Abstract
Organic azides are key motifs in compounds of relevance to chemical biology, medicinal chemistry and materials science. In addition, they also serve as useful building blocks due to their remarkable reactivity. Therefore, the development of efficient protocols to synthesize these compounds is of great significance. This paper reviews the major applications and development of azidation in difunctionalization of olefins using azide reagents.
Keywords: azides, difunctionalization, olefins
1. Introduction
The first organic azide, phenyl azide, discovered by Griess in 1864 opened the door for the use of organic azides in synthesis [1]. Since then, numerous elegant approaches have been developed, such as the aza-Wittig reaction [2], Sundberg rearrangement, Curtius rearrangement [3,4], Schmidt rearrangement [5], Hemetsberger rearrangement [6] and click chemistry [7,8,9,10,11,12]. Organic azides are important building blocks and intermediates, not only due to the fact they are potential precursors of N-containing structural motifs [13,14,15,16,17,18,19], but also for their remarkable biological activity in pharmaceutical chemistry [20]. Moreover, organic azides have also received great attention in other fields, such as supramolecular chemistry [21,22,23,24], medicinal chemistry [25], biotechnology and materials science [26].
Olefins are readily available starting materials in organic synthesis. Recently, the difunctionalization of olefins [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] which could introduce two chemical bonds into substrates simultaneously, has attracted considerable attention. Organic azide-driven difunctionalization of olefins has been a hot topic in this area in recent years. Herein, we summarize the recent developments in the use of azidation reactions in the difunctionalization of olefins.
2. The Application of Organic Azides in Difunctionalization of Olefins
2.1. Construction of C-N3 Bond and C-C Bond
In the past decade, the hydroazidation of olefins has been significantly developed [43,44,45,46,47,48], although this kind of addition reaction has not been generally classified as a traditional difunctionalization of olefins. Therefore, we start here from the construction of C-N3 bonds and C-C bonds.
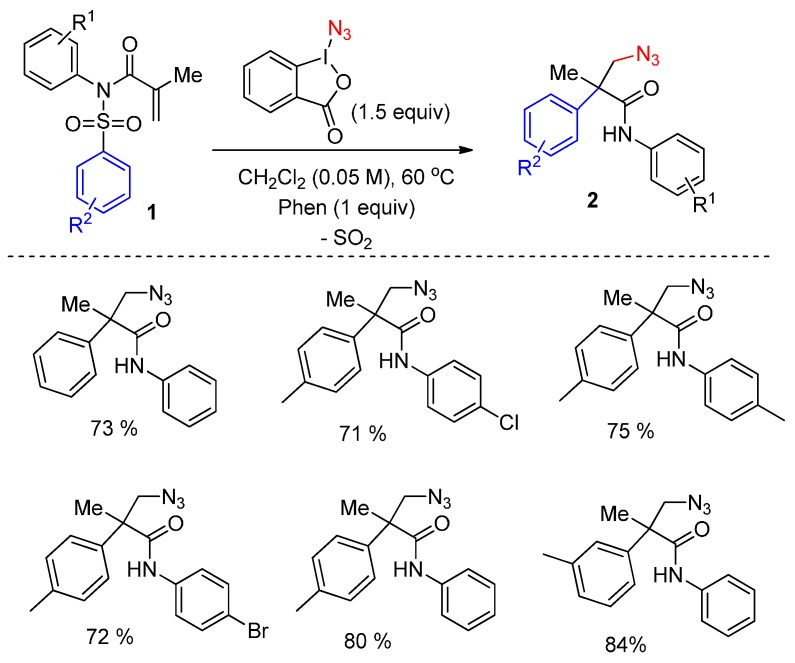
An interesting radical-mediated arylazidation of activated alkenes 1 was reported by Nevado and co-workers (Scheme 1) [49]. This process involves radical addition, Csp2-Csp3 and C-N bond formation, 1,4-aryl migration, and further desulfonylation, producing in good yields the corresponding products 2 bearing a quaternary stereocenter when the substituent on the nitrogen atom is an aryl group.
Scheme 1.
Arylheterofunctionalization of activated alkenes.
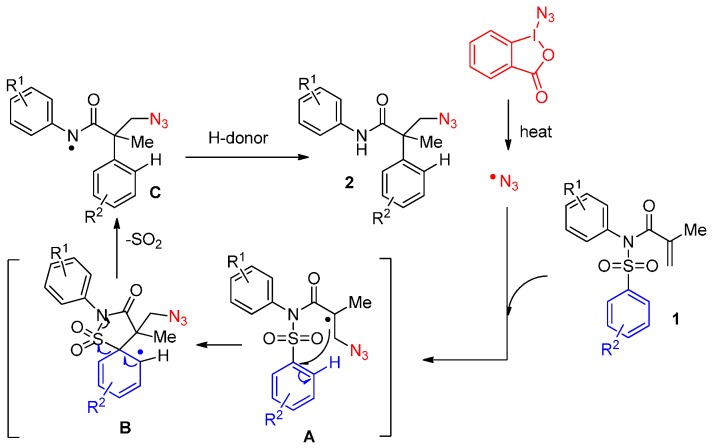
The proposed mechanism is illustrated in Scheme 2. In the first step, the generated azido radical interacts with the activated alkene to give a new C(sp3)-N bond and an alkyl radical intermediate A. A 5-ipso cyclization then takes place on the aromatic ring generating aryl radical B, which leads to amidyl radical C upon rearomatization with concomitant desulfonylation. The subsequent H-abstraction step produces the desired product 2 (Scheme 2).
Scheme 2.
The proposed mechanism of the arylheterofunctionalization of alkenes.
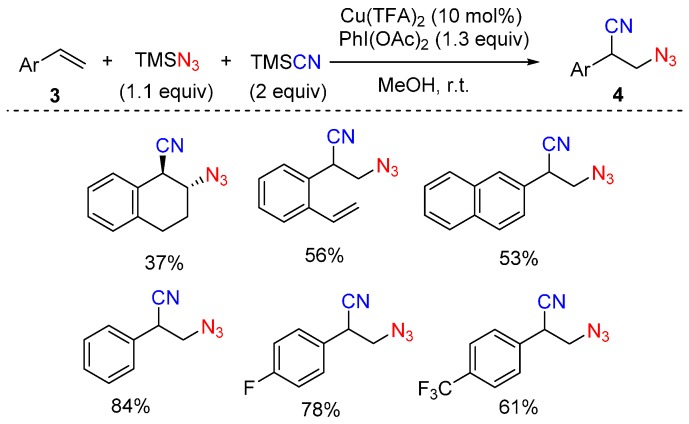
Wang and co-workers reported a copper-catalyzed intermolecular Markovnikov-type azidocyanation reaction of alkenes 3 to construct C-N3 and C-CN bonds simultaneously (Scheme 3) [50], which gives a series of 3-azido-2-arylpropanenitriles 4 in moderate to good yields. These compounds may serve as potential precursors of the corresponding 3-amino-2-arylpropanoic acids. This reaction employs PhI(OAc)2 as oxidant, TMSN3 as N3 source, TMSCN as CN source and it proceeds at room temperature. A mechanistic study revealed that the addition of 2,6-di-tert-butyl-4-methylphenol (BHT) significantly suppressed the reaction, suggesting that a radical process may be involved in this reaction.
Scheme 3.
Azidocyanation of aryl alkenes.
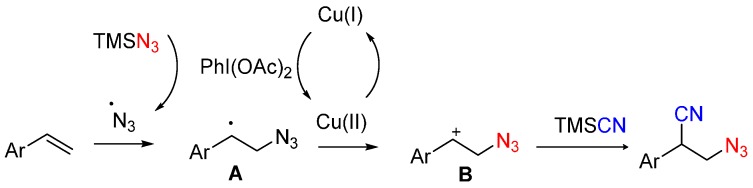
On the basis of the above results, a plausible mechanism is proposed. First, TMSN3 could react with PhI(OAc)2 to generate the N3 radical under the standard reaction conditions. Then the radical addition to alkene gives intermediate A. In the presence of copper(II) catalyst, A could be oxidized to form the intermediate B with a carbocation center, which is trapped by TMSCN to give the final product (Scheme 4).
Scheme 4.
Plausible mechanism of the reaction.
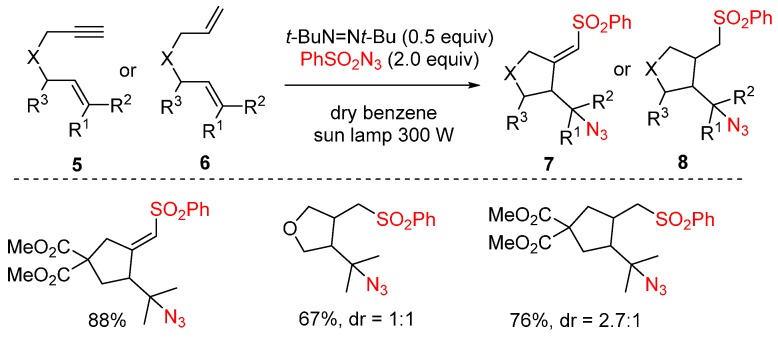
A radical mediated azidosulfonylation of various 1-en-6-ynes 5 or 1,6-dienes 6 that are able to undergo a rapid radical rearrangement was reported by Renaud and co-workers (Scheme 5) [51]. This reaction is initiated by di-tert-butyldiazene upon irradiation with a 300 W sun lamp. Under the standard reaction conditions, the reaction is generally completed within 2–4 h.
Scheme 5.
Azidoarylation of alkenes.
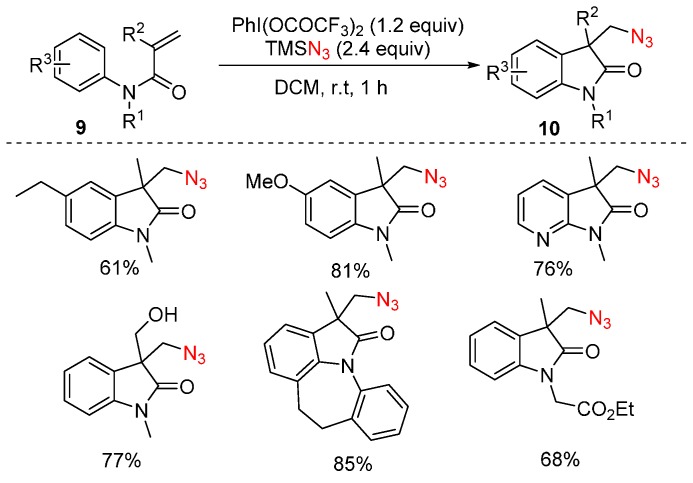
2-Oxindoles not only have unique biological activity, but also are important building blocks in drug design and organic synthesis [52,53]. In order to synthesize these useful intermediates, a rapid approach to oxindoles through C-N3 and C-C bond construction was reported by Antonchick and co-workers [54]. This chemistry proceeds under mild and metal-free conditions, giving biologically interesting 2-oxindoles 10 in good yields (Scheme 6).
