Abstract
This study examined correspondence between timing (onset) and tempo (rate) of sexual maturation prospectively (average ages 11-16 years) measured by gonadal hormones and secondary sex characteristics (Tanner Stage) using dual process models, and associations of these measures with substance use involvement in boys at age 16 years (N=534, 77.5% White/22.5% Non-White). All measures of timing were highly associated. Early Tanner Stage timing often predicted slower increases in gonadal steroids, but not the reverse; patterns varied by ethnicity. Hormone and Tanner Stage measures were similar earlier in development but diverged later in development. In White boys only, early timing of the pubertal rise in testosterone was associated with increased substance use involvement, suggesting a physiological rather than psychosocial mechanism of association.
Keywords: testosterone, DHT, pubertal timing, pubertal tempo, Tanner Stage
Sexual maturation is marked by a sharp increase in adrenal and gonadal hormones in conjunction with physical growth and emergence of secondary sex characteristics (e.g., breast, genital, and pubic hair development, acne, body hair, growth spurt, and voice changes) and differs dramatically for boys and girls (Dorn & Biro, 2011; Grumbach, 2002; Grumbach & Styne, 2003). Findings obtained in cross-sectional research document correspondence between hormone level and developmental status of secondary sex characteristics (e.g., assessing snapshots of a developmental process; Shirtcliff, Dahl, & Pollak, 2009). However, the strength of this association with respect to measures of timing (onset) and tempo (rate of maturation) that index the process of puberty has yet to be prospectively investigated. The present study takes a step toward filling that gap. Understanding the correspondence between timing and tempo of puberty as measured via hormone and physical development measures will help to clarify the mechanisms of associations with behavior, as well as clarify discrepant effects in the literature. We focus specifically on boys, as there remains a dearth of studies on the pubertal process in boys.
Because puberty encompasses not only neuroendocrine changes but also observable physical changes and accompanying social changes, associations of puberty with behavioral and mental health phenotypes may be driven by psychosocial and/or physiological mechanisms, or by confounding factors (Mendle & Ferrero, 2012). The present study is based on the premise that the measurement of the process of puberty (e.g., specifying the measures used to model key components of puberty, including timing and tempo) can help to clarify our empirical and conceptual understanding of the mechanism of associations of puberty with mental health and behavior. Here, we focus on associations of puberty with substance use, because there is theoretical and empirical work linking timing of puberty with substance use in boys (Mendle & Ferrero, 2012), and more recently, linking tempo of puberty with substance use in boys (Castellanos-Ryan, Parent, Vitaro, Tremblay, & Seguin, 2013; Dick, Rose, Pulkkinen, & Kaprio, 2001; Marceau & Jackson, 2017).
Measurement of the Process of Puberty
The developmental process of puberty is often described in terms of timing and tempo. Assessment of the process of puberty (the way timing or tempo is defined) can vary widely across studies. Timing may refer to the age at entry to puberty or at a particular pubertal milestone (e.g., spermarche, growth peak, stage rating), or may refer to the relative development of one adolescent compared to peers or other participants in a study (Marceau, Ram, Houts, Grimm, & Susman, 2011). Tempo may refer to an estimate of a rate of change in development (e.g., stages per year) within a specific time frame (e.g., 10-18 years of age), or the speed of development assessed by the difference in stages between two ages (Marceau et al., 2011).
Similarly, there is variation in measurement of different puberty phenotypes (e.g., adrenarche, gonadarche) across studies (see Dorn & Biro, 2011; Dorn, Dahl, Woodward, & Biro, 2006 for detailed discussion). Adrenarche typically is the earliest phase of puberty, and is marked by rises in adrenal androgens (e.g., dehydroepiandrosterone [DHEA], and its sulfate DHEA-S, and androstenedione; Grumbach & Styne, 2003). The beginnings of adrenarche are best observed by measuring changes in adrenal androgens (Dorn & Biro, 2011). Once the concentrations of adrenal androgens are high enough, they contribute to some of the visible changes of puberty, at which time adrenal development can be measured by observations of body odor and axillary and pubic hair development (secondary sex characteristics; Dorn & Biro, 2011). Gonadarche often begins slightly after adrenarche (Grumbach & Styne, 2003), and is marked by increases in gonadal steroids (e.g., testosterone, dihydrotestosterone) and accompanying changes in other secondary sex characteristics (e.g., genital development; Dorn & Biro, 2011). In sum, adrenal and gonadal development can each be assessed using particular hormone concentrations or secondary sex characteristics.
Finally, the method of measurement (e.g., reporter or observation) can also vary widely across studies, and each measurement method has unique strengths and limitations (Dorn et al., 2006). Self-report measures are easy to collect with minimal burden (Dorn & Biro, 2011) and encompass each individual’s biases and knowledge (or lack thereof) of the whole of pubertal development as well as their expectations, and are influenced by the references with whom they compare themselves (e.g., peers, drawings given with the assessment, adults; Mendle, 2014). Self-report measures can be used to directly assess how youth view themselves relative to peers (e.g., Moore, Harden, & Mendle, 2014), or to create measures of timing and tempo through either repeated measures (e.g., Marceau & Jackson, 2017) or comparisons among a sample (e.g., Dick et al., 2001; Harden & Mendle, 2012). Clinician (e.g., nurse, physician) reports of secondary sex characteristics are considered more valid and less biased for assessing physical changes (Dorn et al., 2006). However, clinician reports can also be influenced by other sources of variation (e.g., nutrition can affect skin changes; individuals may rate pubertal stages differently; Dorn et al., 2006). Finally, hormone concentrations assess the neuroendocrine changes that underlie puberty. However, the timing of hormone collection is incredibly important, due to cyclical changes in hormones and the fact that diverse environmental phenomena (e.g., toxins, stress) can affect the moment-to-moment production of pubertal hormones (Dorn & Biro, 2011). Together, the use of multiple measurement strategies should provide a more complete understanding of the course, causes, and consequences of puberty (Dorn & Biro, 2011; Dorn et al., 2006).
Correspondence among measures
In order to understand the similarities and differences in various assessments of puberty, the correspondence across measures must be understood (Shirtcliff et al., 2009). Cross-sectional studies have generally found moderate correspondence among different measures of self-report (e.g., Brooks-Gunn, Warren, Rosso, & Gargiulo, 1987), and across self- and nurse-reported data on secondary sex characteristics in boys (e.g., Coleman & Coleman, 2002; Jaruratanasirikul, Kreetapirom, Tassanakijpanich, & Sriplung, 2015; Terry et al., 2016; Shirtcliff et al., 2009). Fewer studies have investigated correspondence between nurse-reported secondary sex characteristics and hormone concentrations. In general, there are not specific hormone concentration ranges that can be mapped onto each stage of development across individuals (Dorn & Biro, 2011). However, hormone levels and secondary sex characteristic stages are related. For example, in a cross-sectional study, boys’ genital development stage predicted their testosterone levels, and pubic hair stage predicted both testosterone and DHEA levels (Shirtcliff et al., 2009). No studies have assessed the correspondence of measures of the process of puberty (timing and tempo) ascertained from repeated measures of different data sources. Thus, the first goal of this study was to examine how estimates of pubertal timing and tempo derived from repeated measures of nurse reported Tanner Stages correspond to estimates of timing and tempo derived from repeated hormone assessments as a first step in understanding similarities and differences in the process of puberty as measured via neuroendocrine and physical changes.
Puberty and Substance Use
Individual differences in the timing of puberty have been associated with physical and mental/behavioral health problems (e.g., Ullsperger & Nikolas, 2017). Reasons for this association that have been advanced usually focus on notions such as insufficient readiness or mismatch of early maturing youth to adapt to social demands (e.g., developmental readiness or maturational disparity hypotheses; Ge & Natsuaki, 2009; Mendle, Harden, Brooks-Gunn, & Graber, 2010). These hypotheses have generally been supported in literature examining earlier pubertal timing and internalizing and externalizing problems in boys, although some studies find no effects or the opposite effects (Mendle & Ferrero, 2012; Ullsperger & Nikolas, 2017).
Theoretically, developmental readiness/maturational disparity would predict associations of earlier timing of puberty with emotional and behavioral problems when puberty is measured both by hormone and secondary sex characteristic measures, as at the crux of this theory is a mismatch between physical and emotional development. An important component of the effects of this mismatch is others’ and self-expectations based on the appearance of maturity, which is observed in secondary sex characteristic changes. However, mismatches in the relative development of brain regions (e.g., the prefrontal cortex and limbic regions) across adolescence has also been observed to put youth at risk for substance use (Casey & Jones, 2010). Further, some (but not all) changes in specific brain regions are hormonally-mediated, particularly by testosterone in boys (Dahl & Forbes, 2010; Giedd et al., 2006; Spear, 2013). Thus, developmental readiness/maturational disparity is expected to occur both on a physiological and psychosocial level, and associations of pubertal timing and substance use are therefore hypothesized to be observed using both hormone and secondary sex characteristic measures.
Indeed, a large body of studies using a variety of measures of puberty (though most often self-report) has shown that earlier timing is consistently associated with more substance use and substance use problems in boys (Mendle & Ferrero, 2012; Ullsperger & Nikolas, 2017). Importantly, the early pubertal timing – substance use association has also been shown using hormone levels to index pubertal timing in boys (Dawes et al., 1999; de Water, Braams, Crone, & Peper, 2013). However, there are also several studies that show that later timing of secondary sex characteristic development is associated with substance use in boys (Hummel, Shelton, Heron, Moore, & van den Bree, 2013; Mendle & Ferrero, 2012). Later pubertal timing – substance use associations in boys are often explained by a compensation mechanism whereby less physically mature boys attempt to show they are older by engaging in more mature or risky behaviors, including substance use (Marceau, Abar, & Jackson, 2015). It should be noted that whereas the association of earlier timing and substance use in boys was confirmed, the association of later timing and substance use in boys was not found to be significant in a recent meta-analysis (Ullsperger & Nikolas, 2017).
