D., Doyle L.A., Yang W., Greenberger L.M. Fumitremorgin c reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res. 2000;60:47–50. [PubMed] [Google Scholar]
- 166.Gonzalez-Lobato L., Real R., Prieto J.G., Alvarez A.I., Merino G. Differential inhibition of murine BCRP1/ABCG2 and human BCRP/ABCG2 by the mycotoxin fumitremorgin c. Eur. J. Pharmacol. 2010;644:41–48. doi: 10.1016/j.ejphar.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 167.Kato N., Suzuki H., Takagi H., Asami Y., Kakeya H., Uramoto M., Usui T., Takahashi S., Sugimoto Y., Osada H. Identification of cytochrome p450s required for fumitremorgin biosynthesis in aspergillus fumigatus. ChemBioChem. 2009;10:920–928. doi: 10.1002/cbic.200800787. [DOI] [PubMed] [Google Scholar]
- 168.Allen J.D., van Loevezijn A., Lakhai J.M., van der Valk M., van Tellingen O., Reid G., Schellens J.H., Koomen G.J., Schinkel A.H. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin c. Mol. Cancer Ther. 2002;1:417–425. [PubMed] [Google Scholar]
- 169.Van Loevezijn A., Allen J.D., Schinkel A.H., Koomen G.J. Inhibition of bcrp-mediated drug efflux by fumitremorgin-type indolyl diketopiperazines. Bioorg. Med. Chem. Lett. 2001;11:29–32. doi: 10.1016/S0960-894X(00)00588-6. [DOI] [PubMed] [Google Scholar]
- 170.Weidner L.D., Zoghbi S.S., Lu S., Shukla S., Ambudkar S.V., Pike V.W., Mulder J., Gottesman M.M., Innis R.B., Hall M.D. The inhibitor Ko143 is not specific for ABCG2. J. Pharmacol. Exp. Ther. 2015;354:384–393. doi: 10.1124/jpet.115.225482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Szolomajer-Csikos O., Beery E., Kosa L., Rajnai Z., Jani M., Hetenyi A., Jakab K.T., Krajcsi P., Toth G.K. Synthesis and ABCG2 inhibitory activity of novel fumitremorgin c analogs—Specificity and structure activity correlations. Med. Chem. 2013;9:494–509. doi: 10.2174/1573406411309040003. [DOI] [PubMed] [Google Scholar]
- 172.Hino T., Kawate T., Nakagawa M. A synthesis of so-called fumitremorgin c. Tetrahedron. 1989;45:1941–1944. doi: 10.1016/S0040-4020(01)80057-0. [DOI] [Google Scholar]
- 173.Jiang D., Xu Z., Jia Y. Mg(CLO4)2-catalyzed intramolecular allylic amination: Application to the total synthesis of demethoxyfumitremorgin C. Tetrahedron. 2012;68:4225–4232. doi: 10.1016/j.tet.2012.03.097. [DOI] [Google Scholar]
- 174.Li Y., Hayman E., Plesescu M., Prakash S.R. Synthesis of potent bcrp inhibitor—Ko143. Tetrahedron Lett. 2008;49:1480–1483. doi: 10.1016/j.tetlet.2007.12.130. [DOI] [Google Scholar]
- 175.Kanoh K., Kohno S., Asari T., Harada T., Katada J., Muramatsu M., Kawashima H., Sekiya H., Uno I. (−)-phenylahistin: A new mammalian cell cycle inhibitor produced by aspergillus ustus. Bioorg. Med. Chem. Lett. 1997;7:2847–2852. doi: 10.1016/S0960-894X(97)10104-4. [DOI] [Google Scholar]
- 176.Nicholson B., Lloyd G.K., Miller B.R., Palladino M.A., Kiso Y., Hayashi Y., Neuteboom S.T.C. Npi-2358 is a tubulin-depolymerizing agent: In vitro evidence for activity as a tumor vascular-disrupting agent. Anticancer Drugs. 2006;17:25–31. doi: 10.1097/01.cad.0000182745.01612.8a. [DOI] [PubMed] [Google Scholar]
- 177.Heist R., Aren O., Millward M., Mainwaring P., Mita A., Mita M., Bazhenova L., Blum R., Polikoff J., Gadgeel S., et al. Abstract c30: Phase 1/2 study of the vascular disrupting agent (VDA) plinabulin (NPI-2358) combined with docetaxel in patients with non-small cell lung cancer (NSCLC) Mol. Cancer Ther. 2009;8:C30. doi: 10.1158/1535-7163.TARG-09-C30. [DOI] [Google Scholar]
- 178.Stratmann T., Burgoyne D.L., Moore R.E., Patterson G.M.L., Smith C.D. Hapalosin, a cyanobacterial cyclic depsipeptide with multidrug-resistance reversing activity. J. Org. Chem. 1995;60:2950–2950. doi: 10.1021/jo00114a062. [DOI] [Google Scholar]
