Abstract
Quantifying the similarity of molecules is considered one of the major tasks in virtual screening. There are many similarity measures that have been proposed for this purpose, some of which have been derived from document and text retrieving areas as most often these similarity methods give good results in document retrieval and can achieve good results in virtual screening. In this work, we propose a similarity measure for ligand-based virtual screening, which has been derived from a text processing similarity measure. It has been adopted to be suitable for virtual screening; we called this proposed measure the Adapted Similarity Measure of Text Processing (ASMTP). For evaluating and testing the proposed ASMTP we conducted several experiments on two different benchmark datasets: the Maximum Unbiased Validation (MUV) and the MDL Drug Data Report (MDDR). The experiments have been conducted by choosing 10 reference structures from each class randomly as queries and evaluate them in the recall of cut-offs at 1% and 5%. The overall obtained results are compared with some similarity methods including the Tanimoto coefficient, which are considered to be the conventional and standard similarity coefficients for fingerprint-based similarity calculations. The achieved results show that the performance of ligand-based virtual screening is better and outperforms the Tanimoto coefficients and other methods.
Keywords: chemoinformatics, similarly search, similarity coefficients, virtual screening, drug discovery
1. Introduction
The past few years have witnessed more attention to chemoinformatics and now it has become an active multidisciplinary research area that covers wide aspects of chemistry and drug discovery using different tools and technology. Virtual screening (VS) is considered as one of the most relevant aspects of chemoinformatics; the term screening is given to the selection of molecules for bioactivity testing, and the use of computer methods for the selection of molecules is hence generally referred to as virtual screening [1]. The purpose of VS methods and techniques is to screen a large database of molecules in order to find compounds that fit some established criteria [2]. VS is now one of the important processes of discovering new ligands on the bases of biological structure and it has many definitions, one of which is: “Use of high-performance computing to analyze large databases of chemical compounds in order to identify possible drug candidates” [3]. VS is different and contrasts high-throughput screening (HTS). The experiments are not really done in a chemical laboratory, as HTS, and the compounds do not need to physically exist as they are virtually done by computers programs and methods. They actually rely on computational methods that are used to search molecular databases and identify molecular structures that are most likely to bind to a drug target, typically a protein receptor or enzyme. The VS research challenge is how to develop sophisticated techniques that can easily synthesize and analyze large molecules numbers to help in the drug discovery process [3,4,5,6,7].
For molecular data, there are several types of descriptors, where the molecular descriptor is defined as “The final result of a logical and mathematical procedure which transforms chemical information encoded within a symbolic representation of a molecule into an useful number or the result of some standardized experiment“ [8], the most Ligand-based Virtual Screening(LBVS) methods use 2D fingerprint descriptors [9,10,11], then they calculate the similarity between molecules using similarity coefficients and some others methods. A query involves the specification of the entire structure of a molecule. The drug discovery process faces some challenges and some difficulties, such as complexity, cost and its time-consuming nature. Some studies estimate that the process, and the time and cost of discovering and developing a new drug takes over 10 years and costs approximately $1 billion [12]. To solve the high cost and reduce the time of developing drugs, a lot of researches are being done to provide methods and techniques that could contribute to easing the process. These studies have focused on aiding the efforts of the drug discovery process by using several computational methods. In this paper, we focused on the ligand-based virtual screening (LBVS) approach, which is based on comparative molecular similarity and the analysis of compounds that have known and unknown activities. This approach involves using large molecular databases to find similar molecules that have similar biological properties, as the molecular similarity principle states that “similar compounds tend to have similar properties and activities” [13], and that means molecules that are structurally similar tend to have similar activities. This could be used to identify, analyse and predict the most biologically active compounds and then demonstrate the correlated structural features and chemical properties of molecules with specific activities.
In LBVS, many methods and techniques have been proposed [14,15,16] for instance, Zheng et al. studied the performance of twenty different LBVS methods and they found that the LBVS is a good predictive of lead identification [16], and the work by Ripphausen et al. discussed the LBVS from different viewpoints by analyzing the information that have been provided form peer-reviewed publication [17]. Other research used the Bayesian inference network (BIN) and molecular fragments reweighing to enhance LBVS [14], and another recent work used the quantum based similarity measure (QBS) that provided a clear enhancements for LBVS. In addition, other works applied different fusion rules and techniques using the group fusion and similarity fusion, for instance, Willett [18] discussed and demonstrated most of these fusion rules that available for similarity ranking, Ali and et al. [15] developed a Condorcet fusion which combines the outputs of similarity searches using different distance similarity coefficients. However, more research needs to be done to provide better results for LBVS methods.
The rest of the paper is organized as follows: the next section describes the general concepts of similarity searching. The third section describes the proposed method, the fourth section discusses the experimental design and the results that have been carried out, and the last section concludes the work.
2. Related Work
The similarity measures have been used in different aspects of sciences and for different purposes such as clustering, classification and retrieval problems, the way of finding the appropriate similarity measures for specific domain needs considerable efforts, for chemical area a lot of some many similarity measures have been proposed as earlier we explained, the researchers found that some of these similarity measures which are appropriately work with text and document retrieving also properly work with chemical information [19], for this reason in chemoinformatics researchers give high consideration to the similarity measures proposed in the document and text retrieval area, one of these recently proposed similarity measures for the document retrieval area is the A Similarity Measure for Text Classification and Clustering (SMTP) that proposed by Lin et al. [20], which gained interest in this work, as the SMTP provided a good solution for document classification and clustering based on the flowing similarity proprieties [20]:
The presence or absence of a feature is more essential than the difference between the two values associated with a present feature.
