Abstract
Rice koji, used early in the manufacturing process for many fermented foods, produces diverse metabolites and enzymes during fermentation. Using gas chromatography time-of-flight mass spectrometry (GC-TOF-MS), ultrahigh-performance liquid chromatography linear trap quadrupole ion trap tandem mass spectrometry (UHPLC-LTQ-IT-MS/MS), and multivariate analysis we generated the metabolite profiles of rice koji produced by fermentation with Aspergillus oryzae (RK_AO) or Bacillus amyloliquefaciens (RK_BA) for different durations. Two principal components of the metabolomic data distinguished the rice koji samples according to their fermenter species and fermentation time. Several enzymes secreted by the fermenter species, including α-amylase, protease, and β-glucosidase, were assayed to identify differences in expression levels. This approach revealed that carbohydrate metabolism, serine-derived amino acids, and fatty acids were associated with rice koji fermentation by A. oryzae, whereas aromatic and branched chain amino acids, flavonoids, and lysophospholipids were more typical in rice koji fermentation by B. amyloliquefaciens. Antioxidant activity was significantly higher for RK_BA than for RK_AO, as were the abundances of flavonoids, including tricin, tricin glycosides, apigenin glycosides, and chrysoeriol glycosides. In summary, we have used MS-based metabolomics and enzyme activity assays to evaluate the effects of using different microbial species and fermentation times on the nutritional profile of rice koji.
Keywords: rice koji, fermentation, microbe, metabolomics, enzymatic activity, antioxidant activity
1. Introduction
Fermented food is well-known for its nutritional benefits and biological activities [1]. Fermentation with rice koji, a fermented product, is widely used in the early stages of manufacturing fermented foods such as rice wine (sake and makgeolli), fermented red pepper paste (gochujang), and fermented soybean paste (miso and doenjang) [2,3,4]. Because rice koji is an enzyme source, fermentation with this product affects the quality of the fermented food. During rice koji fermentation, microorganisms produce enzymes involved in metabolite hydrolysis and synthesis, which enhances the flavor, taste, and bioactivities of the fermented foods [5,6]. A nuruk is one of the traditional starter cultures naturally fermented with various airborne microorganisms, whereas koji is fermented by a single microbe under regulated conditions to enhance flavor and enzymatic activity [7].
The filamentous fungus Aspergillus oryzae, an obligate aerobe, is the microorganism used most commonly in the production of koji. A. oryzae not only exhibit strong activity of enzymes such as amylase, protease, and peptidase, but also secretes various hydrolytic enzymes [8]. This fungus is also reported to be a safe fermenter, as under most conditions it does not produce mycotoxins [9]. In addition to Aspergillus, the bacterial genus Bacillus is widely used for fermenting soybean meal. Bacillus is an ideal industrial microorganism because of its high growth rate and strong capacity to produce extracellular enzymes [10]. Bacillus subtilis yields compounds exhibiting various biological functions, and foods fermented by Bacillus spp. have higher digestibility and antioxidant activity than foods fermented with Aspergillus spp. [11,12]. Metabolic and enzymatic differences between the fermenting species affect food qualities such as flavor, taste, and biological activity [4].
Metabolomics is considered a useful and important tool in various fields, including food science, agriculture, and microbiology. Metabolite profiling aims to monitor all metabolites in a sample, facilitating nutritional analysis [13] as well as chemotaxonomic study of plants [14] and microorganisms [15]. Metabolomic approaches have been used to investigate metabolite changes caused by filamentous fungi during rice koji fermentation [7]. Cooked rice fermented with different microorganisms shows different metabolite profiles, enzymatic activities, and other characteristics [2,16]; however, previous investigations have been limited to filamentous fungi and lactic acid bacteria.
Through mass spectrometry (MS)-based metabolite profiling, we identified differences between the metabolites in rice koji inoculated with a fungus (A. oryzae) and that inoculated with a bacterial species (Bacillus amyloliquefaciens). We also determined differences in the enzymatic and antioxidant activities of the two types of rice koji. Furthermore, we used these observations on metabolism to model microorganism-specific metabolic pathways.
2. Results
2.1. Multivariate Analysis of Rice Koji Fermented with Different Microorganisms and Fermentation Times
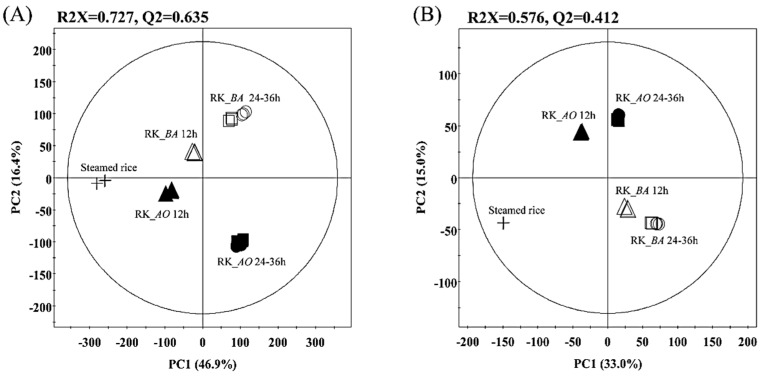
Metabolite profiling of the rice koji data sets was performed using a GC-TOF-MS and UHPLC-LTQ-IT-MS/MS combined multivariate analysis to investigate metabolite differences associated with differences in the inoculated microbes and fermentation times. The principal component analysis (PCA) score plot was obtained from GC-TOF-MS (Figure 1A) and accounted for 63.3% of the total variability. Principal Component 1 (PC1) (46.9%) distinguished the rice koji by fermentation time, and the rice koji samples fermented for 24 h and 36 h were clustered for both the A. oryzae fermentations (RK_AO) and B. amyloliquefaciens fermentations (RK_BA). The fermentation direction of RK_AO was clearly separated from that of RK_BA by Principal Component 2 (PC2) (16.4%). The partial least squares discriminant analysis (PLS-DA) showed the same pattern as the PCA analysis (supplementary meterials Figure S1). Metabolites that contributed to these distinctions in rice koji were identified based on their variable importance in projection values (VIP > 0.7) and p-values (p < 0.05). Forty-seven metabolites, including 10 sugars and sugar alcohols, 11 organic acids, two phenolic acids, 18 amino acids, five fatty acids, and one vitamin were determined to be important variables by PLS1 or PLS2. The metabolites were identified by comparing their mass fragment patterns and retention times with those of standard compounds, the National Institute of Standards and Technology (NIST) database (version 2.0, 2011, FairCom, Gaithersburg, MD, USA), and an in-house library. These metabolites are shown in supplementary materials Table S1, along with their relative contents represented as log10-transformed peak areas.
Figure 1.
Principal component analysis (PCA) score plot for rice koji fermented with A. oryzae (RK_AO) or B. amyloliquefaciens (RK_BA) obtained by GC-TOF-MS (A) and UHPLC-LTQ-IT-MS/MS (B). (+, Steamed rice; unfilled symbols, RK_AO; filled symbols, RK_BA; △, ▲, 12 h; □, ■, 24 h; ○, ●, 36 h).