Scheme 6.
Azidoarylation of alkenes.
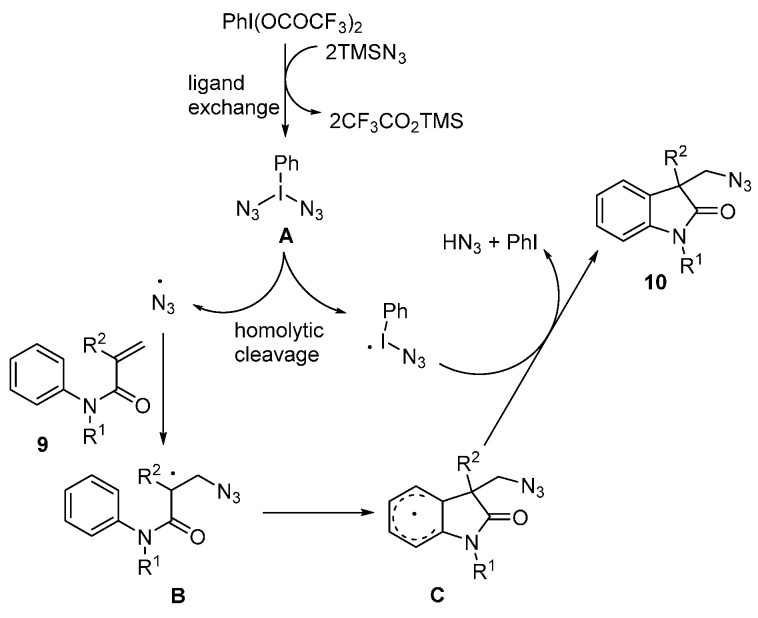
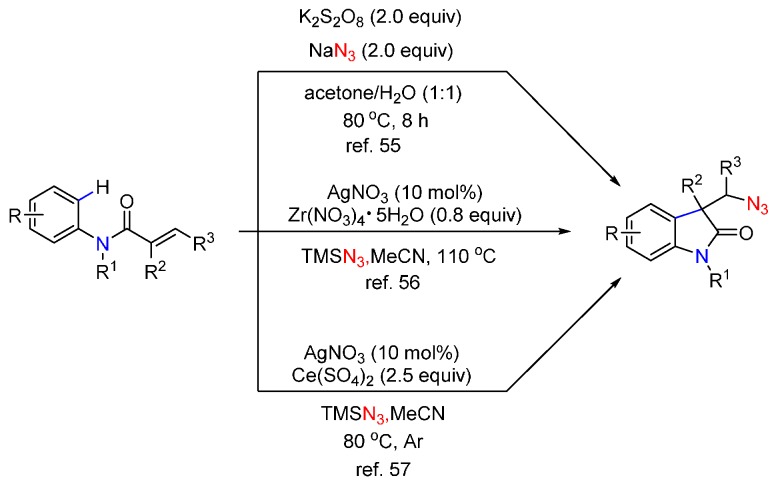
A plausible mechanism for this transformation is illustrated in Scheme 7. This reaction begins with a double ligand exchange between PhI(OCOCF3)2 and TMSN3 to provide intermediate A, which undergoes thermal homolytic cleavage to generate an azido radical. This azido radical attacks alkene 9 to give intermediate B, which is trapped by arene to give intermediate C. Rearomatization of C provides product 10. Similar approaches using different catalysts and oxidants were also reported by the groups of Zhang [55], Yang [56], and Jiao [57] (Scheme 8).
Scheme 7.
Proposed mechanism for the azidoarylation of alkenes.
Scheme 8.
Azidoarylation of alkenes.
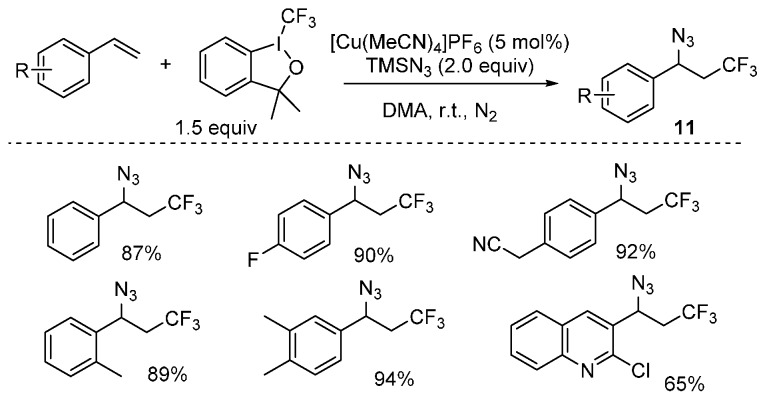
The introduction of a trifluoromethyl group into chemical compounds is an important way to modify their activities and biocompatibilities [58]. In light of their importance in medicinal chemistry, a novel copper-catalyzed intermolecular azidotrifluoromethylation of alkenes has been developed by Liu and co-workers [59]. By using this method, various CF3-containing organoazides 11 were obtained in good yields under mild reaction conditions (Scheme 9). Besides, the resulting products can be readily transformed into other valuable CF3-containing amine compounds.
Scheme 9.
Azidotrifluoromethylation of alkenes.
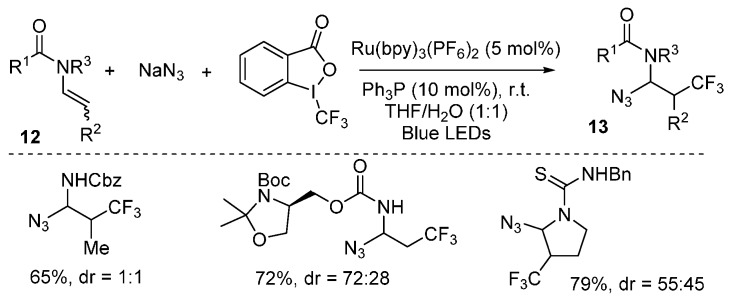
A photoredox-catalyzed azidotrifluoromethylation of enecarbamates 12 was reported by Magnier, Masson and coworkers [60]. This reaction used Tongi’s reagent as CF3 source, and was proposed to follow a radical/cationic pathway. Under the optimized conditions, a wide range of substrates can be readily difunctionalized (Scheme 10).
Scheme 10.
Azidotrifluoromethylation of enecarbamates.
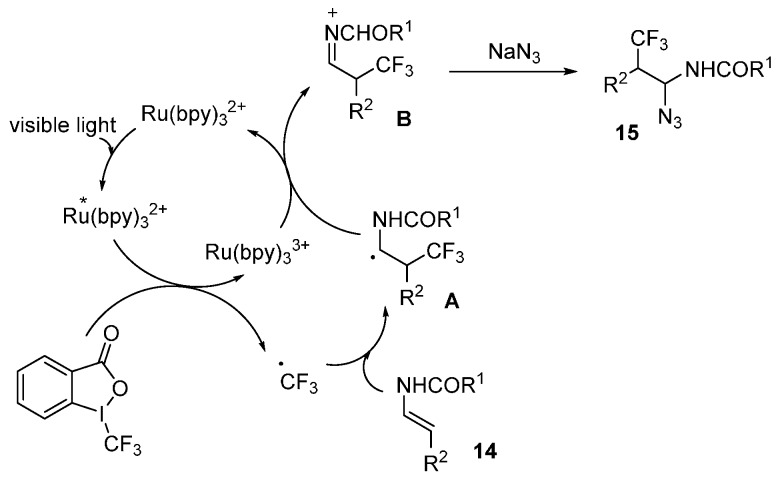
A possible mechanism is shown in Scheme 11. Visible light excites Ru(bpy)32+ into *Ru(bpy)32+, which is a strong reductant species that performs a SET to generate trifluoromethyl radical from Togni’s reagent. Then, addition of trifluoromethyl radical to enecarbamate 14 leads to an α-amido radical intermediate A, which can be oxidized into N-acyliminium cation intermediate B by SET process from Ru(bpy)33+. Final nucleophilic trapping by NaN3 forms the products 15.
Scheme 11.
Proposed mechanism.
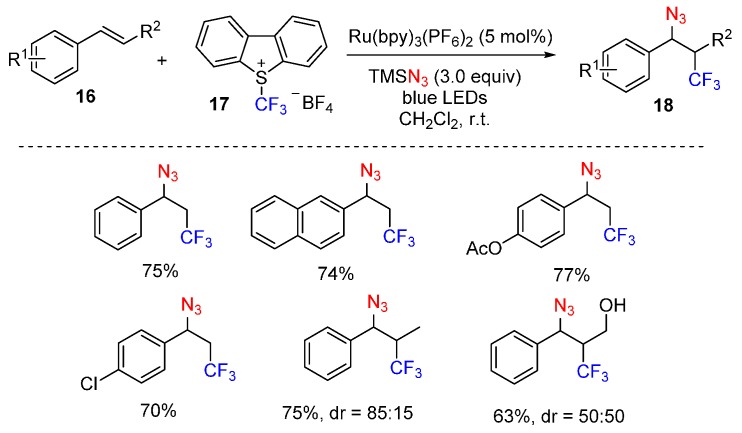
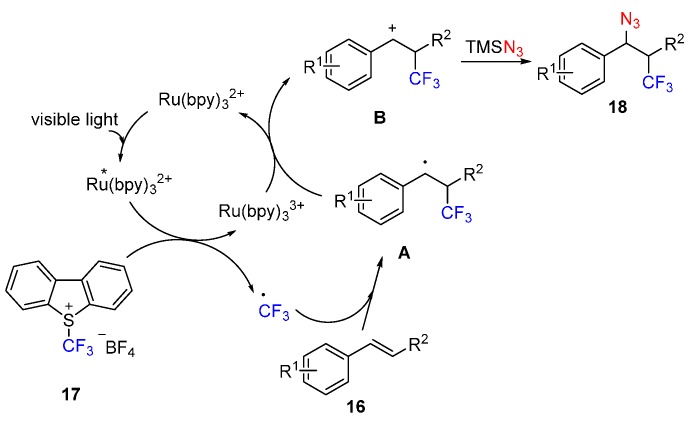
Inspired by their above work, this group also developed a photoredox-catalyzed azidotrifluoromethylation of alkenes 16. Under the optimized conditions, using Umemoto’s reagent as the CF3 source, a wide range of alkenes can be readily difunctionalized (Scheme 12) [61].
Scheme 12.
Azidotrifluoromethylation of alkenes.
A similar mechanism is proposed based on their above work (Scheme 13). Firstly, visible light excites the Ru(bpy)32+ into *Ru(bpy)32+, which performs a SET to generate trifluoromethyl radical from Umemoto’s reagent 17. Then addition of trifluoromethyl radical to alkenes 16 produces carbon radical intermediate A, which can be oxidized into β-trifluoromethylated carbocation intermediate B. Finally, carbocation B is trapped by TMSN3 to form the product 18.
Scheme 13.
Plausible reaction mechanism.
