Abstract
Traditional Chinese Medicine Preparations (TCMPs) contain massive numbers of ingredients responsible for their multiple efficacies. An absorption–based quality control method for complicated TCMPs using Hu–gan–kang–yuan Capsule (HGKYC) as an example was developed. To select proper chemical markers for quality control of HGKYC, an ultra–fast liquid chromatography (UFLC) coupled with electrospray ionization quadrupole time–off light mass spectrometry (UFLC–QTOF–MS/MS) method was used for the rapid separation and structural identification of the constituents in the HGKYC extract and the rat serum after oral administration of HGKYC. As a result, one hundred and seven prototype constituents including flavonoids, organic acid, phenylpropanoids, anthraquinones, saponins, alkaloids, terpenes, phenols and amino acids in HGKYC extract, and 43 compounds found in rat serum after oral administration of HGKYC were unambiguously identified or tentatively characterized by comparing retention times and MS information with those of authentic standards or available literature references. Finally, a simple, low–cost and effective method of simultaneous determination for baicalein, wogonin, paeonol and emodin in HGKYC was developed using high performance liquid chromatography coupled with a diode array detector. In conclusion, an absorption–based quality control pattern was developed and successfully used for evaluating HGKYC.
Keywords: hu–gan–kang–yuan capsule, quality control, UFLC–QTOF–MS/MS, absorption
1. Introduction
Traditional Chinese Medicine Preparations (TCMPs) are usually composed of multiple Chinese medicinal materials that contain massive numbers of ingredients responsible for their multiple efficacies. In the quality control (QC) of TCMPs, the biggest challenge is the choice of chemical markers due to the complicated nature of the compounds in TCMPs. So far, selecting one compound as the quantitative marker like in Western medicine remains the main QC method used for TCMPs. We investigated and found that among 1493 TCMPs in the Chinese Pharmacopoeia (I volume, 2015 version), 849 TCMPs were evaluated by determining only one marker compound [1]. It is no denying that the Western medicine QC platform is somewhat helpful in evaluating the repeatability of TCMP ingredients among different batches, however, this QC platform may not correlate to the TCMPs’ efficacy, because the compounds selected as quantitative markers may not be active or may not be absorbed. Hence, it is inevitable that sometimes the relationship between the “quality” of a TCMP evaluated by some chemical marker and its pharmacological effects can be inconsistent [2].
Conceptually, the active components which are absorbable should be considered as markers for QC evaluation since they are capable of contributing to the pharmacological activities [3,4], and considering the consistency evaluation among the medicinal materials, semi–finished products and TCMPs, proper prototype compounds absorbed in blood should be the preferred chemical markers. Hence, screening the absorbable compounds in TCMPs is of prime importance before confirming rational chemical markers for QC. The frequently used techniques for screening compounds in TCMPs are liquid chromatography–mass spectrometry (LC–MS) with several mass analyzers, such as ion trap, triple quadrupole (QqQ), quadrupole linear ion trap (QTRAP), time–of–flight (TOF). Q–TOF–MS/MS can analyze the fragment ions with accurate mass measurement, which facilitates the elucidation of characteristic fragmentation pathways of the targeted compounds. Its applications in analyzing the chemical profiles of Traditional Chinese Medicines in vivo and in vitro are presently hot topic [5,6,7,8,9].
Hu–gan–kan–kang–yuan Capusule (HGKYC) is a second–generation preparation, displaying pharmacological effects in hepatitis B treatment by decreasing aminopherase and increasing immunity. The preparation consists of 12 traditional Chinese medicinal materials, Schisandrae chinensis fructus, Ligustri lucidi fructus, Epimedii folium, Isatidis Radix, Polygoni cuspidate rhizome et radix, Acanthopanacis senticosi radix et rhizomaseu caulis, Bupleuri radix, Moutan cortex, Scutellariae radix, Astragali radix, Hominis placenta, Taxilli herba. Undoubtedly, there are complicated compounds in HGKYC, and which ones can be absorbed in blood should be investigated. This study tries to develop an absorption–based quality control method for TCMPs using HGKYC as an example.
In this paper, firstly an UFLC–QTOF–MS/MS method was used to identify the compounds in HGKYC extract and in rat serum after oral administration of HGKYC. Secondly, based on the pharmacological bioactivities of the absorbable components, the availabilities of authentic standards and the costs of analysis method, baicalein, wogonin, paeonol and emodin were chosen as chemical markers to evaluate the quality of HGKYC by HPLC–DAD. Finally, a simple, reliable and sensitive method for simultaneous determination of the abovementioned compounds was developed.
2. Materials and Methods
2.1. Chemicals and Materials
The medicinal materials—Schisandrae chinensis fructus, Ligustri lucidi fructus, Epimedii folium, Isatidis Radix, Polygoni cuspidate rhizome et radix, Acanthopanacis senticosi radix et rhizomaseu caulis, Bupleuri radix, Moutan cortex, Scutellariae radix, Astragali radix, Hominis placenta, Taxilli herba—were purchased from Kangsheng Medicinal Company (Guanghzou, China). All medicinal materials were identified by associate professor Zhang Hongwei (Department of Medicinal Plants & Pharmacognosy, Southern Medical University, Guangzhou, China) according to pharmacognostic standards documented in Volume I of 2010 Edition of China Pharmacopoeia. All samples were kept in a desiccator (silica gel as desiccant) at room temperature in Department of Chinese Pharmaceutics, Southern Medical University, Guangzhou, China. Three batches of Hu–gan–kang–yuan capsules were prepared in the laboratories of the Department of Chinese Pharmaceutics, Southern Medical University.
The reference standards of baicalein, wogonin, paeonol and emodin, baicalin, isofraxidin, deoxyschizandrin, schisandrin, paeonol, oroxylin A, salidroside, emodin, paeoniflorin, polydatin, scutellarin, wogonin, resveratrol, oleanic acid, syringing, rhein, ligustroflavone, chrysin, baicalein, ferulic acid, epigoitrin, protocatechuate and astragaloside A were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Arginine, choline, betaine, adenine, nicotinic acid, l–pyroglutamic acid, tyrosine, isoleucine, p–coumaric acid, gallic acid, phenylalanine, tryptophan, protocatechuic aldehyde, methyl gallate, chlorogenic acid, caffeic acid, syringic acid, trans–p–coumaric acid, rutin, scutellarin, hyperoside, quercetrin, ethyl caffeate, kaempferol, schizandrin A and r–schizandrin were purchased from Wei Ke Qi Biological Technology Co., Ltd. (Chengdu, China). The purities of all the standards were greater than 98.0%. Acetonitrile was chromatographic grade, and phosphoric acid, methanol, ethanol were analytical grade. All of them were purchased from Guangzhou Chemical Reagent factory (Guangzhou, China). Ultrapure water was provided by Southern Medical University (Guangzhou, China).
Three male Sprague–Dawley rats weighing 260 ± 20 g were obtained from the Animal Center of Southern Medical University. Animals were bred in a breeding room with a temperature of 23 ± 2 °C humidity of 60% ± 5%, and 12 h dark–light cycle. They were given tap water and fed a normal diet and were acclimatized to the facilities for 3 days. The rats were fasted for 12 h before experimentation, while water was taken freely. The animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of Southern Medical University.
2.2. Instrumentation and Conditions
2.2.1. UFLC–QTOF 5600+MS/MS
UFLC analysis was performed on a Shimadzu UFLC XR instrument (Shimadzu Corp., Kyoto, Japan), consisting of a binary pump, an auto–sampler, a column oven and a diode–array detector. Samples were separated on a Kinetex C18 column (100 mm × 2.1 mm I.D., 2.6 μm, Phenomenex, CA, USA). The mobile phase consisted of acetonitrile (A) and 0.1% aqueous formic acid (v/v) (B). The following gradient elution program was used—linear gradient from 2% to 100% A at 0–30 min), isocratic 100% A at 30–40 min), 100%–2% A at 40–41 min), isocratic 2% A at 41–45 min. The flow rate was kept at 0.3 mL/min. The injected volume was 2 μL and the column temperature was set at 40 °C. The DAD detector scanned from 190 nm to 400 nm. Mass spectrometry was performed on the Triple TOFTM 5600 plus (AB SCIEX, Foster City, CA, USA) a hybrid triple quadrupole time–of–flight mass spectrometer equipped with ESI source. The system was operated with Analyst®TF 1.6 software (AB SCIEX). The conditions of the MS/MS detector were as follows: first ion source gas 60 psi, second ion source gas 60 psi, curtain gas 35 psi, temperature 550 °C, ion spray voltage floating 5500 V, collision energy 35 V, collision energy spread 15 V, declustering potential 80 V. TOF–MS range was set at m/z 100–1000 and product ions mass range was set at m/z 50–1000. Both positive and negative ion modes were used for compound ionization. Nitrogen was used as nebulizer and auxiliary gas.
2.2.2. HPLC–DAD
Quantitative analysis of baicalein, wogonin, paeonol and emodin was carried out in a 1260 HPLC system (Agilent Technologies, Palo Alto, CA, USA) equipped with a diode–array detector The separation was achieved on a Venusil XBL C18 (250 mm × 4.6 mm I.D., 5 µm) (Dalian Elite Analytical Instruments Co., Ltd., Dalian, China) at ambient temperature. The mobile phase consisted of acetonitrile (solvent A) and 0.1% phosphoric acid in water (solvent B). The gradient used is as follows: 30% A at 0–4 min, 30%–25% A at 4–5 min, 25% A at 5–10 min, 25%–40% A at 10–25 min, 40%–70% at 25–32 min, 70%–76% at 32–35 min, 76%–85% at 35–45 min, 85%–30% at 45–50 min. The flow rate was maintained at 1 mL/min, the injection volume was 10 µL and the eluent was monitored at 280 nm.
2.3. Animal Administration
Three SD rats were orally administrated HGKYC powder 10 g/kg. Blood was collected from caudal vein at 4 h after administration and centrifuged at 6000 g for 10 min at 4 °C. Serum was frozen at −20 °C until analysis.
2.4. Sample Preparation
2.4.1. Extract of HGKYC
The contents of HGKYC (0.20 g) were weighed precisely and treated by sonication with 10 mL of methanol for 15 min at 50 °C in an ultrasonic bath (JY92–II, frequency 40 kHz, 250 W, Ningbo Biotechnology Co., Ltd., Ningbo, China). The extract solution was centrifuged at 13,000 rpm for 15 min. Then the supernatant was injected into UFLC and HPLC.
2.4.2. Rat Serum Sample
For identification of multiple constituents in rat, the serum was vortex mixed with 2.5 times amount of acetonitrile (v/v) to precipitate proteins and centrifuged at 13,000 g for 15 min. The supernatant was injected into the UFLC–Q–TOF MS/MS system.
