Abstract
Middle East respiratory syndrome coronavirus, MERS‐CoV, was identified in Saudi Arabia in 2012, and as of January 29, 2018, there were 2,123 laboratory‐confirmed MERS‐CoV cases reported to WHO (WHO, 2018, https://www.who.int/emergencies/mers-cov/en/). Multiple studies suggest that dromedary camels are a source for human MERS‐CoV infection. MERS‐CoV‐specific antibodies have been detected in the serum of dromedary camels across Northern Africa and across the Arabian Peninsula. Israel's geographic location places Israel at risk for MERS‐CoV infection. To date, MERS‐CoV‐related illness has not been reported and the burden of MERS‐CoV infection in the Israeli population is unknown. The seroprevalence of MERS‐CoV‐specific antibodies in Israeli dromedary camels is unknown. The objective of this study was to determine the prevalence of MERS‐CoV seropositivity in dromedary camels in Israel. The prevalence of MERS‐CoV antibodies in Israeli camels was examined in 71 camel sera collected from four farms across Israel by MERS‐CoV‐specific microneutralization (Mnt) assay and confirmed by MERS‐CoV‐specific immunofluorescence assay (IFA). Although this study cannot rule out potential antibody cross‐reactivity by IFA, the presence of bovine coronavirus‐specific antibodies do not appear to impact detection of MERS‐CoV antibodies by Mnt. MERS‐CoV neutralizing antibodies were detectable in 51 (71.8%) camel sera, and no association was observed between the presence of neutralizing antibodies and camel age or gender. These findings extend the known range of MERS‐CoV circulation in Middle Eastern camels. The high rate of MERS‐CoV‐specific antibody seropositivity in dromedary camels in the absence of any reported human MERS cases suggests that there is still much to be learned about the dynamics of camel‐to‐human transmission of MERS‐CoV.
Keywords: coronavirus, dromedary camels, MERS‐CoV, Middle East respiratory syndrome coronavirus
Impacts.
Israel’s geographic location places Israeli citizens at risk for MERS‐CoV infection. To date, MERS‐CoV‐related illness has not been reported in Israel.
Dromedary camels are one potential source of human MERS‐CoV infection; the seroprevalence of MERS‐CoV‐specific antibodies in Israeli dromedary camels is unknown.
In this study, MERS‐CoV seroprevalence in dromedary camels was 72% across four farms in Israel. The high prevalence of MERS‐CoV antibodies in camels and the absence of human MERS cases suggest that there is much to be learned about camel‐to‐human transmission of MERS‐CoV.
1. INTRODUCTION
Middle East respiratory syndrome coronavirus, MERS‐CoV, a member of the Betacoronavirus genus lineage C, was first identified in Saudi Arabia in 2012. As of January 29, 2018, there were 2,123 laboratory‐confirmed human MERS‐CoV cases reported to WHO, including at least 740 MERS‐CoV‐related deaths (WHO, 2018). Multiple studies suggest that dromedary camels are a major source for human MERS‐CoV infection. MERS‐CoV‐specific antibodies have been detected in the serum of dromedary camels across Northern Africa, including Tunisia, Egypt, Sudan, Ethiopia, Nigeria, Kenya and Somalia, and across the Arabian Peninsula, including Jordan, Saudi Arabia, Qatar, Oman and United Arab Emirates (Corman et al., 2014; Hemida et al., 2014; Meyer et al., 2014; Muller et al., 2014). MERS‐CoV neutralizing antibodies have been detected in 30‐year‐old archived camel serum samples, suggesting long‐term circulation of MERS‐CoV in dromedaries in this region (Muller et al., 2014). MERS‐CoV genome has been detected, isolated and sequenced from camel respiratory specimens in Northern Africa, Nigeria and Saudi Arabia, and from an air sample of a camel barn owned by a known MERS‐CoV‐infected human (Alagaili et al., 2014; Azhar et al., 2014; Chu et al., 2015; Haagmans et al., 2014; Raj et al., 2014). Genomic and epidemiologic studies comparing MERS‐CoV sequences from household clusters and camels, and of dromedary farms and human contacts in UAE (Muhairi et al., 2016; Paden et al., 2017), and of patients with corresponding MERS‐CoV‐positive camels in Saudi Arabia (Kasem et al., 2017) demonstrate that camels are a potential source of human MERS‐CoV infection.
