Abstract
OBJECTIVE
Corpus callosotomy is a palliative surgery for drug-resistant epilepsy that reduces the severity and frequency of generalized seizures by disconnecting the two cerebral hemispheres. Unlike with resection, seizure outcomes remain poorly understood. The authors systematically reviewed the literature and performed a meta-analysis to investigate rates and predictors of complete seizure freedom and freedom from drop attacks after corpus callosotomy.
METHODS
PubMed, Web of Science, and Scopus were queried for primary studies examining seizure outcomes after corpus callosotomy published over 30 years. Rates of complete seizure freedom or drop attack freedom were recorded. Variables showing a potential relationship to seizure outcome on preliminary analysis were subjected to formal meta-analysis.
RESULTS
The authors identified 1742 eligible patients from 58 included studies. Overall, the rates of complete seizure freedom and drop attack freedom after corpus callosotomy were 18.8% and 55.3%, respectively. Complete seizure freedom was significantly predicted by the presence of infantile spasms (OR 3.86, 95% CI 1.13–13.23), normal MRI findings (OR 4.63, 95% CI 1.75–12.25), and shorter epilepsy duration (OR 2.57, 95% CI 1.23–5.38). Freedom from drop attacks was predicted by complete over partial callosotomy (OR 2.90, 95% CI 1.07–7.83) and idiopathic over known epilepsy etiology (OR 2.84, 95% CI 1.35–5.99).
CONCLUSIONS
The authors report the first systematic review and meta-analysis of seizure outcomes in both adults and children after corpus callosotomy for epilepsy. Approximately one-half of patients become free from drop attacks, and one-fifth achieve complete seizure freedom after surgery. Some predictors of favorable outcome differ from those in resective epilepsy surgery.
Keywords: complete corpus callosotomy, anterior corpus callosotomy, drop attack, disconnection syndrome, epilepsy
EPILEPSY is a chronic, debilitating disease affecting approximately 4–10 out of 1000 people worldwide.11,68 Most cases are controlled with medication, and seizure freedom can be achieved with adequate treatment.11 However, in up to one-third of cases, seizures are refractory to medication.35 Resection of a localized epileptogenic focus is an effective treatment for appropriate refractory cases.14,77 However, not all patients are candidates for resection, particularly in the case of multifocal or rapidly generalizing seizures without a clearly identified epileptogenic focus. In some of these individuals, corpus callosotomy can be effective at reducing seizures—especially drop attacks (i.e., atonic seizure leading to sudden falls)—by precluding epileptic discharges from traveling between hemispheres (i.e., generalization).2 However, unlike with resection, no large-scale studies or randomized controlled trials have evaluated corpus callosotomy, and the available data arise only from small, heterogeneous case series. Therefore, the rates and predictors of seizure outcome after this procedure remain incompletely understood, and it is not known if factors associated with seizure freedom after corpus callosotomy differ from those in resection.
Here we report the first systematic review and meta-analysis of seizure outcomes following corpus callosotomy for drug-resistant epilepsy in both adults and children. A number of variables, including corpus callosotomy extent, epilepsy etiology, epilepsy duration, electroencephalography (EEG) results, and MRI findings were examined as possible predictors of complete seizure freedom or freedom from drop attacks postoperatively. Corpus callosotomy extent was also examined as a possible predictor of disconnection syndrome. Finally, adverse event rates in the literature were reviewed.
Methods
Literature Review
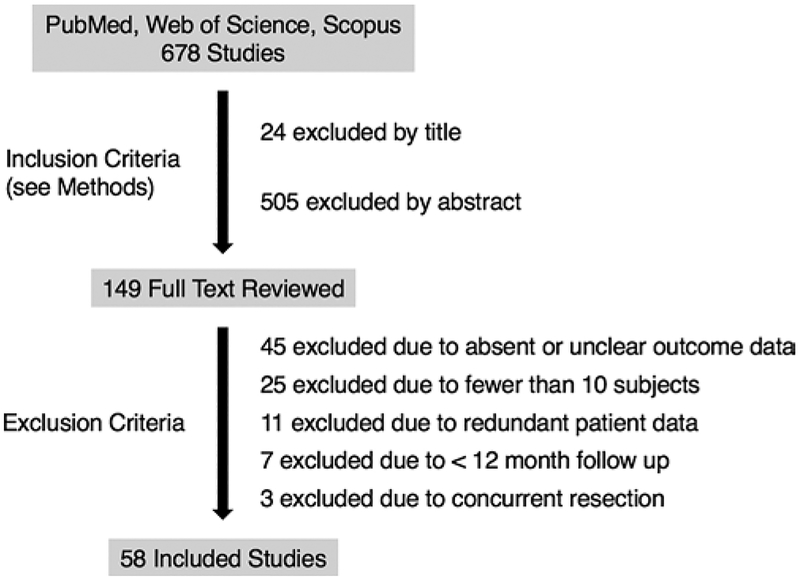
In April 2017 we searched PubMed, Web of Science, and Scopus for articles containing seizure outcomes for corpus callosotomy. Using the search terms “epilepsy” and/or “callosotomy”, we returned 520, 299, and 361 results from PubMed, Web of Science, and Scopus, respectively, totaling 679 unique citations after exclusion of duplicates. The filters used for each search engine were English language only, publication date of 1987 or later, no book chapters or reviews, and only medicine or neuro-science articles. Furthermore, references of related studies were investigated for potential studies that fit the inclusion criteria. Two authors independently reviewed the articles (A.Y.C. and D.J.E.). Data extraction was done by A.Y.C. and reexamined by D.J.E. The following inclusion criteria were used for all databases: 1) a primary clinical study reporting seizure outcomes after corpus callosotomy, excluding book chapters and reviews; 2) English language only; and 3) a publication date no earlier than 1987. Overall, 150 potential studies met our inclusion criteria and were reviewed in full text. Then exclusion criteria were applied, including the following: 1) absent or unclear outcome data (e.g., inability to assign seizure outcomes); 2) data from fewer than 10 patients; 3) patient data redundant with another manuscript; 4) mean or median follow-up shorter than 12 months; and 5) concurrent resection performed in addition to callosotomy. Overall, 58 studies met our inclusion and exclusion criteria, and were examined in detail, as summarized in Fig. 1. The literature search and study were designed using Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.48
FIG. 1.
Flow chart summarizing the manuscript selection process.