The PCA score plot acquired by UHPLC-LTQ-IT-MS/MS also presented distinct patterns associated with fermentation time (PC1: 33.0%) and inoculated microbe (PC2: 15.0%) (Figure 1B). Twenty-seven metabolites were determined by UHPLC-LTQ-IT-MS/MS to be present in significantly different (VIP > 0.7 and p < 0.05) quantities in rice koji. These metabolites included seven flavonoids, two fatty acids, nine lysophospholipids, one siderophore, and eight unknown metabolites. The metabolites were tentatively identified by comparing their molecular weights, retention times, MSn fragment patterns, and UV absorbances to those in published literature and an in-house library. The relative content for each of these metabolites is shown in supplementary materials Table S2, with peak areas log10 transformed.
2.2. Different Metabolites and Metabolic Pathway of Rice Koji According to Microorganisms
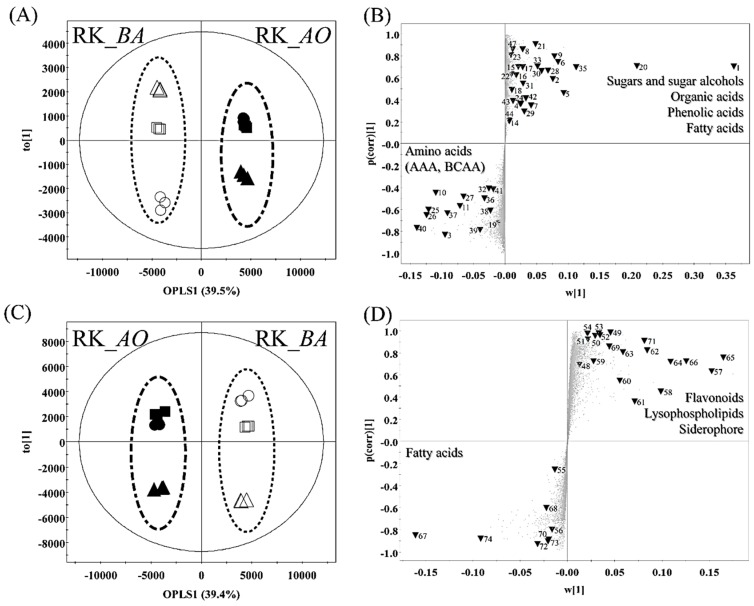
To confirm that the metabolites of rice koji differed according to the inoculated microorganism, RK_AO (12–36 h) and RK_BA (12–36 h) were subjected to an orthogonal partial least square discriminant analysis (OPLS-DA), which showed a clear separation by OPLS component 1, accounting for 39.5% and 39.4% of the variance in data obtained from the GC-TOF-MS (Figure 2A) and UHPLC-LTQ-IT-MS/MS analyses (Figure 2C), respectively.
Figure 2.
The OPLS-DA score plot (A,C) and loading S-plot (B,D) for rice koji fermented with A. oryzae (RK_AO) or B. amyloliquefaciens (RK_BA) obtained by GC-TOF-MS (A,B), and UHPLC-LTQ-IT-MS/MS (C,D). Highlighted metabolites (▼) in the S-plot indicate statistically significant differences between RK_AO and RK_BO (VIP > 1.0 and p < 0.05 in OPLS-DA). Each labeled peak number indicates a metabolite in Tables S1 and S2. The stated super-classes contain the identified metabolites (AAA, aromatic amino acid; BCAA, branched chain amino acid).
We highlight the 69 metabolites selected as variables (VIP > 1.0, p-value < 0.05) in the S-plots obtained from GC-TOF-MS (Figure 2B) and UHPLC-LTQ-IT-MS/MS (Figure 2D). Results showed that the levels of eight sugars and sugar alcohols, seven organic acids, two phenolic acids, seven amino acids, five fatty acids, two nucleotides, one vitamin, and six unknown compounds were higher in RK_AO than in RK_BA. Two sugars and sugar alcohols, two organic acids, 10 amino acids, seven flavonoids, nine lysophospholipids, one siderophore, and two unknown compounds were major metabolites in RK_BA.
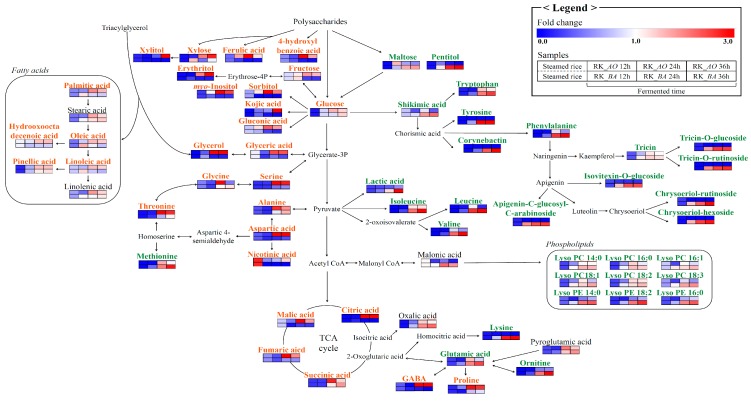
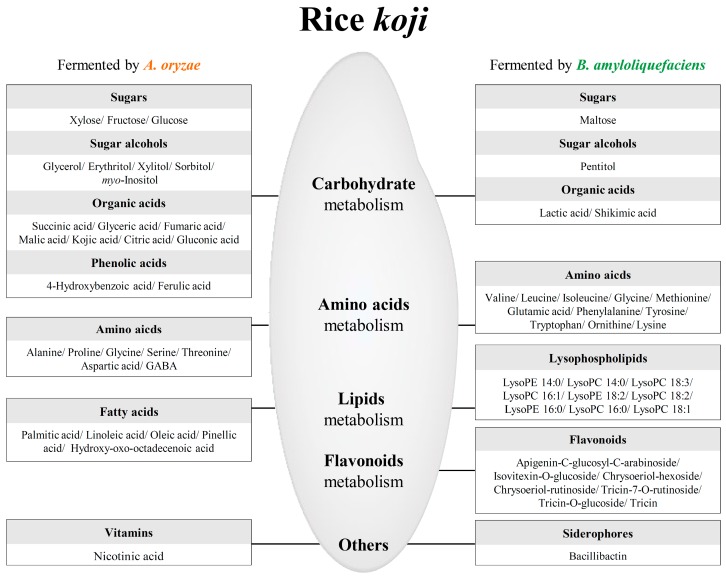
Figure 3 depicts the metabolic pathways shared by the two microbes of interest. Beneath each metabolite, the color on a blue-to-red gradient indicates the mean-normalized relative abundance of each metabolite under each experimental condition, as determined by MS, and metabolites that were significantly different between RK_AO and RK_BA in the OPLS-DA models are shown in orange (RK_AO) and green (RK_BA). Carbohydrate metabolism-related metabolites such as sugars and sugar alcohols (glycerol, erythritol, xylose, xylitol, fructose, glucose, sorbitol, and myo-inositol), organic acids (succinic acid, glyceric acid, fumaric acid, malic acid, kojic acid, citric acid, and gluconic acid), and phenolic acids (4-hydroxybenzoic acid and ferulic acid) were more abundant in the RK_AO samples than they were in RK_BA samples. Related to amino acid metabolism, the aromatic amino acids (AAA; phenylalanine, tyrosine, and tryptophan) derived from shikimic acid and the branched chain amino acids (BCAA; valine, leucine, and isoleucine) were higher in RK_BA, while amino acids related to the serine pathway (alanine, glycine, serine, threonine, and aspartic acid) were higher in RK_AO. The flavonoids (apigenin-C-glucosyl-C-arabinoside, isovitexin-O-glucoside, chrysoeriol-hexoside, chrysoeriol-rutinoside, tricin-7-O-rutinoside, tricin-O-glucoside, and tricin) originated from shikimic acid metabolism and were relatively abundant in RK_BA. In the case of lipid metabolism, the relative content of fatty acids (palmitic acid, linoleic acid, oleic acid, pinellic acid, and hydroxy-oxo-octadecenoic acid) and lysophospholipids (lysoPC (lysophosphatidylcholine) 14:0, LysoPC 18:3, LysoPC 16:1, LysoPC18:2, LysoPC 16:0, LysoPC 18:1, LysoPE (lysophosphatidyl-ethanolamine) 14:0, LysoPE 18:2, and LysoPE16:0) showed opposite trends according to each microorganism.