The similarity degree should increase when the difference between two non-zero values of a specific feature decreases.
The similarity degree should decrease when the number of presence-absence features increases.
Two documents are less similar to each other if none of the features have non-zero values in both documents.
The similarity measure should be symmetric.
The value distribution of a feature is considered, i.e., the standard deviation of the feature is taken into account, for its contribution to the similarity between two documents. A feature with a larger spread offers more contribution to the similarity between d1 and d2.
SMTP is used to measure the similarity measures between two documents, and also it has been extended to measure the similarity between two sets of documents. The SMTP has been derived from different similarity measures that have been used for text such as Dice, Euclidean, Extended Jacard, Cosine which are shown in Table 1 where d1 and d2 are two documents represented as vectors.
Table 1.
Similarity measures most used in text retrieval.
| Similarity Measure | Formula |
|---|---|
| Extended Jaccard coefficient | S= |
| Dice | = |
| Euclidean distance | |
| Cosine similarity | |
| Pairwise-adaptive | = |
| IT-Sim |
where di,K in Pairwise-adaptive is a subset of containing the values of the features which are the union of the K largest features appearing in d1 and d2, respectively.
The SMTP measure the similarity between two documents and . Define a function F as follows in Equation (1) 1below:
| (1) |
where this function numerator and denominator have different cases according to the compared features values as mentioned below.
has three different cases according the value of compared features:
.
.
has two cases:
where: : document 1 and document 2 (compared document)
J: number of features (vectors).
σ: standard deviation of all non-zero values in a vector.
λ : small value of λ, e.g., 0.01–0.0001
Their proposed similarity measure as shown in Equation (2) below:
| (2) |
The measures of SMTP that are shown in Equation (2), are focused to meet all proprieties which provide solution of weakness of text similarity measures which are mentioned in Table 1, for the Euclidean does not meet properties 1, 3, 4, and 6 which are previously stated, and Cosine, Pairwise-adaptive, Extended Jaccard, Dice, and IT-Sim does not satisfy one or more of properties 3, 4 and 6. SMTP provides good results in classification and clustering. In this work we adopted SMTP to be used in ligand-based virtual screening.
3. Similarity Searching
The similarity between any two objects regardless of what that object is can be described as the degree of overlap between characteristic features of these specific objects. These overlaps are calculated using different ways and methods. In cheminformatics, the calculation of similarity measures in order to find matching molecules is not an easy task, as the molecular data representation is quite different from other data representations. For LBVS the similarity searching is considered as an important technique for screening chemical databases in order to identify those molecules that are most similar to other user-defined reference structures using computerized methods and techniques. In virtual screening, there are three main searching methods that are used for compound databases: the structure search, the substructure search and the similarity search. Structure searching focuses on searching a molecular database to check if there are presences or absences of a specific molecule in the database [21,22,23]. The substructure search focuses on the retrieval of molecules that contain a partial structure of a user-defined query [24,25]. Finally, similarity searching is focuses on searching the database to find a similar molecules by look for all the structures in a database that are achieving the highly similar to a given structure [26] for the exact match of molecules and concentrating on the specification of the entire structure of a molecule [27,28,29,30,31,32]. There are some works that combine more than research methods for screening [33]; in VS, the major task of similarity searching is to search databases to find which molecules in a database are similar or contain specific molecular structures and then detect fragments that are shared by the molecules. Many similarity coefficients have been applied in similarity searches and, for classification and clustering purposes, the literature is full of descriptions of these similarity coefficients. The work of Willett [26], and Todeschini et al. [34] discuss most of the proposed similarity measures in chemical databases. Other methods have also been used besides similarity coefficients, such as machine learning methods adapted for VS like the Naive Bayesian classifier [15,35,36], support vector machines (SVMs) [37,38] and voting techniques [37,39,40].
For the molecular similarity searching, many coefficients are introduced and applied with different fingerprint molecular databases. For instance, the Euclidian distance, Cosine, Dice, Forbes and Tanimoto coefficients, which is considered as the standard similarity measure for fingerprint-based similarity calculations [4].
4. Methods
4.1. Tanimoto Similarity Method
Both binary and distance similarity coefficients have been applied, as we mentioned before. A typical coefficient used in chemoinformatics is Jacard-Tanimoto, which is considered the most widely used asymmetric coefficient. It has two formulas for binary and continuous data, as shown in Equations (3) and (4) below, when the molecules A and B are represented by vectors, x, of length N with the number of property having the value .
Equation (3) Tanimoto continuous variable formula:
| (3) |
Equation (4) Tanimoto binary variable formula:
| (4) |
a = bits set to 1 in A; b = bits set to 1 in B; c = number of 1 bits common to both.
Besides that, there are also other measures that have been popularly adopted for computing the similarity between two molecules derived for different areas and these have achieved good results.
4.2. The Adapted Similarity Measure of Text Processing (ASMTP)
The molecules are usually represented in fingerprints, which represent a way of encoding the structure of a molecule. The most common type of fingerprints is a series of binary digits (bits) that represents the presence or absence of particular substructures in the molecule. These features are stored in vectors where each component indicates the value of the corresponding feature in the molecule. The feature values are represented as bit-strings values. The investigation of these bit-strings values show that approximately 90% are zeros, and this sometimes causes difficulty and complexity when quantifying the similarity in drug discovery compared to the other domains of quantifying objects.