Data Collection
The primary outcome measure was seizure outcome at last follow-up. Overall seizure and drop attack outcomes were collected and dichotomized into “completely seizure free” and “not completely seizure free,” or “drop attack free” and “not drop attack free.” Both “drop attack” seizures and “atonic” seizures were considered collectively. Individual patient data were excluded when they were redundant with another publication. The following variables were collected if they could unambiguously be associated with a seizure/drop attack outcome: 1) age at surgery, 2) sex, 3) corpus callosotomy extent, 4) MRI findings, 5) EEG findings, 6) seizure frequency, 7) epilepsy duration, 8) presence of generalized seizures, 9) presence of focal seizures, 10) presence or history of infantile spasms, 11) presence of absence seizures, and 12) epilepsy etiology. Corpus callosotomy extent was also separately related to the presence or absence of disconnection syndrome symptoms after surgery when possible. Disconnection syndrome was assigned individually by the primary studies, but included any of these symptoms: hemispatial neglect, tachistoscopic visual suppression, nondominant hand apraxia, alexia without agraphia, tactile aphasia, dichotic listening suppression, or alien hand syndrome.
Corpus callosotomy extent was dichotomized into complete or partial (i.e., anterior, posterior). Staged disconnections in which an anterior disconnection was followed by a posterior completion were included in some instances. In these cases, if seizure outcomes were reported after the initial anterior section only, this was considered partial section, whereas if seizure outcomes were reported after the completion stage, this was considered complete section. For imaging results, only MRI results were considered, and CT or ambiguous results were excluded. Pre-operative EEG results were considered lateralized if the abnormal electrographic activity was either completely lateralized or predominantly on one side, and results were considered nonlateralized otherwise. Etiology data were collected and categorized as idiopathic, malformation, syndromic, or other, and dichotomized into idiopathic versus known for meta-analysis.
Although sufficient data were not available for systematic analysis of perioperative adverse events, complication rates and types were recorded from a subset of patient series, where available.
Statistical Analysis
Overall seizure and drop attack outcomes were stratified by each variable and examined with preliminary statistical analysis for summary purposes. This was done to help identify which variables were potentially associated with outcomes, to then subject them to formal meta-analysis. Student t-tests and chi-square tests were used to evaluate continuous and categorical data, respectively. Fisher transformation tests were used to examine potential outcome trends over time. Factors demonstrating a potential association with seizure outcome with a significance level of p < 0.05 after preliminary analysis were then subjected to formal meta-analysis. Cochran’s Q and I2 tests were used to evaluate heterogeneity across studies, to ensure that a fixed effects model was appropriate. Mantel-Haenszel testing was used to calculate odds ratios and 95% CI. We investigated the potential bias by visualization of funnel plots and examining the correlations between sample size and both complete seizure freedom and drop attack freedom rates. Wizard Pro 1.8.28 was used for preliminary statistical analysis, and Review Manager v5.3 (Nordic Cochrane Centre, Rigshospitalet, 2008) was used for meta-analysis.
Results
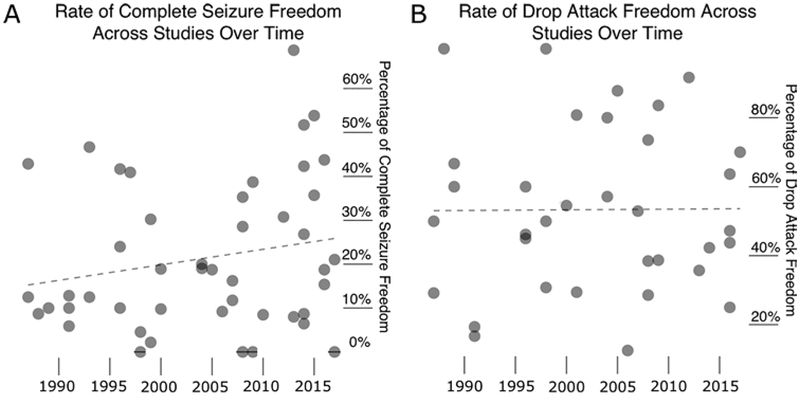
Overall, 1742 patients were included from 58 unique studies (Table 1).1,3–10, 18–21, 23–26, 29, 32–34, 36, 37, 39–47, 49–57,59–62, 66, 69–76, 79–82 All studies were retrospective or prospective case series, and no controlled studies were identified. Overall, the rates of complete seizure freedom and freedom from drop attacks were 18.8% and 55.3%, respectively, at last follow-up. No significant trends in seizure outcome were observed over time (Fig. 2).
TABLE 1. Included studies.
| Study | No. of Pts | Study | No. of Pts |
|---|---|---|---|
| Andersen et al., 1996 | 16 | Maehara et al., 1996 | 12 |
| Baba et al., 1996 | 10 | Makari et al., 1989 | 20 |
| Bower et al., 2013 | 50 | Mamelak et al., 1993 | 15 |
| Carmant et al., 1998 | 13 | Murro et al., 1988 | 23 |
| Chandra et al., 2016 | 16 | Nordgren et al., 1991 | 17 |
| Cohen et al., 1991 | 10 | Oguni et al., 1991 | 31 |
| Cukiert et al., 2006 | 67 | Otsuki et al., 2016 | 13 |
| Cukiert et al., 2009 | 11 | Paglioli et al., 2016 | 36 |
| Daniel et al., 1998 | 10 | Papo et al., 1989 | 10 |
| Fandino-Franky et al., 2000 | 92 | Park et al., 2013 | 14 |
| Fuiks et al., 1991 | 78 | Passamonti et al., 2014 | 26 |
| Garcia-Flores, 1987 | 14 | Phillips & Sakas, 1996 | 13 |
| Gates et al., 1987 | 24 | Ping et al., 2009 | 31 |
| Hodaie et al., 2001 | 17 | Rahimi et al., 2007 | 37 |
| Hong et al., 2018 | 10 | Rathore et al., 2007 | 17 |
| Iwasaki et al., 2012 | 13 | Reutens et al., 1993 | 64 |
| Iwasaki et al., 2016 | 13 | Rossi et al., 1996 | 20 |
| Jea et al., 2008 | 13 | Shim et al., 2008 | 34 |
| Kawai et al., 2004 | 10 | Shimizu, 2005 | 41 |
| Kim et al., 2004 | 42 | Sorenson et al., 1997 | 23 |
| Kokoszka et al., 2017 | 19 | Spencer et al., 1988 | 22 |
| Kwan et al., 2000 | 74 | Sperling et al., 1999 | 46 |
| Kwan et al., 2005 | 48 | Stigsdotter-Broman et al., 2014 | 31 |
| Lee et al., 2013 | 16 | Sunaga et al., 2009 | 73 |
| Lee et al., 2014 | 41 | Tanriverdi et al., 2009 | 62 |
| Liang et al., 2010 | 59 | Turanli et al., 2006 | 16 |
| Liang et al., 2014 | 23 | Yang et al., 1996 | 25 |
| Liang et al., 2015 | 14 | Yang et al., 2014 | 29 |
| Maehara & Shimizu, 2001 | 52 | You et al., 2008 | 14 |
Pts = patients.
