Abstract
A series of novel hybrid compounds between benzofuran and N-aryl piperazine have been synthesized and screened in vitro for anti-inflammatory activity in lipopolysaccharide (LPS)-stimulated RAW-264.7 macrophages and for anticancer activity against three human tumor cell lines. The results demonstrated that derivative 16 not only had inhibitory effect on the generation of NO (IC50 = 5.28 μM), but also showed satisfactory and selective cytotoxic activity against human lung cancer line (A549) and gastric cancer cell (SGC7901) (IC50 = 0.12 μM and 2.75 μM, respectively), which was identified as the most potent anti-inflammatory and anti-tumor agent in this study.
Keywords: benzofuran, N-aryl piperazine moiety, anti-inflammatory activity, anticancer activity
1. Introduction
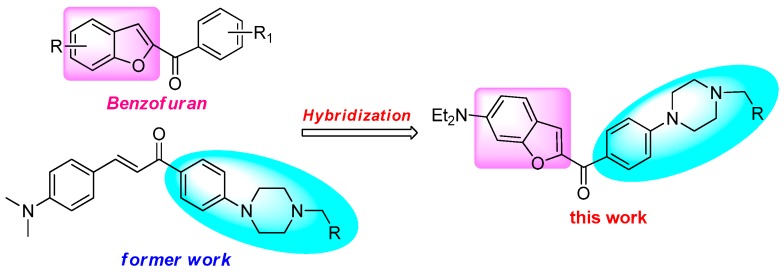
Natural and synthetic benzofuran derivatives are a class of important organic compounds with a broad range of biological activities, such as antioxidant, anti-inflammatory, antibacterial, antitumor, and so on [1,2,3,4,5,6,7,8,9,10,11]. Recently, benzofuran derivatives have attracted considerable interest for their versatile properties in chemistry and pharmacology. In addition, 2-benzoylbenzofuran compounds have been identified to show potent cytotoxic activities, as exemplified in Scheme 1. 2-benzoylphenyl benzofuran compound (A) [12] and 2-(2,4-dimethoxybenzoyl)-phenyl benzofuran derivative (B) [13] showed potent anticancer activities. Furthermore, synthetic 2-benzylbenzofurane-imidazole hybrid (C) [14] and 2-(4-imidazoyl benzoyl)benzofuran (D) [15] were attractive with excellent anti-inflammatory and cytotoxic activities (Scheme 1).
Scheme 1.
Structures of biological benzofuran agents.
Nitrogen heterocycles are an important class of compounds having versatile biological activities, which are used in drug design and synthesis, generally as active units [16,17]. Piperazine—one of the most biological active moieties—represents a series of important organic compounds that make up the core structures in medicines and have been widely used in the development of drug molecular design [18,19].
In previous work, we reported that benzofuran compounds containing N-heterocyclic moieties displayed potent cytotoxic activities [20], and the hybrids between chalcone and N-aryl piperazine bearing acetophenone showed potent anti-inflammatory and anticancer activities [21,22]. In addition, the acetophenone substituent was vital for modulating cytotoxic activities.
Based on these results, in the present research, we have designed and synthesized novel hybrids towards the recombination of benzofuran and N-aryl piperazine (Scheme 2). In order to study the structure–activity relationship (SAR) of hybrid compounds, various R-X were selected, including α-bromoacetophenone, benzyl bromide, and alkyl bromide. The potent in vitro anti-inflammatory activity in lipopolysaccharide (LPS)-stimulated RAW-264.7 macrophages and anticancer activities of compounds against a panel of human cancer cell lines (A549, Hela, and SGC7901) were evaluated, respectively.
Scheme 2.
Designed strategy of benzofuran hybrids.
2. Results
2.1. Chemistry
The general synthetic route used to synthesize hybrid compounds is outlined in Scheme 3. Treatment of commercial 5-diethylaminosalicylaldehyde (1) with 2-bromo-4′-fluoro acetophenone (2) gave the 2-phenzoylbenzofuran compound (3) in the presence of K2CO3 in refluxing acetone. Then, the key benzofuran–piperazine intermediate (4) was prepared by substitution with piperazine from compound (3) in the presence of K2CO3 at 110 °C in DMF. With the desired intermediate (4) in hand, we began the synthesis of amines by treatment of compound (4) with several commercially available R-X. To get further insight into the structure–activity relationship, the tertiary amines (5–25) were prepared with excellent yields by the reaction of intermediate (4) with various R-X. To compare the biological activities of substituents of the NH group of piperazine ring, we prepared the title compounds by substitution of α-bromoacetophenone, benzyl bromide, alkyl bromide, and hetero aromatic bromide. Comparative data for novel hybrid compounds with respect to structures and yield are provided in Table 1. All of the synthesized compounds were characterized by 1H-NMR and 13C-NMR, and some representative compounds were characterized by high-resolution mass spectrometry (HRMS) analysis. The spectra of title compounds were in Supplementary Materials.
Scheme 3.
Synthetic routes of hybrid compounds.
Table 1.
Structures and yields of compounds 5–25.
| Compound | R | m.p. (°C) | Yields (%) a |
|---|---|---|---|
| 5 |  |
171–173 | 51 |
| 6 |  |
171–173 | 57 |
| 7 | 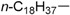 |
185–186 | 68 |
| 8 |  |
174–176 | 83 |
| 9 |  |
176–178 | 81 |
| 10 | 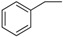 |
179–181 | 83 |
| 11 |  |
186–188 | 79 |
| 12 | 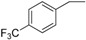 |
180–182 | 76 |
| 13 | 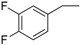 |
178–180 | 82 |
| 14 | 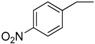 |
187–189 | 81 |
| 15 |  |
156–158 | 91 |
| 16 | 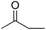 |
161–163 | 86 |
| 17 |  |
188–190 | 82 |
| 18 | 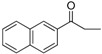 |
202–204 | 70 |
| 19 | 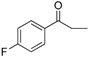 |
192–193 | 84 |
| 20 | 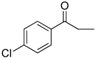 |
194–196 | 75 |
| 21 | 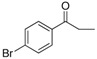 |
198–200 | 83 |
| 22 |  |
181–183 | 85 |
| 23 | 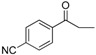 |
204–206 | 77 |
| 24 | 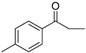 |
184–186 | 82 |
| 25 | 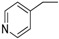 |
135–137 | 86 |
a Yields represent isolated yields.
