Abstract
The present paper reports a complete mass spectrometric characterization of both the phenolic and volatile fractions of a dried spearmint extract. Phenolic compounds were analysed by ultra-high performance liquid chromatography-electrospray ionization-mass spectrometry (UHPLC-ESI-MSn) and a total of 66 compounds were tentatively identified, being the widest phenolic characterisation of spearmint to date. The analysis suggests that the extract is composed of rosmarinic acid and its derivatives (230.5 ± 13.5 mg/g) with smaller amounts of salvianolic acids, caffeoylquinic acids, hydroxybenzoic acids, hydroxycinnamic acids, flavones, and flavanones. Head space solid-phase microextraction (HS-SPME) coupled with gas chromatography-mass spectrometry (GC-MS) technique, that was applied to characterize the volatile fraction of spearmint, identified molecules belonging to different chemical classes, such as p-cymene, isopiperitone, and piperitone, dihydroedulan II, menthone, p-cymen-8-ol, and β-linalool. This comprehensive phytochemical analysis can be useful to test the authenticity of this product rich in rosmarinic acid and other phenolics, and when assessing its biological properties. It may also be applied to other plant-derived food extracts and beverages containing a broad range of phytochemical compounds.
Keywords: spearmint, phenolic composition, volatile fraction, phytochemical characterization, UHPLC-ESI-MSn, HS-SPME/GC-MS
1. Introduction
Among the family of Lamiaceae (Labiatae), mint represents one of the most popular and cultivated officinal and aromatic plants [1]. The cultivation of mint is principally in temperate regions of Europe and Asia, but also in South Africa, Australia, and the United States.
Spearmint (Mentha spicata L.) is an aromatic plant that can be used fresh or as dried leaves or powder, as a seasoning and flavouring herb, or traditionally as an herbal tea. It is commonly used in traditional medicines as a remedy for gastrointestinal and respiratory problems. In addition, spearmint essential oil has economic relevance due to its use in perfumery, confectionary, and pharmaceutical preparations. Besides its flavouring properties, spearmint is also widely used as an antimicrobial agent and as a preservative in food, mainly on account of the phenolic and terpenoid content [2].
The volatile (non-polar) profile of traditional cultivars of spearmint essential oils is mainly constituted by carvone (22%–73%) and limonene (8%–31%), with smaller quantities of 1,8-cineole (4%–7%), menthone (1%–5%), menthol, eucalyptol, and other minor compounds. The profile varies based on plant variety, growth, climate conditions, and harvest time [3,4,5]. The antimicrobial activity of these spearmint essential oil components has been widely described in the literature. Volatile molecules are indeed produced by the plant, serving as a defence mechanism upon predator attack (i.e., pathogens and insects) [5].
Polar extracts of spearmint leaves are, on the contrary, characterised mainly by a high content of phenolic compounds such as rosmarinic acid, luteolin, and apigenin derivatives [6,7]. Some of these components have been shown to have antioxidant properties; therefore, Mentha spicata could also be considered an antioxidant source [7]. In fact, spearmint and spearmint extracts are often used as preservative agents to delay the oxidative degradation that occurs in food during processing or over time with storage [1]. More intriguingly, the anti-inflammatory properties of spearmint extracts rich in phenolic compounds have been demonstrated in vivo in rats [8].
Aqueous extracts from typical commercially grown spearmint lines reportedly contain 0%–6% rosmarinic acid on a dry weight basis [9,10]. However, based on the reported benefits of rosmarinic acid, spearmint lines were developed through selective-breeding techniques to contain higher levels of phenolic compounds such as rosmarinic acid [11]. Therefore, this study aimed to comprehensively characterise the phytochemical profile of a dried aqueous extract from these proprietary spearmint lines. The phenolic composition was fully examined by means of UHPLC-ESI-MSn, while the composition of the volatile fraction was investigated using head space solid-phase microextraction (HS-SPME)/GC-MS technique.
2. Results and Discussion
2.1. Characterization of the Phenolic Profile
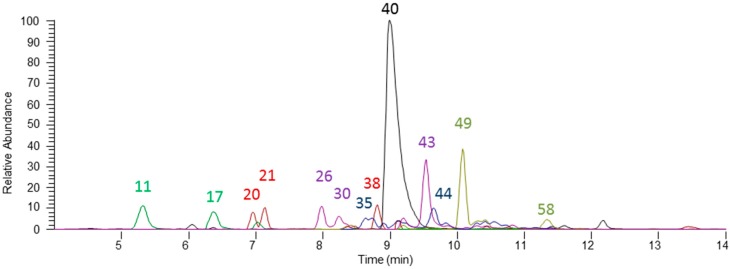
The phenolic fraction of spearmint was fully characterised by means of UHPLC-ESI-MS operating in two complementary conditions. The comprehensive evaluation of the sample allowed for the tentative identification of a total of 66 compounds (Table 1), the widest phenolic characterisation of spearmint to date. More than 200 mass spectrum outputs were analysed for each analytical replicate and experimental condition. Among the classes of identified (poly)phenolic compounds in spearmint, rosmarinic acid derivatives and salvianolic acids were the most prevalent (Figure 1). Different flavones, flavanones, flavonols, phenolic acids, and lignans were also detected. In addition, other phytochemicals, such as organic acids were found.
Table 1.
Identification of phytochemical compounds by UHPLC-MSn in negative mode under different MS operating conditions.
| ID | Compounds | RT (min) | [M − H]− (m/z) | MS2 Ion Fragments (m/z) a | MS3 Ion Fragments (m/z) a | Exp. 1 c | Exp. 2 c | Identification d |
|---|---|---|---|---|---|---|---|---|
| 1 | Quinic acid | 0.57 | 191 | 173 b, 111, 127, 85, 93 | 111, 67 | x | x | Std |
| 2 | l-malic acid | 0.67 | 133 | 115, 87 | x | [12] | ||
| 3 | Citric acid | 0.77 | 191 | 111, 173 | 111, 67 | x | x | [13] |
| 4 | Dihydroxyphenyllactic acid (Danshensu) | 2.61 | 197 | 179, 73, 153 | 135 | x | [14] | |
| 5 | Protocatechuic acid hexoside | 2.75 | 315 | 153, 109, 225 | 109 | x | [15] | |
| 6 | Dihydroxyphenylacetic acid | 3.35 | 167 | 123 | x | [16] | ||
| 7 | Hydroxybenzoic acid | 4.12 | 137 | 137, 93 | x | [17] | ||
| 8 | Caftaric acid | 4.40 | 311 | 149, 179, 243, 135 | 103, 87, 131, 59, 149 | x | Std | |
| 9 | Hydroxyphenyllactic acid | 4.47 | 181 | 163, 135, 73 | 119 | x | x | [18] |
| 10 | Luteolin-8-C-glucoside (orientin) | 4.83 | 447 | 357, 327 | Std | |||
| 11 | 3′-Caffeoylquinic (neochlorogenic acid) | 4.96 | 353 | 191, 179, 135, 173 | 127, 173, 85, 93 | x | Std | |
| 12 | THDBCHMCA f | 5.42 | 295 | 163, 113 | 118 | x | [19] | |
| 13 | Rosmanol | 5.44 | 345 | 299 | 179, 119, 143, 113, 161 | x | [20] | |
| 14 | Coumaric acid | 5.52 | 163 | 119 | x | [17] | ||
| 15 | Salvianolic acid F | 5.56 | 313 | 269, 203, 159 | 159, 109, 254, 269 | x | [14] | |
| 16 | Dicaffeic acid | 5.74 | 341 | 281, 251, 179, 221, 323 | 179, 221, 135 | x | x | [21] |