FIG. 2.
Postoperative seizure outcome rates across all studies by publication date. Z-statistics and p values for Fisher transformation tests are provided. A: Percent of patients with complete seizure freedom per study over time (Z = 1.384; p = 0.166). B: Percent of patients with drop attack freedom per study over time (Z = 0.051; p = 0.959).
Factors potentially associated with seizure outcome were preliminarily examined for summary purposes, as shown in Table 2, to identify variables for formal meta-analysis. Although no relationships between basic demographics and outcome were noted, both complete seizure freedom and drop attack freedom were more common with complete versus partial callosotomy. Complete seizure freedom was also more likely in the setting of normal findings on MRI compared to abnormal findings, and lateralized EEG compared to nonlateralized EEG. Both a shorter duration of epilepsy and the presence of infantile spasms were associated with improved overall seizure outcome, and a higher rate of drop attack freedom was noted in patients with idiopathic versus known epilepsy etiology. There was no significant correlation between study sample and the percentage of complete seizure freedom (p = 0.181) and drop attack freedom (p = 0.827).
TABLE 2. Seizure outcomes stratified by factors of interest.
| Factor | Completely Seizure Free | Persistent Seizures | p Value | Drop Attack Free | Persistent Drop Attacks | p Value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age at op (yrs) | 15.4 ± 2.6 | 16.6 ± 1.2 | 0.402 | 16.0 ± 2.3 | 15.7 ± 2.0 | 0.851 |
| Sex | ||||||
| Female | 17 (19.1%) | 72 (80.9%) | 0.290 | 32 (60.4%) | 21 (39.6%) | 0.700 |
| Male | 20 (13.9%) | 124 (86.1%) | 41 (56.9%) | 31 (43.1%) | ||
| Surgery & diagnostics | ||||||
| Callosotomy extent | ||||||
| Partial | 63 (14.3%) | 377 (85.7%) | 0.030* | 83 (42.6%) | 112 (57.4%) | 0.002* |
| Complete | 34 (21.8%) | 122 (78.2%) | 50 (63.3%) | 29 (36.7%) | ||
| MRI findings | ||||||
| Normal | 19 (36.5%) | 33 (63.5%) | 0.018* | 26 (65.0%) | 14 (35.0%) | 0.117 |
| Abnormal | 17 (18.7%) | 74 (81.3%) | 42 (50.0%) | 42 (50.0%) | ||
| EEG findings | ||||||
| Lateralized | 3 (7.3%) | 38 (92.7%) | 0.020* | 5 (35.7%) | 9 (64.3%) | 0.155 |
| Nonlateralized | 41 (23.6%) | 133 (76.4%) | 68 (55.7%) | 54 (44.3%) | ||
| Disease characteristics | ||||||
| Seizure frequency (monthly) | 258.4 ± 170.0 | 181.7 ± 46.8 | 0.242 | 165.2 ± 54.4 | 248.0 ± 80.3 | 0.096 |
| Epilepsy duration | ||||||
| <15 yrs | 48 (27.4%) | 127 (72.6%) | 0.014* | 52 (52.0%) | 48 (48.0%) | 0.687 |
| ≥15 yrs | 13 (14.1%) | 79 (85.9%) | 31 (55.4%) | 25 (44.6%) | ||
| Generalized seizures | ||||||
| Present | 47 (15.2%) | 263 (84.8%) | 0.873 | 73 (54.5%) | 61 (45.5%) | 0.364 |
| Absent | 12 (14.5%) | 71 (85.5%) | 21 (46.7%) | 24 (53.3%) | ||
| Focal seizures | ||||||
| Present | 23 (13.2%) | 151 (86.8%) | 0.393 | 36 (52.9%) | 32 (47.1%) | 0.325 |
| Absent | 26 (16.6%) | 131 (83.4%) | 34 (44.7%) | 42 (55.3%) | ||
| Infantile spasms | ||||||
| Present | 15 (60.0%) | 10 (40.0%) | <0.001* | 9 (75.0%) | 3 (25.0%) | 0.417 |
| Absent | 12 (16.0%) | 63 (84.0%) | 23 (62.2%) | 14 (37.8%) | ||
| Absence seizures | ||||||
| Present | 8 (11.6%) | 61 (88.4%) | 0.337 | 19 (50.0%) | 19 (50.0%) | 0.711 |
| Absent | 29 (16.5%) | 147 (83.5%) | 53 (53.5%) | 46 (46.5%) | ||
| Epilepsy etiology | ||||||
| Idiopathic | 23 (25.8%) | 66 (74.2%) | 0.085 | 37 (59.7%) | 25 (40.3%) | 0.041* |
| Known | 24 (16.6%) | 121 (83.4%) | 45 (43.3%) | 59 (56.7%) | ||
| Total | 231 (19.0%) | 982 (81.0%) | 373 (55.3%) | 302 (44.7%) | ||
Data are expressed as the mean ± 95% CI for continuous variables and number (%) for categorical variables.
p < 0.05, t-test for continuous variables or chi-square test for categorical variables.
