Abstract
Coumarins are naturally occurring oxygen heterocyclic compounds having multifarious medicinal properties, hence used as lead compounds for designing new potent analogs. The chromene butenoic acid 3 and the benzochromene butenoic acid 4 which are derived from the reaction of glyoxalic acid with 3-acetylcoumarin and 3-acetylbenzocoumarin, respectively, were reacted with different nitrogen and carbon nucleophiles to give new heterocyclic compounds. The structures of the prepared compounds were elucidated by IR, 1H-NMR, and mass spectroscopy. Some of the newly prepared compounds were tested in vitro against a panel of four human tumor cell lines namely; hepatocellular carcinoma (liver) HepG2, colon cancer HCT-116, human prostate cancer PC3, and mammary gland breast MCF-7. Also they were tested as antioxidants. Almost all of the tested compounds showed satisfactory activity.
Keywords: functionalized coumarin, antitumor activity, antioxidant activity
1. Introduction
Coumarins are an important class of compounds of both natural and synthetic origin. Many compounds which contain the coumarin moiety exhibit useful and diverse pharmaceutical and biological activities, often depending on the substituents they bear in the parent benzopyran moiety [1,2] and, there has been a growing interest in their synthesis [3]. Some of these coumarin derivatives have been found useful in photochemotherapy, antitumor [4], anti-HIV therapy [5,6], as CNS-stimulants [7], antibacterial [8,9,10], anticoagulants [11,12,13], antifungal [14,15], antioxidant [16] agents and as dyes [17]. Natural, semi-synthetic and synthetic coumarins are useful substances in drug research [18]. Coumarins can be used not only to treat cancer, but to treat the side effects caused by radiotherapy [19,20]. Coumarin derivatives can possess not only cytostatic, but cytotoxic properties as well [21], as they can inhibit growth in human cancer cell lines [22] such as A549 (lung), ACHN (renal), H727 (lung), MCF7 (breast) and HL-60 (leukemia) and in some clinical trials they exhibited anti-proliferative activity in prostate cancer [23] malignant melanoma [24] and renal cell carcinoma [25]. Coumarin itself also exhibited cytotoxic effects against Hep2 cells (human epithelial type 2) in a dose dependent manner and showed some typical characteristics of apoptosis with loss of membrane microvilli, cytoplasmic hypervacualization and nuclear fragmentation [26].
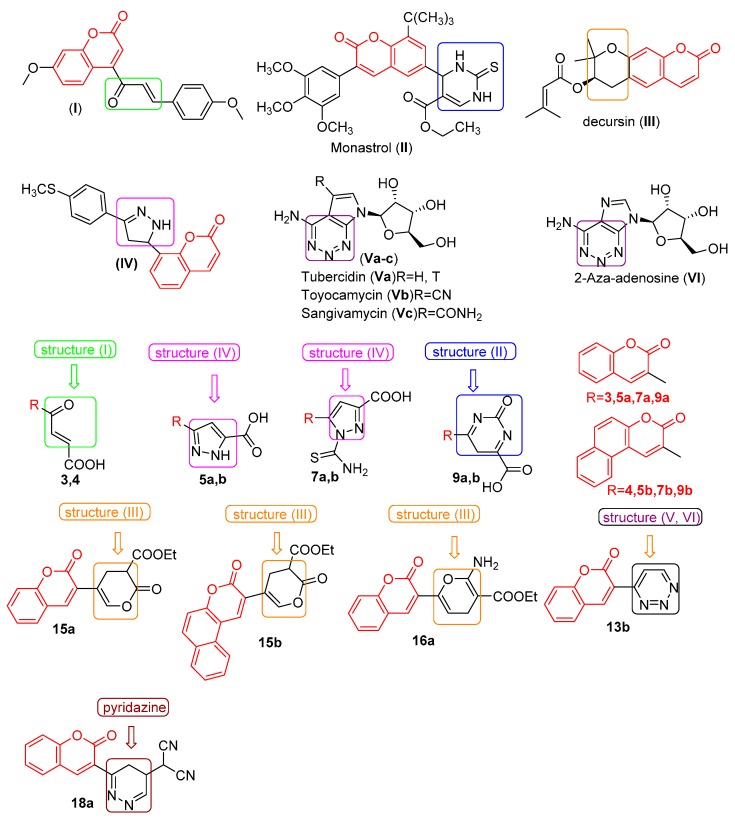
The hormone oestrogen plays the crucial role in the development of the breast cancer, the most frequent malignant disease in women. Therefore many therapies are designed to block its activity [27]. Cinnamoyl-coumarin derivatives were especially effective in oestrogen-dependent cancers, such as breast (MCF7) and ovarian (OVCAR) cancer cell lines. These compounds are selective nonsteroidal inhibitors of 14β-hydroxysteroid dehydrogenase type 1, an enzyme that catalyzes NADPH-dependent reduction of the weak oestrogen, oestrone, into the most potent oestrogen, oestradiol [28]. Seidel et al. [29] synthesized a series of coumarin derivatives which carry α,β-(mono- or bis)-unsaturated ketones at the C3 or C4 position such as compound I (Figure 1) which strongly inhibits proliferation in the chronic myeloid leukemia K-562 and histiocytic lymphoma U-937 cell lines. This compound also inhibited the activity of histone deacetylase (HDAC), an enzyme crucial in cancer development.
Figure 1.
Structure of some anticancer derivatives (I–VI) and some of the designed target compounds 3, 4, 5a,b, 7a,b, 9a,b, 13b, 15a,b, 16a and 18a.
Sashidhara et al. [27] developed new hybrid coumarin-monastrol molecules such as compound II (Figure 1) which showed the most potent selective activity against breast cancer cell lines MCF-7 and MDA–MB-231. This compound induced caspase-3 activation and apoptosis and caused arrest of MCF-7 cell cycle at G1 phase.
From Korean Angelica gigas root Kim et al. [30] isolated pyranocoumarin decursin III (Figure 1), which inhibited proliferation in bladder cancer 235J cells and also in colon cancer HCT-1116 cells. Decursin induced apoptosis in both cancer cell lines through expression of Bax protein and reduced Bcl-2 protein levels. Amin et al. [31] synthesized coumarins IV attached to pyrazoline rings (Figure 1) that have anticancer activity against the HepG2 cell line.
Drugs containing 1,2,3-triazine rings [32] originated from natural and synthetic sources are exemplified by tubercidin (Va), toyocamycin (Vb) and sangivamycin (Vc) (Figure 1), which have significant pharmacological activities. Tubercidin (Va) and its 5-substituted derivatives inhibit both DNA and RNA viruses at the concentrations that inhibit DNA, RNA and protein synthesis in mice and human cell lines. Toyocamycin (Vb) is a known antineoplastic antibiotic with specific antitumor activity. Sangivamycin (Vc) is active against L1210 leukemia, P338 leukemia and Lewis lung carcinoma and under clinical trials against colon cancer, gall bladder cancer and acute myelogenous leukemia in humans. 2-Azaadenosine VI (Figure 1) exhibits five times greater cytotoxicity than 8-azapurine against human epidermis carcinoma cells in vitro.
In continuation of our previous investigations [33,34,35,36,37], some heterocyclic compounds bearing the coumarin moiety were utilized herein for the synthesis of valuable heterocyclic ring systems having satisfactory antitumor and antioxidant activities.
2. Results and Discussion
2.1. Chemistry
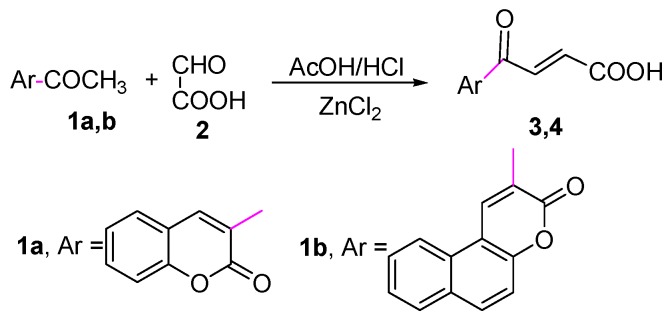
In the present investigation we report the syntheses of some new coumarin derivatives having antitumor and antioxidant activities. 4-Oxo-4-(2-oxo-2H-chromen-3-yl)but-2-enoic acid (3) was synthesized by Tolstoluzhsky et al. [38] using glyoxalic acid and acetic anhydride in the presence of ytterbium triflate as a catalyst under microwave conditions. Herein we synthesized the target compounds 3 and 4 by the reaction of 3-acetyl-2H-chromen-2-one (1a) and 2-acetyl-3H-benzo[f]-chromen-3-one (1b) with glyoxalic acid (2) in acetic acid/HCl to afford 4-oxo-4-(2-oxo-2H-chromen-3-yl)but-2-enoic acid (3) and 4-oxo-4-(3-oxo-3H-benzo[f]chromen-2-yl)but-2-enoic acid (4), respectively (Scheme 1). The IR spectra of compounds 3 and 4 showed characteristic absorption bands at 3446, 3476 cm−1 attributable to OH and also in the range 1730–1656 and 1742–1642 cm−1 attributable to C=O, respectively. 1H-NMR spectra of compounds 3 and 4 showed exchangeable signals at δ 12.51, 9.40 ppm, respectively, assigned to the OH protons. The mass spectra of compounds 3 and 4 showed the molecular ion peak at m/z 244 and a [M + 2]+ peak at m/z 296, respectively which coincide with the molecular weights supporting the proposed identity of the structures.
Scheme 1.
Synthesis of compounds 3 and 4.
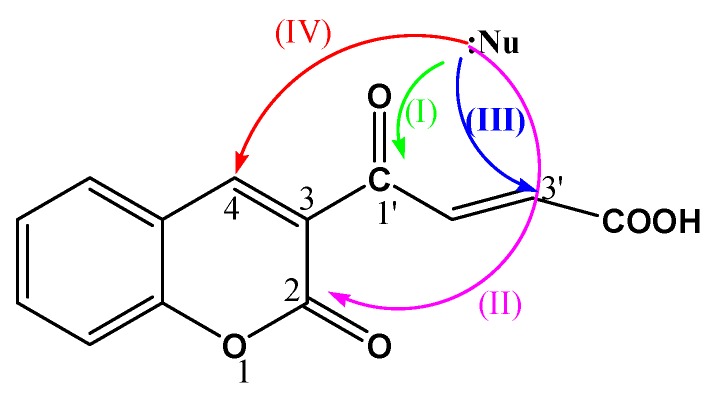
In principle, a nucleophile might be expected to attack a coumarin substrate at any of the electrophilic centers, C-1′ (I) C-2 (II), C-3′ (III) or C-4 (IV) as illustrated in Figure 2.
Figure 2.
Possible sites of nucleophile attack on coumarin derivatives.
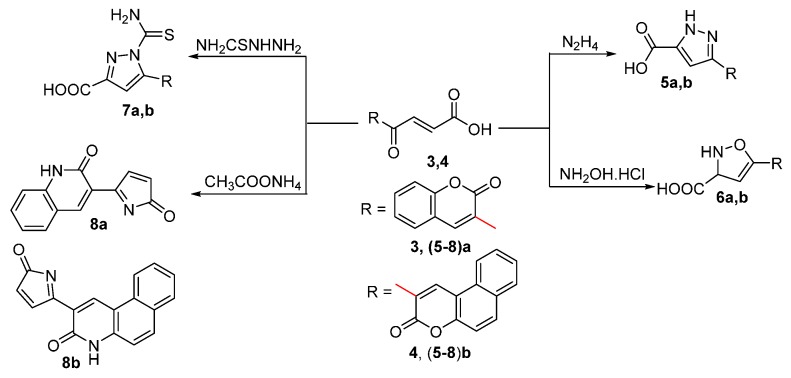
The reactions of compounds 3 and 4 with various nitrogen nucleophiles have been shown to proceed with a high degree of regioselective at the exocyclic acrylic center at C-3′ followed by exo-trig ring closure to afford the substituted coumarin products (Scheme 2). Aroylacrylic acid derivatives can be used in a wide range of the addition reaction. The strong electron-attracting power of the aryl carbonyl group enhances the reactivity of the adjacent double bond-function and promotes the nucleophilic addition at this center. Simultaneous or subsequent cyclization of adducts gives access to various 5- or 6-membered cyclic structures. All the structures were proved by spectroscopic studies.
