Abstract
The general need to slow the depletion of fossil resources and reduce carbon footprints has led to tremendous effort being invested in creating “greener” industrial processes and developing alternative means to produce fuels and synthesize platform chemicals. This work aims to design a microwave-assisted cascade process for a full biomass valorisation cycle. GVL (γ-valerolactone), a renewable green solvent, has been used in aqueous acidic solution to achieve complete biomass lignin extraction. After lignin precipitation, the levulinic acid (LA)-rich organic fraction was hydrogenated, which regenerated the starting solvent for further biomass delignification. This process does not requires a purification step because GVL plays the dual role of solvent and product, while the reagent (LA) is a product of biomass delignification. In summary, this bio-refinery approach to lignin extraction is a cascade protocol in which the solvent loss is integrated into the conversion cycle, leading to simplified methods for biomass valorisation.
Keywords: lignin, biomass, γ-valerolactone, microwaves, bio-refinery
1. Introduction
A number of protocols which convert lignocellulosic biomass to value-added platform molecules and biofuels have been described over the last decade. γ-Valerolactone (GVL) is the most attractive and versatile lignocellulose chemical derivative as it is directly used as a fragrance, liquid fuel and food additive [1,2,3,4]. Furthermore, it is an intermediate in the production of fuel additives, biomass-derived polymers, short chain alkenes (jet fuel) and long chain alkanes (diesel fuel) [5,6,7]. GVL is renewable, safe to store, biodegradable and can be used as a green solvent in fine chemicals synthesis [8] and biomass processing [5]. In fact, GVL is commonly synthesized from levulinic acid (LA) via hydrogenation to 4-hydroxypentanoic acid, which spontaneously condensates to GVL [9]. Several procedures have been carried out under homogeneous and heterogeneous [10] conditions for selective LA hydrogenation [11]. Most processes use molecular H2 as the reducing agent, supported metals as catalysts (Ir, Rh, Pd, Ru, Pt, Re and Ni) and dioxane as the solvent [5]. The highest GVL yields have either been obtained under severe reaction conditions (200 °C and 200 bar H2), in the presence of a co-catalyst (Amberlyst A70 or A15) or an active hydrogen-donor (formic acid, FA) [12]. Recent studies on “greener” methods have moved on from the use of alcohols and ethers as solvents [13,14] to LA hydrogenation in water and milder conditions [15]. Although only a small number of investigations into the solvent-free conversion of LA into GVL have so far been published, a great deal of work to enhance the sustainability of this reaction is still in progress. Ni/Al2O3 catalysts have been tested in batch autoclaves [16], in quite harsh, but solventless, conditions.
It has recently been demonstrated that GVL can completely solubilise the biomass, thus promoting the production of sugars through thermocatalytic saccharification [17]. Wettstein et al. have reported the use of GVL as a solvent in the production of LA and GVL from cellulose [18], and, alternatively, from lignocellulosic biomass [19]. These studies focused on the sugar-derived products, without any further lignin processing, although it is considered the main renewable source of aromatics. Lignin extraction from lignocellulosic biomass (wood, annual plants) is the key step in large scale use for industrial applications. Lignin from the pulp and paper industries is generally only used as fuel to power paper mills and is no longer considered waste. It has, however, attracted increasing interest over the last few years as a means to produce dispersants, adhesives and surfactants, as an antioxidant in plastics and rubbers, as well as the raw material for the synthesis of value-added products [20]. One common method for the extraction of lignin draws on solvent pulping (organosolv lignin). The structure of these lignins is close to those of the native lignins [21,22]. Most common organosolv processes are based on ethanol/water or acetic acid containing a small amount of mineral acid such as hydrochloric or sulfuric acid [23]. A dioxasolv process has recently been used to extract lignin from birch (Betula pendula) sawdust, using a diluted hydrochloric acid solution in dioxane [24]. This process leads to high extraction yields but involve several extraction/precipitation steps with organic solvents.
The last three decades have seen several attempts to improve the sustainability of a variety of chemical processes being performed in non-conventional enabling technologies, such as microwaves (MW) [25] and ultrasound (US) reactors [26]. The peculiar physical properties of MW may significantly promote biopolymers hydrolysis and their further conversion into platform chemicals [27]. Cellulose and lignocellulosic biomass can be converted to LA under MW-assisted protocols and provide full conversion and good selectivity [28,29]. The hydrogenation of LA under MW irradiation has recently been studied with FA being employed as a hydrogen-donor solvent [30]. In this process, 2-methyltetrahydrofuran (MTHF) was produced, with some by-products, in 30 min at 150 °C.
