Abstract
Transient receptor potential ankyrin type-1 (TRPA1) channels are known to actively participate in different pain conditions, including trigeminal neuropathic pain, whose clinical treatment is still unsatisfactory. The aim of this study was to evaluate the involvement of TRPA1 channels by means of the antagonist ADM_12 in trigeminal neuropathic pain, in order to identify possible therapeutic targets. A single treatment of ADM_12 in rats 4 weeks after the chronic constriction injury of the infraorbital nerve (IoN-CCI) significantly reduced the mechanical allodynia induced in the IoN-CCI rats. Additionally, ADM_12 was able to abolish the increased levels of TRPA1, calcitonin gene-related peptide (CGRP), substance P (SP), and cytokines gene expression in trigeminal ganglia, cervical spinal cord, and medulla induced in the IoN-CCI rats. By contrast, no significant differences between groups were seen as regards CGRP and SP protein expression in the pars caudalis of the spinal nucleus of the trigeminal nerve. ADM_12 also reduced TRP vanilloid type-1 (TRPV1) gene expression in the same areas after IoN-CCI. Our findings show the involvement of both TRPA1 and TRPV1 channels in trigeminal neuropathic pain, and in particular, in trigeminal mechanical allodynia. Furthermore, they provide grounds for the use of ADM_12 in the treatment of trigeminal neuropathic pain.
Keywords: neuropathic pain, trigeminal system, allodynia, TRPA1, TRPV1
1. Introduction
Trigeminal neuralgia (TN) is a rare condition characterized by paroxysmal attacks of sharp pain, frequently described as an “electric shock”. Up to 50% of patients with trigeminal neuralgia also have continuous pain in the same territory, which results in greater diagnostic difficulties, higher disability, and lower response to medical and surgical treatments [1]. Three diagnostic categories of TN are identified by the recent classification of headache disorders: Classical (without apparent cause other than neurovascular compression), secondary (caused by an underlying neurological disorder), and idiopathic (no cause is found) [2]. TN has a negative impact on activities of daily living, with up to 45% of patients being absent from usual daily activities for 15 days or more, and one third suffering from mild-to-severe depression [3]. Medications for TN exist, but they are poorly tolerated or ineffective. For this reason, multiple surgical approaches have been developed, but a portion of patients are refractory to both medical and surgical approaches [4,5]. Hence, there is need for further investigation into the mechanisms underlying pain in TN in order to identify new, possibly more effective, therapeutic targets.
In recent years, transient receptor potential (TRP) channels have attracted much attention in the pain field. These channels are non-selective cation channel proteins, widely distributed in many tissues and cell types, localized in the plasma membrane and membranes of intracellular organelles [6]. The TRP ankyrin type-1 (TRPA1) channels, mainly expressed with the vanilloid type-1 (TRPV1), are localized in a subpopulation of C- and Aδ-fibers of neurons located in the dorsal root ganglia (DRG) and trigeminal ganglia (TG) that produce and release neuropeptides, such as substance P (SP), neurokinin A, and calcitonin gene-related peptide (CGRP) [7,8,9]. Many experimental studies, from genetic knockouts to pharmacological manipulation models, reported a critical involvement of TRPA1 channels in different aspects of pain [10] and a role in several models of nerve injury, such as the lumbar spinal nerve ligation [11], and sciatic nerve injury by chronic constriction or transection [12,13,14]. In these models, it was demonstrated that an up-regulation of TRPA1 is associated with mechanical and thermal hyperalgesia, a condition reversed by TRPA1 antagonists [15,16]. In a recent study, Trevisan and colleagues [17] reported that pain-like behaviors are mediated by the TRPA1 channel in an animal model of TN based on the constriction of the infraorbital nerve (IoN) via the increased oxidative stress by-products released from monocytes and macrophages that gather at the site of nerve injury.
The aim of this study was to further investigate the role of TRP channels in trigeminal neuropathic pain induced by the model of a chronic constriction injury of the IoN (IoN-CCI) [18]. More specifically we evaluated: (i) The modulatory effect of TRPA1 antagonism, by means of ADM_12 treatment, on IoN-CCI-induced allodynia; (ii) the levels of TRPA1 and TRPV1 mRNA in specific cerebral and peripheral areas involved in trigeminal sensitization, with particular attention to changes in expression levels of genes coding for CGRP, SP, and cytokines after TRPA1 antagonism; and (iii) the expression of CGRP and SP proteins in the Spinal Nucleus of trigeminal nerve pars caudalis (Sp5C).
2. Results
2.1. ADM_12 Effect on Behavioral Response
In agreement with Deseure and Hans [18], 5 days after surgery, the two groups of rats that underwent IoN-CCI displayed a lack of responsiveness to ipsilateral mechanical stimulation testing (MST) of the IoN territory (Figure 1A). At day 12, the hyporesponsiveness was recovering to be replaced at day +26 by a significant increase in the MST response score as compared to the two Sham groups (Figure 1A). On day +27, the administration of the TRPA1 antagonist treatment in operated rats (IoN-CCI2 group) reduced the response score of the mechanical stimulation compared to the IoN-CCI1 group (injected with saline) (Figure 1B); whereas, ADM_12 treatment in sham-operated rats (Sham2 group) did not change the mechanical response. It is of note that the response to MST in the IoN-CCI2 group was significantly different between day +26 (before ADM_12 injection) and +27 (after drug treatment) (Figure 1C).
Figure 1.
Mechanical stimulation testing (MST): (A) Mean response score to Von Frey hair stimulation of the ligated/sham infraorbital nerve (IoN) territory, on pre-operative day (PO) and on +5, +12, +18, and +26 days post operation. Data is expressed as mean ± SEM. Two-way ANOVA followed by Bonferroni post-hoc test, * p < 0.05 and *** p < 0.001 for chronic constriction injury of the infraorbital nerve (IoN-CCI) groups vs. Sham groups. Drug treatment effect on MST: (B) Mean response score to Von Frey hair stimulation on day +27, 1 h after ADM_12 (or saline) treatment. Data is expressed as mean ± SEM. One-way ANOVA followed by Tukey’s Multiple Comparison Test, * p < 0.05 vs. Sham1 and Sham2, *** p < 0.001 vs. IoN-CCI1. (C) Comparison of the IoN-CCI2 group without treatment (day +26) and after ADM_12 treatment (on day +27). Data is expressed as mean ± SEM. Paired Student’s t test, §§§ p < 0.001 vs. day +26.
2.2. ADM_12 Effect on Gene Expression
The expression of Trpa1, calcitonin-related polypeptide alpha (Calca), and preprotachykinin-A, (PPT-A) was evaluated in the TG and cervical spinal cord (CSC) ipsilateral (ipsi) and contralateral (contra) to the IoN ligation, and in the medulla in toto. Because of the strong relationship between TRPA1 and TRPV1 channels [19,20,21], we also investigated the Trpv1 mRNA expression levels in the same areas.
2.2.1. Trpa1 mRNA Expression
In the ipsilateral TG and CSC, and in medulla region, Trpa1 mRNA expression levels were significantly increased in the IoN-CCI1 group compared with Sham1 and Sham2 groups (Figure 2). The increased mRNA levels were significantly reduced after treatment with ADM_12 in IoN-CCI rats (IoN-CCI2 group) in the same regions (Figure 2). ADM_12 administration did not provoke any changes in sham-operated rats.
Figure 2.
Trpa1 mRNA expression in trigeminal ganglia (TGs) (A), cervical spinal cord (CSC) (B), and medulla (C). Data is expressed as mean + SEM. One way analysis of variance (ANOVA) followed by Tukey’s Multiple Comparison Test or Two-way ANOVA followed by Bonferroni post-hoc test, *** p < 0.001 vs. Sham1 and Sham2 (ipsi), °°° p < 0.001 vs. IoN-CCI1 (ipsi), ^^^ p < 0.001 vs. IoN-CCI1 (contra).
A significant difference in mRNA levels, both in TG and CSC, was detected between sides in the IoN-CCI1 group; whereas, there was no difference between groups when comparing Trpa1 mRNA levels on the contralateral side of TG and CSC (Figure 2A,B).
2.2.2. Trpv1 mRNA Expression
In the ipsilateral TG and CSC, and in the medulla region, Trpv1 mRNA expression levels were significantly increased in the IoN-CCI1 group compared with Sham1 and Sham2 groups (Figure 3). The mRNA levels of Trpv1 were also significantly higher in the IoN-CCI1 group in the contralateral CSC when compared to Sham groups (Figure 3B), though this increase was less marked than the increase observed on the ipsilateral side. The increased mRNA levels in these areas were significantly reduced by ADM_12 treatment in CCI rats (IoN-CCI2 group) in ipsilateral TG and CSC, and in medulla in toto (Figure 3). ADM_12 administration did not provoke any changes in sham-operated rats.
Figure 3.
Trpv1 mRNA expression in TGs (A), CSC (B), and medulla (C). Data is expressed as mean + SEM. One way analysis of variance (ANOVA) followed by Tukey’s Multiple Comparison Test or Two-way ANOVA followed by Bonferroni post-hoc test, *** p < 0.001 vs. Sham1 and Sham 2 (ipsi), °°° p < 0.001 vs. IoN-CCI1 (ipsi), ^^^ p < 0.001 vs. IoN-CCI1 (contra), # p < 0.05 vs. Sham1 and Sham2 (contra).
A significant difference was seen between the ipsi- and contralateral side (both in TG and CSC) in the IoN-CCI1 (Figure 3A,B).
