Abstract
Paeonia is the single genus of ca. 33 known species in the family Paeoniaceae, found in Asia, Europe and Western North America. Up to now, more than 180 compounds have been isolated from nine species of the genus Paeonia, including terpenes, phenols, flavonoids, essential oil and tannins. Terpenes, the most abundant naturally occurring compounds, which accounted for about 57% and occurred in almost every species, are responsible for the observed in vivo and in vitro biological activities. This paper aims to give a comprehensive overview of the recent phytochemical and pharmacological knowledge of the terpenes from Paeonia plants, and enlighten further drug discovery research.
Keywords: Genus Paeonia, terpene, chemical components, pharmacological activities
1. Introduction
Natural products contribute significantly to drug discovery research with a rich source of compounds and provide inherently large-scale of structural diversity than synthetic compounds [1]. The genus Paeonia belongs to the family Paeoniaceae and consists of about thirty-three known species [2]. The roots of P. suffruticosa, P. obovata, and P. lactiflora are important sources of crude drugs in traditional Chinese medication with activities of nitric oxide production inhibitory effects [3,4], anti-tumor activity [5,6], anti-inflammatory effects [7], anti-influenza virus [8], hematopoietic effects [9], anti-aggregatoryand and anti-coagulative effects [10].
2. Plant Distribution
The genus Paeonia naturally distributes in the cold and temperate areas of the Northern Hemisphere. They are mainly distributed in Asia and Europe, and only a few native to Western North America. A total of 11 species are found in China, with wide distribution in southwestern and northwestern areas, central China, northern and northeastern China [11].