Scheme 2.
Synthesis of compounds (5–8)a,b.
Compounds 3 and 4 were reacted with hydrazine hydrate [39] to yield the pyrazolecarboxylic acid derivatives 5a and 5b, respectively, via aza Michael addition followed by cyclization (Scheme 2). The spectroscopic data are consistent with the proposed structures. The IR spectra of compounds 5a and 5b showed bands for acidic OH and NH protons at 3440, 3217, 3400 and 3283 cm−1 respectively. The 1H-NMR spectra showed the appearance of OH protons at δ 11.03 and 12.82 ppm and NH protons at 7.01 and 6.99 ppm for compounds 5a and 5b respectively indicated the presence of the carboxylic and the NH groups.
Similarly, treatment of compounds 3 and 4 with hydroxylamine hydrochloride in pyridine [39] afforded compounds 6a and 6b. The structures of compounds 6a and 6b were deduced from their elemental analysis and spectral data. The IR spectra showed bands for OH, NH and C=O at 3393, 3380, 3204, 3128, 1720, 1728, 1710 and 1718 cm−1 respectively. The 1H-NMR spectra showed the presence of exchangeable OH and NH protons at δ 11.90, 11.52, 10.10 and 6.93 ppm respectively, beside signals for aromatic and =CH protons (cf. Experimental Section).
Compounds 3 and 4 were also reacted with thiosemicarbazide [40] to yield compounds 7a and 7b. The structures of compounds 7a and 7b were deduced by spectroscopic data. The IR spectra showed bands for OH, NH and C=O at 3455, 3382, 3397, 1711, 1712, 1647 and 1653 cm−1, respectively. The 1H-NMR spectra showed the presence of exchangeable OH, NH2 at δ 12.26, 12.0, 5.71 and 6.48 ppm, respectively, beside signals for aromatic and =CH protons (cf. Experimental Section).
Compounds 8a and 8b were formed when a mixture of compounds 3 and/or 4 was fused with ammonium acetate for 3h in the absence of solvent [41]. Proof of the structures of compounds 8a and 8b were based on spectral data. The IR spectra showed bands attributed to NH, and C=O in the range 3181–3385 and 1642–1713 respectively. Also the 1H-NMR showed signals at δ 9.57 and 9.71 ppm for NH protons beside signals in the range of 7.95–8.16, 6.97–8.06 ppm attributed to coumarin, aromatic and HC=CH protons, respectively. The ammonium acetate was reacted with the carboxylic acid group followed by ring closure, and also with the coumarin ring to give the oxopyrrolylquinolinone and the oxopyrrolylbenzoquinolinone derivatives 8a and 8b, respectively.
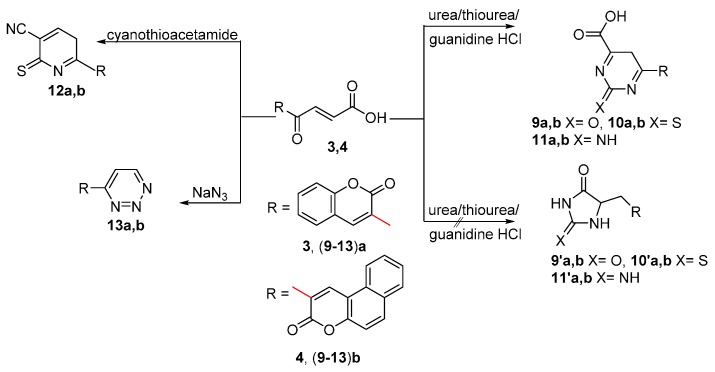
Furthermore, the reaction of compounds 3 and 4 with urea, thiourea [40] and guanidine hydrochloride in DMF afforded products whose spectra (IR, 1H-NMR and MS) and elemental analyses data are consistent with compounds (9–11)a,b rather than compounds (9′–11′)a,b (Scheme 3). The reaction took place via addition of the nitrogen nucleophile on the α-carbon atom followed by exo-trig ring closure on the ketonic carbonyl carbon atom and dehydrogenation to give compounds (9–11)a,b. The structures of compounds (9–11)a,b were confirmed by spectroscopic data. The other products (9′–11′)a,b were discounted on the basis of the spectral data. The IR spectra of compounds (9–11)a,b showed bands for OH and C=O in the range 3403–3455 and 1642–1653 cm−1 respectively. The 1H-NMR spectra showed the appearance of CH2 and OH protons in the range 1.20–1.23 and 9.78–11.23 ppm respectively for compounds (9–11)a,b which indicated the presence of the methylene and the carboxylic groups. The mass spectrum of compound 9a showed the molecular ion peak at m/z 284 (14.42%) which is in a good agreement with the molecular formula C14H8N2O5. Compound 10a failed to react with benzaldehyde in the presence of a mixture of acetic acid, acetic anhydride and zinc chloride, which is a good evidence for the existence of compound 10a and not 10′a.
Scheme 3.
Synthesis of compounds (9–13)a,b.
Cyanothioacetamide reacted with compounds 3 and 4 to give compounds 12a,b. Cyanothioacetamide [42] has three nucleophile sites: the methylene carbon, the amino group and the sulfur atom. On the whole, under cyclocondensation or cycloaddition conditions, cyanothio-acetamide acts as a C,N-, C,S- or S,N- binucleophile. In this case the reaction occurred via Michael addition reaction followed by ring closure, elimination of water and hydrogen molecules to give compounds 12a and 12b. The IR spectra showed bands attributable to C≡N and C=O groups at 2209, 2207, 1713 and 1705 cm−1 respectively, with the absence of bands attributable to OH and NH groups. The 1H-NMR spectra showed the presence of signals for aromatic, =CH, coumarinic hydrogen, and CH2 protons at 6.22–7.94, 6.81–7.95, 1.72 and 1.20 ppm, respectively. The mass spectra of compounds 12a and 12b showed the molecular ion peak at m/z 280 and a [M + 2]+ ion at m/z 332, respectively, which agree well with the suggested structures.
On the other hand, sodium azide reacted with compounds 3 and 4 to yield the triazine derivatives 13a and 13b. The reaction occurred via addition reaction on the carbon double bond followed by ring closure, elimination of water molecule and decarboxylation to give the stable compounds 13a and 13b. The structures of compounds 13a and 13b were confirmed based on spectral data. The IR showed bands for C=O and C=N at 1718, 1707 and 1631, 1624 cm−1, respectively and the absence of bands corresponding to OH and NH groups. The 1H-NMR spectra showed the absence of signals corresponding to NH and OH protons, and showed signals for coumarin, aromatic and two =CH protons in the range 8.89–6.42 ppm.
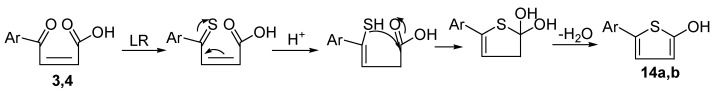
Lawesson’s reagent is a powerful, mild, and versatile thiation agent that efficiently converts oxygen functionalities into their thio-analogs. This reagent has been used for the cyclization of compounds containing at least two oxygen functionalities [43] Lawesson’s reagent acts as a sulfurizing agent as well as dehydrating agent. Compounds 3 and 4 were refluxed with Lawesson’s reagent in acetonitrile to give compounds 14a and 14b (Scheme 4).
Scheme 4.
Mechanistic route of compounds 14a,b.
The reaction seems to produce like Paal-Knorr synthesis mechanism to produce the thiophene ring (Scheme 4). Proof of the structures of compounds 14a and 14b were based on spectral data. The IR spectra showed bands attributed to OH and C=O at 3557, 3428 and 1724 cm−1, respectively. The 1H-NMR showed signals for aromatic, coumarin proton, two =CH and OH protons at 7.61–7.54, 7.37–8.64, 6.91, 6.96, 7.14 and 6.20 ppm respectively. The mass spectra of compounds 14a and 14b showed the molecular ion peaks at m/z 261 and 310, respectively, which coincide with their molecular weights.
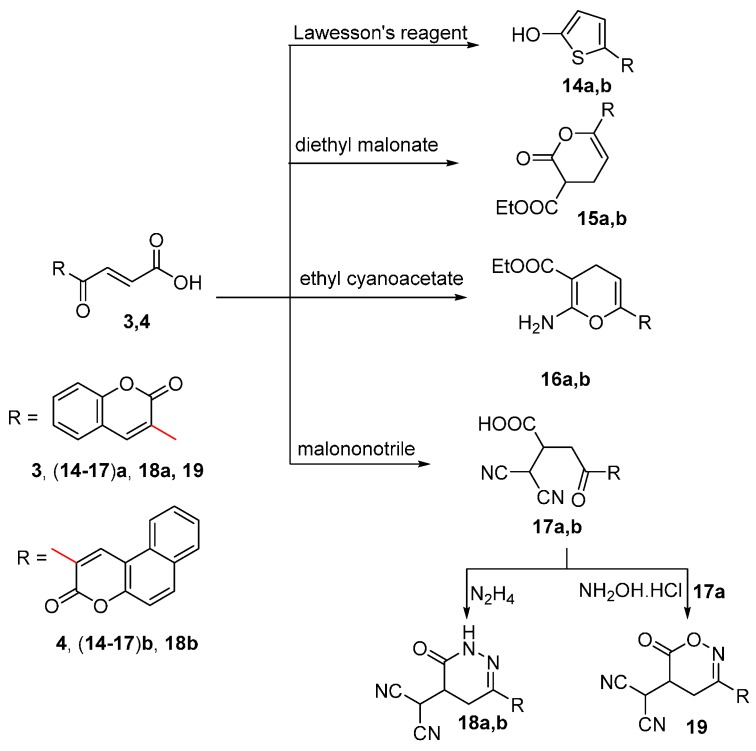
The present study was extended to investigate the chemical behavior of compounds 3 and 4 towards some acyclic carbon nucleophiles containing an active methylene group between two carbonyl groups (Scheme 5). There have been reports on Michael reactions catalyzed by K2CO3 under phase transfer catalysis [44]. To some extent these mild conditions can minimize the reversibility of the Michael addition reaction [45] and other side reactions, thus improved yields can be achieved. Therefore, boiling [39] compounds 3 and 4 with diethyl malonate and/or ethyl cyanoacetate under PTC reaction conditions in the presence of K2CO3 and tetrabutyl-n-ammonium bromide afforded compounds (15,16)a,b, respectively. The pyranone derivatives (15,16)a,b were formed via Michael addition reaction of the carbanion at the exo-double bond followed by cyclization and decarboxylation. The structures of compounds (15,16)a,b were elucidated by spectroscopic data. The IR of compounds 15a and 15b showed bands at 1715, 1716 cm−1 for ester groups, respectively. The 1H-NMR of compounds 15a and 15b showed triplet signals attributed to CH3 at 0.93 ppm and quartet signal for OCH2 at 3.16–3.18 and 3.12–3.18 ppm, respectively beside signals for CH2, CH, =CH and aromatic protons in the range 1.22–1.35, 1.50–1.62, 6.50–8.16 ppm, respectively. Also the spectra showed a signal for the coumarin proton at 8.59 and 8.53 ppm, respectively. The IR of compounds 16a and 16b showed bands at 3418, 3228, 3409 and 3242 cm−1 attributed to NH2 and also bands at 1712 cm−1 for C=O group. The 1H-NMR of compounds 16a and 16b showed signals for CH3, CH2, OCH2, NH2, =CH, aromatic and coumarin protons.
Scheme 5.
Synthesis of compounds 14–19.