The proposed ASMTP algorithm has been derived for the text area, as we found that most of the algorithms developed for textual database processing can be used for processing chemical structure databases [1,19]. This has been applied in several text applications, including single label classification, multi-label classification, k-means like clustering and hierarchical agglomerative clustering, and the results obtained demonstrate the effectiveness of the proposed similarity measure. The documents and text databases are structured typically to the molecular databases, where both use small numbers for representing documents and chemical data by vectors. ASMTP is an adaptation of recently work of [20] which relies on three similarity properties concepts:
-
(a)
The feature appears in both documents.
-
(b)
The feature appears in only one document.
-
(c)
The feature appears in none of the documents.
There are different assumptions for each of the mentioned proprieties concepts. For the first case, the assumption was that similarity increases as the difference between the two involved feature values decreases. For the second case they put an assumption that a fixed value contributed to the similarity. For the last case, if the feature does not appear in the compared objects, this will result in there being no contribution to the similarity. In addition to these similarity properties, the values of the distribution and average of the feature values are taken into account. This has a good effect on calculating the similarity between two molecules. The features with a larger spread make more contributions to the similarity between compared molecules.
For chemical databases we applied SMTP for ligand-based virtual screening by making some modifications to its equation as discussed below.
Suppose there is a molecule with j features . Whereby Fj is represented as a j-dimensional vector. The proposed similarity measure ASMTP, which is based on the above mentioned similarity properties, has function F defined as in Equation (5) below:
| (5) |
where the numerator of F function has three different cases, according to the value of compared features as shown below in Equation (6):
| (6) |
And
The denominator of F function has two different cases:
.
The similarity measure equation of ASMTP is shown in Equation (7) below:
| (7) |
where:
: Molecule1 (query).
: Molecule 2(reference).
J = feature index (vector).
μ: is the average of all non-zero values of vector (feature) j.
λ: small value λ, e.g., 0.01–0.0001.
j: total number of non-zero values in the features index.
The proposed ASMTP measure takes into account the following three cases: (a) The feature that appears in both compared molecules; (b) the feature that appears in only one of the compared molecules; and (c) the feature that appears in none of the compared molecules.
Each one of the mentioned cases has a different way of calculating the first case, so we set a lower bound 0.5 and decrease the similarity as the difference between the feature values of the compared molecules increases. This is scaled by a Gaussian function, as shown in Equation (5) where we have modified to use for representing the average of all non-zero values for a specific feature (feature vector) j in the molecules data set instead of σ which was representing the standard deviation of all non-zero values for feature in the training data in SMTP. In the second case, a negative constant −λ disregarding the magnitude of the non-zero feature value has been added. For the last case, when the feature has not appeared on both compared molecules, this is considered to have no effect on the similarity and no contribution to the similarity. For λ, it has taken a small value for our test using ASMTP, which is the value = 0.0001.
ASMTP is used to enhance the molecular ranking performance in VS by performing similarity calculations that improve the computational efficiency and help to rank and sort molecules in decreasing order of highest probability ratio. The aim is determine the result of the screening in the top 1% and 5%, so the ASMTP will simplify the calculation process and reduce the algorithm programming complexity.
5. Experimental Design
In this section, we investigate the effectiveness of the proposed similarity measure of ASMTP. The investigation is done by conducting several experiments of the simulated VS searches on two different benchmark datasets: the MDL Drug Data Report (MDDR) [41] and the maximum unbiased validation (MUV) [42]. It has been used widely for LBVS, and have also been used by our research group in some previous works [15,43]. All datasets contain 2D structural representations that have been converted to Pipeline Pilot’s ECFC_4 (Extended Connectivity) fingerprints and flooded to 1024 feature sizes [44], whereby the MDDR database contains 102,516 active and inactive molecules. From the MDDR we used two datasets: DS1 and DS2. The DS1 dataset contains 11 activity classes, containing structurally homogeneous and heterogeneous active classes. The DS2 dataset is different from DS1 as it contains more than 10 homogeneous activity classes. The details of selected activity classes of the MDDR are shown in Table 2 and Table 3. Each table contains an activity class index, the activity class name and the number of active molecules that belong to each class. The second dataset is the MUV, which is prepared for VS and has seventeen activity classes that have varying numbers of actives and decoys (Table 4).
Table 2.
MDDR activity classes for the DS1 dataset.
| Activity Index | Activity Class | Active Molecules |
|---|---|---|
| 31420 | Renin inhibitors | 1130 |
| 71523 | HIV protease | 750 |
| 37110 | Thrombin inhibitors | 803 |
| 31432 | Angiotensin II AT1antagonists | 943 |
| 42731 | Substance P antagonists | 1246 |
| 06233 | Substance P antagonists | 752 |
| 06245 | 5HT reuptake inhibitors | 359 |
| 07701 | D2 antagonists | 395 |
| 06235 | 5HT1A agonists | 827 |
| 78374 | Protein kinase C inhibitors | 453 |
Table 3.
DS2 dataset activity classes.
| Activity Index | Activity Class | Active Molecules |
|---|---|---|
| 07707 | Adenosine (AI) agonists | 207 |
| 07708 | Adenosine (A2) agonists | 156 |
| 31420 | Rennin inhibitors 1 | 1300 |
| 42710 | CCK agonists | 111 |
| 64100 | Monocycle_ lactams | 1346 |
| 64200 | Cephalosporin’s | 113 |
| 64220 | Carbacephems | 1051 |
| 64500 | Carbapenems | 126 |
| 64350 | Tribactams | 388 |
| 75755 | Vitamin D analogues | 455 |
Table 4.
