Abstract
Anxiety disorders and schizophrenia are common public health issues. The dried stigma of the plant Crocus sativus L., (C. sativus) commonly known as saffron are used in folk medicine for various purposes. Several lines of evidence suggest that C. sativus, crocins and safranal are implicated in anxiety and schizophrenia. Here, I intend to critically review advances in research of these emerging molecules for the treatment of anxiety and schizophrenia, discuss their advantages over currently used anxiolytics and neuroleptics, as well remaining challenges. Current analysis shows that C. sativus and its components might be a promising class of compounds for the treatment of the above mentioned psychiatric diseases.
Keywords: Crocus sativus L., anxiety, stress, schizophrenia
1. Introduction
Crocus sativus L. (C. sativus), is a perennial herb member of the Iridaceae family, the line of Liliaceae. This plant is cultivated in many countries such as Azerbaijan, China, France, Greece, Egypt, India, Iran, Israel, Italy, Mexico, Morocco, Spain and Turkey. Its product is the well-known spice called saffron. Saffron, in filaments, is the dried dark-red stigmas of C. sativus flower [1]. One stigma of saffron weighs about 2 mg and each flower has three stigmata; 150,000 flowers must be carefully picked one by one to obtain 1 kg of spice. Saffron has a distinct colour, flavour and odour. It is used both as a spice for flavouring and colouring food preparations, and as a perfume. The stigmas of it are also used in folk medicine as an anticatarrhal, eupeptic, antispasmodic, expectorant, emmenagogue and nerve sedative (for review see [2]). Further, saffron has widely been used in Persian traditional medicine for memory problems [3]. The present review was designed to critically assess the role played by C. sativus and its active constituents in anxiety and schizophrenia since the current pharmacotherapy for both these diseases is not satisfactory.
2. Chemistry of C. sativus
Chemical analysis of C. sativus stigmas has shown the presence of about 150 volatile and non-volatile compounds. Fewer than 50 constituents, however, have been identified so far [4]. The volatiles consist of more than 34 components that are terpenes, terpene alcohols and their esters among which safranal is the main component. Non-volatile compounds comprise crocins, crocetin, picrocrocin and flavonoids (quercetin and kaempferol) [5].
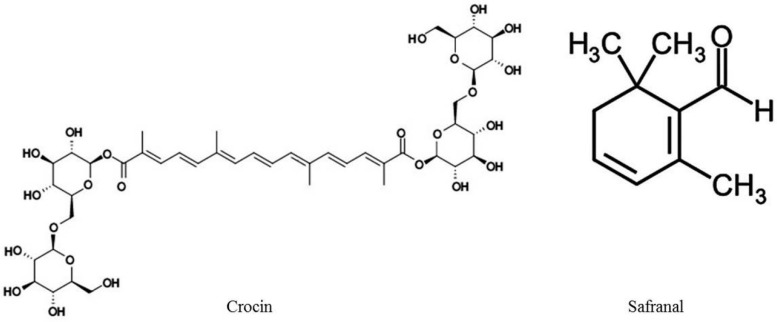
In particular, crocins, glucosyl esters of crocetin, are water-soluble carotenoids and are responsible for saffron’s characteristic colour. Picrocrocin, glycoside of safranal, is responsible for the bitter taste of the spice and is the precursor of safranal. Safranal, the main component of the distilled essential oil, is a monoterpene aldehyde, responsible for its characteristic aroma [6,7]. In Figure 1 the molecular structure of C. sativus components crocin and saffranal is illustrated.
Figure 1.
Molecular structures of C. sativus components crocin and safranal.
3. Effects of C. sativus and Its Constituents on Anxiety
Anxiety may be interpreted as an emotional anticipation of an aversive situation and is reflected by species-specific behavioural fear responses to stressful and threatening stimuli, characteristic for individual trait anxiety. Anxiety disorders including generalized anxiety disorder (GAD), specific and social phobias, post-traumatic stress disorder (PTSD), obsessive-compulsive disorder (OCD) and panic disorder are a major public health issue worldwide.
To date, anxiety disorders have been treated with medications that target γ-aminobutyric acid (GABA) and serotonergic neurotransmission, like benzodiazepines, partial agonists of the serotonergic 5-HT1A receptor and selective serotonin re-uptake inhibitors (SSRIs). Some forms of anxiety, however, are relatively resistant to treatment with these agents [8,9]. In addition, either benzodiazepines or SSRIs can be associated with severe side effects, such as sedation, memory deficits, dependence and withdrawal, sexual dysfunction and weight gain. Further, the 5-HT1A receptor partial agonist buspirone has a somewhat limited use. Although it is generally well tolerated with few side effects, it has lower efficacy and its onset of action is slower than previous drugs such as the benzodiazepines [10]. Thus, there is an urgent need to develop alternative treatment strategies [11].
