Abstract
Interest in essential oils with pesticidal activity against insects and pests is growing. In this study, essential oils from different parts (leaves, twigs and seeds) of Cinnamomum camphora L. Presl were investigated for their chemical composition, and insecticidal and repellent activities against the cotton aphid. The essential oils, obtained by hydrodistillation, were analyzed by GC×GC-TOFMS. A total of 96 components were identified in the essential oils and the main constituents found in the leaves and twigs were camphor, eucalyptol, linalool and 3,7-dimethyl-1,3,7-octatriene. The major components found in the seeds were eucalyptol (20.90%), methyleugenol (19.98%), linalool (14.66%) and camphor (5.5%). In the contact toxicity assay, the three essential oils of leaves, twigs and seeds exhibited a strong insecticidal activity against cotton aphids with LC50 values of 245.79, 274.99 and 146.78 mg/L (after 48 h of treatment), respectively. In the repellent assay, the highest repellent rate (89.86%) was found in the seed essential oil at the concentration of 20 μL/mL after 24 h of treatment. Linalool was found to be a significant contributor to the insecticidal and repellent activities. The results indicate that the essential oils of C. camphora might have the potential to be developed into a natural insecticide or repellent for controlling cotton aphids.
Keywords: Cinnamomum camphora, essential oils, GC×GC-TOFMS, contact toxicity, cotton aphid, repellent activity, linalool
1. Introduction
As an alternative to toxic pesticides, essential oils have attracted particular attention because of their specificity to pests, their biodegradable nature and their potential for commercial application [1]. Cinnamomum camphora (L.) Presl (family: Lauraceae), commonly known as the camphor tree, is a large evergreen tree and is widely distributed in subtropical zones, including southeastern China and northeastern Australia [2]. C. camphora has long been recognized as a source of essential oil. The essential oil of C. camphora can be utilized as a medicine and perfume. According to the previous studies, the essential oil from C. camphora has various bioactive properties, such as antioxidant [3], antibacterial [4,5], antifungal [6], insecticidal [1,7] and repellent activities [1]. The leaves and bark of C. camphora are rich in terpenoids, sesquiterpenes and phenylpropanoids, which are an important group of secondary metabolites and are associated with these bioactivities [6,8].
The cotton aphid (Aphis gossypii Glover) is one of the most serious pests of cotton throughout the world [9]. It can cause damage to the host plant not only by direct feeding, but also through transmission of viral diseases [10]. At present, chemical pesticides are still the primary method for controlling aphids on crop plants [11]. However, the indiscriminate use of these chemical pesticides is very harmful for human health and the environment [12]. It is well known that secondary metabolites of some plants may act as insecticides, such as flavonoids (rotenone) [13], terpenes (azadirachtin) [14,15], alkaloids (oxymatrine) [16] and essential oils [17,18,19,20]. Therefore, botanical pesticides have been considered as an attractive alternative to chemical pesticides. To the best of our knowledge, there is still no reported work on the insecticidal and repellent activities against cotton aphids from the essential oils of C. camphora.
Traditionally, the chemical composition of C. camphora essential oil has been detected by GC and GC-MS. Compared with the traditional GC, two-dimensional GC (2D-GC) technology is an emerging technology that provides higher peak capacity, greater separation capacity and an improved signal-to-noise ratio [21]. Comprehensive two-dimensional GC coupled to time-of-flight mass spectrometry (GC×GC-TOFMS) is a powerful technology, which has been successfully applied for qualitative and quantitative analysis of the chemical composition of different plants [22,23].
Therefore, the aims of this study were to investigate the chemical composition of the essential oils from different parts of C. camphora using GC×GC-TOFMS, and to evaluate the insecticidal and repellent activities of the essential oils against cotton aphids.
2. Results and Discussion
2.1. Chemical Composition of the C. Camphora Essential Oils
The yields of leaf, twig and seed essential oils obtained by water distillation were 0.86% (w/w relative to dry material weight), 0.48% and 2.2%, respectively. In order to obtain higher separation efficiency, GC×GC-TOFMS was used to analyze the essential oils of C. camphora. The identification of compounds was carried out by comparing the mass spectra obtained with those from the NIST2011 mass spectral library or with mass spectra from the literature [22,24,25,26], and by co-injection of available standard compounds, such as eucalyptol (purity 99%), camphor (purity 96%), methyleugenol (purity ≥ 98%) and linalool (purity 97%). Tentative identification of some terpenoids was carried out using retention indices (RI), the zone of elution and mass spectra. The peaks with matching similarity of more than 80% were accepted as candidate compounds. The quantification was carried out by peak area normalization. The GC×GC-TOFMS analysis results for the oils are presented in Table 1.
Table 1.
Chemical compounds identified in the essential oils from C. camphora by two-dimensional gas chromatography coupled to time-of-flight mass spectrometry (GC×GC-TOFMS).
