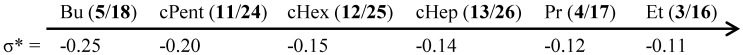Abstract
Series of thirteen 1-[(2-chlorophenyl)carbamoyl]naphthalen-2-yl carbamates and thirteen 1-[(2-nitrophenyl)carbamoyl]naphthalen-2-yl carbamates with alkyl/cycloalkyl/arylalkyl chains were prepared and characterized. Primary in vitro screening of the synthesized compounds was performed against Staphylococcus aureus, two methicillin-resistant S. aureus strains, Mycobacterium marinum, and M. kansasii. 1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl ethylcarbamate and 1-[(2-nitrophenyl)carbamoyl]naphthalen-2-yl ethylcarbamate showed antistaphylococcal (MICs = 42 µM against MRSA) and antimycobacterial (MICs = 21 µM) activity against the tested strains comparable with or higher than that of the standards ampicillin and isoniazid. In the case of bulkier carbamate tails (R > propyl/isopropyl), the activity was similar (MICs ca. 70 µM). Screening of the cytotoxicity of both of the most effective compounds was performed using THP-1 cells, and no significant lethal effect was observed (LD50 >30 µM). The structure-activity relationships are discussed.
Keywords: carbamates, hydroxynaphthalene-carboxamides, in vitro antibacterial activity, in vitro antimycobacterial activity, in vitro cytotoxicity assay, structure-activity relationships
1. Introduction
Infectious diseases represent an increasing worldwide threat. The number of untreatable diseases decreased after the 1950s due to the introduction of new antimicrobial agents. However, since the 1980s, morbidity has risen again. The increase in the number of new infections is caused by general immunosuppression, a significant increase in the number of diabetic or HIV-positive patients, and the development of resistance to commonly used drugs. The resistance of common pathogens to first-choice drugs increased by up to 100% during the last few decades. Moreover, the resistance of some strains to second- or third-choice drugs can be found. Development of cross-resistant and multidrug-resistant strains is a serious global problem. Selection of resistant pathogens is especially caused by irrational and unavailing application of anti-invasive agents in human, veterinary medicine, and in agriculture [1,2,3,4]. Resistance may complicate the treatment of infections regardless of how mild these infections were at the early stage [5,6]. This increasing resistance highlights the urgency of designing new effective anti-invasive drugs and developing strategies focused on overcoming drug resistance [5,6,7,8,9].
The discovery of salicylanilides dates back to early phenol applications; currently, salicylanilides are well-known organic compounds exhibiting a broad spectrum of biological activities such as anthelmintic, antibacterial, antimycobacterial, and antiviral, among others. Salicylanilides have been described to affect a wide range of targets, although the appropriate mechanism of action responsible for overall biological activities of these compounds has not been proposed so far. Thus, salicylanilides seem to be promising candidates for antibacterial agents, which could be a solution to the resistance challenges [10,11,12,13,14,15,16,17].
In addition, the presence of an amide (–CONH–) or carbamate (–OCONH–) group with a hydrophobic residue in its close vicinity is characteristic not only of a number of clinically used drugs [18], but also applied pesticides [19]. These moieties are important functional groups that are able, due to their electron properties, to interact and bind with a number of enzymes/receptors and, by means of these target sites, affect the biological response. The properties of the amide and the carbamate moieties can easily be modified by various substitutions [20,21]. Therefore, the reason for the widespread occurrence of amides and carbamates among new biologically active compounds is obvious [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36].
1-[(2-Substituted phenyl)carbamoyl]naphthalen-2-yl carbamates, described in the present work, can be considered as cyclic analogues of salicylanilides that have expressed promising results as potential antibacterial and antimycobacterial agents ([14,15,16,17], and refs. therein). Pattern compounds of these carbamates–N-(2-chlorophenyl)-2-hydroxynaphthalene-1-carboxamide (1) and N-(2-nitrophenyl)-2-hydroxynaphthalene-1-carboxamide (2) showed antimycobacterial or antibacterial activity with insignificant cytotoxicity on human cells in the previous screening [30]; therefore, the aim of this contribution was to describe the preparation of various alkyl, cycloalkyl, and arylalkyl carbamates and the investigation of their antimicrobial activity.
2. Results and Discussion
2.1. Chemistry
All the studied compounds were prepared according to Scheme 1. In the first step, N-(2-chlorophenyl)-2-hydroxynaphthalene-1-carboxamide (1) and N-(2-nitrophenyl)-2-hydroxy-naphthalene-1-carboxamide (2) were synthesized by the microwave-assisted method [30]. In the second step, a modified method using triethylamine for activation of the phenolic group was used [24]. The addition of activated compounds 1 and 2 to appropriate alkyl, cycloalkyl, and arylalkyl carbamates yielded a series of thirteen 1-[(2-chlorophenyl)carbamoyl]naphthalen-2-yl carbamates 3–15 and thirteen 1-[(2-nitrophenyl)carbamoyl]naphthalen-2-yl carbamates 16–28.
Scheme 1.
Synthesis of 1-[(2-chlorophenyl)carbamoyl]naphthalen-2-yl carbamates 3–15 and 1-[(2-nitrophenyl)carbamoyl]naphthalen-2-yl carbamates 16–28. Reagents and conditions: (a) PCl3, chlorobenzene, MW; (b) TEA, acetonitrile, ambient temperature.
In a number of studies examining the biological activity of potential drugs, the relationship between lipophilicity or other descriptors and their potency have been investigated. In the current investigation, the calculated lipophilicity (log P) of the compounds as well as the electronic parameters and molar volume of R2 substituents (see Table 1) were used to determine if these factors play a role in their biological activity. All the predicted molecular descriptors were calculated using the ACD/Percepta ver. 2012 program, see Table 1. The lipophilicity, expressed as log P values, of the chlorine substituted compounds 1, 3–15 was higher (ranged from 3.94 to 5.75) than that of the nitro substituted derivatives 2, 16–28 (ranged from 3.58 to 5.55). Lipophilicity increases with lengthening of the alkyl tail. Isopropyl showed a lower lipophilicity value than propyl. Significantly lower lipophilicity values were calculated for the cycloalkyl derivatives compared with their N-alkyl isomers. For individual R2 substituents, alkyl/cycloalkyl/arylalkyl tails of the discussed compounds, and also electronic properties expressed as electronic constants σ* were predicted; they ranged from −0.25 to 0.08. The electronic parameters (expressed as Hammett’s σ parameters) of the 2-Cl moiety (compound 1) and the 2-NO2 moiety (compound 2) were 0.22 and 0.77, respectively. Molar volume MV [cm3], a parameter representing the bulk of R2 substituents (i.e., tail length/branching) of each compound, was also calculated for the hydrophobic tail.
Table 1.
Structures of the discussed anilides 1, 2 and carbamates 3–28; calculated values of log P, electronic constants σ*, and molar volume (MV [cm3]) of R2 substituents; in vitro antibacterial activity (MIC) of the compounds in comparison with the ampicillin (APC) standard; in vitro antimycobacterial activity (MIC) of the compounds in comparison with the isoniazid (INH) standard and in vitro cytotoxicity assay (LD50) of the chosen compounds.
| Compd. | R1 | R2 | log P a | MVR2 a [cm3] | σ*R2 a | MIC [µM] | LD50 [µM] | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | MRSA SA 630 | MRSA 3202 | MM | MK | |||||||
| 1 | Cl | − | 5.03 | – | – | 215 | 860 | 860 | 107 | 107 | >30 |
| 3 | Cl | Et | 3.94 | 47.29 | −0.11 | 21.6 | 43.3 | 43.3 | 21.6 | 21.6 | ˃30 |
| 4 | Cl | Pr | 4.41 | 63.80 | −0.12 | 83.5 | 83.5 | 83.5 | 41.7 | 36.5 | ˃30 |
| 5 | Cl | Bu | 4.71 | 80.31 | −0.25 | 645 | 645 | 645 | 80.6 | 80.6 | – |
| 6 | Cl | Pen | 5.47 | 96.81 | −0.23 | 623 | 623 | 623 | 77.8 | 77.8 | – |
| 7 | Cl | Hex | 6.03 | 113.32 | −0.25 | 602 | 602 | 602 | 75.3 | 75.3 | – |
| 8 | Cl | Hep | 6.67 | 129.83 | −0.23 | 583 | 583 | 583 | 72.9 | 72.9 | – |
| 9 | Cl | Oct | 7.19 | 146.33 | −0.23 | 565 | 565 | 565 | 70.6 | 70.6 | – |
| 10 | Cl | i-Pr | 4.20 | 64.18 | −0.19 | 83.5 | 167 | 167 | 41.7 | 41.7 | ˃30 |
| 11 | Cl | c-Pent | 4.60 | 84.10 | −0.20 | 313 | 313 | 626 | 78.2 | 78.2 | – |
| 12 | Cl | c-Hex | 5.03 | 100.87 | −0.15 | 303 | 605 | 303 | 75.6 | 75.6 | – |
| 13 | Cl | c-Hep | 5.46 | 117.56 | −0.14 | 146 | 586 | 586 | 73.2 | 73.2 | – |
| 14 | Cl | PhEt | 5.19 | 108.00 | 0.08 | 288 | 575 | 575 | 71.9 | 71.9 | – |
| 15 | Cl | PhBu | 5.75 | 141.01 | −0.21 | 271 | 541 | 541 | 67.6 | 67.6 | – |
| 2 | NO2 | − | 4.45 | – | – | 26 | 104 | 52 | 104 | 51.9 | >30 |
| 16 | NO2 | Et | 3.58 | 47.29 | −0.11 | 5.27 | 42.1 | 42.1 | 21.0 | 21.0 | ˃30 |
| 17 | NO2 | Pr | 3.96 | 63.80 | −0.12 | 10.1 | 81.3 | 81.3 | 40.6 | 40.6 | ˃30 |
| 18 | NO2 | Bu | 4.32 | 80.31 | −0.25 | 628 | 628 | 628 | 78.5 | 78.5 | – |
| 19 | NO2 | Pen | 5.15 | 96.81 | −0.23 | 607 | 607 | 607 | 75.9 | 75.9 | – |
| 20 | NO2 | Hex | 5.71 | 113.32 | −0.25 | 587 | 587 | 587 | 73.4 | 73.4 | – |
| 21 | NO2 | Hep | 6.81 | 129.83 | −0.23 | 569 | 569 | 569 | 71.1 | 71.1 | – |
| 22 | NO2 | Oct | 7.22 | 146.33 | −0.23 | 552 | 552 | 552 | 69.0 | 69.0 | – |
| 23 | NO2 | i-Pr | 3.80 | 64.18 | −0.19 | 40.6 | 162 | 162 | 40.6 | 40.6 | ˃30 |
| 24 | NO2 | c-Pent | 4.21 | 84.10 | −0.20 | 153 | 610 | 610 | 76.2 | 76.2 | – |
| 25 | NO2 | c-Hex | 4.60 | 100.87 | −0.15 | 74 | 591 | 591 | 73.8 | 73.8 | – |
| 26 | NO2 | c-Hep | 5.05 | 117.56 | −0.14 | 72 | 572 | 572 | 71.5 | 71.5 | – |
| 27 | NO2 | PhEt | 4.67 | 108.00 | 0.08 | 281 | 562 | 562 | 70.2 | 70.2 | – |
| 28 | NO2 | PhBu | 5.55 | 141.01 | −0.21 | 132 | 529 | 529 | 66.1 | 66.1 | – |
| APC | – | − | – | – | 5.72 | 45.8 | 45.8 | – | – | – | |
| INH | – | − | – | – | – | – | – | 467 | 29.2 | – | |
a calculated using ACD/Percepta ver. 2012 (Advanced Chemistry Development, Toronto, ON, Canada); SA = S. aureus ATCC 29213, MRSA = clinical isolates of methicillin-resistant S. aureus SA 630 and 3202 (National Institute of Public Health, Prague, Czech Republic); MM = M. marinum CAMP 5644, MK = M. kansasii DSM 44162.