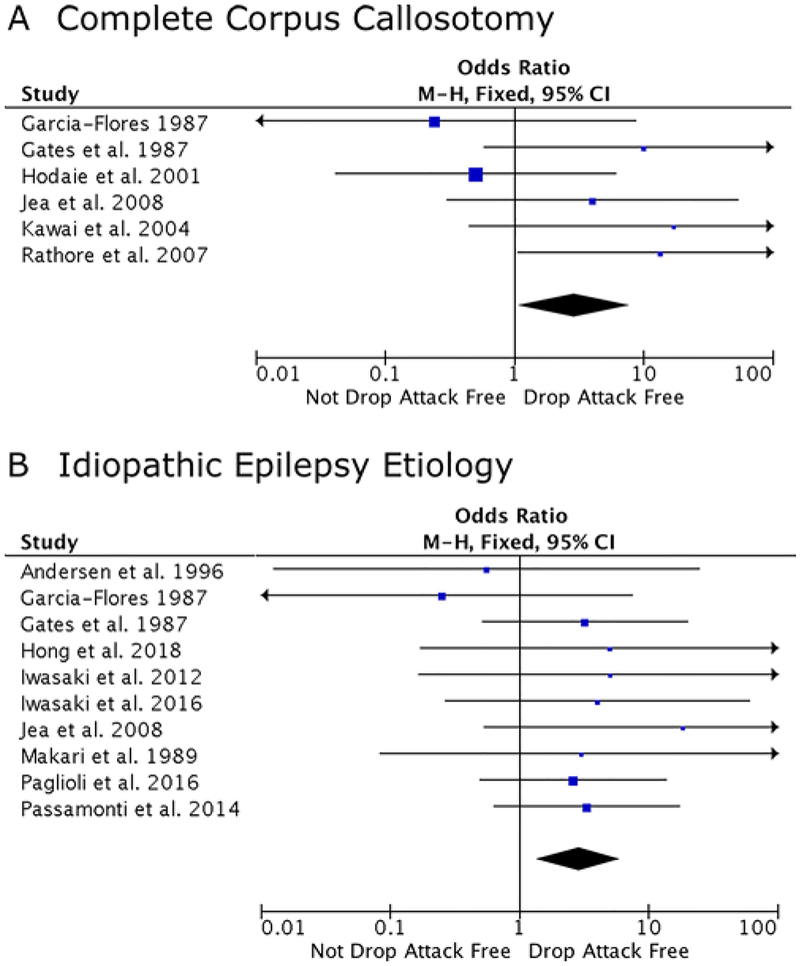
Variables showing potential association with seizure outcome were then subjected to meta-analysis to identify predictors of complete seizure freedom (Fig. 3) and freedom from drop attacks (Fig. 4) after surgery. Overall, there was low heterogeneity observed among studies, and heterogeneity test results are shown in the figure legend, although it is known that heterogeneity statistics can be biased in small meta-analyses such as the present study. Complete seizure freedom was significantly predicted by the presence of infantile spasms over their absence (OR 3.86, 95% CI 1.13–13.23), normal over abnormal MRI findings (OR 4.63, 95% CI 1.75–12.25), and an epilepsy duration of < 15 years (OR 2.57, 95% CI 1.23–5.38), whereas partial versus complete corpus callosotomy (OR 2.02, 95% CI 0.82–4.97) and lateralized versus nonlateralized EEG findings (OR 1.37, 95% CI 0.46–4.09) were not significantly associated with outcome. Next, freedom from drop attacks was significantly predicted by complete over partial callosotomy (OR 2.90, 95% CI 1.07–7.83) and idiopathic over known etiology (OR 2.84, 95% CI 1.35–5.99). Of note, a fixed-effects model was used for all meta-analyses, given presumed prior knowledge of the corpus callosotomy epilepsy patient population, and all tests of heterogeneity were nonsignificant. Funnel plots to evaluate for bias are shown for factors subjected to formal meta-analysis (Supplementary Figs. 1 and 2).
FIG. 3.
Meta-analyses examining factors associated with seizure freedom (p values for heterogeneity tests are provided). A: Complete over partial corpus callosotomy (p = 0.90, I2 = 0%, c2 = 2.83). B: Normal over abnormal MRI findings (p = 0.47, I2 = 0%, c2 = 3.53). C: Lateralized over nonlateralized EEG findings (p = 0.78, I2 = 0%, c2 = 3.20). D: Epilepsy duration < 15 years over ≥ 15 years (p = 0.61, I2 = 0%, c2 = 8.17). E: Presence over absence of infantile spasms (p = 0.043, I2 = 0%, c2 = 2.75). Signifi-cant predictors of complete seizure freedom (p < 0.05) after meta-analysis include normal MRI (B), shorter epilepsy duration (D), and the presence of infantile spasms (E). M-H = Mantel-Haenszel. Figure is available in color online only.
FIG. 4.
Meta-analyses examining factors associated with freedom from drop attacks (p values for heterogeneity tests are provided). A: Complete over partial corpus callosotomy (p = 0.23, I2 = 27%, c2 = 6.81). B: Idiopathic over known epilepsy etiology (p = 0.91, I2 = 0%, c2 = 4.09). Both complete callosotomy (A) and idiopathic epilepsy etiology (B) significantly predict drop attack freedom after meta-analysis (p < 0.05). Figure is available in color online only.
We also investigated a potential relationship between complete versus partial callosotomy and the presence or absence of disconnection syndrome symptoms after surgery. Where data were available (n = 34 studies), disconnection syndrome was reported to be present in 59 (12.4%) but absent in 418 (87.6%) of patients receiving partial callosotomy, and it was present in 17 (8.0%) but absent in 195 (92.0%) of those receiving complete callosotomy (p = 0.093, chi-square). On formal meta-analysis of these data, no significant relationship was noted between callosotomy extent and disconnection syndrome (OR 1.56, 95% CI 0.48–5.07).
Finally, complication rates were nonsystematically reviewed in a subset of studies in which data were available, as summarized in Table 3. Overall, the rate of adverse perioperative events ranged from 8.1% to 12.4%. The most common complications included transient lower-extremity weakness, transient aphasia or mutism, and infection.
TABLE 3. Survey of studies examining complication rates following corpus callosotomy.
| Authors & Year | No. of Pts | Complication Rate | Specific Complications |
|---|---|---|---|
| Bower et al., 2013 | 50 | 5 (10.0%) | 4 (8.0%) mild transient new or worsened hemiparesis; 1 (2.0%) bone flap infection |
| Fandino-Franky et al., 2000 | 97 | 12 (12.4%) | 10 (10.3%) transient lower-extremity weakness; 2 (2.1%) transient transitory mutism |
| Liang et al., 2010 | 59 | 6 (10.2%) | 3 (5.1%) transient urinary incontinence; 2 (3.4%) transient apraxia; 1 (1.7%) transient aphasia |
| Rahimi et al., 2007 | 37 | 3 (8.1%) | 2 (5.4%) hydrocephalus requiring shunt placement; 1 (2.7%) superior mesenteric artery infarction |
| Tanriverdi et al., 2009 | 95 | 11 (11.5%) | 2 (2.1%) transient lower-extremity weakness; 2 (2.1%) transient aphasia; 2 (2.1%) aspiration pneumonia; 1 (1.0%) epidural hematoma requiring reoperation; 1 (1.0%) bone flap infection w/ intracranial abscess; 1 (1.0%) subgaleal hematoma; 1 (1.0%) skin suture detachment; 1 (1.0%) venous thrombosis |
Discussion
Corpus callosotomy is a palliative therapy for drug-resistant epilepsy aiming to prevent epileptic discharges from spreading between hemispheres, and thereby preventing generalization of seizures, particularly drop attacks. We analyzed overall seizure and drop attack outcome data following corpus callosotomy and performed a quantitative meta-analysis. Patients were more likely to be completely seizure free after surgery if they suffered from infantile spasms, the preoperative MRI findings were normal, or epilepsy duration was < 15 years. Patients were more likely to be free of drop attacks if the procedure was a single-stage complete rather than partial callosotomy. No relationship was noted between callosotomy extent and disconnection syndrome, and perioperative adverse events were reported in approximately 8%–12% of patients. Recognizing the rates and predictors of favorable outcome after corpus callosotomy may aid patient selection and preoperative counseling.