In the case of faster tempo, various pubertal milestones are compressed into a shorter timeframe, theoretically exacerbating the effect of earlier timing (e.g., maturational compression hypothesis; Mendle et al., 2010). In contrast to the plethora of studies examining pubertal timing, few studies have examined pubertal tempo in relation to substance use. Faster tempo has been shown to be associated with more substance use problems in boys, as hypothesized by maturational compression (Castellanos-Ryan et al., 2013; Dick et al., 2001), but so has slower development (Marceau & Jackson, 2017). Explanations for associations of slower tempo with substance use parallel theories for timing: boys who appear younger for longer periods of time seek out activities that make them appear older in the eyes of their peers. A rigorous study examining correlates of tempo (estimated via linear and logistic growth models) reported no clear, consistent association of tempo with substance use problems in boys (Beltz, Corley, Bricker, Wadsworth, & Berenbaum, 2014). Thus, the few studies that have examined associations of pubertal tempo and substance use have produced mixed results. These studies each assess puberty using self-report measures, but on different time-scales (from two to seven repeated assessments, with the time between assessments ranging from six months to years). To our knowledge, no studies have examined pubertal tempo as assessed via hormone levels in relation to substance use. Based on the maturational compression hypothesis, faster puberty assessed via hormone changes is expected to be related to substance use via similar brain development mechanisms as described for early timing. That is, faster developing boys would experience a larger dose of testosterone more quickly, potentially leading to faster brain changes in regions influenced by testosterone relative to regions developing with respect to age or other non-pubertal developmental processes. This mismatch could put boys at risk for substance use.
Potential for confounding
The possibility remains that associations between puberty and substance use could arise because of pre-existing risk that is exacerbated by transitions (including off-time or off-tempo pubertal transitions). One class of pre-existing risk that may confound puberty-behavior associations are familial factors (which include genetics and rearing environmental influences) that contribute to both puberty and substance use phenotypes. Twin studies can be used to test whether genetic or familial environmental confounds explain the association of puberty and substance use in boys, although to our knowledge no such studies have been published. However, a recent study found that faster tempo of puberty (assessed via self-report) was related to an increased risk of substance use as indicated by family history of substance use disorder (Mathias et al., 2016; and there was no evidence of an association of tempo with boys’ substance use). This study provides evidence of familial confounding: pre-existing risk factors tapped by family history (e.g., genetics, rearing environment) both put boys at risk for substance use and affected the tempo of their pubertal development, and the association of tempo and substance use was therefore non-significant after accounting for familial confounds. Here, we include risk for substance use (operationalized as fathers having vs. not having a substance use disorder) as a covariate and potential moderator of puberty-substance use associations to partially address the potential of familial confounding accounting for puberty-substance use associations.
Clarifying mechanisms of association through measurement
As noted above, different measures of puberty may be better-suited to tap particular aspects of the pubertal process. For example, self-report measures, most commonly used in the literature, are likely to tap psychosocial mechanisms for associations of puberty and substance use. In contrast, assessing tempo via changes in hormone levels across years may more directly assess physiologically mediated associations of puberty with substance use. Assessing tempo via changes in clinician-rated secondary sex characteristics may index both physiological mechanisms and psychosocial mechanisms for associations with substance use. Therefore, we tested associations of pubertal timing and tempo from nurse reported Tanner Stages and hormone assessments with substance use involvement in order to examine whether associations were specific to a potentially physiological mechanism (e.g., if associations are found for the hormone assessment only), a psychosocial mechanism (e.g., if associations are found for the Tanner Stage assessment only), or a combination (e.g., if associations are found for both measurement strategies).
Present Study
As an initial step in addressing whether timing and tempo derived from Tanner Stages and gonadal steroids are associated with each other and differentially associated with substance use, we assessed timing and tempo in boys at the average ages of 11, 13 and 16 years via nurse reported Tanner Stages and the hormones testosterone and its active metabolite, dihydrotestosterone (DHT). DHT is synthesized from testosterone, and is more potent than testosterone on androgenic receptors, although there is generally more testosterone available to act on receptors (e.g., Grino, Griffin, & Wilson, 1990). We included both hormones as measures of gonadal steroids implicated in pubertal development, and expected results to replicate across hormones. In the current study, adrenal androgens were not available. Further, the age range of the sample was not appropriate for capturing the initial hormonal indications of adrenarche. Thus, here we focus on gonadal steroid hormones and secondary sex characteristic development, with pubic hair development capturing adrenal changes. We hypothesized 1) that the timing and tempo of puberty as assessed via Tanner Stages and gonadal steroids would be associated. We further hypothesized that 2) earlier timing of puberty would be related to more substance use involvement at age 16 years, as assessed by both Tanner Stages and gonadal steroids, and 3) faster tempo of puberty would be related to more substance use involvement at age 16 years, as assessed by both Tanner Stages and gonadal steroids. This pattern of findings would be consistent with both physiological and psychosocial mechanisms of associations of the process of puberty with substance use outcomes.
The sample was drawn from the Center for Education and Drug Abuse Research (CEDAR), and is 71% male. We excluded girls because even though there were measures of estradiol, follicle-stimulating, and luteinizing hormone collected in CEDAR, there was no reliable data on menstrual cycle phase, or whether girls were using oral contraceptives at the time of collection. Therefore, these data were considered to be too unreliable for use in the current study. We studied boys in particular because there continues to be fewer studies of boys’ puberty than girls’. Further, gonadal hormone changes associated with puberty outlast secondary sex characteristic development (Braams, van Duijvenvoorde, Peper, & Crone, 2015), increasing well past age 16 years in boys. Thus, the ages of this sample were appropriate for examining gonadal hormone and secondary sex characteristic development in boys in particular.
Because of recent evidence that boys at risk for substance use may experience accelerated puberty (Mathias et al., 2016), we also examined study hypotheses separately for boys at high and low risk for substance use problems, as indexed by having fathers with and without substance use disorders, respectively. Finally, due to established ethnicity differences in the timing and tempo of puberty (e.g., Susman et al., 2010), we also examined potential differences in study hypotheses by ethnicity. This analysis was entirely exploratory: we were unable to formulate specific hypotheses about potential ethnic differences in associations across measures of puberty or puberty-substance use associations in boys as they have not been explored thus far (e.g., Mendle & Ferrero, 2012, and our review of the literature).
Method
Participants
The sample was drawn from CEDAR, a northern US-based study following 775 families (549 families of boys) longitudinally (Tarter & Vanyukov, 2001). Biological sons (age 11 years, on average) of men with a SU disorder (n = 249), psychiatric (non-SU) disorder (n = 50), or no disorder (n = 250) were included in the present study. The men (fathers) were identified using random digit telephone dialing, advertisement and public service announcements from 1989 – 2009. Approximately 20% of the men with substance use disorder were identified while in treatment for alcohol or drug dependence. Their sons were excluded from the study if any of the following were present: 1) teratological injury indicated by physical anomalies in conjunction with mother’s report of alcohol/drug use during pregnancy, 2) disabling chronic medical or psychiatric illness, 3) history of neurological injury requiring hospitalization, and 4) WISC-III-R full scale IQ lower than 80. Further detail on the CEDAR sample can be found at http://www.pitt.edu/~cedar/design.html.
Data on from the initial three assessments were used in the current study (initial visits occurring between 1990 and 2008): Visit 1 average age 11 years (9.42 - 13.39 years; n = 549); Visit 2, average age 13 years (11.29 - 15.66; n = 469); Visit 3, average age 16 years (15.51 - 17.82; n = 448). The sample was studied for a total of 20 years: after the first three visits used here, they had annual evaluations yearly from ~ age 19 until ~ age 30 years. Several previous reports have examined the role of puberty development on risk for SUD; however, this was the first study directed at examining timing and rate of sexual maturation via neuroendocrine and physical indicators and their independent associations with substance use involvement. The analytic sample (n = 534) excluded boys who were missing all Tanner Stage and hormone assessments.
Demographics
Boys in the analytic sample were primarily Caucasian (77.5%), with some Black or African American (19.5%) and bi/multi-racial participants (3%), largely from middle-upper middle class families. Most (85%) were in families where mothers and fathers were married. Most mothers and fathers had either a high school/GED (mothers: 31%; fathers: 26%), partial college (mothers: 38%, fathers: 34%), college (mothers: 17%, fathers: 19%), or a graduate or professional degree (mothers: 7%, fathers: 11%). Parents’ employment varied: 38% of mothers and 20% of fathers were never or not currently employed, 35% of mothers and 76% of fathers were employed full-time, and 27% of mothers and 4% of fathers were employed part-time. The most common maternal occupations were homemaker/student (22%, Hollingshead rating 0), clerical & sales workers/small farm & business owners (15%, Hollingshead rating 5), technicians/small business owners/semiprofessionals (15%, Hollingshead rating 6), and smaller business owners/farm owner/manager/minor professionals (10%, Hollingshead rating 7). The most common paternal occupations were skilled manual workers/craftsmen & tenant farmers (22%, Hollingshead rating 4), technicians/small business owners/semiprofessionals (15%, Hollingshead rating 6), smaller business owners/farm owner/manager/minor professionals (15%, Hollingshead rating 7), machine operators/semiskilled workers (13%, Hollingshead rating 3), and unskilled workers (11%, Hollingshead rating 2).