3.1. Preclinical Studies
An overview of the preclinical literature regarding the effects of C. sativus and its constituents on anxiety is provided in Table 1. Experimental evidence indicated that crocins displayed an anxiolytic-like effect in procedures assessing anxiety in rats [12,13]. Particularly, in the light/dark test, administration of 50 mg/kg crocins, similarly to the reference compound diazepam (1.5 mg/kg), increased the time to enter the dark compartment, did not affect the number of transitions between the light and the dark chamber of the apparatus and prolonged the time spent in the lit compartment of the light/dark box [12]. In addition, crocins (30 and 50 mg/kg) were found to reduce compulsive behaviour (excessive grooming) induced by the serotonergic 5-HT2c receptor agonist 1-(3-chlorophenyl)piperazine hydrochloride (mCPP) (0.6 mg/kg) [13]. Interestingly, crocins, at any dose tested, did not alter rats’ locomotor activity. Collectively, the results of the above described studies suggest that these active constituents of C. sativus induce anxiolytic-like behaviour in the rat and their anxiolytic effects cannot be attributed to changes in locomotor activity [12,13].
Table 1.
Effects of Crocus sativus L., and its constituents on animal models of anxiety disorders and schizophrenia.
| Species | Agent | Dose Range | Route | Behavioural Test | Effect | Ref. |
|---|---|---|---|---|---|---|
| Rat | Crocins | 15, 30, 50 mg/kg | i.p. acute | Light/dark | Anxiolytic-like (50 mg/kg) effect | [12] |
| Motor activity | No effect | |||||
| Mouse | CsAE | 56, 80, 320, 560 mg/kg | i.p. acute | Elevated plus maze | CsAE (56, 80 mg/kg), safranal (0.15, 0.35 mL/kg) anxiolytic effect. Crocin was ineffective. | [14] |
| Crocin | 50, 200, 600 mg/kg | i.p. acute | Open field | CsAE (dose-dependently), crocin (200–600 mg/kg), reduced motility, grooming, rearing, leaning. | ||
| Safranal | 0.05, 0.15, 0.35 mL/kg | i.p. acute | Rotarod | CsAE (dose-dependently), crocin (200–600 mg/kg), reduced motility, grooming, rearing, leaning. | ||
| Safranal (0.05, 0.15 mL/kg) reduced motility; (0.15, 0.35 mL/kg) increased grooming, leaning, rearing. | ||||||
| CsAE (dose-dependently) decreased motor coordination. Crocin and safranal were ineffective. | ||||||
| Mouse | CsAE | 1, 5, 10 mg/kg | i.p. acute | Food intake | CsAE and crocin decreased stress-induced anorexia. | [15] |
| Plasma corticosterone levels were not increased in CsAE and crocin-treated mice. | ||||||
| CsEE | 1, 5, 10 mg/kg | i.p. acute | CsEE and safranal were ineffective. | |||
| Crocin | 1, 5, 10 mg/kg | i.p. acute | ||||
| Safranal | 1, 5, 10 mg/kg | i.p. acute | ||||
| Rat | Crocins | 15, 30 mg/kg | i.p. acute | Measurement of grooming behaviour | Attenuated mCPP-induced excessive grooming (anxiolytic effect). | [13] |
| m-CPP | 0.6 mg/kg | i.p. acute. | Motor activity | No effect | ||
| Rat | Crocins | 15, 30, 50 mg/kg | i.p. acute | NORT | Crocins (15, 30 mg/kg) counteracted ketamine-induced recognition memory deficits. | [16] |
| Ketamine | 3 mg/kg (NORT) | i.p. acute | NORT | Crocins (15, 30 mg/kg) counteracted ketamine-induced recognition memory deficits. | ||
| SI | Crocins (50 mg/kg) attenuated ketamine-induced social isolation. | |||||
| Ketamine | 8 mg/kg (SI) | i.p. sub-chronic | SI | Crocins (50 mg/kg) attenuated ketamine-induced social isolation. | ||
| Motor activity, stereotypies, ataxia | Crocins (50 mg/kg) attenuated ketamine-induced hypermotility, stereotypies and ataxia. | |||||
| Ketamine | 25 mg/kg (motor activity) | i.p. acute | Motor activity, stereotypies, ataxia | Crocins (50 mg/kg) attenuated ketamine-induced hypermotility, stereotypies and ataxia. |
CsAE, Crocus sativus Aqueous Extracts; CsEE, Crocus sativus Ethanolic Extracts; i.p., intraperitoneally; mCPP, 1-(3-cholorophenyl)piperazine; NORT, novel object recognition task; SI, social interaction.
In a study carried out in mice, it has been revealed that low doses of the aqueous extracts of saffron (56 and 80 mg/kg) and safranal (0.15 and 0.35 mL/kg) induced an anxiolytic-like effect not different from that of diazepam (3 mg/kg) because they increased the time spent in the open arms of an elevated plus maze. At higher doses (320 and 560 mg/kg), the aqueous extracts of saffron did not display any anti-anxiety effect. It is important to underline that the aqueous extracts of saffron, at any dose tested, produced sedation since they reduced mice’ motility and motor coordination evidenced in the open field and rotarod tests, respectively. Safranal at a low dose range (0.05 and 0.15 mL/kg) also induced hypomotility and increased grooming activity and rearing. Crocin (50–600 mg/kg) did not affect mice behaviour in the elevated plus maze test, and at 200 and 600 mg/kg reduced mice motility [14].
The results of this latter study [14] appear to be in contrast with the above described findings [12,13] in which an anxiolytic effect of crocins has been observed. These discrepant findings may be attributable to differences in experimental settings (type of animal, pharmacological design, dose range, behavioural procedure).
