Abstract
Two decades ago successful transfection of antigen presenting cells (APC) in vivo was demonstrated which resulted in the induction of primary adaptive immune responses. Due to the good biocompatibility of plasmid DNA, their cost-efficient production and long shelf life, many researchers aimed to develop DNA vaccine-based immunotherapeutic strategies for treatment of infections and cancer, but also autoimmune diseases and allergies. This review aims to summarize our current knowledge on the course of action of DNA vaccines, and which factors are responsible for the poor immunogenicity in human so far. Important optimization steps that improve DNA transfection efficiency comprise the introduction of DNA-complexing nano-carriers aimed to prevent extracellular DNA degradation, enabling APC targeting, and enhanced endo/lysosomal escape of DNA. Attachment of virus-derived nuclear localization sequences facilitates nuclear entry of DNA. Improvements in DNA vaccine design include the use of APC-specific promotors for transcriptional targeting, the arrangement of multiple antigen sequences, the co-delivery of molecular adjuvants to prevent tolerance induction, and strategies to circumvent potential inhibitory effects of the vector backbone. Successful clinical use of DNA vaccines may require combined employment of all of these parameters, and combination treatment with additional drugs.
Keywords: DNA vaccine, nano carrier, promotor, transgene, adjuvant, antigen presenting cells, dendritic cell, macrophage
1. Introduction
Compared to conventional protein/peptide-based vaccines intended to induce antigen-specific adaptive immune responses, DNA vaccines are more stable, cost-efficient, easy to manufacture and safe in handling [1]. DNA vaccines are being investigated for various applications including therapy of cancer [2], allergies [3], autoimmune [4] and infectious diseases [5]. In the US, at the moment over 500 clinical trials that focus on DNA vaccination are registered, targeting especially viral infections [6] and cancer [7], while bacterial infections and autoimmune diseases are less of a topic. The high number of DNA-vaccines tested in clinical trials emphasizes their important role for future medical approaches. This review aims to summarize current achievements and ongoing developments in the design of optimized DNA vaccines and delivery methods.
2. Course of Action of DNA Vaccines
The classical ways for vaccine delivery are intramuscular, intradermal and subcutaneous injections which address primarily myocytes [8] and keratinocytes [9], respectively, but also antigen presenting cells (APC) residing near the injection side [10] (Figure 1). In case of DNA vaccines, after their internalization the DNA needs to translocate to the nucleus for transcription, followed by translation in the cytoplasm [11].
Figure 1.
DNA vaccines induce adaptive immune responses. A DNA vaccine intended to induce an adaptive immune response needs to encode an antigen and an adjuvant. The according plasmid DNA is applied either systemically or topically, e.g., by intramuscular injection. Transfected keratinocytes or myocytes express transgene and release derived peptide/protein via exosomes or apoptotic bodies. This material is endocytosed by immature dendritic cells (iDC) which subsequently present antigen preferentially via major histocompatibility class (MHCII) to CD4+ T cells in draining lymph nodes. Direct transfection of APC including iDC results in endogenous transgene expression, and hence parallel presentation via MHCI and MHCII, yielding CD8+ and CD4+ T cell responses in parallel. Besides this cellular immune response, a humoral immune response is induced if the B cell receptor recognizes the protein antigen, and acquires help by pre-activated antigen-specific CD4+ T cells.
APC can be transfected directly by DNA vaccines [10]. In this case, the encoded antigen is expressed and after processing antigen-derived peptides are loaded in parallel onto MHCI and MHCII molecules [12]. In case of concomitant activation, antigen-presenting APC migrate into the draining lymph node and can prime CD8+ and CD4+ T helper cells [13].
In case of cutaneous application, transfected keratinocytes may generate antigen which is released by exosomes or apoptotic bodies and is internalized by APC [14]. In general, antigens of exogenous origin are loaded rather exclusively on MHCII resulting in activation of helper CD4+ T cells which in turn contribute to B cell priming to yield a humoral immune response and are required for full activation of CD8+ T cells [15]. So far, only subpopulations of dendritic cells (DC) with so-called cross priming potential are able to load MHCI with internalized antigen [16].
After intramuscular application, transfected myocytes may undergo apoptosis. Apoptotic bodies are engulfed by APC and the exogenous antigen is cross-presented on MHCI resulting in a CD8+ T cell response [17]. Early studies on DNA vaccination demonstrated that priming of CD8+ cytotoxic T lymphocytes (CTL) is mostly dependent on bone marrow-derived DC rather than induced by tissue specific cells [18], and was strictly dependent on CD4+ T cell help [19].
The most serious challenges for DNA vaccines intended to induce an anti-tumor immune response are caused by immune evasion strategies of the tumor [20]. In this regard, tumor cells are often characterized by defective processing of antigens for subsequent presentation via MHCI [21], and impaired expression of MHCI [22]. In addition, within the tumor microenvironment both parenchymal and infiltrated immune cells may overexpress tolerance-promoting non-classical MHCI molecules like human leukocyte antigen (HLA-G) and HLA-E [23]. Besides, tumor-associated macrophages (TAM) protect the tumor from immune responses by various mechanisms including the release of anti-inflammatory interleukin-10 and the expression of surface receptors like programmed death-ligand 1 which inhibit the functions of activated T cells [24]. The effectiveness of DNA vaccines can also be diminished by CD4+CD25+FoxP3+ regulatory T cells (Treg) that promote cancer growth which inhibit T effector cells [25]. Moreover, both TAM- and tumor-derived mediators promote the expansion of myeloid-derived suppressor cells (MDSC) which interfere with the activation of T cells in the tumor microenvironment and attenuate T effector cells as well [26].
3. DNA Vaccines in the Clinic
The use of DNA vaccines has early raised safety concerns mainly concerning the probability of stable integration of transfected DNA into the genome of somatic or even germ cells, causing dysregulated gene expression and mutations. In this regard, Wolff and collegues who demonstrated that a luciferase-encoding vector after intramuscular administration was detectable for more than 19 months in skeletal muscle, but only as an extrachromosomal plasmid [27]. However, Wang and coworkers reported that intramuscular injection followed by elecotroporation strongly enhanced the overall transfection rate, which was associated with a low level chromosomal integration of vector DNA at random sites [28]. However, the authors calculated that the integration frequency was well below the number of spontaneous gene mutations. In a subsequent study, the majority of plasmid DNA administered into skeletal muscles of different rodents was found to remain at the injection site, while minor fractions were also detected in other organs, including the gonads, but not integrated into the genome [29]. Concerning unwanted DNA vaccination-associated immune effects, repeated intramuscular application of a luciferase-encoding reporter vector in primates resulted in long term reporter expression, but induced no anti-DNA antibodies [30]. Potential transfer of prokaryotic elements of DNA vaccines like antibiotic resistance genes into e.g., the gut microbiome has been considered another issue of safety concerns, but so far no such event has been documented [31]. Nonetheless, the aforementioned as well as additional safety concerns of DNA vaccines need to be considered with regard to their translational use in the clinic [32].
Presently, there are no approved DNA vaccines for use in humans. Nevertheless, some DNA-based vaccines were approved by the FDA and the USDA for veterinary use, including a vaccine against West Nile Virus in horses [33] and canine melanoma [34]. One of the first human clinical trials with DNA vaccines evaluated the therapeutic and prophylactic effects against HIV in which no significant immune responses but a potential immunogenicity were detected [35]. However, in the same year Wang and colleagues demonstrated induction of CD8+ T cell responses in primates after immunization with a mixture of for plasmids encoding for different Plasmodium falciparum proteins [36]. Another clinical trial which targeted the Hepatitis B virus showed the induction of a humoral response in patients not responding to conventional vaccination [37]. The overall safety of DNA vaccines has been thoroughly proven in several clinical trials, underlined by the fact that no antibody response against prokaryotic parts of the DNA vaccine itself has been observed and that adverse effects are limited to mild local reactivity at the injection site [2].
One of the first clinical trials employing a DNA vaccine for tumor therapy was conducted in 1998 using prostate membrane antigen as a prostate cancer antigen delivered using an adenoviral vector and granulocyte-macrophage colony–stimulating factor as an adjuvant. In this trial delayed-type I hypersensitivity responses were observed [38]. Therefore, subsequent trials pursued the goal to improve this parameter [39]. Vaccination of melanoma patients with a bivalent DNA encoding Melan-A and tyrosinase as tumor-associated antigens elicited humoral and CTL responses in Stage IV patients [40]. A subsequent clinical trial used the Synchrovax vaccine encoding for four peptide epitopes of Melan-A and melanoma antigen recognized by T cells 1 to induce broad antigen-specific CD8+ T cell responses [41]. This trial showed antigen-specific responses but no induction of tumor regression in the patients. Recent clinical trials showed good results in inducing immune responses against Wilms’ tumor antigen 1 using DNA vaccines encoding for two different CD8+ T cell epitopes [42].
Altogether, clinical trials employing DNA vaccines evoked efficient induction of cellular and humoral responses [2]. However, the level of these responses most often was not sufficient to elicit significant clinical benefits. Therefore, numerous clinical trials focus on DNA vaccine optimization strategies to augment theirimmunogenicity [7] as presented in the following. In addition, it is necessary to compare the suitability of different DNA vaccine delivery routes to yield potent adaptive immune responses. In this regard, the Cutaneous and Mucosal HIV Vaccination (CUTHIVAC) trial is worth to be mentioned since it aimed to comparatively analyze the influence of the combination of different injection sites with or without electroporation [43]. In the following optimization strategies for the design of DNA vaccines and approaches to improve their delivery to APC are presented. Both aspects are important to overcome the poor immunogenicity of DNA vaccines in human.
4. Optimization of DNA Vaccines
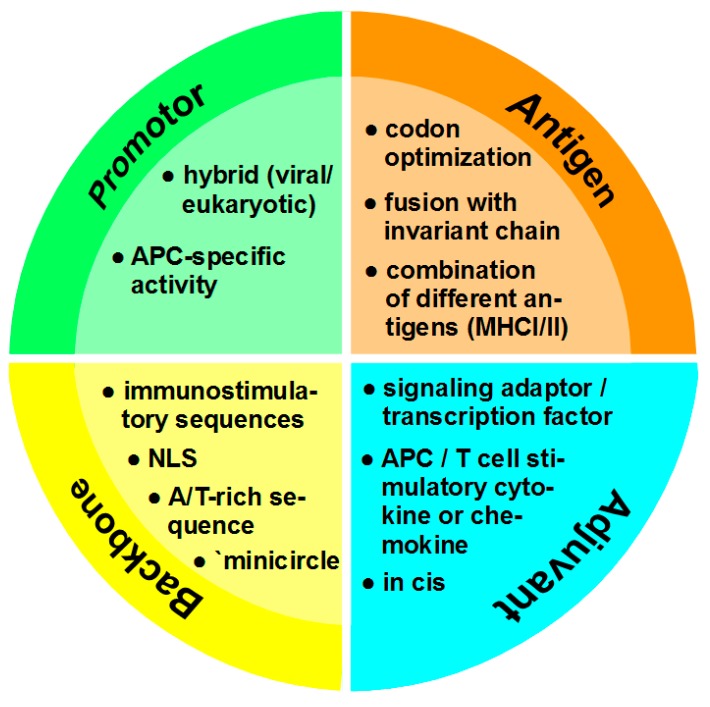
DNA vaccines still face many challenges to become an effective tool as their success achieved in preclinical studies has not been translated into the clinic yet [44]. The biggest challenge is the low immunogenicity of DNA vaccines in bigger animals and humans probably due to the difficulty to upscale the DNA vaccine amounts used in small animal systems [45]. For this, about 5–20 mg of DNA would have to be injected into an average-sized human [46]. If naked i.e., unformulated plasmid DNA is used for vaccination, an important factor that contributes to low therapeutic efficiency is DNA degradation [47]. Studies showed that plasmids, compared to other administration forms like minicircles, are degraded relatively fast within one week [48]. DNA molecules that successfully enter the cell need to pass the barrier of the nuclear membrane to be transcribed [11]. Hence, due to the low amount of DNA that is at disposal for transfection of a given target cell in vivo, a major goal is the optimization of transfection. Optimization parameters of DNA vaccine design are summarized in Figure 2.
Figure 2.
Optimization parameters of DNA vaccine design. Use of a hybrid viral/eukaryotic promoter was shown to prevent transcriptional silencing as observed for viral promotors. Alternatively, a promoter engineered to transcriptinally target APC like DC may be used. To enhance antigen expression, especially in case of pathogens, codon optimization is important. To induce a broad CD4+/CD8+ T cell response, linker-separated sequences encoding different antigens may be used. In addition, fusion with a sequence encoding the invariant chain may enhance loading of antigen onto MHCII. Conventionally, molecular adjuvants intended to enhance the APC activation state and/or T cell attraction and polarization are encoded by expression vectors coadministered with a DNA vaccine. To ensure coexpression of antigen and a molecular adjuvant by a transfected cell, both sequences must be incorporated in the DNA vaccine (in cis), separated by an IRES or T2A sequence. The vector backbone comprises the part of the DNA vaccine which is not required for eukaryotic expression. Inherent or inserted immunostimulatory sequences are detected by danger receptors, and mediate APC activation. Inclusion of a NLS facilitates nuclear entry of the DNA. Intrinsic inhibitory effects of the backbone on the transfection efficiency are limited by insertion of A/T-rich sequences or by recombinase-mediated deletion of the prokaryotic part as a last step after propagation in bacteria to yield minicircle DNA.
4.1. Promotor
Conventionally, viral promoters like the human immediate/early CMV (cytomegalovirus) promoter which are ubiquitously active at high level have been employed to drive transgene expression [49]. However, especially with regard to long term expression, viral promotors are often subjected to methylation-mediated inactivation, whereas eukaryotic promoters and hybrids of eukaryotic/viral promoters remain active [50]. Furthermore, the use of cell type-specific promotors may allow to restrict antigen expression to APC.
To achieve transcriptional targeting of DC which at activated state constitute the most potent APC population [13,51], various promoters of endogenous genes that are expressed predominantly by this APC population were tested to drive DC-focused transgene expression. CD11c has been well established as a DC-specific marker in mouse [52], whereas this ß2 integrin is expressed more broadly in human by different immune cell types [53]. Transgenic mice engineered to express antigens under transcriptional control of a large 5.5 kb promoter fragment showed DC-specific transgene expression [54]. However, when mice were biolistically transfected with a DNA vaccine containing this CD11c promoter fragment insufficient CTL activation was observed [55]. Dendrocyte expressed seven transmembrane protein (DC-STAMP) constitutes an evolutionarily highly conserved transmembrane protein of the endoplasmic reticulum [56]. In the same study, DC-STAMP was reported as primarily expressed in immature human and mouse DC, down-regulated in response to maturation. However, osteoclasts and macrophages were reported to express DC-STAMP as well [57]. The suitability of the DC-STAMP promotor to transcriptionally target DC was evaluated by transduction of hematopoietic stem cells with a lentiviral expression vector harboring the DC-STAMP promoter to drive expression of a fluorescence reporter [58]. Lethally irradiated mice were reconstituted with transductants, and reporter expression was monitored in different immune cell types. Indeed, the reporter was primarily expressed by differentiated conventional and plasmacytoid DC, and at low level in monocytes, B cells, and NK cells. Dectin-2 (CLEC6A) is a C-type lectin receptor (CLR) that binds mannose-rich surface structures of various pathogens [59], and is primarily expressed by Langerhans cells (LC) [60] which constitute the epidermal DC population. In line, biolistic transfection of mice with a reporter plasmid driving luciferase expression by the Dectin-2 promoter yielded predominant reporter expression in LC [61]. In subsequent studies, transduction of mice with a lentiviral reporter expression vector under control of the Dectin-2 promotor resulted in reporter expression in LC as well as macrophages [62]. In a comparative study including the aforementioned gene promoters and a promoter fragment of the dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) gene which encodes for a CLR predominantly expressed by DC and macrophages [59], the DC-STAMP promoter was identified as most potent to induce antigen-specific T cell responses in vitro after transfection of DC [63].
