Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a frequent adverse effect of neurotoxic anticancer medicines. It leads to autonomic and somatic system dysfunction and decreases the patient’s quality of life. This side effect eventually causes chemotherapy non-compliance. Patients are prompted to seek alternative treatment options since there is no conventional remedy for CIPN. A range of medicinal herbs have multifarious effects, and they have shown some evidence of efficacy in various neurological and immunological diseases. While CIPN has multiple mechanisms of neurotoxicity, these phytomedicines might offer neuronal protection or regeneration with the multiple targets in CIPN. Thus far, researchers have investigated the therapeutic benefits of several herbs, herbal formulas, and phytochemicals in preventing the onset and progress of CIPN in animals and humans. Here, we summarize current knowledge regarding the role of phytochemicals, herb extracts, and herbal formulas in alleviating CIPN.
Keywords: phytochemical, medicinal herb, chemotherapy-induced peripheral neuropathy
1. Introduction
Several antineoplastic medicines are reported to cause neurotoxicity and can develop chemotherapy-induced peripheral neuropathy (CIPN) [1]. These drugs have effects on sensory nerves and cause substantial pain, dysfunction, and finally chemotherapy non-compliance [2,3]. This adverse effect damages peripheral nerves and can lead to sensory deficits, gait impairment [4], or severe neuropathic pain [5], and can severely degrade the patient’s quality of life [6]. The most common symptoms reported by patients include sensory symptoms such as numbness, burning, tingling, throbbing, and burning feelings. Moreover, patients may experience motor symptoms, such as dropping items, splaying fingers, and inability to complete normal daily activities [7,8].
CIPN is difficult to prevent and control without dose-reduction or cessation of anticancer drugs [9]. The overall incidence of this adverse effect is remarkably high [10], although the population affected depends on chemotherapy drugs, dose, and exposure time [11,12]. Usually the symptoms of CIPN are reversible; however, sometimes symptoms are irreversible [13] and worsen after withdrawal of drugs, including vincristine, cisplatin, oxaliplatin, or paclitaxel [14,15,16].
Thus far, various pharmacological tactics have been attempted to attenuate CIPN symptoms. These medications include acetyl-l-carnitine, amifostine, glutathione, glutamine, vitamin E, PARP inhibitors, leukemia inhibitory factor, N-acetylcysteine, Ca/Mg, and venlafaxine [17,18,19]. The therapeutic potentials of these drugs are limited by unexpected adverse effects and contradictory results, although these drugs have shown benefits in preventing CIPN [20,21]. Still, no approach has sufficient evidence for recommending use in CIPN treatment. Hence, alternative methods of preventing or treating CIPN are necessary.
Medicinal herbs have been used as therapeutics for centuries throughout the world. Phytochemicals derived from these medicinal plants are used to treat various neurological and immunological disorders. On the basis of recent literature, several phytochemicals, herbs, and herbal formulas exhibiting promising effects on CIPN have been identified. Here, we summarize the therapeutic effects of phytochemicals (see also Table 1), medicinal herbs (Table 2), and herbal formulas (Table 3) against CIPN induced by vincristine, cisplatin, oxaliplatin, and paclitaxel.
Table 1.
Phytochemicals against CIPN.
| Phytochemical | Dose | Chemotherapy | Effects | Refs. |
|---|---|---|---|---|
Auraptenol 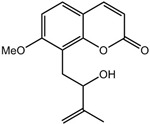
|
0.05–0.8 mg/kg | Vincristine in mice | Suppresses mechanical hyperalgesia and alteration of behavioral and biochemical changes | [22] |
Cannabidiol 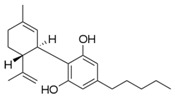
|
2.5–10 mg/kg | Paclitaxel in mice | Inhibits neuropathic pain through 5-HT1A receptor signaling without diminishing chemothferapy efficacy or nervous system function | [23] |
Curcumin 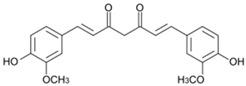
|
10 mg/kg | Cisplatin or oxaliplatin in rats | Reverses the alterations of neurotensin levels in the plasma, protects the sciatic nerve from injury, and reduces drug absorption in the sciatic nerve | [24] |
Rutin 
& quercetin 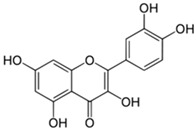
|
25–100 mg/kg | Oxaliplatin in rats | prevent the shrinkage of neurons and inhibit edema | [25] |
Verticinone 
|
1.5–3 mg/kg | Paclitaxel in rats | Has a relatively constant analgesic effect; the analgesic effect of morphine was decreased after repeated medication | [26] |
Xylopic acid 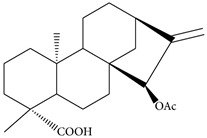
|
10–100 mg/kg | Vincristine in rats | Has anti-allodynic and anti-hyperalgesic properties | [27] |
Table 2.
Medicinal herbs against CIPN.
| Herbs | Dose | Chemotherapy | Effects | Refs. |
|---|---|---|---|---|
| Acorus calamus | 100–200 mg/kg | Vincristine in rats | Attenuates symptoms of neuropathy through serotonin 5-HT1A receptors | [28] |
| Butea monosperma | 200–400 mg/kg | Vincristine in rats | Reverses alterations of behavioral, biochemical, and histopathological changes | [29] |
| Ginkgo biloba L. | 50–150 mg/kg | Vincristine in rats | Decreases paw-withdrawal frequency to cold stimuli and increases the threshold to mechanical stimuli Suppresses NF-κB activation and production of TNF-α and NO Inhibits axonal degradation Improves axonal transportation |
[30] |
| 100–200 mg/kg | Cisplatin in rats, mice, guinea pigs | Protects the inner ear from ototoxicity | [31] | |
| Camellia sinensis | 300 mg/kg | Oxaliplatin in rats | Alleviates mechanical allodynia and thermal hyperalgesia, but does not prevent morphometric or electrophysiological alterations | [32] |
| Ocimum sanctum | 100–200 mg/kg | Vincristine in rats | Attenuates neurotoxicity with the decline in calcium levels and oxidative stress | [33] |
| Salvia officinalis | 100 mg/kg | Cisplatin in mice, | Suppresses a second phase of cisplatin-enhanced pain in the formalin test | [34] |
| Walnut | 6% of diet | Cisplatin in rats | Inhibits an alteration in performance of hippocampus- and cerebellum-related behaviors | [35] |
| Xylopia aethiopica | 30–300 mg/kg | Vincristine in rats | Has anti-allodynic and anti-hyperalgesic properties | [27] |
Table 3.
Herbal formulas against CIPN.
| Herbal Formula | Herbs Composition | Chemotherapy | Effects | Refs. |
|---|---|---|---|---|
| Gyejigachulbu-Tang | Aconiti tuber, Atractylodis lanceae rhizome, Cinnamomi cortex, Glycyrrhizae radix, Paeoniae radix, Zingiberis rhizoma, Zizyphi fructus | Oxaliplatin in rats | Attenuates cold and mechanical allodynia Suppresses spinal glia activation |
[36] |
| Gyeryongtongrac-Bang | Ramulus cinnamomi, Earthworm, Radix astragali, Safflower, Radix angelicae sinensis, Ligusticum, Spatholobus, Radix paeoniae alba, Rhizoma curcumae, Licorice | Oxaliplatin in a randomized, double-blind, placebo-controlled trial | Prevents sensory neurotoxicity and delays its onset | [37] |
| Hwanggiomul-Tang | Zingiberis hizome, Jujubae fructus, Paeonia alba radix, Cinnamomi cortex, Astragalus membranaceus radix | A case study of oxaliplatin-treated 59-year-old man with recurrent colon cancer | Prevents chronic cumulative neurotoxicity | [38] |
| Jakyakgamcho-Tang | Paeoniae radix, Glycyrrhizae radix | Paclitaxel in mice | Relieves allodynia and a hyperalgesia | [39] |
| A retrospective case analysis investigated 23 patients with ovarian cancer treated with paclitaxel and carboplatin combination chemotherapy | Reduces pain in epithelial ovarian carcinoma | [40] | ||
| Jesengsingi-Hwan | Achyranthis bidentatae radix, Alismatis rhizome, Aconiti tuber, Cinnamomi cortex, Corni fructus, Dioscorea opposita rhizoma, Plantaginis semen, Poria alba, Moutan cortex, Rehmannia viride radix | Oxaliplatin in rats | Reduces peripheral neuropathy without influence on anti-cancer potency Ameliorates abnormal sensations and histological damage to the sciatic nerve |
[41,42] |
| Paclitaxel in mice | Inhibits mechanical allodynia | [43] | ||
| Oxaliplatin in a placebo-controlled double-blind randomized study | Delays onset of grade 2 or greater peripheral neurotoxicity without impairing FOLFOX efficacy with an acceptable safety margin | [44,45] | ||
| Prevents exacerbation of peripheral neuropathy | [46,47] | |||
| A clinical trial enrolling 82 patients | Reduces peripheral neuropathy | [48] |
2. Phytochemicals and Medicinal Herbs against Vincristine-Induced Peripheral Neuropathy
Vincristine is one of the most extensively used chemotherapeutic agents to treat diverse types of cancer, including Hodgkin‘s disease, small cell lung cancer, acute myeloid leukemia, acute lymphocytic leukemia, and neuroblastoma. Vincristine inhibits chromosome separation during the metaphase resulting in cell apoptosis [49]. Patients can experience some side effects from vincristine treatment, such as headaches, hair loss, walking difficulty, constipation, and a change in sensation. In serious cases, neuropathic pain, classically resulting in autonomic and peripheral sensory-motor neuropathy limits the dose of vincristine. Vincristine-induced peripheral neuropathy can worsen after therapy has ended [50].