Procedure
Upon arrival at the laboratory between 8:00 and 9:00am, the boys and their parents were oriented to the facility. Next, informed consent was obtained from at least one parent and written assent was obtained from the boys. Breath alcohol and urine drug screens were conducted before the research protocol to ensure that the data were not biased by substance-induced altered physiological state. Tanner Stage was determined by a male nurse employing standard criteria (described below). The blood sample, obtained by a phlebotomist usually within a half hour of arrival, was assayed in the research laboratory at the CEDAR. After completing the evaluations, the participants were debriefed and compensated at the rate of $10/hour. All procedures were approved by the University of Pittsburgh IRB.
Measures
Gonadal Steroids
Plasma testosterone and DHT were ascertained via blood from boys at each visit, at approximately 8:30am (between 8:00 and 9:00am), and were assayed using Amersham’s testosterone/DHT radioimmunoassay (RIA) kit. The Amersham RIA kit employed tritiated DHT and an antibody specific to testosterone and DHT (with 45-50% cross-reactivity between testosterone and DHT). The concentration of testosterone+DHT and the concentration of DHT were determined separately through this procedure. The testosterone concentration was estimated from the difference between the testosterone+DHT and the DHT after accounting for the cross-reactivity. The intra-assay coefficient of variation for testosterone was 5.2 at 356 pg/mL and for DHT was 5.0 at 393 pg/mL. The intra-assay precisions for testosterone were 5.2 and 5.0% CV at 356 pg/mL (SD = 12.3 pg/mL) and 393 pg/mL (SD = 13.0 pg/mL), respectively. The inter-assay precisions for testosterone and DHT were 10.8 and 9.4% CV at 347 pg/mL (SD =25.0 pg/mL) and 396 pg/mL (SD = 24.9 pg/mL), respectively (Dawes et al., 1999; Kirillova et al., 2001). For both testosterone and DHT, outliers past 3SD of the sample mean were windsorized (e.g., their hormone values were changed to be equal to 3SD of the sample mean). Descriptive statistics of testosterone and DHT showed substantial skew at visit 1 (testosterone: 2.11; DHT: 2.09), and less dramatic, but positive skew for the other visits (skewness = .73-.87). Kolmogorov-Smirnov tests suggested significant skewness (D > .07, p < .05) for all hormone variables with the exception of age 16 DHT, for which the p-value was .07. To attenuate skew and maintain comparability over time, all scores were then log transformed. This reduced skew such that all assessments of testosterone and DHT were within acceptable limits (skewness = −.63−.96).
Tanner Stages
Tanner Staging was conducted by a nurse at each visit. This method is generally considered the “gold standard” in assessment of visible changes in secondary sex characteristics marking pubertal development (Dorn & Biro, 2011). Nurses circled a single stage at each wave. Stages of genital development were 1) pre-pubertal; 2) enlargement of scrotum and testes; 3) enlargement in length of penis; further growth of testes; 4) increase in breadth of penis; development of glans; scrotal skin darker; further growth of testes; 5) adult. Stages of pubic hair development were 1) there is not pubic hair; 2) there is sparse growth of long slightly pigmented, downy hair, straight or only slightly curled, primarily at the base of the penis; 3) the hair is considerably darker, coarser, and more curled. The hair spreads sparsely over the junction of the pubes; 4) the hair, now adult in type, covers a smaller area than in the adult; 5) the hair is adult in quantity and type.
Substance Use Involvement
To assess boys’ substance use involvement at age 16 years, the Drug Use Chart (CEDAR, 1989) was used to assess drug exposure of 42 psychoactive substances grouped into ten broad categories similar to National Institute of Mental Health Epidemiologic Catchment Area Study (Anthony & Helzer, 1991). The drug categories were: Alcohol (beer, wine, liquor), cannabis (marijuana), Cocaine/crack, opiates (heroin, codeine, Demerol, morphine, methadone, opium), amphetamines, and methylphenidate (Ritalin), sedatives (barbiturates, quaaludes, Seconal, Xanax, Librium, Valium, etc.), tobacco (smoking tobacco, chewing tobacco, snuff tobacco), hallucinogens (LSD, mescaline, peyote, etc.), PCP and inhalants (amyl nitrate, nitrous oxide, glue, gasoline, etc.). Indicator (1 = yes, 0 = no) variables identifying whether the participant had ever tried a drug in each category were used in item response theory (IRT) models to index substance use involvement. Specifically, all ten drug categories were scaled as indicators of a unidimensional substance use involvement index using a two-parameter logistic item response theory model (Kirisci, Vanyukov, Dunn, & Tarter, 2002). The two-parameter IRT model fit the data better than a one-parameter model, χ2change = 5.26, (df)change = 1, p = .02. Thus, as has been previously found in the CEDAR data (e.g., for parents and youth at age 19 years, Kirisci, Tarter, Reynolds, & Vanyukov, 2006) the two-parameter model was used to estimate item parameters and latent trait scores. The IRT-based reliability coefficient of the measure was calculated with the equation: where is the observed scale score variance and is the average measurement error of variance across levels of the latent substance use severity trait. The IRT based substance use involvement score was reliable, = .84.
Analysis
Preliminary analysis
We conducted a series of preliminary analyses, including descriptive statistics and correlations among study variables, and t-tests to examine potential differences in study variables across risk groups. Next, using Mplus v7.4 (Muthén & Muthén, 2010), we conducted correlations between hormone and secondary sex characteristic measures at each assessment and tested whether the magnitude of correlations among hormone and secondary sex characteristic measures differed across assessments. Specifically, we first tested a model wherein each correlation (testosterone with genital development, testosterone with pubic hair development, DHT with genital development, DHT with pubic hair development) was freely estimated at each wave. Then, we systematically constrained each correlation at visit 1 and 2 to be equal, at visit 2 and 3 to be equal, and at visit 1 and 3 to be equal. A significant decrement in fit according to a chi-square test would indicate significant differences in the magnitude of correlations. Finally, we assessed correlations of each measure of puberty (testosterone, DHT, genital and pubic hair development) at each wave with substance use involvement at age 16 years.
Hypothesis testing
In order to test our hypotheses, we conducted a series of dual-process models in Mplus, using full information maximum likelihood to accommodate missing data. Specifically, to test hypothesis (1), we simultaneously fit linear growth curves of hormone and secondary sex characteristic measures using exact age to index time, and assessed correlations across measures of timing and tempo as well as cross-paths of timing assessed by one measure predicting tempo of development assessed by the other (e.g., with TYPE=RANDOM). Age was centered at the youngest age in the sample (9.42 years), so the intercept (e.g., timing) reflected the estimated gonadal steroid level and Tanner Stage at 9.42 years based on each individual’s trajectory of development. Tempo was quantified as the slope of linear change over time for each individual (e.g., in terms of the Tanner Stages the boy progressed through per year). A series of four dual-process models were fit: 1) testosterone with genital development; 2) testosterone with pubic hair development; 3) DHT with genital development, and 4) DHT with pubic hair development. For each set of analyses, we started with a Base Model estimating latent timing and tempo scores for the measure of hormone and secondary sex characteristics, as well as all six possible correlations among the four latent variables (e.g., timing with tempo within the [1] gonadal steroid and [2] Tanner Stage measures; gonadal steroid timing with Tanner Stage [3] timing and [4] tempo; Tanner Stage timing with gonadal steroid [5] timing and [6] tempo). We then used a nested approach to model fitting, dropping pairs of paths: a) removing correlations of timing with tempo within measure, b) removing correlations of timing across measures and tempo across measures, c) removing cross-correlations of timing from one measure with tempo from the other. A significant decrement in model fit according to a chi-square test of the difference in loglikelihood considering scaling corrections and accompanying degrees of freedom (e.g., see https://www.statmodel.com/chidiff.shtml) would indicate the importance of including those paths in the model. Models with the fewest number of estimated correlations without a decrement in model fit was considered the best-fitting.
In order to test hypotheses (2) and (3), we then added substance use involvement at age 16 as a correlate of the latent timing and tempo factors from the best-fitting model from the previous step (hereafter referred to as Substance Use Risk models). We fit Substance Use Risk models in a two-group framework, testing the moderating role of risk for substance use (boys whose fathers had SUD vs. control). We systematically tested whether means, variances, and correlations among latent timing and tempo factors varied as a function of group, and whether correlations of timing and tempo with substance use involvement varied as a function of group. These tests were carried out by first estimating a model wherein these parameters were allowed to vary across groups, and then estimating a nested model wherein the parameters were constrained to be equal across groups. We again calculated difference tests based on loglikelihood values and scaling corrections; a significant decrement in model fit when constraining parameters to be equal across group indicated significant group differences. The best-fitting model from the Substance Use Risk analysis (e.g., either constraining groups of boys whose fathers has SUD vs. control to have the same estimated parameters or allowing them to differ) was used in the final step.
Finally, to address our exploratory aim, we conducted a second two-group model testing the moderating role of ethnicity (White vs. Non-White [African American and bi/multi-racial groups were combined because the bi/multi-racial group was so small], hereafter referred to as the Ethnicity Differences models). As in the Substance Use Risk models, we systematically tested whether means, variances, and correlations among latent timing and tempo factors varied as a function of group, and whether correlations of timing and tempo with substance use involvement varied as a function of group using the nested model approach. We again calculated difference tests based on loglikelihood values and scaling corrections; a significant decrement in model fit when constraining parameters to be equal across group indicated significant group differences. Model fit statistics for each step are presented in the results section. Only the final, best-fitting models are presented in detail (e.g., Figures).
