Abstract
The synthesis and antiproliferative activity of new benzimidazole derivatives bearing an hydrazone mojety at the 2-position is described. The new N′-(4-arylidene)-1H-benzo[d]imidazole-2-carbohydrazides were evaluated for their cytostatic activity toward the murine leukemia (L1210), human T-cell leukemia (CEM), human cervix carcinoma (HeLa) and human pancreas carcinoma cells (Mia Paca-2). A preliminary structure-activity relationship could be defined. Some of the compounds possess encouraging and consistent antiproliferative activity, having IC50 values in the low micromolar range.
Keywords: benzimidazoles, hydrazones, antiproliferative activity
1. Introduction
As recently reviewed [1], benzimidazole is a privileged structure in medicinal chemistry because of its broad range of biological activities. The benzimidazole ring is present in some clinically used drugs, such as proton pump inhibitors, antihelmintic compounds, the antiviral enviroxime and the antihistaminic astemizole, but it may also display antimycobacterial, antimicrobial, anticonvulsant, analgesic, anti-inflammatory, anti-diabetic, antiprotozoal, antipsychotic, antioxidant and antitumoral properties [1].
Several series of benzimidazole derivatives have shown antiproliferative activity. Moreover, benzimidazole-5-carboxylic acid derivatives induced cell death in leukemic cells [2]. The antiproliferative activity of benzimidazole derivatives has been correlated to multi-target kinase inhibition [3,4]. Furthermore, benzimidazole carbamates showed antitubulin activity [5,6] and benzimidazole-carbazole conjugates were described to stabilize human telomeric DNA and to inhibit telomerase and topoisomerase I in cancer cells [7,8]. Benzimidazole has also been reported as a new scaffold endowed with sirtuin 1 and 2 inhibitory activity. These compounds also showed cytotoxicity against the breast cancer cell lines MCF-7 and MDA-MB-468 [9,10].
Hydrazone is another biologically active pharmacophore group. N-(1′-Naphthyl)-3,4,5-trimethoxybenzohydrazide has been reported as a potential anti-leukemia agent acting as a microtubule destabilizer [11]. 2-N-Heteroaryl caffeine hydrazones demonstrated high activity toward T-lymphoblastic leukemia cells and inhibited both RNA and DNA synthesis and mitosis [12]. 4-(2-Fluorophenoxy)quinolineacylhydrazones showed excellent antiproliferative activity and c-Met kinase inhibitory activity [13]. Salicylaldehyde isonicotinoylhydrazone analogs behaved as iron chelators especially in MCF-7 breast adenocarcinoma cells and this was correlated to their cytotoxic activity [14], while salicylaldehyde 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide hydrazone derivatives inhibited the growth of A549 lung cancer cells [15]. We have previously reported on 2-arylamino-6-trifluoromethyl-3-(hydrazinocarbonyl)pyridines [16] showing in vitro inhibitory activity against human tumour cell lines at low micromolar to nanomolar concentrations. Furthermore 4-(diethylamino)salycilaldehyde hydrazones showed potent cytotoxicity against human tumour cell lines but showed no toxicity in athymic nude mice [17]. Although the biological activity of benzimidazole and hydrazine structures has been well documented, an extensive literature search revealed very few efforts to combine these two important moieties in a single molecular scaffold. Few studies described the anti-inflammatory and antimicrobial activity of benzimidazole-5-carboxylate and its hydrazone derivatives [18] and the antiproliferative activity of N-1-benzoimidazole acetohydrazides against various tumor cell lines [19]. Therefore, in this explorative work, based on the antiproliferative activity of benzimidazole and hydrazone containing structures we decided to investigate, in a dualistic approach [20], new benzimidazole-hydrazone compounds, namely N′-(4-arylidene)-1H-benzo[d]imidazole-2-carbohydrazides and investigated their antiproliferative activity.
2. Results
2.1. Chemistry
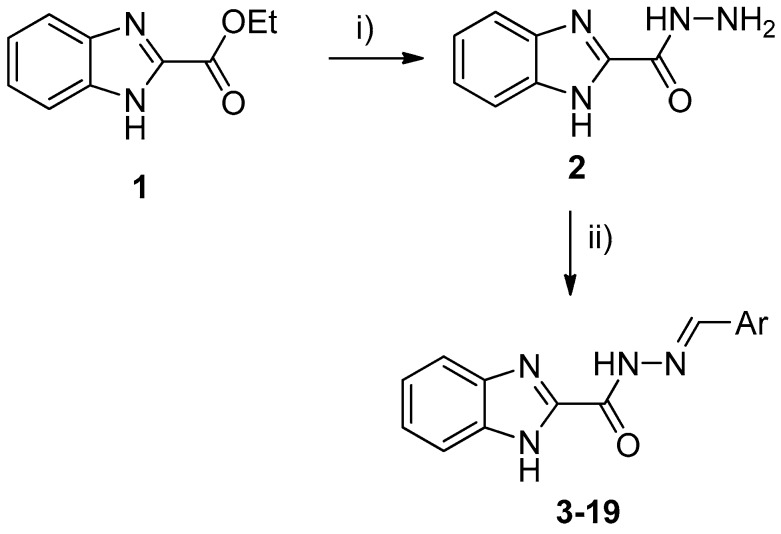
The target hydrazones 3–19 were synthesized as shown in Scheme 1. High yields of 1H-benzo[d]imidazole-2-carbohydrazide (2) were achieved upon refluxing for 3 h in an ethanolic solution of the corresponding ethyl ester 1 and hydrazine hydrate. Hydrazones 3–19 were obtained in good to excellent yield by coupling the hydrazide 2 with the appropriate hydroxyarylaldehydes in ethanol. All the newly synthesized compounds gave corrected analytical data. The IR and NMR spectral data are consistent with the assigned structure. According to the literature, the presence of a single downfield resonating (8.49–9.79 ppm) CH=N signal indicates the exclusive formation of E-isomers [21].
