Abstract
Heterotrimeric G proteins are important molecules for regulating plant architecture and transmitting external signals to intracellular target proteins in higher plants and mammals. The rice genome contains one canonical α subunit gene (RGA1), four extra-large GTP-binding protein genes (XLGs), one canonical β subunit gene (RGB1), and five γ subunit genes (tentatively named RGG1, RGG2, RGG3/GS3/Mi/OsGGC1, RGG4/DEP1/DN1/OsGGC3, and RGG5/OsGGC2). RGG1 encodes the canonical γ subunit; RGG2 encodes the plant-specific type of γ subunit with additional amino acid residues at the N-terminus; and the remaining three γ subunit genes encode the atypical γ subunits with cysteine abundance at the C-terminus. We aimed to identify the RGG3/GS3/Mi/OsGGC1 gene product, Gγ3, in rice tissues using the anti-Gγ3 domain antibody. We also analyzed the truncated protein, Gγ3∆Cys, in the RGG3/GS3/Mi/OsGGC1 mutant, Mi, using the anti-Gγ3 domain antibody. Based on nano-liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, the immunoprecipitated Gγ3 candidates were confirmed to be Gγ3. Similar to α (Gα) and β subunits (Gβ), Gγ3 was enriched in the plasma membrane fraction, and accumulated in the flower tissues. As RGG3/GS3/Mi/OsGGC1 mutants show the characteristic phenotype in flowers and consequently in seeds, the tissues that accumulated Gγ3 corresponded to the abnormal tissues observed in RGG3/GS3/Mi/OsGGC1 mutants.
Keywords: GS3, γ subunit, heterotrimeric G protein, mass spectrometric analysis, RGG3, rice, western blotting
1. Introduction
Heterotrimeric G proteins are well known to consist of three subunits, α, β, and γ, in mammals and yeast [1,2,3,4]. Receptors regulating the heterotrimeric G proteins, such as G protein-coupled receptors (GPCRs), interact with external signals and activate the heterotrimeric G proteins via the intrinsic GDP/GTP exchange factor (GEF) of GPCRs. When GTP binds to the α subunit (Gα-GTP), heterotrimeric G proteins dissociate into the α subunit (Gα-GTP) and βγ dimer. The α subunit and βγ dimer can regulate respective effector molecules. Thus, heterotrimeric G proteins are signal mediators from receptors to effector molecules. In higher plants, heterotrimeric G proteins are important molecules for regulating plant architecture and transmitting external signals to intracellular target proteins [5,6,7]. The biochemical characteristics of the plant heterotrimeric G protein [5] and the signaling mechanism and effector molecules regulating the plant heterotrimeric G protein [6] have been previously reviewed. The plant morphology of heterotrimeric G protein mutants has also been previously summarized [7]. There are three extra-large GTP-binding protein genes (AtXLG1~AtXLG3) [8,9], one canonical α subunit gene (GPA1) [10], one canonical β subunit gene (AGB1) [11], and three γ subunit genes (AGG1~AGG3) [12,13,14], in Arabidopsis; and four extra-large GTP-binding protein genes (prediction by in silico) [15], one canonical α subunit gene (RGA1) [16], one canonical β subunit gene (RGB1) [17], and five γ subunit genes, which we tentatively named RGG1 [18], RGG2 [18], RGG3/GS3/Mi/OsGGC1 [19], RGG4/DEP1/DN1/OsGGC3 [20], and RGG5/OsGGC2 [21], in this paper.
With regard to the γ subunit genes in Arabidopsis, there are AGG1 and AGG2 encoding the canonical γ subunits, and AGG3 encoding the atypical γ subunit with cysteine abundance at the C-terminus. In rice, RGG1 encodes the canonical γ subunit, RGG2 encodes the plant-specific type of γ subunit, and the remaining three γ subunit genes, RGG3/GS3/Mi/OsGGC1, RGG4/DEP1/DN1/OsGGC3, and RGG5/OsGGC2 encode the atypical γ subunits homologous to AGG3. RGG3 corresponds to GRAIN SIZE 3 (GS3) [19] and RGG4 corresponds to DENSE AND ERECT PANICLES 1 (DEP1/DN1) [20]. The genome sequence of RGG5 was predicted by Botella [21]. The diversity and agronomical importance of plant γ subunits have been previously reviewed [21,22].
Mutants of XLG1, XLG2, and XLG3 [23]; GPA1 [24]; AGB1 [25,26]; and AGG1, AGG2 [27], and AGG3 [14] were isolated as heterotrimeric G protein mutants in Arabidopsis. Mutants of RGA1 [28,29], GS3 [30], and DEP1 [20] were isolated as similar G protein mutants in rice. By morphological analysis of gpa1 [24], agb1 [26], d1 [31], and RGB1 knock-down lines [32], it was shown that plant heterotrimeric G proteins modulate cell proliferation.
It has been shown that plant heterotrimeric G proteins are associated with transduction in response to multiple external signals, namely auxin [24,26], abscisic acid [33,34,35,36,37], gibberellin [38,39,40,41], brassinosteroid [24,39,40], sugar [42,43], blue light [44,45], and ozone [46]. It was also shown that the heterotrimeric G proteins of plants are concerned with defense signaling [47,48,49,50].
Based on the characteristics of heterotrimeric G proteins in higher plants, the α subunit is suggested to be contained in a huge complex localized in the plasma membrane fraction of rice [18] and Arabidopsis [51]. In rice, some βγ dimer candidates seem to be present in two different forms: one is a component of a huge complex, and the other is a sole βγ dimer dissociated from a huge complex in the plasma membrane of rice seedlings [18]. Using yeast two-hybrid screening, it was shown that 68 highly interconnected proteins form the core G-protein interactome in Arabidopsis [52], in which the regulators of G protein signaling protein (AtRGS1) [53], THYLAKOID FORMATION 1 (THF1) [43], cupin domain protein (AtPrin1) [35] etc. in addition to α, β, γ1, γ2 subunits, were contained. The huge complexes prepared solubilized plasma membrane fraction in rice [18] and Arabidopsis [51] may represent a part of the G-protein interactome in Arabidopsis [52].
In mammals and yeast, β subunits interact with γ subunits to form the βγ dimer [1,2,3,4]. The βγ dimer has not been purified from the tissues of higher plants so far, but many studies suggest its presence based on the experiments, including an in vitro pull-down assay [12,13], yeast two-hybrid (Y2H) assay [13], split-ubiquitin system [14], and fluorescence response energy transfer (FRET) assay [51,54] in Arabidopsis. Moreover, in rice, the β subunit was shown to interact with the γ1 and γ2 subunits with a Y2H assay [18]. Recently, the interaction of rice β subunit with atypical γ subunits and the localization of these subunits in the plasma membrane were demonstrated with a bi-molecular fluorescence complementation (BiFC) assay [55,56]. These results indicated that both the canonical and atypical γ subunits can interact with the β subunit, and that βγ dimers are localized in the plasma membrane fraction, in Arabidopsis and rice.
GS3 is identified as a major QTL for grain weight and grain length, and as an important gene for agriculture [19,30,56,57,58]. According to the identification of AGG3 in Arabidopsis, GS3 was classified as the atypical γ subunit member, and tentatively named RGG3. In order to understand the mechanism of seed formation in rice, studies on the GS3 protein are important.
To understand the function of RGG3 in the regulation of seed size, identifying the native Gγ3 protein is important. When the native Gγ3 protein is identified, biochemical analysis, namely measuring the subunit stoichiometry and affinity to Gβ, canonical Gα, and XLGs, is possible. Although we tried to identify the native Gγ3 protein using an anti-Gγ3 domain antibody, the antibody recognized multiple proteins. To identify the native Gγ3 protein, we use the RGG3 mutants MINUTE (Mi) and GS3-3, which produce partially defective proteins, as references for subtraction to Taichung 65 (abbreviated as WT [wild-type] hereinafter). Here, we find a candidate of the native RGG3 protein, Gγ3. Finally, we confirmed that the candidate was the native Gγ3 protein using nano-liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis of the immunoprecipitation products using an anti-Gγ3 domain antibody. Using this antibody, the subcellular localization and tissue-specific accumulation of the native Gγ3 protein were studied.
2. Result
2.1. Morphology of Rice Heterotrimeric G Protein γ3 Gene (RGG3/GS3/Mi/OsGGC1) Mutants:
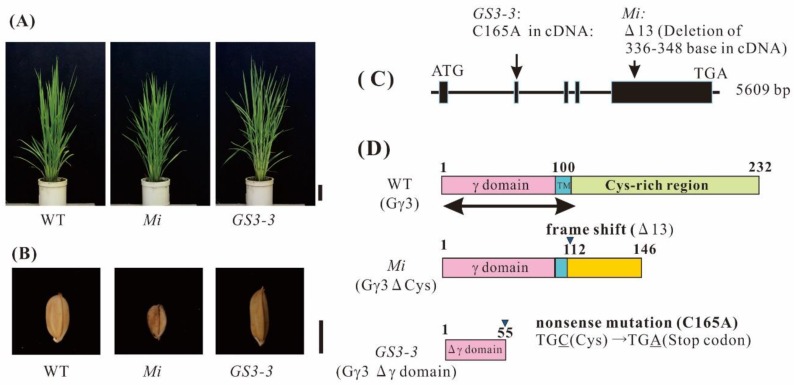
To confirm the functions of rice heterotrimeric G protein γ3 subunit in determining the plant morphology, we prepared plants possessing GS3-3 [30] and Mi [58] mutation with Taichung 65 as a background. The mutant, Mi was slightly dwarfed (Figure 1A) and set small seeds (Figure 1B), compared to those of the WT. GS3-3 had a height similar to that of the WT (Figure 1A) and set large seeds (Figure 1B). These results indicate that the mutations in Mi and GS3-3 clearly affected the seed size.