Figure 3.
Scheme of the metabolic pathway and relative metabolite contents in rice koji fermented with A. oryzae (RK_AO) or B. amyloliquefaciens (RK_BA). The pathway was retrieved from the KEGG database and modified (KEGG. http://www.genome.jp/kegg). The colored squares (blue-to-red) represent fold changes normalized by the average of all values for each metabolite. The orange characterized metabolites had significantly higher relative contents in RK_AO, while the green characterized metabolites had higher contents in RK_BA (VIP > 1.0 and p < 0.05 in OPLS-DA).
2.3. Comparison of Bioactivity and Enzymatic Activity in Different Rice Koji Depending on Microorganisms
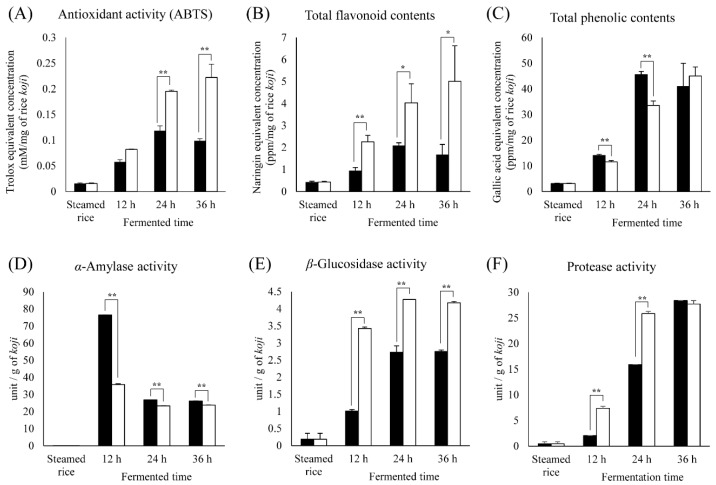
To further examine the differences between rice koji inoculated with A. oryzae and that inoculated with B. amyloliquefaciens during fermentation, we evaluated the antioxidant activity (ABTS), total flavonoid contents (TFC), and total phenolic contents (TPC) (Figure 4A–C). The overall levels of ABTS and TFC were higher for RK_BA than for RK_AO. In case of TPC, RK_AO had higher level than RK_BA at 24 h and 12 h, and there is no significant difference at 36 h. The antioxidant activity of RK_BA increased monotonically with fermentation time, whereas that of RK_AO increased until 24 h and then decreased; we observed an analogous pattern for TFC and TPC. This pattern in TFC and TPC was also almost analogous with the flavonoids and phenolic compounds detected by MS-based analysis.
Figure 4.
Comparison of bioactivities and enzymatic activities for rice koji fermented with A. oryzae (RK_AO, filled columns) or B. amyloliquefaciens (RK_BA, unfilled columns) for different durations. The bioactivities are antioxidant activity (ABTS) (A); total flavonoid contents (B); and total phenolic contents (C); The enzymatic activities are α-amylase activity (D); β-glucosidase activity (E); and protease activity (F). Significant differences between the RK_AO and RK_BA groups were identified by t-test (* p < 0.05, ** p < 0.01).
To understand the differences in metabolites according to the inoculated microorganisms during fermentation, the enzymatic activities in rice koji were measured (Figure 4D–F). The α-amylase activity was highest in the 12-h RK_AO group, and all RK_AO groups exhibited stronger α-amylase activities than the RK_BA groups. β-Glucosidase and protease activities were higher in the RK_BA groups than in the RK_AO groups. Enzyme activities exhibited wider ranges at early-mid fermentation (0–24 h) phase than at later fermentation (24–36 h).
3. Discussion
Enzymes produced by inoculated microorganisms affect the repertoire of metabolites found in koji fermentations. This relationship means that microbial fermenters have decisive effects on the taste, flavor, and nutritional value of the final product [4]. Although there are many scientific studies on rice koji fermentation, only filamentous fungi have been used in non-targeted metabolomic approaches. We investigated differences in microbial metabolites along with antioxidant and enzymatic activities in metabolic pathways between rice koji prepared with two inoculated microbes, a fungus (A. oryzae) and a bacterium (B. amyloliquefaciens), to better understand the nutritional qualities of rice koji. Bacillus species are regarded one of the most potent microbial fermenters because of their rapid growth rate, strong secreted enzymes, and the ability enhancing bioactivity.
The metabolite and enzymatic activities in rice koji are largely distinguished by fermentation time and fermenter species (Figure 1 and Figure 4). The abundances of most metabolites increased with fermentation time and showed remarkable changes in early-mid fermentation (0–24 h), whereas there was no great difference in late fermentation (24–36 h) in both RK_AO and RK_BA. These changes in metabolites and enzymatic activities depending on fermentation time showed patterns that were similar to those found in previous studies [17,18]. This result may indicate that the early-mid fermentation (0–24 h) is the most influential stage for rice koji, as far as the metabolome is concerned. This comparison also revealed great metabolic differences between fermentations with each of the microorganisms. Because many enzymes, encoded by multiple genes and involved in many pathways, participate in the biosynthesis and degradation of a single metabolite, it is difficult to clearly explain the changes in metabolites observed during rice koji fermentation. Nevertheless, the MS-based metabolomic approach reveals that the fungus A. oryzae and the bacterium B. amyloliquefaciens have different metabolic strategies for fermentation of rice koji. Figure 5 showed the metabolic comparison between rice koji by two microbes for 24 h because metabolite difference was noticeable in this sample. Selected metabolites had statistically significant differences in OPLS-DA between RK_AO and RK_BO (VIP > 1.0, p < 0.05).
Figure 5.
The metabolic comparison between rice koji fermented with A. oryzae for 24 h (RK_AO 24 h) or B. amyloliquefaciens for 24 h (RK_BA 24 h). The selected metabolites are variables of OPLS-DA in Figure 2. The metabolite located on left has higher relative content in RK_AO 24 h, while that of located on right was higher in RK_BA 24 h.
