Abstract
AMP-activated kinase (AMPK) is a serine/threonine kinase that is expressed in most cells and activated by a high cellular AMP/ATP ratio (indicating energy deficiency) or by Ca2+. In general, AMPK turns on energy-generating pathways (e.g., glucose uptake, glycolysis, fatty acid oxidation) and stops energy-consuming processes (e.g., lipogenesis, glycogenesis), thereby helping cells survive low energy states. The functional element of the kidney, the nephron, consists of the glomerulus, where the primary urine is filtered, and the proximal tubule, Henle’s loop, the distal tubule, and the collecting duct. In the tubular system of the kidney, the composition of primary urine is modified by the reabsorption and secretion of ions and molecules to yield final excreted urine. The underlying membrane transport processes are mainly energy-consuming (active transport) and in some cases passive. Since active transport accounts for a large part of the cell’s ATP demands, it is an important target for AMPK. Here, we review the AMPK-dependent regulation of membrane transport along nephron segments and discuss physiological and pathophysiological implications.
Keywords: transporter, carrier, pump, membrane, energy deficiency
1. Introduction
The 5′-adenosine monophosphate (AMP)–activated protein kinase (AMPK) is a serine/threonine protein kinase that is evolutionarily conserved and functions as an intracellular energy sensor in mammalian cells [1,2,3,4,5]. It is a central regulator of energy homeostasis and affects many important cellular functions including growth, differentiation, autophagy, and metabolism [1,2,6]. During energy depletion when cellular AMP levels are high relative to the adenosine triphosphate (ATP) concentration, AMPK activates energy-providing pathways including glucose uptake, glycolysis, or fatty acid oxidation [7,8,9,10]. Simultaneously, processes consuming ATP (e.g., gluconeogenesis, lipogenesis, or protein synthesis) are inhibited [7,8,9,10].
Being expressed in most mammalian cells, AMPK is a heterotrimeric protein consisting of a catalytic α (α1 or α2), scaffolding β (β1 or β2), and a regulatory nucleotide-binding γ (γ1, γ2, or γ3) subunit with the expression pattern differing from cell type to cell type [1,2,11,12,13,14]. Induction of AMPK activity involves phosphorylation of the conserved threonine residue Thr172 within the activation loop of the α subunit’s kinase domain by various protein kinases including the tumor suppressor liver kinase B1 (LKB1), Ca2+/calmodulin–dependent protein kinase kinase β (CaMKKβ), and transforming growth factor beta-activated kinase 1 [1,15,16,17,18,19,20,21,22,23,24,25,26,27,28]. AMPK activation in cellular energy depletion is primarily mediated by an increase in the AMP/ATP or ADP/ATP ratio [8,29,30]. Thus, AMP or ADP binding to the subunit at cystathionine-beta-synthase repeats results in conformational changes that allows for the phosphorylation at Thr172 by LKB1. This results in an enhancement of AMPK activity by >100-fold [1,8,12,15,31,32,33,34,35,36]. Moreover, AMP or ADP binding prevents dephosphorylation at Thr172 by protein phosphatases [8,12,37,38]. Additionally, binding of AMP, but not ADP, activates AMPK allosterically [8,11,12,37]. Conversely, ATP binding to the cystathionine-beta-synthase domain results in AMPK dephosphorylation by protein phosphatases [1,8,39].
Besides LKB1-associated regulation of AMPK phosphorylation, an alternative Ca2+-involving activation mechanisms independent of AMP exists [6,12,40,41]. Protein kinase CaMKKβ phosphorylates AMPK at Thr172 in response to elevated intracellular Ca2+ levels which may be caused by mediators such as thrombin or ghrelin [6,12,23,40,42,43]. Intracellular Ca2+ store depletion detected by the Ca2+-sensing protein stromal interacting molecule-1 leads to store-operated Ca2+ entry (SOCE) involving the Ca2+ release-activated Ca2+ channel Orai1 [44,45,46,47,48,49]. Orai1-mediated SOCE impacts on many cellular functions including cell proliferation, differentiation, migration, and cytokine production [44,50,51,52,53,54,55]. SOCE is involved in a sort of feedback mechanism involving AMPK: SOCE activates AMPK through CaMKKβ. AMPK in turn inhibits SOCE [45]. Moreover, AMPK inhibits SOCE by regulating Orai1 membrane abundance (at least in UMR106 cells) [44,56].
AMPK is a major regulator of whole body energy homeostasis [10,12], impacting on a variety of organs including liver [57,58,59,60,61], skeletal [62,63,64,65,66] and cardiac muscle [67,68,69,70,71,72,73], kidney [74,75,76,77], and bone [78,79,80]. In the kidney, AMPK regulates epithelial transport, podocyte function, blood pressure, epithelial-to-mesenchymal transition, autophagy as well as nitric oxide synthesis [75,76,81,82,83]. Not surprisingly, AMPK is highly relevant for renal pathophysiology, including ischemia, diabetic renal hypertrophy, polycystic kidney disease, chronic kidney disease, and hypertension [40,67,74,75,76]. This review summarizes the contribution of AMPK to the regulation of renal transport and hence to the final composition of excreted urine. Moreover, pathophysiological implications are discussed.
2. AMPK and Renal Tubular Transport
The kidney is particularly relevant for fluid, electrolyte, and acid–base homeostasis. In addition, it is an endocrine organ producing different hormones such as erythropoietin, Klotho, and calcitriol, the active form of vitamin D [84,85,86]. The kidneys are made up of about 1 million nephrons, their functional elements. A nephron comprises the glomerulus surrounded by the Bowman´s capsule, the proximal tubule, Henle’s loop, distal tubule, and the collecting duct. The primary urine is filtered in the glomerulus. Its composition is similar to plasma. In general, large molecules and particularly proteins >6000 Dalton are normally filtered to a low extent, if at all. The renal tubular system modifies the primary urine by reabsorbing or secreting ions and molecules, ultimately yielding the final urine [85,86,87]. Epithelial transport is mainly dependent on ATP-dependent pumps (primary-active), secondary-or tertiary-active transporters, as well as carriers and channels (passive, facilitated diffusion). Since active transport consumes energy by definition, it is not surprising that it is subject to regulation by AMPK. Moreover, even passive transport involving glucose transporter (GLUT) carriers is controlled by AMPK [74,75].
2.1. Na+/K+-ATPase
The ubiquitously expressed Na+/K+-ATPase is a primary active ATP-driven pump that mediates the basolateral extrusion of 3Na+ in exchange of 2K+, thereby establishing a transmembrane Na+ gradient, which is the prerequisite for secondary active Na+-dependent transport (e.g., through Na+-dependent glucose cotransporter 1 and 2 (SGLT1/2), Na+/H+ exchanger isoform 1 (NHE1), Na+-coupled phosphate transporter (NaPi-IIa), or Na+-K+-2Cl− cotransporter (NKCC2), as discussed below) [75,88,89,90,91,92,93,94]. Almost one-third of the body’s energy is consumed by this pump [95]. Therefore, it does make sense that it is regulated by AMPK [74,75,76,94]: AMPK inhibits Na+/K+-ATPase in airway epithelial cells by promoting its endocytosis [96,97,98,99,100]. However, AMPK stimulates Na+/K+-ATPase membrane expression in skeletal muscle cells [101] and in renal epithelia [102], thereby counteracting renal ischemia-induced Na+/K+-ATPase endocytosis [103]. Interestingly, AMPKβ1 deficiency was found not to alter outcome in an ischemic kidney injury model in mice [104]. Hence, the effect of AMPK on Na+/K+-ATPase appears to be highly tissue-specific [74,75].
2.2. Proximal Tubule
A wide variety of luminal Na+-dependent cotransporters, which are secondary active, are involved in epithelial transport in the proximal tubule. Secondary active transporters utilize the energy of the transmembrane Na+ gradient generated by the primary active ATP-consuming Na+/K+-ATPase to facilitate transport of a substrate against its concentration gradient [105,106]. These transporters and the basolateral Na+/K+-ATPase consume substantial amounts of total cellular energy [74,75,107]. Hence, AMPK has been demonstrated to be an important regulator of proximal tubule transport [74,75].
2.2.1. Glucose Transport
Since glucose is freely filtered by the glomerulus, glucose concentration in primary urine is similar to the plasma glucose concentration, whereas excreted urine is usually free of glucose [108,109,110]. The sugar is reabsorbed in the proximal tubule by the Na+-dependent glucose cotransporter 1 and 2 (SGLT1 and 2), the different expression patterns and properties of which ensure total glucose reabsorption as long as the plasma glucose concentration is not abnormally high [89,108]. SGLT2 has a high transport capacity but low affinity for glucose and is predominantly expressed in the kidney, while SGLT1 is also expressed in other tissues including the small intestine. SGLT2 contributes to the reabsorption of up to 90% of filtered glucose [108,109,111,112]. On the other hand, AMPK-regulated SGLT1 [7,92,113] has a low transport capacity but high affinity for glucose and reabsorbs the remaining glucose [108,109,110,114,115]. Glucose leaves the basolateral membrane through passive glucose carriers GLUT1 and GLUT2 [108,116,117,118]. AMPK activates SGLT1-dependent glucose transport, presumably by stimulating membrane insertion of the cotransporter as observed in colorectal Caco-2 cells [92,119]. In line with this, AMPK activation is associated with increased SGLT1 expression and glucose uptake in cardiomyocytes [113,120]. Although the AMPK-dependent regulation of SGLT1 in the proximal tubule has not explicitly been addressed, it is tempting to speculate that it is similar to other cell types [92,113,119,120]. The regulation of SGLT by AMPK is a doubled-edged sword: on the one hand, SGLT1-dependent reabsorption of glucose in proximal tubular cells requires energy which is generated by β-oxidation of fatty acids to a large extent [121,122]. On the other hand, it prevents the loss of energy-rich glucose [122,123], thereby maintaining the Na+/K+-ATPase-facilitated Na+ gradient for Na+-dependent transport and many other cellular processes [75,76]. SGLT1-mediated glucose uptake is linked to the GLUT1-dependent efflux at the basolateral side [108,116]. GLUT1 activity is stimulated by AMPK in various cell types [124,125,126,127,128,129,130,131]. Therefore, it is conceivable that renal GLUT1 might also be regulated by AMPK in order to save energy-providing glucose. In line with this, Baldwin et al. (1997) showed enhanced glucose uptake via GLUT1 in baby hamster kidney cells treated with AMPK activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) [132]. Moreover, Sokolovska et al. (2010) reported that metformin, another pharmacological AMPK activator, increased GLUT1 gene expression in rat kidneys [133]. Also, AMPK activation was associated with enhanced activity of GLUT2. These studies, however, found reduced SGLT1 membrane abundance upon AMPK activation, at least in the case of murine intestinal tissue [134,135].
2.2.2. Na+/H+ Exchanger Isoform 1
The ubiquitous Na+/H+ exchanger isoform 1 (NHE1) participates in cell volume and pH regulation by extruding one cytosolic H+ in exchange for one extracellular Na+ [136,137]. NHE1 is expressed in all parts of the nephron, including the proximal tubule. However, it cannot be detected in the macula densa and intercalated cells of the distal nephron [136,138,139]. In the proximal tubule, NHE1 is particularly important for HCO3− reabsorption [140]. In hypoxia, anaerobic glycolysis is predominant, which results in intracellular accumulation of lactate and H+ [90]. Acidosis, however, inhibits glycolysis [90,141,142] and would jeopardize cellular energy generation. AMPK-dependent stimulation of NHE1 activity in human embryonic kidney (HEK) cells therefore helps cells keep up anaerobic glycolysis in oxygen deficiency, as demonstrated by Rotte et al. (2010) [90]. Given that NHE1 is needed for proximal tubular HCO3− reabsorption [140], AMPK may help retain HCO3−, thereby alleviating acidosis in energy deficiency and hypoxia.
2.2.3. Creatine Transporter
In some organs with high metabolic activity, including skeletal muscle, heart, and brain, creatine is used to refuel cellular ATP levels [143,144,145]. In the proximal tubule, creatine, a small molecule that is freely filtered, is also reabsorbed through secondary active Na+-dependent creatine transporter (CRT) (SLC6A8) [7,75,143,146]. AMPK has been demonstrated to downregulate CRT activity and apical membrane expression in a polarized mouse S3 proximal tubule cell line, presumably through mammalian target of rapamycin signaling [147]. The AMPK-dependent inhibition of CRT may help reduce unnecessary energy expenditure [75]. Conversely, AMPK stimulates CRT-mediated creatine transport in cardiomyocytes [148,149]. This again demonstrates that AMPK effects are tissue-specific [148].