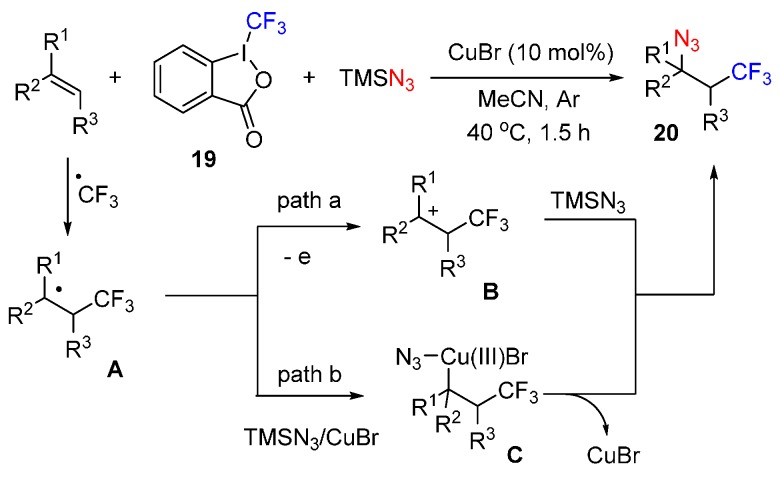
Due to the importance of these compounds, a copper-catalyzed three-component azidotrifluoromethylation of alkenes was designed by Yang (Scheme 14) [62]. This reaction proceeded under mild conditions and gave the corresponding product 20 in good yields. A possible mechanism was also proposed. Togni’s reagent 19 is activated by CuBr, leading to the radical species A by reaction with the alkene. Then, intermediate A is oxidized to intermediate B, which is further trapped by TMSN3 to lead to the product (Path a). Alternatively, intermediate A reacts with TMSN3 and CuBr to afford complex C, further reductive elimination of complex C affords the product 20.
Scheme 14.
Azidotrifluoromethylation of alkenes.
2.2. Construction of C-N3 Bond and C-O Bond
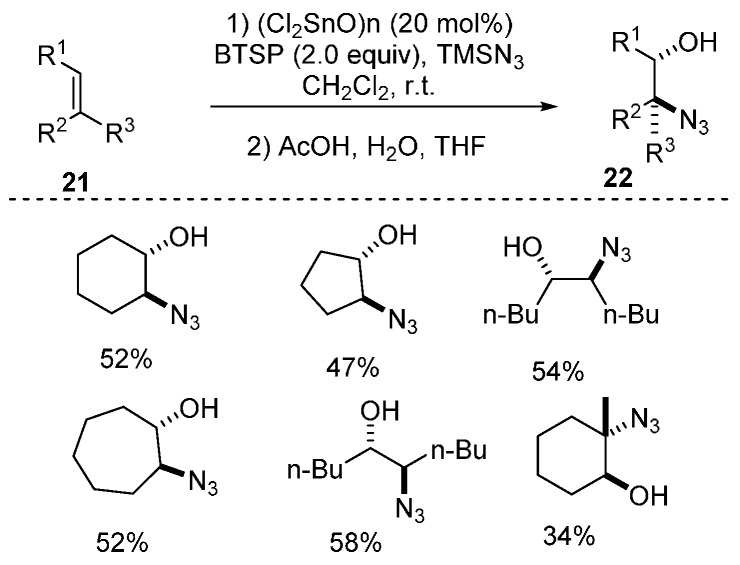
Shibasaki and co-workers reported the first example of (Cl2SnO)n-catalyzed synthesis of trans β-azido alcohols by using readily available alkenes as substrates (Scheme 15) [63].
Scheme 15.
Synthesis of trans β-azido alcohols.
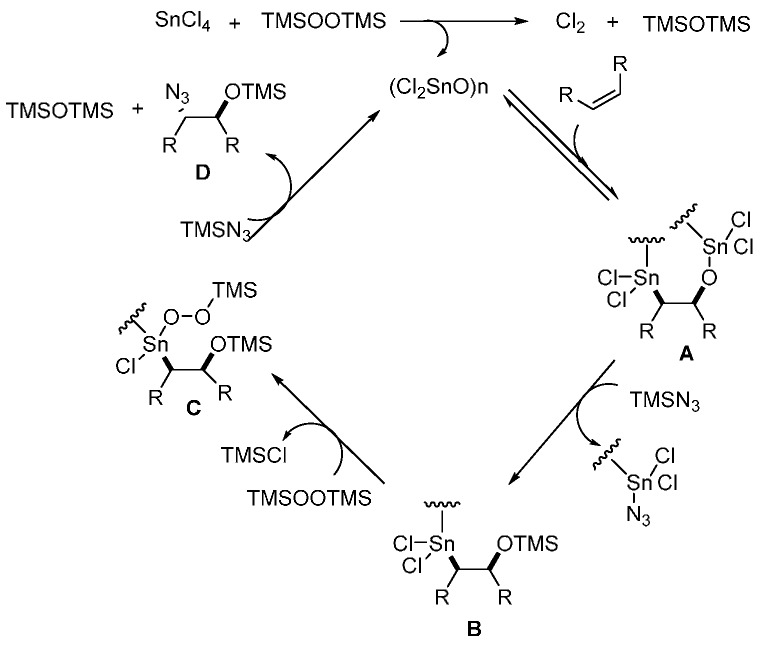
The authors proposed a catalytic cycle that involves the insertion of a C=C double bond to dichlorotin oxide to afford intermediate A, which could react with TMSN3 to afford B. Subsequent nucleophilic attack of BTSP (bis(trimethylsilyl) peroxide) on Sn gives the intermediate C. The regeneration of (Cl2SnO)n by SN2 attack with TMSN3 gives D as the precursor of the product (Scheme 16).
Scheme 16.
Plausible reaction mechanism.
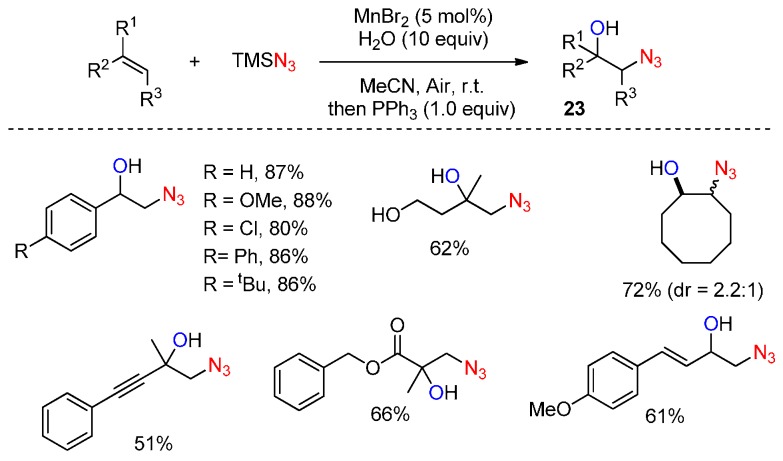
Recently, the Jiao group reported an efficient Mn-catalyzed aerobic oxidative hydroxyazidation of olefins for the synthesis of β-azido alcohols 23 using air as the oxidant (Scheme 17) [64]. This chemistry was notable for its mild reaction conditions, broad substrate scope, and high reaction efficiency. Moreover, the resulting β-azido alcohols are useful precursors of β-amino alcohols, aziridines, and other O- and N-containing heterocyclic compounds.
Scheme 17.
β-Azido alcohol construction.
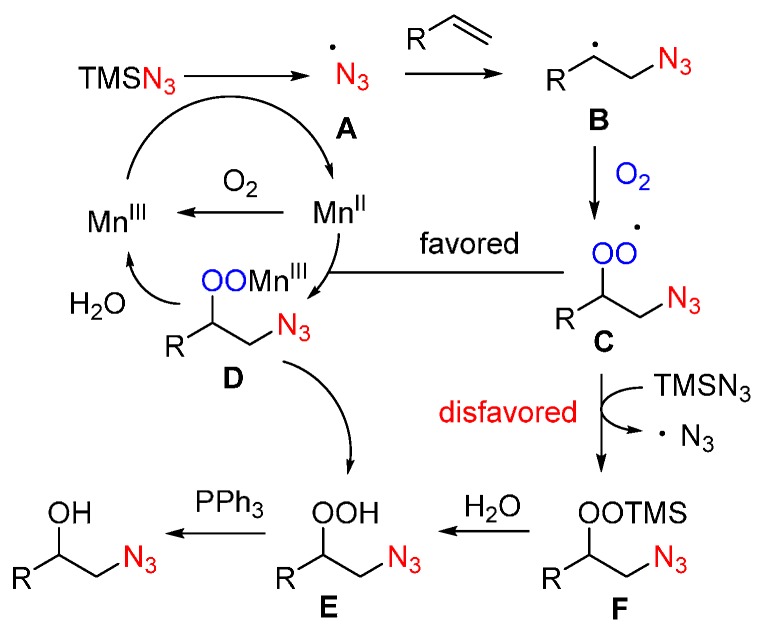
On the basis of the mechanistic study and density functional theory (DFT) calculations, a favored radical process is proposed (Scheme 18). Firstly, the MnII is readily oxidized to MnIII or MnIV by molecule oxygen in the air, then MnIII oxidizes TMSN3 to generate azido radical. MnIV can also oxidize TMSN3 to form the N3 radical A and generate MnIII catalyst. Radical A attacks an alkene at the sterically less hindered position to form the carbon radical B, which is trapped by oxygen to generate the peroxyl radical C. According to the DFT calculations, it is favored for the peroxyl radical C to undergo Mn-participated SET and protonation processes to afford β-azido peroxy alcohols E. In comparison, the pathway through F is disfavored. Finally, β-azido peroxy alcohol E is reduced by PPh3 to form the β-azido alcohol 23.
Scheme 18.
Proposed mechanism.
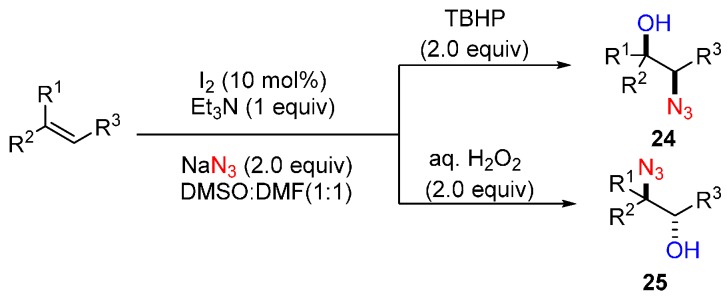
Sudalai and co-workers reported an I2-catalyzed synthesis of 1,2-azidoalcohols by employing NaN3 as N-nuclepohiles and DMF as O-nuclepohiles respectively (Scheme 19) [65]. This approach displayed broad substrate scope and high reaction efficiency with regio- and stereoselectivity. 18O labelling studies proved that DMF served as the O-nucleophile.
Scheme 19.
Synthesis of 1,2-azidoalcohols.