2.2.3. Calca mRNA Expression
In the ipsilateral TG and CSC, and in the medulla region, Calca mRNA expression levels were significantly increased in the IoN-CCI1 group compared with Sham1 and Sham2 groups (Figure 4). Moreover, Calca mRNA levels in IoN-CCI1 and IoN-CCI2 groups were also significantly increased in the contralateral TG as compared to Sham groups (Figure 4A). The increased mRNA levels were significantly reduced after treatment with ADM_12 in IoN-CCI2 rats in ipsilateral TG and CSC, and in medulla in toto (Figure 4). ADM_12 administration did not provoke any changes in sham-operated rats (Figure 4).
Figure 4.
Calca mRNA expression in TGs (A), CSC (B), and medulla (C). Data is expressed as mean + SEM. One way analysis of variance (ANOVA) followed by Tukey’s Multiple Comparison Test or Two-way ANOVA followed by Bonferroni post-hoc test, *** p < 0.001 vs. Sham1 and Sham2 (ipsi), °° p < 0.01 and °°° p < 0.001 vs. IoN-CCI1 (ipsi), ^ p < 0.05 and ^^^ p < 0.001 vs. IoN-CCI1 (contra), # p < 0.05 and ## p < 0.01 vs. Sham1 and Sham2 (contra).
A significant difference was seen between the ipsi- and contralateral side (both in TG and CSC) in the IoN-CCI1 group (Figure 4A,B).
2.2.4. PPT-A mRNA Expression
In the ipsilateral TG and CSC, and in the medulla region, PPT-A mRNA expression levels were significantly increased in the IoN-CCI1 group compared with Sham1 and Sham2 groups (Figure 5). The increased mRNA levels were significantly reduced after treatment with ADM_12 in IoN-CCI rats (IoN-CCI2 group) in the same regions (Figure 5). ADM_12 administration did not cause any changes in sham-operated rats.
Figure 5.
PPT-A mRNA expression in TGs (A), CSC (B), and medulla (C). Data is expressed as mean + SEM. One way analysis of variance (ANOVA) followed by Tukey’s Multiple Comparison Test or Two-way ANOVA followed by Bonferroni post-hoc test, * p < 0.05 and *** p < 0.001 vs. Sham1 and Sham2 groups (ipsi), °°° p < 0.001 vs. IoN-CCI1 group (ipsi), ^^^ p < 0.001 vs. IoN-CCI1 group (contra), §§§ p < 0.001 vs. IoN-CCI2 (contra).
A significant difference was seen between the ipsi- and contralateral side (both in TG and CSC) in the IoN-CCI1 group, as well as in the IoN-CCI2 group at the TG level; whereas, there was no difference between groups on the contralateral side of TG and CSC (Figure 5A,B).
2.2.5. IL-1beta, IL-6, and TNF-alpha mRNA Expression
Since the effects of the surgery, and consequently of the TRPA1 antagonist, on the transcript levels were seen mainly at the ipsilateral side, the cytokines mRNA expression was not evaluated contralaterally.
Interleukin (IL)-1beta, IL-6, and tumor necrosis factor (TNF)-alpha mRNA expression levels were significantly increased in all the areas under evaluation in the IoN-CCI1 group compared with Sham1 and Sham2 groups (Figure 6). Such increases were significantly reduced after treatment with ADM_12 in IoN-CCI rats (IoN-CCI2 group) in the same regions (Figure 6).
Figure 6.
mRNA expression of IL-1beta (A), IL-6 (B), and TNF-alpha (C) in ipsilateral TG and CSC, and in medulla in toto. Data are expressed as mean + SEM. One way analysis of variance (ANOVA) followed by Tukey’s Multiple Comparison Test, *** p < 0.001 vs. Sham1 and Sham2, °°° p < 0.001 vs. IoN-CCI1, # p < 0.05 vs. Sham1.
2.3. ADM_12 Effect on Neuropeptide Protein Expression
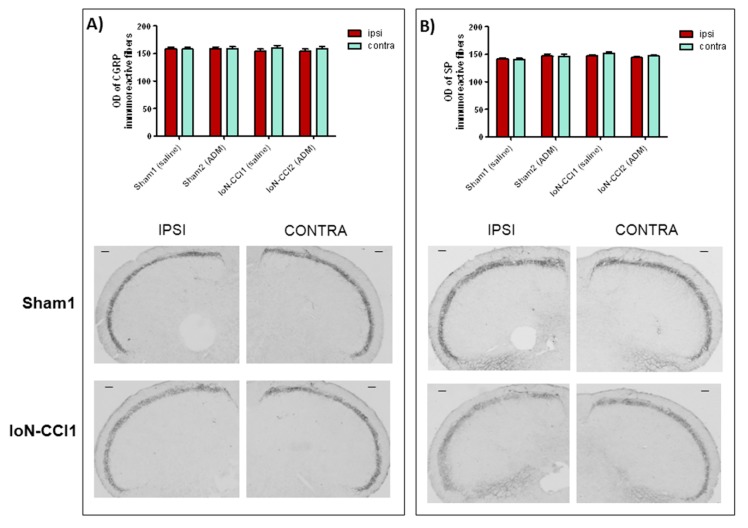
CGRP and SP protein expression was evaluated in Sp5C on both sides. A slight, but not significant difference in the density of immunoreactive fibers for CGRP and SP protein was observed between the ipsilateral and contralateral side in both the IoN-CCI1 and IoN-CCI2 groups (Figure 7). No significant change was seen between sham and operated rats (Figure 7). ADM_12 administration did not induce any change in CGRP and SP expression either in sham or in CCI operated rats (Figure 7).
Figure 7.
(A) Optical density (OD) values of calcitonin gene-related peptide (CGRP) with representative photomicrographs of CGRP immunoreactive fibers in the spinal nucleus of trigeminal nerve pars caudalis (Sp5C) ipsilateral (ipsi) and contralateral (contra) of Sham1 and IoN-CCI1 groups. (B) OD values of substance P (SP) with representative photomicrographs of SP immunoreactive fibers in the Sp5C ipsilateral (ipsi) and contralateral (contra) of Sham1 and IoN-CCI1 groups. Data is expressed as mean + SEM. Two-way analysis of variance (ANOVA) followed by Bonferroni post-hoc test. Scale bar: 100 µm.
3. Discussion
The pathways of trigeminal neuropathic pain are poorly understood. Experimental evidences suggest a strong involvement of TRPA1 in different patterns of neuropathic pain, and recently its role was also demonstrated in a trigeminal neuropathic pain model [17].
Here we evaluated the role of TRPA1 channels in an animal model of trigeminal neuropathic pain (IoN-CCI model), investigating the effects of the TRPA1 antagonist ADM_12 on mechanical allodynia, and neurochemical and transcriptional changes.
ADM_12 was previously shown to revert in vivo the Oxaliplatin-induced neuropathy [22]. At the trigeminal level, ADM_12 was able to reduce orofacial pain in a model of temporomandibular joint inflammation [23], and to counteract trigeminal hyperalgesia in a model of migraine pain, together with decreased Trpa1 and neuropeptide mRNA expression levels in specific areas implicated in trigeminal pain [24].
3.1. Behavioral Response
Infraorbital nerve injury in rats leads to the development, in the ipsilateral side, of a hyporesponsiveness to mechanical stimulation within the first week post operation, followed by a hyperresponsiveness, that according to several studies [18,25], reflects a condition of mechanical allodynia. This biphasic response is probably related to the demyelination process, occurring in the early post-operative period, and remyelination process, that occurs in the late post-operative period [26]. Compared to the above cited papers [18,25,26], the time needed in this study to develop allodynia was somewhat longer. This may have been the result of small differences in the degree of nerve constriction; indeed, different degrees of IoN constriction have been shown to produce different time courses in isolated face grooming behavior [27], and this can also be true for mechanical allodynia.
The allodynic response of operated rats was abolished after treatment with the TRPA1 antagonist ADM_12, suggesting that the blockade of TRPA1 channels located on the trigeminal afferents prevented the release of neuropeptides (CGRP and SP) [28,29], thus resulting in a reduced neurogenic inflammation, and ultimately nociceptor sensitization [30]. Accordingly, Wu and colleagues reported an increase in TRPA1 protein, as well as TRPV1 channels, in the Sp5C region of rats that underwent IoN-CCI surgery [31], confirming their involvement in this process. An additional mechanism is represented by the reduction in the release of pro-inflammatory factors via the inhibition of TRPA1 located on glial cells in the nervous system, as suggested by our results, in which we observed a reduction of the IL-1beta, IL-6, and TNF-alpha transcripts that possibly parallel protein expression [32,33,34], which could account for reduced glial cells activation [32]; or via the inhibition of TRPA1 located on non-neuronal cells, such as keratinocytes and macrophages, in the tissues surrounding the damaged nerve [35]. Pro-inflammatory mediators released in the tissues that surround the damaged nerve, and glial cell activation, are indeed known to play a crucial role in the pathophysiology of neuropathic pain [36,37]. Glial activation and pro-inflammatory cytokines are associated with the onset of neuropathic pain symptoms such as allodynia or hyperalgesia [38,39,40,41,42].
