Abstract
An ultrasound-assisted extraction (UAE) method was developed for the efficient extraction of natural antioxidants from the flowers of Jatropha integerrima. Four independent variables, including ethanol concentration, solvent/material ratio, ultrasound irradiation time and temperature were studied by single factor experiments. Then, the central composite rotatable design and response surface methodology were employed to investigate the effect of three key parameters (ethanol concentration, solvent/material ratio, and ultrasound irradiation time) on the antioxidant activities of the flower extracts. The optimal extraction conditions were an ethanol concentration of 59.6%, solvent/material ratio of 50:1, ultrasound irradiation time of 7 min, and ultrasound irradiation temperature of 40 °C. Under these conditions, the optimized experimental value was 1103.38 ± 16.11 µmol Trolox/g dry weight (DW), which was in accordance with the predicted value (1105.49 µmol Trolox/g DW). Furthermore, the antioxidant activities of flower extracts obtained by UAE were compared with those produced by the traditional maceration and Soxhlet extraction methods, and UAE resulted in higher antioxidant activities after a shorter time at a lower temperature. The results obtained are helpful for the full utilization of Jatropha integerrima, and also indicate that ultrasound-assisted extraction is an efficient method for the extraction of natural antioxidants from plant materials.
Keywords: Jatropha integerrima, flower, natural antioxidant, ultrasound-assisted extraction, response surface methodology
1. Introduction
Antioxidants play an important role in preventing or slowing down autoxidation in biological systems and food, and have attracted much attention [1]. Naturally-occurring antioxidants in plants like flavonoids and phenolic acids are an important part of the human diet, and are of considerable interest due to their capacity for scavenging free radicals like superoxide anion radical and hydroxyl radical, which can cause several diseases, such as cancer, arteriosclerosis, autoimmune and neurodegenerative syndromes [2,3,4,5,6]. Besides, natural antioxidants from herbs and spices could preserve the quality of food products from chemical oxidation and lipid rancidity as more safety and healthy additives compared with synthetic ones [1,6,7]. Thus, evaluation, extraction, separation and purification of natural antioxidants are very important. Furthermore, effective extraction of antioxidants from plant materials is very helpful for full utilization of natural resources.
Jatropha integerrima, a member of the Euphorbiaceae family, is a drought tolerant perennial shrub. It flowers throughout the year, and is one of the most important ornamental species cultivated in the tropics and subtropics for its attractive crimson flowers [8,9]. The essential oils of the leaves and seeds of Jatropha integerrima displayed weak antimicrobial activity against Bacillus cereus and Staphylococcus aureus [10]. In another study, two cyclic peptides isolated from latex of Jatropha integerrima exhibited significant cytotoxic activity against KB human nasopharyngeal carcinoma cells in vitro [11]. Besides, diterpenoids isolated from the trunks of Jatropha integerrima exhibited stronger inhibitory activity against thioredoxin reductase than the positive control (curcumin), which is a potential target for cancer chemotherapy with redox balance and antioxidant functions [12]. Lately, it was found that flower of Jatropha integerrima had the strongest antioxidant capacity among 51 edible and wild flowers from China [13], which implied that the flowers of Jatropha integerrima were a potential rich resource of natural antioxidants. Consequently, effective extraction of antioxidants from flowers of Jatropha integerrima should be helpful for its full utilization.
The conventional methods of antioxidant extraction from plant materials are mainly maceration and Soxhlet extraction, which are very time-consuming and require relatively large quantities of toxic organic solvents. Ultrasound-assisted extraction (UAE) has been found to be a more effective and environmentally friendly way of extracting natural antioxidants from plant materials for its characteristics of shorter extraction time and less use of organic solvents [14,15,16,17,18,19,20]. The acoustic cavitation effect of UAE permits better penetration of the solvent into the sample, increasing the extraction yield of target components. Because the efficiency of an extraction process is usually influenced by several factors, such as solvent concentration, solvent to solid ratio, extraction temperature and time, optimization of these parameters is very important to obtain high extraction yields.
Response surface methodology (RSM) is an efficaceous mathematical and statistical technique for simultaneously evaluating the interaction of several experimental parameters [6,20]. In the present study, in order to maximize the extraction of antioxidants from the flowers of Jatropha integerrima by UAE, the effects of several extraction parameters (ethanol concentration, solvent/material ratio, ultrasound irradiation time and temperature) were investigated and the optimal conditions were obtained using response surface methodology (RSM) by employing a five-level, three-variable central composite rotatable design (CCRD). Additionally, to further confirm the extraction efficiencies, a comparison between UAE and two conventional extraction methods (maceration and Soxhlet extraction) was also conducted.
2. Results and Discussion
2.1. Single Factor Experiment
In the preliminary study, the influence of several factors (ethanol concentration, solvent/material ratio, ultrasonic time and temperature) on antioxidant activities of the extract of Jatropha integerrima flower was detected and analyzed.