In detail, P. emodi grows in the western Himalayas between Nepal and Pakistan [12]. P. obovata naturally distributes in forests ranging from deciduous broad-leaved to coniferous forests and may be found at an altitude from 200 m to 2800 m. In China, it occurs in Anhui, Gansu, Guizhou, Hebei, and Heilongjiang et al. It also grows in Korea, Russia and Japan [13]. In addition, P. lactiflora occurs in northern and northeastern China, Korea, Japan, Mongolia, Russia Far East and Siberia [14]. P. veitchii distributes in western China including Shanxi, Gansu, Ningxia, Qinghai, Sichuan and the eastern rim of Tibet [15]. P. suffruticosa grows in Central to Northern China including Tongling, Heze, Luoyang, Pengzhou, and Beijing [16]. P. delavayi is endemic to southwestern China, where its habitat is limited to Sichuan, Yunnan and the very South-East of Tibet [17].
3. Chemical Constituents
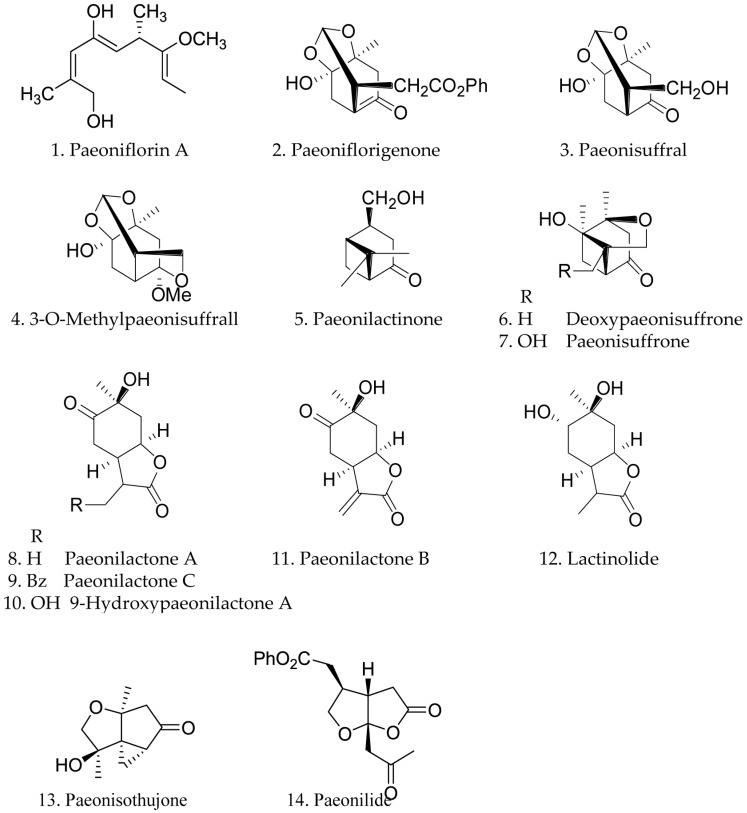
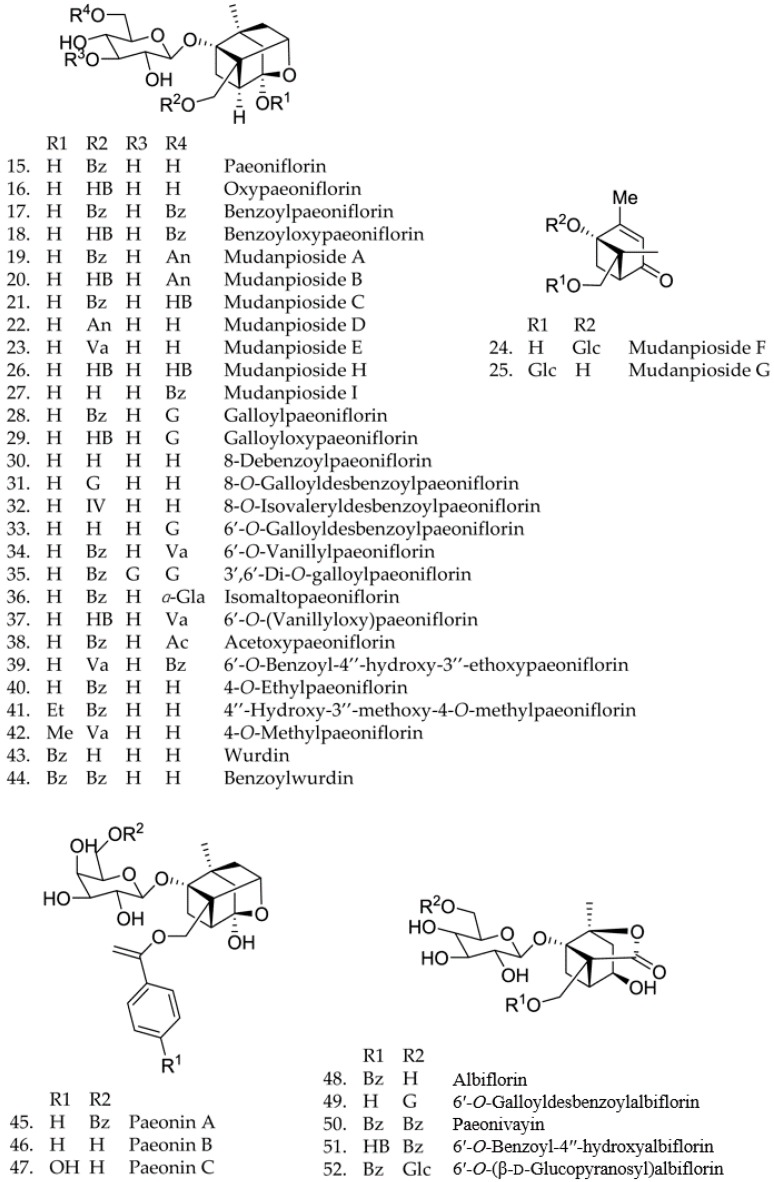
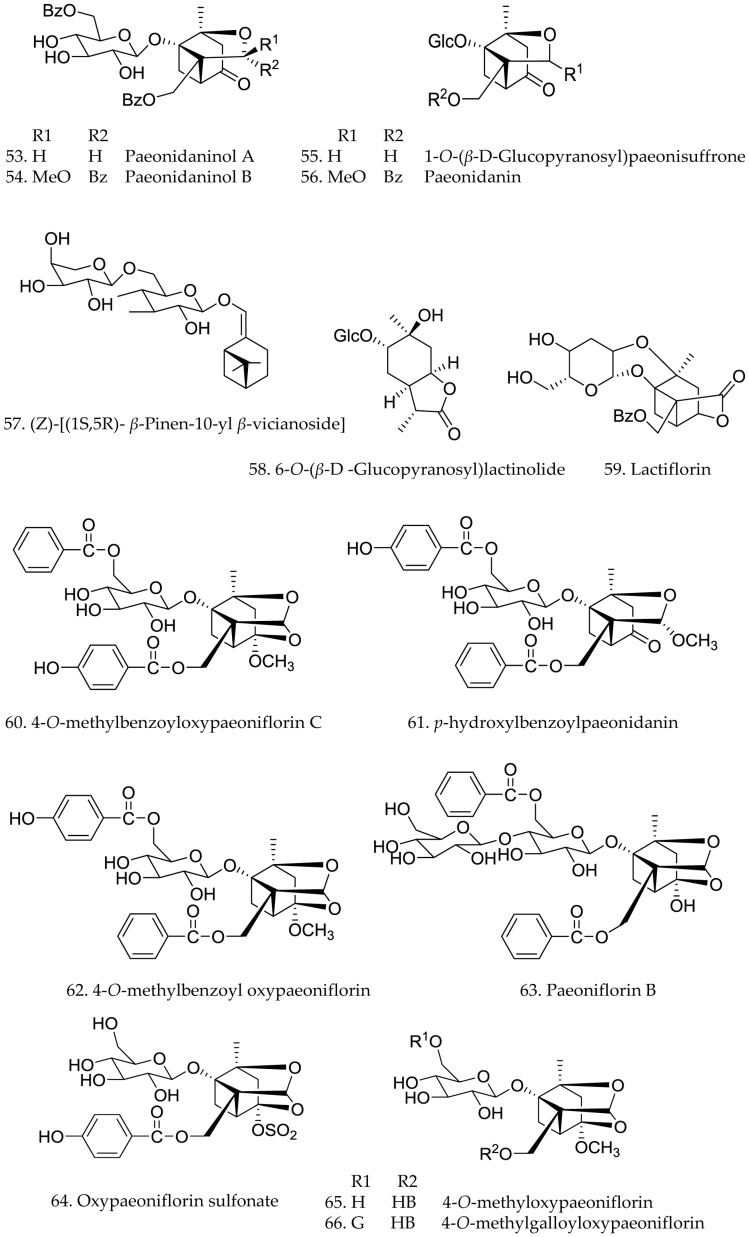
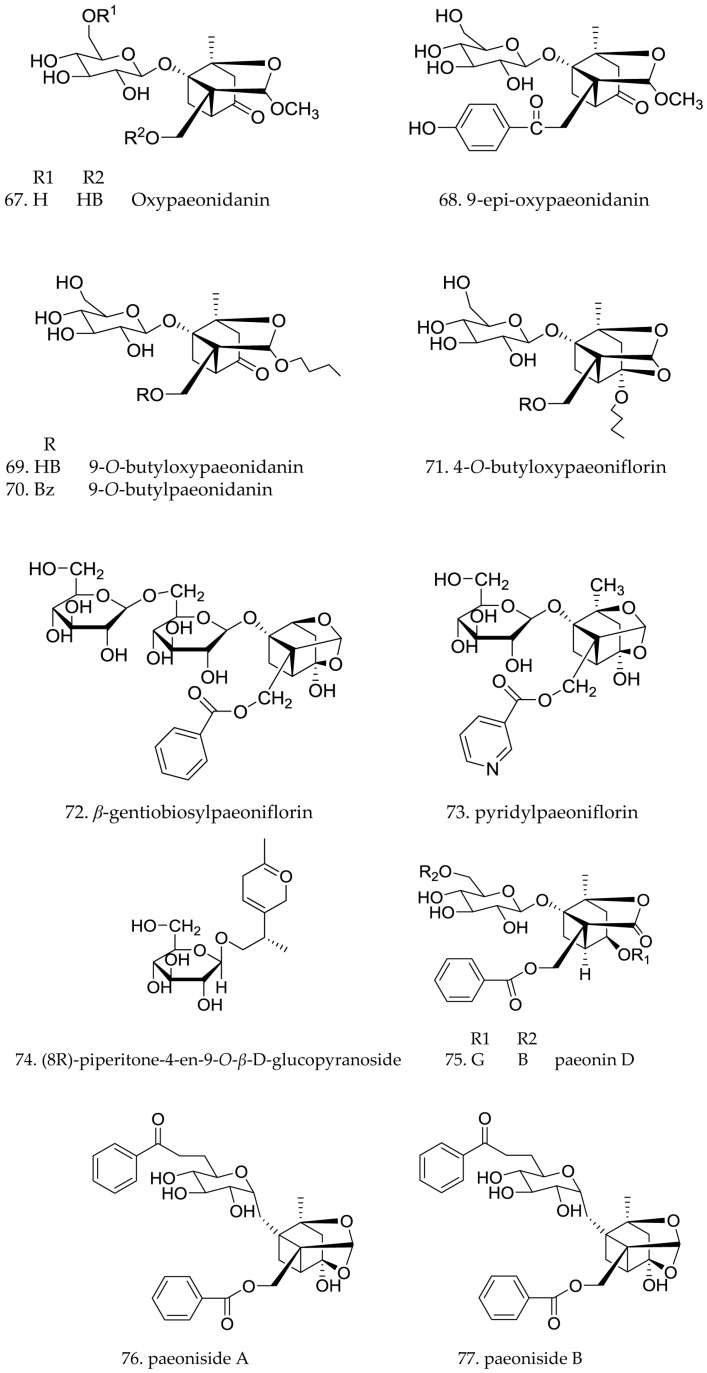
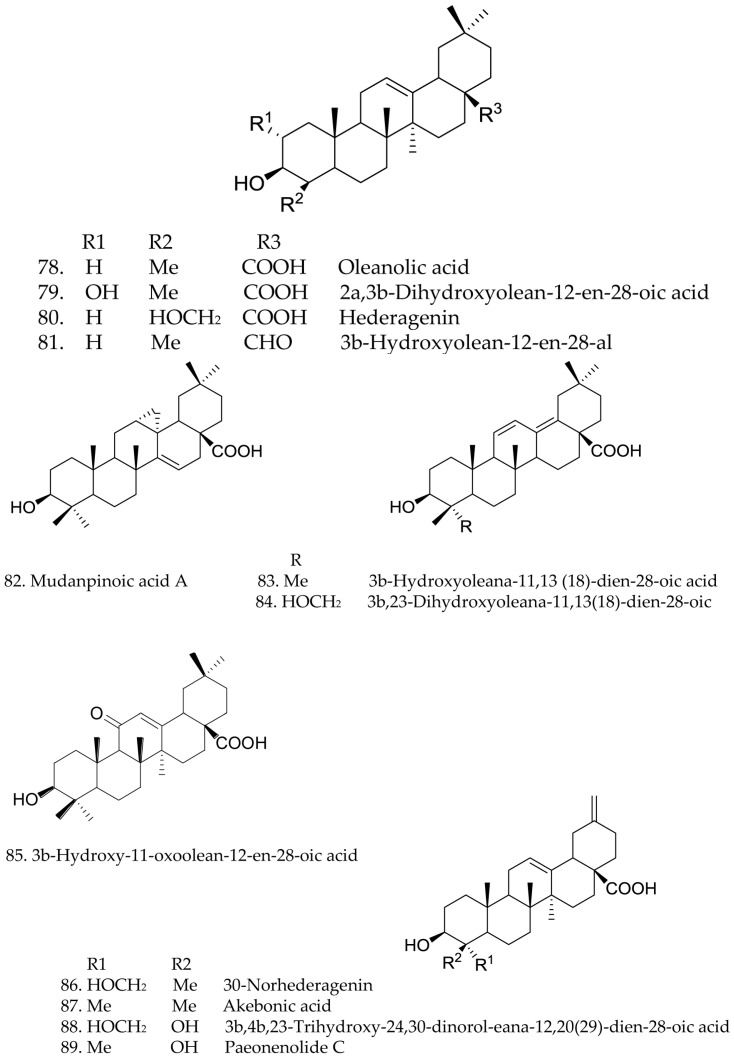
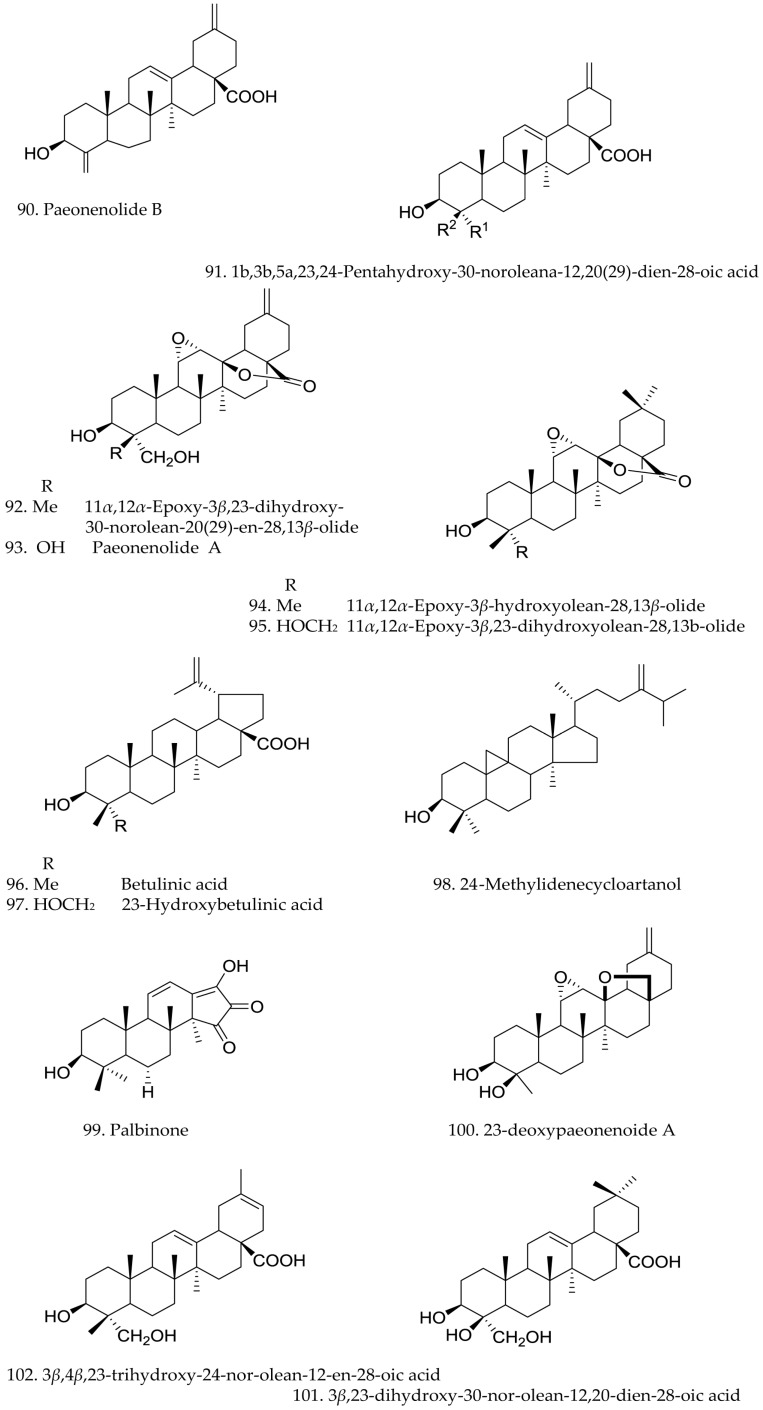
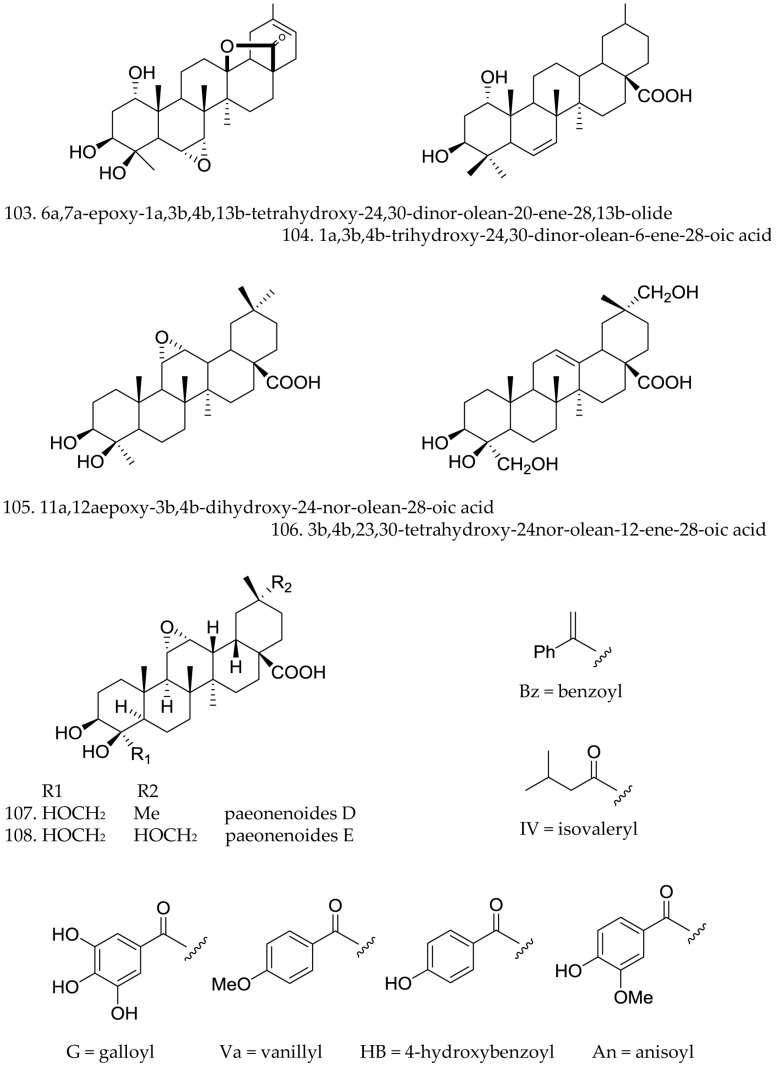
The present chemical studies of Paeonia plants were more focused on the composition of the roots and less on other parts. Since 1753 [18], more than 180 compounds have been isolated. Nine species of the genus Paeonia have been chemically investigated, including P. albiflora, P. delavayi, P. emodi, P. japonica, P. lactiflora, P. obovata, P. peregrina, P. suffruticosa, P. Veitchii. Their flowers are shown below in Figure 1a–i. The skeletons of terpenoid compounds from this genus included monoterpenes, monoterpene glycosides and triterpenes. Their structures and names are summarized below (structures 1–108 and Figure 2, Figure 3 and Figure 4). As shown, monoterpene glycosides are the important components in the genus Paeonia.
Figure 1.
The flowers of the chemically investigated nine species of the genus Paeonia. (a) P. albiflora; (b) P. delavayi; (c) P. emodi; (d) P. japonica; (e) P. lactiflora; (f) P. obovata; (g) P. peregrina; (h) P. suffruticosa; (i) P. veitchii. (https://it.wikipedia.org/wiki/Paeonia).