2.1. Acorus calamus
Acorus calamus is a medicinal herb used to alleviate pain or severe inflammation in Ayurveda. The root of the plant is widely used to treat a number of illnesses such as abdominal tumors, chronic diarrhea, dysentery, epilepsy, fever, mental ailments, kidney and liver issues, and rheumatism [51]. Hydro-alcoholic extracts of A. calamus rhizoma (100–200 mg/kg, p.o. for 14 consecutive days) protect against painful neuropathy induced by vincristine in rats. The extracts inhibit vincristine-induced biochemical (increase in superoxide anion generation level and total calcium level, and myeloperoxidase activity in the sciatic nerve) and behavioral (thermal- and mechano-hyperalgesia) changes to an extent comparable to Lyrica (pregabalin) [28]. The ethanolic extract of A. calamus (up to 600 mg/kg) did not cause lethality and any changes in the general behavior in rats in both acute and chronic toxicity tests [52].
2.2. Auraptenol
Auraptenol (8-(2-hydroxy-3-methylbut-3-enyl)-7-methoxychromen-2-one) is a phytochemical isolated from Angelicae dahuricae radix. The root of the plant is used to treat harmful exterior stimuli on the skin, such as dryness, dampness, heat, and cold in Oriental medicine [53]. It has been shown that its antinociceptive effects are linked to the facilitated release of endogenous opioids [54] and that a single oral administration of A. dahuricae (3.25 g or 6.5 g) decreased cold-induced tonic pain in a dose-dependent manner in clinical trials [55]. It reverted mechanical hyperalgesia induced by vincristine through 5-HT1A receptors in a dose-dependent manner (within the 0.05–0.8 mg/kg range). The highest dose of auraptenol (0.8 mg/kg, i.p.) totally suppressed the mechanical hyperalgesia without affecting the general locomotor activity [22].
2.3. Butea monosperma
Butea monosperma is a medium-sized deciduous tree that has been used as an aphrodisiac, astringent, tonic, and diuretic in Ayurveda [56]. The plant contains many phytocomponents, including saponins, glycosides, mucilage, gums, and fatty acids [57]. Oral intake of ethanolic extract of B. monosperma (200–400 mg/kg for 14 days) suppressed histological, biochemical, and behavioral changes induced by vincristine in rats. Authors have suggested that the therapeutic benefits might originate from its calcium channel inactivating, anti-oxidative, anti-inflammatory, and neuroprotective effects [29].
2.4. Cannabinoids
Historically, Cannabis sativa has used to treat neuropathic pain. Cannabinoids repress neurotransmitter release in the brain by binding on cannabinoid receptors in cells [58] and have anti-inflammatory effects [59]. Rahn et al. investigated the effect of synthetic Δ9-tetrahydrocannabinol analog on vincristine-induced mechanical allodynia in rats. The experiment demonstrated that cannabinoids can inhibit vincristine-induced mechanical allodynia through the cannabinoid receptor 1/2 pathway and the effect is mediated at the level of the spinal cord in part, although these synthetic cannabinoids may have some different pharmacological effects from phytocannabinoids [60]. Cannabinoids have shown anti-cancer activities in animal models of cancer, and they are currently being tested as anti-tumor agents in phase I/II clinical trials [61].
2.5. Ginkgo biloba L.
Ginkgo leaf extract has been used for pharmaceutical purposes since 1965 and is one of the bestselling herbal medicines in the world [62]. Park et al. showed that Ginkgo biloba extract (50–150 mg/kg, p.o.) decreased the paw withdrawal frequency to cold stimuli and increased the withdrawal threshold to mechanical stimuli in peripheral neuropathy-induced rats [30]. They suggested that the anti-hyperalgesic effect of G. biloba extract may be related to suppression of axonal degradation, improved axonal transport, and inhibition of TNF-α and NO production. The few recent studies on the anticancer activity of the extract in in vitro models showed the cell proliferation inhibition, tumor suppression, and DNA damage-repairing effects of the extract [63,64,65]. Biggs et al. analyzed the risk of cancer hospitalization between participants assigned to Ginkgo extract treatment and those assigned to placebo and reported that the data do not support the regular use of G. biloba for reducing the risk of cancer [66].
2.6. Ocimum sanctum L.
In traditional medicine, Ocimum sanctum L. has been recommended for the treatment of skin diseases, bronchitis, diarrhea, malaria, and arthritis. Recent research has also demonstrated its cardioprotective, anti-microbial, anti-fungal, anti-fertility, anti-diabetic, anti-cancer, and analgesic properties [67]. Its leaf oil contains eugenol, eugenic acid, ursolic acid, carvacrol, linalool, limatrol, caryophyllene, and methyl carvacrol [68]. Kaur et al. demonstrated that oral administration of O. sanctum or its saponin-rich fraction (100 and 200 mg/kg, for 14 days) reduced neurotoxicity induced by vincristine in rats with a decline in calcium levels and oxidative stress, thus helping to prevent CIPN symptoms. They estimated that the saponin-rich fraction may mediate the therapeutic effects of O. sanctum in neuropathic pain [33]. Seed oil supplementation of O. sanctum L. (100 μL/kg) reduced 20-methaylcholathrene-induced tumor incidence and tumor volume and enhanced the survival rate in mice [69].
2.7. Xylopic Acid
Traditionally, the fruit of Xylopia aethiopica has been used to manage pain disorders, including neuralgia and headache [70]. Ethanolic extract of X. aethiopica (30–300 mg/kg, p.o.) and its major diterpene xylopic acid (15-(acetyloxy)kaur-16-en-18-oic acid; 10–100 mg/kg, p.o.) exhibit anti-allodynic and anti-hyperalgesic properties in vincristine-induced neuropathic pain. Diterpene xylopic acid from X. aethiopica exhibited greater potency than the ethanolic extract of X. aethiopica itself, while pregabalin (10–100 mg/kg) showed a comparable effect to xylopic acid [27]. Treatment with X. aethiopica extract led to a dose-dependent growth inhibition in many cell lines, including HCT116 colon cancer cells, U937, and KG1a leukemia cells, and the C-33A cervical cancer cell line [71,72], but xylopic acid, unlike kaurenoic acid, has no cytotoxic effects on human cancer cells [73].
3. Phytochemicals and Medicinal Herbs against Cisplatin-Induced Peripheral Neuropathy
Cisplatin is the first member of platinum-based antineoplastic medicine. This platinum complex causes the crosslinking of two DNA strands in cells, which prevents cell division and finally leads to programmed cell death. In addition to nephrotoxicity, neurotoxicity and ototoxicity are dose-limiting adverse effects of cisplatin treatment [74,75].
3.1. Curcumin
Curcumin ((1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a yellow pigment component of Curcuma longa. This phytochemical is well known for its powerful anti-inflammatory and antioxidant properties. It has demonstrated benefits in neuronal diseases such as alcoholic neuropathy and diabetic neuropathy [76,77,78]. In the cisplatin-treated model, curcumin (10 mg/kg, oral) reversed the neurotensin changes in the plasma, reduced cisplatin absorption in the sciatic nerve, and notably ameliorated sciatic nerve histology [24]. Curcumin regulates the growth of cancer cells by the modulation of multiple cell signaling pathways, including protein kinase, mitochondrial, death receptor, caspase activation, cell survival, tumor suppressor, and cell proliferation pathways [79]. Extensive in vivo data support curcumin’s beneficial effects against cancer [80,81,82]; however, there are also conflicting reports that curcumin can promote cancer in mice [83,84].
3.2. Ginkgo biloba L.
Ozturk et al. showed that oral administration of G. biloba alcoholic extract is beneficial in preventing peripheral neuropathy induced by cisplatin in mice. In their experiments, G. biloba extract reduced cisplatin-induced immigrated cell numbers, sensory nerve conduction velocity, and outgrowing of axons [31].