2.2. In Vitro Antibacterial Susceptibility Testing
The in vitro antibacterial activity of the discussed compounds was evaluated against two clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) and S. aureus ATCC 29213 as a reference and quality control strain. All the compounds showed only moderate or negligible activity, except ethylcarbamates 3 (R1 = Cl) and 16 (R1 = NO2). The activity of both ethylcarbamates 3 and 16 was comparable with that of the standard ampicillin; furthermore, the effect of compound 16 against S. aureus was ca. 4-fold higher in comparison with that of the chloro derivative 3. In addition, nitrated propylcarbamate 17 showed ca. 8-fold higher activity than the corresponding chlorinated compound 4, and nitro-anilide 2 had ca. 8-fold higher effectivity against S. aureus than chloro-anilide 1. The observation that anilides substituted by the nitro moiety on the anilide ring showed higher potency only against S. aureus and not against MRSA strains in comparison with chlorine substituted derivatives was also described by Pauk et al. [37]. It can be supposed that nitro derivatives may interact by hydrogen bonding with specific biological structures in S. aureus (that are not present in MRSA strains). These interactions are limited only for spatially small molecules, such as anilide and ethyl-, propyl-, and isopropylcarbamate, while carbamates with bulkier (long or branched) tails are not capable of these interactions. This fact was also confirmed by Zadrazilova et al., where short ethylcarbamates demonstrated higher bacteriostatic activity against various Staphylococcus strains than longer (C9–C12) alkylcarbamates [16]. Due to the moderate activity of the rest of the compounds, no thorough structure-activity relationships could be established. Nevertheless, in general, it can be stated that the activity is influenced by similar factors/parameters as those discussed below.
2.3. In Vitro Antimycobacterial Evaluation
The evaluation of the in vitro antimycobacterial activity of the compounds was performed against Mycobacterium marinum CAMP 5644 (MM) and M. kansasii DSM 44162 (MK), see Table 1. To lower risks and make manipulation in the laboratory easier, surrogate model pathogens for M. tuberculosis can be used in laboratory studies. Mycobacterium marinum is very closely related to M. tuberculosis and is the cause of tuberculosis-like infections in poikilothermic organisms, especially frogs and fish. M. marinum is a good model for study especially because of the lower risk for laboratory workers, genetic relatedness, and pathology similar to human tuberculosis [38]. However, because of M. tuberculosis, the pathogenic role of nontuberculous mycobacteria (NTM) in humans was underestimated for a long time [39]. M. kansasii, the most virulent of the NTM, causes nontuberculous mycobacterial lung infections that are very common nowadays and can be indistinguishable from tuberculosis [40]. Therefore, additionally M. kansasii was chosen as a model species for screening of prospective antimycobacterial drugs to control mycobacterial diseases. The activity of the compounds was expressed as the minimum inhibitory concentration (MIC) that is defined for mycobacteria as a 90% or greater (IC90) reduction of growth in comparison with the control [41].
It can be stated that the potency of almost all discussed compounds is the same against both mycobacterial strains (see Table 1). Similarly, as mentioned above, ethylcarbamates 3 (R1 = Cl) and 16 (R1 = NO2) showed the highest antimycobacterial activity (MICs = 21 µM). In addition, propylcarbamates 4 (R1 = Cl) and 17 (R2 = NO2) expressed substantial activity (MICs ca. 40 µM). The dependences of the antimycobacterial activity expressed as log (1/MIC) on the lipophilicity (log P) of the compounds as well as on the bulkiness/molar volume (MV [cm3]) of individual alkyl/cycloalkyl/arylalkyl chains are illustrated in Figure 1A,B. Practically the same structure-activity relationships can be found for both series; therefore they are illustrated only for M. kansasii. The activity rapidly decreases with lipophilicity and with substituent bulkiness, increasing up to log P ca. 4.4 or MV ca. 80 cm3 (R2 = butyl or cyclopentyl), respectively, and then remains practically constant (or increases insignificantly) with increasing lipophilicity/bulkiness (see Figure 1A,B). For example, correlation factor r = 0.9919, n = 10 (compounds 3–5, 10, 11, 16–18, 23, 24) of the dependence of activity on the bulkiness of the R2 substituent can be calculated. It seems that the activity is also secondarily influenced by the electronic properties of R2 substituents expressed as electronic constants σ*, because it can be stated that the more the σ* values approximate −0.11 (i.e., the less electron donor properties are), the higher the activity is (see Figure 2).
Figure 1.
Dependence of the in vitro antimycobacterial activity against M. kansasii DSM 44162 log (1/MIC [M]) of the tested compounds on lipophilicity, expressed as log P (A) and bulkiness/molar volume (MV [cm3]) of individual R2 substituents (B).
Figure 2.
Increase of the general antimicrobial activity owing to the R2 substituent in dependence on the electronic properties are expressed as electronic constants σ*.
In the previous studies [24,42], the most potent carbamates were substituted by a longer tail, which is connected with their surface activity. In this case, no dependence of antibacterial/antimycobacterial effects on surface activity was observed. As ethylcarbamates showed the highest activity in the discussed screening and propyl- and isopropyl-carbamates showed medium activity, it seems that steric requirements (spatially small molecules) play a significant role for a good biological effect. Similar facts were recently described, for example, by Kratky et al., where carbamates with spatially bulky tails expressed significantly lower activity than short-tail carbamates [43]. On the other hand, the blocking of the phenolic moiety by the carbamate group significantly increased the antimicrobial effect of these compounds. The mode of action (targets) of these compounds is questionable; however, due to the structural similarity with salicylanilides, interactions with vital enzymes, energy metabolism, as well as disturbing of the membrane architecture of prokaryotic cells may be supposed [10,11,13,14,15,16,17,32,33,36,44].
2.4. In Vitro Cytotoxicity Assay
The preliminary in vitro screening of the cytotoxicity of the compounds was performed using the human monocytic leukemia THP-1 cell line. The cytotoxicity was evaluated as the LD50 value (LD50—lethal dose to 50% of the cell population), see Table 1. A compound is considered cytotoxic when it demonstrates a toxic effect on cells at concentrations up to 10 μM [45], and the highest tested concentration that was used for the toxicity assay was 3-fold this value. Treatment with 30 μM compounds did not lead to lethal effects on the THP-1 cells. Based on these observations, it can be concluded that the most potent compounds 3 and 16 can be considered as promising agents for subsequent design of novel antibacterial and antimycobacterial agents.
3. Experimental Section
3.1. General Information
All reagents were purchased from Aldrich (Sigma-Aldrich, St. Louis, MO, USA), and Alfa (Alfa-Aesar, Ward Hill, MA, USA). TLC experiments were performed on alumina-backed silica gel 60 F254 plates (Merck, Darmstadt, Germany). The plates were illuminated under UV light (254 nm) and evaluated in iodine vapour. The melting points were determined on a Kofler hot-plate apparatus HMK (Franz Kustner Nacht KG, Dresden, Germany) and are uncorrected. Infrared (IR) spectra were recorded on a Smart MIRacle™ ATR ZnSe for Nicolet™ Impact 410 FT-IR spectrometer (Thermo Scientific, West Palm Beach, FL, USA). The spectra were obtained by the accumulation of 256 scans with a 2 cm−1 resolution in the region of 4000–650 cm−1. All 1H- and 13C-NMR spectra were recorded on a JEOL ECZR 400 MHz NMR spectrometer (400 MHz for 1H and 100 MHz for 13C, JEOL, Tokyo, Japan) in DMSO-d6. 1H and 13C chemical shifts (δ) are reported in ppm. High-resolution mass spectra were measured using a high-performance liquid chromatograph Dionex UltiMate® 3000 (Thermo Scientific) coupled with a LTQ Orbitrap XL™ Hybrid Ion Trap-Orbitrap Fourier Transform Mass Spectrometer (Thermo Scientific) with injection into HESI II in the positive mode. The lipophilicity (log P) of the final compounds, the surface tension, and the molar volume of R substituents were predicted using ACD/Percepta ver. 2012 (Advanced Chemistry Development, Inc., Toronto, ON, Canada).