The involvement of TRPA1 in mechanosensation has been extensively studied; both genetic deletion of TRPA1 and its pharmacological blockade abrogate mechanical pain-like behaviors [17,43,44]. Recently, Trevisan and colleagues [17] confirmed the critical role played by TRPA1 channels in mechanical allodynia induced by trigeminal neuropathic pain; conversely, in a model of sciatic nerve injury, Lehto and co-workers [45] reported a non-significant involvement of these channels in the mechanical sensitivity. On the other hand, other authors showed that TRPA1 blockade attenuated mechanical hypersensitivity following spinal injury [46,47] or neuropathic pain induced by chemotherapeutic agents [48,49]. Altogether these observations suggest that mechanical allodynia might be differently mediated by TRPA1 channels depending on the type of pain, site of damage, or distribution profile in TG and DRGs [50]. Moreover, the different responses observed in the experimental models could also be related to the different TRPA1 antagonists used, that may inhibit the channel through binding at different sites, with specific regulatory mechanisms [51].
3.2. Trpa1 and Trpv1 mRNA Expression
Chronic constriction injury of the IoN produced a marked increase in the Trpa1 and Trpv1 mRNA expression in central and peripheral areas ipsilaterally, and a slight increase even at the contralateral side, compared to the sham group. This contralateral increase is probably due to activation of inflammatory processes occurring after nerve injury, which can also affect the contralateral side [52]. The elevated TRP transcripts are accompanied by increased IL-1beta, IL-6, and TNF-alpha mRNA levels in the medulla region, and ipsilateral TG and CSC. It is known that TRPA1 and TRPV1 channels can be sensitized by inflammatory agents, causing up-regulation of these channels [53,54,55]. For example, Trpa1 expression has been shown to be up-regulated by TNF-alpha and IL-1 alpha via transcriptional factor hypoxia-inducible factor-1α [56]. Similarly, TNF-alpha can up-regulate TRPV1 protein and mRNA in DRG and TG neurons [57,58]; one of the suggested pathways for Trpv1 regulation is the p38 mitogen-activated protein kinase pathway [59], which may also be partly involved in Trpa1 expression [60]. As regards TRPA1, its activation seems to depend on the activation of the nuclear factor-κB signaling pathway [61].
Furthermore, an important role in neuropathic pain seems to be played by oxidative stress [62,63,64], whose components can directly activate TRPA1 channels [65], thereby contributing to inflammation in a TRPA1-dependent manner. Indeed, it was recently found that trigeminal neuropathic pain behaviors were mediated by TRPA1 targeted by oxidative stress by-products released from monocytes and macrophages surrounding the site of the nerve injury [17].
In agreement with our study, an up-regulation of Trpa1 and Trpv1 mRNA levels, as well as protein levels in TG, DRGs, and dorsal horns, has been seen in different models of neuropathic pain [11,47,60,66,67,68,69,70,71]. Increased mRNA levels may reflect an increase in functional TRPA1 and TRPV1 channels [72,73].
The increased mRNA levels detected in our experiments in CSC and medulla may have different origins: Trpv1 mRNA undergoes bidirectional axon transport along primary afferents [74], and the same could be true for Trpa1, since both TRPV1 and TRPA1 are (co-)expressed, not only on peripheral, but also on central terminals of primary afferent neurons where their activation can lead to the release of transmitters that promote the sensitization of postsynaptic pain transmission pathways [75,76,77,78]. In addition, Trpv1 mRNA could originate from GABAergic interneurons and glial cells in the rat dorsal horn, which are known to express TRPV1 [69,79].
Systemic administration of ADM_12 markedly reduced the mRNA expression levels of both TRPs induced by IoN ligation. The effect of drug treatment on mRNA transcripts is likely to be due to an indirect effect rather than a direct one. It can be reasonably hypothesized that the effect of ADM_12 on TRPA1 mRNA expression is indirectly due to the blockade of the channel, located either on neuronal and non-neuronal cells, which is followed by two events. On one side, the reduction of calcium (Ca2+) entry provokes a reduced activation of second messenger (Ca2+ dependent) molecules (e.g., via the phospholipase C/Ca2+ signaling pathway and Ca(2+)/calmodulin-dependent protein kinase II [CaMKII]) and interfering with the Ca2+-interacting proteins [80,81], with the consequent reduction in transcriptional rate; for example, through the CaMK—cAMP response element-binding protein (CaMK—CREB) cascade. The other event that follows TRPA1 antagonism is the reduction in neuropeptide (CGRP and SP) release [28,29], and pro-inflammatory agents from neuronal fibers and non-neuronal cells. In this frame, we hypothesize that ADM_12 may break off a self-feeding loop in which TRPA1 channels are directly activated or sensitized by Ca2+ [51,81], endogenous substances produced by intracellular Ca2+ elevation [82], and pro-inflammatory molecules [83,84,85], and indirectly by the activation of nociceptive fibers caused by neuropeptide-induced neuroinflammation.
Moreover, we can also speculate that since TRPA1 and TRPV1 functions may be influenced by each other [20,86,87], a re-organization in the expression and nature of these channels after nerve injury [88,89,90] enabled ADM_12 to modulate TRPV1 channels as well. Although a physical interaction between these two channels may be questionable, even if some studies described it in vitro [19,21], many studies reported a functional interaction between them [20,86,91,92,93]. For instance, Masuoka et al. [87] showed in DRG neurons that TRPA1 channels suppress TRPV1 channel activity, possibly through the regulation of basal intracellular calcium concentration, and that the TRPA1 sensitization, induced by inflammatory agents, enhance TRPV1-mediated currents [87].
These observations, including our data, show a relationship between these two TRP channels, although more information and studies are needed to understand the precise mechanisms of this putative interaction.
3.3. Neuropeptide Expression
After nerve injury, an inflammatory process leads to the release of many pro-inflammatory mediators, which participate in peripheral sensitization, promoting an excessive release of neurotransmitters [94]. Together with the inflammatory process, neuropeptides and degenerative changes affecting the nervous fibers are also crucial peripheral mechanisms [95].
In our experimental setting, the mRNA expression levels of genes coding for CGRP (Calca) and SP (PTT-A) markedly increased in the central areas containing the Sp5C, as well as in the TG ipsilateral to the IoN ligation. Interestingly, Calca mRNA expression in IoN ligated rats was also elevated on the contralateral TG. It has been shown that projections from the TG reach the medullary and cervical dorsal horns on both sides [96,97], and that unilateral TG stimulation activates neurons in both ipsi- and contralateral Sp5C [98,99].
One of the mechanisms that could contribute to neuropeptide expression is the CaMK—CREB cascade, which is probably triggered following TRP channel activation [100], and that may represent the target mechanism for the observed inhibitory effect of ADM_12 on the mRNA expression of CGRP and SP. The blockade of TRP channels, which co-localize with CGRP and SP in the trigeminal neurons [7,101], can inhibit Calca and PPT-A mRNA expression, thus reducing the neuropeptide release and the trigeminal sensitization process. The data supports the pivotal involvement of CGRP and SP in the delivery and transmission of pain sensation to the central nervous system, and their role in trigeminal pain syndrome. In fact, an increased concentration of neuropeptides was found in the cerebrospinal fluid and venous blood of patients with trigeminal neuralgia compared to healthy controls [102,103].
In this frame, it was quite surprising that we did not detect any significant difference in neuropeptide protein expression at the Sp5C level, neither among groups, nor between sides. Lynds and co-workers [104] reported no differences in neuropeptide (CGRP and SP) levels between ipsi- and contralateral TG two weeks after IoN transection injury, while Xu and colleagues [105] described a reduction of CGRP and SP protein levels in the ipsilateral caudal medulla eight days after partial IoN ligation. Taken together, these findings suggest that in our model the neuropeptide release at central sites might have taken place at early time points after surgery, and therefore went undetected since we only measured it on day +27, or alternatively, that CGRP and SP are mostly involved at the peripheral terminals [26]. These apparently contrasting findings prompt the need for specifically targeted studies in order to investigate in more depth the role of neuropeptide release in central and peripheral sites in this model of trigeminal neuropathic pain.
3.4. Limitations of the Study and Future Perspectives
We evaluated changes of behavioral responses and mRNA expression after a short period (1 h) of drug exposure. This approach may be questionable, however there are many studies that support our observations. For instance, the mRNA expression of metabotropic glutamate receptors was found to be upregulated 1 h after treatment in mice DRG neurons [106]. Ambalavanar et al. [107] were able to detect changes in CGRP mRNA levels in rat’s TG even 30 minutes after complete Freund’s adjuvant injection. Furthermore, Nesic and co-workers [108] reported change in the mRNA signal of cytokines 1 h after treatment with MK-801, a NMDA receptor antagonist, in the spinal cord of rats subjected to spinal cord injury.
Nevertheless, to elucidate and confirm the present findings, additional experiments with different techniques are necessary. It will be interesting to evaluate in this model the effects of ADM_12 at later time points, as well as after chronic treatment. Another limitation of the present study is the absence of a time course of the expression of CRGP and SP. This was motivated by the ethical and organizational need to keep the number of rats as low as possible. However, based on the present findings, it seems important to address in future studies the parallel evaluation of mRNA and protein expression of CGRP and SP in order to elucidate more clearly the role of these neuropeptides in peripheral and central sites.
4. Materials and Methods
4.1. Animals
Male Sprague-Dawley rats (Charles River, weighing 225–250 g at arrival) were used following the International Association for the Study of Pain (IASP)’s guidelines for pain research in animals [109]. Animals were housed in groups of 2 with water and food available ad libitum, and kept in a colony room (humidity: 45 ± 5%; room temperature: 21 ± 1 °C). Rats were kept under a reversed 12:12 h dark/light cycle (lights on at 20 h). All procedures were in accordance with the European Convention for Care and Use of Laboratory Animals, and were approved by the Ethical Committee for Animal Testing (Ethische Commissie Dierproeven, ECD) of the University of Antwerp (number 2017-16, approval 20/02/2017).