Results
Preliminary Analysis
We began by investigating the distributions of pubertal maturation in the sample. As shown in Table 1, most boys (60%) were in the initial, pre-pubertal stages of genital and pubic hair development at the first assessment. Boys on average increased in Tanner Stage across the visits, as expected, with a majority of boys achieving Tanner Stage 4 (60%) by age 16. It is notable that a substantial proportion of the sample (20%) had only achieved Tanner Stage 3 by age 16. As expected, testosterone and DHT levels also rose across the three visits. The distributions support examining the process of puberty in this sample of boys. T-tests assessing differences between groups (tested both in terms of SUD vs. psychiatric disorder + control fathers, t(164 to 470) = −1.52 to 1.24, p’s > .13, and SUD + psychiatric disorder vs. control fathers, t(164 to 470) = −1.96 to 1.39, p’s > .051) showed no differences in hormone or secondary sex characteristic measures at any wave, or in substance use involvement at age 16. T-tests assessing differences in ethnicity showed differences in pubertal development: non-White boys had more advanced genital and pubic hair development and DHT levels at the first and second assessments and testosterone levels at the first assessment than White boys t(254 to 470) = −2.44 to −4.90, p’s < .05; White and non-White boys did not differ in any measure of puberty at age 16 or testosterone levels at age 13, t(164 to 254) = −0.60 to −1.55, p’s > .12; and White boys had higher substance use involvement at age 16 than non-White boys, t(449) = 2.21, p = .03.
Table 1.
Sample Descriptive Statistics
| Visit 1 | Visit 2 | Visit 3 | ||
|---|---|---|---|---|
| Age | M (SD) | 11.36 (0.92) | 13.41 (0.98) | 16.07 (0.47) |
| [min - max] | [9.42 - 13.39] | [11.29 - 15.66] | [15.51 - 17.82] | |
| Testosterone (ng/ml) | M (SD) | 1204.72 (1470.82) | 3135.41 (2282.42) | 6890.14 (2429.26) |
| [min - max] | [143.00 – 5515.16] | [242.00 – 9959.72] | [1125.00 – 14284.03] | |
| Dihydroxytestosterone (pg/mL) | M (SD) | 250.62 (174.24) | 535.84 (258.58) | 1066.18 (282.02) |
| [min - max] | [56.00 – 786.27] | [90.00 – 1346.97] | [575.00 – 1936.78] | |
| Genital Development | M (SD) | 1.59 (0.83) | 2.82 (1.18) | 3.98 (0.65) |
| Tanner Stage 1 | N (%) | 284 (59.79%) | 57 (15.83%) | 0 |
| Tanner Stage 2 | N (%) | 120 (25.26%) | 88 (24.44%) | 2 (0.58%) |
| Tanner Stage 3 | N (%) | 57 (12%) | 108 (30%) | 68 (19.65%) |
| Tanner Stage 4 | N (%) | 12 (2.53%) | 77 (21.39%) | 205 (59.25%) |
| Tanner Stage 5 | N (%) | 2 (0.42%) | 30 (8.33%) | 74 (20.52%) |
| Pubic Hair Development | M (SD) | 1.75 (0.87) | 2.90 (1.16) | 4.07 (0.66) |
| Tanner Stage 1 | N (%) | 285 (60.13%) | 71 (19.56%) | 1 (0.29%) |
| Tanner Stage 2 | N (%) | 141 (29.75%) | 102 (28.10%) | 2 (0.58%) |
| Tanner Stage 3 | N (%) | 39 (8.23%) | 93 (25.62%) | 71 (20.58%) |
| Tanner Stage 4 | N (%) | 9 (1.9%) | 85 (23.42%) | 208 (60.29%) |
| Tanner Stage 5 | N (%) | 0 | 12 (3.31%) | 63 (18.26%) |
Correlations among study variables are presented in Table 2. Here, we focus on correlations of gonadal steroids with Tanner Stages, and of each with substance use involvement. Analysis of differences in magnitudes across correlations revealed significant differences in every case, χ2 change (1) > 20.33, p < .001. In all cases, the correlation between the gonadal steroid level and secondary sex characteristic stage decreased with age – to non-significance at age 16 years, with the exception of DHT-pubic hair correlations which remained significant though small at age 16 years. Regarding correlations with substance use involvement, higher testosterone, DHT, and genital development stage at age 13 years were each correlated with increased substance use involvement at age 16 years. There was a trend such that age 16 testosterone was nearly concurrently correlated with increased substance use involvement (p = .08). No other point-estimates of gonadal steroid or Tanner Stage was correlated with substance use involvement.
Table 2.
Associations among Study Variables
| Testosterone | Dihydrotestosterone (DHT) | Genital Development Tanner Stage | Pubic Hair Tanner Stage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Age 11 | Age 13 | Age 16 | Age 11 | Age 13 | Age 16 | Age 11 | Age 13 | Age 16 | Age 11 | Age 13 | Age 16 | ||
|
|
|||||||||||||
| Testosterone | Age 11 | 1 | |||||||||||
| 398 | |||||||||||||
| Age 13 | 0.52* | 1 | |||||||||||
| 231 | 256 | ||||||||||||
| Age 16 | 0.25* | 0.34* | 1 | ||||||||||
| 142 | 147 | ||||||||||||
| DHT | Age 11 | 0.90* | 0.47* | 0.26* | 1 | ||||||||
| 356 | 194 | 110 | 356 | ||||||||||
| Age 13 | 0.44* | 0.83* | 0.28* | 0.47* | 1 | ||||||||
| 231 | 256 | 147 | 194 | 256 | |||||||||
| Age 16 | 0.21* | 0.27* | 0.68* | 0.25* | 0.31* | 1 | |||||||
| 142 | 147 | 166 | 110 | 147 | 166 | ||||||||
| Genital Development Tanner Stage | Age 11 | 0.66* | 0.39* | 0.26* | 0.64* | 0.28* | 0.20* | 1 | |||||
| 348 | 246 | 162 | 308 | 246 | 162 | 472 | |||||||
| Age 13 | 0.54* | 0.49* | 0.31* | 0.47* | 0.37* | 0.23* | 0.60* | 1 | |||||
| 289 | 225 | 145 | 252 | 225 | 145 | 321 | 357 | ||||||
| Age 16 | 0.13* | 0.16* | 0.15 | 0.12 | 0.17* | 0.10 | 0.14* | 0.14* | 1 | ||||
| 274 | 199 | 149 | 244 | 199 | 149 | 308 | 275 | 346 | |||||
| Pubic Hair Development Tanner Stage | Age 11 | 0.57* | 0.25* | 0.18* | 0.57* | 0.16* | 0.16* | 0.81* | 0.48* | 0.10 | 1 | ||
| 346 | 246 | 163 | 305 | 246 | 163 | 469 | 320 | 306 | 471 | ||||
| Age 13 | 0.56* | 0.51* | 0.20* | 0.50* | 0.36* | 0.12 | 0.59* | 0.90* | 0.15* | 0.53* | 1 | ||
| 293 | 226 | 145 | 256 | 226 | 145 | 323 | 356 | 277 | 322 | 360 | |||
| Age 16 | 0.16* | 0.21* | 0.13 | 0.14* | 0.20* | 0.16* | 0.19* | 0.20* | 0.85* | 0.16* | 0.22* | 1 | |
| 273 | 198 | 149 | 243 | 198 | 149 | 307 | 274 | 345 | 305 | 276 | 345 | ||
| Substance Use Involvement Age 16 | .04 | .17* | .14 | −.03 | .13* | .03 | .01 | .14* | .04 | −.01 | −.01 | −.0001 | |
| 335 | 239 | 166 | 299 | 239 | 166 | 392 | 330 | 346 | 390 | 332 | 345 | ||
Note.
p < .05. Pearson’s r values are provided, with the N with available data for the association presented below. Emboldened numbers are of particular interest and discussed in text.
Hypothesis Testing
Model Fitting
Model fitting results for all four series of analyses are presented together, as the model selection was highly consistent. Model-fitting results from the Base Model indicated all associations among the latent timing and tempo factors should be retained in all four main analyses (See Table 3, Base Model, for model fit statistics). Thus, the full Base Models were retained as best-fitting in the first step.
Table 3.
Model Fit Statistics
| Testosterone and Genital Development | Testosterone and Pubic Hair Development | DHT and Genital Development | DHT and Pubic Hair Development | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| LL (df) |
Scaling factor | χ2change (df) |
LL (df) |
Scaling factor | χ2change (df) |
LL (df) |
Scaling factor | χ2change (df) |
LL (df) |
Scaling factor | χ2change (df) |
|
| Base Model Full | −1070.15 (20) |
1.34 | −1039.16 (20) |
0.99 | −861.33 (20) |
1.21 | −822.88 (20) |
0.97 | ||||
| a) | −1094.19 (18) |
1.13 | 14.75* (2) |
−1051.53 (18) |
0.98 | 24.61* (2) |
−874.29 (18) |
1.02 | 8.76* (2) |
−828.86 (18) |
0.95 | 10.91* (2) |
| b) | −1100.62 (18) |
1.28 | 27.07* (2) |
−1052.50 (18) |
0.99 | 27.54* (2) |
−844.27 (18) |
1.28 | −56.02* (2) |
−834.77 (18) |
0.85 | 12.06* (2) |
| c) | −2185.90 (18) |
1.27 | 52.03* (2) |
−1046.01 (18) |
0.97 | 12.33* (2) |
−870.84 (18) |
1.34 | 1259.74* (2) |
−927.20 (18) |
0.97 | 9.10* (2) |
| Substance Use Risk | ||||||||||||
| Full | −2631.14 (52) |
1.77 | −2595.82 (52) |
1.53 | −2274.04 (52) |
1.67 | −2228.83 (52) |
1.52 | ||||
| Constrained | −2641.47 (34) |
1.87 | 13.04 (18) |
−2605.39 (34) |
1.84 | 18.40 (18) |
−2283.43 (34) |
1.84 | 13.76 (18) |
−2237.52 (34) |
1.78 | 17.16 (18) |
| Ethnicity Differences | ||||||||||||
| Full | −2798.40 (52) |
1.48 | −2761.46 (52) |
1.35 | −2398.96 (52) |
1.43 | −2350.55 (52) |
1.55 | ||||
| Constrained | −2815.31 (34) |
1.66 | 29.75* (18) |
−2787.88 (34) |
1.56 | 56.23* (18) |
−2418.21 (34) |
1.60 | 34.27* (18) |
−2379.42 (34) |
1.55 | 37.31* (18) |
Note.
p < .05. LL = LogLikelihood value. df = degrees of freedom.
