Abstract
In this review, more than 60 natural canthin-6-one alkaloids and their structures are considered. The biosynthesis, efficient and classic synthetic approaches, and biological activities of canthin-6-one alkaloids, from 1952 to 2015, are discussed. From an analysis of their structural properties and an investigation of the literature, possible future trends for canthin-6-one alkaloids are proposed. The information reported will be helpful in future research on canthin-6-one alkaloids.
Keywords: canthin-6-one, biosynthesis, chemistry, biological activities
1. Introduction
The canthin-6-one alkaloids, a subclass of β-carboline alkaloids with an additional D ring, have been isolated from various plants, principally those in the Rutaceae [1,2,3,4,5,6,7,8] and Simaroubaceae [9,10,11,12,13,14,15,16,17] families, but also those in the Amaranthaceae [18], Caryophyllaceae [19] and Zygophyllaceae [20] families, and more recently from fungi [21] and marine organisms [22]. Canthin-6-one (1) (Figure 1) was first isolated in 1952 by Haynes et al. [23] from the Australian tree Pentaceras australis. A literature search revealed that since then more than 60 members of this class of alkaloids have been isolated from natural sources (Table 1).
Figure 1.
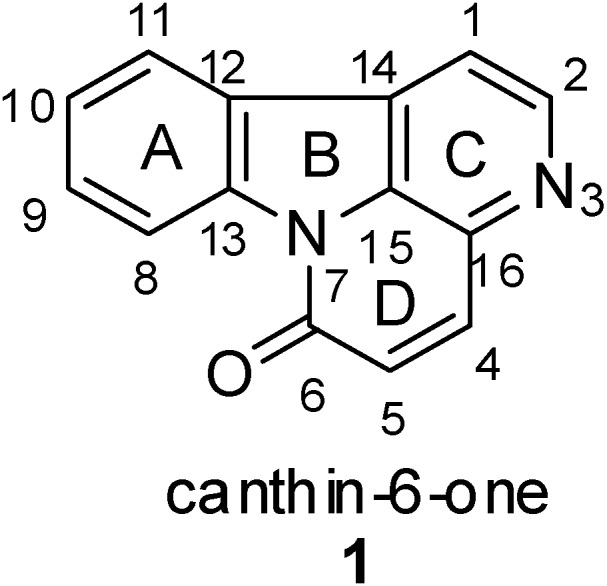
Structure of canthin-6-one 1.
Table 1.
Representative canthin-6-one alkaloids isolated from natural sources.
| When Isolated | Natural Canthin-6-one Alkaloids |
|---|---|
| 1952–1999 (25) | 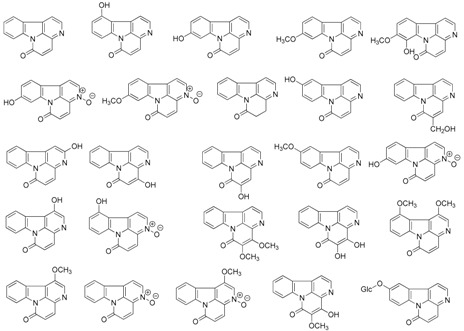 |
| 2000–2004 (16) | 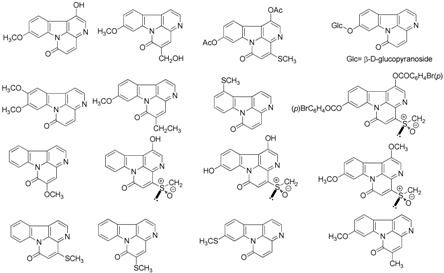 |
| 2005–2015 (19) |  |
In 1986, Guicciardi’s group was the first to demonstrate the biosynthesis of canthinone-type alkaloids through feeding experiments in which 14C-tryptophan was administered to cell cultures of Ailanthus altissima [24], and this was confirmed by a study by Aragozzini’s group a few years later [25]. Based on the biosynthetic line of canthin-6-one alkaloids, the infractine-functionalized and nanoparticle-supported biomimetic synthesis of canthin-6-one was accomplished [26]. The first total synthesis of canthin-6-one (1), which was achieved with a poor overall yield via a classic Bischer-Napieralski method, was reported in 1966 [27]. In 2013, Hollis Showalter et al. reviewed the synthetic approaches to canthin-6-ones and their ring-truncated congeners [28], their review including almost all reported cases. It is noteworthy that these alkaloids have been shown to have broad potential biological activity, such as antitumor [9,11,13,29,30,31,32], antibacterial [33,34,35], antifungal [2,4,22,36,37,38], antiparasitic [16,39,40,41], antiviral [12,19,42,43,44], anti-inflammatory [10], antiproliferative [45,46], and aphrodisiac [47] properties, as well as uses in cancer chemoprevention [48], DNA screening [49], and reducing elevated levels of proinflammatory cytokines and nitric oxide production by lipopolysaccharide-stimulated macrophages [50]. Moreover, excellent photophysical properties were reported by Irikawa et al. in 1987 [51] and, more recently, by Taniguchi et al. in 2012 [15].
This review provides a broad overview of canthin-6-ones. The first section describes the biosynthetic line and the relevant biosynthetic assembly lines of canthin-6-one alkaloids, the second section considers classic and efficient synthetic methods, and the last section summarizes the excellent biological activity of canthin-6-one alkaloids.
2. Biosynthesis
2.1. Biosynthetic Pathway
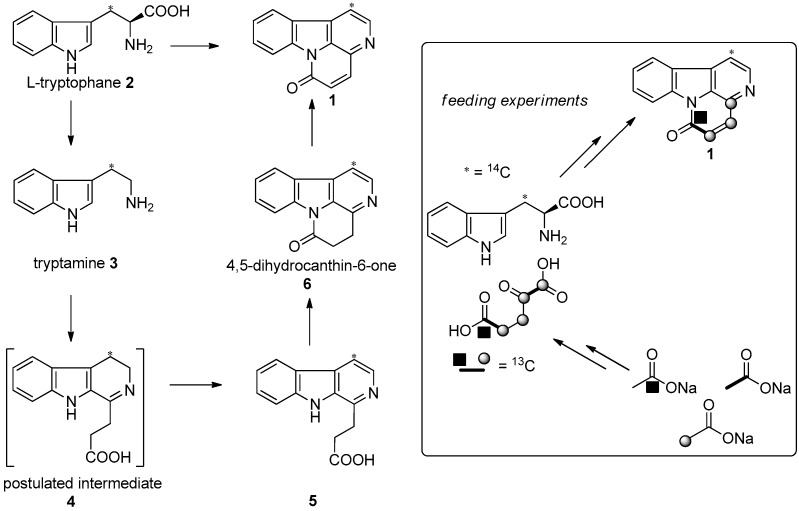
Guicciardi’s and Aragozzini’s groups were the first to demonstrate the biosynthesis of canthin-6-one alkaloids. A general biosynthetic pathway starting from tryptophan (2) is depicted in Scheme 1 [24,25]. All intermediates were characterized by the authors as products of the incorporation of [methylene-14C]-tryptophan (2). Dihydro-β-carboline-1-propionic acid (4) may be the first intermediate, with the decarboxylation of tryptophan (2) into tryptamine (3), and a suitable oxidation may generate β-carboline-1-propionic acid (5), which was isolated in the course of Guicciardi’s feeding experiments. The tricyclic intermediate (5) could be transformed into 4,5-dihydrocanthin-6-one (6), which may sequentially yield canthin-6-one (1) after oxidation. The pathway was confirmed by a supporting feeding experiment carried out in 1988 (inset in Scheme 1).
