Abstract
In this study, a new series of 16 methyl salicylate derivatives bearing a piperazine moiety were synthesized and characterized. The in vivo anti-inflammatory activities of target compounds were investigated against xylol-induced ear edema and carrageenan-induced paw edema in mice. The results showed that all synthesized compounds exhibited potent anti-inflammatory activities. Especially, the anti-inflammatory activities of compounds M15 and M16 were higher than that of aspirin and even equal to that of indomethacin at the same dose. In addition, the in vitro cytotoxicity activities and anti-inflammatory activities of four target compounds were performed in RAW264.7 macrophages, and compound M16 was found to significantly inhibit the release of lipopolysaccharide (LPS)-induced interleukin (IL)-6 and tumor necrosis factor (TNF)-α in a dose-dependent manner. In addition, compound M16 was found to attenuate LPS induced cyclooxygenase (COX)-2 up-regulation. The current preliminary study may provide information for the development of new and safe anti-inflammatory agents.
Keywords: inflammation, salicylate, piperazine, derivatives, anti-inflammatory activity
1. Introduction
Inflammation is a complex biological process that occurs when body tissues are exposed to hazardous stimuli, such as irritants and pathogens [1]. Inflammation seriously threatens human health, and exaggerated and prolonged inflammation may cause various diseases, including arthritis, sepsis, and even cancer [2]. Presently, the most widely used drugs in inflammation treatment are non-steroidal anti-inflammatory drugs (NSAIDs), accounting for 35% of the global market of prescription of pain medications [3,4]. The common NSAIDs, such as aspirin and indomethacin, can inhibit cyclooxygenases (COX-1 and COX-2) [5,6], which are involved in the catalyzation of arachidonic acid to prostaglandins (PGs). Considering the significant toxicity of NSAIDs to the gastrointestinal tract and kidney [7], it is of great importance and urgent need to develop new anti-inflammation drugs [8].
Pharmaceuticals bearing a piperazine moiety are widely used in medicinal and pesticide chemistry [9]. Piperazine can effectively change the physicochemical properties of drugs and improve their pharmacokinetic properties and biological activity. Piperazine derivatives can act as histamine and serotonin receptor antagonists in the control of inflammation [10,11,12].
Salicylates are a class of chemicals with favorable anti-inflammatory effects. A large number of studies have indicated that methyl salicylate derivatives exhibited great anti-inflammatory effects [13,14,15]. In the current study, methyl salicylate and piperazine were combined to synthesize a new series of methyl salicylate derivatives, and their anti-inflammation activities were screened in vitro and in vivo.
2. Results
2.1. Synthesis of Salicylate Derivatives Bearing Piperazine Moiety
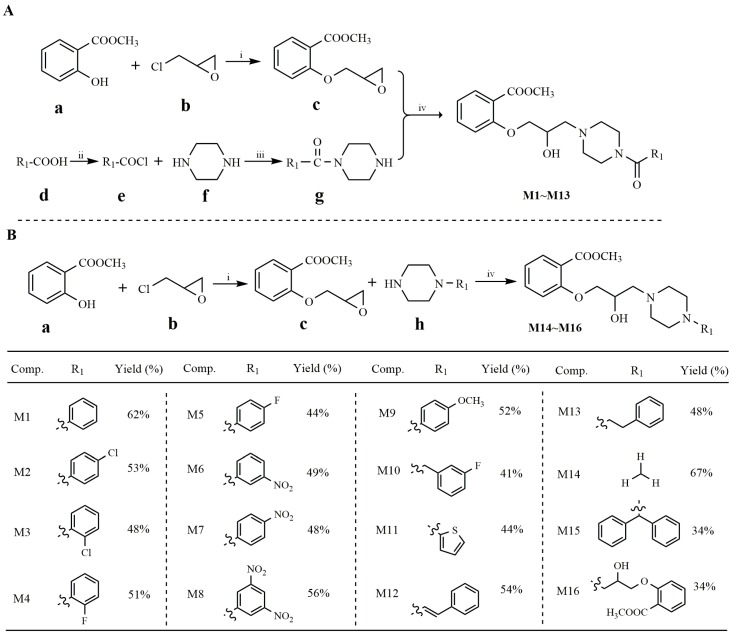
The synthesis of target compounds is outlined in Scheme 1. Methyl salicylate (a) firstly reacted with 3-chloro-1,2-epoxypropane (b) to generate methyl-2-(oxiran-2-ylmethoxy) benzoate (c) (Scheme 1A, upper). Meanwhile, various aryl carboxylic acids (d) reacted with thionyl chloride to obtain the intermediate (e) which subsequently reacted with anhydrous piperazine (f) in the presence of acetic acid at room temperature, generating intermediate (g) (Scheme 1A, bottom). Then, based on combination principles, intermediate c reacted with g to generate products M1–M13 in the presence of toluene, with yields ranging from 41% to 62% (Scheme 1A). For products M14–M16, raw material (h) reacted with methyl-2-(oxiran-2-ylmethoxy) benzoate (c) in the presence of toluene to generate the target methyl salicylate derivatives (M14–M16) (Scheme 1B), with yields ranging from 34% to 67%. All compounds were characterized by microanalytical and spectral data.
Scheme 1.
Synthetic routes of methyl salicylate derivatives. (A) Synthetic routes of compounds M1~M13. (B) Synthetic routes of compounds M14~M16. Reagents and conditions: (i) K2CO3, acetone, reflux, 30 h, yield = 85%; (ii) SOCl2, reflux, 2 h, yield = 71%–87%; (iii) Piperazine, AcOH, r.t., 3 h, yield = 64%–79%; (iv) toluene, reflux, 12 h, yield = 34%–67%.