Israel's geographic location in the Middle East, bordering Jordan where human cases have been reported and MERS‐CoV‐specific antibodies have been detected in the serum of dromedary camels, suggests Israeli citizens may be at risk for MERS‐CoV infection. However, to date, MERS‐CoV‐related illness has not been reported in Israel and the seroprevalence of MERS‐CoV‐specific antibodies in Israeli dromedary camels is unknown. The objective of this study was to determine the prevalence of MERS‐CoV seropositivity in Israeli camels.
2. MATERIALS AND METHODS
2.1. Serum samples
Serum specimens from 71 dromedary camels across four different locations in Israel (Sites A‐D, Tables 1 and 2) were collected between May and June 2013, as previously described (Rasis, Rudoler, Schwartz, & Giladi, 2014). Farm A (n = 9) was located east of Jerusalem; farms B‐D (n = 15, 27 and 20, respectively) were located in the Negev desert, in southern Israel. The origin of these camels prior to their association with these four locations is unknown. These camels were used in the tourism industry. This study included both male (n = 19) and female (n = 52) camels ages 3 to over 20 years old. Blood samples were taken by jugular vein puncture. Serum samples were obtained on the day of collection from unclotted blood using serum separator tubes. All serum specimens were shipped to the CDC and inactivated by gamma irradiation at 5 × 106 rads in a Cobalt irradiator to inactivate potential pathogens, and stored at −80°C until use. The study was approved by the Tel Aviv Sourasky Medical Center Institutional Animal Care and Use Committee (Study 18–6‐13).
Table 1.
Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) Microneutralization and Immunofluorescence (IFA) titres in sera samples collected from dromedary camels in Israel (n = 35): High (≥80) neutralizing antibody titres
| Sample ID | Site | Age (years) | Sex | Microneutralization Reciprocal Titres, MERS‐CoVa | IFA Reciprocal Titres, MERS‐CoVb | IFA results, BCoVc |
|---|---|---|---|---|---|---|
| 1 | C | 10 | F | 25,600 | ≥10,000 | Pos |
| 2 | C | 12 | F | 6,400 | 10,000 | Pos |
| 3 | D | 19 | F | 6,400 | 8,000 | Pos |
| 4 | C | 9 | F | 3,200 | 8,000 | nd |
| 5 | D | 9 | F | 1600 | 4,000 | Pos |
| 6 | D | 3 | F | 1600 | 4,000 | Pos |
| 7 | C | 8 | F | 1600 | 4,000 | nd |
| 8 | B | 15 | F | 800 | 4,000 | Pos |
| 9 | D | 12 | F | 640 | 2,000–4,000 | Pos |
| 10 | D | 15 | F | 640 | 2,000–4,000 | Neg |
| 11 | D | 9 | F | 640 | 2,000 | Pos |
| 12 | D | 11 | F | 640 | 750 | Pos |
| 13 | A | 20 (+) | F | 640 | 750 | Pos |
| 14 | C | 7 | F | 640 | 2,000–4,000 | nd |
| 15 | C | 7 | F | 640 | 4,000 | nd |
| 16 | D | 13 | F | 320 | 100 | Pos |
| 17 | D | 14 | F | 320 | 100 | Pos |
| 18 | D | 24 | F | 320 | 100 | Pos |
| 19 | D | 12 | F | 320 | 100 | Neg |
| 20 | C | 14 | M | 320 | 100 | Pos |
| 21 | C | 14 | M | 320 | 100 | Pos |
| 22 | C | 7 | F | 320 | 100 | nd |
| 23 | D | 9 | F | 160 | 100 | Neg |
| 24 | D | 11 | F | 160 | 100 | Pos |
| 25 | D | 8 | F | 160 | 100 | Neg |
| 26 | B | 9 | F | 160 | 100 | Pos |
| 27 | B | 10 | F | 160 | 100 | Pos |
| 28 | C | 7 | F | 160 | 100 | nd |
| 29 | C | 8 | F | 160 | 100 | Neg |
| 30 | D | 20 | F | 80 | 100 | Neg |
| 31 | D | 10 | F | 80 | Indeterminated | Pos |
| 32 | D | 17 | F | 80 | 100 | Pos |
| 33 | B | 9–10 | F | 80 | 100 | Pos |
| 34 | C | 7 | M | 80 | 100 | Pos |
| 35 | C | 6 | F | 80 | 100 | Pos |
Notes. nd, not done.
