Abstract
Reducing inappropriate prescribing is key to mitigating antibiotic resistance, particularly in acute-care settings. Clinicians’ prescribing decisions are influenced by their judgments and actual or perceived patient expectations. Fuzzy-trace theory predicts that patients and clinicians base such decisions on categorical gist representations that reflect the bottom-line understanding of information about antibiotics. However, due to clinicians’ specialized training, the categorical gists driving clinicians’ and patients’ decisions might differ, which could result in mismatched expectations and inefficiencies in targeting interventions. We surveyed clinicians and patients from two large urban academic hospital emergency departments (EDs), and a sample of non-patient subjects, regarding their gist representations of antibiotic decisions, as well as relevant knowledge and expectations. Results were analyzed using exploratory factor analysis (EFA) and multifactor regression. 149 clinicians (47% female; 74% white), 519 online subjects (45% female; 78% white), and 225 ED patients (61% female; 56% black) completed the survey. While clinicians demonstrated greater knowledge of antibiotics and concern about side effects than patients, the predominant categorical gist for both patients and clinicians was “why not take a risk” which compares the status quo of remaining sick to the possibility of benefit from antibiotics. This gist also predicted expectations and prior prescribing in the non-patient sample. Other representations reflected the gist that “germs are germs” conflating bacteria and viruses, and perceptions of side effects and efficacy. Although individually rational, reliance on the “why not take a risk” representation can lead to socially-suboptimal results including antibiotic resistance and individual patient harm due to adverse events. Changing this representation could alter clinicians’ and patients’ expectations, suggesting opportunities to reduce overprescribing.
Overprescribing of antibiotics is a persistent problem, particularly for upper respiratory infections.1 Inappropriate prescribing can increase healthcare costs, lead to adverse reactions, and accelerate the evolution of antibiotic resistance. Antibiotic resistance has been implicated in over 2 million illnesses and 23,000 deaths, with overall societal costs of $20 billion in direct costs and $35 billion in indirect costs according to the US Centers for Disease Control and Prevention.2,3
Our primary focus is on the decision-making processes of clinicians and patients regarding antibiotic prescribing, which have largely not been addressed, particularly in the acute care setting. Beyond prior work examining characteristics of patients (e.g., their gender, age, insurance status, race/ethnicity, wait time, and comorbidities)4–7, clinicians (e.g., professional specialty, training, and experience)7–16, and healthcare systems (e.g., resources available, environmental factors, and access to and quality of care)17, the most commonly cited reason for inappropriate prescribing is providers’ perceptions of patients’ expectations.2 For example, patient satisfaction is considered a major driver of physician prescribing,18,19 even though physicians are often unable to accurately judge these expectations. Furthermore, prior work suggests that patients’ attitudes about prescribing may be influenced by imperfect information about antibiotics and treatment guidelines20 and impatience,21 while clinicians’ attitudes may be influenced by actual or perceived patient expectations,2,17,21 diagnostic uncertainty,22 impatience, and fear of negative outcomes.21 Ultimately, patients are more satisfied, and diagnoses are more accurate, when their expectations are clear and physicians address them.18,23–25 Thus, a better understanding of clinician and provider decision-making can inform educational interventions that are theoretically-motivated and empirically-validated, with direct implications for practice. Specifically, these interventions can be used to better communicate the risks and consequences of inappropriate prescribing.
Background and rationale for study
The purpose of the current study is to determine whether patients’ rationales for antibiotic use are shared by providers, with the aim of understanding the drivers of effective interventions. Furthermore, we determine whether differences in these rationales account for variations in expectations and prescribing behavior.
Our approach is based on fuzzy trace theory (FTT),24 a theory of medical decisionmaking that we used to generate predictions about patients’ preferences and expectations for antibiotics. FTT postulates that individuals mentally represent information in ways that vary from precise verbatim facts, such as “if I take antibiotics, there is a 0.1% chance of negative side effects” – to the simplest gist – categorical representations of bottom-line meaning, such as “if I take antibiotics, the chance that something bad will happen is nil.” Studies suggest that people encode both types of representations of information into memory but rely mainly on gist for decision-making.26–29 Thus, according to FTT, decisions are based primarily on simple meanings derived from information. FTT has been applied in more than 94 studies to better understand how patients and clinicians make health-related decisions.30
Our main hypothesis about patients’ expectations for antibiotics is motivated by FTT’s predictions for framing effects31. Studies of framing effects typically present decisions between sure options and risky gambles; decision makers usually prefer the sure option for gains but the risky gamble for losses. This is called a “framing” effect because the same options can be phrased as either gains or losses relative to the status quo. Decisions about antibiotics are analogous to decisions in framing problems. That is, a patient’s decision can be characterized as a choice between a certain state of already being sick—the status quo—versus taking a gamble. Given this status quo, at the simplest level of gist – categorical distinctions – patients have two options:
Stay sick for sure (do not take antibiotics)
Stay sick or get better (by taking antibiotics)
Option 2 is a gamble because more than one outcome is possible.24 Getting better is preferred over staying sick; thus, FTT predicts that patients would prefer to take antibiotics since doing so offers the possibility of getting better. We therefore call this gist “why not take a risk” (WNTAR). WNTAR is a strategy that is associated with categorical representations of decision options rather than more precise (verbatim) trading offs of outcomes and probabilities. The underlying premises of the gist representation depicted above are that there is some chance that antibiotics could be effective (e.g., if an infection is bacterial) and that antibiotics are essentially harmless to the individual. Thus, it makes sense-- at the level of the individual--for patients to want antibiotics, and for providers to prescribe antibiotics, when there is a possibility that the patient could get better with nil downside risk (though choosing antibiotics may produce socially suboptimal consequences such as antibiotic resistance at the level of the group).
Current public health interventions (e.g., the CDC’s “Get Smart” program) assume that patients lack knowledge about the differences between viruses and bacteria,25 and therefore will expect antibiotics when they are not warranted. Indeed, prior work32 found that 48% of patients sampled in an inner-city emergency department did not know the differences between bacteria and viruses – a gist that we call “germs are germs” (GAG; i.e., viruses and bacteria are both “bugs” that make people sick). However, consistent with WNTAR, an even greater proportion, of those patients, 76%, agreed with statements indicating that antibiotics might make them better although many knew that antibiotics were unlikely to be effective. Furthermore, possible harms associated with antibiotics were viewed as negligible.
It might be expected that providers would endorse neither GAG nor WNTAR because these representations fail to make precise distinctions (between viruses vs. bacteria or between levels of outcomes and probabilities, respectively). We expect that clinicians are educated about the differences between bacteria and viruses, and thus would not be likely to endorse GAG.
In contrast, FTT makes the surprising prediction that providers should endorse WNTAR33. That is, FTT predicts that developmentally advanced reasoners, such as experts reasoning in their domain of expertise, rely on simple gist representations to make decisions. This finding has been confirmed in prior samples of healthcare providers, but it has never been tested in the domain of antibiotics.24,34 Thus, the main hypothesis we address is whether providers endorse WNTAR much like patients do, despite differences in domain-specific expertise about antibiotics. To explore this hypothesis about how widespread these representations are, we conducted a survey to examine the mental representations (i.e., gists) of decisions about antibiotic prescribing in the ED held by providers, their patients, and the public, and linked their responses to expectations for, and prior receipt of, antibiotics. We also evaluated a variety of other factors that may influence expectations about antibiotics, including beliefs and knowledge of side effects.
Methods
We conducted three observational studies to assess our strategic risk hypotheses across 1) an online sample, 2) ED patients, and 3) health care providers. All three studies were approved by The George Washington University Institutional Review Board (IRB). The provider study was also approved by the Johns Hopkins IRB.
Selection and Description of Participants
Subjects in the online sample were administered an online survey between May 6 and 7, 2015 using Qualtrics software. Subjects were recruited using Amazon’s Mechanical Turk service, which is an online marketplace where individuals can be hired to complete human intelligence tasks (HITs) of short duration for micro-payments. Subjects were eligible to participate if they resided in the U.S., were at least 18 years old, had successfully completed 1000 or more HITs, and had a HIT approval rate of 98% or higher. Subjects received $1 for successful completion of the survey.