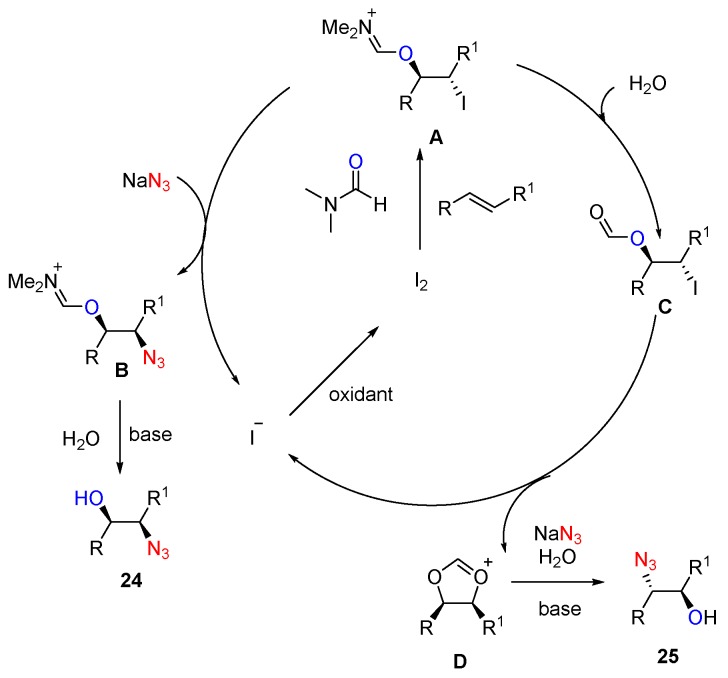
A possible mechanism was proposed (Scheme 20). Firstly, an iodonium ion is formed by the reaction of iodine with alkene, which undergoes subsequent regioselective ring opening with DMF to afford the corresponding iodo intermediate A, followed by stereoselective displacement with NaN3 to give intermediate B. Intermediate B on hydrolysis gives syn azido alcohols 24. Alternatively, under aq. H2O2 conditions, the iodo intermediate A is hydrolyzed in situ to form iodoformate C. The proposed species D is formed from C by the anchimeric assistance from the formate group, it reacts with the azide anion in a regioselective manner to give anti azido alcohols 25 with the liberation of the iodide ion, which is then reoxidized with TBHP/H2O2 to regenerate I2 in the catalytic cycle.
Scheme 20.
Possible mechanism.
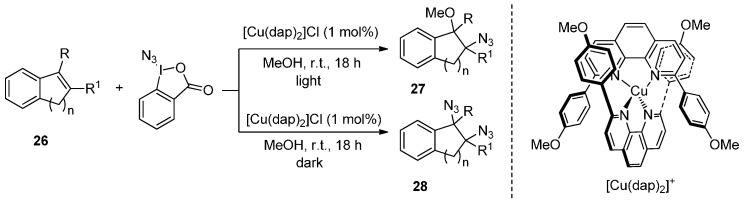
An effective [Cu(dap)2]Cl catalyzed azide addition of styrene-type alkenes 26 using the Zhdankin reagent as N3 source was reported by Greaney and co-workers [66]. This is a light-controlled reaction, and in the presence of light, a photoredox cycle is implicated with polar components such as methanol or bromide adding to a benzylic cation. By contrast, in the absence of light, a double azidation takes place, leading to diazide products. Thus, the degree of azidation can be controlled by switching between light and dark conditions (Scheme 21).
Scheme 21.
Azidation of styrene-type double bonds.
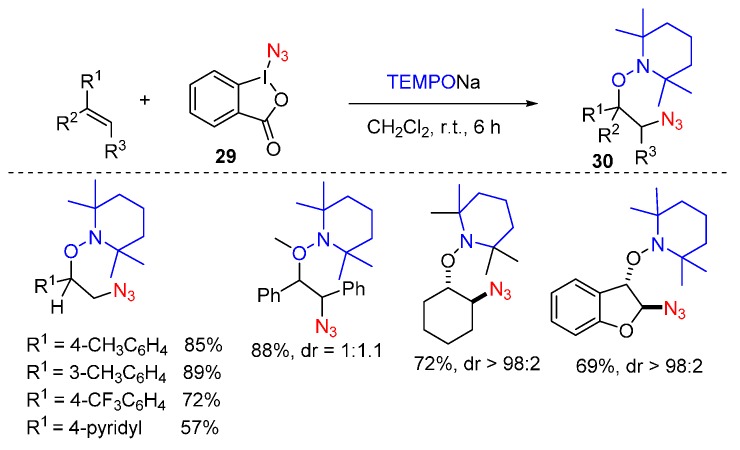
Studer and co-worker achieved oxyazidation reactions of alkenes using sodium TEMPO as O source, reagent 29 as N3 source under mild condition affording product 30 in good yields (Scheme 22) [67]. It is noteworthy that the oxyazidation of cyclic systems proceeded well with excellent diastereoselectivity.
Scheme 22.
Oxyazidation of alkenes.
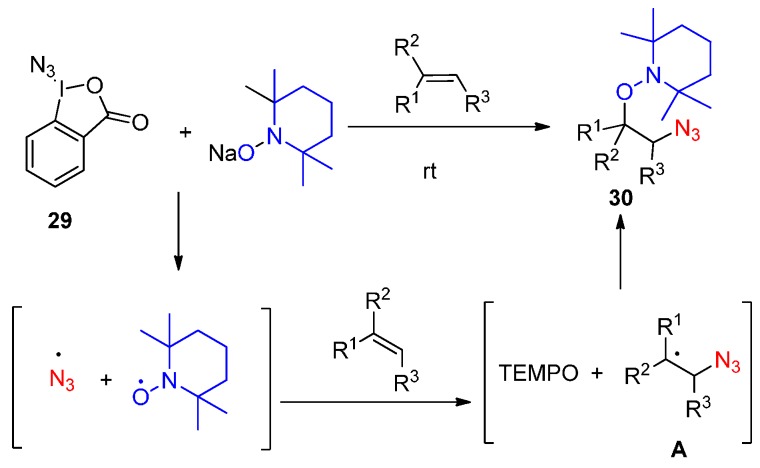
Inspired by their previous work that TEMPONa could reduce CF3-iodine reagent (Togni reagent) to generate CF3-radical [68], a radical process was suggested by the authors (Scheme 23). Under the standard reaction conditions, TEMPONa reduced Togni reagent to generate an N3 radical, and release TEMPO, then the N3 radical is trapped by an olefin to generate species A, which reacts with TEMPO to form products 30.
Scheme 23.
Radical oxyazidation of alkenes.
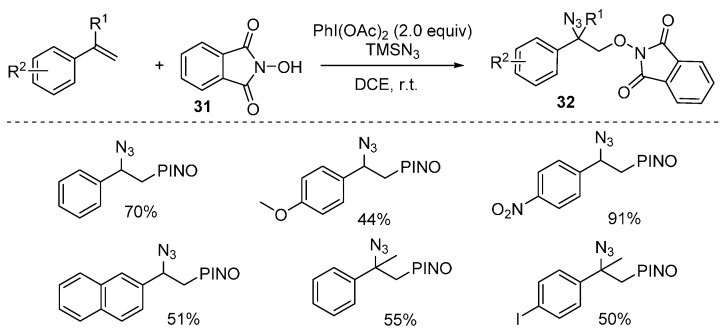
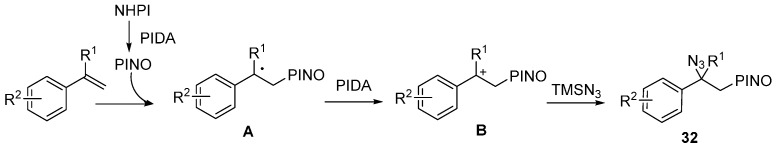
Recently, an efficient oxyazidation of alkenes under metal-free conditions was reported by Xia and co-workers. This reaction could form C-O and C-N bonds in one step by using N-hydroxyphthalimide as an oxygen-radical precursor and TMSN3 as the N3 source. A number of aryl-substituted alkenes was tolerated in this transformation (Scheme 24) [69].
Scheme 24.
Oxyazidation of alkenes.
A possible mechanism is outlined in Scheme 25. In the presence of PIDA, N-hydroxyphthalimide (NHPI) 31 could be oxidized to generate an N-oxyl (PINO) radical, which attacks alkenes to form intermediate A. Intermediate A can be further oxidized to cation intermediate B by PIDA. Finally, intermediate B is trapped by TMSN3 to give products 32.
Scheme 25.
Possible mechanism.
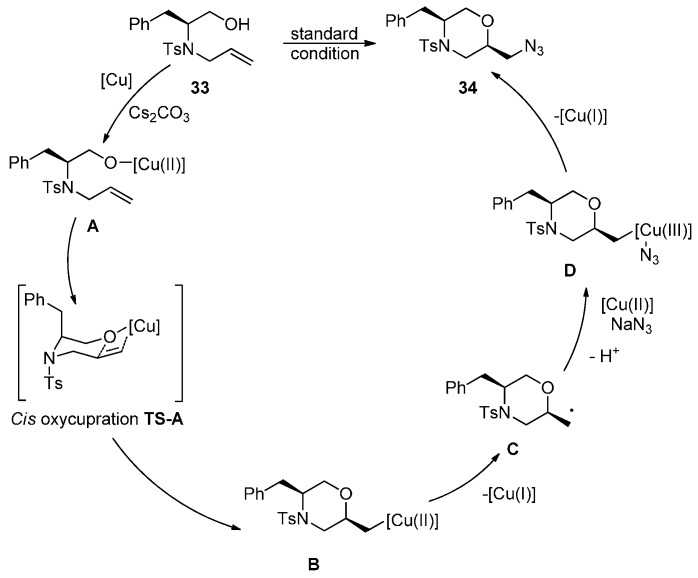
The simultaneous addition of oxygen and nitrogen across an alkene is a convenient way to construct the precursor of 2-aminomethylmorpholines 34. Remarkably, through copper-catalyzed oxyamination of alkene, a novel synthetic method for the preparation of 34 was reported by Chemler and co-workers [70]. Under the reaction conditions, 34 was obtained in good yields with excellent diastereoselectivity (Scheme 26).
Scheme 26.
Oxyazidation of alkenes.
A plausible mechanism is illustrated in Scheme 27. Coordination of alcohol 33 to copper(II) 2-ethylhexanoate gives monomer A. Thermal conditions promote cis-oxycupration via TS-A, then give the copper(II) intermediate B. The diastereoselectivity is rationalized by a chair-like transition state where vicinal tosyl and benzyl substituents adopt pseudoaxial positions. Intermediate B undergoes C-Cu(II) homolytic cleavage to give carbon radical C. In the presence of an azide nucleophile, an organocopper(III) intermediate D is formed, which can undergo reductive elimination to form the product 34.
Scheme 27.
Proposed oxyamination reaction mechanism.