2.4.3. Preparation of Standard Solutions for Qualitative Identification
Known amounts of reference standards arginine, choline, betaine, adenine, nicotinic acid, l–pyroglutamic acid, tyrosine, isoleucine, p–coumaric acid, gallic acid, phenylalanine, tryptophan, protocatechuic aldehyde, methyl gallate, chlorogenic acid, caffeic acid, syringic acid, trans–p–coumaric acid, rutin, scutellarin, hyperoside, quercetrin, ethyl caffeate, kaempferol, schizandrin A, r–schizandrin B, baicalin, isofraxidin, deoxyschizandrin, schisandrin, paeonol, oroxylin A, salidroside, emodin, paeoniflorin, polydatin, scutellarin, wogonin, resveratrol, oleanic acid, syringing, rhein, ligustroflavone, chrysin, baicalein, ferulic acid, epigoitrin, protocatechuate and astragalosfide A were dissolved in methnaol to obtain single stock solultions (about 1.0 mg/mL) and stored at 4 °C until used.
The working solutions were prepared by serial dilution of the stock solutions with methanol. A mixture of all forty–nine reference standards was prepared in methanol and was filtered through a 0.22 μm syringe filter before UFLC–QTOF–MS/MS analysis.
2.4.4. Preparation of Standard Solutions for Quantitative Determination
Standard stock solutions of bacalein, wogonin, paeonol and emodin were precisely weighed and prepared by dissolving them in methanol, respectively. A mixed standard solution was prepared by accurately transferring each–standard stock solution and diluting with methanol. The final concentration of baicalein, wogonin, paeonol and emodin in the mixed standard working solution was 40.25, 46.75, 32.12 and 26.70 μg/mL, respectively. The quality control samples (LQC, MQC and HQC) were 5, 50, and 70 μg/mL for bacalein, 5, 50 and 80 μg/mL for wogonin, 4, 20 and 40 μg/mL for paeonol and emodin, respectively. All solutions were stored at 4 °C until used.
2.4.5. Extract of Negative Control Sample Solution
The negative control samples of HGKYC were prepared by getting rid of one corresponding material medicine from this preparation. The herbs were ground into powders before use, and prepared using the sample preparation protocol (showed in Table 1).
Table 1.
Information and preparation protocols of negative control samples.
| NC Samples | Preparation Method | Specificity Evaluation |
|---|---|---|
| Without Scutellariae radix | ① | baicalein and wogonin |
| Without Moutan cortex | ② | paeonol |
| Without Polygoni cuspidate rhizome et radix | ③ | emodin |
① All medicinal materials in Hu–gan–kang–yuan capsule, except for Scutellariae radix, were exactly weighed and exacted according to the Section 2.4.1. The extract solution was used as negative control sample to evaluate specificity for determining baicalein and wogonin; ② All medicinal materials in Hu–gan–kang–yuan capsule, except for Moutan cortex, were exactly weighed and exacted according to the Section 2.4.2. The extract solution was used as negative control sample to evaluate specificity for determining paeonol; ③ All medicinal materials in Hu–gan–kang–yuan capsule, except for Polygoni cuspidate rhizome et radix, were exactly weighed and exacted according to the Section 2.4.3. The extract solution was used as negative control sample to evaluate specificity for determining emodin.
2.5. Establishment of Peak Identification
The UFLC–Q–TOF–MS/MS data of samples were analyzed by PeakView software version 1.2 (AB SCIEX), mainly with the XIC manager tool which provided the quasi–molecular weight, mass errors and isotope pattern fit. When a reference standard was available, the compound was identified by comparing the retention time and MS/MS spectra. While the identification of compound without available reference standard was mainly based on MS/MS data and available literature reports. The mass error of molecular ions of all identified compounds was less than ±5 ppm.
2.6. Method Validation for Determination of Baicalein, Wogonin, Paeonol and Emodin in HGKYC
A HPLC–DAD method was developed for simultaneous determination of baicalein, wogonin, paeonol and emodin for HGKYC quality evaluation. Specificities were assessed by comparing chromatograms of a standard solution mixture of baicalein, paeonol, wogonin and emodin, extract solution of HGKYC and negative control samples under the same conditions. Linearities were assessed by assaying calibration curves with peak area vs. five different injection amounts of 0.040–0.805 μg, 0.032–0.482 μg, 0.047–0.935 μg and 0.027–0.534 μg for baicalein, paeonol, wogonin and emodin, respectively. The intra–day and inter–day precisions (%, RSD) were established by analyzing QC samples on day 1 and on each of three consecutive days in five replicates. The accuracies were evaluated by means of recovery assays performed by adding known amounts of baicalein, paeonol, wogonin and emodin standards to the sample at the similar concentration in six replicates. The spiked amounts of baicalein, paeonol, wogonin and emodin were 77.10, 96.60, 26.70 and 28.05 μg, respectively. The original amount of baicalein, paeonol, wogonin and emodin in sample solution was 85.23, 104.12, 28.11 and 30.34 μg, respectively. The recoveries were calculated as follows:
| Recovery (%) = [(determined amount–original amount)/amount spiked] × 100 | (1) |
The stabilities were evaluated by RSD (%) of peak area of baicalein, paeonol, wogonin and emodin by analyzing sample solution in six replicates at 4.0 °C within 1 month.
3. Results
3.1. Optimization of UFLC–QTOF–MS/MS Conditions
The ingredients of HGKYC belonged to different chemical families and showed distinct polarities. Thus, to obtain an effective separation, guarantee high ionization, and minimize ion suppression, the mobile phase system consisting of a mixture of 0.1% formic acid water solution and acetonitrile with gradient elution was used. The MS conditions, such as the parameters of gas pressure, ion spray voltage, capillary temperature and voltage of declustering potential were optimized. The optimized conditions were described in Section 2.2.1. The positive and negative ion modes were detected.
3.2. Profiles of Ingredients from HGKYC Extract
The total ion chromatograms (TIC) of HGKYC in positive and negative ion modes were obtained. Figure 1A showed the representative positive signal of samples. To better understand the MS fragmentation patterns of the constituents, forty nine of reference standards, including flavonoids, isoflavonoids, saponins, anthraquinones, organic acid and iridoids were investigated by UFLC–Triple–QTOF–MS/MS firstly.
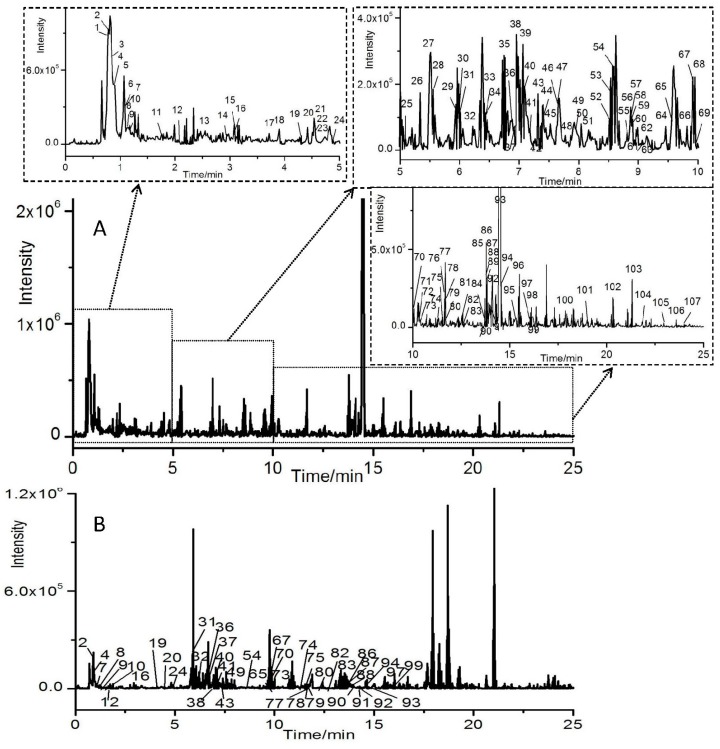
Figure 1.
Total ion chromatography of HGKYC extract (A) and rat serum after oral administration of HGKYC (B) in positive ion mode by UFLC–QTOF–MS/MS. The enlarged view of the area marked was shown on the top and top right.
Under the present conditions, a total of 107 compounds from HGKYC were identified, forty nine of which were unambiguously identified by comparing their retention times, accurate masses and fragment ions with those of the available reference standards, while the others were tentatively identified by elucidating the quasi–molecular ions, fragment ions as well as the available literature reports. The data information of compounds are summarized in Table 2, which includes retention time, molecular formula, measured mass, fragment ions, compound name and related literatures.
Table 2.
Identified compounds in extract of HGKYC and in rat serum after oral administration of HGKYC in positive and negative ion mode.