Finally, administration of aqueous extracts of saffron (1–10 mg/kg), of crocin (1–10 mg/kg) but not of ethanolic extracts of C. sativus (1–10 mg/kg) and safranal (1–10 mg/kg) reduced stress-induced anorexia in the mouse and did not influence plasma corticosterone levels. These results suggest an anti-stress effect of saffron and crocin [15].
3.2. Mechanism of Action of C. sativus and Its Constituents in Anxiety Disorders
The precise mechanism(s) by which saffron and its active components produced their anxiolytic effects, which are of the same magnitude of those produced by the benzodiazepine diazepam, is still under investigation. Benzodiazepines are compounds that produce their anxiolytic effect by acting as indirect agonists on the GABAA receptor. In this context, it has been shown that some flavonoids isolated from plants display an affinity for the benzodiazepine binding site at the GABAA receptor [17,18]. It can be hypothesized that saffron and its components, similarly to other flavonoids, exert their anxiolytic action by interacting with the benzodiazepine binding site at the GABAA receptor. Additional studies are required, however, to address this issue.
The pharmacological mechanism(s) that might account for the potential anti-stress effects of C. sativus and its components has not yet been clarified. Several lines of evidence indicate that stress activates the hypothalamus-pituitary-adrenal (HPA) axis which leads to the plasma corticosterone increment as a response [19]. According to recent findings [15], mice that received saffron aqueous extracts or crocin did not show elevation of plasmatic corticosterone levels under stress. It is likely that crocin may interact with the HPA axis and reduce the stress-induced corticosterone increase [15]. In line with the above results, it has been demonstrated that saffron can inhibit N-methyl-d-aspartate (NMDA) and sigma, St. Louis, MO, USA, opioid receptors [20]. The latter is of importance since NMDA and sigma receptors can regulate corticosterone release from the adrenal cortex in rats [21]. It can be concluded that saffron and crocin may inhibit corticosterone secretion in stressed mice via blockade of NMDA and/or sigma opioid receptors located in the adrenal cortex [15].
4. Effects of C. sativus and Its Constituents in Schizophrenia
Schizophrenia is a serious mental disorder that affects up to 1% of the population worldwide. It is a complex heterogeneous syndrome which impairs social, occupational and individual functioning and results in a remarkable decline in the quality of life of patients. Its aetiology and pathophysiology remain unknown. Schizophrenic patients suffer from enduring and persistent psychotic symptoms, which can be divided in three major types: positive symptoms (f.i., hallucinations, delusions, disordered thought processing, catatonic behaviour), negative symptoms (social withdrawal, anhedonia, avolition) and cognitive disturbances (deficits in attention and memory) [22].
Abnormalities in a number of neurotransmitter systems, most notably the dopamine, glutamate, cholinergic, the serotonergic and the GABAergic systems, are thought to be important for the appearance of this disease [23]. In particular, positive symptoms of schizophrenia are associated with an excess of dopaminergic neurotransmission, in striatal brain regions, while negative symptoms and cognitive deficits are linked to dopaminergic hypofunction in prefrontal brain regions.
Moreover, consistent experimental evidence proposes a role for glutamate hypofunction in the pathophysiology of schizophrenia. NMDA receptor dysfunction is linked to secondary dopaminergic dysregulation in striatal and prefrontal brain regions. In addition, clinical observations have demonstrated that pharmacological blockade of NMDA receptor produced the component symptoms-negative symptoms and cognitive impairment that were neither affected by antipsychotics nor produced by dopaminergic agonists [24]. Further, inhibitory GABAergic neurotransmission appears to be impaired in schizophrenia patients [25]. In this context, it is important to underline that GABAergic firing regulates dopamine transmission in the prefrontal cortex and a GABA interneuron deficit in schizophrenia has been proposed to underlie some of the clinical symptoms [26].
Although traditional antipsychotic drugs have demonstrated utility in treating the positive symptoms of schizophrenia, current treatments are limited in their ability to alleviate the negative and cognitive symptoms’ clusters and often are accompanied by significant side effects which themselves impact the quality of life [27]. Finally, one third of patients are resistant to currently available medication. Therefore, there is an urgent requirement to develop new molecules for the treatment of schizophrenia.
4.1. Preclinical Studies
An overview of the existing literature regarding the effects of C. sativus and its constituents on schizophrenia is provided in Table 1. Acute administration of crocins (15–30 mg/kg) reversed recognition memory deficits produced by the NMDA receptor antagonist ketamine (3 mg/kg) in rats eliciting, thus, the effects of this active constituent of C. sativus in schizophrenia-related cognitive deficits. In addition, crocins (50 mg/kg) attenuated ketamine (25 mg/kg)-induced psychotomimetic effects (hypermotility, stereotypies and ataxia) in the rat. Further, using the social interaction test, a procedure resembling the negative symptoms of schizophrenia, these active constituents of saffron (50 mg/kg) were found to attenuate the social isolation induced by sub-chronic treatment with ketamine (8 mg/kg) in rats [16].
4.2. Clinical Studies
Up to now, only one clinical trial has been performed aiming to assess the safety and tolerability but not the efficacy of saffron extracts and crocin in schizophrenia. This double-blind placebo-controlled study was carried out in 61 schizophrenia patients. Schizophrenics received treatment twice daily (saffron or crocin 15 mg) or placebo for 12 consecutive weeks. In line with prior findings [28,29,30], the results of this study showed that saffron extracts and crocin administered at 15 mg twice daily were safe and well tolerated in schizophrenic patients [31].