2.1.1. Effect of Ethanol Concentration
Methanol, ethanol, acetone and isopropanol, with different levels of water, have been widely used to extract antioxidant components from botanical materials, especially herbs [21,22,23,24,25]. Compared with methanol, acetone and isopropanol, ethanol and water are safer to human beings and the environment. Thus, aqueous ethanol was used in this study, and the results are shown in Figure 1A. Other extraction conditions were set as follows: solvent/material, 100 mL/g; ultrasound irradiation time, 30 min; temperature, 30 °C. When the ethanol concentration increased from 10% to 50% (v/v), the total antioxidant activities of the extracts increased significantly, changing from 774.40 ± 17.44 to 992.36 ± 10.48 µmol Trolox/g DW. An obvious decrease was observed with further increases of the ethanol concentration from 50% to 90%. The total antioxidant activities using 60% and 90% ethanol were 971.05 ± 6.05 µmol Trolox/g DW and 683.34 ± 23.83 µmol Trolox/g DW, respectively. A similar result was reported in the extraction of antioxidant compounds from Zizyphus lotus fruits [6]. The results indicate that 50% ethanol was more suitable in the subsequent experiments.
Figure 1.
Effects of extraction parameters on the antioxidant activities of flower extracts: (A) Effect of ethanol concentration on the antioxidant activities; (B) Effect of the solvent/material ratio on the antioxidant activities; (C) Effect of ultrasound irradiation time on the antioxidant activities; and (D) Effect of temperature on the antioxidant activities.
2.1.2. Effect of Solvent/Material Ratio
To study the effect of different liquid–solid ratios on the antioxidant activities of the extract of Jatropha integerrima flowers, different solvent/material ratios (15:1, 20:1, 25:1, 30:1, 35:1, 40:1, 45:1, 50:1, 60:1, mL/g) were employed, and the other extraction conditions were set as follows: ethanol concentration, 50%; ultrasound irradiation time 30 min; ultrasound irradiation temperature, 30 °C. The results are displayed in Figure 1B. When the ratio of solvent to material increased from 15:1 to 40:1, the total antioxidant activities increased by 33% (from 742.46 ± 2.22 to 990.17 ± 32.96 µmol Trolox/g DW). When the solvent/material ratio exceeded 40:1 (mL/g), the total antioxidant activities almost did not change. The reason was that the higher ratio of solvent to material could accelerate mass transfer and facilitate the diffusion of antioxidants into the medium until the mass transfer process reached its maximum [26,27]. Therefore, 40:1 was selected as optimal solvent/material ratio.
2.1.3. Effect of Ultrasound Irradiation Time
The effect of different ultrasound irradiation times on the total antioxidant activities of the extracts was compared, and the results are shown in Figure 1C. Other extraction conditions were set as follows: ethanol concentration, 50%; ratio of solvent to material, 40 mL/g; ultrasound irradiation temperature, 30 °C. The antioxidant activities increased from 0 to 15 min, and then decreased when the ultrasound irradiation time was longer than 15 min. The maximum antioxidant activities (1053.20 ± 15.66 µmol Trolox/g DW) were obtained after 15 min. The results indicate that under the ultrasound irradiation treatment, the diffusion of the bioactive compounds from material to solvent might be improved and the equilibrium for dissolution might be established in a short time. But the antioxidant components might be degraded after a long exposure to ultrasonic irradiation [28,29]. Thus, 15 min was used in the subsequent experiments.
2.1.4. Effect of Ultrasound Irradiation Temperature
The effect of temperature on antioxidant activities of the extracts was evaluated, and the results are shown in Figure 1D. Other extraction conditions were set as follows: ethanol concentration, 50%; solvent/material ratio, 40 mL/g; ultrasound irradiation time, 15 min. The antioxidant activities was improved when the temperature was raised from 30 to 40 °C, then the antioxidant activities decreased from 40 to 80 °C. The maximum antioxidant activity (1166.22 ± 16.89 µmol Trolox/g) could be obtained at 40 °C. The results indicate that natural antioxidants from Jatropha integerrima reached an equilibrium of desorption and solubility at 40 °C, and some thermally unstable antioxidants from the flowers could be decomposed at higher temperature [21,30]. Therefore, 40 °C was used in the subsequent experiments.
2.2. Response Surface Methodology
In order to evaluate interaction of several experimental parameters, response surface methodology is used.
2.2.1. Experimental Design and Results of CCRD
According to the single factor experiment results, an ethanol concentration of 50% (v/v), solvent/material ratio of 40:1 (mL/g), and ultrasound irrdiation time of 15 min were chosen as the central condition of the central composite experiment design, and the effects of three independent variables on the dependent variable (TEAC value) at five levels were investigated. The 20 experimental designs and the results are shown in Table 1. Results show that the antioxidant activities ranged from 900.78 to 1092.03 μmol Trolox/g DW. The maximum antioxidant activity was recorded under the experimental parameters of ethanol concentration of 50%, solvent/material ratio of 56.8:1 and ultrasound irradiation time of 15 min.
Table 1.