Two previous reviews have investigated the efficacy of corpus callosotomy compared to vagus nerve stimulation. First, a systematic review compared vagus nerve stimulation and corpus callosotomy for atonic seizures and drop attacks.65 The results showed that corpus callosotomy was more likely to produce ≥ 50% reduction in seizure frequency than vagus nerve stimulation, and that adverse events were more likely with vagus nerve stimulation. Second, a meta-analysis that compared the efficacy of vagus nerve stimulation and corpus callosotomy in patients with Lennox-Gastaut syndrome also found that corpus callosotomy was more effective at reducing atonic seizure frequency.38 However, no systematic reviews or meta-analyses examining predictors of seizure outcome after corpus callosotomy had previously been reported.
Predictors of Complete Seizure Freedom
The overall seizure-free rate after corpus callosotomy (19%) is, as expected, much lower than focal resection for epilepsy, given that this is a palliative surgical procedure. For example, results from a systematic review and meta-analysis of epilepsy surgery demonstrated that 66% of patients undergoing temporal resection were seizure free postoperatively,77 whereas approximately 50% of individuals achieve seizure freedom after extratemporal resection for epilepsy.14 However, the primary goal of corpus callosotomy is often to alleviate drop attacks or atonic seizures and thereby prevent falls, and this favorable outcome was seen in 55.3% of cases. Thus, corpus callosotomy should be considered for patients with substantial disability from attacks who are not favorable candidates for focal resection.
Patients were more likely to develop complete seizure freedom following corpus callosotomy if they suffered from infantile spasms. Current first-line therapy for these spasms is administration of corticosteroids and vigabatrin.63 For refractory cases, corpus callosotomy is considered an alternative treatment. In one study, complete callosotomy was shown to eliminate infantile spasms in 80% of subjects.58 Our results suggest that overall seizure outcomes may also be more favorable in this patient sub-population.
Our analysis indicated that patients were less likely to be seizure free if preoperative MRI results were abnormal. This finding differs starkly from literature relating to resective epilepsy surgery, in which abnormal MRI findings are typically associated with more favorable seizure outcome.78 This discrepancy is probably due to the patient population under study. We hypothesize that although an MRI lesion suggests a focal epileptogenic zone, which will respond well to resection, individuals with a focal lesion who undergo corpus callosotomy probably represent the most challenging epilepsy cases, or those with difficult lesions in challenging areas that cannot be safely removed. Thus, outcome predictors in corpus callosotomy do not necessarily mirror those in resection.
A shorter epilepsy duration was also predictive of seizure freedom, which is consistent with the literature relating to epilepsy resections.13,28 Surgery relatively early after epilepsy becomes refractory typically portends better outcomes. For instance, mesial temporal lobe epilepsy is a sometimes progressive disorder, and early surgical intervention is advised to limit possible strengthening of epileptogenic circuits.12,31 This finding implies that early surgical intervention with corpus callosotomy, as with re-section, may produce improved outcomes.
Predictors of Drop Attack Freedom and Extent of Disconnection
Corpus callosotomy recipients with idiopathic epilepsy etiologies were more likely to be free of drop attacks postoperatively than those with known etiologies. Again, this differs from resective surgery, in which a known cause of epilepsy is associated with improved seizure outcomes.14 One possible contributing factor for this discrepancy is that although patients with idiopathic generalized or multifocal epilepsy are not candidates for resection, these individuals may respond to corpus callosotomy. One case series reported that 8 of 9 patients with idiopathic generalized epilepsy had at least 50% seizure reduction after corpus callosotomy.30 Therefore, although resection outcomes are best in patients with a focal and identified epilepsy etiology, individuals with poorly localized or even idiopathic generalized epilepsy may be candidates for corpus callosotomy.
Next, complete callosotomy was more likely than partial callosotomy to be associated with freedom from drop attacks. In one study of 27 pediatric patients, 91% of individuals achieved significant seizure reduction after complete callosotomy, compared to 75% of those who received partial callosotomy.27 However, many surgeons avoid complete callosotomy because of concern for disconnection syndrome. Notably, we did not observe a higher rate of disconnection syndrome among patients with complete (8.0%) compared with partial (12.4%) callosotomy. One recent systematic review did find that disconnection syndrome was more common with complete (12.5%) than partial (0%) callosotomy, but this report was restricted to 12 studies, compared with 57 in this present analysis, and only evaluated children.22 Although most partial callosotomies lesion the anterior corpus callosum, it has been proposed that there is little advantage to sparing only posterior callosal fibers, because interhemispheric tracts related to motor and many important neurocognitive functions cross anteriorly.53 One recent study of 36 patients evaluated selective posterior callosotomies that spared the anterior fibers, and reported good seizure outcomes (83% of patients with > 90% decrease in drop attacks) as well as favorable neuropsychological outcomes.53 Although further study will be required to clarify the optimal extent of resection to avoid disconnection symptoms, data across the literature do suggest a lower rate of seizure freedom with anterior callosotomy compared to a total section.
Adverse Events
We nonsystematically reviewed the rate of adverse events in the studies included in the meta-analysis, and focused on 5 investigations in which these data were given, which reported an overall complication rate between 8.1% and 12.4% (Table 3). Transient neurological deficit was the most common adverse event noted, including in particular lower-extremity weakness or speech problems (e.g., aphasia, mutism). Recently, one group reviewed 236 corpus callosotomies done between 2000 and 2013 (information was obtained from multiple databases), and found that major or minor adverse events occurred in 14.3%, with neurological deficit being most common.64 Another review suggested that adverse events may be more likely with complete versus partial callosotomy.22 Although the risks of permanent or severe complications are low with corpus callosotomy, these data are valuable to consider in patient counseling and surgical decision making. Finally, although neurocognitive outcomes were not examined in the present study, a systematic review of neuropsychological outcomes may be worthwhile in future studies.
Study Limitations
There are several limitations to this study, including many inherent shortcomings of meta-analysis in neuro-surgery that others have previously discussed in detail.67 During our examination of each study, we evaluated for the risk of bias. Typically there was a high risk of bias, given that studies were retrospective and without blinding, control, or randomization. Another limitation was that although we aggregated data from multiple studies to generate a larger patient study group, the validity of our findings depends on the quality of data collected by others, and is thus susceptible to selection or publication bias. For example, we cannot fully evaluate subjectively defined variables for each study, such as individual definitions of disconnection syndrome, and we cannot quantify extent of callosotomy between studies. Furthermore, in a systematic review, we are unable to disaggregate all factors of interest, and cannot perform multivariate analysis to evaluate interactions across variables.