4.3. Mechanism of Action of C. sativus and Its Constituents in Schizophrenia
The mechanism(s) through which crocins exert their effects on ketamine-induced behavioural deficits are not yet clarified. Additional studies should be carried out aiming to elucidate this issue. It is well documented, however, that schizophrenia-like effects of NMDA receptor antagonists (e.g., ketamine) include increased levels of glutamate, hypermotility, stereotypy and cognition deficits [32]. In this context, it has been reported that acute systemic administration of safranal reduced kainic acid-induced increase of extracellular glutamate concentrations in the rat hippocampus [33]. Further, it has been demonstrated that C. sativus extracts inhibited glutamatergic synaptic transmission in rat cortical brain slices [34]. Collectively, these findings suggest that this reduction of glutamate levels by saffron and its constituents might be critical for the beneficial action exerted by crocins on ketamine-induced behavioural deficits.
An alternative hypothesis to explain the beneficial action of crocins in an animal model of schizophrenia is based on the well-known antioxidant properties of crocins [35,36,37,38]. In agreement with the above studies it has been reported that saffron extracts and crocins conferred protection against oxidative stress and spatial learning deficits induced by chronic stress in rats [39]. Although the pathogenesis of schizophrenia remains unknown, a possible relationship between oxidative stress and the disease has been proposed [40], and sub-anesthetic doses of ketamine have been reported to increase oxidative stress in rats’ brain [41]. Specifically, following treatment with different sub-anesthetic doses of ketamine, an increase in oxidative damage marked by an increase in lipid peroxidation, oxidative protein damage and a decrease in enzymatic defense was observed in an animal model of schizophrenia [41]. As a whole, these data indicate that the beneficial effects of crocins on ketamine-induced behavioural deficits might be associated with their antioxidant properties.
5. Effects of C. sativus and Its Constituents on Other Neurological/Neuropsychiatric Diseases
5.1. Anticonvulsant Activity, Neurodegeneration
Studies performed in rodents revealed a certain anticonvulsant activity of aqueous and ethanolic extracts of C. sativus and its active component safranal [42,43]. Saffron and its active constituents affect a number of different neural processes. These molecules conferred protection in a rat model of Parkinson disease (PD) [44] and in animal models of cerebral ischemia [45,46,47].
5.2. Memory
Accumulating evidence indicates that C. sativus and its major component crocin are significantly involved in cognition. Preclinical studies demonstrated their efficacy in attenuating memory disorders in animal models related to Alzheimer disease (AD), cerebral injuries or schizophrenia (for review see [48]).
Clinical research has evaluated the efficacy of saffron in humans suffering from memory problems as are the Alzheimer disease (AD) patients. The results of clinical studies indicate that the effects exerted by ethanolic extracts of saffron on cognition were not different than those expressed by the reference compounds donepezil and memantine in reducing the cognitive decline in patients with mild-to-moderate and moderate to severe AD. In this context, it is important to emphasize the good safety profile of saffron which was revealed in all clinical studies [28,29,30].
5.3. Depression
C. sativus and its active components crocin and safranal have shown antidepressant-like effects in animal models of depression [49]. Importantly, clinical research findings reinforced preclinical results and proposed that saffron is efficacious for the treatment of mild-to-moderate depression [50,51]. Interestingly, it has been demonstrated that saffron antagonized sexual dysfunction in humans induced by the SSRI fluoxetine which is the widest used antidepressant in our days [52,53].
6. Effects of C. sativus and Its Constituents on Other Non-Neurological/Neuropsychiatric Diseases
Preclinical pharmacological studies have demonstrated that C. sativus crude extracts and purified chemicals possess anti-tumour effects (e.g., [54,55,56]). Saffron and its ingredients display antinociceptive, and anti-inflammatory properties [57], reduce atherosclerosis [58] and hepatic damage [59], counteract hyperlipidaemia [60], provide protection from myocardial injury [61] and display antihypertensive action [62,63]. The effects exerted by saffron and its components in the aforementioned pathologies were also extensively discussed in different reviews (e.g., [2,64,65,66]).
The outcome of these preclinical studies indicates that C. sativus and its components exert a beneficial action in several pathologies. It is important to underline, however, that up to now there has been no clinical information on the potential efficacy of saffron and its constituents in the aforementioned pathologies. Future clinical research should address this issue.
7. Safety Evaluation of C. sativus and Its Constituents
Toxicity studies have demonstrated that the hematological and the biochemical parameters were within a normal range in mice treated with saffron extracts [54]. It has also been reported that the oral LD50 of saffron was 20.7 g/kg administered as a decoction in mice [67]. Further, a recent work investigated either the acute (up to 3 g, both orally (p.o.) and intraperitoneally (i.p.)) or the sub-chronic effects of crocin (15–180 mg/kg, i.p.) in different biochemical, hematological and pathological parameters in rodents. The results of this study demonstrated that chronic treatment with crocin did not alter the weight of heart, lung, liver, kidney and spleen. Crocin, at the highest dose (180 mg/kg), increased platelets and creatinine levels, and reduced food intake and body weight. A decline in alveolar size in lungs was observed following the highest dose of crocin (180 mg/kg). The authors concluded that crocin, at pharmacological doses, was not shown to markedly damage any of the major organs of the body [68].