Meanwhile the reaction of compounds 3 and 4 with malononitrile [46] under the same conditions afforded the open structures 17a and 17b, respectively. The structures of compounds 17a and 17b were elucidated by spectroscopic data. The IR spectra showed bands for OH, C≡N and C=O at 3434, 2209, 2210, 1646 (broad) and 1632 (broad) cm−1, respectively. Also the 1H-NMR spectra showed signals for OH protons at 12.22 and 12.56 ppm, respectively, in addition to signals attributed to CH, CH2, aromatic and coumarin protons. The structures of compounds 17a and 17b were deduced chemically by their reaction with hydrazine hydrate [46] and also the reaction of 17a with hydroxylamine hydrochloride to give compounds 18a, 18b and 19, respectively. The reaction was proceeded via condensation reaction of the NH2 group with the ketonic group followed by 6-exo-trig cyclization. The structures of compounds 18a, 18b and 19 were proved by spectroscopic tools. The IR spectra showed bands in the range 2207–2211, 1627–1713 cm−1 attributable to C≡N and C=O groups, respectively, in addition to bands at 3362 and 3399 cm−1 attributable to NH for compounds 18a and 18b, respectively. The 1H-NMR spectra of compounds 18a and 18b showed signals in the range 1.20–1.27, 1.90–1.97 and 2.21–2.75 ppm attributed to CH, CH2 and CH(CN)2, respectively, in addition to signals attributed to aromatic, coumarin and NH protons. The 1H-NMR spectrum of compound 19 showed signals at 1.22–1.25, 2.26–2.30, 2.72, 7.05–8.34 and 8.77 ppm attributed to CH2, 2CH, aromatic and coumarin protons, respectively.
2.2. Pharmacological Activity
2.2.1. Cytotoxic Activity Using an In Vitro Ehrlich Ascites Assay
Out of the newly synthesized compounds, twenty three analogs were selected to be evaluated for their in vitro cytotoxic effect against a panel of four human tumor cell lines namely: hepatocellular carcinoma (liver) HepG2, colon cancer HCT-116, human prostate cancer PC3, and mammary gland breast MCF-7 cancer cell lines (Table 1). In general, the activity observed by all of these molecules ranged from very strongly to weakly cytotoxic. The results revealed that 12 of the tested compounds (5a,b, 7a,b, 9a,b, 11b, 13b, 15a,b, 16a and 18a) exhibited varying degrees of inhibitory activity towards the four tested tumor cell lines, ranging from strong to very strong. As for the activity against hepatocellular carcinoma HePG2, the highest cytotoxic activity was displayed by compounds 7a,b and 15b which showed IC50 values of 10.8 ± 0.88, 9.3 ± 0.58 and 8.2 ± 0.45 μg/mL, respectively. Remarkably strong inhibitory activity ranging from 11.0–19.7 was also seen for compounds 5a, 9a,b, 13b 15a and 18a, while 3, 5b, 10a, 11b, 16a,b and 19 also showed moderate activity and 4, 10b, 11a, 13a, 17a,b and 18b had weak activity.
Table 1.
Cytotoxicity (IC50) of the tested compounds on different cell lines.
| Comp. No. | IC50 (μg/mL) a | |||
|---|---|---|---|---|
| HePG2 | HCT-116 | PC3 | MCF-7 | |
| 3 | 41.2 ± 3.06 | 36.5 ± 02.54 | 42.0 ± 3.24 | 45.8 ± 3.40 |
| 4 | 51.2 ± 3.62 | 33.6 ± 2.64 | 48.9 ± 2.91 | 39.7 ± 2.35 |
| 5a | 12.5 ± 0.69 | 12.8 ± 1.03 | 16.2 ± 1.56 | 15.7 ± 1.24 |
| 5b | 27.6 ± 1.87 | 21.8 ± 2.10 | 34.0 ± 2.65 | 15.8 ± 1.08 |
| 7a | 10.8 ± 0.88 | 10.4 ± 0.94 | 9.6 ± 0.382 | 10.6 ± 0.92 |
| 7b | 9.3 ± 0.58 | 4.8 ± 0.18 | 11.1 ± 1.13 | 7.8 ± 0.67 |
| 9a | 13.1 ± 0.95 | 9.4 ± 0.97 | 14.5 ± 1.30 | 12.0 ± 1.14 |
| 9b | 11.0 ± 0.98 | 11.4 ± 0.87 | 18.0 ± 1.96 | 20.4 ± 1.56 |
| 10a | 35.7 ± 2.54 | 46.3 ± 2.35 | 51.6 ± 3.61 | 29.9 ± 1.97 |
| 10b | 63.5 ± 3.94 | 56.4 ± 3.35 | 83.8 ± 3.58 | 64.4 ± 3.84 |
| 11a | 73.0 ± 4.35 | 78.2 ± 3.98 | 96.8 ± 4.87 | 81.5 ± 4.21 |
| 11b | 22.6 ± 1.36 | 19.8 ± 1.63 | 25.5 ± 1.74 | 5.6 ± 0.43 |
| 13a | 52.9 ± 3.62 | 65.1 ± 4.11 | 76.9 ± 4.65 | 58.3 ± 3.63 |
| 13b | 17.4 ± 1.25 | 14.4 ± 1.01 | 10.8 ± 0.79 | 29.6 ± 1.87 |
| 15a | 17.6 ± 1.05 | 20.0 ± 1.44 | 13.7 ± 0.94 | 17.6 ± 1.37 |
| 15b | 8.2 ± 0.45 | 9.7 ± 0.84 | 8.7 ± 0.45 | 14.1 ± 1.21 |
| 16a | 25.6 ± 1.67 | 15.3 ± 1.13 | 13.4 ± 0.96 | 19.9 ± 2.14 |
| 16b | 26.2 ± 1.13 | 29.6 ± 2.25 | 35.4 ± 2.13 | 40.2 ± 2.64 |
| 17a | 90.6 ± 6.57 | 71.1 ± 4.82 | 87.4 ± 5.14 | 86.7 ± 6.15 |
| 17b | 52.7 ± 3.41 | 44.7 ± 3.12 | 59.8 ± 2.35 | 45.9 ± 2.89 |
| 18a | 19.7 ± 1.20 | 13.8 ± 0.89 | 16.1 ± 1.08 | 13.2 ± 0.76 |
| 18b | 60.1 ± 3.24 | 72.6 ± 3.86 | 82.2 ± 4.32 | 62.5 ± 4.35 |
| 19 | 25.2 ± 2.10 | 22.4 ± 1.37 | 28.8 ± 2.67 | 25.8 ± 1.72 |
| 5-FU | 7.9 ± 0.12 | 5.3 ± 0.14 | 8.3 ± 0.25 | 5.4 ± 0.21 |
a IC50 (μg/mL): 1–10 (very strong), 11–20 (strong), 21–50 (moderate), 51–100 (weak), above 100 (non-cytotoxic).
As for the activity against colon cancer HCT-116 cell line, the highest cytotoxic activities were displayed by compounds 7a,b, 9a and 15b which showed IC50 at 10.4 ± 0.94, 4.8 ± 0.18, 9.4 ± 0.97 and 9.7 ± 0.84 μg/mL, respectively. Compound 7b showed higher activity than the reference (5-Fu 5.3 ± 0.14). Remarkable strong inhibitory activities were also demonstrated by compounds 5a, 9b, 11b, 13b, 15a, 16a and 18a ranging 10.4–20.0 μg/mL. Also compounds 3, 4, 5b, 10a, 16b, 17b and 19 showed moderate activity, while compounds 10b, 11a, 13a, 17a and 18b showed weak activity.
Compounds 7a, 13b and 15b were found to be the most potent derivatives overall the tested compounds against human prostate cancer cell line PC3 with IC50 9.6 ± 0.82, 10.8 ± 0.79 and 8.7 ± 0.45 μg/mL, respectively. Compound 15b is almost equipotent as 5-fluorouracil (IC50 = 8.3 ± 0.25 μg/mL). Also compounds 5a, 7b, 9a,b, 15a, 16a and 18a were strongly active with IC50 = 16.2 ± 1.56, 11.1 ± 1.13, 14.5 ± 1.30, 18.0 ± 1.96 13.7 ± 0.94, 13.4 ± 0.96 and 16.1 ± 1.08 μg/mL, respectively. On the other hand compounds 3, 4, 5b, 11b, 16b and 19 showed moderate activity, while compounds 10a,b, 11a, 13a, 17a,b and 18b showed weak activity.
Whilst compounds 7a,b and 11b showed very strong activity towards mammary gland (breast) MCF-7 with IC50 10.6 ± 0.92, 7.8 ± 0.67 and 5.6 ± 0.43 μg/mL, respectively, compounds 5a,b, 9a,b 15a,b, 16a and 18a displayed strong activity, with IC50 = 15.7 ± 1.24, 15.8 ± 1.08, 12.0 ± 1.14, 20.4 ± 1.56, 17.6 ± 1.37, 14.1 ± 1.21, 19.9 ± 2.14 and 13.2 ± 0.76 μg/mL, respectively. On the other hand compounds 3, 4, 10a, 13b, 16b, 17b and 19 showed moderate activity, while compounds 10b, 11a, 13a, 17a and 18b showed weak activity. Compound 11b is almost equipotent to 5-fluorouracil (IC50 = 5.4 ± 0.21 μg/mL). Compounds containing the coumarin ring showed higher cytotoxic activity than their analogs containing the benzocoumarin ring (the relative viabilities of cells (%) for the reference and the tested compounds are listed in Tables S1 and S2 and Figures S1–S24 as Supplementary Materials).
2.2.2. Antioxidant Activity Using ABTS Inhibition
Twenty three compounds were tested for antioxidant activity reflected as the ability to inhibit oxidation in rat brain and kidney homogenates (Table 2).
Table 2.
Antioxidant activity and bleomycin-dependent DNA damage for the tested compounds a.
| Comp. No. | Antioxidant Activity (ABTS Method) | Bleomycin Dependent DNA Damage | |
|---|---|---|---|
| Absorbance | Inhibition (%) | ||
| 3 | 0.233 | 51.6 | 0.095 |
| 4 | 0.134 | 72.9 | 0.114 |
| 5a | 0.145 | 69.9 | 0.107 |
| 5b | 0.078 | 84.4 | 0.089 |
| 7a | 0.119 | 75.3 | 0.089 |
| 7b | 0.076 | 84.6 | 0.083 |
| 9a | 0.150 | 68.9 | 0.099 |
| 9b | 0.076 | 84.6 | 0.096 |
| 10a | 0.236 | 51.0 | 0.126 |
| 10b | 0.217 | 56.2 | 0.131 |
| 11a | 0.248 | 49.9 | 0.142 |
| 11b | 0.077 | 84.4 | 0.072 |
| 13a | 0.244 | 49.4 | 0.145 |
| 13b | 0.076 | 84.6 | 0.105 |
| 15a | 0.201 | 58.3 | 0.094 |
| 15b | 0.118 | 75.5 | 0.076 |
| 16a | 0.195 | 59.5 | 0.089 |
| 16b | 0.232 | 51.9 | 0.106 |
| 17a | 0.290 | 39.8 | 0.18 |
| 17b | 0.152 | 69.3 | 0.127 |
| 18a | 0.164 | 66.0 | 0.081 |
| 18b | 0.274 | 43.1 | 0.125 |
| 19 | 0.213 | 55.8 | 0.124 |
| Ascorbic acid | 0.053 | 89.0 | 0.073 |
a All experiments were performed three times. The data are expressed as the mean-standard error of the mean (S.E.M.).
Compounds 5b, 7b, 9b, 11b and 13b showed very high inhibitions of 84.4, 84.6, 84.6, 84.4 and 84.6%, respectively. Compounds 4, 5a, 7a, 9a, 15b, 17b and 18a showed high inhibitions of 72.9, 69.9, 75.3, 68.9, 75.5, 69.3 and 66.0%, respectively. In addition the rest of the compounds 3, 10a,b, 11a, 13a, 15a, 16a,b, 17a, 18b and 19 exhibited moderate to weak antioxidant activity ranging from 59.5–39.8%. Compounds containing the benzocoumarin ring showed higher antioxidant activity than their analogs containing the coumarin ring.
2.2.3. Bleomycin-Dependent DNA Damage
Bleomycins are a family of glycopeptide antibiotics routinely used as antitumor agents. The bleomycin assay has been adopted for assessing the pro-oxidant effect of food antioxidants. The antitumor antibiotic bleomycin binds iron ions and DNA. The bleomycin-iron complex degrades DNA when heated with thiobarbituric acid (TBA) to yield a pink chromogen. Upon the addition of suitable reducing agents the antioxidant competes with DNA and diminishes chromogen formation [47].