Scheme 1.
Biosynthetic mechanism leading to canthin-6-one alkaloids, and the supporting feeding experiments.
2.2. Biosynthetic Assembly Line
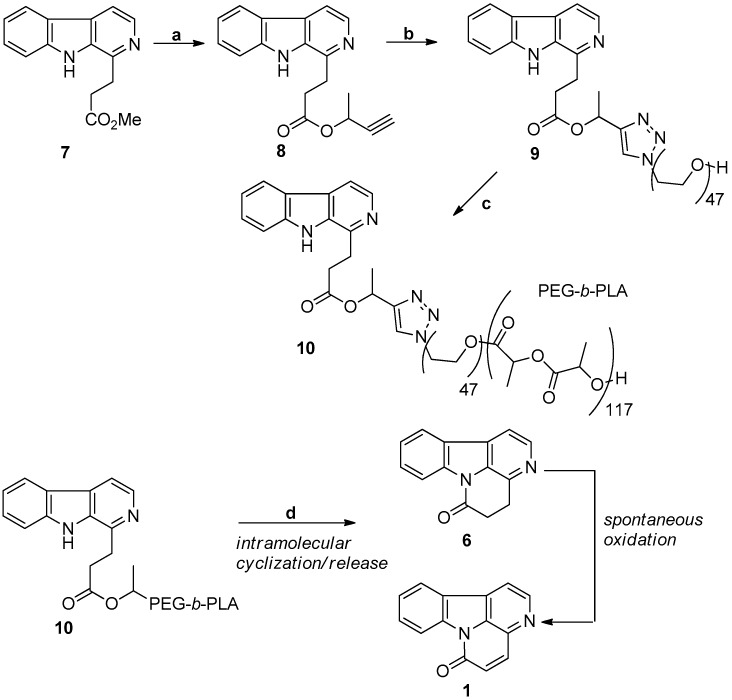
Based on the intimate biochemical mechanisms of the biosynthetic pathways, the first nanoparticle system to mimic the relevant biosynthetic assembly lines to canthin-6-one was elucidated by Cebrián-Torrejón et al. in 2013 (Scheme 2) [26]. The strategy used relied on: (i) the covalent linkage of infractine (7) by “click” chemistry to poly(ethylene glycol); (ii) the use of 7-PEG-OH as a macroinitiator for the ring-opening polymerization of lactide to form 7-PEG-b-PLA copolymer; (iii) the formation of 7-PEG-b-PLA nanoparticles from the self-assembly of 7-PEG-b-PLA in aqueous solution; and (iv) the potential biomimetic release of canthin-6-one (1) from the nanoparticles.
Scheme 2.
The relevant biosynthetic assembly lines. Reagents and conditions: 7-functionalized nanoparticles and the biomimetic release of 1 and 6. (a) DCM, Yb(Otf)3 (20 mol %), reflux, 16 h, 27%; (b) 3-butyn-2-ol (excess), N3-PEG-OH (1 equiv.), CuBr (1 equiv.), dimethylformamide, room temperature (RT), 24 h, PMDTA (N,N,N′,N″,N″-pentamethyldiethylenetriamine), 46%; (c) d,l-lactide (excess), Sn(Oct)2 (0.4 equiv.), toluene, 115 °C, 16 h, 57%; (d) 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (excess), DCM, air, 72 h (identification of 1 and 6 by mass spectrometry and high-performance liquid chromatography-ultraviolet detection studies).
3. Chemistry
The canthin-6-one alkaloids have been synthesized via different approaches by many researchers [2,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75]. In order to use these methods to guide our review more clearly, we categorized classic and efficient synthetic methods according to their key reaction steps.
3.1. Bischer-Napieralski Reaction
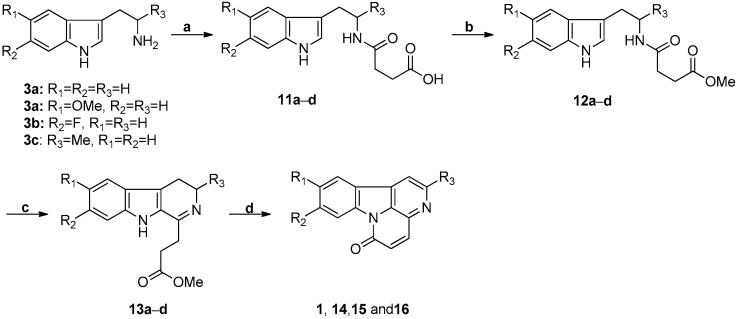
The use of the Bischer-Napieralski reaction to synthesize canthin-6-one alkaloids was first reported in 1966 [27], and Soriano-Agaton et al. reported a more recent synthesis in 2005 (Scheme 3) [2]. The overall yield of canthin-6-one in the later synthesis was increased to 76.83% in four steps by Soriano-Agaton.
Scheme 3.
The synthetic strategy of Soriano-Agaton. Reagents and conditions. (a) Succinic anhydride, DCM, RT, 18 h, 98%, 99%, 98%, and 98% for 11a−d, respectively; (b) Amberlyst 15, MeOH, reflux, 18 h, 98%; (c) POCl3, PhH, reflux, 1 h; (d) DBU, DCM, RT, 18 h, 80%, 70%, 80%, and 20% in two steps for 1, 14, 15, and 16, respectively.
3.2. Pictet-Spengler Reaction
The Pictet-Spengler reaction was first used to synthesize canthin-6-one alkaloids by Mitscher et al. in 1975 [73], and many syntheses of canthin-6-one that employ this reaction have been reported since then. Czerwinski et al. [54] reported an efficient synthetic approach to canthin-6-one via the Pictet-Spengler reaction in 2003 (Scheme 4), obtaining an overall yield of 46.74%.
Scheme 4.
The synthetic strategy of Czerwinski. Reagents and conditions. (a) Anhydrous acetone, PhCOCl, NaOH, −30 °C, 95%; (b) anhydrous tetrahydrofuran (THF), LiAlH4, 0 °C—reflux, 95%; (c) α-ketoglutaric acid, PhH/dioxane (6:4), Dean-Stark trap, reflux, 80%; (d) anhydrous MeOH, Pd/C, HCOONH4, reflux, 83%; (e) PhH/PhMe (3:1), MnO2, reflux, 78%.
3.3. Diels-Alder Reaction
In 1992, Snyder and co-workers reported an elegant strategy for accessing the canthine skeleton through using indole as a dienophile in an intramolecular inverse electron demand Diels-Alder (IEDDA) reaction [68]. Subsequently, in 2003, Lindsley et al. [70] reported a “one-pot” microwave-mediated synthesis of canthin-6-one analogs via the IEDDA reaction (Scheme 5), in which the overall yield was 48% in two steps.
Scheme 5.