We collected a second sample focused on ED patients because they vary significantly from the general population, with important implications for our study. For example, an analysis of nationally representative data on ED visits for common infections found that ED patients were more commonly younger and African American and had Medicaid or no insurance.35 In addition, studies demonstrate that African American and Medicaid patients are more frequent users of the ED for both non-urgent and urgent reasons. Finally, patients with non-commercial insurance coverage use the ED for conditions more likely to be non-urgent.36 These patients also have higher rates of return visits and reduced diagnostic testing.37 Thus, by focusing on ED patients, we can index lower SES and underserved populations. This patient population has been theorized to have different health literacy and viewpoints on antibiotics.38 Furthermore, to the extent that prescribing is driven by patients’ expectations, the decisions of emergency physicians may generalize to primary care providers for illnesses of the same duration in this population.39 Beyond these considerations, there is evidence that patients presenting to the emergency room receive antibiotics at overall higher rates than those who are seen by general practitioners.40
Subjects in the ED patient sample were administered a paper survey between April and July 2015 in the George Washington University Hospital ED. George Washington University Hospital is a Level 1 trauma center, and the ED serves as a primary source of emergency care for the surrounding community. Research staff approached ED patients aged 18 years and older who could understand or read English after they were seen by the ED provider but prior to discharge. Patients received a gift card for $10 upon completion of the survey. We used a paper survey to be consistent with our prior study32 and because of limited staff coverage and access to tablets and/or laptops. This allowed multiple surveys to be distributed in a short period of time while eligible patients waited for evaluation, results, or treatment in the ED.
Finally, in the provider sample, subjects were administered an online survey between May 19 and December 26, 2015 using Qualtrics software. Subjects were attending physicians, residents, physician assistants, and nurse practitioners at The George Washington University Hospital and the Johns Hopkins University Hospital EDs. There were no exclusion criteria. Subjects received a gift card for $25 for completion of the survey.
Technical Information
Subjects were presented with a short scenario of a patient with symptoms of a common upper respiratory infection. The symptoms described are highly familiar to ED patients and clinicians, and prescribing guidelines are clear that antibiotics are not warranted in such cases; yet, they are often prescribed (see Supplemental Material). Subjects were then given 46 Likert scale items to answer (Table 1). All subjects were asked to answer these questions in the context of the scenario with the prompt: “How would you answer the questions below in this situation?” In the online sample, subjects also answered two attention check questions designed to filter out inattentive participants. In the provider sample, one item was dropped due to a labeling reversal. Similar results are obtained if we keep the item. To control for response bias, we included an equal number of positively- and negatively-worded items.
Table 1.
Demographic Characteristics of Each of the Three Samples
| Category | Characteristic | Online (%) N=519 |
ED Patients (%) N=225 |
Providers (%) N=149 |
|---|---|---|---|---|
| Gender | Male | 283 (55) | 134 (60) | 76 (51) |
| Female | 236 (45) | 85 (38) | 70 (47) | |
| No Answer | 0 (0) | 6 (3) | 3 (2) | |
| Race | White | 409 (78) | 79 (35) | 110 (74) |
| Black/African-American | 43 (8) | 119 (53) | 8 (5) | |
| Asian | 47 (10) | 8 (4) | 28 (19) | |
| Mixed/Other | 20 (4) | 17 (8) | 0 (0) | |
| No Answer | 0 (0) | 2 (1) | 3 (2) | |
| Hispanic Ethnicity | No | 478(92) | 181(80) | 135 (91) |
| Yes | 41 (8) | 22 (10) | 11 (7) | |
| No Answer | 0 (0) | 22 (10) | 3 (2) | |
| Age | 18-19 | 7 (1) | 8 (4) | 0 (0) |
| 20-29 | 186 (37) | 74 (33) | 50 (34) | |
| 30-39 | 186 (37) | 55 (24) | 53 (36) | |
| 40-49 | 63 (12) | 35 (16) | 33 (22) | |
| 50-59 | 55 (11) | 24 (11) | 5 (3) | |
| 60+ | 22 (4) | 15 (7) | 5 (3) | |
| No Answer | 0 (0) | 6 (3) | 3 (2) | |
| Native English Speaker | Yes | 508 (98) | 212 (94) | 138 (93) |
| No | 11 (2) | 8 (4) | 8 (5) | |
| No Answer | 0 (0) | 5 (2) | 3 (2) | |
| Education | Did not finish high school | 4 (1) | 15 (7) | 0 (0) |
| Graduated high school | 52 (10) | 51 (23) | 0 (0) | |
| Attended some college but did not finish a 4-year degree | 184 (35) | 52 (23) | 0 (0) | |
| Graduated from a 4-year college or more | 181 (35) | 64 (28) | 0 (0) | |
| Obtained a graduate/professional degree | 49 (9) | 39 (17) | 149 (100) | |
| No Answer | 49 (9) | 4 (2) | 0 (0) | |
| Father’s Education | Did not finish high school | 45 (9) | 25 (11) | 3 (2) |
| Graduated high school | 144 (28) | 51 (23) | 12 (8) | |
| Attended some college but did not finish a 4-year degree | 124 (24) | 27 (12) | 18 (12) | |
| Graduated from a 4-year college or more | 195 (38) | 92 (41) | 113 (76) | |
| Don't know/No Answer | 11 (2) | 30 (13) | 3 (2) | |
| Mother’s Education | Did not finish high school | 47 (9) | 23 (10) | 4 (3) |
| Graduated high school | 185 (36) | 49 (22) | 17 (11) | |
| Attended some college but did not finish a 4-year degree | 122 (24) | 40 (18) | 22 (15) | |
| Graduated from a 4-year college or more | 160 (31) | 90 (40) | 103 (69) | |
| Don't know/no answer | 5 (1) | 23 (10) | 3 (2) | |
| Received Free Lunch | Yes | 149(29) | 130 (58) | 17 (11) |
| No | 358 (69) | 83 (37) | 124 (83) | |
| Don’t know/No Answer | 12 (2) | 12 (5) | 8 (5) | |
| Importance of Religion | Not at all important | 280 (54) | 41 (18) | 42 (28) |
| Slightly important | 64 (12) | 20 (9) | 43 (29) | |
| Somewhat important | 44 (8) | 30 (13) | 32 (21) | |
| Important | 67 (13) | 39 (17) | 17 (11) | |
| Very important | 64 (12) | 94 (42) | 12 (8) | |
| No Answer | 0 (0) | 1 (0) | 15 (10) | |
| Employment Status | Full-time | 301 (58) | 126 (56) | |
| Part-time | 98 (19) | 23 (10) | ||
| Other | 120 (23) | 81 (36) | ||
| Don’t know/No Answer | 0 (0) | 25 (11) | ||
| Occupation | 1st Year Resident | 18 (12) | ||
| 2nd Year Resident | 15 (10) | |||
| 3rd Year Resident | 16 (11) | |||
| 4th Year Resident | 16 (11) | |||
| Attending Physician | 68 (46) | |||
| Physician Assistant | 16 (11) | |||
| Nurse Practitioner | 2 (1) |
To measure subjects’ prior use of and expectations for antibiotics, the online sample included two yes/no questions, (“Did you expect to receive an antibiotic last time you went to the doctor’s office or emergency room?” and “Did you take an antibiotic last time you had symptoms like those described in this situation?”) and the ED patient sample collected information on patients’ final diagnosis, whether antibiotics were indicated, and whether patients received antibiotics. In the provider sample, we collected information on physicians’ overall prescribing behavior (details in41). Finally, in all three studies, subjects responded to two free-response questions, “Why should someone take antibiotics?” and “What is the difference between a viral infection and a bacterial infection?”.
Across all three studies, we collected demographic information, including gender, age, race, ethnicity, level of education, and other indicators of socioeconomic status (e.g. “Do you or did you ever receive a free lunch from school?”). We also collected additional socioeconomic variables such as religious affiliation, level of religious observance, work status, occupation, and parents’ level of educational achievement. In the ED patient sample, we collected the patient’s reason for the visit to the ED.