MUV activity classes.
| Activity Index | Activity Class |
|---|---|
| 466 | S1P1 rec. (agonists) |
| 548 | PKA (inhibitors) |
| 600 | SF1 (inhibitors) |
| 644 | Rho-Kinase2 (inhibitors) |
| 652 | HIV RT-RNase (inhibitors) |
| 689 | Eph rec. A4 (inhibitors) |
| 692 | SF1 (agonists) |
| 712 | HSP 90 (inhibitors) 30 |
| 713 | ER-a-Coact. Bind. (inhibitors) |
| 733 | ER-b-Coact. Bind. (inhibitors) |
| 737 | ER-a-Coact. Bind. (potentiators) |
| 810 | FAK (inhibitors |
| 832 | Cathepsin G (inhibitors) |
| 846 | FXIa (inhibitors) |
| 852 | FXIIa (inhibitors) |
| 858 | D1 rec. (allosteric modulators) |
| 859 | M1 rec. (allosteric inhibitors) |
6. Results and Discussion
The experiments were conducted by performing the proposed ASMTP algorithm. This simulated the VS search by using ten reference structures from each activity class randomly, as the selected references were unified and applied to Tanimoto and the ASMTP. The final output of obtained similarity results of the whole molecules of the database will then be ranked in decreasing order. The average retrieved output of the ten references’ query results mean are calculated in the 1% and 5% cutoffs of the recall data, while the procedure is repeated for the all databases. The common method of evaluating any similarity searching method is achieved by determining where the active compounds appear in the ranked list if the number of known active compounds listed in the top will be an indicator of the effective VS method. The effectiveness of the proposed ASMTP algorithm is evaluated using MUV and MDDR benchmark datasets.
The experiment results obtained by ASMTP, Tanimoto and one of the most recently VS similarity measure technique called Standard Quantum-Based(SQB) that have been proposed by Al-Dabbagh and et al. [43] are shown in Table 5, Table 6 and Table 7. Each table of results shows the database activity classes in the first column, while the second and third columns respectively show the average of recall of the ranking results for all activity classes at the cut off 1% and 5% for the standard similarity coefficient Tanimoto, and the fourth and fifth columns represent the SQB corresponding results and the seventh and eighth columns show ASMTP results. The end of each column shows the overall average recalls results of all classes. The best average recall for each class is highlighted. In the bottom of each column there is a shaded cells row that corresponds to the total number of shaded cells for the Tanimoto and the proposed method that achieved better results. The obtained results DS1 and DS2 are shown in (Table 5 and Table 6), and the results of MUV is shown in (Table 7).
Table 5.
The recall is calculated using the top 1% and top 5% of the DS1 dataset.
| Activity Classes | TAN | SQB | ASMTP | |||
|---|---|---|---|---|---|---|
| 1% | 5% | 1% | 5% | 1% | 5% | |
| 31420 | 57.3 | 85.85 | 73.73 | 87.22 | 78.83 | 96.81 |
| 71523 | 29.96 | 58.09 | 26.84 | 48.7 | 12.82 | 51.94 |
| 37110 | 14.38 | 29.98 | 24.73 | 45.62 | 39.53 | 63.84 |
| 31432 | 36.37 | 76.85 | 36.66 | 70.44 | 45.22 | 97.45 |
| 42731 | 16.89 | 27.74 | 21.17 | 19.35 | 13.95 | 20.88 |
| 6233 | 22.72 | 37.78 | 12.49 | 21.04 | 22.77 | 36.75 |
| 6245 | 5.03 | 14.83 | 6.03 | 13.63 | 11.73 | 26.26 |
| 7701 | 8.45 | 23.07 | 11.35 | 21.85 | 8.95 | 17.26 |
| 6235 | 9.03 | 21 | 10.15 | 19.13 | 21.91 | 37.17 |
| 78374 | 12.08 | 17.81 | 13.08 | 20.55 | 1.77 | 2.65 |
| 78331 | 8.77 | 16.71 | 5.92 | 13.1 | 3.31 | 10.24 |
| Mean | 20.08909 | 37.24636 | 22.01364 | 34.05 | 23.65 | 41.93182 |
| Shaded cells | 3 | 4 | 0 | 0 | 8 | 7 |
Table 6.
The recall is calculated using the top 1% and top 5% of the DS2 dataset.
| Activity Classes | TAN | SQB | Proposed Method | |||
|---|---|---|---|---|---|---|
| 1% | 5% | 1% | 5% | 1% | 5% | |
| 09249 | 61.84 | 70.39 | 58.5 | 74.22 | 72.82 | 73.3 |
| 12455 | 47.03 | 56.58 | 55.61 | 100 | 99.35 | 100 |
| 12464 | 65.1 | 88.19 | 62.22 | 95.24 | 81.66 | 96.46 |
| 31281 | 81.82 | 86.64 | 83 | 93 | 92.73 | 99.09 |
| 43210 | 80.31 | 93.75 | 80.73 | 98.94 | 88.2 | 99.85 |
| 71522 | 53.84 | 77.68 | 53.13 | 98.93 | 81.25 | 99.11 |
| 75721 | 46.8 | 63.94 | 34.61 | 90.9 | 77.27 | 98.67 |
| 78331 | 30.56 | 44.8 | 29.04 | 92.72 | 80 | 96.8 |
| 78348 | 80.18 | 91.71 | 81.86 | 93.75 | 82.17 | 99.74 |
| 78351 | 87.56 | 94.82 | 85.4 | 95.39 | 96.48 | 96.92 |
| Mean | 63.504 | 76.85 | 62.41 | 93.31 | 85.193 | 95.994 |
| Shaded cells | 0 | 0 | 0 | 0 | 10 | 10 |
Table 7.