Figure 1.
Morphology of rice heterotrimeric G protein γ3 gene (RGG3/GS3/Mi/OsGGC1) mutants, and genome and protein structure of RGG3/GS3/Mi/OsGGC1. (A) Gross morphology of the wild-type (WT) (Taichung 65), Mi and GS3-3; Bar = 10 cm. (B) Seed morphologies of the plants in (A); Bar = 5 mm. (C) Genome structure of RGG3/GS3/Mi/OsGGC1 and positions of mutations in RGG3/GS3/Mi/OsGGC1 mutants, Mi and GS3-3. The 13-base deletion (336–348th base in full-length cDNA) and one base substitution (C165A in full-length cDNA) had occurred in Mi and GS3-3, respectively. In GS3-3, a codon, TGC (cysteine) changed to TGA (stop codon). (D) Protein structure of the product of RGG3/GS3/Mi/OsGGC1 in the WT (Gγ3), Mi (Gγ3ΔCys), and GS3-3 (Gγ3Δγ domain). The canonical γ domain region is shown as γ domain (pink bar). The putative transmembrane domain is indicated as TM (blue bar). The region with cysteine abundance is labeled as cysteine-rich region (green bar). The newly produced amino acid sequence by the frame shift resulting from of 13-base deletion is indicated with a yellow bar. An arrow under the WT Gγ3, which covers 120 amino acid residues from N-terminal, is the region used for recombinant proteins, such as the thioredoxin (Trx)-tagged Gγ3 domain protein (Trx-Gγ3 domain protein), used as an antigen, and the glutathione S transferase (GST)-tagged Gγ3 domain protein (GST-Gγ3 domain protein), used for affinity purification of the antibody.
2.2. Genomic Structure of RGG3 and Protein Structure of Gγ3
The genome sequence of RGG3 was found in RAP-DB (Os03g0407400). We reconfirmed the genome sequence of RGG3. RGG3 consists of five exons (Figure 1C) and its translation product, Gγ3, comprises 232 amino acid residues. In order to prepare recombinant proteins, cDNA for RGG3 was isolated. The molecular weight of Gγ3 calculated from the cDNA, was 24249 Da. The Gγ3 consists of the canonical γ domain (about 100 amino acid residues), a short region with hydrophobic amino acid residues (tentatively named transmembrane region: TM), and a region with a large number of cysteines (Cys-rich region) (Figure 1D).
The Mi mutation occurred as a result of the deletion of 13 bases in RGG3. The mutation site corresponds to 336–348th positions in the full-length cDNA of RGG3, resulting in a frame-shift (Figure 1C). We reconfirmed the mutation in Mi. In Mi, the mutated protein, tentatively named Gγ3∆Cys, consists of 146 amino acid residues (Figure 1D). The cysteine-rich region is absent in Gγ3∆Cys. The molecular weight of Gγ3∆Cys, calculated from cDNA, was 15,651 Da.
The GS3-3 mutation occurred as a result of one base substitution. The C at the 165th position in the full-length cDNA of RGG3 was substituted by A (C165A), resulting in the generation of a stop codon (Figure 1C). As the mutation in TCM3-467 was the same as that in GS3-3 [18], we renamed TCM3-467 to GS3-3. The GS3-3 mutation generated a mutated protein with 55 amino acid residues, tentatively named the Gγ3∆γ domain (Figure 1D). The Gγ3∆γ domain is an immature protein lacking about half of the canonical γ domain. The molecular weight of the Gγ3∆γ domain, calculated from cDNA, was 5653 Da. The chemiluminescent intensity of Gγ3ΔCys was more than 7-fold that of Gγ3, when 10 μg of protein of the plasma membranes of the WT and Mi, respectively, was analyzed by western blot.
2.3. Gγ3 Candidates Localized in the Plasma Membrane Fraction
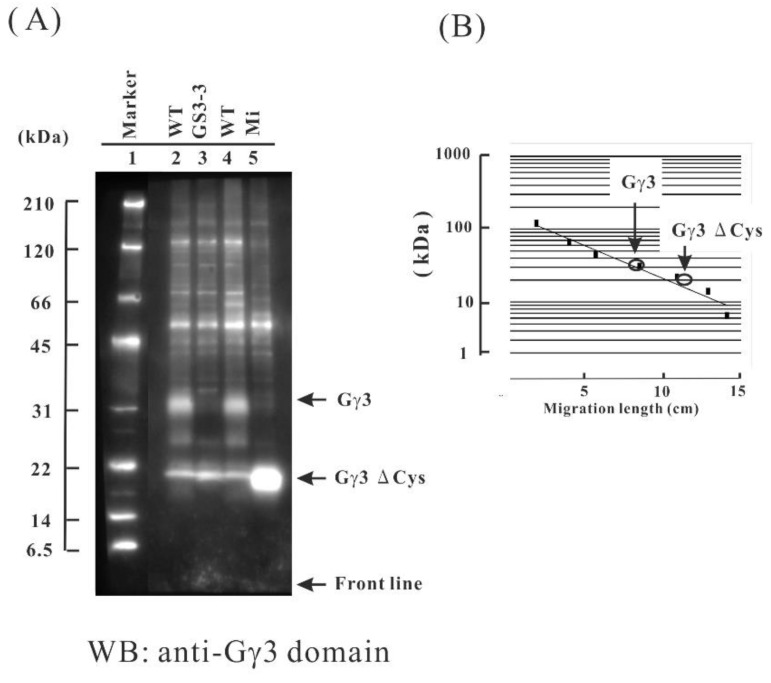
Identification of native Gγ3 was carried out by Western blotting. As mutants have no native full length Gγ3, these were used as references, in order to identify native Gγ3 in WT. The plasma membrane fraction was chosen in this study as it was shown that Gα and Gβ accumulated in plasma membrane fraction in rice. The plasma membrane fractions of WT, GS3-3, and Mi flowers were prepared using an aqueous two-polymer phase system, and Gγ3 candidates were detected by Western blotting using an anti-Gγ3 domain antibody. In the WT, a 32-kDa protein (Gγ3 candidate) was detected (Figure 2A, lanes 2 and 4); this band was not observed in GS3-3 or Mi. The molecular weight of the Gγ3 candidate is much higher than that of Gγ3 calculated from the cDNA of the WT (24 kDa). In GS3-3, the Gγ3∆γ domain was not detected (Figure 2A, lane 3). In Mi, a 20-kDa protein (Gγ3∆Cys candidate) was detected (Figure 2A, lane 5). The molecular weight of the Gγ3∆Cys candidate was much higher than that of Gγ3∆Cys calculated from the cDNA (16 kDa). The molecular weights of Gγ3 and Gγ3∆Cys candidates were measured using molecular weight markers (Figure 2B).
Figure 2.
Immunological study of the Gγ3 candidates in the wild-type (WT), Minute (Mi), and GS3-3 flowers. (A) First, 10 μg of each protein of the plasma membrane fractions of the WT and GS3-3 and 5 μg of the protein of the plasma membrane fractions of Mi were used for the Western blot analysis using an anti-Gγ3 domain antibody. Molecular weight marker (lane 1). The Gγ3 candidate was detected as a broad band with a molecular weight of approximately 32 kDa in the WT (lanes 2 and 4). No Gγ3 was detected in GS3-3 (lane 3). The Gγ3ΔCys candidate was detected as a band with a molecular weight of approximately 20 kDa in Mi (lane 5). (B) The molecular weights of Gγ3 and Gγ3ΔCys candidates were estimated using a molecular weight marker as a standard.
2.4. Immunoprecipitation of Gγ3 and Gγ3∆Cys Using an Anti-Gγ3 Domain Antibody
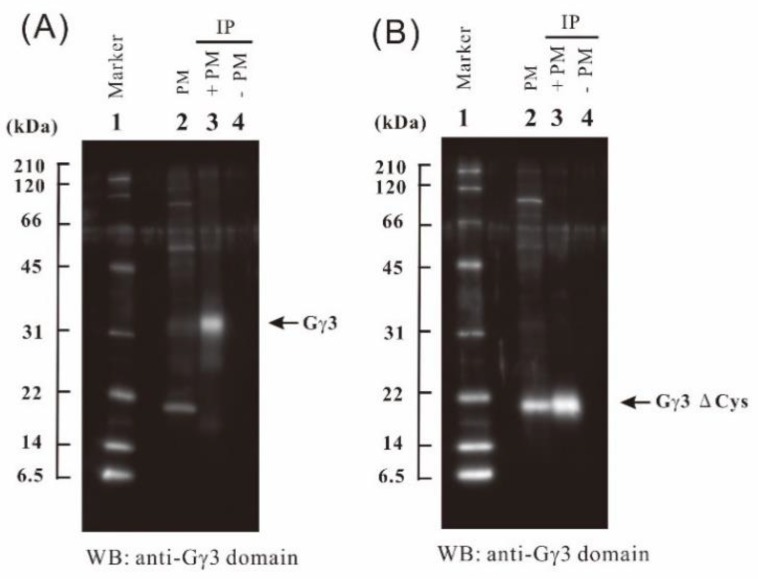
To concentrate Gγ3 and Gγ3∆Cys candidates, immunoprecipitation was carried out using anti-Gγ3 domain antibody. First, 50 μg of the anti-Gγ3 domain antibody was added to 1 mg each of solubilized plasma membrane protein of the WT (Figure 3A) and Mi (Figure 3B) flowers. Gγ3 and Gγ3∆Cys candidates were collected with the antibody cross-linked Protein A bound beads. The 32 kDa protein, a Gγ3 candidate in the WT (Figure 3A, lane 3) and 20-kDa protein, a Gγ3∆Cys candidate in Mi (Figure 3B, lane 3), were immunoprecipitated.