3.3. Salvia officinalis
Salvia officinalis (Sage) is a perennial herb with well-known carminative, antispasmodic, antiseptic, astringent, and antihydrotic properties [85]. The phytocomplexes of S. officinalis contain monoterpenes with a broad range of carbon skeletons, including acyclic, monocyclic, and bicyclic compounds, phenolic compounds, diterpenes, triterpenes [86,87]. An alcoholic extract of S. officinalis leaf (100 mg/kg i.p.) exhibited an anti-nociceptive effect on cisplatin-induced hyperalgesia in mice. In the formalin test, the aqueous extract effectively suppressed the second phase of pain. The extract even showed stronger benefits than morphine [34]. Vujosevic et al. showed anti-mutagenic effects of S. officinalis in a mammalian system in vivo [88], and Keshavarz et al. showed its anti-angiogenic properties for anti-tumor effect in chicken eggs [89].
3.4. Walnut
Walnut is one of the traditional anti-tumor, anti-inflammatory, blood purifying, and antioxidant agents. Shabani et al. investigated whether walnut has a neuroprotective property on neurotoxicity induced by cisplatin. Dietary walnut (6%) altered cerebellum- and hippocampus- related behaviors caused by continuous cisplatin injection in male rats. Dietary walnut also ameliorated motor and memory capacities in cisplatin-treated rats. Cisplatin increased, but walnut decreased the latency to nociceptive stimuli [35]. Dietary walnut suppressed mammary gland tumorigenesis in the C(3)1 TAg mouse [90] and growth of implanted MDA-MB 231 human breast cancer cells in nude mice [91]. It has also been demonstrated that walnut reduces growth of prostate cancer [92,93] and colorectal cancer [94].
4. Phytochemicals and Medicinal Herbs against Oxaliplatin-Induced Peripheral Neuropathy
Oxaliplatin is a platinum-based anti-neoplastic agent used for treating advanced colorectal cancer [95]. Its cytotoxicity is considered to result from the inhibition of DNA synthesis, similar to that of other platinum complexes. Oxaliplatin forms both intra- and inter-strand crosslinks in DNA, which prevent DNA transcription and replication, resulting in programmed cell death [96]. This chemotherapeutic drug is typically used alongside a combination of folinic acid and 5-fluorouracil (FOLFOX). Oxaliplatin has less ototoxicity and nephrotoxicity than cisplatin; however, oxaliplatin treatment can still cause neurotoxicity and ototoxicity [97].
4.1. Curcumin
Al Moundhri et al. showed that oral administration of curcumin (10 mg/kg) reduced drug consistency in the sciatic nerve and prominently ameliorated sciatic nerve injury in oxaliplatin-induced neurotoxicity in rats [24]. Wassem and Parvez showed that curcumin can ameliorate changes in both enzymatic and nonenzymatic antioxidants of mitochondria in vitro. The results reveal the potential of curcumin as a substance that can diminish oxaliplatin-induced peripheral neurotoxicity [98].
4.2. Rutin and Quercetin
Rutin (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-({[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-4H-chromen-4-one) and quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one) are polyphenolic flavonoids found in many medicinal herbs and vegetables. They have been reported to have powerful antioxidant, anti-inflammatory, and anti-nociceptive activities. Rutin is water-soluble and is converted to quercetin once it enters the bloodstream [99]. In alcohol-induced neuropathy, quercetin compound showed remarkable anti-nociceptive and neuroprotective effects [100]. In oxaliplatin-treated rats, rutin and quercetin (25, 50, and 100 mg/kg, i.p.) suppressed neuronal contraction and averted development of edema. Moreover, c-Fos, a marker of neuroplasticity, was decreased by rutin- or quercetin- pretreatment [25]. The neuroprotective mechanism of these phytochemicals is connected to its amelioration of mitochondrial dysfunction [98]. Interestingly, quercetin, but not rutin, inhibited azoxymethane-induced colorectal carcinogenesis in rats [101]. Quercetin has been used in clinical trials in cancer patients. The results demonstrated that quercetin can be safely administered by i.v. and that its anticancer properties are detectable [102].
4.3. Camellia sinensis (Green Tea)
Camellia sinensis is a small tree in which the leaves are used to produce green tea, a popular beverage with therapeutic applications. The key bioactive substances of C. sinensis are catechins. A range of studies have demonstrated that these substances have strong anti-inflammatory, antioxidant, and anticancer properties [103]. The therapeutic property of C. sinensis was tested against oxaliplatin-induced peripheral neuropathy. Oral administration of green tea extract (300 mg/kg for 6 weeks) forcefully attenuated thermal hyperalgesia and mechanical allodynia; however, it did not avert morphometric or electrophysiological changes [32]. The experimental evidence exhibiting the cancer-preventive activity of green tea is increasing rapidly [104]. Nakachi et al. suggested that consumption of green tea prior to cancer development was markedly associated with improved prognosis of stage I/II breast cancer [105].
4.4. Gyeryongtongrac-Bang (Guilongtongluofang in Chinese)
Gyeryongtongrac-Bang is a traditional medicine used to relieve numbness and cold sensation in patients. One hundred twenty colorectal cancer patients who were treated with oxaliplatin were randomly assigned to the Gyeryongtongrac-Bang-treated group (which received aqueous extract from Ramulus cinnamomi, Earthworm, Radix astragali, Safflower, Radix angelicae sinensis, Ligusticum, Spatholobus, Radix paeoniae alba, Rhizoma curcumae, and Licorice once a day) and the control group (which received placebo) in a double-blind trial. A total of 51.7% patients in the Gyeryongtongrac-Bang-treated group showed neurotoxicity, whereas, it was seen in 70.0% of the placebo-treated group after 4 cycles of treatment. The results suggest that Gyeryongtongrac-Bang can be a potent agent that prevents neurotoxic pain without diminishing oxaliplatin-attributed benefits. Additionally, the development of sensory neurotoxicity was delayed in the Gyeryongtongrac-Bang-treated group [37].
4.5. Gyejigachulbu-Tang (Keishikajutsubuto in Japanese)
Gyejigachulbu-Tang is an herbal formula including Aconiti tuber, Atractylodis lanceae rhizome, Cinnamomi cortex, Glycyrrhizae radix, Paeoniae radix, Zingiberis rhizoma, and Zizyphi fructus. In our experiment, oral administration of Gyejigachulbu-Tang (200, 400, and 600 mg/kg for 5 days) markedly ameliorated mechanical- and cold-allodynia induced by oxaliplatin treatment. The formula possibly functions through the suppression of spinal glial activation [36]. We confirmed that the extract of Aconiti tuber could attenuate both cold and mechanical allodynia similar to Gyejigachulbu-Tang treatment. Interestingly, Cinnamomi cortex and coumarin, a phytochemical from C. cortex, also attenuated cold allodynia induced by oxaliplatin treatment in rats (unpublished data). These results suggest that both A. tuber and C. cortex have neuroprotective properties against oxaliplatin-induced neuropathy, thereby playing a major role in the anti-allodynic effect of Gyejigachulbu-Tang.
4.6. Jesengsingi-Hwan (Goshajinkigan in Japanese)
Jesengsingi-Hwan is a traditional herbal formula widely used in Asia. It contains 10 different herbs comprising Achyranthis bidentatae radix, Alismatis rhizome, A. tuber, C. cortex, Corni fructus, Dioscorea opposita rhizoma, Plantaginis semen, Poria alba, Moutan cortex, and Rehmannia viride radix. Recently, the beneficial properties of Jesengsingi-Hwan on CIPN have been widely prospected. In a murine study, Ushino et al. showed that Jesengsingi-Hwan can reduce CIPN without influence on anti-cancer potency [41]. Kono et al. examined a preventive effect of Jesengsingi-Hwan on chronic oxaliplatin-induced hypoesthesia in rats. Oral administration of Jesengsingi-Hwan (0.3 or 1.0 g/kg, 5 times a week for 8 weeks) ameliorated abnormal sensations and histological damage to the sciatic nerve [42]. In a retrospective clinical study, Kono et al. examined the benefits of Jesengsingi-Hwan on oxaliplatin treatment involved in peripheral neuropathy. In the study, the administration of Jesengsingi-Hwan (7.5 g/day) reduced the neurotoxicity of oxaliplatin in colorectal cancer patients [44]. Later, they again reported that daily oral administration of Jesengsingi-Hwan (7.5 g/day) has the capability to delay the development of grade 2 or greater oxaliplatin-induced peripheral neurotoxicity without impairing FOLFOX efficacy in a randomized phase II study [45]. Yoshida et al. also assessed the effects of Jesengsingi-Hwan for oxaliplatin-induced peripheral neurotoxicity in colorectal cancer patients. Twenty-nine colorectal cancer patients received ≥4 weeks of Jesengsingi-Hwan (2.5 g orally 3 times daily before or between meals for a total of 7.5 g/day) for oxaliplatin-induced peripheral neuropathy during chemotherapy. They were compared to 44 patients who had not received Jesengsingi-Hwan during the same period. A Kaplan-Meier analysis showed that Jesengsingi-Hwan could prevent exacerbation of oxaliplatin-induced peripheral neuropathy [46]. Hosokawa et al. assessed the preventive properties of Jesengsingi-Hwan on oxaliplatin-induced neurotoxicity in colorectal cancer patients and found that in the Jesengsingi-Hwan-treated group, 50% of oxaliplatin-induced peripheral neuropathy was prevented without diminishing chemotherapy efficacy [47].