Experimental design of response surface analysis and its experimental values.
| Run | X1 (Concentration of Ethanol, %) | X2 (Solvent/Material Ratio, mL/g) | X3 (Extraction Time, min) | Y (TEAC Value, µmol Trolox/g DW) |
|---|---|---|---|---|
| 1 | 70 | 50 | 7 | 1082.39 |
| 2 | 83.64 | 40 | 15 | 952.35 |
| 3 | 50 | 40 | 15 | 1063.53 |
| 4 | 50 | 40 | 15 | 1086.78 |
| 5 | 30 | 30 | 23 | 977.83 |
| 6 | 50 | 23.18 | 15 | 951.72 |
| 7 | 50 | 40 | 15 | 1051.91 |
| 8 | 70 | 30 | 7 | 960.39 |
| 9 | 50 | 56.82 | 15 | 1092.03 |
| 10 | 30 | 50 | 23 | 1000.05 |
| 11 | 50 | 40 | 28.45 | 1009.28 |
| 12 | 50 | 40 | 15 | 1040.28 |
| 13 | 50 | 40 | 15 | 1071.28 |
| 14 | 70 | 50 | 23 | 1029.11 |
| 15 | 50 | 40 | 15 | 1055.78 |
| 16 | 70 | 30 | 23 | 948.77 |
| 17 | 30 | 30 | 7 | 919.71 |
| 18 | 50 | 40 | 1.55 | 1063.53 |
| 19 | 16.36 | 40 | 15 | 900.78 |
| 20 | 30 | 50 | 7 | 990.36 |
2.2.2. Fitting the Model
Analysis of variance (ANOVA) was performed to evaluate the quality of the fitted model (Table 2). In this model, the second-order polynomial model for the extraction of antioxidants was statistically significant with a small model p-value (p < 0.0001) and satisfactory coefficient of determination (R2 = 0.965). The linear parameters (X1, X2) and quadratic parameters (X12, X22) were significant at the level of p < 0.01, interaction parameters (X1X2, X1X3) and quadratic parameter (X32) were significant at the level of p < 0.05. The “Lack of Fit-Value” of the model is not significant with a p-value of 0.61. The significant regression and non-significant lack of fit indicated that the regression equation is adequate to represent the actual relationship between the response values (Y) and three independent variables. The quadratic regression equation was obtained as follows Equation (1):
| Y = 285.63 + 11.68X1 + 14.21X2 + 14.41X3 + 0.07X1X2 − 0.10X1X3 − 0.14X2X3 − 0.12X12 − 0.15X22 − 0.15X32 | (1) |
Table 2.
ANOVA for the response surface quadratic model.
| Source | Sum of Squares | df | Mean Square | F Value | p Value | Significant |
|---|---|---|---|---|---|---|
| Model | 64580.50 | 9 | 7175.61 | 30.93 | <0.0001 | significant |
| X1 (ethanol concentration) | 3526.02 | 1 | 3526.02 | 15.20 | 0.0030 | |
| X2 (ratio of solvent to material) | 20659.29 | 1 | 20659.29 | 89.04 | <0.0001 | |
| X3 (ultrasonic time) | 571.30 | 1 | 571.30 | 2.46 | 0.1477 | |
| X1X2 | 1497.85 | 1 | 1497.85 | 6.46 | 0.0293 | |
| X1X3 | 2201.69 | 1 | 2201.69 | 9.49 | 0.0116 | |
| X2X3 | 1014.57 | 1 | 1014.57 | 4.37 | 0.063 | |
| X12 | 33523.51 | 1 | 33523.51 | 144.48 | <0.0001 | |
| X22 | 3044.43 | 1 | 3044.43 | 13.12 | 0.0047 | |