2.2.4. Na+-Coupled Phosphate Transporter IIa
Inorganic phosphate is mainly reabsorbed by the secondary active Na+-coupled phosphate transporter (NaPi-IIa) (SLC34A1) in the proximal tubule [93,150,151,152]. Employing electrophysiological recordings in Xenopus oocytes, it was shown that AMPK inhibits NaPi-IIa [93]. Kinetics analysis revealed that AMPK decreases NaPi-IIa membrane expression rather than changing its properties.
The regulation of phosphate metabolism by AMPK is not restricted to NaPi-IIa: Recently, AMPK was demonstrated to control the formation of bone-derived hormone fibroblast growth factor 23 (FGF23) [56], which induces renal phosphate excretion by extracellular-signal regulated kinases 1/2 (ERK1/2)-mediated degradation of membrane NaPi-IIa [150]. AMPK inhibits FGF23 production in cell culture and in mice [56]. Despite markedly elevated FGF23 serum levels in AMPKα1-deficient mice, renal phosphate excretion was not different from wild-type animals [56]. The same holds true for cellular localization of NaPi-IIa and renal ERK1/2 [56]. Thus, it is possible that AMPK deficiency is paralleled with some FGF23 resistance.
2.3. Loop of Henle
2.3.1. Na+-K+-2Cl− Cotransporter
The Na+-K+-2Cl− cotransporter (NKCC2), expressed in the thick ascending limb (TAL) of the loop of Henle and macula densa, is required for the generation of a hypertonic medullary interstitium, a mechanism needed for concentrating urine [75,76,88,91]. NKCC2 is a direct substrate of AMPK which phosphorylates it at its stimulatory serine residue Ser-126 [153]. Moreover, exposure of murine macula densa-like cells to low salt leads to AMPK activation and increased NKCC2 phosphorylation [154]. In addition, increased subapical expression (and apparent reduced apical expression) of NKCC2 in the medullary TAL of the loop of Henle along with elevated urinary Na+ excretion in AMPKβ1-deficient mice on a normal salt diet were observed [155]. This is in line with AMPK being an important regulator of NKCC2-mediated salt retention in the medullary TAL of Henle [155]. Efe et al. (2016) recently observed markedly increased outer medullary expression of NKCC2 in rats treated with the AMPK activator metformin [156]. However, according to a recent in vivo study by Udwan et al. (2017), a low salt diet induced upregulation of NKCC2 surface expression in mouse kidneys but left AMPK activity unchanged [157]. Therefore, the exact role of AMPK in stimulating NKCC2 remains to be established.
2.3.2. Renal Outer Medullary K+ Channel
The apical renal outer medullary K+ channel (ROMK) is required for NKCC2 to work properly, as it allows the recirculation of K+ ions taken up by NKCC2 into the lumen [75,88]. AMPK is an inhibitor of ROMK by downregulating both channel activity and membrane abundance of the channel protein in a heterologous expression system using Xenopus oocytes [158]. In vivo studies revealed that the AMPK effect on ROMK is relevant for the renal excretion of K+ after an acute K+ challenge, as upregulation of renal ROMK1 protein expression and the ability of K+ elimination were more pronounced in AMPKα1-deficient than in wild-type mice [158].
2.4. Distal Tubule
2.4.1. Cystic Fibrosis Transmembrane Conductance Regulator
The ATP-gated and cyclic AMP (cAMP)-dependent Cl− channel cystic fibrosis transmembrane conductance regulator (CFTR) participates in Cl− secretion and is broadly known for its role in cystic fibrosis, the pathophysiology of which is due to channel malfunction [74,75,76,159]. In the kidney, CFTR contributes to Cl− secretion in the distal tubule and the principal cells of the cortical and medullary collecting ducts [74,75,160]. AMPK has been demonstrated to inhibit CFTR-dependent Cl− conductance in Xenopus oocytes [159] and to decrease CFTR channel activity in the lung [161,162] and colon [163]. cAMP-stimulated cell proliferation and CFTR-dependent Cl− secretion play a decisive role for epithelial cyst enlargement in autosomal dominant polycystic kidney disease (ADPKD) [164]. In line with this, AMPK activation inhibits CFTR in Madin-Darby canine kidney (MDCK) cells [165] as well as decreases cystogenesis in murine models of ADPKD [165,166], suggesting a potential role for pharmacological AMPK activation in the treatment of ADPKD [165,166].
2.4.2. Ca2+ Transport
Most Ca2+ is reabsorbed by passive paracellular diffusion along with other ions and water through tight junctions in the proximal tubule and the more distal parts of the nephron [88,167]. Conversely, only 5–10% of filtered Ca2+ is reabsorbed by transcellular transport involving the apical transient receptor potential vanilloid 5 channel TRPV5 in the distal convoluted tubule [88]: Ca2+ enters the cell through TRPV5, whereas basolateral Ca2+ efflux is accomplished by the Na+/Ca2+ exchanger (NCX) and the Ca2+-ATPase [88,167,168]. AMPK has been shown to inhibit NCX and decrease Orai1-mediated SOCE in murine dendritic cells [169]. Therefore, it is tempting to speculate that Ca2+ reabsorption may be downregulated in the distal tubule in ATP deficiency [169,170]. Indeed, AMPK downregulates Orai1-dependent SOCE in T-lymphocytes [171], endothelial cells [45], and in osteoblast-like cells [56]. Since renal Orai1 activity contributes to kidney fibrosis [172], AMPK-mediated Orai1 downregulation may also be therapeutically desirable.
2.5. Collecting Duct
2.5.1. Epithelial Na+ Channel
In the collecting duct, fine tuning of Na+ and K+ homeostasis is accomplished by epithelial Na+ channel (ENaC) and ROMK K+ channel. Both channels are controlled by the renin-angiotensin-aldosterone system [173,174,175] regulating extracellular volume and hence arterial blood pressure [173,174,175,176,177]. Na+ reabsorption by ENaC in the late distal convoluted tubule and cortical collecting duct principal cells is a highly energy-demanding process, as it utilizes the electrochemical driving force generated by the basolateral Na+/K+-ATPase [74,75,76,176,178]. AMPK inhibits epithelial Na+ transport in various tissues, including lung [96,179], colonic [180], and renal cortical collecting duct cells [180,181,182,183]. In line with this, AMPKα1-deficient mice exhibit increased renal ENaC expression [180]. In detail, AMPK downregulates ENaC surface expression by inducing the binding of the ubiquitin ligase neural precursor cell expressed developmentally downregulated protein 4-2 (Nedd4-2) to ENaC subunits, resulting in ENaC ubiquitination with subsequent endocytosis and degradation [177,180,184]. In line with this, activation of AMPK enhances the tubuloglomerular feedback and induces urinary diuresis and Na+ excretion in rats [185]. However, AMPKα1−/− mice with genetic kidney-specific AMPKα2 deletion exhibit a moderate increase in diuresis and natriuresis, possibly because NKCC2 activity is insufficient despite upregulated ENaC activity [186]. Taken together, AMPK activity limits ENaC-dependent energy-consuming Na+ reabsorption [177,180,181,185].
2.5.2. Voltage-Gated K+ Channel
The voltage-gated K+ channel (KCNQ1) is important for the cardiovascular system as well as for electrolyte and fluid homeostasis and is expressed in the distal nephron including the collecting duct [170,187,188,189]. Its exact role is ill-defined, although a contribution to cell volume regulation is postulated [75,187]. Similar to ENaC, AMPK inhibits KCNQ1 via Nedd4-2, as demonstrated in collecting duct principal cells of rat ex vivo kidney slices [187], MDCK cells [190], and Xenopus oocytes [191].
2.5.3. Vacuolar H+-ATPase
The primary active vacuolar H+-ATPase (V-ATPase) is located at the apical membrane of proximal tubule cells and collecting duct type A intercalated cells. It contributes to the regulation of acid–base homeostasis by secreting H+ ions into the tubular lumen [76,192,193]. AMPK inhibits the protein kinase A (PKA)-dependent membrane expression of V-ATPase in collecting duct intercalated cells of rat ex vivo kidney slices [193]. Moreover, epididymal proton-secreting clear cells, developmentally related to intercalated cells, exhibit reduced apical membrane abundance of V-ATPase after in vivo perfusion with the AMPK activator 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) into rats [194]. It appears to be likely that energy deficiency limits highly energy-consuming primary active H+ excretion in the proximal tubule, whereas secondary active NHE1-dependent H+ secretion is maintained, thereby keeping up at least anaerobic glycolysis [192]. The opposing effects of AMPK and PKA on V-ATPase expression and activity in kidney intercalated cells can be explained by different phosphorylation sites, as AMPK and PKA phosphorylate the A subunit at Ser-384 and Ser-175, respectively [195,196]. McGuire and Forgac (2018) further demonstrated that AMPK increases lysosomal V-ATPase assembly and activity in HEK293T cells under conditions of energy depletion [197]. In cells depleted of energy, acidification of autophagic intracellular compartments by V-ATPases enables the lysosomal degradation of proteins and lipids to generate energy substrates for ATP production [197,198]. Thus, it appears to be likely that AMPK-regulated V-ATPase activity depends on its concrete cellular localization and function [197].
2.5.4. Water and Urea Handling
AMPK also regulates renal urea and water handling [76,199]. In the inner medullary collecting duct, osmotic gradients are generated by NKCC2 and urea transporter UT-A1 and water is reabsorbed through aquaporin 2 (AQP2) [76,156,199,200]. The concentration of urine requires the antidiuretic hormone vasopressin, which binds to vasopressin type 2 receptors of collecting duct principal cells, resulting in cAMP-mediated activation of PKA and subsequent phosphorylation and apical membrane insertion of AQP2 and UT-A1 [76,156,199]. Congenital nephrogenic diabetes insipidus (NDI) is a disease primarily caused by mutations of vasopressin type 2 receptors that is characterized by renal resistance to vasopressin and limited urine concentrating capacity [156,201]. According to two in vivo studies using rodent models of congenital NDI, the metformin-stimulated AMPK activation ameliorates the ability of the kidney to concentrate urine by increasing the phosphorylation and apical membrane expression of inner medullary AQP2 and UT-A1 [156,202]. In contrast, an ex vivo treatment of rat kidney slices with AICAR led to reduced apical membrane insertion of AQP2 [203]. Moreover, AMPK antagonizes the desmopressin-induced AQP2 phosphorylation in vitro, thus also suggesting an inhibitory function of AMPK on AQP2 regulation [203]. It appears likely that AMPK-independent effects of the pharmacological AMPK agonists contribute to this discrepancy [156,202,203]. Thus, further studies are clearly required.
3. Conclusions and Perspectives
A growing list of studies indicates the pivotal role of AMPK as a metabolic-sensing regulator of a multitude of transport processes in the kidney [7,74,75,76,170]. Particularly, AMPK activation under conditions of energy deficiency is expected to differentially modulate renal epithelial ion transport in order to preserve cellular energy homeostasis (Figure 1) [7,74,75,76,94,170]. Alongside the above discussed function of AMPK in kidney tubular transport, a variety of other transport proteins, which are expressed in the kidney as well, are regulated by AMPK in extrarenal tissues [7,94,170,204] that are reviewed elsewhere [170] and [7] and summarized in Table 1. Future studies are required to focus on the therapeutic value of pharmacological AMPK manipulation to combat kidney disease [74,75,76,205,206].
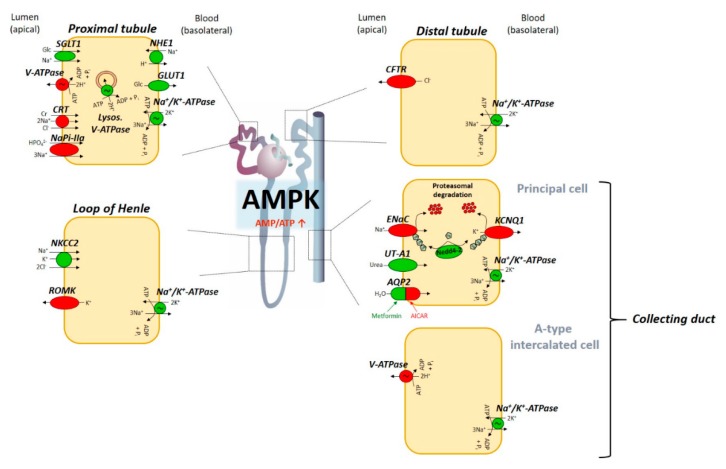
Figure 1.