| 17 | 5′-Caffeoylquinic (chlorogenic acid) | 6.17 | 353 | 191, 179 | 127, 173, 85, 83 | x | Std | |
| 18 | Caffeic acid | 6.25 | 179 | 135 | 135 | x | x | Std |
| 19 | Ferulic acid derivative | 6.88 | 489 | 193, 235, 295, 265 | 149, 134, 178 | x | Std | |
| 20 | Rosmarinic acid derivative | 6.92 | 377 | 359 | 161, 179, 197, 223 | x | x | Std |
| 21 | Rosmarinic acid derivative | 7.08 | 377 | 359 | 161, 179, 197, 223 | x | x | Std |
| 22 | Feruloylquinic acid | 7.15 | 367 | 173, 193, 191 | 93, 111, 155, 71 | x | x | [22] |
| 23 | Tetrahydroxy-dimethoxyflavone-hexoside | 7.29 | 507 | 327, 345, 477, 489 | 312, 167, 295 | x | [23] | |
| 24 | Danshensu derivative | 7.40 | 527 | 197, 179, 483 | 179, 73 | x | [14] | |
| 25 | Rosmarinic acid-O-caffeic acid | 7.61 | 539 | 359, 495, 341, 179 | 161, 179, 197, 223 | x | x | [14] |
| 26 | Salvianolic acid J/isomer | 7.82 | 537 | 339 | 229, 295 | x | x | [14] |
| 27 | Salicylic acid | 7.85 | 137 | 93, 137 | x | [17] | ||
| 28 | Rosmarinic acid-rutinoside | 7.96 | 667 | 359, 487 | 161, 197, 179, 223 | x | Std | |
| 29 | Quercetin-rutinoside (rutin) | 8.07 | 609 | 301, 343, 271, 255, 179 | 179, 151, 257, 273 | x | x | Std |
| 30 | Salvianolic acid J/isomer | 8.08 | 537 | 493, 295, 339 | 295, 313, 383 | x | x | [14] |
| 31 | Luteolin-rutinoside | 8.16 | 593 | 285 | 241, 285, 175, 199, 217 | x | x | [24] |
| 32 | Rosmarinic acid-O-hexoside | 8.25 | 521 | 359 | 161, 197, 179, 223 | x | x | Std |
| 33 | Luteolin-hexoside | 8.26 | 447 | 285 | 285, 241, 199, 175, 217 | x | [24] | |
| 34 | Luteolin-glucuronide | 8.3 | 461 | 285 | 285, 241 | x | x | [20] |
| 35 | Salvianolic acid B/E/isomer | 8.43 | 717 | 519, 475, 339, 537 | 475, 339, 365 | x | x | [14] |
| 36 | Narirutin (Naringenin-7-O-rutinoside) | 8.45 | 625 (579) e | 579 | 271 | x | x | Std |
| 37 | Salvianolic acid D | 8.53 | 417 | 373, 175, 273, 399 | 175, 197, 223 | x | [14] | |
| 38 | Sagerinic acid | 8.66 | 719 | 359, 539, 521, 341 | 161, 179, 197, 223 | x | [16] | |
| 39 | Salvianolic acid E | 8.78 | 717 | 519, 537, 555, 673, 339 | 339, 321, 295, 229 | x | x | [14] |
| 40 | Rosmarinic acid | 8.86 | 359 | 161, 179, 197, 223 | 161, 133 | x | x | Std |
| 41 | Sagerinic acid isomer | 8.99 | 719 | 359 | 161, 179, 197, 223 | x | [25] | |
| 42 | Salvianolic acid A derivative | 9.08 | 897 | 493, 295 | 295, 313, 179 | x | Std | |
| 43 | Lithospermic acid | 9.44 | 537 | 493, 359 | 359, 313, 295 | x | x | Std |
| 44 | Salvianolic acid B | 9.61 | 717 | 519, 321 | 321, 339, 279, 197, 179 | x | x | Std |
| 45 | Dehydro-Rosmarinic acid | 9.70 | 343 | 161, 179, 135, 223, 197 | 161, 133 | x | x | Std |
| 46 | Salvianolic acid B/E/isomer | 9.75 | 717 | 519, 357, 555, 673, 321 | 321, 357, 339 | x | x | [14] |
| 47 | Rosmarinic acid-dihexoside | 9.83 | 683 | 521 | 359, 161, 197, 223 | x | Std | |
| 48 | G(8-O-4)5H | 9.88 | 373 | 179, 161, 135, 355, 197 | 135, 161 | x | [14] | |
| 49 | Salvianolic acid A | 10.02 | 493 | 295, 313, 383, 203 | 159, 277, 109, 267 | x | x | Std |
| 50 | Acacetin derivative | 10.12 | 637 | 591, 283 | 283, 268 | x | x | [18] |
| 51 | Salvianolic acid A isomer | 10.25 | 493 | 295, 331, 383 | 159, 277, 109, 267 | x | x | [19] |
| 52 | Rosmarinic acid derivative | 10.70 | 551 | 519, 359, 313 | 339 | x | x | [20] |
| 53 | Danshensu derivative | 10.87 | 689 | 527, 491 | 197, 179, 347, 161 | x | x | [14] |
| 54 | Danshensu derivative | 10.90 | 691 | 529, 493, 511 | 197, 179, 349, 151 | x | x | [14] |
| 55 | Danshensu derivative | 11.07 | 689 | 527 | 197, 179, 347 | x | x | [14] |
| 56 | Rosmarinic acid derivative | 11.07 | 691 | 359, 511, 341, 529 | 161, 179, 197, 223 | x | Std | |
| 57 | Apigenin | 11.17 | 269 | 269, 225, 149, 241 | 181, 197, 225, 183 | x | [26] | |
| 58 | Salvianolic acid A isomer | 11.22 | 493 | 359, 357, 313 | 161, 179, 197, 223 | x | x | [19] |
| 59 | Cyclolariciresinol | 11.26 | 359 | 345, 161 | 329, 326 | x | x | [27] |
| 60 | Salvianolic acid B derivative | 11.40 | 879 | 519, 699, 339 | 339 | x | [25] | |
| 61 | Rosmarinic acid derivative | 12.33 | 571 | 525 | 341, 359, 161, 179, 221 | x | Std | |
| 62 | Rosmarinic acid derivative | 12.69 | 525 | 359, 341, 161, 179 | 161, 179, 197, 223 | x | Std | |
| 63 | Rosmarinic acid derivative | 13.04 | 507 | 359, 341, 179 | 161, 179, 197, 223 | x | x | Std |
| 64 | Rosmarinic acid derivative | 13.24 | 849 | 359, 687, 669 | 161, 179, 197, 223 | x | x | Std |
| 65 | Acacetin | 13.54 | 283 | 268, 269 | 268, 269, 240 | x | x | [18] |
| 66 | Rosmarinic acid derivative | 13.82 | 507 | 359, 341, 179 | 161, 179, 197, 223 | x | x | Std |
a Fragment ions are listed in order of relative abundances; b MS2 ions in bold were those subjected to MS3 fragmentation; c Exp. 1, detected under experimental condition 1 (epicatechin); Exp. 2, experimental condition 2 (rosmarinic acid); d Identification means identification mode: [Reference number] or Std (compound identified by comparing retention times and MS data with those of reference compounds). Some compounds have been considered “derivatives” since parts of their spectra match those of their corresponding parent compounds but they cannot be fully identified; e The molecular ion is a formic acid adduct (+46); f THDBCHMCA: 1,2,6,7-tetrahydroxy-5H-dibenzo[a,d]cycloheptene-5-methyl-11-carboxylic acid.
Figure 1.
Main spearmint phenolics identified in the extract. Peak numbers are based on Table 1.
The retention times and mass spectrum data, reported as peak assignments for the identified phytochemicals, are included in Table 1. Twelve of the 66 identified compounds were identified and quantified by comparison with reference standards. The remaining 54 compounds were tentatively identified based on the interpretation of their mass spectral behaviour obtained from MS2 and MS3 experiments, and by comparing with data from the literature.
The 54 compounds tentatively identified according to their mass spectral behaviour were quantified by comparison with reference compounds selected based on structural similarity and considering that the functional groups may strongly affect their ionisation properties (i.e., salvianolic acid J was quantified as salvianolic acid A, salvianolic acid E as salvianolic acid B, danshensu and its derivatives as caffeic acid, etc.). Accordingly, in this case, data reported in Table 2 must be considered as semi-quantification. Nevertheless, some compounds responded to the electro-spray ionisation in a unique manner relative to the reference standards used or did not reach the limit of quantification (LOQ) of the corresponding reference compound; therefore, they were not quantified to avoid miscalculation of the phenolic content of the spearmint extract.
Table 2.