In all three studies, survey questions were based on the published literature. The questions were directed at perceptions of antibiotics relative to a common scenario of viral respiratory symptoms (described in detail), rather than at antibiotics prescribed during a specific visit. Responses were recorded using a seven-point Likert scale, ranging from strongly disagree (−3) to strongly agree (+3). To ensure that question wording did not bias responses, each question was presented in both a forward- or reverse-coded version. For example, patients who strongly agree (+3) that antibiotics work against bacteria should strongly disagree (−3) with the statement that antibiotics do NOT work against bacteria. To avoid any bias in question order, the 46 questions were presented in a random order. We did not place any restrictions on which questions could appear together.
Statistics
We computed correlations of item agreement with age, education, and other demographic factors to determine whether these characteristics were associated with knowledge, misconceptions, and risk strategies associated with antibiotics. We also conducted factor analyses (specifically, Exploratory Factor Analyses; EFA) to assess whether questions clustered as predicted (i.e., had been answered similarly across subjects). These clusters (or dimensions) were inspected to identify gist themes underlying responses. There was no forced extraction of components. The varimax rotation method was used with maximum likelihood extraction and Heywood cases were discarded. Six factors were retained for the online sample, and four factors each were retained for the ED patient and provider samples. For all studies, the number of factors retained was based on standard (36) goodness-of-fit criteria (root mean square error of approximation [RMSEA] = 0.060, 0.057, and 0.067; 95% lower confidence bound = 0.056, 0.046, and 0.048; examination of parallel analysis scree plot; Tucker-Lewis Index [TLI] = 0.889, 0.855, and 0.778; minimal Bayesian Information Criterion [BIC] = −2688.33, −3285.95, and −3040.56 for the online, ED patient, and provider samples, respectively; Tables S1, S2, and S3, Supplemental Material). Results were robust across multiple analysis methodologies (Tables S4, S5, and S6, Supplemental Material). To examine the factors predicting antibiotic prescribing, we regressed subjects’ demographic variables against factor loadings. Finally, after controlling for demographic variables, we examined which latent factors predicted subjects’ prior use of, and expectations for, antibiotics in the online sample. We used a combination of forward, backward, and bidirectional regression techniques to select a model with the lowest the Akaike Information Criterion score (see Supplemental Material). All statistical analyses were conducted using version 3.2.0 of the R Project for Statistical Computing.
Role of Funding Source
The funding source had no role in study design or implementation.
Results
Sample Characteristics
In the online sample, data were collected for 540 subjects, of whom 519 (96%) answered both attention check questions correctly and completed the survey. Of these, most self-identified as White (78%), non-Hispanic (92%), with at least some college education (79%). In the ED patient sample, 287 subjects were approached to complete the survey, of whom data were collected for 240 subjects (84%). Of these 240 subjects, 225 (94%) completed the full survey. Most subjects self-identified as African-American (53%), non-Hispanic (80%), and most (53%) had either not attended or not finished college. Finally, in the provider sample, 238 subjects were invited to participate, of whom data were collected for 155 (65%) subjects. Of these 155 subjects, 149 (96%) completed the survey. Most providers self-identified as White (74%), non-Hispanic (91%), with approximately equal numbers of attending physicians and residents (Table 1).
Among all studies, after controlling for multiple comparisons, we found no consistent correlations between age, education (or number of years of practice), or level of religious observance and item agreement (Supplemental Material, Table S7). Also, responses did not differ significantly by gender with one exception: female subjects in the online sample were more likely to agree with items indexing the “going to the ER necessitates antibiotics” gist.
Subjects’ Knowledge and Misconceptions Regarding Antibiotics.
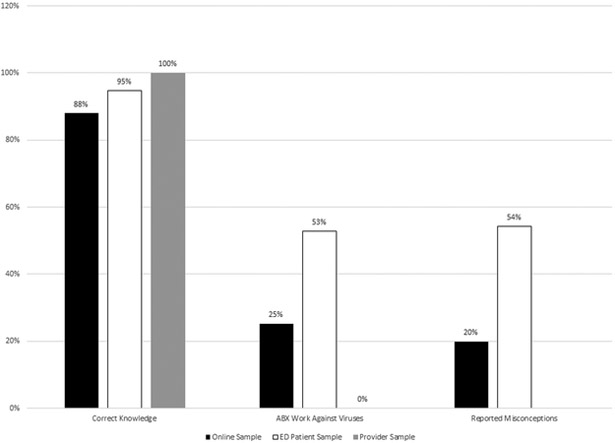
Mean responses for each question are shown in Table 2. Among the online and ED patient samples, most subjects (88%) displayed some correct knowledge: they agreed that antibiotics work against bacteria or that antibiotics should be taken for strep throat (or disagreed that antibiotics do not work against bacteria, etc.; Figure 1). However, many subjects also had misconceptions. For example, some subjects in both samples agreed that antibiotics work against viruses (or disagreed that antibiotics do not work against viruses) and, in free-response questions, several spontaneously reported misconceptions, for example, that bacteria only spread through direct contact whereas viruses spread in the air.
Table 2.
Mean Ratings on a Disagreement-Agreement Scale with Items Describing the Gist of Antibiotics Expectations, Grouped by Gist
| Item short form | Item full text | Sample Mean (SD) |
||
|---|---|---|---|---|
| Online (N=519) |
ED Patients (N=225) |
Providers (N=149) |
||
| Germs are Germs | ||||
| Yes against viruses | Antibiotics work against viruses. | −1.26 (1.88) | −0.25 (2.05) | −2.81 (0.60) |
| No against viruses | Antibiotics do NOT work against viruses. | 1.25 (1.87) | 0.33 (2.01) | 2.74 (0.64) |
| Yes against bacteria | Antibiotics work against bacteria. | 1.84 (1.22) | 1.84 (1.19) | 2.54 (0.83) |
| No against bacteria | Antibiotics do NOT work against bacteria. | −1.76 (1.41) | −1.58 (1.45) | −2.40 (1.29) |
| Viral can turn into bacterial | Sometimes a viral illness turns into something that needs treating with an antibiotic. | 0.73 (1.65) | 1.08 (1.52) | 1.81 (0.98) |
| Viral can’t turn into bacterial | A viral illness never turns into something that needs treating with an antibiotic. | −0.69 (1.56) | −0.78 (1.72) | −2.11 (0.93) |
| Why Not Take a Risk? (Antibiotics Don’t Hurt) | ||||
| Don’t know about ABX, but it can’t hurt/ABX can’t hurt | I don’t know if an antibiotic can make me better, but it can’t hurt to take them./It can’t hurt the patient to take antibiotics. | −0.70 (1.75) | −0.23 (1.87) | −1.50 (1.29) |
| Might not help, but better safe than sorry | Antibiotics might not make (me/the patient) better, but it is better to be safe than sorry so (I/he/she) should take them. | −0.57 (1.83) | 0.23 (1.82) | −1.56 (1.08) |
| Take ABX just in case | Antibiotics might not make (me/the patient) better, but (I/he/she) should take them just in case. | −0.71 (1.78) | −0.19 (1.90) | −1.78 (1.13) |
| ABX might help and can’t hurt | (I/The patient) should take antibiotics because they might help and can’t hurt. | −0.69 (1.74) | −0.15 (1.82) | −1.77 (1.04) |
| It can’t hurt | It can’t hurt to take antibiotics. | −0.81 (1.71) | −0.36 (1.88) | −1.68 (1.29) |
| Better safe than sorry | (I/The patient) should take antibiotics because it’s better to be safe than sorry. | −0.47 (1.81) | 0.13 (1.87) | −1.72 (1.10) |
| Might as well take a chance | (I am already/The patient is) sick, so (I) might as well take a chance that antibiotics will help. | −0.43 (1.85) | 0.30 (1.86) | −1.52 (1.24) |
| Better safe than sorry, so take ABX | It is better to be safe than sorry, so (I/the patient) should take antibiotics. | −0.48 (1.83) | 0.21 (1.84) | −1.56 (1.20) |