Scheme 1.
Synthesis of hydrazones 3–19. Reagents and conditions: (i) NH2NH2·H2O, EtOH, reflux, 3 h; (ii) ArCHO, EtOH reflux, 5 h.
2.2. Antiproliferative Activity
The synthesized hydrazones 3–19 were evaluated in vitro for their inhibitory effects on the proliferation of murine leukemia (L1210), human T-lymphoblastic leukemia (CEM), human cervix carcinoma (HeLa) and human pancreas carcinoma (Mia Paca-2) cells. The presence of a 2-hydroxyl group on the arylidene moiety favourably modulates antiproliferative activity (Table 1).
Table 1.
Antiproliferative activity of N′-(4-arylidene)-1H-benzo[d]imidazole-2-carbohydrazides 3–19.
| Compd. | Ar | IC50 (μM) a | |||
|---|---|---|---|---|---|
| L1210 | CEM | HeLa | Mia-Paca-2 | ||
| 3 | 2-OH-phenyl | 5.7 ± 0.9 | 4.4 ± 0.2 | 12 ± 0.7 | 34 ± 0.9 |
| 4 | 3-OH-phenyl | 94 ± 7 | 98 ± 10 | 88 ± 12.5 | >100 |
| 5 | 4-OH-phenyl | 47 ± 2 | 85 ± 0.01 | >100 | >100 |
| 6 | 2,4-(OH)2-phenyl | 2.6 ± 0.9 | 2.6 ± 1.0 | 4.7 ± 1.6 | 21 ± 12 |
| 7 | 2,5-(OH)2-phenyl | 22 ± 7 | 4.9 ± 1.2 | 77 ± 18 | >100 |
| 8 | 2,3,4-(OH)3-phenyl | 47 ± 7 | 20 ± 8 | 97 ± 28 | >100 |
| 9 | 2,4,6-(OH)3-phenyl | 90 ± 7 | >100 | >100 | >100 |
| 10 | 2-OH-4-OMe-phenyl | 1.6 ± 0.9 | 0.98 ± 0.02 | 4.0 ± 0.4 | 6.3 ± 3.2 |
| 11 | 2-OH-3-OEt-phenyl | 5.9 ± 2.4 | 6.3 ± 1.9 | 22 ± 0.02 | 23 ± 8 |
| 12 | 3-OH-4-OMe-phenyl | 92 ± 4 | 57 ± 3.7 | >100 | >100 |
| 13 | 2-OH-4-N(Et)2-phenyl | 14 ± 2 | 4.8 ± 0.8 | 23 ± 3 | 40 ± 6 |
| 14 | 2-OH-5-Cl-phenyl | 7.4 ± 2.5 | 1.8 ± 0.6 | 4.8 ± 0.9 | 9.2 ± 4.0 |
| 15 | 2-OH-5-Br-phenyl | 5.0 ± 2.8 | 1.8 ± 0.9 | 4.9 ± 0.4 | 35 ± 5 |
| 16 | 2-OH-naphtyl | 2.9 ± 1.3 | 1.0 ± 0.01 | 2.5 ± 1.4 | 7.9 ± 0.3 |
| 17 | phenyl | >250 | >250 | >250 | 220 ± 37 |
| 18 | 4-OMe-phenyl | >250 | >250 | >250 | >250 |
| 19 | napht-1-yl | 240±13 | >250 | >250 | >250 |
a IC50 values (compound concentration that reduces cell growth by 50%) are shown.
3. Discussion
Hydrazones 10 and 16 inhibited the growth of all tested cell lines with low (<10 μM) micromolar IC50 values. The replacement of the 2-hydroxynaphthyl group of compound 16 with the 2-hydroxyphenyl resulted in the hydrazone 3 endowed with reduced antiproliferative activity against HeLa and Mia Paca-2 cells. The shift of the hydroxyl group from the 2-position to the 3- or 4-position led to poorly active compounds 4 and 5. The introduction of a second 4-hydroxyl group (compound 6) caused an increase in activity with respect to the 2-hydroxy analog 3, whereas the presence of the 2,5-dihydroxybenzylidene group (compound 7) led to a clear reduction in inhibitory activity as compared with compounds 4 and 6. The introduction of a third hydroxyl group (compounds 8 and 9) resulted in reduction or loss of cytostatic activity. A comparison of substituent effects revealed that the introduction of a 2-hydroxy-5-halobenzylidene moiety led to compounds 14 and 15 endowed with a better antiproliferative effect as compared with 2,5-dihydroxybenzylidene and 2-hydroxybenzylidene analogs 7 and 4. The hydrazone of 5-chlorosalicylaldehyde 14 showed antiproliferative activity at single digit micromolar IC50 values on all tumor cell lines, while the 5-bromo derivative 15 was less active on Mia Paca-2 cells. The introduction of the 2-hydroxy-4-methoxybenzylidene moiety (compound 10) caused an increase in activity as compared with 2,4-dihydroxybenzylidene and 2-hydroxybenzylidene analogs 6 and 3. A reduction in activity was produced by the shift of the ether group from the 4- to the 3-position (hydrazone 11), while the shift of the hydroxyl group from the 2- to the 3-position (compound 12) led to a drop in activity. The replacement of the 4-methoxy group with a 4-(diethylamino) group was tolerated although hydrazone 13 was less active as compared with hydrazones 3 and 6. The hydrazones 17–19 lacking hydroxyl groups are completely inactive.