| X32 | 1272.59 | 1 | 1272.59 | 5.48 | 0.0412 | |
| Residual | 2320.28 | 10 | 232.03 | |||
| Lack of Fit | 1006.46 | 5 | 201.29 | 0.77 | 0.6115 | Not significant |
| Pure Error | 1313.82 | 5 | 262.76 | |||
| Cor Total | 66900.79 | 19 | ||||
| R-Squared | 0.965 | |||||
| Adj R-Squared | 0.934 |
2.2.3. Analysis of Response Surfaces
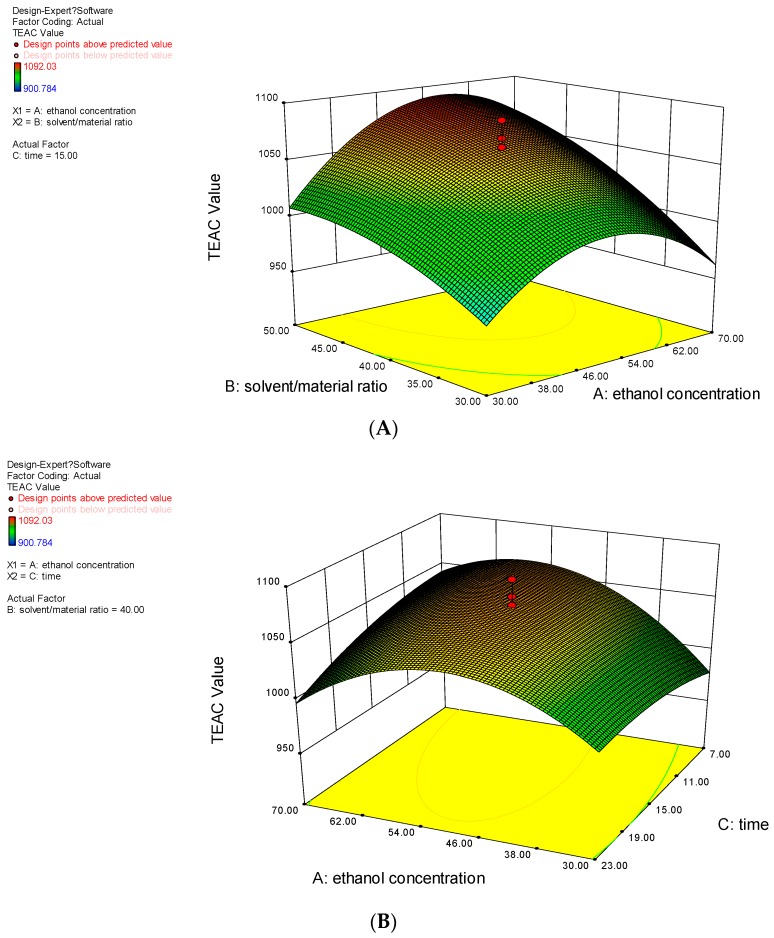
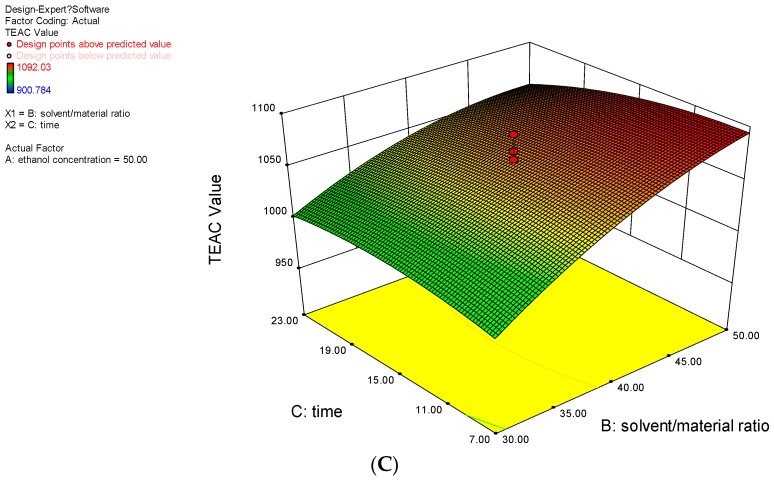
Response surface plots are shown in Figure 2. The interaction between various factors can be seen directly from the response surfaces plots. Figure 2A shows the effect of the interaction of ethanol concentration and solvent/material ratio on the antioxidant activities at a fixed ultrasound irradiation time of 15 min. An increase of liquid-to-solid ratio (X2) resulted in an increase of antioxidant activities to a maximum at a certain level, while an increase of ethanol concentration (X1) resulted in an initial increase of antioxidant activities and then decreased as the concentration continued to increase. Figure 2B shows the effect of the interaction of ethanol concentration and ultrasound irradiation time on the antioxidant activities at a fixed solvent/material ratio of 40:1 mL/g. It could be observed that the concentration of ethanol resulted in similar effects on the antioxidant activities as in Figure 2A, whereas ultrasound irradiation time had only a limited impact on the antioxidant activities. Figure 2C shows the effect of the interaction of solvent/material ratio and ultrasound irradiation time on the antioxidant activities at a fixed ethanol concentration of 50%. It could be observed that the solvent/material ratio demonstrated a strongly positive influence on the antioxidant activities, whereas ultrasound irradiation time had only a slight impact. The combination of the analysis of variance (ANOVA) (Table 2) and response surfaces (Figure 2) indicated that the interaction effect between ethanol concentration and solvent/material ratio (X1X2), and ethanol concentration and ultrasound irradiation time (X1X3) were statistically significant, but the interaction effect between solvent/material ratio and ultrasound irradiation time (X2X3) was non-significant. Furthermore, it was concluded that the effect of ethanol concentration and solvent/material ratio were more significant than ultrasound irradiation time on the antioxidant activities.
Figure 2.
Interaction effects of ethanol concentration and solvent/material ratio (A); ethanol concentration and ultrasound irradiation (B); solvent/material ratio and ultrasound irradiation time (C) on antioxidant activities.