The present work reports a cascade MW-assisted integrated protocol which exploits the biorefinery approach to “green” hydrogenation of biomass-derived LA to GVL, and its recycling for further lignin extraction from biomass.
2. Results and Discussion
2.1. Synthesis of GVL from LA
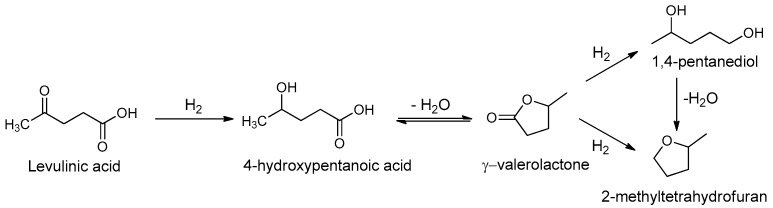
One pathway to GVL relies on the hydrogenation of LA to 4-hydroxypentanoic acid; an unstable intermediate which undergoes intramolecular esterification with loss of a water molecule to afford GVL [31]. The highest yields were achieved using Ru-based catalysts [10]. Other noble metals, such as Pt and Pd, also catalysed GVL hydrogenation to afford 2-methyltetrahydrofuran (MTHF) and 1,4-pentanediol in spite of high GVL production rates (Scheme 1) [11,32].
Scheme 1.
Reaction pathway for the production of GVL and further hydrogenation products from LA.
Bermudez et al. reported the hydrogenation of LA over Pd/C under MW irradiation with FA as the hydrogen source, however the reaction proceeded to MTHF with a LA/catalyst ratio of 1/1 (mL/g) [30]. Our recent findings on the flash MW conversion of biomass into LA provided a base for our experiments into a MW-assisted synthesis of GVL using economical and commercially available Pd/C as catalyst and molecular H2 as the reducing agent. The main target of the present study was to perform a “green” hydrogenation in water or in solvent-free conditions and thus improve the sustainability of the chemical process (Table 1). When the reaction was carried out in water, a significant amount of 4-hydroxypentanoic acid was found (Table 1, entry 1), because of an equilibrium shift in the intramolecular esterification. Contrary to data published by Tan et al. on hydrogenation in water [33], our experiments found that water disfavors intramolecular esterification. We therefore studied the reaction in solvent-free conditions, varying the reaction temperature, the amount of catalyst and hydrogen pressure. The complete conversion and good selectivity were obtained using 50 bar H2 for 4 h at 150 °C (Table 1, entries 3 and 4). Most research on LA hydrogenation to GVL has made use of pure, commercial LA and processing conditions that are not suitable for use in cascade processes that operate with streams of lignocellulosic biomass derived LA. The solvent-free protocol shed light on water’s role in the hydrogenation process, but could not be used in the cascade process where the GVL plays a pivotal role. For this reason, we performed the hydrogenation of biomass-derived LA, using GVL as a solvent, under the optimized conditions (Table 1, entry 7). The LA obtained from the biomass conversion, carried out according to our previous work [29], afforded GVL with complete conversion and selectivity. No further hydrogenation products were detected. Product separation and purification were not required when using GVL as the solvent.
Table 1.
MW-assisted hydrogenation of LA a.
| Entry | LA (mol) | Pd/C (wt %) | Solvent | H2 (bar) | Temperature (°C) | LA Conversion b (%) | GVL Selectivity b (%) |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 35 | H2O | 50 | 150 | 100 | 10 c |
| 2 | 1 | 35 | - | 100 | 90 | 100 | 100 |
| 3 | 1 | 35 | - | 50 | 150 | 100 | 100 |
| 4 | 40 | 10 | - | 50 | 150 | 100 | 100 |
| 5 | 40 | 10 | - | 50 | 90 | 39 | 100 |
| 6 | 40 | 4 | - | 50 | 150 | 86 | 100 |
| 7 d | 10 | 10 | GVL e | 50 | 150 | 100 | 100 |
a Reaction time: 4 h; b Determined by GC-MS; c 4-hydroxypentanoic acid was the major product; d Biomass-derived LA; e LA/GVL = 1/2.
