Abstract
A multi-residue method for the determination of 54 pesticide residues in pollens has been developed and validated. The proposed method was applied to the analysis of 48 crude pollen samples collected from eight provinces of China. The recovery of analytes ranged from 60% to 136% with relative standard deviations (RSDs) below 30%. Of the 54 targeted compounds, 19 pesticides were detected. The major detection rates of each compound were 77.1% for carbendazim, 58.3% for fenpropathrin, 56.3% for chlorpyrifos, 50.0% for fluvalinate, 31.3% for chlorbenzuron, and 29.2% for triadimefon in crude pollen samples. The maximum values of each pesticide were 4516 ng/g for carbendazim, 162.8 ng/g for fenpropathrin, 176.6 ng/g for chlorpyrifos, 316.2 ng/g for fluvalinate, 437.2 ng/g for chlorbenzuron, 79.00 ng/g for triadimefon, and so on. This study provides basis for the research on the risks to honeybee health.
Keywords: pollen, multi-residue, pesticide, UPLC-MS/MS
1. Introduction
Honeybee, belonging to the insect order Hymenoptera [1], is an important pollinator for natural ecosystems and agricultural crops [2]. Today, more than 75% of crop species worldwide—including oil crops, fruits, and vegetables—benefit from insect pollination. Farmers generally rely on the honeybee for providing food production, worth approximately 200 billion U.S. dollars [3]. Meanwhile, a great deal of hive products produced by the honeybee supply humans with various sources of nutrition.
However, a very recent phenomenon of colony collapse disorder (CCD), involving the sudden and massive disappearance of bee colonies around the world, is worrisome [4]. Within the past several years, due to global decline in honeybee population, honeybee health is a matter of public concern [4,5,6]. Since 2006, a large number of colonies have vanished. This phenomenon first emerged in North America. In Europe, the disappearance of the major honeybee colonies occurring in the vicinity of fields sprayed with pesticides was reported in 2012 [7]. CCD was reported in China in 2007 and rapidly spread nationwide. In addition, since 2007, CCD has been suspected to be occurring in Taiwan [8]. According to statistics, the number of colonies have reduced from 7.5 million in the 1990s to 6.8 million. The cause of CCD remains unknown, but there is an agreement among investigators that the possible cause of CCD could be several interacting factors [9]. The leading hypothesis for CCD links sublethal exposure to pesticides and other environmental factors, including parasitic infections and habitat loss, to honeybee losses and pollinator declines in general [10,11,12]. It is noteworthy that the pesticide-related hypothesis has received considerable attention since the emergence of CCD in 2006 [13]. Honeybees are exposed to pesticides in two ways, including foraging and contacting. Honeybees forage in an extensive range, leading them to contact contaminated food containing pollen, nectar, water, beebread, and so on. Consequently, it is particularly essential to investigate pesticide residues in pollens.
To date, a few large multi-residue methods have been developed for the determination of pesticide residues in pollens [9,14,15,16,17,18,19,20]. For example, in 2014, more than 115 pesticides were analyzed in bee pollen by LC-ESI-MS/MS. A sensitive and efficient method for routine pesticide multi-residue analysis in pollen was reported in 2015. The QuEChERS (quick, easy, cheap, effective, rugged, and safe) method was designed and successfully used for the detection of 80 environmental contaminants in pollens, analysed by LC-MS and GC-MS. Two other multi-residue methods, zirconium-based sorbents (Z-Sep) and gel permeation chromatography (GPC), determined by GC-MS, were used in the analysis of 18 pesticides in pollen in 2015. Applications of these methods resulted in crucial information about the magnitude of pesticide contamination in those pollens, particularly in North America and Europe. As a large agricultural country, China faces a high risk of threat to honeybee health because of the wide application of pesticides used for plant protection. However, up to now, there has been no single report on the level of pesticide residue in crude pollens gathered in China. The present study aimed to analyze 48 crude pollen samples, which were collected from eight provinces of China. It provides a basis for studying the risk to honeybee health.
2. Results and Discussion
2.1. Choice of Mobile Phase
Due to a wide spectrum of analyzed pesticides in a pollen matrix and great diversities between their physicochemical properties and acid–base properties, it was quite difficult to acquire a well-defined chromatographic peak and reliable liquid chromatography analysis. So that each compound could be subjected to maximum sensitivity in ultraperformance liquid chromatography (UPLC), the mobile phase composition was optimized. We conducted the test using five kinds of mobile phases for multi-residue analysis (Table 1). The results showed that the sensitivity to all compounds could be maximized at the type I of the mobile phase.
Table 1.
The composition of mobile phases.
| Type | Mobile Phase A | Mobile Phase B |
|---|---|---|
| I | water/methanol (98:2) + 0.05% formic acid | methanol + 0.05% formic acid |
| II | 0.1% formic acid | methanol |
| III | 0.1% formic acid | acetonitrile |
| IV | 0.05% formic acid + 5 mmol/L ammonium acetate | methanol |
| V | 0.05% formic acid + 5 mmol/L ammonium acetate | acetonitrile |
2.2. Validation of the Dispersive Solid-Phase Extraction (dSPE) Clean-Up
To analyze a wide range of compounds, the multi-residue QuEChERS (quick, easy, cheap, effective, rugged, and safe) method was used for pollen samples. Analytes were extracted from the matrix by an organic solvent that was subsequently salted out from an aqueous matrix. Only a large number of proteins, aminophenols, vitamins, and lipids were present in the pollen samples, but some higher polar pigments also existed. Since the pollen matrix is complex, an additional purification step (dSPE) was necessarily used to reduce the presence of interfering substances. Initially, a dSPE step was based on primary and secondary amine-bonded silica (PSA). Since 2006, this step has been further developed. In 2006, Leandro et al. [21] used PSA and octadecyl-bonded silica (PSA/C18) instead of PSA-bonded silica to limit apolar interferences of the matrix. In 2010, Mullin et al. successfully adopted this method and coupled it with analysis using a dual-layer cartridge containing PSA and graphitized carbon black (GCB) to purify components from wax, pollen, bee, and beebread [22]. Here, we designed four compositions to determine the optimal clean-up conditions (Table 2) among the recoveries during this step while considering matrix effects. The test indicated that the most advantageous procedure for the dSPE clean-up is that of level B (Table 2).
Table 2.
Four levels of the composition for dispersive solid-phase extraction (dSPE) clean-up.
| Level | PSA | C18 | GCB | MgSO4 |
|---|---|---|---|---|
| A | 50 mg | 50 mg | 0 mg | 150 mg |
| B | 50 mg | 50 mg | 3.75 mg | 150 mg |
| C | 50 mg | 50 mg | 7.5 mg | 150 mg |
| D | 50 mg | 50 mg | 15 mg | 150 mg |
PSA (primary secondary amine), C18 (octadecyl-bonded silica), GCB (graphitized carbon black).
2.3. Limits of Detection and Quantification
The method limit of detection (LOD) was defined as the lowest concentration tested in which the signal response was three times more than the background noise from the chromatogram in both transitions. The method limit of quantification (LOQ) was defined as the lowest concentration tested in which the signal response was 10 times more than the background noise from the chromatogram in the quantification transition. The ion ratio is established with the respective ratio of a standard [17]. Both LOD and LOQ values are shown in Table 3. The LOD values for all substances were below 0.5 ng/g, with the exception of aldicarb sulfoxide, which had an LOD value of 0.5291 ng/g.
Table 3.