Figure 3.
Immunoprecipitation of Gγ3 and Gγ3ΔCys candidates. (A) Immunoprecipitation of the Gγ3 candidate from solubilized plasma membrane proteins of the wild-type (WT) flower using an anti-Gγ3 domain antibody. Molecular weight marker (lane 1); 10 μg of protein of the plasma membrane fraction of the WT (lane 2); the immunoprecipitation product of solubilized plasma membrane proteins and anti-Gγ3 domain antibody (lane 3); control experiment (buffer in place of the membrane protein; lane 4). (B) Immunoprecipitation of the Gγ3ΔCys candidate from the solubilized plasma membrane proteins of the Minute (Mi) flower using an anti-Gγ3 domain antibody. Molecular weight marker (lane 1); 10 μg of protein of the plasma membrane fraction of Mi (lane 2); the immunoprecipitation product of the solubilized plasma membrane proteins of Mi and the anti-Gγ3 domain antibody (lane 3); control experiment (buffer in place of the membrane protein; lane 4).
2.5. LC-MS/MS Analysis
To demonstrate that Gγ3 and Gγ3ΔCys candidates are actually Gγ3 and Gγ3ΔCys, and that proteins with which the anti-Gγ3 domain antibody reacted, are actually Gγ3 and Gγ3ΔCys, LC-MS/MS analysis was carried out. First, using LC-MS/MS, we checked for Gγ3 and Gγ3ΔCys candidates in the eluate from the gel containing plasma membrane proteins following SDS-PAGE. When the signal intensities of Gγ3 and Gγ3ΔCys candidates detected by LC-MS/MS were not enough, we analyzed immunoprecipitation products, enriched with anti-Gγ3 domain antibody.
First, plasma membrane proteins from the WT and Mi were analyzed by LC-MS/MS. 40 μg of each flower plasma membrane protein from WT and Mi was separated by SDS-PAGE and each lane was separated into 10 pieces to increase the relative amount of target proteins, according to the molecular weight marker. After these gel pieces were digested with trypsin, peptides were analyzed by LC-MS/MS in triplicate. Typical examples are summarized in Table 1. Fragments were assigned to the sequence of Gγ3, and their positions are indicated in Figure 4A.
Table 1.
LC-MS/MS analysis of Gγ3 fragments in in the plasma membrane of the wild-type (WT) and Minute (Mi) flowers.
| (A) Gγ3 fragments in the plasma membrane fraction of the WT flower | |||||
| Fragments | Observed | Mr(expt) | Mr(calc) | Expected | Peptide |
| 1 | 379.2251 | 1134.6536 | 1134.6509 | 0.00078 | R.LQLAVDALHR.E |
| 2 | 714.7006 | 2141.08 | 2141.0753 | 0.00000034 | R.EIGFLEGEINSIEGIHAASR.C |
| 3 | 482.7414 | 963.4682 | 963.4662 | 0.007 | R.EVDEFIGR.T |
| (B) Gγ3 fragments in the plasma membrane fraction of the Mi flower | |||||
| Fragment | Observed | Mr(expt) | Mr(calc) | Expected | Peptide |
| 2 | 714.7014 | 2141.0824 | 2141.0753 | 0.0000024 | R.EIGFLEGEINSIEGIHAASR.C |
| 3 | 482.7408 | 963.4671 | 963.4662 | 0.0077 | R.EVDEFIGR.T |
| 4-1 | 667.8468 | 1333.6791 | 1333.6765 | 0.00015 | R.TPDPFITISSEK.R |
| (C) Gγ3 fragments in the immunoprecipitation products using the plasma membrane fraction of WT flower | |||||
| Fragments | Observed | Mr(expt) | Mr(calc) | Expected | Peptide |
| 1 | 568.3348 | 1134.6551 | 1134.6509 | 5.40 × 10−7 | R.LQLAVDALHR.E |
| 3 | 482.7417 | 963.4688 | 963.4662 | 0.00062 | R.EVDEFIGR.T |
| 4-2 | 497.6015 | 1489.7827 | 1489.7776 | 2.50 × 10−5 | R.TPDPFITISSEKR.S |
| (D) Gγ3 fragments in the immunoprecipitation products using the plasma membrane fraction of Mi flower | |||||
| Fragments | Observed | Mr(expt) | Mr(calc) | Expected | Peptide |
| 1 | 568.3354 | 1134.6562 | 1134.6509 | 8.90 × 10−7 | R.LQLAVDALHR.E |
| 2 | 714.7017 | 2141.0832 | 2141.0753 | 8.60 × 10−7 | R.EIGFLEGEINSIEGIHAASR.C |
| 3 | 482.7427 | 963.4709 | 963.4662 | 0.00072 | R.EVDEFIGR.T |
| 4-1 | 667.8485 | 1333.6825 | 1333.6765 | 7.70 × 10−6 | R.TPDPFITISSEK.R |
Forty micrograms of each protein of the plasma membrane fraction of the wild-type (WT) and Minute (Mi) (A,B) and 5 μL of each eluate in the immunoprecipitation experiment of WT and Mi (C,D) were used for LC-MS/MS. Fragments of the trypsin-digested Gγ3 candidates (p < 0.05) are shown. The fragment numbers correspond to Figure 4A. Mr(expt) and Mr(calc) correspond to the theoretical molecular mass and the molecular mass that was calculated from the observed molecular mass, respectively. The scores from the Mascot search were 91 (A), 90 (B), 164 (C), and 248 (D).
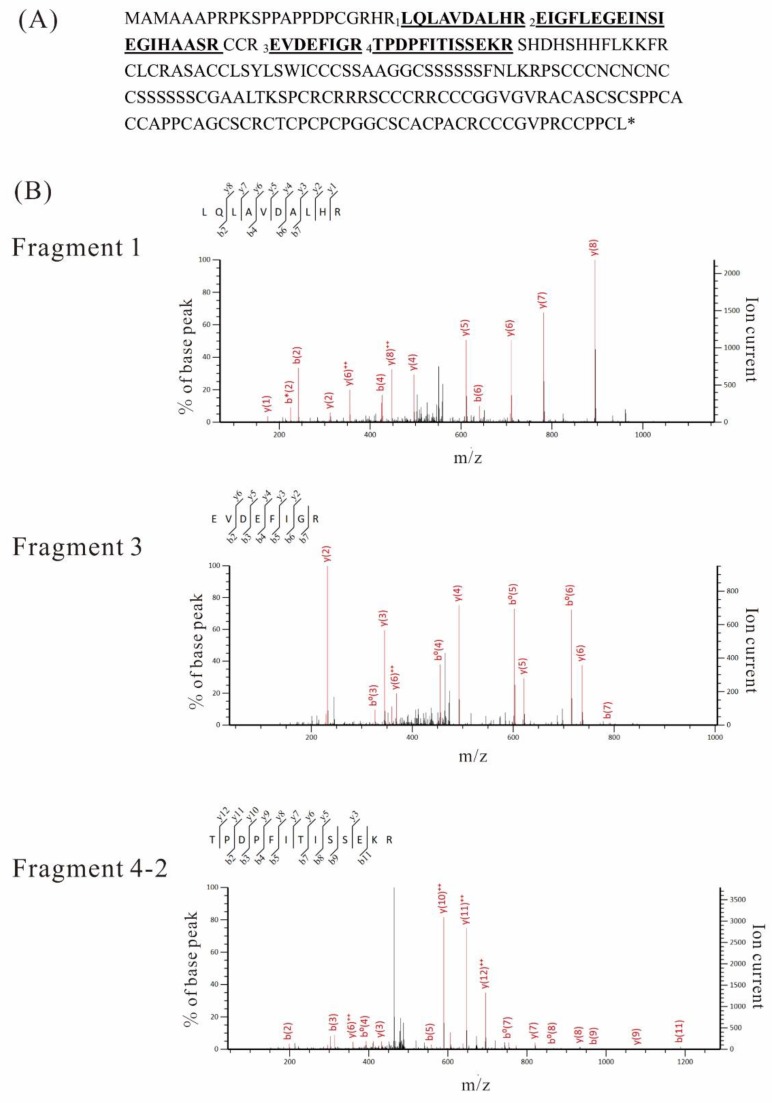
Figure 4.