2.2. Extraction of Lignin from Biomass
The use of GVL/H2O mixtures for the fractionation of lignocellulosic biomass in a MW reactor at 170 °C for 2 h has recently been reported. Acidic catalysis (H2SO4) yielded pure cellulose and a pure lignin fraction [34]. For our own aims, an integrated, MW-assisted flash biomass delignification process (2 min) was carried out using an acidic aqueous system GVL/H2O = 1:1 (HCl, 1M). The biomass was made up of post harvested tomato plants, whose composition was investigated in a previously described procedure (Table 2) [35].
Table 2.
Composition of biomass (wt %).
| Post-Harvested Tomato Plants | |
|---|---|
| Ash | 22% |
| Proteins and lipids | 3% |
| Cellulose | 39% |
| Hemicellulose | 21% |
| Lignin | 15% |
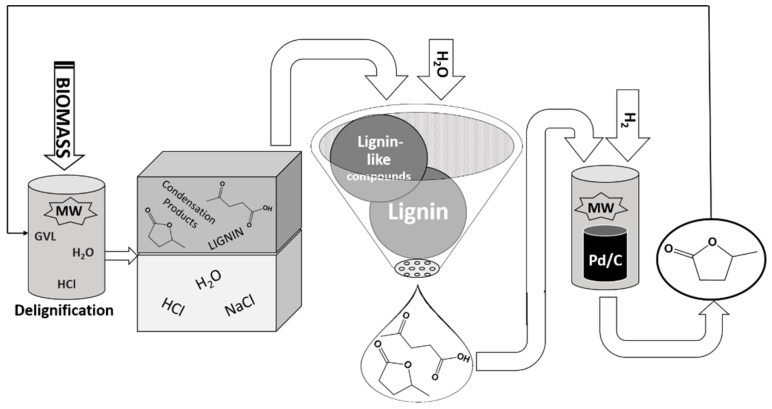
The cellulose fraction was rapidly converted to LA [29], and then subsequently hydrogenated to regenerate and recycle the solvent in a cascade process (Figure 1) where the lignin was extracted via simple precipitation. GVL is stable in acidic systems at high temperatures (225 °C) [17], and the GVL/H2O mixtures facilitate the complete solubilisation of biomass (99.10%). As GVL solvent leads to higher rates of acid-catalyzed reactions than to reactions in water [36], hemicellulose was immediately hydrolyzed to oligomeric and monomeric xylose and the dehydration product furfural. Sugars that are derived from the hydrolysis of cellulose and hemicellulose, and especially furfural, can further react to lignin-like condensation products, which are soluble in the solvent mixture. The addition of NaCl after the MW-assisted process favored the separation of the GVL layer from the HCl water solution.
Figure 1.
Scheme of the cascade process for the extraction of lignin.
In the biphasic system, most of the LA was partitioned in the organic phase (93% of the total LA obtained at the end of the process). Thus, after separation, the organic phase contained GVL, LA, lignin and the lignin-like condensation products, which were eventually formed by cellulose and hemicellulose degradation. Lignin and the lignin-like condensation products, in particular, were recovered by precipitation after dilution, of over 10 times, with distilled water [18]. This procedure yielded a 19 wt % precipitate, compared to the starting biomass, while the lignin in the raw biomass was 15 wt %. This excess comes from the formation of a few lignin-like degradation products.