Another limitation is that this study included both adult and pediatric patients, which may increase heterogeneity. Although we allowed tests of heterogeneity to guide our selection of fixed- versus random-effects models, there may be disagreement about whether this approach is most appropriate in this patient population. We have also used this approach in previous studies of epilepsy surgery.15–17 Next, not all medical literature databases were searched in our study, leading to the possibility that relevant studies were missed. Furthermore, data extraction by one author, with review and verification by a second, may lead to more bias than duplicate data extraction with two independent reviewers. Also, although we used several measures to ensure selection of dependable sources, all studies in this report were retrospective in design and susceptible to recall bias. Nevertheless, the strength of this approach lies in its ability to aggregate a large number of patients across several centers. Replicating these numbers with a prospective case series, even if it were multiinstitutional, would be quite challenging. Finally, PRISMA guidelines were applied to improve the quality of the analysis and findings reported.48
Conclusions
We report a quantitative meta-analysis of overall seizure and drop attack outcomes following corpus callosotomy for refractory epilepsy. The presence of infantile spasms, a normal preoperative MRI study, and a shorter epilepsy duration predicted a higher rate of complete seizure freedom (19% overall), whereas drop attack freedom (55% overall) was predicted by idiopathic epilepsy etiology and complete callosotomy. Interestingly, some predictors of outcome with callosotomy differ from those with resection for epilepsy. We did not find increased risk of disconnection syndrome with complete versus partial callosotomy. Understanding the predictive value of these variables could be beneficial for preoperative corpus callosotomy planning and counseling of potential surgical candidates.
Supplementary Material
Acknowledgments
Dr. Englot is funded by the NIH/NINDS (1R00-NS097618).
ABBREVIATIONS
- EEG
electroencephalography
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analyses
Footnotes
Supplemental Information
Online-Only Content
Supplemental material is available with the online version of the article.
Supplementary Figs. 1 and 2. https://thejns.org/doi/suppl/10.3171/2017.12.JNS172331.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Andersen B, Rogvi-Hansen B, Kruse-Larsen C, Dam M: Corpus callosotomy: seizure and psychosocial outcome. A 39-month follow-up of 20 patients. Epilepsy Res 23:77–85, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Asadi-Pooya AA, Sharan A, Nei M, Sperling MR: Corpus callosotomy. Epilepsy Behav 13:271–278, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Baba H, Ono K, Yonekura M, Teramoto S: Surgical results of anterior callosotomy on medically intractable epilepsy, in Abe O, Inokuchi K, Takasaki K (eds): 30th World Congress of the International College of Surgeons. Bologna: Monduzzi Editore, 1996, pp 1131–1135 (https://pdfs.semanticscholar.org/533b/344ac72145deb5b448a816957a4657aac437.pdf) [Accessed February 14, 2018] [Google Scholar]
- 4.Bower RS, Wirrell E, Nwojo M, Wetjen NM, Marsh WR, Meyer FB: Seizure outcomes after corpus callosotomy for drop attacks. Neurosurgery 73:993–1000, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Carmant L, Holmes GL, Lombroso CT: Outcome following corpus callosotomy. J Epilepsy 11:224–228, 1998 [Google Scholar]
- 6.Chandra SP, Kurwale NS, Chibber SS, Banerji J, Dwivedi R, Garg A, et al. : Endoscopic-assisted (through a mini craniotomy) corpus callosotomy combined with anterior, hippocampal, and posterior commissurotomy in Lennox-Gastaut syndrome: a pilot study to establish its safety and efficacy. Neurosurgery 78:743–751, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Cohen MJ, Holmes GL, Campbell R, Smith JR, Flanigin HF: Cognitive functioning following anterior two-thirds corpus callosotomy in children and adolescents: a one-year prospective report. J Epilepsy 4:63–65, 1991 [Google Scholar]
- 8.Cukiert A, Burattini JA, Mariani PP, Câmara RB, Seda L, Baldauf CM, et al. : Extended, one-stage callosal section for treatment of refractory secondarily generalized epilepsy in patients with Lennox-Gastaut and Lennox-like syndromes. Epilepsia 47:371–374, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Cukiert A, Burattini JA, Mariani PP, Cukiert CM, Argentoni-Baldochi M, Baise-Zung C, et al. : Outcome after extended callosal section in patients with primary idiopathic generalized epilepsy. Epilepsia 50:1377–1380, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Daniel RT, Chandy MJ, Prabhakar S: A prospective study of corpus callosotomy for medically intractable epilepsy. Neurol India 46:189–194, 1998 [PubMed] [Google Scholar]
- 11.Duncan JS, Sander JW, Sisodiya SM, Walker MC: Adult epilepsy. Lancet 367:1087–1100, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Engel J Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. : Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 307:922–930, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Englot DJ, Berger MS, Barbaro NM, Chang EF: Predictors of seizure freedom after resection of supratentorial low-grade gliomas. A review. J Neurosurg 115:240–244, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Englot DJ, Chang EF: Rates and predictors of seizure freedom in resective epilepsy surgery: an update. Neurosurg Rev 37:389–405, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Englot DJ, Chang EF, Auguste KI: Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response. J Neurosurg 115:1248–1255, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Englot DJ, Magill ST, Han SJ, Chang EF, Berger MS, McDermott MW: Seizures in supratentorial meningioma: a systematic review and meta-analysis. J Neurosurg 124:1552–1561, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Englot DJ, Wang DD, Rolston JD, Shih TT, Chang EF: Rates and predictors of long-term seizure freedom after frontal lobe epilepsy surgery: a systematic review and meta-analysis. J Neurosurg 116:1042–1048, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Fandiño-Franky J, Torres M, Nariño D, Fandiño J: Corpus callosotomy in Colombia and some reflections on care and research among the poor in developing countries. Epilepsia 41 (Suppl 4):S22–S27, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Fuiks KS, Wyler AR, Hermann BP, Somes G: Seizure outcome from anterior and complete corpus callosotomy. J Neurosurg 74:573–578, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Flores E: Corpus callosum section for patients with intractable epilepsy. Appl Neurophysiol 50:390–397, 1987 [DOI] [PubMed] [Google Scholar]