2.2. Biological Evaluation
2.2.1. Anti-Inflammatory Activity
RAW 264.7 cells are widely used to establish inflammatory models in vitro. In this work, we investigated the anti-inflammatory activity of synthetic compounds in LPS-induced RAW 264.7 on the generation of NO. The results of the title hybrids are summarized in Table 2.
Table 2.
Anti-inflammatory activities of compounds.
| Compound | NO Generation (IC50, μM) a | Compound | NO Generation (IC50, μM) a |
|---|---|---|---|
| 5 | 14.12 | 16 | 5.28 |
| 6 | 34.24 | 17 | 25.40 |
| 7 | >40 | 18 | 6.53 |
| 8 | >40 | 19 | >40 |
| 9 | 18.52 | 20 | >40 |
| 10 | >40 | 21 | >40 |
| 11 | >40 | 22 | 9.13 |
| 12 | >40 | 23 | 23.56 |
| 13 | 23.06 | 24 | 18.37 |
| 14 | 20.27 | 25 | >40 |
| 15 | 31.68 |
a Values represent the concentration required to produce 50% inhibition of the response.
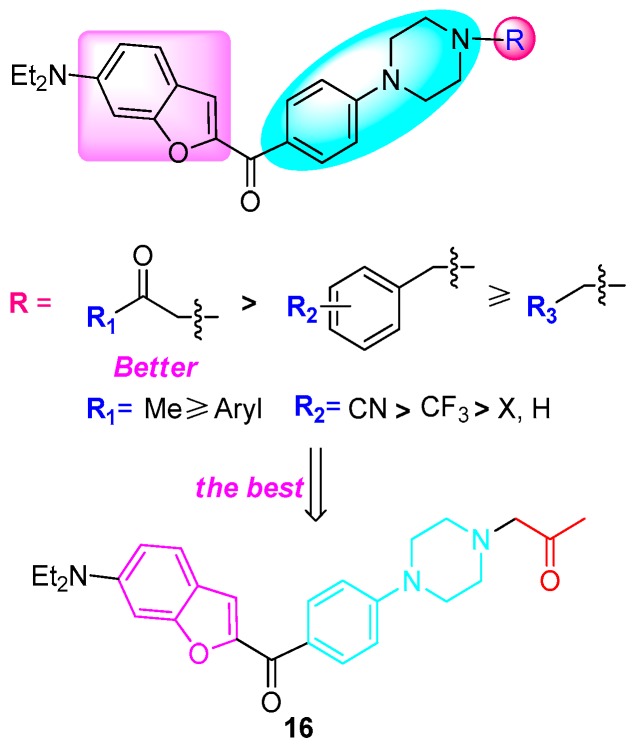
As shown in Table 2, the substituents of the NH group of the piperazine ring have an obvious influence on anti-inflammatory activities. To our delight, benzofuran–piperazine compounds 16, 18, and 22 displayed good anti-inflammatory activity on the generation of NO (IC50 < 10 μM). Especially, compound 16 was found to be the most potent anti-inflammatory agent (IC50 = 5.28 μM). In addition, compounds 5, 9, and 24 showed potent anti-inflammatory activity (IC50 < 20 μM). However, hetero aromatic compound 25 displayed no activity compared to others. Notably, except for the mentioned hybrids, most of the derivatives had little or no inhibitory effects on the release of NO (IC50 > 20 μM). On the other hand, we could find that the substituents of the NH group of the piperazine ring played an important role. Overall, keto- substituents contributed to better activity, alkyl and aryl substituents led to weaker activity, and the pyridyl substituent had no effect on anti-inflammatory activity (keto- > alkyl ≈ aryl > pyridyl). So, in future research, we will focus on the keto- substituents.
2.2.2. Anticancer Activity
The anticancer activities of novel synthesized derivatives were evaluated against human lung cancer cell line (A549), human cervical carcinoma (Hela), and human gastric carcinoma (SGC7901) by MTT [3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2-H-tetrazolium bromide] assay, using cisplatin (DDP) as the reference drug. The anti-tumor results for the hybrids are summarized in Table 3.
Table 3.
In vitro cytotoxic activities of title compounds.
| Compound | Cell Lines (IC50, μM) a | ||
|---|---|---|---|
| A549 | Hela | SGC7901 | |
| 5 | >40 | >40 | 27.24 |
| 6 | >40 | 32.53 | >40 |
| 7 | >40 | >40 | >40 |
| 8 | >40 | >40 | >40 |
| 9 | 19.27 | >40 | >40 |
| 10 | >40 | >40 | >40 |
| 11 | 16.14 | 8.57 | >40 |
| 12 | >40 | 25.14 | 16.27 |
| 13 | >40 | >40 | 25.04 |
| 14 | >40 | 33.24 | >40 |
| 15 | >40 | 22.36 | 30.43 |
| 16 | 0.12 | 26.32 | 2.75 |
| 17 | 27.82 | >40 | 15.41 |
| 18 | 19.34 | >40 | 23.92 |
| 19 | 6.25 | 18.71 | 36.23 |
| 20 | 8.11 | 28.74 | >40 |
| 21 | 23.22 | 15.35 | >40 |
| 22 | 26.07 | >40 | >40 |
| 23 | 34.13 | 12.68 | 7.45 |
| 24 | >40 | 27.58 | >40 |
| 25 | >40 | 26.22 | >40 |
| DDP | 11.54 | 20.52 | 12.44 |
a Cytotoxicity as IC50 values for each cell line, the concentration of compound that inhibits 50% of the cell growth measured by MTT assay. DDP: cisplatin.
As shown in Table 3, the structures of the hybrid compounds have an obvious influence on cytotoxic activities. There were three series of substituents of the piperazine ring, including keto-, alkyl, and aryl. In general, derivatives bearing keto- substituent (16–24) were most active, displaying similar or better cytotoxic activity in vitro compared to cisplatin (DDP). However, hetero aromatic compound 25 had weak cytotoxic activity against Hela, and alkyl-substituted compounds showed no activity, except for hybrid 9 (IC50 = 19.27 μM against A549). Furthermore, the electron withdrawing group or halide substituent at position 4 of the benzene ring of the acetophenone moiety could be more sensitive to cytotoxic activity, such as 4-F (19), 4-Cl (20), and 4-CN (23). Compound 16 showed the most potent cytotoxic activity and pronounced selectivity against A549 (IC50 = 0.12 μM) and SGC7901 (IC50 = 2.75 μM). In addition, some aryl-substituted compounds displayed good inhibitory activity. For example, hybrids 11 and 12 had selective anti-tumor activity against cancer cells (IC50 = 8.57–16.27 μM).