| No. | Compound | RI | Rt (s) 1 | Peak Area (%) | |||
|---|---|---|---|---|---|---|---|
| Leaf | Twig | Seed | |||||
| 1 | methyl isobutyl ketone | <800 | 196, 0.98 | 0.04 | 0.04 | Tr 2 | |
| 2 | hexanal | <800 | 236, 1.19 | 0.03 | 0.02 | - 3 | |
| 3 | 3-hexen-1-ol | 843 | 284, 1.33 | 0.03 | tr | - | |
| 4 | (E)-2-hexenal | 843 | 284, 1.48 | 0.03 | - | - | |
| 5 | 1-hexanol | 858 | 296, 1.25 | 0.03 | - | - | |
| 6 | α-thujene | 924 | 356, 1.10 | 0.11 | 0.10 | 0.27 | |
| 7 | α-pinene | 935 | 368, 1.14 | 1.20 | 0.76 | 1.00 | |
| 8 | camphene | 950 | 384, 1.23 | 0.41 | 0.33 | 0.23 | |
| 9 | sabinene | 973 | 408, 1.30 | 1.95 | 0.68 | 1.36 | |
| 10 | β-pinene | 980 | 416, 1.31 | 1.25 | 0.51 | 1.54 | |
| 11 | α-phellandrene | 1006 | 444, 1.33 | 0.09 | 0.14 | 2.70 | |
| 12 | α-terpinene | 1017 | 456, 1.34 | 0.19 | 0.21 | 0.83 | |
| 13 | p-cymene | 1024 | 464, 1.54 | 0.16 | 0.51 | 1.10 | |
| 14 | limonene | 1028 | 468, 1.38 | 0.92 | 0.65 | 2.32 | |
| 15 | eucalyptol | 1034 | 476, 1.54 | 16.46 | 17.21 | 20.90 | |
| 16 | β-ocimene | 1042 | 484, 1.36 | tr | 0.16 | 0.21 | |
| 17 | γ-terpinene | 1056 | 500, 1.47 | 0.51 | 0.98 | 1.94 | |
| 18 | terpinolene | 1084 | 532, 1.53 | 0.15 | 0.23 | 0.44 | |
| 19 | trans-linalool oxide | 1084 | 532, 1.65 | 0.12 | 0.18 | tr | |
| 20 | 3,7-dimethyl-1,3,7-octatriene | 1095 | 544, 1.67 | 11.07 | 11.47 | - | |
| 21 | linalool | 1099 | 548, 1.57 | 11.58 | 5.13 | 14.66 | |
| 22 | hotrienol | 1099 | 548, 1.68 | 0.59 | 0.79 | - | |
| 23 | 6-methyl-3,5-heptadiene-2-one | 1099 | 548, 2.12 | - | 0.03 | - | |
| 24 | E,E-2,6-dimethyl-1,3,5,7-octatetraene | 1127 | 580, 1.62 | 0.06 | 0.03 | - | |
| 25 | camphor | 1149 | 604, 2.20 | 18.48 | 13.17 | 5.55 | |
| 26 | octanoic acid | 1160 | 616, 1.54 | - | - | 0.03 | |
| 27 | endo-borneol | 1174 | 632, 1.90 | 0.30 | 0.16 | - | |
| 28 | terpinen-4-ol | 1181 | 640, 1.83 | 0.87 | 1.11 | 1.01 | |
| 29 | α-terpineol | 1196 | 656, 1.90 | 5.00 | 4.38 | 2.98 | |
| 30 | estragole | 1196 | 656, 2.16 | - | - | 0.04 | |
| 31 | E,E-2,6-dimethyl-3,5,7-octatriene-2-ol | 1203 | 664, 1.86 | 0.08 | - | - | |
| 32 | trans-3-methyl-6-(1-methylethyl)-2-cyclohexen-1-ol | 1207 | 668, 1.87 | - | 0.04 | 0.03 | |
| 33 | carveol | 1215 | 676, 2.09 | 0.06 | 0.04 | - | |
| 34 | citronellol | 1219 | 680, 1.67 | 0.02 | 0.02 | - | |
| 35 | (Z)-3,7-dimethyl-2,6-octadienal | 1234 | 696, 2.02 | 0.03 | 0.02 | - | |
| 36 | (Z)-3,7-dimethyl-2,6-octadien-1-ol | 1245 | 708, 1.83 | 0.04 | 0.09 | 0.07 | |
| 37 | (E)-3,7-dimethyl-2,6-octadienal | 1264 | 728, 2.04 | 0.03 | 0.02 | - | |
| 38 | bornyl acetate | 1283 | 748, 1.84 | 0.25 | 0.25 | 0.03 | |
| 39 | safrole | 1290 | 756, 2.42 | 0.04 | 0.05 | 3.28 | |
| 40 | thymol | 1294 | 760, 2.20 | - | 0.03 | - | |
| 41 | δ-elemene | 1337 | 804, 1.45 | 0.07 | - | 0.08 | |
| 42 | α-cubebene | 1349 | 816, 1.45 | 0.04 | 0.17 | tr | |
| 43 | 2-methoxy-3-(2-propenyl)-phenol | 1349 | 816, 2.47 | - | 0.13 | - | |
| 44 | n-decanoic acid | 1361 | 828, 1.65 | - | - | 1.72 | |
| 45 | neryl acetate | 1369 | 836, 1.80 | - | - | 0.03 | |
| 46 | unknown | 1369 | 836, 1.82 | 0.02 | tr | - | |
| 47 | ylangene | 1373 | 840, 1.52 | 0.08 | 0.08 | 0.07 | |
| 48 | α-copaene | 1381 | 848, 1.51 | 0.18 | 0.96 | 0.35 | |
| 49 | β-elemene | 1389 | 856, 1.58 | 0.42 | 0.38 | 0.31 | |
| 50 | methyleugenol | 1393 | 860, 2.55 | 0.12 | 0.40 | 19.98 | |
| 51 | dodecanal | 1401 | 868, 1.58 | - | 0.04 | 0.04 | |
| 52 | α-gurjunene | 1414 | 880, 1.60 | 0.04 | 0.21 | 0.24 | |
| 53 | α-santalene | 1418 | 884, 1.52 | - | 0.12 | - | |
| 54 | β-caryophyllene | 1426 | 892, 1.69 | 3.40 | 3.13 | 1.71 | |
| 55 | γ-elemene | 1435 | 900, 1.49 | - | 0.03 | - | |
| 56 | β-famesene | 1447 | 912, 1.48 | - | 0.03 | - | |
| 57 | aromandendrene | 1447 | 912, 1.66 | 0.34 | 0.80 | 0.38 | |
| 58 | β-santalene | 1460 | 924, 1.62 | - | 0.04 | - | |
| 59 | α-humulene | 1464 | 928, 1.75 | 2.62 | 4.12 | 1.23 | |
| 60 | γ-gurjunene | 1473 | 936, 1.83 | 0.08 | - | - | |
| 61 | γ-muurolene | 1477 | 940, 1.72 | 0.29 | 1.24 | 0.54 | |
| 62 | 1-(1,3-dimethyl-3-cyclohexen-1-yl)-ethanone | 1481 | 944, 1.80 | - | 0.02 | - | |
| 63 | germacrene D | 1485 | 948, 1.79 | 3.76 | 0.46 | 0.57 | |
| 64 | α-selinene | 1494 | 956, 1.79 | - | 6.13 | 2.87 | |
| 65 | α-muurolene | 1502 | 964, 1.79 | 1.91 | 0.29 | 0.48 | |
| 66 | β-bisabolene | 1507 | 968, 1.59 | - | 0.04 | - | |
| 67 | δ-cadinene | 1520 | 980, 1.79 | 0.45 | 3.42 | 0.56 | |
| 68 | trans-calamenene | 1524 | 984, 1.98 | 0.07 | 0.68 | 0.04 | |
| 69 | cadina-1(2),4-diene | 1537 | 996, 1.82 | 0.07 | 0.57 | 0.07 | |
| 70 | 1,2,3-trimethoxy-5-(2-propenyl)-benzene | 1537 | 996, 2.68 | - | - | 0.06 | |
| 71 | (E)-α-bisabolene | 1542 | 1000, 1.87 | 0.07 | 0.67 | - | |
| 72 | α-calacorene | 1546 | 1004, 2.13 | tr | 0.08 | 0.02 | |
| 73 | unknown | 1551 | 1008, 1.61 | - | - | 0.16 | |
| 74 | selina-3,7(11)-diene | 1551 | 1008, 1.81 | - | - | 0.12 | |
| 75 | (E)-nerolidol | 1555 | 1012, 1.70 | 2.13 | - | 0.22 | |
| 76 | unknown | 1569 | 1024, 1.94 | 0.57 | 0.48 | 0.16 | |