- 21.Gates JR, Rosenfeld WE, Maxwell RE, Lyons RE: Response of multiple seizure types to corpus callosum section. Epilepsia 28:28–34, 1987 [DOI] [PubMed] [Google Scholar]
- 22.Graham D, Tisdall MM, Gill D: Corpus callosotomy outcomes in pediatric patients: a systematic review. Epilepsia 57:1053–1068, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Hodaie M, Musharbash A, Otsubo H, Snead OC III, Chitoku S, Ochi A, et al. : Image-guided, frameless stereotactic sectioning of the corpus callosum in children with intractable epilepsy. Pediatr Neurosurg 34:286–294, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Hong J, Desai A, Thadani VM, Roberts DW: Efficacy and safety of corpus callosotomy after vagal nerve stimulation in patients with drug-resistant epilepsy. J Neurosurg 128:277–286, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki M, Uematsu M, Hino-Fukuyo N, Osawa S, Shimoda Y, Jin K, et al. : Clinical profiles for seizure remission and developmental gains after total corpus callosotomy. Brain Dev 38:47–53, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki M, Uematsu M, Sato Y, Nakayama T, Haginoya K, Osawa S, et al. : Complete remission of seizures after corpus callosotomy. J Neurosurg Pediatr 10:7–13, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Jalilian L, Limbrick DD, Steger-May K, Johnston J, Powers AK, Smyth MD: Complete versus anterior two-thirds corpus callosotomy in children: analysis of outcome. J Neurosurg Pediatr 6:257–266, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Janszky J, Janszky I, Schulz R, Hoppe M, Behne F, Pannek HW, et al. : Temporal lobe epilepsy with hippocampal sclerosis: predictors for long-term surgical outcome. Brain 128:395–404, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Jea A, Vachhrajani S, Johnson KK, Rutka JT: Corpus callosotomy in children with intractable epilepsy using frameless stereotactic neuronavigation: 12-year experience at the Hospital for Sick Children in Toronto. Neurosurg Focus 25(3):E7, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Jenssen S, Sperling MR, Tracy JI, Nei M, Joyce L, David G, et al. : Corpus callosotomy in refractory idiopathic generalized epilepsy. Seizure 15:621–629, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Jeong SW, Lee SK, Hong KS, Kim KK, Chung CK, Kim H: Prognostic factors for the surgery for mesial temporal lobe epilepsy: longitudinal analysis. Epilepsia 46:1273–1279, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Kawai K, Shimizu H, Yagishita A, Maehara T, Tamagawa K: Clinical outcomes after corpus callosotomy in patients with bihemispheric malformations of cortical development. J Neurosurg 101 (1 Suppl):7–15, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Kim DS, Yang KH, Kim TG, Chang JH, Chang JW, Choi JU, et al. : The surgical effect of callosotomy in the treatment of intractable seizure. Yonsei Med J 45:233–240, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Kokoszka MA, McGoldrick PE, La Vega-Talbott M, Raynes H, Palmese CA, Wolf SM, et al. : Epilepsy surgery in patients with autism. J Neurosurg Pediatr 19:196–207, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Kwan P, Brodie MJ: Early identification of refractory epilepsy. N Engl J Med 342:314–319, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Kwan SY, Lin JH, Wong TT, Chang KP, Yiu CH: Prognostic value of electrocorticography findings during callosotomy in children with Lennox-Gastaut syndrome. Seizure 14:470–475, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Kwan SY, Wong TT, Chang KP, Chi CS, Yang TF, Lee YC, et al. : Seizure outcome after corpus callosotomy: the Taiwan experience. Childs Nerv Syst 16:87–92, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Lancman G, Virk M, Shao H, Mazumdar M, Greenfield JP, Weinstein S, et al. : Vagus nerve stimulation vs. corpus callosotomy in the treatment of Lennox-Gastaut syndrome: a meta-analysis. Seizure 22:3–8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MJ, Kim HD, Lee JS, Kim DS, Lee SK: Usefulness of diffusion tensor tractography in pediatric epilepsy surgery. Yonsei Med J 54:21–27, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee YJ, Lee JS, Kang HC, Kim DS, Shim KW, Eom S, et al. : Outcomes of epilepsy surgery in childhood-onset epileptic encephalopathy. Brain Dev 36:496–504, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Liang C, Tang Y, Mu H, Guo T, Du Y, Yue X, et al. : Corpus callosotomy for patients with intractable seizures: an insight into the rapid relapse. J Craniofac Surg 26:e795–e798, 2015 [DOI] [PubMed] [Google Scholar]
- 42.Liang S, Li A, Jiang H, Meng X, Zhao M, Zhang J, et al. : Anterior corpus callosotomy in patients with intractable generalized epilepsy and mental retardation. Stereotact Funct Neurosurg 88:246–252, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Liang S, Zhang S, Hu X, Zhang Z, Fu X, Jiang H, et al. : Anterior corpus callosotomy in school-aged children with Lennox-Gastaut syndrome: a prospective study. Eur J Paediatr Neurol 18:670–676, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Maehara T, Shimizu H: Surgical outcome of corpus callosotomy in patients with drop attacks. Epilepsia 42:67–71, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Maehara T, Shimizu H, Oda M, Arai N: Surgical treatment of children with medically intractable epilepsy. Outcome of various surgical procedures. Neurol Med Chir (Tokyo) 36:305–309, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Makari GS, Holmes GL, Murro AM, Smith JR, Flanigin HF, Cohen MJ, et al. : Corpus callosotomy for the treatment of intractable epilepsy in children. J Epilepsy 2:1–7, 1989 [DOI] [PubMed] [Google Scholar]
- 47.Mamelak AN, Barbaro NM, Walker JA, Laxer KD: Corpus callosotomy: a quantitative study of the extent of resection, seizure control, and neuropsychological outcome. J Neurosurg 79:688–695, 1993 [DOI] [PubMed] [Google Scholar]
- 48.Moher D, Liberati A, Tetzlaff J, Altman DG: Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murro AM, Flanigin HF, Gallagher BB, King DW, Smith JR: Corpus callosotomy for the treatment of intractable epilepsy. Epilepsy Res 2:44–50, 1988 [DOI] [PubMed] [Google Scholar]
- 50.Nordgren RE, Reeves AG, Viguera AC, Roberts DW: Corpus callosotomy for intractable seizures in the pediatric age group. Arch Neurol 48:364–372, 1991 [DOI] [PubMed] [Google Scholar]