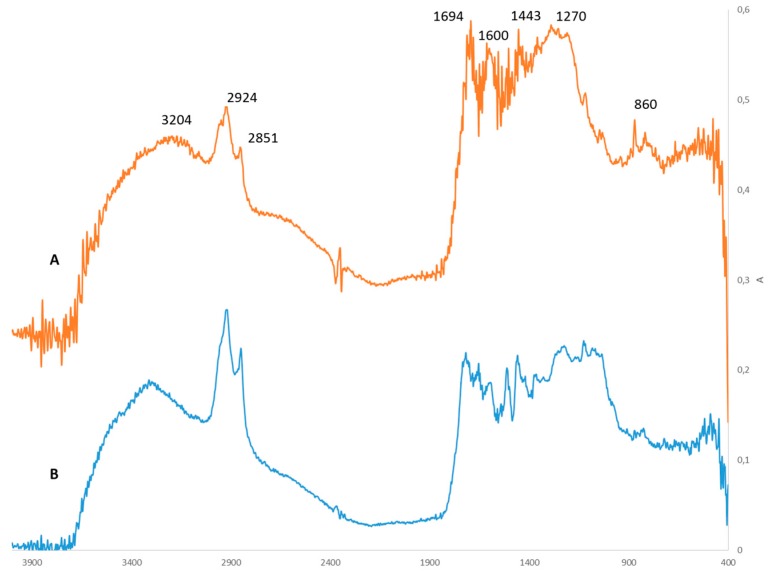
IR spectra of the precipitate (Figure 2A) were very similar to those of the lignin extracted using a dioxasolv process (Figure 2B). Characteristic lignin bands for O–H stretching (3204 cm−1 respectively), C-H stretching (2924 and 2851 cm−1), and aromatic skeletal vibrations (1600 cm−1) were observed. The band at 1694 cm−1 belongs to the stretching of the C=O functional groups. The CH2 deformation of aliphatic chains is also present (1443 cm−1). The broad band at 1270 cm−1 belongs to the guaiacyl unit, as does that at 860 cm−1.
Figure 2.
IR spectra of lignin obtained from biomass deconstruction in GVL/H2O (A) and dioxasolv lignin (B).
The remarkable features of this method are: (i) a one-step flash-treatment for both the solubilisation of the lignin and the conversion of cellulose to LA; (ii) the absence of a purification step for the recovery of LA; (iii) the dual role of GVL as the solvent and product to be recycled. This process complies with the six principles of green extraction [37,38]. In particular, alternative bio-based solvents have been used to extract lignin from a renewable source with an integrated approach by innovative and energy-saving technologies.
2.3. Regeneration of the Solvent
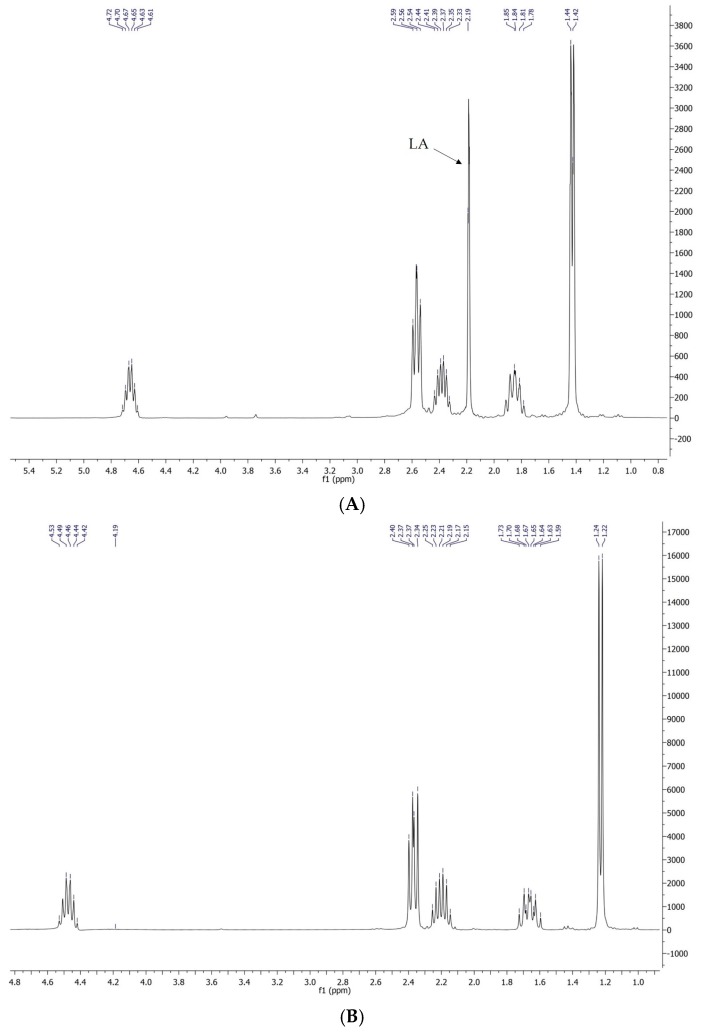
The organic phase that remained after the precipitation of lignin contained only LA and GVL, as confirmed by GC-MS and 1H-NMR (Figure 3A). It was thus used as the feedstock for the regeneration of the solvent. The hydrogenation step was performed using Pd/C as the catalyst, under the optimized conditions reported above for the reduction of biomass-derived LA in GVL (Table 1, entry 7).