2.2.4. Verification of Predicted Value of the Models
The optimal conditions obtained using the model were as follows: ethanol concentration, 59.6%; solvent/material ratio, 50:1; temperature, 40 °C; and ultrasound irradiation time, 7 minutes. Under optimal conditions, the maximum response value of 1105.49 µmol Trolox/g DW was predicted by the model. Verification experiments were performed at the predicted conditions. The result showed that the experimental value (1103.376 ± 16.11 µmol Trolox/g DW; n = 6) was consistent with the predictive value (Table 3). The good correlation between predicted and experimental value demonstrated that response surface methodology is accurate and reliable to find the optimum ultrasound irradiation extraction conditions for antioxidants from flowers of Jatropha integerrima.
Table 3.
Optimum conditions, predicted and experimental value.
| Optimal Condition | TEAC Value (µmol Trolox/g DW) | |||
|---|---|---|---|---|
| Ethanol Concentration (%) | Solvent/Material Ratio (mL/g) | Time (min) | Experimental | Predicted |
| 59.6 | 50 | 7 | 1103.38 ± 16.11 | 1105.49 |
2.3. Comparison of Ultrasound-Assisted Extraction with Maceration and Soxhlet Extraction Methods
As shown in Table 4, the antioxidant activity by UAE (1103.38 ± 16.11 µmol Trolox/g) was 1.88 times that obtained by Soxhlet extraction (588.06 ± 10.47 µmol Trolox/g), and the extraction temperature of UAE (40 °C) was also greatly reduced compared to Soxhlet extraction (95 °C). Besides, the ultrasound irradiation time of UAE (7 min) was significantly shorter than maceration method (24 h) and Soxhlet extraction (4 h). It was concluded that UAE was the most efficient extraction method among the three methods. The results were in agreement with published studies on the extraction of nutraceutical compounds from Dendrobium candidum, flavonoids from grapefruit (Citrus paradisi L.) solid wastes and anthocyanins from blackberry and sweet cherry cultivars [31,32,33], definitely demonstrating that UAE can be applied in the extraction of antioxidants from Jatropha integerrima with higher efficiency and shorter time.
Table 4.
The comparison of UAE with maceration and Soxhlet extraction.
| Extracting Method | Ethanol Concerntration | Temperature | Time | TEAC Value (µmol Trolox/g DW) |
|---|---|---|---|---|
| UAE | 59.63% | 40 °C | 7 min | 1103.38 ± 16.11 |
| Maceration extraction | 59.63% | 25 °C | 24 h | 1022.65 ± 42.32 |
| Soxhlet extraction | 59.63% | 95 °C | 4 h | 588.06 ± 10.47 |
3. Experimental Section
3.1. Chemicals
The compounds 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), and 2,2′-azinobis(3-ethylbenothiazoline-6-sulphonic acid) diammonium salt (ABTS) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Potassium persulfate was purchased from Tianjin Chemical Factory (Tianjin, China). Ethanol and methanol were obtained from Kelong Chemical Factory (Chengdu, China). All chemicals used in the experiments were of analytical grade, and deionized water was used.
3.2. Instruments
The ultrasound-assisted extraction was carried out in a Kj1012B ultrasonic water bath (Guangzhou Kejin Ultrasonic Instrument Factory, Guangzhou, China) with an electric power of 400 W, and 40 kHz frequency, equipped with a digital timer and a temperature controller for the control of time and temperature. The bath consisted of a rectangular vessel (20.2 cm × 17.5 cm × 22.9 cm). The ultrasonic energy was delivered from the bottom to the water with a relatively constant frequency of 40 kHz.
3.3. Sample Preparation
The flowers of Jatropha integerrima were picked from Guangzhou, China. The flowers were washed with deionized water, given an airing at room temperature, and dried to 2.1% residual moisture utilizing freeze-drying technology. Then, the flowers were ground into fine particles (smaller than 0.3 mm) using a special grinder for processing food (RHP-100, Ronghao Industry & Trade Co. Ltd., Yongkang, China), and were stored at 4 °C until used.
3.4. Extraction Procedures of Antioxidants
3.4.1. Ultrasound-assisted Extraction
The ground powder (0.100 g) was placed in a capped tube (15 mL) and mixed with an appropriate amount of ethanol-water solution (according to the experimental design). Next, the mixture was immersed in water in the ultrasonic device at 40 kHz and fixed well in the same position during sonication, for the pre-set ultrasound irradiation time and temperature with a power of 28 W/L. The real temperature of the extraction solution was monitored by a thermometer, and the target temperature was adjusted/controlled using the temperature controller. After extraction, the sample was centrifuged at 4200 g for 30 min, and the supernatant was obtained and stored at 4 °C for the antioxidant activity determination within 48 h.
3.4.2. Maceration Extraction
The ground powder (0.100 g) was mixed with 59.63% ethanol (5 mL), and the antioxidants were extracted at 25 °C for 24 h in a shaking water bath. Then, the sample was centrifuged at 4200 g for 30 min, and the supernatant was collected and stored at 4 °C for the antioxidant activity determination within 48 h.