| No. | TR (min) | Formula | [M + H]+ (Error, ppm) | [M − H]− (Error, ppm) | Fragment Ions in | Identified Compounds | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Positive (+) Ion Mode | Negative (−) Ion Mode | In Extract | In Serum | ||||||
| 1 | 0.77 | C6H14N4O2 | 173.1046 (1.2) | 131.0829 | Arginine s | − | [10] | ||
| 2 | 0.78 | C5H13NO | 104.1070 (0.7) | 60.0832 [M + H − H − C2H5O]+ | Choline s | + | [10] | ||
| 58.0680 | |||||||||
| 3 | 0.85 | C5H9NO2 | 116.0705 (−0.7) | 70.0671 [M + H − HCOOH]+ | Proline | − | [11] | ||
| 4 | 0.9 | C5H11NO2 | 118.0861 (−0.7) | 116.0721 (3.5) | 72.0818 [M + H − HCOOH]+ | Betaine s | + | [10] | |
| 58.0676, 55.0566 | |||||||||
| 5 | 1.07 | C5H5N5 | 136.0717 (−0.2) | 119.0353 [M + H − H − NH3]+ | Adenine s | − | [10] | ||
| 92.0251 [M + H − H − NH3 − HCNN]+ | |||||||||
| 6 | 1.08 | C6H5NO2 | 124.0392 (−0.5) | 106.0278 [M + H − H2O]+ | Nicotinic acid s | − | |||
| 79.0425, 78.0348 | |||||||||
| 7 | 1.08 | C5H7NO3 | 130.0499 (−0.6) | 84.0461 [M + H − HCOOH]+ | l-Pyroglutamic acid s | + | [10] | ||
| 56.0530 [M + H − H − NH3 − HCNOOH]+ | + | ||||||||
| 8 | 1.13 | C9H11NO3 | 182.0806 (−3.3) | 165.0547 [M + H − H − NH3]+ | Tyrosine s | + | [10] | ||
| 136.0761 [M + H − HCOOH]+ | |||||||||
| 119.0487 [M + H − HCOOH − H2O]+ | |||||||||
| 91.0548 | |||||||||
| 9 | 1.17 | C6H13NO2 | 132.1018 (−1.1) | 86.0964 [M + H − HCOOH]+, | Isoleucine s | + | [10] | ||
| 69.0711 [M + H − HCOOH − NH3]+ | |||||||||
| 10 | 1.21 | C9H8O3 | 165.0546 (0.02) | 123.0448 [M + H − H − C2H2O]+ | p-Coumaric acid s | + | [10] | ||
| 95.0500 [M + H − H − C2H2O − CO]+ | |||||||||
| 77.0397 [M + H − H − C2H2O − CO − H2O]+ | |||||||||
| 65.0414 | |||||||||
| 11 | 1.73 | C7H6O5 | 171.0285 (−1.7) | 169.0143 (0.4) | 153.0180 [M + H − H2O]+ | 125.0253 [M − H − CO2]− | Gallic acid s | − | [10] |
| 107.0131 [M + H − H2O − HCOOH]+ | 79.0217 [M − H − CO2 − HCOOH]− | ||||||||
| 12 | 2.04 | C9H11NO2 | 164.0716 (−0.9) | 147.0438 [M − H − NH3]− | Phenylalanine s | + | [10] | ||
| 103.0560 [M − H − NH3 − CO2]− | |||||||||
| 13 | 2.54 | C8H8O4 | 167.0350 (0.2) | 123.0449 [M − H − CO2]− | Vanillic acid | − | [12] | ||
| 93.0358 [M − H − CO2 − OCH3]− | |||||||||
| 97.0283, 69.0341, 65.0399 | |||||||||
| 14 | 2.92 | C7H6O4 | 153.01989 (3.6) | 109.0309 [M − H − CO2]− | Protocatechuic acid | − | [13] | ||
| 91.0208 [M − H − CO2 − H2O]− | |||||||||
| 15 | 3 | C5H7NOS | 130.0316 (−3.9) | 128.0442 (1.0) | 103.0536 [M + H − HCN]+ | Epigoitrin s | − | [14] | |
| 84.0819 [M + H − HCOOH]+ | |||||||||
| 70.0657, 60.9750 | |||||||||
| 16 | 3.08 | C11H12N2O2 | 203.0825 (−0.4) | 142.0664 [M − H − NH3 − CO2]− | Tryptophan s | + | [10] | ||
| 116.0513 [M − H − NH3 − 2OCH2]− | |||||||||
| 17 | 3.83 | C7H6O3 | 137.0248(2.8) | 119.0145 [M − H− H2O]− | Protocatechuic aldehyde s | − | [13] | ||
| 108.0288 [M − H − CO]− | |||||||||
| 92.0284 | |||||||||
| 18 | 3.87 | C14H20O7 | 299.1140 (1.3) | 137.0244 [M − H − Glc]− | Salidroside s | − | [15] | ||
| 119.0335 [M − H − Glc − H2O]− | |||||||||
| 93.0357, 59.0172 | |||||||||
| 19 | 4.31 | C8H8O5 | 183.0300 (0.7) | 168.0065 [M − H − CH3]− | Methyl gallate s | + | [13] | ||
| 124.0175, 78.0136 | |||||||||
| 20 | 4.49 | C15H18O9 | 341.0881 (0.8) | 179.0352 [M − H − Glc]− | Caffeoylglucose | + | [13] | ||
| 135.0454 [M − H − Glc − CO2]− | |||||||||
| 21 | 4.49 | C15H14O6 | 291.0860 (−0.9) | 207.0643, 147.0439 | Catechin s | [13] | |||
| 139.0392, 123.0440 | |||||||||
| 22 | 4.55 | C16H18O9 | 355.1023 (−0.1) | 353.0878 (3.0) | 163.0386 [M + H − H − Glc − OCH2]+ | 191.0564 [M − H − Glc]− | Chlorogenic acid s | − | [16] |
| 145.0280, 117.0329 | |||||||||
| 23 | 4.77 | C17H24O9 | 373.1487 (−1.6) | Syringoside s | − | [17] | |||
| 24 | 4.96 | C9H8O4 | 179.035 4 (2.3) | 135.0459 [M − H − CO2]− | Caffeic acid s | + | [13] | ||
| 25 | 5.15 | C11H10O5 | 223.0595 (−2.5) | 221.0456 (0) | 162.0302 [M + H − H − CH3 − HCOOH]+ | 206.0213 [M − H − CH3]− | Isofraxidin s | − | [16] |
| 190.9989 [M − H − OCH3]− | |||||||||
| 177.0184 [M − H − CO2]− | |||||||||
| 163.0003 [M − H − OCH2 − CO]− | |||||||||
| 26 | 5.36 | C20H23O4N | 342.1695 (−1.3) | 340.1557 (0.8) | 297.1110 [M + H − HCN − H2O]+ | 310.1088 [M − H − OCH2]− | Magnoline | − | [14] |
| 282.0881 [M + H − HCN − H2O − CH3]+ | 252.0404 [M − H − CO2 − NH3 − HCNN]− | ||||||||
| 237.0902, 191.0848, 58.0680 | 224.0509 [M − H − CO2 − NH3 − HCNN − H2O]− | ||||||||
| 162.0180, 118.0288,79.9591 | |||||||||
| 27 | 5.51 | C19H18O11 | 423.0925 (0.7) | 421.0780 (0.8) | 405.0776 [M + H − H2O]+ | 331.0459 [M − H − HCOOH − CO2]− | Mangiferin | − | [18] |
| 339.0520, 327.0493, 303.0478 | 271.0253 [M − H − Xyl]− | ||||||||
| 285.0417 | 259.0230 [M − H − Glc]− | ||||||||
| 273.0395, | |||||||||
| 28 | 5.54 | C17H20N4O6 | 377.1450 (−1.6) | 359.1339, 243.0878, 198.0644 | Riboflavin | − | [19] | ||
| 29 | 5.97 | C17H20O9 | 367.1038 (0.9) | 191.0561 | 3-Feruloyquinic acid | − | [13] | ||
| 30 | 6.07 | C23H28O11 | 479.1569 (2) | 449.1433, 327.1081 | Paeoniflorin s | − | [20] | ||
| 255.0647, 165.0568, 121.0297 | |||||||||
| 31 | 6.08 | C9H10O5 | 197.0458 (1.4) | 169.0147, 124.0175, 78.0178 | Syringic acid s | + | |||
| 32 | 6.2 | C9H8O3 | 163.0400 (−0.4) | 119.0505 [M − H − CO2]− | trans-p-Coumaric acid s | + | |||
| 93.0352 [M − H − CO − C2H2O]− | |||||||||
| 33 | 6.48 | C34H46O18 | 743.2744 (−1.8) | 741.2628 (2.2) | 579.2111 [M − H − Glc]− | Eleutheroside E s | − | [13] | |
| 417.1579 [M − H − 2Glc]− | |||||||||
| 34 | 6.48 | C28H36O13 | 579.2100 (2.9) | Eleutheroside E1 | − | [13] | |||
| 35 | 6.82 | C10H10O4 | 193.0508 (0.8) | 178.0263 [M − H − CH3]− | Ferulic acid s | − | [13] | ||
| 134.0375 [M − H − CH3 − CO2]− | |||||||||
| 36 | 6.9 | C20H22O8 | 391.1372 (−3.9) | 389.1252 (2.7) | 229.0859 [M + H − H − Glc]+ | 227.0718 [M − H − Glc]− | Polydatin s | + | [21] |
| 185.0612 [M − H − Glc − C2H2O]− | |||||||||
| 37 | 6.91 | C14H12O3 | 229.0850 (3.9) | 227.0715 (0.7) | 183.0788 | 185.0601, 143.0508 | Resveratrol s | + | [22] |
| 165.0690 [M + H − H2O − HCOOH]+ | |||||||||
| 107.0489, 91.0540 | |||||||||
| 38 | 6.95 | C26H28O13 | 547.1479 (4) | 6-C-Arabinosyl-8-C-Glucosyl-Chrysin | + | [23] | |||
| 487.1279 | |||||||||
| 457.1169 [M − H − C3H6O − OCH2]− | |||||||||
| 427.1052 | |||||||||
| 409.0946, 367.0839 | |||||||||
| 337.0734 [M − H − Glc − OCH2]− | |||||||||
| 39 | 7.04 | C27H30O16 | 611.1584 (−3.6) | 609.1482 (3.8) | 465.1016 [M + H − H − Rha]+ | 301.0354 [M − H − Rha − Glc]− | Rutin s | − | [18] |
| 303.0501 [M + H − H − Rha − Glc]+ | 271.0228 | ||||||||
| 40 | 7.10 | C21H18O12 | 461.0736 (2.2) | 285.0416 [M − H − Glc acid]− | Scutellarin s | + | [18] | ||
| 41 | 7.19 | C21H20O12 | 463.0891 (1.9) | 301.0360 [M − H − Glc]− | Hyperoside s | + | [24] | ||
| 271.0250 [M − H − Glc − OCH2]− | |||||||||
| 255.0310 [M − H − O − Glc − OCH2]− | |||||||||
| 178.9998, 151.0041 | |||||||||
| 42 | 7.21 | C23H24O13 | 509.1271 (−3.6) | 507.1156 (2.3) | 347.0750 [M + H − H − Glc]+, | 345.0632 [M − H − Glc]− | Viscidulin III- | − | [25] |
| 332.0523 [M + H − H − Glc − CH3]+ | 330.0383 [M − H − Glc − CH3]− | Glucopyranoside | |||||||
| 314.0409 | 315.0174 [M − H − Glc − 2CH3]− | ||||||||
| 43 | 7.32 | C26H28O13 | 547.1479 (4.0) | 487.1279 | 6-C-Glucosyl-8-C-Arabinosyl-Chrysin | [23] | |||
| 457.1169 [M − H − C3H6O − OCH2]− | |||||||||
| 427.1052 | |||||||||
| 409.0946, 367.0839 | |||||||||
| 337.0734 [M − H − Glc − OCH2]− | |||||||||
| 44 | 7.41 | C21H20O13 | 479.0831 (0.1) | 441.0761, 435.0957 | Isomyricitrin | − | [26] | ||
| 313.0570, 165.0548 | |||||||||