Quantitative results (mg/g sample) for polyphenolic fraction of the spearmint extract analyzed.
| ID a | Compounds | Quantified as… | Concentration (mg/g) |
|---|---|---|---|
| 4 | Dihydroxyphenyllactic acid (Danshensu) | Caffeic acid | 0.77 ± 0.09 |
| 5 | Protocatecuic acid hexoside | Caffeic acid | 0.04 ± 0.00 |
| 7 | Hydroxybenzoic acid | Caffeic acid | 0.57 ± 0.07 |
| 8 | Caftaric Acid | Caftaric acid | 2.18 ± 0.30 |
| 9 | Hydroxyphenyllactic acid | Caffeic acid | 0.07 ± 0.00 |
| 10 | Luteolin-8-C-glucoside (orientin) | Luteolin-4-glucoside | 0.02 ± 0.00 |
| 11 | 3′-Caffeoylquinic (neochlorogenic acid) | 3′-Caffeoylquinic b | 1.79 ± 0.22 |
| 14 | Coumaric acid | Caffeic acid | 0.03 ± 0.00 |
| 15 | Salvianolic Acid F | Caffeic acid | 0.01 ± 0.00 |
| 16 | Dicaffeic acid | Caffeic acid | 0.09 ± 0.00 |
| 17 | 5′-Caffeoylquinic (chlorogenic acid) | 5′-Caffeoylquinic b | 1.16 ± 0.08 |
| 18 | Caffeic acid | Caffeic acid | 0.71 ± 0.06 |
| 20 | Rosmarinic acid derivative | Rosmarinic acid | 2.17 ± 0.25 |
| 21 | Rosmarinic acid derivative | Rosmarinic acid | 1.61 ± 0.11 |
| 22 | Feruloylquinic acid | 3′-Caffeoylquinic | 0.11 ± 0.00 |
| 24 | Danshensu derivative | Caffeic acid | 0.01 ± 0.00 |
| 25 | Rosmarinic acid-O-caffeic acid | Rosmarinic acid | 0.05 ± 0.00 |
| 26 | Salvianolic acid J/isomer | Salvianolic acid A | 1.84 ± 0.17 |
| 28 | Rosmarinic acid-rutinoside | Rosmarinic acid | 0.17 ± 0.00 |
| 29 | Quercetin-rutinoside (rutin) | Rutin b | 0.01 ± 0.00 |
| 30 | Salvianolic acid J/isomer | Salvianolic acid A | 0.36 ± 0.05 |
| 31 | Luteolin-rutinoside | Luteolin-4-glucoside | 0.17 ± 0.01 |
| 32 | Rosmarinic acid-O-hexoside | Rosmarinic acid | 0.28 ± 0.03 |
| 33 | Luteolin-hexoside | Luteolin-4-glucoside | 0.02 ± 0.00 |
| 34 | Luteolin-7-glucuronide | Luteolin-4-glucoside | 0.13 ± 0.00 |
| 35 | Salvianolic acid B/E/isomer | Salvianolic acid B | 0.41 ± 0.05 |
| 36 | Narirutin (Naringenin-7-O-rutinoside) | Narirutin b | 0.04 ± 0.01 |
| 37 | Salvianolic Acid D | Rosmarinic acid | 0.29 ± 0.02 |
| 38 | Sagerinic Acid | Rosmarinic acid | 8.93 ± 1.10 |
| 39 | Salvianolic Acid E | Salvianolic acid B | 0.16 ± 0.02 |
| 40 | Rosmarinic Acid | Rosmarinic acid b | 173.76 ± 11.52 |
| 41 | Sagerinic Acid isomer | Rosmarinic acid | 40.05 ± 2.20 |
| 42 | Salvianolic Acid A derivative | Salvianolic acid A | 1.44 ± 0.30 |
| 43 | Lithospermic Acid | Lithospermic acid b | 3.81 ± 0.26 |
| 44 | Salvianolic Acid B | Salvianolic acid B b | 1.35 ± 0.16 |
| 45 | Dehydro-Rosmarinic Acid | Rosmarinic acid | 0.52 ± 0.01 |
| 46 | Salvianolic acid B/E/isomer | Salvianolic acid B | 0.30 ± 0.03 |
| 47 | Rosmarinic acid-dihexoside | Rosmarinic acid | 0.16 ± 0.01 |
| 49 | Salvianolic Acid A | Salvianolic acid A b | 7.79 ± 0.52 |
| 51 | Salvianolic Acid A isomer | Salvianolic acid A | 0.31 ± 0.06 |
| 52 | Rosmarinic acid derivative | Rosmarinic acid | 0.28 ± 0.02 |
| 53 | Danshensu derivative | Caffeic acid | 0.06 ± 0.00 |
| 54 | Danshensu derivative | Caffeic acid | 0.03 ± 0.00 |
| 55 | Danshensu derivative | Caffeic acid | 0.05 ± 0.00 |
| 56 | Rosmarinic acid derivative | Rosmarinic acid | 0.10 ± 0.01 |
| 57 | Apigenin | Daidzein | 0.19 ± 0.01 |
| 58 | Salvianolic Acid A isomer | Salvianolic acid A | 0.69 ± 0.02 |
| 60 | Salvianolic Acid B derivative | Salvianolic acid B | 0.05 ± 0.00 |
| 61 | Rosmarinic acid derivative | Rosmarinic acid | 0.67 ± 0.04 |
| 62 | Rosmarinic acid derivative | Rosmarinic acid | 0.09 ± 0.00 |
| 63 | Rosmarinic acid derivative | Rosmarinic acid | 0.01 ± 0.00 |
| 64 | Rosmarinic acid derivative | Rosmarinic acid | 1.30 ± 0.16 |
| 66 | Rosmarinic acid derivative | Rosmarinic acid | 0.09 ± 0.00 |
| Hydroxybenzoic acids c | 0.61 ± 0.08 | ||
| Hydroxycinnamic acids | 3.00 ± 0.36 | ||
| Caffeoylquinic acids | 3.06 ± 0.27 | ||
| Hydroxyphenylpropanoic acids | 0.99 ± 0.10 | ||
| Rosmarinic acid derivatives | 230.50 ± 13.5 | ||
| Salvianolic acids | 14.70 ± 1.19 | ||
| Flavones | 0.53 ± 0.02 | ||
| Flavonols | 0.01 ± 0.00 | ||
| Flavanones | 0.04 ± 0.01 | ||
| Total Phenolics | 262.97 ± 15.90 |
a See Table 1 for peak assignment; b Quantified by comparison with its corresponding standard; c hydroxybenzoic acids include compound 5 and 7; hydroxycinnamic acids, compounds 8, 14, 16, and 18; caffeoylquinic acids, 11, 17, and 22; hydroxyphenylpropanoic acids, 4, 9, 24, and 53–55; rosmarinic acid derivatives, 20, 21, 25, 28, 32, 37, 38, 40, 41, 45, 47, 52, 56, 61–64, and 66; salvianolic acids, 15, 26, 30, 35, 39, 42, 44, 46, 49, 51, 58, and 60; flavones, 31, 33, 34, and 57; flavonols, 29; and flavanones, 36. Mean (n = 3) ± SD.
The total amount of phenolic compounds of the evaluated spearmint extract calculated on the basis of UHPLC-ESI-MSn data was 262.97 ± 15.90 mg/g, which was in agreement with Dorman et al. [7], who reported a total phenolic content for Mentha spicata L. (spearmint) extract of 214 mg/g, expressed as gallic acid equivalents. More specifically, the sum of rosmarinic acid and other rosmarinic acid derivatives (such as sagerinic acid) in this extract was about the 88% (230.50 ± 13.50 mg/g) of the total amount of detected phenolics, followed by the sum of salvianolic acids (5.6% of total phenolics, 14.70 ± 1.19 mg/g) and caffeoylquinic acids (1.2% of total phenolics, 3.06 ± 0.27 mg/g). Hydroxycinnamic acids, including caftaric acid (an ester of caffeic and tartaric acids), represented about 1.1% of total phenolics (3.00 ± 0.36 mg/g). All of the other detected phenolic groups, such as flavonols, flavanones, flavones, hydroxybenzoic acids, and hydroxyphenylpropanoic acids represented approximately 1% of the total amount of phenolic compounds (0.01 to 0.99 mg/g).