LC-MS/MS analysis of Gγ3 candidates. (A) Four peptides (p < 0.05), which were produced by trypsin-digested Gγ3 candidates in the wild-type (WT) and Mi, were numbered and underlined in the full length Gγ3 amino acid sequence. These peptides are listed in Table 1. (B) MS/MS spectra of the three fragments, which were obtained from the immunoprecipitation product of Gγ3 in the WT (Figure 3A, lane 3). Fragment numbers correspond to Table 1C.
In the analysis of the plasma membrane fraction of the WT, three Gγ3 fragments (fragments 1, 2, and 3) (p < 0.05) were detected in a gel piece containing a 32 kDa protein (Table 1A). In the plasma membrane fraction of Mi, three Gγ3 fragments (fragments 2, 3, and 4-1) (p < 0.05) were detected in a gel piece containing a 20 kDa protein (Table 1B).
Immunoprecipitation products were separated by SDS-PAGE and analyzed by LC-MS/MS. Immunoprecipitation products from the WT and Mi were not detected by silver staining (data not shown). In the immunoprecipitation products of the WT (Figure 3A, lane 3), a gel piece containing a 32 kDa protein was cut and digested with trypsin, and the resultant peptides were analyzed by LC-MS/MS. As a result, three Gγ3 fragments (fragments 1, 3, and 4-2), represented as primary mass (p < 0.05), were obtained (Table 1C). Fragment 4-2 is an incomplete trypsin-digested fragment containing an arginine residue (R) at its C-terminus, making it differ from fragment 4-1. In the immunoprecipitation products of Mi, a gel piece containing a 20-kDa protein was cut and digested by trypsin, and the resultant peptides were analyzed by LC-MS/MS. As a result, four fragments (fragments 1, 2, 3, and 4-1) (p < 0.05) were obtained (Table 1D).
The MS/MS results of fragments 1, 3, and 4-2 are shown in Figure 4B. Based on these results, we concluded that the 32 kDa and 20 kDa polypeptides were Gγ3 and Gγ3∆Cys, respectively. When the immunoprecipitation product of the WT was analyzed by LC-MS/MS, five fragments, SPCRCR, SCCCRR, RCCCGGVGVR, ACASCSCSPPCACCAPPCAGCSCR, and CCPPCL, which were positioned at the C-terminal parts of Gγ3, were detected by the Mascot search, but their scores were very low (Mascot score < 11). Therefore, these five fragments were excluded from Table 1 and Figure 4A.
When the NCBI protein database was used for the analysis of Gγ3 candidates, Gγ3 was annotated using another name, BAH89202.1
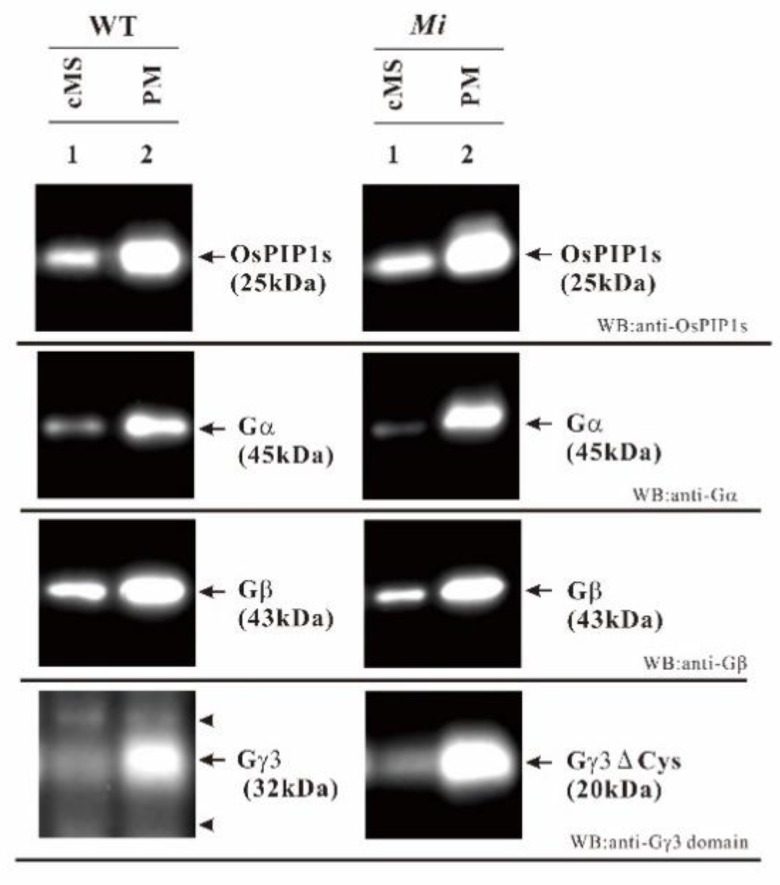
2.6. Gγ3 and Gγ3∆Cys Were Enriched in the Plasma Membrane Fraction
To check whether Gγ3 and Gγ3∆Cys are enriched in the plasma membrane, the amount of Gγ3 and Gγ3∆Cys in the crude microsomal fraction was compared with that in the plasma membrane fraction (Figure 5). Tissue-homogenate was centrifuged at 10,000× g for 10 min and the resulting supernatant was centrifuged at 100,000× g for 1 h. The precipitate (100,000 g ppt) was named the crude microsomal fraction (cMS). The plasma membrane fractions were prepared from cMS, using the aqueous two-polymer phase system.
Figure 5.
Gγ3 and Gγ3ΔCys were enriched in the plasma membrane fraction of the wild-type (WT) and Minute (Mi) flowers. First 10 μg of both the crude microsomal fraction protein and plasma membrane fraction protein from the WT and Mi were analyzed by western blot using anti-OsPIP1s, anti-Gα, anti-Gβ, and anti-Gγ3 domain antibodies. OsPIP1s is an aquaporin, which is a plasma membrane marker. OsPIP1s (25 kDa), Gα (45 kDa), Gβ (4 3kDa), Gγ3 (32 kDa), and Gγ3ΔCys (20 kDa) are indicated by arrows. Non-specific bands are indicated by arrow heads.
OsPIP1s is an aquaporin, which is a plasma membrane marker. Gα and Gβ are the subunits of the heterotrimeric G protein complex in rice. The OsPIP1s, Gα subunit, and Gβ subunit were enriched in the plasma membrane fraction. Furthermore, Gγ3 (32 kDa in WT) and Gγ3∆Cys (20 kDa in Mi) were also enriched in the plasma membrane fraction. These results showed that Gγ3 (32 kDa in WT) and Gγ3∆Cys (20 kDa in Mi) were localized in the plasma membrane fraction.
2.7. Tissue-Specific Accumulation of Gγ3
In order to know the tissues in which Gγ3 accumulates, the accumulation profile of Gγ3 was studied using the plasma membrane fractions of one-week-old etiolated seedlings of WT, developing leaf sheaths, and flowers. The results showed that the Gγ3 protein largely accumulated in the developing flower (Figure 6).
Figure 6.
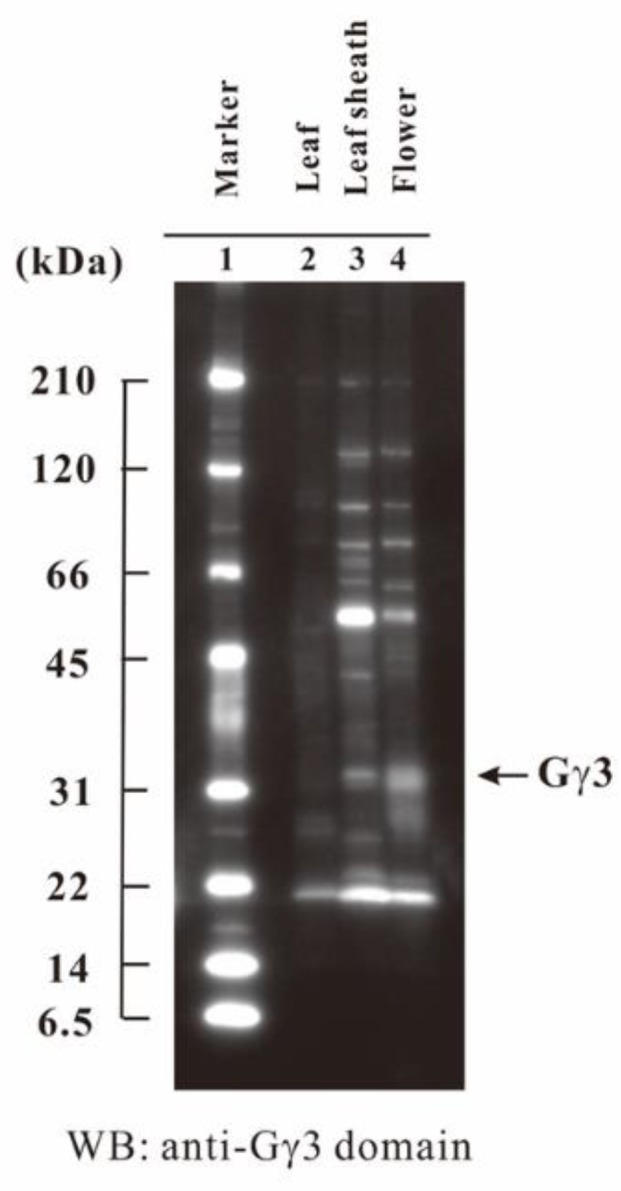
Tissue-specific accumulation of Gγ3 in the wild-type (WT). Ten micrograms of each of the plasma membrane fraction proteins of the leaf, leaf sheath, and flower in the WT was analyzed by SDS-PAGE and Western blotting using an anti-Gγ3 domain antibody. Molecular weight marker (lane 1); leaf from etiolated seedling (lane 2); developing leaf sheath at the eighth leaf stage (lane 3); 1–5 cm flower (lane 4).