To show the mechanism of action of the twenty three tested compounds, their protective activity against DNA damage induced by the bleomycin-iron complex was examined. The results (Table 2) showed that compounds 11b (0.072) and 15b (0.076) were equipotent to ascorbic acid (0.073). Consequently, they have the ability to protect DNA from the damage induced by bleomycin. Compounds 3, 5b, 7a,b, 9a,b, 15a, 16a and 18a meanwhile showed high protection against DNA damage induced by the bleomycin-iron complex ranged from 0.081–0.099. On the other hand, the rest of the compounds exhibited weak activities. Thus, all the tested compounds diminish the chromogen formation between the damage DNA and TBA with different activity. Compounds containing the benzocoumarin ring showed higher activity against DNA damage than their analogs containing the coumarin rings.
2.2.4. Structure Activity Relationships
The antitumor activity of natural and synthetic coumarin derivatives has been extensively explored by many researchers [30,48,49,50] and it has been proven that coumarins, depending on their structure, can act on various tumor cells by different mechanisms, inhibiting the enzyme telomerase, protein kinase activity and downregulating oncogene expression or inducing caspase-9-mediated apoptosis, suppressing cancer cell proliferation by arresting the cell cycle in G0/G1 phase, G2/M phase and affecting the p-groups of cancer cells [51,52].
By comparing the experimental cytotoxicity of the compounds reported in this study to their structures, the following structure activity relationships (SAR) are postulated:
The cytotoxic activity of compounds 3, 4 is due to the presence of the coumarin moiety and also the formation of intermolecular hydrogen bonds between the OH [53] and DNA bases.
The cytotoxic activity of compounds 7a,b is due to the presence of the coumarin moiety and also the formation of intermolecular hydrogen bonds of OH and NH2 groups with DNA bases [52]. Introducing the pyrazole carbothioamide moiety [25] also enhances the cytotoxic activity of compounds 7a,b.
The cytotoxic activity of compounds 5a,b is due to the presence of the coumarin and the pyrazole carboxylic acid moieties [42] and also the formation of intermolecular hydrogen bonds between the OH and DNA bases. Compounds 7a,b demonstrated better activity compared to compounds 5a,b, probably due to the presence of the carbothioamide (S=CNH2) moiety.
Compounds 9a,b showed strong activity due to the presence of the coumarin and the pyridazinone rings.
Compounds 15a,b and 16a showed strong and very strong activity due to the presence of the coumarin and the pyran rings.
Compounds 18a showed strong activity due to the presence of the coumarin and the pyridazine rings, and also the presence of two cyano groups.
3. Experimental Section
3.1. General Information
All melting points were measured on a Gallenkamp melting point apparatus and are uncorrected. The infrared spectra were recorded using potassium bromide disks on a Mattson FTIR infrared spectrophotometer (Mattson, New York, NY, USA). 1H-NMR spectra were run at 300 MHz, on a Varian Mercury VX-300 NMR spectrometer (Bruker, Rheinstetten, Germany), using TMS as an internal standard in deuterated dimethylsulphoxide. Chemical shifts δ are quoted in ppm and J in Hz. The mass spectra were recorded on a GCMS-QP-1000EX mass spectrometer (Shimadzu, Kyoto, Japan) at 70 e.V. All the spectral measurements were carried out at the Microanalytical Center of Cairo University, Cairo, Egypt and the Main Defense Chemical Laboratory, Cairo, Egypt. The elemental analyses were carried out at the Microanalytical center of Ain Shams University, Cairo, Egypt. The pharmaceutical activity assays were carried out at Pharmacology Department, Faculty of Pharmacy, EL-Mansoura University, EL-Mansoura, Egypt. All the chemical reactions were monitored by TLC.
3.2. Synthesis
3.2.1. General Procedure for the Synthesis of Compounds 3 and 4
A mixture of compound 1 and/or 2 (0.012 mol) and glyoxalic acid (0.81 g, 0.011 mol) in glacial acetic acid (20 mL) containing HCl (1–2 mL) in the presence of ZnCl2 (0.5 g) was heated under reflux for 8 h. The reaction mixture was cooled and then poured on hot water to dissolve the ZnCl2. The separated solid was filtered off and washed with water, dried and recrystallized from the proper solvent to give compounds 3 and 4, respectively.
4-Oxo-4-(2-oxo-2H-chromen-3-yl)but-2-enoic Acid (3). Pale brown crystals; yield (1.8 g, 74%); m.p. 200–203 °C; methanol. IR (KBr) cm−1: 3446 (OH), 1730–1656 (C=O), 1609 (C=C). 1H-NMR (DMSO-d6) δ: 7.19–8.66 (m, 6H, 4ArH, 2H olefinic), 9.58 (s, 1H, coumarin), 12.51 (s, 1H, OH D2O exchangeable). MS m/z: 244 ([M]+) (42.9), 199 (42.9), 145 (78.6), 132 (21.4), 131 (71.4), 92 (57.1), 78 (14.3). Anal. Calcd. for C13H8O5: C, 63.94; H, 3.30. Found: C, 63.81; H, 3.32.
4-Oxo-4-(3-oxo-3H-benzo[f]chromen-2-yl)but-2-enoic Acid (4). Brown crystals; yield (2.12 g, 72.2%); m.p. 229–231 °C; dioxane/H2O (3:1). 1H-NMR (DMSO-d6) δ: 7.60–8.11 (m, 8H, 6 ArH, 2H olefinic), 8.34 (s, 1H, coumarin), 9.40 (s, 1H, OH D2O exchangeable). IR (KBr) cm−1: 3476 (OH), 1742–1642 (C=O), 1627 (C=C). MS m/z: 296 ([M + 2]+) (4.78), 249 (7.19), 224 (10.25), 223 (76.82), 198 (14.10), 196 (100.0), 181 (24.30), 168 (63.44), 165 (23.65), 156 (35.56), 153 (17.16), 144 (66.47), 127 (41.76), 72 (23.55), 62 (23.57), 56 (20.29), 46 (25.09). Anal. Calcd. for C17H10O5: C, 69.39; H, 3.43. Found: C, 69.45; H, 3.46.
3.2.2. General Procedure for the Synthesis of Compounds 5a and 5b
A mixture of compound 3 and/or 4 (0.01 mol), hydrazine hydrate, (0.50 mL, 0.01 mol) in DMF (20 mL) was refluxed for 3 h, cool, poured on water, The solid that separated was filtered off, dried and recrystallized from the proper solvent to give compounds 5a and 5b respectively.
3-(2-Oxo-2H-chromen-3-yl)-1H-pyrazole-5-carboxylic Acid (5a). Brown crystals; yield (2.24 g, 87.5%); m.p. > 300 °C; DMF/H2O (3:1). 1H-NMR (DMSO-d6) δ: 7.01 (br.s, 1H, NH D2O exchangeable,), 6.97–7.96 (m, 5H, 4ArH, =CH), 9.01 (s, 1H, coumarin), 11.03 (br.s, 1H, OH D2O exchangeable). IR (KBr) cm−1: 3440 (OH), 3217 (NH), 1720 (C=O coumarin), 1674 (C=O acid), 1625 (C=N). MS m/z: 256 ([M]+) (0.00), 240 (10.52), 149 (10.71), 147 (6.00), 136 (39.95), 122 (8.53), 120 (28.24), 93 (66.40), 91 (100), 74 (8.10), 72 (22.02), 60 (40.16), 58 (12.49), 56 (44.58), 54 (50.41), 46 (20.89). Anal. Calcd. for C13H8N2O4: C, 60.94; H, 3.15; N, 10.93. Found: C, 60.79; H, 18; N, 10.95.
3-(3-Oxo-3H-benzo[f]chromen-2-yl)-1H-pyrazole-5-carboxylic Acid (5b). Brown crystals; yield (2.47 g, 81%); m.p. > 300 °C; methanol. 1H-NMR (DMSO-d6) δ: 6.99 (br.s, 1H, NH, D2O exchangeable,), 7.11–8.10 (m, 7H, 6ArH, =CH), 8.86 (s, 1H, coumarin), 12.82 (s, 1H, OH, D2O exchangeable). IR (KBr) cm−1: 3400 (OH), 3283 (NH), 1722 (C=O coumarin), 1671 (C=O acid), 1625 (C=N). MS m/z (%): 306 ([M]+) (0.00), 262 (0.22), 169 (25.84), 170 (11.60), 144 (28.16), 143 (61.74), 128 (18.61), 127 (11.35), 116 (21.79), 115 (100), 114 (20.20), 68 (0.60). Anal. Calcd. for : C17H10N2O4: C, 66.67; H, 3.29; N, 9.15. Found: C, 66.69; H, 3.30; N, 9.16.
3.2.3. S General Procedure for the Synthesis of Compounds 6a and 6b
A mixture of compound 3 and/or 4 (0.01 mol), and hydroxylamine hydrochloride (0.69 g, 0.01 mol) in pyridine (20 mL) was refluxed for 3 h, cool, poured on ice/HCl, The solid that separated was filtered off, dried and recrystallized from the proper solvent to give compounds 6a and 6b respectively.
5-(2-Oxo-2H-chromen-3-yl)-2,3-dihydroisoxazole-3-carboxylic Acid (6a). Reddish brown crystals; yield (1.49 g, 57.9%); m.p. 230–232°C; methanol/H2O (2:1). 1H-NMR (DMSO-d6) δ: 4.05 (d, 1H, CH), 6.91–7.80 (m, 5H, 4ArH, =CH), 9.05 (s, 1H, coumarin), 10.10 (s, 1H, NH D2O exchangeable), 11.90 (s, 1H, OH, D2O exchangeable). IR (KBr) cm−1: 3393 (OH), 3204 (NH), 1720 (C=O coumarin), 1710 (C=O acid), 1610 (C=N). MS m/z: 260 ([M + 1]+) (4.98), 243 (15.22), 215 (25.33), 146 (39.80), 118 (32.05), 115 (29.27), 104 (19.70), 91 (52.46), 78 (69.66), 71 (47.66), 57 (68.36), 55 (57.48), 46 (43.66), 45 (100). Anal. Calcd. for C13H9NO5: C, 60.24; H, 3.50; N, 5.40. Found: C, 61.12; H, 3.49; N, 5.37.
5-(3-Oxo-3H-benzo[f]chromen-2-yl)-2,3-dihydroisoxazole-3-carboxylic Acid (6b). Pale brown crystals; yield (1.79 g, 58.25%); m.p. 174–175 °C; methanol. 1H-NMR (DMSO-d6) δ: 4.13 (d, 1H, CH), 6.93 (s, 1H, NH D2O exchangeable), 7.18–8.79 (m, 7H, 6ArH, =CH), 9.03 (s, 1H, coumarin), 11.52 (s, 1H, OH D2O exchangeable). IR (KBr) cm−1: 3380 (OH), 3128 (NH), 1728 (C=O coumarin), 1718 (C=O acid), 1627 (C=N). MS m/z: 309 ([M]+) (0.56), 170 (13.27), 144 (11.23), 141 (18.27), 140 (17.02), 115 (22.51), 114 (33.80), 79 (100), 78 (17.90), 71 (16.18), 70 (15.40), 57 (30.65). Anal. Calcd. for C17H11NO5: C, 66.02; H, 3.58; N, 4.53. Found: C, 66.11; H, 3.60; N, 4.52.
3.2.4. General Procedure for the Synthesis of Compounds 7a and 7b
A mixture compound 3 and/or 4 (0.01 mol), thiosemicarbazide hydrochloride, (1.27 g, mL, 0.01 mol) in DMF (20 mL) was refluxed for 3 h, cool, poured on water, The solid that separated was filtered off, dried and recrystallized from the proper solvent to give compounds 7a and 7b respectively.