Figure 2.
Chemical structures of monoterpene.
Figure 3.
Chemical structures of monoterpene glycosides.
Figure 4.
Chemical structures of triterpenes.
3.1. Monoterpenes
Fourteen monoterpenes, 1–14, were isolated from Paeonia species. Paeoniflorin A (1) was obtained from the root cortex P. suffruticosa [19]. Paeoniflorigenone (2), one of the main bioactive constituents, was found in three species, P. suffruticosa, P. peregrina, and P. albiflora [20]. Most monoterpenes were obtained from P. suffruticosa [21]. Three p-menthane monoterpenes, paeonilactone A–C (8, 9, and 11, resp.), were obtained from P. albiflora. In 1996, Paeonilactinone (5) and Lactinolide (12) were reported from P. lactiflora [22]. Later, paeonilide (14) were found in P. delavayi [23]. Their structures, 1–14, are shown below, and their names are collected in Figure 2.
3.2. Monoterpene Glycosides
A total of 58 monoterpene glycosides, 15–77, have been isolated from Paeonia species. Compounds 15–57 are pinane type derivatives with a variety of substituents [8,22,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Later, 6-O-(β-d-glucopyranosyl)lactinolide (58), and lactiflorin (59) were obtained from P. lactiflora [44]. Paeonia species are a rich source of monoterpene constituents possessing a “cage-like” pinane skeleton, which are found as the main biologically active compounds.
In 2012, 11 monoterpene glycosides, including, 4-O-methy-lmoudanpioside C (60), p-hydroxylbenzoyl-paeonidanin (61), 4-O-methylbenzoyl oxypaeoniflorin (62), paeoniflorin B (63), oxypaeoniflorin sulfonate (64), 4-O-methyloxypaeoniflorin (65), 4-O-methylgalloyloxy paeoniflorin (66), oxypaeonidanin (67), 9-epi-oxypaeonidanin (68), 9-O-butyloxypaeonidanin (69), 9-O-butylpaeonidanin (70), and 4-O-butyloxypaeoniflorin (71), were obtained from the ethanol extract of P. suffruticosa Andrews [4]. They equally have “cage-like” pinane skeleton. In