The highly conserved actin-bundling protein Fascin-1 is highly expressed in neuronal cells, and mediates the structural integrity of axons [64]. We have shown that, besides neuronal cells, Fascin-1 is only expressed in activated DC of mouse and human [65]. The Fascin-1 promoter yielded strong and specific activity in DC of both species [66,67]. In subsequent studies we demonstrated that transcriptional targeting of DC using DNA vaccines containing the Fascin-1 promoter to drive antigen expression yielded antigen-specific T helper cell (Th)1-biased immune responses, whereas transfection with CMV promoter-driven DNA vaccines resulted in mixed Th1/Th2 responses [68]. Moreover, Fascin-1 and CMV promotor containing DNA vaccines induced CTL at comparable extent [66]. In various mouse models of allergen-induced airway inflammation and type I allergy [69], as well as a mouse model of multiple sclerosis [70] vaccination with plasmids encoding the relevant allergen/antigen under control of the Fascin-1 promoter showed therapeutic efficacy, especially when co-transfecting expression constructs that encode anti-inflammatory cytokines.
Transcriptional targeting of DC is intended to restrict antigen and adjuvant expression to this APC population, and thereby to prevent addressing of tolerance by regulatory cell types like MDSC and TAM. However, transcriptional targeting of DC will also largely exclude expression of (protein) antigen in B cells and thereby may impair the induction of antigen-specific antibodies. Additional B cell addressing may be achieved by employment of cellular promoters that display activity also in this APC population [51].
4.2. Antigen
The use of DNA vaccines in infectious diseases or for tumor therapy enables to focus on sequences that encode immunogenic peptides of a given pathogen or tumor-specific proteins, and allows to include antigen-encoding sequences derived from different proteins within one minigene to induce a broader T cell response [71]. Antigens that are expressed by tumor cells, so called tumor-associated antigens (TAA) can be divided into two categories: tumor-specific shared antigens and tumor-specific unique antigens [72]. Shared antigens are expressed by different tumors and can also be present in normal cells of different tissues, although at lower amounts. Tumor-specific unique antigens, i.e., neo-antigens, are expressed only by (individual) tumors [73]. Usage of shared antigens is more convenient since the sequence of most proteins is well known and a genetic analysis of the mutanome is not needed to select antigen-encoding sequences [74]. However, a major risk of using shared TAA is the induction of autoimmune responses as the immune system will also be turned against healthy tissues expressing the antigen [75]. Neo-antigens seem to be the first choice if designing a DNA vaccine as studies have shown that effector T cell responses are more potent against mutated antigens [76]. Furthermore, antigens with a higher half-life have been shown to induce stronger cytotoxic T cell responses and thereby increased immunogenicity [77].
Codon optimization is mostly needed if the target antigen is of non-human origin, and can strongly enhance antigen expression [62]. Besides, if immunogenic peptides of an antigen are known, and induction of antibodies specific for the target protein are not an issue, only sequences encoding relevant T cell antigen epitopes may be included [78]. Even if the encoded antigen is expressed at high amounts, antigen presentation/recognition still may be an obstacle to prompt a sufficient immune response. This problem is addressed by introducing epitope-specific changes in the antigen to increase MHC affinity [79]. Similar efforts are being made to increase the affinity of the MHC/peptide complex to T cell receptors [80]. The latest approach to increase antigen presentation is the use of xenogenic antigens [81]. This approach was very successful in the development of the USDA-approved DNA vaccine against canine melanoma.
In current studies, both the strategy to administer a DNA vaccine which encodes for a full length protein as a source of antigen and the administration of a DNA vaccine which encodes for different peptide antigens are followed. While the former approach aims to induce a cellular and concomitant humoral immune response, the latter focuses on the induction of T cell responses at the expense of antibody production. The design of an antigen-encoding expression unit includes the selection of several immunogenic antigens derived from one or different proteins to be presented via MHCI/II to yield parallel CD4+/CD8+ T cell activation, conceivably codon optimization, and eventually strategies to boost MHCII antigen presentation, e.g., by introducing of the invariant chain.
4.3. Adjuvant
To avoid induction of vaccine-mediated antigen-specific tolerance, APC need to be activated by a so-called danger signal which promotes the upregulation of antigen presenting receptors, costimulators and pro-inflammatory mediators for efficient T cell activation and polarization [13]. To this end, antigen-encoding vaccines have been coadministered with established adjuvants like aluminium salt-based Alum [82] or immunostimulatory CpG oligonucleotides which engage the endosomal located toll-like receptor (TLR)9 to stimulate APC [83]. Intradermal application of plasmids was observed to yield Th1-biased immune responses due a CpG-rich element located within the ampicillin resistance gene [84]. Besides TLR9, cytosolic DNA sensors that mediate activation of the stimulator of interferon genes signaling pathway contribute to the intrinsic adjuvant effect of DNA vaccines [85].
Apart from the intrinsic adjuvancy of plasmid DNA, codelivery of plasmids that encode transcription factors as genetic adjuvants has been tested to stimulate APC. Upregulated activation of transcription factors of the nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells (NF-κB) [86] and the interferon-regulatory factor (IRF) [87] family are hallmarks of APC stimulation by danger signals. Shedlock and coworkers have demonstrated that codelivery of a HIV protein encoding DNA vaccine and of an NF-κB p65 expression construct by in vivo electroporation of mice elevated both cellular and humoral immune responses [88]. Codelivery of an influenza antigen encoding DNA vaccine and an IRF-3 encoding vector by biolistic transfection of mice resulted in an increase of activated CD4+ and CD8+ T cells [89]. Cotransfection with an IRF-1 expression vector yielded enhanced viral antigen-specific antibody production, whereas enhanced expression of IRF-7 increased both T cell and B cell responses. However, in combination with a HIV tat antigen DNA vaccine delivered by intramuscular injection, only codelivery of an IRF-1 encoding vector elevated the intended CTL/Th1 response [90]. To achieve coactivation of IRF and NF-κB transcription factors, Luc and co-workers immunized mice with an influenza protein encoding DNA vaccine and a plasmid encoding the virus-induced signaling adaptor protein which resulted in elevated anti-viral T cell responses [91]. In a comparative study, Larsen and colleagues assessed the ability of codelivered expression vectors for numerous different TLR signaling adaptors to enhance immune responses [92]. Of more than seventeen different signaling adaptors tested, only combined coadministration of Tak1 and Tram expression vectors enhanced CLT responses in vivo.
The aforementioned strategies aim to enhance the activation state of transfected APC. In other approaches DNA vaccines were coadministered with plasmids that encode either costimulatory receptors in transfected APC to enhance their T cell stimulatory capacity as reported for CD80 and CD86 used e.g., by de Andrés and colleagues to evoke potent anti-Visna/Maedi virus T cell and antibody responses in immunized sheep [93]. More often, DNA vaccines were codelivered with expression constructs for soluble mediators like cytokines or chemokines that exert stimulatory/chemoattracting effects on APC and other immune cells [13]. For example, IL-12 is produced by activated APC to promote Th1 differentiation, but also stimulates APC themselves [94]. Accordingly, administration of an IL-12 expression construct in combination with a multigene HIV DNA vaccine by intramuscular electroporation resulted in enhanced antigen-specific CD4+ and CD8+ activation in healthy volunteers [95]. IL-15 is also released by activated DC, and exerts broad stimulatory effects on effector CD4+ and CD8+ T cells, B cells, NK cells as well as DC [96]. In several studies a pronounced effect of cotransfected IL-15 was observed as reported e.g., by Sun and coworkers demonstrating that a fusion construct of a Mycobacterium antigen and IL-15 applied intramuscularly into mice yielded pronounced NK activity, an enhanced Th1 and CTL response as well as elevated antibody titers [97]. Altogether, while different cytokines were employed as genetic adjuvants to stimulate both APC and other types of immune cells [45], IL-2 acts mainly on T cells (and NK cells) [98]. Hence, its overexpression is intended to support sustained activation of exhausted T effector cells and to overcome its depletion by Treg [99].
More recently, as an alternative to promote APC and T cell activation by (over)expression of intracellular or secreted factors, the concept of regulating cell activation by RNA interference has gained attention [100]. For this, either specific silencer RNA or plasmids that encode short hairpin RNA which specifically target intracellular mRNA encoding inhibitory factors are applied. By this approach transcription factors like signal transducer and activators of transcription 3 which is known to induce expression of protolerogenic factors [101] or their downstream targets like the coinhibitory surface receptor PD-L1 [102] that counteract APC-mediated costimulation are targeted. Furthermore, micro-RNA (miRNA) species which regulate the activation state of APC have been recognized as interesting targets [103]. For example, delivery of a plasmid harboring multiple miRNA consensus bindings sites, termed miRNA sponge, is intended to limit their inhibitory effect on activation-associated mRNA targets [104].
In current human DNA vaccination approaches antigen-encoding DNA vaccines are coadministered with soluble immunostimulatory agents which often have been approved for anti-tumor adjuvant therapy [105]. Besides, expression vectors that encode cytokines [106] or chemokines [107] are clinically tested. So far, DNA vaccines that integrate both the antigen expression unit and a molecular adjuvant into a single plasmid have been tested in a limited number of studies only.
4.4. Vector Backbone
For transfection the prokaryotic part of a DNA vaccine is not necessary, and early studies have shown that the overall size of a DNA vaccine inversely correlated with transfection efficiency even in mitotically active cell lines [108]. This effect was attributed at least in part to silencing of transgene expression as a consequence of heterochromatin formation of bacterial components spreading into the promoter/vaccine expression cassette [109]. To circumvent this problem, the concept to delete prokaryotic sequences of the plasmid after its propagation in bacteria was developed, yielding so-called minicircle DNA [110]. More recently, Lu and colleagues reported that inclusion of A/T-rich sequences into the vector backbone prevented transcriptional silencing [111]. However, in a growing number of studies, increased efficacy of minicircle DNA vaccines as compared with conventional plasmids has been demonstrated [112].
Concerning the combined administration of DNA vaccines and genetic adjuvants (see above), bicistronic vectors are available which enable expression of both (or more) genes in cis [113]. Such vectors contain either two different promoters to independently express both transgenes or both expression units are separated by sequences like an internal ribosomal entry site (IRES) to mediate cap-independent translation of the downstream expression unit [114]. Especially due to the frequent observation of strongly different expression intensities of both transgenes, as an alternative a virus-derived T2A sequence was introduced instead [115]. After translation, this peptide sequence is recognized by an endogenous protease which mediates posttranslational cleavage of the different transgenes [116]. Usage of an IRES or T2A sequence is interesting when using a cell type-specific promoter for transcriptional targeting of APC.
Efficient transfer of DNA vaccines into the cell nucleus for subsequent expression is an important obstacle for success of transfection, especially in case of mitotically inactive cells like APC [117]. Quite early, certain DNA sequences were identified that mediated nuclear translocation. One of the first identified was the simian virus (SV)40 enhancer region, and transcription factors binding this site in the cytoplasm facilitated active nuclear entry of plasmid DNA due to their nuclear localization signal (NLS) [118]. As a consequence, in some approaches the SV40 enhancer site was included into the vector backbone [11]. Alternatively, the NLS activity of virus-derived peptides like the SV40 large T-antigen was exploited to facilitate nuclear plasmid translocation by attaching these peptides either directly to the vector backbone [119] or to DNA delivery systems [120].
5. Nano-Carriers for Transfer of DNA Vaccines
Nano-carriers (NC) offer the advantage to shield the DNA vaccine from degradation by DNases and other enzymes [121]. Surface modifications of NC with moieties like antibodies or natural ligands like carbohydrates may enable direct targeting of DC [16]. As outlined above, direct transfection of APC would prevent unwanted uptake of antigen and adjuvant by MDSC and TAM that promote tumor tolerance [122]. In addition, internal expression of the transgene ensures that sufficient amounts of antigen are presented via MHCI to yield robust CD8+ T cell responses [16].
NC are defined as particles of 1–1000 nm in size with an interfacial layer that can be composed of different materials [123]. They have gained scientific interest due to their unique properties and the wide range of applications linked to the variance in composition, size, shape and surface modifications [124]. So far, NC are used predominantly as delivery systems for drugs, adjuvants or nucleic acid-based vaccines contributing to the emerging field of nano-vaccines [125]. One of the most frequent applications of NC is the use in tumor therapy [126] as they have many advantages over classical chemotherapeutics and other pharmacology agents like improved bioavailability [127], organ- [128] or cell type- [16] specific targeting, tunable drug release [129], and addressing of organelles [130]. The exact mode of interaction of NC with a cell membrane is strongly influenced by the size, charge, shape, and hydrophilic/hydrophobic surface properties of the former, and uptake most often occurs either by membrane penetration which is associated with transient appearance of holes or by receptor-mediated endocytosis [131]. Both cellular uptake and endosomal release of NC-complexed DNA is enhanced by cell penetrating peptides (CPP) which are either attached directly to DNA [132] or to the DNA-complexing NC [133]. However, most of these studies have been performed under serum-poor conditions in vitro, and the possibility of rapid formation of a protein corona around the NC and engagement of negatively charged serum components by CPP prior to cellular engagement needs to be taken into account [134]. NC surface modifications like dense decoration with polyethylene glycol (PEG) have been introduced to limit serum protein adsorption, and to retain CPP activity [135]. Major safety concerns associated with the use of NC are are biocompatibility, biodistributiion and clearance, and the induction of unwanted immune reactions [136]. For example, repetitive application of PEG-coated NC may result in the arisal of PEG-specific antibodies [137]. The development of new NC-based delivery systems stirred up hope in the field of gene therapy for cancer treatment [138]. APC are assigned to recognize pathogens, so particles that are of similar size as pathogens should be easily engulfed [139]. While DC normally ingest particles that are virus-like in size (20–200 nm) [140], macrophages usually engulf larger particles (0.5–5 µm) [141], but for NC the efficacy of uptake by APC is strongly dependent on the surface properties and shape of the NC [142]. Cationic particles are internalized better by APC, but are also more likely to induce platelet aggregation and hemolysis [143]. Also the shape of the particle is crucial for uptake. For gold NP (AuNP) it was demonstrated that spherical particles are more efficient than any other shape [144]. Additionally, hydrophobicity, surface modification and the delivered cargo can influence the interaction with immune cells [145]. It needs to be considered that all these factors also influence the interaction with serum proteins, i.e., that modifications can have unpredictable effects in the organism [121].
As outlined below, the field of nanomaterials is still emerging resulting in new designs that open new opportunities for use like the development of virus-like particles (VLP) [146] and magnetic particles which may be directed in vivo by applying magnetic fields [147], and the introduction of tumor microenvironment-triggered drug release mechanisms [148]. Still, there are many challenges linked to the use of NC including the issue whether active targeting to specific cells and tissues is possible and needed for therapeutic efficacy [149].
NC-mediated delivery of nucleic acid-based therapeutics is a very promising approach, but several extra- and intracellular barriers still limit transfection efficacy [150] Hence, NC intended for the delivery of nucleic acid-based therapeutics need to fulfill a number of requirements:
-
(i)
The delivery vector has to offer a sufficient capacity to efficiently package DNA/RNA per se, which is an obstacle especially for longer plasmid DNA [151], in order to enable delivery of a sufficient amount of molecules per target cell [152].
-
(ii)
The delivery system has to show stability against serum proteins that may form a protein corona around the NC and thereby affect its targeting and uptake efficiency [134].
-
(iii)
After uptake by the cell, the NC cargo has to evade endo/lysosomal degradation and to enter the cytoplasm by endosomal escape [153]. While released mRNA is translated directly in the cytoplasm, DNA vaccines need to translocate into the nucleus for subsequent transcription which may be enhanced by NLS [154].
5.1. Nano-Carriers Composed of Inorganic Material
There are numerous different types of NC regarding material, size and shape, each having advantages and disadvantages in the field of nanomedicine. Inorganic nanomaterials have a rigid structure and a controllable synthesis allowing simple modification.