3.1. Sugars and Sugar Alcohols
Sugars are generally consumed as carbon sources, yielding energy for proliferation and growth via carbohydrate metabolic pathways such as glycolysis, the pentose phosphate pathway, and the tricarboxylic acid (TCA) cycle. Polysaccharides must be degraded by microbial enzymes such as α- or β-amylase or β-glycosidase before being absorbed [19]. Our results showed that β-glucosidase activity (Figure 4E) was higher in the RK_BA groups than in the RK_AO groups, whereas sugar (Figure 3), sugar alcohol (Figure 3), and α-amylase activities (Figure 4D) were higher in the RK_AO groups. β-Glucosidase liberates glucose by cleaving glucosidic linkages between oligosaccharides and flavonoid glycosides [20]. α-Amylase decomposes rice starch into disaccharides and oligosaccharides, which helps in the production of monosaccharides and sugar alcohols in carbohydrate microbial metabolism [21]. A. oryzae releases various sugar metabolism-related enzymes in addition to β-glucosidase and α-amylase. In the case of xylose metabolism, the xylitol content was far higher in RK_AO than in RK_BA. Fernandes et al. [22] studied pentose metabolism and reported that fungi convert xylose and arabinose to xylitol and arabitol through NADPH-consuming reactions, while bacteria do not transform xylose to xylitol. Other sugar alcohols such as sorbitol and erythritol are also converted from monosaccharides by NADPH-dependent aldose reductase, which is mainly detected in fungi [23,24,25]. The cooperation of enzymes such as amylolytic enzymes and reductases provides microbes, particularly A. oryzae, with the ability to vigorously metabolize carbohydrates.
3.2. Organic Acids
In carbohydrate metabolism, sugar reduction causes organic acid production during fermentation, influencing the acidity of the culture environment and its habitability for microbes [26]. The filamentous fungi produce various organic acids, especially citric acid and gluconic acid [27,28]. Seven organic acids, including TCA cycle intermediates, had higher relative contents in RK_AO than in RK_BA (Figure 3). RK_AO samples exhibited a lower pH than RK_BA samples, because of their heightened organic acid content (supplementary materials Figure S2). This result is consistent with a previous report that the acidity of gochujang made with Bacillus species-fermented koji was lower than that of gochujang made with A. oryzae-fermented koji [18]. A. oryzae tolerates acidic conditions, showing a broad acceptable pH range between 3 and 7, although low pH conditions may inhibit microbial growth [29]. Lactic acid and shikimic acid are organic acids that were predominantly detected in RK_BA (Figure 2). Lactic acid is produced naturally during fermentation by pyruvate metabolism (Figure 3). Lactate dehydrogenase catalyzes the reciprocal conversion of pyruvate and lactic acid; high lactate dehydrogenase activity was demonstrated in Bacillus species, but not in A. oryzae [30]. Lactic acid production by fungus is difficult because near neutral pH conditions must be maintained and the production of ethanol and fumaric acid interrupt the process [31]. In microbial metabolism, shikimic acid is produced via several steps from phosphoenolpyruvate and erythrose 4-phosphate, precursors derived from glycolysis [32]. Shikimic acid is linked to the biosynthesis of AAA such as tryptophan, tyrosine, and phenylalanine. The abundances of AAA were also higher in RK_BA samples than in RK_AO samples (Figure 3).
3.3. Amino Acids
Amino acids serve as the N source for fermenter microorganisms, and the composition and content in the fermented product vary depending on the activity of microbial proteolytic and biosynthetic enzymes such as protease, aminopeptidase, and aminotransferase [33]. Although protease activity was significantly higher in RK_BA samples than in RK_AO samples (Figure 4), and the levels of the larger number amino acids were elevated in RK_BA than in RK_AO (Figure 2), some amino acids were more abundant in RK_AO than in RK_BA. AAA and BCAA exhibited high relative abundances in RK_BA, while five amino acids related to the serine pathway and γ-aminobutyric acid (GABA) showed elevated levels in RK_AO (Figure 3). This result is consistent with previous reports that fermented foods produced using Bacillus spp. have higher AAA and BCAA content than foods fermented by Aspergillus spp. [1,34]. Acetolactate synthase and branched chain aminotransferase catalyze the synthesis of BBCA from pyruvate [35,36], and AAA were synthesized from chorismic acid via 3–6 enzymatic reactions in the shikimic acid pathway [37]. We considered that these pathways and enzymes may be involved in rice koji production by B. amyloliquefaciens.
In glycine, serine, and threonine metabolism, glycine has a reversible relationship with serine by serine-hydroxymethyltransferase and with threonine by threonine-aldolase, both enzymes that are expressed in microorganisms [38]. Aspartic acid is converted to alanine directly by aspartate 4-decarboxylase and to threonine through four enzymatic reaction [39]. GABA is a bioactive compound in rice that is produced from glutamic acid by glutamate decarboxylase (GAD) [40]. The GAD-encoding gene has been cloned in A. oryzae, and GAD purified from A. oryzae was shown to have high activity [41]; however, Bacillus species lack a GAD-related gene, and as such, have relatively weaker capacities to produce GABA [42].
3.4. Lipid Metabolism
Phospholipids composing plant cell membranes are degraded to lysophospholipids and fatty acids by the lipolytic enzyme phospholipase A, which is secreted by the inoculated microbes [43]. Fatty acids were shown to increase with decomposing triacylglycerol in rice koji fermentation [44]. LysoPC/PEs represented higher relative contents in RK_BA; however, fatty acids were higher in RK_AO (Figure 3). Kum et al. [4] reported that long-chain fatty acids were increased in the later stages of rice koji-doenjang fermentation by Aspergillus species and were strongly correlated with lipase activity. Although lipolytic enzymes are expressed in both A. oryzae and B. amyloliquefaciens, the microbial lipid metabolites differed between the rice koji prepared with the two organisms.
3.5. Phenolic Compounds
Fermentation increases the abundance of bioactive phenolic compounds [5,45]. The TFC values were higher in RK_BA than in RK_AO, whereas TPC was slightly more abundant in RK_AO than in RK_BA (Figure 4B,C). Metabolite profiling with the correlation assay confirmed that the detected metabolites contributed to these total contents. With this assay, we detected phenolic acids such as ferulic acid and 4-hydroxybenzoic acid, as well as flavonoids such as two apigenin glycosides, two chrysoeriol glycosides, two tricin glycosides, and tricin.
Phenolic acids such as ferulic acid exist in the bound form in cell wall polysaccharides and are released by feruloyl esterase [46]. Although feruloyl esterase is a common microbial enzyme, it is most commonly secreted by fungi rather than bacteria [47]. Glycosidic flavonoids in cell vacuoles are subjected to reactions such as glycosylation, de-glycosylation, methylation, glucuronidation, and sulfate conjugation [48]. Flavonoid glycosylation is accomplished by glycosyltransferases, whose genes are expressed in B. cereus, B. licheniformis, Streptomyces, and Xanthomonas campestris (e.g., BcGT-1, DSM-13, YjiC, OleD, and XcGT-2). Tricin is considered a deglycosylated metabolite of microbial β-glucosidase from rice koji. β-Glucosidase hydrolyzes flavonoid glycosides into their corresponding aglycons [45]. We found that flavonoid aglycons and glycosides increased with fermentation time, which may have increased the antioxidative effects (Figure 4A). Flavonoids, particularly aglycon, are potential antioxidants due to their redox properties [49].
3.6. Siderophores
Siderophores are iron-chelating compounds secreted by microbes. Bacillibactin is a siderophore secreted by B. amyloliquefaciens to acquire iron [50]. We observed bacillibactin in RK_BA samples but not in any other samples. The dhb gene clusters of Bacillus species encode multi-enzyme metabolic networks that include isochorismatase, isochorismatase synthase, and 2,3-dihydroxybenzoate-AMP ligase; these networks produce bacillibactin derived from chorismic acid [51]. Kojic acid, which is produced from glucose by Aspergillus species, also contains a siderophore structure [5,52]. Relative contents of kojic acid were higher in RK_AO than in RK_BA.
