Abstract
A new pyrrolidine alkaloid named (Z)-3-(4-hydroxybenzylidene)-4-(4-hydroxyphenyl)-1-methylpyrrolidin-2-one was isolated from the ethanol extract of the root barks of Orixa japonica. The structure of the new alkaloid was elucidated on the basis of NMR and MS analysis. The compound exhibited larvicidal activity against the fourth instar larvae of Aedes aegypti (LC50 = 232.09 μg/mL), Anopheles sinensis (LC50 = 49.91 μg/mL), and Culex pipiens pallens (LC50 = 161.10 μg/mL). The new alkaloid also possessed nematicidal activity against Bursaphelenchus xylophilus (LC50 = 391.50 μg/mL) and Meloidogynein congnita (LC50 = 134.51 μg/mL). The results indicate that the crude ethanol extract of O. japonica root barks and its isolated pyrrolidine alkaloid have potential for development into natural larvicides and nematicides.
Keywords: Orixa japonica, mosquito, pyrrolidine alkaloid, larvicidal activity, nematicidal activity
1. Introduction
Mosquito-borne diseases affect at least 500 million people around the world [1]. Dengue fever, mainly transmitted by the yellow fever mosquito (Aedes aegypti L.) and Asian tiger mosquito (Aedes albopictus Skuse), cause 50 million new infections and 24,000 deaths every year [2]. Malaria, mostly transmitted by Anopheles sinensis Wiedemann, used to be a horrible threat to Chinese people for a long period of time. As was reported by the World Health Organization (WHO) in the year of 2010, there were still 225 million people living under the threat of malaria annually [3]. Wuchereriasis and epidemic encephalitis B, causing significant morbidity and mortality, are primarily vectored by the house mosquito, Culex pipiens pallens Coquillett [4]. Currently, the main way to control mosquitoes involves application of synthetic pesticides, organophosphates (e.g., temephos, fenthion, and malathion), and insect growth regulators (e.g., diflubenzuron and methoprene) [5].
Plant-parasitic nematodes are serious worldwide threats to forestry and agriculture because of their wide range of host plants and short biological cycles [6]. The pine wood nematode, Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle, causes pine wilt disease by inducing rapid wilting and leads to death of host pines [7]. The southern root-knot nematode, Meloidogynein congnita (Kofold and White) Chitwood, is one of the most devastating nematode groups, resulting in decreased quality and quantity in fruits, vegetables, and crops [8]. In the past, methods in integrated management of plant-parasitic nematodes have extensively relied on synthetic fumigant and non-fumigant nematicides [6,9].
However, synthetic larvicides and nematicides have shown some side effects, such as being toxic to humans and other nontarget organisms, causing soil and underground water pollution, disrupting biological control systems, and so on [10]. Therefore, it is urgent to research and develop environmentally acceptable larvicides and nematicides. Plants contain a rich source of bioactive chemicals, since many plants have been reported to possess larvicidal and nematicidal activities [8,11,12,13,14].
During our mass screening for bioactive natural products from Chinese medicinal herbs or wild plants, Orixa japonica Thunb. (Rutaceae) showed potential larvicidal and nematicidal activity. Orixa japonica is a shrub distributed in China (Anhui, Fujian, Guizhou, Henan, Hubei, Hunan, Jiangsu, Jiangxi, Shaanxi, Sichuan, Yunnan, and Zhejiang provinces), Japan, and Korea [15]. The whole plant has been used for the treatment of cough, rheumatism, malaria, and dysentery [16]. According to the former studies, its extracts or constituents possess pharmacological and biological activities [17,18,19]. Chemical investigations revealed that several quinoline alkaloids (evolitrine, kokusaginine, orixinone, skimmianine, etc.), coumarins (bergapten, imperatorin, and xanthotoxin), and terpenoids (friedelin, limonene, and γ-terpinene) have been isolated and identified from this plant [10,18]. In continuation of our study for other structural types of bioactive compounds from the ethanol extract of O. japonica root barks, we used the method of repeated column chromatographic separation to yield a new pyrrolidine alkaloid, (Z)-3-(4-hydroxybenzylidene)-4-(4-hydroxyphenyl)-1-methylpyrrolidin-2-one. To our knowledge, this is the first report of a pyrrolidine alkaloid that has been isolated and identified from O. japonica. This paper deals with the isolation and structure determination of this compound, as well as its bioactivities against the three species of mosquitoes and the two species of plant-parasitic nematodes.