The efficacy of any drug depends on its high oral bioavailability, so we assessed the potential bioavailability of the most active compounds, using the adsorption, distribution, metabolism and elimination (ADME) prediction method by molinspiration (http://www.molinspiration.com/cgi-bin/properties).
According to Lipinski’s rule of five [22] a compound to become a successful drug candidate, should have a molecular weight ≤500, a log p ≤ 5, hydrogen bond donor sites ≤5 and hydrogen bond acceptor sites (N and O atoms) ≤10. Predictions of the ADME properties for studied compounds (Table 2) showed that all the active compounds fulfilled this rule, similarly to clinically used drugs. Furthermore, the number of rotable bonds is important for conformational flexibility of the molecule. The total polar surface area (TPSA) is another key property that has been linked to drug bioavailability. These two parameters, i.e., the number of rotable bonds in a molecule ≤10 and polar surface area ≤140 Å are essential for a good oral bioavailability [23]. Theoretically, all hydrazones 3, 6–8, 10, 11, 13–16 should present good passive oral absorption and differences in their bioactivity cannot be attributed to this property.
Table 2.
In silico physico-chemical properties (ADME) of active compounds 3, 6–8, 10, 11, 13–16.
| Compd. | TPSA | n-ROTB | MV | MW | miLogP | n-ON | n-OHNH | n-Viol |
|---|---|---|---|---|---|---|---|---|
| 3 | 90.37 | 3 | 242.94 | 280.29 | 2.64 | 6 | 3 | 0 |
| 6 | 110.60 | 3 | 250.96 | 296.29 | 2.13 | 7 | 4 | 0 |
| 7 | 110.60 | 3 | 250.96 | 296.29 | 2.13 | 7 | 4 | 0 |
| 8 | 130.83 | 3 | 258.97 | 312.29 | 1.67 | 8 | 5 | 0 |
| 10 | 99.61 | 4 | 268.48 | 310.31 | 2.677 | 7 | 3 | 0 |
| 11 | 99.61 | 5 | 285.29 | 324.34 | 2.62 | 7 | 3 | 0 |
| 13 | 93.61 | 6 | 322.45 | 351.41 | 3.47 | 7 | 3 | 0 |
| 14 | 90.37 | 3 | 256.47 | 314.73 | 3.29 | 6 | 3 | 0 |
| 15 | 90.37 | 3 | 260.82 | 359.18 | 3.42 | 6 | 3 | 0 |
| 16 | 90.37 | 3 | 286.93 | 330.35 | 3.79 | 6 | 3 | 0 |
TPSA: topological polar surface area; n-ROTB: number of rotable bonds; MV: molecular volume; MW: molecular weight; milogP: logarithm of partition coefficient between n-octanol and water; n-ON: number of hydrogen bond acceptors; n-OHNH: number of hydrogen bond donors; n-Viol: number of violations of Lipinski’s rule.
4. Materials and Methods
4.1. General Information
All commercially available solvents and reagents were used without further purification. 1H- and 13C-NMR spectra were recorded on an Inova 500 spectrometer (Varian, Palo Alto, CA, USA). The chemical shifts (δ) are reported in part per million downfield from tetramethylsilane (TMS), which was used as internal standard, and the spectra were recorded in hexadeuteriodimethylsulphoxide (DMSO-d6). Infrared spectra were recorded on a Vector 22 spectrometer (Bruker, Bremen, Germany) in Nujol mulls. The main bands are given in cm−1. Positive-ion electrospray ionization (ESI) mass spectra were recorded on a double-focusing MAT 95 instrument (Finnigan, Waltham, MA, USA) with BE geometry. Melting points (mp) were determined on a SMP1 Melting Point apparatus (Stuart Scientific, Stone, UK) and are uncorrected. All products reported showed 1H NMR spectra in agreement with the assigned structures. The purity of the tested compounds was determined by combustion elemental analyses conducted by the Microanalytical Laboratory of the Chemistry Department of the University of Ferrara with a MT-5 CHN recorder elemental analyzer (Yanagimoto, Kyoto, Japan) and the values found were within 0.4% of theoretical values. Hydrazones 17 and 18 were synthesized as previously described [24].
4.2. Synthesis
4.2.1. 1H-benzo[d]imidazole-2-carbohydrazide (2)
A mixture of ethyl 1H-benzo[d]imidazole-2-carboxylate (1, 3.80 g, 20 mmol), and hydrazine monohydrate (3 mL, 61.5 mmol) in EtOH (5 mL) was refluxed for 3 h. After cooling the formed precipitate was filtered off, washed with water (5 × 10 mL) dried and used without further purification. Yield 80%. Mp 240–242 °C (lit. [25] 217–219). IR: 3321, 3265, 3066, 1661, 1609 cm−1. 1H-NMR: δ 4.62 (s, 2H, NH2), 7.28 (m, 2H, Ar), 7.53 (d, J = 8.0 Hz, 1H, Ar), 7.70 (d, J = 8.0 Hz, 1H, Ar), 10.14 (s, 1H, NH), 13.20 (s, 1H, NH). ESI-MS m/z 177 (M + H)+. Anal. Calcd for C8H8N4O: C, 54.54; H, 4.58; N, 31.80. Found: C, 54.57; H, 4.57; N, 31.76.
4.2.2. General Procedure for the Synthesis of Hydrazones 3–19
A mixture of hydrazide 2 (1 mmol) and the appropriate aldehyde (1 mmol) in EtOH (10 mL) was refluxed for 5 h. After cooling the formed precipitate was filtered off and purified by crystallization from the adequate solvent to give the hydrazone derivatives.