The biological results suggested that the existence of a keto- substituent played an important role in the anti-inflammatory and anticancer activity of compounds. In all synthesized derivatives, hybrid 16 had better inhibitory effect on the generation of NO and showed more potent cytotoxic activity against A549 and SGC7901, and could be identified as the most potent anti-inflammatory and anti-tumor agent among those studied. The structure–activity relationship (SAR) results are summarized in Scheme 4.
Scheme 4.
Structure–activity relationship of hybrid compounds.
Overall, although only a few compounds were found to exhibit excellent anti-inflammatory and antitumor activities, we could study the tendency of benzofuran–piperazine compounds and conduct further medicine chemistry research following the results.
3. Materials and Methods
3.1. General Information
Starting materials were commercially available and analytically pure. Melting points were measured on a YANACO microscopic melting point meter and were uncorrected. 1H-NMR and 13C-NMR spectra were recorded on Bruker AV 300 and Bruker AV 400 spectrometers (Bruker, Karlsruhe, Germany) using TMS as internal standard and CDCl3 as solvent, respectively. Thin layer chromatographic (TLC) analysis was carried out on silica gel plates GF254. High-resolution mass spectra were performed on an ESI Q-TOF MS spectrometer (Agilent, Santa Clara, CA, USA).
3.2. Chemistry
3.2.1. General Procedure
General procedure for the synthesis of compound 3: To a solution of acetone (50 mL), 5-diethylamino salicylaldehyde (1.93 g, 10 mmol) and 2-bromo-4′-fluoroacetophenone (2.17 g, 10 mmol), was added K2CO3 (2.76 g, 20 mmol) and left to react for 4 h in reflux. The reaction was poured into 100 mL cold water. After stirring for 10 min, the mixture was filtered, and the filtrate was concentrated in vacuo and dried to afford a yellow solid.
General procedure for the preparation of compound 4: To a stirred solution of compound 3 (1.56 g, 5 mmol) and K2CO3 (1.38 g, 10 mmol) in dried DMF (20 mL), piperazine (0.86 g, 10 mmol) was added, and the reaction mixture was stirred for 12 h at 110 °C. After completion of the reaction as indicated by TLC, the reaction was quenched by the addition of CHCl3 (50 mL) and washed with water (3 × 20 mL). The organic layer was dried by anhydrous sodium sulfate, concentrated in vacuo, and purified by column chromatography to afford a brown solid.
General procedure for the preparation of hybrid derivatives 5–7: To a stirred solution of compound 4 (0.5 mmol) in dried DMF (5 mL), NaH (0.06 g, 60% in oil) was added, and the mixture was stirred at 0 °C for 1 h. Then, R-X (1 mmol) was added, and after completion of the reaction as indicated by TLC, the reaction was quenched by the addition of H2O (30 mL) and was extracted with DCM (3 × 10 mL). The organic layer was dried by anhydrous sodium sulfate, concentrated in vacuo, and purified by column chromatography (1% MeOH/DCM) to afford products.
General procedure for the preparation of derivatives 8–14, 25: To a stirred solution of compound 4 (0.5 mmol) and Cs2CO3 (0.5 g) in dried DCM (15 mL), R-X (0.6 mmol) was added, and the reaction mixture was stirred for 12 h at room temperature (r.t.). After completion of the reaction as indicated by TLC, the mixture was filtered off. Then, the organic layer was concentrated in vacuo and purified by column chromatography (1% MeOH/DCM) to afford products.
General procedure for the preparation of derivatives 15–24: To a stirred solution of compound 4 (0.5 mmol) and K2CO3 (0.2 g) in dried DCM (10 mL), R-X (0.6 mmol) was added, and the reaction mixture was stirred for 12 h at r.t. After completion of the reaction as indicated by TLC, the reaction was quenched by the addition of 5% NaOH (20 mL) and was extracted with DCM (3 × 10 mL). The organic layer was dried using anhydrous sodium sulfate, concentrated in vacuo, and purified by column chromatography (1% MeOH/DCM) to afford products.
3.2.2. The Character of All Compounds
Compound 4: Brown solid; 1H-NMR (300 MHz, CDCl3) δ: 8.02 (d, J = 9.0 Hz, 2H), 7.48 (d, J = 8.7 Hz, 1H), 7.38 (s, 1H), 6.95 (d, J = 9.0 Hz, 2H), 6.72–6.78 (m, 2H), 3.46 (q, J = 7.2 Hz, 4H), 3.35 (t, J = 4.8 Hz, 4H), 3.05 (t, J = 4.8 Hz, 4H), 1.90 (s, 1H), 1.23 (t, J = 6.9 Hz, 6H); 13C-NMR (75 MHz, CDCl3) δ: 181.94, 158.99, 154.37, 151.09, 149.06, 131.51, 128.06, 123.48, 116.76, 116.51, 113.71, 110.93, 93.16, 48.70, 45.99, 45.14, 12.63; HRMS (ESI-TOF): m/z calcd for C23H27N3O2Na [M + Na]+ 400.1995, found 400.1995.
Compound 5: Pale yellow solid; 1H-NMR (400 MHz, CDCl3) δ: 8.02 (d, J = 8.9 Hz, 2H), 7.47 (d, J = 8.8 Hz, 1H), 7.37 (s, 1H), 6.94 (d, J = 8.9 Hz, 2H), 6.78 (s, 1H), 6.73–6.75 (dd, J = 2.2 Hz, 2.2 Hz, 1H), 3.37–3.43 (m, 8H), 2.58 (t, J = 5.2 Hz, 4H), 2.35 (s, 3H), 1.22 (t, J = 7.0 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ: 181.86, 158.99, 153.94, 151.22, 149.10, 131.53, 128.10, 123.45, 116.59, 113.75, 111.01, 93.27, 54.90, 47.56, 46.23, 45.14, 12.64.