2.2. In Vivo Anti-Inflammatory Activities of Salicylate Derivatives Bearing Piperazine Moiety
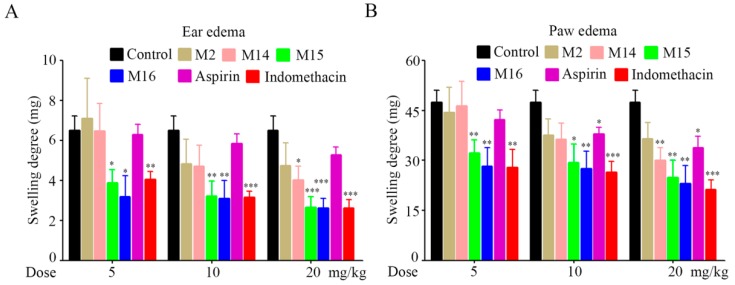
The in vivo anti-inflammatory activities of target compounds were evaluated against xylol-induced ear edema and carrageenan-induced paw edema in mice. All of the target compounds were administered at a dose of 100 mg/kg, and aspirin (100 mg/kg) and indomethacin (5 mg/kg) were used as standard controls. The results indicated that all compounds exhibited favorable anti-inflammatory activity compared to aspirin, except for compound M10 (Table 1). Among the tested compounds, M2, M14, M15, and M16 were much more potent than others, and compound M16 exhibited the strongest anti-inflammatory activity. To further depict the dose-effect relationships of the four compounds (M2, M14, M15, and M16), various doses (5, 10, and 20 mg/kg) of the four compounds, aspirin, and indomethacin were intragastrically administered. The results showed that the anti-inflammatory activities of the four compounds were increased in a dose-dependent manner (Figure 1). Especially, the anti-inflammatory activities of compounds M15 and M16 were better than that of aspirin, and even equal to that of indomethacin at the same dose.
Table 1.
Anti-inflammatory (in vivo) activity of the target compounds against xylol-induced ear edema and carrageenan-induced paw edema in mice.
| Compound | Dose (mg/kg) | Ear Edema | Paw Edema | Dose (mmol/kg) | ||
|---|---|---|---|---|---|---|
| Swelling Degree (mg) | Inhibition (%) | Swelling Degree (mg) | Inhibition (%) | |||
| Control | 8.36 ± 2.50 | - | 94.93 ± 8.46 | - | ||
| M1 | 100 | 2.82 ± 0.56 *** | 66.25 | 59.31 ± 6.25 *** | 37.52 | 0.25 |
| M2 | 100 | 2.29 ± 0.47 *** | 72.65 | 43.44 ± 8.65 *** | 54.24 | 0.23 |
| M3 | 100 | 2.42 ± 0.44 *** | 71.09 | 50.58 ± 7.40 *** | 46.72 | 0.23 |
| M4 | 100 | 2.70 ± 0.49 *** | 67.67 | 54.82 ± 5.03 ** | 42.25 | 0.24 |
| M5 | 100 | 4.61 ± 1.11 ** | 44.82 | 57.98 ± 4.94 ** | 38.92 | 0.24 |
| M6 | 100 | 4.54 ± 1.16 ** | 45.70 | 61.04 ± 5.58 *** | 35.70 | 0.23 |
| M7 | 100 | 5.03 ± 1.77 * | 39.80 | 64.58 ± 6.75 *** | 31.97 | 0.23 |
| M8 | 100 | 4.05 ± 1.55 ** | 51.55 | 59.60 ± 6.86 *** | 37.21 | 0.20 |
| M9 | 100 | 4.63 ± 1.43 ** | 44.65 | 59.22 ± 5.21 *** | 37.62 | 0.23 |
| M10 | 100 | 4.07 ± 1.08 ** | 51.30 | 79.22 ± 7.06 ** | 16.55 | 0.23 |
| M11 | 100 | 3.74 ± 1.13 *** | 55.31 | 49.24 ± 7.79 *** | 48.13 | 0.25 |
| M12 | 100 | 4.31 ± 1.37 ** | 48.40 | 59.91 ± 7.72 *** | 36.89 | 0.24 |
| M13 | 100 | 3.61 ± 1.17 *** | 56.80 | 53.43 ± 6.01 *** | 43.72 | 0.23 |
| M14 | 100 | 2.43 ± 0.75 *** | 70.97 | 43.64 ± 6.37 *** | 54.03 | 0.32 |
| M15 | 100 | 2.13 ± 1.09 *** | 74.48 | 41.42 ± 7.07 *** | 56.37 | 0.24 |
| M16 | 100 | 1.53 ± 0.55 *** | 81.73 | 33.43 ± 6.92 *** | 64.78 | 0.20 |
| Aspirin | 100 | 4.85 ± 1.70 * | 41.96 | 67.24 ± 8.68 *** | 29.16 | 0.56 |
| Indomethacin | 5 | 4.96 ± 1.09 ** | 40.66 | 64.13 ± 8.91 *** | 32.45 | 0.01 |
* p < 0.05, ** p < 0.01, *** p < 0.001 significantly different from the control value.
Figure 1.
Target compounds showed dose-dependent inhibition on (A) ear and (B) paw edema in vivo. * p < 0.05, ** p < 0.01, *** p < 0.001 significantly different from the control value.
Piperazine-derived drugs are considered to be designer drugs which can be divided into two classes: the benzylpiperazines and the phenylpiperazines [16]. Although piperazine-derived drugs have been considered to be safe [16], adverse effects, such as dizziness, headache, hypersensitivity reactions, vomiting, and nausea have been reported from several experimental, clinical, and epidemiological studies [17,18]. Presently, the structure-side-effect relationships of drugs bearing a piperazine moiety with the central nervous system (CNS) have been revealed, indicating that drugs with the least CNS toxicity would be predicted to be those with low γ-aminobutyric acid (GABA)-binding ability and low overall lipophilicity [19]. Considering the potential toxicity of piperazine drugs, it is of great importance to detect their metabolites in humans or animals. Metabolism studies of piperazine designer drugs show that piperazine-derived drugs are mainly metabolized in the liver. The main metabolites are N-(4-hydroxy-3-methoxybenzyl)piperazine for the benzylpiperazines class, and hydroxyl-3-chloroaniline for phenylpiperazines, which finally turned into glucuronides and/or sulfates [16]. Our in vivo study showed that the oral dose of 100 mg/kg did not cause any gross behavioral alterations such as dizziness, respiratory distress, or mortality, indicating that the lethal dose of the target compounds is above 100 mg/kg body weight. Certainly, for future follow ups, we should carry out studies on acute toxicity, pharmacokinetics, and metabolism, especially for compound M16.