3.2. Synthesis
General Procedure for Synthesis of Carbamates 3–28
The synthetic pathway and characterization of N-(2-chlorophenyl)-2-hydroxynaphthalene-1-carboxamide (1) and N-(2-nitrophenyl)-2-hydroxynaphthalene-1-carboxamide (2) were described recently by Gonec et al. [30]. Anilides 1 and 2 (1.0 mmol) and triethylamine (1.1 mmol) were dissolved in dry acetonitrile (10 mL). The solution of the appropriate alkyl isocyanate (1.2 mmol) in acetonitrile (5 mL) was added in four portions within 2 h, and the reacting mixture was stirred for 24 h at ambient temperature. The solvent was evaporated under reduced pressure, and the solid residue was washed with methanol and ethyl acetate to give pure product. All the studied compounds are presented in Table 1.
1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl ethylcarbamate (3). Yield 33%; Mp 137–139 °C; IR (cm−1): 3264, 3214, 1718, 1675, 1540, 1523, 1510, 1481, 1435, 1300, 1256, 1229, 1006, 826, 741, 723, 688, 667; 1H-NMR (DMSO-d6) δ: 10.14 (s, 1H), 7.99–8.06 (m, 3H), 7.88 (t, J = 5.5 Hz, 1H), 7.82 (dd, J = 8.2 Hz, 1.3 Hz, 1H), 7.64 (ddd, J = 8.2 Hz, 6.9 Hz, 1.0 Hz, 1H), 7.55–7.59 (m, 2H), 7.43 (td, J = 7.7 Hz, 1.6 Hz, 1H), 7.42 (d, J = 9.0 Hz, 1H), 7.30 (td, J = 7.8 Hz, 1.3 Hz, 1H), 3.08–3.15 (m, 2H), 1.08 (t, J = 7.3 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.33, 153.97, 142.20, 134.65, 130.54, 130.31, 129.77, 129.60, 128.40, 128.04, 127.58, 127.30, 127.43, 127.28, 126.60, 125.78, 124.67, 122.62, 35.42, 14.83; HR-MS: for C20H16O3N2Cl [M + H]+ calculated 367.08440 m/z, found 367.08545 m/z.
1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl propylcarbamate (4). Yield 26%; Mp 132–134 °C; IR (cm−1): 3259, 3224, 1716, 1659, 1546, 1526, 1510, 1484, 1441, 1298, 1259, 1232, 1060, 991, 809, 741, 694; 1H-NMR (DMSO-d6) δ: 10.10 (s, 1H), 7.99–8.05 (m, 3H), 7.89 (t, J = 5.7 Hz, 1H), 7.83 (dd, J = 8.2 Hz, 1.3 Hz, 1H), 7.64 (ddd, J = 8.2 Hz, 6.9 Hz, 1.0 Hz, 1H), 7.55–7.59 (m, 2H), 7.42 (td, J = 7.7 Hz, 1.6 Hz, 1H), 7.41 (d, J = 9.0 Hz, 1H), 7.30 (td, J = 7.8 Hz, 1.3 Hz, 1H), 3.04 (q, J = 6.4 Hz, 2H), 1.47 (sx, J = 7.2 Hz, 2H), 0.86 (t, J = 7.6 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.33, 154.20, 145.23, 134.67, 130.56, 130.31, 129.81, 129.59, 128.33, 128.04, 127.48, 127.42, 127.30, 127.28, 126.60, 125.78, 124.67, 122.62, 42.35, 22.45, 11.17; HR-MS: for C21H18O3N2Cl [M + H]+ calculated 381.10005 m/z, found 381.10123 m/z.
1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl butylcarbamate (5). Yield 55%; Mp 156–159 °C; IR (cm−1): 3258, 3228, 1724, 1665, 1553, 1526, 1506, 1479, 1439, 1232, 1007, 818, 751; 1H-NMR (DMSO-d6) δ: 10.08 (s, 1H), 7.99–8.05 (m, 3H), 7.88 (t, J = 5.5 Hz, 1H), 7.85 (dd, J = 8.2 Hz, 1.3 Hz, 1H), 7.64 (ddd, J = 8.2 Hz, 6.9 Hz, 1.0 Hz, 1H), 7.55–7.59 (m, 2H), 7.42 (td, J = 7.7 Hz, 1.6 Hz, 1H), 7.41 (d, J = 9.0 Hz, 1H), 7.30 (td, J = 7.8 Hz, 1.3 Hz, 1H), 3.08 (q, J = 6.4 Hz, 2H), 1.44 (qi, J = 7.2 Hz, 2H), 1.28 (sx, J = 7.3 Hz, 2H), 0.85 (t, J = 7.3 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.31, 154.19, 145.23, 134.67, 130.57, 130.31, 129.84, 129.60, 128.20, 128.05, 127.41, 127.32, 127.31, 127.22, 126.59, 125.79, 124.65, 122.60, 40.26, 31.32, 19.37, 13.63; HR-MS: for C22H20O3N2Cl [M + H]+ calculated 395.11570 m/z, found 395.11639 m/z.
1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl pentylcarbamate (6). Yield 36%; Mp 137–138 °C; IR (cm−1): 3227, 3215, 1724, 1669, 1550, 1521, 1508, 1480, 1434, 1233, 1037, 997, 910, 817, 746, 686; 1H-NMR (DMSO-d6) δ: 10.08 (s, 1H), 7.99–8.05 (m, 3H), 7.88 (t, J = 5.5 Hz, 1H), 7.85 (dd, J = 8.2 Hz, 1.3 Hz, 1H), 7.64 (ddd, J = 8.2 Hz, 6.9 Hz, 1.0 Hz, 1H), 7.55–7.59 (m, 2H), 7.39–7.44 (m, 2H), 7.30 (td, J = 7.8 Hz, 1.3 Hz, 1H), 3.07 (q, J = 6.7 Hz, 2H), 1.45 (qi, J = 7.0 Hz, 2H), 1.24–1.28 (m, 4H), 0.84 (t, J = 7.0 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.39, 154.24, 145.26, 134.68, 130.62, 130.36, 129.90, 129.65, 128.27, 128.10, 127.46, 127.40, 127.37, 127.31, 126.64, 125.87, 124.70, 122.65, 40.59, 28.91, 28.42, 21.87, 13.92; HR-MS: for C23H22O3N2Cl [M + H]+ calculated 409.13135 m/z, found 409.13303 m/z.
1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl hexylcarbamate (7). Yield 50%; Mp 103–106 °C; IR (cm−1): 2954, 2924, 1725, 1673, 1587, 1525, 1516, 1463, 1440, 1301, 1241, 1210, 1037, 995, 813, 756, 747, 684; 1H-NMR (DMSO-d6) δ: 10.08 (s, 1H), 7.99–8.05 (m, 3H), 7.88 (t, J = 5.5 Hz, 1H), 7.85 (dd, J = 8.2 Hz, 1.3 Hz, 1H), 7.64 (ddd, J = 8.2 Hz, 6.9 Hz, 1.0 Hz, 1H), 7.55–7.59 (m, 2H), 7.39–7.44 (m, 2H), 7.30 (td, J = 7.8 Hz, 1.3 Hz, 1H), 3.07 (q, J = 6.4 Hz, 2H), 1.45 (qi, J = 7.0 Hz, 2H), 1.20–1.31 (m, 6H), 0.85 (t, J = 6.9 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.39, 154.26, 145.26, 134.70, 130.63, 130.37, 129.93, 129.66, 128.25, 128.13, 127.46, 127.39, 127.36, 127.30, 126.66, 125.88, 124.71, 122.65, 40.64, 31.03, 29.21, 25.92, 22.07, 13.96; HR-MS: for C24H24O3N2Cl [M + H]+ calculated 423.14700 m/z, found 423.14767 m/z.
1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl heptylcarbamate (8). Yield 66%; Mp 89–90 °C; IR (cm−1): 3279, 3227, 2956, 2933, 2923, 1720, 1662, 1538, 1520, 1479, 1463, 1438, 1296, 1266, 1253, 1234, 1192, 1060, 986, 911, 816, 750; 1H-NMR (DMSO-d6) δ: 10.08 (s, 1H), 7.99–8.05 (m, 3H), 7.88 (t, J = 5.5 Hz, 1H), 7.86 (dd, J = 8.2 Hz, 1.3 Hz, 1H), 7.64 (ddd, J = 8.2 Hz, 6.9 Hz, 1.0 Hz, 1H), 7.55–7.59 (m, 2H), 7.39–7.44 (m, 2H), 7.30 (td, J = 7.8 Hz, 1.3 Hz, 1H), 3.07 (q, J = 6.4 Hz, 2H), 1.45 (qi, J = 7.0 Hz, 2H), 1.20–1.29 (m, 8H), 0.85 (t, J = 6.9 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.42, 154.29, 145.28, 134.71, 130.65, 130.39, 129.96, 129.68, 128.27, 128.14, 127.48, 127.41, 127.39, 127.31, 126.67, 125.90, 124.73, 122.67, 40.65, 31.26, 29.26, 28.48, 26.22, 22.11, 14.02; HR-MS: for C25H26O3N2Cl [M + H]+ calculated 437.16265 m/z, found 437.16431 m/z.