In vitro microneutralization assays were performed using the Jordan strain of MERS‐CoV, beginning with a serial dilution range of 1:20–1:640. Samples with a titre ≥ 640 were further examined at serial dilutions out to 1:25,600. Microneutralization titres are reported as the dilution factor at which at least one of three independent wells completely inhibited virus infection.
Immunofluorescence assays (IFAs) were performed against the Jordan strain of MERS‐CoV, beginning at a dilution of 1:100, to a final dilution of 1:10,000.
Sera were evaluated by IFA for the presence of bovine coronavirus antibodies (BCoV) at a dilution of 1:100.
Samples were considered indeterminate when an inconclusive result was obtained by two independent evaluations at a dilution of 1:100.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 2.
Middle East Respiratory Syndrome Coronavirus (MERS‐CoV) Microneutralization and Immunofluorescence (IFA) titres in sera samples collected from dromedary camels in Israel: Low (<80) neutralizing antibody titres (n = 16)
| Sample ID | Site | Age (years) | Sex | Microneutralization Reciprocal Titres, MERS‐CoVa | IFA Reciprocal Titres, MERS‐CoVb | IFA results, BCoVc |
|---|---|---|---|---|---|---|
| 36 | D | 21 | F | 40 | 100 | Pos |
| 37 | B | 10 | F | 40 | Indeterminated | Pos |
| 38 | B | 17 | M | 40 | Indeterminate | Neg |
| 39 | B | 10–12 | M | 40 | <100 | Neg |
| 40 | C | 8 | F | 40 | Indeterminate | Pos |
| 41 | C | 12 | M | 40 | Indeterminate | Indeterminate |
| 42 | C | 10 | M | 40 | Indeterminate | Neg |
| 43 | C | 7 | M | 40 | 100 | Pos |
| 44 | C | 8 | M | 40 | Indeterminate | Indeterminate |
| 45 | C | 11 | F | 40 | 100 | nd |
| 46 | C | 6 | F | 40 | <100 | nd |
| 47 | D | 8 | F | 20 | <100 | Pos |
| 48 | C | 12 | M | 20 | Indeterminate | Indeterminate |
| 49 | C | 9 | F | 20 | <100 | Pos |
| 50 | C | 5 | F | 20 | <100 | Pos |
| 51 | C | 10 | F | 20 | <100 | nd |
Notes. nd, not done.
In vitro microneutralization assays were performed using the Jordan strain of MERS‐CoV, beginning with a serial dilution range of 1:20–1:640. Microneutralization titres are reported as the dilution factor at which at least one of three independent wells completely inhibited virus infection.
Immunofluorescence assays (IFAs) were performed against the Jordan strain of MERS‐CoV, beginning at a dilution of 1:100, to a final dilution of 1:10,000.
Sera were evaluated by IFA for the presence of bovine coronavirus antibodies (BCoV) at a dilution of 1:100.