AuNP showed low cytotoxic effects, were biocompatible and have been used as carriers for virus-derived antigens [155] as well as DNA vaccines since plasmid DNA can be complexed directly on the surface of the particle [156]. In addition, protein-coated AuNP were reported to possess intrinsic immunostimulatory capacity as they mediated DC activation [157]. The transfection efficiency was increased by the use of CPP to coat the AuNPs, thus minimizing influences from the cell environment on the plasmid DNA and facilitating cell uptake [158].
Another interesting candidate for nucleic acid delivery are silica-based NC. These are biocompatible, and can deliver different types of cargo [159]. Of note, such NC were demonstrated to passively target tumor tissues [160]. Mesoporous silica NC have been used for DNA transfection of cell lines in vitro [161]. In general, such NC allow to control the release of cargo by modification of pore size and shape as well as surface functionalization [162].
Graphene oxide is a compound consisting mainly of carbon and oxygen with a layer structure. Because of the electrostatic π-π-stacking interactions it shows a high loading capacity as well as a controlled release of cargo [163]. Additionally, graphene oxide protects nucleic acids from cleavage, making it a very remarkable candidate for gene therapy [164]. For negatively charged carbon NC direct transfer of DNA via the cell membrane into the cytosol was demonstrated [165]. Another type of carbon-based nanomaterial suitable for transfer of nucleic acids are carbon nanotubes (CNT). CNT are small and chemically inert. However, their hydrophobicity limits their use in biomedical application as CNT are poorly soluble in water [166]. To improve their biocompatibility, CNT can be modified covalently, but this often reduces the loading capacity of nucleic acids as well as the intracellular release of the cargo [167].
Magnetic particles like iron oxide core particles have the unique ability not only to provide cargo delivery but also allow detection in vivo by magnetic resonance imaging [168]. An approach tested in the clinic for cancer therapy is to target magnetic particles to the tumor tissue and then to induce magnetic hyperthermia [169]. To improve biocompatibility of magnetic particles, they are often coated with proteins, polymers or polysaccharides, which reduces cytotoxic effects and can improve their DNA transfecting properties [170].
5.2. Lipid-Based Nano-Carriers
Lipids can also be used for gene delivery. Felgner and colleagues were the first to demonstrate that N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTAP) can transfer DNA into cells [171]. Complexes composed of DOTAP and protamine-condensed DNA showed good transfection efficiencies in vivo [172]. Cationic lipids are generally very suitable for transfection as they preferably interact with the anionic cell membrane thus facilitating uptake of the transfection complex into the cell [173]. Charge-driven interaction with cellular receptors initiating endocytosis was shown as the uptake mechanism for cationic liposomes [174]. Furthermore, cationic lipids show great binding of DNA and the forming liposomes protect their cargo from degradation. A positive effect is achieved by the addition of a neutral helper lipid, which stabilizes the lipid-bilayer and may promote endosomal escape of passenger DNA either due to pH buffering of protonatable groups or after fusion with the endosomal membrane [175]. In case of cationic DOTAP-based liposomes containing cholesterol as a helper lipid, macropinocytose was identified as the major uptake mechanism [176]. More recently, incorporation of coiled-coil lipopeptides into the liposomal bilayer was demonstrated to promote interaction with cell membrane receptors and direct release of cargo into the cytosol [177]. Liposomes also can encapsulate protein antigens and are commonly used as adjuvant delivery systems as they spontaneously rearrange into nanostructures [178]. Several liposomal protein-based substances haven been approved decades ago for prophylactic vaccination against herpes simplex virus A [179] and influenza [180].
5.3. Protein-Based Nano-Carriers
Proteins constitute a favorable material for NC generation since they are biocompatible, biodegradable and typically show low cytotoxicity [181]. A good choice for a gene delivery system is gelatin B combined with protamine sulfate [182]. The overall negative charge of the protein switches in the endosome leading to a release of cargo. The major drawback of this system is the relatively low DNA loading capacity. Endogenous proteins can be also used to design NC for gene delivery. A good example is albumin which is generated largely by the liver and constitutes the most abundant serum protein [183]. Albumin has been demonstrated to exert several tasks via transient binding of other extracellular components, including the protection of proteins and fatty acids from peroxidative modifications [184], ion transport, and adsorption of drugs which affects their bioavailability. In line, albumin has many reactive groups on the surface allowing chemical modifications [185]. NC consisting of an albumin core and chitosan as an outer layer successfully transfected different cell lines [186], and when surface-modified with ethylenediamin for introduction of cationic properties served to deliver siRNA into metastatic tumor in vivo [187].
VLP which consist of various self-assembling viral proteins may constitute suitable candidates for gene delivery [188], as they combine the ability of the virus to interact with immune cells while lacking infectious properties [146]. However, VPL proteins were demonstrated to serve as antigens due to their exogenous origin which constitutes an unwanted side effect [189]. Anyway, the first VLP-based vaccine was commercialized in 1986, and by now several anti-viral and one malaria-directed vaccine are clinically used, most often in combination with the classical adjuvant Alum [190].
5.4. Polymeric Nano-Carriers
Polymer-based nanomaterials have gained interest because of their electrostatic interactions with nucleic acids resulting in protection from enzymatic degradation [191]. Furthermore, cationic polymeric nanomaterials are cheap, non-immunogenic, safe and have a greater DNA-loading capacity than viruses. The first polymeric particle reported to enhance transfection was diethylamino-dextran in the late 1960’s [192]. Polymeric systems are often surface-modified to prolong their circulation time in the bloodstream to enable more specific or even cell-targeted delivery. One option to shield NC from the formation of a protein corona determining cellular interactions is the use of PEG [193]. Cationic polylactides are an example of this problem and it was shown that PEGylated particles performed better in serum at high polymer/DNA ratios than commercially available transfection reagents [194]. The importance of polymer-based materials was emphasized when it was early demonstrated that cationic polymers not only bind to DNA but are also very effective in condensing it to form structures resembling viruses [195]. Among the best studied polymeric nanomaterials are poly-d,l-lactide-co-Glycolide (PLG) [196] and poly-d,l-lactic-co-Glycolic acid (PLGA) [197]. PLG- and PLGA-based particles are biocompatible, biodegradable and show a sustained release of a wide range of different cargo molecules [198], including DNA [199]. Poly-L-lysine (PLL) is a natural polymer with a peptidic structure and therefore highly biodegradable. However, the transfection efficiency of PLL particles was lower than obtained using polyethylenimine (PEI) [200]. Polymeric structures achieved a milestone in 1988 as the first receptor targeted transfections were performed. For this, asialoglycoprotein was introduced into PLL targeting its receptor which resulted in increased transfection in vivo [201]. To improve the transfection efficiencies of polymeric systems equipped with targeting moieties, in early approaches inactivated adeno- [202] and rhino- [203] viruses were used in combination with receptor targeting polyplexes. To enhance endosomal escape of the systems, proteins have often been used. In this regard, a sulfhydryl-activatable listeriolysin O/protamine conjugate showed enhanced delivery of DNA into the cytosol [204]. As an alternative, polyamidoamine dendrimers were shown to exert a prominent proton sponge effect as the amines are only slightly protonated at neutral pH [205]. PEI was reported to yield a better transfection capacity as a linear compared to a branched polymer which suggested that unpacking of the DNA is more complicated when complexed with the latter [206]. However, PEI is not biodegradable and shows high toxic effects in a molecular weight-dependent manner [207]. Additionally, cationic polymers in general can be recognized by the immune system and were shown to trigger complement activation, which can lead to an untimely clearance of the NC [208]. In other approaches, the suitability of natural polymers to generate NC has been demonstrated. For example, chitosan was shown to constitute a suitable DNA carrier [209], and bears intrinsic adjuvant activity [210], similar to other polysaccharide-based polymers like inulin [211] and others [212].
6. Route of Vaccination
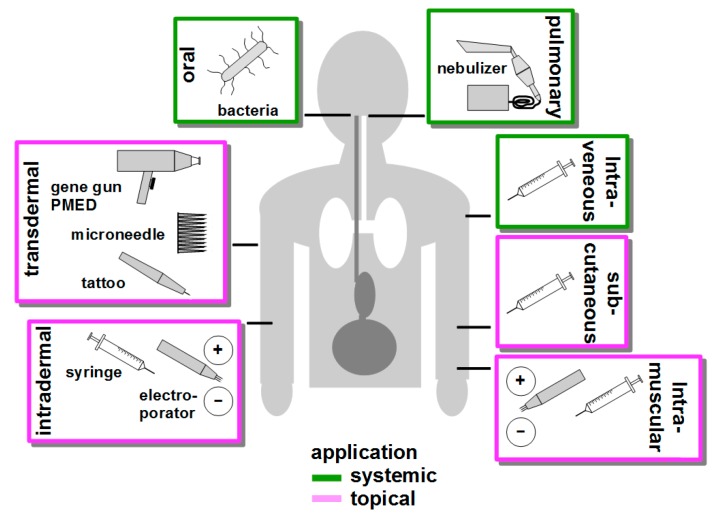
To transfect as many APCs as possible, targeting DNA vaccine delivery to secondary lymphoid organs via systemic application like intravenous injection [213], oral [214] or pulmonary [215] administration is a suitable strategy. Alternatively, DNA vaccines are often applied often topically via the skin [216] or intramuscularly [217]. Figure 3 presents an overview over common DNA vaccination routes.
Figure 3.
Routes of DNA vaccine delikvery. DNA vaccines may be delivered systemically by intraveneous injection to reach secondary lymphatic organs, by oral application of (attenuated) bacteria as a vehicle to confer uptake of DNA by intestinal APC, and by pulmonary administration of nebulized DNA to achive uptake by lung cells. Transdermal delivery primarily adresses LC, and both needle-free delivery of particle-adsorbed DNA vaccines by helium pressure (gene gun, PMED) and needle-based administration via microneedles and tattoo devices are clinically tested. Transfection of cutaneous APC as well as of non-APC by intradermally injected DNA vaccines is enhanced by immediate electroporation. Subcutaneous injection mainly results in transfection of fibroblasts and keratinocyts, which express transgenes and release antigen for uptake APC. Likewise, intramuscular injection of DNA vaccines primarily yields transfection of myocytes that express/release antigen for APC uptake, and myocyte transfection rates are enhanced by electroportion at the site of injection as well.
Intraveneous application of DNA vaccines inprinciple allows to reach APC located in secodnary lymphoid organs. However, the physicochemical characteristics of DNA-complexing NC determine the biodistribution of the DNA vaccine. For example, PEI-complexed reporter expression vectors preferentially transfected lung cells [218], whereas lipsomomal formulations transfected liver cells [219]. In general, numerous types of NC were demonstrated to interact with blood components which thereby form a protein corona around the NC which in turn may strongly affect its cellular binding properties [134]. For example, we have recently shown that NC coated wtih dextran to confer biocompatibility triggered the lectin-dependent pathway of complement activation which resulted in deposition of C3 fragments on the NC surface, and preferential binding to splenic B cells via their complement receptor [220].
For oral administration of DNA, non-pathogenic (L. lactis [221] or attenuated (S. thyphimurium [222] and L. monocytogenes [223]) bacterial strains are often used as vectors, termed bactofection [224]. In the intestine, these bacteria may be phagocytosed directly by mucosal DC/macrophages spreading extensions into the gut lumen or after M cell-mediated transcytosis at the Peyer’s patches [225]. After phagocytosis, plasmid DNA is released from phagolysosome, and the numerous bacteria-associated danger signals result in profound APC activation [226]. More recently, ‘bacterial ghosts’ which constitute only the bacterial envelope have been introduced as carriers for DNA vaccines [227]. Aside oral application, bacteria were shown to confer transfection of APC when applied at other mucosal sites, e.g., when applied intranasally [228]. More recently, the DNA vaccine delivery properties of orally applied bacteria, for example with regard to phagolysosomal escape have been improved by additional coating with NC [229].
Compared with other sytemic routes of DNA vaccination, pulmonary application of aerolized DNA vaccines is a rather new approach [230]. Usage of naked and NC-complexed DNA was demonstrated to yield transfection of lung epithelial cells [231]. Hence, so far research focusses on therapeutic treatment of local gene defects as in case of cystic fibrosis [232].
The skin constitutes an interesting target organ for DNA vaccination due to the rather high frequency of cutaneous DC. For example, human skin, depending on the specific site, contains 200–1000 LC per square millimeter [233]. Furthermore, in skin (activated) DC are the only cell populations showing migratory behavior towards draining lymph nodes to evoke T cell responses [234]. Different transdermakl DNA vaccination strategies have been developed, and their suitability is clinically tested [235]. Needle-free biolistic transfection as mediated by gene gun [69] and PMED (particle-mediated epidermal delivery [236] devices transfers microparticle-adsorbed DNA into the epidermal layer by helium force to transfect LC, dermal DC (and keratinocytes). Of note, the physical stress associated with biolistic transfection, was reported as sufficient to mediate activation and emigration of directly transfected DC [237]. Microneedles which are produced from various materials and techniques display lenghts below one micron [238] and tattooing devices [239] address these cell types as well. Conventional intradermal administration of DNA vaccines by syringes aims to transfect dermal DC (and fibroblasts). Based on the observation that after intradermal injection of DNA a short electrical pulse, termed electroporation, mediates several-fold enhanced transfection has resulted in the development of a number of according devices tested in clinical studies [240]. Similarly, in vaccination studies transfection rates of myocytes after intramuscular injection of DNA, intended to generate antigen for uptake by APC, were found strongly elevated by electroporation as well [106]. In general, electroporation in the context of transdermal [241] and intramuscular [242] DNA vaccination was reported to result in local activation of innate immunity which may be a consequence of e.g., electroporation-induced cellular stress reactions, including necrosis.
Concerning the success of immunization of different DNA vaccination routes, the recent phase I trial CUTHIVEC which assessed in a comparative manner the efficacy of different DNA vaccine administration routes showed increased antigen specific CD4+ and CD8+ responses after combined intramuscular and transcutaneous injection compared to intramuscular plus intradermal injections [43]. The former approach was even more efficient than intramuscular administration followed by electroporation (EP) at the injection side. EP is frequently used to increase the overall transfection efficiency at the injection site and was shown to efficiently enhance immune responses in rhesus macaques after DNA vaccination [243]. In general, the application method itself beyond mediating APC activation may also influence T cell polarization. In a comparative study, intramuscular DNA vaccination resulted in a Th1-biased T cell response (see above), whereas biolistic transfection yielded a Th2 response [244].
With regard to the distribution of DNA vaccines complexed with NC it is noteworthy that small particles are easily transported into the lymph node, while larger particles remain longer at the site of administration [245]. In addition, the route of administration can also account for the fate of the delivery systems. After subcutaneous injection small PEGylated liposomes were found in larger amount in the lymph node than after intravenous or intraperitoneal injection [246]. Concerning NC clearance from the body, NC that are smaller than 8 nm are cleared renally [247], and the extent of renal clearance was shown to correlate with the extent of negative charge [248]. Biliary clearance was observed especially for particles over 200 nm and for strongly charged particles [249].
7. Targeting of Antigen Presenting Cells
Conventional application routes of naked and NC-complexed DNA may result predominantly in transfection of non-APC which in turn may generate and release antigen that may be engulfed by APC [14] [8]. However, only DC populations with cross-presenting potential are able to shuttle a fraction of extracellular antigen towards MHCI which enables stimulation of CD8+ T cells [16]. Therefore, pronounced CTL activation requires direct transfection of APC [10]. Due to their pathogen-like appearance in terms of size and shape, NC-complexed DNA vaccines may passively target APC like (conventional) DC and macrophages since these cell types are specialized in the uptake of ‘foreign’ material [250]. Usage of either natural ligands or antibodies specific for endocytically active receptors strongly expressed on APC may enable cell type-focussed targeting [251]. Such moieties may be coupled to naked DNA or DNA complexing NC.