Tentative model illustrating AMPK-dependent effects on renal transport along the nephron. Cellular energy depletion (e.g., during hypoxia) leads to an elevated AMP/ATP ratio and subsequent AMPK activation. AMPK in turn regulates a multitude of active and passive epithelial transport processes along the renal tubular system in order to maintain cellular energy homeostasis. Ion channels, transport proteins, and ATPases that are activated upon AMPK stimulation are depicted as green icons, whereas red coloring indicates AMPK-dependent inhibition (see text for details). AMP, 5’-adenosine monophosphate; AMPK, AMP-activated protein kinase; SGLT1, Na+-dependent glucose cotransporter 1; V-ATPase, vacuolar H+-ATPase; CRT, creatine transporter; NaPi-IIa, Na+-coupled phosphate transporter IIa; NHE1, Na+/H+ exchanger isoform 1; GLUT1, glucose transporter 1; NKCC2, Na+-K+-2Cl− cotransporter; ROMK, renal outer medullary K+ channel; CFTR, cystic fibrosis transmembrane conductance regulator; ENaC, epithelial Na+ channel; KCNQ1, voltage-gated K+ channel; Nedd4-2, neural precursor cell expressed developmentally downregulated protein 4-2; UT-A1, urea transporter A1; AQP2, aquaporin 2.
Table 1.
Overview of transport proteins regulated by AMPK in extrarenal tissues and evidence for renal expression.
| Ion Channel/Transporter and Method of Modifying AMPK Activity | AMPK Effect | Cell Type of Studied AMPK Effect/Ref. | Evidence for Renal Expression/Ref. |
|---|---|---|---|
| Heterologous expression systems | |||
| Kir2.1 | Reduction of channel activity and membrane abundance via Nedd4-2 mediated endocytosis | Xenopus oocytes [207] | Human proximal tubular cells [208] |
| Kv1.5 | Reduction of channel activity and membrane abundance via Nedd4-2 mediated endocytosis | Xenopus oocytes [209] | Human kidney biopsies [210] |
| Kv11.1 (hERG) | Reduction of channel activity and membrane abundance via Nedd4-2 mediated endocytosis | Xenopus oocytes [211] | Human proximal and distal convoluted tubule [212] |
| SMIT | Reduction of channel activity | Xenopus oocytes [213] | Rat kidney medulla [214] |
| BGT1 | Reduction of channel activity | Xenopus oocytes [213] | Human kidney inner medulla [215] and mouse kidney medulla (basolateral membranes of collecting ducts and TAL of Henle) [216] |
| EAAT3 | Reduction of channel activity and membrane abundance | Xenopus oocytes [217] | Mouse renal proximal tubule [218] |
| NCX | Reduction of channel activity and membrane abundance | Xenopus oocytes [169] | Rat distal convoluted tubule [219] |
| K2P10.1 (TREK-2) | Inhibition of channel activity via phosphorylation at Ser-326 and Ser-359 | HEK293 cells [220] | Human proximal tubule [221] |
| KCa1.1 | Increase in channel activity and membrane abundance | Xenopus oocytes [222] | Human clear cell renal cell carcinoma (ccRCC) and healthy kidney cortex [223] |
| Pharmacological Manipulation | |||
| KCa1.1 | Inhibition of channel activity | Rat carotid body type I cells [224] | |
| Kir6.2 | Upregulation of channel activity Up- or down-regulation of channel activity |
Rat cardiomyocytes [225] Rat pancreatic beta-cells [226,227] |
Rat renal tubular epithelial cells [228] |
| KCa3.1 | Reduction of channel activity | Human airway epithelial cells [229] | Human proximal tubular cells [230] |
| MCT1 and MCT4 | Upregulation of mRNA expression | Rat skeletal muscle [231] | MCT1: basolateral membrane of mouse proximal tubular epithelial cells [232] MCT4: human ccRCC [233] |
| PepT1 | Downregulation of channel activity and brush-border membrane abundance | Caco-2 cells [234] | Rat renal proximal tubule [235] |
| Orai1 | Downregulation of cell membrane abundance and SOCE | Rat UMR106 osteoblast-like cells [56] | Rat glomerular mesangial cells [236] |
| Genetically Modified Mouse Models | |||
| Orai1 | Mouse T-lymphocytes [171] Mouse dendritic cells [169] |
||
Abbreviations
| ADP | Adenosine diphosphate |
| ADPKD | Autosomal dominant polycystic kidney disease |
| AMPK | 5′-adenosine monophosphate (AMP)–activated protein kinase |
| AQP2 | Aquaporin 2 |
| ATP | Adenosine triphosphate |
| BGT1 | Betaine/γ-aminobutyric acid (GABA) transporter 1 |
| CaMKKβ | Ca2+/calmodulin–dependent protein kinase kinase β |
| cAMP | Cyclic adenosine monophosphate |
| ccRCC | Clear cell renal cell carcinoma |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CRT | Creatine transporter |
| EAAT3 | Excitatory amino acid transporter 3 |
| ENaC | Epithelial Na+ channel |
| ERK1/2 | Extracellular-signal regulated kinases 1/2 |
| FGF23 | Fibroblast growth factor 23 |
| GLUT | Glucose transporter |
| HEK | Human embryonic kidney cells |
| hERG | Human ether-a-go-go-related gene |
| Kca | Ca2+ activated K+ channels |
| KCNQ1 | Voltage-gated K+ channel |
| Kir | Inwardly rectifying K+ channels |
| Kv | Voltage gated K+ channels |
| LKB1 | Liver kinase B1 |
| MCT | Monocarboxylate transporters |
| MDCK | Madin-Darby canine kidney cells |
| NaPi-IIa | Na+-coupled phosphate transporter |
| NCX | Na+/Ca2+ exchanger |
| NDI | Nephrogenic diabetes insipidus |
| Nedd4-2 | Neural precursor cell expressed developmentally down-regulated protein 4-2 |
| NHE1 | Na+/H+ exchanger isoform 1 |
| NKCC2 | Na+-K+-2Cl− cotransporter |
| PepT1 | H+-coupled di- and tripeptide transporter 1 |
| PKA | Protein kinase A |
| ROMK | Renal outer medullary K+ channel |
| SGLT | Na+-dependent glucose cotransporter |
| SMIT | Na+ coupled myoinositol transporter |
| SOCE | Store-operated Ca2+ entry |
| TAL | Thick ascending limb |
| TREK-2 | Tandem pore domain K+ channel 2 |
| TRPV5 | Transient receptor potential vanilloid 5 channel |
| UT | Urea transporter |
| V-ATPase | Vacuolar H+-ATPase |
Author Contributions
P.G. and M.F. wrote this review.
Funding
The authors were supported by the Deutsche Forschungsgemeinschaft (DFG; Fo695/2-1).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ramesh M., Vepuri S.B., Oosthuizen F., Soliman M.E. Adenosine Monophosphate-Activated Protein Kinase (AMPK) as a Diverse Therapeutic Target: A Computational Perspective. Appl. Biochem. Biotechnol. 2016;178:810–830. doi: 10.1007/s12010-015-1911-9. [DOI] [PubMed] [Google Scholar]
- 2.Mihaylova M.M., Shaw R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardie D.G. The AMP-activated protein kinase pathway—New players upstream and downstream. J. Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 4.Viollet B. AMPK: Lessons from transgenic and knockout animals. Front. Biosci. 2009;14:19–44. doi: 10.2741/3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viollet B., Andreelli F., Jørgensen S.B., Perrin C., Flamez D., Mu J., Wojtaszewski J.F.P., Schuit F.C., Birnbaum M., Richter E., et al. Physiological role of AMP-activated protein kinase (AMPK): Insights from knockout mouse models. Biochem. Soc. Trans. 2003;31:216–219. doi: 10.1042/bst0310216. [DOI] [PubMed] [Google Scholar]
- 6.Hardie D.G., Schaffer B.E., Brunet A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016;26:190–201. doi: 10.1016/j.tcb.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dërmaku-Sopjani M., Abazi S., Faggio C., Kolgeci J., Sopjani M. AMPK-sensitive cellular transport. J. Biochem. 2014;155:147–158. doi: 10.1093/jb/mvu002. [DOI] [PubMed] [Google Scholar]
- 8.Hardie D.G. AMPK—Sensing energy while talking to other signalling pathways. Cell Metab. 2014;20:939–952. doi: 10.1016/j.cmet.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardie D.G., Carling D., Gamblin S.J. AMP-activated protein kinase: Also regulated by ADP? Trends Biochem. Sci. 2011;36:470–477. doi: 10.1016/j.tibs.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Hardie D.G., Ross F.A., Hawley S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross F.A., Jensen T.E., Hardie D.G. Differential regulation by AMP and ADP of AMPK complexes containing different γ subunit isoforms. Biochem. J. 2016;473:189–199. doi: 10.1042/BJ20150910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie D.G., Lin S.-C. AMP-activated protein kinase—Not just an energy sensor. F1000Research. 2017;6:1724. doi: 10.12688/f1000research.11960.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thornton C., Snowden M.A., Carling D. Identification of a novel AMP-activated protein kinase β subunit isoform that is highly expressed in skeletal muscle. J. Biol. Chem. 1998;273:12443–12450. doi: 10.1074/jbc.273.20.12443. [DOI] [PubMed] [Google Scholar]
- 14.Viollet B., Andreelli F., Jørgensen S.B., Perrin C., Geloen A., Flamez D., Mu J., Lenzner C., Baud O., Bennoun M., et al. The AMP-activated protein kinase α2 catalytic subunit controls whole-body insulin sensitivity. J. Clin. Investig. 2003;111:91–98. doi: 10.1172/JCI16567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawley S.A., Davison M., Woods A., Davies S.P., Beri R.K., Carling D., Hardie D.G. Characterization of the AMP-activated Protein Kinase Kinase from Rat Liver and Identification of Threonine 172 as the Major Site at Which It Phosphorylates AMP-activated Protein Kinase. J. Biol. Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 16.Hong S.-P., Leiper F.C., Woods A., Carling D., Carlson M. Activation of yeast Snf1 and mammalian AMP-activated protein kinase by upstream kinases. Proc. Natl. Acad. Sci. USA. 2003;100:8839–8843. doi: 10.1073/pnas.1533136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley S.A., Boudeau J., Reid J.L., Mustard K.J., Udd L., Mäkelä T.P., Alessi D.R., Hardie D.G. Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25 α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woods A., Johnstone S.R., Dickerson K., Leiper F.C., Fryer L.G.D., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. LKB1 Is the Upstream Kinase in the AMP-Activated Protein Kinase Cascade. Curr. Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Shaw R.J., Kosmatka M., Bardeesy N., Hurley R.L., Witters L.A., DePinho R.A., Cantley L.C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrero-Martín G., Høyer-Hansen M., García-García C., Fumarola C., Farkas T., López-Rivas A., Jäättelä M. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28:677–685. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Momcilovic M., Hong S.-P., Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J. Biol. Chem. 2006;281:25336–25343. doi: 10.1074/jbc.M604399200. [DOI] [PubMed] [Google Scholar]