1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl octylcarbamate (9). Yield 52%; Mp 115–116 °C; IR (cm−1): 3297, 3286, 2924, 2851, 1724, 1673, 1584, 1548, 1540, 1509, 1467, 1441, 1434, 1299, 1246, 1215, 1160, 993, 827, 756, 747, 734, 677; 1H-NMR (DMSO-d6) δ: 10.08 (s, 1H), 7.99–8.05 (m, 3H), 7.88 (t, J = 5.5 Hz, 1H), 7.85 (dd, J = 8.2 Hz, 1.3 Hz, 1H), 7.64 (ddd, J = 8.2 Hz, 6.9 Hz, 1.0 Hz, 1H), 7.54–7.59 (m, 2H), 7.39–7.43 (m, 2H), 7.30 (td, J = 7.8 Hz, 1.3 Hz, 1H), 3.07 (q, J = 6.4 Hz, 2H), 1.45 (qi, J = 7.0 Hz, 2H),1.21–1.30 (m, 10H), 0.86 (t, J = 6.9 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.33, 154.19, 145.23, 134.68, 130.57, 130.33, 129.87, 129.60, 128.22, 128.07, 127.40, 127.35, 127.33, 127.22, 126.61, 125.81, 124.67, 122.60, 40.59, 31.25, 29.20, 28.73, 28.64, 26.21, 22.10, 13.96; HR-MS: for C26H28O3N2Cl [M + H]+ calculated 451.17830 m/z, found 451.18018 m/z.
1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl isoproylcarbamate (10). Yield 39%; Mp 167–169 °C; IR (cm−1): 3249, 3216, 1971, 1716, 1668, 1581, 1544, 1530, 1510, 1481, 1462, 1439, 1324, 1300, 1256, 1234, 1066, 1022, 962, 910, 820, 742, 697; 1H-NMR (DMSO-d6) δ: 10.09 (s, 1H), 7.99–8.06 (m, 3H), 7.86 (t, J = 5.5 Hz, 1H), 7.84 (dd, J = 8.2 Hz, 1.3 Hz, 1H), 7.64 (ddd, J = 8.2 Hz, 6.9 Hz, 1.0 Hz, 1H), 7.56–7.59 (m, 2H), 7.43 (td, J = 7.7 Hz, 1.6 Hz, 1H), 7.41 (d, J = 9.0 Hz, 1H), 7.30 (td, J = 7.8 Hz, 1.3 Hz, 1H), 3.69 (sx, J = 6.6 Hz, 1H), 1.12 (d, J = 6.6 Hz, 6H); 13C-NMR (DMSO-d6), δ: 164.42, 153.41, 145.28, 134.70, 130.60, 130.37, 129.90, 129.65, 128.34, 128.10, 127.49, 127.48, 127.36, 127.35, 126.67, 125.85, 124.70, 122.71, 42.91, 22.40; HR-MS: for C21H18O3N2Cl [M + H]+ calculated 381.10005 m/z, found 381.10123 m/z.
1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl cyclopentylcarbamate (11). Yield 60%; Mp 135–137 °C; IR (cm−1): 3224, 2951, 1713, 1659, 1544, 1525, 1509, 1485, 1467, 1440, 1290, 1256, 1233, 1151, 1060, 1052, 1013, 911, 815, 784, 740, 688, 680; 1H-NMR (DMSO-d6) δ: 10.09 (s, 1H), 7.99–8.05 (m, 3H), 7.93 (d, J = 6.9 Hz, 1H), 7.83 (dd, J = 8.2 Hz, 1.3 Hz, 1H), 7.64 (ddd, J = 8.2 Hz, 6.9 Hz, 1.0 Hz, 1H), 7.55–7.59 (m, 2H), 7.39–7.43 (m, 2H), 7.30 (td, J = 7.8 Hz, 1.3 Hz, 1H), 3.87 (sx, J = 5.9 Hz, 1H), 1.76–1.85 (m, 2H), 1.60–1.70 (m, 2H), 1.46–1.55 (m, 4H); 13C-NMR (DMSO-d6), δ: 164.39, 153.66, 145.26, 134.69, 130.58, 130.34, 129.84, 129.62, 128.44, 128.07, 127.56, 127.43, 127.33, 127.33, 126.69, 125.81, 124.88, 122.74, 52.49, 32.14, 23.91; HR-MS: for C23H20O3N2Cl [M + H]+ calculated 409.13135 m/z, found 409.13258 m/z.
1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl cyclohexylcarbamate (12). Yield 34%; Mp 129–132 °C; IR (cm−1): 3245, 3218, 2931, 2854, 1717, 1664, 1544, 1525, 1510, 1479, 1463, 1436, 1317, 1295, 1272, 1254, 1229, 1150, 1061, 1027, 1016, 911, 816, 778, 747, 682; 1H-NMR (DMSO-d6) δ: 10.08 (s, 1H), 7.99–8.05 (m, 3H), 7.86 (d, J = 7.7 Hz, 1H), 7.84 (dd, J = 8.2 Hz, 1.3 Hz, 1H), 7.64 (ddd, J = 8.2 Hz, 6.9 Hz, 1.0 Hz, 1H), 7.55–7.59 (m, 2H), 7.39–7.45 (m, 2H), 7.30 (td, J = 7.8 Hz, 1.3 Hz, 1H), 3.36–3.44 (m, 1H), 1.77–1.87 (m, 2H), 1.66–1.72 (m, 2H), 1.53–1.58 (m, 1H), 1.21–1.29 (m, 4H), 1.07–1.15 (m, 1H); 13C-NMR (DMSO-d6), δ: 164.38, 153.39, 145.27, 134.69, 130.56, 130.35, 129.85, 129.61, 128.38, 128.07, 127.50, 127.43, 127.32, 127.31, 126.67, 125.80, 124.68, 122.68, 49.99, 32.50, 25.12, 24.60; HR-MS: for C24H22O3N2Cl [M + H]+ calculated 423.14700 m/z, found 423.14818 m/z.
1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl cycloheptylcarbamate (13). Yield 60%; Mp 166–168 °C; IR (cm−1): 3295, 3204, 2919, 2855, 1732, 1708, 1656, 1582, 1544, 1521, 1506, 1475, 1441, 1429, 1342, 1291, 1244, 1217, 1205, 1160, 1039, 1008, 991, 824, 772, 746, 712, 688; 1H-NMR (DMSO-d6) δ: 10.04 (s, 1H), 7.99–8.05 (m, 3H), 7.90 (d, J = 8.2 Hz, 1H), 7.85 (dd, J = 8.2 Hz, 1.3 Hz, 1H), 7.64 (ddd, J = 8.2 Hz, 6.9 Hz, 1.0 Hz, 1H), 7.55–7.59 (m, 2H), 7.39–7.45 (m, 2H), 7.30 (td, J = 7.8 Hz, 1.3 Hz, 1H), 3.52–3.60 (m, 1H), 1.80–1.86 (m, 2H), 1.43–1.65 (m, 8H), 1.32–1.42 (m, 2H); 13C-NMR (DMSO-d6), δ: 164.36, 153.29, 145.29, 134.68, 130.57, 130.34, 129.87, 129.60, 128.24, 128.06, 127.43, 127.34, 127.32, 127.25, 126.66, 125.80, 124.67, 122.70, 52.17, 34.35, 27.82, 23.58; HR-MS: for C25H24O3N2Cl [M + H]+ calculated 437.16265 m/z, found 437.16342 m/z.
1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl (2-phenylethyl)carbamate (14). Yield 38%; Mp 115–118 °C; IR (cm−1): 3316, 3221, 1731, 1661, 1583, 1538, 1511, 1467, 1443, 1307, 1248, 1229, 1221, 1200, 1162, 1059, 1031, 984, 817, 751, 739, 698; 1H-NMR (DMSO-d6) δ: 10.13 (s, 1H), 7.99–8.06 (m, 4H), 7.84 (dd, J = 8.1 Hz, 1.4 Hz, 1H), 7.64 (ddd, J = 8.2 Hz, 6.9 Hz, 1.0 Hz, 1H), 7.55–7.59 (m, 2H), 7.43 (td, J = 7.7 Hz, 1.6 Hz, 1H), 7.37 (d, J = 9.1 Hz, 1H), 7.26–7.32 (m, 4H), 7.21–7.23 (m, 2H), 3.28–3.34 (m, 2H), 2.78 (t, J = 6.4 Hz, 2H); 13C-NMR (DMSO-d6), δ: 164.33, 154.13, 145.16, 139.13, 134.67, 130.57, 130.32, 129.81, 129.63, 128.70, 128.35, 128.28, 128.07, 127.50, 127.47, 127.33, 127.27, 126.55, 126.14, 125.82, 124.69, 122.55, 42.21, 35.23; HR-MS: for C26H20O3N2Cl [M + H]+ calculated 445.13135, found 445.13208 m/z.