addition, in 2012, β-gentiobiosylpaeoniflorin (72), pyridylpaeoniflorin (73), (8R)-piperitone-4-en-9-O-β-d-gluco-pyranoside (74) are isolated from P. suffruticosa [45]. In 2014, a new monoterpene glucoside, paeonin D (75), were obtained from P. lactiflora [46]. In the same year, paeoniside A (76) and paeoniside B (77) were isolated from P. suffruticosa [47]. Their structures, 15–77 are shown below, and their names are collected in Figure 3.
3.3. Triterpenes
A total of 30 triterpenes, 78–108, have been reported from various Paeonia species [44,48,49,50,51,52,53]. Among them, compounds 78–80, 83–86, and 94–97 were isolated from the callus tissues of P. suffruticosa, P. lactiflora, and P. japonica [47,48,54]. Later, four novel 24,30-dinortriterpenoids, 88–90 and 93, were only found in P. delavayi [17]. In 2011, three noroleanane triterpenes 100–102 were obtained from P. rockii [50]. In 2012, four noroleanane triterpenes 103–106 were obtained from P. emodi [55]. In addition, in 2016, two new nortriterpenoids, paeonenoides D (107) and paeonenoides E (108), were obtained from P. lactiflora [56]. Their structures, 78–108 are shown below, and their names are collected in Figure 4.
4. Biological Activities
4.1. Inhibitors of Nitric Oxide Production
Three compounds (1, 17 and 62) was showed significantly suppressed nitric oxide production [4]. Paeoniflorin (15) inhibiting inflammation and inducible nitric oxide synthase signaling pathways, and ameliorates acute myocardial infarction of rat [3]. Compounds 107, 108 were showed inhibitory effects against nitric oxide production in LPS-induced RAW246.7 macrophages [56].
4.2. Anti-Tumor Activity
A few of the compounds showed significant cytotoxicity against a panel of human cancer cell lines. Compound 95 against MCF-7, HT-29, M-14 [57]; Compounds 104–106 against A549, HCT116, HL-60, ZR-75-30; Compounds 104–106 against HL-60, HCT116 and ZR-75-30 [58]; Compounds 107, 108 against Hep-G2, SK-OV-3, HL-60 [56].