Rats were allowed to acclimate for 8 days to the housing conditions before the surgery; they were habituated to the behavioral test procedure daily for three days before pre-operative testing. Habituation and testing were conducted in a darkened room (light provided by a 60 W red light bulb suspended 1 m above the observation area) with a 45 dB background noise.
4.2. Surgery
The IoN-CCI was performed as previously described [18,25,27]. Rats were anaesthetized with pentobarbital (60 mg/kg, intraperitoneally (i.p.)) and treated with atropine (0.1 mg/kg, i.p.). The surgery was performed under direct visual control using a Zeiss operation microscope (×10–25). The rat’s head was fixed in a stereotaxic frame and a mid-line scalp incision was made, exposing the skull and nasal bone. The edge of the orbit was dissected free, and the orbital contents were deflected with a cotton-tipped wooden rod to give access to the left IoN, which was loosely ligated with two chromic catgut ligatures (5-0) (2 mm apart). The scalp incision was closed using polyester sutures (4-0; Ethicon, Johnson & Johnson, Belgium). In sham operated rats, the IoN was exposed using the same procedure, but the nerve was not ligated.
4.3. Mechanical Stimulation Testing (MST)
Baseline data were obtained 1 day before surgery. Following surgery, rats were tested on post-operative days +5, +12, +18, +26, and +27 (Figure 1). A graded series of five Von Frey hairs (0.015 g, 0.127 g, 0.217 g, 0.745 g, and 2.150 g) (Pressure Aesthesiometer®, Stoelting Co, Chicago, IL, USA) were applied by an experimenter who was blind to animal and treatment groups, within the IoN territory, near the center of the vibrissal pad [25,110,111,112,113]. Von Frey hairs were applied in an ascending order of intensity either ipsi- or contralaterally. The scoring system described by Vos [25] was used to evaluate the rats’ response to the stimulation (Table 1). For each rat, and at every designated time, a mean score for the five von Frey filaments was determined.
Table 1.
Response categories with the corresponding score values.
| SCORE | TYPE OF RESPONSE |
|---|---|
| 0 | no response |
| 1 | detection: the rat turns the head toward the stimulating object and the stimulus object is then explored |
| 2 | withdrawal reaction: the rat turns the head slowly away or pulls it briskly backward when the stimulation is applied; sometimes a single face wipe ipsilateral to the stimulated area occurs |
| 3 | escape/attack: the rat avoids further contact with the stimulus object, either passively by moving its body away from the stimulating object to assume a crouching position against the cage wall, or actively by attacking the stimulus object, making biting and grabbing movements |
| 4 | asymmetric face grooming: the rat displays an uninterrupted series of at least three face-wash strokes directed toward the stimulated facial area |
4.4. Drug and Experimental Plan
The TRPA1 antagonist ADM_12, synthesized in the Laboratory of Prof. Cristina Nativi (University of Florence, Italy) and characterized by a high binding constant versus TRPA1 [23], was dissolved in saline and administered intraperitoneally (i.p.) at the dose of 30 mg/kg in a volume of 1 ml/kg [22,23,24].
The animals were randomly allocated in four groups of 12 animals each and assigned to different experimental sets, as shown in Table 2.
Table 2.
Experimental groups and number (N) of animals per group that underwent the mechanical stimulation test (MST). The samples of the subsets were processed for the real time polymerase chain reaction (RT-PCR) or immunohistochemistry (IHC).
| EXPERIMENTAL GROUPS | Surgery | Treatment on Day +27 | MST | RT-PCR | IHC |
|---|---|---|---|---|---|
| Sham1 | Sham | saline | N = 12 | N = 6 | N = 6 |
| Sham2 | Sham | ADM_12 | N = 12 | N = 6 | N = 6 |
| IoN-CCI1 | IoN-CCI | saline | N = 12 | N = 6 | N = 6 |
| IoN-CCI2 | IoN-CCI | ADM_12 | N = 12 | N = 6 | N = 6 |
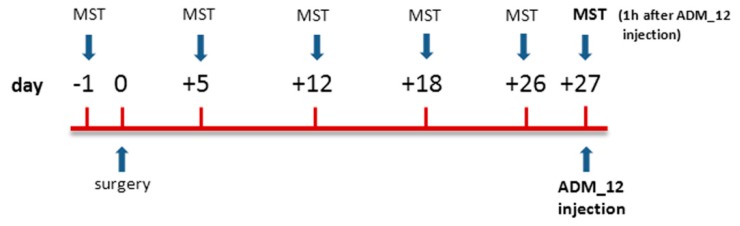
On day +27, sham and operated rats were treated with ADM_12 or saline 1 h prior to the MST (Figure 8). The timing was chosen on the basis of previous studies reporting a significant effect of acute ADM_12 treatment on behavioral responses [22,23,24]. At the end of the behavioral test, each rat was sacrificed with an i.p. overdose of pentobarbital (150 mg/kg). A subset of 6 rats per experimental group served for the detection of gene expression levels by means of real time polymerase chain reaction (RT-PCR); another subset of 6 animals per experimental group underwent the immunohistochemical evaluation of protein expression (Table 2).
Figure 8.
Schematic representation of the experimental design.
4.5. Real Time-PCR
The trigeminal ganglia (TG), cervical spinal cord (CSC, C1-C2 level), and medulla (bregma, −13.30 to −14.60 mm; Paxinos and Watson 4th edition), containing the Sp5C, of each animal were quickly removed after completing the MST on day +27 and frozen at –80 °C. Samples were then processed to evaluate the expression levels of the genes encoding for TRPA1 (Trpa1), TRPV1 (Trpv1), CGRP (Calca), SP (PPT-A), IL-1beta (IL-1beta), IL-6 (IL-6), and TNF-alpha (TNF-alpha). mRNA levels were analyzed by RT-PCR, as previously described [24,114,115]. After tissue homogenization by means of ceramic beads (PRECELLYS, Berthin Pharma, Montigny-le-Bretonneux, France), total RNA was extracted with TRIzol® reagent (Invitrogen, Carlsbad, California, USA) and quantified by measuring the absorbance at 260/280 nm using a nanodrop spectrophotometer (Euroclone, Pero (MI), Italy). Following cDNA generation with the iScript cDNA Synthesis kit (BIO-RAD, Hercules, California, USA), gene expression was analyzed using the Fast Eva Green supermix (BIO-RAD). Primer sequences were obtained from the AutoPrime software (http://www.autoprime.de/AutoPrimeWeb) (Table 3). The amplification was performed through two-step cycling (95–60°C) for 45 cycles with a Light Cycler 480 Instrument RT-PCR Detection System (Roche, Milan, Italy). The expression of the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), remained constant in all the experimental groups considered. All samples were assayed in triplicate.
Table 3.
Primer sequences.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | AACCTGCCAAGTATGATGAC | GGAGTTGCTGTTGAAGTCA |
| Trpa1 | CTCCCCGAGTGCATGAAAGT | TGCATATACGCGGGGATGTC |
| Trpv1 | CTTGCTCCATTTGGGGTGTG | CTGGAGGTGGCTTGCAGTTA |
| Calca | CAGTCTCAGCTCCAAGTCATC | TTCCAAGGTTGACCTCAAAG |
| PPT-A | GCTCTTTATGGGCATGGTC | GGGTTTATTTACGCCTTCTTTC |
| IL-1beta | CTTCCTTGTGCAAGTGTCTG | CAGGTCATTCTCCTCACTGTC |
| IL-6 | TTCTCTCCGCAAGAGACTTC | GGTCTGTTGTGGGTGGTATC |
| TNF-alpha | CCTCACACTCAGATCATCTTCTC | CGCTTGGTGGTTTGCTAC |
4.6. Immunohistochemistry
According to Terayama et al. [116] and Panneton et al. [117], the central afferent innervations of the IoN are mostly distributed in (but not restricted to) the dorsal and lateral part of the Sp5C, projecting to all the laminae. The pattern of CGRP and SP protein related to the painful component of the IoN was investigated in the superficial laminae of the Sp5C.
Immediately after the MST test, animals were anaesthetized and perfused transcardially with phosphate buffered saline (PBS) and 4% paraformaldehyde. The medullary segment containing the Sp5C, between +1 and −5 mm from the obex, was removed and post-fixed for 24 h in the same fixative; subsequently, samples were transferred in solutions of sucrose at increasing concentrations (up to 30%) during the following 72 h. All samples were cut transversely at 30 µm on a freezing sliding microtome. CGRP and SP protein expression was evaluated using the free-floating immunohistochemical technique, as previously reported [24]. For CGRP we used an anti-rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:3200, and an anti-rabbit antibody (Chemicon, Temecula, CA, USA) at a dilution of 1:5000 for SP; both primary antibodies were incubated for 24 h at room temperature. After incubation at room temperature with the secondary biotinylated antibody (Vector Laboratories, Burlingame, CA, USA) and the avidin-biotin complex (Vectastain, Vector Laboratories), sections were stained with the peroxidase substrate kit DAB (3′3′-diaminobenzidine tetrahydrochloride) (Vector Laboratories, Burlingame, CA, USA).
The area covered by CGRP and SP immunoreactive fibers in the Sp5C ipsilateral and contralateral to the surgery (12 sections per animal), was expressed as optical density (OD) values [24,114,118], acquired using an AxioSkop 2 microscope (Zeiss) and a computerized image analysis system (AxioCam, Zeiss, Göttingen, Germany) equipped with dedicated software (AxioVision Rel 4.2, Zeiss, Göttingen, Germany). All sections were averaged and reported as the mean + SEM of OD values.
