Abstract
C-boivinopyranosyl flavones have rarely been isolated from nature. In the search for anti-HBV (hepatitis b virus) constituents of Alternanthera philoxeroides, two new compounds, luteolin-6-C-β-d-boivinopyranosyl-3′-O-β-d-glucopyranoside (1) and chrysoeriol-6-C-β-d-boivinopyranosyl-4′-O-β-d-glucopyranoside (2), along with three known C-boivinopyranosyl flavones (compounds 3–5) were isolated. Their structures were determined by spectroscopic analyses including 1D and 2D NMR, HR-ESI-MS, IR spectra. Compounds 1, 2 and 3 showed significant anti-HBV activities through specifically inhibiting the secretion of HBsAg in HepG2.2.15.
Keywords: C-boivinopyranosyl flavones, Alternanthera philoxeroides, anti-HBV activity
1. Introduction
It is estimated that approximately 350 million people worldwide and 93 million in China alone are hepatitis b virus (HBV) carriers, causing approximately 500,000 deaths every year.
The current treatment strategies involving vaccines, interferons and nucleosides are unsatisfactory due to drug-resistance and adverse side effects [1,2,3].
For many years, herbs have been used as the Traditional Chinese Medicine for treatment of hepatitis B virus infection, such as Salvia miltiorrhiza, Rheum palmatum, Phyllanthi urinariae [4], etc. In recent years, many agents derived from botanical origin have been reported to possess anti-HBV activities, such as niranthin and nirtetralin from Phyllanthus, curcumin from Curcuma, alisol A from Alisma orientalis, oxymatrine from Sophora, etc. [5]. Botanical agents are attractive sources of new anti-HBV drugs.
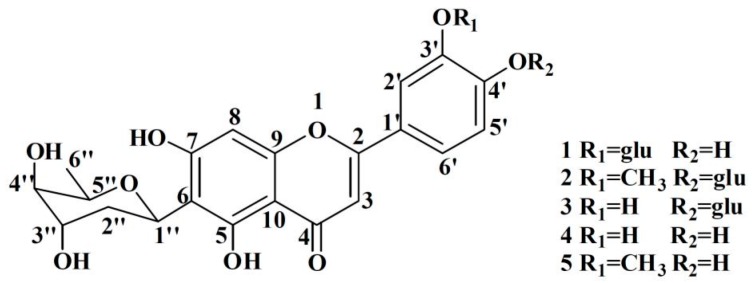
Alternanthera philoxeroides is widely distributed in South China and mainly used in China for the treatment of measles, influenza, encephalitis b. Its major constituents are triterpenoid saponins, flavones, phytosterols, anthraquinones and organic acids [6]. Several oleanolic acid analogues from it were reported to possess anti-HBV activities. In this paper, two new 6-C-boivinopyranosyl flavones together with three known analogues were isolated from Alternanthera philoxeroides (Figure 1), and their anti-HBV activities were evaluated toward HepG2 2.2.15 cells.
Figure 1.
The structures of compounds 1–5.