Interestingly, the findings of clinical studies suggest that both C. sativus extracts and crocin display a relatively safe and normal pharmacological profile. Specifically, in a double-blind, placebo-controlled trial conducted among healthy volunteers, a one-week treatment with saffron (200–400 mg/day) did not evidence particular alterations [69]. The results of another double-blind, placebo-controlled study performed in healthy volunteers also showed that administration for one month of crocin (20 mg/day) did not elicit significant alterations of different hematological, biochemical, hormonal and urinary parameters recorded [70].
8. Conclusions
There is scant experimental evidence, either preclinical or clinical, regarding the involvement of C. sativus and some of its active constituents in anxiety and schizophrenia. In spite of it, the few preclinical results are of certain consistency. Several issues, however, have not been addressed at all. There is no information on the potential efficacy of saffron in other anxiety disorders such as GAD, social phobia, panic and PTSD. There is also poor information regarding the potential antipsychotic action of saffron. There is no experimental evidence whether these compounds can counteract attentional deficits which are considered as a prominent aspect of cognitive dysfunction in schizophrenia. Additional research, using genuine animal behavioural models, is mandatory to establish whether these molecules might be a potential therapeutic tool for the treatment of anxiety disorders and schizophrenia. In this context, it is important to emphasize the good safety profile of saffron which was revealed in all preclinical and clinical studies here presented.
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.International Standard. Saffron-Specification ISO 3632:1980. International Organization for Standardization; Geneva, Switzerland: 1980. [Google Scholar]
- 2.Rios J.L., Recio M.C., Ginger R.M., Manz S. An update review of saffron and its active constituents. Phytother. Res. 1996;10:189–193. doi: 10.1002/(SICI)1099-1573(199605)10:3<189::AID-PTR754>3.0.CO;2-C. [DOI] [Google Scholar]
- 3.Akhondzabeh S. Herbal medicine in the treatment of psychiatric and neurological disorders. In: Abate L., editor. Low-Cost Approaches to Promote Physical and Mental Health: Theory, Research and Practice. Springer; New York, NY, USA: 2007. pp. 119–138. [Google Scholar]
- 4.Winterhalter P., Straubinger M. Saffron-renewed interest in an ancient spice. Food Rev. Int. 2000;16:39–59. doi: 10.1081/FRI-100100281. [DOI] [Google Scholar]
- 5.Liakopoulou-Kyriakides M., Kyriakidis D. Crocus sativus-biological active constituents. Stud. Nat. Prod. Chem. 2002;16:293–312. [Google Scholar]
- 6.Kanakis C.D., Daferera D.J., Tarantilis P.A., Polissiou M.G. Qualitative determination of volatile compounds and quantitative evaluation of safranal and 4-hydroxy-2,6,6-trimethyl-1-cyclohexene-1-carboxaldehyde. J. Agric. Food Chem. 2004;52:4515–4521. doi: 10.1021/jf049808j. [DOI] [PubMed] [Google Scholar]
- 7.Tarantilis P.A., Tsoupras G., Polissiou M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV/Visible photodiode-array detection-mass spectrometry. J. Chromatogr. 1995;699:107–118. doi: 10.1016/0021-9673(95)00044-N. [DOI] [PubMed] [Google Scholar]
- 8.Hammer M.B., Robert S., Fruech B.S. Treatment-resistant posttraumatic stress disorder: Strategies for intervention. CNS Spectr. 2004;9:740–752. doi: 10.1017/s1092852900022380. [DOI] [PubMed] [Google Scholar]
- 9.Van Ameringen M., Mancini C., Pipe B., Bennett M. Optimizing treatment in social phobia: A review of treatment resistance. CNS Spectr. 2004;9:753–762. doi: 10.1017/s1092852900022392. [DOI] [PubMed] [Google Scholar]
- 10.Cryan J.F., Sweeney F.F. The age of anxiety: Role of animal models of anxiolytic action in drug discovery. Br. J. Pharmacol. 2011;164:1129–1161. doi: 10.1111/j.1476-5381.2011.01362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorman J.M. New molecule targets for antianxiety interventions. J. Clin. Psychiatry. 2003;64:28–35. [PubMed] [Google Scholar]
- 12.Pitsikas N., Boultadakis A., Georgiadou G., Tarantilis P.A., Sakellaridis N. Effects of the active constituents of Crocus sativus L.; crocins, in an animal model of anxiety. Phytomedicine. 2008;15:1135–1139. doi: 10.1016/j.phymed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Georgiadou G., Tarantilis P.A., Pitsikas N. Effects of the active constituents of Crocus sativus L.; crocins in an animal model of obsessive-compulsive disorder. Neurosci. Lett. 2012;528:27–30. doi: 10.1016/j.neulet.2012.08.081. [DOI] [PubMed] [Google Scholar]