- 22.Fujiwara Y., Kawaguchi Y., Fujimoto T., Kanayama N., Magari M., Tokumitsu H. Differential AMP-activated Protein Kinase (AMPK) Recognition Mechanism of Ca2+/Calmodulin-dependent Protein Kinase Kinase Isoforms. J. Biol. Chem. 2016;291:13802–13808. doi: 10.1074/jbc.M116.727867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawley S.A., Pan D.A., Mustard K.J., Ross L., Bain J., Edelman A.M., Frenguelli B.G., Hardie D.G. Calmodulin-dependent protein kinase kinase-β is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Hurley R.L., Anderson K.A., Franzone J.M., Kemp B.E., Means A.R., Witters L.A. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 25.Burkewitz K., Zhang Y., Mair W.B. AMPK at the nexus of energetics and aging. Cell Metab. 2014;20:10–25. doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann D. Is TAK1 a Direct Upstream Kinase of AMPK? Int. J. Mol. Sci. 2018;19:2412. doi: 10.3390/ijms19082412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu X., Dahlmans V., Thali R., Preisinger C., Viollet B., Voncken J.W., Neumann D. AMP-activated Protein Kinase Up-regulates Mitogen-activated Protein (MAP) Kinase-interacting Serine/Threonine Kinase 1a-dependent Phosphorylation of Eukaryotic Translation Initiation Factor 4E. J. Biol. Chem. 2016;291:17020–17027. doi: 10.1074/jbc.C116.740498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viollet B., Foretz M. Revisiting the mechanisms of metformin action in the liver. Ann. Endocrinol. 2013;74:123–129. doi: 10.1016/j.ando.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto K., Göransson O., Hardie D.G., Alessi D.R. Activity of LKB1 and AMPK-related kinases in skeletal muscle: Effects of contraction, phenformin, and AICAR. Am. J. Physiol. Endocrinol. Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto K., McCarthy A., Smith D., Green K.A., Grahame Hardie D., Ashworth A., Alessi D.R. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung P.C.F., Salt I.P., Davies S.P., Hardie D.G., Carling D. Characterization of AMP-activated protein kinase γ-subunit isoforms and their role in AMP binding. Biochem. J. 2000;346:659–669. doi: 10.1042/bj3460659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders M.J., Grondin P.O., Hegarty B.D., Snowden M.A., Carling D. Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 2007;403:139–148. doi: 10.1042/BJ20061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao B., Sanders M.J., Underwood E., Heath R., Mayer F.V., Carmena D., Jing C., Walker P.A., Eccleston J.F., Haire L.F., et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oakhill J.S., Chen Z.-P., Scott J.W., Steel R., Castelli L.A., Ling N., Macaulay S.L., Kemp B.E. β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK) Proc. Natl. Acad. Sci. USA. 2010;107:19237–19241. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oakhill J.S., Steel R., Chen Z.-P., Scott J.W., Ling N., Tam S., Kemp B.E. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 36.Viollet B., Mounier R., Leclerc J., Yazigi A., Foretz M., Andreelli F. Targeting AMP-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders. Diabetes Metab. 2007;33:395–402. doi: 10.1016/j.diabet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Gowans G.J., Hawley S.A., Ross F.A., Hardie D.G. AMP Is a True Physiological Regulator of AMP-Activated Protein Kinase by Both Allosteric Activation and Enhancing Net Phosphorylation. Cell Metab. 2013;18:556–566. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies S.P., Helps N.R., Cohen P.T., Hardie D.G. 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C α and native bovine protein phosphatase-2A c. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 39.Chen L., Wang J., Zhang Y.-Y., Yan S.F., Neumann D., Schlattner U., Wang Z.-X., Wu J.-W. AMP-activated protein kinase undergoes nucleotide-dependent conformational changes. Nat. Struct. Mol. Biol. 2012;19:716–718. doi: 10.1038/nsmb.2319. [DOI] [PubMed] [Google Scholar]
- 40.Garcia D., Shaw R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell. 2017;66:789–800. doi: 10.1016/j.molcel.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woods A., Dickerson K., Heath R., Hong S.-P., Momcilovic M., Johnstone S.R., Carlson M., Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Stahmann N., Woods A., Carling D., Heller R. Thrombin activates AMP-activated protein kinase in endothelial cells via a pathway involving Ca2+/calmodulin-dependent protein kinase kinase β. Mol. Cell. Biol. 2006;26:5933–5945. doi: 10.1128/MCB.00383-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y., Atasoy D., Su H.H., Sternson S.M. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lang F., Eylenstein A., Shumilina E. Regulation of Orai1/STIM1 by the kinases SGK1 and AMPK. Cell Calcium. 2012;52:347–354. doi: 10.1016/j.ceca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Sundivakkam P.C., Natarajan V., Malik A.B., Tiruppathi C. Store-operated Ca2+ entry (SOCE) induced by protease-activated receptor-1 mediates STIM1 protein phosphorylation to inhibit SOCE in endothelial cells through AMP-activated protein kinase and p38β mitogen-activated protein kinase. J. Biol. Chem. 2013;288:17030–17041. doi: 10.1074/jbc.M112.411272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang B., Yan J., Umbach A.T., Fakhri H., Fajol A., Schmidt S., Salker M.S., Chen H., Alexander D., Spichtig D., et al. NFκB-sensitive Orai1 expression in the regulation of FGF23 release. J. Mol. Med. 2016;94:557–566. doi: 10.1007/s00109-015-1370-3. [DOI] [PubMed] [Google Scholar]
- 47.Prakriya M., Feske S., Gwack Y., Srikanth S., Rao A., Hogan P.G. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S.L., Kozak J.A., Jiang W., Yeromin A.V., Chen J., Yu Y., Penna A., Shen W., Chi V., Cahalan M.D. Store-dependent and -independent modes regulating Ca2+ release-activated Ca2+ channel activity of human Orai1 and Orai3. J. Biol. Chem. 2008;283:17662–17671. doi: 10.1074/jbc.M801536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tiruppathi C., Ahmmed G.U., Vogel S.M., Malik A.B. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation. 2006;13:693–708. doi: 10.1080/10739680600930347. [DOI] [PubMed] [Google Scholar]
- 50.Yu F., Sun L., Machaca K. Constitutive recycling of the store-operated Ca2+ channel Orai1 and its internalization during meiosis. J. Cell Biol. 2010;191:523–535. doi: 10.1083/jcb.201006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baryshnikov S.G., Pulina M.V., Zulian A., Linde C.I., Golovina V.A. Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes. Am. J. Physiol. Cell Physiol. 2009;297:C1103–C1112. doi: 10.1152/ajpcell.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnstone L.S., Graham S.J.L., Dziadek M.A. STIM proteins: Integrators of signalling pathways in development, differentiation and disease. J. Cell. Mol. Med. 2010;14:1890–1903. doi: 10.1111/j.1582-4934.2010.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang S., Zhang J.J., Huang X.-Y. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15:124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 54.Stathopulos P.B., Ikura M. Store operated calcium entry: From concept to structural mechanisms. Cell Calcium. 2017;63:3–7. doi: 10.1016/j.ceca.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 55.Ambudkar I.S., de Souza L.B., Ong H.L. TRPC1, Orai1, and STIM1 in SOCE: Friends in tight spaces. Cell Calcium. 2017;63:33–39. doi: 10.1016/j.ceca.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glosse P., Feger M., Mutig K., Chen H., Hirche F., Hasan A.A., Gaballa M.M.S., Hocher B., Lang F., Foller M. AMP-activated kinase is a regulator of fibroblast growth factor 23 production. Kidney Int. 2018;94:491–501. doi: 10.1016/j.kint.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 57.Hasenour C.M., Berglund E.D., Wasserman D.H. Emerging role of AMP-activated protein kinase in endocrine control of metabolism in the liver. Mol. Cell. Endocrinol. 2013;366:152–162. doi: 10.1016/j.mce.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y., Xu S., Mihaylova M.M., Zheng B., Hou X., Jiang B., Park O., Luo Z., Lefai E., Shyy J.Y.-J., et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Foretz M., Viollet B. Activation of AMPK for a Break in Hepatic Lipid Accumulation and Circulating Cholesterol. EBioMedicine. 2018;31:15–16. doi: 10.1016/j.ebiom.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merlen G., Gentric G., Celton-Morizur S., Foretz M., Guidotti J.-E., Fauveau V., Leclerc J., Viollet B., Desdouets C. AMPKα1 controls hepatocyte proliferation independently of energy balance by regulating Cyclin A2 expression. J. Hepatol. 2014;60:152–159. doi: 10.1016/j.jhep.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 61.Foretz M., Viollet B. Regulation of hepatic metabolism by AMPK. J. Hepatol. 2011;54:827–829. doi: 10.1016/j.jhep.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 62.Kjøbsted R., Hingst J.R., Fentz J., Foretz M., Sanz M.-N., Pehmøller C., Shum M., Marette A., Mounier R., Treebak J.T., et al. AMPK in skeletal muscle function and metabolism. FASEB J. 2018;32:1741–1777. doi: 10.1096/fj.201700442R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mounier R., Théret M., Lantier L., Foretz M., Viollet B. Expanding roles for AMPK in skeletal muscle plasticity. Trends Endocrinol. Metab. 2015;26:275–286. doi: 10.1016/j.tem.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Kjøbsted R., Munk-Hansen N., Birk J.B., Foretz M., Viollet B., Björnholm M., Zierath J.R., Treebak J.T., Wojtaszewski J.F.P. Enhanced Muscle Insulin Sensitivity After Contraction/Exercise Is Mediated by AMPK. Diabetes. 2017;66:598–612. doi: 10.2337/db16-0530. [DOI] [PubMed] [Google Scholar]
- 65.Cokorinos E.C., Delmore J., Reyes A.R., Albuquerque B., Kjøbsted R., Jørgensen N.O., Tran J.-L., Jatkar A., Cialdea K., Esquejo R.M., et al. Activation of Skeletal Muscle AMPK Promotes Glucose Disposal and Glucose Lowering in Non-human Primates and Mice. Cell Metab. 2017;25:1147–1159. doi: 10.1016/j.cmet.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 66.Fentz J., Kjøbsted R., Birk J.B., Jordy A.B., Jeppesen J., Thorsen K., Schjerling P., Kiens B., Jessen N., Viollet B., et al. AMPKα is critical for enhancing skeletal muscle fatty acid utilization during in vivo exercise in mice. FASEB J. 2015;29:1725–1738. doi: 10.1096/fj.14-266650. [DOI] [PubMed] [Google Scholar]
- 67.Arad M., Seidman C.E., Seidman J.G. AMP-Activated Protein Kinase in the Heart: Role during Health and Disease. Circ. Res. 2007;100:474–488. doi: 10.1161/01.RES.0000258446.23525.37. [DOI] [PubMed] [Google Scholar]
- 68.Voelkl J., Alesutan I., Primessnig U., Feger M., Mia S., Jungmann A., Castor T., Viereck R., Stöckigt F., Borst O., et al. AMP-activated protein kinase α1-sensitive activation of AP-1 in cardiomyocytes. J. Mol. Cell. Cardiol. 2016;97:36–43. doi: 10.1016/j.yjmcc.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Liao Y., Takashima S., Maeda N., Ouchi N., Komamura K., Shimomura I., Hori M., Matsuzawa Y., Funahashi T., Kitakaze M. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovasc. Res. 2005;67:705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 70.Russell R.R., Li J., Coven D.L., Pypaert M., Zechner C., Palmeri M., Giordano F.J., Mu J., Birnbaum M.J., Young L.H. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J. Clin. Investig. 2004;114:495–503. doi: 10.1172/JCI19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gélinas R., Mailleux F., Dontaine J., Bultot L., Demeulder B., Ginion A., Daskalopoulos E.P., Esfahani H., Dubois-Deruy E., Lauzier B., et al. AMPK activation counteracts cardiac hypertrophy by reducing O-GlcNAcylation. Nat. Commun. 2018;9:374. doi: 10.1038/s41467-017-02795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen K., Kobayashi S., Xu X., Viollet B., Liang Q. AMP activated protein kinase is indispensable for myocardial adaptation to caloric restriction in mice. PLoS ONE. 2013;8:e59682. doi: 10.1371/journal.pone.0059682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang P., Hu X., Xu X., Fassett J., Zhu G., Viollet B., Xu W., Wiczer B., Bernlohr D.A., Bache R.J., et al. AMP activated protein kinase-α2 deficiency exacerbates pressure-overload-induced left ventricular hypertrophy and dysfunction in mice. Hypertension. 2008;52:918–924. doi: 10.1161/HYPERTENSIONAHA.108.114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hallows K.R., Mount P.F., Pastor-Soler N.M., Power D.A. Role of the energy sensor AMP-activated protein kinase in renal physiology and disease. Am. J. Physiol. Renal. Physiol. 2010;298:F1067–F1077. doi: 10.1152/ajprenal.00005.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pastor-Soler N.M., Hallows K.R. AMP-activated protein kinase regulation of kidney tubular transport. Curr. Opin. Nephrol. Hypertens. 2012;21:523–533. doi: 10.1097/MNH.0b013e3283562390. [DOI] [PubMed] [Google Scholar]