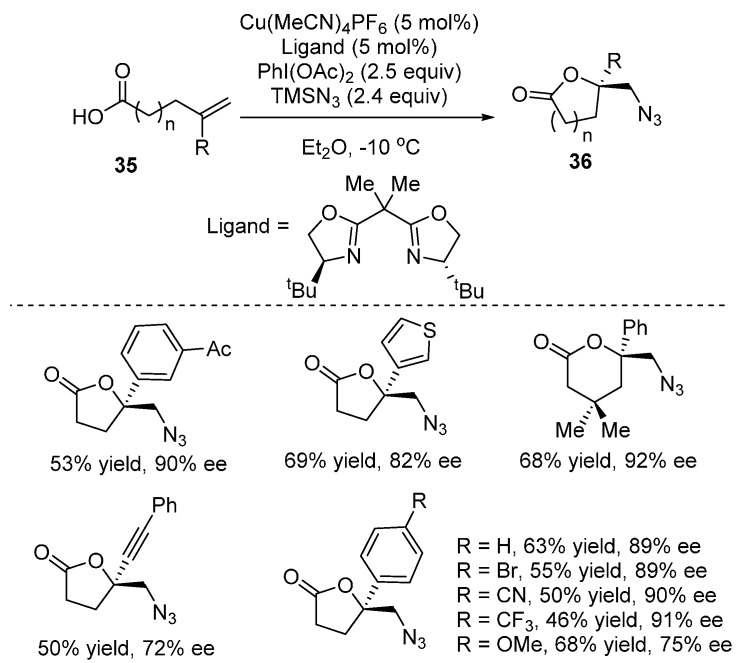
A new approach to synthesize a wide range of chiral lactones 36 based on Cu-catalyzed enantioselective radical oxyfunctionalization of alkenes was developed by Buchwald and co-workers [71]. This method provides a straightforward approach to various lactone building blocks containing tetrasubstituted stereogenic centers, which are hard to access through traditional methods (Scheme 28).
Scheme 28.
Synthesis of chiral lactones.
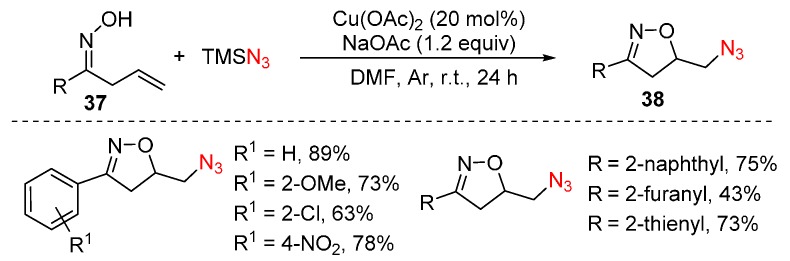
Isoxazolines are useful building blocks in organic chemistry [72]. Recently, an efficient Cu(OAc)2-catalyzed oxyazidation of alkenes 37 was developed by Wang, Xu and co-workers (Scheme 29) [73]. This reaction occurs under mild conditions, forming the azido-substituted isoxazolines 38 in good yields, although the mechanism of this chemistry is still unclear.
Scheme 29.
Oxyazidation of alkenes.
2.3. Construction of C-N3 Bond and C-N Bond
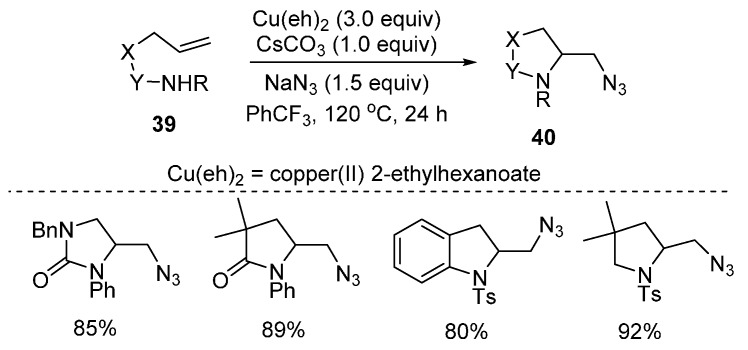
Olefin diamination methods provide powerful access to vicinal diamines that are useful in chemical biology, medicinal chemistry and materials science. Recently, an novel copper(II)-promoted intramolecular azidoamination of alkenes 39 was reported by Chemler and co-workers (Scheme 30) [74]. This method could tolerate a wide range of internal and external amine sources for the formation of differently functionalized nitrogen heterocycles 40.
Scheme 30.
Intermolecular alkene azidoamination.
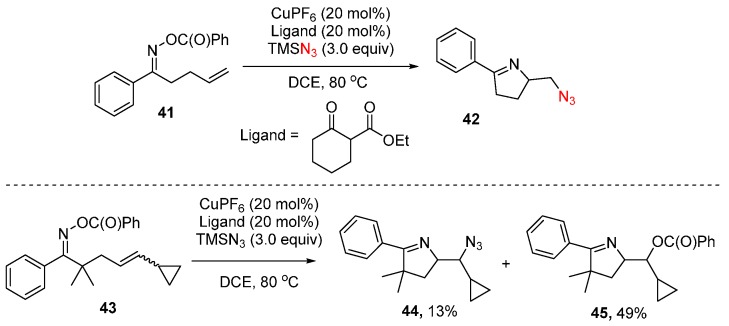
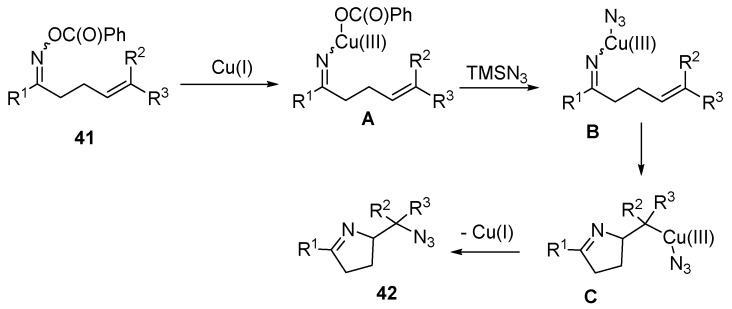
Yu and co-workers reported an intramolecular azidation reaction through copper-mediated N-O cleavage and subsequent C-N bond forming 5-exo cyclization. The forming intermediate is subsequently azidated to afford the corresponding dihydropyrroles (Scheme 31) [75]. To understand this reaction mechanism, compound 43 was treated under standard reaction conditions. However, in this case 44 and 45 are mainly obtained with only trace amounts of ring opening product formation, suggesting that the cyclization step is unlikely a free radical process. Based on these results, a reaction mechanism is proposed (Scheme 32). The first step involves the oxidative addition of Cu(I) to the N-O to give intermediate A, which undergoes ligand exchange and then intramolecular cyclization to afford intermediate C, followed by reductive elimination to afford products 42.
Scheme 31.
Azidation of unsaturated O-acyl oximes and radical clock experiments.
Scheme 32.
Proposed mechanism.
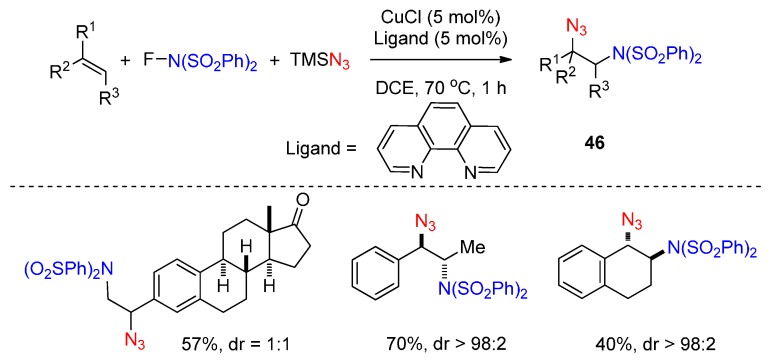
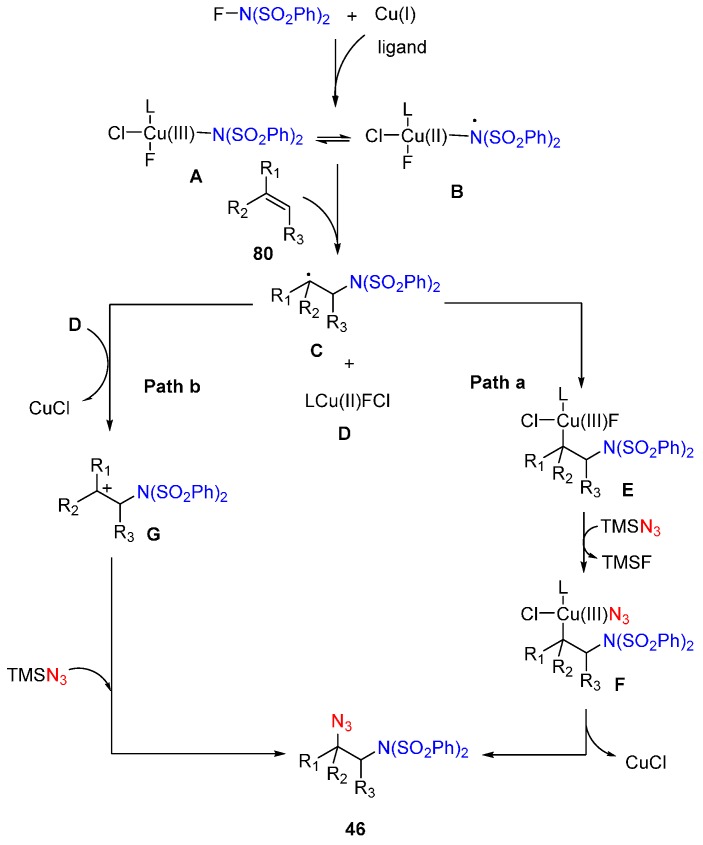
Studer and co-workers described a novel methodology for the efficient synthesis of the precursors of vicinal amino azides 46 (Scheme 33) [76], which can easily be transformed into other important amine derivatives. This chemistry employed Cu(I) as catalyst, TMSN3 as N3 source and available N-fluorobenzenesulfonimide (NFSI) as nitrogen-radical precursor leading to the desired products in moderate to excellent yields with high diastereoselectivity.
Scheme 33.
Aminoazidation of alkenes.
A plausible reaction mechanism based on the reported processes is outlined in Scheme 34 [77,78]. Firstly, Cu(I) reacts with NFSI to form Cu(III) species A, which could exist in equilibrium with a Cu(II)-stabilized N-centered radical B. It is the precursor of bis-sulfonylamidyl radical, which adds to the alkene to generate carbon radical C and Cu(II) species D. Also B species could react as N-radicals with the alkene. Then two pathways are suggested. In path a, trapping of C with D provides Cu(III) species E, which exchanges ligand with TMSN3 to give Cu(III) complex F. Reductive elimination of F affords the products along with the regeneration of the Cu(I) catalyst. In path b, D oxidizes C to cationic intermediate G, which is trapped by TMSN3 to form the products.
Scheme 34.
Proposed reaction mechanism.