- 14.Hosseinzadeh H., Noraei N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituent, crocins and safranal in mice. Phytother. Res. 2009;23:768–774. doi: 10.1002/ptr.2597. [DOI] [PubMed] [Google Scholar]
- 15.Halatei B.S., Khosravi M., Sahrei H., Golmanesch L., Zardooz H., Jalili C., Ghoshoomi H. Saffron (Crocus sativus) acueous extract and its constituent crocin reduces stress-induced anorexia in mice. Phytother. Res. 2011;25:1833–1838. doi: 10.1002/ptr.3495. [DOI] [PubMed] [Google Scholar]
- 16.Georgiadou G., Grivas V., Tarantilis P.A., Pitsikas N. Crocins the active constituents of Crocus sativus L.; counteracted ketamine-induced behavioural deficits in rats. Psychopharmacology. 2014;231:717–726. doi: 10.1007/s00213-013-3293-4. [DOI] [PubMed] [Google Scholar]
- 17.Ai J., Dekermendjian K., Wang X., Nielsen M., Witt M.R. 6-Methylflavone, a benzodiazepine receptor ligand with antagonistic properties on rat brain and human recombinant GABAA receptors in vitro. Drug Dev. Res. 1997;41:99–106. doi: 10.1002/(SICI)1098-2299(199706)41:2<99::AID-DDR7>3.0.CO;2-M. [DOI] [Google Scholar]
- 18.Marder M., Estiu G., Blanch L.B., Viola H., Wasowski C., Medina J.H., Paladini A.C. Molecular modelling and QSAR analysis of the interaction of flavone derivatives with the benzodiazepine binding site of the GABAA receptor complex. Bioorg. Med. Chem. 2001;9:323–335. doi: 10.1016/S0968-0896(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 19.Miller D.B., O’Callaghan J.P. Neuroendocrine aspects of the response to stress. Metabolism. 2002;51:5–10. doi: 10.1053/meta.2002.33184. [DOI] [PubMed] [Google Scholar]
- 20.Lechtenberg M., Schepmann M., Niehues M., Hellenbrand N., Wunsch B., Hensel A. Quality and functionality of saffron: Quality control species assortment and affinity of extract and isolated saffron compounds to NMDA and σ1 (sigma-1) receptors. Planta Med. 2008;74:764–772. doi: 10.1055/s-2008-1074535. [DOI] [PubMed] [Google Scholar]
- 21.Iyengar S., Mick S., Dilworth V., Michel J., Rao T.S., Farah J.M., Wood P.L. Sigma receptors modulate the hypothalamic-pituitary-adrenal (HPA) axis centrally: Evidence for a functional interaction with NMDA receptors in vivo. Neuropharmacology. 1990;29:299–303. doi: 10.1016/0028-3908(90)90017-L. [DOI] [PubMed] [Google Scholar]
- 22.Freedman R. Schizophrenia. N. Engl. J. Med. 2003;349:1738–1749. doi: 10.1056/NEJMra035458. [DOI] [PubMed] [Google Scholar]
- 23.Steeds H., Carhart-Harris R.L., Stone J.M. Drug models of schizophrenia. Ther. Adv. Psychopharmacol. 2015;5:43–58. doi: 10.1177/2045125314557797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Javitt D.C. Glutamate and schizophrenia: Phencyclidine, N-methyl-d-aspartate receptors, and dopamine-glutamate interactions. Int. Rev. Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 25.Pratt J., Winchester C., Dawson N., Morris B. Advancing schizophrenia drug discovery: Optimizing rodent models to bridge the translational gap. Nat. Rev. Drug Discov. 2012;11:560–579. doi: 10.1038/nrd3649. [DOI] [PubMed] [Google Scholar]
- 26.Lewis D., Pierri J., Volk D., Melchitzky D., Woo T. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol. Psychiatry. 1999;46:616–626. doi: 10.1016/S0006-3223(99)00061-X. [DOI] [PubMed] [Google Scholar]
- 27.Field J.R., Walker A.G., Conn P.J. Targeting glutamate synapses in schizophrenia. Trends Mol. Med. 2011;17:689–698. doi: 10.1016/j.molmed.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akhondzadeh S., Shafiee-Sabet M., Harirchian M.H., Togha M., Cheraghmakani H., Razeghi S., Hejazi S.S., Yousefi M.H., Alimardani R., Jamshidi A., et al. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: A 16-week, randomized and placebo-controlled study. J. Clin. Pharm. Ther. 2010;35:581–588. doi: 10.1111/j.1365-2710.2009.01133.x. [DOI] [PubMed] [Google Scholar]
- 29.Akhondzadeh S., Shafiee-Sabet M., Harirchian M.H., Togha M., Cheraghmakani H., Razeghi S., Hejazi S.S., Yousefi M.H., Alimardani R., Jamshidi A., et al. A 22-week, multicentre randomized, double-blind controlled trial of Crocus sativus L.; in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology. 2010;207:637–643. doi: 10.1007/s00213-009-1706-1. [DOI] [PubMed] [Google Scholar]
- 30.Farokhnia M., Shafiee-Sabet M., Iranpour N., Gougol A., Yekehtaz H., Alimardani R., Farsad F., Akhondzadeh S. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer’s disease: A double-blind randomized clinical trial. Hum. Psychopharmacol. Clin. Exp. 2014;29:351–359. doi: 10.1002/hup.2412. [DOI] [PubMed] [Google Scholar]
- 31.Mousavi B., Bathaie S.Z., Fadai F., Ashtari Z., Ali beigi N., Farhang S., Hashempour S., Shahhamzei N., Heidarzadeh H. Safety evaluation of saffron stigma (Crocus sativus L.) aqueous extract and crocin in patients with schizophrenia. Avicenna J. Phytomed. 2015;5:413–419. [PMC free article] [PubMed] [Google Scholar]