| 77 | unknown | 1577 | 1032, 1.48 | - | - | 0.09 | |
| 78 | spathulenol | 1581 | 1036, 2.15 | 0.53 | 0.76 | 0.10 | |
| 79 | unknown | 1586 | 1040, 1.85 | 0.10 | 0.06 | - | |
| 80 | gleenol | 1586 | 1040, 1.89 | - | 0.07 | - | |
| 81 | caryophyllene oxide | 1591 | 1044, 2.16 | 0.39 | 1.38 | - | |
| 82 | β-elemenone | 1595 | 1048, 1.99 | - | - | 0.08 | |
| 83 | viridiflorol | 1599 | 1052, 2.20 | - | 0.12 | - | |
| 84 | tetradecanal | 1604 | 1056, 1.57 | - | 0.03 | - | |
| 85 | trans-2-undecen-1-ol | 1604 | 1056, 1.57 | - | - | 0.02 | |
| 86 | 8,9-dehydro-neoisolongifolene | 1618 | 1068, 2.14 | 0.03 | 0.05 | - | |
| 87 | humulene epoxide II | 1618 | 1068, 2.22 | 0.19 | 0.69 | - | |
| 88 | unknown | 1628 | 1076, 2.15 | 0.60 | - | - | |
| 89 | cubenol | 1632 | 1080, 2.03 | - | 0.57 | - | |
| 90 | ledene oxide-(II) | 1637 | 1084, 2.22 | 0.16 | - | - | |
| 91 | longipinene epoxide | 1642 | 1088, 2.21 | - | 0.46 | - | |
| 92 | α-cadinol | 1661 | 1104, 2.13 | 0.10 | 0.19 | 0.04 | |
| 93 | unknown | 1665 | 1108, 2.22 | - | 0.59 | 0.10 | |
| 94 | bisabolol | 1670 | 1112, 1.91 | - | 0.02 | - | |
| 95 | trans-farnesol | 1708 | 1144, 1.91 | 0.02 | 0.03 | - | |
| 96 | unknown | 1722 | 1156, 2.31 | 1.30 | 1.29 | 0.02 | |
| Total | 92.33 | 90.57 | 94.98 | ||||
1 Rt, retention time in first dimension (s) and retention time in second dimension (s); 2 tr (trace), relative content <0.02%; 3 -, not detected. RI, the retention indices were determined in relation to a homologous series of alkanes (C7–C30) under the same operating conditions.
As seen in Table 1, a total of 96 compounds were identified in leaf, twig and seed essential oils. Among these compounds, 67 compounds were identified in the leaf essential oil of C. camphora, representing 92.33% of the total oil, and the major compounds were camphor (18.48%), eucalyptol (16.46%), linalool (11.58%) and 3,7-dimethyl-1,3,7-octatriene (11.07%). A total of 79 identified constituents which could account for 90.57% of the total essential oil from C. camphora twigs, in which the main compounds were eucalyptol (17.21%), camphor (13.17%) and 3,7-dimethyl-1,3,7-octatriene (11.47%), were identified. Sixty components constituted 94.98% of the seed oil. The major components were eucalyptol (20.90%), methyleugenol (19.98%), linalool (14.66%) and camphor (5.5%).
The chemical composition of C. camphora leaf and twig oils was similar. Fifty-nine compounds were common, among which the main components were camphor, eucalyptol, 3,7-dimethyl-1,3,7-octatriene, linalool and terpineol. At present, no data was reported on the chemical composition of the essential oil of C. camphora seed. In contrast to the leaf and twig oils, the seed oil was characterized by a high content of methyleugenol (19.98%). According to the previous studies, C. camphora could be divided into five chemical types by the main compounds of its leaf oils, such as camphor-type, linalool-type, cineol-type, isonerolidol-type and borneol-type [7]. Therefore, the tested C. camphora sample probably belonged to the camphor-type because it contained rich camphor.
The composition of the essential oils of Cinnamomum species has been widely investigated and the main components were similar. The Cinnamomum species oils were found to mainly contain linalool, eucalyptol, camphor, terpinen-4-ol, limonene, terpineol and safrole [27,28,29,30]. The major compounds from C. camphora oils were similar to the previous reports. However, the relative amounts (based on the peak areas) of the individual components were different. For example, in this study the content of camphor in the essential oil of C. camphora leaf was 18.48% while the content of camphor in the essential oil of C. camphora leaf collected from India was 67.23% [31]. These differences in chemical compounds of the essential oils could be due to several factors such as harvest time, local climate, extraction method and varieties.
2.2. Contact Toxicity
The essential oils from C. camphora leaves, twigs and seeds showed strong contact toxicity against cotton aphids with median lethal concentration (LC50) values of 245.79, 274.99 and 146.78 mg/L after 48 h of treatment, respectively (Table 2).
Table 2.
Contact toxicity of essential oils of C. camphora against cotton aphids.
| Samples | 24 h | 48 h | ||||||
|---|---|---|---|---|---|---|---|---|
| LC50 (mg/L) | 95% CI (mg/L) | Slope ± SE | Chi Square (χ2) | LC50 (mg/L) | 95% CI (mg/L) | Slope ± SE | Chi Square (χ2) | |
| Leaves | 312.42 | 249.93–376.41 | 1.58 ± 0.16 | 1.48 | 245.79 | 191.31–299.85 | 1.61 ± 0.16 | 2.97 |
| Twigs | 376.77 | 283.19–476.81 | 1.15 ± 0.14 | 0.56 | 274.99 | 194.13–356.11 | 1.13 ± 0.15 | 0.24 |
| Seeds | 200.92 | 128.05–272.58 | 1.08 ± 0.15 | 0.48 | 146.78 | 88.77–206.14 | 1.13 ± 0.16 | 0.37 |
| Linalool | 523.66 | 417.69–673.64 | 1.09 ± 0.11 | 1.98 | 262.77 | 202.20–333.83 | 1.02 ± 0.11 | 2.19 |
| Imidacloprid | 12.53 | 10.10–15.46 | 1.17 ± 0.10 | 1.17 | 3.58 | 2.84–4.39 | 1.45 ± 0.12 | 1.72 |
95% CI: 95% confidence interval for each LC50 value.