2.3. Cytotoxicity Assays of Compounds M2, M14, M15, and M16
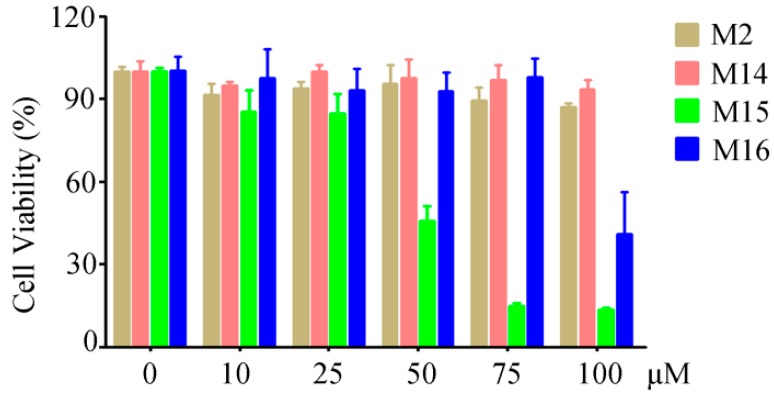
To reveal the cytotoxicity of M2, M14, M15, and M16, a MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was conducted in mouse RAW264.7 macrophages. The results indicated that compounds M2, M14, and M16 showed no significant cytotoxicity with concentrations less than 75 µM (Figure 2). Compound M15 displayed no significant cytotoxicity with concentrations less than 25 µM, but showed a great cytotoxicity at 50 µM (Figure 2). Thus, we used 6.25, 12.5, and 25 µM in the following ELISA (enzyme-linked immunoadsorbent assay) experiments.
Figure 2.
Cytotoxicity activities of M2, M14, M15, and M16 against RAW264.7 macrophages.
2.4. Inhibitory Screening against Lipopolysaccharide (LPS)-Induced Interleukin (IL)-6 and Tumor Necrosis Factor (TNF)-α Release
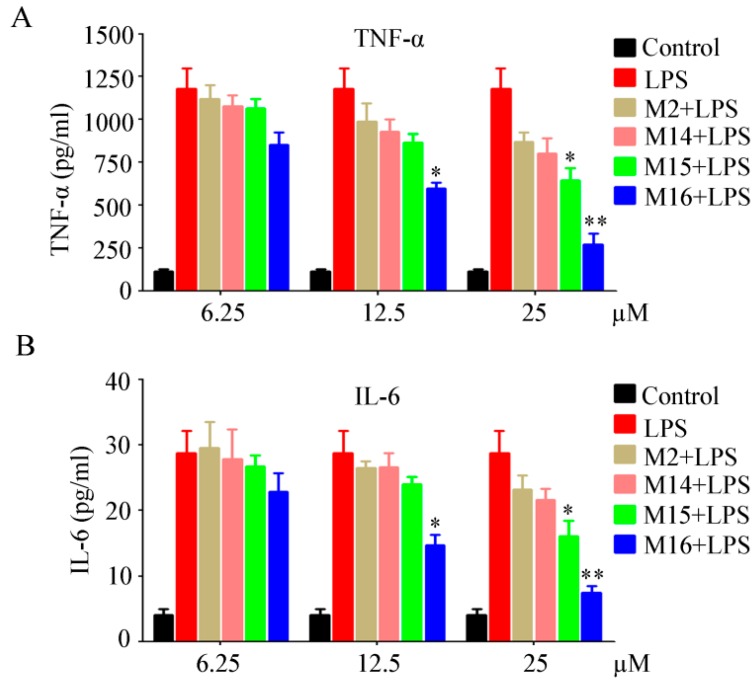
TNF-α and IL-6, which are important pro-inflammatory mediators, can be triggered by lipopolysaccharide (LPS) [20,21]. TNF-α is released in macrophages upon LPS treatment. The secreted TNF-α or LPS subsequently induces the cells to release IL-6 [22]. In this study, the in vitro anti-inflammatory effects of four target compounds were evaluated in the macrophage RAW264.7 cells by measuring the expressions of TNF-α and IL-6 through ELISA assays. RAW264.7 macrophages were pretreated with different doses (6.25, 12.5, and 25 µM) for 2 h and then exposed to LPS (1 µg/mL) for an additional 22 h. The levels of TNF-α and IL-6 in the media were determined. The results displayed that all four target compounds could inhibit the release of TNF-α and IL-6. Especially, compound M16 significantly inhibited TNF-α and IL-6 release in a dose-dependent manner (Figure 3). Compound M15 significantly inhibited TNF-α and IL-6 release at 25 µM. These findings suggested that compounds M15 and M16 exerted their anti-inflammatory effects by suppressing the production of pro-inflammatory factors, which might partly explain their in vivo anti-inflammatory activities.
Figure 3.
Target compounds inhibited lipopolysaccharide (LPS)-induced (A) tumor necrosis factor (TNF)-α and (B) interleukin (IL)-6 release in a dose-dependent manner in vitro. * p < 0.05, ** p < 0.01 significantly different from the LPS value.
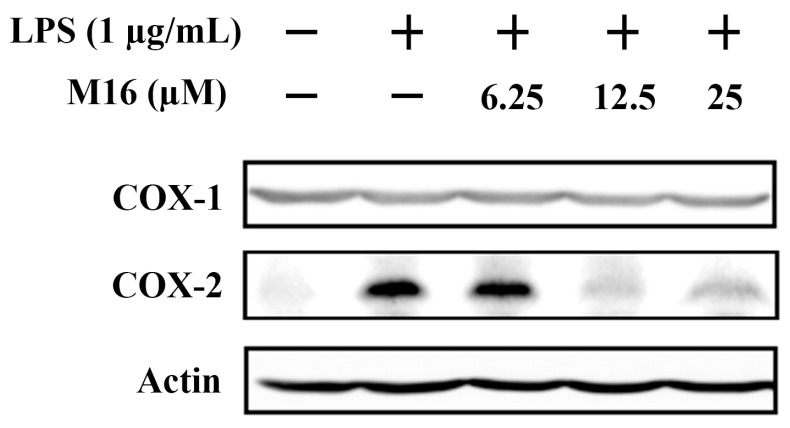
2.5. Compound M16 Attenuates LPS Induced Cyclooxygenase-2 (COX-2) Up-Regulation
COX-2 is recognized as an inducible pro-inflammatory enzyme which is the rate-limiting enzyme of prostanoids synthesis. Many NSAIDs exert their anti-inflammatory activities by inhibiting COX-2 activity [23]. To further elucidate the underlying anti-inflammation mechanism of compound M16, Western blot assays were performed to detect the expression of cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). The results showed that COX-2 was up-regulated in RAW264.7 macrophages upon LPS stimulation. Pretreatment with M16 could attenuate LPS-induced COX-2 up-regulation (Figure 4). These results inferred that compound M16 might exert its anti-inflammatory activities by down-regulating COX-2 expression and inhibiting the release of TNF-α and IL-6.