4.7. Hwanggiomul-Tang (Ogikeishigomotsuto in Japanese)
In Japan, a single case study was reported using Hwanggiomul-Tang for oxaliplatin-induced neuropathic pain. Hwanggiomul-Tang is an herbal mixture containing Zingiberis hizome, Jujubae fructus, Paeonia alba radix, C. cortex, and Astragalus membranaceus radix. The case study of a 59-year old man with recurrent colon cancer suggests that this herbal formula may be useful to reduce or prevent chronic cumulative neurotoxicity due to oxaliplatin [38].
5. Phytochemicals and Medicinal Herbs against Paclitaxel-Induced Peripheral Neuropathy
Paclitaxel is a member of the taxane family of drugs used to treat ovarian, breast, lung, esophageal, prostate, bladder, and pancreatic cancer as well as Kaposi's sarcoma and melanoma [106]. This tubulin-targeting drug protects the microtubule polymer from disassembly by stabilizing it. The stabilization triggers apoptosis through inhibition of mitosis [107]. Paclitaxel-treated cells thus have defects in cell division, chromosome segregation, and mitotic spindle assembly. Paclitaxel can induce phenomena of sensory peripheral neuropathy, such as paresthesia and numbness in the extremities [108]. Symmetrical loss of sensation is also a frequent occurrence. These neuropathy symptoms limit the use of paclitaxel.
5.1. Cannabidiol
Cannabidiol (2-[(1R,6R)-6-isopropenyl-3-methylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol) is a key phytochemical accounting for approximately 40% of the C. sativa extract [109]. This phytocannabinoid is known to have various medical applications on the basis of clinical reports showing the lack of side effects and particularly a lack of psychoactivity with anti-nausea, anti-psychotic, anti-anxiety, and anti-convulsive properties [110]. Cannabidiol (2.5–10 mg/kg, i.p. for 6 days) inhibited paclitaxel-induced neuropathic pain through 5-HT1A receptor signaling without diminishing chemotherapy efficacy or nervous system function in mice [23].
5.2. Verticinone
Verticinone ((3β,5α)-3,20-dihydroxycevan-6-one) is a kind of isosteroidal alkaloid derived from Fritillaria bulbus. Xu et al. [26] examined its analgesic effects using neuropathic pain and inflammation models in rats. The experiments showed that hydro-alcoholic extracted verticinone (1.5–3 mg/kg, p.o.) has a relatively constant analgesic effect in paclitaxel-induced neuropathy; further, the analgesic effect of morphine was decreased after repeated medication. Authors believe verticinone is an anodyne with low tolerance.
5.3. Jesengshingi-Hwan
Bahar et al. reported that paclitaxel-induced allodynia was markedly prevented by Jesengshingi-Hwan (1 g/kg, p.o. daily) in mice, although Jesengshingi-Hwan could not suppress cancer-induced allodynia [43]. Andoh et al. [111] also reported that Jesengsingi-Hwan (0.1–1.0 g/kg, p.o.) markedly inhibited paclitaxel-induced mechanical allodynia in mice. Authors predicted that Achyranthis radix and P. semen in Jesengsingi-Hwan may block the aggravation of paclitaxel-induced neuropathic pain. Yamamoto et al. reported that Jesengsingi-Hwan treatment was beneficial for the treatment of paclitaxel-induced neuropathic pain in eighty-two patients enrolled in clinical trials. The investigators believe its preventive effect may be more potent if it is administered from the start of chemotherapy for breast, colorectal, or gynecological cancer patients [48].
5.4. Jakyakgamcho-Tang (Shakuyakukanzoto in Japanese)
Jakyakgamcho-Tang is an herbal mixture of Paeoniae radix and Glycyrrhizae radix. A mouse study found that this combination (1.75 mg/mouse) remarkably attenuated paclitaxel-induced hyperalgesia and allodynia [39]. Through retrospective case analysis on 23 ovarian cancer patients, Fujii et al. [40] concluded that Jakyakgamcho-Tang (7.5 g/day, p.o. for 8 days) has a remedial value in neuropathic pain after paclitaxel and carboplatin combination chemotherapy. Authors suggest that paclitaxel combination chemotherapy with Jakyakgamcho-Tang taken orally is a safer and more tolerable way to reduce pain in epithelial ovarian carcinoma.
6. Conclusions and Perspectives
Despite the high incidence of CIPN and its dose-limiting effects, there are no current treatments or preventive options for CIPN with conclusive efficacy and safety data. The lack of effective therapeutic methods for CIPN has boosted the need for the use of medicinal herbs and phytochemicals; these have gained increasing attention as a major form of alternative therapy because they are convenient, economical, effective, safe, and therapeutic. Most recently, a number of phytochemicals and herbal medicines have shown potential for protective benefits for CIPN. Owing to the diverse mechanisms of CIPN, the results of phytotherapy using phytochemicals or herbs contributing to the multiple targets of CIPN seem to be encouraging.
To date, however, many of the therapeutic mechanisms of these phytotherapies remain unclear. For example, significant roles of transient receptor potential (TRP) channels for CIPN development have been discovered, but the link between phytotherapy and TRP channels is mostly unknown. An increasing number of studies report the involvement of TRP channels including TRPA1, TRPM8, TRPV1, and TRPV4 in CIPN [112,113,114,115]. Recent studies found that Jesengsingi-Hwan prevents oxaliplatin-induced peripheral neuropathy through the functional alteration in TRPA1 and TRPM8 [116] and can reduce paclitaxel-induced peripheral neuropathy by suppressing TRPV4 expression [117]. Moreover, the TRPV1-mediated anti-cancer effects of cannabidiol have been reported in multiple cancer cell lines, including breast [118], lung [119,120], and colon [121]. Inflammatory and neuro-immune responses also play important roles in the development and progression of CIPN [122]. Phytotherapies, especially cannabinoids, have significant anti-inflammatory and immunomodulatory properties [123]. More studies are necessary to further our understanding of the involved mechanisms such as TRP channels and immunomodulation. In addition, the dose of several phytochemicals and medicinal herbs for the treatment of animals appears high. At high doses, some of them can induce toxicity in the liver or kidneys. Thus, the dose should be interpreted and verified for toxicity. It is essential to find the maximum dose of phytochemicals and herbs for use in humans.
In conclusion, because of their multitarget, multilevel, and integrated benefits, medicinal herbs seem to be a feasible method for the management of CIPN. Phytochemicals, medicinal herbs, and their formulas could be considered for the treatment of CIPN. However, their curative usability should be examined in well-designed clinical trials. In addition, their reciprocal effects with other drugs in humans should be examined in detail.
Acknowledgments
This work was supported by a grant of the Korea Health Technology R & D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C0738).