2. Results and Discussion
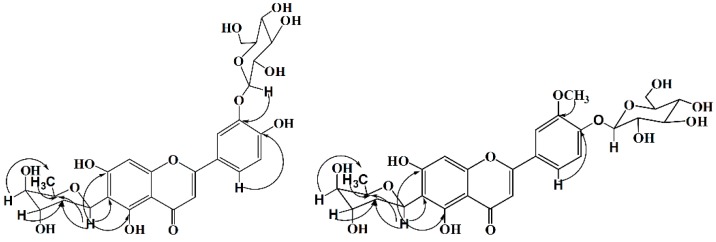
Compound 1 was obtained as a yellow amorphous powder. HR-ESI-MS m/z 579.1728 [M + H]+ (calcd for C27H31O14, 579.1708) showed the molecular formula C27H30O14. IR spectrum indicated the presence of hydroxyl (3447 cm−1), carbonyl (1652 cm−1) and phenyl (1574, 1495 cm−1) groups in the molecule. Fifteen carbon signals in low field of 13C-NMR similar to those of luteolin, together with δ 6.83 (1H, s), 6.58 (1H, s), δ 7.79 (1H, d, J = 1.8 Hz), 6.96 (1H, d, J = 8.4 Hz) and 7.65 (1H, dd, J = 1.8, 8.4 Hz) in 1H-NMR showed an 6, 3′ (or 6, 4′) disubstituted luteolin structure (Table 1). According to the HMQC and HMBC spectra, the NMR chemical shifts of the carbons signals could be assigned unambiguously. The HMBC correlations indicated a 2,6-dideoxygenated pyranosyl directly linked to C-6, which could be identified as boivinopyranosyl by small coupling constants of 3′-H (br.s) and 4′-H (br.s). The connectivity of C3′-O-C1′′′ was deduced by the HMBC correlation of H-1′′′/C-3′ (Figure 2). A β-d-glucopyranosyl was recognized by the anomeric proton signal at 4.89 (d, J = 7.2 Hz) in the 1H-NMR spectrum and also by the characteristic six signals at δ 101.9 (CH), 73.3 (CH), 75.9 (CH), 70.1 (CH), 77.4 (CH), and 60.9 (CH2) in the 13C-NMR spectrum (Table 1). This conclusion was confirmed by comparing acid-hydrolyzed (2N-HCl, 80 °C, 3 h) product of 1 with an authentic d-glucopyranose sample on Si TLC (Rf = 0.4). Therefore, compound 1 was established as luteolin-6-C-β-d-boivinopyranosyl-3′-O-β-d-glucopyranoside.
Table 1.
1H-NMR (400 MHz) and 13C-NMR (100 MHz) data for compound 1 and 2 in DMSO-d6.
| Position | Compound 1 | Compound 2 | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 2 | 163.5 | 163.2 | ||
| 3 | 6.83 (s) | 103.4 | 7.03 (s) | 104.0 |
| 4 | 182.0 | 182.1 | ||
| 5 | 157.1 | 157.2 | ||
| 6 | 110.4 | 110.4 | ||
| 7 | 162.5 | 162.6 | ||
| 8 | 6.58 (s) | 94.8 | 6.63 (s) | 95.0 |
| 9 | 156.1 | 156.2 | ||
| 10 | 103.2 | 103.5 | ||
| 1′ | 121.2 | 123.9 | ||
| 2′ | 7.79 (d, J = 1.8 Hz) | 114.4 | 7.63 (d, J = 2.4 Hz) | 110.2 |
| 3′ | 145.6 | 149.8 | ||
| 4′ | 150.7 | 149.2 | ||
| 5′ | 6.96 (d, J = 8.4 Hz) | 121.9 | 7.25 (d, J = 8.8 Hz) | 115.0 |
| 6′ | 7.65 (dd, J = 1.8, 8.4 Hz) | 116.4 | 7.66 (dd, J = 2.4, 8.8 Hz) | 120.5 |
| boivinose | ||||
| 1′′ | 5.30 (dd, J = 2.7, 12.3 Hz) | 67.3 | 5.34 (dd, J = 2.8, 12.4 Hz) | 67.4 |
| 2′′ | 1.48 (d, J = 13.8 Hz) | 31.4 | 1.50 (d, J = 14.0 Hz) | 31.4 |
| 2.19 (ddd, J = 2.7, 4.2, 13.8 Hz) | 2.21 (ddd, J = 2.4, 3.2, 14.0 Hz) | |||
| 3′′ | 3.83 (br.s) | 66.5 | 3.85 (br.s) | 66.5 |
| 4′′ | 3.23 (br.s) | 68.6 | 3.25 (br.s) | 68.7 |
| 5′′ | 4.02 (q, J = 6.6 Hz) | 70.6 | 3.90 (q, J = 6.0 Hz) | 70.6 |
| 6′′ | 1.14 (d, J = 6.6 Hz) | 17.1 | 1.16 (d, J = 6.0 Hz) | 17.1 |
| glucose | ||||
| 1′′′ | 4.89 (d, J = 7.2 Hz) | 101.9 | 5.08 (d, J = 7.2 Hz) | 99.5 |
| 2′′′ | 3.32 (m) | 73.3 | 3.33 (m) | 73.1 |
| 3′′′ | 3.30 (m) | 75.9 | 3.30 (m) | 76.8 |
| 4′′′ | 3.15 (m) | 70.1 | 3.17 (m) | 69.6 |
| 5′′′ | 3.47 (m) | 77.4 | 3.36 (m) | 77.1 |
| 6′′′ | 3.77 (m) | 60.9 | 3.47 (m) | 60.6 |
| 3.48 (m) | 3.68 (m) | |||
| -OCH3 | 3.90 (s) | 56 | ||
Figure 2.