Among the detected compounds, rosmarinic acid, a caffeic acid dimer, was identified by comparing the mass spectra obtained for the sample with those registered for a rosmarinic acid standard solution. This compound occurred at the highest concentration (173.76 ± 11.52 mg/g) and is approximately four-fold higher than the 4.6 mg/g reported for other water extracted spearmint lines [7]. Differences in the amount of rosmarinic acid of this extract with respect to other spearmint extracts are likely due to the selective-breeding techniques used for its production. However, rosmarinic acid concentrations could vary due to seasonal growth or extraction procedures. Rosmarinic acid is known to exert anti-inflammatory activities mainly due to its ability to inhibit lipoxygenases and cyclooxygenases, but it has also been shown to have anti-acetylcholinesterase, antioxidant, and antibacterial capabilities [28,29,30]. Furthermore, it was possible to observe the presence of several rosmarinic acid derivatives. In particular, significant amounts of sagerinic acid (8.93 ± 1.10 mg/g) and an isomer of sagerinic acid (peak 41; 40.05 ± 2.20 mg/g) were found. This is consistent with results obtained from analysis of lemon balm extracts [25], but have not been reported in the literature in water-extracted spearmint to date.
Other polar compounds in the spearmint extract included additional caffeic acid derivatives, such as salvianolic acids. Among this group of molecules, salvianolic acid A was the most abundant (7.79 ± 0.52 mg/g), followed by salvianolic acid B (1.35 ± 0.16 mg/g). Both were identified by means of reference compounds and served to identify their respective derivatives and isomers. Salvianolic acid D and F (dimers of caffeic acids), salvianolic acid J (a trimer of caffeic acid), and salvianolic acid E (a tetramer of caffeic acid), were all recognised by comparing the obtained fragmentations with those observed following analysis of extracts from Salvia miltiorrhiza roots [14]. All of these compounds displayed the characteristic mass spectra of salvianolic acids: neutral losses of one caffeic acid molecule (m/z 180) and a danshensu unit (m/z 198). Salvianolic acids have been reported in other members of the Lamaciae family although inconsistent between species. Within the Mentha species, data on salvianolic acid concentrations within water extracts is limited, with concentrations of less than 1% observed in some instances and slightly lower than the currently evaluated extract [6]. Danshensu (dyhydroxyphenyllactic acid), another caffeic acid derivative, as well as other danshensu-like compounds (peaks 53, 54, and 55) were identified on the basis of its molecular ion [M − H]− (m/z 197) and its MS2 and MS3 fragments (m/z 179, 153 and 135) [14]. Moreover, a considerable amount of lithospermic acid (3.81 ± 0.26 mg/g), a caffeate trimer, was identified using a reference standard.
The presence of different hydroxycinnamic acids was observed in the first part of the chromatogram. This category was mainly represented by caftaric acid (2.18 ± 0.30 mg/g), followed by caffeic acid (0.71 ± 0.06 mg/g) and other minor components, such as dicaffeic acid and coumaric acid. The phenolic profile contained some compounds in the caffeoylquinic acid family, identified by their respective commercial standards (chlorogenic acid and neochlorogenic acid) or its characteristic fragmentation patterns (feruloylquinic acid). Small amounts of hydroxybenzoic acids were detected (0.57 ± 0.07 mg/g) and the presence of salicylic acid (peak 27) was also observed. Hydroxycinnaminic, hydroxybenzoic, and caffeoylquinic acids have been previously reported to be present in Mentha species with concentrations frequently below 1%, as observed for the current water-extracted spearmint [31].
Small amounts of flavones, flavonols, and flavanones were detected. Among the flavones, the most representative compound, in terms of quantity, was apigenin (0.19 mg/g) which was identified by comparing the obtained mass spectra with those reported in the literature [26]. Rutin, narirutin, and orientin were recognised using their respective commercial standards, while other compounds, such as luteolin-rutinoside, luteolin-hexoside, and luteolin-glucuronide, were identified by comparison of their relative mass spectra to those reported for other vegetables or natural extracts [20,24]. Rutin, luteolin, and several additional flavones have been reported previously in commercially available spearmint at levels similar to those reported for the current extract. However, the apigenin levels reported for the extract was four-fold greater than that previously reported, although less than 1% in both cases [7].
2.2. Characterisation of Volatile Composition
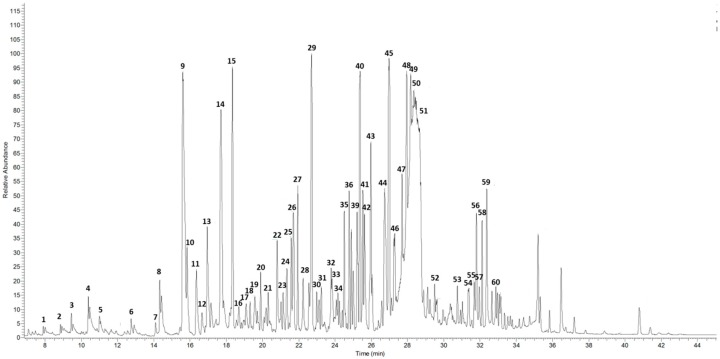
The volatile fraction of dried aqueous spearmint extract was characterised using the HS-SPME/GC-MS technique, which involved obtaining 59 different gas-chromatographic peaks (Figure 2). Peak identification was carried out by comparing recorded mass spectra with those present in the instrument libraries (NIST) and by using the LRI (Linear Retention Index) obtained on two different stationary phase columns (SUPELCOWAX 10 and BP5MS). The detected compounds were semi-quantified using toluene as internal standard (IS). All of the results are listed in Table 3.
Figure 2.
HS-SPME/GC-MS chromatogram of the spearmint extract analyzed. Numbers correspond with the codes indicated at Table 3.
Table 3.
Identification of volatile compounds from the spearmint extract, with relative aromatic notes, calculated LRIs, identification methods, references, and relative amounts.
| ID | Identification | Flavour Note [32] | LRI-Wax | LRI-BP5 a | Identification Method | Ref. | Concentration (µg/100 mg) |
|---|---|---|---|---|---|---|---|
| 1 | Ethylbenzene | Prunus | 1127 | 871 | MS + LRI | [33] | 0.04 ± 0.01 |
| 2 | d-Limonene | Sweet, citrus and peely | 1200 | 1024 | MS + LRI | [34] | 0.04 ± 0.01 |
| 3 | Cosmene | Dahlia, Laurus nobilis | 1219 | 1006 | MS + LRI | NIST | 0.24 ± 0.08 |
| 4 | Cosmene (isomer) | 1252 | 1142 | MS + LRI | NIST | 0.41 ± 0.03 | |
| 5 | o-cymene | Lavander and cypress oil | 1274 | 1022 | MS + LRI | [35] | 0.06 ± 0.01 |
| 6 | Methyl-heptenone | Fruity, apple, musty, ketonic and creamy | 1343 | MS | 0.05 ± 0.01 | ||
| 7 | (z)-3-hexen-1-ol | Green, grassy, melon rind-like | 1387 | 853 | MS + LRI | [36] | 0.07 ± 0.01 |
| 8 | Amyl ethyl carbinol | Earthy | 1395 | 996 | MS | 0.29 ± 0.09 | |
| 9 | p-cymenene | Phenolic | 1444 | 1090 | MS + LRI | [35] | 3.39 ± 0.98 |
| 10 | Amyl vinyl carbinol | Earthy | 1453 | 979 | MS + LRI | [34] | 0.46 ± 0.11 |
| 11 | Furfural | Bready | 1473 | 828 | MS + LRI | [20] | 0.52 ± 0.12 |
| 12 | α-ionene | Plum | 1485 | MS | 0.13 ± 0.01 | ||
| 13 | Dihydroedulan II | (not reported) | 1496 | 1292 | MS + LRI | [37] | 0.69 ± 0.09 |