Author Contributions
G. Lee and S.K. Kim conceived and designed the study. G. Lee wrote the manuscript. Both authors revised and approved this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Miltenburg N.C., Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat. Rev. 2014;40:872–882. doi: 10.1016/j.ctrv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Jaggi A.S., Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Egan M., Burke E., Meskell P., MacNeela P., Dowling M. Quality of life and resilience related to chemotherapy-induced peripheral neuropathy in patients post treatment with platinums and taxanes. J. Res. Nurs. 2015;20:385–398. doi: 10.1177/1744987115574296. [DOI] [Google Scholar]
- 4.Visovsky C., Collins M., Abbott L., Aschenbrenner J., Hart C. Putting evidence into practice: Evidence-based interventions for chemotherapy-induced peripheral neuropathy. Clin. J. Oncol. Nurs. 2007;11:901–913. doi: 10.1188/07.CJON.901-913. [DOI] [PubMed] [Google Scholar]
- 5.Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (cipn): What we need and what we know. J. Peripher. Nerv. Syst. 2014;19:66–76. doi: 10.1111/jns5.12073. [DOI] [PubMed] [Google Scholar]
- 6.Beijers A.J., Vreugdenhil G., Oerlemans S., Eurelings M., Minnema M.C., Eeltink C.M., van de Poll-Franse L.V., Mols F. Chemotherapy-induced neuropathy in multiple myeloma: Influence on quality of life and development of a questionnaire to compose common toxicity criteria grading for use in daily clinical practice. Support. Care Cancer. 2016;24:2411–2420. doi: 10.1007/s00520-015-3032-y. [DOI] [PubMed] [Google Scholar]
- 7.Park S.B., Goldstein D., Krishnan A.V., Lin C.S., Friedlander M.L., Cassidy J., Koltzenburg M., Kiernan M.C. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA A Cancer J. Clin. 2013;63:419–437. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- 8.Argyriou A.A., Kyritsis A.P., Makatsoris T., Kalofonos H.P. Chemotherapy-induced peripheral neuropathy in adults: A comprehensive update of the literature. Cancer Manag. Res. 2014;6:135–147. doi: 10.2147/CMAR.S44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith J.A., Benbow S.J. Meeting report: Inaugural chemotherapy-induced peripheral neuropathy symposium—Santa Barbara, CA, February 2015. Cancer Res. 2015;75:3696–3698. doi: 10.1158/0008-5472.CAN-15-1145. [DOI] [PubMed] [Google Scholar]
- 10.Cavaletti G., Zanna C. Current status and future prospects for the treatment of chemotherapy-induced peripheral neurotoxicity. Eur. J. Cancer. 2002;38:1832–1837. doi: 10.1016/S0959-8049(02)00229-0. [DOI] [PubMed] [Google Scholar]
- 11.Cavaletti G., Frigeni B., Lanzani F., Mattavelli L., Susani E., Alberti P., Cortinovis D., Bidoli P. Chemotherapy-induced peripheral neurotoxicity assessment: A critical revision of the currently available tools. Eur. J. Cancer. 2010;46:479–494. doi: 10.1016/j.ejca.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Cavaletti G., Cornblath D.R., Merkies I.S., Postma T.J., Rossi E., Frigeni B., Alberti P., Bruna J., Velasco R., Argyriou A.A., et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: From consensus to the first validity and reliability findings. Ann. Oncol. 2013;24:454–462. doi: 10.1093/annonc/mds329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hausheer F.H., Schilsky R.L., Bain S., Berghorn E.J., Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin. Oncol. 2006;33:15–49. doi: 10.1053/j.seminoncol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Grothey A. Clinical management of oxaliplatin-associated neurotoxicity. Clin. Colorectal Cancer. 2005;5(Suppl. 1):S38–S46. doi: 10.3816/CCC.2005.s.006. [DOI] [PubMed] [Google Scholar]
- 15.Kushlaf H.A. Emerging toxic neuropathies and myopathies. Neurol. Clin. 2011;29:679–687. doi: 10.1016/j.ncl.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Kuncl R.W., George E.B. Toxic neuropathies and myopathies. Curr. Opin. Neurol. 1993;6:695–704. doi: 10.1097/00019052-199310000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Flatters S.J., Xiao W.H., Bennett G.J. Acetyl-l-carnitine prevents and reduces paclitaxel-induced painful peripheral neuropathy. Neurosci. Lett. 2006;397:219–223. doi: 10.1016/j.neulet.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavaletti G. Calcium and magnesium prophylaxis for oxaliplatin-related neurotoxicity: Is it a trade-off between drug efficacy and toxicity? Oncologist. 2011;16:1667–1668. doi: 10.1634/theoncologist.2011-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ta L.E., Schmelzer J.D., Bieber A.J., Loprinzi C.L., Sieck G.C., Brederson J.D., Low P.A., Windebank A.J. A novel and selective poly(adp-ribose) polymerase inhibitor ameliorates chemotherapy-induced painful neuropathy. PLoS ONE. 2013;8:e54161. doi: 10.1371/journal.pone.0054161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolf S., Barton D., Kottschade L., Grothey A., Loprinzi C. Chemotherapy-induced peripheral neuropathy: Prevention and treatment strategies. Eur. J. Cancer. 2008;44:1507–1515. doi: 10.1016/j.ejca.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 21.Ceresa C., Cavaletti G. Drug transporters in chemotherapy induced peripheral neurotoxicity: Current knowledge and clinical implications. Curr. Med. Chem. 2011;18:329–341. doi: 10.2174/092986711794839160. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Cao S.E., Tian J., Liu G., Zhang X., Li P. Auraptenol attenuates vincristine-induced mechanical hyperalgesia through serotonin 5-ht1a receptors. Sci. Rep. 2013;3:3377. doi: 10.1038/srep03377. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Ward S.J., McAllister S.D., Kawamura R., Murase R., Neelakantan H., Walker E.A. Cannabidiol inhibits paclitaxel-induced neuropathic pain through 5-HT(1A) receptors without diminishing nervous system function or chemotherapy efficacy. Br. J. Pharmacol. 2014;171:636–645. doi: 10.1111/bph.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Moundhri M.S., Al-Salam S., Al Mahrouqee A., Beegam S., Ali B.H. The effect of curcumin on oxaliplatin and cisplatin neurotoxicity in rats: Some behavioral, biochemical, and histopathological studies. J. Med. Toxicol. 2013;9:25–33. doi: 10.1007/s13181-012-0239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azevedo M.I., Pereira A.F., Nogueira R.B., Rolim F.E., Brito G.A., Wong D.V., Lima-Junior R.C., de Albuquerque Ribeiro R., Vale M.L. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol. Pain. 2013;9:53. doi: 10.1186/1744-8069-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu F.Z., Xu S.Z., Wang L.J., Chen C.T., Zhou X.Q., Lu Y.Z., Zhang H.H. Antinociceptive efficacy of verticinone in murine models of inflammatory pain and paclitaxel induced neuropathic pain. Biol. Pharm. Bull. 2011;34:1377–1382. doi: 10.1248/bpb.34.1377. [DOI] [PubMed] [Google Scholar]
- 27.Ameyaw E.O., Woode E., Boakye-Gyasi E., Abotsi W.K., Kyekyeku J.O., Adosraku R.K. Anti-allodynic and anti-hyperalgesic effects of an ethanolic extract and xylopic acid from the fruits of Xylopia aethiopica in murine models of neuropathic pain. Pharm. Res. 2014;6:172–179. doi: 10.4103/0974-8490.129041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muthuraman A., Singh N. Attenuating effect of hydroalcoholic extract of Acorus calamus in vincristine-induced painful neuropathy in rats. J. Nat. Med. 2011;65:480–487. doi: 10.1007/s11418-011-0525-y. [DOI] [PubMed] [Google Scholar]
- 29.Thiagarajan V.R., Shanmugam P., Krishnan U.M., Muthuraman A., Singh N. Antinociceptive effect of Butea monosperma on vincristine-induced neuropathic pain model in rats. Toxicol. Ind. Health. 2013;29:3–13. doi: 10.1177/0748233711432573. [DOI] [PubMed] [Google Scholar]
- 30.Park H.J., Lee H.G., Kim Y.S., Lee J.Y., Jeon J.P., Park C., Moon D.E. Ginkgo biloba extract attenuates hyperalgesia in a rat model of vincristine-induced peripheral neuropathy. Anesthesia Analg. 2012;115:1228–1233. doi: 10.1213/ANE.0b013e318262e170. [DOI] [PubMed] [Google Scholar]