Compound 6: Yellow solid; 1H-NMR (400 MHz, CDCl3) δ: 8.01 (d, J = 9.0 Hz, 2H), 7.46 (d, J = 8.8 Hz, 1H), 7.37 (s, 1H), 6.93 (d, J = 9.0 Hz, 1H), 6.77 (s, 1H), 6.72–6.75 (dd, J = 2.2 Hz, 2.2 Hz, 1H), 3.38–3.44 (m, 8H), 2.63 (t, J = 5.1 Hz, 4H), 2.51 (q, J = 7.2 Hz, 2H), 1.22 (t, J = 7.0 Hz, 6H), 1.15 (t, J = 7.2 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ: 181.84, 158.95, 153.90, 151.11, 149.05, 131.48, 128.01, 123.43, 116.66, 116.50, 113.67, 110.95, 93.16, 52.55, 52.43, 47.46, 45.09, 12.60, 11.97.
Compound 7: Pale brown solid; 1H-NMR (400 MHz, CDCl3) δ: 8.01 (d, J = 9.0 Hz, 2H), 7.45 (d, J = 8.8 Hz, 1H), 7.36 (s, 1H), 6.91 (d, J = 9.0 Hz, 2H), 6.76 (s, 1H), 6.71–6.73 (dd, J = 2.2 Hz, 2.2 Hz, 1H), 3.43 (q, J = 7.1 Hz, 4H), 3.37 (t, J = 5.2 Hz, 4H), 2.57 (t, J = 5.0 Hz, 4H), 2.538 (t, J = 7.6 Hz, 2H), 1.25–1.30 (m, 32H), 1.20 (t, J = 7.0 Hz, 6H), 0.88 (t, J = 6.6 Hz, 3H); 13C-NMR (100 MHz, CDCl3) δ: 181.71, 158.90, 153.96, 151.20, 149.00, 131.46, 127.86, 123.37, 116.53, 116.44, 113.53, 110.92, 93.21, 58.83, 53.03, 47.52, 45.06, 31.99, 29.76, 29.69, 29.67, 29.42, 27.64, 26.95, 22.75, 14.17, 12.58; HRMS (ESI-TOF): m/z calcd for C41H63N3O2 [M + H]+ 630.4993, found 630.4983.
Compound 8: Yellow solid; 1H-NMR (300 MHz, CDCl3) δ: 8.02 (d, J = 8.7 Hz, 2H), 7.48 (d, J = 8.7 Hz, 1H), 7.38 (s, 1H), 6.95 (d, J = 8.7 Hz, 2H), 6.72–6.78 (m, 2H), 5.83–5.97 (m, 1H), 5.26 (d, J = 15.0 Hz, 1H), 5.21 (d, J = 8.1 Hz, 1H), 3.37–3.46 (m, 8H), 3.08 (d, J = 6.6 Hz, 2H), 2.63 (t, J = 5.1 Hz, 4H), 1.23 (t, J = 6.9 Hz, 6H); 13C-NMR (75 MHz, CDCl3) δ:181.94, 158.98, 153.98, 151.11, 149.05, 134.75, 131.53, 128.03, 123.47, 118.60, 116.74, 116.51, 113.70, 110.93, 93.17, 61.86, 52.85, 47.57, 45.14, 12.63; HRMS (ESI-TOF): m/z calcd for C26H31N3O2Na [M + Na]+ 440.2308, found 440.2307.
Compound 9: Pale yellow solid; 1H-NMR (400 MHz, CDCl3) δ: 8.02 (d, J = 9.0 Hz, 2H), 7.47 (d, J = 8.8 Hz, 1H), 7.37 (s, 1H), 6.94 (d, J = 9.0 Hz, 2H), 6.77 (s, 1H), 6.72–6.75 (dd, J = 2.2 Hz, 2.2 Hz, 1H), 3.36–3.43 (m, 10H), 2.73 (t, J = 5.1 Hz, 4H), 2.28 (s, 1H), 1.22 (t, J = 7.1 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ: 181.81, 158.97, 153.86, 151.19, 149.09, 131.50, 128.16, 123.43, 116.60, 116.55, 113.80, 110.99, 93.23, 78.47, 73.65, 51.62, 47.54, 47.00, 45.11, 12.62; HRMS (ESI-TOF): m/z calcd for C26H29N3O2 [M + H]+ 416.2333, found 416.2337.
Compound 10: Yellow solid; 1H-NMR (400 MHz, CDCl3) δ: 8.01 (d, J = 8.3 Hz, 2H), 7.46 (d, J = 8.8 Hz, 1H), 7.25–7.37 (m, 6H), 6.91 (d, J = 8.5 Hz, 2H), 6.78 (s, 1H), 6.74 (d, J = 6.7 Hz, 1H), 3.55 (s, 2H), 3.43 (q, J = 8.8 Hz, 4H), 3.36 (t, J = 4.7 Hz, 4H), 2.60 (t, J = 4.4 Hz, 4H), 1.21 (t, J = 6.9 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ: 181.79, 158.91, 153.97, 151.12, 149.00, 137.88, 131.47, 129.23, 128.39, 127.84, 127.29, 123.41, 116.58, 116.49, 113.57, 110.91, 93.14, 63.07, 52.81, 47.51, 45.07, 12.59.
Compound 11: Pale red solid; 1H-NMR (300 MHz, CDCl3) δ: 8.00 (d, J = 8.7 Hz, 2H), 7.62 (d, J = 8.1 Hz, 2H), 7.44–7.48 (m, 3H), 7.36 (s, 1H), 6.92 (d, J = 9.0 Hz, 2H), 6.76 (s, 1H), 6.71–6.76 (dd, J = 2.1 Hz, 2.1 Hz, 1H), 3.58 (s, 2H), 3.33–3.44 (m, 8H), 2.59 (t, J = 4.8 Hz, 4H), 1.22 (t, J = 6.9 Hz, 6H); 13C-NMR (75 MHz, CDCl3) δ: 181.78, 158.93, 153.83, 150.99, 149.01, 143.91, 132.25, 131.45, 129.56, 128.04, 123.44, 119.00, 116.73, 116.40, 113.68, 111.05, 93.02, 62.39, 52.86, 47.51, 45.07, 12.57.