1-[(2-Chlorophenyl)carbamoyl]naphthalen-2-yl (4-phenylbutyl)carbamate (15). Yield 58%; Mp 132–134 °C; IR (cm−1): 3220, 3024, 2938, 1719,1652, 1582, 1520, 1505, 1478, 1462, 1431, 1290, 1256, 1232, 1060, 985, 822, 760, 745, 699; 1H-NMR (DMSO-d6) δ: 10.10 (s, 1H), 7.99–8.05 (m, 3H), 7.90 (t, J = 5.5 Hz, 1H), 7.83 (dd, J = 8.2 Hz, 1.3 Hz, 1H), 7.63 (ddd, J = 8.2 Hz, 6.9 Hz, 1.0 Hz, 1H), 7.54–7.59 (m, 2H), 7.36–7.43 (m, 3H), 7.24–7.31 (m, 3H), 7.15–7.19 (m, 2H), 3.10 (q, J = 6.0 Hz, 2H), 2.55 (t, J = 7.3 Hz, 2H), 1.59 (qi, J = 7.3 Hz, 2H), 1.48 (qi, J = 7.3 Hz, 2H); 13C-NMR (DMSO-d6), δ: 164.34, 154.19, 145.22, 142.09, 134.68, 130.57, 130.32, 129.83, 129.62, 128.31, 128.23, 128.22, 128.07, 127.44, 127.42, 127.32, 127.26, 126.61, 125.81, 125.64, 124.68, 122.62, 40.39, 34.80, 28.88, 28.19; HR-MS: for C28H24O3N2Cl [M + H]+ calculated 473.16265, found 473.16342 m/z.
1-[(2-Nitrophenyl)carbamoyl]naphthalen-2-yl ethylcarbamate (16). Yield 40%; Mp 157–159 °C; IR (cm−1): 3320, 3216, 2963, 2917, 2843, 1743, 1705, 1656, 1645, 1608, 1580, 1532, 1504, 1462, 1432, 1357, 1341, 1294, 1270, 1250, 1216, 1199, 1162, 1144, 1079, 1034, 993, 822, 791, 779, 732, 667; 1H-NMR (DMSO-d6) δ: 10.94 (s, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.01–8.05 (m, 3H), 7.71–7.80 (m, 3H), 7.65 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.59 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.41–7.48 (m, 2H), 3.04–3.11 (m, 2H), 1.03 (t, J = 7.1 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.12, 153.71, 145.56, 142.98, 133.89, 130.62, 130.51, 130.33, 130.26, 128.10, 127.42, 125.91, 125.82, 125.70, 124.96, 124.90, 124.43, 122.62, 35.39, 14.75; HR-MS: for C20H16O5N3 [M + H]+ calculated 378.10845, found 378.10934 m/z.
1-[(2-Nitrophenyl)carbamoyl]naphthalen-2-yl propylcarbamate (17). Yield 75%; Mp 153–155 °C; IR (cm−1): 3298, 3223, 2960, 2930, 2873, 1727, 1717, 1662, 1589, 1537, 1520, 1505, 1488, 1463, 1440, 1353, 1285, 1257, 1224, 1146, 1106, 1051, 996, 981, 914, 865, 818, 779, 731, 666; 1H-NMR (DMSO-d6) δ: 10.93 (s, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.01–8.05 (m, 3H), 7.72–7.82 (m, 3H), 7.65 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.59 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.41–7.48 (m, 2H), 3.00 (q, J = 6.5 Hz, 2H), 1.42 (sx, J = 7.1 Hz, 2H), 0.80 (t, J = 7.3 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.14, 153.94, 145.61, 142.89, 133.91, 130.69, 130.53, 130.33, 130.33, 128.11, 127.43, 125.93, 125.79, 125.72, 125.64, 124.91, 124.46, 122.62, 42.29, 22.37, 11.10; HR-MS: for C21H18O5N3 [M + H]+ calculated 392.12410, found 392.12521 m/z.
1-[(2-Nitrophenyl)carbamoyl]naphthalen-2-yl butylcarbamate (18). Yield 82%; Mp 171–174 °C; IR (cm−1): 3293, 3222, 2957, 2873, 1724, 1661, 1589, 1549, 1532, 1520, 1505, 1471, 1463, 1439, 1350, 1279, 1257, 1227, 1145, 1109, 1006, 914, 865, 818, 779, 769, 731, 680, 667; 1H-NMR (DMSO-d6) δ: 10.92 (s, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.01–8.05 (m, 3H), 7.73–7.80 (m, 3H), 7.65 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.58 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.45 (td, J = 8.2 Hz, 1.4 Hz, 1H), 7.38 (d, J = 9.1 Hz, 1H), 3.03 (q, J = 6.2 Hz, 2H), 1.38 (qi, J = 7.0 Hz, 2H), 1.23 (sx, J = 7.0 Hz, 2H), 0.81 (t, J = 7.1 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.13, 153.91, 145.58, 142.77, 133.91, 130.74, 130.52, 130.32, 130.31, 128.10, 127.41, 125.91, 125.74, 125.73, 125.56, 124.91, 124.44, 122.59, 40.18, 31.23, 19.27, 13.58; HR-MS: for C22H20O5N3 [M + H]+ calculated 406.13975, found 406.14081 m/z.
1-[(2-Nitrophenyl)carbamoyl]naphthalen-2-yl pentylcarbamate (19). Yield 70%; Mp 141–143 °C; IR (cm−1): 3298, 3226, 2935, 2874, 1723, 1667, 1588, 1548, 1532, 1519, 1505, 1485, 1475, 1436, 1347, 1322, 1284, 1253, 1226, 1142, 1108, 1037, 1025, 996, 913, 866, 819, 778, 767, 730, 680, 660; 1H-NMR (DMSO-d6) δ: 10.93 (s, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.01–8.05 (m, 3H), 7.75–7.81 (m, 3H), 7.65 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.58 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.45 (td, J = 8.2 Hz, 1.4 Hz, 1H), 7.41 (d, J = 9.1 Hz, 1H), 3.02 (q, J = 6.4 Hz, 2H), 1.40 (qi, J = 7.0 Hz, 2H), 1.09–1.33 (m, 4H), 0.82 (t, J = 6.2 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.12, 153.89, 145.58, 142.80, 133.88, 130.72, 130.51, 130.31, 130.30, 128.10, 127.42, 125.91, 125.74, 125.72, 125.58, 124.91, 124.44, 122.61, 40.47, 28.77, 28.29, 21.76, 13.83; HR-MS: for C23H22O5N3 [M + H]+ calculated 420.15540, found 420.15643 m/z.
1-[(2-Nitrophenyl)carbamoyl]naphthalen-2-yl hexylcarbamate (20). Yield 58%; Mp 122–123 °C; IR (cm−1): 3332, 3272, 2929, 2868, 1709, 1665, 1609, 1589, 1541, 1514, 1472, 1443, 1352, 1295, 1250, 1215, 1205, 1158, 1148, 1113, 1046, 993, 978, 911, 863, 825, 779, 761, 738, 697, 672; 1H-NMR (DMSO-d6) δ: 10.93 (s, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.01–8.05 (m, 3H), 7.75–7.81 (m, 3H), 7.65 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.58 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.45 (td, J = 8.2 Hz, 1.4 Hz, 1H), 7.41 (d, J = 9.1 Hz, 1H), 3.02 (q, J = 6.0 Hz, 2H), 1.38 (qi, J = 6.4 Hz, 2H), 1.18–1.25 (m, 6H), 0.83 (t, J = 6.6 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.65, 154.44, 146.08, 143.25, 134.42, 131.24, 131.04, 130.83, 130.82, 128.61, 127.94, 126.44, 126.23, 126.23, 126.06, 125.42, 124.94, 123.10, 41.03, 31.42, 29.56, 26.28, 22.49, 14.38; HR-MS: for C24H24O5N3 [M + H]+ calculated 434.17105, found 434.17236 m/z.
1-[(2-Nitrophenyl)carbamoyl]naphthalen-2-yl heptylcarbamate (21). Yield 46%; Mp 101–102 °C; IR (cm−1): 3333, 3265, 2957, 2925, 2852, 1709, 1665, 1652, 1609, 1588, 1541, 1511, 1472, 1464, 1441, 1350, 1294, 1250, 1215, 1205, 1148, 1045, 979, 911, 824, 779, 755, 737, 696, 667; 1H-NMR (DMSO-d6) δ: 10.93 (s, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.01–8.05 (m, 3H), 7.75–7.81 (m, 3H), 7.65 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.58 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.45 (td, J = 8.2 Hz, 1.4 Hz, 1H), 7.41 (d, J = 9.1 Hz, 1H), 3.02 (q, J = 6.3 Hz, 2H), 1.39 (qi, J = 6.4 Hz, 2H), 1.17–1.28 (m, 8H), 0.85 (t, J = 6.6 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.12, 153.89, 145.58, 142.77, 133.88, 130.74, 130.53, 130.31, 130.31, 128.10, 127.42, 125.91, 125.73, 125.72, 125.58, 124.90, 124.44, 122.61, 40.50, 31.16, 29.11, 28.36, 26.07, 22.04, 13.93; HR-MS: for C25H26O5N3 [M + H]+ calculated 448.18670, found 448.18848 m/z.
1-[(2-Nitrophenyl)carbamoyl]naphthalen-2-yl octylcarbamate (22). Yield 37%; Mp 92–94 °C; IR (cm−1): 3315, 3230, 2924, 2850, 1704, 1653, 1589, 1528, 1508, 1485, 1463, 1435, 1360, 1293, 1271, 1251, 1219, 915, 834, 780, 759, 736, 701, 667; 1H-NMR (DMSO-d6) δ: 10.93 (s, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.01–8.05 (m, 3H), 7.75–7.81 (m, 3H), 7.65 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.59 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.46 (td, J = 8.2 Hz, 1.4 Hz, 1H), 7.41 (d, J = 9.1 Hz, 1H), 3.03 (q, J = 5.5 Hz, 2H), 1.39 (qi, J = 6.0 Hz, 2H), 1.18–1.29 (m, 10H), 0.86 (t, J = 6.4 Hz, 3H); 13C-NMR (DMSO-d6), δ: 164.12, 153.89, 145.58, 142.79, 133.86, 130.72, 130.51, 130.32, 130.31, 128.10, 127.42, 125.91, 125.75, 125.72, 125.58, 124.90, 124.44, 122.59, 40.50, 31.22, 29.09, 28.67, 28.59, 26.12, 22.07, 13.95; HR-MS: for C26H28O5N3 [M + H]+ calculated 462.20235, found 462.20410 m/z.