2. Results and Discussion
2.1. Isolated Bioactive Compound
Purification of the ethanol extract of O. japonica root barks afforded a new pyrrolidine alkaloid (17.9 mg) (Figure 1), the structure of which was elucidated by NMR and MS analysis. NMR and MS spectra are available in supplementary materials.
Figure 1.
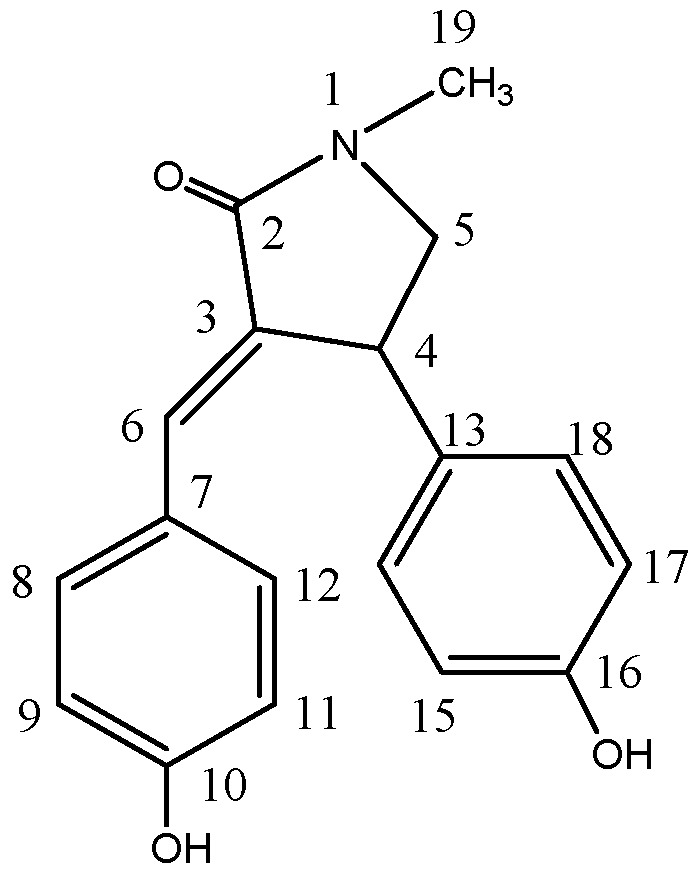
Chemical structure of the pyrrolidine alkaloid isolated from the ethanol extract of O. japonica root barks.
(Z)-3-(4-Hydroxybenzylidene)-4-(4-hydroxyphenyl)-1-methylpyrrolidin-2-one was obtained as white crystal, and displayed a molecular formula of C18H17NO3 ([M − H]−, calcd., m/z 294.1130; found, m/z 294.1135) as determined by high-resolution electrospray ionization mass spectrometry (HRESIMS). The 1H- and 13C-NMR spectra showed 15 proton and 14 carbon signals (Table 1). According to the HSQC spectrum and a previous report, proton signals at δH 6.66 (2H, d, J = 8.1 Hz), 7.23 (2H, d, J = 8.2 Hz), together with their corresponding carbon signals at δC 114.9 and 131.8, respectively, as well as signals at δH 6.72 (2H, d, J = 8.0 Hz) and 7.05 (2H, d, J = 8.0 Hz) with their corresponding carbon signals at δC 115.3 and 127.7, respectively, suggested the presence of two symmetrical para-substituted aromatic protons [20]. Besides, signals at δH 4.48 (1H, d, J = 7.8 Hz), 3.27 (1H, d, J = 9.9 Hz), and 3.98 (1H, d, J = 9.3 Hz), together with their corresponding carbon signals at δC 40.33 and 57.0, respectively, and a carbonyl carbon signal at δC 170.8 revealed a pyrrolidine-2-one ring [21]. Furthermore, the existence of a methyl group at δH 2.98 (3H, s) was noticeable in the 1H-NMR spectrum.
Table 1.