4. Materials and Methods
4.1. Chemicals and Reagents
Water, methanol, and acetonitrile were purchased from Fisher Scientific (Pittsburgh, PA, USA). Dipotassium hydrogen phosphate, potassium dihydrogen phosphate, sodium chloride, diethylene glycol, and sodium carbonate were purchased from Junsei Chemical Co., Ltd. (Tokyo, Japan). Trichloroacetic acid was purchased from Merck Millipore Co. (Darmstadt, Germany). Methoxyamine hydrochloride, pyridine, N-methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA), potassium persulfate, 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), Folin-Ciocalteu’s phenol, soluble starch, potassium sodium tartrate tetrahydrate, 3,5-dinitrosalicylic acid, sodium hydroxide, acetic acid, sodium acetate, and the standards 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), gallic acid, naringin, maltose, tyrosine, and p-nitro-phenol were obtained from Sigma-Aldrich (St. Louis, MO, USA).
4.2. Inoculum and Rice Koji Fermentation
A. oryzae KCCM 11300P and B. amyloliquefaciens KCCM 11718P were used for fermentation of rice. To make rice koji, 1 kg of rice was submerged in water for 30 min, and the water was drained off. The soaked rice was sterilized for 15 min using an autoclave. Steamed rice (50 g) was inoculated with the fungal strain A. oryzae, and incubated at 35 °C for 5 days. Then, the cultured rice was mixed with steam rice again (0.2%, w/w), and fermented at 37 °C for 36 h. The bacterial strain B. amyloliquefaciens was grown in 200 mL of nutrient broth (pH 7.0) at 37 °C with shaking at 200 rpm for 24 h in a 500 mL flask. The cultured broth was also mixed with steamed rice (2.0%, v/w) and fermented at 37 °C for 36 h. Fermented samples were obtained at 12 h intervals and stored at −20 °C before analysis.
4.3. Sample Preparation for Metabolite Profiling
The rice koji was dried using a freeze dryer and ground using a mortar. Each sample powder (3 g) was extracted with 30 mL of 80% aqueous methanol by sonication for 10 min, and then shaken at 200 rpm for 24 h. Next, the sample mixtures were centrifuged at 5000 rpm for 10 min at 4 °C. The supernatants were filtered using Millex®GP 0.22 μm filters (Merck Millipore, Billerica, MA, USA) and dried in a speed vacuum concentrator (Biotron, Seoul, Korea). The extraction yield from each sample was calculated. For the GC-TOF-MS analysis, norvaline was added as an internal standard, and the sample mixture was derivatized. One hundred microliters of methoxyamine hydrochloride (20 mg/mL in pyridine) was added to each dried sample, and the samples were heated at 30 °C for 90 min. Next, 100 μL of the derivatization agent MSTFA was added to each sample, and the derivatization reactions were heated at 37 °C for 30 min. For UHPLC-LTQ-IT-MS/MS, formononetin was added as an internal standard. The dried samples were dissolved in 200 μL of 80% aqueous methanol, and filtered using 0.2 μm polytetrafluoroethylene (PTFE) filters.
4.4. GC-TOF-MS Analysis
The gas chromatography time-of-flight mass spectrometry analysis was performed using an Agilent 7890 A gas chromatograph, a Pegasus HT TOF-MS (Leco Corporation, St. Joseph, MI, USA), and an Agilent 7693 autosampler (Agilent, Atlanta, GA, USA). We used an RTx-5MS GC column (30 m length × 0.25 mm i.d. × 0.25 μm film thickness, J & W Scientific, Folsom, CA, USA), with the carrier gas helium at a constant flow rate of 1.5 mL/min. One microliter of each derivatized sample was injected in split mode (5:1). The temperatures of the injector and ion source were 250 °C and 230 °C, respectively. The column temperature was held constant at 75 °C for 2 min, subsequently increased to 300 °C at a rate of 15 °C/min, and finally held constant for 3 min. The MS data acquisition rate was 10 scans/s, with an m/z range of 50–1000. For the GC-TOF-MS analysis, three replicates of each sample were tested.
4.5. UHPLC-LTQ-IT-MS/MS Analysis
Ultrahigh-performance liquid chromatography linear trap quadrupole ion trap tandem mass spectrometry was performed using an LTQ ion trap mass spectrometer equipped with a binary solvent delivery system, RS autosampler, electrospray interface (Thermo Fisher Scientific, San José, CA, USA), DIONEX UltiMate 3000 RS Pump, RS Column Compartment, and RS Diode Array Detector (Dionex Corporation, Sunnyvale, CA, USA). Each injected sample (10 μL) was separated on a Syncronis C18 column (100 mm × 2.1 mm, 1.7 μm particle size; Thermo Scientific) at a flow rate of 0.3 mL/min. The mobile phases consisted of 0.1% formic acid in water (v/v) (Solution A) and 0.1% formic acid in acetonitrile (v/v) (Solution B). The solvent gradient program began with 10% Solution B/90% Solution A for 1 min, followed by a 14-min constant-rate increase to 100% Solution B, followed by 3 min of 100% Solution B, followed by a 1 min constant-rate decrease to 10% Solution B/90% Solution A, and finally 3 min of 10% Solution B/90% Solution A. The total run time was 22 min. The photodiode array detection range was 200–600 nm. Electron spray ionization was performed in the positive and negative ion modes within an m/z range of 100–1000. Other instrument parameters were as follows: capillary temperature, 275 °C; source voltage, ±5 kV; and capillary voltage, 39 V. Triplicate UHPLC-LTQ-IT-MS/MS runs were performed for each sample.
4.6. Data Processing and Multivariate Statistical Analysis
The GC-TOF-MS and UHPLC-LTQ-IT-MS/MS data were converted to netCDF (*.cdf) format using Leco ChromaTOF and Thermo Xcalibur software. The metAlign software package [53] was used to align the netCDF data. After alignment, the resulting peak list was exported to a Microsoft Excel file (.xls) that included the corrected peak retention times (min), mass to charge ratios (m/z), and peak areas. The peak area values were converted according to the extraction yield of each sample and log10 transformed in Excel. Multivariate statistical analyses were performed using SIMCA-P+ 12.0 software (Umetrics, Umea, Sweden) to compare metabolite differences between rice koji fermented with the fungus and rice koji fermented with the bacterium. We performed a principal component analysis (PCA), partial least-squares discriminant analysis (PLS-DA), and orthogonal partial least square discriminant analysis (OPLS-DA). The data sets were auto-scaled (unit variance scaling) and mean-centered in a column-wise fashion. Metabolites with VIP values greater than 0.7 in PLS1 or PLS2 and p-values less than 0.05 were selected for further analysis.
4.7. Determination of Antioxidant Activity and Total Phenolic and Flavonoid Content
To determine the antioxidant activity of samples, we used an ABTS assay, a total phenolic content (TPC) assay, and a total flavonoid content (TFC) assay. All experiments were performed in triplicate.
The ABTS assay was conducted using a method modified from Re et al. [54]. A stock solution was prepared by dissolving 7 mM ABTS in 2.45 mM potassium persulfate solution, incubating the solution in a water bath at 60 °C for 20 min, and storing this solution for 12 h at room temperature. After the last step, this stock solution was dark blue in color. For analysis, the solution was diluted in distilled water to an absorbance of 0.7 ± 0.02 at 750 nm. We used a spectrophotometer to measure absorbance (Spectronic Genesys 6, Thermo Electron, Madison, WI, USA). Each sample (20 μL) was added along with stock ABTS (180 μL) into a well of a 96-well plate. The plate was incubated at room temperature for 6 min in the dark, and the absorbance of each well was measured at 750 nm. Trolox was used as a standard, and the results are presented as the Trolox equivalent antioxidant capacity (TEAC) concentration (mM) per milligram of koji. The standard curves ranged from 0.0156 mM to 0.5 mM.
