Abstract
A one-pot reaction was described that results in various pyrazole-thiobarbituric acid derivatives as new pharmacophore agents. These new heterocycles were synthesized in high yields with a broad substrate scope under mild reaction conditions in water mediated by NHEt2. The molecular structures of the synthesized compounds were assigned based on different spectroscopic techniques. The new compounds were evaluated for their antibacterial and antifungal activity. Compounds 4h and 4l were the most active compounds against C. albicans with MIC = 4 µg/L. Compound 4c exhibited the best activity against S. aureus and E. faecalis with MIC = 16 µg/L. However, compounds 4l and 4o were the most active against B. subtilis with MIC = 16 µg/L. Molecular docking studies for the final compounds and standard drugs were performed using the OpenEye program.
Keywords: pyrazole, thiobarbituric acid, antimicrobial activity
1. Introduction
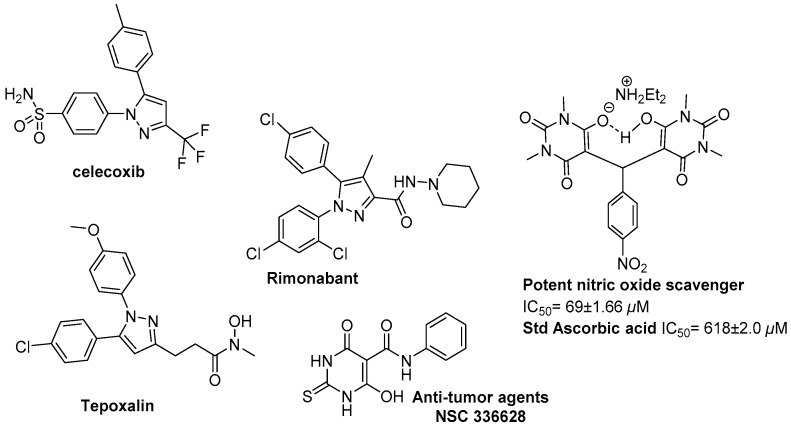
Pyrazole heterocycles are a core structure for pharmaceutical targets in the synthetic and natural products [1,2,3]. For example, Celecoxib, SC-558, and tepoxalin were reported as cyclooxygenase 2 inhibitors [4,5], and rimonabant was identified as reducing obesity for example cannabinoid-1 inverse agonists (Figure 1) [6,7]. Additionally, pyrazole derivatives display usefulness in the fields of luminophores, fluorescence applications, agricultural research, and drug discovery [8,9,10,11,12,13,14,15]. Pyrazoles fused with other privileged structures may possess some promising pharmacological and other activities. Pyrazole and pyrazole-based derivatives have been reported as potential CB2 receptor ligands with antagonist/inverse agonist properties [16], dual inhibition of CCR5/CXCR4 HIV entry [17], and reverse transcriptase HIV-1 non-nucleoside reverse transcriptase inhibitors [18]. Pyrimidine-2,4,6-trione derivatives are an important class of nitrogen heterocycles that have attracted more attention in the last decade: due to their use as a precursor for the construction of condensed heterocyclic systems, they represent an interesting pharmacophore for pharmaceutical products [19,20,21,22,23,24,25,26,27,28,29,30,31,32]. They have utility as anticancer agents, enzyme inhibitors, neuromodulators, antibiotics, herbicides, and plant growth regulators, antibacterial, and anti-oxidant agents (Figure 1). Structure activity relationship SAR development led to the discovery of new, selective, and potent inhibitors that have attracted much interest from chemists. Molecular docking plays an important role in the rational design of drugs. In the field of molecular modeling, docking is a tool that predicts the best orientation of one molecule to a second when bound to each other to form a stable complex. Molecular docking can be defined as an optimization problem that would describe the “best-fit” orientation of a ligand that binds to a particular protein of interest to allow it to perform reliable virtual screening processes, and help us to understand the mechanism of action for tested compounds [33,34].
Figure 1.
Biologically active pyrazole and barbituric acid scaffolds.
In this paper, we synthesized a new series of pyrazole-thiopyrimidine–rione derivatives via a one-pot multi-component reaction in aqueous media to identify new drugs as antimicrobial agents. We subjected our target compounds to molecular docking study with different target proteins to explore their mode of action.
2. Results
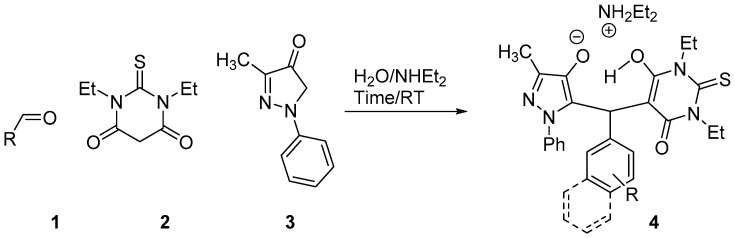
As part of our continuing efforts on the synthesis of bioactive scaffolds using green protocols, we envisioned that pyrazole-thiobarbituric acid derivatives having various substituents were achieved in high yields (63%–88%), as shown in Scheme 1, Table 1. Cascade Aldol-Michael addition of N,N-diethyl thiobarbituric acid, 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one and aldehyde was mediated by aqueous NHEt2 to afford 4a–o. Notably, the present one-pot transformation provides an efficient method for the flexible and rapid synthesis with substrate tolerance of pyrazole-thiobarbituric acid derivatives. The chemical structures of all the synthesized compounds were assigned with the aid of physical and spectroscopic methods.
Scheme 1.
Substrate scope of the cascade reaction: variation of pyrazole-thiobarbituric acid adducts.
Table 1.
Synthesis of 4a–o with different various aldehydes a.
| # | 4 | R | Yield (%) b |
|---|---|---|---|
| 1 | 4a | p-FPh | 76 |
| 2 | 4b | Ph | 83 |
| 3 | 4c | p-ClPh | 84 |
| 4 | 4d | p-CH3Ph | 73 |
| 5 | 4e | m-CH3Ph | 78 |
| 6 | 4f | p-BrPh | 88 |
| 7 | 4g | m-BrPh | 73 |
| 8 | 4h | p-NO2Ph | 73 |
| 9 | 4i | m-NO2Ph | 72 |
| 10 | 4j | p-CH3OPh | 69 |
| 11 | 4k | p-CF3Ph | 63 |
| 12 | 4l | 2,4-Cl2Ph | 68 |
| 13 | 4m | 2,6-Cl2Ph | 65 |
| 14 | 4n | 2-Naphthaldehyde | 67 |
| 15 | 4o | Thiophene | 78 |
a All reactions were carried out with aldehyde 1 (1.5 mmol), 1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione 2, (1.5 mmol), 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (1.5 mmol) and amine (1.5 mmol) in water (1.5 mL) for the specified time. b Yield of isolated product.
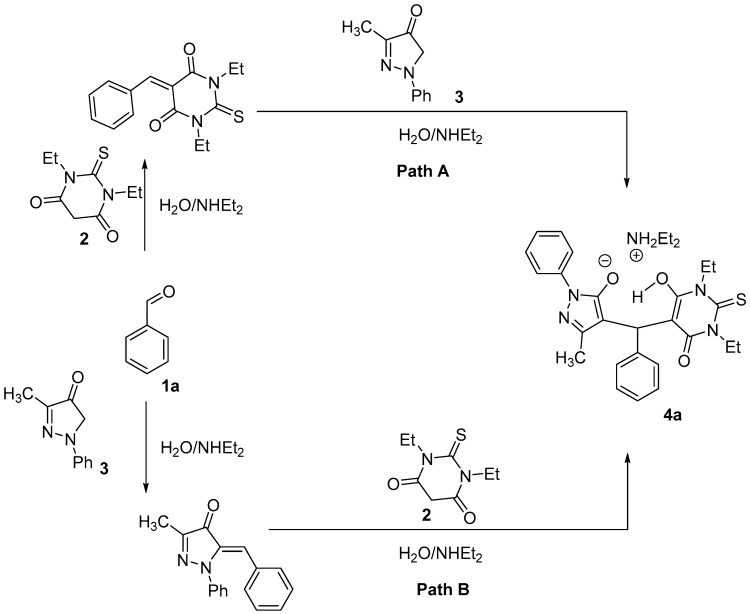
A tandem Aldol-Michael reaction is shown in Scheme 2. In the first step of the reaction, olefin is produced by Aldol condensation between aryl aldehyde 1 and 2 to give an intermediate that acts as a Michael acceptor and is attached by 3 (the Michael donor) to afford the final product 4a (Bath A). Alternatively, Aldol condensation between aryl aldehyde 1 and 3 gives an intermediate that acts as a Michael acceptor and is attached by 2 (the Michael donor) to afford the final product 4a (Bath B). [19].
Scheme 2.
Possible mechanism of the tandem Aldol-Michael reaction.
3. Discussion
3.1. Antimicrobial Activity
Results of the biological activity were displayed in Table 2; results are expressed as µg/L inhibition.
Table 2.