(E)-N′-(2-Hydroxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide (3). Yield 78%. Mp > 250 °C (EtOH). IR: 3191, 1664, 1614, 1556 cm−1. 1H-NMR: δ 6.94 (m, 2H, Ar), 7.31 (m, 3H, Ar) 7.67 (m, 3H, Ar), 8.83 (s, 1H, CH), 11.23 (s, 1H, OH), 12.78 (s, 1H, NH), 13.51 (s, 1H, NH). 13C-NMR: δ 105.4, 109.9, 115.1, 115.9, 123.0, 126.8, 129.7, 136.3, 135.2, 138.4, 141.2, 145.9, 150.1. ESI-MS m/z 281 (M + H)+. Anal. Calcd for C15H12N4O2: C, 64.28; H, 4.32; N, 19.99. Found: C, 64.22; H, 4.33; N, 20.05.
(E)-N′-(3-Hydroxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide (4). Yield 78%. Mp > 250 °C (EtOH). IR: 3221, 1677, 1610, 1576 cm−1. 1H-NMR: δ 6.85 (d, J = 8.0 Hz, 1H, Ar), 7.11 (d, J = 7.5 Hz, 1H, Ar), 7.21 (s, 1H, Ar), 7.26 (d, J = 7.5 Hz, 1H, Ar), 7.29–7.78 (m, 4H, Ar), 8.56 (s, 1H, CH), 9.69 (s, 1H, OH), 12.40 (s, 1H, NH), 13.47 (s, 1H, NH). 13C-NMR: δ 105.3, 105,4, 109.8, 117.1, 117.9, 123.1, 130.1, 129.8, 138.7, 138.8, 144.3, 149.9, 150.2. ESI-MS m/z 281 (M+ H)+. Anal. Calcd for C15H12N4O2: C, 64.28; H, 4.32; N, 19.99. Found: C, 64.34; H, 4.31; N, 20.03.
(E)-N′-(4-Hydroxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide (5). Yield 80%. Mp > 250 °C (1-PrOH). IR: 3289, 1668,1609, 1584 cm−1. 1H-NMR: δ 6.86 (d, J = 8.5 Hz, 2H, Ar), 7.34 (d, J = 7.0 Hz, 2H, Ar), 7.57–7.59 (m, 3H, Ar), 7.77 (d, J = 7.0 Hz, 1H, Ar), 8.53 (s, 1H, CH), 10.03 (s, 1H, OH), 12.23 (s, 1H, NH), 13.43 (s, 1H, NH). 13C-NMR: δ 105.8, 106,3, 116.8, 118.4, 130.3, 139.8, 144.8, 150.9, 152.3. ESI-MS m/z 281 (M + H)+. Anal. Calcd for C15H12N4O2: C, 64.28; H, 4.32; N, 19.99. Found: C, 64.33; H, 4.31; N, 20.04.
(E)-N′-(2,4-Dihydroxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide (6). Yield 75%. Mp > 250 °C (1-PrOH). IR: 3227, 1673, 1638, 1587 cm−1. 1H-NMR: δ 6.34 (m, 2H, Ar), 6.38 (d, J = 6.5 Hz, 1H, Ar), 7.57 (m, 4H, Ar), 8.68 (s, 1H, CH), 10.09 (s, 1H, OH), 11.43 (s, 1H, OH), 12.59 (s, 1H, NH), 13.45 (s, 1H, NH). 13C-NMR: δ 105.0, 109.6, 115.1, 115.9, 123.2, 125.9, 127.6, 134.7, 135.1, 137.8, 145.9, 148.0. ESI-MS m/z 297 (M + H)+. Anal. Calcd for C15H12N4O3: C, 60.81; H, 4.08; N, 18.91. Found: C, 60.76; H, 4.10; N, 18.94.
(E)-N′-(2,5-Dihydroxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide (7). Yield 81%. Mp > 250 °C (EtOH). IR: 3238, 1681, 1620, 1586 cm−1. 1H-NMR: δ 6.77 (d, J = 9.0 Hz, 1H, Ar), 6.79 (d, J = 9.0 Hz, 1H, Ar), 6.96 (s, 1H, Ar), 7.59 (m, 4H, Ar), 8.75 (s, 1H, CH) 9.03 (s, 1H, OH), 10.38 (s, 1H, OH), 12.67 (s, 1H, NH), 13.48 (s, 1H, NH). 13C-NMR: δ 105.7, 110.3, 115.6, 116.0, 123.3, 126.5, 130.2, 134.9, 135.6, 138.1, 145.8, 148.1. ESI-MS m/z 297 (M + H)+. Anal. Calcd for C15H12N4O3: C, 60.81; H, 4.08; N, 18.91. Found: C, 60.86; H, 4.09; N, 18.87.
(E)-N′-(2,3,4-Trihydroxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide (8). Yield 62%. Mp > 250 °C (EtOH). IR: 3228, 3127, 3061, 1672, 1644 cm−1. 1H-NMR: δ 6.42 (d, J = 8.5 Hz, 1H, Ar), 6.76 (d, J = 8.5 Hz, 1H, Ar), 7.64 (m, 4H, Ar), 8.65 (s, 1H, CH), 9.57 (s, 2H, OH), 11.49 (s, 1H, OH), 12.65 (s, 1H, NH), 13.48 (s, 1H, NH). 13C-NMR: δ 106.2, 111.0, 115.7, 116.1, 125.6, 127.1, 135.0, 138.6, 138.7, 139.9, 147.1, 149.2. ESI-MS m/z 313 (M + H)+. Anal. Calcd for C15H12N4O4: C, 57.69; H, 3.87; N, 17.94. Found: C, 57.64; H, 3.88; N, 17.91.