Limit of determination and quantification (LOD and LOQ) of the method, recovery of analytes, and repeatability (relative standard deviations, RSD) obtained in pollens.
| Compound | LOD (ng/g) | LOQ (ng/g) | Recovery (%) | RSD (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Low (ng/g) | Medium (ng/g) | High (ng/g) | Low (ng/g) | Medium (ng/g) | High (ng/g) | |||
| Methamidophos | 0.0556 | 0.1667 | 103 | 85.1 | 89.1 | 5.95 | 1.96 | 1.70 |
| Acephate | 0.2691 | 0.8072 | 123 | 87.7 | 91.2 | 12.5 | 1.39 | 1.52 |
| Omethoate | 0.1383 | 0.4149 | 112 | 89.6 | 93.5 | 3.75 | 1.79 | 1.93 |
| Aldicarb-sulfoxide | 0.5291 | 1.5873 | 120 | 93.3 | 97.5 | 3.33 | 3.01 | 2.02 |
| Aldicarb-sulfone | 0.1343 | 0.4030 | 108 | 98.4 | 98.1 | 3.70 | 0.81 | 2.05 |
| Carbendazim | 0.1064 | 0.3191 | 109 | 93.3 | 93.1 | 8.45 | 5.51 | 2.63 |
| Methomyl | 0.0337 | 0.1010 | 111 | 89.3 | 96.5 | 5.52 | 4.23 | 1.33 |
| Thiamethoxam | 0.0028 | 0.0084 | 125 | 103 | 101 | 4.63 | 1.78 | 0.71 |
| Monocrotophos | 0.0051 | 0.0154 | 112 | 90.9 | 96.9 | 3.57 | 2.21 | 2.68 |
| Imidacloprid | 0.0809 | 0.2427 | 92.7 | 85.6 | 96.2 | 8.99 | 1.40 | 3.50 |
| Trichlorfon | 0.1265 | 0.3794 | 121 | 86.9 | 98.0 | 3.81 | 3.72 | 3.67 |
| Dimethoate | 0.0366 | 0.1098 | 105 | 87.2 | 92.4 | 9.56 | 1.83 | 2.84 |
| Carbofuran-3-hydroxy | 0.0344 | 0.1032 | 94.7 | 90.1 | 99.3 | 6.45 | 1.85 | 2.03 |
| Acetamiprid | 0.0114 | 0.0343 | 103 | 86.9 | 97.7 | 5.95 | 1.41 | 2.25 |
| Aldicarb | 0.0432 | 0.1295 | 93.3 | 86.1 | 98.7 | 6.55 | 4.58 | 1.30 |
| Phosphamidon | 0.0037 | 0.0112 | 117 | 86.9 | 95.5 | 3.94 | 2.13 | 2.56 |
| Dichlorvos | 0.2483 | 0.7450 | 65.3 | 69.7 | 78.4 | 8.66 | 7.26 | 13.60 |
| Carbofuran | 0.0060 | 0.0179 | 88.0 | 87.5 | 95.6 | 4.55 | 4.32 | 1.26 |
| Fenthion-sulfoxide | 0.0202 | 0.0605 | 104 | 89.3 | 96.4 | 3.85 | 1.03 | 1.66 |
| Carbaryl | 0.1087 | 0.3261 | 86.7 | 89.1 | 98.1 | 5.33 | 2.89 | 1.25 |
| Fenthion-sulfone | 0.0369 | 0.1108 | 88.0 | 86.4 | 98.1 | 4.55 | 0.93 | 1.93 |
| Pyrimethanil | 0.0145 | 0.0435 | nd | 82.1 | 89.9 | - | 1.12 | 1.36 |
| Phorate-sulfoxide | 0.0244 | 0.0731 | 112 | 90.9 | 96.5 | 7.14 | 2.54 | 2.09 |
| Phorate-sulfone | 0.0241 | 0.0722 | 98.7 | 86.1 | 94.9 | 2.34 | 3.52 | 0.64 |
| Methidathion | 0.0095 | 0.0286 | 96.0 | 88.4 | 100 | 8.33 | 4.32 | 1.20 |
| Phosmet | 0.0343 | 0.1029 | 94.7 | 85.3 | 90.8 | 2.44 | 3.55 | 10.42 |
| Terbufos-sulfone | 0.0759 | 0.2277 | 112 | 93.6 | 98.0 | 6.19 | 3.08 | 2.45 |
| Terbufos-sulfoxide | 0.0243 | 0.0729 | 109 | 89.9 | 94.5 | 2.11 | 0.51 | 0.65 |
| Azoxystrobin | 0.0062 | 0.0186 | 131 | 91.7 | 97.1 | 9.35 | 2.66 | 1.90 |
| Malathion | 0.0610 | 0.1829 | 101 | 85.0 | 98.5 | 2.28 | 1.44 | 2.00 |
| Triadimefon | 0.0029 | 0.0088 | 133 | 89.6 | 91.2 | 3.46 | 1.79 | 1.52 |
| Dimethomorph | 0.0049 | 0.0147 | 70.7 | 85.3 | 93.6 | 6.54 | 0.54 | 1.48 |
| Triazophos | 0.0082 | 0.0246 | 112 | 93.3 | 101 | 7.14 | 6.49 | 1.82 |
| Ethoprophos | 0.0177 | 0.0530 | 90.7 | 83.5 | 95.3 | 9.18 | 3.08 | 3.81 |
| Iprodione | 0.0611 | 0.1833 | 90.0 | 85.5 | 91.4 | 13.6 | 8.66 | 3.83 |
| Diflubenzuron | 0.0045 | 0.0136 | 81.3 | 81.9 | 92.5 | 11.4 | 5.38 | 1.75 |
| Procholraz | 0.0166 | 0.0499 | 120 | 96.3 | 93.7 | 2.03 | 6.45 | 2.71 |
| Sulfotep | 0.0195 | 0.0585 | 89.3 | 87.2 | 93.9 | 5.17 | 2.43 | 4.94 |
| Chlorbenzuron | 0.0226 | 0.0678 | 68.0 | 89.9 | 92.3 | 30.6 | 6.06 | 1.52 |
| Fenthion | 0.0514 | 0.1542 | 86.7 | 91.7 | 88.3 | 13.3 | 2.19 | 0.94 |
| Coumaphos | 0.0030 | 0.0090 | 88.0 | 103 | 100 | 9.09 | 7.10 | 4.39 |
| Diazinon | 0.0176 | 0.0529 | 101 | 86.4 | 90.7 | 4.56 | 3.21 | 2.70 |
| Phoxim | 0.0135 | 0.0406 | 92.0 | 83.7 | 95.3 | 4.35 | 9.18 | 3.57 |
| Phorate | 0.0154 | 0.0462 | 72.0 | 78.7 | 85.6 | 9.62 | 0.59 | 3.99 |
| Phosalone | 0.0158 | 0.0475 | 97.3 | 86.7 | 95.6 | 8.55 | 3.24 | 3.16 |
| Difenoconazole | 0.0242 | 0.0726 | 104 | 85.9 | 84.4 | 3.85 | 2.34 | 4.52 |
| Emamectin benzoate | 0.0008 | 0.0025 | 85.3 | 81.1 | 76.5 | 7.16 | 1.14 | 1.09 |
| Profenofos | 0.0189 | 0.0568 | 127 | 92.8 | 84.8 | 4.82 | 8.31 | 1.89 |
| Terbufos | 0.1414 | 0.4241 | 97.3 | 77.1 | 86.4 | 8.55 | 5.12 | 1.85 |
| Chlorpyrifos | 0.0638 | 0.1914 | 103 | 74.7 | 81.9 | 12.5 | 3.76 | 2.78 |
| Fenpropathrin | 0.0433 | 0.1300 | 92.0 | 75.5 | 80.0 | 7.53 | 1.62 | 4.77 |
| Pendimethalin | 0.0236 | 0.0708 | 85.3 | 82.4 | 90.1 | 7.16 | 2.57 | 1.56 |
| Pyridaben | 0.0070 | 0.0211 | 136 | 85.3 | 84.8 | 5.88 | 1.08 | 1.89 |
| Fluvalinate | 0.0068 | 0.0203 | 98.7 | 76.8 | 66.0 | 1.17 | 9.16 | 2.12 |
2.4. Linearity
Linearity was evaluated by assessing the detector responses of the objective compounds from matrix-matched calibration solutions, prepared by spiking blank extracts at eight concentration levels. Since there is diversity in the signal responses between each pesticide, the range of concentrations was set at three levels. A range of eight points was used, from 5 to 200 ng/g, with the exception of 2.5–100 ng/g for carbendazim, phosphamidon, pyrimethanil, azoxystrobin, triadimefon, triazophos, and diazinon and 10–1000 ng/g for thiamethoxam, imidacloprid, iprodione, and fluvalinate. The linear ranges of all pesticides are presented in Table 4. Good linearity was observed in all cases, with correlation coefficients better than 0.9902. For all compounds studied, the signal response was linear over the range studies. Therefore, the method had a good linear relationship.