| Might not work but why take a chance? | An antibiotic might not work, but why take a chance on not getting better? | −0.45 (1.68) | 0.10 (1.81) | −1.16 (1.34) |
| Antibiotics Don’t Help | ||||
| Might not help, so better not to take ABX | Antibiotics might not make (me/the patient) better, so (I/he/she) should NOT take them. | −0.16 (1.67) | −0.93 (1.61) | 0.84 (1.28) |
| Don’t take ABX if they don’t make me better | (I/The patient) should NOT take antibiotics if they might not make (me/him/her) better. | 0.51 (1.73) | 0.25 (1.83) | 0.81 (1.40) |
| ABX don’t help and can hurt | (I/The patient) should NOT take antibiotics because they don't help and they can hurt. | −0.61 (1.61) | −1.25 (1.53) | 1.07 (1.37) |
| ABX might not be safe | (I/The patient) should NOT take antibiotics because they might not be safe. | −0.57 (1.50) | −1.01 (1.53) | 0.17 (1.37) |
| Should NOT take a chance | (I am/The patient is) already sick, but (I) should NOT take a chance on getting worse (by taking antibiotics). | −0.54 (1.63) | −1.12 (1.64) | −0.32 (1.51) |
| Better safe than sorry, so no ABX | It is better to be safe than sorry, so (I/the patient) should NOT take antibiotics. | −0.22 (1.70) | −0.91 (1.54) | 0.42 (1.36) |
| Might not work so why take ABX? | An antibiotic might not work, so why take one? | −0.23 (1.66) | −0.88 (1.66) | 0.40 (1.39) |
| Side Effects (Antibiotics Hurt) | ||||
| Don’t know, but (I/pt) might get side effects | I don't know if an antibiotic can make (me/the patient) better, but (I/he/she) might get side effects (if I take/from taking) them. | 0.80 (1.46) | 0.51 (1.65) | 1.35 (1.09) |
| Don’t know but (I/pt) won’t get side effects | I don’t know if an antibiotic can make (me/the patient) better, but (I/he/she) will NOT get side effects (if I take/from taking) them. | −0.93 (1.47) | −0.92 (1.55) | −1.74 (1.01) |
| (I/Pt) might get side effects | (I/The patient) might get side effects if (I/he/she) take antibiotics. | 1.02 (1.41) | 1.11 (1.50) | 1.79 (0.81) |
| (I/Pt) won’t get side effects | (I/The patient) will NOT get side effects if (I/he/she) take antibiotics. | −0.86 (1.36) | −1.06 (1.44) | −1.48 (1.02) |
| ABX might have side effects | Antibiotics might have harmful side effects. | 0.95 (1.41) | 0.93 (1.53) | 1.95 (0.79) |
| ABX don’t have side effects | Antibiotics do NOT have harmful side effects. | −1.09 (1.38) | −1.15 (1.53) | −2.07 (0.96) |
| Don’t know about ABX, but it might hurt/ ABX could hurt pt | I don’t know if an antibiotic can make (me/the patient) better, but it could hurt (the patient) to take them. | 0.44 (1.70) | −0.08 (1.77) | 1.55 (0.90) |
| It could hurt | It could hurt to take antibiotics./ It could hurt the patient to take antibiotics. | 0.59 (1.67) | 0.06 (1.83) | 1.70 (0.99) |
| Antibiotics Help | ||||
| Yes against germs | Antibiotics work against all germs. | −1.61 (1.37) | −0.99 (1.78) | −2.63 (0.66) |
| No against germs | Antibiotics only work against some germs. | 1.68 (1.31) | 1.30 (1.54) | 2.50 (0.87) |
| (I/Pt) (don’t/doesn’t) need ABX | (I/The patient) will get better even if (I don’t/he/she doesn’t) take antibiotics. | 0.88 (1.32) | −0.11 (1.59) | 1.49 (0.93) |
| (I/Pt) need ABX | (I/The patient) won’t get better unless (I take/he/she takes) antibiotics. | 1.18 (1.46) | −0.51 (1.73) | −1.97 (0.95) |
| I know ABX help | I know an antibiotic can make (me/the patient) better | −0.47 (1.68) | 0.63 (1.74) | −1.52 (1.12) |
| I don’t know if ABX help | I don’t know if an antibiotic can make (me/the patient) better. | 0.78 (1.57) | −0.03 (1.65) | 0.28 (1.55) |
| ABX will help | Antibiotics will make (me/the patient) better. | 0.04 (1.33) | 0.74 (1.36) | −1.26 (1.17) |
| ABX might not help | Antibiotics might not make (me/the patient) better. | −1.40 (1.26) | 0.52 (1.77) | 1.85 (0.85) |
| Symptoms go away with ABX | (My/The patient’s) symptoms will only go away with antibiotics. | −0.94 (1.53) | −0.58 (1.72) | −1.95 (0.95) |
| Symptoms don’t go away with ABX | (My/The patient’s) symptoms will NOT go away with antibiotics. | −0.32 (1.43) | −0.63 (1.52) | 0.16 (1.67) |
| Antibiotics Should be Taken for Strep Throat | ||||
| Yes for strep | Antibiotics should be taken for strep throat. | 1.16 (1.50) | 1.30 (1.47) | 1.77 (1.48) |
| No for strep | Antibiotics should NOT be taken for strep throat. | −1.14 (1.52) | −1.24 (1.57) | −1.94 (1.21) |
| The Doctor Doesn’t Believe Me | ||||
| Doctor doesn’t believe me/I don’t believe pt | If a doctor doesn’t give me an antibiotic, the doctor doesn’t believe that I am really sick/If | −1.55 (1.44) | −1.44 (1.64) | −0.89 (1.38) |
| Doctor takes (me/pt) seriously | I don't prescribe an antibiotic the patient won't think I believe that he/she is really sick. A doctor who takes my illness seriously will only give me an antibiotic if I need it./ The patient knows that I will only prescribe antibiotics if he/she needs them. | 1.73 (1.43) | 1.38 (1.70) | 0.34 (1.52) |
| Going to the ER Necessitates Antibiotics | ||||
| ER means ABX | If (I am/ my patient is) sick enough to go to the emergency room, (I/he/she) should get antibiotics. | −0.66 (1.82) | 1.03 (1.77) | −2.02 (1.01) |
| ER doesn’t mean ABX | Just because (I am/the patient is) sick enough to go to the emergency room does NOT mean (I/he/she) should get antibiotics. | 1.15 (1.62) | −0.68 (1.78) | 1.97 (0.98) |
Note. ABX = antibiotics. Pt = Patient. SD = Standard Deviation. Each survey contained both versions of each question. Responses were recorded using a seven-point Likert scale, ranging from strongly disagree (−3) to strongly agree (+3). A source reference for each gist is given in parentheses.
Figure 1.
Subjects’ knowledge and misconceptions about antibiotics across all three studies. Study 1 = Online Sample; Study 2 = ED Patient Sample; Study 3 = Provider Sample. ABX, antibiotics; ED, emergency department.
Why Not Take a Risk?
Across all three studies, many subjects (254, 49%; 176, 78%; 72, 48% in each sample, respectively) agreed with at least one item supporting WNTAR. In addition, EFA results showed that WNTAR captured the largest amount of unique variance in the online and ED patient samples (14% and 17%, respectively), and was tied for largest with subjects’ concerns about side effects (SE) in the provider sample (11% of the variance). All items loading on this dimension highlight the perception of possible gain and negligible downside risk associated with taking antibiotics.
Germs Are Germs.
Compared to the WNTAR gist, fewer subjects agreed that antibiotics work against viruses or that antibiotics do not work against bacteria (or disagreed with their reverse coded variants; 151, 29%; 110, 49%; 11, 7% in the online, ED patient, and provider samples, respectively). Subjects endorsing these items were more likely to endorse WNTAR (76%, 87%, and 58% of subjects who endorsed GAG also endorsed WNTAR in the online, ED patient, and provider samples). Items indicating that viral illnesses can/can’t turn into bacterial illnesses were not included since they do not index confusion between viruses and bacteria. Items expressing the GAG gist captured unique variance in the EFA for the online (6%) and ED patient (9%) samples, but not for the provider sample. As expected, all subjects in the provider sample displayed correct knowledge – none agreed that antibiotics work against viruses (or disagreed that antibiotics do not work against viruses) and all but three subjects (146, 98%) indicated that viral and bacterial illnesses have different etiologies – i.e., they explicitly disagreed with the GAG gist.