Figure 3.
(A) 1H-NMR of the organic phase after the precipitation of lignin; (B) 1H-NMR of the same solution after hydrogenation; (C) GC-MS of the same solution after hydrogenation.
Researchers that used biomass derived LA have observed that mineral acids present in the feed deactivate the catalyst [18]. In our case, no deactivation was observed and LA was fully converted to GVL. As shown in Figure 3, the 1H-NMR signal at 2.19 ppm, which corresponds to the LA methyl group, disappeared after hydrogenation, as confirmed both by NMR (Figure 3B) and GC-MS (Figure 3C), where the only peak detected belongs to GVL. This demonstrates the efficiency of a separation method that ensured the complete washing of HCl in water during the first separation step.
In summary, we report the first example of a cascade process where a real organic feed obtained after biomass deconstruction was applied to regenerate the solvent for the process. Moreover, the MW-assisted flash conversion was able to synergistically improve the efficiency and sustainability of biomass valorisation process.
3. Experimental Section
3.1. General Information
All chemicals and solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA)) and used without further purification. Post-harvest tomato plants were blade milled. MW-assisted reactions were carried out in a high-pressure professional MW reactor (200 bar), SynthWave (Milestone Srl, MLS GmbH, Bergamo, Italy). NMR spectra were recorded on a Avance 300 (300 MHz) spectrometer (Bruker, Madison, WI, USA), at 25 °C; chemical shifts were calibrated to the residual solvent proton. GC-MS analyses were performed in a GC Agilent 6890 (Agilent Technologies, Santa Clara, CA USA) which was fitted with an Agilent Network 5973 mass detector, using a HP-5 column (30 m long capillary column, i.d of 0.25 mm and film thickness 0.25 μm). Infrared spectra (FTIR) were recorded using a KBr powder and a Spectrum BX FT-IR System (Perkin Elmer, Waltham, MA, USA).
3.2. Synthesis of GVL from LA and Analysis
In a typical experiment, the selected volume of LA was directly added to the Pd/C catalyst (10 wt % loading), followed by distilled water or GVL where needed. The required temperature was rapidly achieved in the MW reactor (average power 1500 W), under H2 pressures and magnetic stirring (600 rpm). After cooling, the solution was filtered and the solid was washed three times with CHCl3 (3 × 5 mL).
After solvent evaporation under vacuum, the crude reaction was analyzed by 1H-NMR (CDCl3) and GC-MS. GC-MS chromatograms were recorded after preliminary dilution with chloroform (1:50 for 0.1 mL LA and 1:1000 for 4 mL LA) and derivatization. In a typical procedure, 40 μL N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) were added to 2 mL of product solution. The mixture was heated for 45 min. at 60 °C under magnetic stirring and the product was directly analysed. GC conditions were: injection split 1:20, injector temperature 250 °C, detector temperature 280 °C. Gas carrier: helium (1.2 mL/min), temperature program: From 70 °C (2 min) to 300 °C at 5 °C/min.
3.3. Extraction of Lignin from Biomass and Analysis
The biomass (1 g) was suspended in a GVL/acidic aqueous solution (1:1, 1M HCl, total volume 20 mL). The mixture was irradiated in a MW reactor under N2 at 225 °C (average power 1500 W) with magnetic stirring (600 rpm). The crude reaction was filtered under vacuum after 2 min. The dried solid was weighed (wt % of solubilisation). The liquid fraction was separated into two phases by adding a saturated NaCl solution (30 mL). The organic fraction was then treated with 100 mL water which provided the lignin and lignin-like precipitation. The mixture was filtered and water removed under vacuum. The final solution was analyzed by 1H-NMR (CDCl3) and GC-MS, after prior derivatization of a diluted sample (1:10) in chloroform with BSTFA (as reported above). All the solid residues were accurately washed with a known volume of pure GVL/water solution (three times, 4 mL). The filter was washed with a large amount of distilled water after filtration, assuring the complete elimination of the organic solvent. The precipitate was dried in an oven at 30 °C overnight and then for two hours under vacuum.