Samples were considered indeterminate when an inconclusive result was obtained by two independent evaluations at a dilution of 1:100.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
2.2. MERS‐CoV‐specific neutralization assays
MERS‐CoV‐specific neutralization (MNt) assays were performed to determine the presence of neutralizing antibodies in camel sera using the Jordan strain of MERS‐CoV (Hu/Jordan‐N3/2012), following a previously established method (Sui et al., 2004). Initial MNt assays were performed using a titration range from 20 to 640, and samples with MNt titres of 640 were further titrated. MNt was performed using polyclonal guinea pig anti‐bovine coronavirus (Mebus strain, NIH Biodenfense and Emerging Infections Research Resources Repository) antiserum to evaluate antibody cross‐neutralization.
2.3. Immunofluorescence assay (IFA)
Initial IFA screening was performed using sera diluted at 1:100, following a modified, previously published protocol (Corman et al., 2012). Briefly, MERS‐CoV (Jordan)‐infected Vero cells slides were fixed, permeabilized, blocked with whole camel serum (Abcam, 1:10,000), incubated with serum, and stained with FITC‐conjugated llama anti‐goat IgG (H + L; Bethyl Lab, 1:100; Figure 1). Whole camel blocking serum was screened in the absence of specimens, to verify that blocking did not result in a false positive signal. Specimens indeterminate for the presence of MERS‐CoV antibodies were re‐screened at 1:50 and 1:100. A final indeterminate determination was made after two independent screens by IFA were indeterminate. IFA titres were determined by repeated screening with serial dilutions of camel sera, out to 1:10,000. Reactivity against BCoV Mebus strain was assessed using a commercially available BCoV‐specific IFA kit (Veterinary Medical Research and Development).
Figure 1.
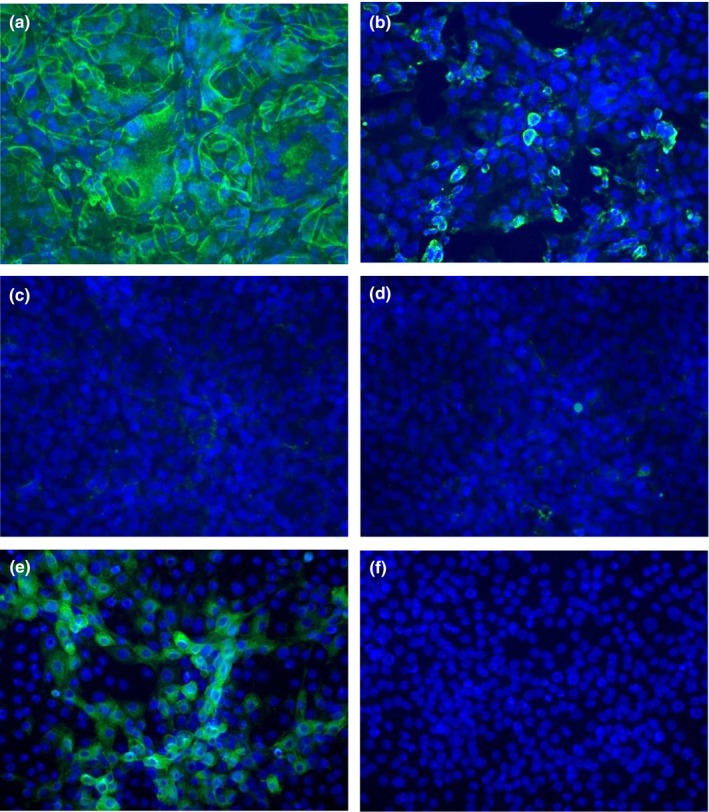
Camel serum antibodies react to Middle East Respiratory Syndrome Coronavirus (MERS‐CoV)‐infected, fixed Vero cells by immunofluorescence assay. Vero cells were infected with MERS‐CoV (Hu/Jordan‐N3/2012, a–c), or mock‐infected (d), fixed, blocked, then incubated with sera from camels with high (a, d), low (b) or no (c) MERS‐CoV neutralizing antibodies and developed for immunofluorescence. Camel sera were determined to be positive (a, b) or negative (c, d) based on the intensity of staining against MERS‐CoV‐infected Vero cells compared to mock‐infected Vero cells (representative samples are shown). Camel sera were also tested against bovine coronavirus (BCoV)‐infected or mock‐infected MDBK cells using a commercially available test kit (Veterinary Medical Research and Development, VMRD). Shown, serum from a camel that did not react in a MERS‐CoV IFA or have MERS‐CoV neutralizing antibodies reacts to BCoV‐infected MDBK cells (e), but not mock‐infected MDBK cells (f). DAPI counterstain was used and cells were imaged using a Zeiss AxioImager microscope at 20X magnification [Colour figure can be viewed at http://www.wileyonlinelibrary.com/]
2.4. Statistical analysis
Statistical analyses were performed using Fisher's exact test and a p‐value <0.05 was considered significant.