Key HMBC correlations of compounds 1 and 2.
Compound 2 was obtained as a yellow amorphous powder. HR-ESI-MS m/z 593.1858 [M + H]+ (calcd for C28H33O14, 593.1865) showed the molecular formula C28H32O14. IR spectrum indicated the presence of hydroxyl group (3395 cm−1), carbonyl group (1655 cm−1) and phenyl group (1591, 1492 cm−1) in the molecule. NMR data (Table 1) similar to those of 1 indicated a 6-C-β-d-boivinopyranosyl flavone glucoside. HMBC correlations proved the locations of -OCH3 at C-3′ and glucopyranosyl at C-4′ (Figure 2). Consequently, the structure of compound 2 was elucidated as chrysoeriol-6-C-β-d-Boivinopyranosyl-4′-O-β-d-glucopyranoside.
Three known 6-C-d-boivinopyranosyl flavones were isolated and their structures were identified as luteolin-6-C-β-d-boivinopyranosyl-4′-O-β-d-glucopyranoside (3) [7], luteolin-6-C-β-d-boivinopyranoside (4) [8], chrysoeriol-6-C-β-d-boivinopyranoside (5) [9] by comparison of their spectroscopic data with those reported in the literature.
These flavones were tested for their potential anti-HBV activities according to inhibitory secretion of HBV surface antigen (HBsAg) and HBV e antigen (HBeAg) in HBV-infected HepG2.2.15 under non-cytotoxic concentration and the results were summarized in Table 2. Compounds 1, 2, and 3 significantly blocked the secretion of HBsAg in a dose dependent manner.
Table 2.
Anti- hepatitis b virus (HBV) activity and cell proliferation of five compounds.
| Compound | IC50 (μM) | CC50 (μM) | |
|---|---|---|---|
| HBsAg | HBeAg | ||
| 1 | 28.65 | NE | >519 |
| 2 | 22.20 | NE | >253 |
| 3 | 31.54 | NE | >519 |
| 4 | 11.39 | 39.78 | 60.10 |
| 5 | NE | NE | <21.81 |
IC50, concentration of 50% inhibition of viral antigen expression; CC50, 50% cytotoxic concentration; NE, no effect.
3. Experimental
3.1. General Experimental Procedures
1H- and 13C-NMR spectra were obtained on a Bruker ECA-400 MHz (Billerica, MA, US) and a Varian UNITYINOVA 600 (Salt Lake City, UT, USA) with TMS as an internal standard. IR spectra were measured on a Bruker Vertex 70. HR-ESI-MS spectra were measured on a 9.4 T Q-FT-MS Apex Qe (Bruker Co.). ESI-MS spectra were measured on a Thermo Finnigan LCQ DECA spectrometer (Madison, WI, USA). Macro porous resin AB-8 (NanKai College Chemical Inc., Tianjin, China), Silica gel (60–120 mesh, 200–300 mesh, Qingdao Marine Chemical Group Co., Qingdao, China), and Sephadex LH-20 (Pharmacia, Uppsala, Sweden) were employed for column chromatography. TLC was carried out using silica gel 60 (>230 mesh, Qingdao Marine Chemical Group Co.) and GF254 plates precoated with silica gel 60.