| 14 | Dihydroedulan II | (not reported) | 1526 | 1297 | MS + LRI | [37] | 2.27 ± 0.66 |
| 15 | β-linalool | Floral | 1551 | 1099 | MS + LRI | [38] | 1.52 ± 0.43 |
| 16 | (R)-(+)-menthofuran | Minty | 1565 | 1159 | MS + LRI | [39] | 0.16 ± 0.05 |
| 17 | 5-methylfurfural | Caramellic | 1582 | 957 | MS + LRI | [38] | 0.18 ± 0.03 |
| 18 | α-ionone | Floral | 1590 | 1428 | MS + LRI | [33] | 0.14 ± 0.02 |
| 19 | (not identified) | 1602 | 0.27 ± 0.08 | ||||
| 20 | Hotrienol | Sweet tropical | 1615 | 1105 | MS + LRI | [40] | 0.38 ± 0.19 |
| 21 | trans-p-metha-2,8-dienol | Minty | 1632 | 1121 | MS + LRI | [35] | 0.12 ± 0.03 |
| 22 | Safranal | Woody, spicy, phenolic, camphoreous | 1653 | 1196 | MS | 0.53 ± 0.13 | |
| 23 | 3-furanmethanol | Tobacco | 1667 | 851 | MS + LRI | [41] | 0.18 ± 0.01 |
| 24 | Tetramethyl-indane | (not reported) | 1676 | MS | 0.42 ± 0.09 | ||
| 25 | (not identified) | 1686 | 0.33 ± 0.04 | ||||
| 26 | Ethyl cyclopentenolone | Caramellic | 1691 | 1087 | MS | 0.75 ± 0.18 | |
| 27 | p-menthen-1-ol | Floral, minty, eucalyptus | 1701 | MS | 0.65 ± 0.19 | ||
| 28 | 4,7-dibenzofuran | (not reported) | 1714 | MS | 0.33 ± 0.06 | ||
| 29 | Menthone | Mentholic | 1735 | 1148 | MS + LRI | [35] | 2.18 ± 0.72 |
| 30 | Camphor | Camphoreous | 1748 | 1145 | MS + LRI | [35] | 0.20 ± 0.02 |
| 31 | 2-piperidin methenamine | (not reported) | 1759 | MS | 0.19 ± 0.08 | ||
| 32 | 1-(1-butenyl)pyrrolidine | (not reported) | 1783 | MS | 0.17 ± 0.05 | ||
| 33 | Methyl salicylate | Minty | 1785 | 1205 | MS + LRI | [33] | 0.21 ± 0.13 |
| 34 | trans-geraniol | Floral | 1804 | 1377 | MS + LRI | NIST | 0.10 ± 0.03 |
| 35 | Teresantalol | Magnolia | 1816 | 1205 | MS | 0.52 ± 0.12 | |
| 36 | β-damascenone | Woody, sweet, fruity, earthy | 1828 | 1381 | MS + LRI | [38] | 0.66 ± 0.17 |
| 37 | 5-isoproprenyl-2-methylcyclopent-1-enecarboxaldehyde | (not reported) | 1834 | MS | 0.43 ± 0.08 | ||
| 38 | Calamenene | Herbal | 1839 | 1525 | MS + LRI | [33] | 0.34 ± 0.11 |
| 39 | Piperitenone | Herbal, minty | 1849 | 1268 | MS + LRI | [35] | 0.69 ± 0.21 |
| 40 | p-cymen-8-ol | Sweet, fruity, cherry, coumarin | 1857 | 1175 | MS + LRI | [33] | 1.96 ± 0.74 |
| 41 | Exo-2-hydroxy cineole | Eucalyptus, basilicum | 1864 | MS | 0.36 ± 0.01 | ||
| 42 | 3,6-dimethyl-phenyl-1,4-diol | (not reported) | 1868 | MS | 0.44 ± 0.02 | ||
| 43 | Longipinene | Hinoki, cypress | 1884 | 1350 | MS + LRI | [42] | 0.74 ± 0.01 |
| 44 | Isopiperitenone | Minty | 1932 | 1340 | MS + LRI | NIST | 2.37 ± 0.94 |
| 45 | Damascenone (isomer) | 1948 | MS | 0.56 ± 0.12 | |||
| 46 | Mint lactone | Sweet, creamy, coumarinic and coconut | 1967 | MS | 0.46 ± 0.03 | ||
| 47 | α,β-dihydro-β-ionone | Woody | 1979 | 1406 | MS | 1.17 ± 0.69 | |
| 48 | Seudenone | Nutty | 1990 | 1050 | MS + LRI | NIST | 0.50 ± 0.19 |
| 49 | Dihydroxy-durene | (not reported) | 1998 | 1322 | MS | 0.31 ± 0.23 | |
| 50 | Cinerolon | Myrthus | 2011 | 1403 | MS | 0.64 ± 0.43 | |
| 51 | Carvone | Minty, licorice | 2054 | 1239 | MS + LRI | [33] | 0.18 ± 0.07 |
| 52 | 1-acetoxy-p-menth-3-one | Minty | 2114 | MS | 0.16 ± 0.05 | ||
| 53 | 2,6-diisopropyl naphtalene | (not reported) | 2144 | MS | 0.33 ± 0.08 | ||
| 54 | (naphtalene derivative) | 2158 | MS | 0.15 ± 0.05 | |||
| 55 | Eugenol | Spicy | 2164 | 1354 | MS + LRI | [35] | 0.75 ± 0.44 |
| 56 | 4-ethylphenol | Phenolic | 2171 | 1175 | MS + LRI | [38] | 0.17 ± 0.01 |
| 57 | Thymol | Herbal | 2179 | 1289 | MS + LRI | [35] | 0.62 ± 0.29 |
| 58 | 2-acetyl-4-methylphenol | Sweet heavy floral herbal | 2190 | 1180 | [43] | 0.95 ± 0.41 | |
| 59 | Carvacrol | Spicy | 2204 | 1298 | MS + LRI | [35] | 0.12 ± 0.03 |
a No value means not found in literature. Mean (n = 2) ± SD.
Quantitatively, the volatile fraction of the spearmint extract examined had 34.64 ± 10.57 µg/100 mg of volatile compounds. In general, since this extract is water-extracted, the volatile fraction analysis yields percentages of components much lower than those reported in the literature for spearmint leaf material. Ketones were the most representative compounds in this fraction, constituting about 32% of the total volatile amount, followed by terpenoids at 20%. Aldehydes, esters, and furans were also detected at 18%–19% of the total volatile fraction. The highest quantitative individual compounds present in the volatile fraction of the tested spearmint were as follows: p-cymene (3.39 ± 0.98 µg/100 mg), isopiperitone and piperitone (2.37 ± 0.94 and 0.69 ± 0.21 µg/100 mg, respectively), dihydroedulan II (two signals: 2.27 ± 0.66 and 0.69 ± 0.09 µg/100 mg), menthone (2.18 ± 0.72 µg/100 mg), p-cymen-8-ol (1.96 ± 0.74 µg/100 mg), and β-linalool (1.52 ± 0.43 µg/100 mg). These molecules confer characteristic aromatic notes to the product, such as minty, phenolic, and floral flavours [32].
Traditional mint presents a really distinctive flavour, mostly due to the presence of a particular alcoholic cyclic terpene: menthol. This molecule, besides being well-known as a primary aromatic compound, is used in medicine for gastro-intestinal disorders [44]. In our sample, menthol was not detected. This can be attributed to the fact that the chemical composition of mint leaves, as the composition of essential oil, can be dependent on different agronomical factors as plant maturity, variety, growth region, climatic conditions, and genetics [3]. In contrast, other typical spearmint volatile fraction components, such as menthone, carvone, eugenol, piperitone, and isopiperitone, were detected. These volatiles have been already reported in peppermint and spearmint essential oils as being responsible for the typical mint notes [45,46].
Carvone and piperitone are two oxygenated terpenoids generated during the biosynthesis of terpenes, which starts from geranyl pyrophosphate, and they are derived from d-limonene. In particular, carvone, with its characteristic aromatic note of mint and liquorice, has different applications, such as repellent, medical, and flavour preparation [5]. However, the carvone level recorded in the spearmint extract is 200-fold lower than that previously reported in an aqueous extract of peppermint (~0.2 vs. 40 µg/100 mg extract), another member of the Lamiaceae family [47]. This low carvone level, in agreement with Narasimhamoorthy et al. [11], may cause lesser mint notes in this line relative to native spearmint lines, which could support its palatability in food and beverage applications.
Among ketones, the most abundant were menthone (2.18 ± 0.72 µg/100 mg) and β-damascenone (0.66 ± 0.17 µg/100 mg), which were consistent with results found by Rohloff et al. [46] and Ka et al. [37] for spearmint and peppermint. The spearmint volatile fraction was also rich in alcohols. In addition to the p-cymen-8-ol (1.96 ± 0.74 µg/100 mg) as identified in Mentha essential oils [4], detectable amounts of 2-acetyl-4-methylphenol, thymol, carvacrol, and p-menthen-1-ol were also observed.