1H (500 MHz) and 13C (125 MHz) NMR data for the isolated compound in MeOD (J in Hz).
| Position | δH | δC |
|---|---|---|
| 2 | 170.8 | |
| 3 | ||
| 4 | 4.48, d, J = 7.8 Hz | 40.3 |
| 5α | 3.98, d, J = 9.3 Hz | 57.0 |
| 5β | 3.27, d, J = 9.9 Hz | |
| 6 | 7.39, s | 132.2 |
| 7 | 125.9 | |
| 8 | 7.23, d, J = 8.2 Hz | 131.8 |
| 9 | 6.66, d, J = 8.1 Hz | 114.9 |
| 10 | 158.2 | |
| 11 | 6.66, d, J = 8.1 Hz | 114.9 |
| 12 | 7.23, d, J = 8.2 Hz | 131.8 |
| 13 | 133.5 | |
| 14 | 7.05, d, J = 8.0 Hz | 127.7 |
| 15 | 6.72, d, J =8.0 Hz | 115.3 |
| 16 | 156.1 | |
| 17 | 6.72, d, J =8.0 Hz | 115.3 |
| 18 | 7.05, d, J = 8.0 Hz | 127.7 |
| 19 | 2.98, s | 29.0 |
In the HMBC spectrum, correlations from H-6 (δH 7.39) to C-8 (δC 131.8) and C-12 (δC 131.8), from H-8 (δH 7.23) to C-6 (δC 132.2) and C-10 (δC 158.2), from H-9 (δH 6.66) to C-7 (δC 125.9) and C-10 (δC 158.2), from H-11 (δH 6.66) to C-7 (δC 125.9) and C-10 (δC 158.2), and from H-12 (δH 7.23) to C-6 (δC 132.2) and C-10 (δC 158.2) revealed fragment A (Figure 2). Besides, HMBC correlations from H-14 (δH 7.05) to C-4 (δC 40.3) and C-16 (δC 156.1), from H-15 (δH 6.72) to C-13 (δC 133.5) and C-16 (δC 156.1), from H-17 (δH 6.72) to C-13 (δC 133.5) and C-16 (δC 156.1), and from H-18 (δH 7.05) to C-14 (δC 127.7) and C-16 (δC 156.1) confirmed fragment B (Figure 2). Furthermore, correlations from H-5 (δH 3.27) to C-2 (δC 170.8) and from H-19 (δH 2.98) to C-2 (δC 170.8) and C-5 (δC 57.0) proved the existence of fragment C (Figure 2). According to HMBC correlations from H-6 (δH 7.39) to C-2 (δC 170.8) and C-4 (δC 40.3), fragment A was attached to C-3 of fragment C (Figure 2). HMBC correlations from H-4 (δH 4.48) to C-14 (δC 127.7) and C-18 (δC 127.7) revealed fragment B connected with C-4 of fragment C. Thus, the compound was determined as (Z)-3-(4-hydroxybenzylidene)-4-(4-hydroxyphenyl)-1-methylpyrrolidin-2-one.
Figure 2.
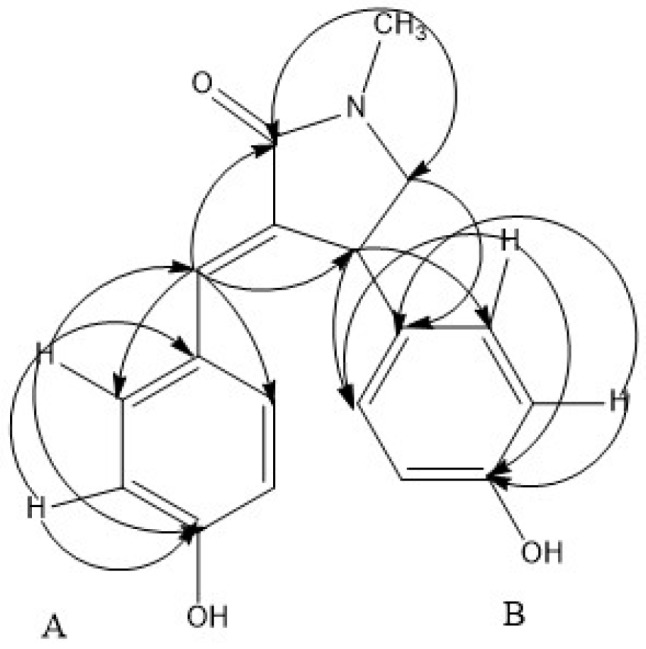
Key HMBC (H→C) correlations of the pyrrolidine alkaloid isolated from the ethanol extract of O. japonica root barks.