4.3. Anti-Inflammatory Effects
Palbinone (99) have a strong inhibitory anti-inflammatory effect NF-κB signal pathway [52]. Paeoniflorin (15) may ameliorate acute renal injury following ANP in rats by inhibiting inflammatory responses and renal cell apoptosis, due to the p38-MAPK and NF-κB [59]. In addition, in 2016, Zhihong M. et al. reported it to have liver protective and anti-inflammatory effects in HCF diet-induced NASH rats, associated with inhibition of the ROCK and NF-κB in the NASH liver [7]. In addition, paeoniside A (76) inhibited against cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) enzymes [47].
4.4. Anti-Oxidative Effects
Compounds 28 and 29 have potent radical-scavenging remarkable effects on DPPH, and compounds 16 have a weak radical-scavenging effect [60]. It is also demonstrated paeonins A (45) and paeonins B (46) exhibited inhibitory activities against lipoxygenase [40]. Paeonin C (47) has potent inhibitory potential against lipoxygenase in a concentration-dependent fashion [61]. Compounds 33 demonstrated a significant scavenging capacity against the DPPH free radical, ROS, the superoxide anion radical, and the hydroxyl radical [62].
4.5. Anti-Aggregatory and Anti-Coagulative Effects
Paeonilide (14) selectively inhibited the platelet aggregation induced by platelet activating factor [23]. Koo Y., et al. reported that paeoniflorin (15) and benzoylpaeoniflorin (17) have obvious inhibitory effect on collagen, endotoxin and adenosine diphosphate (ADP)-induced blood platelet coagulation, but not on blood aggregation in vitro. In addition, compounds 15 and 19 exhibited blood coagulation-inhibitory activity in vivo [10].
4.6. Sedative and Analgesic
Paeoniflorin (15) could modulate sleep behaviors and the mechanisms involved, and increased NREM sleep by inhibiting the histaminergic system via A1 receptors [63]. In addition, it could inhibit formalin-induced nociceptive behavior in mice, these effects may be might be associated with modulation of NMDA receptors, specifically the NR2B subunit [64]. Shimizu et al. found that paeoniflorigenone (2) produced a blocking effect on neuromuscular junction in phrenic nerve diaphragm preparations of mice [65]. In addition, paeonilactone C (9) was showed to suppress stimulated muscle twitchings of frog sciatic nerve-sartorius muscle [66].
4.7. Other Activities
Paeoniflorin (15) and 8-debenzoylpaeoniflorin (30) showed a blood sugar lowering effect in streptozotocin-treated rats [67]. 1-O-(β-d-Glucopyranosyl)paeonisuffrone (55) was found to inhibit histamine release from rat peritoneal exudate cell-induced antigen-antibody reaction [22]. Compound 52 was found to have a direct stimulatory effect on bone formation in vitro and may contribute to the prevention for osteoporosis [42]. Paeoniflorin (15) has previously been reported to alleviate hepatic fibrosis. Paeoniflorin was found to effectively prevent renal interstitial fibrosis [68]. Paeoniflorin (15) has sedative, hypotensive, and weak anti-inflammatory effects, and a preventive effect on stress ulcer [69]. Compound 89 showed inhibitory activity against β-glucuronidase [70].
5. Conclusions
Paeonia is the only genus in the family Paeoniaceae and has significant medicinal importance in traditional Chinese medicine. Researchers have different views on the number of species that can be distinguished ranging from 25 to 40 [71,72], although the current consensus is thirty-three known species. Based on data available, this paper summarizes three types of terpene compositions and exhibited their bioactivities such as inhibitors of nitric oxide production, anti-tumor activity, anti-inflammatory effects, anti-oxidative effects, anti-aggregatoryand and anti-coagulative effects, sedative and analgesic activity. Taken together, the compounds from Paeonia plants have a great potential to be used as new chemical drugs in future. However, only nine species of the genus Paeonia have been chemically studied, it should be urgent to study other species for more potential bioactive components. In addition, the relationships between the species also need to be further clarified.
Acknowledgments
This work was supported by Educational Commission of Heilongjiang Province of China to D.-D.Z. from 2014 (Project Num. 12541643) and Postdoctoral Science Foundation of Heilongjiang Province of China to D.-D.Z. (Project Num. LBH-Z13169).
Author Contributions
D.-D.Z. analyzed, organized and wrote the literature papers; L.-L.J. edited the information of chemical components and pharmacological activities; H.-Y.L. helped to search the botanical information of the Genus Paeonia; P.-F.Y. and Y.-L.Z. analyzed and edited the manuscript. All authors read, revised and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Talmadge J.E. Natural product derived immune-regulatory agents. Int. Immunopharmacol. 2016;37:5–15. doi: 10.1016/j.intimp.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 2.Maarten J.M.C., James W.B. The number of known plants species in the world and its annual increase. Phytotaxa. 2016;261:201–217. [Google Scholar]
- 3.Chen C., Du P., Wang J. Paeoniflorin ameliorates acute myocardial infarction of rats by inhibiting inflammation and inducible nitric oxide synthase signaling pathways. Mol. Med. Rep. 2015;12:3937–3943. doi: 10.3892/mmr.2015.3870. [DOI] [PubMed] [Google Scholar]
- 4.Ding L., Zhao F., Chen L., Jiang Z., Liu Y., Li Z., Qiu F., Yao X. New monoterpene glycosides from Paeonia suffruticosa andrews and their inhibition on NO production in LPS-induced RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2012;22:7243–7247. doi: 10.1016/j.bmcl.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 5.Wu J., Xue X., Zhang B., Jiang W., Cao H., Wang R., Sun D., Guo R. The protective effects of paeonol against epirubicin-induced hepatotoxicity in 4T1-tumor bearing mice via inhibition of the PI3K/Akt/NF-κB pathway. Chem. Biol. Interact. 2016;244:1–8. doi: 10.1016/j.cbi.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Li C., Yazawa K., Kondo S., Mukudai Y., Sato D., Kurihara Y., Kamatani T., Shintani S. The root bark of Paeonia moutan is a potential anticancer agent in human oral squamous cell carcinoma cells. Anticancer Res. 2012;32:2625–2630. [PubMed] [Google Scholar]