3. Discussion
In rice, there are three atypical γ subunit genes (RGG3, RGG4, and RGG5) that are homologous to AGG3. The tentatively named RGG3 corresponds to GRAIN SIZE 3 (GS3), which is a gene that regulates seed length [19,30,56,57,58] and RGG4 corresponds to DENSE AND ERECT PANICLE1 (DEP1), which is a gene that regulates plant architecture including semi-dwarfness, panicle number and panicle erectness [20,55]. RGG5 corresponds to GGC2 [21], which a gene that increases grain length in combination or individually with DEP1 [56]. These genes are important for rice breeding. These have been already cloned, but their native translation products have not yet been studied. In this study, we focused on the native translation products of RGG3/GS3/Mi/OsGGC1.
First, we detected the Gγ3 candidate from the WT and the truncated Gγ3 candidate (Gγ3∆Cys) from Mi by Western blotting using anti-Gγ3 domain antibodies (Figure 2A). In SDS-PAGE, the molecular weights of the Gγ3 and Gγ3∆Cys candidates were estimated as 32 and 20 kDa, respectively, which were larger than the molecular mass calculated using cDNAs, i.e., 24 and 16 kDa, respectively. These results indicate that modifications, such as glycosylation, ubiquitination, phosphorylation, and lipid modification (palmitoylation etc.), may have occurred after translation in the Gγ3 and Gγ3∆Cys candidates. The identification of the modification is a subject requiring further study. In order to obtain concrete evidence on whether the Gγ3 and Gγ3∆Cys candidates detected by western blotting were actually Gγ3 and Gγ3∆Cys proteins, the immunoprecipitation products of the Gγ3 and Gγ3∆Cys candidates were analyzed by LC-MS/MS (Figure 3 and Figure 4). As a result, four fragments, with p < 0.05 by the Mascot search engine, were obtained from the Gγ3 and Gγ3∆Cys candidates. These results indicated that the Gγ3 and Gγ3∆Cys candidates were actually Gγ3 and Gγ3∆Cys, respectively.
Mutants of RGG3, i.e., Mi [58] and GS3-3 [30], set small and large seeds, respectively (Figure 1B). Thus, RGG3 regulates seed morphology. Gγ3 and Gγ3∆Cys were accumulated in the plasma membrane fraction of the flower tissue (Figure 5). The tissue in which Gγ3 and Gγ3∆Cys were accumulated corresponded to the tissue that showed the morphological abnormalities in Mi and GS3-3 (Figure 1 and Figure 6). One of the deletion alleles of GS3 decreased the cell number in the lemma and palea and a knock-down construct of GS3 utilizing RNAi increased the cell number [58]. Gγ3 also modulates cell proliferation, similar to Gα [31] and Gβ [32]. The chemiluminescent intensity of Gγ3ΔCys was more than 7-fold that of Gγ3 (Figure 2). The reason that the amount of Gγ3 was fewer than that of Gγ3ΔCys may be that Gγ3 is degraded by proteases. Another possibility could be that Gγ3ΔCys may stably accumulate in the plasma membrane with other proteins, including Gβ. Hence, further analysis of native and truncated Gγ3s will be important to understanding seed size regulation.
Sun et al. reported that GS3-1 (corresponding to Gγ3) interacted with Gβ using a Y2H assay [56]. Using BiFC, they also revealed that GS3-1 and GS3-4, truncated Gγ3 proteins in GS3-4, interacted with Gβ on the plasma membrane [56]. GS3-4 in GS3-4 [30,56] and Gγ3∆Cys in Mi [58] consisted of 149 and 146 amino acid residues, respectively. GS3-4 and Gγ3∆Cys have the canonical Gγ domain and a putative transmembrane domain, but largely lack a cysteine-rich domain. In this study, native Gγ3 and Gγ3∆Cys were enriched in the rice plasma membrane, similar to the Gβ subunit (Figure 5). We also confirmed that Gγ3 and Gγ3∆Cys interacted with Gβ using a Y2H assay (data not shown). From these results, it is suggested that Gγ3 and Gγ3∆Cys may form a dimer with Gβ on the plasma membrane. As we identified Gγ3 and Gγ3∆Cys by immunological techniques and LC-MS/MS analysis in this study, it will be possible to research whether the Gγ3 protein is a component of the heterotrimeric G protein complex containing the canonical Gα and XLGs.
As the seeds of Mi [58] and GS3-4 [30,56] were shorter than those of the WT, Gγ3∆Cys is the cause of shortened seeds. It will be important to clarify whether Gγ3∆Cys interacts with Gβ. If the βγ dimer composed with Gγ3∆Cys is present in the plasma membrane, it will be interesting to research the interaction between the unusual βγ dimer (GβGγ3∆Cys) and the canonical Gα or XLGs, on the basis of the G protein signaling model [5,6]. As previously reported, some βγ dimers seem to be present in two different fractions in gel filtration: one is a component of a huge complex, and the other is a sole βγ dimer in the plasma membrane of etiolated rice seedlings [18]. Although this may be the result of artificial dissociation during solubilization and gel fractionation, this approach will be important for understanding the heterotrimeric G protein complex. Truncated Gγ3 in GS3-3, namely the Gγ3∆γ domain, consisted of 55 amino acid residues, which is considered as a loss of function of OSR (organ size regulation) [30]. In GS3-3, the Gγ3∆γ domain was not detected in the plasma membrane (Figure 2A). The reason may be due to the lack of the trans-membrane domain in the Gγ3∆γ domain or due to the lack of sites that anti-Gγ3 domain antibody recognizes in the Gγ3∆γ domain. In addition, the Gγ3∆γ domain was not detected in the cytosolic fraction (data not shown). However, it is not ruled out that there is no Gγ3∆γ domain in the cytosolic fraction, due to the detection threshold in Western blot not being met. As seeds of GS3-3 were longer than those of the WT, the lack of a βγ3 dimer may be the cause of enlarged seeds. Hence, as we detected Gγ3 and Gγ3∆Cys proteins in this study, biochemical analysis of the heterotrimeric G protein complex in Mi and GS3-3 will be accelerated. It is of interest to reveal the subunit stoichiometry of the canonical Gα, XLGs, Gβ, and five Gγs, namely γ1, γ2, the Gγ3∆γ domain, γ4, and γ5, and the subsequent subunit composition of the G protein complex in Mi, which sets small grains. It is also important to analyze the subunit stoichiometry of the canonical Gα, XLGs, Gβ, and four Gγs, namely γ1, γ2, γ4, and γ5, and the subsequent subunit composition of the G protein complex in GS3-3, which sets large grains.
4. Materials and Methods
4.1. Plant Materials
A rice cultivar (Oryza sativa L. cv. Taichung 65) and two heterotrimeric G protein γ3 mutants (GS3-3 and Mi) were used in this study. GS3-3 was obtained from the Taichung 65 mutant library, mutagenized by N-methyl-N-nitrosourea treatment, and named TCM-3-467. The Mi mutation was provided from the stocked mutant line, H343 (Oryza sativa L. cv. Akamuro background). H343 was backcrossed four times with Taichung 65, and was used as a near-isogenic line of Mi in this study. All rice plants were grown under a 14-h light (50,000 lux and 28 °C) and 10-h dark (25 °C) cycle, or under natural field conditions.
4.2. Sequencing and Confirmation of RGG3
Genomic DNA was isolated from whole plants of WT, Mi, and GS3-3 using an extraction method with cetyltrimethylammonium bromide (CTAB) [59]. Using this as a template, PCR was performed using > 20 sets of PCR primers to cover 5609 bases of RGG3 (Os03g0407400). The amplified DNA fragments were directly sequenced using the same primers that were used for amplification.
4.3. RNA Isolation, Reverse Transcription, and cDNA Encoding of the Heterotrimeric G Protein Gγ3 Subunit
Total RNA from the flower tissue was directly extracted using RNeasy Plant Mini kits (Qiagen, Hilden, Germany). The first strand of cDNA was synthesized using Super Script First Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA, USA). Total RNA (0.5 μg) and oligo-dT were used as the template and primer, respectively, for the first strand cDNA synthesis.
In order to isolate RGG3 cDNA, the primers were designed based on the database information (Os03g0407400):
RGG3 forward: 5’ atggcaatggcggcggcgcc 3’;
RGG3 reverse: 5’ caagcagggggggcagcaac 3’.
The amplified PCR products were sub-cloned into pCR4 (Invitrogen) and sequenced with a Thermo BigDye Terminator Cycle Sequencing Kit (Amersham Biosciences, Little Chalfont, UK) using a DNA sequencer (Model 377; Applied Biosystems, Foster City, CA, USA).