- 179.Smith C.D. New compounds from cyanobacteria to circumvent mdr. Drug News Perspect. 1995;8:423–425. [Google Scholar]
- 180.Dinh T.Q., Smith C.D., Du X., Armstrong R.W. Design, synthesis, and evaluation of the multidrug resistance-reversing activity of d-glucose mimetics of hapalosin. J. Med. Chem. 1998;41:981–987. doi: 10.1021/jm970709p. [DOI] [PubMed] [Google Scholar]
- 181.Okuno T., Ohmori K., Nishiyama S., Yamamura S., Nakamura K., Houk K.N., Okamoto K. Chemical study on hapalosin, a cyclic depsipeptide possessing multidrug resistance reversing activities: Synthesis, structure and biological activity. Tetrahedron. 1996;52:14723–14734. doi: 10.1016/0040-4020(96)00951-9. [DOI] [Google Scholar]
- 182.Dinh T.Q., Smith C.D., Armstrong R.W. Analogs incorporating trans-4-hydroxy-l-proline that reverse multidrug resistance better than hapalosin. J. Org. Chem. 1997;62:790–791. doi: 10.1021/jo962180u. [DOI] [Google Scholar]
- 183.Kashihara N., To-e S., Nakamura K., Umezawa K., Yamamura S., Nishiyama S. Synthesis and biological activities of hapalosin derivatives with modification at the c12 position. Bioorg. Med. Chem. Lett. 2000;10:101–103. doi: 10.1016/S0960-894X(99)00647-2. [DOI] [PubMed] [Google Scholar]
- 184.Dinh T.Q., Du X., Smith C.D., Armstrong R.W. Synthesis, conformational analysis, and evaluation of the multidrug resistance-reversing activity of the triamide and proline analogs of hapalosin. J. Org. Chem. 1997;62:6773–6783. doi: 10.1021/jo9708396. [DOI] [Google Scholar]
- 185.Dinh T.Q., Du X.H., Armstrong R.W. Synthesis and conformational analysis of the multidrug resistance-reversing agent hapalosin and its non-n-methyl analog. J. Org. Chem. 1996;61:6606–6616. doi: 10.1021/jo9608329. [DOI] [PubMed] [Google Scholar]
- 186.Ohmori K., Okuno T., Nishiyama S., Yamamura S. Synthetic study on hapalosin, a cyclic depsipeptide possessing multidrug resistance reversing activities. Tetrahedron Lett. 1996;37:3467–3470. doi: 10.1016/0040-4039(96)00592-8. [DOI] [Google Scholar]
- 187.Hermann C., Giammasi C., Geyer A., Maier M.E. Syntheses of hapalosin analogs by solid-phase assembly of acyclic precursors. Tetrahedron. 2001;57:8999–9010. doi: 10.1016/S0040-4020(01)00903-6. [DOI] [Google Scholar]
- 188.Hermann C., Pais G.C.G., Geyer A., Kuhnert S.M., Maier M.E. Total synthesis of hapalosin and two ring expanded analogs. Tetrahedron. 2000;56:8461–8471. doi: 10.1016/S0040-4020(00)00772-9. [DOI] [Google Scholar]
- 189.Palomo C., Oiarbide M., García J.M., González A., Pazos R., Odriozola J.M., Bañuelos P., Tello M., Linden A. A practical total synthesis of hapalosin, a 12-membered cyclic depsipeptide with multidrug resistance-reversing activity, by employing improved segment coupling and macrolactonization†. J. Org. Chem. 2004;69:4126–4134. doi: 10.1021/jo0497499. [DOI] [PubMed] [Google Scholar]
- 190.Pais G.C.G., Maier M.E. Efficient synthesis of the γ-amino-β-hydroxy acid subunit of hapalosin. J. Org. Chem. 1999;64:4551–4554. doi: 10.1021/jo990200x. [DOI] [Google Scholar]
- 191.Maier M.E., Hermann C. Synthesis of the γ-amino-β-hydroxy acid of hapalosin via an asymmetric dihydroxylation route. Tetrahedron. 2000;56:557–561. doi: 10.1016/S0040-4020(99)01052-2. [DOI] [Google Scholar]
- 192.Kumar H., Reddy A.S., Yadav J.S., Reddy B.V.S. A highly stereoselective formal synthesis of hapalosin. Synlett. 2013;24:1415–1419. [Google Scholar]
- 193.Parkes K.E.B., Bushnell D.J., Crackett P.H., Dunsdon S.J., Freeman A.C., Gunn M.P., Hopkins R.A., Lambert R.W., Martin J.A. Studies toward the large-scale synthesis of the hiv proteinase inhibitor ro 31-8959. J. Org. Chem. 1994;59:3656–3664. doi: 10.1021/jo00092a026. [DOI] [Google Scholar]