3.4. Regeneration of the Solvent and Analysis
The solution recovered after lignin precipitation was hydrogenated under MW irradiation without any further purification or separation step, according to the optimized procedure for biomass-derived LA. The catalyst excess was calculated for the complete conversion of cellulose. After 4 h, the crude reaction was cooled down, filtered, and the solution analyzed using 1H-NMR (CDCl3) and GC-MS after prior derivatization.
4. Conclusions
A cascade MW-assisted integrated process has been developed for the extraction of lignin from biomass by mean of a “green” and renewable solvent produced and regenerated in the same process. The acidic aqueous GVL solution afforded the complete solubilisation of the biomass deconstruction products and the facile recovery of lignin by simple precipitation. In addition, the MW-assisted hydrogenation of the obtained LA to GVL eliminates the need for a separation step, as GVL is both solvent and product. The lignin extracted from the biomass was very similar to samples obtained using other extraction methods involving organic solvents and numerous steps. This method for lignin extraction may well lead to an upgrade in the subsequent lignin valorisation processes.
Acknowledgments
The authors acknowledge the financial support of the University of Turin (fondi ricerca locale 2013) and H2020 project US4GREENCHEM, which is strongly related to this investigation.
Author Contributions
S.T. and G.C. conceived and designed the experiments; G.G. performed the experiments; G.G. and E.C.G. performed the analyses; D.C. contributed the set-up of microwave equipments; S.T. wrote the paper, G.C. revised the paper. All the authors read and approved the paper.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Footnotes
Sample Availability: Samples of extracted lignin are available from the authors.
References
- 1.Bozell J.J., Petersen G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010;12:539–554. doi: 10.1039/b922014c. [DOI] [Google Scholar]
- 2.Corma A., Iborra S., Velty A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007;107:2411–2502. doi: 10.1021/cr050989d. [DOI] [PubMed] [Google Scholar]
- 3.Huber G.W., Corma A. Synergies between bio- and oil refineries for the production of fuels from biomass. Angew. Chem. Int. Ed. 2007;46:7184–7201. doi: 10.1002/anie.200604504. [DOI] [PubMed] [Google Scholar]
- 4.Clark J., Luque H.R., Matharu A.S. Green chemistry, biofuels and biorefinery. Ann. Rev. Chem. Biomol. Eng. 2012;3:183–207. doi: 10.1146/annurev-chembioeng-062011-081014. [DOI] [PubMed] [Google Scholar]
- 5.Alonso D.M., Wettstein S.G., Dumesic J.A. γ-Valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 2013;15:584–595. doi: 10.1039/c3gc37065h. [DOI] [Google Scholar]
- 6.Jaimes C., Dobreva-Schué R., Giani-Beaune O., Schué F., Amass W., Amass A. Ring-opening homopolymerization and copolymerization of lactones. Part 2. Enzymatic degradability of poly(β-hydroxybutyrate) stereoisomers and copolymers of β-butyrolactone with ε-caprolactone and δ-valerolactone. Polym. Int. 1999;48:23–32. doi: 10.1002/(SICI)1097-0126(199901)48:1<23::AID-PI97>3.0.CO;2-Y. [DOI] [Google Scholar]
- 7.Manzer L.E. Catalytic synthesis of-Methylene-Valerolactone: A biomass-derived acrylic monomer. Appl. Catal. A. 2004;272:249–256. doi: 10.1016/j.apcata.2004.05.048. [DOI] [Google Scholar]
- 8.Strappaveccia G., Luciani L., Bartollini E., Marrocchi A., Pizzo F., Vaccaro L. γ-Valerolactone as an alternative biomass-derived medium for the Sonogashira reaction. Green Chem. 2015;17:1071–1076. doi: 10.1039/C4GC01728E. [DOI] [Google Scholar]