In addition to ketones, terpenoids, and alcohols, several compounds belonging to different chemical classes represented the remaining 18%–19% of the volatile fraction of the dried spearmint powder. Among these minor volatile compounds, dihydroedulan II (two signals: 2.27 ± 0.66 and 0.69 ± 0.09 µg/100 mg) was identified. Dihydroedulan II is a benzopyran compound that has already been detected in the essential oil of Ocimum basilicum (basil), another member of the Lamiaceae family [48] but not previously reported in Mentha spicata. In accordance with data from Rohloff [46] in peppermint, detectable amounts of R-(+)-menthofuran (0.16 ± 0.05 µg/100 mg) were observed. Slight quantities of aldehydes, in particular furfural (0.52 ± 0.12 µg/100 mg) and 5-methyl furfural (0.18 ± 0.03 µg/100 mg), were also detected. Similarly, Ka et al. [37] identified these compounds in distilled extracts from some medicinal plants, such as Angelica tenuissimae, pine needles from Pinus sylvestris, and leaves of sweet flags (Acorus gramineus).
3. Materials and Methods
3.1. Materials
Methanol, acetonitrile, formic acid, toluene, and C8–C20 alcane solution were purchased from Sigma-Aldrich (Milan, Italy). Ultrapure water from MilliQ system (Millipore, Bedford, MA, USA) was used throughout the experiment. The proprietary spearmint extract was manufactured by Kemin Foods, L.C. (Des Moines, IA, USA) as described [11,49]. In brief, the spearmint extract was prepared by microwave drying within one hour of harvest followed by extraction of the dried spearmint leaf with acidified water.
3.2. Characterization and Quantification of Phenolic Fraction by UHPLC-ESI-MSn
The extraction of phenolic compounds was performed on 200 mg of spearmint extract by adding 1 mL of 80% aqueous methanol acidified with formic acid (1%), according to Sánchez-Salcedo et al. (2015) [50]. The solution was shaken in an ultrasonic bath at room temperature for 25 min. The mixture was then centrifuged at 10,480 g for 5 min at room temperature. In order to obtain an exhaustive extraction of the phenolic fraction, two additional extractions were performed on the same sample. The three supernatants were pooled before UHPLC-ESI-MSn analyses. Each sample was extracted in quadruplicate.
Methanolic extracts of spearmint were analyzed using an Accela UHPLC 1250 equipped with a linear ion trap-mass spectrometer (MS) (LTQ XL, Thermo Fisher Scientific Inc., San Jose, CA, USA) fitted with a heated-electrospray ionization probe (H-ESI-II; Thermo Fisher Scientific Inc.). Separations were performed using a BlueOrchid C18 column (50 × 2 mm, 1.8 µm particle size, Knauer, Berlin, Germany). The total volume injected was 5 µL and the column oven temperature was 30 °C. Two MS experiments in negative mode were performed according to a previous protocol [51]. Optimal parameters for epicatechin analysis (Experimental Conditions 1) were carried out using the following conditions. The MS was operated using a capillary temperature equal to 275 °C, while the source heater temperature was set to 200 °C. The sheath gas flow was operated at 40 units, while both auxiliary and sweep gas were set to 5 units. The source voltage was 4 kV. The capillary and tube lens voltages were −42 and −118 V, respectively. Elution was performed at a flow rate of 0.3 mL/min. The gradient started with 99% of 0.1% aqueous formic acid, keeping isocratic conditions for 2 min, followed by a 10 min linear gradient of acetonitrile in 0.1% formic acid which started at 1% and was increased to 40%. The acidified acetonitrile was increased to 80% between minutes 12 and 13 min, and maintained for 3 min, followed by 4 min at the starting conditions to re-equilibrate the column. Analyses were carried out using full scan, data-dependent MS3 scanning from m/z 100–1500, with collision-induced dissociation (CID) equal to 30 (arbitrary units). Pure helium gas was used for CID.
The second experimental framework utilized MS with conditions optimized for rosmarinic acid analysis (Experimental Conditions 2). The capillary temperature was set to 275 °C, while the source heater temperature was 50 °C. The sheath gas flow was operated at 40 units, while auxiliary and sweep gas were set to 5 and 0 units, respectively. The source voltage was operated at 4 kV. The capillary and tube lens voltages were −26 and −78 V, respectively. Analyses were carried out using full scan, data-dependent MS3 scanning from m/z 100–1500, with CID equal to 30 (arbitrary units). The chromatographic conditions were identical to those used for the preliminary phenolic analyses.
Quantification was performed using selected ion monitoring mode (SIM) by selecting the relative base peak at the corresponding mass to charge ratio (m/z) under Experimental Conditions 2, based on rosmarinic acid. Different dilutions of the extract in 0.1% aqueous formic acid (dilution factors ranging from 10–1000) were used to avoid signal saturation and quantify within the linearity range of the reference compounds.
3.3. Volatile Extraction and Characterization by Head Space Solid Phase Microextraction (HS-SPME) Coupled with GC-MS Technique
The volatile fraction of the spearmint sample was characterized following the protocol of Cirlini et al. (2012) [34] with slight modifications. Briefly, 100 mg of spearmint extract were placed in a 30 mL glass vial. For each SPME analysis, 100 µL of an aqueous toluene standard solution (348 mg/L) were added to the sample. The vial was stirred in a warm water bath at 35 °C for 45 min. For each sample, a SPME fibre was inserted in the sample head space and the sample was stirred at constant speed. The fibre was then removed and inserted into the GC-MS injector for 2 min for the desorption of the volatiles. The analysis was done in duplicate.
The silica fibre adopted for the analysis was coated with 50/30 µm of divinylbenzene-carboxen-polymethylsiloxane (DVB/Carboxen/PDMS; Supelco, Bellefonte, PA, USA). Before starting the analyses, the fibre was conditioned by inserting it into the GC/MS injector at 230 °C for at least 10 min. All the analyses were performed on a Thermo Scientific Trace 1300 gas-chromatograph coupled to a Thermo Scientific ISQ mass spectrometer equipped with electronic impact (EI) source. The separation of analytes was performed on a SUPELCOWAX 10 capillary column (Supelco, 30 m × 0.25 mm, f.t. 0.25 µm) using helium as carrier gas. The injector temperature was set at 230 °C and splitless mode was used as the injection modality keeping the valve closed for 2 min. The oven temperature started at 50 °C for 3 min and was increased to 200 °C (5 °C/min). The final oven temperature (200 °C) was maintained for 18 min and the auxiliary temperature was set at 230 °C. Full scan mode was chosen as the acquisition mode (m/z 41–500).
The tentative identification of the volatiles was performed by comparison of the obtained mass spectra with those present in the instrument libraries (NIST). Furthermore, in order to obtain a more confident identification, the linear retention indices (LRI) were calculated on the basis of a C8–C20 alcane solution analyses. The same procedure was repeated utilizing a different stationary phase column, BP5MS (30 m × 0.25 mm, with 0.25 µm film thickness, SGE Analytical Science, Milan, Italy), on which both the alcane standard solution and spearmint sample were analysed maintaining the same extraction and instrumental conditions as previously described. The semi-quantification of all detected gas-chromatographic signals was performed on the basis of the use of an internal standard (toluene).
4. Conclusions
This study reported the comprehensive characterisation of a spearmint extract developed utilizing selective breeding to yield high rosmarinic acid and other phenolic components, with a particular emphasis on the (poly)phenolic and volatile fraction. The use of two different chromatographic techniques, UHPLC, and GC, both coupled to mass spectrometry, allowed for the elucidation of the fingerprint of these two different fractions.
In particular, the use of the UHPLC-ESI-MSn technique allowed us to fully unravel the (poly)phenolic profile of dried spearmint. A total of 66 different molecules were identified on the basis of their characteristic MSn spectra, with 53 of them semi-quantified. The total amount of phenolic compounds was about 260 mg/g extract, which demonstrated that the spearmint extract is a matrix rich in phenolics. The major phenolic compounds in the spearmint extract were represented by rosmarinic acid and its derivatives (88% of the total phenolics). Among the other molecules identified, different salvianolic, caffeoylquinic, hydroxybenzoic, and hydroxycinnamic acids were detected, as well as small amounts of flavones, flavanones, and flavonols. The results of the spearmint extract volatile profile, analysed using the HS-SPME/GC-MS technique, suggested the extract was mainly represented by 59 volatile compounds belonging to different chemical classes, in particular ketones and terpenoids. Attending to the characteristics of plant extracts, the phytochemical composition of this matrix could vary from season to season and even from lot to lot. Regardless of normal variation, these particularly sensitive techniques would allow testing of the authenticity of the product and assist when evaluating its biological and essential properties. On the other hand, the analysis of a higher number of samples, considering factors such as seasonality as well as agricultural practices and crop location would be quite interesting. This fact could be tackled in further studies, although a reductive approach would be needed since it is not feasible to perform this kind of comprehensive identification for large batches of samples.