Side Effects.
Virtually all subjects agreed that antibiotics have side effects (477, 92%; 208, 92%; 149, 100% in the online, ED patient, and provider samples, respectively). SE captured a significant source of variance in the EFA across all three samples (11% in the online sample, 9% in the ED patient sample, and 11% in the provider sample).
Antibiotics Do Not Help.
Subjects who agreed with this gist endorsed items indicating that they did not believe antibiotics are efficacious. Subjects endorsing these items were less likely to endorse WNTAR, especially in the online and ED patient samples (respectively, 36%, 70%, and 47% of subjects who agreed that antibiotics don’t help also endorsed WNTAR). This dimension also captured a significant source of variance in the EFA across all three samples (11% in the online sample, 11% in the ED patient sample, and 5% in the provider sample).
Other Gists.
In addition to the gists listed above, items indicating perceptions of antibiotic efficacy (“ABX Help”) captured significant variance in the online and provider samples (9% and 8%, respectively), but not in the ED patient sample. In addition, a factor indicating antibiotics should be taken for strep throat captured significant variance (4%) in the online sample.
Factors Affecting Antibiotic Prescribing
Relations between demographic variables and factor loadings are reported in Table 3. Demographics associated with endorsement of each factor diverged between samples and none of these factors consistently predicted endorsement of a gist across all three samples. After controlling for demographic factors, we found that endorsement of WNTAR was associated with subjects’ prior use of antibiotics (OR=3.08, p<0.001) and their expectations for antibiotics (OR=1.99, p<0.001) in the online sample. Additionally, endorsement of the “Antibiotics Don’t Help” gist was associated with lower expectations for antibiotics (OR=0.56, p<0.001). The “ABX Help” gist and a factor associated with strep throat also significantly predicted prior use of and expectations for antibiotics. Neither SE nor GAG significantly predicted these responses.
Table 3:
Results of Linear Regression Analyses for the Studies in our Sample
| Predictor | WNTAR |
GAG |
SE |
ABX Don’t Help |
ABX Help |
Strep Throat |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | β | B | SE B | β | B | SE B | β | B | SE B | β | B | SE B | β | B | SE B | β | |
| Online Sample | ||||||||||||||||||
| Male Gender | −0.09 | 0.09 | −0.09 | 0.02 | 0.09 | 0.02 | 0.02 | 0.09 | 0.02 | −0.18 | 0.08 | −0.18* | −0.09 | 0.09 | −0.09 | 0.00 | 0.09 | 0.00 |
| Age | −0.01 | 0.00 | −0.14** | −0.01 | 0.00 | −0.10* | 0.00 | 0.00 | −0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | −0.04 | 0.00 | 0.00 | 0.03 |
| Hispanic Ethnicity | −0.03 | 0.19 | −0.03 | −0.11 | 0.19 | −0.11 | 0.05 | 0.19 | 0.05 | −0.19 | 0.17 | −0.19 | 0.06 | 0.17 | 0.06 | 0.26 | 0.19 | 0.26 |
| Race: Asian | −0.17 | 0.17 | −0.17 | 0.01 | 0.17 | 0.01 | 0.09 | 0.16 | 0.09 | 0.55 | 0.15 | 0.55*** | 0.05 | 0.15 | 0.05 | −0.28 | 0.17 | −0.28 |
| Race: African-American | −0.50 | 0.62 | −0.50 | 0.20 | 0.18 | 0.20 | 0.14 | 0.18 | 0.14 | −0.12 | 0.16 | −0.12 | 0.07 | 0.17 | 0.07 | −0.24 | 0.18 | −0.24 |
| Race: Mixed/Other | −0.36 | 0.27 | −0.36 | −0.11 | 0.27 | −0.11 | 0.49 | 0.27 | 0.49 | 0.24 | 0.25 | 0.24 | −0.59 | 0.25 | −0.59* | −0.11 | 0.28 | −0.11 |
| English Native Language? | 0.07 | 0.37 | 0.07 | 0.05 | 0.37 | 0.05 | −0.05 | 0.37 | −0.05 | −0.40 | 0.34 | −0.40 | −0.22 | 0.35 | −0.22 | 0.14 | 0.38 | 0.14 |
| Education | −0.02 | 0.06 | −0.02 | −0.04 | 0.06 | −0.03 | −0.01 | 0.06 | −0.01 | 0.03 | 0.05 | 0.03 | −0.02 | 0.05 | −0.02 | 0.06 | 0.06 | 0.05 |
| Father’s Education | −0.03 | 0.05 | −0.03 | −0.09 | 0.05 | −0.09 | −0.03 | 0.05 | −0.03 | −0.01 | 0.04 | −0.01 | −0.06 | 0.04 | −0.06 | 0.06 | 0.05 | 0.06 |
| Mother’s Education | −0.01 | 0.05 | −0.01 | 0.03 | 0.05 | 0.03 | −0.05 | 0.05 | −0.05 | 0.02 | 0.04 | 0.02 | 0.01 | 0.04 | 0.01 | 0.04 | 0.05 | 0.04 |
| Received Free Lunch? | 0.06 | 0.10 | 0.06 | 0.15 | 0.10 | 0.15 | 0.05 | 0.10 | 0.05 | −0.02 | 0.09 | −0.02 | −0.03 | 0.10 | −0.03 | 0.12 | 0.11 | 0.12 |
| Religiosity | −0.06 | 0.03 | −0.08 | 0.06 | 0.03 | 0.09 | 0.01 | 0.03 | 0.02 | −0.02 | 0.03 | −0.03 | −0.05 | 0.03 | −0.07 | −0.04 | 0.03 | −0.05 |
| Work Status: Other | 0.27 | 0.11 | 0.27* | −0.22 | 0.11 | −0.22 | −0.01 | 0.11 | −0.01 | 0.06 | 0.10 | 0.06 | −0.07 | 0.10 | −0.07 | 0.00 | 0.11 | 0.00 |
| Work Status: Part Time | 0.08 | 0.12 | 0.08 | 0.04 | 0.12 | 0.04 | 0.06 | 0.12 | 0.06 | −0.19 | 0.11 | −0.19 | −0.09 | 0.11 | −0.09 | −0.16 | 0.12 | −0.16 |
| (Intercept) | 0.58 | 0.45 | −0.07 | 0.39 | 0.45 | −0.08 | 0.19 | 0.45 | −0.01 | 0.34 | 0.41 | 0.44 | 0.64 | 0.42 | 0.31 | −0.47 | 0.46 | −0.10 |
| ED Patient Sample | ||||||||||||||||||
| Male Gender | 0.06 | 0.15 | 0.06 | −0.18 | 0.15 | −0.18 | 0.05 | 0.14 | 0.05 | 0.10 | 0.17 | 0.10 | ||||||
| Age | −0.01 | 0.01 | −0.15 | 0.00 | 0.01 | 0.05 | 0.01 | 0.01 | 0.09 | 0.01 | 0.01 | 0.09 | ||||||
| Hispanic Ethnicity | 0.31 | 0.23 | 0.31 | −0.15 | 0.23 | −0.15 | 0.23 | 0.21 | 0.23 | 0.00 | 0.26 | 0.00 | ||||||
| Race: Asian | 0.26 | 0.39 | 0.26 | −0.59 | 0.40 | −0.59 | 0.11 | 0.36 | 0.11 | −0.20 | 0.44 | −0.20 | ||||||
| Race: African-American | 0.39 | 0.21 | 0.39 | −0.46 | 0.22 | −0.46* | 0.35 | 0.19 | 0.35 | −0.34 | 0.24 | −0.34 | ||||||
| Race: Mixed/Other | 0.15 | 0.27 | 0.15 | −0.38 | 0.28 | −0.38 | 0.37 | 0.25 | 0.37 | −0.22 | 0.31 | −0.22 | ||||||
| English Native Language? | −0.08 | 0.31 | −0.08 | 0.13 | 0.31 | 0.13 | 0.05 | 0.28 | 0.05 | −0.40 | 0.34 | −0.40 | ||||||
| Education | −0.26 | 0.07 | −0.31*** | −0.10 | 0.07 | −0.12 | −0.16 | 0.07 | −0.19* | −0.10 | 0.08 | −0.12 | ||||||
| Father’s Education | 0.07 | 0.04 | 0.13 | 0.04 | 0.04 | 0.07 | 0.00 | 0.04 | 0.00 | 0.07 | 0.05 | 0.13 | ||||||
| Mother’s Education | −0.01 | 0.05 | −0.02 | −0.01 | 0.05 | −0.02 | 0.01 | 0.04 | 0.02 | 0.03 | 0.05 | 0.06 | ||||||
| Received Free Lunch? | −0.26 | 0.18 | −0.26 | −0.28 | 0.18 | −0.28 | 0.17 | 0.16 | 0.17 | 0.21 | 0.20 | 0.21 | ||||||
| Religiosity | 0.06 | 0.05 | 0.09 | −0.06 | 0.06 | −0.09 | 0.00 | 0.05 | 0.00 | 0.05 | 0.06 | 0.07 | ||||||