For the TFC assay, we followed a method outlined by Davis [55] with slight modifications. Twenty microliters of each sample, 20 μL of 1 N NaOH, and 180 μL of 90% diethylene glycol were mixed in a 96-well plate. The mixture was incubated for 60 min at room temperature, and the absorbance was measured at 405 nm using the spectrophotometer. TFC is expressed as the naringin equivalent (NE) concentration (ppm) per milligram of koji. The standard concentration curve ranged from 1.56 to 200 ppm.
The TPC determination followed a method used by Yildirim et al. [56] with slight modifications. Briefly, 20 μL of each sample and 100 μL of 0.2 N Folin-Ciocalteu reagent were added to a 96-well plate and incubated at room temperature for 6 min. Next, 80 μL of 7.5% sodium carbonate (Na2CO3) solution was added to the mixture, reacted for 60 min at room temperature, and evaluated at 750 nm. The results are presented as gallic acid equivalent (GE) concentrations (ppm) per milligram of koji in a standard concentration range of 3.91–500 ppm.
4.8. Determination of Enzymatic Activities
Enzyme activity assays were conducted for α-amylase, protease, and β-glycosidase. To determine enzyme activity, each rice koji sample (10 g) was extracted with 90 mL of distilled water by shaking at 120 rpm and 30 °C for 1 h. The mixture was centrifuged at 5000 rpm and 4 °C for 5 min. Next, the supernatants were filtered using 0.2 μm PTFE filters.
To assess α-amylase activity, 1 mL of 1% soluble starch solution in 20 mM sodium phosphate buffer with 6.7 mM sodium chloride (pH 6.9) was added to the extracted enzyme solution. The mixture was incubated at 55 °C for 10 min. Next, each sample was mixed with a color reagent solution (96 mM 3,5-dinitrosalicylic acid solution added to sodium potassium tartrate solution) and boiled at 100 °C for 15 min. The heated samples were cooled on ice and diluted with 9 mL of distilled water. The absorbance of each sample was read at 540 nm. One unit of α-amylase activity was defined as the quantity of α-amylase that induced a change of 1 mg of maltose in a 1% soluble starch solution in 1 min [8].
Protease activity was assayed using a modification on the method described by Kum et al. [4]. One milliliter of the extracted enzyme solution was mixed with 5 mL of 0.6% casein solution, dissolved in 0.1 M phosphate buffer (pH 7), and reacted at 37 °C for 10 min. After 10 min, the reaction was stopped by adding 5 mL of 0.4 M trichloroacetic acid and incubating at 37 °C for 30 min. The precipitate was filtered with a 0.2 μm PTFE filter. Next, 5 mL of 0.4 M sodium carbonate and 1 mL of thrice-diluted 2 N folin reagent were added to 2 mL of filtrate. The mixture was incubated at 37 °C for 30 min, and its absorbance measured at 660 nm. One unit of protease activity was defined as the amount of protease required to release 1 μg of tyrosine per minute from 0.6% casein under corresponding conditions.
The β-glucosidase activity assay followed the method of Zhang et al. [20], with slight modifications. Nine millimolar p-nitrophenol β-d-glucopyranoside (p-NPG) in 0.1 M sodium acetate buffer (pH 4.6) was prepared as a substrate solution. One milliliter of extracted enzyme sample was added to 1 mL of substrate solution and 8 mL of sodium acetate buffer, and then reacted at 37 °C for 30 min. After 30 min, 10 mL of 0.4 M sodium carbonate was added to stop the reaction, and the absorbance was measured at 400 nm. One unit of β-glucosidase activity refers to the quantity of β-glucosidase needed to liberate 1 nmol p-nitrophenol from p-NPG in 1 min under the specified conditions. All experiments were performed in triplicate.
5. Conclusions
In conclusion, we investigated the fermentative behavior of A. oryzae (a fungus) and B. amyloliquefaciens (a bacterium) over different fermentation times. The rice koji samples exhibited distinct metabolites and enzymatic activities based on fermentation duration and fermenter species. The RK_AO groups had relatively high contents of carbohydrate metabolism intermediates such as sugars and sugar alcohols, organic acids, and phenolic acids and lipid metabolism intermediate, fatty acids. The RK_BA groups had relatively high abundances of flavonoids, lysophospholipids, and amino acids, especially AAA and BCAA. The existence and expression levels of certain genes affected the metabolisms in these two microorganisms. The heightened flavonoid content of RK_BA led to higher antioxidant activity in these samples relative to that of RK_AO. These metabolic, enzymatic, and bioactivity characteristics of rice koji may be used to optimize choices of fermenter species and fermentation durations to enhance the quality of fermented food.
Acknowledgments
This work was supported by the National Research Foundation of Korea(NRF) grant funded by the Korea government(MSIP) (No. NRF-2014R1A2A1A11050884), and by a grant from the Next-Generation BioGreen 21 Program (grant No. PJ01109403), Rural Development Administration, Republic of Korea.
Supplementary Materials
The following are available online at http://www.mdpi.com/1420-3049/21/6/773/s1, Figure S1: Partial least square-discriminate analysis (PLS-DA) score plot for rice koji fermented with A. oryzae (RK_AO) or B. amlyoliquefaciens (RK_BA) during fermentation times obtained from GC-TOF-MS (a) and UHPLC-LTQ-IT-MS/MS (b), Figure S2: Comparison of pH and total acidity of rice koji fermented with A. oryzae (RK_AO, closed circle) or B. amlyoliquefaciens (RK_BA, open circle) during fermentation times, Table S1: Discriminative metabolites and their relative contents in rice koji fermented with A. oryzae (RK_AO) or B. amyloliquefaciens (RK_BA) during fermentation using GC-TOF-MS, Table S2: Discriminative metabolites and their relative contents in rice koji fermented with A. oryzae (RK_AO) or B. amyloliquefaciens (RK_BA) during fermentation using UHPLC-LTQ-IT-MS/MS.