Acknowledgments
This study was partly supported by Kemin Food, L.C.
Abbreviations
The following abbreviations are used in this manuscript:
| CID | collision-induced dissociation |
| GC-MS | gas chromatography-mass spectrometry |
| LIR | linear retention indices |
| HS-SPME | head space solid-phase microextraction |
| UHPLC-ESI-MSn | ultra-high performance liquid chromatography-electrospray ionization-mass spectrometry |
Author Contributions
D.D.R., K.A.H. and K.M.N. conceived and designed the experiments; M.C., P.M. and M.T. performed the experiments; M.C., P.M. and M.T. analyzed the data; D.D.R., K.A.H., K.M.N. and C.D. contributed reagents/materials/analysis tools; M.C., P.M., M.T. and D.D.R. wrote the paper. K.M.N is currently with Midwest Center for Metabolic and Cardiovascular Research, 489 Taft Ave., Suite 202, Glen Ellyn, IL, 60137, USA; knieman@mbclinicalresearch.com.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the collection, analyses, or interpretation of data, and in the writing of the manuscript.
Footnotes
Sample Availability: Samples are available from the authors.
References
- 1.Kanatt S.R., Chander R., Sharma A. Antioxidant potential of mint (Mentha spicata L.) in radiation-processed lamb meat. Food Chem. 2007;100:451–458. doi: 10.1016/j.foodchem.2005.09.066. [DOI] [Google Scholar]
- 2.Kivilompolo M., Hyotylainen T. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: Characterisation and quantification of antioxidant phenolic acids. J. Chromatogr. A. 2007;1145:155–164. doi: 10.1016/j.chroma.2007.01.090. [DOI] [PubMed] [Google Scholar]
- 3.Telci I., Demirtas I., Bayram E., Arabaci O., Kacar O. Environmental variation on aroma components of pulegone/piperitone rich spearmint (Mentha spicata L.) Ind. Crops Prod. 2010;32:588–592. doi: 10.1016/j.indcrop.2010.07.009. [DOI] [Google Scholar]
- 4.Tyagi A.K., Malik A. Antimicrobial potential and chemical composition of Mentha piperita oil in liquid and vapour phase against food spoiling microorganisms. Food Control. 2011;22:1707–1714. doi: 10.1016/j.foodcont.2011.04.002. [DOI] [Google Scholar]
- 5.Silva C.L., Câmara J.S. Profiling of volatiles in the leaves of Lamiaceae species based on headspace solid phase microextraction and mass spectrometry. Food Res. Int. 2013;51:378–387. doi: 10.1016/j.foodres.2012.12.040. [DOI] [Google Scholar]
- 6.Caboni P., Saba M., Tocco G., Casu L., Murgia A., Maxia A., Menkissoglu-Spiroudi U., Ntalli N. Nematicidal activity of mint aqueous extracts against the root-knot nematode Meloidogyne incognita. J. Agric. Food Chem. 2013;61:9784–9788. doi: 10.1021/jf403684h. [DOI] [PubMed] [Google Scholar]
- 7.Dorman H.J.D., Koşar M., Khahlos K., Holm Y., Hitunen R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003;51:4563–4569. doi: 10.1021/jf034108k. [DOI] [PubMed] [Google Scholar]
- 8.Arumugan P., Gayatri Priya N., Subathra M., Ramesh A. Anti-Inflammatory activity of four solvent fractions of ethanol extract of Mentha spicata L. investigated on acute and chronic inflammation induced rats. Environ. Toxicol. Pharm. 2008;26:92–95. doi: 10.1016/j.etap.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Shekarchi M., Hajimehdipoor H., Saeidnia S., Gohari A.R., Hamedani M.P. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacogn. Mag. 2012;8:37–41. doi: 10.4103/0973-1296.93316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H., Provan G.J., Helliwell K. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem. 2004;87:307–311. doi: 10.1016/j.foodchem.2003.12.029. [DOI] [Google Scholar]
- 11.Narasimhamoorthy B., Zhao L.Q., Liu W., Yang W., Greaves J.A. Differences in the chemotype of two native spearmint clonal lines selected for rosmarnic acid accumulation in comparison to commercially grown native spearmint. Ind. Crops Prod. 2015;63:87–91. doi: 10.1016/j.indcrop.2014.10.044. [DOI] [Google Scholar]
- 12.Sawada Y., Akiyama K., Sakata A., Kuwahara A., Otsuki H., Sakurai T., Saito K., Hirai M.Y. Widely targeted metabolomics based on large-scale MS/MS data for elucidating metabolite accumulation patterns in plants. Plant. Cell Physiol. 2009;50:37–47. doi: 10.1093/pcp/pcn183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cristofori V., Caruso D., Latini G., Dell’Agli M., Cammilli C., Rugini E., Bignami C., Muleo R. Fruit quality of Italian pomegranate (Punica granatum L.) autochthonous varieties. Eur. Food Res. Technol. 2011;232:397–403. doi: 10.1007/s00217-010-1390-8. [DOI] [Google Scholar]
- 14.Hu P., Liang Q.L., Luo G.A., Zhao Z.Z., Jiang Z.H. Multi-Component HPLC fingerprinting of Radix Salviae Miltiorrhizae and its LC-MS-MS identification. Chem. Pharm. Bull. 2005;53:677–683. doi: 10.1248/cpb.53.677. [DOI] [PubMed] [Google Scholar]
- 15.Perestrelo R., Lu Y., Santos S.A.O., Silvestre A.J.D., Neto C.P., Câmara J.S., Rocha S.M. Phenolic profile of Sercial and Tinta Negra Vitis vinifera L. grape skins by HPLC-DAD-ESI-MSn: Novel phenolic compounds in Vitis vinifera L. grape. Food Chem. 2012;135:94–104. doi: 10.1016/j.foodchem.2012.04.102. [DOI] [Google Scholar]
- 16.Dall’Asta M., Calani L., Tedeschi M., Jechiu L., Brighenti F., del Rio D. Identification of microbial metabolites derived from in vitro fecal fermentation of different polyphenolic food sources. Nutrition. 2012;28:197–203. doi: 10.1016/j.nut.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Patán F., Monagas M., Moreno-Arribas M.V., Bartolomé B. Determination of microbial phenolic acids in human faeces by UPLC-ESI-TQ MS. J. Agric. Food Chem. 2011;59:2241–2247. doi: 10.1021/jf104574z. [DOI] [PubMed] [Google Scholar]
- 18.Sawada Y., Nakabayashi R., Yamada Y., Suzuki M., Sato M., Sakata A., Akiyama K., Sakurai T., Matsuda F., Aoki T., Hirai M.Y., Saito K. RIKEN tandem mass spectral database (ReSpect) for phytochemicals: A plant-specific MS/MS-based data resource and database. Phytochem. 2012;82:38–45. doi: 10.1016/j.phytochem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Lee H.J., Cho J.Y., Moon J.H. Chemical conversions of salvianolic acid B by decoction in aqueous solution. Fitoterapia. 2012;83:1196–1204. doi: 10.1016/j.fitote.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Santos J., Oliveira M.B.P.P., Ibáñez E., Herrero M. Phenolic profile evolution of different ready-to-eat baby-leaf vegetables during storage. J. Chromatogr. A. 2014;1327:118–131. doi: 10.1016/j.chroma.2013.12.085. [DOI] [PubMed] [Google Scholar]
- 21.Dugo P., Donato P., Cacciola F., Germanò M.P., Rapisarda A., Mondello L. Characterization of the polyphenolic fraction of Morus alba leaves extracts by HPLC coupled to a hybrid IT-TOF MS system. J. Sep. Sci. 2009;32:3627–3634. doi: 10.1002/jssc.200900348. [DOI] [PubMed] [Google Scholar]
- 22.Clifford M.N., Johnston K.L., Knight S., Kuhnert N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003;51:2900–2911. doi: 10.1021/jf026187q. [DOI] [PubMed] [Google Scholar]