2.2. Bioactivities
The isolated pyrrolidine alkaloid was tested for its larvicidal activity against the fourth instar larvae of Ae. aegypti, An. sinensis, and C. pipiens pallens. The fourth instar larvae of An. sinensis was the most susceptible with a 24 h LC50 value of 49.91 μg/mL (Table 2), while C. pipiens pallens and Ae. aegypti were 3.2 and 4.7 times less sensitive than An. sinensis, respectively. However, such differences in sensitivity to chemicals need to be further investigated. When compared with a positive control, commercial rotenone, the isolated compound was 62–85 times less toxic to the three species of mosquitoes.
Table 2.
Larvicidal activity of the isolated pyrrolidine alkaloid against the fourth instar larvae of Aedes aegypti, Anopheles sinensis, and Culex pipiens pallens.
| Mosquitoes | Treatment | LC50 (μg/mL) (95% CL) |
LC90 (μg/mL) (95% CL) |
Slope ± SD | χ2 | p |
|---|---|---|---|---|---|---|
| Ae. aegypti | Compound | 232.09 (209.05–254.74) |
293.19 (265.30–321.58) |
12.63 ± 1.43 | 8.69 | 0.0130 * |
| Rotenone | 3.75 (3.39–4.11) |
9.64 (8.64–10.56) |
3.22 ± 0.33 | 18.20 | 0.0001 * | |
| An. sinensis | Compound | 49.91 (45.61–54.51) |
82.31 (74.12–90.46) |
5.90 ± 0.68 | 11.05 | 0.0040 * |
| Rotenone | 0.73 (0.66–0.80) |
1.13 (1.02–1.24) |
4.00 ± 0.51 | 10.55 | 0.0051 * | |
| C. pipiens pallens | Compound | 161.10 (145.37–177.04) |
211.80 (192.01–232.97) |
10.78 ± 1.24 | 9.28 | 0.0097 * |
| Rotenone | 1.88 (1.69–2.01) |
3.74 (3.38–4.09) |
6.90 ± 0.88 | 15.80 | 0.0004 * |
* Values were significant at the p < 0.05 level.
The isolated alkaloid also possessed nematicidal activity against juveniles of Bursaphelenchus xylophilus and Meloidogynein congnita with 72 h LC50 values of 391.50 μg/mL and 134.51 μg/mL (Table 3), respectively; M. incongnita was 2.9 times more susceptible than B. xylophilus. However, this susceptibility difference is not yet clear. In comparison to a positive control, commercial avermectin, the isolated compound was 5000 times less toxic to the two species of nematodes.
Table 3.
Nematicidal activity of the isolated pyrrolidine alkaloid against juveniles of Bursaphelenchus xylophilus and Meloidogynein congnita.
| Nematodes | Treatment | LC50 (μg/mL) (95% CL) |
LC90 (μg/mL) (95% CL) |
Slope ± SD | χ2 | p |
|---|---|---|---|---|---|---|
| B. xylophilus | Compound | 391.50 (353.90–429.18) |
>500 | 2.37 ± 0.20 | 15.15 | 0.0005 * |
| Avermectin | 0.071 (0.068–0.075) |
0.24 (0.22–0.26) |
2.45 ± 0.20 | 14.76 | 0.0006 * | |
| M. incongnita | Compound | 134.51 (121.91–146.24) |
>500 | 1.18 ± 0.12 | 12.00 | 0.0025 * |
| Avermectin | 0.025 (0.023–0.027) |
0.13 (0.12–0.14) |
2.08 ± 0.16 | 28.21 | 0.0000 * |
* Values were significant at the p < 0.05 level.
On the basis of a literature survey, compounds of the pyrrolidine type have exhibited various biological activities, such as antioxidative, antifungal, and antibacterial activity; antitumor properties; cytotoxicity; and glycosidase inhibitory; and platelet aggregation inhibitory effects [22,23,24]. It is worth mentioning that pyrrolidines have been found to possess larvicidal activity against Ae. aegypti at a concentration less than 140 ppm [25]. Pyrrolidines also possess considerable insecticidal activity when evaluated against termite workers [26]. Moreover, pyrrolidine alkaloids have also been found to possess DNA-binding affinity and cytotoxicity [27], which might be the mechanism of action of the new alkaloid against larval mosquitoes and plant-parasitic nematodes. The above findings suggested the new alkaloid with a pyrrolidine ring isolated from the ethanol extract of O. japonica has the potential to be developed into an alternative larvicide and nematicide.