4.7. Statistical Evaluation
Data from recent studies [18,27] was used to calculate the required number of animals per experimental group to obtain a statistical power of 0.80 at an alpha level of 0.05, and a difference of at least 20% in behavioral responses after IoN-CCI surgery. The calculations were done using software (Lenth RV. Java Applets for Power and Sample Size) retrieved on 8 April 2013, from http://www.stat.uiowa.edu/~rlenth/Power, which estimated a sample size of 12 rats per experimental group.
Statistical analysis was performed with the GraphPad Prism program (GraphPad Software, San Diego, California, USA). In the MST, for each rat and at every designated time, a mean score for the five Von Frey hairs was determined. The IoN-CCI rats were compared to the sham-operated rats. For mRNA levels, results were analyzed using the ΔΔCt method to compare expression of genes of interest with that of GAPDH, used as control gene. All data was tested for normality using the Kolmogorov–Smirnov normality test and considered normal. Differences between groups, or between ipsilateral and contralateral sides, were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s Multiple Comparison Test, or by means of two-way ANOVA followed by Bonferroni post-hoc test, respectively. Differences between two groups were analyzed by the Paired student’s t test. A probability level of less than 5% was regarded as significant.
5. Conclusions
Antagonism of the TRPA1 channel by means of ADM_12 attenuates experimentally-induced mechanical allodynia [17,119] in a reliable animal model of trigeminal neuropathic pain. Allodynia is one of the major clinical features of trigeminal neuropathic pain [120,121], thus the modulation of the TRPA1 channel may represent a suitable therapeutic target [122,123], and ADM_12 a possible tool, in trigeminal neuropathic pain management. As a corollary, our data also suggests a possible role for TRPV1 channels in the behavioral and biomolecular responses related to trigeminal neuropathic pain. Further exploration of the mechanisms underlying the antinociceptive effects of TRPA1, and studies directed to better understand the relationship between TRPA1 and TRPV1, would improve our understanding of the complex nociceptive processing in trigeminal neuropathic pain.
Acknowledgments
The authors would like to thank Stefania Ceruti for her precious suggestions during the writing of the manuscript.
Abbreviations
| CaMKII | Ca(2+)/calmodulin-dependent protein kinase II |
| Calca | calcitonin-related polypeptide alpha |
| CGRP | calcitonin gene-related peptide |
| CREB | cAMP response element-binding protein |
| CSC | cervical spinal cord |
| DRG | dorsal root ganglia |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| IL | interleukin |
| IoN | infraorbital nerve |
| IoN-CCI | chronic constriction injury of the infraorbital nerve |
| MST | mechanical stimulation testing |
| OD | optical density |
| PPT-A | preprotachykinin-A |
| RT-PCR | real time polymerase chain reaction |
| SP | substance P |
| Sp5C | spinal nucleus of trigeminal nerve pars caudalis |
| TG | trigeminal ganglia |
| TN | trigeminal neuralgia |
| TNF-alpha | tumor necrosis factor alpha |
| TRP | transient receptor potential |
Author Contributions
C.D.: Conceptualization, Investigation, Formal analysis, Writing—original draft; R.G.: Conceptualization, Writing—review & editing; A.M.Z.: Investigation; O.F.: Methodology; C.N.: Writing—review & editing; C.T.: Funding acquisition, Writing—review & editing; K.D.: Conceptualization, Supervision, Investigation, Writing—review & editing. All authors read and approved the final manuscript.
Funding
This research was funded by a grant of the Italian Ministry of Health (Ricerca Corrente, 2016) to the IRCCS Mondino Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Cruccu G. Trigeminal Neuralgia. Continuum. 2017;23:396–420. doi: 10.1212/CON.0000000000000451. [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 3.Zakrzewska J.M., Wu J., Mon-Williams M., Phillips N., Pavitt S.H. Evaluating the impact of trigeminal neuralgia. Pain. 2017;158:1166–1174. doi: 10.1097/j.pain.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 4.Obermann M., Katsarava Z. Update on trigeminal neuralgia. Expert Rev. Neurother. 2009;9:323–329. doi: 10.1586/14737175.9.3.323. [DOI] [PubMed] [Google Scholar]
- 5.Zakrzewska J.M., Akram H. Neurosurgical interventions for the treatment of classical trigeminal neuralgia. Cochrane Database Syst. Rev. 2011;9:CD007312. doi: 10.1002/14651858.CD007312.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong X.P., Wang X., Xu H. TRP channels of intracellular membranes. J. Neurochem. 2010;113:313–328. doi: 10.1111/j.1471-4159.2010.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Story G.M., Peier A.M., Reeve A.J., Eid S.R., Mosbacher J., Hricik T.R., Earley T.J., Hergarden A.C., Andersson D.A., Hwang S.W., et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/S0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya M.R., Bautista D.M., Wu K., Haeberle H., Lumpkin E.A., Julius D. Radial stretch reveals distinct populations of mechanosensitive mammalian somatosensory neurons. Proc. Natl. Acad. Sci. USA. 2008;105:20015–20020. doi: 10.1073/pnas.0810801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quartu M., Serra M.P., Boi M., Poddighe L., Picci C., Demontis R., Del Fiacco M. TRPV1 receptor in the human trigeminal ganglion and spinal nucleus: Immunohistochemical localization and comparison with the neuropeptides CGRP and SP. J. Anat. 2016;229:755–767. doi: 10.1111/joa.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jardín I., López J.J., Diez R., Sánchez-Collado J., Cantonero C., Albarrán L., Woodard G.E., Redondo P.C., Salido G.M., Smani T., et al. TRPs in Pain Sensation. Front Physiol. 2017;8:392. doi: 10.3389/fphys.2017.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obata K., Katsura H., Mizushima T., Yamanaka H., Kobayashi K., Dai Y., Fukuoka T., Tokunaga A., Tominaga M., Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J. Clin. Investig. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsura H., Obata K., Mizushima T., Yamanaka H., Kobayashi K., Dai Y., Fukuoka T., Tokunaga A., Sakagami M., Noguchi K. Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Exp. Neurol. 2006;200:112–123. doi: 10.1016/j.expneurol.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Caspani O., Zurborg S., Labuz D., Heppenstall P.A. The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS ONE. 2009;4:e7383. doi: 10.1371/journal.pone.0007383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staaf S., Oerther S., Lucas G., Mattsson J.P., Ernfors P. Differential regulation of TRP channels in a rat model of neuropathic pain. Pain. 2009;144:187–199. doi: 10.1016/j.pain.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Eid S.R., Crown E.D., Moore E.L., Liang H.A., Choong K.C., Dima S., Henze D.A., Kane S.A., Urban M.O. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathyinduced mechanical hypersensitivity. Mol. Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J., Joshi S.K., DiDomenico S., Perner R.J., Mikusa J.P., Gauvin D.M., Segreti J.A., Han P., Zhang X.F., Niforatos W., et al. Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain. 2011;152:1165–1172. doi: 10.1016/j.pain.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 17.Trevisan G., Benemei S., Materazzi S., De Logu F., De Siena G., Fusi C., Fortes Rossato M., Coppi E., Marone I.M., Ferreira J., et al. TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain. 2016;139:1361–1377. doi: 10.1093/brain/aww038. [DOI] [PubMed] [Google Scholar]
- 18.Deseure K., Hans G.H. Chronic Constriction Injury of the Rat’s Infraorbital Nerve (IoN-CCI) to Study Trigeminal Neuropathic Pain. J. Vis. Exp. 2015:103. doi: 10.3791/53167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staruschenko A., Jeske N.A., Akopian A.N. Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J. Biol. Chem. 2010;285:15167–15177. doi: 10.1074/jbc.M110.106153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akopian A.N. Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions. Curr. Pharm. Biotechnol. 2011;12:89–94. doi: 10.2174/138920111793937952. [DOI] [PubMed] [Google Scholar]
- 21.Fischer M.J., Balasuriya D., Jeggle P., Goetze T.A., McNaughton P.A., Reeh P.W., Edwardson J.M. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Arch. 2014;466:2229–2241. doi: 10.1007/s00424-014-1497-z. [DOI] [PubMed] [Google Scholar]
- 22.Fragai M., Comito G., Di Cesare Mannelli L., Gualdani R., Calderone V., Louka A., Richichi B., Francesconi O., Angeli A., Nocentini A., et al. Lipoyl-Homotaurine Derivative (ADM_12) Reverts Oxaliplatin-Induced Neuropathy and Reduces Cancer Cells Malignancy by Inhibiting Carbonic Anhydrase IX (CAIX) J. Med. Chem. 2017;60:9003–9011. doi: 10.1021/acs.jmedchem.7b01237. [DOI] [PubMed] [Google Scholar]