Table 4.
Matrix effects (ME), retention time (tR), linear range, linear regression equation, and linearity.
| Compound | ME (%) | tR (min) | Linear Range (ng/g) | Linear Regression Equation | Linearity |
|---|---|---|---|---|---|
| Methamidophos | −6 | 1.17 | 5–200 | Y = 140.5X + 99.14 | 0.9984 |
| Acephate | 0 | 1.50 | 5–200 | Y = 61.56X − 69.02 | 0.9990 |
| Omethoate | −21 | 1.74 | 5–200 | Y = 266.0X + 94.55 | 0.9963 |
| Aldicarb-sulfoxide | −5 | 1.92 | 5–200 | Y = 38.60X + 39.18 | 0.9954 |
| Aldicarb-sulfone | −10 | 2.10 | 5–200 | Y = 112.0X + 38.23 | 0.9994 |
| Carbendazim | −10 | 2.26 | 2.5–100 | Y = 941.6X + 11.03 | 0.9993 |
| Methomyl | 3 | 2.38 | 5–200 | Y = 98.08X − 28.38 | 0.9997 |
| Thiamethoxam | −24 | 2.54 | 10–400 | Y = 66.00X + 134.2 | 0.9975 |
| Monocrotophos | −20 | 2.67 | 5–200 | Y = 1022X + 889.6 | 0.9967 |
| Imidacloprid | −13 | 3.04 | 10–400 | Y = 71.91X − 48.57 | 0.9996 |
| Trichlorfon | −80 | 3.26 | 5–200 | Y = 138.7X + 12.85 | 0.9991 |
| Dimethoate | −76 | 3.29 | 5–200 | Y = 154.1X + 79.95 | 0.9986 |
| Carbofuran-3-hydroxy | −80 | 3.35 | 5–200 | Y = 179.0X + 101.9 | 0.9991 |
| Acetamiprid | −50 | 3.37 | 5–200 | Y = 645.1X + 279.1 | 0.9986 |
| Aldicarb | −24 | 3.97 | 5–200 | Y = 578.5X + 620.2 | 0.9954 |
| Phosphamidon | 15 | 4.33 | 2.5–100 | Y = 207.4X − 73.14 | 0.9996 |
| Dichlorvos | −1 | 4.48 | 5–200 | Y = 232.7X − 24.55 | 0.9998 |
| Carbofuran | −38 | 4.58 | 5–200 | Y = 854.1X − 164.8 | 0.9995 |
| Fenthion-sulfoxide | −51 | 4.76 | 5–200 | Y = 780.0X + 66.08 | 0.9999 |
| Carbaryl | −30 | 4.80 | 5–200 | Y = 139.9X + 21.77 | 0.9985 |
| Fenthion-sulfone | −15 | 4.91 | 5–200 | Y = 165.1X − 26.61 | 0.9991 |
| Pyrimethanil | −25 | 5.03 | 2.5–100 | Y = 1810X − 63.22 | 0.9999 |
| Phorate-sulfoxide | −27 | 5.05 | 5–200 | Y = 938.3X + 576.2 | 0.9990 |
| Phorate-sulfone | −24 | 5.14 | 5–200 | Y = 302.9X − 5.827 | 0.9998 |
| Methidathion | −4 | 5.39 | 5–200 | Y = 93.08X + 9.293 | 0.9998 |
| Phosmet | −50 | 5.52 | 5–200 | Y = 106.4X + 64.63 | 0.9976 |
| Terbufos-sulfone | 16 | 5.62 | 5–200 | Y = 96.91X − 66.94 | 0.9975 |
| Terbufos-sulfoxide | 20 | 5.64 | 5–200 | Y = 192.5X − 80.41 | 0.9983 |
| Azoxystrobin | 25 | 5.67 | 2.5–100 | Y = 363.1X + 48.65 | 0.9990 |
| Malathion | 13 | 5.89 | 5–200 | Y = 151.5X − 111.6 | 0.9952 |
| Triadimefon | 15 | 5.99 | 2.5–100 | Y = 310.0X − 135.0 | 0.9955 |
| Dimethomorph | −20 | 6.01 | 5–200 | Y = 168.5X − 169.4 | 0.9903 |
| Triazophos | 16 | 6.05 | 2.5–100 | Y = 747.9X + 45.03 | 0.9995 |
| Ethoprophos | −1 | 6.19 | 5–200 | Y = 305.2X − 87.03 | 0.9992 |
| Iprodione | −46 | 6.33 | 10–400 | Y = 55.78X − 106.2 | 0.9952 |
| Diflubenzuron | −6 | 6.34 | 5–200 | Y = 86.59X − 52.92 | 0.9941 |
| Prochloraz | −27 | 6.44 | 5–200 | Y = 587.4X − 606.3 | 0.9902 |
| Sulfotep | −33 | 6.44 | 5–200 | Y = 1144X − 1042 | 0.9955 |
| Chlorbenzuron | −27 | 6.48 | 5–200 | Y = 89.28X − 107.6 | 0.9940 |
| Fenthion | −66 | 6.51 | 5–200 | Y = 101.6X − 19.60 | 0.9983 |
| Coumaphos | −35 | 6.54 | 5–200 | Y = 63.55X − 95.86 | 0.9914 |
| Diazinon | 9 | 6.55 | 2.5–100 | Y = 1220X − 596.6 | 0.9907 |
| Phoxim | −20 | 6.63 | 5–200 | Y = 49.86X + 5.412 | 0.9969 |
| Phorate | −12 | 6.67 | 5–200 | Y = 33.04X − 65.92 | 0.9996 |
| Phosalone | −26 | 6.69 | 5–200 | Y = 49.75X − 34.82 | 0.9944 |
| Difenoconazole | −10 | 6.84 | 5–200 | Y = 520.7X − 417.0 | 0.9949 |
| Emamectin benzoate | −12 | 6.83 | 5–200 | Y = 1177X − 804.8 | 0.9931 |
| Profenofos | −25 | 7.05 | 5–200 | Y = 182.7X − 171.3 | 0.9935 |
| Terbufos | −34 | 7.10 | 5–200 | Y = 83.82X − 1.636 | 0.9989 |
| Chlorpyrifos | −44 | 7.32 | 5–200 | Y = 327.6X + 67.46 | 0.9995 |
| Fenpropathrin | −46 | 7.32 | 5–200 | Y = 407.6X + 67.03 | 0.9999 |
| Pendimethalin | −26 | 7.34 | 5–200 | Y = 230.1X − 83.93 | 0.9987 |
| Pyridaben | −73 | 7.67 | 5–200 | Y = 1811X − 172.8 | 0.9984 |
| Fluvalinate | −77 | 7.74 | 10–400 | Y = 472.3X + 334.3 | 0.9997 |
2.5. Matrix Effects
In this study, one of the aims was to apply the multi-residue method to a great quantity of samples to receive a summarization of environmental contamination, so standard addition calibration could not be used [23,24,25]. The matrix effect in the mass spectrometric analysis was calculated by comparing the peak areas of the standards in the mobile phase with those of the same quantities of standards, which were added to the spiked samples following the extraction. The response of each pesticide in the mobile phase was designated as the 100% response value. Table 4 shows the spectrum with matrix effects for each compound determined in pollen. The diversification with matrix effects was dependent on the physicochemical properties of the compound and the matrix. These data indicated that the concentrations of the major pesticides could be affected by the matrix effect. The external calibration curve using matrix-matched standards was an efficacious method to overcome the matrix effects when a great quantity of complex samples such as pollens are to be determined.