- 76.Rajani R., Pastor-Soler N.M., Hallows K.R. Role of AMP-activated protein kinase in kidney tubular transport, metabolism, and disease. Curr. Opin. Nephrol. Hypertens. 2017;26:375–383. doi: 10.1097/MNH.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 77.Lee M.-J., Feliers D., Mariappan M.M., Sataranatarajan K., Mahimainathan L., Musi N., Foretz M., Viollet B., Weinberg J.M., Choudhury G.G., et al. A role for AMP-activated protein kinase in diabetes-induced renal hypertrophy. Am. J. Physiol. Renal. Physiol. 2007;292:F617–F627. doi: 10.1152/ajprenal.00278.2006. [DOI] [PubMed] [Google Scholar]
- 78.Jeyabalan J., Shah M., Viollet B., Chenu C. AMP-activated protein kinase pathway and bone metabolism. J. Endocrinol. 2012;212:277–290. doi: 10.1530/JOE-11-0306. [DOI] [PubMed] [Google Scholar]
- 79.McCarthy A.D., Cortizo A.M., Sedlinsky C. Metformin revisited: Does this regulator of AMP-activated protein kinase secondarily affect bone metabolism and prevent diabetic osteopathy. World J. Diabetes. 2016;7:122–133. doi: 10.4239/wjd.v7.i6.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kanazawa I. Interaction between bone and glucose metabolism. Endocr. J. 2017;64:1043–1053. doi: 10.1507/endocrj.EJ17-0323. [DOI] [PubMed] [Google Scholar]
- 81.Tain Y.-L., Hsu C.-N. AMP-Activated Protein Kinase as a Reprogramming Strategy for Hypertension and Kidney Disease of Developmental Origin. Int. J. Mol. Sci. 2018;19:1744. doi: 10.3390/ijms19061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsai C.-M., Kuo H.-C., Hsu C.-N., Huang L.-T., Tain Y.-L. Metformin reduces asymmetric dimethylarginine and prevents hypertension in spontaneously hypertensive rats. Transl. Res. 2014;164:452–459. doi: 10.1016/j.trsl.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 83.Allouch S., Munusamy S. AMP-activated Protein Kinase as a Drug Target in Chronic Kidney Disease. Curr. Drug Targets. 2018;19:709–720. doi: 10.2174/1389450118666170601130947. [DOI] [PubMed] [Google Scholar]
- 84.Curthoys N.P., Moe O.W. Proximal tubule function and response to acidosis. Clin. J. Am. Soc. Nephrol. 2014;9:1627–1638. doi: 10.2215/CJN.10391012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wallace M.A. Anatomy and Physiology of the Kidney. AORN J. 1998;68:799–820. doi: 10.1016/S0001-2092(06)62377-6. [DOI] [PubMed] [Google Scholar]
- 86.Mount D.B. Thick ascending limb of the loop of Henle. Clin. J. Am. Soc. Nephrol. 2014;9:1974–1986. doi: 10.2215/CJN.04480413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang J.L., Rusinek H., Chandarana H., Lee V.S. Functional MRI of the kidneys. J. Magn. Reson. Imaging. 2013;37:282–293. doi: 10.1002/jmri.23717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blaine J., Chonchol M., Levi M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015;10:1257–1272. doi: 10.2215/CJN.09750913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee Y.J., Han H.J. Regulatory mechanisms of Na+/glucose cotransporters in renal proximal tubule cells. Kidney Int. Suppl. 2007:S27–S35. doi: 10.1038/sj.ki.5002383. [DOI] [PubMed] [Google Scholar]
- 90.Rotte A., Pasham V., Eichenmüller M., Bhandaru M., Föller M., Lang F. Upregulation of Na+/H+ exchanger by the AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2010;398:677–682. doi: 10.1016/j.bbrc.2010.06.135. [DOI] [PubMed] [Google Scholar]
- 91.Palmer L.G., Schnermann J. Integrated control of Na transport along the nephron. Clin. J. Am. Soc. Nephrol. 2015;10:676–687. doi: 10.2215/CJN.12391213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sopjani M., Bhavsar S.K., Fraser S., Kemp B.E., Föller M., Lang F. Regulation of Na+-coupled glucose carrier SGLT1 by AMP-activated protein kinase. Mol. Membr. Biol. 2010;27:137–144. doi: 10.3109/09687681003616870. [DOI] [PubMed] [Google Scholar]
- 93.Dërmaku-Sopjani M., Almilaji A., Pakladok T., Munoz C., Hosseinzadeh Z., Blecua M., Sopjani M., Lang F. Down-regulation of the Na+-coupled phosphate transporter NaPi-IIa by AMP-activated protein kinase. Kidney Blood Press. Res. 2013;37:547–556. doi: 10.1159/000355735. [DOI] [PubMed] [Google Scholar]
- 94.Hallows K.R. Emerging role of AMP-activated protein kinase in coupling membrane transport to cellular metabolism. Curr. Opin. Nephrol. Hypertens. 2005;14:464–471. doi: 10.1097/01.mnh.0000174145.14798.64. [DOI] [PubMed] [Google Scholar]
- 95.Noske R., Cornelius F., Clarke R.J. Investigation of the enzymatic activity of the Na+, K+-ATPase via isothermal titration microcalorimetry. Biochim. Biophys. Acta. 2010;1797:1540–1545. doi: 10.1016/j.bbabio.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 96.Woollhead A.M., Scott J.W., Hardie D.G., Baines D.L. Phenformin and 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) activation of AMP-activated protein kinase inhibits transepithelial Na+ transport across H441 lung cells. J. Physiol. 2005;566:781–792. doi: 10.1113/jphysiol.2005.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Woollhead A.M., Sivagnanasundaram J., Kalsi K.K., Pucovsky V., Pellatt L.J., Scott J.W., Mustard K.J., Hardie D.G., Baines D.L. Pharmacological activators of AMP-activated protein kinase have different effects on Na+ transport processes across human lung epithelial cells. Br. J. Pharmacol. 2007;151:1204–1215. doi: 10.1038/sj.bjp.0707343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vadász I., Dada L.A., Briva A., Trejo H.E., Welch L.C., Chen J., Tóth P.T., Lecuona E., Witters L.A., Schumacker P.T., et al. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na, K-ATPase endocytosis. J. Clin. Investig. 2008;118:752–762. doi: 10.1172/JCI29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gusarova G.A., Dada L.A., Kelly A.M., Brodie C., Witters L.A., Chandel N.S., Sznajder J.I. α1-AMP-activated protein kinase regulates hypoxia-induced Na, K-ATPase endocytosis via direct phosphorylation of protein kinase C zeta. Mol. Cell. Biol. 2009;29:3455–3464. doi: 10.1128/MCB.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gusarova G.A., Trejo H.E., Dada L.A., Briva A., Welch L.C., Hamanaka R.B., Mutlu G.M., Chandel N.S., Prakriya M., Sznajder J.I. Hypoxia leads to Na, K-ATPase downregulation via Ca2+ release-activated Ca(2+) channels and AMPK activation. Mol. Cell. Biol. 2011;31:3546–3556. doi: 10.1128/MCB.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Benziane B., Björnholm M., Pirkmajer S., Austin R.L., Kotova O., Viollet B., Zierath J.R., Chibalin A.V. Activation of AMP-activated protein kinase stimulates Na+, K+-ATPase activity in skeletal muscle cells. J. Biol. Chem. 2012;287:23451–23463. doi: 10.1074/jbc.M111.331926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alves D.S., Farr G.A., Seo-Mayer P., Caplan M.J. AS160 associates with the Na+, K+-ATPase and mediates the adenosine monophosphate-stimulated protein kinase-dependent regulation of sodium pump surface expression. Mol. Biol. Cell. 2010;21:4400–4408. doi: 10.1091/mbc.e10-06-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Seo-Mayer P.W., Thulin G., Zhang L., Alves D.S., Ardito T., Kashgarian M., Caplan M.J. Preactivation of AMPK by metformin may ameliorate the epithelial cell damage caused by renal ischemia. Am. J. Physiol. Renal. Physiol. 2011;301:F1346–F1357. doi: 10.1152/ajprenal.00420.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mount P.F., Gleich K., Tam S., Fraser S.A., Choy S.-W., Dwyer K.M., Lu B., van Denderen B., Fingerle-Rowson G., Bucala R., et al. The outcome of renal ischemia-reperfusion injury is unchanged in AMPK-β1 deficient mice. PLoS ONE. 2012;7:e29887. doi: 10.1371/journal.pone.0029887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fitzgerald G.A., Mulligan C., Mindell J.A. A general method for determining secondary active transporter substrate stoichiometry. eLife. 2017;6 doi: 10.7554/eLife.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Forrest L.R., Krämer R., Ziegler C. The structural basis of secondary active transport mechanisms. Biochim. Biophys. Acta. 2011;1807:167–188. doi: 10.1016/j.bbabio.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 107.Mandel L.J., Balaban R.S. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am. J. Physiol. 1981;240:F357–F371. doi: 10.1152/ajprenal.1981.240.5.F357. [DOI] [PubMed] [Google Scholar]
- 108.Mather A., Pollock C. Glucose handling by the kidney. Kidney Int. Suppl. 2011;79:S1–S6. doi: 10.1038/ki.2010.509. [DOI] [PubMed] [Google Scholar]
- 109.Bakris G.L., Fonseca V.A., Sharma K., Wright E.M. Renal sodium-glucose transport: Role in diabetes mellitus and potential clinical implications. Kidney Int. 2009;75:1272–1277. doi: 10.1038/ki.2009.87. [DOI] [PubMed] [Google Scholar]
- 110.Wright E.M., Hirayama B.A., Loo D.F. Active sugar transport in health and disease. J. Intern. Med. 2007;261:32–43. doi: 10.1111/j.1365-2796.2006.01746.x. [DOI] [PubMed] [Google Scholar]
- 111.Hawley S.A., Ford R.J., Smith B.K., Gowans G.J., Mancini S.J., Pitt R.D., Day E.A., Salt I.P., Steinberg G.R., Hardie D.G. The Na+/Glucose Cotransporter Inhibitor Canagliflozin Activates AMPK by Inhibiting Mitochondrial Function and Increasing Cellular AMP Levels. Diabetes. 2016;65:2784–2794. doi: 10.2337/db16-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.You G., Lee W.-S., Barros E.J.G., Kanai Y., Huo T.-L., Khawaja S., Wells R.G., Nigam S.K., Hediger M.A. Molecular Characteristics of Na+-coupled Glucose Transporters in Adult and Embryonic Rat Kidney. J. Biol. Chem. 1995;270:29365–29371. doi: 10.1074/jbc.270.49.29365. [DOI] [PubMed] [Google Scholar]
- 113.Banerjee S.K., Wang D.W., Alzamora R., Huang X.N., Pastor-Soler N.M., Hallows K.R., McGaffin K.R., Ahmad F. SGLT1, a novel cardiac glucose transporter, mediates increased glucose uptake in PRKAG2 cardiomyopathy. J. Mol. Cell. Cardiol. 2010;49:683–692. doi: 10.1016/j.yjmcc.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wright E.M. Renal Na+-glucose cotransporters. Am. J. Physiol. Renal. Physiol. 2001;280:F10–F18. doi: 10.1152/ajprenal.2001.280.1.F10. [DOI] [PubMed] [Google Scholar]
- 115.Pajor A.M., Wright E.M. Cloning and functional expression of a mammalian Na+/nucleoside cotransporter. A member of the SGLT family. J. Biol. Chem. 1992;267:3557–3560. [PubMed] [Google Scholar]
- 116.Linden K.C., DeHaan C.L., Zhang Y., Glowacka S., Cox A.J., Kelly D.J., Rogers S. Renal expression and localization of the facilitative glucose transporters GLUT1 and GLUT12 in animal models of hypertension and diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 2006;290:F205–F213. doi: 10.1152/ajprenal.00237.2004. [DOI] [PubMed] [Google Scholar]