3. Experimental Section
3.1. General
NMR spectra were recorded on Bruker (Billerica, MA, USA) Avance 500 instruments using MeOD as solvents. HRESIMS analysis was performed on an APEX II spectrometer (Bruker Daltonic Inc., Billerica, MA, USA).
3.2. Mosquitoes
Eggs of Ae. aegypti and An. sinensis and egg masses of C. pipiens pallens were obtained from the Department of Vector Biology and Control, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention. Adult mosquitoes were reared in a cage (60 cm × 30 cm × 30 cm) which was placed in a growth chamber (14:10 h light:dark (L:D), 26–28 °C, 70%–80% RH). Adults were maintained on a 10% glucose solution and were allowed to blood-feed on live mice. Adults of both Ae. aegypti and An. sinensis deposited on strips of moistened filter paper, which were kept in glass beakers. Paper strips with Ae. aegypti eggs were kept wet for 24 h and then dehydrated, while An. sinensis eggs need to be kept wet. However, egg masses of C. pipiens pallens were oviposited in distilled water directly. Then, the egg masses were transported to a clean, white porcelain basin containing distilled water. Eggs or egg masses were kept in distilled water under the same conditions described above. Mosquito larvae were provided with a mixture of pig liver, fish food, and yeast powder (1:1:1) and were not utilized for experiments until they reached the fourth instar.
3.3. Nematodes
Colonies of B. xylophilus were maintained on Botrytis cinerea cultures. The fungus B. cinerea was cultured on potato dextrose agar (PDA) in a growth chamber (25–28 °C in dark). When B. cinerea was fully grown on PDA, B. xylophilus were collected through the modified Baermann funnel technique, washed with a mixture of 0.1% streptomycin sulfate and 0.002% actinone three times to remove any surface bacterial or fungal contaminants and then inoculated on the plate [28]. The plate was maintained in the growth chamber (25–28 °C in dark) until the fungal mycelium were completely consumed by B. xylophilus. Then, B. xylophilus were collected, washed thoroughly with sterilized distilled water, and used for bioassays immediately. All the bioassays were performed under laboratory conditions at 25–28 °C.
Eggs of M. incongnita were extracted from infected roots of tomato (Lycopersicone sculentum Mill.). All the tomato plants were reared in a growth chamber (16:8 h L:D, 25–28 °C, 75%–80% RH). When they reached a five-leaf stage, the tomatoes were used for inoculations. After 42 days, infected tomatoes were uprooted and the roots were washed free of soil with distilled water. Egg masses were hand-picked using sterilized tweezers from infected roots and then placed on a mesh nylon filter (openings 30 μm in diameter) [29]. J2s that passed through the filter were collected daily and used for bioassays immediately.
3.4. Plant Material
Fresh root barks of O. japonica were collected in Xixiu District, Anshun City, Guizhou Province, China (39.90°N and 116.41°E) in August 2014, and identified by Dr. Liu QR, College of Life Sciences, Beijing Normal University, Beijing. A voucher specimen of O. japonica (Rutaceae-Orixa japonica-Guizhou-2014-08) was deposited at the museum of the Department of Entomology, China Agricultural University, Beijing, China.
3.5. Extraction and Isolation
Root barks of O. japonica (20 kg) were air-dried, cut into pieces, and then successively extracted with 40L of different concentrations of ethanol/distilled water mixtures (95%, 75%, 50%, by volume) and distilled water at room temperature for 3 days. The extracts were then filtered, mixed and removed from the solvent under reduced pressure to afford crude extract (200 g). After that, the crude extract was suspended in distilled water (2 L) and then successively partitioned with the same volume of n-hexane, chloroform, ethyl acetate, and n-butyl alcohol to obtain four solvent fractions (37 g, 69 g, 25 g, 33 g). The n-butyl alcohol fraction was subjected to chromatography on a macroporous resin (AB-8, 1000 g) column (85 mm i.d., 850 mm length), eluting with distilled water containing increasing amounts of ethanol (up to 90%, by volume) to yield eight fractions. Fraction 8 (5.3 g) was then subjected to silica gel (Merck 9385) column (50 mm i.d., 500 mm length), eluting with CH2Cl2–EtOH (100:0–100:50) to afford 25 fractions. Fraction 10 (114 mg) was separated by a silica gel (Merck 9385) column (25 mm i.d., 500 mm length), eluting with CH2Cl2–EtOH (100:5–100:20) to afford 13 fractions. Fraction 6 (9.2 mg) was further purified by Sephadex LH-20 column (15 mm i.d., 500 mm length) chromatography, eluting with ethanol alone to yield the new pyrrolidine alkaloid (17.9 mg).