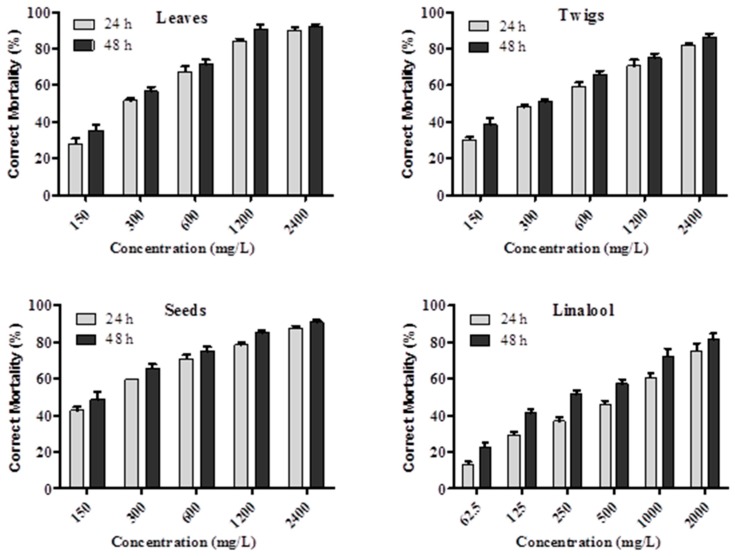
As seen from Table 2, the essential oil of C. camphora seeds (LC50 = 146.78 mg/L) showed higher contact toxicity against cotton aphids than that of C. camphora leaves (LC50 = 245.79 mg/L) and twigs (LC50 = 274.99 mg/L). Compared with the commercial insecticide imidacloprid (LC50 = 3.58 mg/L), the essential oil of C. camphora seeds demonstrated 41 times less toxicity after 48 h of treatment. The imidacloprid also showed acute contact toxicity to cotton aphids with an LC50 value of 12.53 mg/L after 24 h of treatment. However, compared with the other plant essential oils in the published reports, for example essential oils of Cynanchum mongolicum (LC50 = 38.4 μL/mL, after 48 h of treatment) and essential oils of Rosmarinus officinalis, Schinus areira and Tagetes terniflora (LC50 = 15.2, 58.3, and 76.2 mg/mL after 24 h of treatment, respectively) [32,33], the essential oil of C. camphora seeds revealed a stronger level of contact toxicity against cotton aphids. In Figure 1, it can be seen that the contact toxicity of essential oils from C. camphora and linalool against cotton aphids was concentration-dependent.
Figure 1.
Contact toxicity of C. camphora essential oils and linalool on cotton aphids at different concentrations after 24 h and 48 h of treatment. Error bar represents the standard deviation of the mean (n = 3).
Linalool, a monoterpene alcohol, has been proved to possess insecticidal activities [34]. For example, linalool isolated from Zanthoxylum schinifolium essential oils exhibited contact activity against Sitophilus zeamais with an LD50 value of 13.90 μg/adult [35]. In this study, linalool also exhibited contact toxicity against cotton aphids with an LC50 value of 262.77 mg/L after 48 h of treatment. Therefore, linalool could be one of the active compounds in the essential oils of C. camphora. Additionally, the major compounds from C. camphora oils such as eucalyptol, terpineol, caryophyllene and limonene also exhibited stronger contact toxicity against various pests [36,37,38].
2.3. Repellent Activity
The repellent activity of the essential oils of C. camphora against cotton aphids was evaluated. The results are presented in Table 3.
Table 3.
Repellent rate for C. camphora essential oils and linalool against cotton aphids at different concentrations after 12 h and 24 h of treatment.
| Time (h) | Concentration (μL/mL) | Repellent Rate (%) | Concentration (μL/mL) | Repellent Rate (%) | |||
|---|---|---|---|---|---|---|---|
| Leaves | Twigs | Seeds | Linalool | DEET | |||
| 12 | 20 | 75.44 ± 1.76a | 70.97 ± 2.79a | 76.19 ± 2.75a | / | 10 | 88.71 ± 4.27a |
| 10 | 47.37 ± 3.03b | 51.61 ± 2.79b | 53.40 ± 3.17b | 70.18 ± 3.51a | 5 | 77.42 ± 4.41ab | |
| 5 | 31.58 ± 5.26c | 30.65 ± 1.61c | 36.51 ± 1.59c | 56.14 ± 4.64b | 2 | 72.58 ± 4.34b | |
| 2 | 19.30 ± 1.75d | 19.35 ± 1.62d | 20.63 ± 4.20c | 49.12 ± 4.34bc | 0.5 | 54.84 ± 4.63c | |
| 1 | 10.53 ± 3.04d | 6.45 ± 1.61e | 12.70 ± 1.59d | 36.84 ± 3.83cd | 0.1 | 38.71 ± 4.27d | |
| 0.5 | / 1 | / | / | 26.32 ± 3.04d | 0.01 | 20.97 ± 1.61e | |
| 24 | 20 | 83.83 ± 1.47a | 72.13 ± 1.64a | 89.86 ± 1.45a | / | 10 | 80.70 ± 1.75a |
| 10 | 60.29 ± 4.41b | 59.01 ± 5.91b | 69.57 ± 2.51b | 76.56 ± 2.70a | 5 | 71.93 ± 4.64ab | |
| 5 | 42.65 ± 4.31c | 52.46 ± 4.34b | 55.07 ± 3.83c | 65.63 ± 4.14b | 2 | 66.67 ± 1.75b | |
| 2 | 25.00 ± 2.56d | 36.07 ± 5.68c | 36.23 ± 1.45d | 59.38 ± 4.13bc | 0.5 | 54.38 ± 3.51c | |
| 1 | 17.65 ± 3.89d | 29.51 ± 5.91d | 21.74 ± 0e | 48.44 ± 2.71d | 0.1 | 43.86 ± 6.32c | |
| 0.5 | / | / | / | 37.50 ± 1.56e | 0.01 | 19.3 ± 3.51d | |
1 / without treatment at the given concentration; Mean (± standard error) of three replicates for each sample. Percentage values followed by the same letter are not significantly different in the same group at p ≤ 0.05 (Duncan’s test).