We then conducted the Substance Use Risk analysis by adding substance use involvement into the models, predicted by the latent timing and tempo variables. First, we estimated all parameters (means, variances, correlations) separately for high risk and control groups (Table 3, Substance Use Risk: Full), and then we constrained all parameters across substance use risk groups (Table 3, Substance Use Risk: Constrained). All parameters could be constrained across groups, revealing no substance use risk group differences in timing, tempo, or correlations across measures in all four main analyses. Thus, the constrained models were retained as best-fitting for the final step.
Finally, we conducted the Ethnicity Differences analysis, by first estimating all parameters separately for White and Non-White boys (Table 3, Ethnicity Differences: Full), and then constraining all parameters across ethnicity groups (Table 3, Ethnicity Differences: Constrained). We found that we could not constrain parameters across ethnicity in any of the four main analyses. However, because of measurement invariance (e.g., means and variances could not be constrained across groups) we could not formally test whether there were differences among correlations across ethnicity groups. Thus, the final models for each main set of analyses allowed for differences in ethnicity across model parameters. Unstandardized parameter estimates and standard errors for the final models are included in Figures (Figure 1: testosterone and genital development; Figure 2: testosterone and pubic hair development; Figure 3: DHT and genital development; Figure 4: DHT and pubic hair development).
Figure 1.
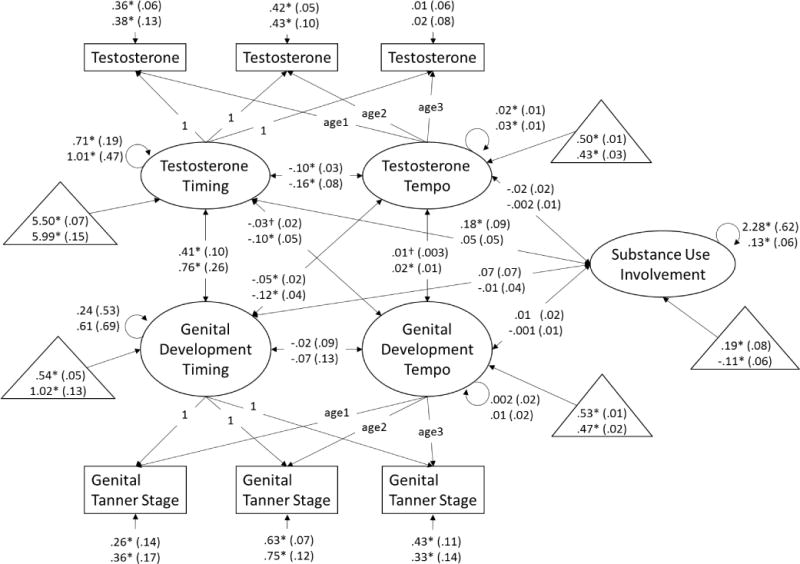
Dual process model of testosterone and genital development and associations with substance use involvement at age 16 years. Age1 is a variable containing each boys’ exact age at visit 1, age2 is a variable containing each boys’ exact age at visit 2, and age3 is a variable containing each boys’ exact age at visit 3. Unstandardized beta-weights are presented with standard errors in parentheses. Results for White boys appear above results for non-White boys. Estimated means for measures of timing (estimated log transformed hormone level or stage at 9.42 years) and tempo (change in log transformed hormone levels or number of stages per 2-year interval) and substance use (where 0 = the full sample mean) are presented in triangles. * p < .05, † p < .10.
Figure 2.
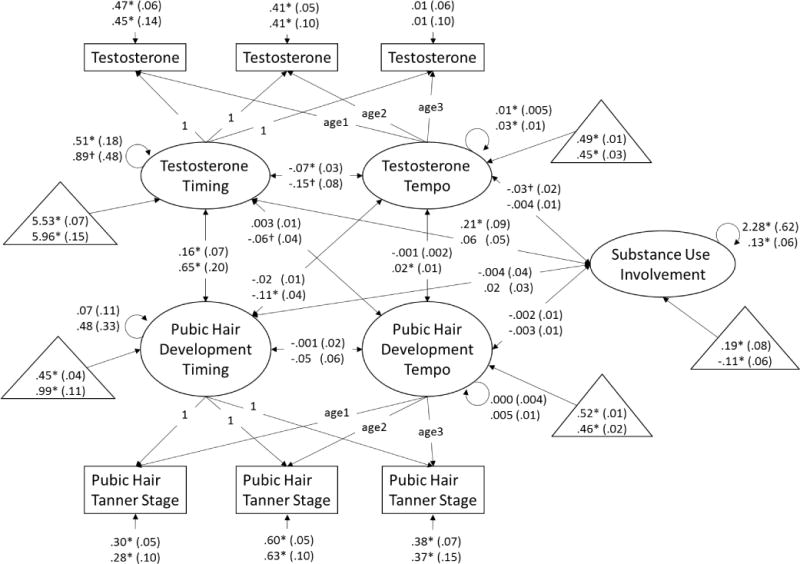
Dual process model of testosterone and pubic hair development and associations with substance use involvement at age 16 years. Age1 is a variable containing each boys’ exact age at visit 1, age2 is a variable containing each boys’ exact age at visit 2, and age3 is a variable containing each boys’ exact age at visit 3. Unstandardized beta-weights are presented with standard errors in parentheses. Results for White boys appear above results for non-White boys. Estimated means for measures of timing (estimated log transformed hormone level or stage at 9.42 years) and tempo (change in log transformed hormone levels or number of stages per 2-year interval) and substance use (where 0 = the full sample mean) are presented in triangles. * p < .05, † p < .10.
Figure 3.
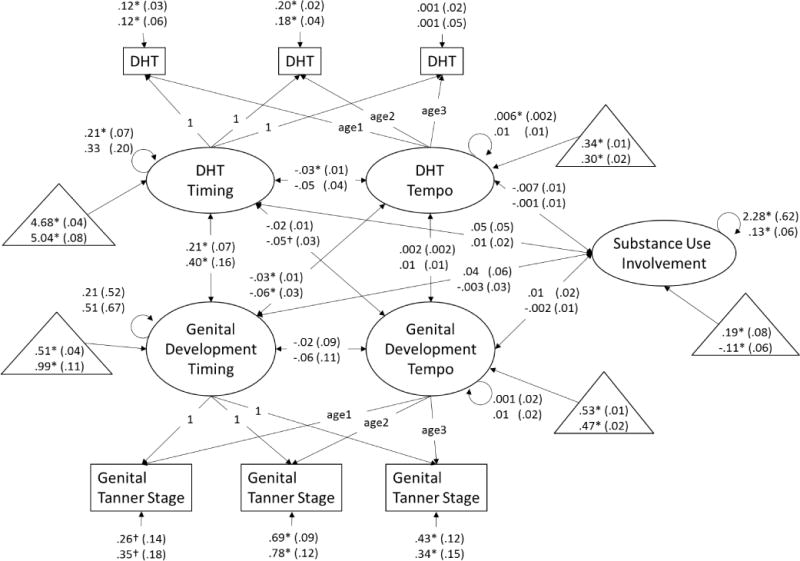
Dual process model of dihydrotestosterone and genital development and associations with substance use involvement at age 16 years. Age1 is a variable containing each boys’ exact age at visit 1, age2 is a variable containing each boys’ exact age at visit 2, and age3 is a variable containing each boys’ exact age at visit 3. Unstandardized beta-weights are presented with standard errors in parentheses. Results for White boys appear above results for non-White boys. Estimated means for measures of timing (estimated log transformed hormone level or stage at 9.42 years) and tempo (change in log transformed hormone levels or number of stages per 2-year interval) and substance use (where 0 = the full sample mean) are presented in triangles. * p < .05, † p < .10.
Figure 4.
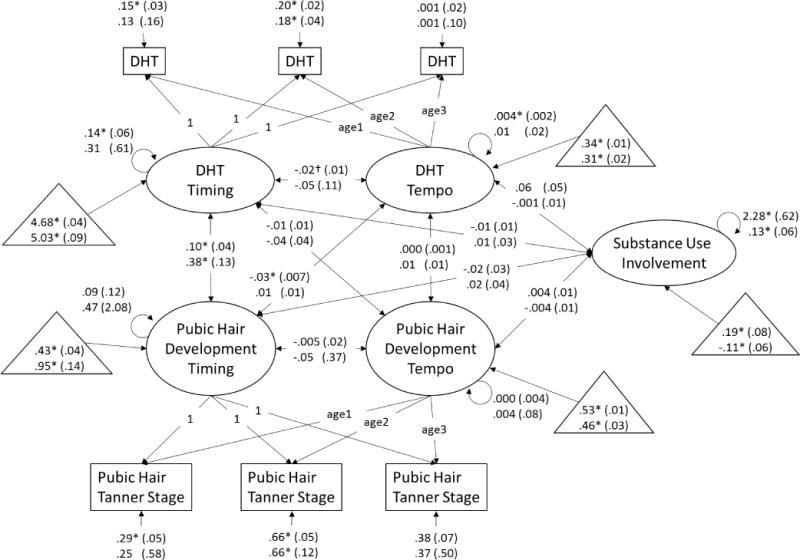
Dual process model of dihydrotestosterone and pubic hair development and associations with substance use involvement at age 16 years. Age1 is a variable containing each boys’ exact age at visit 1, age2 is a variable containing each boys’ exact age at visit 2, and age3 is a variable containing each boys’ exact age at visit 3. Unstandardized beta-weights are presented with standard errors in parentheses. Results for White boys appear above results for non-White boys. Estimated means for measures of timing (estimated log transformed hormone level or stage at 9.42 years) and tempo (change in log transformed hormone levels or number of stages per 2-year interval) and substance use (where 0 = the full sample mean) are presented in triangles. * p < .05, † p < .10.