- 117.Dominguez J.H., Camp K., Maianu L., Garvey W.T. Glucose transporters of rat proximal tubule: Differential expression and subcellular distribution. Am. J. Physiol. 1992;262:F807–F812. doi: 10.1152/ajprenal.1992.262.5.F807. [DOI] [PubMed] [Google Scholar]
- 118.Thorens B., Lodish H.F., Brown D. Differential localization of two glucose transporter isoforms in rat kidney. Am. J. Physiol. 1990;259:C286–C294. doi: 10.1152/ajpcell.1990.259.2.C286. [DOI] [PubMed] [Google Scholar]
- 119.Castilla-Madrigal R., Barrenetxe J., Moreno-Aliaga M.J., Lostao M.P. EPA blocks TNF-α-induced inhibition of sugar uptake in Caco-2 cells via GPR120 and AMPK. J. Cell. Physiol. 2018;233:2426–2433. doi: 10.1002/jcp.26115. [DOI] [PubMed] [Google Scholar]
- 120.Di Franco A., Cantini G., Tani A., Coppini R., Zecchi-Orlandini S., Raimondi L., Luconi M., Mannucci E. Sodium-dependent glucose transporters (SGLT) in human ischemic heart: A new potential pharmacological target. Int. J. Cardiol. 2017;243:86–90. doi: 10.1016/j.ijcard.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 121.Portilla D. Energy metabolism and cytotoxicity. Semin. Nephrol. 2003;23:432–438. doi: 10.1016/S0270-9295(03)00088-3. [DOI] [PubMed] [Google Scholar]
- 122.Le Hir M., Dubach U.C. Peroxisomal and mitochondrial β-oxidation in the rat kidney: Distribution of fatty acyl-coenzyme A oxidase and 3-hydroxyacyl-coenzyme A dehydrogenase activities along the nephron. J. Histochem. Cytochem. 1982;30:441–444. doi: 10.1177/30.5.7200500. [DOI] [PubMed] [Google Scholar]
- 123.Uchida S., Endou H. Substrate specificity to maintain cellular ATP along the mouse nephron. Am. J. Physiol. 1988;255:F977–F983. doi: 10.1152/ajprenal.1988.255.5.F977. [DOI] [PubMed] [Google Scholar]
- 124.Fryer L.G.D., Foufelle F., Barnes K., Baldwin S.A., Woods A., Carling D. Characterization of the role of the AMP-activated protein kinase in the stimulation of glucose transport in skeletal muscle cells. Biochem. J. 2002;363:167–174. doi: 10.1042/bj3630167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Al-Bayati A., Lukka D., Brown A.E., Walker M. Effects of thrombin on insulin signalling and glucose uptake in cultured human myotubes. J. Diabetes Complicat. 2016;30:1209–1216. doi: 10.1016/j.jdiacomp.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 126.Andrade B.M., Cazarin J., Zancan P., Carvalho D.P. AMP-activated protein kinase upregulates glucose uptake in thyroid PCCL3 cells independent of thyrotropin. Thyroid. 2012;22:1063–1068. doi: 10.1089/thy.2012.0041. [DOI] [PubMed] [Google Scholar]
- 127.Takeno A., Kanazawa I., Notsu M., Tanaka K.-I., Sugimoto T. Glucose uptake inhibition decreases expressions of receptor activator of nuclear factor-kappa B ligand (RANKL) and osteocalcin in osteocytic MLO-Y4-A2 cells. Am. J. Physiol. Endocrinol. Metab. 2018;314:E115–E123. doi: 10.1152/ajpendo.00159.2017. [DOI] [PubMed] [Google Scholar]
- 128.Wang Y., Zhang Y., Wang Y., Peng H., Rui J., Zhang Z., Wang S., Li Z. WSF-P-1, a novel AMPK activator, promotes adiponectin multimerization in 3T3-L1 adipocytes. Biosci. Biotechnol. Biochem. 2017;81:1529–1535. doi: 10.1080/09168451.2017.1336923. [DOI] [PubMed] [Google Scholar]
- 129.Yamada S., Kotake Y., Sekino Y., Kanda Y. AMP-activated protein kinase-mediated glucose transport as a novel target of tributyltin in human embryonic carcinoma cells. Metallomics. 2013;5:484–491. doi: 10.1039/c3mt20268b. [DOI] [PubMed] [Google Scholar]
- 130.Yu H., Zhang H., Dong M., Wu Z., Shen Z., Xie Y., Kong Z., Dai X., Xu B. Metabolic reprogramming and AMPKα1 pathway activation by caulerpin in colorectal cancer cells. Int. J. Oncol. 2017;50:161–172. doi: 10.3892/ijo.2016.3794. [DOI] [PubMed] [Google Scholar]
- 131.Abbud W., Habinowski S., Zhang J.Z., Kendrew J., Elkairi F.S., Kemp B.E., Witters L.A., Ismail-Beigi F. Stimulation of AMP-activated protein kinase (AMPK) is associated with enhancement of Glut1-mediated glucose transport. Arch. Biochem. Biophys. 2000;380:347–352. doi: 10.1006/abbi.2000.1935. [DOI] [PubMed] [Google Scholar]
- 132.Baldwin S.A., Barros L.F., Griffiths M., Ingram J., Robbins E.C., Streets A.J., Saklatvala J. Regulation of GLUTI in response to cellular stress. Biochem. Soc. Trans. 1997;25:954–958. doi: 10.1042/bst0250954. [DOI] [PubMed] [Google Scholar]
- 133.Sokolovska J., Isajevs S., Sugoka O., Sharipova J., Lauberte L., Svirina D., Rostoka E., Sjakste T., Kalvinsh I., Sjakste N. Influence of metformin on GLUT1 gene and protein expression in rat streptozotocin diabetes mellitus model. Arch. Physiol. Biochem. 2010;116:137–145. doi: 10.3109/13813455.2010.494672. [DOI] [PubMed] [Google Scholar]
- 134.Walker J., Jijon H.B., Diaz H., Salehi P., Churchill T., Madsen K.L. 5-aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: A possible role for AMPK. Biochem. J. 2005;385:485–491. doi: 10.1042/BJ20040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sakar Y., Meddah B., Faouzi M.A., Cherrah Y., Bado A., Ducroc R. Metformin-induced regulation of the intestinal d-glucose transporters. J. Physiol. Pharmacol. 2010;61:301–307. [PubMed] [Google Scholar]
- 136.Parker M.D., Myers E.J., Schelling J.R. Na+–H+ exchanger-1 (NHE1) regulation in kidney proximal tubule. Cell. Mol. Life Sci. 2015;72:2061–2074. doi: 10.1007/s00018-015-1848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Odunewu A., Fliegel L. Acidosis-mediated regulation of the NHE1 isoform of the Na⁺/H⁺ exchanger in renal cells. Am. J. Physiol. Renal. Physiol. 2013;305:F370–F381. doi: 10.1152/ajprenal.00598.2012. [DOI] [PubMed] [Google Scholar]
- 138.Biemesderfer D., Reilly R.F., Exner M., Igarashi P., Aronson P.S. Immunocytochemical characterization of Na+-H+ exchanger isoform NHE-1 in rabbit kidney. Am. J. Physiol. 1992;263:F833–F840. doi: 10.1152/ajprenal.1992.263.5.F833. [DOI] [PubMed] [Google Scholar]
- 139.Peti-Peterdi J., Chambrey R., Bebok Z., Biemesderfer D., St John P.L., Abrahamson D.R., Warnock D.G., Bell P.D. Macula densa Na+/H+ exchange activities mediated by apical NHE2 and basolateral NHE4 isoforms. Am. J. Physiol. Renal. Physiol. 2000;278:F452–F463. doi: 10.1152/ajprenal.2000.278.3.F452. [DOI] [PubMed] [Google Scholar]
- 140.Baum M., Moe O.W., Gentry D.L., Alpern R.J. Effect of glucocorticoids on renal cortical NHE-3 and NHE-1 mRNA. Am. J. Physiol. 1994;267:F437–F442. doi: 10.1152/ajprenal.1994.267.3.F437. [DOI] [PubMed] [Google Scholar]
- 141.Hue L., Beauloye C., Marsin A.-S., Bertrand L., Horman S., Rider M.H. Insulin and Ischemia Stimulate Glycolysis by Acting on the Same Targets Through Different and Opposing Signaling Pathways. J. Mol. Cell. Cardiol. 2002;34:1091–1097. doi: 10.1006/jmcc.2002.2063. [DOI] [PubMed] [Google Scholar]
- 142.Marsin A.-S., Bouzin C., Bertrand L., Hue L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J. Biol. Chem. 2002;277:30778–30783. doi: 10.1074/jbc.M205213200. [DOI] [PubMed] [Google Scholar]
- 143.Wyss M., Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol. Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 144.García-Delgado M., Peral M.J., Cano M., Calonge M.L., Ilundáin A.A. Creatine transport in brush-border membrane vesicles isolated from rat kidney cortex. J. Am. Soc. Nephrol. 2001;12:1819–1825. doi: 10.1681/ASN.V1291819. [DOI] [PubMed] [Google Scholar]
- 145.Wallimann T., Wyss M., Brdiczka D., Nicolay K., Eppenberger H.M. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: The ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Neumann D., Schlattner U., Wallimann T. A molecular approach to the concerted action of kinases involved in energy homoeostasis. Biochem. Soc. Trans. 2003;31:169–174. doi: 10.1042/bst0310169. [DOI] [PubMed] [Google Scholar]
- 147.Li H., Thali R.F., Smolak C., Gong F., Alzamora R., Wallimann T., Scholz R., Pastor-Soler N.M., Neumann D., Hallows K.R. Regulation of the creatine transporter by AMP-activated protein kinase in kidney epithelial cells. Am. J. Physiol. Renal. Physiol. 2010;299:F167–F177. doi: 10.1152/ajprenal.00162.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Darrabie M.D., Arciniegas A.J.L., Mishra R., Bowles D.E., Jacobs D.O., Santacruz L. AMPK and substrate availability regulate creatine transport in cultured cardiomyocytes. Am. J. Physiol. Endocrinol. Metab. 2011;300:E870–E876. doi: 10.1152/ajpendo.00554.2010. [DOI] [PubMed] [Google Scholar]
- 149.Santacruz L., Arciniegas A.J.L., Darrabie M., Mantilla J.G., Baron R.M., Bowles D.E., Mishra R., Jacobs D.O. Hypoxia decreases creatine uptake in cardiomyocytes, while creatine supplementation enhances HIF activation. Physiol. Rep. 2017;5 doi: 10.14814/phy2.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Erben R.G., Andrukhova O. FGF23-Klotho signaling axis in the kidney. Bone. 2017;100:62–68. doi: 10.1016/j.bone.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 151.Biber J., Hernando N., Forster I., Murer H. Regulation of phosphate transport in proximal tubules. Pflugers Arch. 2009;458:39–52. doi: 10.1007/s00424-008-0580-8. [DOI] [PubMed] [Google Scholar]
- 152.Murer H., Forster I., Biber J. The sodium phosphate cotransporter family SLC34. Pflugers Arch. 2004;447:763–767. doi: 10.1007/s00424-003-1072-5. [DOI] [PubMed] [Google Scholar]
- 153.Fraser S.A., Gimenez I., Cook N., Jennings I., Katerelos M., Katsis F., Levidiotis V., Kemp B.E., Power D.A. Regulation of the renal-specific Na+-K+-2Cl− co-transporter NKCC2 by AMP-activated protein kinase (AMPK) Biochem. J. 2007;405:85–93. doi: 10.1042/BJ20061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cook N., Fraser S.A., Katerelos M., Katsis F., Gleich K., Mount P.F., Steinberg G.R., Levidiotis V., Kemp B.E., Power D.A. Low salt concentrations activate AMP-activated protein kinase in mouse macula densa cells. Am. J. Physiol. Renal. Physiol. 2009;296:F801–F809. doi: 10.1152/ajprenal.90372.2008. [DOI] [PubMed] [Google Scholar]
- 155.Fraser S.A., Choy S.-W., Pastor-Soler N.M., Li H., Davies M.R.P., Cook N., Katerelos M., Mount P.F., Gleich K., McRae J.L., et al. AMPK couples plasma renin to cellular metabolism by phosphorylation of ACC1. Am. J. Physiol. Renal. Physiol. 2013;305:F679–F690. doi: 10.1152/ajprenal.00407.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Efe O., Klein J.D., LaRocque L.M., Ren H., Sands J.M. Metformin improves urine concentration in rodents with nephrogenic diabetes insipidus. JCI Insight. 2016;1 doi: 10.1172/jci.insight.88409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Udwan K., Abed A., Roth I., Dizin E., Maillard M., Bettoni C., Loffing J., Wagner C.A., Edwards A., Feraille E. Dietary sodium induces a redistribution of the tubular metabolic workload. J. Physiol. 2017;595:6905–6922. doi: 10.1113/JP274927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Siraskar B., Huang D.Y., Pakladok T., Siraskar G., Sopjani M., Alesutan I., Kucherenko Y., Almilaji A., Devanathan V., Shumilina E., et al. Downregulation of the renal outer medullary K+ channel ROMK by the AMP-activated protein kinase. Pflugers Arch. 2013;465:233–245. doi: 10.1007/s00424-012-1180-1. [DOI] [PubMed] [Google Scholar]
- 159.Hallows K.R., Raghuram V., Kemp B.E., Witters L.A., Foskett J.K. Inhibition of cystic fibrosis transmembrane conductance regulator by novel interaction with the metabolic sensor AMP-activated protein kinase. J. Clin. Invest. 2000;105:1711–1721. doi: 10.1172/JCI9622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Morales M.M., Falkenstein D., Lopes A.G. The Cystic Fibrosis Transmembrane Regulator (CFTR) in the kidney. An. Acad. Bras. Ciênc. 2000;72:399–406. doi: 10.1590/S0001-37652000000300013. [DOI] [PubMed] [Google Scholar]