The recall is calculated using the top 1% and top 5% of the MUV 17 activity classes data sets.
| Activity Index | Tanimoto | SQB | Proposed Method | |||
|---|---|---|---|---|---|---|
| 1% | 5% | 1% | 5% | 1% | 5% | |
| 466 | 3.1 | 5.86 | 1.38 | 8.62 | 5.86 | 9.66 |
| 548 | 8.62 | 22.76 | 11.38 | 24.14 | 10.34 | 17.93 |
| 600 | 3.79 | 11.38 | 5.52 | 16.21 | 6.21 | 13.45 |
| 644 | 7.59 | 17.59 | 8.97 | 17.93 | 7.24 | 12.41 |
| 652 | 2.76 | 7.93 | 3.79 | 9.66 | 5.86 | 11.38 |
| 689 | 3.79 | 9.66 | 4.48 | 11.72 | 5.86 | 9.71 |
| 692 | 0.69 | 4.83 | 1.38 | 4.83 | 3.79 | 6.55 |
| 712 | 4.14 | 10.34 | 5.17 | 11.03 | 6.21 | 8.97 |
| 713 | 3.1 | 7.24 | 2.76 | 5.86 | 6.21 | 9.31 |
| 733 | 3.45 | 8.97 | 4.14 | 8.62 | 5.86 | 9.31 |
| 737 | 2.41 | 8.28 | 1.72 | 8.28 | 7.59 | 14.14 |
| 810 | 2.07 | 6.9 | 1.72 | 11.03 | 7.24 | 13.1 |
| 832 | 6.55 | 13.1 | 8.28 | 14.83 | 13.1 | 20 |
| 846 | 9.66 | 28.62 | 12.41 | 26.9 | 10.69 | 25.52 |
| 852 | 12.41 | 21.38 | 9.66 | 20 | 13.45 | 21.03 |
| 858 | 1.72 | 5.86 | 1.38 | 6.21 | 6.21 | 7.93 |
| 859 | 1.38 | 8.97 | 2.41 | 8.62 | 5.86 | 10.69 |
| Avg | 4.54 | 11.70 | 5.09 | 12.61 | 7.50 | 12.991 |
| Shaded cells | 0 | 2 | 3 | 5 | 14 | 11 |
The overall average results of all datasets achieved good results and outperformed Tanimoto and SQB. The DS1 achieved good results in six out of 11 classes in a cut off 1% and seven out of 11 in a cut off 5%. The DS2 achieved good results in nine out of 10 classes in a cut off 1%, and 10 out of 10 in cut off 5%. This is considered a good indicator of the high performance of the ASMTP algorithm. The MUV results also outperformed Tanimoto and SQB in both cut offs 1% and 5%, and 11 out of 17 MUV classes achieved good results in cut offs 1% and 5% respectively.
In addition, to examine the effectiveness of the proposed method ASMTP ,we used some of the most widely used evaluation method for VS, firstly we applied the Receiver Operating Characteristic (ROC) curve which has been used in various fields (medicine, meteorology, etc.) and also in the drug discovery field [45,46]. A ROC curve describes the tradeoff between sensitivity and specificity, which are the main characteristic features of any test. In the drug design context, sensitivity (Se) would be the percentage of truly active compounds being selected from the virtual screening workflow: the number of true positive (TP) results divided by the sum of true positives and false negatives (FN):
| (8) |
The Specificity (Sp) represents the percentage of truly inactive compounds being correctly identified by the computer test and therefore being discarded, that is, the number of true negative results (TN) divided by the sum of true negatives and false positives (FP):
| (9) |
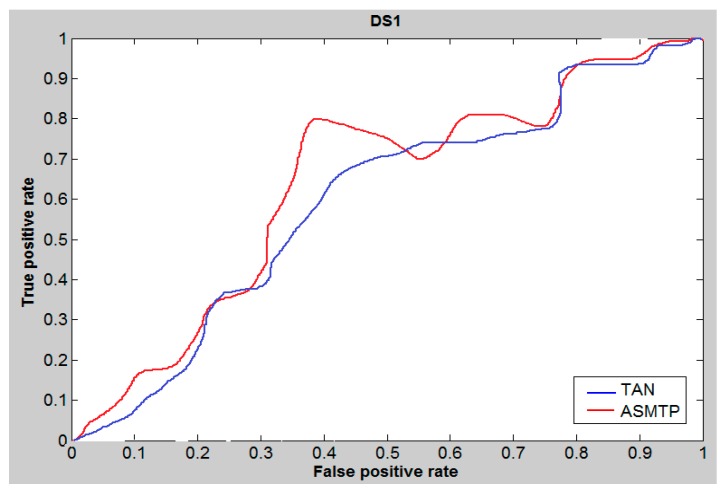
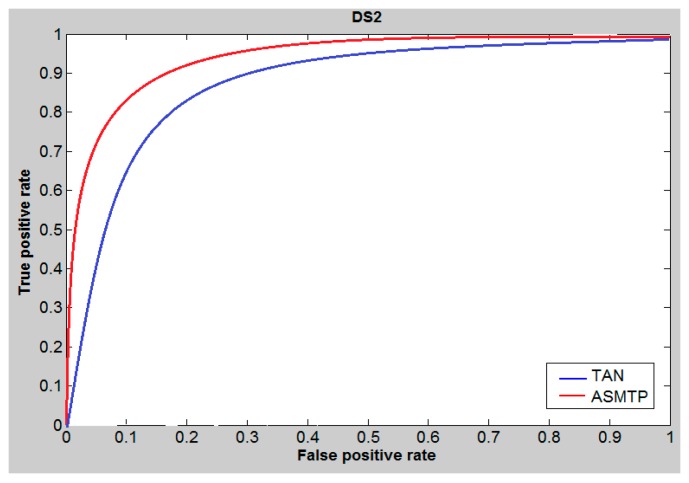
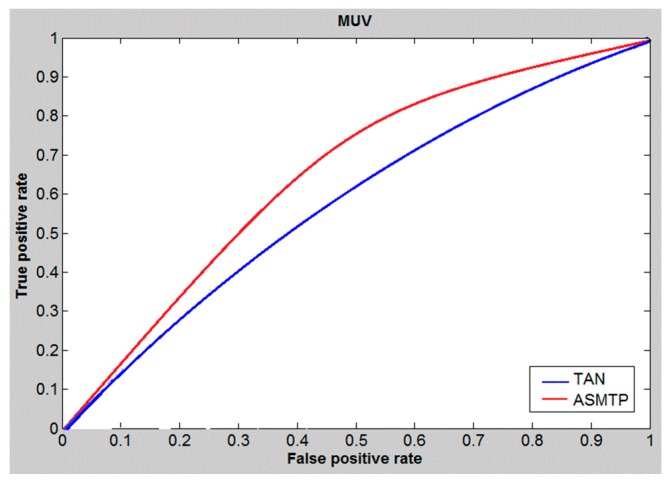
The area under the ROC curve (AUC) is a measure used to measure the test performance for the he closer AUC is to 1, the better is the performance of the prediction. For ASMTP we applied the ROC curve to study and evaluate the performance of the proposed ASMTP at cutoff 5%. Figure 1, Figure 2 and Figure 3, illustrate and provides a preliminary indication about the quality of the proposed method for data set DS1, DS2, and MUV compared to conventional similarity measures Tanimoto, and we can say that the conclusion derived from these tables (Table 5, Table 6 and Table 7) provides the same conclusion that derived from Figure 1, Figure 2 and Figure 3 that confirms the superior of ASMTP method.