Results of cup-plate method and minimal inhibitory concentrations of the compounds that show antimicrobial activity.
| # | Compounds | Gram-Positive Bacteria | Yeast | ||||||
|---|---|---|---|---|---|---|---|---|---|
| S. aureus ATCC 29213 | E. faecalis ATCC 29212 | B. subtilis ATCC 10400 | C. albicans ATCC 2091 | ||||||
| CPM (mm) | MIC (µg/L) | CPM (mm) | MIC (µg/L) | CPM (mm) | MIC (µg/L) | CPM (mm) | MIC (µg/L) | ||
| 1 | 4a | 13 | 32 | 15 | 32 | 12 | 32 | 18 | 8 |
| 2 | 4b | 12 | 32 | 12 | 32 | 10 | 32 | 16 | 16 |
| 3 | 4c | 14 | 16 | 12 | 16 | 11 | 32 | 16 | 16 |
| 4 | 4d | Nil | 128 | 9 | 128 | 11 | 64 | 11 | 64 |
| 5 | 4e | Nil | 128 | 10 | 64 | 11 | 64 | 12 | 64 |
| 6 | 4f | Nil | 128 | 10 | 64 | 11 | 64 | 15 | 32 |
| 7 | 4g | 13 | 32 | 12 | 64 | 11 | 64 | 14 | 32 |
| 8 | 4h | 13 | 32 | 16 | 32 | 11 | 64 | 20 | 4 |
| 9 | 4i | 14 | 32 | 16 | 32 | 13 | 32 | 14 | 32 |
| 10 | 4j | 14 | 32 | 18 | 16 | 13 | 32 | 17 | 16 |
| 11 | 4k | 13 | 64 | 10 | 32 | 11 | 32 | 16 | 16 |
| 12 | 4l | 13 | 32 | 20 | 32 | 15 | 16 | 21 | 4 |
| 13 | 4m | 13 | 32 | 24 | 32 | 16 | 32 | 15 | 16 |
| 14 | 4n | 14 | 32 | 11 | 32 | 11 | 32 | 16 | 16 |
| 15 | 4o | 14 | 32 | Nil | 32 | 11 | 16 | 17 | 8 |
| Standard | Ciprofloxacin | 27 | ≤0.25 | 24 | ≤0.25 | 25 | ≤0.25 | ND | ND |
| Fluconazole | ND | ND | ND | ND | ND | ND | 28 | 0.5 | |
3.1.1. Antibacterial Activity against Gram-Positive Bacteria
The antibacterial activity of synthesized compounds was elucidated against six strains including E. faecalis ATCC29212, S. aureus ATCC 29213, E. coli ATCC 25922, B. subtilis ATCC 10400, P. aeruginosa ATCC 27857, and P. s vulagris ATCC 6380. Their activities were compared with the known Ciprofloxacin.
The results summarized in Table 2 show that all compounds are sensitive to the tested strains including E. faecalis, S. aureus, and B. subtilis except for compounds 4d–f and 4o, which were not active against S. aureus and E. faecalis, respectively. Compound 4c was the most active compound against S. aureus with an MIC value of 16 µg/L. In addition, it exhibited good activity against E. faecalis and B. subtilis, with MIC values of 16 µg/L and 32 µg/L, respectively. Compound 4j showed activity against E. faecalis (similar to 4c) with MIC values of 16 µg/L. Compounds 4l and 4o showed activity against B. subtilis with MIC values of 16 µg/L. Compounds 4a, 4b, 4i, 4m, and 4n showed moderate activity against all bacteria with MIC values of 32 µg/L. Compound 4h exhibited moderate activity against E. faecalis and S. aureus with MIC values of 32 µg/L and weak activity against B. subtilis with MIC values of 64 µg/L. Compound 4k exhibited moderate activity against E. faecalis and B. subtilis with MIC values of 32 µg/L and weak activity against S. aureus with MIC values of 64 µg/L. Compounds 4d, 4e, and 4f were the least active compounds against the tested bacteria. The synthesized compounds showed no activity against P. aeruginosa, E. coli, or P. vulgaris.
3.1.2. Antifungal Activity
The new pyrazole-thiobarbituric acid derivatives were evaluated for their antifungal activity against fungi) C. albicans ATCC 2091) by the diffusion method and serial dilution method (BSAC, 2015). Their activities were compared with the known antifungal agent Fluconazole. Compounds 4h and 4l were the most active compounds against C. albicans, with MIC values of 4 µg/L, followed by compounds 4a and 4o with MIC values of 8 µg/L. The order of activity was compounds 4b, 4c, 4k, 4m, 4n, and 4j with MIC values of 16 µg/L, then compounds 4f, 4g, and 4i with MIC values of 32 µg/L. Compounds 4d and 4e were the least active compounds with MIC values of 36 µg/L. Among those compounds most active as antimicrobial, we can conclude that the aryl part is either incorporated by the electron withdrawing group (EWG) at the para position or is replaced by heterocyles (compound 4o).
3.1.3. Molecular Docking as Antifungal
Comparative consensus score of synthesized compounds were listed in Table 3. The synthesized compounds and fluconazole were docked with Lanosterol 14 α-demethylase (CYP51A1) (PDB ID 4WMZ) [35].
Table 3.
Molecular modeling consensus score for the tested compounds and Fluconzole.
| No. | R | Consensus Score | |
|---|---|---|---|
| 4WMZ | 3Q70 | ||
| Fluconazole | - | 57 | 17 |
| 4l | 2,4-Cl2Ph | 48 | 24 |
| 4h | p-NO2Ph | 55 | 25 |
| 4o | Thiophene | 21 | 42 |
| 4n | 2-Naphthalde | 23 | 21 |
| 4i | m-NO2Ph | 35 | 28 |
| 4a | p-FPh | 14 | 28 |
| 4g | m-BrPh | 15 | 31 |
| 4d | p-CH3Ph | 36 | 35 |
| 4b | Ph | 20 | 37 |
| 4e | m-CH3Ph | 11 | 37 |
| 4f | p-BrPh | 22 | 38 |
| 4m | 2,6-Cl2Ph | 28 | 41 |
| 4c | p-ClPh | 33 | 45 |
| 4k | p-CF3Ph | 34 | 47 |
| 4j | p-CH3OPh | 40 | 5 |
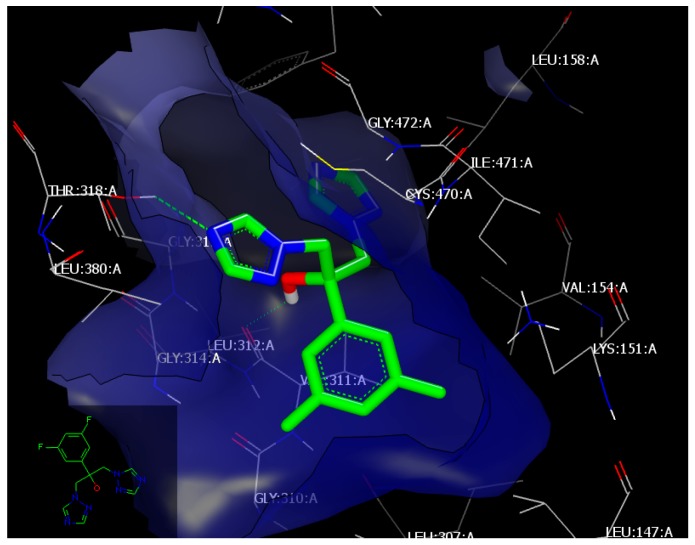
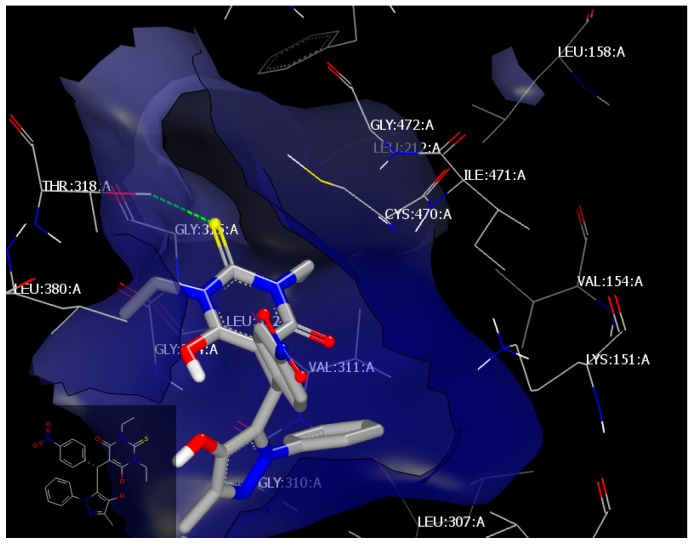
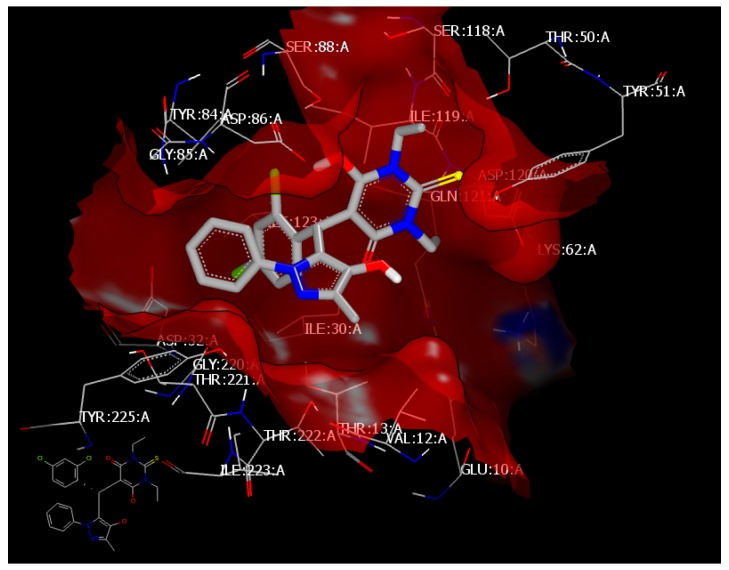
The standard drug, fluconazole, with a consensus score of 57, forms two hydrogen bonds with the receptor; with THR318:A through the nitrogen of triazole moiety (HB acceptor) and with Val 311:A through the hydroxyl functionality (HB donor) (Figure 2). Compound 4l, with a consensus score of 48, interacts with the same receptor through hydrophobic–hydrophobic interaction without HB formation. Compound 4h, with a consensus score of 55, forms a hydrogen bond with receptor THR 318:A (as fluconazole did) through the sulfur of the pyrimidin moiety (HB acceptor), (Figure 3).