- 9.Ali Abdelrahman O., Heyden A., Bond J.Q. Analysis of kinetics and reaction pathways in the aqueous-phase hydrogenation of levulinic acid to form γ-valerolactone over Ru/C. ACS Catal. 2014;4:1171–1181. doi: 10.1021/cs401177p. [DOI] [Google Scholar]
- 10.Wright W.R.H., Palkovits R. Development of heterogeneous catalysts for the conversion of levulinic acid to γ-valerolactone. ChemSusChem. 2012;5:1657–1667. doi: 10.1002/cssc.201200111. [DOI] [PubMed] [Google Scholar]
- 11.Yan K., Yang Y., Chai J., Lu Y. Catalytic reactions of γ-valerolactone: A platform to fuels and value-added chemicals. Appl. Catal. B Environmental. 2015;179:292–304. doi: 10.1016/j.apcatb.2015.04.030. [DOI] [Google Scholar]
- 12.Deuss P.J., Barta K., de Vries J.G. Homogeneous catalysis for the conversion of biomass and biomass-derived platform chemicals. Catal. Sci. Technol. 2014;4:1174–1196. doi: 10.1039/c3cy01058a. [DOI] [Google Scholar]
- 13.Yan Z.P., Lin L., Liu S. Synthesis of γ-valerolactone by hydrogenation of biomass-derived levulinic acid over Ru/C Catalyst. Energy Fuels. 2009;23:3853–3858. doi: 10.1021/ef900259h. [DOI] [Google Scholar]
- 14.Al-Shaal M.G., Wright W.R.H., Palkovits R. Exploring the ruthenium catalysed synthesis of γ-valerolactone in alcohols and utilisation of mild solvent-free reaction conditions. Green Chem. 2012;14:1260–1263. doi: 10.1039/c2gc16631c. [DOI] [Google Scholar]
- 15.Raspolli Galletti A.M., Antonetti C., de Luise V., Martinelli M. A sustainable process for the production of γ-valerolactone by hydrogenation of biomass-Derived levulinic acid. Green Chem. 2012;14:688–694. doi: 10.1039/c2gc15872h. [DOI] [Google Scholar]
- 16.Hengst K., Schubert M., Carvalho H.W.P., Lu C., Kleist W., Grunwaldt J.-D. Synthesis of γ-valerolactone by hydrogenation of levulinic acid over supported nickel catalysts. Appl. Catal. A: Gen. 2015;502:18–26. doi: 10.1016/j.apcata.2015.05.007. [DOI] [Google Scholar]
- 17.Luterbacher J.S., Rand J.M., Alonso D.M., Han J., Youngquist J.T., Maravelias C.T., Pfleger B.F., Dumesic J.A. Nonenzymatic sugar production from biomass using biomass-derived γ-valerolactone. Science. 2014;343:277–280. doi: 10.1126/science.1246748. [DOI] [PubMed] [Google Scholar]
- 18.Wettstein S.G., Alonso D.M., Chong Y., Dumesic J.A. A sulfuric acid management strategy for the production of liquid hydrocarbon fuels via catalytic conversion of biomass-derived levulinic acid. Energy Environ. Sci. 2012;5:8199–8203. doi: 10.1039/c2ee22111j. [DOI] [Google Scholar]
- 19.Alonso D.M., Wettstein S.G., Mellmer M.A., Gurbuzab E.I., Dumesic J.A. Integrated conversion of hemicellulose and cellulose from lignocellulosic biomass. Energy Environ. Sci. 2013;6:76–80. doi: 10.1039/C2EE23617F. [DOI] [Google Scholar]
- 20.Laurichesse S., Avérous L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014;39:1266–1290. doi: 10.1016/j.progpolymsci.2013.11.004. [DOI] [Google Scholar]
- 21.Sannigrahi P., Ragauskas A.J., Miller S.J. Lignin structural modifications resulting from ethanol organosolv treatment of loblolly pine. Energy Fuels. 2009;24:683–689. doi: 10.1021/ef900845t. [DOI] [Google Scholar]
- 22.El Hage R., Brosse N., Chrusciel L., Sanchez C., Sannigrahi P., Ragauskas A. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym. Degrad. Stab. 2009;94:1632–1638. doi: 10.1016/j.polymdegradstab.2009.07.007. [DOI] [Google Scholar]