For targeting, CLR that are predominantly expressed by DC and macrophages constitute suitable candidates [252]. For example, DC-SIGN [253] and the mannose receptor [254] are expressed by either cell type at differential intensity, respectively, and mediate internalization of mannosylated particles. Qiao and coworkers demonstrated that vaccination of mice with a mannosylated cationic liposome that complexed HIV protein-encoding plasmid DNA resulted in improved immune responses in mice [255]. Intramuscular application of a DNA vaccine encoding a botulinum neurotoxin fused with a single chain antibody fragment (scFv) specific for the CLR DEC-205 resulted in improved cellular and humoral immune responses, and protected vaccinated animals from botulinus infection [256]. Fusion of a tumor antigen encoding DNA vaccine with a CD11c-specific scFv was protective in a mouse breast cancer model and slowed tumor growth in a therapeutic setting [257].
8. Concluding Remarks
The immunogenicity of DNA vaccines in human ist still to low to yield therapeutically convincing results. However, in the last 15 years different approaches have shown that optimization of different parameters contributes to enhanced transfection and hence immunogenicity of DNA vaccines also in human. Hence, an ideal DNA vaccine will need to be complexed with an APC-targeting NC to prevent extracellular degradation and to enable direct APC transfection, and will contain a NLS to facilitate nuclear entry. The DNA vaccine should contain a promoter that facilitates transcriptional targeting of APC to prevent unwanted antigen expression in tolerance-promoting cell types like MDSC. Furthermore, the DNA vaccine should include a genetic adjuvant which promotes activation of the transfected APC to prevent antigen-specific tolerance induction. Of note, several types of NC bear intrinsic immunostimulatory activity, and the mode of DNA delivery may also result in local inflammation. Both factors need to be considered since they contribute to shape the character of the induced immune response, especially with regard to T cell polarization [258]. With regard to tumor therapy, recent progress in cost effective deep sequencing now allows to identify patients-specific tumor antigens [259].
While administration of a DNA vaccine as outlined above aims to induce antigens-specific immune responses, additional application of agents that inhibit immuno-regulatory myeloid cells like MDSC and Treg as well as the tumor itself may have synergistic effects [260]. Besides chemotherapeutics, checkpoint inhibitors that have recently been introduced into clinical therapy are likely candidates for cotreatment [261].
Abbreviations
| APC | antigen presenting cell |
| AuNP | gold nanoparticle |
| CLR | C-type lectin receptor |
| CPP | cell penetrating peptide |
| CNT | carbon nanotube |
| CTL | cytotoxic T lymphocyte Cutaneous and Mucosal HIV Vaccination |
| CUTHIVAC | cutaneous and mucosal HIV vaccination |
| DC | dendritic cell |
| DC-SIGN | dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin |
| DC-STAMP | dendrocyte expressed seven transmembrane protein |
| DOTAP | N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride |
| IRF | interferon-regulatory factor |
| HLA | human leukocyte antigen |
| LC | Langerhans cell |
| MDSC | myeloid-derived suppressor cell |
| MHC | major histocompatibility class |
| NC | nano-carrier |
| NK | natural killer cell |
| miRNA | micro-RNA |
| NF-κB | nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells |
| NLS | nuclear localization signal |
| PEG | polyethyleneglycol |
| PEI | polyethylenimine |
| PLGA | poly-d,l-lactic-coglycolic acid |
| scFv | single chain antibody fragment |
| SV40 | Simian virus 40 |
| TAA | tumor-associated antigen |
| TAM | tumor-associated macrophage |
| Th | T helper cell |
| TLR | toll-like receptor |
| Treg | regulatory T cell |
| VLP | virus-like particle |
Funding
DH and MB are funded by the Deutsche Forschungsgemeinschaft (SFB1066, project B05).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Prazeres D.M.F., Monteiro G.A. Plasmid Biopharmaceuticals. Microbiol. Spectrum. 2014;2 doi: 10.1128/microbiolspec.PLAS-0022-2014. [DOI] [PubMed] [Google Scholar]
- 2.Fioretti D., Iurescia S., Rinaldi M. Recent advances in design of immunogenic and effective naked DNA vaccines against cancer. Recent Pat. Anti-Canc. Drug Discov. 2014;9:66–82. doi: 10.2174/1574891X113089990037. [DOI] [PubMed] [Google Scholar]
- 3.Scheiblhofer S., Thalhamer J., Weiss R. DNA and mRNA vaccination against allergies. Pediat. Allergy Immu. 2018 doi: 10.1111/pai.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang N., Nandakumar K.S. Recent advances in the development of vaccines for chronic inflammatory autoimmune diseases. Vaccine. 2018;36:3208–3220. doi: 10.1016/j.vaccine.2018.04.062. [DOI] [PubMed] [Google Scholar]
- 5.Maslow J.N. Vaccines for emerging infectious diseases: Lessons from MERS coronavirus and Zika virus. Hum. Vacc. Immunother. 2017;13:2918–2930. doi: 10.1080/21645515.2017.1358325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weniger B.G., Anglin I.E., Tong T., Pensiero M., Pullen J.K. Workshop report: Nucleic acid delivery devices for HIV vaccines: Workshop proceedings, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA, May 21, 2015. Vaccine. 2018;36:427–437. doi: 10.1016/j.vaccine.2017.10.071. [DOI] [PubMed] [Google Scholar]
- 7.Tiptiri-Kourpeti A., Spyridopoulou K., Pappa A., Chlichlia K. DNA vaccines to attack cancer: Strategies for improving immunogenicity and efficacy. Pharmacol. Therapeut. 2016;165:32–49. doi: 10.1016/j.pharmthera.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Marino M., Scuderi F., Provenzano C., Bartoccioni E. Skeletal muscle cells: From local inflammatory response to active immunity. Gene Ther. 2011;18:109–116. doi: 10.1038/gt.2010.124. [DOI] [PubMed] [Google Scholar]
- 9.Hengge U.R., Chan E.F., Foster R.A., Walker P.S., Vogel J.C. Cytokine gene expression in epidermis with biological effects following injection of naked DNA. Nat. Genet. 1995;10:161–166. doi: 10.1038/ng0695-161. [DOI] [PubMed] [Google Scholar]
- 10.Porgador A., Irvine K.R., Iwasaki A., Barber B.H., Restifo N.P., Germain R.N. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J. Exp. Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai H., Lester G.M.S., Petishnok L.C., Dean D.A. Cytoplasmic transport and nuclear import of plasmid DNA. Biosci. Rep. 2017;37 doi: 10.1042/BSR20160616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coban C., Kobiyama K., Jounai N., Tozuka M., Ishii K.J. DNA vaccines: A simple DNA sensing matter? Hum. Vacc. Immunother. 2013;9:2216–2221. doi: 10.4161/hv.25893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joffre O., Nolte M.A., Sporri R., Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 14.Sudowe S., Dominitzki S., Montermann E., Bros M., Grabbe S., Reske-Kunz A.B. Uptake and presentation of exogenous antigen and presentation of endogenously produced antigen by skin dendritic cells represent equivalent pathways for the priming of cellular immune responses following biolistic DNA immunization. Immunology. 2009;128:e193–e205. doi: 10.1111/j.1365-2567.2008.02947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Den Haan J.M., Arens R., van Zelm M.C. The activation of the adaptive immune system: Cross-talk between antigen-presenting cells, T cells and B cells. Immunol. Lett. 2014;162:103–112. doi: 10.1016/j.imlet.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Macri C., Dumont C., Johnston A.P., Mintern J.D. Targeting dendritic cells: A promising strategy to improve vaccine effectiveness. Clin. Transl. Immunol. 2016;5:e66. doi: 10.1038/cti.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazzaro S., Giovani C., Mangiavacchi S., Magini D., Maione D., Baudner B., Geall A.J., De Gregorio E., D’Oro U., Buonsanti C. CD8 T-cell priming upon mRNA vaccination is restricted to bone-marrow-derived antigen-presenting cells and may involve antigen transfer from myocytes. Immunology. 2015;146:312–326. doi: 10.1111/imm.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwasaki A., Torres C.A., Ohashi P.S., Robinson H.L., Barber B.H. The dominant role of bone marrow-derived cells in CTL induction following plasmid DNA immunization at different sites. J. Immunol. 1997;159:11–14. [PubMed] [Google Scholar]
- 19.Maecker H.T., Umetsu D.T., DeKruyff R.H., Levy S. Cytotoxic T cell responses to DNA vaccination: Dependence on antigen presentation via class II MHC. J. Immunol. 1998;161:6532–6536. [PubMed] [Google Scholar]
- 20.Shimizu K., Iyoda T., Okada M., Yamasaki S., Fujii S.I. Immune suppression and reversal of the suppressive tumor microenvironment. Int. Immunol. 2018;30:445–454. doi: 10.1093/intimm/dxy042. [DOI] [PubMed] [Google Scholar]
- 21.Concha-Benavente F., Srivastava R., Ferrone S., Ferris R.L. Immunological and clinical significance of HLA class I antigen processing machinery component defects in malignant cells. Oral Oncol. 2016;58:52–58. doi: 10.1016/j.oraloncology.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garrido F., Aptsiauri N., Doorduijn E.M., Garcia Lora A.M., van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 2016;39:44–51. doi: 10.1016/j.coi.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wischhusen J., Waschbisch A., Wiendl H. Immune-refractory cancers and their little helpers--an extended role for immunetolerogenic MHC molecules HLA-G and HLA-E? Semin. Cancer Biol. 2007;17:459–468. doi: 10.1016/j.semcancer.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Petty A.J., Yang Y. Tumor-associated macrophages: Implications in cancer immunotherapy. Immunotherapy. 2017;9:289–302. doi: 10.2217/imt-2016-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ahrends T., Borst J. The opposing roles of CD4(+) T cells in anti-tumour immunity. Immunology. 2018 doi: 10.1111/imm.12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber R., Fleming V., Hu X., Nagibin V., Groth C., Altevogt P., Utikal J., Umansky V. Myeloid-Derived Suppressor Cells Hinder the Anti-Cancer Activity of Immune Checkpoint Inhibitors. Front. Immunol. 2018;9:1310. doi: 10.3389/fimmu.2018.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolff J.A., Ludtke J.J., Acsadi G., Williams P., Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Hum. Mol. Genet. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z., Troilo P.J., Wang X., Griffiths T.G., Pacchione S.J., Barnum A.B., Harper L.B., Pauley C.J., Niu Z., Denisova L., et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004;11:711–721. doi: 10.1038/sj.gt.3302213. [DOI] [PubMed] [Google Scholar]
- 29.Manam S., Ledwith B.J., Barnum A.B., Troilo P.J., Pauley C.J., Harper L.B., Griffiths T.G., 2nd, Niu Z., Denisova L., Follmer T.T., et al. Plasmid DNA vaccines: Tissue distribution and effects of DNA sequence, adjuvants and delivery method on integration into host DNA. Intervirology. 2000;43:273–281. doi: 10.1159/000053994. [DOI] [PubMed] [Google Scholar]
- 30.Jiao S., Williams P., Berg R.K., Hodgeman B.A., Liu L., Repetto G., Wolff J.A. Direct gene transfer into nonhuman primate myofibers in vivo. Hum. Gene Ther. 1992;3:21–33. doi: 10.1089/hum.1992.3.1-21. [DOI] [PubMed] [Google Scholar]
- 31.Mairhofer J., Lara A.R. Advances in host and vector development for the production of plasmid DNA vaccines. Methods Mol. Biol. 2014;1139:505–541. doi: 10.1007/978-1-4939-0345-0_38. [DOI] [PubMed] [Google Scholar]
- 32.Myhr A.I. DNA Vaccines: Regulatory Considerations and Safety Aspects. Curr. Issues Mol. Biol. 2017;22:79–88. doi: 10.21775/cimb.022.079. [DOI] [PubMed] [Google Scholar]
- 33.Dauphin G., Zientara S. West Nile virus: Recent trends in diagnosis and vaccine development. Vaccine. 2007;25:5563–5576. doi: 10.1016/j.vaccine.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Atherton M.J., Morris J.S., McDermott M.R., Lichty B.D. Cancer immunology and canine malignant melanoma: A comparative review. Vet. Immunol. Immunop. 2016;169:15–26. doi: 10.1016/j.vetimm.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 35.MacGregor R.R., Boyer J.D., Ugen K.E., Lacy K.E., Gluckman S.J., Bagarazzi M.L., Chattergoon M.A., Baine Y., Higgins T.J., Ciccarelli R.B., et al. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: Safety and host response. J. Infect. Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- 36.Wang R., Doolan D.L., Le T.P., Hedstrom R.C., Coonan K.M., Charoenvit Y., Jones T.R., Hobart P., Margalith M., Ng J., et al. Induction of antigen-specific cytotoxic T lymphocytes in humans by a malaria DNA vaccine. Science. 1998;282:476–480. doi: 10.1126/science.282.5388.476. [DOI] [PubMed] [Google Scholar]
- 37.Rottinghaus S.T., Poland G.A., Jacobson R.M., Barr L.J., Roy M.J. Hepatitis B DNA vaccine induces protective antibody responses in human non-responders to conventional vaccination. Vaccine. 2003;21:4604–4608. doi: 10.1016/S0264-410X(03)00447-X. [DOI] [PubMed] [Google Scholar]
- 38.Mincheff M., Tchakarov S., Zoubak S., Loukinov D., Botev C., Altankova I., Georgiev G., Petrov S., Meryman H.T. Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: A phase I/II clinical trial. Eur. Urol. 2000;38:208–217. doi: 10.1159/000020281. [DOI] [PubMed] [Google Scholar]
- 39.McNeel D.G., Becker J.T., Johnson L.E., Olson B.M. DNA Vaccines for Prostate Cancer. Curr. Cancer Ther. Rev. 2012;8:254–263. doi: 10.2174/157339412804143113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tagawa S.T., Lee P., Snively J., Boswell W., Ounpraseuth S., Lee S., Hickingbottom B., Smith J., Johnson D., Weber J.S. Phase I study of intranodal delivery of a plasmid DNA vaccine for patients with Stage IV melanoma. Cancer. 2003;98:144–154. doi: 10.1002/cncr.11462. [DOI] [PubMed] [Google Scholar]
- 41.Weber J., Boswell W., Smith J., Hersh E., Snively J., Diaz M., Miles S., Liu X., Obrocea M., Qiu Z., et al. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J. Immunother. 2008;31:215–223. doi: 10.1097/CJI.0b013e3181611420. [DOI] [PubMed] [Google Scholar]
- 42.Ottensmeier C., Bowers M., Hamid D., Maishman T., Regan S., Wood W., Cazaly A., Stanton L. Wilms’ Tumour Antigen 1 Immunity via DNA Fusion Gene Vaccination in Haematological Malignancies by Intramuscular Injection Followed by Intramuscular Electroporation: A Phase II Non-Randomised Clinical Trial (WIN) NIHR Journals Library.; Southampton, UK: 2016. Efficacy and Mechanism Evaluation. [DOI] [PubMed] [Google Scholar]