| Work Status: Other | 0.16 | 0.16 | 0.16 | −0.11 | 0.16 | −0.11 | −0.10 | 0.14 | −0.10 | 0.23 | 0.18 | 0.23 | ||||||
| Work Status: Part Time | 0.07 | 0.24 | 0.07 | −0.79 | 0.24 | −0.79** | −0.44 | 0.22 | −0.44* | 0.15 | 0.27 | 0.15 | ||||||
| Antibotics Indicated? | 0.73 | 0.20 | 0.73*** | −0.03 | 0.20 | −0.03 | −0.45 | 0.18 | −0.45* | −0.05 | 0.22 | −0.05 | ||||||
| (Intercept) | 0.52 | 0.59 | −0.33 | 0.83 | 0.60 | 0.52 | −0.04 | 0.54 | −0.27 | −0.08 | 0.66 | 0.35 | ||||||
| Provider Sample | ||||||||||||||||||
| Hospital: JHU | −0.37 | 0.16 | −0.37* | −0.70 | 0.15 | −0.70*** | 0.05 | 0.15 | 0.05 | 0.14 | 0.16 | 0.14 | ||||||
| Male Gender | 0.06 | 0.16 | 0.06 | −0.09 | 0.15 | −0.09 | 0.16 | 0.15 | 0.16 | −0.04 | 0.16 | −0.04 | ||||||
| Age | 0.14 | 0.13 | 0.15 | 0.23 | 0.12 | 0.23 | −0.09 | 0.13 | −0.09 | −0.13 | 0.13 | −0.13 | ||||||
| Hispanic Ethnicity | −0.29 | 0.30 | −0.29 | 0.53 | 0.28 | 0.53 | 0.26 | 0.29 | 0.26 | 0.01 | 0.29 | 0.01 | ||||||
| Race: Asian | 0.30 | 0.20 | 0.30 | −0.17 | 0.19 | −0.17 | −0.14 | 0.20 | −0.14 | −0.09 | 0.20 | −0.09 | ||||||
| Race: African-American | 0.02 | 0.36 | 0.02 | 0.27 | 0.34 | 0.27 | −0.50 | 0.35 | −0.50 | 0.27 | 0.36 | 0.27 | ||||||
| English Native Language? | 0.43 | 0.35 | 0.43 | −0.09 | 0.33 | −0.09 | 0.30 | 0.34 | 0.30 | −0.17 | 0.35 | −0.17 | ||||||
| Father’s Education | 0.08 | 0.13 | 0.05 | 0.01 | 0.13 | 0.00 | −0.21 | 0.13 | −0.15 | 0.20 | 0.13 | 0.14 | ||||||
| Mother’s Education | 0.03 | 0.12 | 0.02 | −0.09 | 0.12 | −0.07 | 0.04 | 0.12 | 0.03 | −0.19 | 0.12 | −0.15 | ||||||
| Received Free Lunch? | 0.55 | 0.22 | 0.55* | 0.03 | 0.20 | 0.03 | −0.38 | 0.21 | −0.38 | 0.16 | 0.22 | 0.16 | ||||||
| Religiosity | −0.07 | 0.07 | −0.08 | −0.11 | 0.06 | −0.14 | −0.03 | 0.06 | −0.04 | −0.01 | 0.07 | −0.01 | ||||||
| Years Experience | −0.03 | 0.02 | −0.20 | −0.01 | 0.02 | −0.06 | 0.03 | 0.02 | 0.20 | −0.01 | 0.02 | −0.07 | ||||||
| (Intercept) | −1.64 | 0.69 | −1.33 | 0.63 | 0.64 | 0.47 | 1.03 | 0.67 | 0.36 | 0.08 | 0.68 | −0.22 | ||||||
Note. = p<0.001
= p<0.01
= p<0.05.
B = Linear regression coefficient. SE B = Standard Error of B. β = Standardized linear regression coefficient. WNTAR = Why Not Take a Risk?; GAG = Germs are Germs; SE = Side Effects; DR = Downside Risk; ABX = Antibiotics
Discussion
Our findings suggest that WNTAR – the strategic rationale that one should take antibiotics when already sick because there is a potential for them to help and negligible downside risk – is widespread among the public, patients, and, surprisingly, healthcare providers. Whereas ABX Help involves agreeing with the misconception that antibiotics will help, WNTAR requires only that subjects acknowledge the possibility that they might help. Thus, WNTAR, though socially suboptimal, may be perceived as individually justifiable when it comes to treating patients because there is a real, though small, chance that the patients may have a bacterial illness that will not spontaneously resolve without antibiotics.42–45 Furthermore, the risks associated with antibiotic use, although potentially life-threatening,46–49 are believed to be essentially nil. Across all three samples, WNTAR accounted for the largest amount of variance in the data. This implies that both clinicians and patients may benefit from interventions focusing on gist descriptions of risk and potential for patient harm associated with taking antibiotics.
Agreement with WNTAR was positively associated with both prior use of and expectations for antibiotics in the online sample. Given that patients’ expectations have been shown to drive antibiotic prescribing2, our results suggest that agreement with WNTAR may predict prescribing rates. Furthermore, widespread provider agreement with WNTAR highlights that this gist also accounts for physician prescribing, as we have shown elsewhere.41 The latter result is consistent with FTT’s predictions: that simple categorical gists characterize experts’ mental representations in medical decision-making34 and decision-making under risk.33
To the extent that other gists support WNTAR, they may also be associated with increased prescribing. For example, patients who believe that antibiotics work against viruses would be more likely to expect a possible upside from antibiotic therapy and, indeed, we observe that patients who endorse items indexing GAG are more likely to also endorse WNTAR. Despite this association, GAG and WNTAR are distinct misconceptions, as indicated by the fact that GAG captured unique variance as did WNTAR in the online and ED patient samples. However, among ED patients – our least-educated sample – agreement with both GAG and WNTAR was high. In contrast, the vast majority of providers disagreed with GAG but almost half of them agreed with WNTAR. In addition, WNTAR captured unique variance in the factor analysis of provider data whereas GAG did not capture any unique variance. GAG was not associated with prior use of or expectations for antibiotics in the online sample. Although educational campaigns to improve judicious prescribing, such as the CDC’s “Get Smart” program, are indeed targeting an existing knowledge discrepancy, our results suggest that lack of knowledge is not the sole or even the main driver of prescribing.
Among all samples, acknowledgment of side effects associated with antibiotic therapy also captured unique variance. Although virtually all subjects endorsed SE, many still agreed with WNTAR. Moreover, this gist did not reduce expectations for or prior use of antibiotics in the online sample suggesting that knowledge of side effects does not necessarily change the strategic gist associated with WNTAR. Specifically, one may be aware of the theoretical possibility of side effects while still considering their impact negligible. Thus, simply informing patients about side effects may be insufficient to change patients’ expectations unless it is done in such a way as to communicate the gist of non-negligible downside risks.
Many subjects endorse WNTAR even though they also believe that antibiotics will not help them. Thus, a communication intervention emphasizing that antibiotics will not help may have limited efficacy unless it also explicitly addresses the WNTAR gist.