3.6. Bioactivity Assays
All bioactivity assays were performedunder laboratory conditions at 25–28 °C. The compound was dissolved in ethanol and diluted with tap water. The larvicidal bioassays were as recommended by WHO [30]. Solutions (250 mL) of the tested material at various concentrations were placed in glass beakers, and then 10 larvae were delivered toeach beaker. Each test was composed of six concentrations with five replicates. Commercial rotenone (purchased from Aladdin-Reagent Company, Shanghai, China) served as a positive control and ethanol was used as a negative control. Both treated and control larvae were placed in the growth chamber where mosquitoes were reared. Mortality recordings were taken after 24 h of exposure. Larvae that showed no movements were considered to be dead.
The standard nematode suspensions were prepared by appropriate dilution with sterilized distilled water to get approximately 100 juveniles/mL. Then, each well of 24-well tissue culture plates were added with 500 μL standard juvenile suspension. Numbers of active juveniles in every well were counted under a stereoscope at 10× and 5× before 500 μL stock solution was added to the corresponding well. However, the final concentration of ethanol in each treatment never exceeded 1% (by volume) [31]. Plates were then covered with “Xuan paper” (a high-quality rice paper made for traditional Chinese painting and calligraphy) to avoid evaporation. Each test was composed of five concentrations with three replicates. Commercial avermectin (purchased from Aladdin-Reagent Company, Shanghai, China) was used as a positive control, and distilled water containing ethanol (1%, by volume) served as a negative control. Both treated and control juveniles were placed in a growth chamber at 25–28 °C in dark. Mortality recordings were taken 72 h after treatment. Juveniles that showed no movements when stimulated with a fine needle were considered to be dead.
4. Conclusions
A new pyrrolidine alkaloid was obtained from ethanol extract of O. japonica root barks, identified as (Z)-3-(4-hydroxybenzylidene)-4-(4-hydroxyphenyl)-1-methylpyrrolidin-2-one. It possessed larvicidal activity against fourth instar larvae of Ae. aegypti, An. sinensis, and C. pipiens pallens, as well as nematicidal activity against juveniles of M. incognita and B. xylophilus. However, further investigations should be conducted to explore its action mechanism and safety issues, so as to develop a fundamental structure with potential bioactivities.
Acknowledgments
This project was supported by National Key Technology Research and Development Program of Ministry of Science and Technology of China (Grant No. 2014BAD23B02). We thank Liu Q.R. from the College of Life Sciences, Beijing Normal University, Beijing 100875, for the identification of the investigated plant.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/12/1665/s1.
Author Contributions
Z.L.L. conceived and designed the experiments; X.C.L. performed the experiments; D.L. analyzed the NMR spectra data; Q.Z.L., L.Z. and Q.L. contributed reagents/materials/analysis tools; X.C.L. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the crude extracts and pure compounds are available from the authors.