Author Contributions
C.H.L. conceived and designed the experiments; D.E.L. performed the experiment and analyzed the data; D.E.L. and S.L. conducted the data interpretation. E.S.J., H.W.S. and B.S.M. participated in design for experiments and sample preparation. D.E.L. wrote the paper. Authorship must be limited to those who have contributed substantially to the work reported. All authors approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Tamang J.P. Health Benefits of Fermented Foods and Beverages. CRC Press; New York, NY, USA: 2015. [Google Scholar]
- 2.Blandino A., Al-Aseeri M.E., Pandiella S.S., Cantero D., Webb C. Cereal-based fermented foods and beverages. Food Res. Int. 2003;36:527–543. doi: 10.1016/S0963-9969(03)00009-7. [DOI] [Google Scholar]
- 3.Shin H.W., Jang E.S., Moon B.S., Lee J.J., Lee D.E., Lee C.H., Shin C.S. Anti-obesity effects of gochujang products prepared using rice koji and soybean meju in rats. J. Food Sci. Technol. 2016;53:1004–1013. doi: 10.1007/s13197-015-2162-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kum S.J., Yang S.O., Lee S.M., Chang P.S., Choi Y.H., Lee J.J., Hurh B.S., Kim Y.S. Effects of Aspergillus species inoculation and their enzymatic activities on the formation of volatile components in fermented soybean paste (doenjang) J. Agric. Food Chem. 2015;63:1401–1418. doi: 10.1021/jf5056002. [DOI] [PubMed] [Google Scholar]
- 5.Kim A.J., Choi J.N., Kim J., Yeo S.H., Choi J.H., Lee C.H. Metabolomics-based optimal koji fermentation for tyrosinase inhibition supplemented with Astragalus Radix. Biosci. Biotechnol. Biochem. 2012;76:863–869. doi: 10.1271/bbb.110171. [DOI] [PubMed] [Google Scholar]
- 6.Onuma K., Kanda Y., Suzuki Ikeda S., Sakaki R., Nonomura T., Kobayashi M., Osaki M., Shikanai M., Kobayashi H., Okada F. Fermented brown rice and rice bran with aspergillus oryzae (FBRA) prevents inflammation-related carcinogenesis in mice, through inhibition of inflammatory cell infiltration. Nutrients. 2015;7:10237–10250. doi: 10.3390/nu7125531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim A.J., Choi J.N., Kim J., Park S.B., Yeo S.H., Choi J.H., Lee C.H. GC-MS based metabolite profiling of rice koji fermentation by various fungi. Biosci. Biotechnol. Biochem. 2010;74:2267–2272. doi: 10.1271/bbb.100488. [DOI] [PubMed] [Google Scholar]
- 8.Bechman A., Phillips R.D., Chen J. Changes in selected physical property and enzyme activity of rice and barley koji during fermentation and storage. J. Food Sci. 2012;77:M318–M322. doi: 10.1111/j.1750-3841.2012.02691.x. [DOI] [PubMed] [Google Scholar]
- 9.Sugiyama S.I. Selection of micro-organisms for use in the fermentation of soy sauce. Food Microbiol. 1984;1:339–347. doi: 10.1016/0740-0020(84)90067-4. [DOI] [Google Scholar]
- 10.Syed R., Roja Rani S., Masoodi T.A., Shafi G., Alharbi K. Functional analysis and structure determination of alkaline protease from Aspergillus flavus. Bioinformation. 2012;8:175–180. doi: 10.6026/97320630008175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juan M.Y., Wu C.H., Chou C.C. Fermentation with Bacillus spp. as a bioprocess to enhance anthocyanin content, the angiotensin converting enzyme inhibitory effect, and the reducing activity of black soybeans. Food Microbiol. 2010;27:918–923. doi: 10.1016/j.fm.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Teng D., Gao M., Yang Y., Liu B., Tian Z., Wang J. Bio-modification of soybean meal with Bacillus subtilis or Aspergillus oryzae. Biocatal. Agric. Biotechnol. 2012;1:32–38. doi: 10.1016/j.bcab.2011.08.005. [DOI] [Google Scholar]
- 13.Wishart D.S. Metabolomics: Applications to food science and nutrition research. Trends Food Sci. Technol. 2008;19:482–493. doi: 10.1016/j.tifs.2008.03.003. [DOI] [Google Scholar]
- 14.Lee S., Oh D.G., Lee S., Kim G.R., Lee J.S., Son Y.K., Bae C.H., Yeo J., Lee C.H. Chemotaxonomic metabolite profiling of 62 Indigenous plant species and its correlation with bioactivities. Molecules. 2015;20:19719–19734. doi: 10.3390/molecules201119652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee M.Y., Son G.H. Liquid chromatography-mass spectrometry-based chemotaxonomic classification of Aspergillus spp. and evaluation of the biological activity of its unique metabolite, neosartorin. J. Microbiol. Biotechnol. 2013;23:932–941. doi: 10.4014/jmb.1212.12068. [DOI] [PubMed] [Google Scholar]
- 16.Lv X.C., Huang Z.Q., Zhang W., Rao P.F., Ni L. Identification and characterization of filamentous fungi isolated from fermentation starters for Hong Qu glutinous rice wine brewing. J. Gen. Appl. Microbiol. 2012;58:33–42. doi: 10.2323/jgam.58.33. [DOI] [PubMed] [Google Scholar]
- 17.Chutmanop J., Chuichulcherm S., Chisti Y., Srinophakun P. Protease production by Aspergillus oryzae in solid-state fermentation using agroindustrial substrates. J. Chem. Technol. Biotechnol. 2008;83:1012–1018. doi: 10.1002/jctb.1907. [DOI] [Google Scholar]
- 18.Kim D.H., Choi H.J. Physicochemical properties of kochujang prepared by Bacillus sp. koji. Korean J. Food Sci. Technol. 2003;35:1174–1181. [Google Scholar]
- 19.Gänzle M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014;37:2–10. doi: 10.1016/j.fm.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y.C., Lee J.H., Vodovotz Y., Schwartz S.J. Changes in distribution of isoflavones and β-glucosidase activity during soy bread proofing and baking. Cereal. Chem. 2004;81:741–745. doi: 10.1094/CCHEM.2004.81.6.741. [DOI] [Google Scholar]
- 21.Baek J.G., Shim S.M., Kwon D.Y., Choi H.K., Lee C.H., Kim Y.S. Metabolite profiling of Cheonggukjang, a fermented soybean paste, inoculated with various Bacillus strains during fermentation. Biosci. Biotechnol. Biochem. 2010;74:1860–1868. doi: 10.1271/bbb.100269. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes S., Murray P.G. Metabolic engineering for improved microbial pentose fermentation. Bioeng. Bugs. 2010;1:424–428. doi: 10.4161/bbug.1.6.12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrash J.M. All in the family: Aldose reductase and closely related aldo-keto reductases. Cell Mol. Life Sci. 2004;61:737–749. doi: 10.1007/s00018-003-3402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seiboth B., Gamauf C., Pail M., Hartl L., Kubicek C.P. The d-xylose reductase of Hypocrea jecorina is the major aldose reductase in pentose and d-galactose catabolism and necessary for beta-galactosidase and cellulase induction by lactose. Mol. Microbiol. 2007;66:890–900. doi: 10.1111/j.1365-2958.2007.05953.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee J.K., Kim S.Y., Ryu Y.W., Seo J.H., Kim J.H. Purification and characterization of a novel erythrose reductase from Candida magnoliae. Appl. Environ. Microbiol. 2003;69:3710–3718. doi: 10.1128/AEM.69.7.3710-3718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponnusamy K., Lee S., Lee C.H. Time-dependent correlation of the microbial community and the metabolomics of traditional barley nuruk starter fermentation. Biosci. Biotechnol. Biochem. 2013;77:683–690. doi: 10.1271/bbb.120665. [DOI] [PubMed] [Google Scholar]