3.4.3. Soxhlet Extraction
The ground powder (2.000 g) were kept over a Whatman filter paper. The antioxidants were extracted with 59.63% ethanol (400 mL) during percolation. The solvent was heated at 95 °C in a Soxhlet extractor [27,34]. After 4 h, the extract was taken out and stored at 4 °C for the antioxidant activity determination within 48 h.
3.5. Determination of Antioxidant Capacity
The antioxidant activity of the extract was evaluated using Trolox equivalent antioxidant capacity assay according to the procedure described previously with slight modification [35]. Firstly, the ABTS•+ stock solution was prepared through mixing 7 mmol/L ABTS with 2.45 mmol/L potassium persulfate in a volume ratio of 1:1, and incubating in the dark for 16 h at room temperature and used within 2 days. Secondly, the ABTS•+ stock solution were dilute to make sure that the absorbance of ABTS•+ working solution was 0.70 ± 0.05 at 734 nm. Finally, 100 μL of the dilute sample was mixed with 3.8 mL ABTS•+ working solution and the absorbance was measured at 734 nm after 6 min of incubation at room temperature. The results were calculated and expressed as µmol Trolox/g dry weight.
3.6. Experimental Design
3.6.1. Single-Factor Experiments
To evaluate the effect of each factor under ultrasound treatment on antioxidant activity of the extract of Jatropha integerrima flower, ethanol concentration (0%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%), solvent/material ratio (15:1, 20:1, 25:1, 30:1, 35:1, 40:1, 45:1, 50:1, 60:1 mL/g), ultrasound irradiation time (0, 5, 10, 15, 20, 25, 30 min) and ultrasound irradiation temperature (30, 40, 50, 60, 70, 80 °C) were investigated as single factor variables in the experimental design. Three variables that affected the extraction efficiencies significantly were selected in this part for the subsequent experiments.
3.6.2. Response Surface Methodology Experiments
Response surface method was used to find the optimal condition of ultrasound-assisted extraction. According to the results of single factor optimization, three variables were selected. Each variable was coded as X1–X3 and examined in five levels (Table 5). The 20 experimental runs including six replicates at the center point were employed. The Design-Expert (DE) design assumes that the main effects of the variables have interactions and are based on a second-order polynomial model [36,37], as follows:
| Y = β0 + ∑βiXi + ∑βii Xi2 + ∑βij Xi Xj | (2) |
where Y is the response value; β0 is the constant; βi is the linear regression coefficient; βii is the quadratic regression coefficient; βij is the interaction regression coefficient; Xi and Xj are the independent variables.
Table 5.
Variables and their five levels employed in central composite rotatable design.
| Variable | Units | Symbol | Code Levels | ||||
|---|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | |||
| Ethanol concentration | % (v/v) | X1 | 16.36 | 30 | 50 | 70 | 83.64 |
| Solvent/material ratio | mL/g | X2 | 23.18 | 30 | 40 | 50 | 56.82 |
| Ultrasonic time | min | X3 | 1.55 | 7 | 15 | 23 | 28.45 |
3.7. Statistical Analysis
All the experiments were performed in triplicate, and the average value ± SD (standard deviation) was reported. Statistical analysis was performed using Design Expert 8.06.1, and Excel 2007.
4. Conclusions
In this study, an ultrasound-assisted extraction method was developed for the extraction of natural antioxidants from the flower of Jatropha integerrima, and the optimal extraction conditions were obtained by response surface methodology. The high correlation (R2 = 0.965) of the model showed that the second-order polynomial model could successfully express the influence of independent variables on the response. The results showed that the extraction conditions including ethanol concentration, solvent/material ratio and ultrasound irradiation time markedly influenced the antioxidant activities of the extract of Jatropha integerrima flower. The optimal UAE conditions were as follows: ethanol concentration of 59.6%, solvent/material ratio of 50:1, ultrasound irradiation time of 7 min, and temperature of 40 °C, which resulted in 1103.38 ± 16.11 µmol Trolox/g DW for antioxidant activities of the extract of Jatropha integerrima flower. The comparison of UAE and two conventional extraction methods (maceration and Soxhlet extraction) showed the superiority of UAE for extracting antioxidants from the flower of Jatropha integerrima. It has been evidenced that UAE combined with RSM is an effective technique for the extraction of natural antioxidants from the flower of Jatropha integerrima. The results should be helpful for full utilization of Jatropha integerrima, and also indicate that UAE is an efficient method for the extraction of natural antioxidants from plant materials.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81372976), Key Project of Guangdong Provincial Science and Technology Program (No. 2014B020205002), and the Hundred-Talents Scheme of Sun Yat-Sen University.