- 23.Gualdani R., Ceruti S., Magni G., Merli D., Di Cesare Mannelli L., Francesconi O., Richichi B., la Marca G., Ghelardini C., Moncelli M.R., et al. Lipoic-based TRPA1/TRPV1 antagonist to treat orofacial pain. ACS Chem. Neurosci. 2015;6:380–385. doi: 10.1021/cn500248u. [DOI] [PubMed] [Google Scholar]
- 24.Demartini C., Tassorelli C., Zanaboni A.M., Tonsi G., Francesconi O., Nativi C., Greco R. The role of the transient receptor potential ankyrin type-1 (TRPA1) channel in migraine pain: Evaluation in an animal model. J. Headache Pain. 2017;18:9. doi: 10.1186/s10194-017-0804-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vos B.P., Strassman A.M., Maciewicz R.J. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J. Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa G.M.F., de Oliveira A.P., Martinelli P.M., da Silva Camargos E.R., Arantes R.M.E., de Almeida-Leite C.M. Demyelination/remyelination and expression of interleukin-1β, substance P, nerve growth factor, and glial-derived neurotrophic factor during trigeminal neuropathic pain in rats. Neurosci. Lett. 2016;612:210–218. doi: 10.1016/j.neulet.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Deseure K., Hans G. Behavioral study of non-evoked orofacial pain following different types of infraorbital nerve injury in rats. Physiol. Behav. 2015;138:292–296. doi: 10.1016/j.physbeh.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura Y., Une Y., Miyano K., Abe H., Hisaoka K., Morioka N., Nakata Y. Activation of transient receptor potential ankyrin 1 evokes nociception through substance P release from primary sensory neurons. J. Neurochem. 2012;120:1036–1047. doi: 10.1111/j.1471-4159.2011.07628.x. [DOI] [PubMed] [Google Scholar]
- 29.Benemei S., Fusi C., Trevisan G., Geppetti P. The TRPA1 channel in migraine mechanism and treatment. Br. J. Pharmacol. 2014;171:2552–2567. doi: 10.1111/bph.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gold M.S., Gebhart G.F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 2010;16:1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu C., Xie N., Lian Y., Xu H., Chen C., Zheng Y., Chen Y., Zhang H. Central antinociceptive activity of peripherally applied botulinum toxin type A in lab rat model of trigeminal neuralgia. SpringerPlus. 2016;5:431. doi: 10.1186/s40064-016-2071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Latrémolière A., Mauborgne A., Masson J., Bourgoin S., Kayser V., Hamon M., Pohl M. Differential implication of proinflammatory cytokine interleukin-6 in the development of cephalic versus extracephalic neuropathic pain in rats. J. Neurosci. 2008;28:8489–8501. doi: 10.1523/JNEUROSCI.2552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi B.M., Lee S.H., An S.M., Park D.Y., Lee G.W., Noh G.J. The time-course and RNA interference of TNF-α, IL-6, and IL-1β expression on neuropathic pain induced by L5 spinal nerve transection in rats. Korean J. Anesthesiol. 2015;68:159–169. doi: 10.4097/kjae.2015.68.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang B., Yu Y., Aori G., Wang Q., Kong D., Yang W., Guo Z., Zhang L. Tanshinone IIA Attenuates Diabetic Peripheral Neuropathic Pain in Experimental Rats via Inhibiting Inflammation. Evid.-Based Complement. Altern. Med. 2018;2018:2789847. doi: 10.1155/2018/2789847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atoyan R., Shander D., Botchkareva N.V. Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J. Invest. Dermatol. 2009;129:2312–2315. doi: 10.1038/jid.2009.58. [DOI] [PubMed] [Google Scholar]
- 36.Thacker M.A., Clark A.K., Marchand F., McMahon S.B. Pathophysiology of peripheral neuropathic pain: Immune cells and molecules. Anesth. Analg. 2007;105:838–847. doi: 10.1213/01.ane.0000275190.42912.37. [DOI] [PubMed] [Google Scholar]
- 37.Mika J., Zychowska M., Popiolek-Barczyk K., Rojewska E., Przewlocka B. Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 2013;716:106–119. doi: 10.1016/j.ejphar.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 38.Ledeboer A., Sloane E.M., Milligan E.D., Frank M.G., Mahony J.H., Maier S.F., Watkins L.R. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115:71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Zhuang Z.Y., Gerner P., Woolf C.J., Ji R.R. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Lees J.G., Duffy S.S., Moalem-Taylor G. Immunotherapy targeting cytokines in neuropathic pain. Front. Pharmacol. 2013;4:142. doi: 10.3389/fphar.2013.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zanjani T.M., Sabetkasaei M., Karimian B., Labibi F., Farokhi B., Mossafa N. The attenuation of pain behaviour and serum interleukin-6 concentration by nimesulide in a rat model of neuropathic pain. Scand. J. Pain. 2010;1:229–234. doi: 10.1016/j.sjpain.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Piao Z.G., Cho I.H., Park C.K., Hong J.P., Choi S.Y., Lee S.J., Lee S., Park K., Kim J.S., Oh S.B. Activation of glia and microglial p38 MAPK in medullary dorsal horn contributes to tactile hypersensitivity following trigeminal sensory nerve injury. Pain. 2006;121:219–231. doi: 10.1016/j.pain.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 43.Kerstein P.C., del Camino D., Moran M.M., Stucky C.L. Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol. Pain. 2009;5:19. doi: 10.1186/1744-8069-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwan K.Y., Glazer J.M., Corey D.P., Rice F.L., Stucky C.L. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J. Neurosci. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lehto S.G., Weyer A.D., Youngblood B.D., Zhang M., Yin R., Wang W., Teffera Y., Cooke M., Stucky C.L., Schenkel L., et al. Selective antagonism of TRPA1 produces limited efficacy in models of inflammatory- and neuropathic-induced mechanical hypersensitivity in rats. Mol. Pain. 2016;12 doi: 10.1177/1744806916677761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei H., Koivisto A., Saarnilehto M., Chapman H., Kuokkanen K., Hao B., Huang J.L., Wang Y.X., Pertovaara A. Spinal transient receptor potential ankyrin 1 channel contributes to central pain hypersensitivity in various pathophysiological conditions in the rat. Pain. 2011;152:582–591. doi: 10.1016/j.pain.2010.11.031. [DOI] [PubMed] [Google Scholar]
- 47.Park J., Zheng L., Acosta G., Vega-Alvarez S., Chen Z., Muratori B., Cao P., Shi R. Acrolein contributes to TRPA1 up-regulation in peripheral and central sensory hypersensitivity following spinal cord injury. J. Neurochem. 2015;135:987–997. doi: 10.1111/jnc.13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Materazzi S., Fusi C., Benemei S., Pedretti P., Patacchini R., Nilius B., Prenen J., Creminon C., Geppetti P., Nassini R. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflugers Arch. 2012;463:561–569. doi: 10.1007/s00424-011-1071-x. [DOI] [PubMed] [Google Scholar]
- 49.Trevisan G., Materazzi S., Fusi C., Altomare A., Aldini G., Lodovici M., Patacchini R., Geppetti P., Nassini R. Novel Therapeutic Strategy to Prevent Chemotherapy-Induced Persistent Sensory Neuropathy By TRPA1 Blockade. Cancer Res. 2013;73:3120–3131. doi: 10.1158/0008-5472.CAN-12-4370. [DOI] [PubMed] [Google Scholar]
- 50.Vandewauw I., Owsianik G., Voets T. Systematic and quantitative mRNA expression analysis of TRP channel genes at the single trigeminal and dorsal root ganglion level in mouse. BMC Neurosci. 2013;14:21. doi: 10.1186/1471-2202-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paulsen C.E., Armache J.P., Gao Y., Cheng Y., Julius D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature. 2015;525:552. doi: 10.1038/nature14871. [DOI] [PubMed] [Google Scholar]
- 52.Jancalek R. Signaling mechanisms in mirror image pain pathogenesis. Ann. Neurosci. 2011;18:123–127. doi: 10.5214/ans.0972.7531.111810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amaya F., Oh-hashi K., Naruse Y., Iijima N., Ueda M., Shimosato G., Tominaga M., Tanaka Y., Tanaka M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003;963:190–196. doi: 10.1016/S0006-8993(02)03972-0. [DOI] [PubMed] [Google Scholar]
- 54.Devesa I., Planells-Cases R., Fernández-Ballester G., González-Ros J.M., Ferrer-Montiel A., Fernández-Carvajal A. Role of the transient receptor potential vanilloid 1 in inflammation and sepsis. J. Inflamm. Res. 2011;4:67–81. doi: 10.2147/JIR.S12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diogenes A., Akopian A.N., Hargreaves K.M. NGF up-regulates TRPA1: Implications for orofacial pain. J. Dent. Res. 2007;86:550–555. doi: 10.1177/154405910708600612. [DOI] [PubMed] [Google Scholar]