- 31.Ozturk G., Anlar O., Erdogan E., Kosem M., Ozbek H., Turker A. The effect of ginkgo extract EGb761 in cisplatin-induced peripheral neuropathy in mice. Toxicol. Appl. Pharmacol. 2004;196:169–175. doi: 10.1016/j.taap.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Lee J.S., Kim Y.T., Jeon E.K., Won H.S., Cho Y.S., Ko Y.H. Effect of green tea extracts on oxaliplatin-induced peripheral neuropathy in rats. BMC Complement. Altern. Med. 2012;12:124. doi: 10.1186/1472-6882-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaur G., Jaggi A.S., Singh N. Exploring the potential effect of Ocimum sanctum in vincristine-induced neuropathic pain in rats. J. Brachial Plex. Peripher. Nerve Inj. 2010;5:3. doi: 10.1186/1749-7221-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namvaran-Abbas-Abad A., Tavakkoli F. Antinociceptive effect of salvia extract on cisplatin-induced hyperalgesia in mice. Neurophysiology. 2012;43:452–458. doi: 10.1007/s11062-012-9249-1. [DOI] [Google Scholar]
- 35.Shabani M., Nazeri M., Parsania S., Razavinasab M., Zangiabadi N., Esmaeilpour K., Abareghi F. Walnut consumption protects rats against cisplatin-induced neurotoxicity. Neurotoxicology. 2012;33:1314–1321. doi: 10.1016/j.neuro.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Ahn B.S., Kim S.K., Kim H.N., Lee J.H., Lee J.H., Hwang D.S., Bae H., Min B.I., Kim S.K. Gyejigachulbu-tang relieves oxaliplatin-induced neuropathic cold and mechanical hypersensitivity in rats via the suppression of spinal glial activation. Evid. Based Complement. Altern. Med. 2014;2014:436482. doi: 10.1155/2014/436482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Zhu G., Han L., Liu J., Ma T., Yu H. Clinical study on the prevention of oxaliplatin-induced neurotoxicity with guilongtongluofang: Results of a randomized, double-blind, placebo-controlled trial. Evid. Based Complement. Altern. Med. 2013;2013:541217. doi: 10.1155/2013/541217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sima L., Pan L. Influence of chinese herb ah on chemotherapy-induced peripheral neuropathy. Ann. Oncol. 2009;20:46. [Google Scholar]
- 39.Hidaka T., Shima T., Nagira K., Ieki M., Nakamura T., Aono Y., Kuraishi Y., Arai T., Saito S. Herbal medicine shakuyaku-kanzo-to reduces paclitaxel-induced painful peripheral neuropathy in mice. Eur. J. Pain. 2009;13:22–27. doi: 10.1016/j.ejpain.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Fujii K., Okamoto S., Saitoh K., Sasaki N., Takano M., Tanaka S., Kudoh K., Kita T., Tode T., Kikuchi Y. The efficacy of shakuyaku-kanzo-to for peripheral nerve dysfunction in paclitaxel combination chemotherapy for epithelial ovarian carcinoma. Gan To Kagaku Ryoho. Cancer Chemother. 2004;31:1537–1540. [PubMed] [Google Scholar]
- 41.Ushio S., Egashira N., Sada H., Kawashiri T., Shirahama M., Masuguchi K., Oishi R. Goshajinkigan reduces oxaliplatin-induced peripheral neuropathy without affecting anti-tumour efficacy in rodents. Eur. J. Cancer. 2012;48:1407–1413. doi: 10.1016/j.ejca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Kono T., Suzuki Y., Mizuno K., Miyagi C., Omiya Y., Sekine H., Mizuhara Y., Miyano K., Kase Y., Uezono Y. Preventive effect of oral goshajinkigan on chronic oxaliplatin-induced hypoesthesia in rats. Sci. Rep. 2015;5:16078. doi: 10.1038/srep16078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bahar M.A., Andoh T., Ogura K., Hayakawa Y., Saiki I., Kuraishi Y. Herbal medicine goshajinkigan prevents paclitaxel-induced mechanical allodynia without impairing antitumor activity of paclitaxel. Evid. Based Complement. Altern. Med. 2013 doi: 10.1155/2013/849754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kono T., Mamiya N., Chisato N., Ebisawa Y., Yamazaki H., Watari J., Yamamoto Y., Suzuki S., Asama T., Kamiya K. Efficacy of goshajinkigan for peripheral neurotoxicity of oxaliplatin in patients with advanced or recurrent colorectal cancer. Evid. Based Complement. Altern. Med. 2011;2011:418481. doi: 10.1093/ecam/nep200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kono T., Hata T., Morita S., Munemoto Y., Matsui T., Kojima H., Takemoto H., Fukunaga M., Nagata N., Shimada M., et al. Goshajinkigan oxaliplatin neurotoxicity evaluation (gone): A phase 2, multicenter, randomized, doubleblind, placebocontrolled trial of goshajinkigan to prevent oxaliplatininduced neuropathy. Cancer Chemother. Pharmacol. 2013;72:1283–1290. doi: 10.1007/s00280-013-2306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida N., Hosokawa T., Ishikawa T., Yagi N., Kokura S., Naito Y., Nakanishi M., Kokuba Y., Otsuji E., Kuroboshi H., et al. Efficacy of goshajinkigan for oxaliplatin-induced peripheral neuropathy in colorectal cancer patients. J. Oncol. 2013;2013:139740. doi: 10.1155/2013/139740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosokawa A., Ogawa K., Ando T., Suzuki N., Ueda A., Kajiura S., Kobayashi Y., Tsukioka Y., Horikawa N., Yabushita K., et al. Preventive effect of traditional japanese medicine on neurotoxicity of folfox for metastatic colorectal cancer: A multicenter retrospective study. Anticancer Res. 2012;32:2545–2550. [PubMed] [Google Scholar]
- 48.Yamamoto T., Murai T., Ueda M., Katsuura M., Oishi M., Miwa Y., Okamoto Y., Uejima E., Taguchi T., Noguchi S., et al. Clinical features of paclitaxel-induced peripheral neuropathy and role of gosya-jinki-gan. Gan To kagaku ryoho. Cancer Chemother. 2009;36:89–92. [PubMed] [Google Scholar]
- 49.Jordan M.A. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr. Med. Chem. Anti-Cancer Agent. 2002;2:1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
- 50.Verstappen C.C., Koeppen S., Heimans J.J., Huijgens P.C., Scheulen M.E., Strumberg D., Kiburg B., Postma T.J. Dose-related vincristine-induced peripheral neuropathy with unexpected off-therapy worsening. Neurology. 2005;64:1076–1077. doi: 10.1212/01.WNL.0000154642.45474.28. [DOI] [PubMed] [Google Scholar]
- 51.Sharma V., Singh I., Chaudhary P. Acorus calamus (the healing plant): A review on its medicinal potential, micropropagation and conservation. Nat. Prod. Res. 2014;28:1454–1466. doi: 10.1080/14786419.2014.915827. [DOI] [PubMed] [Google Scholar]
- 52.Shah P.D., Ghag M., Deshmukh P.B., Kulkarni Y., Joshi S.V., Vyas B.A., Shah D.R. Toxicity study of ethanolic extract of Acorus calamus rhizome. Int. J. Green Pharm. 2014;6:38–44. doi: 10.4103/0973-8258.97119. [DOI] [Google Scholar]
- 53.Li H., Dai Y., Zhang H., Xie C. Pharmacological studies on the chinese drug radix Angelicae dahuricae. Zhongguo Zhong Yao Za Zhi. 1991;16:560–562, 576. [PubMed] [Google Scholar]
- 54.Nie H., Shen Y.J. Effect of essential oil of radix Angelicae dahuricae on beta-endorphin, acth, no and proopiomelanocortin of pain model rats. Zhongguo Zhong Yao Za Zhi. 2002;27:690–693. [PubMed] [Google Scholar]
- 55.Yuan C.S., Mehendale S.R., Wang C.Z., Aung H.H., Jiang T., Guan X., Shoyama Y. Effects of Corydalis yanhusuo and Angelicae dahuricae on cold pressor-induced pain in humans: A controlled trial. J. Clin. Pharmacol. 2004;44:1323–1327. doi: 10.1177/0091270004267809. [DOI] [PubMed] [Google Scholar]
- 56.Akram M., Akhtar N., Asif H.M., Shah P.A., Saeed T., Mahmood A., Malik N.S. Butea monosperma lam.: A review. J. Med. Plants Res. 2011;5:3994–3996. [Google Scholar]
- 57.Madhavi A. An overview of Butea monosperma (flame of forest) World J. Pharm. Pharm. Sci. 2013;3:307–319. [Google Scholar]
- 58.Rahn E.J., Hohmann A.G. Cannabinoids as pharmacotherapies for neuropathic pain: From the bench to the bedside. Neurotherapeutics. 2009;6:713–737. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagarkatti P., Pandey R., Rieder S.A., Hegde V.L., Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009;1:1333–1349. doi: 10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahn E.J., Makriyannis A., Hohmann A.G. Activation of cannabinoid cb1 and cb2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br. J. Pharmacol. 2007;152:765–777. doi: 10.1038/sj.bjp.0707333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Velasco G., Hernandez-Tiedra S., Davila D., Lorente M. The use of cannabinoids as anticancer agents. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2016;64:259–266. doi: 10.1016/j.pnpbp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 62.Isah T. Rethinking Ginkgo biloba L.: Medicinal uses and conservation. Pharm. Rev. 2015;9:140–148. doi: 10.4103/0973-7847.162137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang W., Qiu W.L., Wang Y.S., Cong Q., Edwards D., Ye B., Xu C.J. Ginkgo may prevent genetic-associated ovarian cancer risk: Multiple biomarkers and anticancer pathways induced by ginkgolide b in brca1-mutant ovarian epithelial cells. Eur. J. Cancer Prev. 2011;20:508–517. doi: 10.1097/CEJ.0b013e328348fbb7. [DOI] [PubMed] [Google Scholar]