- 161.Hallows K.R., McCane J.E., Kemp B.E., Witters L.A., Foskett J.K. Regulation of channel gating by AMP-activated protein kinase modulates cystic fibrosis transmembrane conductance regulator activity in lung submucosal cells. J. Biol. Chem. 2003;278:998–1004. doi: 10.1074/jbc.M210621200. [DOI] [PubMed] [Google Scholar]
- 162.King J.D., Fitch A.C., Lee J.K., McCane J.E., Mak D.-O.D., Foskett J.K., Hallows K.R. AMP-activated protein kinase phosphorylation of the R domain inhibits PKA stimulation of CFTR. Am. J. Physiol. Cell Physiol. 2009;297:C94–C101. doi: 10.1152/ajpcell.00677.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kongsuphol P., Hieke B., Ousingsawat J., Almaca J., Viollet B., Schreiber R., Kunzelmann K. Regulation of Cl− secretion by AMPK in vivo. Pflugers Arch. 2009;457:1071–1078. doi: 10.1007/s00424-008-0577-3. [DOI] [PubMed] [Google Scholar]
- 164.Li H., Findlay I.A., Sheppard D.N. The relationship between cell proliferation, Cl-secretion, and renal cyst growth: A study using CFTR inhibitors. Kidney Int. 2004;66:1926–1938. doi: 10.1111/j.1523-1755.2004.00967.x. [DOI] [PubMed] [Google Scholar]
- 165.Takiar V., Nishio S., Seo-Mayer P., King J.D., Li H., Zhang L., Karihaloo A., Hallows K.R., Somlo S., Caplan M.J. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc. Natl. Acad. Sci. USA. 2011;108:2462–2467. doi: 10.1073/pnas.1011498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yuajit C., Muanprasat C., Gallagher A.-R., Fedeles S.V., Kittayaruksakul S., Homvisasevongsa S., Somlo S., Chatsudthipong V. Steviol retards renal cyst growth through reduction of CFTR expression and inhibition of epithelial cell proliferation in a mouse model of polycystic kidney disease. Biochem. Pharmacol. 2014;88:412–421. doi: 10.1016/j.bcp.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 167.Jeon U.S. Kidney and calcium homeostasis. Electrolyte Blood Press. 2008;6:68–76. doi: 10.5049/EBP.2008.6.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Na T., Peng J.-B. TRPV5: A Ca2+ channel for the fine-tuning of Ca2+ reabsorption. Handb. Exp. Pharmacol. 2014;222:321–357. doi: 10.1007/978-3-642-54215-2_13. [DOI] [PubMed] [Google Scholar]
- 169.Nurbaeva M.K., Schmid E., Szteyn K., Yang W., Viollet B., Shumilina E., Lang F. Enhanced Ca2⁺ entry and Na+/Ca2+ exchanger activity in dendritic cells from AMP-activated protein kinase-deficient mice. FASEB J. 2012;26:3049–3058. doi: 10.1096/fj.12-204024. [DOI] [PubMed] [Google Scholar]
- 170.Lang F., Föller M. Regulation of ion channels and transporters by AMP-activated kinase (AMPK) Channels (Austin) 2014;8:20–28. doi: 10.4161/chan.27423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bhavsar S.K., Schmidt S., Bobbala D., Nurbaeva M.K., Hosseinzadeh Z., Merches K., Fajol A., Wilmes J., Lang F. AMPKα1-sensitivity of Orai1 and Ca2+ entry in T-lymphocytes. Cell. Physiol. Biochem. 2013;32:687–698. doi: 10.1159/000354472. [DOI] [PubMed] [Google Scholar]
- 172.Mai X., Shang J., Liang S., Yu B., Yuan J., Lin Y., Luo R., Zhang F., Liu Y., Lv X., et al. Blockade of Orai1 Store-Operated Calcium Entry Protects against Renal Fibrosis. J. Am. Soc. Nephrol. 2016;27:3063–3078. doi: 10.1681/ASN.2015080889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shigaev A., Asher C., Latter H., Garty H., Reuveny E. Regulation of sgk by aldosterone and its effects on the epithelial Na+ channel. Am. J. Physiol. Renal. Physiol. 2000;278:F613–F619. doi: 10.1152/ajprenal.2000.278.4.F613. [DOI] [PubMed] [Google Scholar]
- 174.Zaika O., Mamenko M., Staruschenko A., Pochynyuk O. Direct activation of ENaC by angiotensin II: Recent advances and new insights. Curr. Hypertens. Rep. 2013;15:17–24. doi: 10.1007/s11906-012-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Staruschenko A. Regulation of transport in the connecting tubule and cortical collecting duct. Compr. Physiol. 2012;2:1541–1584. doi: 10.1002/cphy.c110052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Bhalla V., Hallows K.R. Mechanisms of ENaC regulation and clinical implications. J. Am. Soc. Nephrol. 2008;19:1845–1854. doi: 10.1681/ASN.2008020225. [DOI] [PubMed] [Google Scholar]
- 177.Bhalla V., Oyster N.M., Fitch A.C., Wijngaarden M.A., Neumann D., Schlattner U., Pearce D., Hallows K.R. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J. Biol. Chem. 2006;281:26159–26169. doi: 10.1074/jbc.M606045200. [DOI] [PubMed] [Google Scholar]
- 178.Hager H., Kwon T.H., Vinnikova A.K., Masilamani S., Brooks H.L., Frøkiaer J., Knepper M.A., Nielsen S. Immunocytochemical and immunoelectron microscopic localization of α-, β-, and γ-ENaC in rat kidney. Am. J. Physiol. Renal. Physiol. 2001;280:F1093–F1106. doi: 10.1152/ajprenal.2001.280.6.F1093. [DOI] [PubMed] [Google Scholar]
- 179.Myerburg M.M., King J.D., Oyster N.M., Fitch A.C., Magill A., Baty C.J., Watkins S.C., Kolls J.K., Pilewski J.M., Hallows K.R. AMPK agonists ameliorate sodium and fluid transport and inflammation in cystic fibrosis airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2010;42:676–684. doi: 10.1165/2009-0147OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Almaça J., Kongsuphol P., Hieke B., Ousingsawat J., Viollet B., Schreiber R., Amaral M.D., Kunzelmann K. AMPK controls epithelial Na+ channels through Nedd4-2 and causes an epithelial phenotype when mutated. Pflugers Arch. 2009;458:713–721. doi: 10.1007/s00424-009-0660-4. [DOI] [PubMed] [Google Scholar]
- 181.Carattino M.D., Edinger R.S., Grieser H.J., Wise R., Neumann D., Schlattner U., Johnson J.P., Kleyman T.R., Hallows K.R. Epithelial sodium channel inhibition by AMP-activated protein kinase in oocytes and polarized renal epithelial cells. J. Biol. Chem. 2005;280:17608–17616. doi: 10.1074/jbc.M501770200. [DOI] [PubMed] [Google Scholar]
- 182.Yu H., Yang T., Gao P., Wei X., Zhang H., Xiong S., Lu Z., Li L., Wei X., Chen J., et al. Caffeine intake antagonizes salt sensitive hypertension through improvement of renal sodium handling. Sci. Rep. 2016;6:25746. doi: 10.1038/srep25746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Weixel K.M., Marciszyn A., Alzamora R., Li H., Fischer O., Edinger R.S., Hallows K.R., Johnson J.P. Resveratrol inhibits the epithelial sodium channel via phopshoinositides and AMP-activated protein kinase in kidney collecting duct cells. PLoS ONE. 2013;8:e78019. doi: 10.1371/journal.pone.0078019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ho P.-Y., Li H., Pavlov T.S., Tuerk R.D., Tabares D., Brunisholz R., Neumann D., Staruschenko A., Hallows K.R. β1Pix exchange factor stabilizes the ubiquitin ligase Nedd4-2 and plays a critical role in ENaC regulation by AMPK in kidney epithelial cells. J. Biol. Chem. 2018;293:11612–11624. doi: 10.1074/jbc.RA118.003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Huang D.Y., Gao H., Boini K.M., Osswald H., Nürnberg B., Lang F. In vivo stimulation of AMP-activated protein kinase enhanced tubuloglomerular feedback but reduced tubular sodium transport during high dietary NaCl intake. Pflugers Arch. 2010;460:187–196. doi: 10.1007/s00424-010-0803-7. [DOI] [PubMed] [Google Scholar]
- 186.Lazo-Fernández Y., Baile G., Meade P., Torcal P., Martínez L., Ibañez C., Bernal M.L., Viollet B., Giménez I. Kidney-specific genetic deletion of both AMPK α-subunits causes salt and water wasting. Am J. Physiol. Renal. Physiol. 2017;312:F352–F365. doi: 10.1152/ajprenal.00169.2016. [DOI] [PubMed] [Google Scholar]
- 187.Alzamora R., Gong F., Rondanino C., Lee J.K., Smolak C., Pastor-Soler N.M., Hallows K.R. AMP-activated protein kinase inhibits KCNQ1 channels through regulation of the ubiquitin ligase Nedd4-2 in renal epithelial cells. Am. J. Physiol. Renal. Physiol. 2010;299:F1308–F1319. doi: 10.1152/ajprenal.00423.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vallon V., Grahammer F., Richter K., Bleich M., Lang F., Barhanin J., Völkl H., Warth R. Role of KCNE1-dependent K+ fluxes in mouse proximal tubule. J. Am. Soc. Nephrol. 2001;12:2003–2011. doi: 10.1681/ASN.V12102003. [DOI] [PubMed] [Google Scholar]
- 189.Vallon V., Grahammer F., Volkl H., Sandu C.D., Richter K., Rexhepaj R., Gerlach U., Rong Q., Pfeifer K., Lang F. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc. Natl. Acad. Sci. USA. 2005;102:17864–17869. doi: 10.1073/pnas.0505860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Andersen M.N., Krzystanek K., Jespersen T., Olesen S.-P., Rasmussen H.B. AMP-activated protein kinase downregulates Kv7.1 cell surface expression. Traffic. 2012;13:143–156. doi: 10.1111/j.1600-0854.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 191.Alesutan I., Föller M., Sopjani M., Dërmaku-Sopjani M., Zelenak C., Fröhlich H., Velic A., Fraser S., Kemp B.E., Seebohm G., et al. Inhibition of the heterotetrameric K+ channel KCNQ1/KCNE1 by the AMP-activated protein kinase. Mol. Membr. Biol. 2011;28:79–89. doi: 10.3109/09687688.2010.520037. [DOI] [PubMed] [Google Scholar]
- 192.Al-Bataineh M.M., Gong F., Marciszyn A.L., Myerburg M.M., Pastor-Soler N.M. Regulation of proximal tubule vacuolar H+-ATPase by PKA and AMP-activated protein kinase. Am. J. Physiol. Renal. Physiol. 2014;306:F981–F995. doi: 10.1152/ajprenal.00362.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gong F., Alzamora R., Smolak C., Li H., Naveed S., Neumann D., Hallows K.R., Pastor-Soler N.M. Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am. J. Physiol. Renal. Physiol. 2010;298:F1162–F1169. doi: 10.1152/ajprenal.00645.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hallows K.R., Alzamora R., Li H., Gong F., Smolak C., Neumann D., Pastor-Soler N.M. AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am. J. Physiol. Cell Physiol. 2009;296:C672–C681. doi: 10.1152/ajpcell.00004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Alzamora R., Al-bataineh M.M., Liu W., Gong F., Li H., Thali R.F., Joho-Auchli Y., Brunisholz R.A., Satlin L.M., Neumann D., et al. AMP-activated protein kinase regulates the vacuolar H+-ATPase via direct phosphorylation of the A subunit (ATP6V1A) in the kidney. Am. J. Physiol. Renal. Physiol. 2013;305:F943–F956. doi: 10.1152/ajprenal.00303.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Alzamora R., Thali R.F., Gong F., Smolak C., Li H., Baty C.J., Bertrand C.A., Auchli Y., Brunisholz R.A., Neumann D., et al. PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the a subunit in kidney cells. J. Biol. Chem. 2010;285:24676–24685. doi: 10.1074/jbc.M110.106278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McGuire C.M., Forgac M. Glucose starvation increases V-ATPase assembly and activity in mammalian cells through AMP kinase and phosphatidylinositide 3-kinase/Akt signaling. J. Biol. Chem. 2018;293:9113–9123. doi: 10.1074/jbc.RA117.001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Collins M.P., Forgac M. Regulation of V-ATPase Assembly in Nutrient Sensing and Function of V-ATPases in Breast Cancer Metastasis. Front. Physiol. 2018;9:902. doi: 10.3389/fphys.2018.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sands J.M., Klein J.D. Physiological insights into novel therapies for nephrogenic diabetes insipidus. Am. J. Physiol. Renal. Physiol. 2016;311:F1149–F1152. doi: 10.1152/ajprenal.00418.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Denton J.S., Pao A.C., Maduke M. Novel diuretic targets. Am. J. Physiol. Renal. Physiol. 2013;305:F931–F942. doi: 10.1152/ajprenal.00230.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Bech A.P., Wetzels J.F.M., Nijenhuis T. Effects of sildenafil, metformin, and simvastatin on ADH-independent urine concentration in healthy volunteers. Physiol. Rep. 2018;6:e13665. doi: 10.14814/phy2.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Klein J.D., Wang Y., Blount M.A., Molina P.A., LaRocque L.M., Ruiz J.A., Sands J.M. Metformin, an AMPK activator, stimulates the phosphorylation of aquaporin 2 and urea transporter A1 in inner medullary collecting ducts. Am. J. Physiol. Renal. Physiol. 2016;310:F1008–F1012. doi: 10.1152/ajprenal.00102.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Al-bataineh M.M., Li H., Ohmi K., Gong F., Marciszyn A.L., Naveed S., Zhu X., Neumann D., Wu Q., Cheng L., et al. Activation of the metabolic sensor AMP-activated protein kinase inhibits aquaporin-2 function in kidney principal cells. Am. J. Physiol. Renal. Physiol. 2016;311:F890–F900. doi: 10.1152/ajprenal.00308.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Andersen M.N., Rasmussen H.B. AMPK: A regulator of ion channels. Commun. Integr. Biol. 2012;5:480–484. doi: 10.4161/cib.21200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nickolas T.L., Jamal S.A. Bone kidney interactions. Rev. Endocr. Metab. Disord. 2015;16:157–163. doi: 10.1007/s11154-015-9314-3. [DOI] [PubMed] [Google Scholar]