4.4. Preparation of the Microsomal and Plasma Membrane Fractions in Rice
Crude microsomal fractions were prepared from 2–5 cm flowers of the WT, Mi, and GS3-3, as described previously [18], and plasma membrane fractions were purified from the crude microsomal fraction using an aqueous two-polymer phase system [60]. From the etiolated seedlings, which were grown for 5 d at 28 °C, and developing leaf sheaths at the eighth leaf stage, crude microsomal fractions and plasma membrane fractions were prepared, respectively.
4.5. SDS-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Electrophoresis was carried out on 12.5% and 10/20% gradient polyacrylamide gels containing 0.1% SDS, as described previously [61].
For LC-MS/MS analysis, 40 μg of flower plasma membrane proteins from both the WT and Mi were analyzed using 15% SDS-PAGE. Electrophoresis was stopped at a position where the Bromophenol Blue was 3 cm away from the stacking gel. The 3-cm long gel was divided into 10 pieces according to the molecular weight marker (Precision Plus ProteinTM KaleidoscopeTM; Bio-Rad Laboratories), without staining. These gel pieces were used for trypsin digestion. In some cases, gels were silver-stained using Pierce Silver Stain for Mass Spectrometry (Thermo Scientific).
4.6. Preparation of Trx-Gγ3 and GST-Gγ3 Domain Proteins
cDNA encoding 120 amino acid residues from the N-terminal of the rice Gγ3 protein was amplified by PCR using primers. The cDNA contains the Gγ3 domain and the putative transmembrane region:
RGG3 domain forward: 5’ccttggctcatatggatatcatggcaatggcggcggcgccccggcccaag3’;
RGG3 domain reverse: 5’aagcttcccgggtcaggaggaggatgagcagccgccggcggcgctgctg3’.
Amplified cDNA was sub-cloned in pET32a containing thioredoxin (Trx) and histidine (His) tags (Novagen). The resultant clone, the Trx-Gγ3 domain vector, was transformed in T7 Express lysY/Iq E. coli (New England Biolabs), and the recombinant protein was synthesized and designated as the Trx-Gγ3 domain protein. The cDNA covering the Gγ3 domain was also sub-cloned in pET41 containing glutathione S-transferase (GST) and His tags (Novagen). The resultant clone, the GST-Gγ3 domain vector, was transformed in T7 Express lysY/Iq E. coli (New England Biolabs), and the recombinant protein was synthesized and designated as the GST-Gγ3 domain protein.
The overexpression of the Trx-Gγ3 domain protein and GST-Gγ3 domain protein in T7 Express lysY/Iq E. coli was carried out as described elsewhere [61]. Inductions were performed at 37 °C. Induction was initiated by the addition of IPTG (final IPTG concentration, 1 mM). After 3 h, E. coli was harvested after centrifugation at 10,000 × g for 5 min at 4 °C, and stocked at −80 °C before use.
As the Trx-Gγ3 domain protein and GST-Gγ3 domain protein were included in the body, both proteins were solubilized in 6 M guanidine hydrochloride, 10 mM Tris HCl, pH 8.0. Solubilized proteins were applied to Ni-NTA agarose (Qiagen, Hilden, Germany). The purification of both proteins was performed according to the protocols recommended by the manufacturers.
The antibody was raised against the Trx-Gγ3 domain protein in rabbits. Affinity purification of the antibody was performed using a polyvinylidene fluoride (PVDF) filter (Millipore, Burlington, MA, USA), immobilized with the GST-Gγ3 domain protein.
4.7. Western Blot Analysis (WB)
Proteins were separated by 12.5% or 10/20% gradient SDS-PAGE, and blotted onto a PVDF membrane (Millipore). The antibody against the rice Gγ3 domain was affinity-purified in this study. Antibodies against the rice heterotrimeric G protein α and β subunits, namely the anti-Gα and anti-Gβ antibodies, were used as described previously [18]. The antibody against aquaporin (a plasma membrane marker), namely, anti-OsPIP1s, was purchased from Operon Biotechnologies. The Chemi-Lumi One Markers Kit (Nacalai Tesque, Kyoto, Japan) was used as a molecular weight marker for western blotting.
ECLTM peroxidase labelled anti-rabbit antibody was purchased as second antibody from GE Healthcare, Little Chalfont, UK. ECL ImmobilonTM Western Chemiluminescent HRP Substrate (Millipore, Burlington, MA, USA) was used as the western blotting detection reagent. The chemiluminescent signal was measured using a Fusion SL (MS instruments).
4.8. Immunoprecipitation
First, 50 μg of affinity-purified anti-Gγ3 domain antibody was bound to 50 mg of Protein A bound magnetic beads (Millipore, Burlington, MA, USA). After washing them thrice with 1× PBS, the anti-Gγ3 domain antibody and Protein A were cross-linked with dimethyl pimelimidate dihydrochloride (DMP). The conditions followed for cross-linking were according to the protocols recommended by the manufacturers. After quenching the magnetic cross-linked beads with the anti-Gγ3 domain antibody, they were stored at 4 °C until use.
Next, 0.1 mL of 10% SDS was added to 0.9 mL of plasma membrane fraction (1 mg protein/10 mg SDS/mL) and denatured for 5 min at 90 °C. After diluting the solubilized fraction with 10 mL of 1× TBS containing 1% Tween 20, the magnetic beads cross-linked with 50 μg of the anti-Gγ3 domain antibody were added. After incubation for 2 h at 25 °C, the magnetic beads were collected into a 1.5 mL tube and washed thrice each with 0.5 mL of 1× TBS containing 0.1% Tween 20 and 0.5 mL of 1× TBS. Proteins were eluted using 40 μL of dissociation buffer (Bio-rad) without a reducing agent, from the beads. In total, 5 μL of each eluate was used for LC-MS/MS.
4.9. Protein Reduction, Alkylation, and Trypsin Digestion for LC-MS/MS Analysis
Gel pieces were resuspended in 50 mM NH4HCO3, reduced with 50 mM dithiothreitol for 30 min at 56 °C, and alkylated with 50 mM iodoacetamide for 30 min at 37 °C in the dark. Alkylated proteins in the gels were digested with 10 μg/mL of trypsin solution (Promega, Madison, WI, USA) for 16 h at 37 °C. The resultant peptides were concentrated and suspended in 0.1% formic acid and analyzed by LC-MS/MS.
4.10. Protein Identification Using Nano-LC-MS/MS
The peptides were loaded onto the LC system (EASY-nLC 1000; Thermo Fisher Scientific, Waltham, MA, USA) equipped with a trap column (EASY-Column, C18-A1 5 µm, 100 µm ID × 20 mm; Thermo Fisher Scientific), equilibrated with 0.1% formic acid, and eluted with a linear acetonitrile gradient (0–50%) in 0.1% formic acid at a flow rate of 200 nL/min. The eluted peptides were loaded and separated on the column (C18 capillary tip column, 75 µm ID × 120 mm; Nikkyo Technos, Tokyo, Japan) with a spray voltage of 1.5 kV. The peptide ions were detected using MS (LTQ Orbitrap Elite MS; Thermo Fisher Scientific) in the data-dependent acquisition mode with Xcalibur software (version 2.2; Thermo Fisher Scientific). Full-scan mass spectra were acquired in MS over 400–1500 m/z with a resolution of 60,000. The 10 most intense precursor ions were selected for collision-induced fragmentation in the linear ion trap, at a normalized collision energy of 35%. Dynamic exclusion was employed within 90 s to prevent the repetitive selection of peptides.
4.11. MS Data Analysis
Protein identification was performed using the Mascot search engine (version 2.5.1, Matrix Science, London, UK) and the in-house database, which constructed the amino acid sequences of rice heterotrimeric G protein subunits. For both the searches, the carbamidomethylation of cysteine was set as a fixed modification, and oxidation of methionine was set as a variable modification. Trypsin was specified as the proteolytic enzyme and one missed cleavage was allowed. The peptide mass tolerance was set at 10 ppm, fragment mass tolerance was set at 0.8 Da, and peptide charges were set at +2, +3, and +4. An automatic decoy database search was performed as part of the search. Mascot results were filtered with the Percolator function to improve the accuracy and sensitivity of peptide identification. The minimum requirement for the identification of a protein was two matched peptides. Significant changes in the abundance of proteins between samples were determined (p < 0.05).
4.12. Gene ID
The accession numbers of the rice heterotrimeric G proteins α, β, and γ3 subunit genes (RGA1, RGB1, and RGG3, respectively) are Os05g0333200, Os03g0669200, and Os03g0407400, respectively.
Acknowledgments
We thank Yasuo Nagato for providing TCM3-467 and Iturou Takamure for H343. Part of the work was performed at the Biological Resource Research and Development Center, Fukui Prefectural University (Fukui, Japan).