| 45 | 7.59 | C25H24O12 | 517.1326 (−2.8) | 515.1199 (0.7) | 499.1177 [M + H − H2O]+ | 353.0889 | Cynarin | − | [13] |
| 163.0382 | 191.0562 | ||||||||
| 179.0347 | |||||||||
| 46 | 7.63 | C33H40O18 | 723.2144 (0.2) | Ligustroflavone s | − | [27] | |||
| 47 | 7.63 | C31H42O17 | 687.24992 (0.6) | 685.2372 (3.4) | 523.1831 [M − H − Glc]− | Specnuezhenide | − | [15] | |
| 454.1404, 421.1508 | |||||||||
| 299.1137, 223.0603 | |||||||||
| 48 | 7.74 | C20H18O11 | 435.0913 (−2) | 433.0784 (1.7) | 303.0493 [M + H − H−Arab]+ | 301.0294 [M − H − Arab]− | Quercetin-3-arabinoside | − | [23] |
| 271.0257 [M − H − Arab − OCH2]− | |||||||||
| 255.0314, 178.9989, 151.0036 | |||||||||
| 49 | 7.93 | C21H20O9 | 417.1169 (−2.6) | 415.1044 (2.2) | 399.1071 [M + H − H2O]+ | 325.0692 [M − H − C3H6O3]− | Chrysin-8-C-glucoside | + | [23] |
| 297.0758 [M + H − H − C4H8O4]+ | 295.0624 [M − H − C4H8O4]− | ||||||||
| 279.0639 [M + H − H − C4H8O4−H2O]+ | 267.0676 [M − H − C4H8O4 − CO]− | ||||||||
| 267.0644 [M + H − H − C4H8O4 − 2H2O]+ | 149.0237 | ||||||||
| 50 | 7.97 | C21H20O11 | 449.1063 (−3.3) | 447.0947 (3.1) | 303.0488 [M + H − H − Rha]+ | 301.0379 [M − H − Rha]− | Quercetrin s | − | [18] |
| 287.0549 [M + H − H − O − Rha]+ | 271.0265 [M − H − Rha − OCH2]− | ||||||||
| 129.0543, 85.0289 | 255.0315, 178.9983 | ||||||||
| 85.0286 | 151.0041 | ||||||||
| 51 | 8.22 | C32H38O16 | 679.2215 (−2.6) | 533.1669 [M + H − H − Rha]+ | hexandraside E | − | [14] | ||
| 371.1096 [M + H − H − Rha − Glc]+ | |||||||||
| 315.0457 [M + H − H − Rha − Glc − C4H8]+ | |||||||||
| 52 | 8.52 | C38H48O20 | 825.2794 (−2.1) | 663.2238 [M + H − H − Glc]+ | Diphylloside B | − | [14] | ||
| 517.1674 [M + H − H − Rha − Glc]+ | |||||||||
| 355.1199 [M + H − H − Rha−2Glc]+ | |||||||||
| 299.0562 [M + H − H − Rha−2Glc − C4H8]+ | |||||||||
| 53 | 8.53 | C26H28O11 | 517.1693 (−2.2) | 355.1169 [M + H − H − Glc]+ | Icarrin C | − | [14] | ||
| 299.0524 [M + H − H—Glc − C4H8]+ | |||||||||
| 54 | 8.58 | C21H18O11 | 447.0912 (−2.2) | 445.07858 (2.1) | 271.0595 [M + H − H − O − Glc]+ | 269.0459 [M − H − O − Glc]− | Baicalin s | + | [23] |
| 55 | 8.82 | C38H48O19 | 809.28357 (−3.3) | 807.2011 (0.33) | 663.2266 [M + H − H − Xyl]+ | Epimedin B | − | [14] | |
| 517.1687 [M + H − H − Xyl − Rha]+ | |||||||||
| 355.1182 [M + H − H − Xyl − Rha − Glc]+ | |||||||||
| 299.0542 [M + H − H − Xyl − Rha − Glc − C4H8]+ | |||||||||
| 56 | 8.86 | C22H22O9 | 431.1318 (−4.2) | 429.1101 (0.3) | 269.0802 [M + H − H − Glc]+ | Ononin | − | ||
| 57 | 8.87 | C21H22O9 | 417.1191 (0) | 255.0678 [M − H − Glc]− | Polygonimitin B | [18] | |||
| 211.0773 [M − H − Glc − CO2]− | |||||||||
| 58 | 8.89 | C21H20O11 | 449.1063 (−3.3) | 447.0946 (3.1) | 303.0488 [M + H − H − Rha]+ | 301.0379 [M − H − Rha]− | Isoquercetrin | − | |
| 287.0549 [M + H − H − O − Rha]+ | 271.0265 [M − H − Rha − CH2]− | ||||||||
| 129.0543,85.0289 | 255.0315,178.9983 | ||||||||
| 59 | 8.9 | C32H38O15 | 663.2265 (−2.7) | 661.2169 (4.7) | 517.1667 [M + H − H − Rha]+ | 515.1907 | Epimedoside A | − | |
| 355.1180 [M + H − H − Rha − Glc]+ | 353.1050 | ||||||||
| 299.0559 [M + H − H − Rha − Glc − C4H8]+ | |||||||||
| 60 | 9.0 | C17H14O8 | 347.0751 (−3.1) | 332.0523, 317.0288, 314.0414 | Viscidulin III | − | [23] | ||
| 289.0340, 169.0125 | |||||||||
| 61 | 9.04 | C39H50O20 | 839.2967 (−0.2) | 677.2346 [M + H − H − Glc]+ | Epimedin A | − | |||
| 531.1881 [M + H − H − Glc − Rha]+ | |||||||||
| 369.1342 [M + H − H − 2Glc − Rha]+ | |||||||||
| 313.0691 [M + H − H − 2Glc − Rha − C4H8]+ | |||||||||
| 62 | 9.15 | C33H40O16 | 693.2365 (−3.4) | 547.1791 [M + H − H − Rha]+ | anhydroicaritin | − | [14] | ||
| 531.1895 [M + H − H − Glc]+ | -3,7-di-O-glucoside | ||||||||
| 385.1284 [M + H − H− Rha − Glc]+ | |||||||||
| 369.1321 [M + H − H − O − Rha − Glc]+ | |||||||||
| 313.0687 [M + H − H − O − Rha − Glc − C4H8]+ | |||||||||
| 63 | 9.15 | C30H32O13 | 599.1787 (2.8) | 581.1699 [M − H − H2O]− | Benzoyloxypaeoniflorin | − | [20] | ||
| 477.1593 [M − H − C7H6O2]− | |||||||||
| 449.7983 [M − H − C7H6O2 − CO]− | |||||||||
| 431.1372 [M − H − H2O − Xyl]− | |||||||||
| 281.0676 [M − H − H2O − Xyl − C5H10O5]− | |||||||||
| 64 | 9.45 | C22H22O10 | 447.1269 (−3.8) | 445.1143 (0.6) | 285.0757 [M + H − H − Glc]+, | 430.0906 [M − H − CH3]− | Wogonin-7-O-glucoside | − | [23] |
| 270.0526 [M + H − H − Glc − CH3]+ | 268.0456 [M − H − CH3 − Glc]− | ||||||||
| 65 | 9.51 | C22H20O12 | 477.1026 (−2.3) | 475.0892 (2) | 301.0706 [M + H − H − Glucuronic acid]+ | 299.0573 [M − H − Glucuronic acid]− | 5,4′-Dihydroxy-8-methoxy-flavone-7-O-glucuronide | + | [23] |
| 286.0470 [M + H − H − Glucuronic acid − CH3]+ | 284.0324 [M − H − Glucuronic acid − CH3]− | ||||||||
| 66 | 9.8 | C15H10O7 | 301.0356 (0.9) | 273.0396 [M − H − CO]− | Quercetin | − | [18] | ||
| 245.0407 [M − H − CO − H2O]− | |||||||||
| 178.9978 | |||||||||
| 151.0035, 121.0292 | |||||||||
| 107.0144 | |||||||||
| 67 | 9.9 | C21H18O11 | 447.0919 (−0.7) | 271.0595 | Baicalein-6-O-glucuronide | + | [23] | ||
| 68 | 9.96 | C11H12O4 | 207.0667 (2.1) | 179.0345, 135.0469 | Ethyl Caffeate s | [23] | |||
| 69 | 9.99 | C22H22O11 | 461.1088 (−0.4) | 459.0949 (3.4) | 285.0759, 270.0522 | 283.0624, 268.0387, 175.0256, 113.0261 | Wogonoside s | − | [23] |
| 70 | 10.03 | C22H20O11 | 461.1069 (−2) | 459.0949 (3.4) | 285.0759 [M + H − H − Glucuronic acid]+ | 283.0624 [M − H − Glucuronic acid]− | Oroxyloside s | + | [23] |
| 270.0522 [M + H − H − Glucuronic acid − CH3]+ | 268.0387 [M − H − Glucuronic acid − CH3]− | ||||||||
| 71 | 10.33 | C39H50O19 | 823.30066 (−1.5) | 677.2421 [M + H − H − Rha]+ | Epmedin C | − | [14] | ||
| 531.1840 [M + H − H−2Rha]+ | |||||||||
| 369.1326 [M + H − H−2Rha − Glc]+ | |||||||||
| 313.0708 [M + H − H−2Rha − Glc − C4H8]+ | |||||||||
| 72 | 10.34 | C27H30O11 | 531.1846 (−2.8) | 369.1326 [M + H − H − Glc]+ | Icariside I s | − | [14] | ||
| 313.0695 [M + H − H − Glc − C4H8]+ | |||||||||
| 73 | 10.55 | C33H40O15 | 677.24256 (−2.1) | 675.2326 (4.7) | 531.1847 [M + H − H − Rha]+ | 367.1099 | Icariin s | + | [14] |
| 369.1332 [M + H − H − Rha − Glc]+ | 269.0461 [M − H − Rha − Glc]− | ||||||||
| 313.0712 [M + H − H − Rha − Glc − C4H8]+ | |||||||||
| 74 | 11.3 | C9H10O3 | 165.0557 (0) | 150.0333 [M − H − CH3]− | Paeonol s | + | [28] | ||
| 135.0094 [M − H − OCH2]− | |||||||||
| 122.0385, 91.0208 | |||||||||
| 75 | 11.44 | C15H10O6 | 285.0407 (0.8) | 241.0517 [M − H − CO2]− | Kaempferol s | + | [18] | ||
| 211.0408 [M − H − CO2−OCH2]− | |||||||||
| 195.0463,167.0509 | |||||||||
| 76 | 11.56 | C16H12O6 | 299.0563 (0.7) | 284.0327 [M − H − CH3]− | 5,7,4’-Trihydroxy-8- | − | [23] | ||
| 240.0424 [M − H − CH3 − CO2]− | methoxyflavone | ||||||||
| 171.0452,153.9909 | |||||||||
| 125.9968 | |||||||||
| 77 | 11.59 | C16H12O7 | 315.0510 (0) | 300.0279 [M − H − CH3]− | Isorhamnetin s | + | [29] | ||
| 282.0178 [M − H − CH3 − H2O]− | |||||||||
| 255.0240, 151.0016 | |||||||||
| 78 | 11.71 | C32H38O15 | 663.3011 (3.1) | 661.2163 (−3.5) | 517.1667 [M + H − HCOOH]+ | 353.0978 [M − H − Rha − Glc]− | Ikarisoside B | + | [26] |
| 355.1165 [M + H − H − Rha − Glc]+ | |||||||||
| 299.0599 [M + H − H − Rha − Glc − C4H8]+ | |||||||||
| 79 | 11.73 | C15H10O5 | 271.0597 (−1.6) | 269.0456(1.9) | 253.0500 [M + H − H2O]+ | 251.036 [M − H − H2O]− | Baicalein s | + | [23] |
| 123.0080 | 223.0407, 169.0664 | ||||||||
| 80 | 12.15 | C31H36O14 | 631.2050 (2.8) | 481.1616 [M − H − Xyl]− | Ikarisoside F | + | [26] | ||
| 353.1076, 352.0928, 341.0528 | |||||||||