- 56.Hatano N., Itoh Y., Suzuki H., Muraki Y., Hayashi H., Onozaki K., Wood I.C., Beech D.J., Muraki K. Hypoxia-inducible factor-1α (HIF1α) switches on transient receptor potential ankyrin repeat 1 (TRPA1) gene expression via a hypoxia response element-like motif to modulate cytokine release. J. Biol. Chem. 2012;287:31962–31972. doi: 10.1074/jbc.M112.361139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hensellek S., Brell P., Schaible H.G., Bräuer R., Segond von Banchet G. The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol. Cell Neurosci. 2007;36:381–391. doi: 10.1016/j.mcn.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 58.Khan A.A., Diogenes A., Jeske N.A., Henry M.A., Akopian A., Hargreaves K.M. Tumor necrosis factor alpha enhances the sensitivity of rat trigeminal neurons to capsaicin. Neuroscience. 2008;155:503–509. doi: 10.1016/j.neuroscience.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 59.Ikeda-Miyagawa Y., Kobayashi K., Yamanaka H., Okubo M., Wang S., Dai Y., Yagi H., Hirose M., Noguchi K. Peripherally increased artemin is a key regulator of TRPA1/V1 expression in primary afferent neurons. Mol. Pain. 2015;11:8. doi: 10.1186/s12990-015-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto K., Chiba N., Chiba T., Kambe T., Abe K., Kawakami K., Utsunomiya I., Taguchi K. Transient receptor potential ankyrin 1 that is induced in dorsal root ganglion neurons contributes to acute cold hypersensitivity after oxaliplatin administration. Mol. Pain. 2015;11:69. doi: 10.1186/s12990-015-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang J., Ding Y., Li B., Liu H., Yang X., Chen M. TRPA1 mediated aggravation of allergic contact dermatitis induced by DINP and regulated by NF-κB activation. Sci. Rep. 2017;7:43586. doi: 10.1038/srep43586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim D., You B., Jo E.K., Han S.K., Simon M.I., Lee S.J. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc. Natl. Acad. Sci. USA. 2010;107:14851–14856. doi: 10.1073/pnas.1009926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim H.K., Park S.K., Zhou J.L., Taglialatela G., Chung K., Coggeshall R.E., Chung J.M. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 64.Naik A.K., Tandan S.K., Dudhgaonkar S.P., Jadhav S.H., Kataria M., Prakash V.R., Kumar D. Role of oxidative stress in pathophysiology of peripheral neuropathy and modulation by N-acetyl-L-cysteine in rats. Eur. J. Pain. 2006;10:573–579. doi: 10.1016/j.ejpain.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Andersson D.A., Gentry C., Moss S., Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hudson L.J., Bevan S., Wotherspoon G., Gentry C., Fox A., Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur. J. Neurosci. 2001;13:2105–2114. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- 67.Fukuoka T., Tokunaga A., Tachibana T., Dai Y., Yamanaka H., Noguchi K. VR1, but not P2X(3), increases in the spared L4 DRG in rats with L5 spinal nerve ligation. Pain. 2002;99:111–120. doi: 10.1016/S0304-3959(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 68.Frederick J., Buck M.E., Matson D.J., Cortright D.N. Increased TRPA1, TRPM8, and TRPV2 expression in dorsal root ganglia by nerve injury. Biochem. Biophys. Res. Commun. 2007;358:1058–1064. doi: 10.1016/j.bbrc.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 69.Kim Y.H., Back S.K., Davies A.J., Jeong H., Jo H.J., Chung G., Na H.S., Bae Y.C., Kim S.J., Kim J.S., et al. TRPV1 in GABAergic interneurons mediates neuropathic mechanical allodynia and disinhibition of the nociceptive circuitry in the spinal cord. Neuron. 2012;74:640–647. doi: 10.1016/j.neuron.2012.02.039. [DOI] [PubMed] [Google Scholar]
- 70.Urano H., Ara T., Fujinami Y., Hiraoka B.Y. Aberrant TRPV1 expression in heat hyperalgesia associated with trigeminal neuropathic pain. Int. J. Med. Sci. 2012;9:690–697. doi: 10.7150/ijms.4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quartu M., Carozzi V.A., Dorsey S.G., Serra M.P., Poddighe L., Picci C., Boi M., Melis T., Del Fiacco M., Meregalli C., et al. Bortezomib treatment produces nocifensive behavior and changes in the expression of TRPV1, CGRP, and substance P in the rat DRG, spinal cord, and sciatic nerve. Biomed. Res. Int. 2014;2014:180428. doi: 10.1155/2014/180428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwartz E.S., Christianson J.A., Chen X., La J.H., Davis B.M., Albers K.M., Gebhart G.F. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology. 2011;140:1283–1291. doi: 10.1053/j.gastro.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nummenmaa E., Hämäläinen M., Moilanen L.J., Paukkeri E.L., Nieminen R.M., Moilanen T., Vuolteenaho K., Moilanen E. Transient receptor potential ankyrin 1 (TRPA1) is functionally expressed in primary human osteoarthritic chondrocytes. Arthritis Res. Ther. 2016;18:185. doi: 10.1186/s13075-016-1080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tohda C., Sasaki M., Konemura T., Sasamura T., Itoh M., Kuraishi Y. Axonal transport of VR1 capsaicin receptor mRNA in primary afferents and its participation in inflammation-induced increase in capsaicin sensitivity. J. Neurochem. 2001;76:1628–1635. doi: 10.1046/j.1471-4159.2001.00193.x. [DOI] [PubMed] [Google Scholar]
- 75.Kosugi M., Nakatsuka T., Fujita T., Kuroda Y., Kumamoto E. Activation of TRPA1 channel facilitates excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. J. Neurosci. 2007;27:4443–4451. doi: 10.1523/JNEUROSCI.0557-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koivisto A., Chapman H., Jalava N., Korjamo T., Saarnilehto M., Lindstedt K., Pertovaara A. TRPA1: A transducer and amplifier of pain and inflammation. Basic Clin. Pharmacol. Toxicol. 2014;114:50–55. doi: 10.1111/bcpt.12138. [DOI] [PubMed] [Google Scholar]
- 77.Kim Y.S., Chu Y., Han L., Li M., Li Z., LaVinka P.C., Sun S., Tang Z., Park K., Caterina M.J., et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron. 2014;81:873–887. doi: 10.1016/j.neuron.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yeo E.J., Cho Y.S., Paik S.K., Yoshida A., Park M.J., Ahn D.K., Moon C., Kim Y.S., Bae Y.C. Ultrastructural analysis of the synaptic connectivity of TRPV1-expressing primary afferent terminals in the rat trigeminal caudal nucleus. J. Comp. Neurol. 2010;518:4134–4146. doi: 10.1002/cne.22369. [DOI] [PubMed] [Google Scholar]
- 79.Doly S., Fischer J., Salio C., Conrath M. The vanilloid receptor-1 is expressed in rat spinal dorsal horn astrocytes. Neurosci. Lett. 2004;357:123–126. doi: 10.1016/j.neulet.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 80.Mandadi S., Armati P.J., Roufogalis B.D. Protein kinase C modulation of thermo-sensitive transient receptor potential channels: Implications for pain signaling. J. Nat. Sci. Biol. Med. 2011;2:13–25. doi: 10.4103/0976-9668.82311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zurborg S., Yurgionas B., Jira J.A., Caspani O., Heppenstall P.A. Direct activation of the ion channel TRPA1 by Ca2+ Nat. Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 82.Takahashi N., Mizuno Y., Kozai D., Yamamoto S., Kiyonaka S., Shibata T., Uchida K., Mori Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels. 2008;2:287–298. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- 83.Bandell M., Story G.M., Hwang S.W., Viswanath V., Eid S.R., Petrus M.J., Earley T.J., Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/S0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 84.Bautista D.M., Jordt S.E., Nikai T., Tsuruda P.R., Read A.J., Poblete J., Yamoah E.N., Basbaum A.I., Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 85.Wang S., Dai Y., Fukuoka T., Yamanaka H., Kobayashi K., Obata K., Cui X., Tominaga M., Noguchi K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: A molecular mechanism of inflammatory pain. Brain. 2008;131:1241–1251. doi: 10.1093/brain/awn060. [DOI] [PubMed] [Google Scholar]
- 86.Lee L.Y., Hsu C.C., Lin Y.J., Lin R.L., Khosravi M. Interaction between TRPA1 and TRPV1: Synergy on pulmonary sensory nerves. Pulm. Pharmacol. Ther. 2015;35:87–93. doi: 10.1016/j.pupt.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Masuoka T., Kudo M., Yamashita Y., Yoshida J., Imaizumi N., Muramatsu I., Nishio M., Ishibashi T. TRPA1 Channels Modify TRPV1-Mediated Current Responses in Dorsal Root Ganglion Neurons. Front. Physiol. 2017;8:272. doi: 10.3389/fphys.2017.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matzner O., Devor M. Hyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channels. J. Neurophysiol. 1994;72:349–359. doi: 10.1152/jn.1994.72.1.349. [DOI] [PubMed] [Google Scholar]
- 89.McCallum J.B., Wu H.E., Tang Q., Kwok W.M., Hogan Q.H. Subtype-specific reduction of voltage-gated calcium current in medium-sized dorsal root ganglion neurons after painful peripheral nerve injury. Neuroscience. 2011;179:244–255. doi: 10.1016/j.neuroscience.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Waxman S.G., Kocsis J.D., Black J.A. Type III Na+ channel mRNA is expressed in embryonic but not adult spinal sensory neurons, and is re-expressed following axotomy. J. Neurophysiol. 1994;72:466–470. doi: 10.1152/jn.1994.72.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruparel N.B., Patwardhan A.M., Akopian A.N., Hargreaves K.M. Desensitization of transient receptor potential ankyrin 1 (TRPA1) by the TRP vanilloid 1-selective cannabinoid arachidonoyl-2 chloroethanolamine. Mol. Pharmacol. 2011;80:117–123. doi: 10.1124/mol.110.068940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Andrade E.L., Meotti F.C., Calixto J.B. TRPA1 antagonists as potential analgesic drugs. Pharmacol. Ther. 2012;133:189–204. doi: 10.1016/j.pharmthera.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 93.Honda K., Shinoda M., Furukawa A., Kita K., Noma N., Iwata K. TRPA1 contributes to capsaicin-induced facial cold hyperalgesia in rats. Eur. J. Oral Sci. 2014;122:391–396. doi: 10.1111/eos.12157. [DOI] [PubMed] [Google Scholar]