- 206.Graciolli F.G., Neves K.R., Barreto F., Barreto D.V., Dos Reis L.M., Canziani M.E., Sabbagh Y., Carvalho A.B., Jorgetti V., Elias R.M., et al. The complexity of chronic kidney disease-mineral and bone disorder across stages of chronic kidney disease. Kidney Int. 2017;91:1436–1446. doi: 10.1016/j.kint.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 207.Alesutan I., Munoz C., Sopjani M., Dërmaku-Sopjani M., Michael D., Fraser S., Kemp B.E., Seebohm G., Föller M., Lang F. Inhibition of Kir2.1 (KCNJ2) by the AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2011;408:505–510. doi: 10.1016/j.bbrc.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 208.Derst C., Karschin C., Wischmeyer E., Hirsch J.R., Preisig-Müller R., Rajan S., Engel H., Grzeschik K.-H., Daut J., Karschin A. Genetic and functional linkage of Kir5.1 and Kir2.1 channel subunits. FEBS Lett. 2001;491:305–311. doi: 10.1016/S0014-5793(01)02202-5. [DOI] [PubMed] [Google Scholar]
- 209.Mia S., Munoz C., Pakladok T., Siraskar G., Voelkl J., Alesutan I., Lang F. Downregulation of Kv1.5 K channels by the AMP-activated protein kinase. Cell. Physiol. Biochem. 2012;30:1039–1050. doi: 10.1159/000341480. [DOI] [PubMed] [Google Scholar]
- 210.Bielanska J., Hernandez-Losa J., Perez-Verdaguer M., Moline T., Somoza R., Cajal S., Condom E., Ferreres J., Felipe A. Voltage-Dependent Potassium Channels Kv1.3 and Kv1.5 in Human Cancer. Curr. Cancer Drug Targets. 2009;9:904–914. doi: 10.2174/156800909790192400. [DOI] [PubMed] [Google Scholar]
- 211.Almilaji A., Munoz C., Elvira B., Fajol A., Pakladok T., Honisch S., Shumilina E., Lang F., Föller M. AMP-activated protein kinase regulates hERG potassium channel. Pflugers Arch. 2013;465:1573–1582. doi: 10.1007/s00424-013-1299-8. [DOI] [PubMed] [Google Scholar]
- 212.Wadhwa S., Wadhwa P., Dinda A.K., Gupta N.P. Differential expression of potassium ion channels in human renal cell carcinoma. Int. Urol. Nephrol. 2009;41:251–257. doi: 10.1007/s11255-008-9459-z. [DOI] [PubMed] [Google Scholar]
- 213.Munoz C., Sopjani M., Dërmaku-Sopjani M., Almilaji A., Föller M., Lang F. Downregulation of the osmolyte transporters SMIT and BGT1 by AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2012;422:358–362. doi: 10.1016/j.bbrc.2012.04.092. [DOI] [PubMed] [Google Scholar]
- 214.Yamauchi A., Nakanishi T., Takamitsu Y., Sugita M., Imai E., Noguchi T., Fujiwara Y., Kamada T., Ueda N. In vivo osmoregulation of Na/myo-inositol cotransporter mRNA in rat kidney medulla. J. Am. Soc. Nephrol. 1994;5:62–67. doi: 10.1681/ASN.V5162. [DOI] [PubMed] [Google Scholar]
- 215.Rasola A., Galietta L.J., Barone V., Romeo G., Bagnasco S. Molecular cloning and functional characterization of a GABA/betaine transporter from human kidney. FEBS Lett. 1995;373:229–233. doi: 10.1016/0014-5793(95)01052-G. [DOI] [PubMed] [Google Scholar]
- 216.Zhou Y., Holmseth S., Hua R., Lehre A.C., Olofsson A.M., Poblete-Naredo I., Kempson S.A., Danbolt N.C. The betaine-GABA transporter (BGT1, slc6a12) is predominantly expressed in the liver and at lower levels in the kidneys and at the brain surface. Am. J. Physiol. Renal. Physiol. 2012;302:F316–F328. doi: 10.1152/ajprenal.00464.2011. [DOI] [PubMed] [Google Scholar]
- 217.Sopjani M., Alesutan I., Dërmaku-Sopjani M., Fraser S., Kemp B.E., Föller M., Lang F. Down-regulation of Na+-coupled glutamate transporter EAAT3 and EAAT4 by AMP-activated protein kinase. J. Neurochem. 2010;113:1426–1435. doi: 10.1111/j.1471-4159.2010.06678.x. [DOI] [PubMed] [Google Scholar]
- 218.Hu Q.X., Ottestad-Hansen S., Holmseth S., Hassel B., Danbolt N.C., Zhou Y. Expression of Glutamate Transporters in Mouse Liver, Kidney, and Intestine. J. Histochem. Cytochem. 2018;66:189–202. doi: 10.1369/0022155417749828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schmitt R., Ellison D.H., Farman N., Rossier B.C., Reilly R.F., Reeves W.B., Oberbäumer I., Tapp R., Bachmann S. Developmental expression of sodium entry pathways in rat nephron. Am. J. Physiol. Renal. Physiol. 1999;276:F367–F381. doi: 10.1152/ajprenal.1999.276.3.F367. [DOI] [PubMed] [Google Scholar]
- 220.Kréneisz O., Benoit J.P., Bayliss D.A., Mulkey D.K. AMP-activated protein kinase inhibits TREK channels. J. Physiol. 2009;587:5819–5830. doi: 10.1113/jphysiol.2009.180372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gu W., Schlichthörl G., Hirsch J.R., Engels H., Karschin C., Karschin A., Derst C., Steinlein O.K., Daut J. Expression pattern and functional characteristics of two novel splice variants of the two-pore-domain potassium channel TREK-2. J. Physiol. 2002;539:657–668. doi: 10.1113/jphysiol.2001.013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Föller M., Jaumann M., Dettling J., Saxena A., Pakladok T., Munoz C., Ruth P., Sopjani M., Seebohm G., Rüttiger L., et al. AMP-activated protein kinase in BK-channel regulation and protection against hearing loss following acoustic overstimulation. FASEB J. 2012;26:4243–4253. doi: 10.1096/fj.12-208132. [DOI] [PubMed] [Google Scholar]
- 223.Rabjerg M., Oliván-Viguera A., Hansen L.K., Jensen L., Sevelsted-Møller L., Walter S., Jensen B.L., Marcussen N., Köhler R. High expression of KCa3.1 in patients with clear cell renal carcinoma predicts high metastatic risk and poor survival. PLoS ONE. 2015;10:e0122992. doi: 10.1371/journal.pone.0122992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wyatt C.N., Mustard K.J., Pearson S.A., Dallas M.L., Atkinson L., Kumar P., Peers C., Hardie D.G., Evans A.M. AMP-activated protein kinase mediates carotid body excitation by hypoxia. J. Biol. Chem. 2007;282:8092–8098. doi: 10.1074/jbc.M608742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yoshida H., Bao L., Kefaloyianni E., Taskin E., Okorie U., Hong M., Dhar-Chowdhury P., Kaneko M., Coetzee W.A. AMP-activated protein kinase connects cellular energy metabolism to KATP channel function. J. Mol. Cell. Cardiol. 2012;52:410–418. doi: 10.1016/j.yjmcc.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lim A., Park S.-H., Sohn J.-W., Jeon J.-H., Park J.-H., Song D.-K., Lee S.-H., Ho W.-K. Glucose deprivation regulates KATP channel trafficking via AMP-activated protein kinase in pancreatic β-cells. Diabetes. 2009;58:2813–2819. doi: 10.2337/db09-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Chang T.-J., Chen W.-P., Yang C., Lu P.-H., Liang Y.-C., Su M.-J., Lee S.-C., Chuang L.-M. Serine-385 phosphorylation of inwardly rectifying K+ channel subunit (Kir6.2) by AMP-dependent protein kinase plays a key role in rosiglitazone-induced closure of the K(ATP) channel and insulin secretion in rats. Diabetologia. 2009;52:1112–1121. doi: 10.1007/s00125-009-1337-4. [DOI] [PubMed] [Google Scholar]
- 228.Tan X.-H., Zheng X.-M., Yu L.-X., He J., Zhu H.-M., Ge X.-P., Ren X.-L., Ye F.-Q., Bellusci S., Xiao J., et al. Fibroblast growth factor 2 protects against renal ischaemia/reperfusion injury by attenuating mitochondrial damage and proinflammatory signalling. J. Cell. Mol. Med. 2017;21:2909–2925. doi: 10.1111/jcmm.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Klein H., Garneau L., Trinh N.T.N., Privé A., Dionne F., Goupil E., Thuringer D., Parent L., Brochiero E., Sauvé R. Inhibition of the KCa3.1 channels by AMP-activated protein kinase in human airway epithelial cells. Am. J Physiol. Cell Physiol. 2009;296:C285–C295. doi: 10.1152/ajpcell.00418.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Huang C., Shen S., Ma Q., Chen J., Gill A., Pollock C.A., Chen X.-M. Blockade of KCa3.1 ameliorates renal fibrosis through the TGF-β1/Smad pathway in diabetic mice. Diabetes. 2013;62:2923–2934. doi: 10.2337/db13-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Takimoto M., Takeyama M., Hamada T. Possible involvement of AMPK in acute exercise-induced expression of monocarboxylate transporters MCT1 and MCT4 mRNA in fast-twitch skeletal muscle. Metab. Clin. Exp. 2013;62:1633–1640. doi: 10.1016/j.metabol.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 232.Becker H.M., Mohebbi N., Perna A., Ganapathy V., Capasso G., Wagner C.A. Localization of members of MCT monocarboxylate transporter family Slc16 in the kidney and regulation during metabolic acidosis. Am. J. Physiol. Renal. Physiol. 2010;299:F141–F154. doi: 10.1152/ajprenal.00488.2009. [DOI] [PubMed] [Google Scholar]
- 233.Fisel P., Kruck S., Winter S., Bedke J., Hennenlotter J., Nies A.T., Scharpf M., Fend F., Stenzl A., Schwab M., et al. DNA methylation of the SLC16A3 promoter regulates expression of the human lactate transporter MCT4 in renal cancer with consequences for clinical outcome. Clin. Cancer Res. 2013;19:5170–5181. doi: 10.1158/1078-0432.CCR-13-1180. [DOI] [PubMed] [Google Scholar]
- 234.Pieri M., Christian H.C., Wilkins R.J., Boyd C.A.R., Meredith D. The apical (hPepT1) and basolateral peptide transport systems of Caco-2 cells are regulated by AMP-activated protein kinase. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;299:G136–G143. doi: 10.1152/ajpgi.00014.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Shen H., Smith D.E., Yang T., Huang Y.G., Schnermann J.B., Brosius F.C. Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am. J. Physiol. Renal. Physiol. 1999;276:F658–F665. doi: 10.1152/ajprenal.1999.276.5.F658. [DOI] [PubMed] [Google Scholar]
- 236.Shen B., Zhu J., Zhang J., Jiang F., Wang Z., Zhang Y., Li J., Huang D., Ke D., Ma R., et al. Attenuated mesangial cell proliferation related to store-operated Ca2+ entry in aged rat: The role of STIM 1 and Orai 1. Age. 2013;35:2193–2202. doi: 10.1007/s11357-013-9511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]