Figure 2.
Visual representation of fluconzol docked with 4WMZ, showing two hydrogen bonding interactions with THR 318:A and LEU 312:A, as shown by Vida.
Figure 3.
Visual representation of 4h docked with 4WMZ, showing hydrogen bonding interactions with THR 318:A, as shown by Vida.
Our hypothesis was directed at the study of another kind of protein as these compounds may have a mode of action that differs from the fluconazole mechanism. After intensive study, synthesized compounds presented docking interaction with dihydrofolate reductase (DHFR), correlated with their biological activity.
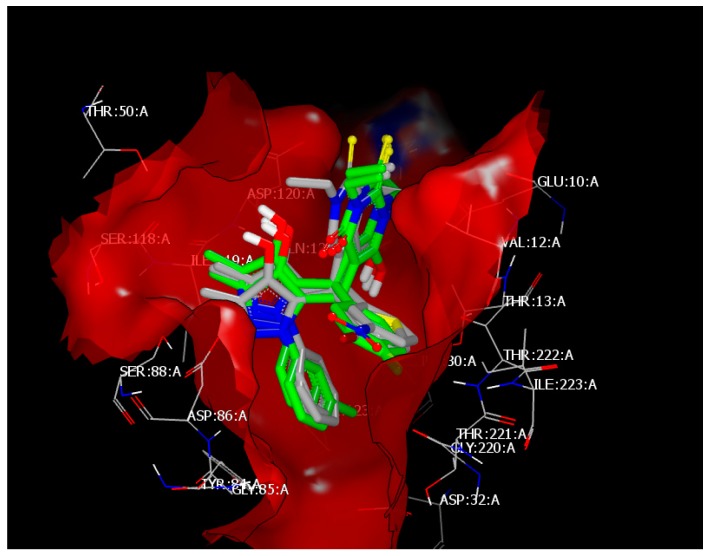
Compound 4l interacts with the receptor of dihydrofolate reductase (DHFR) (ID 3Q70) [36] through hydrophobic–hydrophobic interaction, with a consensus score of 24 (Figure 4). Similarly, compounds 4o and 4a, with consensus scores of 26 and 28, respectively, interact with the same receptor through hydrophobic–hydrophobic interaction and overlay with compounds 4c, 4d, 4g, and 4i (Figure 5).
Figure 4.
Visual representation of 4l showed the hydrophobic–hydrophobic interaction with the binding site of 3Q70, as shown by Vida.
Figure 5.
Visual representation of 4a overlay with 4o, 4d, 4g, and 4i, showing the hydrophobic–hydrophobic interaction at the binding site of 3Q70, as shown by Vida.
3.1.4. Molecular Docking as Gram-Positive Bacteria Antagonist
Molecular modeling gave us the comparative consensus scores of synthesized compounds with two targets: DNA topisomerase II (PDB ID 5BTC) [37] and gyrase B (PDB ID 4URM) proteins [38]. The consensus scores for the tested compounds with these two proteins are listed in Table 4.
Table 4.
Molecular modeling consensus score for the tested compounds and Ciprofloxacin.
| No. | R | Consensus Score | |
|---|---|---|---|
| 5BTC | 4URM | ||
| Ciprofloxacin | - | 1 | 4 |
| 4o | Thiophene | 10 | 24 |
| 4b | Ph | 17 | 24 |
| 4j | p-CH3OPh | 18 | 30 |
| 4e | m-CH3Ph | 19 | 33 |
| 4d | p-CH3Ph | 23 | 22 |
| 4h | p-NO2Ph | 23 | 55 |
| 4c | p-ClPh | 29 | 24 |
| 4f | p-BrPh | 29 | 29 |
| 4a | p-FPh | 36 | 31 |
| 4g | m-BrPh | 46 | 29 |
| 4i | m-NO2Ph | 47 | 37 |
| 4k | p-CF3Ph | 48 | 59 |
| 4l | 2,4-Cl2Ph | 54 | 17 |
| 4n | 2-Naphthalde | 54 | 37 |
| 4m | 2,6-Cl2Ph | 60 | 41 |
3.1.5. Docking with DNA Topisomerase II (5BTC)
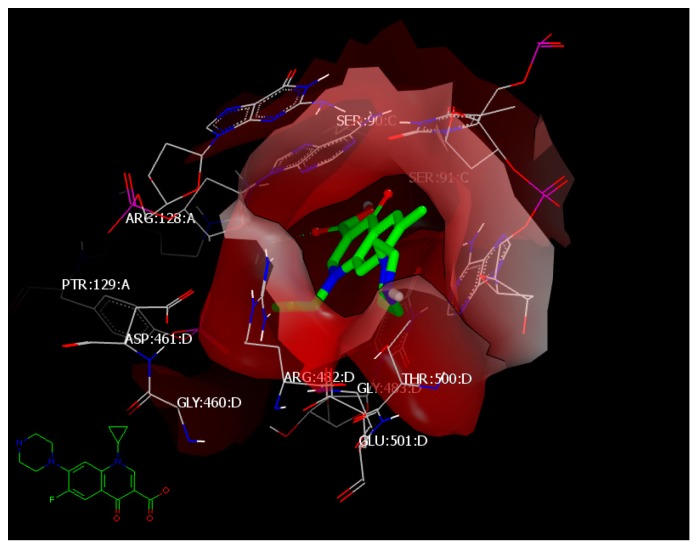
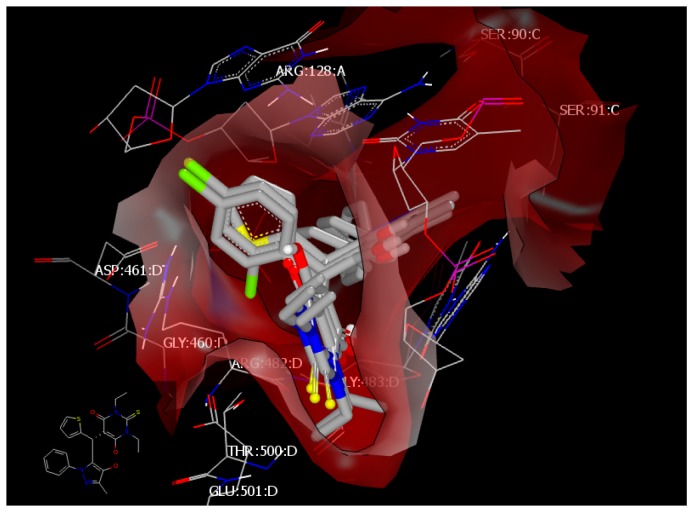
The standard drug ciprofloxacin has a consensus score of 1 through hydrophobic–hydrophobic interaction and forms HB with ARG:128:A through the oxygen of its carbonyl (Figure 6). The docking mode for the most active compounds showed hydrophobic–hydrophobic interaction with the receptor. Compound 4c with a consensus score of 29, compound 4o with a consensus score of 10, and compound 4l with a consensus score of 54 showed exhibited hydrophobic–hydrophobic interactions and overlay each other (Figure 7). Our attention was directed to explore another kind of protein.
Figure 6.
Visual representation of ciprofloxacin docked with 5BTC, showing hydrophobic–hydrophobic interaction and hydrogen bonding with ARG 128:A, as shown by Vida.
Figure 7.
Visual representation of compounds 4c, 4o, and 4l docked with 5BTC, showing no hydrogen bond interaction, as shown by Vida.
3.1.6. With Gyrase B (PDB ID 4URM)
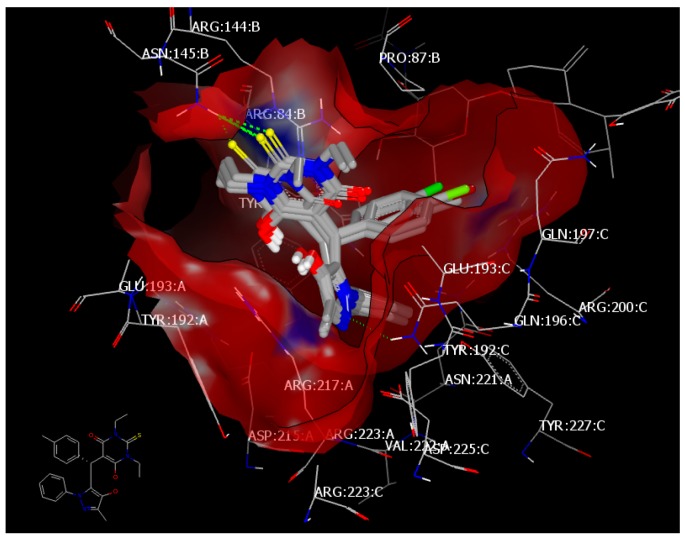
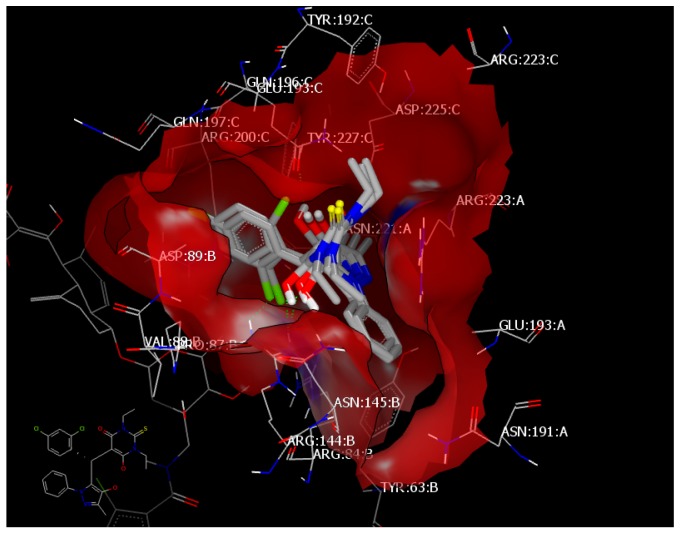
Ciprofloxacin forms a hydrogen bond with ASP 225:A through the NH of the piperidine moiety with a consensus score of 4. Compound 4c (consensus score: 24) showed HB interaction with ASN 145:A through the sulfur atom of the thiobarbiturate ring and overlay with 4a, 4d, and 4f with the same HB. However, 4a showed extra HB with GLN 196:A through the N of pyrazole (Figure 8). Compounds 4l (consensus score 17) and 4m (consensus score 41) showed hydrophobic–hydrophobic interaction and overlay with 4b; however, compound 4b (consensus score: 24) exhibits three kinds of HB (Figure 9).