Figure 1.
ROC curves and AUCs at 5% cutoff of DS1 data set.
Figure 2.
ROC curves and AUCs at 5% cutoff of DS2 data set.
Figure 3.
ROC curves and AUCs at 5% cutoff of MUV data set.
Beside ROC curve, we also used Boltzmann enhanced discrimination of receiver operating characteristic (BEDROC) [45] which is based on the idea of exponentially weighted active ranks. And it is a little bit different from the enrichment factor (EF) or the receiver operating characteristic (ROC), for it concentrates on the beginning of a ranked list, and giving more weight to the compounds that will be ranked early the BEDROC scores are bounded between 0 and 1, where higher scores indicating more known actives being ranked on earlier ranks. Table 8, shows the results of EF and the BEDROC, The conclusion which can be drawn from all above evaluation methods confirm that ASMTP search outperformed the conventional similarity measure and SQB performance or at least it is not worse than them.
Table 8.
Comparison results of enrichment values of (BEDROC α = 20) and (EF 1%) using ASMTP on MDDR1, MDDR2, and MUV data sets.
| Methods | DS1 | DS2 | MUV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BEDROC( α 20) | EF (1%) | BEDROC( α 20) | EF (1%) | BEDROC( α 20) | EF (1%) | |||||||
| Mean | Median | Mean | Median | Mean | Median | Mean | Median | Mean | Median | Mean | Median | |
| Tan | 0.48 | 0.46 | 80.01 | 86.01 | 0.33 | 0.34 | 23.01 | 23.01 | 0.37 | 0.37 | 16.69 | 17.92 |
| SQB | 0.53 | 0.57 | 90.01 | 89.31 | 0.44 | 0.39 | 29.01 | 22.01 | 0.41 | 0.39 | 18.01 | 19.74 |
| ASMTP | 0.61 | 0.64 | 92.9 | 90.23 | 0.46 | 0.50 | 28.27 | 25.32 | 0.44 | 0.42 | 18.93 | 20.14 |
In spite of the recent work done by Nagwani [47], where he reported some limitations of SMTP in document retrieving. Conversely, by applying the ASMTP in LBVS we found that it works properly, and it has achieved good results which prove that the ASMTP is working properly with both datasets that contain most similar molecules (homogeneous) and also it enhanced the retrieval performance of diversity data (heterogeneous) data sets.
7. Conclusions
In this study, we presented a new LBVS similarity measure that has been derived from the document and text searching areas. The adapted ASMTP algorithm focuses on the preferred selected similarity properties, we conduct the experiments on two benchmark datasets the MDDR and MUV, and compared the achieved results with the Tanimoto coefficient which is considered as the conventional similarity measure in VS, and also we compared the results with QSB which is most recently proposed similarity measure for LBVS , We also have investigated the effectiveness of ASMTP performances by applying a set of VS mostly used evaluation methods. The overall achieved results from conducting screening and evaluation results show that the performance obtained by the proposed measure is improved LBVS with heterogeneous molecules data, and achieved superior results with data that are structurally homogeneous.
Acknowledgments
This work is supported by the Ministry of Higher Education (MOHE) and Research Management Centre (RMC) at the Universiti Teknologi Malaysia (UTM) under the Research University Grant Category (VOT R.J130000.7828.4F741).