- 7.Ma Z., Chu L., Liu H., Li J., Zhang Y., Liu W., Dai J., Yi J., Gao Y. Paeoniflorin alleviates non-alcoholic steatohepatitis in rats: Involvement with the rock/NF-κB pathway. Int. Immunopharmacol. 2016;38:377–384. doi: 10.1016/j.intimp.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Li J., Yang X., Huang L. Anti-influenza virus activity and constituents. Characterization of Paeonia delavayi extracts. Molecules. 2016;21:1133–1143. doi: 10.3390/molecules21091133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y., Wang L., Yang Z., Wang J., Li W., Zhou J., Zhang J. Hematopoietic effects of paeoniflorin and albiflorin on radiotherapy-induced myelosuppression mice. Evid. Complement. Altern. Med. eCAM. 2016;2016 doi: 10.1155/2016/5789381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo Y.K., Kim J.M., Koo J.Y., Kang S.S., Bae K., Kim Y.S., Chung J.H., Yun-Choi H.S. Platelet anti-aggregatory and blood anti-coagulant effects of compounds isolated from Paeonia lactiflora and Paeonia suffruticosa. Die Pharm. 2010;65:624–628. [PubMed] [Google Scholar]
- 11.He C., Peng B., Dan Y., Peng Y., Xiao P. Chemical taxonomy of tree peony species from China based on root cortex metabolic fingerprinting. Phytochemistry. 2014;107:69–79. doi: 10.1016/j.phytochem.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Jugran A.K., Chaudhary W.Y., Bahukhandi A., Bhatt I.D., Rawal R.S., Dhyani P.P. Effect of processing and storage methods on the nutritional, anti-nutritional, and anti-oxidant properties of Paeonia emodi, wall. Ex. Royle. Appl. Biochem. Biotechnol. 2016;180:322–337. doi: 10.1007/s12010-016-2101-0. [DOI] [PubMed] [Google Scholar]
- 13.Bae J.Y., Kim C.Y., Kim H.J., Park J.H., Ahn M.J. Differences in the chemical profiles and biological activities of Paeonia lactiflora and Paeonia obovata. J. Med. Food. 2015;18:224–232. doi: 10.1089/jmf.2014.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu S., Yu X., Wu Y., Shiraishi F., Kawahara N., Komatsu K. Genetic and chemical characterization of white and red peony root derived from Paeonia lactiflora. J. Nat. Med. 2015;69:35–45. doi: 10.1007/s11418-014-0857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang R., Chou G.X., Zhu E.Y., Wang Z.T., Bi K.S. A new phenolic glycoside from the roots of Paeonia veitchii. J. Asian Nat. Prod. Res. 2006;8:277–280. doi: 10.1080/10286020500172608. [DOI] [PubMed] [Google Scholar]
- 16.Zhou S.L., Zou X.H., Zhou Z.Q., Liu J., Xu C., Yu J., Wang Q., Zhang D.M., Wang X.Q., Ge S., et al. Multiple species of wild tree peonies gave rise to the “king of flowers”, Paeonia suffruticosa andrews. Proc. R. Soc. Biol. Sci. 2014;281 doi: 10.1098/rspb.2014.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasegawa Y., Gong X., Kuroda C. Chemical diversity of iridal-type triterpenes in iris delavayi collected in Yunnan province of China. Nat. Prod. Commun. 2011;6:789–792. [PubMed] [Google Scholar]
- 18.Horikoshi T., Homma N., Hatakeyama Y., Hemmi S. Studies on the cultivation of medicinal plants. V. On the growth of Paeonia suffruticosa Andr. Especially multiplication and yield (author’s transl) Bull. Natl. Inst. Hyg. Sci. 1975:109–113. [PubMed] [Google Scholar]
- 19.Ding L., Jiang Z., Liu Y., Chen L., Zhao Q., Yao X., Zhao F., Qiu F. Monoterpenoid inhibitors of no production from Paeonia suffruticosa. Fitoterapia. 2012;83:1598–1603. doi: 10.1016/j.fitote.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Luo N.C., Ding W., Wu J., Qian D.W., Li Z.H., Qian Y.F., Guo J.M., Duan J.A. UPLC-Q-TOF/MS coupled with multivariate statistical analysis as a powerful technique for rapidly exploring potential chemical markers to differentiate between radix paeoniae alba and radix paeoniae rubra. Nat. Prod. Commun. 2013;8:487–491. [PubMed] [Google Scholar]
- 21.Yoshikawa M., Ohta T., Kawaguchi A., Matsuda H. Bioactive constituents of Chinese natural medicines. V. Radical scavenging effect of moutan cortex. (1): Absolute stereostructures of two monoterpenes, paeonisuffrone and paeonisuffral. Chem. Pharm. Bull. 2000;48:1327–1331. doi: 10.1248/cpb.48.1327. [DOI] [PubMed] [Google Scholar]
- 22.Murakami N., Saka M., Shimada H., Matsuda H., Yamahara J., Yoshikawa M. New bioactive monoterpene glycosides from paeoniae radix. Chem. Pharm. Bull. 1996;44:1279–1281. doi: 10.1248/cpb.44.1279. [DOI] [PubMed] [Google Scholar]
- 23.Liu J.K., Ma Y.B., Wu D.G., Lu Y., Shen Z.Q., Zheng Q.T., Chen Z.H. Paeonilide, a novel anti-PAF-active monoterpenoid-derived metabolite from Paeonia delavayi. Biosci. Biotechnol. Biochem. 2000;64:1511–1514. doi: 10.1271/bbb.64.1511. [DOI] [PubMed] [Google Scholar]
- 24.Wang S.J., Yang Y.C., Li S., Shi J.G. A new paeoniflorin derivative isolated from the root bark ethanol extract of Paeonia suffruticosa. China J. Chin. Mater. Med. 2005;30:759–761. [PubMed] [Google Scholar]
- 25.Wu S.H., Chen Y.W., Yang L.Y., Li S.L., Li Z.Y. Monoterpene glycosides from Paeonia delavayi. Fitoterapia. 2007;78:76–78. doi: 10.1016/j.fitote.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 26.An R.B., Kim H.C., Lee S.H., Jeong G.S., Sohn D.H., Park H., Kwon D.Y., Lee J.H., Kim Y.C. A new monoterpene glycoside and antibacterial monoterpene glycosides from Paeonia suffruticosa. Arch. Pharm. Res. 2006;29:815–820. doi: 10.1007/BF02973899. [DOI] [PubMed] [Google Scholar]
- 27.Xiao C., Wu M., Chen Y., Zhang Y., Zhao X., Zheng X. Revealing metabolomic variations in cortex moutan from different root parts using HPLC-MS method. Phytochem. Anal. PCA. 2015;26:86–93. doi: 10.1002/pca.2539. [DOI] [PubMed] [Google Scholar]