As shown in Table 3, the essential oil of C. camphora seeds was more effective compared to the essential oils of C. camphora leaves and twigs in terms of repellent rate, but it was less effective than linalool at the concentration of 10 µL/mL. At the tested concentrations, the positive control, DEET, exhibited strong repellency at 12 h after treatment. Compared with the positive control, only at the highest concentration of 20 µL/mL, the repellent rate (76.19%) of the seed oil of C. camphora was almost the same as that of DEET (repellent rate = 77.42%, tested at the concentration of 5 µL/mL). Therefore, a higher concentration (more than 10 µL/mL) is recommended when C. camphora essential oils are used as a repellent.
Meanwhile, many essential oils have been evaluated for repellency against insects mentioned in the literature. For example, in a certain range of concentration (1.25–10.0 µL/mL), the essential oil from C. mongolicum showed high repellent activity against soybean aphids (Aphis glycines) at 2 h exposure [32]. The essential oil of Tagetes terniflora and Schinus areira leaves exhibited repellency against aphids with a repellent index of 66.66% and 73.33% at 24 h exposure, respectively [33]. The essential oils of Rosmarinus officinalis L. and Mentha spicata L. showed a strong repellency effect against Ixodes ricinus Nymphs in the laboratory bioassay. Furthermore, R. officinalis and M. spicata exhibited good repellency (68.29% and 59.38%, respectively) in the field trial [39].
In general, the insecticidal and repellent activity of essential oils of could not be easily correlated with one specific compound. In this study, linalool also showed strong repellent activity against cotton aphids with a repellent rate in the range of 37.50% to 76.56% at the given concentrations after 24 h of treatment. Therefore, as one of the major components in the essential oils of C. camphora, linalool could be one of the active compounds.
Above all, this is the first report that the C. camphora essential oils showed strong insecticidal and repellent activities against cotton aphids. Linalool may be one of the important activity components, as it was proved to possess insecticidal and repellent activity against cotton aphids. The essential oils of C. camphora could be potential alternatives to the traditional chemical control of cotton aphids. For the practical use of C. camphora oils and their constituents as novel insecticides, further studies on the insecticidal and repellent mechanisms and safety evaluations of C. camphora essential oils are needed. Additionally, the characteristic components (such as methyl eugenol, eucalyptol and camphor) are needed to evaluate their contribution to the insecticidal and repellent activities.
3. Materials and Methods
3.1. Plant Material
The leaves, twigs and seeds of C. camphora were collected in Nanchang, Jiangxi Province, China on 25 September 2014. The C. camphora trees were cultivated in the garden. A total of nine trees were randomly selected for samples collection (650 g of seeds from nine trees; 3080 g of leaves collected from each tree; 2560 g of small twigs collected from each tree). The plant material was authenticated by Prof. Yimin Hu from Anhui Academy of Forestry, China. The samples were dried in the shade and ground into powder, and stored at −20 °C.
3.2. Chemicals
The HPLC-grade methanol and hexane were purchased from fisher scientific (Fair Lawn, NJ, USA). Analytical-grade chemical was obtained from Beijing Chemical Works (China). Water was purified with an ultrapure water system (Purelab Plus, Pall, Port Washington, NY, USA). The standard compound of imidacloprid (purity, 96%, a commercial aphidicide) was obtained from National Pesticide Quality Supervision and Inspection Centre (Beijing, China). N,N-Diethyl-3-methylbenzamide (DEET), eucalyptol (purity 99%), camphor (purity 96%), methyleugenol (purity ≥98%) and linalool (purity 97%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Alkanes (C7–C30) was purchased from Supelco (Bellefonte, PA, USA). All other analytical-grade chemicals were obtained from Beijing Chemical Works (Beijing, China).
3.3. Insect
A continuous culture of A. gossypii is maintained in a temperature-controlled incubator at 28 ± 1 °C, 70% ± 5% relative humidity (RH) and exposed to a long photoperiod (16 h light:8 h dark, 16:8 LD) in the laboratory. The cotton aphids were reared on the leaf blades of cotton seedlings, and all bioassays were carried out using apterous adult aphids.
3.4. Essential Oils
Essential oils were prepared according to the method described in previous studies [6,7] with a slight modification. The dried plant powder (50 g) was subjected to hydrodistillation in a Clevenger-type apparatus for six hours. The obtained essential oils were dried by anhydrous sodium sulphate (Na2SO4). The essential oils were accurately weighed. All experiments were carried out in triplicate. The oil yields were calculated on the basis of the dry weight of plant material. The formula was as follows:
| Essential oils yield (%) = W1/W2 × 100 | (1) |
where W1 was the net weight of oils (grams) and W2 was the total weight of dry samples (grams).
3.5. GC×GC-TOFMS Analysis
The essential oil samples from three parts (leaves, twigs and seeds) of C. camphora (10 μL) were diluted with n-hexane (1 mL), respectively. The GC×GC-TOF/MS analysis was carried out on an Agilent 7890B gas chromatography system (Agilent Technologies, Santa Clara, CA, USA), equipped with a Pegasus 4D TOFMS (LECO Corporation, St. Joseph, MI, USA). The GC×GC system contained two chromatography columns. The first column was a nonpolar Rxi-5 Sil MS (5% phenyl-95% dimethyl arylene polysiloxane, 30 m × 0.25 mm i.d. × 0.25 μm film thickness) and the second column was a medium polarity Rxi-17 Sil MS with dimensions of 2 m × 0.18 mm i.d. × 0.18 μm film thickness, 50% phenyl-50% dimethyl arylene polysiloxane (Restek Corp., Bellefonte, PA, USA). Helium was used as a carrier gas at a constant flow rate of 1 mL/min. The initial temperature of the first column was set at 50 °C for 12 s and then ramped to 280 °C at a rate of 8 °C/min. The secondary oven was set at a 5 °C offset above the primary oven. The modulator temperature offset and transfer line temperature were 15 °C and 280 °C, respectively. The modulation period was 4 s and the hot pulse was set at 0.8 s. The injection volume was 1 μL in split mode, at a ratio of 50:1. The temperature of the injector was kept at 240 °C. The mass spectrometer was set to scan in the range of m/z 33–550 at an acquisition rate of 100 spectra/s. The detector voltage was set at 1420 V, and the electron energy was set to 70 eV. The ion source temperature was kept at 250 °C. All the operations and analysis of data were controlled using LECO ChromaTOF software version 4.52.