3. RESULTS
Fifty‐one of the 71 (71.8%) camel sera had MERS‐CoV neutralizing antibodies titres (Tables 1 and 2). Thirty‐five serum samples (49.3%) had high MERS‐CoV neutralizing antibody titres ranging from 80 to 25,600 (Table 1) with IFA titres ranging from 100 to greater than 10,000 (Table 1). As MERS‐CoV neutralization titres increased to ≥640 (n = 15), MERS‐CoV‐specific titres determined by IFA also increased above 100. Sixteen of the 71 (22.5%) camels had lower MERS‐CoV serum neutralizing antibody titres, ranging from 20 to 40 (Table 2). For these 16 camels, the IFA titres were equal to 100 for three camels (Table 2 and Figure 1b), less than 100 for six camels, and indeterminate for the remaining seven camels. For the remaining 20 (28.2%) camels, serum neutralizing antibody titres were less than 20 or below the level of detection, with MERS‐CoV antibody titres, by IFA, either <100 (n = 15; Figure 1c) or indeterminate (n = 5; data not shown). Attempts to detect coronavirus genomic material by RT‐PCR from the camel sera were unsuccessful. The inability to detect genomic material may in part be due to three key factors in this study; one, specimen collection was not optimized for nucleic acid preservation; two, prior to study, sera were irradiated at 5 × 106 rads upon arrival at the CDC per importation requirements; and three, camels may not have had acute infections at the time of serum collection.
The presence of BCoV‐reactive antibodies was determined in a randomly selected subset of camel sera with MERS‐CoV neutralizing antibody titres (n = 42) and with no titres (n = 20) by BCoV IFA, using a 1:100 dilution of camel sera. Nine of 42 (21.4%) camels with MERS‐CoV microneutralization titres were negative for BCoV‐reactive serum antibodies, including one camel with a MERS‐CoV‐specific neutralization titre of 640, three were indeterminate (7%) with the remaining 30 sera (71.4%) demonstrating reactivity to BCoV. Fourteen of the 20 camels (70%) with no detectable MERS‐CoV neutralizing antibody titres (titres <20) were positive for BCoV‐reactive antibodies at a 1:100 dilution. Serum antibodies from dromedary camels in Saudi Arabia demonstrated reactivity to both MERS‐CoV and bovine coronavirus (BCoV) (Hemida et al., 2013; Perera et al., 2013), suggesting that the presence of BCoV antibodies may impair the ability to specifically detect MERS‐CoV‐specific antibodies. However, antibodies specific to MERS‐CoV did not neutralize BCoV or SARS‐CoV infection, nor did BCoV‐specific antibodies neutralize MERS‐CoV infection (Hemida et al., 2013; Perera et al., 2013). Consistent with those findings, antiserum against BCoV did not cross‐neutralize MERS‐CoV in the Mnt used in this study, confirming the specificity of the assay to discriminate between the two viruses (data not shown).
There was no association of MERS‐CoV neutralizing antibodies with gender or age of the camels. By location, the number of MERS‐CoV neutralizing antibody positive camels was significantly higher at sites C and D (p = 0.008 and 0.002, respectively), compared to site B, significantly higher at site C than A (p < 0.001) and significantly higher at site D than A (p < 0.001). Of those specimens tested for BCoV, all indeterminate specimens (n = 12) originated from site D and were all positive for antibodies against MERS‐CoV.