Figure 4.
Compound M16 attenuated LPS induced cyclooxygenase (COX)-2 up-regulation.
3. Experimental Section
3.1. Synthetic Details of Intermediates and Target Compounds
For intermediate c, 20 mL methyl salicylate (0.15 mol) was dissolved in 250 mL acetone, and then 40 g anhydrous potassium carbonate (0.30 mol) was added into the solution. The solution was kept stirring at room temperature, and 100 mL 3-chloro-1,2-epoxypropane (1.26 mol) was added and stirred at reflux for 30 h at 60 °C. The excess solvent was removed in vacuo, and then the crude product c was dissolved in 200 mL toluene. Then, the solvent was sequentially washed with water (150 mL × 2), 5% sodium hydroxide (200 mL × 2), and water (150 mL × 2). Finally, the organic phase was dried over anhydrous sodium sulfate overnight and then evaporated under vacuum.
For intermediate g, 0.02 mol aryl carboxylic acid was dissolved in 20 mL thionyl chloride, and the solution was stirred at reflux for 4 h. Then, 20 mL anhydrous chloroform was added into the solution, and thionyl chloride was removed in vacuo under reduced pressure to give intermediate e. Subsequently, intermediate e was dissolved in 10 mL chloroform and added dropwise into anthalazine dissolved in acetic acid. The solution was kept stirring at room temperature for 3 h, and then was alkalized in an ice bath with 20% sodium hydroxide till the pH value reached 9~10. Finally, the organic phase was extracted with chloroform (50 mL × 5), dried over anhydrous sodium sulfate overnight, and then evaporated under vacuum.
For products M1–M13, intermediates c and g were dissolved in 80 mL toluene with a material ratio (1:1.1, mol ratio). The solution was stirred at reflux for 10 h at 95 °C. Finally, the excess solvent was evaporated off, and the residues were purified by silica gel-column chromatography with acetic ether/petroleum ether (7:3, v:v) as eluent to afford the corresponding products M1–M13.
For M14 and M15, monosubstituted alkyl piperazine and intermediate c were dissolved in 80 mL toluene with a material ratio (1:1.1, mol ratio). The solution was stirred at reflux for 10 h at 95 °C. Finally, the excess solvent was evaporated off and the residues were filtered by silica gel-column chromatography with acetic ether/petroleum ether (6:4, v:v) as eluent to afford the corresponding products M14–M15.
For M16, anhydrous piperazine and intermediate c were dissolved in 80 mL toluene with a material ratio (1:2.2, mol ratio). The solution was stirred at reflux for 10 h at 95 °C. Finally, the excess solvent was evaporated off and the residues were filtered by silica gel-column chromatography with acetic ether/petroleum ether (6:4, v:v) as eluent to afford the corresponding product M16.
Compounds characterization data:
Methyl 2-(3-(4-benzoylpiperazin-1-yl)-2-hydroxypropoxy)benzoate M1: yellow liquid. IR (KBr, ν cm−1): 3442 (νO-H); 3064 (νAr-H); 2943, 1498, 1444 (νC-H); 1721, 1628 (νC=O); 1H-NMR (CD3Cl, 600 MHz, δ ppm): 2.42~2.67 (m, 6H, -CH2-), 3.44 (m, 2H, -CH2-), 3.80 (m, 2H, -NCH2-), 3.87 (s, 3H, -CH3), 4.02 (m, 2H, -OCH2-), 4.14 (m, 1H, -OH), 4.19 (m, 1H, -CH-), 7.00 (m, 2H, ArH), 7.39 (m, 5H, ArH), 7.45 (m, 1H, ArH), 7.81 (m, 1H, ArH). MS (ESI, m/z): 399.35 ([M + H]+). Elemental analysis: calcd. for C22H26N2O5: C, 66.32; H, 6.58; N, 7.03; Found: C, 66.38; H, 6.90; N, 6.56.
Methyl 2-(3-(4-(4-chlorobenzoyl)piperazin-1-yl)-2-hydroxypropoxy)benzoate M2: yellow liquid. IR (KBr, ν cm−1): 3431 (νO-H); 3055 (νAr-H); 2943, 1498, 1445 (νC-H); 1718 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.57~2.68 (m, 6H, -CH2-), 3.44 (m, 2H, -CH2-), 3.79 (m, 2H, -NCH2-), 3.87 (s, 3H, -CH3), 4.02 (m, 2H, -OCH2-), 4.18~4.21 (m, 2H, -CH(OH)-), 7.01 (m, 2H, ArH), 7.34 (d, J = 8.4 Hz, 2H, ArH), 7.38 (d, J = 8.4 Hz, 2H, ArH),7.46 (m, 1H, ArH), 7.81 (t, J = 3.9 Hz, 1H, ArH). MS (ESI, m/z): 433.35 ([M + H]+). Elemental analysis: calcd. for C22H25ClN2O5: C, 61.04; H, 5.82; N, 6.47; Found: C, 61.08; H, 5.84; N, 6.49.
Methyl 2-(3-(4-(2-chlorobenzoyl)piperazin-1-yl)-2-hydroxypropoxy)benzoate M3: yellow liquid. IR (KBr, ν cm−1): 3439 (νO-H); 3072 (νAr-H); 2934, 1486, 1453 (νC-H); 1727, 1633 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.57~2.63 (m, 6H, -CH2-), 3.18~3.32 (m, 2H, -CH2-), 3.71 (m, 2H, -CH2-), 3.85 (s, 3H, -CH3), 4.02 (d, J = 7.2 Hz, 2H, -CH2-), 4.12 (m, 1H, -OH), 4.16 (d, J = 2.4 Hz, 1H, -CH-), 6.98 (m, 2H, ArH), 7.26~7.32 (m, 3H, ArH), 7.37 (m, H, ArH), 7.44 (t, J = 7.8 Hz, 1H, ArH), 7.79 (m, 1H, ArH). MS (ESI, m/z): 433.29 ([M + H]+). Elemental analysis: calcd. for C22H25ClN2O5: C, 61.04; H, 5.82; N, 6.47; Found: C, 61.02; H, 5.88; N, 6.44.