3.6. Contact Toxicity Bioassay
The contact toxicity was measured using topical application method as previously described [10,40,41] and with a slight modification. In each test, a total of 50 aphids were selected on clean cotton seedlings. Sample solutions were deposited on the dorsum of the thorax of each aphid by an auto micro-applicator (900-X, Burkard Manufacturing Co. Ltd., Rickmansworth, UK). Based on the preliminary screening result, the essential oils and standard chemicals were serially diluted in acetone to evaluate the dose-effect relationships against cotton aphid. Each essential oil was diluted into different concentrations (150, 300, 600, 1200 and 2400 µg/mL). Imidacloprid, as a positive control, was diluted into five concentrations (1, 5, 10, 50 and 100 µg/mL). Linalool was diluted into six concentrations (62.5, 125, 250, 500, 1000 and 2000 µg/mL). Control aphids were treated only with acetone. Each treatment was replicated three times. After application, the cotton seedlings with treated aphids were transported to petri dishes (9.0 cm i.d. × 1.5 cm) and maintained at controlled temperature (28 ± 1 °C) and humidity (70% ± 5%) conditions in the light incubator (16:8 LD). Aphid mortality was assessed 24 h and 48 h after application. The corrected mortality rates were measured using Abbott’s formula [42].
| Correct mortality (%) = (M1 − Mc)/(100 − Mc ) × 100 | (2) |
where M1 (%) was the mortality of the treated groups and Mc (%) was the mortality of the control groups.
3.7. Repellent Activity Bioassay
Repellent activity bioassay was used to assess behavioral response of cotton aphid to essential oil volatiles. The repellency of C. camphora essential oils against cotton aphids was determined as previously described and with a slight modification [43,44]. The essential oils were diluted in ethanol to serial concentrations (1, 2, 5, 10 and 20 µL/mL). A commercial repellent, DEET, as a positive control, was diluted to six concentrations (0.01, 0.1, 0.5, 2, 5 and 10 µL/mL). Linalool was diluted to five concentrations (0.5, 1, 2, 5 and 10 µL/mL). Fresh leaf discs of cotton (1.5 cm in diameter) were dipped into the prepared sample solutions for 5 s. The leaf discs were air dried in fuming hood and then transferred to the Petri dishes (9.0 cm in diameter, with 2% solidified agar beds and filter paper). Leaf discs soaked identically in ethanol served as the controls. The filter paper (6.5 cm in diameter, Whatman No. 2) was placed in the center of Petri plates (Figure 2).
Figure 2.
Layout of leaf discs of cotton in the Petri dish.
Thirty cotton aphids were released at the center of each filter paper disc and the lid was sealed in place with parafilm. Petri dishes were maintained at 28 ± 1 °C and 70% ± 5% RH with a 16:8 h light:dark photocycle in a light incubator. Three replicates were used for each concentration, so that a total number of 90 cotton aphids were tested at each concentration.
Aphids were calculated to move on the cotton leaf after 12 h and 24 h. The repellent rate (Rr) of each essential oil/compound was calculated using the formula
| Repellent rate (Rr) (%) = (NC − NT)/(Nc ) × 100 | (3) |
where NC was the number of cotton aphids on the leaf disc in the negative control groups and NT was the number of cotton aphids on the leaf disc in the treated groups.
3.8. Statistical Analysis
Statistical significance was carried out applying one-way ANOVA followed by Duncan’s test at p = 0.05, using SPSS version 19.0 (IBM Corp., Armonk, NY, USA). Median Lethal Concentration (LC50 with 95% confidence intervals) is expressed in milligrams of the material per liter. Probit analysis of concentration and aphid mortality data was performed to evaluate the LC50 value [35].
4. Conclusions
The GC×GC-TOFMS analysis results revealed that camphor, eucalyptol, linalool, 3,7-dimethyl-1,3,7-octatriene and methyleugenol were the major components in the essential oils of C. camphora. Linalool has proved to be a significant contributor to the insecticidal and repellent activities against cotton aphids. This study indicates that the essential oils of C. camphora seeds possess potent insecticidal and repellent activities. C. camphora can be a promising source of natural insecticide or repellent for controlling cotton aphids.
Acknowledgments
The authors would like to acknowledge the financial support from the Project of the State Forestry Administration of China (2014-4-33), the Central Public-Interest Scientific Institution Basal Research Fund, China (No.1632014009) and Key Projects in the National Science & Technology Pillar Program in the Twelfth Five-year Plan Period (No. 2012BAD23B0304).
Author Contributions
H.J. performed all experimental work, data analysis and manuscript preparation. J.W. participated in the design of the study and writing the manuscript. L.S. and X.C. performed essential oil extraction. X.Y. and F.T. contributed to manuscript preparation. Y.Y. supervised the experimental work.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the essential oils and linalool are available from the authors.