Author Contributions
M.H and M.M.A.D. are Ph.D. candidates and conducted the research and performed the experiments under the supervision of N.S. and collaboration with F.S. and A.A. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Willett P. Similarity methods in chemoinformatics. Annu. Rev. Inf. Sci. Technol. 2009;43:1–117. doi: 10.1002/aris.2009.1440430108. [DOI] [Google Scholar]
- 2.Jorgensen W.L. The many roles of computation in drug discovery. Science. 2004;303:1813–1818. doi: 10.1126/science.1096361. [DOI] [PubMed] [Google Scholar]
- 3.Walters W.P., Stahl M.T., Murcko M.A. Virtual screening—An overview. Drug Discov. Today. 1998;3:160–178. doi: 10.1016/S1359-6446(97)01163-X. [DOI] [Google Scholar]
- 4.Bajusz D., Rácz A., Héberger K. Why is tanimoto index an appropriate choice for fingerprint-based similarity calculations? J. Cheminform. 2015;7 doi: 10.1186/s13321-015-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bajorath J. Integration of virtual and high-throughput screening. Nat. Rev. Drug Discov. 2002;1:882–894. doi: 10.1038/nrd941. [DOI] [PubMed] [Google Scholar]
- 6.Cano G., García-Rodríguez J., Pérez-Sánchez H. Improvement of Virtual Screening Predictions using Computational Intelligence Methods. Lett. Drug Des. Discov. 2014;11:33–39. doi: 10.2174/15701808113109990054. [DOI] [Google Scholar]
- 7.Jain A. Virtual screening in lead discovery and optimization. Curr. Opin. Drug Discov. Dev. 2004;7:396–403. [PubMed] [Google Scholar]
- 8.Todeschini R., Consonni V. Molecular Descriptors for Chemoinformatics, Volume 41 (2 Volume Set) Volume 41 John Wiley & Sons; New York, NY, USA: 2009. [Google Scholar]
- 9.Sheridan R.P. Alternative global goodness metrics and sensitivity analysis: Heuristics to check the robustness of conclusions from studies comparing virtual screening methods. J. Chem. Inf. Model. 2008;48:426–433. doi: 10.1021/ci700380x. [DOI] [PubMed] [Google Scholar]
- 10.Von Korff M., Freyss J., Sander T. Comparison of ligand-and structure-based virtual screening on the DUD data set. J. Chem. Inf. Model. 2009;49:209–231. doi: 10.1021/ci800303k. [DOI] [PubMed] [Google Scholar]
- 11.Hu G., Kuang G., Xiao W., Li W., Liu G., Tang Y. Performance evaluation of 2D fingerprint and 3D shape similarity methods in virtual screening. J. Chem. Inf. Model. 2012;52:1103–1113. doi: 10.1021/ci300030u. [DOI] [PubMed] [Google Scholar]
- 12.Hughes J., Rees S., Kalindjian S., Philpott K. Principles of early drug discovery. Br. J. Pharmacol. 2011;162:1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson M.A., Maggiora G.M. Concepts and Applications of Molecular Similarity. Wiley; England, UK: 1990. [Google Scholar]
- 14.Ahmed A., Abdo A., Salim N. Ligand-based virtual screening using Bayesian inference network and reweighted fragments. Sci. World J. 2012;2012 doi: 10.1100/2012/410914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed A., Saeed F., Salim N., Abdo A. Condorcet and borda count fusion method for ligand-based virtual screening. J. Cheminform. 2014;6:19. doi: 10.1186/1758-2946-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng M., Liu Z., Yan X., Ding Q., Gu Q., Xu J. LBVS: An online platform for ligand-based virtual screening using publicly accessible databases. Mol. Divers. 2014;18:829–840. doi: 10.1007/s11030-014-9545-3. [DOI] [PubMed] [Google Scholar]
- 17.Ripphausen P., Nisius B., Bajorath J. State-of-the-art in ligand-based virtual screening. Drug Discov. Today. 2011;16:372–376. doi: 10.1016/j.drudis.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Willett P. Fusing similarity rankings in ligand-based virtual screening. Comput. Struct. Biotechnol. J. 2013;5 doi: 10.5936/csbj.201302002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willett P. Textual and Chemical Information Processing: Different Domains but Similar Algorithms. [(accessed on 20 February 2015)];Inf. Res. 2000 5 Available online: http://www.informationr.net/ir/5-2/paper69.html. [Google Scholar]
- 20.Lin Y.-S., Jiang J.-Y., Lee S.-J. A similarity measure for text classification and clustering. IEEE Trans. Knowl. Data Eng. 2014;26:1575–1590. doi: 10.1109/TKDE.2013.19. [DOI] [Google Scholar]
- 21.Downs G.M., Willett P., Fisanick W. Similarity searching and clustering of chemical-structure databases using molecular property data. J. Chem. Inf. Comput. Sci. 1994;34:1094–1102. doi: 10.1021/ci00021a011. [DOI] [Google Scholar]
- 22.Lyne P.D. Structure-based virtual screening: An overview. Drug Discov. Today. 2002;7:1047–1055. doi: 10.1016/S1359-6446(02)02483-2. [DOI] [PubMed] [Google Scholar]
- 23.Lionta E., Spyrou G., K Vassilatis D., Cournia Z. Structure-based virtual screening for drug discovery: Principles, applications and recent advances. Curr. Top. Med. Chem. 2014;14:1923–1938. doi: 10.2174/1568026614666140929124445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnard J.M. Substructure searching methods: Old and new. J. Chem. Inf. Comput. Sci. 1993;33:532–538. doi: 10.1021/ci00014a001. [DOI] [Google Scholar]