Limitations and Directions for Future Work
Some subjects were recruited through an online marketplace rather than an emergency department. These subjects were not actually acute care patients at the time of the survey, which may affect their perceptions about prescribing when asked to imagine an acute care scenario. However, the scenario described an upper respiratory infection with symptoms that are highly likely to be familiar to most respondents. Furthermore, the scenario was not intended to refer to an individual patient’s immediate symptoms. Indeed, only 10 of the patients in the ED sample who completed the survey were diagnosed with an upper respiratory infection during that visit, and results for that subset resembled the larger sample. A follow-up study should be conducted of gist representations among patients with respiratory symptoms, controlling for the confound that antibiotics will sometimes be indicated.
We do not claim that our results are nationally representative. For example, the online sample oversamples some groups (Asians and individuals with two or more races) and undersamples others (Blacks, American Indians and Alaskan Natives), when compared to the overall US population (see Table S11). Our ED patient sample located in an inner-city urban center has a higher proportion of minorities when compared to the average ED population. Thus, ED results may not generalize to other acute care settings, though they were in good agreement with a similar survey in a different ED32. Finally, the provider sample was limited to an analysis of two hospital systems in the DC-MD-VA area. Future research should therefore examine generalizability in a nationally representative sample, including relationships between sociodemographic factors and gist representations of antibiotic use.
One might object that other factors, which we did not test for in our surveys, are important determinants of expectations for antibiotics. Prior work17,19,25 has been conducted using quantitative surveys and in-depth interviews that motivate the survey items we selected. In addition, this study and our prior studies32,41 have tested some of these factors for relative strengths of association with expectations/behaviors. In all cases, we found that WNTAR captured the most variance and, where expectations and behaviors were tested, we found that WNTAR predicted strong effects even after controlling for other factors. However, there are factors that could have affected subjects’ responses that we did not explicitly test for, such as the role of financial cost in patients’ decisions (although we examined, and found no consistent effect of, the relationship between measures of socioeconomic status and patients’ responses or the provider’s ability to follow up in a short time if a patient did not get better). In general, one might speculate that the gists endorsed by different subjects would vary with their personal experiences. Although we did not collect data on the personal experiences of subjects, the fact that our results replicate across multiple samples (an online sample, two clinical sites, and two EDs, including our previous study), suggests that the results are robust across a range of personal experiences.
Similarly, we did not explicitly include items indexing providers’ awareness of antimicrobial resistance (AMR). This is because, in prior analyses of factors motivating prescribing by ED clinicians,17 AMR was not a major factor. In addition, we conducted interviews with clinicians prior to survey generation and AMR was never mentioned as a major decision factor in deciding whether to prescribe to the patient. Importantly, FTT predicts that discussions of AMR that cue relevant social values may be an effective communication strategy to reduce inappropriate prescribing. Therefore, future work should test the relationship between antibiotic prescribing and providers’ awareness of AMR.
Although our results show that both patients and providers endorse WNTAR, and that WNTAR captures significant variance in responses in both samples, we did not directly examine providers’ beliefs about patients’ expectations. Although the literature supports this link,25 explicitly examining whether providers’ endorsement of the WNTAR gist is associated with patients’ endorsement of the same gist is an important direction for future work.
Conclusions
A categorical choice between the patient remaining sick or possibly getting better through antibiotic therapy is the primary strategy characterizing both prescribing by clinicians and expectations by patients. Despite differences in prescribing among clinicians and lack of understanding among some patients about the difference between bacteria and viruses, the dominant strategy for decision making used by both groups appears to be “why not take a risk,” which assumes that, for individuals, the side effects from antibiotics are essentially nil. Educational strategies for patients and providers that focus on reframing the strategic choice to be one between the patient remaining sick or potentially being qualitatively worse off (e.g., by developing an antibiotic-resistant serious illness or an adverse event such as a clostridium difficile infection) may be more effective in altering prescribing patterns than current education focused on knowledge of the difference between bacteria and viruses. Theory suggests that the goal of education should be to capture the simple bottom line of antibiotic decisions at the individual level, whether that is “going from bad to worse,” “it only takes once”50,51 or some other categorical gist, remains a question for future research.
Supplementary Material
Acknowledgments
Preparation of this manuscript was supported in part by the National Institute of General Medical Sciences R01GM114771 to DAB and by National Institute of Nursing Research R01NR014368 to VFR.
References
- 1.Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010-2011. JAMA. 2016. May 3;315(17):1864–73. [DOI] [PubMed] [Google Scholar]
- 2.Sirota M, Round T, Samaranayaka S, Kostopoulou O. Expectations for antibiotics increase their prescribing: Causal evidence about localized impact. Health Psychol. 2017;36(4):402. [DOI] [PubMed] [Google Scholar]
- 3.US Centers for Disease Control and Prevention. CDC - Coverage - NIS Child Table Data for 2014 - Imz Managers - Vaccines [Internet]. 2014. [cited 2015 September 22]. Available from: http://www.cdc.gov/vaccines/imz-managers/coverage/nis/child/data/tables-2014.html
- 4.Gerber JS, Prasad PA, Localio AR, Fiks AG, Grundmeier RW, Bell LM, et al. Racial Differences in Antibiotic Prescribing by Primary Care Pediatricians. Pediatrics. 2013. April 1;131(4):677–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Taylor TH, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;civ076. [DOI] [PubMed] [Google Scholar]
- 6.Xu KT, Roberts D, Sulapas I, Martinez O, Berk J, Baldwin J. Over-prescribing of antibiotics and imaging in the management of uncomplicated URIs in emergency departments. BMC Emerg Med. 2013; 13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barlam TF, Morgan JR, Wetzler LM, Christiansen CL, Drainoni M-L. Antibiotics for Respiratory Tract Infections: A Comparison of Prescribing in an Outpatient Setting. Infect Control Hosp Epidemiol. 2015. Feb;36(2):153–9. [DOI] [PubMed] [Google Scholar]
- 8.Bharathiraja R, Sridharan S, Chelliah LR, Suresh S, Senguttuvan M. Factors affecting antibiotic prescribing pattern in pediatric practice. Indian J Pediatr. 2005. October 1;72(10):877–9. [DOI] [PubMed] [Google Scholar]
- 9.Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors Influencing Antibiotic-Prescribing Decisions Among Inpatient Physicians: A Qualitative Investigation. Infect Control Amp Hosp Epidemiol. 2015. September;36(9):1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menendez R, Torres A, Zalacain R, Aspa J, Martín-Villasclaras JJ, Borderias L, et al. Guidelines for the Treatment of Community-acquired Pneumonia. Am J Respir Crit Care Med. 2005. September 15;172(6):757–62. [DOI] [PubMed] [Google Scholar]
- 11.Halm EA, Switzer GE, Mittman BS, Walsh MB, Chang C-CH, Fine MJ. What Factors Influence Physicians’ Decisions to Switch from Intravenous to Oral Antibiotics for Community-acquired Pneumonia? J Gen Intern Med. 2001. September 1;16(9):599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halm EA, Atlas SJ, Borowsky LH, Benzer TI, Metlay JP, Chang Y, et al. Understanding Physician Adherence With a Pneumonia Practice Guideline: Effects of Patient, System, and Physician Factors. Arch Intern Med. 2000. January 10;160(1):98–104. [DOI] [PubMed] [Google Scholar]