4. DISCUSSION
These findings demonstrate high MERS‐CoV‐specific neutralizing antibody titres suggest that MERS‐CoV, or a related virus, has circulated through dromedary camels in Israel, extending the known geographic range of MERS‐CoV circulation in camels. While the results do not rule out antibody cross‐reactivity, the inability of BCoV immune sera to neutralize MERS‐CoV suggests that the presence of BCoV‐specific antibodies did not appear to impact the ability to specifically detect MERS‐CoV‐specific by Mnt. The circulation of MERS‐CoV or a closely related virus in dromedary camels in Israel in the absence of any reported clinical cases of MERS‐CoV in the Israeli population suggests that there may be other factors involved in the dynamics of camel‐to‐human transmission of MERS‐CoV beyond circulation within camel herds.
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
ACKNOWLEDGEMENTS
We would like to acknowledge Jessica Rudd and Aaron Curns, at the Centers for Disease Control and Prevention, for performing the statistical analyses for this study. The authors thank Dr. Kathleen Tatti and Dr. Aron Hall for review of the manuscript and for providing critical comments. The following reagent was obtained through the NIH Biodenfense and Emerging Infections Research Resources Repository, NIAID, NIH: Polyclonal anti‐Bovine Coronavirus, Mebus (antiserum, Guinea Pig), NR‐455.
Harcourt JL, Rudoler N, Tamin A, et al. The prevalence of Middle East respiratory syndrome coronavirus (MERS‐CoV) antibodies in dromedary camels in Israel. Zoonoses Public Health. 2018;65:749–754. 10.1111/zph.12482
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- Alagaili, A. N. , Briese, T. , Mishra, N. , Kapoor, V. , Sameroff, S. C. , Burbelo, P. D. , … Lipkin, W. I. (2014) Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio, 5, e00884–00814 10.1128/mBio.00884-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar, E. I. , Hashem, A. M. , El‐Kafrawy, S. A. , Sohrab, S. S. , Aburizaiza, A. S. , Farraj, S. A. , … Madani, T. A. (2014) Detection of the Middle East respiratory syndrome coronavirus genome in an air sample originating from a camel barn owned by an infected patient. mBio, 5, e01450–01414 10.1128/mBio.01450-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, D. K. , Oladipo, J. O. , Perera, R. A. , Kuranga, S. A. , Chan, S. M. , Poon, L. L. , & Peiris, M. (2015). Middle East respiratory syndrome coronavirus (MERS‐CoV) in dromedary camels in Nigeria, 2015. Euro Surveillance, 20(49), pii: 30086. 10.2807/1560-7917.ES.2015.20.49.30086 [DOI] [PubMed] [Google Scholar]
- Corman, V. M. , Jores, J. , Meyer, B. , Younan, M. , Liljander, A. , Said, M. Y. , … Muller, M. A. (2014). Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992–2013. Emerging Infectious Diseases, 20, 1319–1322. 10.3201/eid2008.140596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman, V. M. , Muller, M. A. , Costabel, U. , Timm, J. , Binger, T. , Meyer, B. , … Drosten, C. (2012). Assays for laboratory confirmation of novel human coronavirus (hCoV‐EMC) infections. Euro Surveillance, 17, pii: 20334. [DOI] [PubMed] [Google Scholar]