- 43.Haidari G., Cope A., Miller A., Venables S., Yan C., Ridgers H., Reijonen K., Hannaman D., Spentzou A., Hayes P., et al. Combined skin and muscle vaccination differentially impact the quality of effector T cell functions: The CUTHIVAC-001 randomized trial. Sci. Rep. 2017;7:13011. doi: 10.1038/s41598-017-13331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pierini S., Perales-Linares R., Uribe-Herranz M., Pol J.G., Zitvogel L., Kroemer G., Facciabene A., Galluzzi L. Trial watch: DNA-based vaccines for oncological indications. Oncoimmunology. 2017;6:e1398878. doi: 10.1080/2162402X.2017.1398878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suschak J.J., Williams J.A., Schmaljohn C.S. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum. Vacc. Immunother. 2017;13:2837–2848. doi: 10.1080/21645515.2017.1330236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dupuis M., Denis-Mize K., Woo C., Goldbeck C., Selby M.J., Chen M., Otten G.R., Ulmer J.B., Donnelly J.J., Ott G., et al. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J. Immunol. 2000;165:2850–2858. doi: 10.4049/jimmunol.165.5.2850. [DOI] [PubMed] [Google Scholar]
- 47.Lechardeur D., Verkman A.S., Lukacs G.L. Intracellular routing of plasmid DNA during non-viral gene transfer. Adv. Drug Deliv. Rev. 2005;57:755–767. doi: 10.1016/j.addr.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Osborn M.J., McElmurry R.T., Lees C.J., DeFeo A.P., Chen Z.Y., Kay M.A., Naldini L., Freeman G., Tolar J., Blazar B.R. Minicircle DNA-based gene therapy coupled with immune modulation permits long-term expression of alpha-L-iduronidase in mice with mucopolysaccharidosis type I. Mol. Ther. 2011;19:450–460. doi: 10.1038/mt.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grubor-Bauk B., Yu W., Wijesundara D., Gummow J., Garrod T., Brennan A.J., Voskoboinik I., Gowans E.J. Intradermal delivery of DNA encoding HCV NS3 and perforin elicits robust cell-mediated immunity in mice and pigs. Gene Ther. 2016;23:26–37. doi: 10.1038/gt.2015.86. [DOI] [PubMed] [Google Scholar]
- 50.Krinner S., Heitzer A., Asbach B., Wagner R. Interplay of Promoter Usage and Intragenic CpG Content: Impact on GFP Reporter Gene Expression. Hum. Gene Ther. 2015;26:826–840. doi: 10.1089/hum.2015.075. [DOI] [PubMed] [Google Scholar]
- 51.Vanniasinkam T., Reddy S.T., Ertl H.C. DNA immunization using a non-viral promoter. Virology. 2006;344:412–420. doi: 10.1016/j.virol.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 52.Metlay J.P., Witmer-Pack M.D., Agger R., Crowley M.T., Lawless D., Steinman R.M. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J. Exp. Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erdei A., Lukacsi S., Macsik-Valent B., Nagy-Balo Z., Kurucz I., Bajtay Z. Non-identical twins: Different faces of CR3 and CR4 in myeloid and lymphoid cells of mice and men. Semin. Cell Dev. Biol. 2017 doi: 10.1016/j.semcdb.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 54.Brocker T., Riedinger M., Karjalainen K. Driving gene expression specifically in dendritic cells. Adv. Exp. Med. Biol. 1997;417:55–57. doi: 10.1007/978-1-4757-9966-8_9. [DOI] [PubMed] [Google Scholar]
- 55.Lauterbach H., Gruber A., Ried C., Cheminay C., Brocker T. Insufficient APC capacities of dendritic cells in gene gun-mediated DNA vaccination. J. Immunol. 2006;176:4600–4607. doi: 10.4049/jimmunol.176.8.4600. [DOI] [PubMed] [Google Scholar]
- 56.Eleveld-Trancikova D., Triantis V., Moulin V., Looman M.W., Wijers M., Fransen J.A., Lemckert A.A., Havenga M.J., Figdor C.G., Janssen R.A., et al. The dendritic cell-derived protein DC-STAMP is highly conserved and localizes to the endoplasmic reticulum. J. Leukocyte Biol. 2005;77:337–343. doi: 10.1189/jlb.0804441. [DOI] [PubMed] [Google Scholar]
- 57.Yagi M., Miyamoto T., Toyama Y., Suda T. Role of DC-STAMP in cellular fusion of osteoclasts and macrophage giant cells. J. Bone Miner. Metab. 2006;24:355–358. doi: 10.1007/s00774-006-0697-9. [DOI] [PubMed] [Google Scholar]
- 58.Dresch C., Edelmann S.L., Marconi P., Brocker T. Lentiviral-mediated transcriptional targeting of dendritic cells for induction of T cell tolerance in vivo. J. Immunol. 2008;181:4495–4506. doi: 10.4049/jimmunol.181.7.4495. [DOI] [PubMed] [Google Scholar]
- 59.Goyal S., Castrillon-Betancur J.C., Klaile E., Slevogt H. The Interaction of Human Pathogenic Fungi With C-Type Lectin Receptors. Front. Immunol. 2018;9:1261. doi: 10.3389/fimmu.2018.01261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ariizumi K., Shen G.L., Shikano S., Ritter R., 3rd, Zukas P., Edelbaum D., Morita A., Takashima A. Cloning of a second dendritic cell-associated C-type lectin (dectin-2) and its alternatively spliced isoforms. J. Biol. Chem. 2000;275:11957–11963. doi: 10.1074/jbc.275.16.11957. [DOI] [PubMed] [Google Scholar]
- 61.Bonkobara M., Zukas P.K., Shikano S., Nakamura S., Cruz P.D., Jr., Ariizumi K. Epidermal Langerhans cell-targeted gene expression by a dectin-2 promoter. J. Immunol. 2001;167:6893–6900. doi: 10.4049/jimmunol.167.12.6893. [DOI] [PubMed] [Google Scholar]
- 62.Lopes A., Vanvarenberg K., Preat V., Vandermeulen G. Codon-Optimized P1A-Encoding DNA Vaccine: Toward a Therapeutic Vaccination against P815 Mastocytoma. Mol. Ther.-Nucl. Acids. 2017;8:404–415. doi: 10.1016/j.omtn.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moulin V., Morgan M.E., Eleveld-Trancikova D., Haanen J.B., Wielders E., Looman M.W., Janssen R.A., Figdor C.G., Jansen B.J., Adema G.J. Targeting dendritic cells with antigen via dendritic cell-associated promoters. Cancer Gene Ther. 2012;19:303–311. doi: 10.1038/cgt.2012.2. [DOI] [PubMed] [Google Scholar]
- 64.Yamashiro S. Functions of fascin in dendritic cells. Crit. Rev. Immunol. 2012;32:11–21. doi: 10.1615/CritRevImmunol.v32.i1.20. [DOI] [PubMed] [Google Scholar]
- 65.Ross R., Jonuleit H., Bros M., Ross X.L., Yamashiro S., Matsumura F., Enk A.H., Knop J., Reske-Kunz A.B. Expression of the actin-bundling protein fascin in cultured human dendritic cells correlates with dendritic morphology and cell differentiation. J. Investig. Dermatol. 2000;115:658–663. doi: 10.1046/j.1523-1747.2000.00112.x. [DOI] [PubMed] [Google Scholar]
- 66.Ross R., Sudowe S., Beisner J., Ross X.L., Ludwig-Portugall I., Steitz J., Tuting T., Knop J., Reske-Kunz A.B. Transcriptional targeting of dendritic cells for gene therapy using the promoter of the cytoskeletal protein fascin. Gene Ther. 2003;10:1035–1040. doi: 10.1038/sj.gt.3301968. [DOI] [PubMed] [Google Scholar]
- 67.Bros M., Ross X.L., Pautz A., Reske-Kunz A.B., Ross R. The human fascin gene promoter is highly active in mature dendritic cells due to a stage-specific enhancer. J. Immunol. 2003;171:1825–1834. doi: 10.4049/jimmunol.171.4.1825. [DOI] [PubMed] [Google Scholar]
- 68.Sudowe S., Ludwig-Portugall I., Montermann E., Ross R., Reske-Kunz A.B. Transcriptional targeting of dendritic cells in gene gun-mediated DNA immunization favors the induction of type 1 immune responses. Mol. Ther. 2003;8:567–575. doi: 10.1016/S1525-0016(03)00242-9. [DOI] [PubMed] [Google Scholar]
- 69.Raker V., Maxeiner J., Reske-Kunz A.B., Sudowe S. Efficiency of biolistic DNA vaccination in experimental type I allergy. Methods Mol. Biol. 2013;940:357–370. doi: 10.1007/978-1-62703-110-3_26. [DOI] [PubMed] [Google Scholar]
- 70.Castor T., Yogev N., Blank T., Barwig C., Prinz M., Waisman A., Bros M., Reske-Kunz A.B. Inhibition of experimental autoimmune encephalomyelitis by tolerance-promoting DNA vaccination focused to dendritic cells. PLoS ONE. 2018;13:e0191927. doi: 10.1371/journal.pone.0191927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nazarkina Zh K., Khar’kova M.V., Antonets D.V., Morozkin E.S., Bazhan S.I., Karpenko L.I., Vlasov V.V., Ilyichev A.A., Laktionov P.P. Design of Polyepitope DNA Vaccine against Breast Carcinoma Cells and Analysis of Its Expression in Dendritic Cells. Bull. Exp. Biol. Med. 2016;160:486–490. doi: 10.1007/s10517-016-3203-y. [DOI] [PubMed] [Google Scholar]
- 72.Hirayama M., Nishimura Y. The present status and future prospects of peptide-based cancer vaccines. Int. Immunol. 2016;28:319–328. doi: 10.1093/intimm/dxw027. [DOI] [PubMed] [Google Scholar]
- 73.Brennick C.A., George M.M., Corwin W.L., Srivastava P.K., Ebrahimi-Nik H. Neoepitopes as cancer immunotherapy targets: Key challenges and opportunities. Immunotherapy. 2017;9:361–371. doi: 10.2217/imt-2016-0146. [DOI] [PubMed] [Google Scholar]
- 74.Kiyotani K., Chan H.T., Nakamura Y. Immunopharmacogenomics towards personalized cancer immunotherapy targeting neoantigens. Cancer Sci. 2018;109:542–549. doi: 10.1111/cas.13498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sultan H., Trillo-Tinoco J., Rodriguez P., Celis E. Effective antitumor peptide vaccines can induce severe autoimmune pathology. Oncotarget. 2017;8:70317–70331. doi: 10.18632/oncotarget.19688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lu Y.C., Yao X., Crystal J.S., Li Y.F., El-Gamil M., Gross C., Davis L., Dudley M.E., Yang J.C., Samuels Y., et al. Efficient identification of mutated cancer antigens recognized by T cells associated with durable tumor regressions. Clin. Cancer Res. 2014;20:3401–3410. doi: 10.1158/1078-0432.CCR-14-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bins A.D., Wolkers M.C., van den Boom M.D., Haanen J.B., Schumacher T.N. In vivo antigen stability affects DNA vaccine immunogenicity. J. Immunol. 2007;179:2126–2133. doi: 10.4049/jimmunol.179.4.2126. [DOI] [PubMed] [Google Scholar]
- 78.Rosa D.S., Ribeiro S.P., Fonseca S.G., Almeida R.R., Santana V.C., Apostolico Jde S., Kalil J., Cunha-Neto E. Multiple Approaches for Increasing the Immunogenicity of an Epitope-Based Anti-HIV Vaccine. AIDS Res. Hum. Retrov. 2015;31:1077–1088. doi: 10.1089/aid.2015.0101. [DOI] [PubMed] [Google Scholar]
- 79.Hoppes R., Oostvogels R., Luimstra J.J., Wals K., Toebes M., Bies L., Ekkebus R., Rijal P., Celie P.H., Huang J.H., et al. Altered peptide ligands revisited: Vaccine design through chemically modified HLA-A2-restricted T cell epitopes. J. Immunol. 2014;193:4803–4813. doi: 10.4049/jimmunol.1400800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pielak R.M., O’Donoghue G.P., Lin J.J., Alfieri K.N., Fay N.C., Low-Nam S.T., Groves J.T. Early T cell receptor signals globally modulate ligand:receptor affinities during antigen discrimination. Proc. Natl. Acad. Sci. USA. 2017;114:12190–12195. doi: 10.1073/pnas.1613140114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Seledtsova G.V., Shishkov A.A., Kaschenko E.A., Goncharov A.G., Gazatova N.D., Seledtsov V.I. Xenogeneic cell-based vaccine therapy for stage III melanoma: Safety, immune-mediated responses and survival benefits. Eur. J. Dermatol. 2016;26:138–143. doi: 10.1684/ejd.2016.2733. [DOI] [PubMed] [Google Scholar]
- 82.Garg R., Kaur M., Saxena A., Prasad R., Bhatnagar R. Alum adjuvanted rabies DNA vaccine confers 80% protection against lethal 50 LD50 rabies challenge virus standard strain. Mol. Immunol. 2017;85:166–173. doi: 10.1016/j.molimm.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 83.Hemmi H., Takeuchi O., Kawai T., Kaisho T., Sato S., Sanjo H., Matsumoto M., Hoshino K., Wagner H., Takeda K., et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 84.Tudor D., Dubuquoy C., Gaboriau V., Lefevre F., Charley B., Riffault S. TLR9 pathway is involved in adjuvant effects of plasmid DNA-based vaccines. Vaccine. 2005;23:1258–1264. doi: 10.1016/j.vaccine.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 85.Suschak J.J., Wang S., Fitzgerald K.A., Lu S. A cGAS-Independent STING/IRF7 Pathway Mediates the Immunogenicity of DNA Vaccines. J. Immunol. 2016;196:310–316. doi: 10.4049/jimmunol.1501836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Delft M.A., Huitema L.F., Tas S.W. The contribution of NF-kappaB signalling to immune regulation and tolerance. Eur. J. Clin. Investig. 2015;45:529–539. doi: 10.1111/eci.12430. [DOI] [PubMed] [Google Scholar]
- 87.Sasaki I., Kaisho T. Transcriptional control of dendritic cell differentiation. Curr. Topics Microbiol. 2014;381:257–278. doi: 10.1007/82_2014_378. [DOI] [PubMed] [Google Scholar]
- 88.Shedlock D.J., Tingey C., Mahadevan L., Hutnick N., Reuschel E.L., Kudchodkar S., Flingai S., Yan J., Kim J.J., Ugen K.E., et al. Co-Administration of Molecular Adjuvants Expressing NF-Kappa B Subunit p65/RelA or Type-1 Transactivator T-bet Enhance Antigen Specific DNA Vaccine-Induced Immunity. Vaccines. 2014;2:196–215. doi: 10.3390/vaccines2020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sasaki S., Amara R.R., Yeow W.S., Pitha P.M., Robinson H.L. Regulation of DNA-raised immune responses by cotransfected interferon regulatory factors. J. Virol. 2002;76:6652–6659. doi: 10.1128/JVI.76.13.6652-6659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castaldello A., Sgarbanti M., Marsili G., Brocca-Cofano E., Remoli A.L., Caputo A., Battistini A. Interferon regulatory factor-1 acts as a powerful adjuvant in tat DNA based vaccination. J. Cell. Physiol. 2010;224:702–709. doi: 10.1002/jcp.22169. [DOI] [PubMed] [Google Scholar]
- 91.Luo M., Qu X., Pan R., Zhu D., Zhang Y., Wu J., Pan Z. The virus-induced signaling adaptor molecule enhances DNA-raised immune protection against H5N1 influenza virus infection in mice. Vaccine. 2011;29:2561–2567. doi: 10.1016/j.vaccine.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 92.Larsen K.C., Spencer A.J., Goodman A.L., Gilchrist A., Furze J., Rollier C.S., Kiss-Toth E., Gilbert S.C., Bregu M., Soilleux E.J., et al. Expression of tak1 and tram induces synergistic pro-inflammatory signalling and adjuvants DNA vaccines. Vaccine. 2009;27:5589–5598. doi: 10.1016/j.vaccine.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 93.De Andres X., Reina R., Ciriza J., Crespo H., Glaria I., Ramirez H., Grillo M.J., Perez M.M., Andresdottir V., Rosati S., et al. Use of B7 costimulatory molecules as adjuvants in a prime-boost vaccination against Visna/Maedi ovine lentivirus. Vaccine. 2009;27:4591–4600. doi: 10.1016/j.vaccine.2009.05.080. [DOI] [PubMed] [Google Scholar]