- 27.Song H., Lee S.Y. Production of succinic acid by bacterial fermentation. Enzyme Microb. Technol. 2006;39:352–361. doi: 10.1016/j.enzmictec.2005.11.043. [DOI] [Google Scholar]
- 28.Magnuson J.K., Lasure L.L. Advances in Fungal Biotechnology for Industry, Agriculture, and Medicine. Springer; New York, NY, USA: 2004. Organic acid production by filamentous fungi; pp. 307–340. [Google Scholar]
- 29.Carlsen M., Spohr A.B., Nielsen J., Villadsen J. Morphology and physiology of an α-amylase producing strain of Aspergillus oryzae during batch cultivations. Biotechnol. Bioeng. 1996;49:266–276. doi: 10.1002/(SICI)1097-0290(19960205)49:3<266::AID-BIT4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Dennis D., Kaplan N.O. d-and l-lactic acid dehydrogenases in Lactobacillus plantarum. J. Biol. Chem. 1960;235:810–818. [PubMed] [Google Scholar]
- 31.Dave K.K., Punekar N.S. Expression of Lactate Dehydrogenase in Aspergillus niger for l-Lactic Acid Production. PLoS ONE. 2015;10:e0145459. doi: 10.1371/journal.pone.0145459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Licona-Cassani C., Lara A.R., Cabrera-Valladares N., Escalante A., Hernandez-Chavez G., Martinez A., Bolivar F., Gosset G. Inactivation of pyruvate kinase or the phosphoenolpyruvate: Sugar phosphotransferase system increases shikimic and dehydroshikimic acid yields from glucose in Bacillus subtilis. J. Mol. Microbiol. Biotechnol. 2013;24:37–45. doi: 10.1159/000355264. [DOI] [PubMed] [Google Scholar]
- 33.Ko B.K., Ahn H.J., van den Berg F., Lee C.H., Hong Y.S. Metabolomic insight into soy sauce through 1H NMR spectroscopy. J. Agric. Food Chem. 2009;57:6862–6870. doi: 10.1021/jf901454j. [DOI] [PubMed] [Google Scholar]
- 34.Ham S.S., Choi K.K., Cui C.B., Lee B.G., Joo D.S., Lee D.S. Quality characteristics of soy sauce fermented by Bacillus licheniformis NH20 isolated from traditional meju and Aspergillus oryzae. Food Sci. Biotechnol. 2004;13:537–543. [Google Scholar]
- 35.Lee J.E., Hwang G.S., Lee C.H., Hong Y.S. Metabolomics reveals alterations in both primary and secondary metabolites by wine bacteria. J. Agric. Food Chem. 2009;57:10772–10783. doi: 10.1021/jf9028442. [DOI] [PubMed] [Google Scholar]
- 36.Hutson S. Structure and function of branched chain aminotransferases. Prog. Nucleic Acid Res. Mol. Biol. 2001;70:175–206. doi: 10.1016/s0079-6603(01)70017-7. [DOI] [PubMed] [Google Scholar]
- 37.Maeda H., Dudareva N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012;63:73–105. doi: 10.1146/annurev-arplant-042811-105439. [DOI] [PubMed] [Google Scholar]
- 38.Woldman Y., Appling D.R. A general method for determining the contribution of split pathways in metabolite production in the yeast Saccharomyces cerevisiae. Metab. Eng. 2002;4:170–181. doi: 10.1006/mben.2001.0221. [DOI] [PubMed] [Google Scholar]
- 39.Oikawa T. Amino Acid Biosynthesis-Pathways, Regulation and Metabolic Engineering. Springer; Berlin, Germany; Heidelberg, Germany: 2006. Alanine, aspartate, and asparagine metabolism in microorganisms; pp. 273–288. [Google Scholar]
- 40.Moongngarm A., Saetung N. Comparison of chemical compositions and bioactive compounds of germinated rough rice and brown rice. Food Chem. 2010;122:782–788. doi: 10.1016/j.foodchem.2010.03.053. [DOI] [Google Scholar]
- 41.Tsuchiya K., Nishimura K., Iwahara M. Purification and characterization of glutamate decarboxylase from Aspergillus oryzae. Food Sci. Technol. Res. 2003;9:283–287. doi: 10.3136/fstr.9.283. [DOI] [Google Scholar]
- 42.Park K.B., Oh S.H. Enhancement of γ-aminobutyric acid production in Chungkukjang by applying a Bacillus subtilis strain expressing glutamate decarboxylase from Lactobacillus brevis. Biotechnol. Lett. 2006;28:1459–1463. doi: 10.1007/s10529-006-9112-9. [DOI] [PubMed] [Google Scholar]
- 43.Zhan J., Jiang S., Pan L., Zhang Y. Purification, characterization and application of a cold-adapted phospholipase A1 from Bacillus cereus sp. AF-1. Biotechnol. Biotechnol. Equip. 2013;27:3972–3976. doi: 10.5504/BBEQ.2013.0044. [DOI] [Google Scholar]
- 44.Kim J., Choi J.N., John K.M., Kusano M., Oikawa A., Saito K., Lee C.H. GC–TOF-MS-and CE–TOF-MS-based metabolic profiling of cheonggukjang (fast-fermented bean paste) during fermentation and its correlation with metabolic pathways. J. Agric. Food Chem. 2012;60:9746–9753. doi: 10.1021/jf302833y. [DOI] [PubMed] [Google Scholar]
- 45.Liu S.X., Yang H.Y., Li S.Y., Zhang J.Y., Li T., Zhu B.Q., Zhang B.L. Polyphenolic compositions and chromatic characteristics of bog bilberry syrup wines. Molecules. 2015;20:19865–19877. doi: 10.3390/molecules201119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braga C.M.P., Delabona Pda S., Lima D.J., Paixao D.A.A., Pradella J.G., Farinas C.S. Addition of feruloyl esterase and xylanase produced on-site improves sugarcane bagasse hydrolysis. Bioresour. Technol. 2014;170:316–324. doi: 10.1016/j.biortech.2014.07.115. [DOI] [PubMed] [Google Scholar]
- 47.Topakas E., Vafiadi C., Christakopoulos P. Microbial production, characterization and applications of feruloyl esterases. Process Biochem. 2007;42:497–509. doi: 10.1016/j.procbio.2007.01.007. [DOI] [Google Scholar]
- 48.Huynh N.T., van Camp J., Smagghe G., Raes K. Improved release and metabolism of flavonoids by steered fermentation processes: A review. Int. J. Mol. Sci. 2014;15:19369–19388. doi: 10.3390/ijms151119369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rao A.S., Reddy S.G., Babu P.P., Reddy A.R. The antioxidant and antiproliferative activities of methanolic extracts from Njavara rice bran. BMC Complem. Altern. Med. 2010;10:4. doi: 10.1186/1472-6882-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X.H., Koumoutsi A., Scholz R., Schneider K., Vater J., Süssmuth R., Piel J., Borriss R. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J. Biotechnol. 2009;140:27–37. doi: 10.1016/j.jbiotec.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Hertlein G., Müller S., Garcia-Gonzalez E., Poppinga L., Süssmuth R.D., Genersch E. Production of the catechol type siderophore bacillibactin by the honey bee pathogen Paenibacillus larvae. PLoS ONE. 2014;9:e108272. doi: 10.1371/journal.pone.0108272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terabayashi Y., Sano M., Yamane N., Marui J., Tamano K., Sagara J., Dohmoto M., Oda K., Ohshima E., Tachibana K., et al. Identification and characterization of genes responsible for biosynthesis of kojic acid, an industrially important compound from Aspergillus oryzae. Fungal. Genet. Biol. 2010;47:953–961. doi: 10.1016/j.fgb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 53.WAGENINGEN. [(accessed on 2 April 2016)]. Available online: http://www.metalign.nl.
- 54.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 55.Davis W.B. Determination of flavanones in citrus fruits. Anal. Chem. 1947;19:476–478. doi: 10.1021/ac60007a016. [DOI] [Google Scholar]
- 56.Yildirim A., Mavi A., Kara A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.