Methyl 2-(3-(4-(2-fluorobenzoyl)piperazin-1-yl)-2-hydroxypropoxy)benzoate M4: yellow liquid. IR (KBr, ν cm−1): 3456 (νO-H); 3080 (νAr-H); 2942, 1498, 1427 (νC-H); 1735, 1642 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.47~2.62 (m, 6H, -CH2-), 3.30 (m, 2H, -CH2-), 3.77 (m, 2H, -CH2-), 3.81 (s, 3H, -CH3), 3.98 (m, 1H, -OH), 4.11~4.15 (m, 3H, -CHCH2-), 6.95 (t, J = 7.5 Hz, 2H, ArH), 7.03 (t, J = 9 Hz, 1H, ArH), 7.15 (t, J = 7.5 Hz, 1H, ArH), 7.32 (m, 2H, ArH), 7.41 (t, J = 7.8 Hz, 1H, ArH), 7.52 (d, J = 7.8 Hz, 1H, ArH). MS (ESI, m/z): 417.33 ([M + H]+). Elemental analysis: calcd. for C22H25FN2O5: C, 63.45; H, 6.05; N, 6.73; Found: C, 63.48; H, 6.01; N, 6.6.
Methyl 2-(3-(4-(4-fluorobenzoyl)piperazin-1-yl)-2-hydroxypropoxy)benzoate M5: yellow liquid. IR (KBr, ν cm−1): 3439 (νO-H); 3072 (νAr-H); 2942, 1506, 1452 (νC-H); 1718, 1642 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.50~2.70 (m, 6H, -CH2-), 3.41 (m, 2H, -CH2-), 3.75 (m, 2H, -CH2-), 3.84 (s, 3H, -CH3), 3.99 (m, 1H, -CH-), 4.07~4.14 (m, 2H, -CH2-), 4.17 (m, 1H, -OH), 6.97 (m, 2H, ArH), 7.05 (t, J = 8.4 Hz, 2H, ArH), 7.38 (m, 2H, ArH), 7.43 (t, J = 7.8 Hz, 1H, ArH), 7.78 (d, J = 7.8 Hz, 1H, ArH). MS (ESI, m/z): 417.26 ([M + H]+). Elemental analysis: calcd. for C22H25FN2O5: C, 63.45; H, 6.05; N, 6.73; Found: C, 63.51; H, 6.08; N, 6.78.
Methyl 2-(2-hydroxy-3-(4-(3-nitrobenzoyl)piperazin-1-yl)propoxy)benzoate M6: yellow liquid. IR (KBr, ν cm−1): 3448 (νO-H); 3080 (νAr-H); 2925, 1532, 1452 (νC-H); 1726, 1642 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.54~2.70 (m, 6H, -CH2-), 3.42 (m, 2H, -CH2-), 3.81 (m, 2H, -CH2-), 3.85 (s, 3H, -CH3), 4.01 (t, J = 7.5 Hz, 1H, -CH-), 4.08 (d, J = 3.6 Hz, 1H, -OH), 4.14~4.19 (m, 2H, -CH2-), 6.98 (t, J = 9.33 Hz, 2H, ArH), 7.44 (t, J = 7.8 Hz, 1H, ArH), 7.59 (t, J = 7.8 Hz, 1H, ArH), 7.72 (d, J = 7.8 Hz, 1H, ArH), 7.79 (d, J = 7.8 Hz, 1H, ArH), 8.25 (d, J = 8.4 Hz, 2H, ArH). MS (ESI, m/z): 444.12 ([M + H]+). Elemental analysis: calcd. for C22H25N3O7: C, 59.59; H, 5.68; N, 9.48; Found: C, 59.62; H, 5.65; N, 9.52.
Methyl 2-(2-hydroxy-3-(4-(4-nitrobenzoyl)piperazin-1-yl)propoxy)benzoate M7: yellow liquid. IR (KBr, ν cm−1): 3439 (νO-H); 3080 (νAr-H); 2942, 1524, 1444 (νC-H); 1727, 1634 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.58~2.74 (m, 6H, -CH2-), 3.38 (m, 2H, -CH2-), 3.81 (m, 2H, -CH2-), 3.86 (s, 3H,-CH3), 4.02 (m, 1H, -OH), 4.10~4.21 (m, 3H, -CHCH2-), 7.01 (m, 2H, ArH), 7.46 (m, 1H, ArH), 7.56 (m, 2H, ArH), 7.81 (m, 1H, ArH), 8.26 (m, 2H, ArH). MS (ESI, m/z): 444.14 ([M + H]+). Elemental analysis: calcd. for C22H25N3O7: C, 59.59; H, 5.68; N, 9.48; Found: C, 59.63; H, 5.64; N, 9.53.
Methyl 2-(3-(4-(3,5-dinitrobenzoyl)piperazin-1-yl)-2-hydroxypropoxy)benzoate M8: yellow liquid. IR (KBr, ν cm−1): 3456 (νO-H); 3097 (νAr-H); 2926, 1548, 1445 (νC-H); 1727, 1650 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.61~2.78 (m, 6H, -CH2-), 3.45 (m, 2H, -CH2-), 3.84 (m, 2H, -CH2-), 3.87 (s, 3H, -CH3), 4.02~4.07 (m, 2H, -CH2-), 4.17 (s, 1H, -CH-), 4.22 (m, 1H, -OH), 7.02 (m, 2H ArH,), 7.26 (s, 1H, ArH), 7.48 (t, J = 7.8 Hz, 1H, ArH), 7.82 (d, J = 7.8 Hz, 1H, ArH), 8.60 (s, 2H, ArH), 9.09 (s, 1H, ArH). MS (ESI, m/z): 489.28 ([M + H]+). Elemental analysis: calcd. for C22H24N4O9: C, 54.10; H, 4.95; N, 11.47; Found: C, 54.12; H, 4.98; N, 11.44.
Methyl 2-(2-hydroxy-3-(4-(4-methoxybenzoyl)piperazin-1-yl)propoxy)benzoate M9: yellow liquid. IR (KBr, ν cm−1): 3431 (νO-H); 3072 (νAr-H); 2942, 1515, 1446 (νC-H); 1727, 1625 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.48~2.70 (m, 8H, -CH2-), 3.79 (m, 3H, -CH3), 3.84 (s, 3H, CH3), 4.00 (m, 1H, -OH), 4.15 (m, 3H, -CHCH2-), 6.87 (d, J = 7.8 Hz, 2H, ArH), 6.97 (t, J = 8.1 Hz, 2H, ArH), 7.34 (d, J = 7.8 Hz, 2H, ArH), 7.43 (t, J = 7.8 Hz, 1H, ArH), 7.78 (d, J = 7.8 Hz, 1H, ArH). MS (ESI, m/z): 429.30 ([M + H]+). Elemental analysis: calcd. for C23H28N2O6: C, 64.47; H, 6.59; N, 6.54; Found: C, 64.43; H, 6.54; N, 6.58.