- 25.Willett P., Winterman V., Bawden D. Implementation of nonhierarchic cluster analysis methods in chemical information systems: Selection of compounds for biological testing and clustering of substructure search output. J. Chem. Inf. Comput. Sci. 1986;26:109–118. doi: 10.1021/ci00051a005. [DOI] [Google Scholar]
- 26.Willett P. Similarity-based approaches to virtual screening. Biochem. Soc. Trans. 2003;31:603–606. doi: 10.1042/bst0310603. [DOI] [PubMed] [Google Scholar]
- 27.Willett P. Similarity-based virtual screening using 2D fingerprints. Drug Discov. Today. 2006;11:1046–1053. doi: 10.1016/j.drudis.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Whittle M., Gillet V.J., Willett P., Alex A., Loesel J. Enhancing the effectiveness of virtual screening by fusing nearest neighbor lists: A comparison of similarity coefficients. J. Chem. Inf. Comput. Sci. 2004;44:1840–1848. doi: 10.1021/ci049867x. [DOI] [PubMed] [Google Scholar]
- 29.Willett P. Chemoinformatics and Computational Chemical Biology. Springer; New York, NY, USA: 2011. Similarity searching using 2D structural fingerprints; pp. 133–158. [DOI] [PubMed] [Google Scholar]
- 30.Cereto-Massagué A., Ojeda M.J., Valls C., Mulero M., Garcia-Vallvé S., Pujadas G. Molecular fingerprint similarity search in virtual screening. Methods. 2014;71:58–63. doi: 10.1016/j.ymeth.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Bender A., Jenkins J.L., Scheiber J., Sukuru S.C.K., Glick M., Davies J.W. How similar are similarity searching methods? A principal component analysis of molecular descriptor space. J. Chem. Inf. Model. 2009;49:108–119. doi: 10.1021/ci800249s. [DOI] [PubMed] [Google Scholar]
- 32.Downs G.M., Willett P. Similarity searching in databases of chemical structures. Rev. Comput. Chem. 1996;7:1–66. [Google Scholar]
- 33.Drwal M.N., Griffith R. Combination of ligand-and structure-based methods in virtual screening. Drug Discov. Today Technol. 2013;10:e395–e401. doi: 10.1016/j.ddtec.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Todeschini R., Consonni V., Xiang H., Holliday J., Buscema M., Willett P. Similarity coefficients for binary chemoinformatics data: Overview and extended comparison using simulated and real data sets. J. Chem. Inf. Model. 2012;52:2884–2901. doi: 10.1021/ci300261r. [DOI] [PubMed] [Google Scholar]
- 35.Bender A., Mussa H.Y., Glen R.C., Reiling S. Similarity searching of chemical databases using atom environment descriptors (MOLPRINT 2D): Evaluation of performance. J. Chem. Inf. Comput. Sci. 2004;44:1708–1718. doi: 10.1021/ci0498719. [DOI] [PubMed] [Google Scholar]
- 36.Wang B., Ekins S. Computer Applications in Pharmaceutical Research and Development. Volume 2 John Wiley & Sons; New York, NY, USA: 2006. [Google Scholar]
- 37.Han L., Ma X., Lin H., Jia J., Zhu F., Xue Y., Li Z., Cao Z., Ji Z., Chen Y. A support vector machines approach for virtual screening of active compounds of single and multiple mechanisms from large libraries at an improved hit-rate and enrichment factor. J. Mol. Gr. Model. 2008;26:1276–1286. doi: 10.1016/j.jmgm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Jorissen R.N., Gilson M.K. Virtual screening of molecular databases using a support vector machine. J. Chem. Inf. Model. 2005;45:549–561. doi: 10.1021/ci049641u. [DOI] [PubMed] [Google Scholar]
- 39.Hert J., Willett P., Wilton D.J., Acklin P., Azzaoui K., Jacoby E., Schuffenhauer A. New methods for ligand-based virtual screening: Use of data fusion and machine learning to enhance the effectiveness of similarity searching. J. Chem. Inf. Model. 2006;46:462–470. doi: 10.1021/ci050348j. [DOI] [PubMed] [Google Scholar]
- 40.Chen J., Holliday J., Bradshaw J. A machine learning approach to weighting schemes in the data fusion of similarity coefficients. J. Chem. Inf. Model. 2009;49:185–194. doi: 10.1021/ci800292d. [DOI] [PubMed] [Google Scholar]
- 41.Symyx technologies Mdl drug data report: Sci Tegic Accelrys Inc., the MDL Drug Data Report (MDDR) [(accessed on 12 March 2016)]. Available online: http://www.accelrys.com/
- 42.Rohrer S.G., Baumann K. Maximum unbiased validation (MUV) data sets for virtual screening based on PubChem bioactivity data. J. Chem. Inf. Model. 2009;49:169–184. doi: 10.1021/ci8002649. [DOI] [PubMed] [Google Scholar]
- 43.Al-Dabbagh M.M., Salim N., Himmat M., Ahmed A., Saeed F. A quantum-based similarity method in virtual screening. Molecules. 2015;20:18107–18127. doi: 10.3390/molecules201018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pipeline Pilot Software. Scitegic Accelrys Inc.; San Diego, CA, USA: 2008. [Google Scholar]
- 45.Truchon J.-F., Bayly C.I. Evaluating virtual screening methods: Good and bad metrics for the “early recognition” problem. J. Chem. Inf. Model. 2007;47:488–508. doi: 10.1021/ci600426e. [DOI] [PubMed] [Google Scholar]
- 46.Riniker S., Landrum G.A. Open-source platform to benchmark fingerprints for ligand-based virtual screening. J. Cheminform. 2013;5:1–17. doi: 10.1186/1758-2946-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagwani N.K. A comment on “a similarity measure for text classification and clustering”. IEEE Trans. Knowl. Data Eng. 2015;27:2589–2590. doi: 10.1109/TKDE.2015.2451616. [DOI] [Google Scholar]