| 81 | 12.37 | C16H12O4 | 269.0805 (−1.2) | 267.0664 (0.4) | 254.0576, 237.0532 | 252.0430, 223.0396, 195.0445 | Formononetin s | − | [10] |
| 197.0599 | 132.0221 | ||||||||
| 181.0644, 118.0416 | |||||||||
| 82 | 12.65 | C26H28O10 | 501.1744 (−2.3) | 499.1618 (1.6) | 355.1169 [M + H − H − Rha]+ | 353.1043 [M − H − Rha]− | Baohuoside II s | + | [14] |
| 299.0559 [M + H − H − Rha − C4H8]+ | 309.0445 [M − H − Rha − CO2]− | ||||||||
| 147.0645,129.0533 | |||||||||
| 83 | 13.34 | C33H40O15 | 677.2417 (−3.4) | 675.2326 (4.7) | 531.1847 [M + H − H − Rha]+ | 367.1099 [M − H − Rha − Glc]− | Baohuoside VII | + | [26] |
| 369.1324 [M + H − H − Rha − Glc]+ | 352.0997 | ||||||||
| 313.0712 [M + H − H − Rha − Glc − C4H8]+ | |||||||||
| 84 | 13.49 | C43H70O15 | 827.4768 (−2.3) | 455.3660, 437.335 | Astragaloside II | − | [10] | ||
| 175.0610,157.0474,143.1087 | |||||||||
| 85 | 13.74 | C42H68O13 | 781.4736 (0.4) | 745.4447 | Saikosaponin a | − | [30] | ||
| 619.4203, 583.3921 | |||||||||
| 455.3486, 437.3374 | |||||||||
| 419.3231 | |||||||||
| 86 | 13.79 | C16H12O5 | 285.0754 (−1.3) | 283.0615 (0.9) | 270.0514 [M + H − H − CH3]+ | 268.0380 [M − H − CH3]− | Wogonin s | + | [23] |
| 179.0488 | 163.0036 | ||||||||
| 87 | 13.80 | C32H38O14 | 647.2323 (−1.7) | 501.1726 [M + H − H − Rha]+ | Sagittatoside B | + | [26] | ||
| 465.1557 [M + H − H − Rha − HCOOH]+ | |||||||||
| 409.0917, 355.1171 | |||||||||
| 299.0594 | |||||||||
| 88 | 13.81 | C16H12O5 | 285.0754 (−1.3) | 283.0615 (0.9) | 270.0514 [M + H − H − CH3]+ | 268.0380 [M − H − CH3]− | Oroxylin A s | + | [23] |
| 179.0488 | 163.0036 | ||||||||
| 89 | 13.82 | C32H38O14 | 647.2323 (−1.7) | 629.2323, 501.1726, | 2′′-O–Rhamnosyl-ikarisoside A | [26] | |||
| 465.1557, 355.1171, 299.0059 | |||||||||
| 90 | 13.9 | C33H40O14 | 661.2470 (−3.1) | 659.2374 (4.4) | 515.1897 [M + H − H − C6H10O4]+ | 366.1127 | 2′′-O-Rhamnosyl-ikariside II | + | [14] |
| 369.1333, 355.0809, 313.0703 | |||||||||
| 91 | 13.96 | C15H10O4 | 255.0642 (−3.9) | 253.0708 (0.7) | 153.0177 [M + H − H − C5H8O − H2O]+ | 209.0609 [M − H − CO2]− | Chrysin s | + | [23] |
| 103.0544 | 143.0505, 63.0276 | ||||||||
| 92 | 14.01 | C17H14O6 | 315.0856 (−2.3) | 313.0718 (0.2) | 300.0618, 285.0396 | 298.0485, 283.0252, 211.0396 | Kumatakenin | [29] | |
| 257.0440, 182.9919, 154.9964 | 155.0506 | ||||||||
| 93 | 14.5 | C24H32O7 | 433.2215 (−1.3) | 415.2106 [M + H − H2O]+ | Schisandrin s | + | [31] | ||
| 400.1869 [M + H − H2O − CH3]+ | |||||||||
| 384.1923 [M + H − H − OCH3 − H2O]+ | |||||||||
| 359.14907 [M + H − H2O − C4H8]+ | |||||||||
| 315.1223 | |||||||||
| 94 | 14.63 | C27H30O10 | 515.1901 (−2) | 513.1780 (2.7) | 369.1321 [M + H − H − Rha]+ | 367.1134 [M − H − Rha]− | Icarisid II s | + | [14] |
| 313.0700 [M + H − H − Rha − C4H8]+ | 351.0882 [M − H − O − Rha]−, 323.0912 | ||||||||
| 95 | 15.2 | C45H72O16 | 869.4881 (−1.4) | 689.4234, 437.3396 | Astragaloside I s | − | [10] | ||
| 217.0720, 157.0506, 143.1059 | |||||||||
| 96 | 15.5 | C23H28O7 | 417.1890 (−4.2) | 399.1815 [M + H − H2O]+ | Schisandrol B | − | [31] | ||
| 384.1501 [M + H − H2O − CH3]+ | |||||||||
| 368.1618 [M + H − H2O−OCH3]+ | |||||||||
| 357.1394 [M + H − H2O − C3H6]+ | |||||||||
| 343.1187 [M + H − H2O − C4H8]+ | |||||||||
| 97 | 16.02 | C30H48O5 | 489.3566 (−1.8) | 487.3434 (1) | 453.3324 [M + H − H − 2H2O]+ | 469.3361 [M − H − H2O]− | Tormentic acid | + | [15] |
| 407.3254 [M + H − HCOOH−2H2O]+ | 407.1533 | ||||||||
| 201.1702, 127.0767 | |||||||||
| 98 | 16.07 | C21H20O6 | 369.1323 (−2.6) | 313.0693 | Icaritin | − | [14] | ||
| 99 | 16.08 | C15H10O5 | 271.0596 (−2) | 269.0460 (1.5) | 225.0560, 241.0514, 197.0608, 182.0375 | Emodin s | + | [18] | |
| 100 | 17.88 | C23H30O6 | 403.2112 (−0.8) | 388.1848 [M + H − H − CH3]+, | Schisanhenol | − | [31] | ||
| 372.1919 [M + H − H − OCH3]+ | |||||||||
| 371.1859 [M + H − H − CH3OH]+ | |||||||||
| 333.1326, 302.1149 | |||||||||
| 101 | 19.09 | C28H34O9 | 515.2264 (−2.3) | 415.2009 [M + H − H − C4H6COOH]+ | Schisantherin B | − | [31] | ||
| 385.1637 [M + H − H − C4H6COOH − OCH2]+ | |||||||||
| 355.1534, 343.1160, 316.0939 | |||||||||
| 102 | 20.31 | C24H32O6 | 417.2259 (−3) | 402.1999 [M + H − H − CH3]+ | Schizandrin A s | − | [31] | ||
| 386.2059 [M + H − H − OCH3]+ | |||||||||
| 347.1480 [M + H − H − C5H10]+ | |||||||||
| 316.1289 [M + H − H − C5H10 − OCH3]+ | |||||||||
| 301.1058, 285.1104, 273.1091, 242.0911 | |||||||||
| 103 | 21.3 | C23H28O6 | 401.1948 (−2.6) | 386.1728 [M + H − H − CH3]+ | γ-Schizandrin B s | − | [31] | ||
| 331.1168 [M + H − H − C5H10]+ | |||||||||
| 300.0987, 242.0929 | |||||||||
| 104 | 21.87 | C22H24O6 | 385.1638 (−1.9) | 355.1561 [M + H − H − OCH2]+ | Schizandrin C | − | [31] | ||
| 315.0895 [M + H − H − C5H10]+ | |||||||||
| 285.0757 [M + H − H − C5H10−OCH2]+ | |||||||||
| 257.0809, 242.0588, 227.0703, 153.0663 | |||||||||
| 105 | 22.98 | C32H50O5 | 515.3723 (−1.6) | 513.3594 (1.7) | 409.3466, 191.1793 | 495.3496 [M − H − H2O]− | 19α−Hydroxyl−3−acetyl ursolic acid | − | [15] |
| 469.3720 [M − H − CO2]− | |||||||||
| 106 | 23.56 | C30H48O3 | 455.3537 (1.3) | Oleanic acid s | − | [15] | |||
| 107 | 23.73 | C30H48O3 | 455.3537 (1.3) | Ursolic acid | − | [15] | |||
“s” Identified with reference compounds; “+”: detected and “−”not detected.
3.2.1. Identification of Flavonoids and Isoflavonoids
There are notable amounts of flavonoids and flavonoid derivatives in Scutellariae radix and Epimedii folium. Especially there are amounts of prenylated flavones in Epimedii folium, which have characteristic prenylated group at C–8 position. In MS analysis, the consecutive losses of sugar, H2O, carbonyl and isopentene group (C4H8) were observed as typical fragmentation profiles of these prenylated flavones. A total of 45 flavones or isoflavones were identified in HGKYC, with 18 definitely elucidated and the others tentatively identified.
The fragmentation patterns of ordinary flavones obey the Retro–Diels–Alder (RDA) fragmentation mechanism and the losses of a glucose residue (162 Da), rhamnose residue (146 Da), a methyl group (15 Da), H2O (18 Da), CO (28 Da) which are often the initial breakdown syeps of flavonoids [32]. For instance, Peak 54 gave an [M + H]+ ion at m/z 447.0912 (C21H18O11) and yielded a high intensity fragment ion at m/z 271.0595 by loss of one glucuronic acid moiety (176 Da), and peak 79 gave an [M + H]+ ion at m/z 271.0596 and a fragment ion at m/z 123.0080 by loss of H2O (18 Da). It indicated that peak 54 might be the glucuronide of peak 79, by comparing retention time, UV spectra and the fragment ions with the reference standards, so peaks 54 and 79 were identified as baicalin and baicalein, respectively [23]. Peak 81 gave an [M + H]+ ion at m/z 269.0805 (C16H12O4) in positive ion mode, and by comparing the retention time and fragmentation pathways with the authentic standard, peak 81was identified as formononetin. Peak 56 gave an [M + H]+ ion at m/z 431.1318 (C22H22O9) and yielded a characteristic fragment ion at m/z 269.0802 by losing one glucose residue, which indicated peak 56 possibly was the glycoside of peak 81. By comparing MS/MS data with peak 81 and the available literature, peak 56 was tentatively identified as formononetinglucoside (ononin) [10]. Peaks 39, 40, 41, 46, 54, 78, 69, 70, 75, 77, 81, 86, 88, 91 were definitely identified as rutin, scutellarin, hyperoside, ligustroflavone, baicalin, baicalein, wogonoside, oroxyloside, kaempferol, isorhamnetin, formononetin, wogonin, oroxylin A and chrysin, respectively, by comparing their MS and UV spectra information with those of reference standards.