1-Carbamothioyl-5-(2-oxo-2H-chromen-3-yl)-1H-pyrazole-3-carboxylic Acid (7a). Brown crystals; yield (2.32 g, 73.8%); m.p. 253–255 °C; DMF/H2O (3:1). 1H-NMR (DMSO-d6) δ: 55.71 (s, 2H, NH2, D2O exchangeable), 6.93–7.52 (m, 5H, ArH, =CH), 7.94 (s, 1H, coumarin), 12.26 (s, 1H, OH, D2O exchangeable). IR (KBr) νmax/cm−1: 3455 (OH), 3406, 3382 (NH2), 1711 (C=O coumarin), 1647 (C=O acid), 1607 (C=N). MS m/z: 315 ([M]+) (0.00), 314 ([M − 1]+) (3.07) 299 (2.35), 145 (3.65), 117 (3.85), 74 (6.31), 73 (100), 60 (25.43), 58 (15.94), 57 (82.07). Anal. Calcd for C14H9N3O4S: C, 53.33; H, 2.88; N, 13.33; S, 10.17. Found: C, 53.70; H, 2.78; N, 13.40; S, 10.05.
1-Carbamothioyl-5-(3-oxo-3H-benzo[f]chromen-2-yl)-1H-pyrazole-3-carboxylic Acid (7b). Brown crystals; yield (2.38 g, 65.4%); m.p. 220–223 °C; DMF/H2O (3:1). 1H-NMR (DMSO-d6) δ: 6.48 (s, 2H, NH2, D2O exchangeable), 7.26–8.62 (m, 7H, 6ArH, =CH) 8.65 (s,1H coumarin), 12.0 (s, 1H, OH, D2O exchangeable). IR (KBr) cm−1: 3455 (OH), 3419, 3397 (NH2), 1712 (C=O coumarin), 1653 (C=O acid), 1625 (C=N). MS m/z: 367 ([M + 2]+) (0.93), 340 (12.65), 115 (18.67), 73 (100), 58 (9.22). Anal. Calcd for C18H11N3O4S: C, 59.17; H, 3.03; N, 11.50; S, 8.78. Found: C, 59.28; H, 3.05; N, 11.53; S, 8.80.
3.2.5. General Procedure for the Synthesis of Compounds 8a and 8b
A mixture of compound 3 and/or 4 (0.01 mol) and ammonium acetate (1.54 g, 0.02 mol) was fused at 150–170 °C for 4 h. The reaction mixture poured onto ice/HCl. The solid that separated was filtered off, dried and recrystallized from the proper solvent to give compounds 8a and 8b respectively.
3-(5-Oxo-5H-pyrrol-2-yl)quinolin-2(1H)-one (8a). Brown crystals; yield (1.69 g, 75.4%); m.p. > 300 °C; DMF/H2O (3:1). 1H-NMR (DMSO-d6) δ: 4.91 (d, 1H, CH=CHCO), 5.12 (d, 1H, CH=CHCO), 7.00–7.95 (m, 5H, 4ArH,1H, coumarin); 9.57 (s, 1H, NH, D2O exchangeable). IR (KBr) cm−1:3181 (NH), 1710, 1660 (C=O), 1607 (C=N). MS m/z: 224 ([M]+) (0.00), 225 ([M + 1]+) (21.10), 145 (20.13), 104 (12.53), 81 (24.42), 73 (100), 57 (41.93). Anal. Calcd for C13H8N2O2: C, 69.64; H, 3.60; N, 12.49. Found: C, 69.80; H, 3.78; N, 12.46.
2-(5-Oxo-5H-pyrrol-2-yl)benzo[f]quinolin-3(4H)-one (8b). Brown crystals; yield (1.89 g, 69%); m.p. 254–256 °C; DMF/EtOH (2:1). 1H-NMR (DMSO-d6) δ: 7.06–8.16 (m, 8H, 6ArH, 1H, coumarin, 2H, 2=CH), 9.71 (s, 1H, NH, D2O exchangeable). IR (KBr) cm−1:1713, 1642 (C=O), 1626 (C=N). MS m/z: 274 ([M]+) (0.00), 196 (46.29), 195 (13.87), 170 (16.14), 156 (22.43), 128 (33.78), 80 (100), 74 (6.75). Anal. Calcd. for C17H10N2O2: C, 74.44; H, 3.67; N, 10.21. Found: C, 74.63; H, 3.64; N, 10.31.
3.2.6. General Procedure for the Synthesis of Compounds (9–12)a,b
A mixture of compound 3 and/or 4 (0.01 mol), urea, thiourea, guanidine hydrochloride and cyano thioactamide (0.01 mol) in DMF (20 mL) was refluxed for 3 h, cool, poured on water, The solid that separated was filtered off, dried and recrystallized from the proper solvent to give compounds 9–12(a,b), respectively.
2-Oxo-6-(2-oxo-2H-chromen-3-yl)-2,5-dihydropyrimidine-4-carboxylic Acid (9a). Brown crystals; yield (2.08 g, 73.4%); m.p. 237–239 °C; dioxane/H2O (3:1). 1H-NMR (DMSO-d6) δ: 1.22 (s, 2H, CH2 methylene), 7.03–8.59 (m, 4H, ArH), 9.01 (s, 1H, coumarin), 11.23 (s, 1H, OH exchangeable with D2O). IR (KBr) cm−1: 3455 (OH), 1720–1674 (C=O), 1624 (C=N), 1610 (C=C). MS m/z: 284 ([M]+, 14.42), 240 (15.34), 212 (23.51), 190 (14.49), 184 (16.77), 170 (14.97), 98 (37.20), 60 (100). Anal. Calcd. for C14H8N2O5: C, 59.16; H, 2.84; N, 9.86. Found: C, 59.20; H, 2.90; N, 9.75.
2-Oxo-6-(3-oxo-3H-benzo[f]chromen-2-yl)-2,5-dihydropyrimidine-4-carboxylic Acid (9b). Brown crystals; yield (2.18 g, 65.47%); m.p. 274–276 °C; DMF/H2O (3:1). 1H-NMR (DMSO-d6) δ: 1.20 (s, 2H, CH2 methylene), 6.61–8.93 (m, 6H, ArH), 9.05 (s, 1H, coumarin), 10.25 (s, 1H, OH, D2O exchangeable). IR (KBr) cm−1: 3437 (OH), 1720–1642 (C=O), 1626 (C=N). MS m/z: 334 ([M]+, 0.00), 289 (47.5), 196 (27.51), 170 (10.0), 158 (42.5), 96 (44.3), 80 (100). Anal. Calcd. for C18H10N2O5: C, 64.67; H, 3.02; N, 8.38. Found: C, 64.82; H, 3.04; N, 8.35.
6-(2-Oxo-2H-chromen-3-yl)-2-thioxo-2,5-dihydropyrimidine-4-carboxylic Acid (10a). Brown crystals; m.p. 150–153 °C; yield (2.38 g, 79.4%); dioxane/H2O (3:1). 1H-NMR (DMSO-d6) δ: 1.22 (s, 2H, CH2 methylene), 6.89–8.59 (m, 4H, ArH), 9.00 (s, 1H, coumarin), 11.23 (s, 1H, OH exchangeable with D2O). IR (KBr) cm−1: 3384 (OH), 1720 (C=O coumarin), 1708 (C=O acid), 1627 (C=N), 1610 (C=C), 1223 (C=S). MS m/z: 300 ([M]+) (0.96), 257 (13.01), 256 (16.03), 225 (11.13), 213 (12.16), 185 (10.60), 150 (21.69), 149 (17.38), 136 (18.33), 119 (15.30), 111 (14.99), 79 (12.65), 72 (13.48), 70 (37.28), 68 (14.13), 67 (21.49), 69 (100). Anal. Calcd. for C14H8N2O4S: C, 56.00; H, 2.69; N, 9.33; S, 10.68. Found: C, 55.98; H, 2.70; N, 9.32; S, 10.67.
6-(3-Oxo-3H-Benzo[f]chromen-2-yl)-2-thioxo-2,5-dihydropyrimidine-4-carboxy-lic Acid (10b). Brown crystals; yield (3.08 g, 88%); m.p. 249–251 °C; DMF/H2O (3:1). 1H-NMR (DMSO-d6) δ: 1.23 (s, 2H), 6.90–8.05 (m, 6H), 8.75 (s, 1H), 9.78 (s, 1H). IR (KBr) cm−1: 3368, 1714, 1708, 1627, 1213. MS m/z: 352 ([M + 2]+), 322 (10.21), 310 (8.57), 287 (15.06), 212 (22.06), 197 (21.27), 186 (17.49), 170 (15.20), 155 (13.28), 98 (99.00), 72 (25.98), 65 (100), 56 (46.47). Anal. Calcd. for C18H10N2O4S: C, 61.71; H, 2.88; N, 8.00; S, 9.15. Found: C, 61.80; H, 2.87; N, 7.89; S, 9.14.
2-Imino-6-(2-oxo-2H-chromen-3-yl)-2,5-dihydropyrimidine-4-carboxylic Acid (11a). Deep brown crystals; yield (1.88 g, 66.4%); m.p. 228–231 °C; dioxane/H2O (3:1); 1H-NMR (DMSO-d6) δ: 1.23 (s, 2H, CH2 methylene), 6.90–8.05 (m, 6H, ArH), 8.75 (s, 1H, coumarin), 9.78 (s, 1H, OH, exchangeable with D2O). IR (KBr) cm−1: 3368 (OH), 1714(C=O coumarin), 1708 (C=O acid), 1627 (C=N), 1213 (C=S). MS m/z: 352 ([M + 2]+), (7.49), 322 (10.21), 310 (8.57), 287 (15.06), 212 (22.06), 197 (21.27), 186 (17.49), 170 (15.20), 155 (13.28), 98 (99.00), 72 (25.98), 65 (100), 56 (46.47). Anal. Calcd. for C14H9N3O4: C, 59.37; H, 3.20; N, 14.84. Found: C, 59.35; H, 3.21; N, 14.85.
2-Imino-6-(3-oxo-3H-benzo[f]chromen-2-yl)-2,5-dihydropyrimidine-4-carboxylic Acid (11b). Deep brown crystals; yield (2.48 g, 74.6%); m.p. 155–157 °C; DMF. 1H-NMR (DMSO-d6) δ: 1.21 (s, 2H, CH2), 2.46 (s, 1H, NH, D2O exchangeable), 7.17–8.21 (m, 8H, 7Ar-H, 1H coumarin). IR (KBr) cm−1: 3418 (OH), 3063 (NH), 1728 (C=O coumarin), 1709 (C=O acid). MS m/z: 335 ([M + 2]+), (0.95), 196 (46.29), 198 (38.27), 139 (46.56), 128 (38.16), 116 (19.92), 74 (31.27), 63 (100), 58 (70.77). Anal. Calcd. for C18H11N3O4: C, 64.86; H, 3.33; N, 12.61. Found: C, 64.80; H, 3.29; N, 12.54.
6-(2-Oxo-2H-chromen-3-yl)-2-thioxo-2,5-dihydropyridine-3-carbonitrile (12a). Brown crystals; yield (1.67 g, 59.8%); m.p. > 300 °C. 1H-NMR (DMSO-d6) δ: 1.27 (d, 2H, CH2), 6.22–7.94 (m, 6H, 4ArH, =CH, 1H, coumarin). IR (KBr) cm−1: 2209 (C≡N), 1713 (C=O). MS m/z: 280 ([M]+) (2.14), 167 (33.41), 149 (100), 71 (30.33). Anal. Calcd. for C15H8N2O2S: C, 64.27; H, 2.88; N, 9.99; S, 11.44. Found: C, 64.30; H, 2.87; N, 10.02; S, 11.43.
6-(3-Oxo-3H-benzo[f]chromen-2-yl)-2-thioxo-2,5-dihydropyridine-3-carbonitrile (12b). Brown crystals; yield (1.49 g, 45.2%); m.p. > 300 °C; DMF/H2O (3:1). 1H-NMR (DMSO-d6) δ: 1.20 (d, 2H, CH2), 6.81–7.95 (m, 8H, 6ArH, =CH, 1H, coumarin). IR (KBr) cm−1: 2207 (C≡N), 1705 (C=O). MS m/z: 332 ([M + 2]+) (2.97), 211 (13.22), 139 (20.64), 57 (92.51), 55 (100). Anal. Calcd. for C19H10N2O2S: C, 69.08; H, 3.05; N, 8.48; S, 9.71. Found: C, 69.15; H, 3.03; N, 8.46; S, 9.70.