Compound 12: Brown solid; 1H-NMR (400 MHz, CDCl3) δ: 8.02 (d, J = 9.0 Hz, 2H), 7.59 (d, J = 8.1 Hz, 2H), 7.48 (d, J = 3.1 Hz, 2H), 7.46 (d, J = 3.9 Hz, 1H), 7.38 (s, 1H), 6.91 (d, J = 9.0 Hz, 2H), 6.78 (s, 1H), 6.72–6.75 (dd, J = 2.2 Hz, 2.2 Hz, 1H), 3.58 (s, 2H), 3.43 (q, J = 7.0 Hz, 4H), 3.36 (t, J = 4.9 Hz, 4H), 2.58 (t, J = 5.0 Hz, 4H), 1.21 (t, J = 7.0 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ: 181.68, 158.90, 153.87, 151.08, 149.01, 142.32, 131.43, 129.24, 127.92, 125.32, 125.29, 125.25, 125.22, 123.40, 116.57, 116.44, 113.60, 110.91, 93.06, 62.34, 52.78, 47.46, 45.02, 12.53; HRMS (ESI-TOF): m/z calcd for C31H32F3N3O2 [M + H]+ 536.2519, found 536.2526.
Compound 13: Pale yellow solid; 1H-NMR (400 MHz, CDCl3) δ: 8.01 (d, J = 9.0 Hz, 2H), 7.46 (d, J = 8.8 Hz, 1H), 7.37 (s, 1H), 7.19- 7.22 (m, 1H), 7.03–7.17 (m, 2H), 8.01 (d, J = 9.0 Hz, 2H), 6.77 (s, 1H), 6.72–6.74 (dd, J = 2.2 Hz, 2.2 Hz, 1H), 3.46 (s, 2H), 3.43 (q, J = 7.1 Hz, 4H), 3.35 (t, J = 4.9 Hz, 4H), 2.56 (t, J = 5.0 Hz, 4H), 1.21 (t, J = 7.1 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ: 181.67, 158.88, 153.84, 151.05, 148.99, 135.27, 135.22, 135.18, 131.41, 127.89, 124.76, 124.72, 124.70, 124.66, 123.39, 117.69, 117.52, 117.02, 116.85, 116.57, 116.42, 113.58, 110.90, 93.04, 61.76, 52.66, 47.45, 45.01, 12.53; HRMS (ESI-TOF): m/z calcd for C30H32F2N3O2 [M + H]+ 504.2457, found 504.2453.
Compound 14: Pale brown solid; 1H-NMR (400 MHz, CDCl3) δ: 8.20 (d, J = 8.8 Hz, 2H), 8.02 (d, J = 9.0 Hz, 2H), 7.55 (d, J = 8.8 Hz, 2H), 7.47 (d, J = 8.8 Hz, 1H), 7.37 (s, 1H), 6.93 (d, J = 9.0 Hz, 2H), 6.77 (s, 1H), 6.73–6.76 (dd, J = 2.2 Hz, 2.2 Hz, 1H), 3.64 (s, 2H), 3.36–3.45 (m, 8H), 2.62 (t, J = 5.1 Hz, 4H), 1.23 (t, J = 7.0 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ: 181.79, 158.99, 153.88, 151.18, 149.13, 147.41, 146.08, 131.52, 129.59, 128.20, 123.71, 123.45, 116.63, 116.54, 113.77, 111.03, 93.21, 62.17, 52.97, 47.63, 45.12, 12.63.
Compound 15: Yellow solid; 1H-NMR (300 MHz, CDCl3) δ: 8.01 (d, J = 8.7 Hz, 2H), 7.47 (d, J = 8.7 Hz, 1H), 7.37 (s, 1H), 6.94 (d, J = 8.7 Hz, 2H), 6.77 (s, 1H), 6.75 (d, J = 9.0 Hz, 1H), 4.24 (q, J = 6.9 Hz, 2H), 3.45 (q, J = 6.9 Hz, 8H), 3.27 (s, 2H), 2.76 (t, J = 5.1 Hz, 4H), 1.31 (t, J = 7.2 Hz, 3H), 1.22 (t, J = 7.2 Hz, 6H); 13C-NMR (75 MHz, CDCl3) δ: 181.91, 170.21, 158.97, 153.85, 151.06, 149.04, 131.50, 128.15, 123.47, 116.77, 113.79, 110.92, 93.12, 60.88, 59.46, 52.75, 47.47, 45.12, 14.37, 12.61; HRMS (ESI-TOF): m/z calcd for C27H34N3O4 [M + H]+ 464.2543, found 464.2545.
Compound 16: Pale red solid; 1H-NMR (400 MHz, CDCl3) δ: 7.99 (d, J = 8.9 Hz, 2H), 7.45 (d, J = 8.8 Hz, 1H), 7.35 (s, 1H), 6.90 (d, J = 9.0 Hz, 2H), 6.75 (s, 1H), 6.70–6.73 (dd, J = 2.2 Hz, 2.2 Hz, 1H), 3.36–3.40 (m, 8H), 3.23 (s, 2H), 2.62 (t, J = 5.0 Hz, 4H), 2.14 (s, 3H), 1.19 (t, J = 7.0 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ: 206.03, 181.69, 158.87, 153.75, 151.00, 148.98, 131.39, 128.00, 123.38, 116.60, 116.39, 113.65, 110.89, 93.02, 67.93, 52.98, 47.33, 45.00, 27.81, 12.52; HRMS (ESI-TOF): m/z calcd for C26H31N3O3 [M + H]+ 434.2438, found 434.2444.
Compound 17: Pale yellow solid; 1H-NMR (300 MHz, CDCl3) δ: 8.03 (d, J = 9.0 Hz, 4H), 7.59 (d, J = 7.2 Hz, 1H), 7.45–7.50 (m, 3H), 7.38 (s, 1H), 6.96 (d, J = 9.0 Hz, 2H), 6.78 (s, 1H), 6.73–6.77 (dd, J = 2.1 Hz, 2.1 Hz, 1H), 3.90 (s, 2H), 3.39–3.48 (m, 8H), 2.80 (t, J = 4.8 Hz, 4H), 1.24 (t, J = 7.2 Hz, 6H); 13C-NMR (75 MHz, CDCl3) δ: 196.18, 181.89, 158.96, 153.88, 151.10, 149.05, 136.01, 133.52, 131.50, 128.73, 128.20, 123.45, 116.72, 116.50, 113.76, 110.94, 93.16, 64.36, 53.26, 47.50, 45.10, 12.61; HRMS (ESI-TOF): m/z calcd for C31H34N3O3 [M + H]+ 496.2595, found 496.2598.