Associations among measures of timing
Earlier pubertal timing as measured via testosterone (e.g., higher initial testosterone level) was correlated with earlier genital development timing in White and non-White boys (Figure 1). Earlier pubertal timing as measured via testosterone was correlated with earlier pubic hair development timing in White and non-White boys (Figure 2). Paralleling findings from the models including testosterone, earlier pubertal timing as measured via DHT (e.g., higher initial DHT level) was correlated with earlier genital development timing in White and non-White boys (Figure 3). Consistent with all previous models, earlier pubertal timing as measured via DHT was correlated with earlier pubic hair development timing in White and non-White boys (Figure 4). Thus, hypothesis 1 was confirmed for pubertal timing in all models, for White and non-White boys.
Associations among measures of tempo
Faster pubertal tempo as measured by changes in testosterone (e.g., greater testosterone rise over the course of the study) was correlated with faster genital development tempo in non-White boys, and was correlated at the trend-level for White boys (Figure 1). Similarly, faster pubertal tempo as measured by changes in testosterone was correlated with faster pubic hair development tempo in non-White boys, but not White boys (Figure 2). However, faster pubertal tempo as measured by changes in DHT (e.g., greater DHT rise over the course of the study) was not correlated with faster genital development tempo (Figure 3) or pubic hair development tempo for White or non-White boys (Figure 4). Thus, hypothesis 1 was only partially confirmed for pubertal tempo: only in models of testosterone with secondary sex characteristics, and differing for White and non-White boys.
Associations of timing and tempo of gonadal steroids
Earlier timing of testosterone development was correlated with slower tempo of testosterone development in White and non-White boys (Figure 1), though this association only reached trend-level for non-White boys in the model including pubic hair development (Figure 2). Earlier timing of DHT development was correlated with slower tempo of DHT development in White boys in the model including genital development (Figure 3). However, this association only reached trend-level for White boys in the model including pubic hair development (Figure 4). Timing of DHT development was uncorrelated with tempo of DHT development for non-White boys (Figures 3, 4).
Associations of timing and tempo of secondary sex characteristics
Timing and tempo of genital development were not correlated in either White or non-White boys (Figures 1, 3), nor was there was a correlation of timing with tempo of pubic hair development in White or non-White boys (Figures 2, 4).
Associations of gonadal steroid timing with secondary sex characteristic tempo
Earlier testosterone timing was correlated with slower genital development tempo in non-White boys and at trend-level (p ≤ .10) in White boys (Figure 1). Testosterone timing was not correlated with pubic hair development tempo in White or non-White boys (Figure 2). For White and non-White boys, DHT timing was uncorrelated with genital development tempo (Figure 3). For White and non-White boys, DHT timing was also uncorrelated with pubic hair development tempo (Figure 4).
Associations of secondary sex characteristic timing with gonadal steroid tempo
Earlier genital development timing was correlated with slower testosterone development tempo in White and non-White boys (Figure 1). Earlier pubic hair development timing was correlated with slower testosterone development tempo in non-White but not White boys (Figure 2). Earlier genital development timing was correlated with slower DHT development tempo in White and non-White boys (Figure 3). Earlier pubic hair development timing was correlated with slower DHT development tempo in White but not non-White boys (Figure 4).
Associations with substance use involvement
Partially confirming hypothesis (2), earlier timing of testosterone development was correlated with substance use involvement for White boys but not non-White boys (Figures 1, 2). There were no other correlations of timing or tempo of puberty with substance use involvement in any models (Figures 1–4), contrary to hypothesis (3).
Discussion
The present study was the first to confirm associations between the timing and tempo of pubertal development as measured via hormones (indexing the underlying neuroendocrine changes) and Tanner Stages (indexing visible physical changes). This work builds on previous studies examining different ways to measure pubertal stage in adolescence, and extends this literature by examining a larger swath of the pubertal process as opposed to specific snapshots of that process in time. We further showed that even though the measures of timing and tempo were correlated across measurement strategy, they were differentially related to substance use involvement at age 16: only early timing of testosterone was associated with increased substance use involvement. This finding suggests that early timing of puberty may be related to substance use in White boys through physiological rather than psychosocial mechanisms. Further, although we could not formally test for ethnicity differences due to measurement invariance, patterns of findings suggest that there may be ethnicity differences in relations among measures of puberty and puberty-substance use associations.
Associations of timing and tempo across measures
Findings showing associations of pubertal timing as measured by testosterone and DHT with genital and pubic hair timing are essentially replications of cross-sectional findings showing associations of pubertal stage with hormone measures, and confirmed our first hypothesis. This is not surprising, since timing in these models corresponds to the predicted level of development at 9.42 years for each boy. As in Shirtcliff et al., (2009), both genital and pubic hair development were associated with testosterone levels early in the pubertal process. We extended these findings to show the same associations with DHT, and that these associations hold for White and non-White boys.
Tempo of genital development and tempo of pubic hair development were each associated with tempo of testosterone but not DHT in non-White boys. This provides some confirmation of our first hypothesis with regard to tempo, but associations of tempo across measures were not found in several cases (e.g., secondary sex characteristics with DHT tempo, any associations in White boys). Similarly, the effect of initial levels of testosterone on genital development tempo was attenuated to trend-level (e.g., non-significance) a) when examining the effect of testosterone on pubic hair (rather than genital) development and b) examining the effect of DHT (rather than testosterone) on genital development tempo. This is perhaps not surprising: testosterone is the hormone driving genital development, whereas DHT is synthesized from testosterone. Therefore, associations of DHT with secondary sex characteristics may be residual or non-primary. Indeed, there were generally fewer associations of DHT with secondary sex characteristics across models and ethnicity, relative to testosterone.
The initial association of gonadal steroids and pubic hair development stage was generally attenuated over time, as noted by the lack of associations among measures of initial hormone levels with pubic hair tempo and measures of tempo of gonadal hormones and pubic hair (except non-White boys’ testosterone and pubic hair development). Interestingly, there were no associations of gonadal steroid tempo and secondary sex characteristic tempo in White boys in any model, suggesting that there could be ethnicity differences in the attenuation of initial associations of multiple aspects of pubertal maturation over time (although statistical tests of these differences were not possible in this sample due to measurement invariance). Ethnicity differences in tempo and associations with behavioral phenotypes particularly in boys continue to be an important area for future research, as more work is needed to a) replicate, and b) explain these findings.
Pubic hair timing was associated with tempo of testosterone (for non-White boys) and DHT (for White boys), but not the opposite. This finding may suggest that the timing of adrenal changes has more of an influence on the progression of gonadal steroid increases across pubertal development than the opposite direction of effects (for the moment, setting aside the possible ethnic differences). It is important to note, however, that this analysis is not suited to testing hypotheses about the direction of effects explicitly. However, there is evidence from adrenal insufficiency patients who progress through gonadarche on-time that suggests that adrenarche does not initiate gonadarche (Havelock, Auchus, & Rainey, 2004; Urban, Lee, Gutai, & Migeon, 1980). Another examination of typically developing boys highlights the variability in synchrony and the order of pubertal development: approximately 25% of boys showed pubic hair development prior to testicular development, 59% of boys showed testicular development prior to pubic hair development, and 16% showed synchronous development (Mouritsen et al., 2013), further suggesting that adrenarche is unlikely to cause gonadarche or vice versa in normal puberty. Future work is needed to determine a plausible explanation for why earlier pubic hair development timing may be associated with slower rises in gonadal steroids, and why different gonadal steroids are influenced for White and non-White boys, if this effect is replicated.
We generally found that earlier timing was associated with slower tempo. This finding occurred within hormone measures for White boys, and in associations of secondary sex characteristic timing with hormone tempo for White and non-White boys. Some studies of secondary sex characteristic development assessing change in pubertal stage over time in boys (in samples comprised of mostly White boys) have found similar associations of higher stage at a given age (e.g., earlier timing) with slower maturation (Cance, Ennett, Morgan-Lopez, Foshee, & Talley, 2013; Mendle et al., 2010), whereas others have found null associations (secondary sex characteristics in the present study; Marceau et al., 2015). However, other studies have found associations of earlier timing with faster development of secondary sex characteristics (Beltz et al., 2014; Marceau et al., 2011). Patterns of findings suggesting that earlier timing predicts slower tempo, as found in analyses of gonadal steroids here, may be explained by a statistical artifact: a ceiling effect whereby youth who present at a higher stage initially in the study may have a lower slope simply because they have less development remaining (Mendle et al., 2010). However, this is less likely to be the case when considering that the rise in gonadal steroids which have no specific cap based on the measure (as opposed to Tanner Stages which cap at 5), or based on the age/development at the end of the study (as gonadal steroids are expected to rise for about 4 more years past the end of this study; Braams et al., 2015). Thus, we find novel evidence that earlier onset of the pubertal rise in gonadal steroids and related secondary sex characteristics may be related to slower (or decelerating) rises in gonadal steroid across adolescence in boys. This effect, including its pertinence to particularly White boys, must be replicated and the potential molecular mechanisms investigated, before it can be interpreted without speculation.