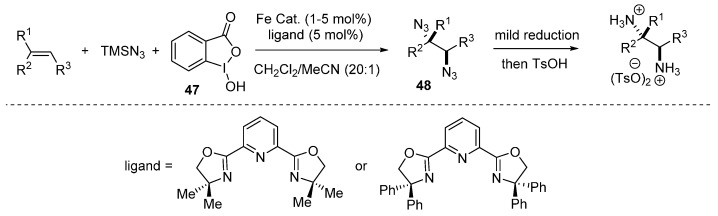
Snider and co-workers reported a Mn(OAc)3-mediated diazidation of alkenes and glycals for the formation of 1,2-diazides compounds [79]. Very recently, Xu and co-workers reported a novel iron-catalyzed diastereoselective olefin diazidation method which tolerates a broad range of olefins (Scheme 35) [80]. This method also provides a convenient approach to vicinal primary diamines and other synthetically valuable nitrogen-containing compounds.
Scheme 35.
Diazidation of olefins.
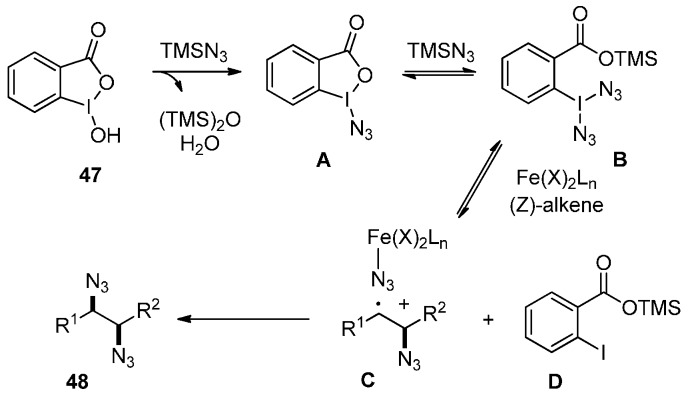
A proposed mechanism is shown in Scheme 36. TMSN3 reacts with 47 to give intermediate A, which is further activated by TMSN3 to generate intermediate B. In the presence of iron catalysts, intermediate B reacts with alkene to afford intermediate C and release intermediate D. It is likely that the high-valent iron species may be further oxidize intermediate C through inner-sphere azido ligand transfer to afford the diazide product.
Scheme 36.
Possible mechanism.
2.4. Construction of C-N3 Bond and C-P Bond
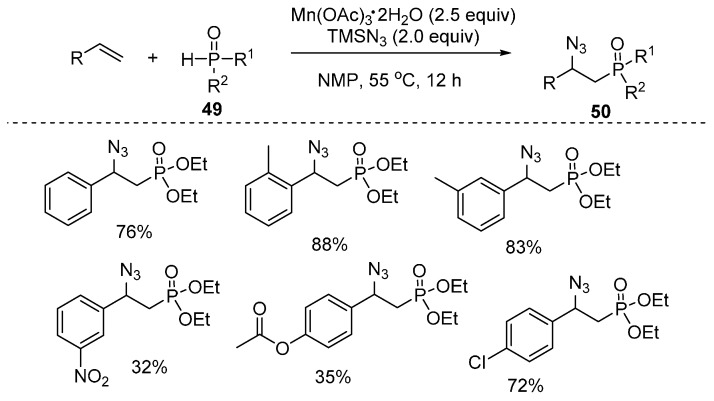
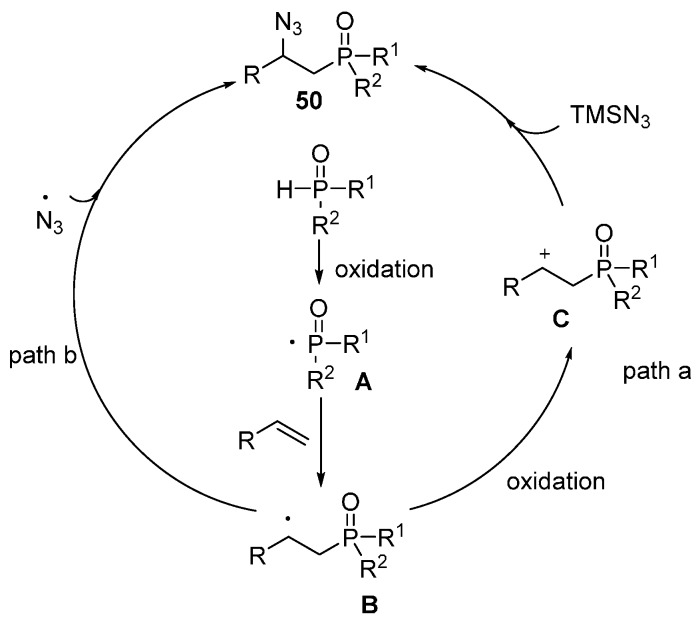
An efficient method for the synthesis of β-azidophosphonates 49 through Mn(OAc)3-mediated radical oxidative phosphonation-azidation of alkenes under relatively mild reaction conditions was reported by Tang and co-workers. This reaction displayed a broad substrate scope and can be easily scaled up. The products can be obtained in a one-pot operation (Scheme 37) [81].
Scheme 37.
Phosphonation–azidation of alkenes.
Based on their previous studies in P–C bond formation, and reaction of organophosphorus radicals [82], a possible mechanism was proposed (Scheme 38). The reaction is initiated by the addition of phosphorous radical A to alkene to generate radical B, which undergoes further oxidation to afford cationic intermediate C. The intermediate C is then trapped by TMSN3 to afford the final product (path a). Alternatively, the radical B could be directly trapped by an azido radical to form the product (path b) (Scheme 38).
Scheme 38.
Possible reaction pathways.
2.5. Construction of C-N3 Bond and C-Se Bond
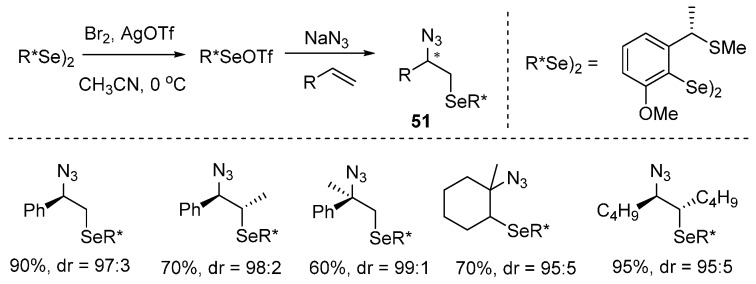
Tiecco and co-workers reported the first example of a highly asymmetric electrophilic azidoselenenylation of olefins for the preparation of azido selenium derivatives 51. This reaction occurs with a high level of facial selectivity, which was made possible by the use of chiral, non-racemic selenium reagents (Scheme 39) [83].
Scheme 39.
Azidoselenenylation of alkenes.
2.6. Construction of C-N3 Bond and C-Halogen Bond
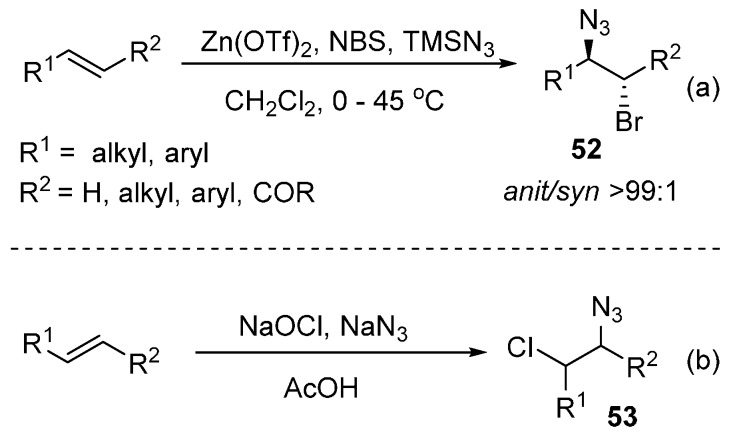
1,2-Haloazidation of alkenes represents an important transformation in organic synthesis. By using Zn(OTf)2 as catalyst, a metal-catalyzed bromoazidation of alkenes was reported by Hajra and co-workers. The corresponding bromoazidation products were obtained in good yields by using this protocol (Scheme 40a) [84]. Phukan’s group also achieved the bromoazidation of alkenes by using other bromine sources [85,86]. Moreover, a route to 1,2-azidochlorides from alkenes was developed by Finn and co-workers (Scheme 40b) [87].
Scheme 40.
Bromoazidation or chloroazidation of alkenes.
3. Conclusions
In conclusion, this review has summarized recent difunctionalization reactions of olefins with organic and inorganic azides through C-N3 bond formation. An oxidative single electron transfer (SET) process is involved in most cases. These approaches provide efficient protocols for the preparation of various organic azido compounds, which can then be further applied in many transformations to synthesize various valuable nitrogen-containing compounds.
Acknowledgments
Financial support from National Basic Research Program of China (973 Program) (grant No. 2015CB856600) and National Natural Science Foundation of China (Nos. 21325206, 21172006), and National Young Top-notch Talent Support Program are greatly appreciated.
Conflicts of Interest
Declare conflicts of interest or state “The authors declare no conflict of interest."