- 13.Avorn J Cultural and Economic Factors That (Mis)Shape Antibiotic Use: The Nonpharmacologic Basis of Therapeutics. Ann Intern Med. 2000. July 18; 133(2): 128. [DOI] [PubMed] [Google Scholar]
- 14.Nyquist A-C, Gonzales R, Steiner JF, Sande MA. Antibiotic Prescribing for Children With Colds, Upper Respiratory Tract Infections, and Bronchitis. JAMA. 1998. March 18;279(11):875–7. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues AT, Ferreira M, Pineiro-Lamas M, Falcao A, Figueiras A, Herdeiro MT. Determinants of physician antibiotic prescribing behavior: a 3 year cohort study in Portugal. Curr Med Res Opin. 2016. May 3;32(5):949–57. [DOI] [PubMed] [Google Scholar]
- 16.Parker HM, Mattick K. The determinants of antimicrobial prescribing among hospital doctors in England: a framework to inform tailored stewardship interventions. Br J Clin Pharmacol. 2016. August 1;82(2):431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.May L, Gudger G, Armstrong P, Brooks G, Hinds P, Bhat R, et al. Multisite exploration of clinical decision making for antibiotic use by emergency medicine providers using quantitative and qualitative methods. Infect Control Hosp Epidemiol. 2014;35(09): 1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shapiro E Injudicious antibiotic use: An unforeseen consequence of the emphasis on patient satisfaction? Clin Ther. 2002. January 1;24(1):197–204. [DOI] [PubMed] [Google Scholar]
- 19.Stearns CR, Gonzales R, Camargo J Carlos A, Maselli J, Metlay JP. Antibiotic Prescriptions Are Associated with Increased Patient Satisfaction With Emergency Department Visits for Acute Respiratory Tract Infections. Acad Emerg Med. 2009. October 1;16(10): 934–41. [DOI] [PubMed] [Google Scholar]
- 20.Vaz LE, Kleinman KP, Lakoma MD, Dutta-Linn MM, Nahill C, Hellinger J, et al. Prevalence of Parental Misconceptions About Antibiotic Use. Pediatrics. 2015. July 1;peds.2015–0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigues AT, Roque F, Falcao A, Figueiras A, Herdeiro MT. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents. 2013. March 1;41(3):203–12. [DOI] [PubMed] [Google Scholar]
- 22.Meropol SB, Votruba ME. Decision-Making and the Barriers to Judicious Antibiotic Use. Pediatrics. 2015. August 1;136(2):387–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ong S, Nakase J, Moran GJ, Karras DJ, Kuehnert MJ, Talan DA. Antibiotic Use for Emergency Department Patients With Upper Respiratory Infections: Prescribing Practices, Patient Expectations, and Patient Satisfaction. Ann Emerg Med. 2007. September;50(3):213–20. [DOI] [PubMed] [Google Scholar]
- 24.Reyna VF. A Theory of Medical Decision Making and Health: Fuzzy Trace Theory. Med Decis Mak Int J Soc Med Decis Mak. 2008;28(6): 850–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler CC, Rollnick S, Pill R, Maggs-Rapport F, Stott N. Understanding the culture of prescribing: qualitative study of general practitioners’ and patients’ perceptions of antibiotics for sore throats. BMJ. 1998. September 5;317(7159):637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adam MB, Reyna VF. Coherence and correspondence criteria for rationality: Experts’ estimation of risks of sexually transmitted infections. J Behav Decis Mak. 2005;18(3):169–186. [Google Scholar]
- 27.Reyna VF, Adam MB. Fuzzy-Trace Theory, Risk Communication, and Product Labeling in Sexually Transmitted Diseases. Risk Anal. 2003. April 1;23(2):325–42. [DOI] [PubMed] [Google Scholar]
- 28.Reyna VF. Risk perception and communication in vaccination decisions: A fuzzy-trace theory approach. Vaccine. 2012. May 28;30(25):3790–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reyna VF, Nelson WL, Han PK, Dieckmann NF. How Numeracy Influences Risk Comprehension and Medical Decision Making. Psychol Bull. 2009. November;135(6):943–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blalock SJ, Reyna VF. Using fuzzy-trace theory to understand and improve health judgments, decisions, and behaviors: A literature review. Health Psychol. 2016;35(8):781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reyna VF. A new intuitionism: Meaning, memory, and development in Fuzzy-Trace Theory. Judgm Decis Mak. 2012. May;7(3):1–45. [PMC free article] [PubMed] [Google Scholar]
- 32.Broniatowski DA, Klein EY, Reyna VF. Germs Are Germs, and Why Not Take a Risk? Patients’ Expectations for Prescribing Antibiotics in an Inner-City Emergency Department. Med Decis Making. 2015. January 1;35(1):60–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reyna VF, Chick CF, Corbin JC, Hsia AN. Developmental Reversals in Risky Decision Making Intelligence Agents Show Larger Decision Biases Than College Students. Psychol Sci. 2014. January 1;25(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reyna VF, Lloyd FJ. Physician decision making and cardiac risk: effects of knowledge, risk perception, risk tolerance, and fuzzy processing. J Exp Psychol Appl. 2006;12(3):179. [DOI] [PubMed] [Google Scholar]
- 35.Mannix R, Stack AM, Chiang V. Insurance status and the care of adult patients 19 to 64 years of age visiting the emergency department. Acad Emerg Med. 2012;19(7):808–815. [DOI] [PubMed] [Google Scholar]
- 36.Chen BK, Hibbert J, Cheng X, Bennett K. Travel distance and sociodemographic correlates of potentially avoidable emergency department visits in California, 2006-2010: an observational study. Int J Equity Health. 2015. March 21;14:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen BK, Cheng X, Bennett K, Hibbert J. Travel distances, socioeconomic characteristics, and health disparities in nonurgent and frequent use of Hospital Emergency Departments in South Carolina: a population-based observational study. BMC Health Serv Res. 2015. May 16;15:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bush K, Courvalin P, Dantas G, Davies J, Eisenstein B, Huovinen P, et al. Tackling antibiotic resistance. Nat Rev Microbiol. 2011;9(12):894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Safdar N, Tape TG, Fox BC, Svenson JE, Wigton RS. Factors Affecting Antibiotic Prescribing for Acute Respiratory Infection by Emergency Physicians. Health (N Y). 2014. March 13;06(08):774. [Google Scholar]
- 40.Nadeem Ahmed M, Muyot MM, Begum S, Smith P, Little C, Windemuller FJ. Antibiotic prescription pattern for viral respiratory illness in emergency room and ambulatory care settings. Clin Pediatr (Phila). 2010;49(6):542–547. [DOI] [PubMed] [Google Scholar]
- 41.Klein EY, Martinez EM, May L, Saheed M, Reyna V, Broniatowski DA. Categorical Risk Perception Drives Variability in Antibiotic Prescribing in the Emergency Department: A Mixed Methods Observational Study. J Gen Intern Med. 2017. October;32(10):1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wessels MR. Streptococcal pharyngitis. N Engl J Med. 2011;364(7):648–655. [DOI] [PubMed] [Google Scholar]
- 43.Wilson JF. Acute Sinusitis. Ann Intern Med. 2010;153(5):ITC3–1. [DOI] [PubMed] [Google Scholar]
- 44.Heald A, Auckenthaler R, Borst F, Delaspre O, Cermann D, Matter L, et al. Adult bacterial nasopharyngitis. J Gen Intern Med. 1993;8(12):667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaiser L, Lew D, Hirschel B, Auckenthaler R, Morabia A, Heald A, et al. Effects of antibiotic treatment in the subset of common-cold patients who have bacteria in nasopharyngeal secretions. The Lancet. 1996;347(9014):1507–1510. [DOI] [PubMed] [Google Scholar]
- 46.Antibiotic Resistance Threats in the United States, 2013. ∣ Antibiotic/Antimicrobial Resistance ∣ CDC [Internet]. [cited 2016 December 26]. Available from: https://www.cdc.gov/drugresistance/threat-report-2013/
- 47.Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. Bmj. 2007;335(7627):982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linder JA. Editorial commentary: antibiotics for treatment of acute respiratory tract infections: decreasing benefit, increasing risk, and the irrelevance of antimicrobial resistance. Clin Infect Dis. 2008;47(6):744–746. [DOI] [PubMed] [Google Scholar]
- 49.Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735–743. [DOI] [PubMed] [Google Scholar]
- 50.Reyna VF, Estrada SM, DeMarinis JA, Myers RM, Stanisz JM, Mills BA. Neurobiological and memory models of risky decision making in adolescents versus young adults. J Exp Psychol Learn Mem Cogn. 2011;37(5):1125. [DOI] [PubMed] [Google Scholar]
- 51.Cohn LD, Macfarlane S, Yanez C, Imai WK. Risk-perception: differences between adolescents and adults. Health Psychol Off J Div Health Psychol Am Psychol Assoc. 1995. May;14(3):217–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.