- 32.Moghaddam B., Adams B., Verma A., Daly D. Activation of glutamatergic transmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J. Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosseinzadeh H., Sadeghnia H.R., Rahimi A. Effects of safranal on extracellular hippocampal levels of glutamate and aspartate during kainic acid treatment in anesthetized rats. Planta Med. 2008;74:1441–1445. doi: 10.1055/s-2008-1081335. [DOI] [PubMed] [Google Scholar]
- 34.Berger F., Hensel A., Nieber K. Saffron extracts and trans-crocetin inhibit glutamatergic synaptic transmission in rat cortical brain slices. Neuroscience. 2011;180:238–247. doi: 10.1016/j.neuroscience.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 35.Naghizadeh B., Mansouri M.T., Ghorbanzadeh B., Farbood Y., Sarkaki A. Protective effects of oral crocin against intracerebroventricular streptozotocin-induced spatial memory deficit and oxidative stress in rats. Phytomedicine. 2013;20:537–543. doi: 10.1016/j.phymed.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Ochiai T., Soeda S., Ohno S., Tanaka H., Shoyama Y., Shimeno H. Crocin prevent the death of PC-12 cells through sphingomyelinase-ceramide signaling by increasing glutathione synthesis. Neurochem. Int. 2004;44:321–330. doi: 10.1016/S0197-0186(03)00174-8. [DOI] [PubMed] [Google Scholar]
- 37.Papandreou M.A., Tsachaki M., Efthimiopoulos S., Cordopatis P., Lamari F.N., Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav. Brain Res. 2011;219:197–204. doi: 10.1016/j.bbr.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y.Q., Liu J.X., Wang J.N., Xu L. Effects of crocin on reperfusion induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007;1138:86–94. doi: 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]
- 39.Ghadrdoost B., Vafaei A., Rashidy-Pour A., Hajisoltani R., Bandegi A.R., Motamedi F., Haghighi S., Sameni H.R., Pahlvan S. Protective effect of saffron extracts and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur. J. Pharmacol. 2011;667:222–229. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Bitanihirwe B.K., Woo T.U. Oxidative stress in schizophrenia: An integrated approach. Neurosci. Biobehav. Rev. 2011;35:878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Oliveira L., Spiazzi C.M., Bortolin T., Canever L., Petronilho F., Mina F.G., Dal-Pizzol F., Quevedoa J., Zugno A.I. Different sub-anesthetic doses of ketamine increase oxidative stress in brain of rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:1003–1008. doi: 10.1016/j.pnpbp.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Hosseinzadeh H., Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L.; stigmas in mice. Arch. Iran Med. 2005;5:44–47. [Google Scholar]
- 43.Hosseinzadeh H., Sadeghnia H.R. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat: Involvement of the GABAergic and opioids systems. Phytomedicine. 2007;14:256–262. doi: 10.1016/j.phymed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad A.S., Ansari M.A., Ahmad M., Saleem S., Yousuf S., Hoda M.N., Islam F. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol. Biochem. Behav. 2005;81:805–813. doi: 10.1016/j.pbb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Hosseinzadeh H., Sadeghnia H.R. Safranal a constituent of Crocus sativus (saffron) attenuated cerebral ischemia-induced oxidative damage in rat hippocampus. J. Pharm. Pharm. Sci. 2005;8:394–399. [PubMed] [Google Scholar]
- 46.Hosseinzadeh H., Sadeghnia H.R., Ghaeni F.A., Motamedshariaty V.S., Mohajeri S.A. Effects of saffron (Crocus sativus L.) and its active constituent crocin, on recognition and spatial memory after chronic cerebral hypofunction in rats. Phytother. Res. 2012;26:381–386. doi: 10.1002/ptr.3566. [DOI] [PubMed] [Google Scholar]
- 47.Saleem S., Ahmad M., Ahmad A.S., Yousuf F., Ansari M.A., Khan M.B., Ishrat T., Islam F. Effect of Saffron (Crocus sativus) on neurobehavioral and neurochemical changes in cerebral ischemia in rats. J. Med. Food. 2006;9:246–253. doi: 10.1089/jmf.2006.9.246. [DOI] [PubMed] [Google Scholar]
- 48.Pitsikas N. The effects of Crocus sativus L. and its constituents on memory: Basic studies and clinical applications. Evid. Based Complement. Altern. Med. 2015;2015 doi: 10.1155/2015/926284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hosseinzadeh H., Karimi G., Niapoor M. Antidepressant effects of Crocus sativus stigma extracts and its constituents, crocins and safranal in mice. J. Med. Plants. 2004;3:48–58. [Google Scholar]