- 94.Langrish C.L., McKenzie B.S., Wilson N.J., de Waal Malefyt R., Kastelein R.A., Cua D.J. IL-12 and IL-23: Master regulators of innate and adaptive immunity. Immunol. Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 95.Li S.S., Kochar N.K., Elizaga M., Hay C.M., Wilson G.J., Cohen K.W., De Rosa S.C., Xu R., Ota-Setlik A., Morris D., et al. DNA Priming Increases Frequency of T-Cell Responses to a Vesicular Stomatitis Virus HIV Vaccine with Specific Enhancement of CD8(+) T-Cell Responses by Interleukin-12 Plasmid DNA. Clin. Vaccine Immunol. 2017;24 doi: 10.1128/CVI.00263-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Steel J.C., Waldmann T.A., Morris J.C. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol. Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sun L., Yuan Q., Xu T., Yao L., Feng J., Ma J., Wang L., Lv C., Wang D. Novel adjuvant for immunization against tuberculosis: DNA vaccine expressing Mycobacterium tuberculosis antigen 85A and interleukin-15 fusion product elicits strong immune responses in mice. Biotechnol. Lett. 2017;39:1159–1166. doi: 10.1007/s10529-017-2342-1. [DOI] [PubMed] [Google Scholar]
- 98.Spolski R., Li P., Leonard W.J. Biology and regulation of IL-2: From molecular mechanisms to human therapy. Nat. Rev. Immunol. 2018;18:648–659. doi: 10.1038/s41577-018-0046-y. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y., Liang S., Li X., Wang L., Zhang J., Xu J., Huo S., Cao X., Zhong Z., Zhong F. Mutual enhancement of IL-2 and IL-7 on DNA vaccine immunogenicity mainly involves regulations on their receptor expression and receptor-expressing lymphocyte generation. Vaccine. 2015;33:3480–3487. doi: 10.1016/j.vaccine.2015.05.068. [DOI] [PubMed] [Google Scholar]
- 100.Abedini F., Ebrahimi M., Hosseinkhani H. Technology of RNA Interference in Advanced Medicine. MicroRNA. 2018;7:74–84. doi: 10.2174/2211536607666180129153307. [DOI] [PubMed] [Google Scholar]
- 101.Luo Z., Wang C., Yi H., Li P., Pan H., Liu L., Cai L., Ma Y. Nanovaccine loaded with poly I:C and STAT3 siRNA robustly elicits anti-tumor immune responses through modulating tumor-associated dendritic cells in vivo. Biomaterials. 2015;38:50–60. doi: 10.1016/j.biomaterials.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 102.Luo X., Peng X., Hou J., Wu S., Shen J., Wang L. Folic acid-functionalized polyethylenimine superparamagnetic iron oxide nanoparticles as theranostic agents for magnetic resonance imaging and PD-L1 siRNA delivery for gastric cancer. Int. J. Nanomed. 2017;12:5331–5343. doi: 10.2147/IJN.S137245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Self-Fordham J.B., Naqvi A.R., Uttamani J.R., Kulkarni V., Nares S. MicroRNA: Dynamic Regulators of Macrophage Polarization and Plasticity. Front. Immunol. 2017;8:1062. doi: 10.3389/fimmu.2017.01062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Migault M., Donnou-Fournet E., Galibert M.D., Gilot D. Definition and identification of small RNA sponges: Focus on miRNA sequestration. Methods. 2017;117:35–47. doi: 10.1016/j.ymeth.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 105.Scheiermann J., Klinman D.M. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine. 2014;32:6377–6389. doi: 10.1016/j.vaccine.2014.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elizaga M.L., Li S.S., Kochar N.K., Wilson G.J., Allen M.A., Tieu H.V.N., Frank I., Sobieszczyk M.E., Cohen K.W., Sanchez B., et al. Safety and tolerability of HIV-1 multiantigen pDNA vaccine given with IL-12 plasmid DNA via electroporation, boosted with a recombinant vesicular stomatitis virus HIV Gag vaccine in healthy volunteers in a randomized, controlled clinical trial. PLoS ONE. 2018;13:e0202753. doi: 10.1371/journal.pone.0202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thomas S.K., Cha S.C., Smith D.L., Kim K.H., Parshottam S.R., Rao S., Popescu M., Lee V.Y., Neelapu S.S., Kwak L.W. Phase I study of an active immunotherapy for asymptomatic phase Lymphoplasmacytic lymphoma with DNA vaccines encoding antigen-chemokine fusion: Study protocol. BMC Cancer. 2018;18:187. doi: 10.1186/s12885-018-4094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kreiss P., Cameron B., Rangara R., Mailhe P., Aguerre-Charriol O., Airiau M., Scherman D., Crouzet J., Pitard B. Plasmid DNA size does not affect the physicochemical properties of lipoplexes but modulates gene transfer efficiency. Nucleic Acids Res. 1999;27:3792–3798. doi: 10.1093/nar/27.19.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gracey Maniar L.E., Maniar J.M., Chen Z.Y., Lu J., Fire A.Z., Kay M.A. Minicircle DNA vectors achieve sustained expression reflected by active chromatin and transcriptional level. Mol. Ther. 2013;21:131–138. doi: 10.1038/mt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hardee C.L., Arevalo-Soliz L.M., Hornstein B.D., Zechiedrich L. Advances in Non-Viral DNA Vectors for Gene Therapy. Genes. 2017;8:65. doi: 10.3390/genes8020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lu J., Zhang F., Fire A.Z., Kay M.A. Sequence-Modified Antibiotic Resistance Genes Provide Sustained Plasmid-Mediated Transgene Expression in Mammals. Mol. Ther. 2017;25:1187–1198. doi: 10.1016/j.ymthe.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stenler S., Blomberg P., Smith C.I. Safety and efficacy of DNA vaccines: Plasmids vs. minicircles. Hum. Vacc. Immunother. 2014;10:1306–1308. doi: 10.4161/hv.28077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vargas J.E., Salton G., Sodre de Castro Laino A., Pires T.D., Bonamino M., Lenz G., Delgado-Canedo A. pLR: A lentiviral backbone series to stable transduction of bicistronic genes and exchange of promoters. Plasmid. 2012;68:179–185. doi: 10.1016/j.plasmid.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 114.Terenin I.M., Smirnova V.V., Andreev D.E., Dmitriev S.E., Shatsky I.N. A researcher’s guide to the galaxy of IRESs. Cell. Mol. Life Sci. 2017;74:1431–1455. doi: 10.1007/s00018-016-2409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chng J., Wang T., Nian R., Lau A., Hoi K.M., Ho S.C., Gagnon P., Bi X., Yang Y. Cleavage efficient 2A peptides for high level monoclonal antibody expression in CHO cells. MAbs. 2015;7:403–412. doi: 10.1080/19420862.2015.1008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim J.H., Lee S.R., Li L.H., Park H.J., Park J.H., Lee K.Y., Kim M.K., Shin B.A., Choi S.Y. High cleavage efficiency of a 2A peptide derived from porcine teschovirus-1 in human cell lines, zebrafish and mice. PLoS ONE. 2011;6:e18556. doi: 10.1371/journal.pone.0018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lechardeur D., Lukacs G.L. Nucleocytoplasmic transport of plasmid DNA: A perilous journey from the cytoplasm to the nucleus. Hum. Gene Ther. 2006;17:882–889. doi: 10.1089/hum.2006.17.882. [DOI] [PubMed] [Google Scholar]
- 118.Dean D.A., Dean B.S., Muller S., Smith L.C. Sequence requirements for plasmid nuclear import. Exp. Cell Res. 1999;253:713–722. doi: 10.1006/excr.1999.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kanazawa T., Yamazaki M., Fukuda T., Takashima Y., Okada H. Versatile nuclear localization signal-based oligopeptide as a gene vector. Biol. Pharm. Bull. 2015;38:559–565. doi: 10.1248/bpb.b14-00706. [DOI] [PubMed] [Google Scholar]
- 120.Bogacheva M., Egorova A., Slita A., Maretina M., Baranov V., Kiselev A. Arginine-rich cross-linking peptides with different SV40 nuclear localization signal content as vectors for intranuclear DNA delivery. Bioorg. Med. Chem. Lett. 2017;27:4781–4785. doi: 10.1016/j.bmcl.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 121.Cai P., Zhang X., Wang M., Wu Y.L., Chen X. Combinatorial Nano-Bio Interfaces. ACS Nano. 2018 doi: 10.1021/acsnano.8b03285. [DOI] [PubMed] [Google Scholar]
- 122.Schupp J., Krebs F.K., Zimmer N., Trzeciak E., Schuppan D., Tuettenberg A. Targeting myeloid cells in the tumor sustaining microenvironment. Cell. Immunol. 2017 doi: 10.1016/j.cellimm.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 123.Deshantri A.K., Varela Moreira A., Ecker V., Mandhane S.N., Schiffelers R.M., Buchner M., Fens M. Nanomedicines for the treatment of hematological malignancies. J. Control. Release. 2018;287:194–215. doi: 10.1016/j.jconrel.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 124.Lorden E.R., Levinson H.M., Leong K.W. Integration of drug, protein, and gene delivery systems with regenerative medicine. Drug Deliv. Transl. Res. 2015;5:168–186. doi: 10.1007/s13346-013-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Angell C., Xie S., Zhang L., Chen Y. DNA Nanotechnology for Precise Control over Drug Delivery and Gene Therapy. Small. 2016;12:1117–1132. doi: 10.1002/smll.201502167. [DOI] [PubMed] [Google Scholar]
- 126.Shao K., Singha S., Clemente-Casares X., Tsai S., Yang Y., Santamaria P. Nanoparticle-based immunotherapy for cancer. ACS Nano. 2015;9:16–30. doi: 10.1021/nn5062029. [DOI] [PubMed] [Google Scholar]
- 127.Blackman L.D., Varlas S., Arno M.C., Houston Z.H., Fletcher N.L., Thurecht K.J., Hasan M., Gibson M.I., O’Reilly R.K. Confinement of Therapeutic Enzymes in Selectively Permeable Polymer Vesicles by Polymerization-Induced Self-Assembly (PISA) Reduces Antibody Binding and Proteolytic Susceptibility. ACS Central Sci. 2018;4:718–723. doi: 10.1021/acscentsci.8b00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Steen E.J.L., Edem P.E., Norregaard K., Jorgensen J.T., Shalgunov V., Kjaer A., Herth M.M. Pretargeting in nuclear imaging and radionuclide therapy: Improving efficacy of theranostics and nanomedicines. Biomaterials. 2018;179:209–245. doi: 10.1016/j.biomaterials.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 129.Rather A.M., Mahato S., Maji K., Gogoi N., Manna U. ‘Reactive’ nano-complex coated medical cotton: A facile avenue for tailored release of small molecules. Nanoscale. 2017;9:16154–16165. doi: 10.1039/C7NR03990E. [DOI] [PubMed] [Google Scholar]
- 130.Wolfram R.K., Heller L., Csuk R. Targeting mitochondria: Esters of rhodamine B with triterpenoids are mitocanic triggers of apoptosis. Eur. J. Med. Chem. 2018;152:21–30. doi: 10.1016/j.ejmech.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 131.Malekkhaiat Haffner S., Malmsten M. Membrane interactions and antimicrobial effects of inorganic nanoparticles. Adv. Colloid Interface Sci. 2017;248:105–128. doi: 10.1016/j.cis.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 132.Radis-Baptista G., Campelo I.S., Morlighem J.R.L., Melo L.M., Freitas V.J.F. Cell-penetrating peptides (CPPs): From delivery of nucleic acids and antigens to transduction of engineered nucleases for application in transgenesis. J. Biotechnol. 2017;252:15–26. doi: 10.1016/j.jbiotec.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 133.Falanga A., Galdiero S. Peptide chemistry encounters nanomedicine: Recent applications and upcoming scenarios in cancer. Future Med. Chem. 2018;10:1877–1880. doi: 10.4155/fmc-2018-0182. [DOI] [PubMed] [Google Scholar]
- 134.Bros M., Nuhn L., Simon J., Moll L., Mailander V., Landfester K., Grabbe S. The Protein Corona as a Confounding Variable of Nanoparticle-Mediated Targeted Vaccine Delivery. Front. Immunol. 2018;9:1760. doi: 10.3389/fimmu.2018.01760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khalil I.A., Harashima H. An efficient PEGylated gene delivery system with improved targeting: Synergism between octaarginine and a fusogenic peptide. Int. J Pharm. 2018;538:179–187. doi: 10.1016/j.ijpharm.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 136.Hua S., de Matos M.B.C., Metselaar J.M., Storm G. Current Trends and Challenges in the Clinical Translation of Nanoparticulate Nanomedicines: Pathways for Translational Development and Commercialization. Front. Pharm. 2018;9:790. doi: 10.3389/fphar.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang P., Sun F., Liu S., Jiang S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Release. 2016;244:184–193. doi: 10.1016/j.jconrel.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Das S.K., Menezes M.E., Bhatia S., Wang X.Y., Emdad L., Sarkar D., Fisher P.B. Gene Therapies for Cancer: Strategies, Challenges and Successes. J. Cell. Physiol. 2015;230:259–271. doi: 10.1002/jcp.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu Q., Chen X., Jia J., Zhang W., Yang T., Wang L., Ma G. pH-Responsive Poly(d,l-lactic-co-glycolic acid) Nanoparticles with Rapid Antigen Release Behavior Promote Immune Response. ACS Nano. 2015;9:4925–4938. doi: 10.1021/nn5066793. [DOI] [PubMed] [Google Scholar]
- 140.Foged C., Brodin B., Frokjaer S., Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int. J. Pharm. 2005;298:315–322. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 141.Xiang S.D., Scholzen A., Minigo G., David C., Apostolopoulos V., Mottram P.L., Plebanski M. Pathogen recognition and development of particulate vaccines: Does size matter? Methods. 2006;40:1–9. doi: 10.1016/j.ymeth.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 142.Jiang Y., Huo S., Mizuhara T., Das R., Lee Y.W., Hou S., Moyano D.F., Duncan B., Liang X.J., Rotello V.M. The Interplay of Size and Surface Functionality on the Cellular Uptake of Sub-10 nm Gold Nanoparticles. ACS Nano. 2015;9:9986–9993. doi: 10.1021/acsnano.5b03521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Goodman C.M., McCusker C.D., Yilmaz T., Rotello V.M. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug. Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 144.Niikura K., Matsunaga T., Suzuki T., Kobayashi S., Yamaguchi H., Orba Y., Kawaguchi A., Hasegawa H., Kajino K., Ninomiya T., et al. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 145.Thomann-Harwood L.J., Kaeuper P., Rossi N., Milona P., Herrmann B., McCullough K.C. Nanogel vaccines targeting dendritic cells: Contributions of the surface decoration and vaccine cargo on cell targeting and activation. J. Control. Release. 2013;166:95–105. doi: 10.1016/j.jconrel.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 146.Mohsen M.O., Zha L., Cabral-Miranda G., Bachmann M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin. Immunol. 2017;34:123–132. doi: 10.1016/j.smim.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 147.Chang D., Lim M., Goos J., Qiao R., Ng Y.Y., Mansfeld F.M., Jackson M., Davis T.P., Kavallaris M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharm. 2018;9:831. doi: 10.3389/fphar.2018.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ghassami E., Varshosaz J., Taymouri S. Redox sensitive polysaccharide based nanoparticles for improved cancer treatment: A comprehensive review. Curr. Pharm. Des. 2018 doi: 10.2174/1381612824666180813114841. [DOI] [PubMed] [Google Scholar]
- 149.Lammers T., Kiessling F., Ashford M., Hennink W., Crommelin D., Storm G. Cancer nanomedicine: Is targeting our target? Nat. Rev. Mater. 2016;1 doi: 10.1038/natrevmats.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lachelt U., Wagner E. Nucleic Acid Therapeutics Using Polyplexes: A Journey of 50 Years (and Beyond) Chem. Rev. 2015;115:11043–11078. doi: 10.1021/cr5006793. [DOI] [PubMed] [Google Scholar]