(E)-N′-(2,4,6-Trihydroxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide (9). Yield 60%. Mp > 250 °C (EtOH). IR: 3225, 1672, 1592 cm−1. 1H-NMR: δ 5.80 (s, 1H, Ar) 5.86 (s, 1H, Ar), 7.26 (d, J = 7.0 Hz, 1H, Ar), 7.30–7.78 (m, 3H, Ar), 8.98 (s, 1H, CH), 10.14 (s, 1H, OH), 11.14 (s, 2H, OH), 12.68 (s, 1H, NH), 13.43 (s, 1H, NH). 13C-NMR: δ 99.2, 103.9, 119.7, 127.9, 138.9, 140.1, 145.2, 147.4, 151.0. ESI-MS m/z 313 (M + H)+. Anal. Calcd for C15H12N4O4: C, 57.69; H, 3.87; N, 17.94. Found: C, 57.73; H, 3.86; N, 17.92.
(E)-N′-(2-Hydroxy-4-methoxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide (10). Yield 79%. Mp > 250 °C (EtOH). IR: 3214, 1665, 1633, 1607, 1567 cm−1. 1H-NMR: δ 3.79 (s, 3H, OCH3), 6.54 (m, 2H, Ar), 7.48 (m, 5H, Ar), 8.74 (s, 1H, CH), 11.55 (s, 1H, NH), 12.69 (s, 1H, OH), 13.47 (s, 1H, NH). 13C-NMR: δ 58.5, 104.4, 109.7, 114.9, 115.8, 123.1, 126.0, 127.6, 134.5, 135.2, 137.7, 145.7, 147.6. δ ESI-MS m/z 311 (M + H)+. Anal. Calcd for C16H14N4O3: C, 61.93; H, 4.55; N, 18.06. Found: C, 61.99; H, 4.53; N, 18.02.
(E)-N′-(3-Ethoxy-2-hydroxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide (11). Yield 70%. Mp > 250 °C (EtOH). IR: 3322, 1694, 1610, 1583 cm−1. 1H-NMR: δ 1.36 (t, J = 7.0 Hz, 3H, CH3), 4.07 (q, J = 7.0 Hz, 2H, CH2), 6.86 (m, 1H, Ar), 7.03 (d, J = 8.0 Hz, 1H, Ar), (d, J = 8.0 Hz, 1H, Ar), 7.59 (m, 4H, Ar), 8.84 (s, 1H, CH), 10.99 (s, 1H, NH), 12.81 (s, 1H, OH), 13.53 (s, 1H, NH). 13C-NMR: δ 14.9, 65.0, 106.3, 107.7, 115.6, 115.8, 123.1, 124.5, 129.9, 135.8, 137.9, 146.2, 147.4, 149.2. ESI-MS m/z 325 (M + H)+. Anal. Calcd for C17H16N4O3: C, 62.95; H, 4.97; N, 17.27. Found: C, 63.01; H, 4.99; N, 17.23.
(E)-N′-(3-Hydroxy-4-methoxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide (12). Yield 71%. Mp > 250 °C (EtOH). IR: 3277, 3219, 1673, 1612, 1567 cm−1. 1H-NMR: δ 3.96 (s, 3H, OCH3), 6.99 (d, J = 8.5 Hz, 1H, Ar), 7.0 (d, J = 8.5 Hz, 1H, Ar), 7.29 (s, 1H, Ar), 7.49 (m, 4H, Ar), 8.49 (s, 1H, CH), 9.39 (br s, 1H, OH), 12.25 (br s, 1H, NH), 13.20 (br s, 1H, NH). 13C-NMR: δ 58.5, 106.0, 106.3, 110.1, 115.9, 116.7, 124.8, 128.5, 129.7, 135.4, 136.1, 145.4, 146.9. ESI-MS m/z 311 (M + H)+. Anal. Calcd for C16H14N4O3: C, 61.93; H, 4.55; N, 18.06. Found: C, 61.88; H, 4.56; N, 18.10.
(E)-N′-(4-(Diethylamino)-2-hydroxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide (13). Yield 78%. Mp > 250 °C (EtOH). IR: 1668, 1631, 1586 cm−1. 1H-NMR: δ 1.10 (t, J = 7.0 Hz, 6H, CH3), 3.35 (q, J = 7.0 Hz, 4H, CH2), 6.12 (s, 1H, Ar), 6.27 (d, J = 6.0 Hz, 1H, Ar), 7.14 (d, J = 6.0 Hz, 1H, Ar), 7.32 (m, 2H, Ar), 7.57(m, 1H, Ar), 7.77 (m, 1H, Ar), 8.60 (s, 1H, CH), 11.42 (s, 1H, OH), 12.52 (s, 1H, NH), 13.42 (s, 1H, NH). 13C-NMR: δ 13.2, 48.9, 99.2, 103.6, 106.7, 109.9, 115.9, 122.7, 125.9, 131.4, 137.7, 140.0, 142.3, 146.2, 150.6. ESI-MS m/z 352 (M + H)+. Anal. Calcd for C19H21N5O2: C, 64.94; H, 6.02; N, 19.93. Found: C, 65.01; H, 5.99; N, 19.97.