References
- 1.Bräse S., Gil C., Knepper K., Zimmermann V. Organic azides: An exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. 2005;44:5188–5240. doi: 10.1002/anie.200400657. [DOI] [PubMed] [Google Scholar]
- 2.Palacios F., Alonso C., Aparicio D., Rubiales G., Santos J.M.D.L. The aza-wittig reaction: An efficient tool for the construction of carbon-nitrogen double bonds. Tetrahedron. 2007;63:523–575. doi: 10.1016/j.tet.2006.09.048. [DOI] [Google Scholar]
- 3.Curtius T. Ueber Stickstoffwasserstoffsäure (Azoimid) N3H. Ber. Dtsch. Chem. Ges. 1890;23:3023–3033. doi: 10.1002/cber.189002302232. [DOI] [Google Scholar]
- 4.Curtius T. Hydrazide und Azide organischer Säuren. J. Prakt. Chem. 1894;50:275–294. doi: 10.1002/prac.18940500125. [DOI] [Google Scholar]
- 5.Grecian S., Aubé J. Schmidt rearrangement reactions with alkyl azides. In: Bräse S., Banert K., editors. Organic Azides, Syntheses and Applications. John Wiley & Sons; Chichester, UK: 2010. pp. 191–237. [Google Scholar]
- 6.Vital P., Tanner D. Efficient and highly enantioselective formation of the all-carbon quaternary stereocentre of lyngbyatoxin A. Org. Biomol. Chem. 2006;4:4292–4298. doi: 10.1039/b612578f. [DOI] [PubMed] [Google Scholar]
- 7.Kolb H.C., Finn M.G., Sharpless K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Rostovtsev V.V., Green L.G., Fokin V.V., Sharpless K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Tornøe C.W., Christensen C., Meldal M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 10.Ritschel J., Sasse F., Maier M.E. Synthesis of a benzolactone collection using click chemistry. Eur. J. Org. Chem. 2007;2007:78–87. doi: 10.1002/ejoc.200600681. [DOI] [Google Scholar]
- 11.Franc G., Kakkar A.K. "Click" methodologies: Efficient, simple and greener routes to design dendrimers. Chem. Soc. Rev. 2010;39:1536–1544. doi: 10.1039/b913281n. [DOI] [PubMed] [Google Scholar]
- 12.Aragão-Leoneti V., Campo V.L., Gomes A.S., Field R.A., Carvalho I. Application of copper(I)-catalysed azide/alkyne cycloaddition (CuAAC) ‘click chemistry’ in carbohydrate drug and neoglycopolymer synthesis. Tetrahedron. 2010;66:9475–9492. doi: 10.1016/j.tet.2010.10.001. [DOI] [Google Scholar]
- 13.Wang T., Jiao N. Direct approaches to nitriles via highly efficient nitrogenation strategy through C-H or C-C bond cleavage. Acc. Chem. Res. 2014;47:1137–1145. doi: 10.1021/ar400259e. [DOI] [PubMed] [Google Scholar]
- 14.Chiba S. Application of organic azides for the synthesis of nitrogen-containing molecules. Synlett. 2012;23:21–44. doi: 10.1055/s-0031-1290108. [DOI] [Google Scholar]
- 15.Shin K., Kim H., Chang S. Transition-metal-catalyzed C-N bond forming reactions using organic azides as the nitrogen source: A journey for the mild and versatile C-H amination. Acc. Chem. Res. 2015;48:1040–1052. doi: 10.1021/acs.accounts.5b00020. [DOI] [PubMed] [Google Scholar]
- 16.Intrieri D., Zardi P., Caselli A., Gallo E. Organic azides: "Energetic reagents" for the intermolecular amination of C-H bonds. Chem. Commun. 2014;50:11440–11453. doi: 10.1039/C4CC03016H. [DOI] [PubMed] [Google Scholar]
- 17.Sebeika M.M., Jones G.B. Safer, greener, and more facile alternatives for synthesis with organic azides. Curr. Org. Synth. 2014;11:732–750. doi: 10.2174/1570179411666140328221938. [DOI] [Google Scholar]
- 18.Tanimoto H., Kakiuchi K. Recent applications and developments of organic azides in total synthesis of natural products. Nat. Prod. Commun. 2013;8:1021–1034. [PubMed] [Google Scholar]
- 19.Tuktarov A.R., Akhmetov A.R., Dzhemilev U.M. Organic and inorganic azides in fullerene chemistry: Achievements and trends. Mater. Sci. Res. J. 2014;8:123–166. [Google Scholar]
- 20.Thirumurugan P., Matosiuk D., Jozwiak K. Click chemistry for drug development and diverse chemical-biology applications. Chem. Rev. 2013;113:4905–4979. doi: 10.1021/cr200409f. [DOI] [PubMed] [Google Scholar]
- 21.Hahn U., Elhabiri M., Trabolsi A., Herschbach H., Leize E., Dorsselaer A.V., Albrecht-Gary A.M., Nierengarten J.F. Supramolecular click chemistry with a bisammonium-C60 substrate and a ditopic crown ether host. Angew. Chem. Int. Ed. 2005;44:5338–5341. doi: 10.1002/anie.200501249. [DOI] [PubMed] [Google Scholar]
- 22.Trabolsi A., Elhabiri M., Urbani M., Delgado de la Cruz J.L., Ajamaa F., Solladié N., Albrecht-Gary A.M., Nierengarten J.F. Supramolecular click chemistry for the self-assembly of a stable Zn(II)–porphyrin–C60 conjugate. Chem. Commun. 2005;46:5736–5738. doi: 10.1039/b510782b. [DOI] [PubMed] [Google Scholar]
- 23.Hoogenboom R., Moore B.C., Schubert U.S. Synthesis of star-shaped poly(ε-caprolactone) via “click” chemistry and “supramolecular click” chemistry. Chem. Commun. 2006;38:4010–4012. doi: 10.1039/B608313G. [DOI] [PubMed] [Google Scholar]
- 24.Tang J., Costa J.S., Aromí G., Mutikainen I., Turpeinen U., Gamez P., Reedijk J. Supramolecular click assembly of a fused double-stranded [MnII3] dihelicate. Eur. J. Inorg. Chem. 2007;26:4119–4122. doi: 10.1002/ejic.200700697. [DOI] [Google Scholar]
- 25.Tron G.C., Pirali T., Billington R.A., Canonico P.L., Sorba G., Genazzani A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008;28:278–308. doi: 10.1002/med.20107. [DOI] [PubMed] [Google Scholar]
- 26.Kluger R. Click chemistry for biotechnology and materials science. J. Am. Chem. Soc. 2010;132:6611–6612. doi: 10.1021/ja102850q. [DOI] [Google Scholar]
- 27.Muñiz K., Martínez C. Development of intramolecular vicinal diamination of alkenes: From palladium to bromine catalysis. J. Org. Chem. 2013;78:2168–2174. doi: 10.1021/jo302472w. [DOI] [PubMed] [Google Scholar]
- 28.Kolb H.C., VanNieuwenhze M.S., Sharpless K.B. Catalytic asymmetric dihydroxylation. Chem. Rev. 1994;94:2483–2547. doi: 10.1021/cr00032a009. [DOI] [Google Scholar]
- 29.Zeni G., Larock R.C. Synthesis of heterocycles via palladium pi-olefin and pi-alkyne chemistry. Chem. Rev. 2004;104:2285–2309. doi: 10.1021/cr020085h. [DOI] [PubMed] [Google Scholar]
- 30.Muñiz K. Imido-osmium(VIII) compounds in organic synthesis: Aminohydroxylation and diamination reactions. Chem. Soc. Rev. 2004;33:166–174. doi: 10.1039/B307102M. [DOI] [PubMed] [Google Scholar]
- 31.Minatti A., Muñiz K. Intramolecular aminopalladation of alkenes as a key step to pyrrolidines and related heterocycles. Chem. Soc. Rev. 2007;36:1142–1152. doi: 10.1039/B607474J. [DOI] [PubMed] [Google Scholar]
- 32.Jensen K.H., Sigman M.S. Mechanistic approaches to palladium-catalyzed alkene difunctionalization reactions. Org. Biomol. Chem. 2008;6:4083–4088. doi: 10.1039/b813246a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heinrich M.R. Intermolecular olefin functionalisation involving aryl radicals generated from arenediazonium salts. Chem. Eur. J. 2009;15:820–833. doi: 10.1002/chem.200801306. [DOI] [PubMed] [Google Scholar]
- 34.Cardona F., Goti A. Metal-catalysed 1,2-diamination reactions. Nat. Chem. 2009;1:269–275. doi: 10.1038/nchem.256. [DOI] [PubMed] [Google Scholar]
- 35.Mcdonald R.I., Liu G., Stahl S.S. Palladium(II)-catalyzed alkene functionalization via nucleopalladation: Stereochemical pathways and enantioselective catalytic applications. Chem. Rev. 2011;111:2981–3019. doi: 10.1021/cr100371y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donohoe T.J., Callens C.K.A., Flores A., Lacy A.R., Rathi A.H. Recent developments in methodology for the direct oxyamination of olefins. Chem. Eur. J. 2011;17:58–76. doi: 10.1002/chem.201002323. [DOI] [PubMed] [Google Scholar]
- 37.Merino E., Nevado C. Addition of CF3 across unsaturated moieties: A powerful functionalization tool. Chem. Soc. Rev. 2014;43:6598–6608. doi: 10.1039/C4CS00025K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chemler S.R., Bovino M.T. Catalytic aminohalogenation of alkenes and alkynes. ACS Catal. 2013;3:1076–1091. doi: 10.1021/cs400138b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egami H., Sodeoka M. Trifluoromethylation of alkenes with concomitant introduction of additional functional groups. Angew. Chem. Int. Ed. 2014;53:8294–8308. doi: 10.1002/anie.201309260. [DOI] [PubMed] [Google Scholar]
- 40.Romero R.M., Wöste T.H., Muñiz K. Vicinal difunctionalization of alkenes with iodine (III) reagents and catalysts. Chem. Asian J. 2014;9:972–983. doi: 10.1002/asia.201301637. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu Y., Kanai M. Recent progress in copper-catalyzed difunctionalization of unactivated carbon-carbon multiple bonds. Tetrahedron Lett. 2014;55:3727–3737. doi: 10.1016/j.tetlet.2014.05.077. [DOI] [Google Scholar]
- 42.Wolfe J.P. Intramolecular alkoxycyanation and alkoxyacylation reactions: New types of alkene difunctionalizations for the construction of oxygen heterocycles. Angew. Chem. Int. Ed. 2012;51:10224–10225. doi: 10.1002/anie.201204470. [DOI] [PubMed] [Google Scholar]
- 43.Waser J., Nambu H., Carreira E.M. Cobalt-catalyzed hydroazidation of olefins. J. Am. Chem. Soc. 2005;127:8294–8295. doi: 10.1021/ja052164r. [DOI] [PubMed] [Google Scholar]
- 44.Waser J., Gaspar B., Nambu H., Carreira E.M. Hydrazines and azides via the metal-catalyzed hydrohydrazination and hydroazidation of olefins. J. Am. Chem. Soc. 2006;128:11693–11712. doi: 10.1021/ja062355+. [DOI] [PubMed] [Google Scholar]
- 45.Kapat A., König A., Montermini F., Renaud P. A radical procedure for the anti-Markovnikov hydroazidation of alkenes. J. Am. Chem. Soc. 2011;133:13890–13893. doi: 10.1021/ja2054989. [DOI] [PubMed] [Google Scholar]