- 28.Ryu G., Park E.K., Joo J.H., Lee B.H., Choi B.W., Jung D.S., Lee N.H. A new antioxidant monoterpene glycoside, α-benzoyloxypaeoniflorin from Paeonia suffruticosa. Arch. Pharm. Res. 2001;24:105–108. doi: 10.1007/BF02976476. [DOI] [PubMed] [Google Scholar]
- 29.Ding H.Y., Lin H.C., Chang T.S. Tyrosinase inhibitors isolated from the roots of Paeonia suffruticosa. J. Cosmet. Sci. 2009;60:347–352. [PubMed] [Google Scholar]
- 30.Furuya R., Hu H., Zhang Z., Shigemori H. Suffruyabiosides A and B, two new monoterpene diglycosides from moutan cortex. Molecules. 2012;17:4915–4923. doi: 10.3390/molecules17054915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song W.H., Cheng Z.H., Chen D.F. Anticomplement monoterpenoid glucosides from the root bark of Paeonia suffruticosa. J. Nat. Prod. 2014;77:42–48. doi: 10.1021/np400571x. [DOI] [PubMed] [Google Scholar]
- 32.Okubo T., Nagai F., Seto T., Satoh K., Ushiyama K., Kano I. The inhibition of phenylhydroquinone-induced oxidative DNA cleavage by constituents of moutan cortex and paeoniae radix. Biol. Pharm. Bull. 2000;23:199–203. doi: 10.1248/bpb.23.199. [DOI] [PubMed] [Google Scholar]
- 33.Matsuda H., Ohta T., Kawaguchi A., Yoshikawa M. Bioactive constituents of Chinese natural medicines. VI. Moutan cortex. (2): Structures and radical scavenging effects of suffruticosides A, B, C, D, and e and galloyl-oxypaeoniflorin. Chem. Pharm. Bull. 2001;49:69–72. doi: 10.1248/cpb.49.69. [DOI] [PubMed] [Google Scholar]
- 34.Yoshikawa M., Uchida E., Kawaguchi A., Kitagawa I., Yamahara J. Galloyl-oxypaeoniflorin, suffruticosides A, B, C, and D, five new antioxidative glycosides, and suffruticoside E, A paeonol glycoside, from Chinese moutan cortex. Chem. Pharm. Bull. 1992;40:2248–2250. doi: 10.1248/cpb.40.2248. [DOI] [PubMed] [Google Scholar]
- 35.He C., Xiao W., Li M., Peng Y., Xu L., Gu J., Xiao P. Chemical constituents from seeds of Paeonia suffruticosa. China J. Chin. Mater. Med. 2010;35:1428–1431. [PubMed] [Google Scholar]
- 36.Tanaka T., Kataoka M., Tsuboi N., Kouno I. New monoterpene glycoside esters and phenolic constituents of paeoniae radix, and increase of water solubility of proanthocyanidins in the presence of paeoniflorin. Chem. Pharm. Bull. 2000;48:201–207. doi: 10.1248/cpb.48.201. [DOI] [PubMed] [Google Scholar]
- 37.Wu S.H., Luo X.D., Ma Y.B., Hao X.J., Wu D.G. Monoterpenoid derivatives from Paeonia delavayi. J. Asian Nat. Prod. Res. 2002;4:135–140. doi: 10.1080/10286020290027425. [DOI] [PubMed] [Google Scholar]
- 38.Fu Q., Wang S.B., Zhao S.H., Chen X.J., Tu P.F. Three new monoterpene glycosides from the roots of Paeonia lactiflora. J. Asian Nat. Prod. Res. 2013;15:697–702. doi: 10.1080/10286020.2013.794420. [DOI] [PubMed] [Google Scholar]
- 39.Washida K., Yamagaki T., Iwashita T., Nomoto K. Two new galloylated monoterpene glycosides, 4-O-galloylalbiflorin and 4′-O-galloylpaeoniflorin, from the roots of Paeonia lactiflora (Paeoniae radix) grown and processed in Nara Prefecture, Japan. Chem. Pharm. Bull. 2009;57:1150–1152. doi: 10.1248/cpb.57.1150. [DOI] [PubMed] [Google Scholar]
- 40.Braca A., Kiem P.V., Yen P.H., Nhiem N.X., Quang T.H., Cuong N.X., Minh C.V. New monoterpene glycosides from Paeonia lactiflora. Fitoterapia. 2008;79:117–120. doi: 10.1016/j.fitote.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Riaz N., Anis I., Malik A., Ahmed Z., Muhammad P., Nawaz S.A., Choudhary M.I. Paeonins A and B, lipoxygenase inhibiting monoterpene galactosides from Paeonia emodi. Chem. Pharm. Bull. 2003;51:252–254. doi: 10.1248/cpb.51.252. [DOI] [PubMed] [Google Scholar]
- 42.Yen P.H., Kiem P.V., Nhiem N.X., Tung N.H., Quang T.H., Minh C.V., Kim J.W., Choi E.M., Kim Y.H. A new monoterpene glycoside from the roots of Paeonia lactiflora increases the differentiation of osteoblastic MC3T3-E1 cells. Arch. Pharm. Res. 2007;30:1179–1185. doi: 10.1007/BF02980258. [DOI] [PubMed] [Google Scholar]
- 43.Lang H.Y., Li S.Z., McCabe T., Clardy J. A new monoterpene glycoside of Paeonia lactiflora. Planta Med. 1984;50:501–504. doi: 10.1055/s-2007-969783. [DOI] [PubMed] [Google Scholar]
- 44.Ahmad S., Ullah F., Sadiq A., Ayaz M., Imran M., Ali I., Zeb A., Shah M.R. Chemical composition, antioxidant and anticholinesterase potentials of essential oil of Rumex hastatus D. Don collected from the North West of Pakistan. BMC Complement. Altern. Med. 2016;16:29. doi: 10.1186/s12906-016-0998-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He C.N., Zhang Y.C., Peng Y., Yang J.S., Xiao P.G. Monoterpene glycosides from the seeds of Paeonia suffruticosa protect HEK 293 cells from irradiation-induced DNA damage. Phytochem. Lett. 2012;5:128–133. doi: 10.1016/j.phytol.2011.11.008. [DOI] [Google Scholar]
- 46.Li P., Zhang Z.M., Li T., Zhang Y.B., Sze S.C., Wang G.C., Li Y.L., Ye W.C. Monoterpene derivatives from the roots of Paeonia lactiflora and their anti-proliferative activity. Fitoterapia. 2014;98:124–129. doi: 10.1016/j.fitote.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 47.Zhu X., Fang Z.H. New monoterpene glycosides from the root cortex of Paeonia suffruticosa and their potential anti-inflammatory activity. Nat. Prod. Res. 2014;28:301–305. doi: 10.1080/14786419.2013.858340. [DOI] [PubMed] [Google Scholar]
- 48.Liu W., Li D.D., Yang H.S., Chen Y.Y., Wei J.F., Kang W.Y., Guo X.C. Determination of oleanic acid and paeoniflorin in Paeonia lactiflora by ultrasound-assisted ionic liquid-reversed phase liquid chromatography. China J. Chin. Mater. Med. 2015;40:443–449. [PubMed] [Google Scholar]
- 49.Gao H., Zhang X., Wang N.L., Liu H.W., Zhang Q.H., Song S.S., Yu Y., Yao X.S. Triterpenoid saponins from Stauntonia chinensis. J. Asian Nat. Prod. Res. 2007;9:175–182. doi: 10.1080/10286020500480787. [DOI] [PubMed] [Google Scholar]