The synthetic strategy of Lindsley. Reagents and conditions. (a) 1,2-Diphenylethanedione, AcONH4, AcOH, 220 °C, 40 min, 80%; (b) 4,5-bis(thioacetamido)pentanoyl (BTAP), AcOH, 70 °C, 4 h, 60%.
3.4. Aldol Reaction
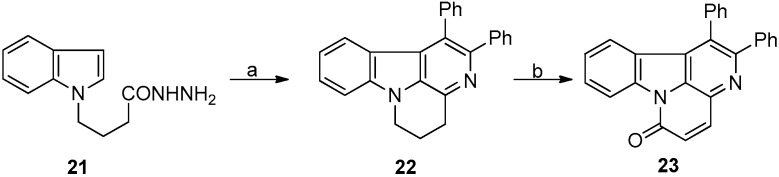
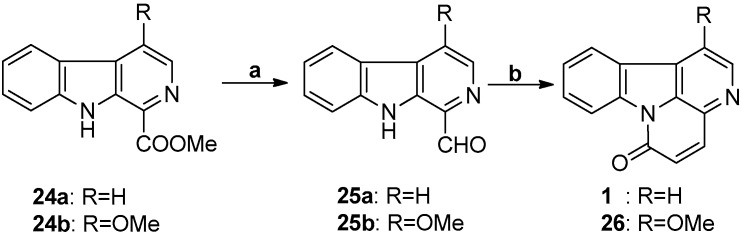
An efficient synthesis of canthin-6-one from β-carboline-1-carbaldehyde via the aldol reaction was reported by Suzuki et al. in 2005 (Scheme 6) [56]. Using a two-step reaction, canthin-6-one was obtained with an overall yield of 70.55%.
Scheme 6.
The synthetic strategy of Suzuki. Reagents and conditions. (a) Dibal-H, DCM, −40 °C, 5 min, 85% for 25a and 70% for 25b; (b) EtOAc/LiHMDS/THF, stirred for 15 min at −78 °C, then EtOH quench for 30 min at RT, 83% for 1 and 88% for 26.
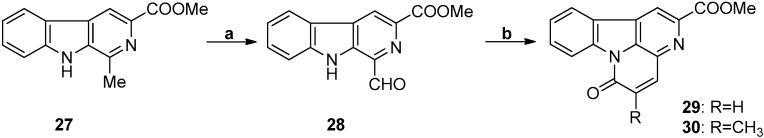
3.5. Perkin Reaction
The use of the Perkin reaction to synthesize canthin-6-one alkaloid analogs was reported by Giudice et al. in 1990 (Scheme 7) [75] and, more recently, by Brahmbhatt et al. in 2010 [42]. Despite the relatively low overall yield (48.96% for 29 and 46.24% for 30), the synthesis strategy is relatively simple and low-cost.
Scheme 7.
The synthetic strategy of Giudice. Reagents and conditions. (a) SeO2, dioxane, reflux, 2 h, 68%; (b) (RCO)2O, pyridine, heat, 3 h, 72% for 29 and 68% for 30.
3.6. Non-Classic Strategy
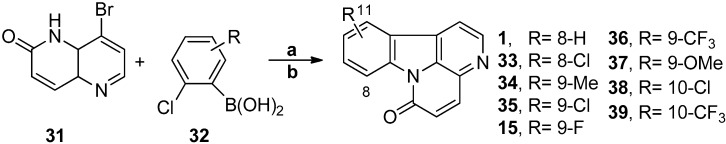
In 2010, Gollner et al. reported a “non-classic” strategy that focused on the construction of the central B ring (Scheme 8) [61]. The strategy relies on a palladium-catalyzed Suzuki-Miyaura C-C coupling followed by a copper-catalyzed C-N coupling that can be achieved either stepwise or in a new one-pot protocol starting from the appropriate 8-bromo-1,5-naphthyridine. Canthin-6-one (1) and nine analogues were prepared rapidly and in high yields (71%–95%). Ethyl canthin-6-one-1-carboxylate has also been efficiently synthesized, by Ioannidou et al. in 2011, from readily prepared ethyl 4-bromo-6-methoxy-1,5-naphthyridine-3-carboxylate in a three-step “non-classic” reaction that focuses on the construction of the central pyrrole (B ring) via a palladium-catalyzed Suzuki-Miyaura coupling followed by a copper-catalyzed C-N coupling; the overall yield was 85% [62].
Scheme 8.
The synthetic strategy of Gollner. Reagents and conditions. (a) ArB(OH)2, K2CO3, Pd(dppf)Cl2, dioxane/H2O (3:1); (b) CuI, N,N′-dimethylethylendiamine (DMEDA), Cs2CO3, dioxane/H2O (3:1). Overall yields: 1, 95%; 33, 82%; 34, 92%; 35, 82%; 15, 88%; 36, 78%; 37, 92%; 38, 77%; 39, 71%.
4. Biological Activity
Canthin-6-one alkaloids have been reported to have a wide range of potential therapeutic applications, including, but not limited to, use as antitumor [9,11,13,29,30,31,32], antibacterial [33,34,35], antifungal [2,4,22,36,37,38], antiparasitic [16,39,40,41], antiviral [12,19,42,43,44], anti-inflammatory [10,76], antiproliferative [45,46], and aphrodisiac [47] agents, and use in cancer chemoprevention [48], DNA screening [49], reducing elevated levels of proinflammatory cytokines and nitric oxide production by lipopolysaccharide-stimulated macrophages [50], and so on. In this review, favorable biological activities of canthin-6-one alkaloids that are equal to or better than those of standard drugs are discussed, and their potential is highlighted.
4.1. Antibacterial
In 2007, O’Donnell et al. reported the in vitro antibacterial activity of canthin-6-one alkaloids, and found minimum inhibitory concentrations (MICs) in the range of 8–82 µg/mL against a panel of fast-growing Mycobacterium species and 8–64 µg/mL against multidrug-resistant and methicillin-resistant strains of Staphylococcus aureus [33]. In the same year, Ostrov et al. [34] reported the use of structure-based molecular docking to identify novel drug-like small molecules. They found that canthin-6-one could dock to two sites of Escherichia coli DNA gyrase, targeting and inhibiting the DNA supercoiling activity of purified E. coli DNA gyrase, although it could not effectively accumulate inside E. coli. Our group has designed and synthesized a series of 3-N-alkylated and 3-N-benzylated canthin-6-ones, and evaluated their in vitro antibacterial activities. Of these compounds, eleven 3-N-substituted canthin-6-ones were found to be the most potent, with MIC values lower than 1.95 (μg/mL) against S. aureus [77].
4.2. Antitumor
The significant antitumor activity of canthin-6-one alkaloids has been reported by many researchers. For example, in 2014, Devkota et al. [11] reported a study in which canthin-6-one alkaloids were tested in an Nf1- and p53-defective mouse malignant glioma tumor cell line engineered to express a dual reporter (Table 2). The results indicated that the majority of the canthin-6-one alkaloids inhibited cell growth and exhibited some toxicity. The antitumor mechanism was reported by Dejos et al. in the same year [45]. They found that the primary effect of canthin-6-one is as an antiproliferative, possibly by interfering with the G2/M transition. In 2015, Cebrian-Torrejon et al. [78] presented an approach for studying the performance of novel targets able to overcome cancer stem cell chemoresistance. The approach was based on voltammetric data for microparticulate films of natural or synthetic alkaloids from the canthin-6-one series.