2.6. Recovery Studies
Recoveries and relative standard deviations (RSDs, measurement of precision) of the target substances were determined by spiking blank pollen samples with three different concentrations (all compounds were spiked at 5, 50, and 500 ng/g, with the exception of carbendazim, phosphamidon, pyrimethanil, azoxystrobin, triadimefon, triazophos, and diazinon, which were spiked at 2.5, 25, and 250 ng/g, and thiamethoxam, imidacloprid, iprodione, and fluvalinate were spiked at 10, 100, and 1000 ng/g) and then analyzing five replicates for three levels named low, medium, and high. Results are exhibited in Table 3. The method showed excellent performance since recoveries for the majority of the compounds were within the satisfactory range of 70%–120%. Only dichlorvos, azoxystrobin, triadimefon, chlorbenzuron, profenofos, and pyridaben had accuracies not in the acceptable range of 60%–136%. The RSD values in all cases were below 20%; in addition, when chlorbenzuron was spiked in the low range, the RSD value was more than 30%. Consequently, the procedure described in the Section 3.2 is an accurate, sensitive, and efficient method for multi-residue analysis in pollens.
2.7. Real Sample
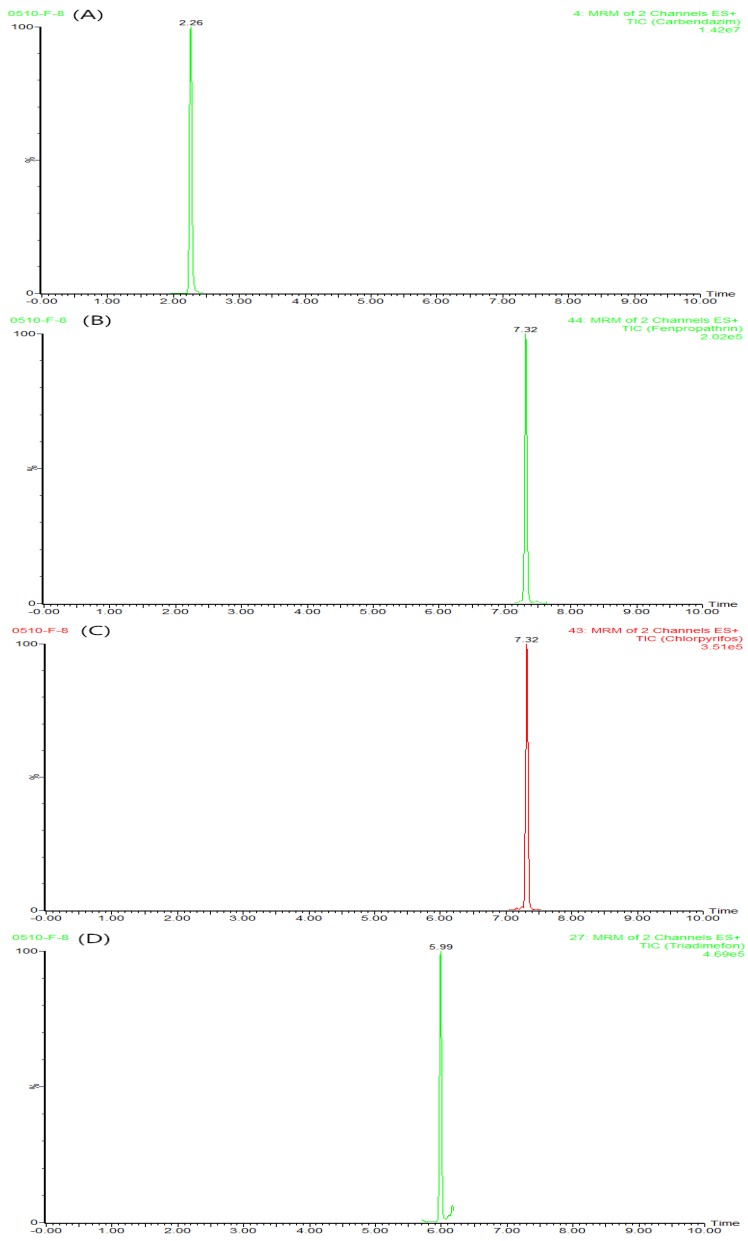
The multi-residue analytical method established above was applied to measure pesticide concentrations in 48 pollen samples collected from 11 apiaries in 8 provinces of China. The selected regions are characterized by agricultural events, and therefore they are prone to pollen polluted with pesticides. One hundred percent of the samples analyzed included at least one pesticide with the concentration ranging from 3.6 to 4516.4 ng/g. Of the 54 targeted compounds, 19 of them were detected. Table 5 presents the pesticides detected in 48 samples of pollen. The pesticides most commonly found in the pollens were: carbendazim (77.1%), fenpropathrin (58.3%), chlorpyrifos (56.3%), fluvalinate (50.0%), chlorbenzuron (31.3%), and triadimefon (29.2%). An emblematical chromatogram of a pollen sample including carbendazim, fenpropathrin, chlorpyrifos, and triadimefon is exhibited in Figure 1.
Table 5.
Several typical pesticides in pollen samples.
| Compound | Positive Sample | Total Number of Sample | Detection Rate (%) | Detected Concentration Ranges (ng/g) | Max Value (ng/g) | Central Values (ng/g) |
|---|---|---|---|---|---|---|
| carbendazim | 37 | 48 | 77.1 | 3.200–4516 | 4516 | 44.00 |
| fenpropathrin | 28 | 48 | 58.3 | 5.000–162.8 | 162.8 | 21.70 |
| chlorpyrifos | 27 | 48 | 56.3 | 5.000–176.6 | 176.6 | 23.60 |
| fluvalinate | 27 | 48 | 50.0 | 6.600–316.2 | 316.2 | 33.00 |
| chlorbenzuron | 15 | 48 | 31.3 | 5.000–437.2 | 437.2 | 27.00 |
| triadimefon | 14 | 48 | 29.2 | 2.600–79.00 | 79.00 | 19.70 |
| acetamiprid | 8 | 48 | 1.7 | 5.200–63.60 | 63.60 | 8.300 |
| imidacloprid | 7 | 48 | 1.5 | 17.60–49.80 | 49.80 | 27.60 |
Figure 1.