Author Contributions
D.-P.X. and H.-B.L. conceived and designed the experiments; D.-P.X., Y.Z. and J.Z. performed the experiments; D.-P.X. analyzed the data and wrote the paper; S.L., A.-N.L. and H.-B.L. contributed to valuable discussion and revising manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Shahidi F., Zhong Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010;112:930–940. doi: 10.1002/ejlt.201000044. [DOI] [Google Scholar]
- 2.Pellegrini N., Serafini M., Colombi B., Del Rio D., Salvatore S., Bianchi M., Brighenti F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003;133:2812–2819. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- 3.Balasundram N., Sundram K., Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- 4.Ksouri R., Ksouri W.M., Jallali I., Debez A., Magne C., Hiroko I., Abdelly C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012;32:289–326. doi: 10.3109/07388551.2011.630647. [DOI] [PubMed] [Google Scholar]
- 5.Sahin S., Aybastıer O., Isik E. Optimisation of ultrasonic-assisted extraction of antioxidant compounds from Artemisia absinthium using response surface methodology. Food Chem. 2013;141:1361–1368. doi: 10.1016/j.foodchem.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Hammi K.M., Jdey A., Abdelly C., Majdoub H., Ksouri R. Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Zizyphus lotus fruits using response surface methodology. Food Chem. 2015;184:80–89. doi: 10.1016/j.foodchem.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Estaca J., Lopez-de-Dicastillo C., Hernandez-Munoz P., Catala R., Gavara R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014;35:42–51. doi: 10.1016/j.tifs.2013.10.008. [DOI] [Google Scholar]
- 8.Sujatha M., Dhingra M. Rapid plant regeneration from various explants of Jatropha integerrima. Plant Cell Tissue Organ Cult. 1993;35:293–296. doi: 10.1007/BF00037284. [DOI] [Google Scholar]
- 9.Sujatha M., Sivaraj N., Prasad M.S. Biochemical and histological changes during in vitro organogenesis in Jatropha integerrima. Biol. Plant. 2000;43:167–171. doi: 10.1023/A:1002775420715. [DOI] [Google Scholar]
- 10.Eshilokun A.O., Kasali A.A., Ogunwande I.A., Walker T.M., Setzer W.N. Chemical composition and antimicrobial studies of the essential oils of Jatropha integerrima Jacq (leaf and seeds) Nat. Prod. Commun. 2007;2:853–855. [Google Scholar]
- 11.Wele A., Baragueye C., Ndiaye W., Fall D., Ndoye I., Diop Y., Dubosq Y., Bodo B. Cytotoxic activity of two cyclic peptides from the latex of Jatropha integerrima Euphorbiaceae. Dakar Med. 2006;52:209–215. [PubMed] [Google Scholar]
- 12.Zhu J.Y., Lou L.L., Guo Y.Q., Li W., Guo Y.H., Bao J.M., Tang G.H., Bu X.Z., Yin S. Natural thioredoxin reductase inhibitors from Jatropha integerrima. RSC Adv. 2015;5:47235–47243. doi: 10.1039/C5RA07274C. [DOI] [Google Scholar]
- 13.Li A.N., Li S., Li H.B., Xu D.P., Xu X.R., Chen F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Food. 2014;6:319–330. doi: 10.1016/j.jff.2013.10.022. [DOI] [Google Scholar]
- 14.Hamdaoui O., Naffrechoux E. An investigation of the mechanisms of ultrasonically enhanced desorption. AICHE J. 2007;53:363–373. doi: 10.1002/aic.11090. [DOI] [Google Scholar]
- 15.Vilkhu K., Mawson R., Simons L., Bates D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008;9:161–169. doi: 10.1016/j.ifset.2007.04.014. [DOI] [Google Scholar]
- 16.Garcia-Salas P., Morales-Soto A., Segura-Carretero A., Fernandez-Gutierrez A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules. 2010;15:8813–8826. doi: 10.3390/molecules15128813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majd M.H., Rajaei A., Bashi D.S., Mortazavi S.A., Bolourian S. Optimization of ultrasonic-assisted extraction of phenolic compounds from bovine pennyroyal (Phlomidoschema parviflorum) leaves using response surface methodology. Ind. Crop. Prod. 2014;57:195–202. doi: 10.1016/j.indcrop.2014.03.031. [DOI] [Google Scholar]
- 18.Yolmeh M., Najafi M.B.H., Farhoosh R. Optimisation of ultrasound-assisted extraction of natural pigment from annatto seeds by response surface methodology (RSM) Food chem. 2014;155:319–324. doi: 10.1016/j.foodchem.2014.01.059. [DOI] [PubMed] [Google Scholar]
- 19.Rosello-Soto E., Galanakis C.M., Brncic M., Orlien V., Trujillo F.J., Mawson R., Knoerzerf K., Tiwari B.K., Barba F.J. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci. Technol. 2015;42:134–149. doi: 10.1016/j.tifs.2015.01.002. [DOI] [Google Scholar]