Table 2.
Cell viability and inhibition of proliferation (%) in an Nf1- and p53-defective mouse Central Nervous System (CNS) tumor cell line by canthines at 2.0 mg/mL.
| Compound | Cell Viability (Untreated Controls = 100) | Inhibition of Proliferation (%) |
|---|---|---|
| Canthin-6-one-9-methoxy-5-O-β-d-glucopyranoside | 51 | 71 |
| 9-Methoxycanthin-6-one | 41 | 76 |
| 8-Hydroxy-9-methoxycanthin-6-one | 51 | 59 |
| 9-Hydroxycanthin-6-one | 56 | 48 |
| canthin-6-one-3-N-oxide | 59 | 70 |
| 9-Hydroxycanthin-6-one-3-N-oxide | 54 | 76 |
| 11-Hydroxycanthin-6-one-3-N-oxide | 54 | 70 |
4.3. Antifungal
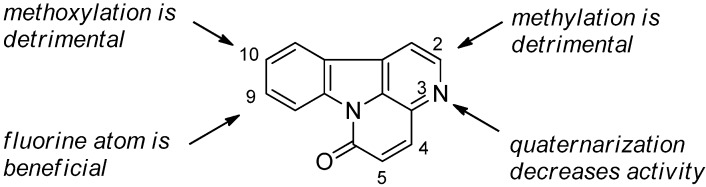
Canthin-6-one (1) was reported by Thouvenel et al. in 2003 to exhibit a broad spectrum of activity against Aspergillus fumigatus, A. niger, A. terreus, Candida albicans, C. tropicalis, C. glabrata, C. neoformans, Geotrichum candidum, Saccharomyces cerevisiae, Trichosporon beigelii, T. cutaneum, and T. mentagrophytes var. interdigitale, with MICs between 5.3 and 46 µM [36]. Moreover, in 2005, Soriano-Agaton et al. [2] described the structure -activity relationships for the antifungal activity of canthin-6-one (Figure 2). The mechanism of action of the antifungal canthin-6-one series was investigated in Saccharomyces cerevisiae by Loiseau et al. in 2008 [79]. In 2013, the antifungal mechanism of canthin-6-one was also reported on by Dejos et al. [38]. Although no novel clues to the mechanism were found, they demonstrated that the major-facilitator-superfamily (MFS)-type transporter Flr1 may be able to reduce sensitivity to canthin-6-one when it is overproduced via a higher gene dosage, and that Flr1-mediated tolerance to canthin-6-one is strictly dependent on the transcription factor Yap1. As Dejos et al. stated, “Although the Yap1-Flr1 pair is not naturally involved in yeast tolerance to canthin-6-one, this study demonstrates that their overexpression can lead to resistance to the chemical stress generated by this drug.”
Figure 2.
Structure-activity relationships for antifungal activity.
4.4. Anti-Inflammatory
The transcription factor NF-κB is a key regulator of many proinflammatory pathways, and therefore its inhibition results in anti-inflammatory effects. Canthin-6-one alkaloids were first found to be NF-κB inhibitors by Tran et al. in 2014 [10]. The IC50 values of 9-hydroxycanthin-6-one and 9-methoxycanthin-6-one were 3.8 and 7.4 µM, respectively. However, the IC50 value of the standard drug parthenolide was only 1.5 µM.
4.5. Wnt Signaling Inhibitors
Numerous diseases have been attributed to the aberrant transduction of Wnt signaling, which regulates various processes such as cell proliferation and differentiation, and embryo development. In 2015, 9-hydroxycanthin-6-one was screened for its activity in targeting TCF/β-catenin transcriptional modulating activity with a cell-based luciferase assay by Ohishi et al. [80]. The degradation of β-catenin by 9-hydroxycanthin-6-one was suppressed by GSK3β-siRNA, while 9-hydroxycanthin-6-one decreased β-catenin even in the presence of CK1α-siRNA. These results suggest that 9-hydroxycanthin-6-one inhibits Wnt signaling through the activation of GSK3β, independently of CK1α.
4.6. Protein Tyrosine Phosphatase 1B Inhibitors
As a potential therapy for diabetes, protein tyrosine phosphatase 1B (PTP1B) inhibitors have attracted considerable attention. In 2015, Sasaki et al. [81] reported that compound 40 (Figure 3) is the competitive PTP1B inhibitor, with the best inhibitory selectivity of PTP1B and other protein tyrosine phosphatase (PTPs), and showed in cell-based assays that it promotes activity in the insulin signaling pathway.
Figure 3.
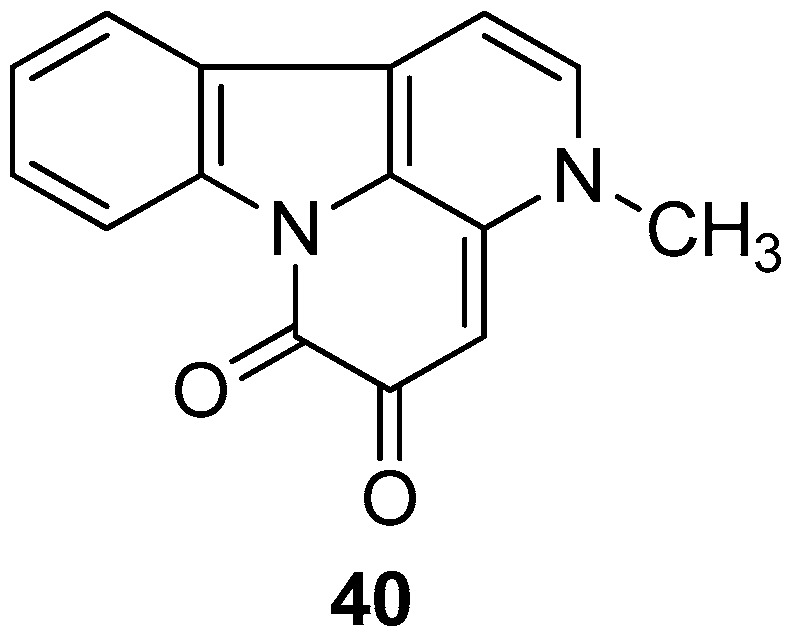
Structure of canthine 40.
5. Conclusions and Future Prospects
The main achievements in the study of canthin-6-one alkaloids over the period of 1952 to 2015 have been reviewed, with emphasis on the biosynthesis, chemistry, and biological activities of these compounds. The low toxicity and good biological activities of canthin-6-one alkaloids mean that there is potential for them to be developed into new drugs. New research by Doménech-Carbó et al. has shown that microparticulate films of canthin-6-one on glassy carbon electrodes could yield separate voltammetric signals for dsDNA, ssDNA, and G-quadruplex DNA, different degrees of DNA methylation, and biomimetic nucleosomal DNA, with a detection limit of 10−5 M [49]. Moreover, excellent photophysical properties of canthin-6-one have been recently reported by Taniguchi et al. [15].