An example of the extracted Quantification ion (MRM1) chromatograms of a pollen sample, indicating the presence of (A) carbendazim; (B) fenpropathrin; (C) chlorpyrifos; and (D) triadimefon.
Since there are not any reported data about the pesticide residues in crude pollens in China, these results are interesting. As shown in Table 5, some neonicotinoid pesticides containing imidacloprid and acetamiprid were still found in pollens, but both detection rates and maximum values were of a relatively low range. This indicates that, with the increase of the reports related to the poisoning of honeybee with neonicotinoids, people use pesticides more and more cautiously during the flowering time of plants [2,3,26,27,28]. However, some pesticides highly toxic to honeybees were detected as before, such as fenpropathrin and chlorpyrifos. These would cause a huge impact on honeybees, including their behavior [29], enzyme activity [30,31,32], and so on. This suggests that the risk of pesticides highly toxic to honeybee health cannot be ignored. Thus, the investigators who focus on studying the honeybee’s health should accelerate the progress of similar researches. Meanwhile, as a compound with the highest detection rate, the maximum concentration of carbendazim reached 4516.4 ng/g. The possible reason for this is that, as a broad-spectrum pesticide for fungal disease management, carbendazim has been widely used to combat against some nectar plant diseases, including the disease caused by the fungus Sclerotinia sclerotiorum. Therefore, with the end of oilseed rape flowering, the detection rate of carbendazim also decreased. Currently, there are a few concerns [33] about the impact of fungicides on bees due to the absence of the acute lethal effect off fungicides on honeybees. Nevertheless, based on our results, a hypothesis that fungicides could bring some chronic effects on honeybees, including behavior, heredity, and so forth, should be proposed. The research studying how pesticides bring a high risk to honeybee health will be a long-term process.
3. Experimental Section
3.1. Chemicals and Standards
LC-grade acetonitrile and methanol were obtained from Tedia (Shanghai, China). Acetic acid, formic acid, magnesium sulfate anhydrous (MgSO4), and sodium acetate (NaOAc) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). C18, GCB, and PSA were obtained from Agilent Technologies (Santa Clara, CA, USA).
The compounds used in this study were selected following the requirement of Ministry of Agriculture of China (Beijing, China). All standard solutions of pesticides including methamidophos, methomyl, acephate, omethoate, carbendazim, methomyl, thiamethoxam, monocrotophos, imidacloprid, trichlorfon, dimethoate, acetamiprid, aldicarb, phosphamidon, dichlorvos, carbofuran, carbaryl, pyrimethanil, methidathion, phosmet, azoxystrobin, malathion, triadimefon, dimethomorph, triazophos, ethoprophos, iprodione, diflubenzuron, prochloraz, sulfotep, chlorbenzuron, fenthion, coumaphos, diazinon, phoxim, phorate, phosalone, difenoconazole, emamectin benzoate, profenofos, chlorpyrifos, fenpropathrin, pendimethalin, pyridaben, and fluvalinate (with purity equal than 1000 mg/L) were obtained from Agro-Environmental Protection Institute, Ministry of Agriculture of China. In addition, some pesticides with purity higher than or equal to 98.5%, including aldicarb-sulfoxide, aldicarb-sulfone, carbofuran-3-hydroxy, fenthion-sulfoxide, fenthion-sulfone, phorate-sulfoxide, phorate-sulfone, terbufos-sulfone, and terbufos-sulfoxide, were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). The standard stock solution of each compound at 100 mg/L was prepared in acetone or methanol, except carbendazim in dimethylformamide, and stored at −20 °C.
3.2. Sample Preparation
We followed QuEChERS method and made some modifications. First, 1 g of pollen samples were weighed in a 50 mL centrifuge tube, and 4 mL of water was added into the tube. The tube was shaken to blend the pollen. Then, 2 g portions of glass beads and 10 mL of 1% acetic acid mixture in acetonitrile were added and vortexed for 2 min at room temperature and for 10 min at −20 °C. Second, 0.5 g of MgSO4 and 2 g of NaOAc were added into each tube. The mixture was immediately hand-shaken for 1 min and centrifuged at 3500 rpm for 5 min. Subsequently, 5 mL of the acetonitrile fraction was transferred to a 15 mL centrifuge tube containing 1.25 g of the salt kits level B, described in Section 2.2. The tube was shaken by hand for 1 min and then centrifuged at 3500 rpm for 3 min. Finally, 2.5 mL of the extract was sampled in a 10 mL glass cone and was evaporated at 30 °C until dried under a gentle stream of nitrogen. Five hundred microliters of methanol was added, while the methanol layer was filtered through a 0.22 μm filter membrane and introduced into an autosampler vial for UPLC-MS/MS analysis [4,34,35,36].
3.3. UPLC-MS/MS Analysis
The UPLC-MS/MS instrument consists of a Waters Acquity ultraperformance liquid chromatograph (UPLC) equipped with a 1.7 μm, 2.1 mm × 100 mm particle size and Acquity BEH (ethylene bridged hybrid) C18 column (Waters, Milford, MA, USA) coupled to a Waters Xevo TQ triple quadrupole mass spectrometer operated in the positive electrospray ionization mode. The LC was operated under gradient conditions with mobile phases of water/methanol (98:2) + 0.05% formic acid (A) and methanol + 0.05% formic acid (B) at 40 °C [37]. It was run at 0.45 mL/min starting with 5% component B during a 0.25 min period of the injection time. Then, the composition changed to 100% component B and was maintained until 8.5 min after running. Ultimately, the mobile phase B decreased to 5% in 8.51 min and was held for 10 min to achieve re-equilibration. The total running time of analysis was 10 min. The injection volume was 3 μL.
The MS source temperature was set at 150 °C with nitrogen flow rates of 50 and 900 L/h for the cone and desolvation gases, respectively. The desolvation temperature was 500 °C. Argon was used as the collision gas with a flow of 0.15 mL/min. The mass spectrometer was operated in the multiple reaction monitoring mode (MRM) for monitoring two precursor/products ion transitions for each analyte. The target ion transition with the highest intensity (primary ion transition) was used for quantitation, whereas the second target ion transition was used for confirmation. Further confirmation was obtained through a product ion scan (PIC) for each peak, which was matched to a reference spectrum for each analyte. The quantification and confirmation calculations were determined using the software Target Lynx 4.1 (Waters Corp, Milford, MA, USA) implemented in the instrument. Ion transitions, cone voltages, collision energies, and dwell times for the analytes were shown in Table 6 [16,38].
Table 6.