Figure 8.
Visual representation of compound 4d docked with 4URM and overlay with 4c, 4a and 4f. The compounds showed hydrogen bonding between the sulfur of the pyrimidine ring and ASN 145:A, as shown by Vida.
Figure 9.
Visual representation of compounds 4l and 4m docked with 4URM without HB interaction and overlay with 4b.
The antimicrobial activity of these compounds might be attributed to the presence of different pharmacophores in the molecules. The sulfur and OH groups in the substrates actively participate in the biological activity, as is corroborated by the formation of a hydrogen bond with the amino acid in the receptor. The site and kind of substituent located in the aldehyde part affects the dissociation rate, required for the liberation of free compounds from its salt form, and participates in the ligand–protein interaction. In this regard, the presence of an electron donating group (EDG) retards activity, while an electron withdrawing group (EWG) represents the best activity. Among the compounds having EWG, the para position is the best site for activity and the electronic and steric effect has a role in activity (compound 4f is inactive). However, the difference in activity between compounds 4l and 4m could be explained by their 3D structures during the docking study by using Omega application. In compound 4m, the chloride atom in position 6 was incorporated closely with the hydroxyl groups, while in the case of compound 4l its presence at position para meant the chloride atom was not incorporated near the hydroxyl group. This evidence represents the importance of EWG at the para position (see the Supplementary Materials).
4. Materials and Methods
“All the chemicals were purchased from Sigma-Aldrich (Riedstraße, Germany) Fluka (Buchs, Switzerland), etc., and were used without further purification, unless otherwise stated. All melting points were measured on a Gallenkamp melting point apparatus (Bibby Scientific Limited, Beacon Road, Stone, Staffordshire, UK) in open glass capillaries and are uncorrected. IR Spectra were measured as KBr pellets on a Nicolet 6700 FT-IR spectrophotometer (Thermo Fisher Scientific, Madison, WI, USA). The NMR spectra were recorded on a Jeol-400 NMR spectrometer (Tokyo, Japan). 1H-NMR (400 MHz), and 13C-NMR (100 MHz) were run in deuterated dimethylsulphoxide (DMSO-d6). Chemical shifts (δ) are referred to in terms of ppm and J-coupling constants are given in Hz. Mass spectra were recorded on a Jeol of JMS-600 H (Santa Clara, CA, USA). Elemental analysis was carried out on an Elmer 2400 Elemental Analyzer (Waltham, MA, USA) in the CHN mode”.
4.1. General Procedure for Knoevenagel Condensation–Michael Addition for the Synthesis of 4a–o (GP1)
A mixture of aldehyde 1 (1.5mmol), 1,3-diethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione 2, (1.5 mmol), 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one (1.5mmol) and Et2NH (1.5 mmol, 155 μL) in 3 mL of degassed H2O was stirred at room temperature for 1–5 h until TLC showed complete disappearance of the reactants. The precipitate was removed by filtration and washed with ether (3 × 20 mL). The solid was dried to afford pure product 4a–o.
1,3-Diethyl-5-((4-fluorophenyl)(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)methyl)-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4a: 4a was prepared according to the general procedure (GP1) from p-flurobenzaldehyde, yielding a pink powder materilas. m.p.: 118 °C; IR (KBr, cm−1): 3445, 2989, 272, 2511, 1646, 1602, 1498, 1392, 1305, 1269; 1H-NMR (400 MHz, DMSO-d6): δ 17.65 (s, 1H, OH), 9.14 (bs, NH, NHEt2), 7.55–7.20 (m, 9H, Ph), 5.52 (s, 1H, benzyl-H), 4.57 (m, 4H, CH2CH3), 2.52 (q, 4H, J = 7.3 Hz, CH2CH3), 2.04 (s, 3H, CH3), 1.03 (t, 6H, J = 7.3 Hz, CH2CH3), 1.00 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 193.2, 174.7, 164.0, 148.9, 146.5, 138.5, 129.0, 128.9, 128.2, 126.3, 122.2, 114.7, 114.5, 96.6, 43.4, 42.0, 15.2, 12.6, 12.5, 11.2; LC/MS (ESI): 280.1 [M]+ For C18H17FN2; Anal. for C29H36FN5O3S; calcd. C, 59.69; H, 6.01; F, 9.44; N, 11.60; S, 5.31; Found: C, 59.67; H, 6.01; F, 9.44; N, 11.63; S, 5.32.
1,3-Diethyl-6-hydroxy-5-((5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)(phenyl)methyl)-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4b: 4b was prepared according to the general procedure (GP1) from benzaldehyde, yielding faint orange materials. m.p.: 116 °C; IR (KBr, cm−1): 3448, 3023, 2982, 2785, 2508, 1580, 1502, 1410, 1389, 1264; 1H-NMR (400 MHz, DMSO-d6): δ 17.60 (s, 1H, OH), 7.55–7.13 (m, 10H, Ph), 5.53 (s, 1H, benzyl-H), 4.57 (m, 4H, CH2CH3), 2.32 (q, 4H, J = 7.3 Hz, CH2CH3), 2.10 (s, 3H, CH3), 1.21 (t, 6H, J = 7.3 Hz, CH2CH3), 0.90 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 198.0, 174.8, 164.0, 163.6, 163.2, 151.4, 146.9, 138.0, 128.8, 127.9, 127.5, 126.9, 125.9, 121.9, 121.8, 91.2, 65.8, 42.1, 12.6, 12.2, 10.7; LC/MS (ESI): 262.1 [M]+ for C18H18N2; Anal. for C29H37N5O3S; calcd. C, 65.02; H, 6.96; N, 13.07; S, 5.99; Found: C, 65.03; H, 6.94; N, 13.05; S, 6.01.
5-((4-Chlorophenyl)(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)methyl)-1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4c: 4c was prepared according to the general procedure (GP1) from p-cholorbenzaldehyde, yielding white materials. m.p.: 178 °C; IR (KBr, cm−1): 3453, 2981, 2873, 2495, 1686, 1582, 1487, 1438, 1386, 1267; 1H-NMR (400 MHz, DMSO-d6): δ 17.62 (s, 1H, OH), 9.33 (bs, NH, NHEt2), 7.24–6.96 (m, 10H, Ph), 5.80 (s, 1H, benzyl-H), 4.59 (m, 4H, CH2CH3), 3.32 (q, 4H, J = 7.3 Hz, CH2CH3), 3.29 (s, 3H, CH3), 3.03 (t, 6H, J = 7.3 Hz, CH2CH3), 1.29 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 198.2, 174.8, 164.0, 163.6, 163.2, 151.4, 139.7, 139.1, 131.4, 128.3, 127.8, 96.8, 91.2, 44.1, 42.1, 34.2, 28.6, 12.4, 12.3, 11.3; LC/MS (ESI): 296.1 [M]+ for C18H17ClN2; Anal. for C29H36ClN5O3S; calcd. C, 61.09; H, 6.36; Cl, 6.22; N, 12.28; S, 5.62; Found: C, 61.11; H, 6.34; Cl, 6.21; N, 12.31; S, 5.62.
1,3-Diethyl-6-hydroxy-5-((5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)(p-tolyl)methyl)-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4d: 4d was prepared according to the general procedure (GP1) from p-toulaldehyde, yielding orange materials (1.4 g, 2.97 mmol, 99%). m.p.: 193 °C; IR (KBr, cm−1): 3456, 3046, 2981, 2873, 2501, 1674, 1600, 1437, 1385, 1268; 1H-NMR (400 MHz, DMSO-d6): δ 17.63 (s, 1H, OH), 9.30 (bs, NH, NHEt2), 7.24–6.93 (m, 10H, Ph), 5.82 (s, 1H, benzyl-H), 4.59 (m, 4H, CH2CH3), 3.32 (q, 4H, J = 7.3 Hz, CH2CH3), 3.29 (s, 3H, CH3), 3.03 (t, 6H, J = 7.3 Hz, CH2CH3), 2.24 (s, 3H, CH3), 1.27 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 196.2, 174.6, 164.0, 163.6, 163.1, 159.4, 151.4, 139.6, 139.3, 131.4, 128.8, 128.8, 126.2, 126.1, 97.1, 91.1, 44.3, 41.9, 34.2, 28.8, 20.9, 12.4, 12.2, 11.3; LC/MS (ESI): 276.1 [M]+ for C19H20N2; Anal. for C30H39N5O3S; calcd. C, 65.55; H, 7.15; N, 12.74; S, 5.83; Found: C, 65.54; H, 7.15; N, 12.73; S, 5.83.