The loss of an isopentene group was observed as a typical fragmentation profile of prenylated flavones except the consecutive neutral losses of sugar moieties. For example, Peak 72 gave an [M + H]+ ion at m/z 531.1846 and yielded characteristic fragment ions at m/z 369.1326 and 313.0695 by successive losses of glucose residue (162 Da) and isopentene group (56 Da). By comparing with the information of authentic standard, peak 72 was identified as icariside I [14]. Peak 73 gave an [M + H]+ ion at m/z 677.2427 and gave fragment ions at m/z 531.184, 369.1332 and 313.0712 by the successive losses of one rhamnose, glucose residue and isopentene group, peak 73 was identified as icariin by comparing with the authentic standard [14]. Based on the characteristic fragmentation profiles of flavones and prenylated flavones as well as the available literatures, 27 flavones (peaks 38, 43, 44, 48, 49, 60, 64, 65, 66, 67, 76, 51, 52, 53, 59, 61, 62, 71, 78, 80, 83, 87, 89, 90, 92, 94 and 98) were tentatively identified (Table 2).
Peak 51 gave an [M + H]+ ion at m/z 679.2215 (C32H38O16) in positive ion mode and yielded fragment ions at m/z 533.1669, 371.1096 and 315.0457 by successive losses of one rhmnose residue, glucose residue and isopentene group, it was tentatively identified as hexandraside E by comparison with the available literature [14]. Peak 53 gave an [M + H]+ ion at m/z 517.1693 (C26H28O11) in positive ion mode and yielded a high intensity fragment at m/z 355.1191 by loss of one glucose, while another fragment ion at m/z 299.0524 originated from the ion at m/z 355.1191 by losing one isopentene group. Additionally, peak 53 had the same fragment patterns as diphylloside B after the latter lost the two–connected rhamnoses, so by comparison with the available literature, peak 53 was speculated to be icarrin C [14]. Peak 61 gave an [M + H]+ ion at m/z 839.2967 (C39H50O20) in positive ion mode and yielded characteristic fragment ions at m/z 677.2346, 531.1881, 369.1342 and 313.0691 by the successive losses of one glucose, rhamnose, C–7–O–glucose and isopentene group, and by comparison with the available literature, peak 61 was tentatively identified as epimedin A [14]. Peak 62 gave an [M + H]+ ion at m/z 693.2365 (C33H40O16) in positive ion mode and yielded fragment ions at m/z 547.1791 and 531.1895 by the losses of one rhamnose and glucose residue, respectively. The fragment ion at m/z 385.1284 was originated by the simultaneous losses of one rhamnose and glucoseresidue from the molecular ion. The characteristic fragment ion at m/z 313.0687 originated from the fragment ion at m/z 369.1321 by the loss of an isopentene group. By comparison with the available literature, peak 62 was tentatively identified as anhydroicaritin–3,7–di–O–glucoside [14].
3.2.2. Identification of Organic Acids
Because of the presence of COOH groups in organic acid structures, their fragment ions were usually generated by the losses of H2O (18 Da), CO2 (44 Da) and HCOOH (44 Da). Seventeen organic acids have been identified from HGKYC, nine of which were confirmed by reference standards, peaks 6, 10, 11, 22, 24, 31, 32, 35 and 106 were identified as nicotinic acid, p–coumaric acid, gallic acid, chlorogenic acid, caffeic acid, syringic acid, trans–p–coumaric acid, ferulic acid and oleanic acid, respectively. The other organic acids (peaks 13, 14, 20, 29, 45, 97, 105, 107) were tentatively assigned. Peak 14 exhibited an [M − H]− ion at m/z 153.0199 and yielded high intensity fragments ions at m/z 109.0309 and 91.0208 by the successive losses of CO2 and H2O, by comparing with the literature report, peak 14 was identified as protocatechuic acid [13]. Peak 20 gave an [M − H]− ion at m/z 341.0881 (C15H18O9) and yielded fragment ions at m/z 179.0352 and 135.0454 by the successive losses of one glucose residue and CO2, so was inferred that peak 20 was caffeoylglucose by comparison with the available literature [13]. Peak 97 gave an [M + H]+ ion at m/z 489.3566 (C30H48O5) in positive ion mode and yielded major fragment ions at m/z 453.3324 and 407.3254 by the successive losses of 2H2O and HCOOH, respectively. By comparison with the available literature, peak 97 was tentatively identified as tormentic acid [15]. Peak 107 had the same [M − H]− ion at m/z 455.3537 (C30H48O3) with oleanic acid in negative ion mode. Oleanic acid and ursolic acid were reported in Ligustri lucidi fructus, therefore, peak 107 was tentatively characterized as ursolic acid [15]
3.2.3. Identification of Phenylpropanoids
Fifteen phenylpropanoids have been identified from HGKYC, seven of which were identified by comparison with the available reference standards. Peaks 23, 25, 33, 37, 93, 102 and 103 were identified as syringoside, isofraxidin, eleutheroside E, resveratrol, schizandrin A and γ–schizandrin B by comparing with their reference standards, respectively. Peak 96 gave an [M + H]+ ion at m/z 417.1890 (C23H28O7) in positive ion mode and yielded fragment ions at m/z 399.1815 and 384.1501 by the successive losses of H2O, CH3 or OCH3. The [M + H − H − 18 − 42]+ ion at m/z 357.1394 originated from the ion at m/z 417.1890 by loss of H2O and C3H6. By comparison with the available literature, peak 96 was tentatively identified as schisandrol B [31]. Peak 100 gave an [M + H]+ ion at m/z 403.2112 (C23H30O6) in positive ion mode and yielded fragment ions at m/z 388.1848, 372.1919 and 371.1859 by the losses of CH3, OCH3 and CH3OH, respectively. Peak 100 was tentatively identified as schisanhenol based on the available literature [31]. Peak 101 gave an [M + H]+ ion at m/z 515.32264 (C28H34O9) in positive mode and yielded characteristic fragment ion at m/z 415.2009 by the loss of C4H6COOH. The ion at m/z 385.1637 was originated from the ion at m/z 415 by the loss of one formaldehyde. By comparison with the available literature, peak 101 was tentatively identified as schisantherin B [31]. Peak 104 gave an [M + H]+ ion at m/z 385.1638 in positive mode and yielded fragment ions at m/z 355.1561 and 315.0895 by the loss of OCH2 and C5H10. The ion at m/z 285.0757 was originated from ion at m/z 315.0895 by the loss of OCH2. By comparison with the available literature [31], peak 104 was identified as schizandrin C.
3.2.4. Identification of Anthraquinones
Three anthraquinones have been identified from HKGYC, one of which was identified by comparing with the authentic standard, namely peak 99 identified as emodin [18]. Peaks 27 and 57 were tentatively assigned as mangiferin and polygonimitin B based on the available literature reports [18].
3.2.5. Identification of Saponins
Three saponins have been identified from HKGYC. Peak 95 gave an [M + H]+ ion at m/z 869.4881 and yielded fragment ions at m/z 689.4234, 437.3396, 217.0720 and 157.0506. By comparison with an authentic standard, peak 95 was identified as astragaloside A [10]. Peak 84 gave an [M + H]+ ion at m/z 827.4768 (C43H70O15) in positive ion mode and gave a series of fragment ions typical of triterpenoid saponins at m/z 455,437,175 and 143; peak 85 gave an [M + H]+ ion at m/z 781.4736 (C42H68O13) and yielded fragment ions at m/z 745.4447,619.4203, 583.3921, by comparison with the available literature, peaks 84 and 85 were tentatively identified as astragaloside II and saikosaponin a, respectively [18,23].
3.2.6. Identification of Alkaloids
Four alkaloids were identified from HGKYC, of which peaks 2, 4 and 15 were definitely identified as choline, betaine and epigoitrin by comparison with their authentic standards [18,19]. Peak 26 gave an [M + H]+ ion at m/z 342.1695 (C20H23O4N) and gave an [M − H]− ion at m/z 340.1550 in negative mode, and yielded [M + H − H − HCN − H2O]+ at m/z 297.1110 by losing HCN and H2O, so peak 26 was tentatively identified as magnoline on the basis of the available literature [14].
3.2.7. Identification of Terpenes and Phenols
Peaks 17, 18, 19, 21, 30, 36, 68, 74 were identified as protocatechuic aldehyde, salidroside, methyl gallate, catechin, paeoniflorin, polydatin, caffeate and paeonol by comparison with authentic standards. Peak 63 gave an [M − H]− ion at m/z 599.1787 (C30H32O13) and yielded fragment ions at m/z 581.1699, 477.1593, 449.7983 by the losses of H2O, benzoyloxy and CO. The [M − H − 122 − 28]− ion at m/z 449.7983 originated from the ion at m/z 599 by losing benzoyloxy and CO, which indicated the presence of a benzoyloxy group. Based on the fragmentation pathways of peak 30 and literature data [20], peak 63 was tentatively identified as benzoyloxypaeoniflorin. Peak 47 gave an [M − H]− ion at m/z 685.2372 in negative ion mode and had fragment ions at m/z 685.2439, 523.1866, 453.1445 and 421.1530. Peak 47 was tentatively identified as specnuezhenide based on the published literature [15].
3.2.8. Identification of Other Compounds
By comparing the retention times and MS spectra with those of authentic standards, peaks 1, 5, 7, 8, 9, 12 and 16 were identified as arginine, adenine, l–pyroglutamic acid, tyrosine, isoleucine, phenylalanine and tryptophan, respectively. Peak 3 gave an [M + H]+ ion at m/z 116.0706 (C5H9NO2) in positive ion mode and yielded a characteristic ion at m/z 70.0671 by the loss of HCOOH; peak 28 gave an [M − H]− ion at m/z 377.1450 (C17H20N4O6) and yielded fragment ions at m/z 359.1339 and 243.0878 by the losses of H2O and C5H10O4 group, because of the unavailability of authentic standards, peak 3 and peak 28 were tentatively identified as proline and riboflavin, respectively, based on the available literature [32,33].
3.3. Profiles of Ingredients in Rat Serum after Oral Administration HGKYC
By comparing retention times, UV spectra and MS/MS data with those of compounds analyzed in HGKYC extract sample, forty three prototype compounds were found in rat serum (shown in Table 2 and Figure 1B). As shown in Table 2, the compounds absorbed in blood were far fewer than those in HGKYC extract, which indicated the number of compounds absorbed in blood is limited due to poor absorption or low concentration. On the basis of the availabilities of authentic standards and pharmacological effects reported, baicalein, wogonin, paeonol and emodin were then chosen as the chemical markers for quality control of HGKYC.