(E)-N′-(5-Chloro-2-hydroxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide (14). Yield 85%. Mp > 250 °C (EtOH). IR: 3215, 1680, 1605, 1591 cm−1. 1H-NMR: δ 6.96 (d, J = 8.0 Hz, 1H, Ar), 7.33–7.65 (m, 6H, Ar), 8.82 (s, 1H, CH), 11.15 (br s, 1H, OH), 12.90 (br s, 1H, NH), 13.30 (br s, 1H, NH). 13C-NMR: δ 105.6, 107.4, 109.9, 115.9, 117.2, 122.8, 123.0, 128.0, 129.5, 134.4, 141.5, 141.9, 147.6, 151.2. ESI-MS m/z 315 (M + H)+. Anal. Calcd for C15H11ClN4O2: C, 57.24; H, 3.52; N, 17.80. Found: C, 57.30; H, 3.51; N, 17.83.
(E)-N′-(5-Bromo-2-hydroxybenzylidene)-1H-benzo[d]imidazole-2-carbohydrazide (15). Yield 82%. Mp > 250 °C (EtOH). IR: 3200, 1680, 1602, 1588 cm−1. 1H-NMR: δ 6.91 (d, J = 9.0 Hz, 1H, Ar), 7.33 (d, J = 6.0 Hz, 1H, Ar), 7.35 (d, J = 6.0 Hz, 1H, Ar), 7.60 (m, 4H, Ar), 8.81 (s, 1H, CH), 11.15 (s, 1H, OH), 12.85 (s,1H, NH), 13.50 (s, 1H, NH). 13C-NMR: δ 105.2, 107.5, 109.9, 116.0, 117.3, 122.9, 123.0, 128.2, 129.4, 134.5, 141.5, 142.0, 147.7, 151.1. ESI-MS m/z 359 (M + H)+. Anal. Calcd for C15H11BrN4O2: C, 50.16; H, 3.09; N, 15.60. Found: C, 50.22; H, 3.11; N, 15.57.
(E)-N′-((2-Hydroxynaphthalen-1-yl)methylene)-1H-benzo[d]imidazole-2-carbohydrazide (16). Yield 85%. Mp > 250 °C (EtOH). IR: 3254, 1679, 1625, 1576 cm−1. 1H-NMR: δ 7.26 (d, J = 9.0 Hz, 1H, Ar), 7.35 (d, J = 6.5 Hz, 1H, Ar), 7.38 (d, J = 6.5 Hz, 1H, Ar), 7.54 (m, 3H, Ar), 7.82 (d, J = 7.0 Hz, 1H, Ar), 8.11 (m, 3H, Ar), 9.79 (s, 1H, CH), 12.77 (s, 1H, NH), 12.86 (s, 1H, OH), 13.58 (s, 1H, NH). 13C-NMR: δ 111.6, 115.9, 122.0, 123.2, 123.6, 126.1, 126.8, 127.8, 131.0, 132.1, 135.0, 136.2, 137.8, 145.7, 147.3, 151.8, 158.0, 161.3. MS m/z 331 (M + H)+. Anal. Calcd for C19H14N4O2: C, 69.08; H, 4.27; N, 16.96. Found: C, 69.14; H, 4.29; N, 17.01.
(E)-N′-(Naphthalen-1-ylmethylene)-1H-benzo[d]imidazole-2-carbohydrazide (19). Yield 92%. Mp > 250 °C (EtOH). IR: 3208, 3044, 1618, 1558 cm−1. 1H-NMR: δ 7.32 (m, 2H, Ar), 7.65 (m, 4H, Ar), 7.81 (m, 1H, Ar), 8.01–8.16 (m, 3H, Ar), 8.74 (m, 1H, Ar), 9.44 (s, 1H, CH), 12.54 (s, 1H, NH), 13.55 (s, 1H, NH). 13C-NMR: 115.0, 122.9, 123.1, 126.0, 126.2, 127.5, 128.4, 128.5, 130.2, 130.6, 130.7, 138.6, 143.3, 144.8, 155.6. ESI-MS m/z 315 (M + H)+. Anal. Calcd for C19H14N4O: C, 72.60; H, 4.49; N, 17.82. Found: C, 72.66; H, 4.47; N, 17.78.
4.3. Antiproliferative Activity
4.3.1. Cell Lines
Human cervical carcinoma (HeLa), human T-lymphoblast (CEM) and mouse leukema (L1210) cells were obtained from ATCC (Middlesex, UK). Human pancreatic carcinoma (Mia-Paca 2) cells were kindly provided by Prof. Anna Karlsson (Karolinska Institute, Stockholm, Sweden). All cell lines were grown in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS, Gibco), 0.01M Hepes (Gibco) and 1 mM sodium pyruvate (Gibco) in a humidified 5% CO2 incubator at 37 °C.
4.3.2. Cell Proliferation
Suspension (L1210 and CEM cells) were seeded in 96-well microtiter plates at 60,000 cells/well in the presence of different concentrations of the compounds. The cells were allowed to proliferate for 48 h or 96 h, respectively and then counted in a Coulter counter. The 50% inhibitory concentration (IC50) was defined as the compound concentration required to reduce cell proliferation by 50%. HeLa and Mia-Paca2 cells were seeded in 96-well plates at 15,000 cells/well in the presence of different concentrations of the compounds. After 4 days of incubation, the cells were trypsinized and counted in a Coulter counter.
4.3.3. IC50 Determination
The compounds were dissolved in DMSO at 20 mM (stock solution) and kept in the refrigerator until use. Then, compound dilutions were made in cell culture medium, and serial compound concentrations were tested starting at 100 μM as the highest concentration. The DMSO concentration, present in the highest compound concentration was 0.5% that is a concentration that did not affect the tumor cell proliferation. The IC50 values were calculated using following formula: C1 − [ 50 − N1%/N2% − N1% ] × ( C1 − C2) wherein C1 is the compound concentration that inhibits cell proliferation more than 50%; C2 is the compound concentration that inhibits cell proliferation less than 50%; N1% represents the cell number (in percent of control in the absence of compound) obtained in the presence of C1 and N2% represents the cell number (in percent of control in the absence of compound) obtained in the presence of C2.