- 194.McDonald L.A., Christopher Swersey J., Ireland C.M., Carroll A.R., Coll J.C., Bowden B.F., Fairchild C.R., Cornell L. Botryllamides A–D, new brominated tyrosine derivatives from styelid ascidians of the genus botryllus. Tetrahedron. 1995;51:5237–5244. doi: 10.1016/0040-4020(95)00202-J. [DOI] [Google Scholar]
- 195.Rao M.R., Faulknert D.J. Botryllamides E–H, four new tyrosine derivatives from the ascidian botrylloides tyreum. J. Nat. Prod. 2004;67:1064–1066. doi: 10.1021/np0499618. [DOI] [PubMed] [Google Scholar]
- 196.Henrich C.J., Robey R.W., Takada K., Bokesch H.R., Bates S.E., Shukla S., Ambudkar S.V., McMahon J.B., Gustafson K.R. Botryllamides: Natural product inhibitors of ABCG2. ACS Chem. Biol. 2009;4:637–647. doi: 10.1021/cb900134c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Takada K., Imamura N., Gustafson K.R., Henrich C.J. Synthesis and structure-activity relationship of botryllamides that block the ABCG2 multidrug transporter. Bioorg. Med. Chem. Lett. 2010;20:1330–1333. doi: 10.1016/j.bmcl.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Aoki S., Cao L., Matsui K., Rachmat R., Akiyama S.I., Kobayashi M. Kendarimide a, a novel peptide reversing P-glycoprotein-mediated multidrug resistance in tumor cells, from a marine sponge of haliclona sp. Tetrahedron. 2004;60:7053–7059. doi: 10.1016/j.tet.2003.07.020. [DOI] [Google Scholar]
- 199.Kotoku N., Cao L., Aoki S., Kobayashi M. Absolute stereo-structure of kendarimide a, a novelmdr modulator, from a marine sponge. Heterocycles. 2005;65:563–578. [Google Scholar]
- 200.Fu X., Do T., Schmitz F.J., Andrusevich V., Engel M.H. New cyclic peptides from the ascidian lissoclinum patella. J. Nat. Prod. 1998;61:1547–1551. doi: 10.1021/np9802872. [DOI] [PubMed] [Google Scholar]
- 201.Schmidt E.W., Nelson J.T., Rasko D.A., Sudek S., Eisen J.A., Haygood M.G., Ravel J. Patellamide a and c biosynthesis by a microcin-like pathway in prochloron didemni, the cyanobacterial symbiont of lissoclinum patella. Proc. Natl. Acad. Sci. USA. 2005;102:7315–7320. doi: 10.1073/pnas.0501424102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.García-Reynaga P., VanNieuwenhze M.S. A new total synthesis of patellamide a. Org. Lett. 2008;10:4621–4623. doi: 10.1021/ol801895y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kawakami A., Miyamoto T., Higuchi R., Uchiumi T., Kuwano M., Van Soest R.W.M. Structure of a novel multidrug resistance modulator, irciniasulfonic acid, isolated from a marine sponge, ircinia sp. Tetrahedron Lett. 2001;42:3335–3337. doi: 10.1016/S0040-4039(01)00426-9. [DOI] [Google Scholar]
- 204.Emura C., Higuchi R., Miyamoto T. Irciniasulfonic acid B, a novel taurine conjugated fatty acid derivative from a japanese marine sponge, Ircinia sp. Tetrahedron. 2006;62:5682–5685. doi: 10.1016/j.tet.2006.03.087. [DOI] [Google Scholar]
- 205.Kim C.K., Song I.H., Park H.Y., Lee Y.J., Lee H.S., Sim C.J., Oh D.C., Oh K.B., Shin J. Suvanine sesterterpenes and deacyl irciniasulfonic acids from a tropical coscinoderma sp. Sponge. J. Nat. Prod. 2014;77:1396–1403. doi: 10.1021/np500156n. [DOI] [PubMed] [Google Scholar]
- 206.Emura C., Higuchi R., Miyamoto T. Synthetic studies on the natural multidrug resistance modulator, irciniasulfonic acid B. Chem. Lett. 2010;39:1002–1003. doi: 10.1246/cl.2010.1002. [DOI] [Google Scholar]
- 207.Adrian P., Dobbs A.V., Butler L.A., Parker R.J. Document first total synthesis of the irciniasulfonic acids. Synlett. 2005;4:652–654. [Google Scholar]
- 208.Steyn P.S. The isolation, structure and absolute configuration of secalonic acid D, the toxic metabolite of penicillium oxalicum. Tetrahedron. 1970;26:51–57. doi: 10.1016/0040-4020(70)85006-2. [DOI] [PubMed] [Google Scholar]