Methyl 2-(3-(4-(2-(3-fluorophenyl)acetyl)piperazin-1-yl)-2-hydroxypropoxy)benzoate M10: yellow liquid. IR (KBr, ν cm−1): 3448 (νO-H); 3074 (νAr-H); 2951, 1486, 1445 (νC-H); 1718, 1642 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.36~2.61 (m, 6H, -CH2-), 3.42 (t, J = 5.1 Hz, 2H, -CH2-), 3.58~3.70 (m, 5H, -CHCH2-,-CH2-), 3.83 (s, 3H, -CH3), 3.98 (m, 1H, -OH), 4.08~4.15 (m, 2H, -CH2-), 6.89~6.98 (m, 5H, ArH), 7.24 (d, J = 6.0 Hz, 1H, ArH), 7.42 (m, 1H, ArH), 7.78 (t, J = 3.9 Hz, 1H, ArH). MS (ESI, m/z): 431.40 ([M + H]+). Elemental analysis: calcd. for C23H27FN2O5: C, 64.17; H, 6.32; N, 6.51; Found: C, 64.15; H, 6.38; N, 6.48.
Methyl 2-(2-hydroxy-3-(4-(thiophene-2-carbonyl)piperazin-1-yl)propoxy)benzoate M11: yellow liquid. IR (KBr, ν cm−1): 3447 (νO-H); 3089 (νAr-H); 2934, 1523, 1452 (νC-H); 1727, 1625 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.53~2.64 (m, 6H, -CH2-), 3.68~3.80 (m, 4H, -CH2-), 3.83 (s, 3H, -CH3), 4.00 (m, 1H, -OH), 4.05~4.18 (m, 3H, -CHCH2-), 6.98 (m, 3H, ArH), 7.24 (m, 1H, ArH), 7.40 (m, 2H, ArH), 7.77 (m, 1H, ArH). MS (ESI, m/z): 405.16 ([M + H]+). Elemental analysis: calcd. for C20H24N2O5S: C, 59.39; H, 5.98; N, 6.93; Found: C, 59.44; H, 6.02; N, 6.89.
Methyl 2-(3-(4-cinnamoylpiperazin-1-yl)-2-hydroxypropoxy)benzoate M12: yellow liquid. IR (KBr, ν cm−1): 3448 (νO-H); 3097 (νAr-H); 2934, 1527, 1453 (νC-H); 1718, 1642 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.50~2.68 (m, 6H, -CH2-), 3.50~3.78 (m, 4H, -CH2-), 3.86 (s, 3H, -CH3), 4.01~4.12 (m, 2H, -CH(OH)-), 4.15~4.21 (m, 2H, -CH2-), 6.85 (d, J = 15.6 Hz, 1H, ArH), 6.97~7.00 (t, J = 7.2 Hz, 2H, -CH = CH-), 7.31~7.36 (m, 3H, ArH), 7.44 (t, J = 7.8 Hz, 1H, ArH), 7.49 (d, J = 7.2 Hz, 2H, ArH), 7.64 (d, J = 15 Hz, 1H, ArH), 7.80 (d, J = 7.8 Hz, H, ArH). MS (ESI, m/z): 425.31 ([M + H]+). Elemental analysis: calcd. for C24H28N2O5: C, 67.91; H, 6.65; N, 6.60; Found: C, 67.88; H, 6.64; N, 6.64.
Methyl 2-(2-hydroxy-3-(4-(3-phenylpropanoyl)piperazin-1-yl)propoxy)benzoate M13: yellow liquid. IR (KBr, ν cm−1): 3456 (νO-H); 3038 (νAr-H); 2951, 1498, 1452 (νC-H); 1718, 1642 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.41~2.61 (m, 8H, -CH2-), 2.95 (t, J = 7.8 Hz, 2H, -CH2-), 3.8 (t, J = 5.1 Hz, 2H, -CH2-), 3.63 (m, 2H, -CH2-), 3.86 (s, 3H, -CH3), 4.01 (m, 1H, -OH), 4.10~4.19 (m, 3H, -CHCH2-), 6.98 (m, 2H, ArH), 7.19 (t, J = 8.7 Hz, 3H, ArH), 7.27 (t, J = 7.5 Hz, 2H, ArH), 7.45 (m, 1H, ArH), 7.80 (d, J = 7.2 Hz, 1H, ArH). MS (ESI, m/z): 427.34 ([M + H]+). Elemental analysis: calcd. for C24H30N2O5: C, 67.59; H, 7.09; N, 6.57; Found: C, 67.56; H, 7.06; N, 6.61.
Methyl 2-(2-hydroxy-3-(4-methylpiperazin-1-yl)propoxy)benzoate M14: yellow liquid. IR (KBr, ν cm−1): 3439 (νO-H); 3072 (νAr-H); 2951, 1486, 1453 (νC-H); 1718, 1608 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.26 (s, 3H, -CH3), 2.30~2.70 (m, 10H, -CH2-), 3.85 (s, 3H, -CH3), 4.00 (m, 1H, -OH), 4.10~4.14 (m, 3H, -CHCH2-), 6.97 (m, 2H, ArH), 7.43 (t, J = 7.8 Hz, 1H, ArH), 7.79 (m, 1H, ArH). MS (ESI, m/z): 309.20 ([M + H]+). Elemental analysis: calcd. for C16H24N2O4: C, 62.32; H, 7.84; N, 9.08; Found: C, 62.29; H, 7.88; N, 9.04.
Methyl 2-(3-(4-benzhydrylpiperazin-1-yl)-2-hydroxypropoxy)benzoate M15: yellow liquid. IR (KBr, ν cm−1): 3456 (νO-H); 3114 (νAr-H); 2942, 1489, 1452 (νC-H); 1718, 1642 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.36~2.70 (m, 10H, -CH2-), 3.87 (s, 3H, -CH3), 3.93~4.08 (m, 2H, -CH(OH)-), 4.09~4.16 (m, 2H, -CH2-), 4.23 (s, 1H, -CH-), 6.99 (t, J = 7.5 Hz, 2H, ArH), 7.17 (t, J = 7.8 Hz, 2H, ArH), 7.27 (m, 4H, ArH), 7.40~7.46 (m, 5H, ArH), 7.81 (d, J = 7.8 Hz, 1H, ArH). MS (ESI, m/z): 416.43 ([M + H]+). Elemental analysis: calcd. for C28H32N2O4: C, 73.02; H, 7.00; N, 6.08; Found: C: 73.08; H, 7.03; N, 6.11.