- 50.Mencherini T., Picerno P., Festa M., Russo P., Capasso A., Aquino R. Triterpenoid constituents from the roots of Paeonia rockii ssp. rockii. J. Nat. Prod. 2011;74:2116–2121. doi: 10.1021/np200359v. [DOI] [PubMed] [Google Scholar]
- 51.Lin H.C., Ding H.Y., Wu Y.C. Two novel compounds from Paeonia suffructicosa. J. Nat. Prod. 1998;61:343–346. doi: 10.1021/np9704258. [DOI] [PubMed] [Google Scholar]
- 52.Gao H., Wang Z., Yang L., Yu Y., Yao Z.-H., Wang N.-L., Zhou G.-X., Ye W.-C., Yao X.-S. Five new bidesmoside triterpenoid saponins from Stauntonia chinensis. Magn. Reson. Chem. MRC. 2008;46:630–637. doi: 10.1002/mrc.2222. [DOI] [PubMed] [Google Scholar]
- 53.Kadota S., Terashima S., Basnet P., Kikuchi T., Namba T. Palbinone, a novel terpenoid from Paeonia albiflora; potent inhibitory activity on 3α-hydroxysteroid dehydrogenase. Chem. Pharm. Bull. 1993;41:487–490. doi: 10.1248/cpb.41.487. [DOI] [PubMed] [Google Scholar]
- 54.Furukawa M., Makino M., Ohkoshi E., Uchiyama T., Fujimoto Y. Terpenoids and phenethyl glucosides from Hyssopus cuspidatus (labiatae) Phytochemistry. 2011;72:2244–2252. doi: 10.1016/j.phytochem.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Tantry M.A., Dar J.A., Khuroo M.A., Shawl A.S. Nortriterpenoids from the roots of Paeonia emodi. Phytochem. Lett. 2012;5:253–257. doi: 10.1016/j.phytol.2012.01.006. [DOI] [Google Scholar]
- 56.Fu Q., Qiu L., Yuan H.M., Yu T., Zou L. Paeonenoides D and E: Two new nortriterpenoids from Paeonia lactiflora and their inhibitory activities on NO production. Helv. Chim. Acta. 2016;99:46–49. doi: 10.1002/hlca.201500130. [DOI] [Google Scholar]
- 57.Ambroz M., Hanusova V., Skarka A., Bousova I., Kralova V., Langhasova L., Skalova L. Essential oil from Myrica rubra leaves potentiated antiproliferative and prooxidative effect of doxorubicin and its accumulation in intestinal cancer cells. Planta Med. 2016;82:89–96. doi: 10.1055/s-0035-1558083. [DOI] [PubMed] [Google Scholar]
- 58.Bou-Abdallah F., Giffune T.R. The thermodynamics of protein interactions with essential first row transition metals. Biochim. Biophys. Acta. 2016;1860:879–891. doi: 10.1016/j.bbagen.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato T., Kawamoto A., Tamura A., Tatsumi Y., Fujii T. Mechanism of antioxidant action of pueraria glycoside (PG)-1 (an isoflavonoid) and mangiferin (a xanthonoid) Chem. Pharm. Bull. 1992;40:721–724. doi: 10.1248/cpb.40.721. [DOI] [PubMed] [Google Scholar]
- 60.Wang P., Wang W., Shi Q., Zhao L., Mei F., Li C., Zuo T., He X. Paeoniflorin ameliorates acute necrotizing pancreatitis and pancreatitisinduced acute renal injury. Mol. Med. Rep. 2016;14:1123–1131. doi: 10.3892/mmr.2016.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riaz N., Malik A., Rehman A.U., Ahmed Z., Muhammad P., Nawaz S.A., Siddiqui J., Choudhary M.I. Lipoxygenase inhibiting and antioxidant oligostilbene and monoterpene galactoside from Paeonia emodi. Phytochemistry. 2004;65:1129–1135. doi: 10.1016/j.phytochem.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 62.Yao C.W., Piao M.J., Kim K.C., Zheng J., Cha J.W., Hyun J.W. 6′-O-galloylpaeoniflorin protects human keratinocytes against oxidative stress-induced cell damage. Biomol. Ther. 2013;21:349–357. doi: 10.4062/biomolther.2013.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kimura M., Kimura I., Takahashi K., Muroi M., Yoshizaki M., Kanaoka M., Kitagawa I. Blocking effects of blended paeoniflorin or its related compounds with glycyrrhizin on neuromuscular junctions in frog and mouse. Jpn. J. Pharmacol. 1984;36:275–282. doi: 10.1254/jjp.36.275. [DOI] [PubMed] [Google Scholar]
- 64.Chen C.R., Sun Y., Luo Y.J., Zhao X., Chen J.F., Yanagawa Y., Qu W.M., Huang Z.L. Paeoniflorin promotes non-rapid eye movement sleep via adenosine a1 receptors. J. Pharmacol. Exp. Ther. 2016;356:64–73. doi: 10.1124/jpet.115.227819. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y.F., Lee M.M., Fang H.L., Yang J.G., Chen Y.C., Tsai H.Y. Paeoniflorin inhibits excitatory amino acid agonist-and high-dose morphine-induced nociceptive behavior in mice via modulation of N-methyl-d-aspartate receptors. BMC Complement. Altern. Med. 2016;16:240. doi: 10.1186/s12906-016-1230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim S.H., Lee M.K., Lee K.Y., Sung S.H., Kim J., Kim Y.C. Chemical constituents isolated from Paeonia lactiflora roots and their neuroprotective activity against oxidative stress in vitro. J. Enzym. Inhib. Med. Chem. 2009;24:1138–1140. doi: 10.1080/14756360802667977. [DOI] [PubMed] [Google Scholar]
- 67.Hsu F.L., Lai C.W., Cheng J.T. Antihyperglycemic effects of paeoniflorin and 8-debenzoylpaeoniflorin, glucosides from the root of Paeonia lactiflora. Planta Med. 1997;63:323–325. doi: 10.1055/s-2006-957692. [DOI] [PubMed] [Google Scholar]
- 68.Zeng J., Dou Y., Guo J., Wu X., Dai Y. Paeoniflorin of Paeonia lactiflora prevents renal interstitial fibrosis induced by unilateral ureteral obstruction in mice. Phytomedicine. 2013;20:753–759. doi: 10.1016/j.phymed.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Takagi K., Harada M. Pharmacological studies on herb paeony root. II. Anti-inflammatory effect, inhibitory effect on gastric juice secretion, preventive effect on stress ulcer, antidiuretic effect of paeoniflorin and combined effects with licorice component Fm 100. J. Pharm. Soc. Jpn. 1969;89:887–892. doi: 10.1248/yakushi1947.89.7_887. [DOI] [PubMed] [Google Scholar]
- 70.Nawaz H.R., Malik A., Khan P.M., Shujaat S., Rahman A. A novel β-glucuronidase inhibiting triterpenoid from Paeonia emodi. Chem. Pharm. Bull. 2000;48:1771–1773. doi: 10.1248/cpb.48.1771. [DOI] [PubMed] [Google Scholar]
- 71.Josef J.H., James W.W. The Genus Paeonia. Timber Press; Oregon, OR, USA: 2004. [Google Scholar]
- 72.Michio T. Paeoniaceae. In: Kubitzki K., editor. The Families and Genera of Vascular Plants. Volume 10. Springer; Berlin/Heidelberg, Germany: 2007. pp. 265–269. [Google Scholar]