References
- 1.Liu C.H., Mishra A.K., Tan R.X., Tang C., Yang H., Shen Y.F. Repellent and insecticidal activities of essential oils from Artemisia princeps and Cinnamomum camphora and their effect on seed germination of wheat and broad bean. Bioresource Technol. 2006;97:1969–1973. doi: 10.1016/j.biortech.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Imai S., Ogawa K. Quantitative analysis of carbon balance in the reproductive organs and leaves of Cinnamomum camphora (L.) Presl. J. Plant Res. 2009;122:429–437. doi: 10.1007/s10265-009-0233-9. [DOI] [PubMed] [Google Scholar]
- 3.Su J., Chen J., Liao S., Li L., Zhu L., Chen L. Composition and biological activities of the essential oil extracted from a novel plant of Cinnamomum camphora Chvar. Borneol. J. Med. Plants Res. 2012;6:3487–3494. [Google Scholar]
- 4.Yeh R.Y., Shiu Y.L., Shei S.C., Cheng S.C., Huang S.Y., Lin J.C., Liu C.H. Evaluation of the antibacterial activity of leaf and twig extracts of stout camphor tree, Cinnamomum kanehirae, and the effects on immunity and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immun. 2009;27:26–32. doi: 10.1016/j.fsi.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Marasini B.P., Baral P., Aryal P., Ghimire K.R., Neupane S., Dahal N., Singh A., Ghimire L., Shrestha K. Evaluation of antibacterial activity of some traditionally used medicinal plants against human pathogenic bacteria. Biomed. Res. Int. 2015;2015:265425. doi: 10.1155/2015/265425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pragadheesh V.S., Saroj A., Yadav A., Chanotiya C.S., Alam M., Samad A. Chemical characterization and antifungal activity of Cinnamomum camphora essential oil. Ind. Crop. Prod. 2013;49:628–633. doi: 10.1016/j.indcrop.2013.06.023. [DOI] [Google Scholar]
- 7.Chen H.P., Yang K., You C.X., Lei N., Sun R.Q., Geng Z.F., Ma P., Cai Q., Du S.S., Deng Z.W. Chemical constituents and insecticidal activities of the essential oil of Cinnamomum camphora leaves against Lasioderma serricorne. J. Chem. 2014;2014:963729. doi: 10.1155/2014/963729. [DOI] [Google Scholar]
- 8.Singh P., Srivastava B., Kumar A., Dubey N.K. Fungal contamination of raw materials of some herbal drugs and recommendation of Cinnamomum camphora oil as herbal fungitoxicant. Microb. Ecol. 2008;56:555–560. doi: 10.1007/s00248-008-9375-x. [DOI] [PubMed] [Google Scholar]
- 9.Koo H.N., An J.J., Park S.E., Kim J.I., Kim G.H. Regional susceptibilities to 12 insecticides of melon and cotton aphid, Aphis gossypii (Hemiptera: Aphididae) and a point mutation associated with imidacloprid resistance. Crop. Prot. 2014;55:91–97. doi: 10.1016/j.cropro.2013.09.010. [DOI] [Google Scholar]
- 10.Sy Mohamad S.F., Mohamad S., Aziz A.A. The susceptibility of aphids, Aphis gossypii Glover to lauric acid based natural pesticide. Procedia Eng. 2013;53:20–28. doi: 10.1016/j.proeng.2013.02.004. [DOI] [Google Scholar]
- 11.Cao C.W., Zhang J., Gao X.W., Liang P., Guo H.L. Overexpression of carboxylesterase gene associated with organophosphorous insecticide resistance in cotton aphids, Aphis gossypii (Glover) Pestic. Biochem. Physiol. 2008;90:175–180. doi: 10.1016/j.pestbp.2007.11.004. [DOI] [Google Scholar]
- 12.Skevas T., Stefanou S.E., Lansink A.O. Pesticide use, environmental spillovers and efficiency: A DEA risk-adjusted efficiency approach applied to Dutch arable farming. Eur. J. Oper. Res. 2014;237:658–664. doi: 10.1016/j.ejor.2014.01.046. [DOI] [Google Scholar]
- 13.Carriere C.H., Kang N.H., Niles L.P. Neuroprotection by valproic acid in an intrastriatal rotenone model of Parkinson’s disease. Neuroscience. 2014;267:114–121. doi: 10.1016/j.neuroscience.2014.02.028. [DOI] [PubMed] [Google Scholar]
- 14.Kreutzweiser D., Thompson D., Grimalt S., Chartrand D., Good K., Scarr T. Environmental safety to decomposer invertebrates of azadirachtin (neem) as a systemic insecticide in trees to control emerald ash borer. Ecotoxicol. Environ. Saf. 2011;74:1734–1741. doi: 10.1016/j.ecoenv.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Jadeja G.C., Maheshwari R.C., Naik S.N. Extraction of natural insecticide azadirachtin from neem (Azadirachta ndica A. Juss) seed kernels using pressurized hot solvent. J. Supercrit. Fluid. 2011;56:253–258. doi: 10.1016/j.supflu.2011.01.004. [DOI] [Google Scholar]
- 16.Yang J., Zhang L., Zhu G., Li L. Separation and enrichment of major quinolizidine type alkaloids from Sophora alopecuroides using macroporous resins. J. Chromatogr. B. 2014;945–946:17–22. doi: 10.1016/j.jchromb.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Bachrouch O., Ferjani N., Haouel S., Jemâa J.M.B. Major compounds and insecticidal activities of two Tunisian Artemisia essential oils toward two major coleopteran pests. Ind. Crop. Prod. 2015;65:127–133. doi: 10.1016/j.indcrop.2014.12.007. [DOI] [Google Scholar]
- 18.Zandi-Sohani N., Ramezani L. Evaluation of five essential oils as botanical acaricides against the strawberry spider mite Tetranychus turkestani Ugarov and Nikolskii. Int. Biodeterior. Biodegrad. 2015;98:101–106. doi: 10.1016/j.ibiod.2014.12.007. [DOI] [Google Scholar]
- 19.Nenaah G.E. Bioactivity of powders and essential oils of three Asteraceae plants as post-harvest grain protectants against three major coleopteran pests. J. Asia-Pac. Entomol. 2014;17:701–709. doi: 10.1016/j.aspen.2014.07.003. [DOI] [Google Scholar]
- 20.El-Seedi H.R., Khattab A., Gaara A.H.M., Mohamed T.K., Hassan N.A., El-kattan A.E. Essential oil analysis of Micromeria nubigena H.B.K. and its antimicrobial activity. J. Essent. Oil Res. 2008;20:452–456. [Google Scholar]
- 21.Prebihalo S., Brockman A., Cochran J., Dorman F.L. Determination of emerging contaminants in wastewater utilizing comprehensive two-dimensional gas-chromatography coupled with time-of-flight mass spectrometry. J. Chromatogr. A. 2015;1419:109–115. doi: 10.1016/j.chroma.2015.09.080. [DOI] [PubMed] [Google Scholar]
- 22.Dos Santos A.L., Polidoro A.d.S., Schneider J.K., da Cunha M.E., Saucier C., Jacques R.A., Cardoso C.A.L., Mota J.S., Caramão E.B. Comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (GC×GC/TOFMS) for the analysis of volatile compounds in Piper regnellii (Miq.) C. DC. essential oils. Microchem. J. 2015;118:242–251. doi: 10.1016/j.microc.2014.07.007. [DOI] [Google Scholar]