- 151.Keeney M., Ong S.G., Padilla A., Yao Z., Goodman S., Wu J.C., Yang F. Development of poly(beta-amino ester)-based biodegradable nanoparticles for nonviral delivery of minicircle DNA. ACS Nano. 2013;7:7241–7250. doi: 10.1021/nn402657d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cohen R.N., van der Aa M.A., Macaraeg N., Lee A.P., Szoka F.C., Jr. Quantification of plasmid DNA copies in the nucleus after lipoplex and polyplex transfection. J. Control. Release. 2009;135:166–174. doi: 10.1016/j.jconrel.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Selby L.I., Cortez-Jugo C.M., Such G.K., Johnston A.P.R. Nanoescapology: Progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9 doi: 10.1002/wnan.1452. [DOI] [PubMed] [Google Scholar]
- 154.Xu Y., Liang W., Qiu Y., Cespi M., Palmieri G.F., Mason A.J., Lam J.K. Incorporation of a Nuclear Localization Signal in pH Responsive LAH4-L1 Peptide Enhances Transfection and Nuclear Uptake of Plasmid DNA. Mol. Pharm. 2016;13:3141–3152. doi: 10.1021/acs.molpharmaceut.6b00338. [DOI] [PubMed] [Google Scholar]
- 155.Chen Y.S., Hung Y.C., Lin W.H., Huang G.S. Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnology. 2010;21:195101. doi: 10.1088/0957-4484/21/19/195101. [DOI] [PubMed] [Google Scholar]
- 156.Peng L.H., Huang Y.F., Zhang C.Z., Niu J., Chen Y., Chu Y., Jiang Z.H., Gao J.Q., Mao Z.W. Integration of antimicrobial peptides with gold nanoparticles as unique non-viral vectors for gene delivery to mesenchymal stem cells with antibacterial activity. Biomaterials. 2016;103:137–149. doi: 10.1016/j.biomaterials.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 157.Fogli S., Montis C., Paccosi S., Silvano A., Michelucci E., Berti D., Bosi A., Parenti A., Romagnoli P. Inorganic nanoparticles as potential regulators of immune response in dendritic cells. Nanomedicine. 2017;12:1647–1660. doi: 10.2217/nnm-2017-0061. [DOI] [PubMed] [Google Scholar]
- 158.Peng L.H., Niu J., Zhang C.Z., Yu W., Wu J.H., Shan Y.H., Wang X.R., Shen Y.Q., Mao Z.W., Liang W.Q., et al. TAT conjugated cationic noble metal nanoparticles for gene delivery to epidermal stem cells. Biomaterials. 2014;35:5605–5618. doi: 10.1016/j.biomaterials.2014.03.062. [DOI] [PubMed] [Google Scholar]
- 159.Niut Y., Popatt A., Yu M., Karmakar S., Gu W., Yu C. Recent advances in the rational design of silica-based nanoparticles for gene therapy. Ther. Deliv. 2012;3:1217–1237. [PubMed] [Google Scholar]
- 160.Pasqua L., Leggio A., Sisci D., Ando S., Morelli C. Mesoporous Silica Nanoparticles in Cancer Therapy: Relevance of the Targeting Function. Mini Rev. Med. Chem. 2016;16:743–753. doi: 10.2174/1389557516666160321113620. [DOI] [PubMed] [Google Scholar]
- 161.Xiong L., Qiao S.Z. A mesoporous organosilica nano-bowl with high DNA loading capacity - a potential gene delivery carrier. Nanoscale. 2016;8:17446–17450. doi: 10.1039/C6NR06777H. [DOI] [PubMed] [Google Scholar]
- 162.Mody K.T., Popat A., Mahony D., Cavallaro A.S., Yu C., Mitter N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale. 2013;5:5167–5179. doi: 10.1039/c3nr00357d. [DOI] [PubMed] [Google Scholar]
- 163.Zhou T., Zhou X., Xing D. Controlled release of doxorubicin from graphene oxide based charge-reversal nanocarrier. Biomaterials. 2014;35:4185–4194. doi: 10.1016/j.biomaterials.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 164.Singh D.P., Herrera C.E., Singh B., Singh S., Singh R.K., Kumar R. Graphene oxide: An efficient material and recent approach for biotechnological and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;86:173–197. doi: 10.1016/j.msec.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 165.Arayachukiat S., Seemork J., Pan-In P., Amornwachirabodee K., Sangphech N., Sansureerungsikul T., Sathornsantikun K., Vilaivan C., Shigyou K., Pienpinijtham P., et al. Bringing macromolecules into cells and evading endosomes by oxidized carbon nanoparticles. Nano Lett. 2015;15:3370–3376. doi: 10.1021/acs.nanolett.5b00696. [DOI] [PubMed] [Google Scholar]
- 166.Mehra N.K., Jain N.K. Development, characterization and cancer targeting potential of surface engineered carbon nanotubes. J. Drug Target. 2013;21:745–758. doi: 10.3109/1061186X.2013.813028. [DOI] [PubMed] [Google Scholar]
- 167.Sajid M.I., Jamshaid U., Jamshaid T., Zafar N., Fessi H., Elaissari A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int. J. Pharm. 2016;501:278–299. doi: 10.1016/j.ijpharm.2016.01.064. [DOI] [PubMed] [Google Scholar]
- 168.Angelakeris M. Magnetic nanoparticles: A multifunctional vehicle for modern theranostics. Biochim. Biophys. Acta. 2017;1861:1642–1651. doi: 10.1016/j.bbagen.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 169.Seemann K.M., Luysberg M., Revay Z., Kudejova P., Sanz B., Cassinelli N., Loidl A., Ilicic K., Multhoff G., Schmid T.E. Magnetic heating properties and neutron activation of tungsten-oxide coated biocompatible FePt core-shell nanoparticles. J. Control. Release. 2015;197:131–137. doi: 10.1016/j.jconrel.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 170.Svoboda O., Fohlerova Z., Baiazitova L., Mlynek P., Samouylov K., Provaznik I., Hubalek J. Transfection by Polyethyleneimine-Coated Magnetic Nanoparticles: Fine-Tuning the Condition for Electrophysiological Experiments. J. Biomed. Nanotechnol. 2018;14:1505–1514. doi: 10.1166/jbn.2018.2602. [DOI] [PubMed] [Google Scholar]
- 171.Felgner P.L., Gadek T.R., Holm M., Roman R., Chan H.W., Wenz M., Northrop J.P., Ringold G.M., Danielsen M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Li S., Huang L. In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene Ther. 1997;4:891–900. doi: 10.1038/sj.gt.3300482. [DOI] [PubMed] [Google Scholar]
- 173.Rezaee M., Oskuee R.K., Nassirli H., Malaekeh-Nikouei B. Progress in the development of lipopolyplexes as efficient non-viral gene delivery systems. J. Control. Release. 2016;236:1–14. doi: 10.1016/j.jconrel.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 174.Zuhorn I.S., Hoekstra D. On the mechanism of cationic amphiphile-mediated transfection. To fuse or not to fuse: Is that the question? J. Membr. Biol. 2002;189:167–179. doi: 10.1007/s00232-002-1015-7. [DOI] [PubMed] [Google Scholar]
- 175.Mevel M., Haudebourg T., Colombani T., Peuziat P., Dallet L., Chatin B., Lambert O., Berchel M., Montier T., Jaffres P.A., et al. Important role of phosphoramido linkage in imidazole-based dioleyl helper lipids for liposome stability and primary cell transfection. J. Gene Med. 2016;18:3–15. doi: 10.1002/jgm.2869. [DOI] [PubMed] [Google Scholar]
- 176.Cardarelli F., Pozzi D., Bifone A., Marchini C., Caracciolo G. Cholesterol-dependent macropinocytosis and endosomal escape control the transfection efficiency of lipoplexes in CHO living cells. Mol. Pharm. 2012;9:334–340. doi: 10.1021/mp200374e. [DOI] [PubMed] [Google Scholar]
- 177.Yang J., Bahreman A., Daudey G., Bussmann J., Olsthoorn R.C., Kros A. Drug Delivery via Cell Membrane Fusion Using Lipopeptide Modified Liposomes. ACS Central Sci. 2016;2:621–630. doi: 10.1021/acscentsci.6b00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Alving C.R., Beck Z., Matyas G.R., Rao M. Liposomal adjuvants for human vaccines. Expert Opin. Drug Del. 2016;13:807–816. doi: 10.1517/17425247.2016.1151871. [DOI] [PubMed] [Google Scholar]
- 179.Chappuis F., Farinelli T., Deckx H., Sarnecki M., Go O., Salzgeber Y., Stals C. Immunogenicity and estimation of antibody persistence following vaccination with an inactivated virosomal hepatitis A vaccine in adults: A 20-year follow-up study. Vaccine. 2017;35:1448–1454. doi: 10.1016/j.vaccine.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 180.Levin Y., Kochba E., Shukarev G., Rusch S., Herrera-Taracena G., van Damme P. A phase 1, open-label, randomized study to compare the immunogenicity and safety of different administration routes and doses of virosomal influenza vaccine in elderly. Vaccine. 2016;34:5262–5272. doi: 10.1016/j.vaccine.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 181.Lohcharoenkal W., Wang L., Chen Y.C., Rojanasakul Y. Protein nanoparticles as drug delivery carriers for cancer therapy. BioMed Res. Int. 2014;2014:180549. doi: 10.1155/2014/180549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Moran M.C., Rosell N., Ruano G., Busquets M.A., Vinardell M.P. Gelatin-based nanoparticles as DNA delivery systems: Synthesis, physicochemical and biocompatible characterization. Colloids Surf. B Biointerfaces. 2015;134:156–168. doi: 10.1016/j.colsurfb.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 183.Merlot A.M., Kalinowski D.S., Richardson D.R. Unraveling the mysteries of serum albumin-more than just a serum protein. Front. Physiol. 2014;5:299. doi: 10.3389/fphys.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huang Y., Shuai Y., Li H., Gao Z. Tyrosine residues play an important role in heme detoxification by serum albumin. Biochim. Biophys. Acta. 2014;1840:970–976. doi: 10.1016/j.bbagen.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 185.Karimi M., Avci P., Mobasseri R., Hamblin M.R., Naderi-Manesh H. The novel albumin-chitosan core-shell nanoparticles for gene delivery: Preparation, optimization and cell uptake investigation. J. Nanopart. Res. 2013;15:1651. doi: 10.1007/s11051-013-1651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kumari M., Liu C.H., Wu W.C. Efficient gene delivery by oligochitosan conjugated serum albumin: Facile synthesis, polyplex stability, and transfection. Carbohyd. Polym. 2018;183:37–49. doi: 10.1016/j.carbpol.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 187.Han J., Wang Q., Zhang Z., Gong T., Sun X. Cationic bovine serum albumin based self-assembled nanoparticles as siRNA delivery vector for treating lung metastatic cancer. Small. 2014;10:524–535. doi: 10.1002/smll.201301992. [DOI] [PubMed] [Google Scholar]
- 188.Neddermeyer A.H., Hultenby K., Paidikondala M., Schuchman R.M., Bidokhti M.R.M. Investigating Tick-borne Flaviviral-like Particles as a Delivery System for Gene Therapy. Curr. Ther. Res. Clin. Exp. 2018;88:8–17. doi: 10.1016/j.curtheres.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Fric J., Marek M., Hruskova V., Holan V., Forstova J. Cellular and humoral immune responses to chimeric EGFP-pseudocapsids derived from the mouse polyomavirus after their intranasal administration. Vaccine. 2008;26:3242–3251. doi: 10.1016/j.vaccine.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 190.Cimica V., Galarza J.M. Adjuvant formulations for virus-like particle (VLP) based vaccines. Clin. Immunol. 2017;183:99–108. doi: 10.1016/j.clim.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Quader S., Kataoka K. Nanomaterial-Enabled Cancer Therapy. Mol. Ther. 2017;25:1501–1513. doi: 10.1016/j.ymthe.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.McCutchan J.H., Pagano J.S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J. Natl. Cancer Inst. 1968;41:351–357. [PubMed] [Google Scholar]
- 193.Hatakeyama H., Akita H., Harashima H. The polyethyleneglycol dilemma: Advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol. Pharm. Bull. 2013;36:892–899. doi: 10.1248/bpb.b13-00059. [DOI] [PubMed] [Google Scholar]
- 194.Chen C.K., Jones C.H., Mistriotis P., Yu Y., Ma X., Ravikrishnan A., Jiang M., Andreadis S.T., Pfeifer B.A., Cheng C. Poly(ethylene glycol)-block-cationic polylactide nanocomplexes of differing charge density for gene delivery. Biomaterials. 2013;34:9688–9699. doi: 10.1016/j.biomaterials.2013.08.063. [DOI] [PubMed] [Google Scholar]
- 195.Laemmli U.K. Characterization of DNA condensates induced by poly(ethylene oxide) and polylysine. Proc. Natl. Acad. Sci. USA. 1975;72:4288–4292. doi: 10.1073/pnas.72.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jang H.J., Jeong E.J., Lee K.Y. Carbon Dioxide-Generating PLG Nanoparticles for Controlled Anti-Cancer Drug Delivery. Pharm. Res. 2018;35:59. doi: 10.1007/s11095-018-2359-8. [DOI] [PubMed] [Google Scholar]
- 197.Li Z., Xiong F., He J., Dai X., Wang G. Surface-functionalized, pH-responsive poly(lactic-co-glycolic acid)-based microparticles for intranasal vaccine delivery: Effect of surface modification with chitosan and mannan. Eur. J. Pharm. Biopharm. 2016;109:24–34. doi: 10.1016/j.ejpb.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 198.Danhier F., Ansorena E., Silva J.M., Coco R., Le Breton A., Preat V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. 2012;161:505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 199.Lin W.J., Lee W.C. Polysaccharide-modified nanoparticles with intelligent CD44 receptor targeting ability for gene delivery. Int. J. Nanomed. 2018;13:3989–4002. doi: 10.2147/IJN.S163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Merdan T., Kopecek J., Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv. Drug Deliv. Rev. 2002;54:715–758. doi: 10.1016/S0169-409X(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 201.Wu G.Y., Wu C.H. Receptor-mediated gene delivery and expression in vivo. J. Biol. Chem. 1988;263:14621–14624. [PubMed] [Google Scholar]
- 202.Curiel D.T., Agarwal S., Wagner E., Cotten M. Adenovirus enhancement of transferrin-polylysine-mediated gene delivery. Proc. Natl. Acad. Sci. USA. 1991;88:8850–8854. doi: 10.1073/pnas.88.19.8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zauner W., Blaas D., Kuechler E., Wagner E. Rhinovirus-mediated endosomal release of transfection complexes. J. Virol. 1995;69:1085–1092. doi: 10.1128/jvi.69.2.1085-1092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Saito G., Amidon G.L., Lee K.D. Enhanced cytosolic delivery of plasmid DNA by a sulfhydryl-activatable listeriolysin O/protamine conjugate utilizing cellular reducing potential. Gene Ther. 2003;10:72–83. doi: 10.1038/sj.gt.3301859. [DOI] [PubMed] [Google Scholar]
- 205.Tang M.X., Szoka F.C. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 1997;4:823–832. doi: 10.1038/sj.gt.3300454. [DOI] [PubMed] [Google Scholar]
- 206.Itaka K., Harada A., Yamasaki Y., Nakamura K., Kawaguchi H., Kataoka K. In situ single cell observation by fluorescence resonance energy transfer reveals fast intra-cytoplasmic delivery and easy release of plasmid DNA complexed with linear polyethylenimine. J. Gene Med. 2004;6:76–84. doi: 10.1002/jgm.470. [DOI] [PubMed] [Google Scholar]