- 50.Akhondzadeh S., Fallah-Pour H., Afkham K., Jamshidi A.H., Khalighi-Cigaroudi F. Comparison of Crocus sativus L.; and imipramine in the treatment of mild to moderate depression: A pilot double-blind, randomized trial. BMC Complement. Altern. Med. 2004;4:12–16. doi: 10.1186/1472-6882-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noorbala A.A., Akhondzadeh S., Tahmacebi-Pour N., Jamshidi A.H. Hydro-alcoholic extract of Crocus sativus L.; versus fluoxetine in the treatment of mild to moderate depression: A double-blind, randomized trial. J. Ethnopharmacol. 2005;97:281–284. doi: 10.1016/j.jep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Kashani L., Raisi F., Saroukhani S., Sohrabi H., Modabbernia A., Nasehi A.-A., Jamshidi A., Ashrafi M., Mansouri P., Ghaeli P., et al. Saffron for treatment of fluoxetine-induced sexual dysfunction in women: Randomized double-blind placebo-controlled study. Hum. Psychopharmacol. 2012;28:54–60. doi: 10.1002/hup.2282. [DOI] [PubMed] [Google Scholar]
- 53.Modabbernia A., Sohrabi H., Nasehi A.A., Raisi F., Saroukhani S., Jamshidi A., Tabrizi M., Ashrafi M., Akhondzadeh S. Effect of saffron on fluoxetine-induced sexual impairment in men: Randomized double-blind placebo-controlled trial. Psychopharmacology. 2013;223:381–388. doi: 10.1007/s00213-012-2729-6. [DOI] [PubMed] [Google Scholar]
- 54.Nair S.C., Panikkar B., Panikkar K.R. Antitumor activity of saffron. Cancer Lett. 1991;57:109–114. doi: 10.1016/0304-3835(91)90203-T. [DOI] [PubMed] [Google Scholar]
- 55.Salomi M.J., Nair S.C., Panikkar K.R. Inhibitory effects of nigella sativa and saffron on chemical carcinogenesis in mice. Nutr. Cancer. 1991;16:67–72. doi: 10.1080/01635589109514142. [DOI] [PubMed] [Google Scholar]
- 56.Tarantilis P.A., Morjani H., Manfait M., Polissiou M. Inhibition of growth and induction of differentiation of promyelocytic leukemia (HL-60) by carotenoids from Crocus sativus L. Anticancer Res. 1994;14:1913–1918. [PubMed] [Google Scholar]
- 57.Hosseinzadeh H., Younesi H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;15:2–7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gainer J.L., Jones J.R. The use of crocetin in experimental atherosclerosis. Experientia. 1975;31:548–549. doi: 10.1007/BF01932451. [DOI] [PubMed] [Google Scholar]
- 59.Wang C.J., Shiow S.J., Lin J.K. Effects of crocetin on the hepatotoxicity and hepatic DNA binding of aflatoxin B1 in rats. Carcinogenesis. 1991;12:459–462. doi: 10.1093/carcin/12.3.459. [DOI] [PubMed] [Google Scholar]
- 60.Lee I.A., Lee J.H., Baek N.I., Kim D.H. Antihyperilipidemic effect of crocin isolated from the fructus of Gardenia jasminoides. J. Microbiol. Biotechnol. 2005;16:1084–1089. doi: 10.1248/bpb.28.2106. [DOI] [PubMed] [Google Scholar]
- 61.Du P., Qian Z., Shen X., Rao S., Wen N. Effectiveness of crocin against myocardial injury. Chin. J. New Drugs. 2005;14:1424–1427. [Google Scholar]
- 62.Fatehi M., Rashidabady T., Fatehi-Hassanabad Z. Effects of Crocus sativus petal’s extract on rat blood pressure and on responses induced by electrical field stimulation in the rat isolated vas deferens and guinea-pig-ileum. J. Ethnopharmacol. 2003;84:199–203. doi: 10.1016/S0378-8741(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 63.Imenshahidi M., Hosseinzadeh H., Javadpour Y. Hypotensive effect of aqueous saffron extract (Crocus sativus L.) and its constituents, safranal and crocin, in normotensive and hypertensive rats. Phytother. Res. 2010;24:990–994. doi: 10.1002/ptr.3044. [DOI] [PubMed] [Google Scholar]
- 64.Abdullaev F.I., Espinosa-Aguirre J.J. Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect. Prev. 2004;28:426–432. doi: 10.1016/j.cdp.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Alavizadeh S.H., Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 66.Bathaie S.Z., Mousavi S.Z. New applications and mechanisms of action of saffron and its important ingredients. Crit. Rev. Food Sci. Nutr. 2010;50:761–786. doi: 10.1080/10408390902773003. [DOI] [PubMed] [Google Scholar]
- 67.Abdullaev F.I. Cancer chemoprotective and tumoricidial properties of saffron (Crocus sativus L.) Exp. Biol. Med. 2002;227:20–25. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 68.Hosseinzadeh H., Motamedshariaty V.S., Sameni A.K., Vahabzadeh M. Acute and sub-acute toxicity of crocin, a constituent of Crocus sativus L.; (saffron), in mice and rats. Pharmacologyonline. 2010;2:943–951. [Google Scholar]
- 69.Modagheghi M.H., Shahabian M., Esmaeli H.A., Rajbai O., Hosseinzadeh H. Safety evaluation of saffron (Crocus sativus) tablets in healthy volunteers. Phytomedicine. 2008;15:1032–1037. doi: 10.1016/j.phymed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Mohamadpour A.H., Ayati Z., Parizadeh M.R., Rajbai O., Hosseinzadeh H. Safety evaluation of crocin (a constituent of saffron) tablets in healthy volunteers. Iran J. Basic Med. Sci. 2013;16:39–46. [PMC free article] [PubMed] [Google Scholar]