- 20.Vuong Q.V., Thanh D.T., Bhuyan D.J., Goldsmith C.D., Sadeqzadeh E., Scarlett C.J., Bowyer M.C. Optimization of ultrasound-assisted extraction conditions for euphol from the medicinal plant, Euphorbia tirucalli, using response surface methodology. Ind. Crop. Prod. 2015;63:197–202. doi: 10.1016/j.indcrop.2014.09.057. [DOI] [Google Scholar]
- 21.Ma Y., Ye X., Hao Y., Xu G., Xu G., Liu D. Ultrasound-assisted extraction of hesperidin from Penggan (Citrus reticulata) peel. Ultrason. Sonochem. 2008;15:227–232. doi: 10.1016/j.ultsonch.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Wang J., Sun B., Cao Y., Tian Y., Li X. Optimisation of ultrasound-assisted extraction of phenolic compounds from wheat bran. Food Chem. 2008;106:804–810. doi: 10.1016/j.foodchem.2007.06.062. [DOI] [Google Scholar]
- 23.Vankar P.S., Srivastava J. Ultrasound-assisted extraction in different solvents for phytochemical study of Canna indica. Int. J. Food Eng. 2010;6:1556–3758. doi: 10.2202/1556-3758.1599. [DOI] [Google Scholar]
- 24.Zou T.B., Xia E.Q., He T.P., Huang M.Y., Jia Q., Li H.W. Ultrasound-assisted Extraction of Mangiferin from Mango (Mangifera indica L.) leaves using response surface methodology. Molecules. 2014;19:1411–1421. doi: 10.3390/molecules19021411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghitescu R.E., Volf I., Carausu C., Bühlmann A. M., Gilca I. A., Popa V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015;22:535–541. doi: 10.1016/j.ultsonch.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Yang L., Cao Y.L., Jiang J.G., Lin Q.S., Chen J., Zhu L. Response surface optimization of ultrasound-assisted flavonoids extraction from the flower of Citrus aurantium L. var. amara Engl. J. Sep. Sci. 2010;33:1349–1355. doi: 10.1002/jssc.200900776. [DOI] [PubMed] [Google Scholar]
- 27.Li A.N., Li S., Xu D.P., Xu X.R., Chen Y.M., Ling W.H., Chen F., Li H.B. Optimization of ultrasound-assisted extraction of lycopene from papaya processing waste by response surface methodology. Food Anal. Meth. 2015;8:1207–1214. doi: 10.1007/s12161-014-9955-y. [DOI] [Google Scholar]
- 28.Ghafoor K., Choi Y.H., Jeon J.Y., Jo I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food Chem. 2009;57:4988–4994. doi: 10.1021/jf9001439. [DOI] [PubMed] [Google Scholar]
- 29.Tiwari B.K., O’Donnell C.P., Cullen P.J. Effect of sonication on retention of anthocyanins in blackberry juice. J. Food Eng. 2009;93:166–171. doi: 10.1016/j.jfoodeng.2009.01.027. [DOI] [Google Scholar]
- 30.Zou Y., Xie C., Fan G., Gu Z., Han Y. Optimization of ultrasound-assisted extraction of melanin from Auricularia auricula fruit bodies. Innov. Food Sci. Emerg. Technol. 2010;11:611–615. doi: 10.1016/j.ifset.2010.07.002. [DOI] [Google Scholar]
- 31.Oancea S., Grosu C., Ketney O., Stoia M. Conventional and ultrasound-assisted extraction of anthocyanins from blackberry and sweet cherry cultivars. Acta Chim. Slov. 2013;60:383–389. [PubMed] [Google Scholar]
- 32.Cui H.Y., Murthy H.N., Moh S.H., Cui Y.Y., Lee E.J., Paek K.Y. Comparison of conventional and ultrasound-assisted methods for extraction of nutraceutical compounds from Dendrobium candidum. CyTA J. Food. 2014;12:355–359. doi: 10.1080/19476337.2014.888482. [DOI] [Google Scholar]
- 33.Garcia-Castello E.M., Rodriguez-Lopez A.D., Mayor L., Ballesteros R., Conidi C., Cassano A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT-Food Sci. Technol. 2015;64:1114–1122. doi: 10.1016/j.lwt.2015.07.024. [DOI] [Google Scholar]
- 34.Chemat F., Li Y., Tomao V., Ginies C., Cravotto G. Optimization of procedures for in-line extraction of lipids and polyphenols from grape seeds. Food Anal. Meth. 2014;7:459–464. doi: 10.1007/s12161-013-9646-0. [DOI] [Google Scholar]
- 35.Jing C.L., Dong X.F., Tong J.M. Optimization of ultrasonic-assisted extraction of flavonoid compounds and antioxidants from Alfalfa using response surface method. Molecules. 2015;20:15550–15571. doi: 10.3390/molecules200915550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Celli G.B., Ghanem A., Brooks M.S.L. Optimization of ultrasound-assisted extraction of anthocyanins from haskap berries (Lonicera caerulea L.) using Response Surface Methodology. Ultrason. Sonochem. 2015;27:449–455. doi: 10.1016/j.ultsonch.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 37.Wang W., Ma X., Xu Y., Cao Y., Jiang Z., Ding T., Ye X., Liu D. Ultrasound-assisted heating extraction of pectin from grapefruit peel: Optimization and comparison with the conventional method. Food chem. 2015;178:106–114. doi: 10.1016/j.foodchem.2015.01.080. [DOI] [PubMed] [Google Scholar]