- 207.Grandinetti G., Ingle N.P., Reineke T.M. Interaction of poly(ethylenimine)-DNA polyplexes with mitochondria: Implications for a mechanism of cytotoxicity. Mol. Pharm. 2011;8:1709–1719. doi: 10.1021/mp200078n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chan C.L., Ewert K.K., Majzoub R.N., Hwu Y.K., Liang K.S., Leal C., Safinya C.R. Optimizing cationic and neutral lipids for efficient gene delivery at high serum content. J. Gene Med. 2014;16:84–96. doi: 10.1002/jgm.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Erbacher P., Zou S., Bettinger T., Steffan A.M., Remy J.S. Chitosan-based vector/DNA complexes for gene delivery: Biophysical characteristics and transfection ability. Pharm. Res. 1998;15:1332–1339. doi: 10.1023/A:1011981000671. [DOI] [PubMed] [Google Scholar]
- 210.Slutter B., Plapied L., Fievez V., Sande M.A., des Rieux A., Schneider Y.J., Van Riet E., Jiskoot W., Preat V. Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J. Control. Release. 2009;138:113–121. doi: 10.1016/j.jconrel.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 211.Saade F., Honda-Okubo Y., Trec S., Petrovsky N. A novel hepatitis B vaccine containing Advax, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine. 2013;31:1999–2007. doi: 10.1016/j.vaccine.2012.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sun B., Yu S., Zhao D., Guo S., Wang X., Zhao K. Polysaccharides as vaccine adjuvants. Vaccine. 2018;36:5226–5234. doi: 10.1016/j.vaccine.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 213.Glass J.J., Kent S.J., De Rose R. Enhancing dendritic cell activation and HIV vaccine effectiveness through nanoparticle vaccination. Exp. Rev. Vac. 2016;15:719–729. doi: 10.1586/14760584.2016.1141054. [DOI] [PubMed] [Google Scholar]
- 214.Zhang L., Yang W., Hu C., Wang Q., Wu Y. Properties and applications of nanoparticle/microparticle conveyors with adjuvant characteristics suitable for oral vaccination. Int. J. Nanomed. 2018;13:2973–2987. doi: 10.2147/IJN.S154743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Laube B.L. Aerosolized Medications for Gene and Peptide Therapy. Respir. Care. 2015;60:806–821. doi: 10.4187/respcare.03554. [DOI] [PubMed] [Google Scholar]
- 216.Fernando G.J., Zhang J., Ng H.I., Haigh O.L., Yukiko S.R., Kendall M.A. Influenza nucleoprotein DNA vaccination by a skin targeted, dry coated, densely packed microprojection array (Nanopatch) induces potent antibody and CD8(+) T cell responses. J. Control. Release. 2016;237:35–41. doi: 10.1016/j.jconrel.2016.06.045. [DOI] [PubMed] [Google Scholar]
- 217.Jorritsma S.H.T., Gowans E.J., Grubor-Bauk B., Wijesundara D.K. Delivery methods to increase cellular uptake and immunogenicity of DNA vaccines. Vaccine. 2016;34:5488–5494. doi: 10.1016/j.vaccine.2016.09.062. [DOI] [PubMed] [Google Scholar]
- 218.Dif F., Djediat C., Alegria O., Demeneix B., Levi G. Transfection of multiple pulmonary cell types following intravenous injection of PEI-DNA in normal and CFTR mutant mice. J. Gene Med. 2006;8:82–89. doi: 10.1002/jgm.831. [DOI] [PubMed] [Google Scholar]
- 219.Ukawa M., Akita H., Hayashi Y., Ishiba R., Tange K., Arai M., Kubo K., Higuchi Y., Shimizu K., Konishi S., et al. Neutralized nanoparticle composed of SS-cleavable and pH-activated lipid-like material as a long-lasting and liver-specific gene delivery system. Adv. Healthc. Mater. 2014;3:1222–1229. doi: 10.1002/adhm.201300629. [DOI] [PubMed] [Google Scholar]
- 220.Shen L., Tenzer S., Storck W., Hobernik D., Raker V.K., Fischer K., Decker S., Dzionek A., Krauthauser S., Diken M., et al. Protein corona-mediated targeting of nanocarriers to B cells allows redirection of allergic immune responses. J. Allergy Clin. Immunol. 2018 doi: 10.1016/j.jaci.2017.08.049. [DOI] [PubMed] [Google Scholar]
- 221.Wang M., Gao Z., Zhang Y., Pan L. Lactic acid bacteria as mucosal delivery vehicles: A realistic therapeutic option. Appl. Microbiol. Biotechnol. 2016;100:5691–5701. doi: 10.1007/s00253-016-7557-x. [DOI] [PubMed] [Google Scholar]
- 222.Chen W.Z., Li Y.M., Yu X., Li Y., Li W.K., Wang Q.L., Liang A.X., Li X., Yang L.G., Han L. The efficacy, biodistribution and safety of an inhibin DNA vaccine delivered by attenuated Salmonella choleraesuis. Microbiol. Biotechnol. 2018;11:248–256. doi: 10.1111/1751-7915.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Deng W., Lira V., Hudson T.E., Lemmens E.E., Hanson W.G., Flores R., Barajas G., Katibah G.E., Desbien A.L., Lauer P., et al. Recombinant Listeria promotes tumor rejection by CD8(+) T cell-dependent remodeling of the tumor microenvironment. Proc. Natl. Acad. Sci. USA. 2018;115:8179–8184. doi: 10.1073/pnas.1801910115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Celec P., Gardlik R. Gene therapy using bacterial vectors. Front. Biosci. 2017;22:81–95. doi: 10.2741/4473. [DOI] [PubMed] [Google Scholar]
- 225.Lin I.Y., Van T.T., Smooker P.M. Live-Attenuated Bacterial Vectors: Tools for Vaccine and Therapeutic Agent Delivery. Vaccines. 2015;3:940–972. doi: 10.3390/vaccines3040940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jiao H., Yang H., Zhao D., Chen J., Zhang Q., Liang J., Yin Y., Kong G., Li G. Design and immune characterization of a novel Neisseria gonorrhoeae DNA vaccine using bacterial ghosts as vector and adjuvant. Vaccine. 2018;36:4532–4539. doi: 10.1016/j.vaccine.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 227.Hou R., Li M., Tang T., Wang R., Li Y., Xu Y., Tang L., Wang L., Liu M., Jiang Y., et al. Construction of Lactobacillus casei ghosts by Holin-mediated inactivation and the potential as a safe and effective vehicle for the delivery of DNA vaccines. BMC Microbiol. 2018;18:80. doi: 10.1186/s12866-018-1216-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mancha-Agresti P., de Castro C.P., Dos Santos J.S.C., Araujo M.A., Pereira V.B., LeBlanc J.G., Leclercq S.Y., Azevedo V. Recombinant Invasive Lactococcus lactis Carrying a DNA Vaccine Coding the Ag85A Antigen Increases INF-gamma, IL-6, and TNF-alpha Cytokines after Intranasal Immunization. Front. Microbiol. 2017;8:1263. doi: 10.3389/fmicb.2017.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hu Q., Wu M., Fang C., Cheng C., Zhao M., Fang W., Chu P.K., Ping Y., Tang G. Engineering nanoparticle-coated bacteria as oral DNA vaccines for cancer immunotherapy. Nano Lett. 2015;15:2732–2739. doi: 10.1021/acs.nanolett.5b00570. [DOI] [PubMed] [Google Scholar]
- 230.Mottais A., Le Gall T., Sibiril Y., Ravel J., Laurent V., d’Arbonneau F., Montier T. Enhancement of lung gene delivery after aerosol: A new strategy using non-viral complexes with antibacterial properties. Biosci. Rep. 2017;37 doi: 10.1042/BSR20160618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rajapaksa A.E., Ho J.J., Qi A., Bischof R., Nguyen T.H., Tate M., Piedrafita D., McIntosh M.P., Yeo L.Y., Meeusen E., et al. Effective pulmonary delivery of an aerosolized plasmid DNA vaccine via surface acoustic wave nebulization. Respir. Res. 2014;15:60. doi: 10.1186/1465-9921-15-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Davies L.A., Nunez-Alonso G.A., McLachlan G., Hyde S.C., Gill D.R. Aerosol delivery of DNA/liposomes to the lung for cystic fibrosis gene therapy. Hum. Gene Ther. Clin. Dev. 2014;25:97–107. doi: 10.1089/humc.2014.019. [DOI] [PubMed] [Google Scholar]
- 233.Chen H., Yuan J., Wang Y., Silvers W.K. Distribution of ATPase-positive Langerhans cells in normal adult human skin. Br. J. Dermatol. 1985;113:707–711. doi: 10.1111/j.1365-2133.1985.tb02406.x. [DOI] [PubMed] [Google Scholar]
- 234.Russo E., Nitschke M., Halin C. Dendritic cell interactions with lymphatic endothelium. Lymphatic Res. Biol. 2013;11:172–182. doi: 10.1089/lrb.2013.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yu D., Liu H., Shi S., Dong L., Wang H., Wu N., Gao H., Cheng Z., Zheng Q., Cai J., et al. A novel dendritic-cell-targeting DNA vaccine for hepatitis B induces T cell and humoral immune responses and potentiates the antivirus activity in HBV transgenic mice. Immunol. Lett. 2015;168:293–299. doi: 10.1016/j.imlet.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 236.Alvarez R.D., Huh W.K., Bae S., Lamb L.S., Jr., Conner M.G., Boyer J., Wang C., Hung C.F., Sauter E., Paradis M., et al. A pilot study of pNGVL4a-CRT/E7(detox) for the treatment of patients with HPV16+ cervical intraepithelial neoplasia 2/3 (CIN2/3) Gynecol. Oncol. 2016;140:245–252. doi: 10.1016/j.ygyno.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Larregina A.T., Watkins S.C., Erdos G., Spencer L.A., Storkus W.J., Beer Stolz D., Falo L.D., Jr. Direct transfection and activation of human cutaneous dendritic cells. Gene Ther. 2001;8:608–617. doi: 10.1038/sj.gt.3301404. [DOI] [PubMed] [Google Scholar]
- 238.Li J., Zeng M., Shan H., Tong C. Microneedle Patches as Drug and Vaccine Delivery Platform. Curr. Med. Chem. 2017;24:2413–2422. doi: 10.2174/0929867324666170526124053. [DOI] [PubMed] [Google Scholar]
- 239.Platteel A.C.M., Nieuwenhuizen N.E., Domaszewska T., Schurer S., Zedler U., Brinkmann V., Sijts A., Kaufmann S.H.E. Efficacy Testing of H56 cDNA Tattoo Immunization against Tuberculosis in a Mouse Model. Front. Immunol. 2017;8:1744. doi: 10.3389/fimmu.2017.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lee S.H., Danishmalik S.N., Sin J.I. DNA vaccines, electroporation and their applications in cancer treatment. Hum. Vacc. Immunotherap. 2015;11:1889–1900. doi: 10.1080/21645515.2015.1035502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Schultheis K., Smith T.R.F., Kiosses W.B., Kraynyak K.A., Wong A., Oh J., Broderick K.E. Delineating the Cellular Mechanisms Associated with Skin Electroporation. Hum. Gene Ther. Methods. 2018;29:177–188. doi: 10.1089/hgtb.2017.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lamolinara A., Stramucci L., Hysi A., Iezzi M., Marchini C., Mariotti M., Amici A., Curcio C. Intradermal DNA Electroporation Induces Cellular and Humoral Immune Response and Confers Protection against HER2/neu Tumor. J. Immunol. Res. 2015;2015:159145. doi: 10.1155/2015/159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Kulkarni V., Rosati M., Jalah R., Ganneru B., Alicea C., Yu L., Guan Y., LaBranche C., Montefiori D.C., King A.D., et al. DNA vaccination by intradermal electroporation induces long-lasting immune responses in rhesus macaques. J. Med. Primatol. 2014;43:329–340. doi: 10.1111/jmp.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Weiss R., Scheiblhofer S., Freund J., Ferreira F., Livey I., Thalhamer J. Gene gun bombardment with gold particles displays a particular Th2-promoting signal that over-rules the Th1-inducing effect of immunostimulatory CpG motifs in DNA vaccines. Vaccine. 2002;20:3148–3154. doi: 10.1016/S0264-410X(02)00250-5. [DOI] [PubMed] [Google Scholar]
- 245.Dane K.Y., Nembrini C., Tomei A.A., Eby J.K., O’Neil C.P., Velluto D., Swartz M.A., Inverardi L., Hubbell J.A. Nano-sized drug-loaded micelles deliver payload to lymph node immune cells and prolong allograft survival. J. Control. Release. 2011;156:154–160. doi: 10.1016/j.jconrel.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 246.Allen T.M., Hansen C.B., Guo L.S. Subcutaneous administration of liposomes: A comparison with the intravenous and intraperitoneal routes of injection. Biochim. Biophys. Acta. 1993;1150:9–16. doi: 10.1016/0005-2736(93)90115-G. [DOI] [PubMed] [Google Scholar]
- 247.Longmire M., Choyke P.L., Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine. 2008;3:703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Hu J., Sheng Y., Shi J., Yu B., Yu Z., Liao G. Long circulating polymeric nanoparticles for gene/drug delivery. Curr. Drug Metab. 2017 doi: 10.2174/1389200219666171207120643. [DOI] [PubMed] [Google Scholar]
- 249.He C., Hu Y., Yin L., Tang C., Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 250.Blank F., Stumbles P.A., Seydoux E., Holt P.G., Fink A., Rothen-Rutishauser B., Strickland D.H., von Garnier C. Size-dependent uptake of particles by pulmonary antigen-presenting cell populations and trafficking to regional lymph nodes. Am. J. Respir. Cell Mol. Biol. 2013;49:67–77. doi: 10.1165/rcmb.2012-0387OC. [DOI] [PubMed] [Google Scholar]
- 251.Delcassian D., Patel A.K., Cortinas A.B., Langer R. Drug delivery across length scales. J. Drug Target. 2018:1–15. doi: 10.1080/1061186X.2018.1438440. [DOI] [PubMed] [Google Scholar]
- 252.Frenz T., Grabski E., Duran V., Hozsa C., Stepczynska A., Furch M., Gieseler R.K., Kalinke U. Antigen presenting cell-selective drug delivery by glycan-decorated nanocarriers. Eur. J. Pharm. Biopharm. 2015;95:13–17. doi: 10.1016/j.ejpb.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 253.Appelmelk B.J., van Die I., van Vliet S.J., Vandenbroucke-Grauls C.M., Geijtenbeek T.B., van Kooyk Y. Cutting edge: Carbohydrate profiling identifies new pathogens that interact with dendritic cell-specific ICAM-3-grabbing nonintegrin on dendritic cells. J. Immunol. 2003;170:1635–1639. doi: 10.4049/jimmunol.170.4.1635. [DOI] [PubMed] [Google Scholar]
- 254.Burgdorf S., Lukacs-Kornek V., Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J. Immunol. 2006;176:6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 255.Qiao C., Liu J., Yang J., Li Y., Weng J., Shao Y., Zhang X. Enhanced non-inflammasome mediated immune responses by mannosylated zwitterionic-based cationic liposomes for HIV DNA vaccines. Biomaterials. 2016;85:1–17. doi: 10.1016/j.biomaterials.2016.01.054. [DOI] [PubMed] [Google Scholar]
- 256.Chen B.Y., Zhou G., Li Q.L., Lu J.S., Shi D.Y., Pang X.B., Zhou X.W., Yu Y.Z., Huang P.T. Enhanced effects of DNA vaccine against botulinum neurotoxin serotype A by targeting antigen to dendritic cells. Immunol. Let. 2017;190:118–124. doi: 10.1016/j.imlet.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 257.Wang Q., Cao W., Yang Z.G., Zhao G.F. DC targeting DNA vaccines induce protective and therapeutic antitumor immunity in mice. Int. J. Clin. Exp. Med. 2015;8:17565–17577. [PMC free article] [PubMed] [Google Scholar]
- 258.Walsh K.P., Mills K.H. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013;34:521–530. doi: 10.1016/j.it.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 259.Polini A., Mercato L.L.D., Barra A., Zhang Y.S., Calabi F., Gigli G. Towards the development of human immune-system-on-a-chip platforms. Drug Discov. Today. 2018 doi: 10.1016/j.drudis.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Marshall H.T., Djamgoz M.B.A. Immuno-Oncology: Emerging Targets and Combination Therapies. Front. Oncol. 2018;8:315. doi: 10.3389/fonc.2018.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yan X., Zhang S., Deng Y., Wang P., Hou Q., Xu H. Prognostic Factors for Checkpoint Inhibitor Based Immunotherapy: An Update With New Evidences. Front. Pharm. 2018;9:1050. doi: 10.3389/fphar.2018.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]