5. Conclusions
This study started with the aim to explore the possible antiproliferative properties of dualistic molecules bearing a combination of the hydrazone and benzimidazole moieties. Based on compounds 3–19 the in vitro activity on murine leukemia (L1210), human T-lymphoblastic leukemia (CEM), human cervix carcinoma (HeLa) and human pancreas carcinoma (Mia Paca-2) cells, we have observed that the presence of a 2-hydroxyl group on the arylidene moiety favourably modulates antiproliferative activity. In particular, hydrazones 10 and 16 inhibited the growth of all tested cell lines with low (<10 µM) micromolar IC50 values. Predictions of the ADME properties for studied compounds showed that all hydrazones 3, 6–8, 10, 11, 13–16 might present good passive oral absorption. In conclusion by combination of benzimidazole and hydrazone pharmacophores we obtained a new class of N′-(4-arylidene)-1H-benzo[d]imidazole-2-carbohydrazides endowed with significant antiproliferative activity as well as good potential absorption properties. These results further encourage us in developing an enlarged synthetic study in order to highlight SAR in this interesting class of molecules.
Acknowledgments
This study was supported by the Italian Ministero dell’Istruzione, Università e della Ricerca (PRIN 2010-2011, Prot. No. 20105YY2HL_002), the Regione Autonoma della Sardegna (Project L.R. 7/2007, No. 2012_CRP-59473) and the KU Leuven (GOA 15/19 TBA). AD gratefully acknowledges the Sardinia Regional Government for the financial support of his Ph.D. scholarship (P.O.R. Sardegna F.S.E. Operational Programme of the Autonomous Region of Sardinia, European Social Fund 2007–2013—Axis IV Human Resources, Objective l.3, Line of Activity l.3.1). We acknowledge Lizette van Berckelaer for excellent technical assistance.
Abbreviations
The following abbreviations are used in this manuscript:
| ADME | Adsorption, distribution, metabolism and elimination |
| IR | infrared spectra |
| NMR | Nuclear magnetic resonance |
| MS | Mass spectra |
| TPSA | Total polar surface area |
Author Contributions
V.O., G.B., S.M. and J.B. conceived and designed the experiments; M.D., A.D., A.B., S.P. and S.L. performed the experiments; V.O., G.B., S.M. and J.B. analyzed the data; V.O. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 3–19 are available from the authors.
References
- 1.Yadav G., Ganguly S. Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: A mini-review. Eur. J. Med. Chem. 2015;97:419–443. doi: 10.1016/j.ejmech.2014.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Gowda N.R., Kavitha C.V., Chiruvella K.K., Joy O., Rangappa K.S., Raghavan S.C. Synthesis and biological evaluation of novel 1-(4-methoxyphenethyl)-1H-benzimidazole-5-carboxylic acid derivatives and their precursors as antileukemic agents. Bioorg. Med. Chem. Lett. 2009;19:4594–4600. doi: 10.1016/j.bmcl.2009.06.103. [DOI] [PubMed] [Google Scholar]
- 3.Li Y., Tan C., Gao C., Zhang C., Luan X., Chen X., Liu H., Chen Y., Jiang Y. Discovery of benzimidazole derivatives as novel multi-target EGFR, VEGFR-2 and PDGFR kinase inhibitors. Bioorg. Med. Chem. 2011;19:4529–4535. doi: 10.1016/j.bmc.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Determann R., Dreher J., Baumann K., Preu L., Jones P.G., Totzke F., Schächtele C., Kubbutat M.H., Kunick C. 2-Anilino-4-(benzimidazol-2-yl)pyrimidines—A multikinase inhibitor scaffold with antiproliferative activity toward cancer cell lines. Eur. J. Med. Chem. 2012;53:254–263. doi: 10.1016/j.ejmech.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Gao C., Li B., Zhang B., Sun Q., Li L., Li X., Chen C., Tan C., Liu H., Jiang Y. Synthesis and biological evaluation of benzimidazole acridine derivatives as potential DNA-binding and apoptosis-inducing agents. Bioorg. Med. Chem. 2015;23:1800–1807. doi: 10.1016/j.bmc.2015.02.036. [DOI] [PubMed] [Google Scholar]
- 6.Guan Q., Han C., Zuo D., Zhai M., Li Z., Zhang Q., Zhai Y., Jiang X., Bao K., Wu Y., et al. Synthesis and evaluation of benzimidazole carbamates bearing indole moieties for antiproliferative and antitubulin activities. Eur. J. Med. Chem. 2014;87:306–315. doi: 10.1016/j.ejmech.2014.09.071. [DOI] [PubMed] [Google Scholar]
- 7.Maji B., Kumar K., Kaulage M., Muniyappa K., Bhattacharya S. Design and Synthesis of New Benzimidazole–Carbazole Conjugates for the Stabilization of Human Telomeric DNA, Telomerase Inhibition, and Their Selective Action on Cancer Cells. J. Med. Chem. 2014;57:6973–6988. doi: 10.1021/jm500427n. [DOI] [PubMed] [Google Scholar]