1,3-Diethyl-6-hydroxy-5-((5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)(m-tolyl)methyl)-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4e: 4e was prepared according to the general procedure (GP1) from m-toulaldehyde, yielding white materials. m.p.: 200 °C; IR (KBr, cm−1): 3453, 3039, 2980, 2506, 1688, 1599, 1437, 1408, 1348, 1267; 1H-NMR (400 MHz, DMSO-d6): δ 17.59 (s, 1H, OH), 9.33 (bs, NH, NHEt2), 7.24–6.79 (m, 10H, Ph), 5.80 (s, 1H, benzyl-H), 4.62 (m, 4H, CH2CH3), 3.34 (q, 4H, J = 7.3 Hz, CH2CH3), 3.29 (s, 3H, CH3), 3.04 (t, 6H, J = 7.3 Hz, CH2CH3), 2.27 (s, 3H, CH3), 1.27 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 196.2, 174.7, 163.7, 163.2, 152.3, 140.2, 137.5, 128.0, 126.9, 126.5, 123.4, 96.8, 91.5, 44.4, 44.1, 41.9, 34.6, 21.4, 12.4, 12.3, 11.3; LC/MS (ESI): 276.1 [M]+ for C19H20N2; Anal. for C30H39N5O3S; calcd. C, 65.55; H, 7.15; N, 12.74; S, 5.83; Found: C, 65.55; H, 7.15; N, 12.74; S, 5.85.
5-((4-Bromophenyl)(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)methyl)-1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4f: 4f was prepared according to the general procedure (GP1) from p-bromobenzaldehyde, yielding beige materials. m.p.: 156 °C; IR (KBr, cm−1): 3431, 3006, 2981, 2509, 1603, 1579, 1501, 1484, 1411, 1390, 264; 1H-NMR (400 MHz, DMSO-d6): δ 17.61 (s, 1H, OH), 9.34 (bs, NH, NHEt2), 7.52–7.02 (m, 10H, Ph), 5.43 (s, 1H, benzyl-H), 4.54 (m, 4H, CH2CH3), 3.48 (q, 4H, J = 7.3 Hz, CH2CH3), 2.15 (q, 4H, J = 7.3 Hz, CH2CH3), 1.97 (s, 3H, CH3), 1.21 (t, 6H, J = 7.3 Hz, CH2CH3), 0.78 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 146.7, 138.2, 131.1, 129.4, 128.8, 126.2, 121.9, 98.2, 65.8, 41.3, 15.2, 12.6, 12.2, 10.7; LC/MS (ESI): 340.1 [M]+ for C18H17BrN2; Anal. for C29H36BrN5O3S; calcd. C, 56.67; H, 5.90; Br, 13.00; N, 11.40; S, 5.22; Found: C, 56.67; H, 5.89; Br, 13.01; N, 11.43; S, 5.20.
5-((3-Bromophenyl)(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)methyl)-1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4g: 4g was prepared according to the general procedure (GP1) from m-bromobenzaldehyde, yielding a pink powder materials. m.p.: 144 °C; IR (KBr, cm−1): 3432, 3060, 2981, 2743, 2505, 1611, 1591, 1501, 1404, 1264; 1H-NMR (400 MHz, DMSO-d6): δ 17.61 (s, 1H, OH), 9.34 (bs, NH, NHEt2), 7.52–7.02 (m, 10H, Ph), 5.43 (s, 1H, benzyl-H), 4.54 (m, 4H, CH2CH3), 3.48 (q, 4H, J = 7.3 Hz, CH2CH3), 2.15 (q, 4H, J = 7.3 Hz, CH2CH3), 1.97 (s, 3H, CH3), 1.21 (t, 6H, J = 7.3 Hz, CH2CH3), 0.78 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 146.7, 138.2, 131.1, 129.4, 128.8, 126.2, 121.9, 98.2, 65.8, 41.3, 15.2, 12.6, 12.2, 10.7; LC/MS (ESI): 340.1 [M]+ for C18H17BrN2;Anal. for C29H36BrN5O3S; calcd. C, 56.67; H, 5.90; Br, 13.00; N, 11.40; S, 5.22; Found: C, 56.67; H, 5.89; Br, 13.01; N, 11.43; S, 5.20.
1,3-Diethyl-6-hydroxy-5-((5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)(4-nitrophenyl)methyl)-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4h: 4h was prepared according to the general procedure (GP1) from p-nitrobenzaldehyde, yielding yellow materials. m.p.: 108 °C; IR (KBr, cm−1): 3448, 2982, 2732, 2502, 1705, 1580, 1514, 1411, 1345, 1264; 1H-NMR (400 MHz, DMSO-d6): δ 17.61 (s, 1H, OH), 10.14 (bs, NH, NHEt2), 8.01 (dd, 2H, J = 7.3 Hz, 1.5Hz, Ph), 7.53 (dd, 2H, J = 7.3 Hz, 1.5 Hz, Ph) 7.48–7.2 (m, 5H, Ph), 5.55 (s, 1H, benzyl-H), 4.54 (m, 4H, CH2CH3), 2.37 (q, 4H, J = 7.3 Hz, CH2CH3), 2.05 (s, 3H, CH3), 1.27 (t, 6H, J = 7.3 Hz, CH2CH3), 0.92 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 192.2, 174.7, 163.7, 163.2, 152.3, 146.6, 130.5, 129.0, 128.9, 128.4, 127.8, 126.3, 124.3, 123.2, 122.1, 103.3, 96.1, 43.4, 41.7, 34.7, 34.2, 12.5, 12.3, 10.7 ; LC/MS (ESI): 307.1 [M]+ for C18H17N3O2; Anal. for C29H36N6O5S; calcd. C, 59.98; H, 6.25; N, 14.47; S, 5.52; Found: C, 60.00; H, 6.26; N, 14.45; S, 5.53.
1,3-Diethyl-6-hydroxy-5-((5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)(3-nitrophenyl)methyl)-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4i: 4i was prepared according to the general procedure (GP1) from m-nitrobenzaldehyde, yielding yellow materials. m.p.: 136 °C; IR (KBr, cm−1): 3433, 2982, 2787, 2508, 1581, 1526, 1503, 1410, 1348, 1264; 1H-NMR (400 MHz, DMSO-d6): δ 17.61 (s, 1H, OH), 10.14 (bs, NH, NHEt2), 7.97 (s, 1H, Ph), 7.58–7.2 (m, 7H, Ph), 5.55 (s, 1H, benzyl-H), 4.60 (m, 4H, CH2CH3), 2.61 (q, 4H, J = 7.3 Hz, CH2CH3), 2.08 (s, 3H, CH3), 1.22 (t, 6H, J = 7.3 Hz, CH2CH3), 1.11 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 192.2, 175.0, 163.7, 163.2, 152.3, 148.6, 130.5, 129.0, 128.9, 128.4, 127.8, 126.3, 124.3, 123.2, 122.1, 103.3, 96.1, 43.4, 42.2, 15.2, 12.6, 12.5, 11.1; LC/MS (ESI): 307.1 [M]+ for C18H17N3O2; Anal. for C29H36N6O5S; calcd. C, 59.98; H, 6.25; N, 14.47; S, 5.52; Found: C, 60.01; H, 6.26; N, 14.45; S, 5.54.
1,3-Diethyl-6-hydroxy-5-((5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)(4-methoxyphenyl)methyl)-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4j: 4j was prepared according to the general procedure (GP1) from anisaldehyde, yielding white crystalline materials. m.p: 112 °C; IR (KBr, cm−1): 3424, 2981, 2735, 2508, 1610, 1506, 1406, 1388, 1265; 1H-NMR (400 MHz, DMSO-d6): δ 17.71 (s, 1H, OH), 9.14 (bs, NH, NHEt2), 7.58–6.80 (m, 9H, Ph), 5.46 (s, 1H, benzyl-H), 4.60 (m, 4H, CH2CH3), 2.36 (q, 4H, J = 7.3 Hz, CH2CH3), 2.19 (s, 3H, CH3), 1.21 (t, 6H, J = 7.3 Hz, CH2CH3), 0.92 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 194.2, 175.0, 163.7, 163.2, 152.3, 141.8, 138.5, 136.6, 128.8, 128.5, 127.8, 125.7, 121.8, 119.2, 114.2, 113.3. 113.2, 96.4, 91.5, 41.5, 34.5, 15.2, 12.6, 12.4, 11.0; LC/MS (ESI): 292.1 [M]+ for C19H20N2O; Anal. for C30H39N5O4S; calcd. C, 63.69; H, 6.95; N, 12.38; S, 5.67; Found: C, 63.70; H, 6.97; N, 12.41; S, 5.68.
1,3-Diethyl-5-((4-trifluoromethylphenyl)(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)methyl)-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4k: 4k was prepared according to the general procedure (GP1) from p-trifluoromethyl benzaldehyde, yielding a pink powder materials. m.p.: 138 °C; IR (KBr, cm−1): 3441, 2982, 2787, 2505, 1615, 1579, 1420, 1390, 1265; 1H-NMR (400 MHz, DMSO-d6): δ 17.65 (s, 1H, OH), 9.14 (bs, NH, NHEt2), 7.55–7.24 (m, 9H, Ph), 5.52 (s, 1H, benzyl-H), 4.57 (m, 4H, CH2CH3), 2.52 (q, 4H, J = 7.3 Hz, CH2CH3), 2.04 (s, 3H, CH3),1.03 (t, 6H, J = 7.3 Hz, CH2CH3), 1.00 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 193.2, 174.7, 164.0, 148.9, 146.5, 138.5, 129.0, 128.9, 128.2, 126.3, 122.2, 114.7, 114.5, 96.6, 43.4, 42.0, 15.2, 12.6, 12.5, 11.2; LC/MS (ESI): 330.13 [M]+ for C19H17F3N2; Anal. for C30H36F3N5O3S; calcd. C, 59.69; H, 6.01; F, 9.44; N, 11.60; S, 5.31; Found: C, 59.67; H, 6.01; F, 9.44; N, 11.63; S, 5.32.