Ion transitions used for the quantification (MRM1) and confirmation (MRM2), dwell time, cone voltages, and collision energies for MS.
| Compound | Transitions | Dwell Time (s) | Cone Voltage (V) | Collision Energy (eV) |
|---|---|---|---|---|
| Methamidophos | Quantification ion 142 > 93.9 | 0.050 | 17 | 13 |
| Confirmation ion 142 > 124.9 | 17 | 13 | ||
| Acephate | Quantification ion 184.1 > 143 | 0.036 | 8 | 8 |
| Confirmation ion 184.1 > 125.1 | 8 | 18 | ||
| Omethoate | Quantification ion 214.1 > 125.1 | 0.028 | 16 | 22 |
| Confirmation ion 214.1 > 183.1 | 16 | 11 | ||
| Aldicarb sulfoxide | Quantification ion 207 > 89 | 0.028 | 13 | 14 |
| Confirmation ion 207 > 132 | 13 | 10 | ||
| Aldicarb sulfone | Quantification ion 223 > 86 | 0.028 | 22 | 14 |
| Confirmation ion 223 > 148 | 22 | 10 | ||
| Carbendazim | Quantification ion 192.1 > 160.1 | 0.028 | 24 | 18 |
| Confirmation ion 192.1 > 132.1 | 24 | 28 | ||
| Methomyl | Quantification ion 163 > 88 | 0.028 | 17 | 10 |
| Confirmation ion 163 > 106 | 17 | 10 | ||
| Thiamethoxam | Quantification ion 292.1 > 210.9 | 0.044 | 18 | 12 |
| Confirmation ion 292.1 > 181 | 18 | 24 | ||
| Monocrotophos | Quantification ion 224.1 > 127.1 | 0.044 | 15 | 16 |
| Confirmation ion 224.1 > 98.1 | 15 | 12 | ||
| Imidacloprid | Quantification ion 256.1 > 209.1 | 0.028 | 23 | 15 |
| Confirmation ion 256.1 > 175.1 | 23 | 20 | ||
| Trichlorfon | Quantification ion 257 > 109 | 0.028 | 22 | 18 |
| Confirmation ion 257 > 79 | 22 | 30 | ||
| Dimethoate | Quantification ion 230.1 > 199 | 0.028 | 12 | 10 |
| Confirmation ion 230.1 > 125 | 12 | 20 | ||
| Carbofuran-3-hydroxy | Quantification ion 238 > 163 | 0.028 | 25 | 16 |
| Confirmation ion 238 > 181 | 25 | 10 | ||
| Acetamiprid | Quantification ion 223 > 126 | 0.028 | 23 | 20 |
| Confirmation ion 223 > 56.1 | 23 | 15 | ||
| Aldicarb | Quantification ion 212.8 > 88.9 | 0.078 | 20 | 16 |
| Confirmation ion 212.8 > 115.9 | 20 | 12 | ||
| Phosphamidon | Quantification ion 300.1 > 174.1 | 0.028 | 17 | 14 |
| Confirmation ion 300.1 > 127.1 | 17 | 25 | ||
| Dichlorvos | Quantification ion 221 > 109 | 0.022 | 23 | 22 |
| Confirmation ion 221 > 79 | 23 | 34 | ||
| Carbofuran | Quantification ion 222.1 > 165.1 | 0.022 | 25 | 16 |
| Confirmation ion 222.1 > 123 | 25 | 16 | ||
| Fenthion-sulfoxide | Quantification ion 295 > 109 | 0.022 | 29 | 32 |
| Confirmation ion 295 > 280 | 29 | 18 | ||
| Carbaryl | Quantification ion 202 > 145 | 0.022 | 19 | 22 |
| Confirmation ion 202 > 117 | 19 | 28 | ||
| Fenthion-sulfone | Quantification ion 311 > 125 | 0.022 | 29 | 22 |
| Confirmation ion 311 > 109 | 29 | 28 | ||
| Pyrimethanil | Quantification ion 200.2 > 107 | 0.022 | 42 | 24 |
| Confirmation ion 200.2 > 82 | 42 | 24 | ||
| Phorate-sulfoxide | Quantification ion 277 > 96.9 | 0.022 | 15 | 32 |
| Confirmation ion 277 > 143 | 15 | 20 | ||
| Phorate-sulfone | Quantification ion 293 > 96.9 | 0.022 | 15 | 30 |
| Confirmation ion 293 > 115 | 15 | 24 | ||
| Methidathion | Quantification ion 303 > 145 | 0.022 | 10 | 10 |
| Confirmation ion 303 > 85.1 | 10 | 20 | ||
| Phosmet | Quantification ion 318 > 160 | 0.018 | 20 | 14 |
| Confirmation ion 340 > 214.1 | 30 | 14 | ||
| Terbufos-sulfone | Quantification ion 321.2 > 171 | 0.018 | 19 | 12 |
| Confirmation ion 321.2 > 97 | 19 | 40 | ||
| Terbufos-sulfoxide | Quantification ion 305 > 187 | 0.018 | 10 | 11 |
| Confirmation ion 305 > 97 | 10 | 40 | ||
| Azoxystrobin | Quantification ion 404 > 372 | 0.017 | 17 | 15 |
| Confirmation ion 404 > 329 | 17 | 30 | ||
| Malathion | Quantification ion 331 > 127 | 0.018 | 18 | 12 |
| Confirmation ion 331 > 79 | 18 | 40 | ||
| Triadimefon | Quantification ion 294.1 > 197.2 | 0.018 | 22 | 15 |
| Confirmation ion 294.1 > 69.3 | 22 | 20 | ||
| Dimethomorph | Quantification ion 388.1 > 300.9 | 0.018 | 30 | 20 |
| Confirmation ion 388.1 > 165 | 30 | 30 | ||
| Triazophos | Quantification ion 314.1 > 161.9 | 0.013 | 22 | 18 |
| Confirmation ion 314.1 > 118.9 | 22 | 35 | ||
| Ethoprophos | Quantification ion 243.2 > 131 | 0.008 | 18 | 20 |
| Confirmation ion 243.2 > 97 | 18 | 31 | ||
| Iprodione | Quantification ion 330 > 244.7 | 0.008 | 12 | 16 |
| Confirmation ion 330 > 288 | 12 | 15 | ||
| Diflubenzuron | Quantification ion 310.9 > 157.9 | 0.008 | 20 | 14 |
| Confirmation ion 310.9 > 140.9 | 20 | 36 | ||
| Procholraz | Quantification ion 376 > 308 | 0.008 | 20 | 15 |
| Confirmation ion 376 > 266 | 20 | 15 | ||
| Sulfotep | Quantification ion 323 > 97 | 0.008 | 17 | 32 |
| Confirmation ion 323 > 171 | 17 | 15 | ||
| Chlorbenzuron | Quantification ion 309 > 155.9 | 0.008 | 22 | 26 |
| Confirmation ion 309 > 138.8 | 22 | 18 | ||
| Fenthion | Quantification ion 279 > 168.9 | 0.008 | 30 | 18 |
| Confirmation ion 279 > 105 | 30 | 28 | ||
| Coumaphos | Quantification ion 363.1 > 307 | 0.008 | 21 | 16 |
| Confirmation ion 363.1 > 289 | 21 | 24 | ||
| Diazinon | Quantification ion 305.1 > 169 | 0.008 | 20 | 22 |
| Confirmation ion 305.1 > 96.9 | 20 | 35 | ||
| Phoxim | Quantification ion 299 > 129 | 0.008 | 12 | 13 |
| Confirmation ion 299 > 153 | 12 | 7 | ||
| Phorate | Quantification ion 261 > 97 | 0.008 | 14 | 28 |
| Confirmation ion 261 > 75 | 14 | 10 | ||
| Phosalone | Quantification ion 367.9 > 181.9 | 0.008 | 12 | 14 |
| Confirmation ion 367.9 > 110.9 | 12 | 42 | ||
| Difenoconazole | Quantification ion 406 > 251.1 | 0.026 | 37 | 25 |
| Confirmation ion 406 > 111.1 | 37 | 60 | ||
| Emamectin benzoate | Quantification ion 886.5 > 158.1 | 0.022 | 20 | 32 |
| Confirmation ion 886.5 > 81.9 | 20 | 64 | ||
| Profenofos | Quantification ion 372.9 > 302.6 | 0.022 | 25 | 20 |
| Confirmation ion 372.9 > 127.9 | 25 | 40 | ||
| Terbufos | Quantification ion 289 > 103 | 0.022 | 12 | 8 |
| Confirmation ion 289 > 57.2 | 12 | 22 | ||
| Chlorpyrifos | Quantification ion 350 > 97 | 0.022 | 27 | 32 |
| Confirmation ion 350 > 198 | 27 | 20 | ||
| Fenpropathrin | Quantification ion 350.1 > 97 | 0.022 | 15 | 34 |
| Confirmation ion 350.1 > 125 | 15 | 14 | ||
| Pendimethalin | Quantification ion 252.2 > 212.2 | 0.022 | 12 | 10 |
| Confirmation ion 252.2 > 194.1 | 12 | 17 | ||
| Pyridaben | Quantification ion 365.1 > 147.1 | 0.022 | 19 | 24 |
| Confirmation ion 365.1 > 309.1 | 19 | 12 | ||
| Fluvalinate | Quantification ion 507 > 181.1 | 0.022 | 15 | 30 |
| Confirmation ion 507 > 208.1 | 15 | 12 |
3.4. Sample Collection
The pollen samples were provided in March, April, May, June, and July of 2016 by individual apiaries located in eight regions of China. For each month, the samples were collected in several colonies of individual apiary and repacked to obtain one crisper per apiary. All pollen samples were stored at −20 °C until further use. Figure 2 presents the location of the region of the sample collected in China.