References
- 1.Perumalsamy H., Jang M.J., Kim J.R., Kadarkarai M., Ahn Y.J. Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasite Vector. 2015;8:237–250. doi: 10.1186/s13071-015-0848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shetty V., Sanila D., Shetty N.J. Insecticide susceptibility status in three medically important species of mosquitoes, Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus, from Bruhat Bengaluru Mahanagara Palike, Karnataka, India. Pest Manag. Sci. 2013;69:257–267. doi: 10.1002/ps.3383. [DOI] [PubMed] [Google Scholar]
- 3.World Malaria Report 2010. World Health Organization; Geneva, Switzerland: 2010. [Google Scholar]
- 4.Sun L., Dong H., Guo C., Qian J., Sun J., Ma L., Zhu C. Larvicidal activity of extracts of Ginkgo biloba exocarp for three different strains of Culex pipiens pallens. J. Med. Entomol. 2006;43:258–261. doi: 10.1603/0022-2585(2006)043[0258:LAOEOG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z.L., Liu Q.Z., Du S.S., Deng Z.W. Mosquito larvicidal activity of alkaloids and limonoids derived from Evodia rutaecarpa unripe fruits against Aedes albopictus (Diptera: Culicidae) Parasitol. Res. 2012;111:991–996. doi: 10.1007/s00436-012-2923-9. [DOI] [PubMed] [Google Scholar]
- 6.Akhtar M. Current options in integrated management of plant-parasitic nematodes. Integ. Pest Manag. Rev. 1997;2:187–197. doi: 10.1023/A:1018409303298. [DOI] [Google Scholar]
- 7.Cui H., Jin H., Liu Q., Yan Z., Ding L., Qin B. Nematicidal metabolites from roots of Stellera chamaejasme against Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. Pest Manag. Sci. 2014;70:827–835. doi: 10.1002/ps.3625. [DOI] [PubMed] [Google Scholar]
- 8.Caboni P., Saba M., Oplos C., Aissani N., Maxia A. Nematicidal activity of furanocoumarins from parsley against Meloidogyne spp. Pest Manag. Sci. 2014;71:1099–1105. doi: 10.1002/ps.3890. [DOI] [PubMed] [Google Scholar]
- 9.Neville C.F., Grundon M.F., Ramachandran V.N., Reisch J. Quinoline alkaloids. Part 27. Synthesis of the Ptelea alkaloids pteleflorine, neohydroxylunine, O-methylhydroxyluninium salt and hydroxylunine. J. Chem. Soc. Perkin Trans. 1. 1991;2:259–262. [Google Scholar]
- 10.Ito C., Itoigawa M., Furukawa A., Hirano T., Murata T., Kaneda N., Hisada Y., Okuda K., Furukawa H. Quinolone alkaloids with nitric oxide production inhibitory activity from Orixa japonica. J. Nat. Prod. 2004;67:1800–1803. doi: 10.1021/np0401462. [DOI] [PubMed] [Google Scholar]
- 11.Liu X.C., Zhou L., Liu Q., Liu Z.L. Laboratory evaluation of larvicidal activity of the essential oil of Allium tuberosum roots and its selected major constituent compounds against Aedes albopictus (Diptera: Culicidae) J. Med. Entomol. 2015;52:437–441. doi: 10.1093/jme/tjv016. [DOI] [PubMed] [Google Scholar]
- 12.Liu X.C., Liu Q., Chen X.B., Zhou L., Liu Z.L. Larvicidal activity of the essential oil from Tetradium glabrifolium fruits and its constituents against Aedes albopictus. Pest Manag. Sci. 2015;71:1582–1586. doi: 10.1002/ps.3964. [DOI] [PubMed] [Google Scholar]
- 13.Choi N.H., Kwon H.R., Son S.W., Choi Y.H., Jang K.S., Lee S.O., Choi J.E., Ngoc L.H., Kim J.C. Nematicidal activity of malabaricones isolated from Myristica malabarica fruitrinds against Bursaphelenchus xylophilus. Nematology. 2008;10:801–807. doi: 10.1163/156854108786161454. [DOI] [Google Scholar]
- 14.Faria J.M.S., Barbosa P., Bennett R.N., Mota M., Figueiredo A.C.S. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from essential oils, essential oils fractions and decoction waters. Phytochemistry. 2013;94:220–228. doi: 10.1016/j.phytochem.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Wu Z.Y., Peter H.R., Hong D.Y. Orixa japonica, Flora of China. [(accessed on 25 October 2016)]. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200012465.