5-((2,4-Dichlorophenyl)(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)methyl)-1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4l: 4l was prepared according to the general procedure (GP1) from 2,4-dicholrobenzaldehyde, yielding an orange powder materials. m.p.: 132 °C; IR (KBr, cm−1): 3444, 2981, 2736, 2506, 1582, 1503, 1455, 1410, 1388, 1264; 1H-NMR (400 MHz, DMSO-d6): δ 17.65 (s, 1H, OH), 7.55–7.21 (m, 9H, Ph), 5.41 (s, 1H, benzyl-H), 4.49 (m, 4H, CH2CH3), 2.53 (q, 4H, J = 7.3 Hz, CH2CH3), 2.17 (s, 3H, CH3), 1.80 (bs, NH, NHEt2), 1.20 (t, 6H, J = 7.3 Hz, CH2CH3), 1.00 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 195.2, 174.8, 164.0, 148.7, 146.5, 138.5, 129.0, 128.9, 128.2, 126.3, 122.2, 114.7, 114.5, 96.6, 43.2, 41.5, 15.4, 12.9, 12.7, 11.2; LC/MS (ESI): 330.07 [M]+ for C18H16Cl2N2; Anal. for C29H35Cl2N5O3S; calcd. C, 57.61; H, 5.84; Cl, 11.73; N, 11.58; S, 5.30; Found: C, 57.60; H, 5.85; Cl, 11.70; N, 12.01; S, 5.31.
5-((2,4-Dichlorophenyl)(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)methyl)-1,3-diethyl-6-hydroxy-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4m: 4m was prepared according to the general procedure (GP1) from 2,6-dicholrobenzaldehyde, yielding a pink powder materials. m.p.: 171 °C; IR (KBr, cm−1): 3448, 2932, 2980, 2755, 2506, 1582, 1499, 1407, 1371, 1263; 1H-NMR (400 MHz, DMSO-d6): δ 17.30 (s, 1H, OH), 11.9 (bs, NH, NHEt2), 7.74 (d, 2H, J = 7.3 Hz, Ph), 7.37 (t, 1H, J = 7.3 Hz, Ph), 7.24–7.01 (m, 9H, Ph), 5.74 (s, 1H, benzyl-H), 4.37 (m, 4H, CH2CH3), 2.90 (q, 4H, J = 7.3 Hz, CH2CH3), 1.96 (s, 3H, CH3), 1.11 (t, 12H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 193.8, 173.9, 161.7, 153.1, 146.6, 138.5, 135.7, 129.5, 128.7, 127.0, 124.4, 120.0, 94.7, 43.1, 42.0, 31.8, 12.5, 12.4, 11.0; LC/MS (ESI): 330.07 [M]+ for C18H16Cl2N2; Anal. for C29H35Cl2N5O3S; calcd. C, 57.61; H, 5.84; Cl, 11.73; N, 11.58; S, 5.30; Found: C, 57.60; H, 5.85; Cl, 11.70; N, 12.01; S, 5.31.
1,3-Diethyl-6-hydroxy-5-((5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)(naphthalen-2-yl)methyl)-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4n: 4n was prepared according to the general procedure (GP1) from naphthaldehyde, yielding white crystalline materials. m.p.: 118 °C; IR (KBr, cm−1): 3443, 3052, 2981, 2872, 2737, 2507, 1582, 1503, 1454, 1410, 1388, 1264; 1H-NMR (400 MHz, DMSO-d6): δ 17.65 (s, 1H, OH), 9.30 (bs, NH, NHEt2), 7.75–7.05 (m, 12H, Ph), 5.68 (s, 1H, benzyl-H), 4.61 (m, 4H, CH2CH3), 2.40 (q, 4H, J = 7.3 Hz, CH2CH3), 2.13 (s, 3H, CH3), 1.21 (t, 6H, J = 7.3 Hz, CH2CH3), 0.87 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 193.8, 173.9, 161.7, 153.1, 146.6, 138.5, 135.7, 129.5, 128.7, 127.0, 124.4, 120.0, 94.7, 43.1, 42.0, 31.8, 12.5, 12.4, 11.0; LC/MS (ESI): 312 [M]+ for C22H20N2; Anal. for C33H39N5O3S; calcd C, 67.66; H, 6.71; N, 11.96; S, 5.47; Found: C, 67.67; H, 6.70; N, 11.95; S, 5.48.
1,3-Diethyl-6-hydroxy-5-((5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)(thiophen-2-yl)methyl)-2-thioxo-2,3-dihydropyrimidin-4(1H)-one diethylaminium salt 4o: 4o was prepared according to the general procedure (GP1) from thiophenldehyde, yielding a brown powder materials. m.p.: 122 °C; IR (KBr, cm−1): 3437, 2932, 2980, 2736, 2509, 1598, 1502, 1410, 1389, 1329, 1264; 1H-NMR (400 MHz, DMSO-d6): δ 17.30 (s, 1H, OH), 8.38 (bs, NH, NHEt2), 7.87 (d, 2H, J = 7.3 Hz, thiophene), 7.70 (d, 1H, J = 7.3 Hz, thiophene), 7.45–7.07 (m, 6H, Ph), 5.55 (s, 1H, benzyl-H), 4.44 (m, 4H, CH2CH3), 2.89 (q, 4H, J = 7.3 Hz, CH2CH3), 2.28 (s, 3H, CH3), 2.19 (t, 6H, J = 7.3 Hz, CH2CH3), 1.12 (t, 6H, J = 7.3 Hz, CH2CH3); 13C-NMR (100 MHz, DMSO-d6): δ = 193.8, 173.9, 163.2, 161.7, 152.2, 148.1, 148.0, 147.5, 137.3, 136.3, 135.7, 128.9, 128.6, 128.4, 128.2, 119.4, 101.9, 101.8, 97.3, 79.8, 43.2, 41.2, 34.5, 21.0, 12.8, 12.6, 11.012.4, 10.8; LC/MS (ESI): 268.1 [M]+ for : C16H16N2S; Anal. for C27H35N5O3S2; calcd. C, 59.86; H, 6.51; N, 12.93; S, 11.84; Found: C, 59.86; H, 6.51; N, 12.90; S, 11.83.
4.2. Agar Cup Plate Method
“The tested bacterial strains were grown in Cation Adjustment Mueller–Hinton (CAMH) broth (Merck®, Darmstadt, Germany), while C. albicans strain was grown in Sabauraud Dextrose Broth (SDB) to mid-log phase. The bacterial and fungal suspension was measured by spectrophotometery using Spectrophotometer (LKB® Ultrospec, Madison, WI, USA) t 625 nm to give absorbance 0.12 (1 × 108 CFU/mL). The suspension was diluted 1:100 in CAMH broth to obtain 1 × 106 CFU/mL. This suspension was swabbed on a CAMH agar plate (Merck®, Darmstadt, Germany) and allowed to dry completely. Mueller–Hinton Agar and Sabauraud Dextrose Agar were used for bacteria and fungi, respectively. Four wells (7 mm in diameter) were made in agar plate using a cork borer. One milliliter of stock solution (5120 μg/mL) was diluted two-fold in 1 mL DMSO to obtain 2560 μg/mL. One hundred microliters (256 µg) of the tested compound was poured into the well using a calibrated pipette. The plates were kept in a refrigerator at 4 °C for half an hour to allow diffusion of the compound in the agar. Then, the plates were incubated at 37 °C for 24 h. After the incubation period, the diameter of the inhibition zone was measured and recorded in mm with the aid of a ruler. Ciprofloxacin (10 µg/cup) and fluconazole (10 µg/mL) were used as positive controls for antibacterial and antifungal activity, respectively. The experiment was carried out in duplicate and the mean diameter was taken” [39].
4.3. Determination of MIC
“MIC was determined for the compounds that showed antimicrobial activity by cup plate method. Briefly, 2 mL of CAMH broth (for bacterial strains) and 2 mL of SAB (for fungal strain) were dispensed into 7-mL Peju sterile tubes. For each compound, 14 tubes were used. Tubes 13 and 14 were used as a positive growth control (no tested compound) and a negative control for medium sterility (no microorganisms), respectively. One milliliter of stock solution (5120 μg/mL) was 10-fold diluted in 9 mL CAMH to obtain 512 μg/mL. Two milliliters of the tested compounds (512 μg/mL) were pipetted into the first tube and mixed well. Then 2 mL were withdrawn from the 1st tube and added to the 2nd tube to make a two-fold dilution. This procedure was repeated down to the 12th tube to reach a concentration of 0.125 μg/mL. Two milliliters were discarded from the 12th tube. A volume of 2 mL of inoculums (1 × 106 CFU/mL) was added to all tubes except number 14 to give a final concentration of 1 × 106 CFU/mL. Ciprofloxacin and fluconazole were used as positive controls for antibacterial and antifungal activity, respectively. The inoculated tubes were incubated at 37 °C for 20 h. After the incubation period, the results of MIC were recorded manually and interpreted according to the guidelines of the British Society of Antimicrobial Chemotherapy (BSAC)” [39].