Dimethyl 2,2′-((piperazine-1,4-diylbis(2-hydroxypropane-3,1-diyl))bis(oxy))dibenzoate M16: yellow liquid. IR (KBr, ν cm−1): 3430 (νO-H); 3080 (νAr-H); 2934, 1506, 1453 (νC-H); 1727, 1633 (νC=O); 1H-NMR (CD3Cl, δ ppm): 2.54~2.72 (m, 2 × 6H, -CH2-), 3.85 (s, 2 × 3H, -CH3), 4.00 (t, J = 4.5 Hz, 2 × 1H, -OH), 4.13 (m, 2 × 2H, -CH2-), 6.97 (m, 2 × 2H, ArH), 7.42 (m, 2 × 1H, ArH), 7.79 (m, 2 × 1H, ArH). MS (ESI, m/z): 503.35 ([M + H]+). Elemental analysis: calcd. for C26H34N2O8: C, 62.14; H, 6.82; N, 5.57; Found: C, 62.18; H, 6.79; N, 5.54.
3.2. Cell Culture and Cytotoxicity Assays
Mouse RAW264.7 macrophages were purchased from American Type Culture Collection (ATCC) and cultured in DMEM (Dulbecco modified Eagle medium) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin–streptomycin at 37 °C, in an atmosphere of 95% air and 5% CO2 under humidified conditions. For cytotoxicity assay, cells were seeded in 96-well plates at the density of 2 × 104 cells per well and incubated for 16 h. The medium was removed, and the cells were then incubated with different concentrations of synthesized compounds for indicated times, followed by 2–3 h treatment with MTT. After MTT removal, 150 µL dimethyl sulfoxide (DMSO) was added into each well and absorption values was measured at 490 nm using a SpectraMAX M5 plate reader (Molecular Devices Inc. San Francisco, CA, USA). Three independent experiments were conducted, and samples were analyzed in triplicate for each experiment.
3.3. In Vivo Anti-Inflammatory Activity Assays in Ear and Paw Edema
The animal studies were approved by the Institutional Review Board of Guangxi Medical University. All animal studies were conducted according to protocols approved by the Animal Ethics Committee of Guangxi Medical University (Nanning, China). The anti-inflammatory activity was evaluated on xylene-induced ear edema and carrageenan-induced mice paw edema [24]. Briefly, the mice (20 ± 2 g, 10/group) were fasted with free access to water for at least 16 h, and then randomly divided into two groups: the dosage and control groups. Ear edema and paw edema was induced by applying xylene on the left ear and subcutaneous injection of carrageenan in the left toe of each mouse, respectively. One hour before edema induction, the mice were intragastrically administered with target drugs in 1% carboxymethyl cellulose solution or control solvent. Three hours later, the mice were euthanized by cervical dislocation, and right ears or paws were cut and weighed for swelling degree and swelling inhibition calculation.
3.4. ELISA Assay
Mouse RAW264.7 macrophages were pretreated with compounds (M2, M14, M15, and M16) or vehicle control for 2 h, then treated with LPS (1 µg/mL) for 22 h. After treatment, the cells and media were collected separately. The levels of IL-6 and TNF-α in the media were determined using ELISA kit (eBioscience, San Diego, CA, USA). The total protein was extracted from the collected cells and determined using Bio-Rad protein assay. The amount of IL-6 and TNF-α was normalized to the total protein amount.
3.5. Western Blot Assays
For Western blot assay, cells were lysed in RIPA (Radio-Immunoprecipitation Assay) buffer supplemented with protease inhibitors. Proteins (20 μg) were subjected to 6%–15% SDS-PAGE, electrophoresed, and transferred on to a nitrocellulose membrane. After blocking with 5% non-fat milk in tris-buffered saline, the membrane was washed and incubated with the indicated primary and secondary antibodies and detected using the Luminescent Image Analyser LSA 4000 (GE, Fairfield, CO, USA).
3.6. Statistical Analysis
The data are presented as the mean ± SD. Differences between data groups were evaluated for significance using Student’s t-test of unpaired data. p values < 0.05 were considered statistically significant.
4. Conclusions
In summary, a new series of methyl salicylate derivatives were synthesized and characterized. The in vivo anti-inflammatory results revealed that all target compounds exhibited potent anti-inflammatory activity compared to aspirin, except compound M10. In addition, compounds M2, M14, M15, and M16 exhibited strong anti-inflammatory activity in a dose-dependent manner in vivo. Especially, the anti-inflammatory activities of compounds M15 and M16 were higher than that of aspirin, and even equal to that of indomethacin at the same dose. Furthermore, compound M16 could significantly inhibit LPS-induced IL-6 and TNF-α release and down-regulate the expression of COX-2 in a dose-dependent manner. Our findings may provide information on potentially new and safe anti-inflammatory agents for further studies.
Acknowledgments
The work was supported by the National Natural Science Foundation of Zhejiang Province of China (No. Y12H300003), the high level innovation team and outstanding scholar project of Guangxi institutions of higher education (gui jiao ren [2014] 49 hao), and the Postdoctoral Science Foundation of Guangxi Province of China (Y201001928).
Author Contributions
The project was conceived by Lichuan Wu and Hua Yang. The experiments were designed by Lichuan Wu. The experiments were conducted by Jingfen Li, Yong Yin, Lichuan Wu, Pengyun Liang, Hua Yang, Menghua Li, and Xu Liu The manuscript was written by Lisheng Wang and Lichuan Wu.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds M2, M14, M15, and M16 are available from the authors.