3.2. Plant Materials
Alternanthera philoxeroides was bought from Qixin decoction pieces Co.Ltd, Hebei province, China and identified by Bin Li, Department of Pharmaceutical Chemistry, Beijing Institute of Radiation Medicine.
3.3. Extraction and Isolation
The dried and powdered material (20 kg) was extracted with 90% ethanol for two times under reflux. The concentrated extract (2.4 kg) was suspended in water and then partitioned with petroleum ether, chloroform, EtOAc, and n-BuOH successively. The n-BuOH extract (400 g) was subsequently separated into five fractions by macro porous resin AB-8. Among them, 50% ethanol fraction (60 g) was subjected to silica gel column chromatography (CC) eluted with CHCl3/MeOH gradient (from 10:1 to 0:1) to yield five fractions A–E. Fraction A eluted with CHCl3/MeOH gradient (from 10:1 to 3:1) on silica gel again to yield fractions A1 and A2. They were chromatographed respectively by Sephadex LH-20 (with CHCl3/MeOH, 1:1) to obtain compound 1 (58 mg), and compound 3 (63 mg). Fraction B eluted with CHCl3/MeOH gradient (from 10:1 to 5:1) on silica gel again to yield fractions B1, B2 and B3. They were chromatographed respectively by Sephadex LH-20 (with CHCl3/MeOH, 1:1) to obtain compound 2 (65 mg), compound 4 (15 mg) and compound 5 (85 mg).
Compound 1: yellow amorphous powder. IR (KBr) νmax 3447, 1652, 1574, 1495 cm−1; HR-ESI-MS m/z 579.1728 [M + H]+ (calcd for C27H31O14, 579.1708); 1H-NMR (DMSO-d6, 400 MHz) and 13C-NMR (DMSO-d6, 100 MHz) spectroscopic data, see Table 1.
Compound 2: yellow amorphous powder.IR (KBr) νmax 3395, 1655, 1591, 1492 cm−1; HR-ESI-MS m/z 593.1858 [M + H]+ (calcd for C28H33O14, 593.1865); 1H-NMR (DMSO-d6, 400 MHz) and 13C-NMR (DMSO-d6, 100 MHz) spectroscopic data, see Table 1.
3.4. Anti-HBV Assay
The anti-HBV assay was performed according to the previous report [10]. The inhibition of the secretions of HBsAg and HBeAg was assayed by ELISA method; and the cytotoxicity was assessed by the MTT method.
3.4.1. Inhibition Assay of HBsAg and HBeAg Secretions HepG
The HepG2.2.15 cells were plated in 96-well cell plates at a density of 5 × 104 cells·mL−1 in 200 μL of DMEM medium, and routinely cultured at 37 °C under 5% CO2. Different concentrations of the studied compounds were supplemented to the medium after cells were plated. Control cultures received the carrier solvent (DMEM with 0.2% DMSO). Cells were grown in the presence of the studied compounds for 8 days with changing the medium on the 4th day. The suspension and the cells were separated and collected for HBsAg and HBeAg level tests immediately. The inhibiting rates (%) were calculated by comparing the treatment group with the tested compounds and the solvent control group with DMSO. The percent of inhibition (%) = [1 − OD value of sample well/OD value of DMSO well] × 100.
3.4.2. MTT-Based Cytotoxicity Assay
HepG2.2.15 cells were cultured with test compounds for 8 days. After cultivation, cell proliferation was determined by MTT assay. Briefly, 10 μL of MTT (5 g·mL−1) was added to each well and further incubated for 4 h. Then, the culture medium was removed from each well and DMSO was added to dissolve the purple formazan of MTT. The absorbance at 540 nm was read in the Multiskan MK3 (Thermo).