- 46.Leggans E.K., Barker T.J., Duncan K.K., Boger D.L. Iron(III)/NaBH4-mediated additions to unactivated alkenes: Synthesis of novel 200-vinblastine analogues. Org. Lett. 2012;14:1428–1431. doi: 10.1021/ol300173v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glavee G.N., Klabunde K.J., Sorensen C.M., Hadjipanayis G.C. Chemistry of borohydride reduction of iron(II) and iron(III) ions in aqueous and nonaqueous media. Formation of nanoscale Fe, FeB, and Fe2B powders. Inorg. Chem. 1995;34:28–35. doi: 10.1021/ic00105a009. [DOI] [Google Scholar]
- 48.Bellavista T., Meninno S., Lattanzi A., Sala G.D. Asymmetric hydroazidation of nitroalkenes promoted by a secondary amine-thiourea catalyst. Adv. Synth. Catal. 2015;357:3365–3373. doi: 10.1002/adsc.201500403. [DOI] [Google Scholar]
- 49.Kong W., Merino E., Nevado C. Arylphosphonylation and arylazidation of activated alkenes. Angew. Chem. Int. Ed. 2014;53:5078–5082. doi: 10.1002/anie.201311241. [DOI] [PubMed] [Google Scholar]
- 50.Xu L., Mou X.Q., Chen Z.M., Wang S.H. Copper-catalyzed intermolecular azidocyanation of aryl alkenes. Chem. Commun. 2014;50:10676–10679. doi: 10.1039/C4CC04640D. [DOI] [PubMed] [Google Scholar]
- 51.Mantrand N., Renaud P. Azidosulfonylation of alkenes, dienes, and enynes. Tetrahedron. 2008;64:11860–11864. doi: 10.1016/j.tet.2008.09.096. [DOI] [Google Scholar]
- 52.Klein J.E.M.N., Taylor R.J.K. Transition-metal-mediated routes to 3,3-disubstituted oxindoles through anilide cyclisation. Eur. J. Org. Chem. 2011;2011:6821–6841. doi: 10.1002/ejoc.201100836. [DOI] [Google Scholar]
- 53.Zhou F., Liu Y.L., Zhou J. Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position. Adv. Synth. Catal. 2010;352:1381–1407. doi: 10.1002/adsc.201000161. [DOI] [Google Scholar]
- 54.Matcha K., Narayan R., Antonchick A.P. Metal-free radical azidoarylation of alkenes: Rapid access to oxindoles by cascade C-N and C-C bond-forming reactions. Angew. Chem. Int. Ed. 2013;52:7985–7989. doi: 10.1002/anie.201303550. [DOI] [PubMed] [Google Scholar]
- 55.Qiu J., Zhang R. Transition-metal-free oxidative carboazidation of acrylamides via cascade C-N and C-C bond forming reactions. Org. Biomol. Chem. 2014;12:4329–4334. doi: 10.1039/c4ob00720d. [DOI] [PubMed] [Google Scholar]
- 56.Wei X.H., Li Y.M., Zhou A.X., Yang T.T., Yang S.D. Silver-catalyzed carboazidation of arylacrylamides. Org. Lett. 2013;15:4158–4161. doi: 10.1021/ol402138y. [DOI] [PubMed] [Google Scholar]
- 57.Yuan Y., Shen T., Wang K., Jiao N. Ag-promoted azido-carbocyclization of activated alkenes via C-H bond cleavage. Chem. Asian J. 2013;8:2932–2935. doi: 10.1002/asia.201300960. [DOI] [PubMed] [Google Scholar]
- 58.Yamazaki T., Taguchi T., Ojima I. Fluorine in Medical Chemistry and Chemical Biology. Wiley-Blackwell; Chichester, UK: 2009. Unique properties of fluorine and their relevance to medicinal chemistry and chemical biology; pp. 1–46. [Google Scholar]
- 59.Wang F., Qi X., Liang Z., Chen P., Liu G. Copper-catalyzed intermolecular trifluoromethylazidation of alkenes: Convenient access to CF3-containing alkyl azides. Angew. Chem. Int. Ed. 2014;126:1912–1917. doi: 10.1002/ange.201309991. [DOI] [PubMed] [Google Scholar]
- 60.Carboni A., Dagousset G., Magnier E., Masson G. Photoredox-induced three-component oxy-, amino-, and carbotrifluoromethylation of enecarbamates. Org. Lett. 2014;16:1240–1243. doi: 10.1021/ol500374e. [DOI] [PubMed] [Google Scholar]
- 61.Dagousset G., Carboni A., Magnier E., Masson G. Photoredox-induced three-component azido- and aminotrifluoromethylation of alkenes. Org. Lett. 2014;16:4340–4343. doi: 10.1021/ol5021477. [DOI] [PubMed] [Google Scholar]
- 62.Yang M., Wang W., Liu Y., Feng L., Ju X., Yang M. Copper-catalyzed three-component azidotrifluoromethylation/difunctionalization of alkenes. Chin. J. Chem. 2014;32:833–837. doi: 10.1002/cjoc.201400167. [DOI] [Google Scholar]
- 63.Sakurada I., Yamasaki S., Kanai M., Shibasaki M. Dichlorotin oxide-catalyzed new direct functionalization of olefins: Synthesis of trans β-azidohydrins and 1,2-diols. Tetrahedron Lett. 2000;41:2415–2418. doi: 10.1016/S0040-4039(00)00176-3. [DOI] [Google Scholar]
- 64.Sun X., Li X., Song S., Zhu Y., Liang Y.F., Jiao N. Mn-catalyzed highly efficient aerobic oxidative hydroxyazidation of olefins: A direct approach to β-azido alcohols. J. Am. Chem. Soc. 2015;137:6059–6066. doi: 10.1021/jacs.5b02347. [DOI] [PubMed] [Google Scholar]
- 65.Prasad P.K., Reddi R.N., Sudalai A. Oxidant controlled regio- and stereodivergent azidohydroxylation of alkenes via I2 catalysis. Chem. Commun. 2015;51:10276–10279. doi: 10.1039/C5CC02374B. [DOI] [PubMed] [Google Scholar]
- 66.Fumagalli G., Rabet P.T.G., Boyd S., Greaney M.F. Three-component azidation of styrene-type double bonds: Light-switchable behavior of a copper photoredox catalyst. Angew. Chem. Int. Ed. 2015;54:11481–11484. doi: 10.1002/anie.201502980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang B., Studer A. Stereoselective radical oxyazidation of alkenes. Org. Lett. 2013;15:4548–4551. doi: 10.1021/ol402106x. [DOI] [PubMed] [Google Scholar]
- 68.Li Y., Studer A. Transition-metal-free trifluoromethylaminoxylation of alkenes. Angew. Chem. Int. Ed. 2012;51:8221–8224. doi: 10.1002/anie.201202623. [DOI] [PubMed] [Google Scholar]
- 69.Xia X.F., Gu Z., Liu W., Wang H., Xia Y., Gao H., Liu X., Liang Y.M. Metal-free three-component oxyazidation of alkenes with trimethylsilyl azide and N-hydroxyphthalimide. J. Org. Chem. 2015;80:290–295. doi: 10.1021/jo502327r. [DOI] [PubMed] [Google Scholar]
- 70.Sequeira F.C., Chemler S.R. Stereoselective synthesis of morpholines via copper-promoted oxyamination of alkenes. Org. Lett. 2012;14:4482–4485. doi: 10.1021/ol301984b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu R., Buchwald S.L. Versatile enantioselective synthesis of functionalized lactones via copper-catalyzed radical oxyfunctionalization of alkenes. J. Am. Chem. Soc. 2015;137:8069–8077. doi: 10.1021/jacs.5b04821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carre G., Ottoli G. Stereoselective synthesis of drugs and drug precursors by hydrolases and oxidoreductases. Biocatal. Biotransfor. 2000;18:119–132. doi: 10.3109/10242420009015241. [DOI] [Google Scholar]
- 73.Zhu L., Yu H., Xu Z., Jiang X., Lin L., Wang R. Copper-catalyzed oxyazidation of unactivated alkenes. Org. Lett. 2014;16:1562–1565. doi: 10.1021/ol403687k. [DOI] [PubMed] [Google Scholar]
- 74.Sequeira F.C., Turnpenny B.W., Chemler S.R. Copper-promoted and copper-catalyzed intermolecular alkene diamination. Angew. Chem. Int. Ed. 2010;49:6365–6368. doi: 10.1002/anie.201003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su H., Li W., Xuan Z., Yu W. Copper-catalyzed cyclization and azidation of γ, δ-unsaturated ketone o-benzoyl oximes. Adv. Synth. Catal. 2015;357:64–70. doi: 10.1002/adsc.201400681. [DOI] [Google Scholar]
- 76.Zhang B., Studer A. Copper-catalyzed intermolecular aminoazidation of alkenes. Org. Lett. 2014;16:1790–1793. doi: 10.1021/ol500513b. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H., Pu W., Xiong T., Li Y., Zhou X., Sun K., Liu Q., Zhang Q. Copper-catalyzed intermolecular aminocyanation and diamination of alkenes. Angew. Chem. Int. Ed. 2013;52:2529–2533. doi: 10.1002/anie.201209142. [DOI] [PubMed] [Google Scholar]
- 78.Kaneko K., Yoshino T., Matsunaga S., Kanai M. Sultam synthesis via Cu-catalyzed intermolecular carboamination of alkenes with N-fluorobenzenesulfonimide. Org. Lett. 2013;15:2502–2505. doi: 10.1021/ol4009848. [DOI] [PubMed] [Google Scholar]
- 79.Snider B.B., Lin H. An improved procedure for the conversion of alkenes and glycals to 1,2-diazides using Mn(OAc)3·2H2O in acetonitrile containing trifluoroacetic acid. Synth. Commun. 1998;28:1913–1922. doi: 10.1080/00397919808007024. [DOI] [Google Scholar]
- 80.Yuan Y.A., Lu D.F., Chen Y.R., Xu H. Iron catalyzed direct diazidation of a broad range of olefins. Angew. Chem. Int. Ed. 2016;55:534–538. doi: 10.1002/anie.201507550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu J., Li X., Gao Y., Zhang L., Chen W., Fang H., Tang G., Zhao Y. Mn(III)-mediated phosphonation–azidation of alkenes: A facile synthesis of β-azidophosphonates. Chem. Commun. 2015;51:11240–11243. doi: 10.1039/C5CC03995A. [DOI] [PubMed] [Google Scholar]
- 82.Xu J., Zhang P., Li X., Gao Y., Wu J., Tang G., Zhao Y. Tetrabutylammonium iodide-catalyzed phosphorylation of benzyl C-H bonds via a cross-dehydrogenative coupling reaction. Adv. Synth. Catal. 2014;356:3331–3335. doi: 10.1002/adsc.201400436. [DOI] [Google Scholar]
- 83.Tiecco M., Testaferri L., Santi C., Tomassini C., Marini F., Bagnoli L., Temperini A. Asymmetric azidoselenenylation of alkenes: A key step for the synthesis of enantiomerically enriched nitrogen-containing compounds. Angew. Chem. Int. Ed. 2003;42:3131–3133. doi: 10.1002/anie.200351229. [DOI] [PubMed] [Google Scholar]
- 84.Hajra S., Sinha D., Bhowmick M. Metal triflate catalyzed highly regio- and stereoselective 1,2-bromoazidation of alkenes using NBS and TMSN3 as the bromine and azide sources. Tetrahedron Lett. 2006;47:7017–7019. doi: 10.1016/j.tetlet.2006.07.110. [DOI] [Google Scholar]
- 85.Saikia I., Phukan P. Catalyst-free vicinal bromoazidation of olefins using TMSN3 and NBS. C. R. Chim. 2012;15:688–692. doi: 10.1016/j.crci.2012.05.001. [DOI] [Google Scholar]
- 86.Saikia I., Phukan P. Facile generation of vicinal bromoazides from olefins using TMSN3 and TsNBr2 without any catalyst. Tetrahedron Lett. 2009;50:5083–5087. doi: 10.1016/j.tetlet.2009.06.059. [DOI] [Google Scholar]
- 87.Valiulin R.A., Mamidyala S., Finn M.G. Taming ghlorine azide: access to 1,2-azidochlorides from alkenes. J. Org. Chem. 2015;80:2740–2755. doi: 10.1021/acs.joc.5b00009. [DOI] [PubMed] [Google Scholar]