3.2.7. General Procedure for the Synthesis of Compounds 13a,b
A mixture of compound 3 and/or 4 (0.01 mol), and sodium azide (0.65 g, 0.01 mol) in DMF (30 mL) was refluxed for 3 h, The mixture was poured onto iced water and the solid obtained was filtered off, dried and recrystallized from the proper solvent to give compounds 13a and 13b, respectively.
3-(1,2,3-Triazin-4-yl)-2H-chromen-2-one (13a). Pale brown crystals; yield (2.00 g, 89.2%); m.p. 270–272 °C; dioxane/H2O (3:1). 1H-NMR (DMSO-d6) δ: 6.60–8.20 (m, 6H, 4ArH, 2=CH), 8.89 (s, 1H, coumarin). IR (KBr) cm−1: 1718 (C=O), 1631 (C=N). MS m/z: 227 ([M + 2]+) (16.37), 200 (24.66), 199 (25.61), 189 (22.85), 175 (21.17), 157 (20.19), 147 (23.03), 145 (28.23), 133 (25.42), 124 (19.35), 110 (24.98), 103 (27.33), 92 (23.60), 85 (19.39), 74 (13.75), 73 (100). Anal. Calcd. for C12H7N3O2: C, 64.00; H, 3.13; N, 18.66. Found: C, 64.16; H, 3.14; N, 18.69.
2-(1,2,3-Triazin-4-yl)-3H-benzo[f]chromen-3-one (13b). Brown crystals; yield (1.72 g, 62.7%); m.p. > 300 °C; DMF/H2O (3:1). 1H-NMR (DMSO-d6) δ: 6.42–8.16 (m, 8H, 6ArH, 2=CH), 8.22 (s, 1H, coumarin). IR (KBr) cm−1:1707 (C=O), 1624(C=N). MS m/z: 275 ([M]+) (0.09), 196 (0.98), 98 (98.39), 80 (100), 66 (12.96), 64 (97.95). Anal. Calcd. for C16H9N3O2: C, 69.81; H, 3.30; N, 15.27. Found: C, 69.87; H, 3.29; N, 15.24.
3.2.8. General Procedure for the Synthesis of Compounds 14a,b
A mixture of compound 3 and/or 4 (0.01 mol) and Lawesson’s reagent (4.0 g, 0.01 mol) was refluxed in acetonitrile (30 mL) for 28 h. The reaction mixture was filtered while hot and the filtrate was left to cool at room temperature. The obtained solid was filtered off, dried and recrystallized from the proper solvent to give compounds 14a and 14b respectively.
3-(5-Hydroxythiophen-2-yl)-2H-thiochromen-2-one (14a) Pale brown crystals; yield (1.99 g, 76.9%); m.p. 250–253 °C; ethanol/DMF (2:1). 1H-NMR (DMSO-d6) δ: 6.91 (d, 1H, =CH), 7.14 (s, 1H, OH D2O exchangeable), 7.54–7.61 (m, 6H, 4ArH, =CH, 1H coumarin). IR (KBr) cm−1 3557 (OH), 1724 (C=O), 1602 (C=C). MS m/z: 261 ([M + 1]+) (0.13), 188 (100), 108 (62.03), 94 (19.60), 92 (16.20), 77 (39.43), 62 (33.50), 46 (28.69). Anal. Calcd. for C13H8O2S2: C, 59.98; H, 3.10; S, 24.63. Found: C, 59.91; H, 3.13; S, 24.66.
2-(5-Hydroxythiophen-2-yl)-3H-benzo[f]thiochromen-3-one (14b). Brown crystals; yield (1.76 g, 56.9%); m.p. > 300 °C; DMF/H2O (3:1). 1H-NMR (DMSO-d6) δ: 6.20 (s, 1H, OH, D2O exchangeable), 6.93 (d, 1H, J = 3.00, =CH), 6.96 (d, 1H, J = 3.00, =CH), 7.37–8.64 (m, 6H, ArH), 9.28 (s, 1H, coumarin). IR (KBr) cm−1 3428 (OH), 1724 (C=O), 1630 (C=C). MS m/z (%): 310 ([M]+) (8.69), 282 (13.31), 172 (12.06), 128 (11.51), 86 (22.08), 84 (48.17), 70 (25.06), 57 (100). Anal. Calcd. for C17H10O2S2: C, 65.78; H, 3.25; S, 20.66. Found: C, 59.95; H, 3.23; S, 20.65.
3.2.9. General Procedure for the Synthesis of Compounds (15–17)a,b
A mixture of compound 3 and/or 4 (0.01 mol), diethyl malonate, ethyl cyanoacetate and malononitrile (0.01 mol) in dry acetone (30 mL) was refluxed on a water bath for 24 h in the presence of K2CO3 (2.76 g, 0.02 mole) and tetrabutyl-n-ammonium bromide (0.64 g, 0.002 mol). The excess solvent was evaporated and the reaction mixture was poured into water. The separated solid was filtered off, dried and recrystallized from the suitable solvent to give compounds (15–17)a,b.
Ethyl 2-Oxo-5-(2-oxo-2H-chromen-3-yl)-3,4-dihydro-2H-pyran-3-carboxylate (15a). Brown crystals; yield (2.12 g, 67.8%); m.p. > 300 °C; acetic acid/water (2:1). 1H-NMR (DMSO-d6) δ: 0.93 (t, 3H, CH3), 1.22–1.35 (m, 2H, CH2), 1.50–1.62 (m, 1H, CH), 3.16–3.18 (q, 2H, OCH2), 6.50–8.16 (m, 5H, 4ArH, =CH), 8.59 (s, 1H, coumarin). IR (KBr) cm−1 1715 (C=O). Anal. Calcd. for C17H14O6: C, 64.97; H, 4.49. Found: C, 64.90; H, 4.47.
Ethyl 2-Oxo-5-(3-oxo-3H-benzo[f]chromen-2-yl)-3,4-dihydro-2H-pyran-3-carboxylate (15b). Brown crystals; yield (2.35 g, 64.8%); m.p. > 300 °C; toluene. 1H-NMR (DMSO-d6) δ: 0.93 (t, 3H, CH3, J = 7.5 Hz), 1.23–1.34 (m, 2H, CH2), 1.50–1.63 (m, 1H, CH), 3.10–3.18 (q, 2H, OCH2, J = 7.5 Hz), 7.15 (t, 1H, =CH), 7.12–8.08 (m, 6H, ArH), 8.53 (s, 1H, coumarin). IR (KBr) cm−1 1716, broad 1625 (C=O). Anal. Calcd. for C21H16O6: C, 69.23; H, 4.43. Found: C, 69.25; H, 4.41.
Ethyl 2-amino-6-(2-oxo-2H-chromen-3-yl)-4H-pyran-3-carboxylate (16a). Brown crystals; yield (2.31 g, 74%); m.p. > 300 °C; ethanol/toluene (2:1). 1H-NMR (DMSO-d6) δ: 1.05 (t, 3H, CH3, J = 7.2 Hz), 2.72–2.74 (m, 2H, CH2), 3.14–3.18 (q, 2H, OCH2, J = 7.2 Hz), 4.00 (s, 2H, NH2, D2O exchangeable), 6.96–7.90 (m, 6H, 4ArH, =CH, CH coumarin). IR (KBr) cm−1: 3418, 3228 (NH2), 1712 (C=O). Anal. Calcd. for C17H15NO5: C, 65.17; H, 4.83; N, 4.47. Found: C, 65.10; H, 4.81; N, 4.48.
Ethyl 2-Amino-6-(3-oxo-3H-benzo[f]chromen-2-yl)-4H-pyran-3-carboxylate (16b). Brown crystals; yield (2.61 g, 72%); m.p. > 300 °C; acetic acid/water (2:1). 1H-NMR (DMSO-d6) δ: 0.92 (t, 3H, CH3, J = 7.2 Hz), 1.23–1.33 (m, 2H, CH2), 2.57 (s, 2H, NH2, D2O exchangeable), 3.12–3.18 (q, 2H, OCH2, J = 7.2 Hz), 6.58 (t, 1H, =CH), 7.00–8.50 (m, 6H, ArH), 8.95 (s, 1H, coumarin). IR (KBr) cm−1: 3409, 3242 (NH2), 1712 (C=O). Anal. Calcd. for C21H17NO5: C, 69.41; H, 4.72; N, 3.85. Found: C, 69.46; H, 4.71; N, 3.86.
2-(Dicyanomethyl)-4-oxo-4-(2-Oxo-2H-chromen-3-yl)butanoic Acid (17a). Brown crystals; yield (2.41 g, 78%); m.p. > 300 °C; ethanol. 1H-NMR (DMSO-d6) δ: 2.24–2.28 (m, 1H, CH), 2.72–2.76 (m, 2H,CH2), 2.83–2.88 (m, 1H, CH), 7.41–8.44 (m, 4H, ArH), 8.56 (s, 1H, coumarin), 12.22 (s, 1H, OH, D2O exchangeable). IR (KBr) cm−1: 3434 (OH), 2209 (C≡N), 1708, 1646 (C=O). Anal. Calcd. for C16H10N2O5: C, 61.94; H, 3.25; N, 9.03. Found: C, 61.99; H, 3.24; N, 9.13.
2-(Dicyanomethyl)-4-oxo-4-(3-oxo-3H-benzo[f]chromen-2-yl)butanoic Acid (17b). Brown crystals; yield (2.59 g, 72.2%); m.p. > 300 °C; DMF. 1H-NMR (DMSO-d6) δ: 2.63–2.67 (m, 1H, CH), 2.72–2.79 (m, 2H, CH2), 2.88–2.91 (m, 1H, CH), 7.04–8.64 (m, 4H, ArH), 8.93 (s, 1H, coumarin), 12.56 (s, 1H, OH, D2O exchangeable). IR (KBr) cm−1: 3434 (OH), 2210 (C≡N), 1713, 1632 (C=O). Anal. Calcd. for C20H12N2O5: C, 66.67; H, 3.36; N, 7.77. Found: C, 66.64; H, 3.37; N, 7.79.
3.2.10. General Procedure for the Synthesis of Compounds 18a and 18b
A mixture of compounds 17a,b (3.10 g, 0.01 mol), hydrazine hydrate (0.50 mL, 0.01 mol) in DMF (20 mL) was refluxed for 3 h, cool, poured on water, The solid that separated was filtered off, dried and recrystallized from the proper solvent to give compounds 18a and 18b respectively.
2-(3-Oxo-6-(2-oxo-2H-chromen-3-yl)-2,3,4,5-tetrahydropyridazin-4-yl)malononitrile (18a). Brown crystals; yield (2.40 g, 83%); m.p. > 300 °C; AcOH/H2O (3:1). 1H-NMR (DMSO-d6) δ: 1.23–1.27 (m, 1H, CH), 1.90–1.95 (m, 2H, CH2), 2.71 (d, 1H, CH), 6.50–8.00 (m, 4H, ArH), 8.13 (s, 1H, coumarin), 8.60 (s, 1H, NH, D2O exchangeable). IR (KBr) cm−1: 3362 (NH), 2211 (C≡N), 1627 (C=O). Anal. Calcd. for C16H10N4O3: C, 62.74; H, 3.29; N, 18.29. Found: C, 62.75; H, 3.27; N, 18.28.
2-(3-Oxo-6-(3-oxo-3H-benzo[f]chromen-2-yl)-2,3,4,5-tetrahydropyridazin-4-yl)malononitrile (18b). Brown crystals; yield (2.70 g, 79.6%); m.p. > 300 °C; DMF. 1H-NMR (DMSO-d6) δ: 1.20–1.22 (m, 1H, CH), 1.92–1.97 (m, 2H, CH2), 2.21 (d, 1H, CH), 6.56–8.25 (m, 6H, ArH), 8.13 (s, 1H, coumarin), 8.54 (s, 1H, NH, D2O exchangeable). IR (KBr) cm−1: 3399 (NH), 2207 (C≡N), 1627 (C=O). Anal. Calcd. for C20H12N4O3: C, 67.41; H, 3.39; N, 15.72. Found: C, 67.42; H, 3.38; N, 15.73.