- 23.Ferrer A., Vega A., Rodríguez L., Jiménez L. Acetosolv pulping for the fractionation of empty fruit bunches from palm oil industry. Bioresour. Technol. 2013;132:115–120. doi: 10.1016/j.biortech.2012.12.189. [DOI] [PubMed] [Google Scholar]
- 24.Lancefield C.S., Ojo O.S., Tran F., Westwood N.J. Isolation of functionalized phenolic monomers through selective oxidation and CO bond cleavage of the β-O-4 linkages in lignin. Angew. Chem. Int. Ed. 2015;54:258–262. doi: 10.1002/anie.201409408. [DOI] [PubMed] [Google Scholar]
- 25.De La Hoz A., Loupy A. Microwaves in Organic Synthesis. 3rd ed. Springer Science; New York, NY, USA: 2012. pp. 541–562. [Google Scholar]
- 26.Cravotto G., Cintas P. The combined use of microwaves and ultrasound: Improved tools in process chemistry and organic synthesis. Chem. Eur. J. 2007;13:1902–1909. doi: 10.1002/chem.200601845. [DOI] [PubMed] [Google Scholar]
- 27.Tabasso S., Carnaroglio D., Calcio Gaudino E., Cravotto G. Microwave, ultrasound and ball mill procedures for bio-waste valorisation. Green Chem. 2015;17:684–693. doi: 10.1039/C4GC01545B. [DOI] [Google Scholar]
- 28.Szabolcs A., Molnár M., Dibó G., Mika L. Microwave-assisted conversion of carbohydrates to levulinic acid: An essential step in biomass conversion. Green Chem. 2013;15:439–445. doi: 10.1039/C2GC36682G. [DOI] [Google Scholar]
- 29.Tabasso S., Montoneri E., Carnaroglio D., Caporaso M., Cravotto G. Microwave-assisted flash conversion of non-edible polysaccharides and post-harvest tomato plant waste to levulinic acid. Green Chem. 2014;16:73–76. doi: 10.1039/C3GC41103F. [DOI] [Google Scholar]
- 30.Bermudez J.M., Menéndez J.A., Romero A.A., Serrano E., Garcia-Martinez J., Luque R. Continuous flow nanocatalysis: Reaction pathways in the conversion of levulinic acid to valuable chemicals. Green Chem. 2013;15:2786–2792. doi: 10.1039/c3gc41022f. [DOI] [Google Scholar]
- 31.Morrison R.T., Boyd R.N. Organic. Chemistry. Allyn and Bacon; Boston, MA, USA: 1983. pp. 813–885. [Google Scholar]
- 32.Mehdi H., Fábos V., Tuba R., Bodor A., Mika L.T., Horváth I.T. Integration of homogeneous and heterogeneous catalytic processes for a multi-step conversion of biomass: From sucrose to levulinic acid, γ-valerolactone, 1,4-pentanediol, 2-methyl-tetrahydrofuran, and alkanes. Top. Catal. 2008;48:49–54. doi: 10.1007/s11244-008-9047-6. [DOI] [Google Scholar]
- 33.Tan J., Cui J., Deng T., Cui X., Ding G., Zhu Y., Li Y. Water-promoted hydrogenation of levulinic acid to γ-valerolactone on supported ruthenium catalyst. ChemCatChem. 2015;7:508–512. doi: 10.1002/cctc.201402834. [DOI] [Google Scholar]
- 34.Fang W., Sixta H. Advanced biorefinery based on the fractionation of biomass in γ-valerolactone and water. ChemSusChem. 2015;8:73–76. doi: 10.1002/cssc.201402821. [DOI] [PubMed] [Google Scholar]
- 35.Genevini P.L., Adani F., Villa C. Rice hull degradation by co-composting with dairy cattle slurry. Soil Sci. Plant. Nutr. 1997;43:135–147. doi: 10.1080/00380768.1997.10414722. [DOI] [Google Scholar]
- 36.Mellmer M.A., Alonso D.M., Luterbacher J.S., Gallo J.M.R., Dumesic J.A. Effects of γ-valerolactone in hydrolysis of lignocellulosic biomass to monosaccharides. Green Chem. 2014;16:4659–4662. doi: 10.1039/C4GC01768D. [DOI] [Google Scholar]
- 37.Chemat F., Maryline Abert Vian M.A., Cravotto G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012;13:8615–8627. doi: 10.3390/ijms13078615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rombaut N., Tixier A.-S., Bily A., Chemat F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Biorefin. 2014;8:530–544. doi: 10.1002/bbb.1486. [DOI] [Google Scholar]