- 64.Marques F., Azevedo F., Johansson B., Oliveira R. Stimulation of DNA repair in saccharomyces cerevisiae by Ginkgo biloba leaf extract. Food Chem. Toxicol. 2011;49:1361–1366. doi: 10.1016/j.fct.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 65.Esmekaya M.A., Aytekin E., Ozgur E., Guler G., Ergun M.A., Omeroglu S., Seyhan N. Mutagenic and morphologic impacts of 1.8 ghz radiofrequency radiation on human peripheral blood lymphocytes (hPBLs) and possible protective role of pre-treatment with Ginkgo biloba (EGb 761) Sci. Total Environ. 2011;410:59–64. doi: 10.1016/j.scitotenv.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 66.Biggs M.L., Sorkin B.C., Nahin R.L., Kuller L.H., Fitzpatrick A.L. Ginkgo biloba and risk of cancer: Secondary analysis of the ginkgo evaluation of memory (gem) study. Pharmacoepidemiol. Drug Saf. 2010;19:694–698. doi: 10.1002/pds.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pattanayak P., Behera P., Das D., Panda S.K. Ocimum sanctum linn. A reservoir plant for therapeutic applications: An overview. Pharm. Rev. 2010;4:95–105. doi: 10.4103/0973-7847.65323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kelm M.A., Nair M.G., Strasburg G.M., DeWitt D.L. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum linn. Phytomedicine. 2000;7:7–13. doi: 10.1016/S0944-7113(00)80015-X. [DOI] [PubMed] [Google Scholar]
- 69.Prakash J., Gupta S.K. Chemopreventive activity of Ocimum sanctum seed oil. J. Ethnopharmacol. 2000;72:29–34. doi: 10.1016/S0378-8741(00)00194-X. [DOI] [PubMed] [Google Scholar]
- 70.Igwe S.A., Afonne J.C., Ghasi S.I. Ocular dynamics of systemic aqueous extracts of Xylopia aethiopica (african guinea pepper) seeds on visually active volunteers. J. Ethnopharm. 2003;86:139–142. doi: 10.1016/S0378-8741(02)00371-9. [DOI] [PubMed] [Google Scholar]
- 71.Adaramoye O.A., Sarkar J., Singh N., Meena S., Changkija B., Yadav P.P., Kanojiya S., Sinha S. Antiproliferative action of Xylopia aethiopica fruit extract on human cervical cancer cells. Phytother. Res. 2011;25:1558–1563. doi: 10.1002/ptr.3551. [DOI] [PubMed] [Google Scholar]
- 72.Choumessi A.T., Danel M., Chassaing S., Truchet I., Penlap V.B., Pieme A.C., Asonganyi T., Ducommun B., Valette A. Characterization of the antiproliferative activity of Xylopia aethiopica. Cell Div. 2012;7:8. doi: 10.1186/1747-1028-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cavalcanti B.C., Bezerra D.P., Magalhaes H.I., Moraes M.O., Lima M.A., Silveira E.R., Camara C.A., Rao V.S., Pessoa C., Costa-Lotufo L.V. Kauren-19-oic acid induces DNA damage followed by apoptosis in human leukemia cells. J. Appl. Toxicol. 2009;29:560–568. doi: 10.1002/jat.1439. [DOI] [PubMed] [Google Scholar]
- 74.Karasawa T., Steyger P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015;237:219–227. doi: 10.1016/j.toxlet.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tsang R.Y., Al-Fayea T., Au H.J. Cisplatin overdose: Toxicities and management. Drug Saf. 2009;32:1109–1122. doi: 10.2165/11316640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 76.Ataie A., Sabetkasaei M., Haghparast A., Moghaddam A.H., Kazeminejad B. Neuroprotective effects of the polyphenolic antioxidant agent, curcumin, against homocysteine-induced cognitive impairment and oxidative stress in the rat. Pharmacol. Biochem. Behav. 2010;96:378–385. doi: 10.1016/j.pbb.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 77.Attia H.N., Al-Rasheed N.M., Al-Rasheed N.M., Maklad Y.A., Ahmed A.A., Kenawy S.A. Protective effects of combined therapy of gliclazide with curcumin in experimental diabetic neuropathy in rats. Behav. Pharmacol. 2012;23:153–161. doi: 10.1097/FBP.0b013e3283512c00. [DOI] [PubMed] [Google Scholar]
- 78.Kandhare A.D., Raygude K.S., Ghosh P., Ghule A.E., Bodhankar S.L. Therapeutic role of curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neurosci. Lett. 2012;511:18–22. doi: 10.1016/j.neulet.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 79.Ravindran J., Prasad S., Aggarwal B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lopez-Lazaro M. Anticancer and carcinogenic properties of curcumin: Considerations for its clinical development as a cancer chemopreventive and chemotherapeutic agent. Mol. Nutr. Food Res. 2008;52(Suppl 1):S103–S127. doi: 10.1002/mnfr.200700238. [DOI] [PubMed] [Google Scholar]
- 81.Sharma R.A., Euden S.A., Platton S.L., Cooke D.N., Shafayat A., Hewitt H.R., Marczylo T.H., Morgan B., Hemingway D., Plummer S.M., et al. Phase i clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 82.Kunnumakkara A.B., Guha S., Krishnan S., Diagaradjane P., Gelovani J., Aggarwal B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappab-regulated gene products. Cancer Res. 2007;67:3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- 83.Dance-Barnes S.T., Kock N.D., Moore J.E., Lin E.Y., Mosley L.J., D’Agostino R.B., Jr., McCoy T.P., Townsend A.J., Miller M.S. Lung tumor promotion by curcumin. Carcinogenesis. 2009;30:1016–1023. doi: 10.1093/carcin/bgp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.National Toxicology Program NTP toxicology and carcinogenesis studies of turmeric oleoresin (cas no. 8024-37-1) (major component 79%–85% curcumin, cas no. 458-37-7) in F344/N rats and B6C3F1 mice (feed studies) Nat. Toxicol. Program. Tech. Rep. Ser. 1993;427:1–275. [PubMed] [Google Scholar]
- 85.Miroddi M., Navarra M., Quattropani M.C., Calapai F., Gangemi S., Calapai G. Systematic review of clinical trials assessing pharmacological properties of salvia species on memory, cognitive impairment and alzheimer’s disease. CNS Neurosci. Ther. 2014;20:485–495. doi: 10.1111/cns.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perry N.B., Anderson R.E., Brennan N.J., Douglas M.H., Heaney A.J., McGimpsey J.A., Smallfield B.M. Essential oils from dalmatian sage (Salvia officinalis L.): Variations among individuals, plant parts, seasons, and sites. J. Agric. Food Chem. 1999;47:2048–2054. doi: 10.1021/jf981170m. [DOI] [PubMed] [Google Scholar]
- 87.Abu-Darwish M.S., Cabral C., Ferreira I.V., Goncalves M.J., Cavaleiro C., Cruz M.T., Al-bdour T.H., Salgueiro L. Essential oil of common sage (Salvia officinalis L.) from jordan: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. BioMed Res. Int. 2013;2013:538940. doi: 10.1155/2013/538940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vujosevic M., Blagojevic J. Antimutagenic effects of extracts from sage (Salvia officinalis) in mammalian system in vivo. Acta Vet. Hung. 2004;52:439–443. doi: 10.1556/AVet.52.2004.4.6. [DOI] [PubMed] [Google Scholar]
- 89.Keshavarz M., Bidmeshkipour A., Mostafaie A., Mansouri K., Mohammadi-Motlagh H.R. Anti tumor activity of Salvia officinalis is due to its anti-angiogenic, anti-migratory and anti-proliferative effects. Yakhteh. 2011;12:477–482. [Google Scholar]
- 90.Hardman W.E., Ion G., Akinsete J.A., Witte T.R. Dietary walnut suppressed mammary gland tumorigenesis in the c(3)1 tag mouse. Nutr. Cancer. 2011;63:960–970. doi: 10.1080/01635581.2011.589959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hardman W.E., Ion G. Suppression of implanted mda-mb 231 human breast cancer growth in nude mice by dietary walnut. Nutr. Cancer. 2008;60:666–674. doi: 10.1080/01635580802065302. [DOI] [PubMed] [Google Scholar]