1-[(2-Nitrophenyl)carbamoyl]naphthalen-2-yl isoproylcarbamate (23). Yield 69%; Mp 172–176 °C; IR (cm−1): 3355, 3320, 2974, 1738, 1665, 1586, 1532, 1505, 1489, 1440, 1362, 1245, 1213, 1173, 1149, 1056, 1018, 953, 924, 826, 778, 763, 732; 1H-NMR (DMSO-d6) δ: 10.93 (s, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.01–8.05 (m, 3H), 7.75–7.80 (m, 3H), 7.66 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.59 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.46 (td, J = 8.2 Hz, 1.4 Hz, 1H), 7.42 (d, J = 9.1 Hz, 1H), 3.65 (sx, J = 6.4 Hz, 2H), 1.07 (d, J = 6.4 Hz, 6H); 13C-NMR (DMSO-d6), δ: 164.18, 153.10, 145.61, 142.94, 133.92, 130.69, 130.54, 130.37, 130.33, 128.14, 127.46, 125.96, 125.85, 125.80, 125.72, 124.94, 124.46, 122.71, 42.86, 22.32; HR-MS: for C21H18O5N3 [M + H]+ calculated 394.13975, found 394.14058 m/z.
1-[(2-Nitrophenyl)carbamoyl]naphthalen-2-yl cyclopentylcarbamate (24). Yield 28%; Mp 138–142 °C; IR (cm−1): 3295, 3219, 2960, 1719, 1661, 1588, 1519, 1487, 1438, 1351, 1283, 1255, 1222, 1144, 1078, 998, 822, 779, 732, 667; 1H-NMR (DMSO-d6) δ: 10.92 (s, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.01–8.05 (m, 3H), 7.84 (d, J = 6.9 Hz, 1H), 7.72–7.79 (m, 2H), 7.65 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.59 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.46 (td, J = 8.2 Hz, 1.4 Hz, 1H), 7.42 (d, J = 9.1 Hz, 1H), 3.83 (sx, J = 5.5 Hz, 1H), 1.72–1.80 (m, 2H), 1.56–1.63 (m, 2H), 1.42–1.49 (m, 4H); 13C-NMR (DMSO-d6), δ: 164.18, 153.41, 145.64, 142.94, 133.92, 130.69, 130.55, 130.36, 130.34, 128.14, 127.46, 125.97, 125.86, 125.80, 125.72, 124.95, 124.45, 122.73, 52.47, 32.06, 23.29; HR-MS: for C23H20O5N3 [M + H]+ calculated 420.15540, found 420.15620 m/z.
1-[(2-Nitrophenyl)carbamoyl]naphthalen-2-yl cyclohexylcarbamate (25). Yield 54%; Mp 140–143 °C; IR (cm−1): 3328, 3297, 2937, 2911, 2858, 1705, 1662, 1589, 1536, 1514, 1505, 1472, 1440, 1354, 1312, 1292, 1209, 1146, 1049, 1014, 823, 779, 761, 733, 697; 1H-NMR (DMSO-d6) δ: 10.91 (s, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.01–8.05 (m, 3H), 7.72–7.79 (m, 3H), 7.65 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.58 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.45 (td, J = 8.2 Hz, 1.4 Hz, 1H), 7.41 (d, J = 9.1 Hz, 1H), 3.26–3.33 (m, 1H), 1.71–1.76 (m, 2H), 1.63–1.68 (m, 2H), 1.51–1.56 (m, 1H), 1.02–1.27 (m, 5H); 13C-NMR (DMSO-d6), δ: 164.17, 153.14, 145.65, 142.90, 133.91, 130.70, 130.53, 130.36, 130.35, 128.13, 127.46, 125.96, 125.82, 125.79, 125.69, 124.93, 124.45, 122.67, 49.96, 32.39, 25.10, 24.56; HR-MS: for C24H22O5N3 [M + H]+ calculated 434.17105, found 434.17208 m/z.
1-[(2-Nitrophenyl)carbamoyl]naphthalen-2-yl cycloheptylcarbamate (26). Yield 58%; Mp 142–145 °C; IR (cm−1): 3324, 2912, 2855, 1703, 1662, 1588, 1528, 1505, 1472, 1440, 1353, 1291, 1245, 1218, 1203, 1141, 1041, 997, 909, 823, 779, 758, 737, 697; 1H-NMR (DMSO-d6) δ: 10.91 (s, 1H), 8.06 (d, J = 9.1 Hz, 1H), 8.01–8.05 (m, 3H), 7.81 (d, J = 8.2 Hz, 1H), 7.72–7.78 (m, 2H), 7.65 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.58 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.45 (td, J = 8.2 Hz, 1.4 Hz, 1H), 7.41 (d, J = 9.1 Hz, 1H), 3.46–3.54 (m, 1H), 1.73–1.80 (m, 2H), 1.39–1.60 (m, 8H), 1.29–1.38 (m, 2H); 13C-NMR (DMSO-d6), δ: 164.18, 153.01, 145.67, 142.82, 133.92, 130.73, 130.54, 130.35, 130.35, 128.13, 127.45, 125.95, 125.80, 125.80, 125.63, 124.93, 124.45, 122.70, 52.13, 34.26, 27.80, 23.54; HR-MS: for C25H24O5N3 [M + H]+ calculated 448.18670, found 448.18784 m/z.
1-[(2-Nitrophenyl)carbamoyl]naphthalen-2-yl (2-phenylethyl)carbamate (27). Yield 32%; Mp 127–130 °C; IR (cm−1): 3336, 1742, 1708, 1683, 1608, 1578, 1535, 1510, 1495, 1434, 1398, 1334, 1270, 1211, 1144, 1130, 967, 824, 758, 743, 698; 1H-NMR (DMSO-d6) δ: 10.96 (s, 1H), 8.07 (d, J = 9.1 Hz, 1H), 8.01–8.05 (m, 3H), 7.91 (t, J = 5.7 Hz, 1H), 7.71–7.79 (m, 2H), 7.65 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.59 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.46 (td, J = 8.2 Hz, 1.4 Hz, 1H), 7.38 (d, J = 9.1 Hz, 1H), 7.17–7.26 (m, 5H), 3.26 (q, J = 6.4 Hz, 2H), 2.73 (t, J = 7.3 Hz, 2H); 13C-NMR (DMSO-d6), δ: 164.10, 153.87, 145.54, 143.01, 139.13, 133.94, 130.63, 130.54, 130.35, 130.31, 128.88, 128.72, 128.31, 128.14, 127.46, 126.10, 125.98, 125.82, 123.69, 124.94, 124.48, 122.58, 42.16, 35.11; HR-MS: for C26H20O5N3 [M + H]+ calculated 456.15540, found 456.15712 m/z.
1-[(2-Nitrophenyl)carbamoyl]naphthalen-2-yl (4-phenylbutyl)carbamate (28). Yield 47%; Mp 109–110 °C; IR (cm−1): 3351, 3233, 2934, 1714, 1677, 1593, 1519, 1505, 1482, 1458, 1435, 1352, 1287, 1244, 1222, 1051, 1007, 816, 779, 752, 731, 967; 1H-NMR (DMSO-d6) δ: 10.94 (s, 1H), 8.07 (d, J = 8.7 Hz, 1H), 8.00–8.05 (m, 3H), 7.82 (t, J = 5.7 Hz, 1H), 7.70–7.72 (m, 2H), 7.65 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.58 (ddd, J = 8.2 Hz, 6.9 Hz, 1.4 Hz, 1H), 7.43–7.46 (m, 1H), 7.41 (d, J = 9.1 Hz, 1H), 7.24–7.28 (m, 2H), 7.15–7.18 (m, 3H), 3.06 (q, J = 5.9 Hz, 2H), 2.52 (t, J = 7.3 Hz, 2H), 1.54 (qi, J = 7.3 Hz, 2H), 1.43 (qi, J = 7.3 Hz, 2H); 13C-NMR (DMSO-d6), δ: 164.13, 153.93, 145.59, 142.96, 142.09, 133.88, 130.65, 130.54, 130.35, 130.32, 128.30, 128.22, 128.13, 127.45, 125.95, 125.80, 125.72, 125.64, 125.64, 124.93, 124.47, 122.63, 40.32, 34.77, 28.80, 28.11; HR-MS: for C28H24O5N3 [M + H]+ calculated 484.18670, found 484.18801 m/z.
3.3. In Vitro Antibacterial Susceptibility Testing
The synthesized compounds were evaluated for in vitro antibacterial activity against representatives of multidrug-resistant bacteria and clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) SA 630 and SA 3202, that were obtained from the National Institute of Public Health (Prague, Czech Republic). Staphylococcus aureus ATCC 29213 was used as a reference and quality control strain. Ampicillin (Sigma-Aldrich) was used as the standard. Prior to testing, each strain was passaged onto nutrient agar (Oxoid, Hampshire, UK) with 5% of bovine blood, and bacterial inocula were prepared by suspending a small portion of bacterial colony in sterile phosphate buffered saline (pH 7.2–7.3). The cell density was adjusted to 0.5 McFarland units using a densitometer (Densi-La-Meter, LIAP, Riga, Latvia). The final inoculum was made to a 1:20 dilution of the suspension with the Mueller-Hinton broth (MH broth). The compounds were dissolved in DMSO (Sigma), and the final concentration of DMSO in the MH broth (Oxoid) did not exceed 2.5% of the total solution composition. The final concentrations of the evaluated compounds ranged from 256 μg/mL to 0.008 μg/mL. The broth dilution micro-method, modified according to NCCLS (National Committee for Clinical Laboratory Standards) guidelines [46,47] in MH broth, was used to determine the minimum inhibitory concentration (MIC). Drug-free controls, sterility controls, and controls consisting of MH broth and DMSO alone were included. The determination of results was performed visually after 24 h of static incubation in the darkness at 37 °C in an aerobic atmosphere. The MICs were defined as the lowest concentration of the compound at which no visible bacterial growth was observed. The results are summarized in Table 1.