Abbreviations
| agb1 | mutant of heterotrimeric G protein β subunit gene in Arabidopsis |
| AGB1 | heterotrimeric G protein β subunit gene in Arabidopsis |
| AGG1 | heterotrimeric G protein γ1 subunit gene in Arabidopsis |
| AGG2 | heterotrimeric G protein γ2 subunit gene in Arabidopsis |
| AGG3 | heterotrimeric G protein γ3 subunit gene in Arabidopsis |
| cMS | crude microsomal fraction |
| d1 | mutant of heterotrimeric G protein α subunit gene in rice |
| DEP1 | DENCE AND ERECT PANICLES 1 gene |
| DN1 | DENCE PANICLE 1 gene |
| gpa1 | mutant of heterotrimeric G protein α subunit gene in Arabidopsis |
| GPA1 | heterotrimeric G protein α subunit gene in Arabidopsis |
| GS3 | GRAIN SIZE 3 gene |
| Mi | MINUTE, a mutant of GS3/RGG3 |
| OsGGC1 | a gene of heterotrimeric G protein γ subunit Type-C in rice, which corresponds to GS3/RGG3 |
| OsGGC2 | a gene of heterotrimeric G protein γ subunit Type-C in rice, which corresponds to RGG5 |
| OsGGC3 | a gene of heterotrimeric G protein γ subunit Type-C in rice, which corresponds to which corresponds to DEP1/RGG4 |
| PM | plasma membrane |
| RGA1 | heterotrimeric G protein α subunit gene in rice |
| RGB1 | heterotrimeric G protein β subunit gene in rice |
| RGG1 | heterotrimeric G protein γ1 subunit gene in rice |
| RGG2 | heterotrimeric G protein γ2 subunit gene in rice |
| RGG3 | heterotrimeric G protein γ3 subunit gene in rice |
| RGG4 | heterotrimeric G protein γ4 subunit gene in rice |
| RGG5 | heterotrimeric G protein γ5 subunit gene in rice |
| WB | western blot |
| WT | wild-type |
| XLG | extra-large GTP-binding protein |
Author Contributions
Investigation and formal analysis, A.N. and S.M.; methodology, T.I.; resources, G.C. and K.M.; writing and funding acquisition, Y.I.
Funding
This work was supported by a grant for Scientific Research from Fukui Prefectural University and the JSPS KAKENHI, Grant Number 26712001.
Conflicts of Interests
The authors declare no conflicts of interest.
References
- 1.Offermanns S. Mammalian G-protein function in vivo: New insights through altered gene expression. Rev. Physiol. Biochem. Pharmacol. 2000;140:63–133. doi: 10.1007/BFb0035551. [DOI] [PubMed] [Google Scholar]
- 2.Gomperts B.D., Kramer I.J.M., Tatham P.E.R., editors. Signal Transduction. Elsevier Inc.; Amsterdam, The Netherlands: 2002. [Google Scholar]
- 3.Wettschureck N., Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 4.Milligan G., Kostenis E. Heterotrimeric G-proteins: A short history. Br. J. Pharmacol. 2006;147:S46–S55. doi: 10.1038/sj.bjp.0706405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Temple B.R.S., Jones A.M. The Plant Heterotrimeric G-Protein Complex. Annu. Rev. Plant Biol. 2007;58:249–266. doi: 10.1146/annurev.arplant.58.032806.103827. [DOI] [PubMed] [Google Scholar]
- 6.Urano D., Chen J.-G., Botella J.R., Jones A.M. Heterotrimeric G protein signalling in the plant kingdom. Open Biol. 2013;3:120–186. doi: 10.1098/rsob.120186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urano D., Miura K., Wu Q., Iwasaki Y., Jackson D., Jones A.M. Plant morphology of heterotrimeric G protein mutants. Plant Cell Physiol. 2016;57:437–445. doi: 10.1093/pcp/pcw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y.-R.J., Assmann S.M. Arabidopsis thaliana ‘extra-large GTP-binding protein’ (AtXLG1): A new class of G-protein. Plant Mol. Biol. 1999;40:55–64. doi: 10.1023/A:1026483823176. [DOI] [PubMed] [Google Scholar]
- 9.Assmann S.M. Heterotrimeric and unconventional GTP binding proteins in plant cell signaling. Plant Cell. 2002:355S–S373. doi: 10.1105/tpc.001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma H., Yanofsky M.F., Meyerowitz E.M. Molecular cloning and characterization of GPA1, a G protein α subunit gene from Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 1990;87:3821–3825. doi: 10.1073/pnas.87.10.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss C.A., Garnaat C.W., Mukai K., Hu Y., Ma H. Isolation of cDNAs encoding guanine nucleotide-binding protein β-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1) Proc. Natl. Acad. Sci. USA. 1994;91:9554–9558. doi: 10.1073/pnas.91.20.9554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mason M.G., Botella J.R. Completing the heterotrimer: Isolation and characterization of an Arabidopsis thaliana G protein γ-subunit cDNA. Proc. Natl. Acad. Sci. USA. 2000;97:14784–14788. doi: 10.1073/pnas.97.26.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mason M.G., Botella J.R. Isolation of a novel G-protein γ-subunit from Arabidopsis thaliana and its interaction with Gβ. Biochim. Biophys. Acta. 2001;1520:147–153. doi: 10.1016/S0167-4781(01)00262-7. [DOI] [PubMed] [Google Scholar]
- 14.Chakravorty D., Trusov Y., Zhang W., Acharya B.R., Sheahan M.B., McCurdy D.W., Assmann S.M., Botella J.R. An atypical heterotrimeric G-protein γ-subunit is involved in guard cell K+-channel regulation and morphological development in Arabidopsis thaliana. Plant J. 2011;67:840–851. doi: 10.1111/j.1365-313X.2011.04638.x. [DOI] [PubMed] [Google Scholar]
- 15.Wu Q., Regan M., Furukawa H., Jackson D. Role of heterotrimeric Gα proteins in maize development and enhancement of agronomic traits. PLOS Genet. 2018;14:e1007374. doi: 10.1371/journal.pgen.1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa A., Tsubouchi H., Iwasaki Y., Asahi T. Molecular cloning and characterization of a cDNA for the α subunit of a G protein from rice. Plant Cell Physiol. 1995;36:353–359. doi: 10.1093/oxfordjournals.pcp.a078767. [DOI] [PubMed] [Google Scholar]
- 17.Ishikawa A., Iwasaki Y., Asahi T. Molecular cloning and characterization of a cDNA for the β subunit of a G protein from rice. Plant Cell Physiol. 1996;37:223–228. doi: 10.1093/oxfordjournals.pcp.a028935. [DOI] [PubMed] [Google Scholar]
- 18.Kato C., Mizutani T., Tamaki H., Kumagai H., Kamiya T., Hirobe A., Fujisawa Y., Kato H., Iwasaki Y. Characterization of heterotrimeric G protein complexes in rice plasma membrane. Plant J. 2004;38:320–331. doi: 10.1111/j.1365-313X.2004.02046.x. [DOI] [PubMed] [Google Scholar]
- 19.Fan C., Xing Y., Mao H., Lu T., Han B., Xu C., Li X., Zhang Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006;112:1164–1171. doi: 10.1007/s00122-006-0218-1. [DOI] [PubMed] [Google Scholar]
- 20.Huang X., Qian Q., Liu Z., Sun H., He S., Luo D., Xia G., Chu C., Li J., Fu X. Natural variation at the DEP1 locus enhances grain yield in rice. Nat. Genet. 2009;41:494–497. doi: 10.1038/ng.352. [DOI] [PubMed] [Google Scholar]
- 21.Botella J.R. Can heterotrimeric G proteins help to feed the world? Trend Plant Sci. 2012;17:563–568. doi: 10.1016/j.tplants.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Trusov Y., Chakravorty D., Botella J.R. Diversity of heterotrimeric G-protein γ subunits in plants. BMC Res. Notes. 2012;5:608. doi: 10.1186/1756-0500-5-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding L., Pandey S., Assmann S.M. Arabidopsis extra-large G proteins (XLGs) regulate root morphogenesis. Plant J. 2008;53:248–263. doi: 10.1111/j.1365-313X.2007.03335.x. [DOI] [PubMed] [Google Scholar]
- 24.Ullah H., Chen J.-G., Young J.C., Im K.-H., Sussman M.R., Jones A.M. Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science. 2001;292:2066–2069. doi: 10.1126/science.1059040. [DOI] [PubMed] [Google Scholar]
- 25.Lease K.A., Wen J., Li J., Doke J.T., Liscum E., Walker J.C. A mutant Arabidopsis heterotrimeric G-protein β subunit affects leaf, flower, and fruit development. Plant Cell. 2001;13:2631–2641. doi: 10.2307/3871524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ullah H., Chen J.-G., Temple B., Boyes D.C., Alonso J.M., Davis K.R., Ecker J.R., Jones A.M. The β-subunit of Arabidopsis G protein negatively regulates auxin-induced cell division and affects multiple developmental processes. Plant Cell. 2003;15:393–409. doi: 10.1105/tpc.006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trusov Y., Rookes J.E., Tilbrook K., Chakravorty D., Mason M.G., Anderson D., Chen J.-G., Jones A.M., Botella J.R. Heterotrimeric G protein γ subunits provide functional selectivity in Gβγ dimer signaling in Arabidopsis. Plant Cell. 2007;19:1235–1250. doi: 10.1105/tpc.107.050096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujisawa Y., Kato T., Ohki S., Ishikawa A., Kitano H., Sasaki T., Asahi T., Iwasaki Y. Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc. Natl. Acad. Sci. USA. 1999;96:7575–7580. doi: 10.1073/pnas.96.13.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashikari M., Wu J., Yano M., Sasaki T., Yoshimura A. Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the α-subunit of GTP-binding protein. Proc. Natl. Acad. Sci. USA. 1999;96:10284–10289. doi: 10.1073/pnas.96.18.10284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mao H., Sun S., Yao J., Wang C., Yu S., Xu C., Li X., Zhang Q. Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc. Natl. Acad. Sci. USA. 2010;107:19579–19584. doi: 10.1073/pnas.1014419107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izawa Y., Takayanagi Y., Inaba N., Abe Y., Minami M., Fujisawa Y., Kato H., Ohki S., Kitano H., Iwasaki Y. Function and expression pattern of the α subunit of the heterotrimeric G protein in rice. Plant Cell Physiol. 2010;51:271–281. doi: 10.1093/pcp/pcp186. [DOI] [PubMed] [Google Scholar]