3.4. Optimization of HPLC Conditions
In this study, different mobile phases, elution modes and detection wavelengths were investigated. Mobile phases of acetonitrile–water and methanol–water with different modifiers including acetic acid, formic acid and phosphoric acid were tested under different gradient elution modes. The detection wavelength was selected according to the maximum absorption wavelengths of baicalein, wogonin, paeonol and emodin shown in UV spectra from DAD. The optimized conditions were described in Section 2.2.2. Finally, excellent separations were achieved and typical chromatograms are shown in Figure 2.
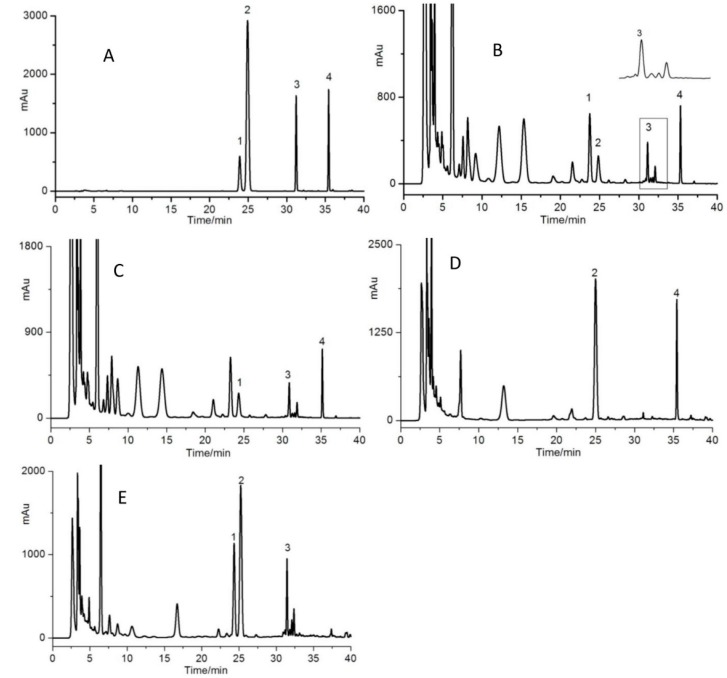
Figure 2.
HPLC chromatographic profiles of standards mixture (A); sample of Hu–gan–kang–yuan capsule (B); negative control sample without Moutan cortex (C); without Scutellariae radix (D); and without Polygoni cuspidate rhizome et radix (E). 1: baicalein, 2: paeonol, 3: wogonin, 4: emodin.
3.5. Optimization of Extraction Conditions
Ultrasound–assisted extraction (UAE) has been widely used in sample preparation for the quality control of TCMPs. In the extraction process, extraction solvent, sample–solvent ratio, extraction time and temperature are critical for high extraction effectiveness. Pure and aqueous methanol or ethanol solutions are often used as extraction solvents. In the present study, based on the physicochemical properties of the targeted compounds, different concentrations (50%, 80%, and 100%) of methanol water solutions were examined to extract the four compounds in HGKYC. Considering extract rates of targeted compounds, contents of interfering components and extract time, samples were extracted for 15 min by UAE using 50–time of methanol, 50 °C as extraction temperature.
3.6. Method Validation
Typical HPLC chromatograms of the authentic standards of the four compounds, the sample, and the negative control samples were shown in Figure 2. This showed that there are no peaks of baicalein, paeonol, wogonin and emodin in the corresponding negative control samples. In addition, targeted compounds in the reference standard sample and the tested sample showed good resolution with the adjacent peaks. The calibration curves, coefficients of determination (r) and concentration ranges were y = 27858x–115.36 (r = 1.0) for baicalein in 0.040–0.805 μg, y = 32233x + 38.381 (r = 0.9998) for paeonol in 0.032–0.482 μg, y = 30503 x + 96.12 (r = 1.0) for wogonin in 0.047–0.935 μg and y = 46970 x + 321.12 (r = 0.9994) for emodin in 0.027–0.534 μg, respectively. The mean recoveries of baicalein, paeonol, wogonin and emodin were 98.45% (RSD = 2.53%), 97.55% (RSD = 2.84%), 99.13% (RSD = 1.54%) and 101.08% (RSD = 2.04%), respectively. The intra–day precisions (RSD values) of baicalein, paeonol, wogonin and emodin were in the range of 0.33%–1.82%, 1.21%–1.97%, 0.63%–1.52% and 0.73%–1.92%, respectively, the inter–day precisions were in the range of 1.16%–1.65%, 1.52%–1.95%, 1.33%–1.59% and 1.13%–1.79%, respectively. The RSD values of baicalein, paeonol, wogonin and emodin solution at 4.0 °C within 1 month were in the range of 1.1%–2.17%, 1.58%–2.95%, 1.22%–2.15% and 0.98%–2.99%, respectively.
3.7. Sample Determination
The contents of baicalein, paeonol, wogonin and emodin in HGKYC were determined using the validated methods in triplicates. The results are shown in Table 3.
Table 3.
Contents of baicalein, paeonol, wogonin and emodin in HGKYC (μg/g, n = 3, mean ± SD).
| Batch | Baicalein | Paeonol | Wogonin | Emodin |
|---|---|---|---|---|
| 1 | 1893 ± 32 | 160 ± 2.2 | 542 ± 6.7 | 590 ± 7.2 |
| 2 | 17557.2 | 14557.2 | 48357.2 | 55757.2 |
| 3 | 19247.2 | 17547.2 | 56747.2 | 60247.2 |
4. Discussion
UFLC–QTOF–MS/MS, a high–efficiency analytical technique, can provide qualitative and quantitative information about samples, but the expensive instrument price and analysis cost limits its applicability in quality control of TCMPs for pharmaceutical companies. HPLC–DAD is a high quality and inexpensive analysis technique, commonly used to analyze quantitatively chemical markers in TCMPs. At present, HPLC–DAD is irreplaceable in QC of TCMPs though it is not as powerful as UFLC–QTOF–MS/MS in functional analysis. Ideally, the more chemical markers selected to evaluate the quality of TCMPs, the better to ensure the consistency between the quality of TCMPs and their pharmacological activities, but that is unpractical because of the limited availability of authentic standards. In this paper, on the basis of the prototype compounds detected in rat serum after oral administration HGKYC, considering the following reasons: (1) the analysis cost; (2) the popularity of analysis equipment; (3) the availabilities of authentic standards; and (4) the reported anti–inflammatory activities and protective effects on hepatocytes of baicalein and wogonin in Scutellariae radix [34,35,36,37], paeonol in Moutan Cortex [38,39] and emodin in Polygoni Cuspidate Rhizome et Radix [40,41], then baicalein, wogonin, emodin and paeonol were selected as chemical markers for evaluating the quality of HGKYC by HPLC–DAD. Hence, the quality control scheme for HGKYC using the absorption–based chemical markers is warranted.
5. Conclusions
In summary, a reliable and powerful twenty–five minute long analytical method by UFLC–Q–TOF–MS/MS was successfully established for identifying compound profiles in HGKYC extract and in rat serum after oral administration of HGKYC. A total of 107 compounds in HGKYC, 43 compounds of which were also present in rat serum, were identified or tentatively identified based on their retention times, UV spectra, and MS information. The absorption–based quality control scheme for HGKYC provides a valuable demonstration for the quality control of TCMPs.
Acknowledgments
We express our appreciation to Hongwei Zhang for identifying the samples and Xuan Zeng for the usage to UFLC–QTOF–MS/MS equipment. The work was supported by Science and Technology Planning Project of Guangdong Province, China (No. 2011B032200003) and Science and Technology Planning Project of Guangzhou, China (No. 2012Y2-00018-4).