- 23.Fischer U.A., Dettmann J.S., Carle R., Kammerer D.R. Impact of processing and storage on the phenolic profiles and contents of pomegranate (Punica granatum L.) juices. Eur. Food Res. Technol. 2011;233:797–816. doi: 10.1007/s00217-011-1560-3. [DOI] [Google Scholar]
- 24.McNab H., Ferreira E.S.B., Hulme A.N., Quye A. Negative ion ESI-MS analysis of natural yellow dye flavonoids—An isotopic labelling study. Int. J. Mass Spectrum. 2009;284:57–65. doi: 10.1016/j.ijms.2008.05.039. [DOI] [Google Scholar]
- 25.Miron T.L., Herrero M., Ibáñez E. Enrichment of antioxidant compounds from lemon balm (Melissa officinalis) by pressurized liquid extraction and enzyme-assisted extraction. J. Chromatogr. A. 2013;1288:1–9. doi: 10.1016/j.chroma.2013.02.075. [DOI] [PubMed] [Google Scholar]
- 26.Fabre N., Rustan I., de Hoffmann E., Quetin-Leclercq J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001;12:707–715. doi: 10.1016/S1044-0305(01)00226-4. [DOI] [PubMed] [Google Scholar]
- 27.Eklund P.C., Backman M.J., Kronberg L.Å., Smeds A.I., Sjöholm R.E. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 2008;43:97–107. doi: 10.1002/jms.1276. [DOI] [PubMed] [Google Scholar]
- 28.Petersen M., Simmonds M.S. Rosmarinic acid. Phytochemistry. 2003;62:121–125. doi: 10.1016/S0031-9422(02)00513-7. [DOI] [PubMed] [Google Scholar]
- 29.Costa P., Goncalves S., Valentao P., Andrade P.B., Romano A. Accumulation of phenolic compounds in in vitro cultures and wild plants of Lavandula viridis L’Her and their antioxidant and anti-cholinesterase potential. Food Chem. Toxicol. 2013;57:69–74. doi: 10.1016/j.fct.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Mushtaq N., Schmatz R., Pereira L.B., Ahmad M., Stefanello N., Vieira J.M., Abdalla F., Rodrigues M.V., Baldissarelli J., Pelinson L.P., et al. Rosmarinic acid prevents lipid peroxidation and increase in acetylcholinesterase activity in brain of streptozotocin-induced diabetic rats. Cell Biochem. Funct. 2014;32:287–293. doi: 10.1002/cbf.3014. [DOI] [PubMed] [Google Scholar]
- 31.Dvorackova E., Snoblova M., Hrdlicka P. Content of phenolic compounds in herbs used in the Czech Republic. Int. Food Res. J. 2014;21:1495–1500. [Google Scholar]
- 32.Acree T., Arn H. Flavornet and Human Odor Space. [(accessed on 28 October 2014)]. Availiable online: http://www.flavornet.org/
- 33.Pino J.A., Mesa J., Muñoz Y., Martì M.P., Marbot R. Volatile components from mango (Mangifera indica L.) cultivars. J. Agric. Food Chem. 2005;53:2213–2223. doi: 10.1021/jf0402633. [DOI] [PubMed] [Google Scholar]
- 34.Cirlini M., Dall’Asta C., Silvanini A., Beghè D., Fabbri A., Galaverna G., Ganino T. Volatile fingerprinting of chestnut flours from traditional Emilia Romagna (Italy) cultivars. Food Chem. 2012;134:662–668. doi: 10.1016/j.foodchem.2012.02.151. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Solana R., Salgado J.M., Domínguez J.M., Cortés-Diéguez S. Comparison of Soxhlet, Accelerated Solvent and Supercritical Fluid Extraction Techniques for Volatile (GC–MS and GC/FID) and Phenolic Compounds (HPLC–ESI/MS/MS) from Lamiaceae Species. Phytochem. Anal. 2015;26:61–71. doi: 10.1002/pca.2537. [DOI] [PubMed] [Google Scholar]
- 36.Ruther J. Retention index database for identification of general green leaf volatiles in plants by coupled capillary gas chromatography-mass spectrometry. J. Chromatogr. A. 2000;890:313–319. doi: 10.1016/S0021-9673(00)00618-X. [DOI] [PubMed] [Google Scholar]
- 37.Ka M.-H., Choi E.H., Chun H.-S., Lee K.-G. Antioxidative activity of volatile extracts isolated from Angelica tenuissimae roots, Peppermint leaves, Pine needles, and Sweet Flag leaves. J. Agric. Food Chem. 2005;53:4124–4129. doi: 10.1021/jf047932x. [DOI] [PubMed] [Google Scholar]
- 38.Dall’Asta C., Cirlini M., Morini E., Galaverna G. Brand-Dependent volatile fingerprinting of Italian wines from Valpolicella. J. Chromatogr. A. 2011;1218:7557–7565. doi: 10.1016/j.chroma.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 39.Couladis M., Tsortanidou V., Francisco-Ortega J., Santos-Guerra A., Harvala C. Composition of the essential oils of Argyranthemum species growing in the Canary Islands. Flavour Fragr. J. 2001;16:103–106. doi: 10.1002/ffj.954. [DOI] [Google Scholar]
- 40.Engel K.H., Tressl R. Formation of aroma components from nonvolatile precursors in passion fruit. J. Agric. Food Chem. 1983;31:998–1002. doi: 10.1021/jf00119a019. [DOI] [Google Scholar]
- 41.Umano K., Nakahara K., Shoji A., Shibamoto T. Aroma chemicals isolated and identified from leaves of Aloe arborescens Mill. Var. natalensis Berger. J. Agric. Food Chem. 1999;47:3702–3705. doi: 10.1021/jf990116i. [DOI] [PubMed] [Google Scholar]
- 42.Couladis M., Baziou P., Petrakis P.V., Harvala C. Essential oil composition of Hypericum perfoliatum L. growing in different locations in Greece. Flavour Fragr. J. 2001;16:204–206. doi: 10.1002/ffj.979. [DOI] [Google Scholar]
- 43.Sardashti A., Ganjali A., Kordi A. Effect of humic substances on the quality of essential oils of medicinal plants. J. Med. Plants Res. 2012;6:2644–2654. [Google Scholar]
- 44.Patil T., Ishiuji Y., Yosipovitch G. Menthol: A refreshing look at this compound. J. Am. Acad. Dermatol. 2007;57:873–878. doi: 10.1016/j.jaad.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Dìaz-Maroto M.C., Perez-Coello M.S., Gonzales Vinas M.A., Cabezudo M.D. Influence of drying on the flavor quality of Spearmint (Mentha spicata L.) J. Agric. Food Chem. 2003;51:1265–1269. doi: 10.1021/jf020805l. [DOI] [PubMed] [Google Scholar]
- 46.Rohloff J. Monoterpene composition of essential oil from Peppermint (Mentha piperita L.) with regard to leaf position using Solid-Phase Microextraction and Gas Chromatography/Mass Spectrometry analysis. J. Agric. Food Chem. 1999;47:3782–3786. doi: 10.1021/jf981310s. [DOI] [PubMed] [Google Scholar]
- 47.Riachi L.G., Abi-Zaid I.E., Moreira R.F., de Maria C.A. Volatile composition of peppermint (Mentha piperita L.) commercial teas through solid phase extraction. Arch. Latinoam. Nutr. 2012;62:389–392. [PubMed] [Google Scholar]
- 48.Bozin B., Mimica-Dunik N., Simin N., Anackov G. Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006;54:1822–1828. doi: 10.1021/jf051922u. [DOI] [PubMed] [Google Scholar]
- 49.Lasrado J.A., Trinker D., Ceddia M.A., Herrlinger K.A. The safety of a dry spearmint extract in vitro and in vivo. Regul. Toxicol. Pharmacol. 2015;71:213–224. doi: 10.1016/j.yrtph.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Sánchez-Salcedo E.M., Mena P., García-Viguera C., Martínez J.J., Hernández F. Phytochemical evaluation of white (Morus alba L.) and black (Morus nigra L.) mulberry fruits, a starting point for the assessment of their beneficial properties. J. Funct. Foods. 2015;12:399–408. [Google Scholar]
- 51.Mena P., Calani L., Dall’Asta C., Galaverna G., García-Viguera C., Bruni R., Crozier A., del Rio D. Rapid and comprehensive evaluation of (Poly)phenolic compounds in pomegranate (Punica granatum L.) juice by UHPLC-MSn. Molecules. 2012;17:14821–14840. doi: 10.3390/molecules171214821. [DOI] [PMC free article] [PubMed] [Google Scholar]