- Haagmans, B. L. , Al Dhahiry, S. H. , Reusken, C. B. , Raj, V. S. , Galiano, M. , Myers, R. , … Koopmans, M. P. (2014). Middle East respiratory syndrome coronavirus in dromedary camels: An outbreak investigation. The Lancet. Infectious Diseases, 14, 140–145. 10.1016/S1473-3099(13)70690-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida, M. G. , Perera, R. A. , Al Jassim, R. A. , Kayali, G. , Siu, L. Y. , Wang, P. , … Peiris, M. (2014). Seroepidemiology of Middle East respiratory syndrome (MERS) coronavirus in Saudi Arabia (1993) and Australia (2014) and characterisation of assay specificity. Euro Surveillance, 19(23), pii: 20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida, M. G. , Perera, R. A. , Wang, P. , Alhammadi, M. A. , Siu, L. Y. , Li, M. , … Peiris, M. (2013). Middle East Respiratory Syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveillance, 18, 20659. [DOI] [PubMed] [Google Scholar]
- Kasem, S. , Qasim, I. , Al‐Hufofi, A. , Hashim, O. , Alkarar, A. , Abu‐Obeida, A. , … Peiris, M. (2017). Cross‐sectional study of MERS‐CoV‐specific RNA and antibodies in animals that have had contact with MERS patients in Saudi Arabia. Journal of Infection and Public Health, 11(3), 331–338. 10.1016/j.jiph.2017.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. , Muller, M. A. , Corman, V. M. , Reusken, C. B. , Ritz, D. , Godeke, G. J. , … Drosten, C. (2014). Antibodies against MERS coronavirus in dromedary camels, United Arab Emirates, 2003 and 2013. Emerging Infectious Diseases, 20, 552–559. 10.3201/eid2004.131746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhairi, S. A. , Hosani, F. A. , Eltahir, Y. M. , Mulla, M. A. , Yusof, M. F. , Serhan, W. S. , … Abdelazim, A. S. (2016). Epidemiological investigation of Middle East respiratory syndrome coronavirus in dromedary camel farms linked with human infection in Abu Dhabi Emirate, United Arab Emirates. Virus Genes, 52, 848–854. 10.1007/s11262-016-1367-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, M. A. , Corman, V. M. , Jores, J. , Meyer, B. , Younan, M. , Liljander, A. , … Drosten, C. (2014). MERS Coronavirus Neutralizing Antibodies in Camels, Eastern Africa, 1983–1997. Emerging Infectious Diseases, 20, 1983–1997. 10.3201/eid2012.141026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paden, C. R. , Yusof, M. , Al Hammadi, Z. M. , Queen, K. , Tao, Y. , Eltahir, Y. M. , … Al Muhairi, S. S. M. (2017). Zoonotic origin and transmission of Middle East respiratory syndrome coronavirus in the UAE. Zoonoses Public Health, 65(3), 322–333. 10.1111/zph.12435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera, R. A. , Wang, P. , Gomaa, M. R. , El‐Shesheny, R. , Kandeil, A. , Bagato, O. , & … Kayali, G. (2013) Seroepidemiology for MERS coronavirus using microneutralisation and pseudoparticle virus neutralisation assays reveal a high prevalence of antibody in dromedary camels in Egypt, June 2013. Euro Surveillance, 18, pii=20574. [DOI] [PubMed] [Google Scholar]
- Raj, V. S. , Farag, E. A. , Reusken, C. B. , Lamers, M. M. , Pas, S. D. , Voermans, J. , … Haagmans, B. L. (2014). Isolation of MERS coronavirus from a dromedary camel, Qatar, 2014. Emerging Infectious Diseases, 20, 1339–1342. 10.3201/eid2008.140663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasis, M. , Rudoler, N. , Schwartz, D. , & Giladi, M. (2014). Bartonella dromedarii sp. nov. isolated from domesticated camels (Camelus dromedarius) in Israel. Vector Borne and Zoonotic Diseases, 14, 775–782. 10.1089/vbz.2014.1663 [DOI] [PubMed] [Google Scholar]
- Sui, J. , Li, W. , Murakami, A. , Tamin, A. , Matthews, L. J. , Wong, S. K. , … Marasco, W. A. (2004). Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proceedings of the National Academy of Sciences of the United States of America, 101, 2536–2541. 10.1073/pnas.0307140101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2018). Middle East Respiratory Syndrome Coronavirus (MERS‐CoV). Retrieved from http://www.who.int/emergencies/mers-cov/en/