Figure 2.
Location of the region of the sample collected in China: A—Jilin Province; B—Shanxi Province; C—Shandong Province; D—Henan Province; E—Anhui Province; F—Hubei Province; G—Chongqing Province; H—Hainan Province.
4. Conclusions
In this study, an accurate and efficient modified QuEChERS protocol was established for determining 54 pesticide residues in crude pollen samples by UPLC-MS/MS analysis. More than 19 different compounds were detected in the samples collected in China. Although the maximum value of several pesticides detected in pollen is slightly lower than the LC50 that is reported for honeybee, the accumulation of pesticides would still endanger honeybee heath. With the appearance of the higher detection rates of carbendazim, fenpropathrin, chlorpyrifos, fluvalinate, chlorbenzuron, and triadimefon, evaluating the risk to honeybee health in the process of spraying pesticides will rely on this study. Further research on pesticide residue in other honeybee food will be necessary to better assess the risk to honeybee health.
Acknowledgments
This work was supported by the Earmarked Fund for China Agriculture Research System (No. CARS-45-KXJ9).
Author Contributions
Hai-Qun Cao, Yan-Hong Shi, and Lin-Sheng Yu conceived and designed the study. Zhou Tong, Yan-Can Wu, and Qiong-Qiong Liu performed the experiments. Yan-Can Wu and Zhou Tong analyzed data. Zhou Tong wrote the manuscript. Zhen-Yu Liu, Hai-Qun Cao and Li-Jun Zhou edited and revised the manuscript. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Farooqui T. A potential link among biogenic amines-based pesticides, learning and memory, and colony collapse disorder: A unique hypothesis. Neurochem. Int. 2013;62:122–136. doi: 10.1016/j.neuint.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Tan K., Chen W., Dong S., Liu X., Wang Y., Nieh J.C. A neonicotinoid impairs olfactory learning in Asian honey bees (Apis cerana) exposed as larvae or as adults. Sci. Rep. 2015;5:10989. doi: 10.1038/srep10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hladik M.L., Vandever M., Smalling K.L. Exposure of native bees foraging in an agricultural landscape to current-use pesticides. Sci. Total Environ. 2016;542(Pt A):469–477. doi: 10.1016/j.scitotenv.2015.10.077. [DOI] [PubMed] [Google Scholar]
- 4.Kiljanek T., Niewiadowska A., Semeniuk S., Gawel M., Borzecka M., Posyniak A. Multi-residue method for the determination of pesticides and pesticide metabolites in honeybees by liquid and gas chromatography coupled with tandem mass spectrometry—Honeybee poisoning incidents. J. Chromatogr. A. 2016;1435:100–114. doi: 10.1016/j.chroma.2016.01.045. [DOI] [PubMed] [Google Scholar]
- 5.Schmuck R., Schöning R., Stork A., Schramel O. Risk posed to honeybees (Apis mellifera L, Hymenoptera) by an imidacloprid seed dressing of sunflowers. Pest Manag. Sci. 2001;57:225–238. doi: 10.1002/ps.270. [DOI] [PubMed] [Google Scholar]
- 6.Swinton S.M., Lupi F., Robertson G.P., Hamilton S.K. Ecosystem services and agriculture: Cultivating agricultural ecosystems for diverse benefits. Ecol. Econ. 2007;64:245–252. doi: 10.1016/j.ecolecon.2007.09.020. [DOI] [Google Scholar]
- 7.Stokstad E. Agriculture. Field research on bees raises concern about low-dose pesticides. Science. 2012;335:1555. doi: 10.1126/science.335.6076.1555. [DOI] [PubMed] [Google Scholar]
- 8.Yu C., Lin R., Fu M., Zhou Y., Zong F., Hui J., Ning L., Piao X., Jia Z., Liu Y. Impact of imidacloprid on life-cycle development of Coccinella septempunctata in laboratory microcosms. Ecotoxicol. Environ. Saf. 2014;110:168–173. doi: 10.1016/j.ecoenv.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Tette P.A., Guidi L.R., Gloria M.B., Fernandes C. Pesticides in honey: A review on chromatographic analytical methods. Talanta. 2016;149:124–141. doi: 10.1016/j.talanta.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 10.Brittain C.A., Vighi M., Bommarco R., Settele J., Potts S.G. Impacts of a pesticide on pollinator species richness at different spatial scales. Basic Appl. Ecol. 2010;11:106–115. doi: 10.1016/j.baae.2009.11.007. [DOI] [Google Scholar]
- 11.Bryden J., Gill R.J., Mitton R.A.A., Raine N.E., Jansen V.A.A. Chronic sublethal stress causes bee colony failure. Ecol. Lett. 2013;16:1463–1469. doi: 10.1111/ele.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cressey D. Europe debates risk to bees. Nature. 2013;496:408. doi: 10.1038/496408a. [DOI] [PubMed] [Google Scholar]
- 13.Ciarlo T.J., Mullin C.A., Frazier J.L., Schmehl D.R. Learning impairment in honey bees caused by agricultural spray adjuvants. PLoS ONE. 2012;7:e40848. doi: 10.1371/journal.pone.0040848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M., Collins E.M., Tao L., Lu C. Simultaneous determination of residues in pollen and high-fructose corn syrup from eight neonicotinoid insecticides by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2013;405:9251–9264. doi: 10.1007/s00216-013-7338-7. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Chao M., Agruna M.J., Calvete G.F., Sakkas V., Llompart M., Dagnac T. Validation of an off line solid phase extraction liquid chromatography-tandem mass spectrometry method for the determination of systemic insecticide residues in honey and pollen samples collected in apiaries from NW Spain. Anal. Chim. Acta. 2010;672:107–113. doi: 10.1016/j.aca.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Kamel A. Refined methodology for the determination of neonicotinoid pesticides and their metabolites in honey bees and bee products by liquid chromatography-tandem mass spectrometry (LC-MS/MS) J. Agric. Food Chem. 2010;58:5926–5931. doi: 10.1021/jf904120n. [DOI] [PubMed] [Google Scholar]