Further research by international groups is required to: (i) develop efficient and environmentally sustainable synthetic strategies for producing canthin-6-one alkaloids on a large scale; (b) elucidate the structure-activity relationships and mechanisms of the different biological activities of these compounds; (c) explore their biological activities; and (d) identify electrochemical and photochemical applications.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31270388), Fundamental Research Funds for the Central Universities (Grant No. QN2011066), and International S&T Cooperation Foundation of NWAFU (A213021501).
Author Contributions
The manuscript was written through the contributions of Jiangkun Dai, Na Li, Junru Wang and Uwe Schneider. All of the authors have approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tessier A., Campbell P.G., Bisson M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979;51:844–851. doi: 10.1021/ac50043a017. [DOI] [Google Scholar]
- 2.Soriano-Agatón F., Lagoutte D., Poupon E., Roblot F., Fournet A., Gantier J.C., Hocquemiller R. Extraction, hemisynthesis, and synthesis of canthin-6-one analogues. Evaluation of their antifungal activities. J. Nat. Prod. 2005;68:1581–1587. doi: 10.1021/np050250z. [DOI] [PubMed] [Google Scholar]
- 3.Mukhlesur R.M., Anwarul I.M., Khondkar P., Gray A.I. Alkaloids and lignans from Zanthoxylum budrunga (Rutaceae) Biochem. Syst. Ecol. 2005;33:91–96. doi: 10.1016/j.bse.2004.04.016. [DOI] [Google Scholar]
- 4.He W., Puyvelde L.V., Kimpe N.D., Verbruggen L., Anthonissen K., Flaas M.V., Bosselaers J., Mathenge S.G., Mudida F.P. Chemical constituents and biological activities of Zanthoxylum usambarense. Phytother. Res. 2002;16:66–70. doi: 10.1002/ptr.849. [DOI] [PubMed] [Google Scholar]
- 5.Cebrián-Torrejón G., Kablan L., Ferreira M.E., Cruz D.R., Doménech-Carbó A., Bilbao N.V., Arias A.R., Figadère B., Poupon E., Fournet A. Harvesting canthinones: Identification of the optimal seasonal point of harvest of Zanthoxylum chiloperone leaves as a source of 5-methoxycanthin-6-one. Nat. Prod. Res. 2015;29:2054–2058. doi: 10.1080/14786419.2015.1022774. [DOI] [PubMed] [Google Scholar]
- 6.Min Y.D., Kwon H.C., Yang M.C., Lee K.H., Choi S.U., Lee K.R. Isolation of limonoids and alkaloids from Phellodendron amurense and their multidrug resistance (MDR) reversal activity. Arch. Pharm. Res. 2007;30:58–63. doi: 10.1007/BF02977779. [DOI] [PubMed] [Google Scholar]
- 7.Shi M.J., Liu W.W., Wang H.Y., Cheng H., Hu L. Effect of canthin-6-one experimental arrhythmia. Chin. Tradit. Herba. Drugs. 2000;31:37–39. [Google Scholar]
- 8.Ferreira M.E., Cebrián-Torrejón G., Corrales A.S., de Bilbao N.V., Rolón M., Gomez C.V., Leblanc K., Yaluf G., Schinini A., Torres S., et al. Zanthoxylum chiloperone leaves extract: First sustainable Chagas disease treatment. J. Ethnopharmacol. 2011;133:986–993. doi: 10.1016/j.jep.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Rivero-Cruz J.F., Lezutekong R., Lobo-Echeverri T., Ito A., Mi Q., Chai H.B., Soejarto D.D., Cordell G.A., Pezzuto J.M., Swanson S.M., et al. Cytotoxic constituents of the twigs of Simarouba glauca collected from a plot in southern florida. Phytother. Res. 2005;19:136–140. doi: 10.1002/ptr.1642. [DOI] [PubMed] [Google Scholar]
- 10.Tran T.V.A., Malainer C., Schwaiger S., Atanasov A.G., Heiss E.H., Dirsch V.M., Stuppner H. NF-κB inhibitors from Eurycoma longifolia. J. Nat. Prod. 2014;77:483–488. doi: 10.1021/np400701k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devkota K.P., Wilson J.A., Henrich C.J., McMahon J.B., Reilly K.M., Beutler J.A. Compounds from Simarouba berteroana which inhibit proliferation of NF1-defective cancer cells. Phytochem. Lett. 2014;7:42–45. doi: 10.1016/j.phytol.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J., Yan X.H., Dong J.H., Sang P., Fang X., Di Y.T., Zhang Z.K., Hao X.J. Tobacco mosaic virus (TMV) inhibitors from Picrasma quassioides Benn. J. Agric. Food Chem. 2009;57:6590–6595. doi: 10.1021/jf901632j. [DOI] [PubMed] [Google Scholar]
- 13.Kuo P.C., Shi L.S., Damu A.G., Su C.R., Huang C.H., Ke C.H., Wu .B., Lin A.J., Bastow K.F., Lee K.H., et al. Cytotoxic and antimalarial β-carboline alkaloids from the roots of Eurycoma longifolia. J. Nat. Prod. 2003;66:1324–1327. doi: 10.1021/np030277n. [DOI] [PubMed] [Google Scholar]
- 14.Handa S.S., Kinghorn A.D., Cordell G.A., Farnsworth N.R. Plant anticancer agents XXV. Constituents of Soulamea soulameoides. J. Nat. Prod. 1983;46:359–364. doi: 10.1021/np50027a011. [DOI] [PubMed] [Google Scholar]
- 15.Taniguchi K., Takizawa S., Hirano T., Murata S., Kagechika H., Kishida A., Ohsaki A. Amarastelline A: A fluorescent alkaloid from Quassia amara and its properties in living cells. ChemPlusChem. 2012;77:427–431. doi: 10.1002/cplu.201200016. [DOI] [Google Scholar]
- 16.Kardono L.B., Angerhofer C.K., Tsaur S., Padmawinata K., Pezzuto J.M., Kinghorn A.D. Cytotoxic and antimalarial constituents of the roots of Eurycoma longifolia. J. Nat. Prod. 1991;54:1360–1367. doi: 10.1021/np50077a020. [DOI] [PubMed] [Google Scholar]
- 17.Kanchanapoom T., Kasai R., Chumsri P., Hiraga Y., Yamasaki K. Canthin-6-one and β-carboline alkaloids from Eurycoma harmandiana. Phytochemistry. 2001;56:383–386. doi: 10.1016/S0031-9422(00)00363-0. [DOI] [PubMed] [Google Scholar]