Author Contributions
F.W. designed research; M.C., C.L., F.C. and Q.S. performed research and analyzed the data; F.W. wrote the paper; Z.M. checked the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Editorial Committee of Pharmacopoeia of Ministry of Health, China. The Pharmacopeoia of People’s Republic of China (Part I) Chemical Industry Press; Beijing, China, 2015; pp. 425–: 1749. [Google Scholar]
- 2.Yin T.J., Yang G.Y., Ma Y., Xu B.B., Hu M., You M., Gao S. Developing an activity and absorption-based quality control platform for Chinese traditional medicine: Application to Zeng-Sheng-Ping (Antitumor B) J. Ethnopharmacol. 2015;172:195–201. doi: 10.1016/j.jep.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu Y.H., Jiang P., Wang S.P., Yan S.K., Xiang L., Zhang W.D., Liu R.H. Plasma pharmacochemistry based approach to screening potential bioactive components in Huang-Lian-Jie-Du-Tang using high performance liquid chromatography coupled with mass spectrometric detection. J. Ethnopharmacol. 2012;141:728–735. doi: 10.1016/j.jep.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q., Jiang P., Ye F.Y., Shi R., Ma Y.M., Zhong J., Wu J.S., Liu P., Liu C.H., Jia Y.Q. Identification and pharmacokinetics of multiple constituents in rat plasma after oral administration of yin chen zhu fu decoction. J. Ethnopharmacol. 2014;153:714–724. doi: 10.1016/j.jep.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Huang C., Xu Q.M., Chen C., Song C.W., Xu Y., Xiang Y., Feng Y.L., Ouyang H., Zhang Y., Jiang H.L. The rapid discovery and identification of physalins in the calyx of Physalisalkekengil.var franchetii (Mast.) Makino using ultra-highperformance liquid chromatography-quadrupole time of flight tandem mass spectrometry together with a novel three-step data mining strategy. J. Chromatogr A. 2014;1361:139–152. doi: 10.1016/j.chroma.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Li Z.F., Wang Y.W., Ouyang H. ; Lu,Y.; Qiu, Y.; Feng,Y.L.; Jiang, H.L.; Zhou, X.; Yang, S.L. A novel dereplication strategy for the identification of two new trace compounds in the extract of Gastrodiaelata using UHPLC/Q–TOF–MS/MS. J. Chromatogr. B. 2015;988:45–52. doi: 10.1016/j.jchromb.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Xu J.D., Mao Q., Shen H., Zhu L.Y., Li S.L., Yan R. Ultra-high performance liquid chromatography coupled with photo–diode array and quadrupole/time-of-flight mass spectrometry based chemical profiling approach to evaluate the influence of preparation methods on the holistic quality of Qiong-Yu-Gao, a traditional complex herbal medicine. J. Chromatogr. A. 2013;1304:154–168. doi: 10.1016/j.chroma.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Li P.L., Liu M.H., Hu J.H., Su W.W. Systematic chemical profiling of Citrus grandis ‘‘Tomentosa’’ byultra-fast liquid chromatography/diode-array detector/quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2014;90:167–179. doi: 10.1016/j.jpba.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Li Y., Zhou G.S., Peng Y., Tu P.F., Li X.B. Screening and identification of three typical phenylethanoid glycosides metabolites from Cistanches Herba by human intestinal bacteria using UPLC/Q–TOF–MS. J. Pharm. Biomed. Anal. 2016;118:167–176. doi: 10.1016/j.jpba.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 10.Liu M.H., Tong X., Wang J.X., Zou W., Cao H., Su W.W. Rapid separation and identification of multiple constituents in traditional Chinese medicine formula shenqi fuzheng injection by ultra-fast liquid chromatography combined with quadrupole-time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2013;74:141–155. doi: 10.1016/j.jpba.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y.J., Gou Q., Xia Z.N. Coordination interaction capillary electrophoresis detection of arginine and proline in radix isatidis and its injection. J. Chongqing Univ. 2009;32:1357–1362. [Google Scholar]
- 12.Liu E.H., Qi L.W., Peng Y.B., Cheng X.L., Wu Q., Li P., Li C.Y. Rapid separation and identification of 54 major constituents in Bu yang huan wu decoction by ultra-fast HPLC system coupled with DAD-TOF/MS. Biomed. Chromatogr. 2009;23:828–842. doi: 10.1002/bmc.1193. [DOI] [PubMed] [Google Scholar]
- 13.Huang J., Shao Q., Xiang Y.H., Ge Z.W., Fan X.H. Identification of phenylpropanoids in Ciwujia injection by HPLC–MS. China J. Chin. Mater. Med. 2014;39:2513–2520. [PubMed] [Google Scholar]
- 14.Gan J.S., Ma Y., Wang Z.Y., Liu X.S., Liu Y. Analysis on chemical constituents in Epimedii Herba by UPLC/Q–TOF–MS. Drugs Clin. 2014;29:351–352. [Google Scholar]
- 15.Jiang Y.J., Yao W.F., Zhang L., Ding A.W. Anaylsis of chemical components of Ligustrum lucidum by ultra-performance liquid chromatography-electrospray ionization-quadrupole-time of flight-mass spectrometry. China J. Chin. Mater. Med. 2012;37:2304–2308. [PubMed] [Google Scholar]
- 16.Sun H., Han Y., Zhang A.H., Meng X.C., Wang Z.Y., Sun W.J., Sun H.F., Wang X.J. UPLC–MS based metabolic profiling of the phenotypes of Acanthopanax senticosus reveals the changes in active metabolites distinguishing the diversities of the plant grown in northeast area of China. Chin. J. Nat. Med. 2012;10:196–206. doi: 10.3724/SP.J.1009.2012.00196. [DOI] [Google Scholar]
- 17.Yang L., Ge H.S., Wang W.J., Zu Y.G., Yang F.J., Zhao C.J., Zhang L., Zhang Y. Development of sample preparation method for eleutheroside B and E analysis in Acanthopanax senticosus by ionic liquids-ultrasound based extraction and high-performance liquid chromatography detection. Food Chem. 2013;141:2426–2433. doi: 10.1016/j.foodchem.2013.05.094. [DOI] [PubMed] [Google Scholar]
- 18.P. Yu H. Zhang, Simultaneous analysis of 17 compounds from the extract of Giant knotweed R. by HPLC–ESI–MS. J. Shenyang. Pharm. Univ. 2011;28:963–968. [Google Scholar]
- 19.Chen T., Tian F., Tang Y.N., Liu Y., Lin Z.Y., Wang Y.C. Quick separation and identification of 24 chemical constituents in Radix Astragali by HPLC–ESI–TOF/MS. China Pharm. 2014;17:593–596. [Google Scholar]
- 20.Wu H., Zhu Z.Y., Zhang G.Q., Zhao L., Zhang H., Zhu D.L., Chai Y.F. Comparative pharmacokinetic study of paeoniflorin after oral administration of pure paeoniflorin, extract of Cortex Moutan and Shuang-Dan prescription to rats. J. Ethnopharmacol. 2009;125:444–449. doi: 10.1016/j.jep.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Yu S., Guo Q.S., Wang H.L., Gao J.P., Xu X. Simultaneous determination of resveratrol and polydatin in Polygonum Cuspidatum by quantitative nuclear magnetic resonance spectroscopy. Chin. J. Anal. Chem. 2015;43:69–74. [Google Scholar]
- 22.Ye X.L., Song L., Ren G.F., Ye W.F., Wang J. Pharmacokinetics study of bioactive constituents of Polygonumcuspidatum in rat by HPLC–MS. Chin. J. Pharm. Anal. 2013;33:749–754. [Google Scholar]
- 23.Chen Z.W., Tong L., Li S.M., Li D.X., Liu X.L., Ding L., Zhu Y.H., Zhou S.P., Sun H. Identification of major parent compounds and metabolites in bile, plasma and urine of rats after oral administration of Radix Scutellariae extract by UFLC–IT–TOF/MS. J. Chin. Pharm. Sci. 2013;22:319–328. doi: 10.5246/jcps.2013.04.046. [DOI] [Google Scholar]
- 24.Gong J.R., Wang S.F. Chemical constituents of Acanthopanax senticosus. Chin. Tradit. Herb. Drugs. 2012;43:2337–2341. [Google Scholar]
- 25.Zhou X.Q., Liang H., Lu X.H., Cai S.Q., Wang B., Zhao Y.Y. Flavonoids from Scutellaria baicalensis and their bioactivities. J. Peking. Univ. Health. Sci. 2009;41:578–584. [PubMed] [Google Scholar]
- 26.Lu L., Zhang H., Zhao L., Jia J., Li Y.Y., Zhang G.Q. RRLC–TOF/MS in rapid identification of 43 chemical constituents of epimedium. Acad. J. Second. Mil. Med. Univ. 2011;32:306–310. doi: 10.3724/SP.J.1008.2011.00306. [DOI] [Google Scholar]
- 27.Huang W., Su Z.R., Bi W.C., Li J., Shi L.Q., Wen Y.Q., Zhan H.Q. HPLC determination of nuezhenide in Fructus Ligustri Lucidi. Chin. J. Pharm. Anal. 2009;29:824–826. [Google Scholar]
- 28.Lau C.H., Chan C.M., Chan Y.W., Lau K.M., Lau T.W., Lam F.C., Law W.T., Che C.T., Leung P.C., Fung K.P., et al. Pharmacological investigations of the anti-diabetic effect of Cortex Moutan and its active component paeonol. Phytomedicine. 2007;14:778–784. doi: 10.1016/j.phymed.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Rui W.F., Yi F., Shi Z.F., Jiang M.M. UPLC/Q–TOF–MS study on fingerpint of Astragalus membranaceus obtained from 7 different origin. Chin. J. Pharm. Anal. 2012;32:607–611. [Google Scholar]
- 30.Liu X.F., Lou Z.Y., Zhu Z.Y., Zhang H., Zhao L., Chai Y.F. HPLC–TOF/MS in rapid identification of chemical compositions in Xiao chai hu decoction. Acad. J. Second. Mil. Med. Univ. 2009;30:941–946. doi: 10.3724/SP.J.1008.2009.00941. [DOI] [Google Scholar]
- 31.Huang X., Song F.R., Liu Z.Q., Liu S.Y. Studies on the lignans in extract of the fruits of Schisandra chinensis and Schisandra sphenanthera by high performance liquid chromatography-electrospray ionization mass spectrometry. Acta Chim. Sin. 2008;66:1059–1066. [Google Scholar]
- 32.Abad-Garcia B., Garmon-Lobato S., Berrueta B., Gallo L.A., Vicente F. On line characterization of 58 phenolic compounds in Citrus fruit juices from Spanish cultivars by high-performance liquid chromatography with photodiode-array detection coupled to electrospray ionization triple quadrupole mass spectrometry. Talanta. 2012;99:213–224. doi: 10.1016/j.talanta.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 33.Xiao P., Huang H.Z., Chen J.W., Li X. In vitro antioxidant and anti-inflammatory activities of Radix Isatidis extract and bioaccessibility of six bioactive compounds after simulated gastro-intestinal digestion. J. Ethnopharmacol. 2014;157:55–61. doi: 10.1016/j.jep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y., Shan L., Hua Y.P., Wang D., Zeng H.W., Liu R.H., Zhang W.D., Hu Z.L. Baicalein selectively induces apoptosis in activated lymphocytes and ameliorates concanavalin A-induced hepatitis in mice. PLoS ONE. 2013;8:e69592. doi: 10.1371/journal.pone.0069592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W.P., Xia T.S., Yu X.P. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-B pathway in vitro. Inflamm. Res. 2015;64:423–431. doi: 10.1007/s00011-015-0822-0. [DOI] [PubMed] [Google Scholar]
- 36.Lee J.Y., Park W. Anti-inflammatory effect of wogonin on RAW 264.7 mouse macrophages induced with polyinosinic-polycytidylicacid. Molecules. 2015;20:6888–6900. doi: 10.3390/molecules20046888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y., Yao J., Wu X.P., Zhao L., Zhou Y.X., Zhang Y., You Q.D., Guo Q.L., Lu N. Wogonin suppresses human alveolar adenocarcinoma cell A549 migration in inflammatory microenvironment by modulating the IL–6/STAT3 signaling pathway. Mol. Carcinog. 2015;54:E81–E93. doi: 10.1002/mc.22182. [DOI] [PubMed] [Google Scholar]
- 38.Huang H., Chang E.J., Lee Y., Kim J.S., Kang S.S., Kim H.H. A genome–Wide microarray analysis reveals anti-inflammatory target genes of paeonol in macrophages. Inflamm. Res. 2008;57:189–198. doi: 10.1007/s00011-007-7190-3. [DOI] [PubMed] [Google Scholar]
- 39.Chen N., Liu D., Soromou L.W., Sun J., Zhong W., Guo W., Huo M., Li H., Guan S., Chen Z., et al. Paeonol suppresses lipopolysaccharide-induced inflammatory cytokines in macrophage cells and protects mice from lethal endotoxin shock. Fundam. Clin. Pharmacol. 2014;28:268–76. doi: 10.1111/fcp.12019. [DOI] [PubMed] [Google Scholar]
- 40.Ding Y., Zhao L., Mei H., Zhang S.L., Huang Z.H., Du Y.Y., Ye P. Exploration of e modin to treat alpha-naphthylisothiocyanate-induced cholestatic hepatitis via anti-inflammatory pathway. Eur. J. Pharmacol. 2008;590:377–386. doi: 10.1016/j.ejphar.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 41.Xue J.H., Chen F., Wang J., Wu S.S., Zheng M., Zhu H.H., Liu Y.N., He J.L., Chen Z. Emodin protects against concanavalin A-Induced hepatitis in mice through inhibiting activation of the p38 MAPK–NF–κB signaling pathway. Cell. Physiol. Biochem. 2015;35:1557–1570. doi: 10.1159/000373971. [DOI] [PubMed] [Google Scholar]