- 92.Davis P.A., Vasu V.T., Gohil K., Kim H., Khan I.H., Cross C.E., Yokoyama W. A high-fat diet containing whole walnuts (Juglans regia) reduces tumour size and growth along with plasma insulin-like growth factor 1 in the transgenic adenocarcinoma of the mouse prostate model. Br. J. Nutr. 2012;108:1764–1772. doi: 10.1017/S0007114511007288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reiter R.J., Tan D.X., Manchester L.C., Korkmaz A., Fuentes-Broto L., Hardman W.E., Rosales-Corral S.A., Qi W.B. A walnut-enriched diet reduces the growth of lncap human prostate cancer xenografts in nude mice. Cancer Investig. 2013;31:365–373. doi: 10.3109/07357907.2013.800095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nagel J.M., Brinkoetter M., Magkos F., Liu X., Chamberland J.P., Shah S., Zhou J.R., Blackburn G., Mantzoros C.S. Dietary walnuts inhibit colorectal cancer growth in mice by suppressing angiogenesis. Nutrition. 2012;28:67–75. doi: 10.1016/j.nut.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Becouarn Y., Ychou M., Ducreux M., Borel C., Bertheault-Cvitkovic F., Seitz J.F., Nasca S., Nguyen T.D., Paillot B., Raoul J.L., et al. Phase ii trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. J. Clin. Oncol. 1998;16:2739–2744. doi: 10.1200/JCO.1998.16.8.2739. [DOI] [PubMed] [Google Scholar]
- 96.Graham J., Muhsin M., Kirkpatrick P. Oxaliplatin. Nat. Rev. Drug Discov. 2004;3:11–12. doi: 10.1038/nrd1287. [DOI] [PubMed] [Google Scholar]
- 97.Pasetto L.M., D’Andrea M.R., Rossi E., Monfardini S. Oxaliplatin-related neurotoxicity: How and why? Crit. Rev. Oncol. Hemat. 2006;59:159–168. doi: 10.1016/j.critrevonc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 98.Waseem M., Parvez S. Neuroprotective activities of curcumin and quercetin with potential relevance to mitochondrial dysfunction induced by oxaliplatin. Protoplasma. 2016;253:417–430. doi: 10.1007/s00709-015-0821-6. [DOI] [PubMed] [Google Scholar]
- 99.Morand C., Manach C., Crespy V., Remesy C. Respective bioavailability of quercetin aglycone and its glycosides in a rat model. BioFactors. 2000;12:169–174. doi: 10.1002/biof.5520120127. [DOI] [PubMed] [Google Scholar]
- 100.Raygude K.S., Kandhare A.D., Ghosh P., Ghule A.E., Bodhankar S.L. Evaluation of ameliorative effect of quercetin in experimental model of alcoholic neuropathy in rats. Inflammopharmacology. 2012;20:331–341. doi: 10.1007/s10787-012-0122-z. [DOI] [PubMed] [Google Scholar]
- 101.Ruiz P.A., Braune A., Haller D.R. Quercetin inhibits tnf-induced irf-1 but not nf-kappa b recruitment to the ip-10 gene promoter in intestinal epithelial cells through the modulation of histone acetyl transferase activity. Gastroenterology. 2006;130:A693–A693. [Google Scholar]
- 102.Ferry D.R., Smith A., Malkhandi J., Fyfe D.W., DeTakats P.G., Anderson D., Baker J., Kerr D.J. Phase i clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996;2:659–668. [PubMed] [Google Scholar]
- 103.Zaveri N.T. Green tea and its polyphenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 104.Khan N., Mukhtar H. Cancer and metastasis: Prevention and treatment by green tea. Cancer Metast. Rev. 2010;29:435–445. doi: 10.1007/s10555-010-9236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakachi K., Suemasu K., Suga K., Takeo T., Imai K., Higashi Y. Influence of drinking green tea on breast cancer malignancy among japanese patients. Jpn. J. Cancer Res. 1998;89:254–261. doi: 10.1111/j.1349-7006.1998.tb00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saville M.W., Lietzau J., Pluda J.M., Feuerstein I., Odom J., Wilson W.H., Humphrey R.W., Feigal E., Steinberg S.M., Broder S., et al. Treatment of hiv-associated kaposi’s sarcoma with paclitaxel. Lancet. 1995;346:26–28. doi: 10.1016/S0140-6736(95)92654-2. [DOI] [PubMed] [Google Scholar]
- 107.Bharadwaj R., Yu H. The spindle checkpoint, aneuploidy, and cancer. Oncogene. 2004;23:2016–2027. doi: 10.1038/sj.onc.1207374. [DOI] [PubMed] [Google Scholar]
- 108.Grisold W., Cavaletti G., Windebank A.J. Peripheral neuropathies from chemotherapeutics and targeted agents: Diagnosis, treatment, and prevention. Neuro-Oncology. 2012;14:45–54. doi: 10.1093/neuonc/nos203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Campos A.C., Moreira F.A., Gomes F.V., del Bel E.A., Guimaraes F.S. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. R. Soc. B. 2012;367:3364–3378. doi: 10.1098/rstb.2011.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mechoulam R., Parker L.A., Gallily R. Cannabidiol: An overview of some pharmacological aspects. J. Clin. Pharmacol. 2002;42(Suppl. 1):11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- 111.Andoh T., Kitamura R., Fushimi H., Komatsu K., Shibahara N., Kuraishi Y. Effects of goshajinkigan, hachimijiogan, and rokumigan on mechanical allodynia induced by paclitaxel in mice. J. Tradit. Complement. Med. 2014;4:293–297. doi: 10.4103/2225-4110.128906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Materazzi S., Fusi C., Benemei S., Pedretti P., Patacchini R., Nilius B., Prenen J., Creminon C., Geppetti P., Nassini R. Trpa1 and trpv4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflugers Arch. Eur. J. Physiol. 2012;463:561–569. doi: 10.1007/s00424-011-1071-x. [DOI] [PubMed] [Google Scholar]
- 113.Alessandri-Haber N., Dina O.A., Joseph E.K., Reichling D.B., Levine J.D. Interaction of transient receptor potential vanilloid 4, integrin, and src tyrosine kinase in mechanical hyperalgesia. J. Neurosci. 2008;28:1046–1057. doi: 10.1523/JNEUROSCI.4497-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goswami C. Trpv1-tubulin complex: Involvement of membrane tubulin in the regulation of chemotherapy-induced peripheral neuropathy. J. Neurochem. 2012;123:1–13. doi: 10.1111/j.1471-4159.2012.07892.x. [DOI] [PubMed] [Google Scholar]
- 115.Gauchan P., Andoh T., Kato A., Kuraishi Y. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neurosci. Lett. 2009;458:93–95. doi: 10.1016/j.neulet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 116.Mizuno K., Kono T., Suzuki Y., Miyagi C., Omiya Y., Miyano K., Kase Y., Uezono Y. Goshajinkigan, a traditional japanese medicine, prevents oxaliplatin-induced acute peripheral neuropathy by suppressing functional alteration of trp channels in rat. J. Pharmacol. Sci. 2014;125:91–98. doi: 10.1254/jphs.13244FP. [DOI] [PubMed] [Google Scholar]
- 117.Matsumura Y., Yokoyama Y., Hirakawa H., Shigeto T., Futagami M., Mizunuma H. The prophylactic effects of a traditional japanese medicine, goshajinkigan, on paclitaxel-induced peripheral neuropathy and its mechanism of action. Mol. Pain. 2014;10:61. doi: 10.1186/1744-8069-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bifulco M., Malfitano A.M., Pisanti S., Laezza C. Endocannabinoids in endocrine and related tumours. Endocr. Relat. Cancer. 2008;15:391–408. doi: 10.1677/ERC-07-0258. [DOI] [PubMed] [Google Scholar]
- 119.McKallip R.J., Lombard C., Fisher M., Martin B.R., Ryu S.H., Grant S., Nagarkatti P.S., Nagarkatti M. Targeting cb2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood. 2002;100:627–634. doi: 10.1182/blood-2002-01-0098. [DOI] [PubMed] [Google Scholar]
- 120.Li R., Wang H., Bekele B.N., Yin Z., Caraway N.P., Katz R.L., Stass S.A., Jiang F. Identification of putative oncogenes in lung adenocarcinoma by a comprehensive functional genomic approach. Oncogene. 2006;25:2628–2635. doi: 10.1038/sj.onc.1209289. [DOI] [PubMed] [Google Scholar]
- 121.Ramer R., Rohde A., Merkord J., Rohde H., Hinz B. Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm. Res. 2010;27:2162–2174. doi: 10.1007/s11095-010-0219-2. [DOI] [PubMed] [Google Scholar]
- 122.Wang X.M., Lehky T.J., Brell J.M., Dorsey S.G. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine. 2012;59:3–9. doi: 10.1016/j.cyto.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chiurchiu V., Leuti A., Maccarrone M. Cannabinoid signaling and neuroinflammatory diseases: A melting pot for the regulation of brain immune responses. J. Neuroimmune Pharmacol. 2015;10:268–280. doi: 10.1007/s11481-015-9584-2. [DOI] [PubMed] [Google Scholar]