Compound 18: Pale red solid; 1H-NMR (300 MHz, CDCl3) δ: 8.59 (s, 1H), 7.97–8.09 (m, 4H), 7.93 (t, J = 8.7 Hz, 2H), 7.54–7.64 (m, 2H), 7.49 (d, J = 8.7 Hz, 1H), 7.39 (s, 1H), 6.97 (d, J = 8.7 Hz, 2H), 6.78 (s, 1H), 6.73–6.77 (dd, J = 2.1 Hz, 2.1 Hz, 1H), 4.04 (s, 2H), 3.40–3.50 (m, 8H), 2.85 (t, J = 5.1 Hz, 4H), 1.24 (t, J = 6.9 Hz, 6H); 13C-NMR (75 MHz, CDCl3) δ: 196.21, 182.00, 159.02, 153.94, 149.11, 135.87, 132.60, 131.56, 129.96, 129.76, 128.80, 128.65, 128.21, 127.97, 127.03, 123.96, 123.49, 116.75, 116.57, 113.83, 110.99, 93.24, 64.56, 53.38, 47.60, 45.16, 12.66; HRMS (ESI-TOF): m/z calcd for C35H35N3O3Na [M + Na]+ 568.2570, found 568.2569.
Compound 19: Orange solid; 1H-NMR (300 MHz, CDCl3) δ: 8.04–8.08 (m, 2H), 8.01 (d, J = 8.7 Hz, 2H), 7.47 (d, J = 8.7 Hz, 1H), 7.37 (s, 1H), 7.16 (t, J = 8.4 Hz, 2H), 6.94 (d, J = 8.7 Hz, 2H), 6.77 (s, 1H), 6.72–6.75 (dd, J = 2.1 Hz, 2.1 Hz, 1H), 3.84 (s, 2H), 3.38–3.45 (m, 8H), 2.77 (t, J = 5.1 Hz, 4H), 1.22 (t, J = 6.9 Hz, 6H); 13C-NMR (75 MHz, CDCl3) δ: 194.77, 181.92, 159.00, 153.86, 151.05, 149.08, 132.41, 131.51, 131.10, 130.97, 128.19, 123.49, 116.82, 116.49, 116.01, 115.72, 113.81, 110.95, 93.12, 64.51, 53.26, 47.51, 45.12, 12.62.
Compound 20: Pale brown solid; 1H-NMR (300 MHz, CDCl3) δ: 8.01 (d, J = 9.3 Hz, 2H), 7.98 (d, J = 9.0 Hz, 2H), 7.47 (d, J = 8.4 Hz, 1H), 7.42 (d, J = 8.4 Hz, 2H), 7.37 (s, 1H), 6.94 (d, J = 9.0 Hz, 2H), 6.77 (s, 1H), 6.72–6.77 (dd, J = 2.1 Hz, 2.1 Hz, 1H), 3.83 (s, 2H), 3.43 (t, J = 7.2 Hz, 8H), 2.76 (t, J = 4.8 Hz, 4H), 1.22 (t, J = 7.2 Hz, 6H); 13C-NMR (75 MHz, CDCl3) δ: 195.17, 181.86, 158.96, 153.82, 151.01, 149.04, 139.94, 134.21, 131.48, 129.75, 129.03, 128.15, 123.47, 116.79, 116.44, 113.78, 110.92, 93.07, 64.50, 53.22, 47.47, 45.10, 12.60; HRMS (ESI-TOF): m/z calcd for C31H32N3O3NaCl [M + Na]+ 552.2024, found 552.2023.
Compound 21: Pale green solid; 1H-NMR (300 MHz, CDCl3) δ: 8.03 (d, J = 8.7 Hz, 2H), 7.92 (d, J = 8.4 Hz, 2H), 7.63 (d, J = 8.7 Hz, 2H), 7.48 (d, J = 8.7 Hz, 1H), 7.38 (s, 1H), 6.96 (d, J = 9.0 Hz, 2H), 6.78 (s, 1H), 6.73–6.77 (dd, J = 2.1 Hz, 2.1 Hz, 1H), 3.84 (s, 2H), 3.44 (t, J = 7.2 Hz, 8H), 2.78 (t, J = 5.1 Hz, 4H), 1.24 (t, J = 6.9 Hz, 6H); HRMS (ESI-TOF): m/z calcd for C31H32BrN3O3 [M + H]+ 574.1700, found 574.1699.
Compound 22: Orange solid; 1H-NMR (400 MHz, CDCl3) δ: 8.00 (d, J = 8.9 Hz, 2H), 7.99 (d, J = 8.8 Hz, 2H), 7.45 (d, J = 8.8 Hz, 2H), 7.36 (s, 1H), 6.92 (d, J = 8.8 Hz, 2H), 6.75 (s, 1H), 6.73 (d, J = 8.8 Hz, 1H), 3.83 (s, 3H), 3.82 (s, 2H), 3.37–3.43 (m, 8H), 2.76 (t, J = 5.0 Hz, 4H), 1.20 (t, J = 7.0 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ: 194.62, 181.78, 163.78, 158.92, 153.85, 151.08, 149.05, 131.46, 130.52, 129.05, 128.00, 123.42, 116.66, 116.48, 113.83, 113.69, 110.96, 93.12, 64.02, 55.53, 53.14, 47.37, 45.05, 12.57; HRMS (ESI-TOF): m/z calcd for C32H36N3O4 [(M + H)]+ 526.2700, found 526.2700.
Compound 23: Pale yellow solid; 1H-NMR (400 MHz, CDCl3) δ: 8.11 (d, J = 8.4 Hz, 2H), 8.00 (d, J = 8.9 Hz, 2H), 7.76 (d, J = 8.4 Hz, 2H), 7.46 (d, J = 8.8 Hz, 1H), 7.36 (s, 1H), 6.92 (d, J = 9.0 Hz, 2H), 6.72–6.75 (m, 2H), 3.86 (s, 2H), 3.44 (q, J = 7.1 Hz, 8H), 2.76 (t, J = 4.9 Hz, 4H), 1.21 (t, J = 7.0 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ: 195.18, 181.85, 159.01, 153.77, 151.06, 149.15, 138.84, 132.57, 131.51, 128.84, 128.30, 123.49, 117.96, 116.83, 116.74, 116.50, 113.87, 111.05, 93.14, 64.70, 53.15, 47.48, 45.11, 12.61; HRMS (ESI-TOF): m/z calcd for C32H33N4O3 [M + H]+ 521.2547, found 521.2547.