- 51.Oguni H, Olivier A, Andermann F, Comair J: Anterior callosotomy in the treatment of medically intractable epilepsies: a study of 43 patients with a mean follow-up of 39 months. Ann Neurol 30:357–364, 1991 [DOI] [PubMed] [Google Scholar]
- 52.Otsuki T, Kim HD, Luan G, Inoue Y, Baba H, Oguni H, et al. : Surgical versus medical treatment for children with epileptic encephalopathy in infancy and early childhood: results of an international multicenter cohort study in Far-East Asia (the FACE study). Brain Dev 38:449–460, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Paglioli E, Martins WA, Azambuja N, Portuguez M, Frigeri TM, Pinos L, et al. : Selective posterior callosotomy for drop attacks: a new approach sparing prefrontal connectivity. Neurology 87:1968–1974, 2016 [DOI] [PubMed] [Google Scholar]
- 54.Papo I, Quattrini A, Provinciali L, Rychlicki F, Paggi A, Del Pesce M, et al. : Callosotomy for the management of intractable non-focal epilepsy: a preliminary personal assessment. Acta Neurochir (Wien) 96:46–53, 1989 [DOI] [PubMed] [Google Scholar]
- 55.Park MS, Nakagawa E, Schoenberg MR, Benbadis SR, Vale FL: Outcome of corpus callosotomy in adults. Epilepsy Behav 28:181–184, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Passamonti C, Zamponi N, Foschi N, Trignani R, Luzi M, Cesaroni E, et al. : Long-term seizure and behavioral outcomes after corpus callosotomy. Epilepsy Behav 41:23–29, 2014 [DOI] [PubMed] [Google Scholar]
- 57.Phillips J, Sakas DE: Anterior callosotomy for intractable epilepsy: outcome in a series of twenty patients. Br J Neurosurg 10:351–356, 1996 [DOI] [PubMed] [Google Scholar]
- 58.Pinard JM, Delalande O, Chiron C, Soufflet C, Plouin P, Kim Y, et al. : Callosotomy for epilepsy after West syndrome. Epilepsia 40:1727–1734, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Ping Z, Ji-Wen X, Gui-Song W, Hong-Yu Z, Xin T: Evaluation of efficacy and safety of anterior corpus callosotomy with keyhole in refractory seizures. Seizure 18:417–419, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Rahimi SY, Park YD, Witcher MR, Lee KH, Marrufo M, Lee MR: Corpus callosotomy for treatment of pediatric epilepsy in the modern era. Pediatr Neurosurg 43:202–208, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Rathore C, Abraham M, Rao RM, George A, Sankara Sarma P, Radhakrishnan K: Outcome after corpus callosotomy in children with injurious drop attacks and severe mental retardation. Brain Dev 29:577–585, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Reutens DC, Bye AM, Hopkins IJ, Danks A, Somerville E, Walsh J, et al. : Corpus callosotomy for intractable epilepsy: seizure outcome and prognostic factors. Epilepsia 34:904–909, 1993 [DOI] [PubMed] [Google Scholar]
- 63.Riikonen R: Combination therapy for treatment of infantile spasms. Lancet Neurol 16:19–20, 2017 [DOI] [PubMed] [Google Scholar]
- 64.Rolston JD, Englot DJ, Knowlton RC, Chang EF: Rate and complications of adult epilepsy surgery in North America: analysis of multiple databases. Epilepsy Res 124:55–62, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rolston JD, Englot DJ, Wang DD, Garcia PA, Chang EF: Corpus callosotomy versus vagus nerve stimulation for atonic seizures and drop attacks: a systematic review. Epilepsy Behav 51:13–17, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rossi GF, Colicchio G, Marchese E, Pompucci A: Callosotomy for severe epilepsies with generalized seizures: outcome and prognostic factors. Acta Neurochir (Wien) 138:221–227, 1996 [DOI] [PubMed] [Google Scholar]
- 67.Sampson JH, Barker FG II: Methodology and reporting of meta-analyses in the neurosurgical literature. J Neurosurg 120:791–794, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Sander JW: The epidemiology of epilepsy revisited. Curr Opin Neurol 16:165–170, 2003 [DOI] [PubMed] [Google Scholar]
- 69.Shim KW, Lee YM, Kim HD, Lee JS, Choi JU, Kim DS: Changing the paradigm of 1-stage total callosotomy for the treatment of pediatric generalized epilepsy. J Neurosurg Pediatr 2:29–36, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Shimizu H: Our experience with pediatric epilepsy surgery focusing on corpus callosotomy and hemispherotomy. Epilepsia 46 (Suppl 1):30–31, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Sorenson JM, Wheless JW, Baumgartner JE, Thomas AB, Brookshire BL, Clifton GL, et al. : Corpus callosotomy for medically intractable seizures. Pediatr Neurosurg 27:260–267, 1997 [DOI] [PubMed] [Google Scholar]
- 72.Spencer SS, Spencer DD, Williamson PD, Sass K, Novelly RA, Mattson RH: Corpus callosotomy for epilepsy. I. Seizure effects. Neurology 38:19–24, 1988 [DOI] [PubMed] [Google Scholar]
- 73.Sperling MR, Feldman H, Kinman J, Liporace JD, O’Connor MJ: Seizure control and mortality in epilepsy. Ann Neurol 46:45–50, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Stigsdotter-Broman L, Olsson I, Flink R, Rydenhag B, Malmgren K: Long-term follow-up after callosotomy—a prospective, population based, observational study. Epilepsia 55:316–321, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sunaga S, Shimizu H, Sugano H: Long-term follow-up of seizure outcomes after corpus callosotomy. Seizure 18:124–128, 2009 [DOI] [PubMed] [Google Scholar]
- 76.Tanriverdi T, Olivier A, Poulin N, Andermann F, Dubeau F: Long-term seizure outcome after corpus callosotomy: a retrospective analysis of 95 patients. J Neurosurg 110:332–342, 2009 [DOI] [PubMed] [Google Scholar]
- 77.Téllez-Zenteno JF, Dhar R, Wiebe S: Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain 128:1188–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Tonini C, Beghi E, Berg AT, Bogliun G, Giordano L, Newton RW, et al. : Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res 62:75–87, 2004 [DOI] [PubMed] [Google Scholar]
- 79.Turanli G, Yalnizoğlu D, Genç-Açikgöz D, Akalan N, Topçu M: Outcome and long term follow-up after corpus callosotomy in childhood onset intractable epilepsy. Childs Nerv Syst 22:1322–1327, 2006 [DOI] [PubMed] [Google Scholar]
- 80.Yang PF, Lin Q, Mei Z, Chen ZQ, Zhang HJ, Pei JS, et al. : Outcome after anterior callosal section that spares the splenium in pediatric patients with drop attacks. Epilepsy Behav 36:47–52, 2014 [DOI] [PubMed] [Google Scholar]
- 81.Yang TF, Wong TT, Kwan SY, Chang KP, Lee YC, Hsu TC: Quality of life and life satisfaction in families after a child has undergone corpus callosotomy. Epilepsia 37:76–80, 1996 [DOI] [PubMed] [Google Scholar]
- 82.You SJ, Kang HC, Ko TS, Kim HD, Yum MS, Hwang YS, et al. : Comparison of corpus callosotomy and vagus nerve stimulation in children with Lennox-Gastaut syndrome. Brain Dev 30:195–199, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.