4.4. Docking Study
The docking study was performed using the OpenEye program [40] and its applications Omega, Oedcocking, Fred, and Vida. This software package is able to perform consensus scoring, which is an essential filtering technique used to obtain more accurate predictions, i.e., the lower the consensus score, the better the binding affinity of the ligands towards the receptor. Both the ligand input file (compile all .pdb files) and the receptor input file were passed into Fred to perform the molecular docking simulations. Multiple scoring functions were employed in order to obtain a consensus structure and score in the final output. A virtual library of target compounds and fluconazole as the standard drug for antifungal activity and ciprofloxacin as the standard for Gram-positive antibacterial activity was prepared using Chem Office 2012 (OpenEye Scientific software Inc. (Santa Fe, NM, USA) and energy minimized with PM3 minimization. All calculations were performed on a PC running Windows 7 ultimate. Two different target proteins were downloaded from the Protein Data Bank, namely Lanosterol 14 α-demethylase (CYP51A1) (PDB ID 4WMZ) and secreted aspartic protease (PDB ID 3Q70), and were chosen as antifungal targets against Candia albicans. In order to understand the antibacterial activities of these newly synthesized compounds, DNA topisomerase II (PDB ID 5BTC) and gyrase B (PDB ID 4URM) were selected as antibacterial targets. The catalytic domain for each protein was prepared for docking using Open Eye (OpenEye Scientific software Inc. (Santa Fe, NM, USA).
4.4.1. Generation of Conformers Using OMEGA
OMEGA (OpenEye Scientific software Inc., Santa Fe, NM, USA) was used to generate multi-conformer structure databases with high speed and reliability, in order to achieve flexible ligand docking, to be used in the docking simulations. The energy-minimized structures were then converted into .pdb files, maintaining all heavy atoms. Each .pdb file was concatenated into one continuous .pdb file to be used as an input for Omega.
4.4.2. Receptor (PDB file) Preparation Using OEDCKING Application
The receptor PDB files were downloaded from the Protein Data Bank (PDB): Lanosterol 14 α-demethylase (CYP51A1) (PDB ID 4WMZ), and secreted aspartic protease (PDB ID 3Q70), and were chosen as antifungal targets against Candia albicans. Two different target proteins were downloaded from the Protein Data Bank in order to understand the antibacterial activities of these newly synthesized compounds. DNA topisomerase II (PDB ID 5BTC), and gyrase B (PDB ID 4URM) were selected as antibacterial targets and were prepared using the make_receptor command. This step will be followed by finding the potential binding sites on the receptor of interest. The application uses a molecular probe to comb the receptor molecule, identifying all the possible binding interactions. The application allows you to create a grid box in the mode selection pane and adjust its size using the mode controls. The box size should never exceed 50,000–60,000 A°.
4.4.3. FRED Docking Application
Both the ligand input file and the receptor input file were passed into FRED to perform the molecular docking simulations. Multiple scoring functions were employed in order to obtain a consensus structure and score in the final output.
4.4.4. Vida Application
Vida is a graphical user interface that visualizes, analyzes, and manages corporate collections of molecular structures and information. Snapshots were taken to visualize and obtain the main interaction forces between the analogs and the receptor of interest.
5. Conclusions
A series of pyrazole-thiobarbituric acid derivatives (4a–o) were synthesized using a one-pot method with a broad substrate scope under mild reaction conditions in water, mediated by NHEt2. The compounds were evaluated for their antifungal and antibacterial activity. The chemical structures of the newly synthesized molecules were characterized by spectroscopic methods (IR, 1H-NMR, 13C-NMR, MS) and elemental analysis. The results of the current study revealed that compounds 4h and 4l were the most active against C. albicans, with an MIC value of 4 µg/L. Next were compounds 4a and 4o, which showed activity of MIC 8 µg/L, as compared with the reference drug Fluconazole with a MIC value of 0.5 µg/L. Furthermore, all synthesized compounds were docked inside the active site for two kinds of proteins, namely Lanosterol 14 α-demethylase (CYP51A1) (PDB ID 4WMZ) and secreted aspartic protease (PDB ID 3Q70). Compound 4h docked with 4WMZ and showed hydrogen bonding interactions with THR 318:A, a binding interaction similar to that of standard Fluconazole. Most of the compounds showed hydrophobic–hydrophobic interaction towards the binding site of 3Q70 and overlay each other.
However, among the synthesized compounds, compound 4c exhibited marked activity against S. aureus and E. faecalis, with MIC values of 16 µg/L. Compounds 4l and 4o were the most active compounds against B. subtilis with MIC = 16 µg/L in comparison to standard ciprofloxacin, with MIC values of <0.25 µg/L against all tested bacteria. The target compounds were subjected to dock with DNA topisomerase II (PDB ID 5BTC) and gyrase B (PDB ID 4URM) proteins to explore their mode of interaction in Grampositive bacteria.
Based on a docking study of the antimicrobial activity of the tested compounds, the sulfur atom and OH functionality in the thiobarbiturate moiety, the OH group in the pyrazole fragment, and the presence of an EWG group in the p-position of the aryl part participate in the compound’s interaction with the tested receptors. These pharmacophores may contribute, at least in part, to the activity of the tested compounds as antimicrobial agents.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research group NO (RG-257-1435-1436). Yaseen acknowledges Fathi Halaweish of the Chemistry & Biochemistry Department, South Dakota State University, Brookings 57007, SD, USA for providing the academic license for the OpenEye Scientific Software Inc. (Santa Fe, NM, USA) that helped in performing the docking study.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/10/1337/s1.
Author Contributions
A.B. conceived and designed the experiments; B.M.A.-Q. performed the experiments; A.M.A.-M. analyzed the data; A.B. contributed reagents/materials/analysis tools; M.H.A.-A. carried out the antimicrobial activity; Y.A.M.M.E. carried out the molecular docking studies; A.B. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 4a–o are available from the authors.
References
- 1.Abunada N.M., Hassaneen H.H., Kandile N.G., Miqdad O.A. Synthesis and antimicrobial activity of some new pyrazole, fused pyrazolo[3,4-d]-pyrimidine and pyrazolo[4,3-e][1,2,4]-triazolo[1,5-c]pyrimidine derivatives. Molecules. 2008;13:1501–1517. doi: 10.3390/molecules13071501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamberth C. Pyrazole chemistry in crop protection. Heterocycles. 2007;71:1467–1502. doi: 10.3987/REV-07-613. [DOI] [Google Scholar]
- 3.Dressen D., Garofalo A.W., Hawkinson J., Hom D., Jagodzinski J., Marugg J.L., Neitzel M.L., Pleiss M.A., Szoke B., Tung J.S., et al. Preparation and optimization of a series of 3-carboxamido-5-phenacylaminopyrazole bradykinin B1 receptor antagonists. J. Med. Chem. 2007;50:5161–5167. doi: 10.1021/jm051292n. [DOI] [PubMed] [Google Scholar]
- 4.Chavatte P., Yous S., Marot C., Baurin N., Lesieur D. Three-dimensional quantitative structure-activity relationships of cyclo-oxygenase-2 (COX-2) inhibitors: A comparative molecular field analysis. J. Med. Chem. 2001;44:3223–3230. doi: 10.1021/jm0101343. [DOI] [PubMed] [Google Scholar]
- 5.Woessner K.M., Simon R.A., Stevenson D.D. The safety of celecoxib in patients with aspirin-sensitive asthma. Arthritis Rheum. 2002;46:2201–2206. doi: 10.1002/art.10426. [DOI] [PubMed] [Google Scholar]
- 6.Fong T.M., Heymsfield S.B. Cannabinoid-1 receptor inverse agonists: Current understanding of mechanism of action and unanswered questions. Int. J. Obes. 2009;33:947–955. doi: 10.1038/ijo.2009.132. [DOI] [PubMed] [Google Scholar]
- 7.Menozzi G., Fossa P., Cichero E., Spallarossa A., Ranise A., Mosti L. Rational design, synthesis and biological evaluation of new 1,5-diarylpyrazole derivatives as CB1 receptor antagonists, structurally related to rimonabant. Eur. J. Med. Chem. 2008;43:2627–2638. doi: 10.1016/j.ejmech.2008.01.043. [DOI] [PubMed] [Google Scholar]
- 8.Gudmundsson K.S., Johns B.A., Allen S.H. Pyrazolopyridines with potent activity against herpesviruses: Effects of C5 substituents on antiviral activity. Bioorg. Med. Chem. Lett. 2008;18:1157–1161. doi: 10.1016/j.bmcl.2007.11.120. [DOI] [PubMed] [Google Scholar]
- 9.Kendre D.B., Toche R.B., Jachak M.N. Synthesis of novel Dipyrazolo [3,4-b:3,4-d] pyridines and study of their fluorescence behavior. Tetrahedron. 2007;63:11000–11004. doi: 10.1016/j.tet.2007.08.052. [DOI] [Google Scholar]