3.4. In Vitro Antimycobacterial Evaluation
The evaluation of the in vitro antimycobacterial activity of the compounds was performed against Mycobacterium marinum CAMP 5644 and M. kansasii DSM 44162. The broth dilution micro-method in Middlebrook 7H9 medium (Difco, Lawrence, KS, USA) supplemented with ADC Enrichment (Becton, Dickinson & Comp., Franklin Lakes, NJ, USA) was used to determine the minimum inhibitory concentration (MIC), as previously described [48]. The compounds were dissolved in DMSO (Sigma-Aldrich), and the final concentration of DMSO did not exceed 2.5% of the total solution composition. The final concentrations of the evaluated compounds, ranging from 256 μg/mL to 0.125 μg/mL, were obtained by twofold serial dilution of the stock solution in a microtiter plate with sterile medium. Bacterial inocula were prepared by transferring colonies from the culture to sterile water. The cell density was adjusted to 0.5 McFarland units using a densitometer (Densi-La-Meter, LIAP, Riga, Latvia). The final inoculum was made by 1:1000 dilution of the suspension with sterile water. Drug-free controls, sterility controls, and controls consisting of the medium and DMSO alone were included. The determination of results was performed visually after 7 days of static incubation in the darkness at 37 °C in an aerobic atmosphere for M. kansasii and after 21 days of static incubation in the darkness at 28 °C in an aerobic atmosphere for M. marinum. The minimum inhibitory concentration (MIC) was defined as the lowest concentration of the compound at which no visible bacterial growth was observed. The MIC value is routinely and widely used in bacterial assays and is a standard detection limit according to the Clinical and Laboratory Standards Institute (CLSI) [49]. Isoniazid (Sigma-Aldrich) was used as the reference antibacterial drug. The results are summarized in Table 1.
3.5. In Vitro Cytotoxicity Assay
Human monocytic leukemia THP-1 cells were obtained from the European Collection of Cell Cultures (ECACC, Salisbury, UK; Methods of characterization: DNA Fingerprinting (Multilocus probes) and isoenzyme analysis). These cells were routinely cultured in RPMI 1640 (Lonza, Verviers, Belgium) medium supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich), 2% l-glutamine, 1% penicillin, and streptomycin (Lonza, Verviers, Belgium) at 37 °C with 5% CO2. Cells were passaged at approximately 1-week intervals. Cells were routinely tested for the absence of mycoplasma (Hoechst 33258 staining method). The tested compounds were dissolved in DMSO (Sigma-Aldrich) and added in five increasing concentrations to the cell suspension in the culture medium. The maximum concentration of DMSO in the assays never exceeded 0.1%. Subsequently, the cells were incubated for 24 h at 37 °C with 5% CO2 at various compound concentrations ranging from 0.37 to 30 μmol/L in RPMI 1640 medium. Cell toxicity was determined using a Cytotoxicity Detection KitPLUS Lactate dehydrogenase (LDH) assay kit (Roche Diagnostics, Mannheim, Germany), and used according to the manufacturer’s instructions, as described previously [14,30,32,33]. For LDH assays, cells were seeded into 96-well plates (5 × 104 cells·well−1 in 100 μL culture medium) in triplicate in serum-free RPMI 1640 medium, and measurements at 492 nm wavelength (Synergy 2 Multi-Mode Microplate Reader, BioTek, Winooski, VT, USA) were taken 24 h after the treatment with tested compounds. The median lethal dose values, LD50, were deduced through the production of a dose-response curve. All data were evaluated using GraphPad Prism 5.00 software (GraphPad Software, San Diego, CA, USA). The results are summarized in Table 1.
4. Conclusions
Series of thirteen 1-[(2-chlorophenyl)carbamoyl]naphthalen-2-yl carbamates and thirteen 1-[(2-nitrophenyl)carbamoyl]naphthalen-2-yl carbamates were prepared and subsequently characterized. All compounds were tested for their in vitro antimicrobial activity against S. aureus, two methicillin-resistant S. aureus strains, M. marinum, and M. kansasii. 1-[(2-Chlorophenyl)-carbamoyl]naphthalen-2-yl ethylcarbamate (3) and 1-[(2-nitrophenyl)carbamoyl]naphthalen-2-yl ethylcarbamate (16) showed MICs = 42 µM against all methicillin-resistant S. aureus strains. In addition, both compounds expressed MICs = 21 µM against M. marinum and M. kansasii. Propyl- (4, 17) and isopropyl- (10, 23) carbamates demonstrated medium antimicrobial activity, while bulkier-substituted carbamates showed only moderate or no activity, which was similar in both the chlorinated and nitrated series. Screening of the cytotoxicity of the most potent and discussed compounds, performed using the THP-1 cells, proved no significant lethal effect (LD50 >30 µM). The biological activity of the compounds is dependent on the alkyl substitution of carbamate nitrogen. Spatially small N-alkyl substituents are important for the activity. Antimicrobial activity rapidly decreases with chain prolongation or with a bulkier N-cycloalkyl, N-arylalkyl substituent.
Acknowledgments
This study was supported by IGA VFU Brno 311/2016/FaF, 328/2016/FaF, 302/2015/FaF, 320/2015/FaF and by the Slovak Research and Development Agency (Grant No. APVV-0516-12). The HPLC/HRMS system forms a part of the National Infrastructure CzeCOS (LM2015061); Michal Oravec was supported by the National Sustainability Program (NPU I; Grant No. LO1415 POLYMAT).
Author Contributions
Tomas Gonec, Josef Stranik, Jiri Kos, Josef Jampilek—design, synthesis of the compounds, SAR, writing of the paper. Michal Oravec, Aneta Cernikova—analysis/characterization of the compounds. Sarka Pospisilova, Valeria Pudelkova, Alois Cizek—antimicrobial evaluation. Lucie Holanova, Peter Kollar—cytotoxicity assay.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of compounds 1–28 are available from authors T. Gonec, J. Stranik, J. Kos, J. Jampilek.
References
- 1.World Health Organization . World Health Statistics 2016: Monitoring Health for the SDGs. WHO Press; Geneva, Switzerland: 2016. [Google Scholar]
- 2.World Health Organization . Global Tuberculosis Report 2015. WHO Press; Geneva, Switzerland: 2015. [Google Scholar]
- 3.World Health Organization . Global Antimicrobial Resistance Surveillance System 2015. WHO Press; Geneva, Switzerland: 2015. [Google Scholar]
- 4.European Centre for Disease Prevention and Control. [(accessed on 30 July 2016)]. Available online: http://ecdc.europa.eu/en/Pages/home.aspx.
- 5.Thampi N., Showler A., Burry L., Bai A.D., Steinberg M., Ricciuto D.R., Bell C.M., Morris A.M. Multicenter study of health care cost of patients admitted to hospital with Staphylococcus aureus bacteremia: Impact of length of stay and intensity of care. Am. J. Infect. Control. 2015;43:739–744. doi: 10.1016/j.ajic.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Kaku N., Yanagihara K., Morinaga Y., Yamada K., Harada Y., Migiyama Y., Nagaoka K., Matsuda J., Uno N., Hasegawa H., et al. Influence of antimicrobial regimen on decreased in-hospital mortality of patients with MRSA bacteremia. J. Infect. Chemother. 2014;20:350–355. doi: 10.1016/j.jiac.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 7.Tavares L.S., Silva C.S.F., de Souza V.C., da Silva V.L., Diniz C.G., Santos M.O. Strategies and molecular tools to fight antimicrobial resistance: Resistome, transcriptome, and antimicrobial peptides. Front. Microbiol. 2013;4:412. doi: 10.3389/fmicb.2013.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jampilek J. Potential of agricultural fungicides for antifungal drug discovery. Expert Opin. Drug Dis. 2016;11:1–9. doi: 10.1517/17460441.2016.1110142. [DOI] [PubMed] [Google Scholar]
- 9.Jampilek J. How Can We Bolster the Antifungal Drug Discovery Pipeline? Future Med. Chem. 2016;8:1393–1397. doi: 10.4155/fmc-2016-0124. [DOI] [PubMed] [Google Scholar]
- 10.Kratky M., Vinsova J. Salicylanilide ester prodrugs as potential antimicrobial agents—A review. Curr. Pharm. Des. 2011;17:3494–3505. doi: 10.2174/138161211798194521. [DOI] [PubMed] [Google Scholar]
- 11.Imramovsky A., Pauk K., Pejchal V., Hanusek J. Salicylanilides and their derivates as perspective anti-tuberculosis drugs: Synthetic routes and biological evaluations. Mini Rev. Org. Chem. 2011;8:211–220. doi: 10.2174/157019311795177808. [DOI] [Google Scholar]
- 12.Kratky M., Vinsova J. Antiviral activity of substituted salicylanilides—A review. Mini Rev. Med. Chem. 2011;11:956–967. doi: 10.2174/138955711797068382. [DOI] [PubMed] [Google Scholar]
- 13.Kratky M., Vinsova J. Advances in mycobacterial isocitrate lyase targeting and inhibitors. Curr. Med. Chem. 2012;19:6126–6137. doi: 10.2174/0929867311209066126. [DOI] [PubMed] [Google Scholar]
- 14.Gonec T., Kos J., Zadrazilova I., Pesko M., Keltosova S., Tengler J., Bobal P., Kollar P., Cizek A., Kralova K., et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013;21:6531–6541. doi: 10.1016/j.bmc.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Zadrazilova I., Pospisilova S., Pauk K., Imramovsky A., Vinsova J., Cizek A., Jampilek J. In vitro bactericidal activity of 4- and 5-chloro-2-hydroxy-N-[1-oxo-1-(phenylamino)alkan-2-yl]benzamides against MRSA. BioMed Res. Int. 2015;2015:349534. doi: 10.1155/2015/349534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zadrazilova I., Pospisilova S., Masarikova M., Imramovsky A., Monreal-Ferriz J., Vinsova J., Cizek A., Jampilek J. Salicylanilide carbamates: Promising antibacterial agents with high in vitro activity against methicillin-resistant Staphylococcus aureus. Eur. J. Pharm. Sci. 2015;77:197–207. doi: 10.1016/j.ejps.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Kushkevych I., Kollar P., Ferreira A.L., Palma D., Duarte A., Lopes M.M., Bartos M., Pauk K., Imramovsky A., Jampilek J. Antimicrobial effect of salicylamide derivatives against intestinal sulfate-reducing bacteria. J. Appl. Biomed. 2016;14:125–130. doi: 10.1016/j.jab.2016.01.005. [DOI] [Google Scholar]
- 18.Steinhilber D., Schubert-Zsilavecz M., Roth H.J. Medizinische Chemie: Targets, Arzneistoffe, Chemische Biologie. Deutscher Apotheker Verlag; Stutgart, Germany: 2010. [Google Scholar]
- 19.US Environmental Protection Agency–Pesticide Registration: Pesticide Data Submitters List (PDSL), 2016. [(accessed on 30 July 2016)]; Available online: https://www.epa.gov/sites/production/files/2016-04/documents/dslchem_0.pdf.