- 209.Ren H., Tian L., Gu Q.Q., Zhu W.M. Secalonic acid D; a cytotoxic constituent from marine lichen-derived fungus Gliocladium sp. T31. Arch. Pharm. Res. 2006;29:59–63. doi: 10.1007/BF02977469. [DOI] [PubMed] [Google Scholar]
- 210.Hong R. Secalonic acid d as a novel DNA topoisomerase I inhibitor from marine lichen-derived fungus Gliocladium sp. T31. Pharm. Biol. 2011;49:796–799. doi: 10.3109/13880209.2010.548817. [DOI] [PubMed] [Google Scholar]
- 211.Hu Y.-P., Tao L.-Y., Wang F., Zhang J.-Y., Liang Y.-J., Fu L.-W. Secalonic acid d reduced the percentage of side populations by down-regulating the expression of ABCG2. Biochem. Pharmacol. 2013;85:1619–1625. doi: 10.1016/j.bcp.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 212.Guru S.K., Pathania A.S., Kumar S., Ramesh D., Kumar M., Rana S., Kumar A., Malik F., Sharma P.R., Chandan B.K., et al. Secalonic acid-D represses HIF1A/VEGF-mediated angiogenesis by regulating the Akt/mTOR/p70S6K signaling cascade. Cancer Res. 2015;75:2886–2896. doi: 10.1158/0008-5472.CAN-14-2312. [DOI] [PubMed] [Google Scholar]
- 213.Wezeman T., Masters K.S., Bräse S. Double trouble—The art of synthesis of chiral dimeric natural products. Angew. Chem. Int. Ed. 2014;53:4524–4526. doi: 10.1002/anie.201402384. [DOI] [PubMed] [Google Scholar]
- 214.Li X., Jiang H., Uffman E.W., Guo L., Zhang Y., Yang X., Birman V.B. Kinetic resolution of secondary alcohols using amidine-based catalysts. J. Org. Chem. 2012;77:1722–1737. doi: 10.1021/jo202220x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Qin T., Porco J.A., Jr. Total syntheses of secalonic acids a and d. Angew. Chem. Int. Ed. 2014;53:3107–3110. doi: 10.1002/anie.201311260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nising C.F., Schmid U.K., Nieger M., Bräse S. A new protocol for the one-pot synthesis of symmetrical biaryls. J. Org. Chem. 2004;69:6830–6833. doi: 10.1021/jo0490393. [DOI] [PubMed] [Google Scholar]
- 217.Chen Y.F., Wang S.Y., Shen H., Yao X.F., Zhang F.L., Lai D. The marine-derived fungal metabolite, terrein, inhibits cell proliferation and induces cell cycle arrest in human ovarian cancer cells. Int. J. Mol. Med. 2014;34:1591–1598. doi: 10.3892/ijmm.2014.1964. [DOI] [PubMed] [Google Scholar]
- 218.Kim D.S., Cho H.J., Lee H.K., Lee W.H., Park E.S., Youn S.W., Park K.C. Terrein, a fungal metabolite, inhibits the epidermal proliferation of skin equivalents. J. Dermatol. Sci. 2007;46:65–68. doi: 10.1016/j.jdermsci.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 219.Park S.H., Kim D.S., Kim W.G., Ryoo I.J., Lee D.H., Huh C.H., Youn S.W., Yoo I.D., Park K.C. Terrein: A new melanogenesis inhibitor and its mechanism. Cell. Mol. Life Sci. 2004;61:2878–2885. doi: 10.1007/s00018-004-4341-3. [DOI] [PubMed] [Google Scholar]
- 220.Liao W.Y., Shen C.N., Lin L.H., Yang Y.L., Han H.Y., Chen J.W., Kuo S.C., Wu S.H., Liaw C.C. Asperjinone, a nor-neolignan, and terrein, a suppressor of ABCG2-expressing breast cancer cells, from thermophilic aspergillus terreus. J. Nat. Prod. 2012;75:630–635. doi: 10.1021/np200866z. [DOI] [PubMed] [Google Scholar]
- 221.Mandai H., Omori K., Yamamoto D., Tsumura T., Murota K., Yamamoto S., Mitsudo K., Ibaragi S., Sasaki A., Maeda H., et al. Synthetic (+)-terrein suppresses interleukin-6/soluble interleukin-6 receptor induced-secretion of vascular endothelial growth factor in human gingival fibroblasts. Bioorg. Med. Chem. 2014;22:5338–5344. doi: 10.1016/j.bmc.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 222.Khalil Z.G., Huang X.-C., Raju R., Piggott A.M., Capon R.J. Shornephine a: Structure, chemical stability, and p-glycoprotein inhibitory properties of a rare diketomorpholine from an australian marine-derived aspergillus sp. J. Org. Chem. 2014;79:8700–8705. doi: 10.1021/jo501501z. [DOI] [PMC free article] [PubMed] [Google Scholar]