- 8.Singh M., Tandon V. Synthesis and biological activity of novel inhibitors of topoisomerase I: 2-Aryl-substituted 2-bis-1H-benzimidazoles. Eur. J. Med. Chem. 2011;46:659–669. doi: 10.1016/j.ejmech.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Yoon Y.K., Ali M.A., Wei A.C., Shirazi A.N., Parang K., Choon T.S. Benzimidazoles as new scaffold of sirtuin inhibitors: Green synthesis, in vitro studies, molecular docking analysis and evaluation of their anti-cancer properties. Eur. J. Med. Chem. 2014;83:448–454. doi: 10.1016/j.ejmech.2014.06.060. [DOI] [PubMed] [Google Scholar]
- 10.Yoon Y.K., Ali M.A., Wei A.C., Choon T.S., Osman H., Parang K., Shirazi A.N. Synthesis and evaluation of novel benzimidazole derivatives as sirtuin inhibitors with antitumor activities. Bioorg. Med. Chem. 2014;22:703–710. doi: 10.1016/j.bmc.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Salum L.B., Mascarello A., Canevarolo R.R., Altei W.F., Laranjeira A.B., Neuenfeldt P.D., Stumpf T.R., Chiaradia-Delatorre L.D., Vollmer L.L., Daghestani H.N., et al. N-(1′-naphthyl)-3,4,5-trimethoxybenzohydrazide as microtubule destabilizer: Synthesis, cytotoxicity, inhibition of cell migration and in vivo activity against acute lymphoblastic leukemia. Eur. J. Med. Chem. 2015;96:504–518. doi: 10.1016/j.ejmech.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 12.Kaplánek R., Jakubek M., Rak J., Kejík Z., Havlík M., Dolenský B., Frydrych I., Hajdúch M., Kolář M., Bogdanová K., et al. Caffeine–hydrazones as anticancer agents with pronounced selectivity toward T-lymphoblastic leukaemia cells. Bioorg. Chem. 2015;60:19–29. doi: 10.1016/j.bioorg.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Liao W., Xu C., Ji X., Hu G., Ren L., Liu Y., Li R., Gong P., Sun T. Design and optimization of novel 4-(2-fluorophenoxy)quinoline derivatives bearing a hydrazone moiety as c-Met kinase inhibitors. Eur. J. Med. Chem. 2014;87:508–518. doi: 10.1016/j.ejmech.2014.09.095. [DOI] [PubMed] [Google Scholar]
- 14.Potůčková E., Hrušková K., Bureš J., Kovaříková P., Špirková I.A., Pravdíková K., Kolbabová L., Hergeselová T., Hašková P., Jansová H., et al. Structure-activity relationships of novel salicylaldehyde isonicotinoyl hydrazone (SIH) analogs: Iron chelation, anti-oxidant and cytotoxic properties. PLoS ONE. 2014;9:e112059. doi: 10.1371/journal.pone.0112059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xia Y., Fan C.-D., Zhao B.-X., Shin D.-S., Miao J.-Y. Synthesis and structure–activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide hydrazone derivatives as potential agents against A549 lung cancer cells. Eur. J. Med. Chem. 2008;43:2347–2353. doi: 10.1016/j.ejmech.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Onnis V., Cocco M.T., Fadda R., Congiu C. Synthesis and evaluation of anticancer activity of 2-arylamino-6-trifluoromethyl-3-(hydrazonocarbonyl)pyridines. Bioorg. Med. Chem. 2009;17:6158–6165. doi: 10.1016/j.bmc.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 17.Congiu C., Onnis V. Synthesis and biological evaluation of novel acylhydrazone derivatives as potential antitumor agents. Bioorg. Med. Chem. 2013;21:6592–6599. doi: 10.1016/j.bmc.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Kumar V., Basavarajaaswamy G., Rai M.V., Poojary B., Pai V.R., Shruthi N., Bhat M. Rapid ‘one-pot’ synthesis of a novel benzimidazole-5-carboxylate and its hydrazone derivatives as potential anti-inflammatory and antimicrobial agents. Bioorg. Med. Chem. Lett. 2015;25:1420–1426. doi: 10.1016/j.bmcl.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 19.Liu T., Sun C., Xing X., Jing L., Tan R., Luo Y., Zhao Y. Synthesis and evaluation of 2-[2-(phenylthiomethyl)-1H-benzo[d]imidazol-1-yl)acetohydrazide derivatives as antitumor agents. Bioorg. Med. Chem. Lett. 2012;22:3122–3125. doi: 10.1016/j.bmcl.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 20.Manfredini S., Vertuani S., Scalambra E. Dualistic Molecules Having UV Radiation Filtrating Ability at Wide Spectrum and Potent Damping Activity of the Reactivity of Free Radicals (Radicals Scavenging) WO/2013/102843. International Patent Application. 2013 Jul 11;
- 21.Lima P.C., Lima L.M., da Silva K.C.M., Leda P.H.O., de Miranda A.L.P., Fraga C.A.M., Eliezer J., Barreiro E.J. Synthesis and analgesic activity of novel N-acylarylhydrazones and isosters, derived from natural safrole. Eur. J. Med. Chem. 2000;35:187–203. doi: 10.1016/S0223-5234(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 22.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 23.Veber D.F., Johnson S.R., Cheng H.Y., Smith B.R., Ward K.W., Kapple K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 24.Graubaum H., Martin D. Reactions of benzimidazole-2-carbohydrazide with electrophilic compounds. J. Prakt. Chem. 1986;328:515–521. doi: 10.1002/prac.19863280408. [DOI] [Google Scholar]
- 25.Harisha R.S., Hosamani K.M., Keri R.S. Synthesis, in vitro microbial and cytotoxic studies of new benzimidazole derivatives. Arch. Pharm. 2009;342:412–419. doi: 10.1002/ardp.200900022. [DOI] [PubMed] [Google Scholar]