- 20.Pattabiraman V.R., Bode J.W. Rethinking amide bond synthesis. Nature. 2011;480:471–479. doi: 10.1038/nature10702. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh A.K., Brindisi M. Organic carbamates in drug design and medicinal chemistry. J. Med. Chem. 2015;58:2895–2940. doi: 10.1021/jm501371s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otevrel J., Mandelova Z., Pesko M., Guo J., Kralova K., Sersen F., Vejsova M., Kalinowski D., Kovacevic Z., Coffey A., et al. Investigating the spectrum of biological activity of ring-substituted salicylanilides and carbamoylphenylcarbamates. Molecules. 2010;15:8122–8142. doi: 10.3390/molecules15118122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imramovsky A., Pesko M., Kralova K., Vejsova M., Stolarikova J., Vinsova J., Jampilek J. Investigating spectrum of biological activity of 4- and 5-chloro-2-hydroxy-N-[2-(arylamino)-1-alkyl-2-oxoethyl]-benzamides. Molecules. 2011;16:2414–2430. doi: 10.3390/molecules16032414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imramovsky A., Pesko M., Monreal-Ferriz J., Kralova K., Vinsova J., Jampilek J. Photosynthesis-inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkyl-carbamates. Bioorg. Med. Chem. Lett. 2011;21:4564–4567. doi: 10.1016/j.bmcl.2011.05.118. [DOI] [PubMed] [Google Scholar]
- 25.Jampilek J., Brychtova K. Azone analogues: Classification, design, and transdermal penetration principles. Med. Res. Rev. 2012;32:907–947. doi: 10.1002/med.20227. [DOI] [PubMed] [Google Scholar]
- 26.Malik I., Bukovsky M., Andriamainty F., Galisinova J. Anti-microbial activity of meta-alkoxyphenylcarbamates containing substituted N-phenylpiperazine fragment. Braz. J. Microbiol. 2012;43:959–965. doi: 10.1590/S1517-838220120003000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malik I., Bukovsky M., Andriamainty F., Galisinova J. Antimicrobial effect of para-alkoxyphenylcarbamic acid esters containing substituted N-phenylpiperazine moiety. Braz. J. Microbiol. 2013;44:457–463. doi: 10.1590/S1517-83822013000200018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zumla A., Nahid P., Cole S.T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discov. 2013;12:388–404. doi: 10.1038/nrd4001. [DOI] [PubMed] [Google Scholar]
- 29.Kos J., Zadrazilova I., Pesko M., Keltosova S., Tengler J., Gonec T., Bobal P., Kauerova T., Oravec M., Kollar P., et al. Antibacterial and herbicidal activity of ring-substituted 3-hydroxynaphthalene-2-carboxanilides. Molecules. 2013;18:7977–7997. doi: 10.3390/molecules18077977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonec T., Kos J., Zadrazilova I., Pesko M., Govender R., Chambel B., Pereira D., Kollar P., Imramovsky A., O’Mahony J., et al. Antibacterial and herbicidal activity of ring-substituted 2-hydroxynaphthalene-1-carboxanilides. Molecules. 2013;18:9397–9419. doi: 10.3390/molecules18089397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jampilek J. Recent advances in design of potential quinoxaline anti-infectives. Curr. Med. Chem. 2014;21:4347–4373. doi: 10.2174/0929867321666141011194825. [DOI] [PubMed] [Google Scholar]
- 32.Gonec T., Kos J., Nevin E., Govender R., Pesko M., Kushkevych I., Oravec M., Kollar P., O’Mahony J., Kralova K., et al. Preparation and biological properties of ring-substituted naphthalene-1-carboxanilides. Molecules. 2014;19:10386–10409. doi: 10.3390/molecules190710386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonec T., Zadrazilova I., Nevin E., Kauerova T., Pesko M., Kos J., Oravec M., Kollar P., Coffey A., O’Mahony J., et al. Synthesis and biological evaluation of N-alkoxyphenyl-3-hydroxynaphthalene-2-carboxanilides. Molecules. 2015;20:9767–9787. doi: 10.3390/molecules20069767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kralova K., Perina M., Waisser K., Jampilek J. Structure-activity relationships of N-benzylsalicylamides for inhibition of photosynthetic electron transport. Med. Chem. 2015;11:156–164. doi: 10.2174/1573406410666140815125004. [DOI] [PubMed] [Google Scholar]
- 35.Kauerova T., Kos J., Gonec T., Jampilek J., Kollar P. Antiproliferative and pro-apoptotic effect of novel nitro-substituted hydroxynaphthanilides on human cancer cell lines. Int. J. Mol. Sci. 2016;17:1219. doi: 10.3390/ijms17081219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonec T., Pospisilova S., Kauerova T., Kos J., Dohanosova J., Oravec M., Kollar P., Coffey A., Liptaj T., Cizek A., et al. N-alkoxyphenylhydroxynaphthalenecarboxamides and their antimycobacterial activity. Molecules. 2016;21:1068. doi: 10.3390/molecules21081068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pauk K., Zadrazilova I., Imramovsky A., Vinsova J., Pokorna M., Masarikova M., Cizek A., Jampilek J. New derivatives of salicylamides: Preparation and antimicrobial activity against various bacterial species. Bioorg. Med. Chem. 2013;21:6574–6581. doi: 10.1016/j.bmc.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 38.Alnimr A.M. Dormancy models for Mycobacterium tuberculosis: A mini review. Braz. J. Microbiol. 2015;46:641–647. doi: 10.1590/S1517-838246320140507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Ingen J., Boeree M.J., van Soolingen D., Mouton J.W. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist. Updates. 2012;15:149–161. doi: 10.1016/j.drup.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Matveychuk A., Fuks L., Priess R., Hahim I., Shitrit D. Clinical and radiological features of Mycobacterium kansasii and other NTM infections. Resp. Med. 2012;106:1472–1477. doi: 10.1016/j.rmed.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 41.National Committee for Clinical Laboratory Standards . M24-A2 Susceptibility Testing of Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes; Approved Standard. 2nd ed. NCCLS; Wayne, PA, USA: 2011. [PubMed] [Google Scholar]
- 42.Tengler J., Kapustikova I., Pesko M., Govender R., Keltosova S., Mokry P., Kollar P., O’Mahony J., Coffey A., Kralova K., et al. Synthesis and biological evaluation of 2-hydroxy-3-[(2-aryloxyethyl)amino]propyl 4-[(alkoxycarbonyl)amino]benzoates. Sci. World J. 2013;2013:274570. doi: 10.1155/2013/274570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kratky M., Vinsova J. Salicylanilide N-monosubstituted carbamates: Synthesis and in vitro antimicrobial activity. Bioorg. Med. Chem. 2016;24:1322–1330. doi: 10.1016/j.bmc.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Kos J., Nevin E., Soral M., Kushkevych I., Gonec T., Bobal P., Kollar P., Coffey A., O’Mahony J., Liptaj T., et al. Synthesis and antimycobacterial properties of ring-substituted 6-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2015;23:2035–2043. doi: 10.1016/j.bmc.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Suffness M., Douros J. Current status of the NCI plant and animal product program. J. Nat. Prod. 1982;45:1–14. doi: 10.1021/np50019a001. [DOI] [PubMed] [Google Scholar]
- 46.National Committee for Clinical Laboratory Standards . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard. 5th ed. NCCLS; Wayne, PA, USA: 2000. CLSI Document M7-A5. [Google Scholar]
- 47.National Committee for Clinical Laboratory Standards . Performance Standards for Antimicrobial Susceptibility Testing. NCCLS; Wayne, MI, USA: 2002. 12th Informational Supplement M100-S12. [Google Scholar]
- 48.Schwalbe R., Steele-Moore L., Goodwin A.C. Antimicrobial Susceptibility Testing Protocols. CRC Press; Boca Raton, FL, USA: 2007. [Google Scholar]
- 49.Clinical and Laboratory Standards Institute. [(accessed on 5 September 2016)]. Available online: http://www.clsi.org.