- 17.Kasiotis K.M., Anagnostopoulos C., Anastasiadou P., Machera K. Pesticide residues in honeybees, honey and bee pollen by LC-MS/MS screening: Reported death incidents in honeybees. Sci. Total Environ. 2014;485–486:633–642. doi: 10.1016/j.scitotenv.2014.03.042. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Kelley R.A., Anderson T.D., Lydy M.J. Development and comparison of two multi-residue methods for the analysis of select pesticides in honey bees, pollen, and wax by gas chromatography-quadrupole mass spectrometry. Talanta. 2015;140:81–87. doi: 10.1016/j.talanta.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 19.Vazquez P.P., Lozano A., Ucles S., Ramos M.M., Fernandez-Alba A.R. A sensitive and efficient method for routine pesticide multiresidue analysis in bee pollen samples using gas and liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A. 2015;1426:161–173. doi: 10.1016/j.chroma.2015.11.081. [DOI] [PubMed] [Google Scholar]
- 20.Wiest L., Bulete A., Giroud B., Fratta C., Amic S., Lambert O., Pouliquen H., Arnaudguilhem C. Multi-residue analysis of 80 environmental contaminants in honeys, honeybees and pollens by one extraction procedure followed by liquid and gas chromatography coupled with mass spectrometric detection. J. Chromatogr. A. 2011;1218:5743–5756. doi: 10.1016/j.chroma.2011.06.079. [DOI] [PubMed] [Google Scholar]
- 21.Leandro C.C., Bishop D.A., Fussell R.J., Smith F.D., Keely B.J. Semiautomated determination of pesticides in water using solid phase extraction disks and gas chromatography-mass spectrometry. J. Agric. Food Chem. 2006;54:645–649. doi: 10.1021/jf051874d. [DOI] [PubMed] [Google Scholar]
- 22.Mullin C.A., Frazier M., Frazier J.L., Ashcraft S., Simonds R., Vanengelsdorp D., Pettis J.S. High levels of miticides and agrochemicals in North American apiaries: Implications for honey bee health. PLoS ONE. 2010;5:e9754. doi: 10.1371/journal.pone.0009754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajšlová J., Zrostlíková J. Matrix effects in (ultra)trace analysis of pesticide residues in food and biotic matrices. J. Chromatogr. A. 2003;1000:181–197. doi: 10.1016/S0021-9673(03)00539-9. [DOI] [PubMed] [Google Scholar]
- 24.Kruve A., Kunnapas A., Herodes K., Leito I. Matrix effects in pesticide multi-residue analysis by liquid chromatography-mass spectrometry. J. Chromatogr. A. 2008;1187:58–66. doi: 10.1016/j.chroma.2008.01.077. [DOI] [PubMed] [Google Scholar]
- 25.Niessen W.M., Manini P., Andreoli R. Matrix effects in quantitative pesticide analysis using liquid chromatography-mass spectrometry. Mass Spectrom. Rev. 2006;25:881–899. doi: 10.1002/mas.20097. [DOI] [PubMed] [Google Scholar]
- 26.Hatjina F., Papaefthimiou C., Charistos L., Dogaroglu T., Bouga M., Emmanouil C., Arnold G. Sublethal doses of imidacloprid decreased size of hypopharyngeal glands and respiratory rhythm of honeybees in vivo. Apidologie. 2013;44:467–480. doi: 10.1007/s13592-013-0199-4. [DOI] [Google Scholar]
- 27.Gonalons C.M., Farina W.M. Effects of Sublethal Doses of Imidacloprid on Young Adult Honeybee Behaviour. PLoS ONE. 2015;10:e0140814. doi: 10.1371/journal.pone.0140814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang E., Nieh J.C. The neonicotinoid imidacloprid impairs honey bee aversive learning of simulated predation. J. Exp. Biol. 2015;218:3199–3205. doi: 10.1242/jeb.127472. [DOI] [PubMed] [Google Scholar]
- 29.Frost E.H., Shutler D., Hillier N.K. Effects of fluvalinate on honey bee learning, memory, responsiveness to sucrose, and survival. J. Exp. Biol. 2013;216(Pt 15):2931–2938. doi: 10.1242/jeb.086538. [DOI] [PubMed] [Google Scholar]
- 30.David A., Botias C., Abdul-Sada A., Nicholls E., Rotheray E.L., Hill E.M., Goulson D. Widespread contamination of wildflower and bee-collected pollen with complex mixtures of neonicotinoids and fungicides commonly applied to crops. Environ. Int. 2016;88:169–178. doi: 10.1016/j.envint.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Renzi M.T., Amichot M., Pauron D., Tchamitchian S., Brunet J.L., Kretzschmar A., Maini S., Belzunces L.P. Chronic toxicity and physiological changes induced in the honey bee by the exposure to fipronil and Bacillus thuringiensis spores alone or combined. Ecotoxicol. Environ. Saf. 2016;127:205–213. doi: 10.1016/j.ecoenv.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 32.Berenbaum M.R., Johnson R.M. Xenobiotic detoxification pathways in honey bees. Curr. Opin. Insect Sci. 2015;10:51–58. doi: 10.1016/j.cois.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Thompson H.M., Fryday S.L., Harkin S., Milner S. Potential impacts of synergism in honeybees (Apis mellifera) of exposure to neonicotinoids and sprayed fungicides in crops. Apidologie. 2014;45:545–553. doi: 10.1007/s13592-014-0273-6. [DOI] [Google Scholar]
- 34.González-Curbelo M.Á., Socas-Rodríguez B., Herrera-Herrera A.V., González-Sálamo J., Hernández-Borges J., Rodríguez-Delgado M.Á. Evolution and applications of the QuEChERS method. TrAC Trends Anal. Chem. 2015;71:169–185. doi: 10.1016/j.trac.2015.04.012. [DOI] [Google Scholar]
- 35.Barganska Z., Slebioda M., Namiesnik J. Determination of pesticide residues in honeybees using modified QUEChERS sample work-up and liquid chromatography-tandem mass spectrometry. Molecules. 2014;19:2911–2924. doi: 10.3390/molecules19032911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fidente P., Seccia S., Vanni F., Morrica P. Analysis of nicotinoid insecticides residues in honey by solid matrix partition clean-up and liquid chromatography-electrospray mass spectrometry. J. Chromatogr. A. 2005;1094:175–178. doi: 10.1016/j.chroma.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Wang J., Chow W., Chang J., Wong J.W. Ultrahigh-performance liquid chromatography electrospray ionization Q-Orbitrap mass spectrometry for the analysis of 451 pesticide residues in fruits and vegetables: Method development and validation. J. Agric. Food Chem. 2014;62:10375–10391. doi: 10.1021/jf503778c. [DOI] [PubMed] [Google Scholar]
- 38.Walorczyk S., Gnusowski B. Development and validation of a multi-residue method for the determination of pesticides in honeybees using acetonitrile-based extraction and gas chromatography-tandem quadrupole mass spectrometry. J. Chromatogr. A. 2009;1216:6522–6531. doi: 10.1016/j.chroma.2009.07.045. [DOI] [PubMed] [Google Scholar]