- 18.Bharitkar Y.P., Hazra A., Apoorva Poduri N.S., Ash A., Maulik P.R., Mondal N.B. Isolation, structural elucidation and cytotoxicity evaluation of a new pentahydroxy-pimarane diterpenoid along with other chemical constituents from Aerva lanata. Nat. Prod. Res. 2015;29:253–261. doi: 10.1080/14786419.2014.971794. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh P.W., Chang F.R., Lee K.H., Hwang T.L., Chang S.M., Wu Y.C. A new anti-HIV alkaloid, drymaritin, and a new c-glycoside flavonoid, diandraflavone, from Drymaria diandra. J. Nat. Prod. 2004;67:1175–1177. doi: 10.1021/np0400196. [DOI] [PubMed] [Google Scholar]
- 20.Ma Z.Z., Hano Y., Nomura T., Chen Y.J. Alkaloids and phenylpropanoids from Peganum nigellastrum. Phytochemistry. 2000;53:1075–1078. doi: 10.1016/S0031-9422(99)00440-9. [DOI] [PubMed] [Google Scholar]
- 21.Bröckelmann M.G., Dasenbrock J., Steffan B., Steglich W., Wang Y., Raabe G., Fleischhauer J. An unusual series of thiomethylated canthin-6-ones from the North American mushroom Boletus curtisii. Eur. J. Org. Chem. 2004:4856–4863. doi: 10.1002/ejoc.200400519. [DOI] [Google Scholar]
- 22.Tanaka N., Momose R., Takahashi Y., Kubota T., Takahashi-Nakaguchi A., Gonoi T., Fromont J., Kobayashi J. Hyrtimomines D and E, bisindole alkaloids from a marine sponge Hyrtios sp. Tetrahedron Lett. 2013;54:4038–4040. doi: 10.1016/j.tetlet.2013.05.073. [DOI] [Google Scholar]
- 23.Haynes H.F., Nelson E.R., Price J.R. Alkaloids of the Australian Rutaceae: Pentaceras australis Hook. F.I. Isolation of the Alkaloids and Identification of Canthin-6-one. Aust. J. Sci. Res. Ser. A. 1952;5:387–400. [Google Scholar]
- 24.Crespi-Perellino N., Guicciardi A., Malyszko G., Minghetti A. Biosynthetic relationship between indole alkaloids produced by cell cultures of Ailanthus altissima. J. Nat. Prod. 1986;49:814–822. doi: 10.1021/np50047a009. [DOI] [Google Scholar]
- 25.Aragozzini F., Maconi E., Gualandris R. Evidence for involvement of ketoglutarate in the biosynthesis of canthin-6-one from cell cultures of Ailanthus altissima. Plant Cell Rep. 1988;7:213–215. doi: 10.1007/BF00269327. [DOI] [PubMed] [Google Scholar]
- 26.Cebrián-Torrejón G., Mackiewicz N., Vázquez-Manrique R.P., Fournet A., Figadère B., Nicolas J., Poupon E. Solution phase and nanoparticular biosynthetically inspired interconnections in the canthi-6-one β-carboline series and study of phenotypic properties on C. elegans. Eur. J. Org. Chem. 2013;2013:5821–5828. doi: 10.1002/ejoc.201300770. [DOI] [Google Scholar]
- 27.Rosenkranz H.J., Botyos G., Schmid H. Synthese von tuboflavin, 4-Äthyl-canthin-6-one and canthin-6-one. Justus Liebigs Ann. Chem. 1966;691:159–164. doi: 10.1002/jlac.19666910120. [DOI] [Google Scholar]
- 28.Hollis Showalter H.D. Progress in the synthesis of canthine alkaloids and ring-truncated congeners. J. Nat. Prod. 2013;76:455–467. doi: 10.1021/np300753z. [DOI] [PubMed] [Google Scholar]
- 29.Peduto A., More V., de Caprariis P., Festa M., Capasso A., Piacente S., de Martino L., de Feo V., Filosa R. Synthesis and cytotoxic activity of new β-carboline derivatives. Mini-Rev. Med. Chem. 2011;11:486–491. doi: 10.2174/138955711795843383. [DOI] [PubMed] [Google Scholar]
- 30.Miyake K., Tezuka Y., Awale S., Li F., Kadota S. Canthin-6-one alkaloids and a tirucallanoid from Eurycoma longifolia and their cytotoxic activity against a human HT-1080 fibrosarcoma cell line. Nat. Prod. Commun. 2010;5:17–22. [PubMed] [Google Scholar]
- 31.Ammirante M., di Giacomo R., de Martino L., Rosati A., Festa M., Gentilella A., Pascale M.C., Belisario M.A., Leone A., Turco M.C., et al. 1-Methoxy-canthin-6-one induces c-Jun NH2-terminal kinase-dependent apoptosis and synergizes with tumor necrosis factor-related apoptosis-inducing ligand activity in human neoplastic cells of hematopoietic or endodermal origin. Cancer Res. 2006;66:4385–4393. doi: 10.1158/0008-5472.CAN-05-3895. [DOI] [PubMed] [Google Scholar]
- 32.Kuo P.C., Damu A.G., Lee K.H., Wu T.S. Cytotoxic and antimalarial constituents from the roots of Eurycoma longifolia. Bioorg. Med. Chem. 2004;12:537–544. doi: 10.1016/j.bmc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 33.O’Donnell G., Gibbons S. Antibacterial activity of two canthin-6-one alkaloids from Allium neapolitanum. Phytother. Res. 2007;21:653–657. doi: 10.1002/ptr.2136. [DOI] [PubMed] [Google Scholar]
- 34.Ostrov D.A., Prada J.A.H., Corsino P.E., Finton K.A., Le N., Rowe T.C. Discovery of novel DNA gyrase inhibitors by high-throughput virtual screening. Antimicrob. Agents Chemother. 2007;51:3688–3698. doi: 10.1128/AAC.00392-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen M., Fan H.Y., Dai S.J., Liu K. Alkaloids from twigs and leaves of Picrasma quassioides. Chin. Tradit. Herbal Drugs. 2007;38:807–810. [Google Scholar]
- 36.Thouvenel C., Gantier J.C., Duret P., Fourneau C., Hocquemiller R., Ferreira M.E., de Arias A.R., Fournet A. Antifungal compounds from Zanthoxylum chiloperone var. angustifolium. Phytother. Res. 2003;17:678–680. doi: 10.1002/ptr.1137. [DOI] [PubMed] [Google Scholar]
- 37.Li H.Y., Koike K., Ohmoto T. New alkaloids, picrasidines W, X and Y, from Picrasma quassioides and X-ray crystallographic analysis of picrasidine Q. Chem. Pharm. Bull. 1993;41:1807–1811. doi: 10.1248/cpb.41.1807. [DOI] [Google Scholar]
- 38.Dejos C., Régnacq M., Bernard M., Voisin P., Bergès T. The MFS-type efflux pump Flr1 induced by Yap1 promotes canthin-6-one resistance in yeast. Fed. Eur. Biochem. Soc. Lett. 2013;587:3045–3051. doi: 10.1016/j.febslet.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira M.E., Nakayama H., de Arias A.R., Schinini A., de Bilbao N.V., Serna E., Lagoutte D., Soriano-Agatón F., Poupon E., Hocquemiller R., et al. Effects of canthin-6-one alkaloids from Zanthoxylum chiloperone on trypanosoma cruzi-infected mice. J. Ethnopharmacol. 2007;109:258–263. doi: 10.1016/j.jep.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 40.Takasu K., Shimogama T., Saiin C., Kim H.S., Wataya Y., Brun R., Ihara M. Synthesis and evaluation of β-carbolinium cations as new antimalarial agents based on π-delocalized lipophilic cation (DLC) hypothesis. Chem. Pharm. Bull. 2005;53:653–661. doi: 10.1248/cpb.53.653. [DOI] [PubMed] [Google Scholar]