Associations with Substance Use
Beltz et al., (2014) used a split-half replication approach in a large sample and found that earlier timing and faster tempo of secondary sex characteristic development were sometimes associated with higher levels of substance use symptoms for boys (not found in both replicates). Considering their findings for earlier timing in light of the present findings, we draw the following hypothesis: perhaps the timing of hormone changes, as opposed to social changes, drives vulnerability to substance use in White boys. This hypothesis fits with evidence that hormone changes may drive behavioral changes related to 1) sensation-seeking and 2) sexual interest, that then can lead youth to engage in substance use either directly or through affiliation with deviant peer groups (perhaps driven by motivation for social dominance; Forbes & Dahl, 2010). Self-reported measures may only able to pick up this effect inconsistently, and because self-report measures are somewhat overlapping with hormone measures, as indicated by our findings.
As noted in previous literature (Mendle & Ferrero, 2012) there is a particular dearth in studies examining puberty-behavior associations in non-White boys. One study did show increased anxiety, depression, and externalizing problems for (self-reported) early maturing African American boys (Ge, Brody, Conger, & Simons, 2006), however, these early maturing African American boys did not show more substance use (Ge, Jin, et al., 2006). These findings and our new findings together suggest that the literature on boys’ puberty and substance use may not be relevant for non-White populations. It should be noted, however, that one other study did suggest that there were no racial/ethnic differences in perceived pubertal timing and substance initiation in boys (Lee et al., 2014).
We found no associations of tempo with substance use in White or non-White boys. Prior studies finding associations of slower (Marceau et al., 2015; Marceau & Jackson, 2017) or faster pubertal tempo with substance use (Castellanos-Ryan et al., 2013; Dick et al., 2001) used self-report measures of puberty. Further, the most rigorous prior study (also using self-reported measures of puberty) found tenuous (positive) links that were not replicated (Beltz et al., 2014). The reasons for non-replication of this effect, including sample characteristics, measurement and modeling strategies, and potential moderators (especially that may systematically vary across samples) is certainly an important area for future research to investigate. The maturational deviance hypothesis (Petersen & Taylor, 1980) hypothesizes nonlinear associations of puberty and behavior, such that both early and late maturation relative to peers marks vulnerability to maladjustment. The findings in the literature may be explained by the failure of these studies to test for nonlinear associations (a limitation we also suffer, as we were limited by only 3 assessments of puberty). Future work may investigate this possibility as well.
Limitations and future directions
There are several limitations in the current study. First, we had few repeated measures. Three assessments is sufficient to extrapolate a linear growth trajectory, but not enough to model the nonlinear nature of pubertal development that has been described for decades (Greulich, Dorfman, Catchpole, Solomon, & Culotta, 1942; Tanner, 1962). More repeated measures would also help to clarify the synchrony/asynchrony of adrenal and gonadal development. Relatedly, for assessments of testosterone and DHT, data were log transformed prior to fitting the linear growth curves. Thus, although linear changes were modeled, the real form of change estimated is exponential – that is, a significant linear slope reflects data that is actually increasing at an accelerating rate. Indeed, plots of our raw hormone data showed an accelerating increasing pattern over time, and growth models of the log transformed data fit better than growth curves of the raw data (available upon author request). For those reasons, this strategy was judged to be appropriate in this case (and also is consistent with the modeling choices made by Braams et al., 2015). Consequently, the estimated parameters for timing and tempo presented in Figures 1–4 reflect the median for hormone assessments (because of the log transformation, which is appropriate because medians are better estimates of center than means for skewed data) but reflect the mean for secondary sex characteristic assessments (because these data were not log transformed). The general interpretation of earlier timing and faster tempo is still appropriate for both measurement strategies, but alternative modeling choices should be considered in other data in the future.
Second, time of awakening was not assessed on the visit day, and as such we were unable to adjust for time since waking for hormone assessments. Because diurnal cycle was not of interest here, rather changes over time was, it is unlikely that this limitation introduced systematic bias related to the main study hypotheses. Nonetheless, more accurate timing, and more assessments (as opposed to the single day available) to obtain more robust measures of testosterone and DHT at each age would greatly increase the accuracy of results. Third, we had no measures of adrenal hormones. We might expect a stronger association of the tempo of changes in adrenal hormones influencing puberty (e.g., DHEA) and pubic hair development changes across pubertal development, relative to gonadal steroids. Adrenal hormone development is thus crucial to include in future studies, as adrenarche and gonadarche are separate processes, although linked in typical pubertal maturation (Grumbach & Styne, 2003). Fourth, our age-bands at each assessment were somewhat wide. The use of exact age at each assessment to some extent attenuates this limitation, but clearer results may emerge from studies including narrower age bands at each assessment. Fifth, we were unable to include girls in the present analysis due to low sample size and imprecise measurement of pubertal hormones. The present analysis would be interesting and important to conduct on females as well, especially given differences in puberty across the sexes. Finally, there is no reliability information available for nurse Tanner staging, as only one nurse conducted the evaluation of each participant. Nurses were trained by a physician and conducted evaluations with picture references to improve accuracy. However, combined with particularly late development in this sample as compared with others (e.g., Marceau et al., 2011), the accuracy of the Tanner staging is unclear.
Conclusions
Despite these limitations, the present study takes an important step towards understanding the correspondence between hormonal (gonadal steroid) and visible (secondary sex characteristic) changes associated with the process of puberty in boys. Indeed, timing (indexed as the estimated stage/hormone level at 9.42 years of age) of gonadal steroid development was positively associated with the timing of secondary sex characteristic development, replicating cross-sectional findings examining stage-hormone level associations at a given age, particularly early in development. Our findings also showed a decrease in the correspondence of gonadal steroids and Tanner stages over time. And, this pattern of correspondence was extended to the tempo of puberty only as measured by testosterone and Tanner Stages in non-White boys hinting at the intriguing possibility of ethnicity differences in the process of puberty in boys. This pattern of findings may indicate that studies of pubertal milestones later in the pubertal process and studies of pubertal tempo through the use of secondary sex characteristic development (as is most prevalent in the current literature) may increasingly diverge from hormone assessments in findings and/or interpretation, particularly when indexing gonadarche vs. adrenarche, and particularly in White boys. This effect would help to explain why findings regarding associations of the tempo of puberty and behavioral outcomes are so mixed in the literature, which uses several different measurement strategies.
We also showed that despite associations of timing and tempo of puberty as assessed by hormones and secondary sex characteristics, particularly earlier in the pubertal process, it was specifically the early timing of pubertal maturation as assessed via testosterone that marked risk for substance use involvement, in White boys, at age 16 years. Understanding the role of the tempo of pubertal development for physical and mental health is a burgeoning field, and the present study underscores and extends an important message: that the ways in which puberty is measured (and whether the measures index adrenarche or gonadarche) has implications for whether and when significant associations of puberty with mental/behavioral health outcomes emerge. Here, we offer preliminary evidence that testosterone changes are of primary importance for predicting (and thus identifying youth at-risk for) substance use outcomes in White boys. That is, physiological mechanisms may better explain this association than the more commonly tested psychosocial mechanisms. Especially in instances where pubertal maturation may better index development than chronological age (e.g., some aspects of brain development; Herting & Sowell, 2017), it will be important to consider the measurement of the pubertal process, beyond cross-sectional snapshots, and to measure the aspects of puberty (e.g., adrenarche, gonadarche, secondary sex characteristics visible to the individual or the casual observer) that best index development for the process under study.
Acknowledgments
We are grateful to the CEDAR staff and participating families. The funding for this study was provided by the National Institute on Drug Abuse: data collection: P50 DA005605 (PI: Tarter), data analysis and manuscript preparation: K05 DA031248 (PI: Tarter), and K01 DA039288 (PI: Marceau).
Contributor Information
Kristine Marceau, Purdue University.
Levent Kirisci, University of Pittsburgh.