Compound 24: Pale green solid; 1H-NMR (400 MHz, CDCl3) δ: 8.04 (q, J = 8.6 Hz, 2H), 7.93 (q, J = 7.9 Hz, 2H), 7.49 (q, J = 8.7 Hz, 1H), 7.40 (d, J = 15.3 Hz, 1H), 7.28 (q, J = 7.7 Hz, 2H), 6.95 (q, J = 8.6 Hz, 2H), 6.79 (d, J = 13.3 Hz, 1H), 6.73 (d, J = 8.9 Hz, 1H), 3.88 (d, J = 15.4 Hz, 2H), 3.39–3.44 (m, 8H), 2.79 (t, J = 4.1 Hz, 4H), 2.42 (t, J = 6.2 Hz, 3H), 1.16–1.24 (m, 6H); 13C-NMR (100 MHz, CDCl3) δ: 195.75, 181.80, 158.95, 153.88, 151.15, 149.08, 144.33, 133.55, 131.49, 129.37, 128.30, 128.05, 123.42, 116.62, 116.52, 113.72, 110.98, 93.17, 64.15, 53.17, 47.43, 45.07, 21.74, 12.59.
Compound 25: Brown solid; 1H-NMR (400 MHz, CDCl3) δ: 8.56 (d, J = 6.0 Hz, 2H), 8.01 (d, J = 8.9 Hz, 2H), 7.46 (d, J = 8.8 Hz, 1H), 7.37 (s, 1H), 7.31 (d, J = 5.9 Hz, 1H), 6.93 (d, J = 9.0 Hz, 2H), 6.77 (s, 1H), 6.75 (d, J = 8.8 Hz, 1H), 3.56 (s, 2H), 3.36–3.44 (m, 8H), 2.61 (t, J = 5.0 Hz, 4H), 1.22 (t, J = 7.0 Hz, 6H); 13C-NMR (100 MHz, CDCl3) δ: 181.81, 158.98, 153.89, 151.15, 150.57, 150.01, 149.09, 147.36, 131.50, 128.15, 123.92, 123.44, 121.35, 116.64, 116.52, 114.50, 113.74, 110.98, 93.19, 61.76, 52.97, 47.60, 45.11, 12.62; HRMS (ESI-TOF): m/z calcd for C29H33N4O2 [M + H]+ 469.2598, found 469.2601.
3.3. Biological Activity Experiments
3.3.1. Anti-Inflammatory Activity
Murine RAW264.7 macrophages were plated in 96-well plate at a density of 1 × 105 cells/well and stimulated with 1 μg/mL LPS in the presence or absence of various concentrations of compound for 24 h. The production of NO was determined by assaying culture supernatant for NO2−. Supernatant (100 μL) was mixed with an equal volume of Griess reagent at r.t. for 10 min. Absorbance was measured at 540 nm in a microplate reader.
3.3.2. Antitumor Activity
The assay was carried out using the method described previously. About 1 × 104 cell/well were seeded into 96-well microtiter plates. At twenty-four hours post-seeding, cells were treated with vehicle control or various concentrations of samples for 48 h. Twenty microliters of MTT solution (5 mg/mL) was added to each well, and the tumor cells were incubated at 37 °C in a humidified atmosphere of 5% CO2 air for 4 h. Upon removal of MTT/medium, 150 μL of DMSO was added to each well, and the plate was agitated at oscillator for 5 min to dissolve the MTT-formazan. The assay plate was read at a wavelength of 570 nm using a microplate reader.
4. Conclusions
In summary, a series of novel hybrid compounds between benzofuran and N-aryl piperazine have been synthesized and screened in vitro for anti-inflammatory and anticancer activity. The results demonstrated that derivative 16 not only had an inhibitory effect on the generation of NO (IC50 = 5.28 μM), but also displayed good cytotoxic activity against A549 and SGC7901 (IC50 = 0.12 μM and 2.75 μM, respectively), which was considered to be the most potent anti-inflammatory and anti-tumor agent in this study. Further research is currently underway, and the results will be reported in due course.
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (81460624, 81560620), the Application Basic Research Program of Yunnan Province (2014FZ078, 2014FZ087) and the Yunnan Province doctoral newcomer Award (YN2015015). We acknowledge the Key Discipline of Pharmacy and Yi medicine (Yunnan University of Traditional Chinese Medicine, China) for support on research.
Supplementary Materials
The 1H-NMR, 13C-NMR and HRMS spectra are available online at: http://www.mdpi.com/1420-3049/21/12/1684/s1.
Author Contributions
Yulu Ma and Zewei Mao designed and carried out the experiments and wrote the paper; Xi Zheng assisted in experiment; Hui Gao analyzed the data; Chunping Wan supervised and directed the biological assays; Gaoxiong Rao supervised the whole experiment and provided technical guidance. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 3–25 are available from the authors.