- 41.Ferreira M.E., de Arias A.R., de Ortiz S.T., Inchausti A., Nakayama H., Thouvenel C., Hocquemiller R., Fournet A. Leishmanicidal activity of two canthin-6-one alkaloids, two major constituents of Zanthoxylum chiloperone var. angustifolium. J. Ethnopharmacol. 2002;80:199–202. doi: 10.1016/S0378-8741(02)00025-9. [DOI] [PubMed] [Google Scholar]
- 42.Brahmbhatt K.G., Ahmed N., Sabde S., Mitra D., Singh I.P., Bhutani K.K. Synthesis and evaluation of β-carboline derivatives as inhibitors of human immunodeficiency virus. Bioorg. Med. Chem. Lett. 2010;20:4416–4419. doi: 10.1016/j.bmcl.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 43.Xu Z., Chang F.R., Wang H.K., Kashiwada Y., McPhail A.T., Bastow K.F., Tachibana Y., Cosentino M., Lee K.H. Anti-HIV agents 451 and antitumor agents 205.2 two new sesquiterpenes, Leitneridanins A and B, and the cytotoxic and anti-HIV principles from Leitneria floridana. J. Nat. Prod. 2000;63:1712–1715. doi: 10.1021/np000260u. [DOI] [PubMed] [Google Scholar]
- 44.Ohomoto T., Koike K. Studies on the constituents of Ailanthus altissima Swingle. III. The alkaloidal constituents. Chem. Pharm. Bull. 1984;32:170–173. doi: 10.1248/cpb.32.170. [DOI] [Google Scholar]
- 45.Dejos C., Voisin P., Bernard M., Régnacq M., Bergès T. Canthin-6-one displays antiproliferative activity and causes accumulation of cancer cells in the G2/M phase. J. Nat. Prod. 2014;77:2481–2487. doi: 10.1021/np500516v. [DOI] [PubMed] [Google Scholar]
- 46.De Feo V., Martino L.D., Santoro A., Leone A., Pizza C., Franceschelli S., Pascale M. Antiproliferative effects of tree of heaven (Ailanthus altissima Swingle) Phytother. Res. 2005;19:226–230. doi: 10.1002/ptr.1670. [DOI] [PubMed] [Google Scholar]
- 47.Chiou W.F., Wu T.S. 9-Hydroxycanthin-6-one induces penile erection and delays ejaculation. J. Sex. Med. 2012;9:1027–1036. doi: 10.1111/j.1743-6109.2011.02296.x. [DOI] [PubMed] [Google Scholar]
- 48.Murakami C., Fukamiya N., Tamura S., Okano M., Bastow K.F., Tokuda H., Mukainaka T., Nishino H., Lee K.H. Multidrug-resistant cancer cell susceptibility to cytotoxic quassinoids, and cancer chemopreventive effects of quassinoids and canthine alkaloids. Bioorg. Med. Chem. 2004;12:4963–4968. doi: 10.1016/j.bmc.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 49.Doménech-Carbó A., Cebrián-Torrejón G., de Miguel L., Tordera V., Rodrigues-Furtado D., Assad-Kahn S., Fournet A., Figadère B., Vázquez-Manrique R.P., Poupon E. DsDNA, ssDNA, G-quadruplex DNA, and nucleosomal DNA electrochemical screening using canthin-6-one alkaloid-modified electrodes. Electrochim. Acta. 2014;115:546–552. doi: 10.1016/j.electacta.2013.11.025. [DOI] [Google Scholar]
- 50.Siveen K., Kuttan G. Modulation of humoral immune responses and inhibition of proinflammatory cytokines and nitric oxide production by 10-methoxycanthin-6-one. Immunopharmacol. Immunotoxicol. 2012;34:116–125. doi: 10.3109/08923973.2011.586703. [DOI] [PubMed] [Google Scholar]
- 51.Irikawa H., Okumura Y. Autoxidation of indolo [3,2,1-de][1,5]naphthyridines. Bull. Chem. Soc. Jpn. 1987;60:3797–3798. doi: 10.1246/bcsj.60.3797. [DOI] [Google Scholar]
- 52.Hagen T.J., Cook J.M. Synthesis of 1-methoxycanthine-6-one. Tetrahedron Lett. 1988;29:2421–2424. doi: 10.1016/S0040-4039(00)87897-1. [DOI] [Google Scholar]
- 53.Hagen T.J., Narayanan K., Names J., Cook J.M. DDQ oxidations in the indole area. Synthesis of 4-alkoxy-β-carbolines including the natural products crenatine and 1-methoxycanthin-6-one. J. Org. Chem. 1989;54:2170–2178. doi: 10.1021/jo00270a029. [DOI] [Google Scholar]
- 54.Czerwinski K.M., Zificsak C.A., Stevens J., Oberbeck M., Randlett C., King M., Mennen S. An improved synthesis of canthin-6-one. Synth. Commun. 2003;33:1225–1231. doi: 10.1081/SCC-120017199. [DOI] [Google Scholar]
- 55.Rößler U., Blechert S., Steckhan E. Single electron transfer induced total synthesis of canthin-6-one. Tetrahedron Lett. 1999;40:7075–7078. doi: 10.1016/S0040-4039(99)01472-0. [DOI] [Google Scholar]
- 56.Suzuki H., Adachi M., Ebihara Y., Gyoutoku H., Furuya H., Murakami Y., Okuno H. A total synthesis of 1-methoxycanthin-6-one: An efficient one-pot synthesis of the canthin-6-one skeleton from β-Carboline-1-carbaldehyde. Synthesis. 2005;1:28–32. doi: 10.1055/s-2004-834915. [DOI] [Google Scholar]
- 57.Markgraf J.H., Dowst A.A., Hensley L.A., Jakobsche C.E., Kaltner C.J., We P.J., Zimmerman P.W. A versatile route to benzocanthinones. Tetrahedron. 2005;61:9102–9110. doi: 10.1016/j.tet.2005.07.034. [DOI] [Google Scholar]
- 58.Bergman J., Lidgren G., Gogoll A. Synthesis and reactions of oxazolones from l-tryptophan and α-haloacetic anhydrides. Bull. Soc. Chim. Belg. 1992;101:643–660. doi: 10.1002/bscb.19921010712. [DOI] [Google Scholar]
- 59.Condie G.C., Bergman J. Reactivity of β-carbolines and cyclopenta[b]indolones prepared from the intramolecular cyclization of 5(4H)-oxazolones derived from l-tryptophan. Eur. J. Org. Chem. 2004;2004:1286–1297. doi: 10.1002/ejoc.200300673. [DOI] [Google Scholar]
- 60.Singh V., Hutait S., Batra S. Baylis-Hillman reaction of 1-formyl-β-carboline: One-step synthesis of the canthin-6-one framework by an unprecedented cascade cyclization reaction. Eur. J. Org. Chem. 2009;2009:6211–6216. doi: 10.1002/ejoc.200900962. [DOI] [Google Scholar]