- 32.Utsunimiya U., Samejima C., Takayanagi Y., Izawa Y., Yoshida T., Sawada Y., Fijisawa Y., Kato H., Iwasaki Y. Suppression of the rice heterotrimeric G protein β-subunit gene, RGB1, causes dwarfism and browning of internodes and lamina joint regions. Plant J. 2011;67:907–916. doi: 10.1111/j.1365-313X.2011.04643.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang X.Q., Ullah H., Jones A.M., Assmann S.M. G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science. 2001;292:2070–2072. doi: 10.1126/science.1059046. [DOI] [PubMed] [Google Scholar]
- 34.Coursol S., Fan L.M., Le Stunff H., Spiegel S., Gilroy S., Assmann S.M. Sphingolipid signalling in Arabidopsis guard cells involves heterotrimeric G proteins. Nature. 2003;423:651–654. doi: 10.1038/nature01643. [DOI] [PubMed] [Google Scholar]
- 35.Lapik Y.R., Kaufman L.S. The Arabidopsis cupin domain protein AtPirin1 interacts with the G protein α-Subunit GPA1 and regulates seed germination and early seedling development. Plant Cell. 2003;15:1578–1590. doi: 10.1105/tpc.011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandey S., Assmann S.M. The Arabidopsis putative G protein-coupled receptor GCR1 interacts with the G protein α subunit GPA1 and regulates abscisic acid signaling. Plant Cell. 2004;16:1616–1632. doi: 10.1105/tpc.020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra G., Zhang W., Deng F., Zhao J., Wang X. A bifurcating pathway directs abscisic acid effects on stomatal closure and opening in Arabidopsis. Science. 2006;312:264–266. doi: 10.1126/science.1123769. [DOI] [PubMed] [Google Scholar]
- 38.Ueguchi-Tanaka M., Fujisawa Y., Kobayashi M., Ashikari M., Iwasaki Y., Kitano H., Matsuoka M. Rice dwarf mutant d1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc. Natl. Acad. Sci. USA. 2000;97:11638–11643. doi: 10.1073/pnas.97.21.11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ullah H., Chen J.G., Wang S., Jones A.M. Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol. 2002;129:897–907. doi: 10.1104/pp.005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J.G., Pandey S., Huang J., Alonso J.M., Ecker J.R., Assmann S.M., Jones A.M. GCR1 can act independently of heterotrimeric G-protein in response to brassinosteroids and gibberellins in Arabidopsis seed germination. Plant Physiol. 2004;135:907–915. doi: 10.1104/pp.104.038992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bethke P.C., Hwang Y.S., Zhu T., Jones R.L. Global patterns of gene expression in the aleurone of wild-type and dwarf1 mutant rice. Plant Physiol. 2006;140:484–498. doi: 10.1104/pp.105.074435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen J.-G., Jones A.M. AtRGS1 function in Arabidopsis thaliana. Method. Enzymol. 2004;389:338–350. doi: 10.1016/S0076-6879(04)89020-7. [DOI] [PubMed] [Google Scholar]
- 43.Huang J., Taylor J.P., Chen J.G., Uhrig J.F., Schnell D.J., Nakagawa T., Korth K.L., Jones A.M. The plastid protein THYLAKOID FORMATION1 and the plasma membrane G-protein GPA1 interact in a novel sugar-signaling mechanism in Arabidopsis. Plant Cell. 2006;18:1226–1238. doi: 10.1105/tpc.105.037259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warpeha K.M., Hamm H.E., Rasenick M.M., Kaufman L.S. A blue-light-activated GTP-binding protein in the plasma membranes of etiolated peas. Proc. Natl. Acad. Sci. USA. 1991;88:8925–8929. doi: 10.1073/pnas.88.20.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Warpeha K.M., Lateef S.S., Lapik Y., Anderson M., Lee B.S., Kaufman L.S. G-protein-coupled receptor 1, G-protein Gα-subunit 1, and Prephenate dehydratase 1 are required for blue light-induced production of phenylalanine in etiolated Arabidopsis. Plant Physiol. 2006;140:844–855. doi: 10.1104/pp.105.071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joo J.H., Wang S., Chen J.G., Jones A.M., Fedoroff N.V. Different signaling and cell death roles of heterotrimeric G protein α and β subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell. 2005;17:957–970. doi: 10.1105/tpc.104.029603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suharsono U., Fujisawa Y., Kawasaki T., Iwasaki Y., Satoh H., Shimamoto K. The heterotrimeric G protein α subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc. Natl. Acad. Sci. USA. 2002;99:13307–13312. doi: 10.1073/pnas.192244099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komatsu S., Yang G., Hayashi N., Kaku H., Umemura K., Iwasaki Y. Alterations by a defect in a rice G protein α subunit in probenazole and pathogen-induced responses. Plant Cell Environ. 2004;27:947–957. doi: 10.1111/j.1365-3040.2004.01202.x. [DOI] [Google Scholar]
- 49.Iwata M., Umemura K., Teraoka T., Usami H., Fujisawa Y., Iwasaki Y. Role of the α subunit of heterotrimeric G-protein in probenazole-inducing defense signaling in rice. J. Gen. Plant Pathol. 2003;69:83–86. doi: 10.1007/s10327-002-0008-9. [DOI] [Google Scholar]
- 50.Lieberherr D., Thao N.P., Nakashima A., Umemura K., Kawasaki T., Shimamoto K. A sphingolipid licitor-inducible mitogen-activated protein kinase is regulated by the small GTPase OsRac1 and heterotrimeric G-protein in rice. Plant Physiol. 2005;138:1644–1652. doi: 10.1104/pp.104.057414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S., Assmann S.M., Fedoroff N.V. Characterization of the Arabidopsis heterotrimeric G protein. J. Biol. Chem. 2008;283:13913–13922. doi: 10.1074/jbc.M801376200. [DOI] [PubMed] [Google Scholar]
- 52.Klopffleish K., Phan N., Augstin K., Bayne R., Booker K.S., Bolella J., Carpita N.C., Carr T., Chen J.-C., Cooke T.R., et al. Arabidopsis G-protein interactome reveals connections to cell wall carbohydrates and morphogenesis. Mol. Syst. Biol. 2011;7:532. doi: 10.1038/msb.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J.-G., Willard F.S., Huang J., Liang J., Chasse S.A., Jones A.M., Siderovski D.P. A seven-transmembrane RGS protein that modulates plant cell proliferation. Science. 2003;301:1728–1731. doi: 10.1126/science.1087790. [DOI] [PubMed] [Google Scholar]
- 54.Adjobo-Hermans M.J., Goedhart J., Gadella T.W., Jr. Plant G protein heterotrimers require dual lipidation motifs of Gα and Gγ and do not dissociate upon activation. J. Cell Sci. 2006;119:5087–5097. doi: 10.1242/jcs.03284. [DOI] [PubMed] [Google Scholar]
- 55.Sun H., Qian Q., Wu K., Lou J., Wang S., Zhang C., Ma Y., Lie Q., Huang X., Yuan Q., et al. Heterotrimeric G proteins regulate nitrogen-use efficiency in rice. Nat. Genet. 2014;46:652–656. doi: 10.1038/ng.2958. [DOI] [PubMed] [Google Scholar]
- 56.Sun S., Wang L., Mao H., Shao L., Li X., Xiao J., Ouyang Y., Zhang Q. A G-protein pathway determines grain size in rice. Nat. Commun. 2018;9:815–824. doi: 10.1038/s41467-018-03141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takano-Kai N., Jiang H., Kubo T., Sweeney M., Matsumoto T., Kanamori H., Padhukasahasram B., Bustamante C., Yoshimura A., Doi K., et al. Evolutionary history of GS3, a gene conferring grain length in rice. Genetics. 2009;182:1–12. doi: 10.1534/genetics.109.103002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takano-Kai N., Jiang H., Powell A., McCouch S., Takamure I., Furuya N., Doi K., Yoshimura A. Multiple and independent origins of short seeded alleles of GS3 in rice. Breed. Sci. 2013;63:77–85. doi: 10.1270/jsbbs.63.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sambrook J., Russell D.W., editors. Molecular Cloning. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2001. [Google Scholar]
- 60.Yoshida S., Uemura M., Niki T., Sakai A., Gusta L.V. Partition of membrane particles in aqueous two-polymer phase system and its partial use for purification of plasma membranes from plants. Plant Physiol. 1983;72:105–114. doi: 10.1104/pp.72.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iwasaki Y., Kato T., Kaidoh T., Ishikawa A., Asahi T. Characterization of the putative α subunit of a heterotrimeric G protein in rice. Plant Mol. Biol. 1997;34:563–572. doi: 10.1023/A:1005807010811. [DOI] [PubMed] [Google Scholar]