- 23.Da Cunha M.E., Schneider J.K., Brasil M.C., Cardoso C.A., Monteiro L.R., Mendes F.L., Pinho A., Jacques R.A., Machado M.E., Freitas L.S., et al. Analysis of fractions and bio-oil of sugar cane straw by one-dimensional and two-dimensional gas chromatography with quadrupole mass spectrometry (GC×GC/qMS) Microchem. J. 2013;110:113–119. doi: 10.1016/j.microc.2013.03.004. [DOI] [Google Scholar]
- 24.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 25.Gong H.Y., Liu W.H., Lv G.Y., Zhou X. Analysis of essential oils of Origanum vulgare from six production areas of China and Pakistan. Rev. Bras. Farmacogn. 2014;24:25–32. doi: 10.1590/0102-695X2014241434. [DOI] [Google Scholar]
- 26.Brokl M., Fauconnier M.L., Benini C., Lognay G., du Jardin D., Focant J.F. Improvement of ylang-ylang essential oil characterization by GC×GC-TOFMS. Molecules. 2013;18:1783–1797. doi: 10.3390/molecules18021783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng S.L., Cui Z.X., Huang X.C., Chen Y.F., Xu D., Xiong P. Variations in essential oil yield and composition during Cinnamomum cassia bark growth. Ind. Crop. Prod. 2011;33:248–252. doi: 10.1016/j.indcrop.2010.10.018. [DOI] [Google Scholar]
- 28.Abdelwahab S.I., Zaman F.Q., Mariod A.A., Yaacob M., Abdelmageed A.H.A., Khamis S. Chemical composition, antioxidant and antibacterial properties of the essential oils of Etlingera elatior and Cinnamomum pubescens kochummen. J. Sci. Food. Agric. 2010;90:2682–2688. doi: 10.1002/jsfa.4140. [DOI] [PubMed] [Google Scholar]
- 29.Li Y., Kong D., Huang R., Liang H., Xu C., Wu H. Variations in essential oil yields and compositions of Cinnamomum cassia leaves at different developmental stages. Ind. Crop. Prod. 2013;47:92–101. doi: 10.1016/j.indcrop.2013.02.031. [DOI] [Google Scholar]
- 30.Subki S.Y.M., Jamal J.A., Husain K., Manshoor N. Characterisation of leaf essential oils of three Cinnamomum species from Malaysia by gas chromatography and multivariate data analysis. Pharmacogn. J. 2013;5:22–29. doi: 10.1016/j.phcgj.2012.12.004. [DOI] [Google Scholar]
- 31.Garg S.N., Gupta D., Charles R., Yadav A., Naavi A.A. Volatile oil constituents of leaf, stem and bark of Cinnamomum camphora (Linn.) Nees and Eberm. Indian Perfum. 2002;46:41–44. [Google Scholar]
- 32.Wang Y., An Z., Zhen C.G., Liu Q.Z., Shi W.P. Composition of the essential oil of Cynanchum mongolicum (Asclepiadaceae) and insecticidal activities against Aphis glycines (Hemiptera: Aphidiae) Pharmacogn. Mag. 2014;10:S130–S134. doi: 10.4103/0973-1296.127362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chopa C.S., Descamps L.R. Composition and biological activity of essential oils against Metopolophium dirhodum (Hemiptera: Aphididae) cereal crop pest. Pest. Manag. Sci. 2012;68:1492–1500. doi: 10.1002/ps.3334. [DOI] [PubMed] [Google Scholar]
- 34.Yang F., Long E., Wen J., Cao L., Zhu C., Hu H., Ruan Y., Okanurak K., Hu H., Wei X., et al. Linalool, derived from Cinnamomum camphora (L.) Presl leaf extracts, possesses molluscicidal activity against Oncomelania hupensis and inhibits infection of Schistosoma japonicum. Parasit. Vector. 2014;7:407. doi: 10.1186/1756-3305-7-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C.F., Yang K., Zhang H.M., Cao J., Fang R., Liu Z.L., Du S.S., Wang Y.Y., Deng Z.W., Zhou L.G. Components and insecticidal activity against the maize weevils of Zanthoxylum schinifolium fruits and leaves. Molecules. 2011;16:3077–3088. doi: 10.3390/molecules16043077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo S.S., You C.X., Liang J.Y., Zhang W.J., Geng Z.F., Wang C.F., Du S.S., Lei N. Chemical composition and bioactivities of the essential oil from Etlingera yunnanensis against two stored product insects. Molecules. 2015;20:15735–15747. doi: 10.3390/molecules200915735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z.L., Zhao N.N., Liu C.M., Zhou L.G., Du S.S. Identification of insecticidal constituents of the essential oil of Curcuma wenyujin rhizomes active against Liposcelis bostrychophila badonnel. Molecules. 2012;17:12049–12060. doi: 10.3390/molecules171012049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X.C., Li Y.P., Li H.Q., Deng Z.W., Zhou L.G., Liu Z.L., Du S.S. Identification of repellent and insecticidal constituents of the essential oil of Artemisia rupestris L. Aerial parts against Liposcelis bostrychophila badonnel. Molecules. 2013;18:10733–10746. doi: 10.3390/molecules180910733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Seedi H.R., Khalil N.S., Azeem M., Taher E.A., Göransson U., Pålsson K., Borg-Karlson A.-K. Chemical composition and repellency of essential oils from four medicinal plants against Ixodes ricinus nymphs (Acari: Ixodidae) J. Med. Entomol. 2012;49:1067–1075. doi: 10.1603/ME11250. [DOI] [PubMed] [Google Scholar]
- 40.Pavela R., Zabka M., Vrchotova N., Triska J., Kazda J. Selective effects of the extract from Angelica archangelica L. against Harmonia axyridis (Pallas)-an important predator of aphids. Ind. Crop. Prod. 2013;51:87–92. doi: 10.1016/j.indcrop.2013.08.073. [DOI] [Google Scholar]
- 41.Overgaard H.J., Sirisopa P., Mikolo B., Malterud K.E., Wangensteen H., Zou Y.F., Paulsen B.S., Massamba D., Duchon S., Corbel V., et al. Insecticidal activities of bark, leaf and seed extracts of Zanthoxylum heitzii against the african malaria vector Anopheles gambiae. Molecules. 2014;19:21276–21290. doi: 10.3390/molecules191221276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abbott W. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 43.Ikeura H., Kobayashi F., Hayata Y. Repellent effect of herb extracts on the population of wingless green peach aphid, Myzus persicae Sulzer (Hemiptera: Aphididae) J. Agric. Sci. 2012;4:139–144. doi: 10.5539/jas.v4n5p139. [DOI] [Google Scholar]
- 44.Mann R.S., Tiwari S., Smoot J.M., Rouseff R.L., Stelinski L.L. Repellency and toxicity of plant-based essential oils and their constituents against Diaphorina citri kuwayama (Hemiptera: Psyllidae) J. Appl. Entomol. 2012;136:87–96. doi: 10.1111/j.1439-0418.2010.01592.x. [DOI] [Google Scholar]