Ralph E. Tarter, University of Pittsburgh
References
- Anthony JC, Helzer JE. Syndromes of drug abuse and dependence. Psychiatric disorders in America: The epidemiologic catchment area study. 1991:116–154. [Google Scholar]
- Beltz AM, Corley RP, Bricker JB, Wadsworth SJ, Berenbaum SA. Modeling pubertal timing and tempo and examining links to behavior problems. Developmental Psychology. 2014;50(12):2715–2726. doi: 10.1037/a0038096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde AC, Peper JS, Crone EA. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. Journal of Neuroscience. 2015;35(18):7226–7238. doi: 10.1523/JNEUROSCI.4764-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls’ pubertal status. Child Dev. 1987;58(3):829–841. [PubMed] [Google Scholar]
- Cance JD, Ennett ST, Morgan-Lopez AA, Foshee VA, Talley AE. Perceived pubertal timing and recent substance use among adolescents: a longitudinal perspective. Addiction. 2013;108(10):1845–1854. doi: 10.1111/add.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49(12):1189–1201. doi: 10.1016/j.jaac.2010.08.017. quiz 1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos-Ryan N, Parent S, Vitaro F, Tremblay RE, Seguin JR. Pubertal development, personality, and substance use: a 10-year longitudinal study from childhood to adolescence. J Abnorm Psychol. 2013;122(3):782–796. doi: 10.1037/a0033133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman L, Coleman J. The measurement of puberty: a review. J Adolesc. 2002;25(5):535–550. doi: 10.1006/jado.2002.0494. [DOI] [PubMed] [Google Scholar]
- Dahl RE, Forbes EE. Pubertal Development and Behavior: Hormonal Activation of Social and Motivational Tendencies. Brain and cognition. 2010;72(1):66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes MA, Dorn LD, Moss HB, Yao JK, Kirisci L, Ammerman RT, Tarter RE. Hormonal and behavioral homeostasis in boys at risk for substance abuse. Drug Alcohol Depend. 1999;55(1–2):165–176. doi: 10.1016/S0376-8716(99)00003-4. [DOI] [PubMed] [Google Scholar]
- de Water E, Braams BR, Crone EA, Peper JS. Pubertal maturation and sex steroids are related to alcohol use in adolescents. Horm Behav. 2013;63(2):392–397. doi: 10.1016/j.yhbeh.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Dick DM, Rose RJ, Pulkkinen L, Kaprio J. Measuring puberty and understanding its impact: A longitudinal study of adolescent twins. Journal of Youth & Adolescence. 2001;30(4):385–400. [Google Scholar]
- Dorn LD, Biro FM. Puberty and Its Measurement: A Decade in Review. Journal of Research on Adolescence. 2011;21(1):180–195. doi: 10.1111/j.1532-7795.2010.00722.x. [DOI] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10(1):30–56. [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: Hormonal activation of social and motivational tendencies. Brain and cognition. 2010;72(1):66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Brody G, Conger R, Simons R. Pubertal Maturation and African American Children’s Internalizing and Externalizing Symptoms. Journal of Youth and Adolescence. 2006;35(4):528–537. [Google Scholar]
- Ge X, Jin R, Natsuaki MN, Gibbons FX, Brody GH, Cutrona CE, Simons RL. Pubertal maturation and early substance use risks among African American children. Psychology of Addictive Behaviors. 2006;20(4):404–414. doi: 10.1037/0893-164X.20.4.404. [DOI] [PubMed] [Google Scholar]
- Ge X, Natsuaki MN. In Search of Explanations for Early Pubertal Timing Effects on Developmental Psychopathology. Current Directions in Pscyhological Science. 2009;18(6):327–331. doi: 10.1111/j.1467-8721.2009.01661.x. [DOI] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Chrousos GP. Puberty-related influences on brain development. Molecular and Cellular Endocrinology. 2006:254–255. 154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Greulich WW, Dorfman RI, Catchpole HR, Solomon CI, Culotta CS. Somatic and Endocrine Studies of Puberal and Adolescent Boys. Monogr Soc Res Child Dev. 1942;7(3):i–85. doi: 10.2307/1165411. [DOI] [Google Scholar]
- Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990;126(2):1165–1172. doi: 10.1210/endo-126-2-1165. [DOI] [PubMed] [Google Scholar]
- Grumbach MM. The Neuroendocrinology of Human Puberty Revisited. Horm Res Paediatr. 2002;57(Suppl. 2):2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- Grumbach MM, Styne DM. Puberty: ontogeny, neuroendocrinology, physiology, and disorders. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. 10. 2003. pp. 1115–1286. [Google Scholar]
- Harden KP, Mendle J. Gene-environment interplay in the association between pubertal timing and delinquency in adolescent girls. J Abnorm Psychol. 2012;121(1):73–87. doi: 10.1037/a0024160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelock JC, Auchus RJ, Rainey WE. The rise in adrenal androgen biosynthesis: adrenarche. Semin Reprod Med. 2004;22(4):337–347. doi: 10.1055/s-2004-861550. [DOI] [PubMed] [Google Scholar]
- Herting MM, Sowell ER. Puberty and structural brain development in humans. Front Neuroendocrinol. 2017;44:122–137. doi: 10.1016/j.yfrne.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel A, Shelton KH, Heron J, Moore L, van den Bree MBM. A systematic review of the relationships between family functioning, pubertal timing and adolescent substance use. Addiction. 2013;108(3):487–496. doi: 10.1111/add.12055. [DOI] [PubMed] [Google Scholar]
- Jaruratanasirikul S, Kreetapirom P, Tassanakijpanich N, Sriplung H. Reliability of pubertal maturation self-assessment in a school-based survey. Journal of Pediatric Endocrinology and Metabolism. 2015;28(3–4):367–374. doi: 10.1515/jpem-2014-0053. [DOI] [PubMed] [Google Scholar]
- Kirillova GP, Vanyukov MM, Gavaler JS, Pajer K, Dunn M, Tarter RE. Substance abuse in parents and their adolescent offspring: The role of sexual maturation and sensation seeking. Journal of Child & Adolescent Substance Abuse. 2001;10(4):77–89. [Google Scholar]
- Kirisci L, Tarter RE, Reynolds M, Vanyukov M. Individual differences in childhood neurobehavior disinhibition predict decision to desist substance use during adolescence and substance use disorder in young adulthood: A prospective study. Addict Behav. 2006;31(4):686–696. doi: 10.1016/j.addbeh.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Kirisci L, Vanyukov M, Dunn M, Tarter R. Item response theory modeling of substance use: an index based on 10 drug categories. Psychology of Addictive Behaviors. 2002;16(4):290. [PubMed] [Google Scholar]
- Lee JS, McCarty CA, Ahrens K, King KM, Vander Stoep A, McCauley EA. Pubertal Timing and Adolescent Substance Initiation. Journal of Social Work Practice in the Addictions. 2014;14(3):286–307. doi: 10.1080/1533256X.2014.935648. [DOI] [Google Scholar]
- Marceau K, Abar C, Jackson K. Parental Knowledge is a Contextual Amplifier of Associations of Pubertal Maturation and Substance Use. Journal of Youth and Adolescence. 2015;44(9):1720–1734. doi: 10.1007/s10964-015-0335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Jackson K. Deviant Peers as a Mediator of Pubertal Timing–Substance Use Associations: The Moderating Role of Parental Knowledge. Journal of Adolescent Health. 2017;61(1):53–60. doi: 10.1016/j.jadohealth.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Ram N, Houts RM, Grimm KJ, Susman EJ. Individual differences in boys’ and girls’ timing and tempo of puberty: Modeling development with nonlinear growth models. Developmental Psychology. 2011;47(5):1389–1409. doi: 10.1037/a0023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias CW, Charles NE, Liang Y, Acheson A, Lake SL, Ryan SR, Dougherty DM. Pubertal Maturation Compression and Behavioral Impulsivity Among Boys at Increased Risk for Substance Use. Addictive Disorders & Their Treatment. 2016;15(2):61–73. doi: 10.1097/adt.0000000000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J. Beyond pubertal timing: New directions for studying individual differences in development. Current Directions in Psychological Science. 2014;23(3):215–219. doi: 10.1177/0963721414530144. [DOI] [Google Scholar]
- Mendle J, Ferrero J. Detrimental psychological outcomes associated with pubertal timing in adolescent boys. Developmental Review. 2012;32(1):49–66. doi: 10.1016/j.dr.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Harden KP, Brooks-Gunn J, Graber JA. Development’s tortoise and hare: Pubertal timing, pubertal tempo, and depressive symptoms in boys and girls. Developmental Psychology. 2010;46(5):1341–1353. doi: 10.1037/a0020205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SR, Harden KP, Mendle J. Pubertal timing and adolescent sexual behavior in girls. Dev Psychol. 2014;50(6):1734–1745. doi: 10.1037/a0036027. [DOI] [PubMed] [Google Scholar]
- Mouritsen A, Aksglaede L, Soerensen K, Hagen CP, Petersen JH, Main KM, Juul A. The pubertal transition in 179 healthy Danish children: associations between pubarche, adrenarche, gonadarche, and body composition. European Journal of Endocrinology. 2013;168(2):129–136. doi: 10.1530/eje-12-0191. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide: Statistical Analysis with Latent Variables: User’ss Guide. Muthén & Muthén; 2010. [Google Scholar]
- Negriff S, Blankson AN, Trickett PK. Pubertal Timing and Tempo: Associations With Childhood Maltreatment. Journal of research on adolescence : the official journal of the Society for Research on Adolescence. 2015;25(2):201–213. doi: 10.1111/jora.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research, C. f. E. a. D. A. Drug use chart 1989 [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal Development: Correspondence Between Hormonal and Physical Development. Child Dev. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The role of brain development in drug effect and drug response. Biological Research on Addiction. 2013;2:271–280. [Google Scholar]
- Susman EJ, Houts RM, Steinberg L, Belsky J, Cauffman E, DeHart G, for the Eunice Kennedy Shriver, N. E. C. C. R. N. Longitudinal Development of Secondary Sexual Characteristics in Girls and Boys Between Ages 91/2 and 151/2 Years. 2010;164:166–173. doi: 10.1001/archpediatrics.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JM. Growth at adolescence. 2nd. Thomas; Springfield, Ill: 1962. [Google Scholar]
- Tarter RE, Vanyukov MM. Introduction: Theoretical and Operational Framework for Research into the Etiology of Substance Use Disorders. Journal of Child & Adolescent Substance Abuse. 2001;10(4):1–12. doi: 10.1300/J029v10n04_01. [DOI] [Google Scholar]
- Terry MB, Goldberg M, Schechter S, Houghton LC, White ML, O’Toole K, Andrulis IL. Comparison of Clinical, Maternal, and Self Pubertal Assessments: Implications for Health Studies. Pediatrics. 2016;138(1):e20154571. doi: 10.1542/peds.2015-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger JM, Nikolas MA. A meta-analytic review of the association between pubertal timing and psychopathology in adolescnece: Are there sex differences? Psychological Bulletin. 2017;143(9):903–938. doi: 10.1037/bul0000106. [DOI] [PubMed] [Google Scholar]
- Urban MD, Lee PA, Gutai JP, Migeon CJ. Androgens in pubertal males with Addison’s disease. J Clin Endocrinol Metab. 1980;51(4):925–929. doi: 10.1210/jcem-51-4-925. [DOI] [PubMed] [Google Scholar]