- 94.Kim K.H., Kim J.I., Han J.A., Choe M.A., Ahn J.H. Upregulation of neuronal nitric oxide synthase in the periphery promotes pain hypersensitivity after peripheral nerve injury. Neuroscience. 2011;8:367–378. doi: 10.1016/j.neuroscience.2011.05.064. [DOI] [PubMed] [Google Scholar]
- 95.Costa G.M.F., Leite C.M.A. Trigeminal neuralgia: Peripheral and central mechanisms. Rev. Dor São Paulo. 2015;16:297–301. doi: 10.5935/1806-0013.20150061. [DOI] [Google Scholar]
- 96.Pfaller K., Arvidsson J. Central distribution of trigeminal and upper cervical primary afferents in the rat studied by anterograde transport of horseradish peroxidase conjugated to wheat germ agglutinin. J. Comp. Neurol. 1988;268:91–108. doi: 10.1002/cne.902680110. [DOI] [PubMed] [Google Scholar]
- 97.Jacquin M.F., Chiaia N.L., Rhoades R.W. Trigeminal projections to contralateral dorsal horn: Central extent, peripheral origins, and plasticity. Somatosens. Mot. Res. 1990;7:153–183. doi: 10.3109/08990229009144705. [DOI] [PubMed] [Google Scholar]
- 98.Ingvardsen B.K., Laursen H., Olsen U.B., Hansen A.J. Possible mechanism of c-fos expression in trigeminal nucleus caudalis following cortical spreading depression. Pain. 1997;72:407–415. doi: 10.1016/S0304-3959(97)00069-9. [DOI] [PubMed] [Google Scholar]
- 99.Samsam M., Coveñas R., Csillik B., Ahangari R., Yajeya J., Riquelme R., Narváez J.A., Tramu G. Depletion of substance P, neurokinin A and calcitonin gene-related peptide from the contralateral and ipsilateral caudal trigeminal nucleus following unilateral electrical stimulation of the trigeminal ganglion; a possible neurophysiological and neuroanatomical link to generalized head pain. J. Chem. Neuroanat. 2001;21:161–169. doi: 10.1016/S0891-0618(01)00088-6. [DOI] [PubMed] [Google Scholar]
- 100.Nakanishi M., Hata K., Nagayama T., Sakurai T., Nishisho T., Wakabayashi H., Hiraga T., Ebisu S., Yoneda T. Acid activation of Trpv1 leads to an up-regulation of calcitonin gene-related peptide expression in dorsal root ganglion neurons via the CaMK-CREB cascade: A potential mechanism of inflammatory pain. Mol. Biol. Cell. 2010;21:2568–2577. doi: 10.1091/mbc.e10-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang D., Li S., Dhaka A., Story G.M., Cao Y.Q. Expression of the transient receptor potential channels TRPV1, TRPA1 and TRPM8 in mouse trigeminal primary afferent neurons innervating the dura. Mol. Pain. 2012;8:66. doi: 10.1186/1744-8069-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qin Z.L., Yang L.Q., Li N., Yue J.N., Wu B.S., Tang Y.Z., Guo Y.N., Lai G.H., Ni J.X. Clinical study of cerebrospinal fluid neuropeptides in patients with primary trigeminal neuralgia. Clin. Neurol. Neurosurg. 2016;143:111–115. doi: 10.1016/j.clineuro.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 103.Strittmatter M., Grauer M., Isenberg E., Hamann G., Fischer C., Hoffmann K.H., Blaes F., Schimrigk K. Cerebrospinal fluid neuropeptides and monoaminergic transmitters in patients with trigeminal neuralgia. Headache. 1997;37:211–216. doi: 10.1046/j.1526-4610.1997.3704211.x. [DOI] [PubMed] [Google Scholar]
- 104.Lynds R., Lyu C., Lyu G.W., Shi X.Q., Rosén A., Mustafa K., Shi T.J.S. Neuronal plasticity of trigeminal ganglia in mice following nerve injury. J. Pain Res. 2017;10:349–357. doi: 10.2147/JPR.S120092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu M., Aita M., Chavkin C. Partial infraorbital nerve ligation as a model of trigeminal nerve injury in the mouse: Behavioral, neural, and glial reactions. J. Pain. 2008;9:1036–1048. doi: 10.1016/j.jpain.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chiechio S., Copani A., De Petris L., Morales M.E., Nicoletti F., Gereau R.W., 4th Transcriptional regulation of metabotropic glutamate receptor 2/3 expression by the NF-kappaB pathway in primary dorsal root ganglia neurons: A possible mechanism for the analgesic effect of L-acetylcarnitine. Mol. Pain. 2006;2:20. doi: 10.1186/1744-8069-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ambalavanar R., Dessem D., Moutanni A., Yallampalli C., Yallampalli U., Gangula P., Bai G. Muscle inflammation induces a rapid increase in calcitonin gene-related peptide (CGRP) mRNA that temporally relates to CGRP immunoreactivity and nociceptive behavior. Neuroscience. 2006;143:875–884. doi: 10.1016/j.neuroscience.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 108.Nesic O., Svrakic N.M., Xu G.Y., McAdoo D., Westlund K.N., Hulsebosch C.E., Ye Z., Galante A., Soteropoulos P., Tolias P., et al. DNA microarray analysis of the contused spinal cord: Effect of NMDA receptor inhibition. J. Neurosci. Res. 2002;68:406–423. doi: 10.1002/jnr.10171. [DOI] [PubMed] [Google Scholar]
- 109.Zimmerman M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 110.Deseure K., Koek W., Colpaert F.C., Adriaensen H. The 5-HT(1A) receptor agonist F 13640 attenuates mechanical allodynia in a rat model of trigeminal neuropathic pain. Eur. J. Pharmacol. 2002;456:51–57. doi: 10.1016/S0014-2999(02)02640-7. [DOI] [PubMed] [Google Scholar]
- 111.Deseure K., Koek W., Adriaensen H., Colpaert F.C. Continuous administration of the 5-hydroxytryptamine1A agonist (3-Chloro-4-fluoro-phenyl)-[4-fluoro-4-[[(5-methyl-pyridin-2-ylmethyl) -amino]-methyl]piperidin-1-yl]-methadone (F 13640) attenuates allodynia-like behavior in a rat model of trigeminal neuropathic pain. J. Pharmacol. Exp. Ther. 2003;306:505–514. doi: 10.1124/jpet.103.050286. [DOI] [PubMed] [Google Scholar]
- 112.Deseure K.R., Adriaensen H.F., Colpaert F.C. Effects of the combined continuous administration of morphine and the high-efficacy 5-HT1A agonist, F 13640 in a rat model of trigeminal neuropathic pain. Eur. J. Pain. 2004;8:547–554. doi: 10.1016/j.ejpain.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 113.Deseure K., Bréand S., Colpaert F.C. Curative-like analgesia in a neuropathic pain model: Parametric analysis of the dose and the duration of treatment with a high-efficacy 5-HT(1A) receptor agonist. Eur. J. Pharmacol. 2007;568:134–141. doi: 10.1016/j.ejphar.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 114.Greco R., Demartini C., Zanaboni A.M., Redavide E., Pampalone S., Toldi J., Fülöp F., Blandini F., Nappi G., Sandrini G., et al. Effects of kynurenic acid analogue 1 (KYNA-A1) in nitroglycerin-induced hyperalgesia: Targets and anti-migraine mechanisms. Cephalalgia. 2017;37:1272–1284. doi: 10.1177/0333102416678000. [DOI] [PubMed] [Google Scholar]
- 115.Greco R., Ferrigno A., Demartini C., Zanaboni A., Mangione A.S., Blandini F., Nappi G., Vairetti M., Tassorelli C. Evaluation of ADMA-DDAH-NOS axis in specific brain areas following nitroglycerin administration: Study in an animal model of migraine. J. Headache Pain. 2015;16:560. doi: 10.1186/s10194-015-0560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Terayama R., Nagamatsu N., Ikeda T., Nakamura T., Rahman O.I., Sakoda S., Shiba R., Nishimori T. Differential expression of Fos protein after transection of the rat infraorbital nerve in the trigeminal nucleus caudalis. Brain Res. 1997;768:135–146. doi: 10.1016/S0006-8993(97)00633-1. [DOI] [PubMed] [Google Scholar]
- 117.Panneton W.M., Pan B., Gan Q. Somatotopy in the Medullary Dorsal Horn As a Basis for Orofacial Reflex Behavior. Front. Neurol. 2017;8:522. doi: 10.3389/fneur.2017.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Greco R., Tassorelli C., Sandrini G., Di Bella P., Buscone S., Nappi G. Role of calcitonin gene-related peptide and substance P in different models of pain. Cephalalgia. 2008;28:114–126. doi: 10.1111/j.1468-2982.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- 119.Green D., Ruparel S., Gao X., Ruparel N., Patil M., Akopian A., Hargreaves K. Central activation of TRPV1 and TRPA1 by novel endogenous agonists contributes to mechanical allodynia and thermal hyperalgesia after burn injury. Mol. Pain. 2016;12 doi: 10.1177/1744806916661725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ossipov M.H., Lai J., Malan T.P., Jr., Porreca F. Spinal and supraspinal mechanisms of neuropathic pain. Ann. N. Y. Acad. Sci. 2000;909:12–24. doi: 10.1111/j.1749-6632.2000.tb06673.x. [DOI] [PubMed] [Google Scholar]
- 121.Zakrzewska J.M. Differential diagnosis of facial pain and guidelines for management. Br. J. Anaesth. 2013;111:95–104. doi: 10.1093/bja/aet125. [DOI] [PubMed] [Google Scholar]
- 122.Garrison S.R., Stucky C.L. The dynamic TRPA1 channel: A suitable pharmacological pain target? Curr. Pharm. Biotechnol. 2011;12:1689–1697. doi: 10.2174/138920111798357302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen J., Hackos D.H. TRPA1 as a drug target—promise and challenges. N.-S. Arch. Pharmacol. 2015;388:451–463. doi: 10.1007/s00210-015-1088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]