3.2.11. Synthesis of 2-(6-Oxo-3-(2-oxo-2H-chromen-3-yl)-5,6-dihydro-4H-1,2-oxazin-5-yl)malon-onitrile 19
A mixture of compound 17 (3.10 g, 0.01 mol), and hydroxylamine hydrochloride (0.69 g, 0.01 mol) in pyridine (20 mL) was refluxed for 3 h, cool, poured onto ice/HCl, The solid that separated was filtered off, dried and recrystallized from ethanol/benzene to give compound 19 as brown crystals; yield (2.01 g, 65.5%); m.p. > 300 °C; ethanol/benzene (2:1). 1H-NMR (DMSO-d6) δ: 1.22–1.25 (d, 2H, CH2), 2.26–2.30 (m, 1H, CH), 2.72 (d, 1H, CH), 7.05–8.34 (m, 4H, ArH), 8.77 (s, 1H, coumarin). IR (KBr) cm−1: 2209 (C≡N), 1713, 1627 (C=O). Anal. Calcd. for C16H9N3O4: C, 62.54; H, 2.95; N, 13.68; Found: C, 62.51; H, 2.93; N, 13.66.
3.3. Pharmacological Activity
3.3.1. Cytotoxicity Assay
The cytotoxic activity of twenty two compounds was tested against four human tumor cell lines namely: hepatocellular carcinoma (liver) HePG2, colon cancer HCT-116, human (prostate) cancer PC3 and mammary gland (breast) MCF-7. The cell lines were obtained from ATCC via the Holding company for biological products and vaccines (VACSERA, Cairo, Egypt). 5-Fluorouracil was used as a standard anticancer drug for comparison. The reagents used were RPMI-1640 medium, MTT, DMSO and 5-fluorouracil (Sigma Co., St. Louis, MO, USA), and fetal bovine serum (GIBCO, Paisely, UK).
MTT Assay
The different cell lines [54,55] mentioned above were used to determine the inhibitory effects of compounds on cell growth using the MTT assay. This colorimetric assay is based on the conversion of the yellow tetrazolium bromide (MTT) to a purple formazan derivative by mitochondrial succinate dehydrogenase in viable cells. The cells were cultured in RPMI-1640 medium with 10% fetal bovine serum. Antibiotics added were 100 units/mL penicillin and 100 µg/mL streptomycin at 37 °C in a 5% CO2 incubator. The cells lines were seeded [56] in a 96-well plate at a density of 1.0 × 104 cells/well at 37 °C for 48 h under 5% CO2. After incubation the cells were treated with different concentration of compounds and incubated for 24 h. After 24 h of drug treatment, 20 µL of MTT solution at 5 mg/mL was added and incubated for 4 h. Dimethyl sulfoxide (DMSO) in volume of 100 µL is added into each well to dissolve the purple formazan formed. The colorimetric assay is measured and recorded at absorbance of 570 nm using a plate reader (EXL 800, BioTech, Winooski, VT, USA). The relative cell viability in percentage was calculated as (A570 of treated samples/A570 of untreated sample) × 100.
3.3.2. Antioxidant Assay
ABTS Method
For each of the investigated compounds [57,58,59] of ABTS solution (60 µM, 2 mL) was added to MnO2 suspension (25 mg/mL, 3 mL), all prepared in aqueous phosphate buffer solution (pH 7, 0.1 M, 5 mL). The mixture was shaken, centrifuged, filtered and the absorbance of the resulting green blue solution (ABTS radical solution) at 734 nm was adjusted to approx. ca. 0.5. Then, asolution (50 µL of (2 mM) of the tested compound in spectroscopic grade MeOH/phosphate buffer (1:1) was added. The absorbance was measured and the reduction in color intensity was expressed as inhibition percentage. L–ascorbic acid was used as standard antioxidant (positive control). Blank sample was run without ABTS and using MeOH/phosphate buffer (1:1) instead of the tested compounds. Negative control was run with ABTS and MeOH/phosphate buffer (1:1) only.
Bleomycin—Dependent DNA Damage Assay
To the reaction mixtures [60,61] in a final volume of 1.0 mL, the following reagents were added: DNA (0.2 mg/mL), bleomycin sulfate (0.05 mg/mL), FeCl3 (0.025 mM), magnesium chloride (5 mM), KH2PO4–KOH buffer pH 7.0 (30 mM), and ascorbic acid (0.24 mM) or the test fractions diluted in MeOH to give a concentration of (0.1 mg/mL). The reaction mixtures were incubated in a water bath at 37 °C for 1 h. At the end of the incubation period, 0.1 mL of ethylenediaminetetraacetic acid (EDTA) (0.1 M) was added to stop the reaction (the iron-EDTA complex is unreactive in the bleomycin assay). DNA damage was assessed by adding 1 mL 1% (w/v) thiobarbituric acid (TBA) and 1 mL of 25% (v/v) hydrochloric acid followed by heating in a water-bath maintained at 80 °C for 15 min. The chromogenic formed was extracted into 1-butanol, and the absorbance was measured at 532 nm.
4. Conclusions
The objective of the present study was to synthesize the coumarin scaffold-based compounds and evaluate their cytotoxicity, antioxidant and bleomycin dependent DNA damage protection activities. The tested compounds showed very strong to non-cytotoxic activity against four anticancer cell lines. The best results were observed for compounds 5a, 7a,b, 9a,b, 13b, 15a,b, 16a and 18a. Compound 15b showed activity approximately equal to that of 5-FU as a standard against PC3 and compound 7b showed higher activity than the 5-FU against HCT-116.
Acknowledgments
Technical support from Department of Chemistry, Faculty of Science, Ain Shams University is gratefully acknowledged.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/2/249/s1.
Author Contributions
The authors contributed equally to this work.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Musa M.A., Badisa V.L., Latinwo L.M., Cooperwood J., Sinclair A., Abdullah A. Cytotoxic activity of new acetoxycoumarin derivatives in cancer cell lines. Anticancer Res. 2011;31:2017–2022. [PMC free article] [PubMed] [Google Scholar]
- 2.Borah P., Naidu P.S., Bhuyan P.J. Synthesis of some tetrazole fused pyrido[2,3-c]coumarin derivatives from a one-pot three-component reaction via intramolecular 1,3-dipolar cycloaddition reaction of azide to nitriles. Tetrahedron Lett. 2012;53:5034–5037. doi: 10.1016/j.tetlet.2012.07.060. [DOI] [Google Scholar]
- 3.El-Ansary S.L., Abbas S.E., Mikhael A.N., El-Banna H.A. Synthesis and biological activity of some new coumarins. Egypt. J. Pharm. Sci. 1992;33:639–650. [Google Scholar]
- 4.Manfredini S., Daniele S., Ferroni R., Bazzanini R., Vertuani S., Hatse S., Balzarini J., de Clercq E. retinoic acid conjugates as potential antitumor agents: synthesis and biological activity of conjugates with Ara-A, Ara-C, 3(2H)-furanone, and aniline mustard moieties. J. Med. Chem. 1997;40:3851–3857. doi: 10.1021/jm9602322. [DOI] [PubMed] [Google Scholar]
- 5.Wattenberg L.W., Lam K.T., Fladmoe A.V. Inhibition of chemical carcinogen-induced neoplasia by coumarins and α-angelicalactone. Cancer Res. 1979;39:1651–1654. [PubMed] [Google Scholar]
- 6.Kashman Y., Gustafson K.R., fuller R.W., Cardellina J.H., McMahon J.B., Currens M.J., Buckheit R.W., Hughes S.H., Craqq G.M., Boyd M.R. The calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum. J. Med. Chem. 1992;35:2735–2743. doi: 10.1021/jm00093a004. [DOI] [PubMed] [Google Scholar]
- 7.Mckee T.C., Fuller R.W., Covington C.D., Cardellina J.H., Gulakowski R.J., Krepps B.L., McMahon J.B., Boyd M.R. New pyranocoumarins isolated from Calophyllum lanigerum and Calophyllum teysmannii. J. Nat. Prod. 1996;59:754–758. doi: 10.1021/np9603784. [DOI] [PubMed] [Google Scholar]
- 8.Anjum N.F., Aleem A., Nayeem N., Asdaq S.M. Synthesis and antibacterial activity of substituted 2-phenyl-4-chromones. Der Pharma Chem. 2011;3:56–62. [Google Scholar]
- 9.De Souza S.M., Delle Monache F., Smânia A., Jr. Antibacterial activity of coumarins. Z. Naturforsch. C. 2005;60:693–700. doi: 10.1515/znc-2005-9-1006. [DOI] [PubMed] [Google Scholar]
- 10.Behrami A. Antibacterial activity of coumarine derivatives synthesized from 4-chloro-chromen-2-one. The comparison with standard drug. Orient. J. Chem. 2014;30:1747–1752. doi: 10.13005/ojc/300433. [DOI] [Google Scholar]
- 11.Jung J., Kim J., Park O. Simple and cost effective syntheses of 4-hydroxycoumarin. Synth. Commun. 1999;29:3587–3595. doi: 10.1080/00397919908085993. [DOI] [Google Scholar]
- 12.Barker W.M., Hermodson M.A., Link K.P. 4-Hydroxycoumarins. Synthesis of the metabolites and some other derivatives of warfarin. J. Med. Chem. 1971;14:167–169. doi: 10.1021/jm00284a022. [DOI] [PubMed] [Google Scholar]
- 13.Greaves M. Pharmacogenetics in the management of coumarin anticoagulant therapy: The way forward or an expensive diversion? PLoS Med. 2005;2:e342. doi: 10.1371/journal.pmed.0020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montagner C., de Souzaa S.M., Groposo C., Delle Monacheb F., Smaˆnia E.F.A., Smaˆnia A., Jr. Antifungal activity of coumarins. Z. Naturforsch. C. 2008;63:21–28. doi: 10.1515/znc-2008-1-205. [DOI] [PubMed] [Google Scholar]
- 15.De Araújo R.S.A., Guerra F.Q.S., Lima E., De Simone C.A., Tavares J.F., Scotti L., Scotti M.T., De Aquino T.M., De Moura R.O., Mendonça F.J.B., et al. Synthesis, structure-activity relationships (SAR) and in silico studies of coumarin derivatives with antifungal activity. Int. J. Mol. Sci. 2013;14:1293–1309. doi: 10.3390/ijms14011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzone G., Malaj N., Galano A., Russo N., Toscano M. Antioxidant properties of several coumarin–chalcone hybrids from theoretical insights. RSC Adv. 2015;5:565–575. doi: 10.1039/C4RA11733F. [DOI] [Google Scholar]
- 17.Raboin J., Beley M., Kirsch G. Pyridine-fused coumarins: A new class of ligands for ruthenium complexes with enhanced spectral absorption. Tetrahedron Lett. 2000;4:1175–1177. doi: 10.1016/S0040-4039(99)02255-8. [DOI] [Google Scholar]
- 18.Karatzas N.B. Coumarins, a class of drugs with a unique contribution to medicine: The tale of their discovery. Hellenic J. Cardiol. 2014;55:89–91. [PubMed] [Google Scholar]
- 19.Agarwal R. Synthesis and biological screening of some novel coumarin derivatives. Biochem. Pharmacol. 2000;6:1042–1051. [Google Scholar]
- 20.Marshall M.E., Butler K., Hermansen D. Treatment of hormone-refractory stage D carcinoma of prostate with coumarin (1,2-benzopyrone) and cimetidine: A pilot study. Prostate. 1990;17:95–99. doi: 10.1002/pros.2990170203. [DOI] [PubMed] [Google Scholar]