- 16.He Q.S., Feng Y., Peng Q.C., Yang Z.N. Analysis of volatile chemical constituents in entire plants of Orixa japonica by GC-MS spectrometry. Chin. J. Exp. Tradit. Med. Form. 2010;16:83–87. (In Chinese) [Google Scholar]
- 17.Kang C.H., Choi Y.H., Choi I.W., Lee J.D., Kim G.Y. Inhibition of lipopolysaccharide-induced iNOS, COX-2, and TNF-α expression by aqueous extract of Orixa japonica in RAW 264.7 cells via suppression of NF-κB activity. Trop. J. Pharm. Res. 2011;10:161–168. [Google Scholar]
- 18.Ono H., Kuwahara Y., Nishida R. Hydroxybenzoic acid derivatives in a nonhostrutaceous plant, Orixa japonica, deter both oviposition and larval feeding in a Rutaceae-feeding swallowtail butterfly, Papilio xuthus L. J. Chem. Ecol. 2004;30:287–301. doi: 10.1023/B:JOEC.0000017978.73061.a0. [DOI] [PubMed] [Google Scholar]
- 19.Sharma N., Sharma V.K., Seo S.Y. Screening of some medicinal plants for anti-lipase activity. J. Ethnopharmacol. 2005;97:453–456. doi: 10.1016/j.jep.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Guo L.N., Pei Y.H., Chen G., Cong H., Liu J.C. Two new compounds from Dictamnus dasycarpus. J. Asian Nat. Prod. Res. 2012;14:105–110. doi: 10.1080/10286020.2011.628316. [DOI] [PubMed] [Google Scholar]
- 21.Crucianelli E., Galeazzi R., Martelli G., Orena M., Rinaldi S., Sabatino P. A novel conformationally restricted analogue of 3-methylaspartic acid via stereoselective methylation of chiral pyrrolidin-2-ones. Tetrahedron. 2010;66:400–405. doi: 10.1016/j.tet.2009.10.004. [DOI] [Google Scholar]
- 22.Dade J.M.E., Irie-N’Guessan G., Komlaga G., Say M., Okpekon T.A., Boti J.B., Kablan B.J., Bamba E.H.S. Pyrrolidine alkaloids and their glycosylated derivatives from the root bark of Dichrostachys cinerea (L.) Wight & Arn. (Fabaceae) Phytochem. Lett. 2016;16:268–276. [Google Scholar]
- 23.Majik M.S., Naik D., Bhat C., Tilve S., Tilvi S., D’Souza L. Synthesis of (R)-norbgugaine and its potential as quorum sensing inhibitor against Pseudomonas aeruginosa. Bioorg. Med. Chem. Lett. 2013;23:2353–2356. doi: 10.1016/j.bmcl.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 24.Girgis A.S. Regioselective synthesis of dispiro[1H-indene-2,3′-pyrrolidine-2′,3′′-[3H]indole]-1,2′′(1′′H)-diones of potential antitumor properties. Eur. J. Med. Chem. 2009;44:91–100. doi: 10.1016/j.ejmech.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Carreño Otero A.L., Vargas Méndez L.Y., Duque L.J.E., Kouznetsov V.V. Design, synthesis, acetylcholinesterase inhibition and larvicidal activity of girgensohnine analogs on Aedes aegypti, vector of dengue fever. Eur. J. Med. Chem. 2014;78:392–400. doi: 10.1016/j.ejmech.2014.03.067. [DOI] [PubMed] [Google Scholar]
- 26.Jones T.H., Blum M.S., Andersen A.N., Fales H.M., Escoubas P. Novel 2-ethyl-5-alkylpyrrolidines in the venom of an Australian ant of the genus Monomorium. J. Chem. Ecol. 1988;14:35–45. doi: 10.1007/BF01022529. [DOI] [PubMed] [Google Scholar]
- 27.Mizushina Y., Xu X., Asano N., Kasai N., Kato A., Takemura M., Asahara H., Linn S., Sugawara F., Yoshida H., et al. The inhibitory action of pyrrolidine alkaloid, 1,4-dideoxy-1,4-imino-d-ribitol, on eukaryotic DNA polymerases. Biochem. Biophys. Res. Commun. 2003;304:78–85. doi: 10.1016/s0006-291x(03)00540-0. [DOI] [PubMed] [Google Scholar]
- 28.Viglierchio D.R., Schmitt R.V. On the methodology of nematode extraction from field samples: Baermann funnel modifications. J. Nematol. 1983;15:438–444. [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer S.L., Zasada I.A., Roberts D.P., Vinyard B.T., Lakshman D.K., Lee J.K., Chitwood D.J., Carta L.K. Plantago lanceolata and Plantago rugelii extracts are toxic to Meloidogyne incognita but not to certain microbes. J. Nematol. 2006;38:333–338. [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization (WHO) Report of the WHO Informal Consultation on the “Evaluation and Testing of Insecticides”. World Health Organization; Geneva, Switzerland: 1996. pp. 32–36. Technical Report Number: CTD/WHOPES/IC/96.1. [Google Scholar]
- 31.Caboni P., Ntalli N.G., Aissani N., Cavoski I., Angioni A. Nematicidal activity of (E,E)-2,4-decadienal and (E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. J. Agric. Food Chem. 2012;60:1146–1151. doi: 10.1021/jf2044586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