- 61.Gollner A., Koutentis P.A. Two-step total syntheses of canthin-6-one alkaloids: New one-pot sequential Pd-catalyzed Suzuki-Miyaura coupling and Cu-catalyzed amidation reaction. Org. Lett. 2010;12:1352–1355. doi: 10.1021/ol100300s. [DOI] [PubMed] [Google Scholar]
- 62.Ioannidou H.A., Martin A., Gollner A., Koutentis P.A. Three-step synthesis of ethyl canthinone-3-carboxylates from ethyl 4-bromo-6-methoxy-1,5-naphthyridine-3-carboxylate via a Pd-catalyzed Suzuki-Miyaura coupling and a Cu-catalyzed amidation reaction. J. Org. Chem. 2011;76:5113–5122. doi: 10.1021/jo200824b. [DOI] [PubMed] [Google Scholar]
- 63.Markgraf J.H., Snyder S.A., Vosburg D.A. A concise route to isocanthin-6-one. Tetrahedron Lett. 1998;39:1111–1112. doi: 10.1016/S0040-4039(97)10794-8. [DOI] [Google Scholar]
- 64.Snyder S.A., Vosburg D.A., Jarvis M.G., Markgraf J.H. Intramolecular hetero Diels-Alder routes to γ-carboline alkaloids. Tetrahedron. 2000;56:5329–5335. [Google Scholar]
- 65.Hájíček J., Trojánek J. Synthesis of optically active 4,4- and 5,5-disubstituted 4,5-dihydro-6H-canthin-6-ones and their CD spectra. Collect. Czechoslov. Chem. Commun. 1982;47:3306–3311. doi: 10.1135/cccc19823306. [DOI] [Google Scholar]
- 66.Hájíček J., Holubek J., Trojánek J. Synthesis of 4,4- and 5,5-disubstituted 4,5-dihydro-6H-canthin-6-ones. Collect. Czechoslov. Chem. Commun. 1982;47:2749–2762. doi: 10.1135/cccc19822749. [DOI] [Google Scholar]
- 67.Chughtai M., Eagan J.M., Padwa A. Intramolecular [4 + 2]-cycloaddition of 5-amino-substituted oxazoles as an approach toward the left-hand segment of haplophytine. Synlett. 2011;2:215–218. [Google Scholar]
- 68.Benson S.C., Li J.H., Snyder J.K. Indole as a dienophile in inverse electron demand Diels-Alder reactions. intramolecular reactions with 1,2,4-triazines to access the canthine skeleton. J. Org. Chem. 1992;57:5285–5287. doi: 10.1021/jo00046a005. [DOI] [Google Scholar]
- 69.Li J.H., Snyder J.K. Selective oxidation of canthines to canthin-6-ones with triethylbenzylammonium permanganate. Tetrahedron Lett. 1994;35:1485–1488. doi: 10.1016/S0040-4039(00)76738-4. [DOI] [Google Scholar]
- 70.Lindsley C.W., Wisnoski D.D., Wang Y., Leister W.H., Zhao Z. A “one pot” microwave-mediated synthesis of the basic canthine skeleton: Expedient access to unnatural β-carboline alkaloids. Tetrahedron Lett. 2003;44:4495–4498. doi: 10.1016/S0040-4039(03)01019-0. [DOI] [Google Scholar]
- 71.De Bruyn A., Eeckhaut G., Villaneuva J., Hannart J. Synthesis and 1H-NMR study of 3,4-diethyl-1,2,3,3a,4,5-hexahydro-canthinone-6. Tetrahedron. 1985;41:5553–5561. doi: 10.1016/S0040-4020(01)91356-0. [DOI] [Google Scholar]
- 72.Puzik A., Bracher F. A convenient approach to the canthin-4-one ring system: Total synthesis of the alkaloids tuboflavine and norisotuboflavine. J. Heterocycl. Chem. 2009;46:770–773. doi: 10.1002/jhet.126. [DOI] [Google Scholar]
- 73.Mitscher L., Shipchandler M., Showaleter H., Bathala M. Antimicrobial agents from higher-plants-synthesis in canthin-6-one (6H-indolo[3,2,1-de][1,5]naphthyridin-6-one) series. Heterocycles. 1975;3:7–14. doi: 10.3987/R-1975-01-0007. [DOI] [Google Scholar]
- 74.Fang H.W., Liao Y.R., Hwang T.L., Shieh P.C., Lee K.H., Hung H.Y., Wu T.S. Total synthesis of cordatanine, structural reassignment of drymaritin, and anti-inflammatory activity of synthetic precursors. Bioorg. Med. Chem. Lett. 2015;25:3822–3824. doi: 10.1016/j.bmcl.2015.07.074. [DOI] [PubMed] [Google Scholar]
- 75.Giudice M.R.D., Settimj F.G.G. New tetracyclic compounds containing the β-carboline moiety. J. Heterocycl. Chem. 1990;27:967–973. doi: 10.1002/jhet.5570270427. [DOI] [Google Scholar]
- 76.Kim H.M., Kim S.J., Kim H.Y., Ryu B., Kwak H., Hur J., Choi J.H., Jang D.S. Constituents of the stem barks of Ailanthus altissima and their potential to inhibit LPS-induced nitric oxide production. Bioorg. Med. Chem. Lett. 2015;25:1017–1020. doi: 10.1016/j.bmcl.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 77.Dai J.K., Dan W.J., Li N., Du H.T., Zhang J.W., Wang J.R. Synthesis, in vitro antibacterial activities of a series of 3-N-substituted canthin-6-ones. Bioorg. Med. Chem. Lett. 2016;26:580–583. doi: 10.1016/j.bmcl.2015.11.070. [DOI] [PubMed] [Google Scholar]
- 78.Cebrian-Torrejon G., Domenech-Carbo A., Scotti M.T., Fournet A., Figadere B., Poupon E. Experimental and theoretical study of possible correlation between the electrochemistry of canthin-6-one and the anti-proliferative activity against human cancer stem cells. J. Mol. Struct. 2015;1102:242–246. doi: 10.1016/j.molstruc.2015.08.042. [DOI] [Google Scholar]
- 79.Lagoutte D., Nicolas V., Poupon E., Fournet A., Hocquemiller R., Libong D., Chaminade P., Loiseau P.M. Antifungal canthin-6-one series accumulate in lipid droplets and affect fatty acid metabolism in Saccharomyces cerevisiae. Biomed. Pharmacother. 2008;62:99–103. doi: 10.1016/j.biopha.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 80.Ohishi K., Toume K., Arai M.A., Koyano T., Kowithayakorn T., Mizoguchi T., Itoh M., Ishibashi M. 9-Hydroxycanthin-6-one, a β-Carboline Alkaloid from Eurycoma longifolia, Is the First Wnt Signal Inhibitor through Activation of Glycogen Synthase Kinase 3β without Depending on Casein Kinase 1α. J. Nat. Prod. 2015;78:1139–1146. doi: 10.1021/acs.jnatprod.5b00153. [DOI] [PubMed] [Google Scholar]
- 81.Sasaki T., Li W., Higai K., Koike K. Canthinone alkaloids are novel protein tyrosine phosphatase 1B inhibitors. Bioorg. Med. Chem. Lett. 2015;25:1979–1981. doi: 10.1016/j.bmcl.2015.03.014. [DOI] [PubMed] [Google Scholar]