- 21.Benci K., Mandić L., Suhina T., Sedić M., Klobučar M., Pavelić S.K., Pavelić K., Wittine K., Mintas M. Novel coumarin derivatives containing 1,2,4-triazole, 4,5-dicyanoimidazole and purine moieties: Synthesis and evaluation of their cytostatic activity. Molecules. 2012;17:11010–11025. doi: 10.3390/molecules170911010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marshall M.E., Kervin K., Benefield C., Umerani A., Albainy-Jenei S., Zhao Q., Khazaeli M.B. Growth-inhibitory effects of coumarin (1,2-benzopyrone) and 7-hydroxycoumarin on human malignant cell lines in vitro. J. Cancer Res. Clin. Oncol. 1994;120:S3–S10. doi: 10.1007/BF01377114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohler J.L., Gomella L.G., Crawford E.D., Glode L.M., Zippe C.D., Fair W.R., Marshall M.E. Phase II evaluation of coumarin (1,2-benzopyrone) in metastatic prostatic carcinoma. Prostate. 1992;20:123–131. doi: 10.1002/pros.2990200208. [DOI] [PubMed] [Google Scholar]
- 24.Thornes R.D., Daly L., Lynch G., Breslin B., Browne H., Browne H.Y., Corrigan T., Daly P., Edwards G., Gaffney E., et al. Treatment with coumarin to prevent or delay recurrence of malignant melanoma. J. Cancer Res. Clin. Oncol. 1994;120:S32–S34. doi: 10.1007/BF01377122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall M.E., Butler K., Fried A. Phase I evaluation of coumarin (1,2-benzopyrone) and cimetidine in patients with advanced malignancies. Mol. Biother. 1991;3:170–178. [PubMed] [Google Scholar]
- 26.Mirunalini S., Deepalakshmi K., Manimozhi J. Antiproliferative effect of coumarin by modulating oxidant/antioxidant status and inducing apoptosis in Hep2 cells. Biomed. Aging Pathol. 2014;4:131–135. doi: 10.1016/j.biomag.2014.01.006. [DOI] [Google Scholar]
- 27.Sashidhara K.V., Avula S.R., Sharma K., Palnati G.R., Bathula S.R. Discovery of coumarin monastrol hybrid as potential antibreast tumor-specific agent. Eur. J. Med. Chem. 2013;60:120–127. doi: 10.1016/j.ejmech.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 28.Jamier V., Marut W., Valente S., Chereau C., Chouzenoux S., Nicco C., Lemarechal H., Weill B., Kirsch G., Jacob C., et al. Chalcone-coumarin derivatives as potential anticancer drugs: An in vitro and in vivo investigation. Anticancer Agents Med. Chem. 2014;14:963–974. doi: 10.2174/1871520613666131224124445. [DOI] [PubMed] [Google Scholar]
- 29.Seidel C., Schnekenburger M., Zwergel C., Gaascht F., Mai A., Dicato M., Kirsch G., Valente S., Diederich M. Novel inhibitors of human histone deacetylases: Design, synthesis and bioactivity of 3-alkenoylcoumarines. Bioorg. Med. Chem. Lett. 2014;24:3797–3801. doi: 10.1016/j.bmcl.2014.06.067. [DOI] [PubMed] [Google Scholar]
- 30.Kim W.J., Lee S.J., Choi Y.D., Moon S.K. Decursin inhibits growth of human bladder and colon cancer cells via apoptosis, G1-phase cell cycle arrest and extracellular signal-regulated kinase activation. Int. J. Mol. Med. 2010;25:635–641. doi: 10.3892/ijmm_00000386. [DOI] [PubMed] [Google Scholar]
- 31.Amin K.M., Abou-Seri S.M., Awadallah F.M., Eissa A.A.M., Hassan G.S., Abdulla M.M. Synthesis and anticancer activity of some 8-substituted-7-methoxy-2H chromen-2-one derivatives toward hepatocellular carcinoma HepG2 cells. Eur. J. Med. Chem. 2015;90:221–231. doi: 10.1016/j.ejmech.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 32.Migawa M.T., Drach J.C., Townsend L.B. Design, synthesis and antiviral activity of novel 4,5-disubstituted 7-(β-d-Ribofuranosyl)pyrrolo[2,3-d][1,2,3]triazines and the novel 3-amino-5-methyl-1-(β-d-ribofuranosyl)- and 3-amino-5-methyl-1-(2-deoxy-β-d-ribofuranosyl)-1,5-dihydro-1,4,5,6,7,8-hexaazaace-naphthylene as an-alogues of triciribine. J. Med. Chem. 2005;48:3840–3851. doi: 10.1021/jm0402014. [DOI] [PubMed] [Google Scholar]
- 33.El-Kasaby M.A., Salem M.A.I. Synthesis and reactions of 6,7(4′-alkyl-α-pyrano)-4-alkyl coumarin. Rev. Roum. Chem. 1981;26:717–723. [Google Scholar]
- 34.Salem M.A.I., El-Kasaby M.A. Reaction of 3-carbethoxy-5,6-benzocoumarin with anthranilic acid, synthesis and some reactions. J. Chem. Soc. Pak. 1987;19:177–189. [Google Scholar]
- 35.Abdou W.M., Salem M.A.I., Sediek A.A. The reactivity of 2-acetyl(3H)naphtha[2,1-b]pyran-3-one, synthesis of coummarinyl[2,1-b]fused cyclic compounds. Heterocycl. Commun. 1998;4:145–150. doi: 10.1515/HC.1998.4.2.145. [DOI] [Google Scholar]
- 36.Marzouk M.I. Study on 2-Cyanobenzo[f]chromen-3-one as Michael acceptors. Int. J. Chem. 2002;12:1–7. [Google Scholar]
- 37.Nofal Z.M., EL-Zahar M.I., Salem M.A.I., Madkour H.M.F., Abd EL-Karim S.S. Synthesis and chemophylatic effect of novel coumarin derivatives. Egypt. J. Chem. 2005;48:587–704. [Google Scholar]
- 38.Tolstoluzhsky N.V., Gorobets N.Y., Kolos N.N., Desenko S.M. Efficient ytterbium triflate catalyzed microwave-assisted synthesis of 3-acylacrylic acid building blocks. J. Comb. Chem. 2008;10:893–896. doi: 10.1021/cc800103f. [DOI] [PubMed] [Google Scholar]
- 39.El-Hashash M.A., Rizk S.A., Atta-Allah S.R. Synthesis and regioselective reaction of some unsymmetrical heterocyclic chalcone derivatives and spiro heterocyclic compounds as antibacterial agents. Molecules. 2015;20:22069–22083. doi: 10.3390/molecules201219827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharshira E.M., Hamada N.M.M. Synthesis, antibacterial and antifungal activities of some pyrazole-1-carbothioamides and pyrimidine-2(1H)-thiones. Am. J. Org. Chem. 2012;2:26–31. doi: 10.5923/j.ajoc.20120202.05. [DOI] [Google Scholar]
- 41.Salem M.S., Marzouk M.I., Ali S.N., Madkour H.M.F. Synthesis, structure characterization and biological evaluation of new 6,8-dichloro-2-methyl-4H-chromen-4-one derivatives. Eur. J. Chem. 2012;3:220–227. doi: 10.5155/eurjchem.3.2.220-227.592. [DOI] [Google Scholar]
- 42.Dotsenko V.V., Krivokolysko S.G., Litvinov B.P. Reaction of diketene with cyanothioacetamide: A convenient and regioselective method for the preparation of new 4(1H)-pyridone derivatives. Chem. Heterocycl. Compd. 2007;43:599–607. doi: 10.1007/s10593-007-0094-x. [DOI] [Google Scholar]
- 43.Minetto G., Raveglia L.F., Sega A., Taddei M. Microwave-assisted Paal-Knorr reaction-three-step Regio controlled synthesis of polysubstituted furans, pyrroles and thiophenes. Eur. J. Org. Chem. 2005;2005:5277–5288. doi: 10.1002/ejoc.200500387. [DOI] [Google Scholar]
- 44.Dere R.T., Pal R.R., Patil P.S., Salunkhe M.M. Influence of ionic liquids on the phase transfer-catalysed enantioselective Michael reaction. Tetrahedron Lett. 2003;44:5351–5353. doi: 10.1016/S0040-4039(03)01198-5. [DOI] [Google Scholar]
- 45.Zhang Z., Dong Y.-W., Wang G.-W., Komatsu K. Highly efficient mechanochemical reactions of 1,3-dicarbonyl compounds with chalcones and azachalcones catalyzed by potassium carbonate. Synlett. 2004;1:61–64. doi: 10.1002/chin.200421076. [DOI] [Google Scholar]
- 46.Salem M.S., Guirguis D.B., El-Helw E.A.E., Ghareeb M.A., Derbala H.A.Y. Antioxidant activity of heterocyclic compounds derived from 4-(4-acetamidophenyl)-4-oxobut-2-enoic acid. Int. J. Sci. Res. 2014;3:1274–1282. [Google Scholar]
- 47.Gutteridge J.M., Rowley D.A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of “free” iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem. J. 1981;199:263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh K.R., Lange S.T., Kim K.K., Brard L. A coumarin derivative (RKS262) inhibits cell-cycle progression, causes pro-apoptotic signaling and cytotoxicity in ovarian cancer cells. Investig. New Drugs. 2011;29:63–72. doi: 10.1007/s10637-009-9335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J., Lu M.L., Dai H.L., Zhang S.P., Wang H.X., Wei N. Esculetin, a coumarin derivative, exerts in vitro and in vivo antiproliferative activity against hepatocellular carcinoma by initiating a mitochondrial-dependent apoptosis pathway. Braz. J. Med. Biol. Res. 2015;48:245–253. doi: 10.1590/1414-431X20144074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang W., Li Z., Zhou M., Wu F., Hou X., Luo H., Liu H., Han X., Yan G., Ding Z., et al. Synthesis and biological evaluation of 4-(1,2,3-triazol-1-yl)coumarin derivatives as potential antitumor agents. Bioorg. Med. Chem. Lett. 2014;24:799–807. doi: 10.1016/j.bmcl.2013.12.095. [DOI] [PubMed] [Google Scholar]
- 51.Amin K.M., Eissa A.M., Abou-Seri S.M., Awadallah F.M., Hassan G.S. Synthesis and biological evaluation of novel coumarin–pyrazoline hybrids endowed with phenylsulfonyl moiety as antitumor agents. Eur. J. Med. Chem. 2013;60:187–198. doi: 10.1016/j.ejmech.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Nasr T., Bondock S., Youns M. Anticancer activity of new coumarin substituted hydrazide–hydrazone derivatives. Eur. J. Med. Chem. 2014;76:539–548. doi: 10.1016/j.ejmech.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 53.Bohon J., Santos C.R. Structural effect of the anticancer agent 6-thioguanine on duplex DNA. Nucleic Acids Res. 2003;31:1331–1338. doi: 10.1093/nar/gkg203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 55.Denizot F., Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 56.Mauceri H.J., Hanna N.N., Beckett M.A., Gorski D.H., Staba M., Stellato K.A., Bigelow K., Heimann R., Gately S., Dhanabal M., et al. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:287–291. doi: 10.1038/28412. [DOI] [PubMed] [Google Scholar]
- 57.Lissi E.A., Modak B., Torres R., Escobar J., Urzua A. Total antioxidant potential of resinous exudates from Heliotropium Species and a comparison of ABTS and DPPH methods. Free Radic. Res. 1999;30:471–477. doi: 10.1080/10715769900300511. [DOI] [PubMed] [Google Scholar]
- 58.El-Gazar A.B.A., Youssef M.M., Youssef A.M.S., Abu-Hashem A.A., Badria F.A. Design and synthesis of azolopyrimidoquinolines, pyrimidoquinazolines as antioxidant, anti-inflammatory and analgesic activities. Eur. J. Med. Chem. 2009;44:609–624. doi: 10.1016/j.ejmech.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 59.Aeschbach R., Löliger J., Scott B.C., Murcia A., Butler J., Halliwell B., Aruoma O.I. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994;32:31–36. doi: 10.1016/0278-6915(84)90033-4. [DOI] [PubMed] [Google Scholar]
- 60.Abdel-Wahab B.F., EL-Ahl A.S., Badria F.A. synthesis of new 2-naphthyl ethers and their protective activities against DNA damage induced by bleomycin–iron. Chem. Pharm Bull. 2009;57:1348–1351. doi: 10.1248/cpb.57.1348. [DOI] [PubMed] [Google Scholar]
- 61.Badria F.A., Ameen M., Akl M.R. Evaluation of cytotoxic compounds from Calligonum comosum L. growing in Egypt. Z. Naturforsch. C. 2007;62:656–660. doi: 10.1515/znc-2007-9-1005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.