References
- 1.Xue S.T., Guo H.F., Liu M.J., Jin J., Ju D.H., Liu Z.Y., Li Z.R. Synthesis of a novel class of substituted benzothiophene or benzofuran derivatives as BMP-2 up-regulators and evaluation of the BMP-2-up-regulating effects in vitro and the effects on glucocorticoid-induced osteoporosis in rats. Eur. J. Med. Chem. 2015;96:151–161. doi: 10.1016/j.ejmech.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Hassan G.S., Abou-Seri S.M., Kamel G., Ali M.M. Celecoxib analogs bearing benzofuran moiety as cyclooxygenase-2 inhibitors: Design, synthesis and evaluation as potential anti-inflammatory agents. Eur. J. Med. Chem. 2014;76:482–493. doi: 10.1016/j.ejmech.2014.02.033. [DOI] [PubMed] [Google Scholar]
- 3.Carrër A., Brinet D., Florent J.C., Rousselle P., Bertounesque E. Palladium-Catalyzed Direct Arylation of Polysubstituted Benzofurans. J. Org. Chem. 2012;77:1316–1327. doi: 10.1021/jo202060k. [DOI] [PubMed] [Google Scholar]
- 4.Meshram H.M., Reddy B.C., Prasad B.R.V., Goud P.R., Kumar G.S., Kumar R.N. DABCO-Promoted Efficient and Convenient Synthesis of Benzofurans. Synth. Commun. 2012;42:1669–1676. doi: 10.1080/00397911.2010.542862. [DOI] [Google Scholar]
- 5.Kumaraswamy G., Ramakrishna G., Raju R., Padmaja M. An expedient synthesis of enantioenriched substituted (2-benzofuryl)arylcarbinols via tandem Rap–Stoermer and asymmetric transfer hydrogenation reactions. Tetrahedron. 2010;66:9814–9818. doi: 10.1016/j.tet.2010.10.074. [DOI] [Google Scholar]
- 6.Watanabe H., Kawasaki A., Sano K., Ono M., Saji H. Synthesis and evaluation of copper-64 labeled benzofuran derivatives targeting β-amyloid aggregates. Bioorg. Med. Chem. 2016;24:3618–3623. doi: 10.1016/j.bmc.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Na M., Hoang D.M., Njamen D., Mbafor J.T., Fomum Z.T., Thuong P.T., Ahn J.S., Oh W.K. Inhibitory effect of 2-arylbenzofurans from Erythrina addisoniae on protein tyrosine phosphatase-1B. Bioorg. Med. Chem. Lett. 2007;17:3868–3871. doi: 10.1016/j.bmcl.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Tian Y., Liang Z., Xu H., Mou Y., Guo C. Design, Synthesis and Cytotoxicity of Novel Dihydroartemisinin-Coumarin Hybrids via Click Chemistry. Molecules. 2016;21:758–772. doi: 10.3390/molecules21060758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Z., Xu H., Tian Y., Guo M., Su X., Guo C. Design, Synthesis and Antifungal Activity of Novel Benzofuran-Triazole Hybrids. Molecules. 2016;21:732–742. doi: 10.3390/molecules21060732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X.Q., Liu L.X., Li Y., Sun C.J., Chen W., Li L., Zhang H.B., Yang X.D. Design, synthesis and biological evaluation of novel hybrid compounds of imidazole scaffold-based 2-benzylbenzofuran as potent anticancer agents. Eur. J. Med. Chem. 2013;62:111–121. doi: 10.1016/j.ejmech.2012.12.040. [DOI] [PubMed] [Google Scholar]
- 11.Vinh T.K., Yee S.W., Kirby1 A.J., Nicholls1 P.J., Simons C. 1-[(Benzofuran-2-yl) phenylmethyl] triazoles as steroidogenic inhibitors: Synthesis and in vitro inhibition of human placental CYP19 aromatase. Anti-Cancer Drug Des. 2001;16:217–225. [PubMed] [Google Scholar]
- 12.Romagnoli R., Baraldi P.G., Sarkar T., Carrion M.D., Cruz-Lopez O., Cara C.L., Tolomeo M., Grimaudo S., Cristina A.D., Pipitone M.R., et al. Synthesis and biological evaluation of 2-(3′,4′,5-trimethoxy benzoyl)-3-N,N-dimethylamino benzo[b]furan derivatives as inhibitors of tubulin polymerization. Bioorg. Med. Chem. 2008;16:8419–8426. doi: 10.1016/j.bmc.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pevet I., Brulé C., Tizot A., Gohier A., Cruzalegui F., Boutin J.A., Goldstein S. Synthesis and pharmacological evaluation of thieno[2,3-b]pyridine derivatives as novel c-Src inhibitors. Bioorg. Med. Chem. 2011;19:2517–2528. doi: 10.1016/j.bmc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Yang X.D., Wan W.C., Deng X.Y., Li Y., Yang L.J., Li L., Zhang H.B. Design, synthesis and cytotoxic activities of novel hybrid compounds between 2-phenylbenzofuran and imidazole. Bioorg. Med. Chem. Lett. 2012;22:2726–2729. doi: 10.1016/j.bmcl.2012.02.094. [DOI] [PubMed] [Google Scholar]
- 15.Mao Z.W., Wan C.P., Jiang Y., Guo W.L., Rao G.X. Synthesis and Anti-tumor activity in vitro of N-hetercycle substitued benzofuran derivatives. J. China Pharm. Univ. 2015;46:58–61. [Google Scholar]
- 16.Vergelli C., Ciciani G., Cilibrizzi A., Crocetti L., Mannelli L.D.C., Ghelardini C., Guerrini G., Iacovone A., Giovannoni M.P. Synthesis of five and six-membered heterocycles bearing an arylpiperazinylalkyl side chain as orally active antinociceptive agents. Bioorg. Med. Chem. 2015;23:6237–6245. doi: 10.1016/j.bmc.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 17.Biswas N.N., Kutty S.K., Iskander G.M., Mielczarek M., Bhadbhade M.M., Gardner C.R., Black D.S., Kumar N. Synthesis of brominated novel N-heterocycles: New scaffolds for antimicrobial discovery. Tetrahedron. 2016;72:539–546. doi: 10.1016/j.tet.2015.12.018. [DOI] [Google Scholar]
- 18.Chaudhary P., Kumar R., Verma A.K., Singh D., Yadav V., Chhillar A.K., Sharma G.L., Chandra R. Synthesis and antimicrobial activity of N-alkyl and N-aryl piperazine derivatives. Bioorg. Med. Chem. 2006;14:1819–1826. doi: 10.1016/j.bmc.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 19.Zhao H.Y., Prosser A.R., Liottaa D.C., Wilson L.J. Discovery of novel N-aryl piperazine CXCR4 antagonists. Bioorg. Med. Chem. Lett. 2015;25:4950–4955. doi: 10.1016/j.bmcl.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao Z.W., Zheng X., Lin Y.P., Hu C.Y., Wang X.L., Wan C.P., Rao G.X. Design, synthesis and anticancer activity of novel hybrid compounds between benzofuran and N-aryl piperazine. Bioorg. Med. Chem. Lett. 2016;26:3421–3424. doi: 10.1016/j.bmcl.2016.06.055. [DOI] [PubMed] [Google Scholar]
- 21.Mao Z.W., Zheng X., Qi Y., Zhang M.D., Huang Y., Wan C.P., Rao G.X. Synthesis and biological evaluation of novel hybrid compounds between chalcone and piperazine as potential antitumor agents. RSC Adv. 2016;6:7723–7727. doi: 10.1039/C5RA20197G. [DOI] [Google Scholar]
- 22.Mao Z.W., Zheng X., Lin Y.P., Qi Y., Hu C.Y., Wan C.P., Rao G.X. Concise synthesis and biological evaluation of chalcone derivatives bearing N-heterocyclic moieties. Heterocycles. 2016;92:1102–1110. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.