References
- 1.Ferrero-Miliani L., Nielsen O.H., Andersen P.S., Girardin S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. Clin. Exp. Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilg H., Moschen A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 3.Melnikova I. Pain market. Nat. Rev. Drug Discov. 2010;9:589–590. doi: 10.1038/nrd3226. [DOI] [PubMed] [Google Scholar]
- 4.Velazquez C.A., Chen Q.H., Citro M.L., Keefer L.K., Knaus E.E. Second-generation aspirin and indomethacin prodrugs possessing an O2-(acetoxymethyl)-1-(2-carboxypyrrolidin-1-yl)diazenium-1,2-diolate nitric oxide donor moiety: Design, synthesis, biological evaluation, and nitric oxide release studies. J. Med. Chem. 2008;51:1954–1961. doi: 10.1021/jm701450q. [DOI] [PubMed] [Google Scholar]
- 5.Dannhardt G., Kiefer W., Kramer G., Maehrlein S., Nowe U., Fiebich B. The pyrrole moiety as a template for COX-1/COX-2 inhibitors. Eur. J. Med. Chem. 2000;35:499–510. doi: 10.1016/S0223-5234(00)00150-1. [DOI] [PubMed] [Google Scholar]
- 6.Dubost J.J., Soubrier M., Sauvezie B. Treatment of rheumatoid polyarthritis: Evolution of concepts and strategies. Rev. Med. Interne. 1999;20:171–178. doi: 10.1016/S0248-8663(99)83037-9. [DOI] [PubMed] [Google Scholar]
- 7.Uchoa Fde T., da Silva T.G., de Lima Mdo C., Galdino S.L., Pitta Ida R., Dalla Costa T. Preclinical pharmacokinetic and pharmacodynamic evaluation of thiazolidinone PG15: An anti-inflammatory candidate. J. Pharm. Pharmacol. 2009;61:339–345. doi: 10.1211/jpp.61.03.0008. [DOI] [PubMed] [Google Scholar]
- 8.Nagarapu L., Mateti J., Gaikwad H.K., Bantu R., Sheeba Rani M., Prameela Subhashini N.J. Synthesis and anti-inflammatory activity of some novel 3-phenyl-N-[3-(4-phenylpiperazin-1yl)propyl]-1H-pyrazole-5-carboxamide derivatives. Bioorg. Med. Chem. Lett. 2011;21:4138–4140. doi: 10.1016/j.bmcl.2011.05.105. [DOI] [PubMed] [Google Scholar]
- 9.Malandrino S., Melillo G., Bestetti A., Borsa M., Giuliani P., Tonon G.C. Antitussive properties of levodropropizine. Arzneim. Forsch. 1988;38:1141–1143. [PubMed] [Google Scholar]
- 10.Chen Y., Wang G., Xu X., Liu B.F., Li J., Zhang G. Design, synthesis and biological activity evaluation of arylpiperazine derivatives for the treatment of neuropathic pain. Molecules. 2011;16:5785–5806. doi: 10.3390/molecules16075785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang W., Lim H.D., Zhang M., Desai P., Dai H., Colling P.M., Leurs R., Thurmond R.L. Cloning and pharmacological characterization of the dog histamine H4 receptor. Eur. J. Pharmacol. 2008;592:26–32. doi: 10.1016/j.ejphar.2008.06.095. [DOI] [PubMed] [Google Scholar]
- 12.Thurmond R.L., Desai P.J., Dunford P.J., Fung-Leung W.P., Hofstra C.L., Jiang W., Nguyen S., Riley J.P., Sun S., Williams K.N., et al. A potent and selective histamine H4 receptor antagonist with anti-inflammatory properties. J. Pharmacol. Exp. Ther. 2004;309:404–413. doi: 10.1124/jpet.103.061754. [DOI] [PubMed] [Google Scholar]
- 13.Mao P., Liu Z., Xie M., Jiang R., Liu W., Wang X., Meng S., She G. Naturally occurring methyl salicylate glycosides. Mini Rev. Med. Chem. 2014;14:56–63. doi: 10.2174/1389557513666131211110004. [DOI] [PubMed] [Google Scholar]
- 14.Xin W., Huang C., Zhang X., Xin S., Zhou Y., Ma X., Zhang D., Li Y., Zhou S., Zhang D., et al. Methyl salicylate lactoside inhibits inflammatory response of fibroblast-like synoviocytes and joint destruction in collagen-induced arthritis in mice. Br. J. Pharmacol. 2014;171:3526–3538. doi: 10.1111/bph.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X., Sun J., Xin W., Li Y., Ni L., Ma X., Zhang D., Zhang D., Zhang T., Du G. Anti-inflammation effect of methyl salicylate 2-O-β-d-lactoside on adjuvant induced-arthritis rats and lipopolysaccharide (LPS)-treated murine macrophages RAW264.7 cells. Int. Immunopharmacol. 2015;25:88–95. doi: 10.1016/j.intimp.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Arbo M.D., Bastos M.L., Carmo H.F. Piperazine compounds as drugs of abuse. Drug Alcohol. Depend. 2012;122:174–185. doi: 10.1016/j.drugalcdep.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Maurer H.H., Kraemer T., Springer D., Staack R.F. Chemistry, pharmacology, toxicology, and hepatic metabolism of designer drugs of the amphetamine (ecstasy), piperazine, and pyrrolidinophenone types: A synopsis. Ther. Drug Monit. 2004;26:127–131. doi: 10.1097/00007691-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Anonymous. Drugs for parasitic infections. Med. Lett. Drugs Ther. 1993;35:111–122. [PubMed] [Google Scholar]
- 19.Domagala J.M. Structure-activity and structure-side-effect relationships for the quinolone antibacterials. J. Antimicrob. Chemother. 1994;33:685–706. doi: 10.1093/jac/33.4.685. [DOI] [PubMed] [Google Scholar]
- 20.Corriveau C.C., Danner R.L. Endotoxin as a therapeutic target in septic shock. Infect. Agents Dis. 1993;2:35–43. [PubMed] [Google Scholar]
- 21.Hayden M.S., Ghosh S. Signaling to NF-κB. Genes. Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 22.West M.A., Seatter S.C., Bellingham J., Clair L. Mechanisms of reprogrammed macrophage endotoxin signal transduction after lipopolysaccharide pretreatment. Surgery. 1995;118:220–228. doi: 10.1016/S0039-6060(05)80327-7. [DOI] [PubMed] [Google Scholar]
- 23.Yuan C., Rieke C.J., Rimon G., Wingerd B.A., Smith W.L. Partnering between monomers of cyclooxygenase-2 homodimers. Proc. Natl. Acad. Sci. USA. 2006;103:6142–6147. doi: 10.1073/pnas.0601805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winter C.A., Risley E.A., Nuss G.W. Carrageenin-induced edema in hind paw of the rat as an assay for anti-iflammatory drugs. Exp. Biol. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]