- 10.Bazgira A., Khanaposhtani M.M., Soorki A.A. One-pot synthesis and antibacterial activities of pyrazolo [4′,3′:5,6] pyrido [2,3-d] pyrimidine-dione derivatives. Bioorg. Med. Chem. Lett. 2008;18:5800–5803. doi: 10.1016/j.bmcl.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 11.Varma R.S. Solvent-free organic syntheses. Using supported reagents and microwave irradiation. Green Chem. 1999;1:43–55. doi: 10.1039/a808223e. [DOI] [Google Scholar]
- 12.Ahluwalia V.K., Dahiya A., Garg V.J. Reaction of 5-amino-4-formyl-3-methyl (or phenyl)-1-phenyl-1H-pyrazoles with active methylene compounds: Synthesis of fused heterocyclic rings. Indian J. Chem. 1997;36B:88–90. doi: 10.1002/chin.199740182. [DOI] [Google Scholar]
- 13.Trost B.M., Brennan M.K. Asymmetric syntheses of oxindole and indole spirocyclic alkaloid natural products. Synthesis. 2009;18:3003–3025. doi: 10.1055/s-0029-1216975. [DOI] [Google Scholar]
- 14.Ranjith Kumar R., Perumal S., Senthilkumar P., Yogeeswari P., Sriram D. A facile synthesis and antimycobacterial evaluation of novel spiro-pyrido-pyrrolizines and pyrrolidines. Eur. J. Med. Chem. 2009;44:3821–3829. doi: 10.1016/j.ejmech.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y.S., Cai J., Hu Z.Y., Lin G.H. A new class of metal-free catalysts for direct diastereo-and regioselective Mannich reactions in aqueous media. Tetrahedron Lett. 2004;45:8949–8952. doi: 10.1016/j.tetlet.2004.09.174. [DOI] [Google Scholar]
- 16.Deiana V., Gómez-Cañas M., Pazos M.R., Fernández-Ruiz J., Asproni B., Cichero E., Fossa P., Muñoz E., Deligia F., Murineddu G., et al. Tricyclic pyrazoles. Part 8. Synthesis, biological evaluation and modelling of tricyclic pyrazole carboxamides as potential CB2 receptor ligands with antagonist/inverse agonist properties. Eur. J. Med. Chem. 2016;112:66–80. doi: 10.1016/j.ejmech.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Cox B.D., Prosser A.R., Sun Y., Li Z., Lee S., Huang M.B., Bond V.C., Snyder J.P., Krystal M., Wilson L.J., et al. Pyrazolo-Piperidines Exhibit Dual Inhibition of CCR5/CXCR4 HIV Entry and Reverse Transcriptase. ACS Med. Chem. Lett. 2015;6:753–757. doi: 10.1021/acsmedchemlett.5b00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cichero E., Fossa P. Docking-based 3D-QSAR analyses of pyrazole derivatives as HIV-1 non-nucleoside reverse transcriptase inhibitors. J. Mol. Model. 2012;18:1573–1582. doi: 10.1007/s00894-011-1190-5. [DOI] [PubMed] [Google Scholar]
- 19.Barakat A., Al-Majid A.M., Al-Najjar H.J., Mabkhot Y.N., Javaid S., Yousuf S., Choudhary M.I. Zwitterionic pyrimidinium adducts as antioxidants with therapeutic potential as nitric oxide scavenger. Eur. J. Med. Chem. 2014;84:146–154. doi: 10.1016/j.ejmech.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Asiri A.M., Khan S.A., Ng S.W. Pyridinium 5-[(1,3-diethyl-6-hydroxy-4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl)(2-methoxyphenyl) methyl]-1,3-diethyl-4,6-dioxo-2-thioxopyrimidin-5-ide. Acta Crystallogr. Sect. E Struct. Rep. Online. 2009;65:o1860–o1861. doi: 10.1107/S160053680902618X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elinson M.N., Nasybullin R.F., Sokolova O.O., Zaimovskaya T.A., Egorov M.P. Non-catalytic multicomponent rapid and efficient approach to 10-(2,4,6-trioxohexahydropyrimidin-5-yl)-3,3-dimethyl-2, 3,4,9-tetrahydro-1H-xanthen-1-ones from salicylaldehydes, dimedone, and barbituric acids. Mon. Chem. Chem. Mon. 2015;146:1689–1694. doi: 10.1007/s00706-015-1512-x. [DOI] [Google Scholar]
- 22.Ahluwalia V.K., Goyal B., Das U. One-pot Syntheses of 5-Oxo-1,4,5,6,7,8-hexahydroquinolines and Pyrimido [4,5-b] quinolines using Microwave Irradiation and Ultrasound. J. Chem. Res. Synop. 1997;7 doi: 10.1002/chin.199752062. [DOI] [Google Scholar]
- 23.Ghahremanzadeh R., Fereshtehnejad F., Bazgir A. Chromeno [2,3-d] pyrimidine-triones synthesis by a three-component coupling reaction. Chem. Pharm. Bull. 2010;58:516–520. doi: 10.1248/cpb.58.516. [DOI] [PubMed] [Google Scholar]
- 24.Jursic B.S., Neumann D.M. Preparation of 5,5′-pyrilidene and 5,5′-quinolidene bis-barbituric acid derivatives. J. Heterocycl. Chem. 2003;40:465–474. doi: 10.1002/jhet.5570400310. [DOI] [Google Scholar]
- 25.Barakat A., Al-Majid A.M., Al-Ghamdi A.M., Mabkhot Y.N., Rafiq Siddiqui M., Ghabbour H.A., Fun H.K. Tandem Aldol-Michael reactions in aqueous diethylamine medium: A greener and efficient approach to dimedone-barbituric acid derivatives. Chem. Cent. J. 2014;8 doi: 10.1186/1752-153X-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barakat A., Al-Majid A.M., Al-Najjar H.J., Mabkhot Y.N., Ghabbour H.A., Fun H.K. An efficient and green procedure for synthesis of rhodanine derivatives by Aldol-Thia-Michael protocol using aqueous diethylamine. RSC Adv. 2014;4:4909–4916. doi: 10.1039/c3ra46551a. [DOI] [Google Scholar]
- 27.Brewer A.D., Minatelli J.A., Plowman J., Paull K.D., Narayanan V.L. 5-(N-phenylcarboxamido)-2-thiobarbituric acid (NSC 336628), a novel potential antitumor agent. Biochem. Pharm. 1985;34:2047–2050. doi: 10.1016/0006-2952(85)90335-1. [DOI] [PubMed] [Google Scholar]
- 28.Al-Najjar H.J., Barakat A., Al-Majid A.M., Mabkhot Y.N., Weber M., Ghabbour H.A., Fun H.-K. A Greener, Efficient Approach to Michael Addition of Barbituric acid to Nitroalkene in Aqueous Diethylamine Medium. Molecules. 2014;19:1150–1162. doi: 10.3390/molecules19011150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Majid A.M., Barakat A., Al-Najjar H.J., Mabkhot Y.N., Ghabbour H.A., Fun H.K. Tandem Aldol-Michael reactions in aqueous diethylamine medium: A greener and efficient approach to bis-pyrimidine derivatives. Int. J. Mol. Sci. 2013;14:23762–23773. doi: 10.3390/ijms141223762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barakat A., Al-Majid A.M., Shahidu M.I., Warad I., Masand V.H., Yousuf S., Choudhary M.I. Molecular structure investigation and biological evaluation of Michael adducts derived from dimedone. Res. Chem. Intermed. 2016;42:4041–4053. doi: 10.1007/s11164-015-2257-1. [DOI] [Google Scholar]
- 31.Barakat A., Islam M.S., Al-Majid A.M., Al-Othman Z., Soliman S.M., Ghabbour H.A., Fun H.K. Synthesis of novel 5-monoalkylbarbiturates derivatives-new access to 1,2-oxazepines. Tetrahedron Lett. 2015;56:6984–6987. doi: 10.1016/j.tetlet.2015.10.108. [DOI] [Google Scholar]
- 32.Barakat A., Al-Majid A.M., Lotfy G., Arshad F., Yousuf S., Choudhary M.I., Ashraf S., Ul-Haq Z. Synthesis and dynamics studies of barbituric acid derivatives as urease inhibitors. Chem. Cent. J. 2015;9 doi: 10.1186/s13065-015-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaserer T., Obermoser V., Weninger A., Gust R., Schuster D. Evaluation of selected 3D virtual screening tools for the prospective identification of peroxisome proliferator-activated receptor (PPAR) γ partial agonists. Eur. J. Med. Chem. 2016;124:49–62. doi: 10.1016/j.ejmech.2016.07.072. [DOI] [PubMed] [Google Scholar]
- 34.Cichero E., Espinoza S., Franchini S., Guariento S., Brasili L., Gainetdinov R.R., Fossa P. Further insights into the pharmacology of the human trace amine-associated receptors: Discovery of novel ligands for TAAR1 by a virtual screening approach. Chem. Biol. Drug Des. 2014;84:712–720. doi: 10.1111/cbdd.12367. [DOI] [PubMed] [Google Scholar]
- 35.Sagatova A.A., Keniya M.V., Wilson R.K., Monk B.C., Tyndall J.D. Structural insights into binding of the antifungal drug fluconazole to Saccharomyces cerevisiae lanosterol 14α-demethylase. Antimicrob. Agents Chemother. 2015;59:4982–4989. doi: 10.1128/AAC.00925-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. [(accessed on 29 April 2016)]. Available online: http://www.rcsb.org/pdb/explore/explore.do?structureId=3Q70.
- 37.Blower T.R., Williamson B.H., Kerns R.J., Berger J.M. Crystal structure and stability of gyrase-fluoroquinolone cleaved complexes from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 2016;113:1706–1713. doi: 10.1073/pnas.1525047113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu J., Patel S., Sharma N., Soisson S.M., Kishii R., Takei M., Fukuda Y., Lumb K.J., Singh S.B. Structures of kibdelomycin bound to Staphylococcus aureus GyrB and ParE showed a novel U-shaped binding mode. ACS Chem. Biol. 2014;19:2023–2031. doi: 10.1021/cb5001197. [DOI] [PubMed] [Google Scholar]
- 39.BSAC Methods for Antimicrobial Susceptibility Testing—Version 14. [(accessed on 9 April 2015)]. Available online: http://bsac.org.uk/wp-content/uploads/2012/02/BSAC-Susceptibility-testing-version-14.pdf.
- 40.Fast Rigid Exhaustive Docking (FRED) Receptor. Federal Reserve Bank of St. Louis; Santa Fe, NM, USA: [(accessed on 29 April 2016)]. Version 2.2.5; OpenEye Scientific Software. Available online: http://www.eyesopen.com. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.