4. Conclusions
C-boivinopyranosyl flavones have rarely been isolated from nature; fewer than 20 compounds could be found by SCifinder. In this article, two new C-boivinopyranosyl flavones, along with three known compounds were isolated and identified. Compounds 1, 2, and 3 significantly blocked the secretion of HBsAg in a dose dependent manner. They inhibited HBsAg secretion respectively by 70.6% (compound 1), 74.1% (compound 2) and 67.3% (compound 3) at non-cytotoxic concentration of 129 μM (compounds 1 and 3), 127 μM (compound 2).
Acknowledgments
We are grateful to Yan Xue, Mei-feng Xu and Yu-mei Zhao of the National Center of Biomedical Analysis for the measurements of the MS and NMR spectra.
Author Contributions
Bin Li and Jun-Xing Dong conceived and designed the experiments; Qing-Lan Guo performed the experiments; Li Chen and Ying Tian analyzed the data; Qiong Wang and Shi-Jun Liu contributed reagents/materials/analysis tools; Bin Li wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Not available.
References
- 1.Bhattacharya D., Thio C.L. Review of hepatitis B therapeutics. Clin. Infect. Dis. 2010;51:1201–1208. doi: 10.1086/656624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liaw Y.F., Brunetto M.R., Hadziyannis S. The natural history of chronic HBV infection and geographical differences. Antivir. Ther. 2010;15:25–33. doi: 10.3851/IMP1621. [DOI] [PubMed] [Google Scholar]
- 3.Mirandola S., Campagnolo D., Bortoletto G., Franceschini L., Marcolongo M., Al-berti A. Large-scale survey of naturally occurring HBV polymerase mutations associated with anti-HBV drug resistance in untreated patients with chronic hepatitis B. J. Viral Hepat. 2011;18:212–216. doi: 10.1111/j.1365-2893.2011.01435.x. [DOI] [PubMed] [Google Scholar]
- 4.Cui X.L., Wang Y., Kokudo N. Traditional Chinese medicine and related active compounds against hepatitis B virus infection. BioSci. Trends. 2010;2:39–47. [PubMed] [Google Scholar]
- 5.Qiu L.P., Chen K.P. Anti-HBV agents derived from botanical origin. Fitoterapia. 2013;84:140–157. doi: 10.1016/j.fitote.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Fang J.B., Duan H.Q., Zhang Y.W., Yoshihisa T. Chemical constituents from herb of Alternanthera philoxeroides. China J. Chin. Mater. Med. 2006;13:1072–1075. [PubMed] [Google Scholar]
- 7.Van H.L., Karalic I., Van C.S., Defore D., Heyerick A. Antioxidant flavone glycosides from the leaves of Fargesia robusta. J. Nat. Prod. 2010;9:1573–1577. doi: 10.1021/np100220g. [DOI] [PubMed] [Google Scholar]
- 8.Wang G.J., Chen Y.M., Wang T.M., Lee C.K., Chen K.J., Lee T.H. Flavonoids with iNOS inhibitory activity from Pogonatherum crinitum. J. Ethnopharmacol. 2008;1:71–78. doi: 10.1016/j.jep.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Fan W.Q., Xiong M.X., Ma Z., Li Q.Y., Liu Y.W. Chemical constituents of Alternanthera philoxeroides. Chin. J. Nat. Med. 2008;2:112–115. doi: 10.3724/SP.J.1009.2008.00112. [DOI] [Google Scholar]
- 10.Tian Y., Sun L.M., Li B., Liu X.Q., Dong J.X. New anti-HBV caryophyllane-type sesquiterpenoids from Euphorbia humifusa Willd. Fitoterapia. 2011;82:251–254. doi: 10.1016/j.fitote.2010.10.005. [DOI] [PubMed] [Google Scholar]